Abstract
Aflatoxin B1 (AFB1) is the most toxic mycotoxin and is widespread in moldy feed. The use of biological removal methods to reduce AFB1 has become a research hotspot. This study aimed to isolate lactic acid bacteria (LAB) capable of removing AFB1 from moldy feeds and assessed the removal capacity under various environmental conditions. A strain named Lactobacillus brevis DN-1 was isolated from feed samples and showed 71.38% AFB1 percent removal. Furthermore, DN-1 showed good antifungal activity against Aspergillus flavus BNCC336156 and Aspergillus parasiticus BNCC335939. The optimum growth temperature and pH of DN-1 were 37 °C and 6.0, respectively, and DN-1 grew well in the concentration range of 0–20 µg/L AFB1. Under a temperature of 20–40 °C, pH of 3.0–9.0, and anaerobic conditions, the percent removal of AFB1 was more than 60%. An analysis of the different components of DN-1 showed that cell wall adsorption was the main removal method and suggested the pathway for AFB1 removal by LAB. In addition, strain DN-1 was used as a biological preservative in artificially contaminated peanut and sunflower cakes, which significantly inhibited the growth of mold and production of AFB1. In brief, this study highlights the potential use of DN-1 as a preventive agent against aflatoxicosis via strong removal capability in the application of fermented feed or food.
1. Introduction
Aflatoxins (AFs) are toxic secondary metabolites produced primarily by A. flavus and A. parasiticus, which are widely distributed, diverse, and environmentally resilient [1,2]. Feed ingredients, grains, and silage are susceptible to contamination with AFs [3]. AFs are categorized as B1, B2, G1, and G2 (toxicity grade: AFB1 > AFG1 > AFB2 > AFG2) [4]. Among them, AFB1 is the most toxic and has been classified as a class I carcinogen by the International Agency for Research on Cancer [5].
AFB1 is a toxic compound that has been extensively studied and reported in various animals, such as cattle, rats, and trout. It is known to be hepatotoxic, immunotoxic, carcinogenic, and DNA mutagenic [6]. When consumed through contaminated food and feeds, it can have detrimental effects on human and animal health. This is because its cytochrome P450 (CYP450) activates in the liver and reduces the expression of important enzymes like glutathione dismutase and superoxide dismutase [7,8]. AFB1-DNA adducts can cause mutations in codons, which may result in the loss of function of tumor suppressor genes and the development of liver cancer in both humans and animals [9]. Furthermore, AFB1 has also been found to have reproductive toxicity as it reduces sperm concentration in mice and affects fertility and hormone levels [10,11].
Peanut and sunflower cakes are by-products of the food and agricultural industries, and they contain many nutrients needed by animals and humans, such as protein and fatty acids [12]. It can not only be used as a good source of antioxidants, protein, and fiber but can also be used in the feed or food industry [13]. However, peanut and sunflower cakes can be easily infected with fungi and produce mycotoxins during storage.
In feed, microbial removal technology is considered to be a promising method. The addition of bacterial or fungal microorganisms can significantly reduce AFB1 concentrations. Fusarium sp. WCQ3361, isolated from moldy peanuts, successfully detoxified up to 95.38% of AFB1 [14]. Similarly, the recombinant protein lac2 derived from Pichia pastoris X33 achieved an impressive percent removal of 99.82% for AFB1 [15]. The furan ring of AFB1 can be targeted by aflatoxin-oxidase (AFO) from Armillariella tabescens, leading to the conversion of AFB1 to epoxide. In the hydrolysis process, the production of AFB1-8,9-dihydrodiol was the primary objective to accomplish removal [16].
Fermentation by LAB is a new strategy that has been employed to remove AFB1. Previous research has focused on evaluating the removal potential of Lactobacillus spp., Bifidobacterium spp., and Schizosaccharomyces spp. [17]. A strain of Lactobacillus plantarum was isolated by Zhu et al. [18] with 89.5% removal of AFB1 in a de Man, Rogosa, and Sharpe (MRS) medium. Recently, researchers have described the use of LAB to inhibit fungal growth and prolong the shelf life of feed or food [19,20,21]. However, the use of LAB for the anaerobic fermentation of high-moisture feed is underdeveloped due to low efficiency, speed, and scope of application. Therefore, promising LAB strain resources need to be further developed before utilization. Taking into account the growing interest in using naturally removed AFB1 compounds and the potential of LAB, the objective of this study is to screen LAB for removing AFB1 and evaluate their ability as biocontrol agents to reduce the mold growth and mycotoxin production of peanut and sunflower cakes.
2. Materials and Methods
2.1. Sample Collection and Chemical Reagents
The samples for screening LAB were collected from the vicinity of Tongliao City (122°24′ E, 43°62′ N). These samples mainly included moldy silage corn, corn stover, and corn kernel. Standard AFB1 was purchased from Yuanye Biotechnology Co., Ltd. (Shanghai, China) and dissolved in methanol (20 mg/mL) to form a stock solution for this study. Methanol and acetonitrile of chromatographic grade were purchased from ROE Sciences (Newark, NJ, USA). Standard A. flavus BNCC336156 and A. parasiticus BNCC335939 lyophilized powder were obtained from the BeNa Culture Collection (Xinyang, China). The media used were an MRS medium and potato dextrose agar (PDA), purchased from Golden Clone Biotechnology Co., Ltd. (Beijing, China). Ultrapure water was prepared using a Milli-Q system acquired from Millipore (Billerica, MA, USA). Before use, all media were autoclaved at 121 °C for 20 min.
2.2. Determination of the Potential LAB Isolates Capable of Removing AFB1
The assay was conducted following the method described by Kim et al. [22], with some modifications. Each sample (approximately 10 g) was suspended in 90 mL of sterile water for 20 min, and the entire procedure was carried out under anaerobic conditions. Next, 0.5 mL of the supernatant was diluted to 10−5 and inoculated into an MRS solid medium for incubation. Single colonies with different morphologies and sizes were selected, delineated, and purified three times using this method. Based on colony morphology, Gram staining, and physiological and biochemical reactions, the isolate was identified as LAB [23]. Subsequently, the bacterial concentration was estimated using the live cell counting method of Hazan et al. [24]. The bacterial cell concentration was adjusted to 1.0 × 106 CFU/mL, and a final concentration of 5 μg/L AFB1 standard solution was added. The mixture was then incubated at 37 °C for 24 h, while the MRS medium without inoculum (containing AFB1) served as the control. The supernatant was collected through centrifugation, and the AFB1 content was determined using high-performance liquid chromatography (HPLC, Agilent1200, Santa Clara, CA, USA).
2.3. Identifying the Selected Potential Strain
The potential strains screened were named DN-1. They were identified by examining the cell morphology, physiological and biochemical characteristics, and 16S rRNA using the universal primers 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5′-TACGGYTACCTTGTTACGACTT-3′) to amplify 16S rRNA through polymerase chain reaction (PCR, MG96+, Hangzhou, China). The PCR reaction system (50 μL) included 1 μL forward primer 27F, 1 μL reverse primer 1492R, 2 μL DNA template, 5 μL 10× buffer, 0.5 μL r Tap enzyme, and 40.5 μL ddH2O. The PCR reaction procedure involved pre-denaturation at 94 °C for 5 min, denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, extension at 72 °C for 80 s, and 34 cycles and extension at 72 °C for 10 min. The PCR amplified products were separated by electrophoresis on a 1.0% agarose gel at 80 V for 30 min and then analyzed using a gel-imaging system. The 16S rRNA sequence analysis was performed at Meiyou Anuo Biotechnology Co., Ltd. (Beijing, China). The sequence was then compared with other sequences in the GenBank database using the Basic Local Alignment Search Tool (BLAST) program (http://blast.ncbi.nlm.nih.gov) (accessed on 1 March 2024).
2.4. Inhibition of Fungi by DN-1
A. flavus BNCC336156 and A. parasiticus BNCC335939, which are known to produce AFB1, were chosen as the indicator fungi for this study. To explore the antifungal activity of DN-1, we used a modified version of the two-layer plate method described by Wang et al. [14]. In this method, a 10-mL MRS solid medium was poured into a sterile Petri dish to create the base plate. Once the medium solidified, the DN-1 strain was inoculated on the substrate in biparallel lines. The plate was then incubated at the optimum temperature (37 °C) for 24 h. For the top plate, we used 8 mL of PDA medium mixed with 0.2 mL of fungal spore suspension (containing 1 × 105 conidia/mL) and incubated it at 30 °C for 72 h. The plate without DN-1 strain was used as a negative control, and each treatment group had 3 replicates, giving a total of 9 plates.
2.5. Effect of Culture Conditions on Strain Growth and Removal of AFB1
The above strains were activated and inoculated in the MRS medium to investigate the effects of different incubation temperatures (20, 25, 30, 35, 37, and 40 °C), various initial pH values (3.0, 5.0, 6.0, 7.0, 9.0, and 11.0), different AFB1 concentrations (0, 2.5, 5, 10, and 20 µg/L), as well as aerobic and anaerobic influences on the growth of the strains and their ability to remove AFB1. All test materials were made in triplicate. The removal ability of AFB1 was determined by using the culture medium without inoculated strains as the control. The growth was quantified by measuring the OD600 value of the solution with a microplate reader (MultiskanTM FC, Thermo Fisher Scientific, Dreieich, Germany), and the removal ability was quantified by measuring the residual AFB1 concentration with HPLC.
2.6. Different Active Components of AFB1 Removal
The DN-1 strain with the highest removal ability was selected as the test organism, following the experimental method of Xiong et al. [25], with appropriate modifications. The culture solution, containing 1.0 × 1010 CFU/mL, was centrifuged to obtain the supernatant and bacterial precipitate. The supernatant was filtered using a 0.22-μm aqueous filter membrane (EY-H491, Beijing, China) and stored at −20 °C for reserve. The bacterial precipitate was rinsed with sterile phosphate buffer solution (PBS) to obtain the bacterial suspension. The bacterial suspension was then crushed using a hand-held ultrasonic cell crusher (HUP-100, Beijing, China) and subsequently centrifuged at 4 °C and 4000× g for 20 min. The resulting supernatant of the cell lysate was passed through a 0.22-μm aqueous filter membrane. The bacterial suspension was treated by autoclaving at 121 °C and 0.12 MPa for 20 min. The above isolates were incubated with AFB1 solution at a final concentration of 5 μg/L, along with an equal amount of sterile PBS solution as a blank control at a constant temperature of 37 °C for 24 h. At the end of the incubation period, the supernatant was collected by centrifugation and used for the assay. The remaining bacterial-AFB1 complex precipitate was also collected and treated with 5 mL of acetonitrile and methanol, respectively, with constant shaking for 1 h to elute the precipitated adsorbed AFB1. Finally, the supernatant was recovered for assay.
Cell wall separations were prepared following the method described by Lahtinen et al. [26], with some modifications. The culture medium, containing 1.0 × 1010 CFU/mL, was centrifuged and resuspended in a PBS solution (50 mM, pH 7.8) with 50 mg/mL lysozyme for 48 h. The resulting precipitates were then centrifuged at 4 °C and 4000× g for 35 min, followed by washing with a PBS solution. Next, the precipitate was suspended in 3 mL of tris-HCl buffer containing 10 mM EDTA and 1 mg of protease. After 12 h of incubation and centrifugation, the precipitate was again suspended in tris-HCl buffer containing 50 µg/L DNAse and RNAse. Following 2 h of incubation and centrifugation, it was washed with cold Milli-Q water and suspended in a 20 g/L sodium dodecyl sulfate (SDS) solution. The final suspension was incubated at 85 °C for 2.5 h. After centrifugation, the precipitate containing the cell walls was washed with Milli-Q water and stored at 4 °C.
Exopolysaccharides (EPSs) were isolated from cells using a modified method based on Hernandez et al. [27]. First, cultures containing 1.0 × 1010 CFU/mL of the strain were centrifuged and resuspended in 1 M NaCl. Next, ultrasonication was employed for 3 min to separate the EPSs bound to the cells. The supernatant was then treated with 2-fold cold ethanol and incubated at 4° C for 12 h to precipitate the EPSs. After centrifugation at 4 °C and 4000× g for 10 min, the isolated exopolysaccharides were stored at −20 °C.
2.7. Biopreservation of Peanut and Sunflower Cakes
To evaluate the antifungal and anti-AFB1 activities of DN-1, two feed samples were used: peanut and sunflower cakes from the Qinghua Oil Pressing Factory in Tongliao, Inner Mongolia. The fresh samples were baked in an oven at 65 °C for 48 h, and the initial moisture content (MC) was calculated using gravimetric methodology. The MC of both feed samples was adjusted to 65%. Each feed sample, weighing 400 g, was separately inoculated with a suspension containing 1 mL of A. flavus BNCC336156 and 1 mL of A. parasiticus BNCC335939 (containing 1 × 104 conidia/mL) to artificially contaminate them with molds. Next, the peanut cake samples (400 g) were divided into two groups: one group was sprayed with 5 mL of DN-1 solution (OD600 ≈ 0.8), while the control group was sprayed with 5 mL of sterile water. Similarly, the same treatment, including a control group, was applied to the sunflower cake. The samples were placed in 36 polyethylene bags (2 species cakes × 2 groups × 3 replications × 3 time points (5, 10, and 30 d)) for vacuum-sealed fermentation. Mold counts and AFB1 production were determined by taking samples on days 0, 5, 10, and 30.
2.8. Analytical Methods
Appropriate modifications were made according to the method described by Muaz et al. [28]. AFB1 analysis was conducted using HPLC. The sample was centrifuged at 3500× g for 5 min in a 50-mL centrifuge tube. Then, 1 mL of the supernatant was transferred into the centrifuge tube, diluted to 20 mL with water, and passed through an immunoaffinity column (IAC-006, Dalian, China). The elution was performed using methanol, and the sample was dried using nitrogen. The resulting sample was then adjusted to 1 mL with the initial mobile phase and passed through a 0.22-μm organic-phase filter membrane. The chromatographic column used was an Agilent C18 column (2.1 mm × 100 mm, 3 μm), with a mobile phase consisting of methanol and water in a ratio of 70:30 (v/v). The flow rate was set to 0.3 mL/min, and the injection volume was 5 µL. A fluorescence detector (G1321B, Agilent, Waldbronn, Germany) was used to detect AFB1. Fluorescence detection was performed at an excitation wavelength of 362 nm and an emission wavelength of 435 nm. The standard curve of AFB1 was prepared with concentrations of 0.5, 1, 2, 5, 10, and 20 μg/L. The AFB1 percent removal was calculated using the following formula: W1 (%) = 100 × (V1 − N1)/V1, where N1 represents the concentration of AFB1 added and inoculated with LAB and V1 is the concentration of the AFB1 control sample.
2.9. Statistical Analysis
The experimental data were collated in Excel 2019 and analyzed by one-way analysis of variance (ANOVA) with LSD multiple comparisons using SPSS 26.0 statistical software with a significant level of p < 0.05 and plotted using GraphPad Prism 8.0 statistical software (GraphPad Software Inc., La Jolla, CA, USA).
3. Results and Discussion
3.1. Isolation and Identification of AFB1 Removal by LAB
To evaluate the removal of AFB1 by LAB, the percent removal was determined by adding an AFB1 standard solution and comparing the results. A total of 56 LAB strains were isolated sequentially from the samples, of which eight strains showed the ability to remove AFB1. Among these strains, strain DN-1 exhibited the highest percent removal (71.38%), while the remaining strains had lower percent removal (Table 1). The HPLC chromatograms of DN-1 and control samples are shown in Figure 1F. DN-1 was selected for further study. In the MRS medium, strain DN-1 appeared as creamy white and slightly elevated colonies. After 48 h of incubation at 37 °C, the colonies were round and had a diameter of 0.6–0.8 mm (Figure 1A). The bacteria were Gram-staining-positive and had rod-shaped cells. An analysis of the 16S rRNA gene sequence and phylogenetic tree revealed that strain DN-1 shared 99% similarity with Lactobacillus brevis ATCC14869 (Figure 1E). Based on the physiological and biochemical response characteristics (Table S1), as well as the morphological and phylogenetic features, the strain was identified as Lactobacillus brevis (the GenBank accession number is PP033635). It was deposited in the China General Microbiological Culture Collection Center (CGMCC) on 20 June 2023, with the strain deposit number CGMCC No. 27664. The physiological and biochemical characteristics and 16S rRNA identification were conducted following the guidelines of Berge’s Manual of Systematic Bacteriology [29] and the International Journal of Systematic and Evolutionary Microbiology [30].

Table 1.
Percentages of AFB1 removal by different LAB strains.
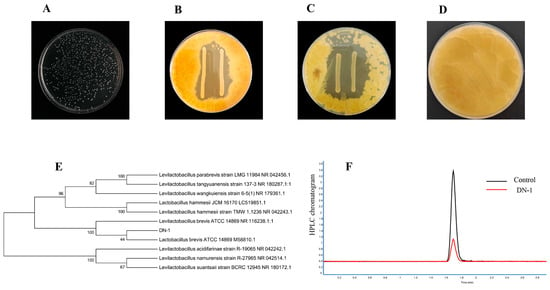
Figure 1.
Characterization of DN-1. (A) Colony morphology of DN-1 on MRS solid medium. (B) Antifungal properties of DN-1 against A. parasiticus BNCC335939. (C) Antifungal properties of DN-1 against A. flavus BNCC336156. (D) Negative control of antifungal properties experiment. (E) Phylogenetic tree of DN-1 based on 16S rRNA gene sequences. (F) HPLC chromatogram of DN-1 removal.
Multiple strains of the obligatory heterofermentative LAB L. brevis, which have been isolated from various sources such as food, plants, and the human microbiome, exhibit probiotic qualities [31,32]. In a study by Son et al. [33], L. brevis was isolated from kimchi and demonstrated pathogen antagonism and antidiabetic activity, making it an ideal candidate for a probiotic product. Another study by Yakabe et al. [34] revealed that heat-inactivated L. brevis can enhance the host cellular immune response by increasing the expression of IL-8, a proinflammatory chemokine. Additionally, L. brevis is commonly used as a silage additive due to its ability to produce organic acids [35].
Recently, various LAB have been identified as effective agents for removing AFB1 through adsorption or degradation. Ondiek et al. [36] successfully isolated Lactobacillus acidophilus AS1.3342 from dairy products, which exhibited 46% removal of AFB1. Another strain, Lactobacillus kefiri KFLM3 isolated from kefir, demonstrated the ability to remove 80% of AFB1 in milk [37]. The ameliorative effect of Lactobacillus salivarius against AFB1 was also studied in broilers [38], showing promising results. Furthermore, removal effects were observed in mice, beer, peanut oil, and cornmeal. Previous studies have established the safety profile of Lactobacillus spp. as a probiotic, suggesting that DN-1, the strain isolated in this study, can be added to feed to mitigate AFB1-induced toxicity.
3.2. Inhibitory Effect of DN-1 Strain on Toxin-Producing Fungi
Subsequent mold inhibition tests were conducted on the highly effective removal strain DN-1 mentioned earlier. The results showed that DN-1 exhibited good inhibition of both A. flavus BNCC336156 and A. parasiticus BNCC335939 and formed inhibitory zones in the vicinity of the fungi (Figure 1B,C). The inhibition zone of DN-1 around A. flavus BNCC336156 was greater than 3 mm, and the inhibition zone around A. parasiticus BNCC335939 was 1–2 mm. The difference in inhibition zones may be attributed to the strain type and culture conditions. Multi-locus variable number tandem repeat analysis (MLVA) has been used to analyze the genetic diversity of 13 strains of Lactobacillus plantarum with antifungal activity from different sources, and 10 different genotypes have been found, which shows that the strains are genetically heterogeneous [39]. LAB secrete compounds with antifungal properties such as organic acids, fatty acids, and cyclic dipeptides. These compounds form disulfide bonds on the fungal surface, disrupting the permeability of fungal cell membranes and causing leakage of proteins, reducing sugars, and DNA [40]. Guimaraes et al. [41] reported a 50% inhibition of Penicillium nosocomialis by LAB supernatant-containing organic acids.
3.3. Effect of Culture Conditions on the Growth of DN-1
To investigate the impact of culture conditions on the growth and viability of DN-1 strains, we monitored LAB growth under various temperatures, pH levels, and AFB1 concentration media. Figure 2A illustrates that DN-1 exhibited robust growth across a wide temperature range. The highest growth rate and OD600 value (1.65) were observed after 48 h of incubation at 37 °C. Comparatively, the growth rates were slower at 25 °C and 40 °C, with lower final bacterial densities. These findings indicate that the optimal incubation temperature for DN-1 is 37 °C.
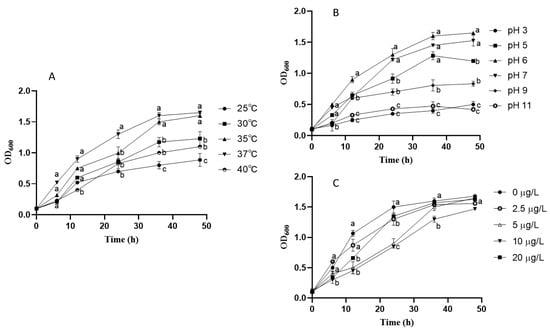
Figure 2.
Effect of temperature (A), pH (B), and different AFB1 concentrations (C) on DN-1 growth. OD600 is used to indicate DN-1 growth. Results are expressed as mean and standard deviation of three replications (n = 3), and different lowercase letters (a–c) for each time point indicate significant differences.
Strain DN-1 exhibited robust growth within the pH range of 5.0 to 9.0, as depicted in Figure 2B. Notably, pH 6.0 emerged as the optimal pH for DN-1 growth, yielding a maximum OD600 value of 1.63. In addition, DN-1 growth was impeded under strong acidic (pH 3.0) and alkaline (pH 11.0) conditions.
The addition of different concentrations of AFB1 to the medium, as shown in Figure 2C, resulted in a positive growth trend of the strain. After 36 h of incubation, the growth of all strains entered a stabilization period, with the OD600 values of all strains reaching 1.4 at 48 h. This suggests that DN-1 thrives within this concentration range.
3.4. Effects of Culture Conditions on AFB1 Removal
Environmental factors, including pH, temperature, and oxygen, on DN-1 removal of AFB1 are illustrated in Figure 3A. pH had an impact on the removal ability of DN-1. A pH range of 5 to 9 exhibited good AFB1 removal ability (>60%), with no significant differences in removal ability observed after 48 h of incubation at pH 6 and 7, which were 71.5% and 72.5%, respectively. However, the DN-1 strain showed a lower percent removal after 24 h of incubation at pH 3, with a significant increase in percent removal observed only after 48 h of incubation. The percent removal showed almost no removal ability at pH 11.
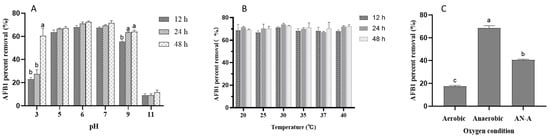
Figure 3.
Effect of pH (A), temperature (B), and oxygen (C) on the percent removal of AFB1. AN-A: 3 d of anaerobic culture after 5 d of aerobic culture. Results are expressed as the mean and standard deviation of three replications (n = 3), and different lowercase letters (a–b) in each pH treatment group indicate significant differences. Different lowercase letters (a–c) between oxygen-conditioned groups indicate significant differences.
The removal ability of DN-1 was monitored in the temperature range of 20–40 °C, as the temperature during feed fermentation can reach as high as 40 °C. The results indicated that there were no significant differences in percent removal after 24 h of incubation at all tested temperatures (Figure 3B). Furthermore, extending the incubation time to 48 h did not show any differences, suggesting that the removal ability of DN-1 is thermally stable.
The feed fermentation process consisted of two phases: aerobic (start of fermentation and opening phase) and anaerobic. We evaluated the effect of oxygen demand on percent removal. Incubating under aerobic conditions resulted in less than 20% removal of AFB1 (Figure 3C). However, this percent removal could be restored to its initial state under anaerobic conditions. After 5 days of incubation under aerobic conditions, the samples were transferred to anaerobic conditions for 3 days, resulting in a percent removal of 40%. This improvement may be attributed to the fact that DN-1 is an anaerobic bacterium. Although this strain may have a limited role under aerobic conditions, it can reach its full potential during feed fermentation.
3.5. Efficiency of Removal of AFB1 by Different Active Components
Previous studies have demonstrated that bacteria can remove AFB1 through two main mechanisms: biodegradation or cell wall adsorption. To determine whether our LAB strain removes AFB1 using one or both of these mechanisms, we conducted experiments using different fractions of DN-1 fermentation broth, along with an inactivation treatment and desorption experiments (Table 2).

Table 2.
The difference in AFB1 removal ability among diverse cell components.
The results indicated that the bacterial suspension had a significantly higher AFB1 percent removal (72.82%) compared to the no-cell supernatant (12.17%) and bacterial lysis (8.52%). The heat treatment of bacterial suspension resulted in a significant increase in percent removal to 77.28%, which indicated that removal activity still existed, and it had an even greater capacity of removing AFB1. This is consistent with the research results of Ondiek et al. [36]. On the contrary, Liew et al. [42] found that the removal efficiency of Lactobacillus casei Shirota cells after heat treatment was the lowest.
Furthermore, compared to the control, methanol and acetonitrile reagents were able to dissolve a small amount of AFB1, and the percent removal after dissolution was 25.96% and 20.28%. However, AFB1 bound to the cell wall could be released back into solution when using polar solvents. These findings are consistent with those of Peltonen et al. [43], who tested 20 LAB. Among them, L. amylovorus CSCC 5160 was found to be the most effective. The bacterial precipitate of this LAB was able to bind 59.7% of the AFB1 present in a solution with a concentration of 5 μg/mL within 24 h. However, it is important to note that this binding was reversible, as 48.6% of the AFB1 dissociated from the bacterium after being incubated in a toxin-free solution. Yasmeen et al. [38] found that L. salivarius removed up to 99.97% of AFB1, with the cell wall playing an important role, and application in broilers showed that the strain reduced the toxicity of AFB1 and that the intestinal environment did not invalidate the strain.
These findings indicate that the AFB1 removal component is located in the cell wall and not within the cell or outside of it. To confirm the presence of removal components in the cell wall, we investigated the percent removal of DN-1 cell walls and EPSs. The results revealed that EPSs exhibited a lower removal capacity (35.50%) compared to their cell wall isolates (70.59%). Similarly, Lahtinen et al. [26] isolated EPSs and cell walls containing peptidoglycan from Lactobacillus rhamnosus GG. The results showed that there was no evidence of EPSs, Ca2+, or Mg2+ being involved in AFB1 binding, and AFB1 binding appeared to be the cell wall isolate.
3.6. Possible Removal Pathways for AFB1
According to our results and previous studies [19,36,42], it is considered that the removal of AFB1 by DN-1 mainly occurs through cell wall adsorption. The mechanism by which LAB remove AFB1 is summarized in Figure 4. The typical cell wall of LAB consists of thick multilayered peptidoglycan balloons, surrounded by a cytoplasmic membrane decorated with polysaccharides, proteins, teichoic acids, and lipoteichoic acids [17]. Several studies have attempted to identify the specific binding sites of AFB1 on the LAB cell surface by subjecting the cells to various physical, chemical, and enzymatic treatments. These components contribute differently to the cells, resulting in varying removal capacities [44]. This could explain the relatively low removal efficiency of the EPS component observed in this study.
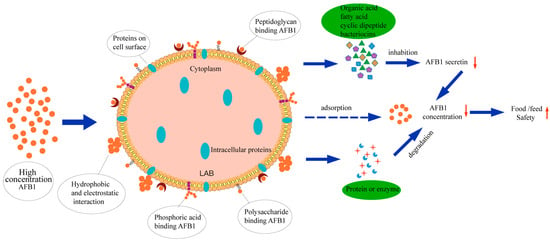
Figure 4.
The ways of removing AFB1 by LAB.
Heat treatment and acid treatment improve the ability of LAB to remove AFB1 [36]. The results of electron microscope observations show that heat treatment and acid treatment change the cell wall morphology and increase the adsorption area, which leads to the obvious enhancement of adsorption capacity [45]. Heat treatment may disrupt cell wall polysaccharides, leading to the formation of additional bonds with AFB1, and the affinity for AFB1 molecules will increase accordingly. Acid treatment causes protein denaturation, breaking it down into smaller peptides by disrupting peptide bonds. This process increases hydrophobicity and exposes more binding sites.
Bacteriocin plays an important role in the removal of AFB1. Sezer et al. [46] filtered the bacteriocin of L. plantarum and L. lactis through a 1000-Da dialysis membrane to obtain a crude protein extract, and the extract had a higher AFB1 removal ability (90%) than the two strains in mixed culture (81%). In this study, bacteriocin in a no-cell supernatant and bacterial lysis supernatant may also played a role.
Gomaa et al. [40] treated the screened L. brevis NM101-1 and L. paracasei ABRIINW.F58 with protease. The results indicated that the removal effect was lost after protease treatment. Furthermore, the study revealed a significant inhibition in the transcription level of the omt-A gene, which encodes a key enzyme involved in AFB1 biosynthesis. This suggests that proteins in LAB also play an important role in removing AFB1. In a word, the ways by which LAB remove AFB1 include antifungal activities, adsorption to AFB1 on the cell surface, and the decomposition and utilization of AFB1 by cells.
3.7. The Potential of DN-1 for Biopreservation
The antifungal activity of DN-1 was assessed on solid media, and its ability to preserve feed was also examined on peanut and sunflower cakes. The fermentation broth of DN-1 inhibited the growth of A. flavus BNCC336156 and A. parasiticus BNCC335939 in both peanut and sunflower cakes (Table 3) compared to the control. The inadequate amino acid composition of the peanut cakes makes them susceptible to A. flavus. In this study, DN-1 demonstrated a reduction in fungal growth, with a complete inhibition of AFB1 production at day 5 and a 96.4% reduction at day 10 (Figure 5A). Although DN-1 did not prevent AFB1 production, its prophylactic treatment effectively suppressed its production for at least 5 d.

Table 3.
Fungal growth of peanut and sunflower cakes contaminated with A. flavus BNCC336156 and A. parasiticus BNCC335939 monitored during storage.
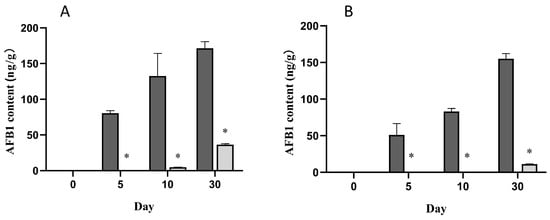
Figure 5.
Effect of DN-1 on AFB1 production: (A) AFB1 content (ng/g) of the peanut cakes contaminated with molds at different fermentation times (0, 5, 10, and 30 days); control samples are shown in dark gray, and treated samples are shown in light gray. (B) AFB1 content (ng/g) in sunflower cakes contaminated by molds at different fermentation times (0, 5, 10, and 30 days); control samples are shown in dark gray, and treated samples are shown in light gray. * Represents significant differences between treatments (p < 0.05).
In the case of sunflower cakes, DN-1 exhibited excellent antifungal activity. After 30 d, the mold count in the group treated with DN-1 spray was only 3.67 lg cfu/g FM (Table 3) compared to the control. Moreover, DN-1 completely inhibited AFB1 production by day 10 (Figure 5B). However, the efficacy of DN-1 in inhibiting AFB1 production decreased over time, to 92.7%, after 30 d of storage. This study suggests that the reduction in AFB1 production could be attributed to the ability of the DN-1 treatment to slow down fungal growth, which in turn required more time for secondary metabolism.
The antifungal effect in combination with the inhibition of mycotoxin production seems to be the main mechanism of action of LAB in feeds, according to our data. Various sources of LAB have demonstrated antifungal mycotoxin production inhibition and biopreservation potential [19]. Among the different Lactobacillus species, L. plantarum is widely recognized for its ability to inhibit the growth of a wide range of fungi and to inhibit mycotoxin production [47].
Some LAB can effectively reduce the concentration of ZEA in maize meal, maize porridge, and corn porridge by more than 50% after 4 days of incubation [20,48]. The DN-1 strain can inhibit the growth of spoilage bacteria in fermented peanut and sunflower cakes and prevent the feed from mildew. Consequently, the use of DN-1 in peanut and sunflower cakes can not only reduce the production of AFB1 and promote nutritive value but also be conducive to animal health.
4. Conclusions
In the past, LAB have shown the potential of biopreservation to avoid the production of molds and related mycotoxins in food and agricultural products. In this study, we obtained a strain of DN-1 that showed a high capacity for removing AFB1 and inhibiting A. flavus BNCC336156 and A. parasiticus BNCC335939. DN-1 exhibits robust pH and thermal stability such that it can adapt well to feed fermentation environments. The components of DN-1 cells (living cells, heat-treated cells, and cell wall fractions) have been shown to remove AFB1, and the binding of the cell wall is the main removal method. DN-1 treatment did not completely inhibit fungal growth and AFB1 production in peanut and sunflower cakes, but it significantly reduced both. Taking into account the limitations of this study, further research is needed to clarify the mechanism of removal of AFB1 by DN-1. In conclusion, L. brevis DN-1 could effectively inhibit fungi and reduce mycotoxins in the application of fermented feed or food.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/agriculture14050698/s1, Table S1: Physiological and biochemical test results of stain DN-1.
Author Contributions
Conceptualization, H.N. and X.W.; methodology, X.W.; software, X.W.; validation, X.W., J.X. and S.W.; formal analysis, X.W.; investigation, Z.H.; resources, Z.H.; writing—original draft preparation, X.W. and S.W.; writing—review and editing, X.W.; visualization, B.W.; supervision, H.N.; project administration, H.N.; funding acquisition, H.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the program for Young Talents of Science and Technology of the Universities of Inner Mongolia Autonomous Region (NJYT22054), Natural Science Foundation of Inner Mongolian Autonomous Region (2022MD03074), and Inner Mongolia Autonomous Region Science and Technology Research Projects (2020GG0108, 2021GG0035).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The datasets generated for this study are available upon request from the corresponding author.
Acknowledgments
We thank Kai Liu and his team for helping us determine aflatoxin B1.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Neme, K.; Mohammed, A. Mycotoxin occurrence in grains and the role of postharvest management as a mitigation strategies. A review. Food Control 2017, 78, 412–425. [Google Scholar] [CrossRef]
- Sadighara, P.; Ghanati, K. The aflatoxin B1 content of peanut-based foods in Iran: A systematic review. Rev. Environ. Health 2022, 37, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Driehuis, F.; Spanjer, M.C.; Scholten, J.M.; Te Giffel, M.C. Occurrence of mycotoxins in maize, grass and wheat silage for dairy cattle in the Netherlands. Food Addit. Contam Part B Surveill 2008, 1, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, A.; Mozaffari Nejad, A.S.; Mehri, F. Occurrence, dietary exposure, and risk assessment of aflatoxins in wheat flour from Iran. Int. J. Environ. Anal. Chem. 2021, 103, 9395–9408. [Google Scholar] [CrossRef]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens-the IARC Monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Yu, P.; Yang, K.; Cao, D. Aflatoxin B1: Metabolism, toxicology, and its involvement in oxidative stress and cancer development. Toxicol. Mech. Methods 2022, 32, 395–419. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Pulford, D.J.; Ellis, E.M.; McLeod, R.; James, R.F.; Seidegård, J.; Mosialou, E.; Jernström, B.; Neal, G.E. Regulation of rat glutathione S-transferase A5 by cancer chemopreventive agents: Mechanisms of inducible resistance to aflatoxin B1. Chem.-Biol. Interact. 1998, 111–112, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, Z.; Shi, Y.; Sun, H.; Yuan, B.; Cai, J.; Chen, J.; Long, M. Role of epigenetics in mycotoxin toxicity: A review. Environ. Toxicol. Pharmacol. 2023, 100, 104154. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Huang, K.; Zhang, B.; Zhu, L.; Xu, W. Aflatoxin B1-induced epigenetic alterations: An overview. Food Chem. Toxicol. 2017, 109, 683–689. [Google Scholar] [CrossRef]
- Fasihi-Ramandi, M.; Bayat, G.; Kachuei, R.; Golmohammadi, R. Effects of aflatoxin B1 exposure on sperm in rodents: A systematic review and meta-analysis. Int. J. Environ. Health Res. 2022, 33, 1629–1639. [Google Scholar] [CrossRef]
- González-Jartín, J.M.; Ferreiroa, V.; Rodríguez-Cañás, I.; Alfonso, A.; Sainz, M.J.; Aguín, O.; Vieytes, M.R.; Gomes, A.; Ramos, I.; Botana, L.M. Occurrence of mycotoxins and mycotoxigenic fungi in silage from the north of Portugal at feed-out. Int. J. Food Microbiol. 2022, 365, 109556. [Google Scholar] [CrossRef] [PubMed]
- Abedini, A.; Alizadeh, A.M.; Mahdavi, A.; Golzan, S.A.; Salimi, M.; Tajdar-Oranj, B.; Hosseini, H. Oilseed Cakes in the Food Industry; A Review on Applications, Challenges, and Future Perspectives. Curr. Nutr. Food Sci. 2022, 18, 345–362. [Google Scholar] [CrossRef]
- Gültekin Subaşı, B.; Vahapoğlu, B.; Capanoglu, E.; Mohammadifar, M.A. A review on protein extracts from sunflower cake: Techno-functional properties and promising modification methods. Crit. Rev. Food Sci. Nutr. 2022, 62, 6682–6697. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Wang, H.; Qiu, H.; Zhang, M.; Li, S.; Luo, X.; Song, Y.; Zhou, H.; Ma, W.; et al. Rapid biodegradation of aflatoxin B1 by metabolites of Fusarium sp. WCQ3361 with broad working temperature range and excellent thermostability. J. Sci. Food Agric. 2017, 97, 1342–1348. [Google Scholar] [PubMed]
- Song, Y.; Wang, Y.; Guo, Y.; Qiao, Y.; Ma, Q.; Ji, C.; Zhao, L. Degradation of zearalenone and aflatoxin B1 by Lac2 from Pleurotus pulmonarius in the presence of mediators. Toxicon 2021, 201, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Z.; Lu, F.P.; Jiang, H.L.; Tan, C.P.; Yao, D.S.; Xie, C.F.; Liu, D.L. The furofuran-ring selectivity, hydrogen peroxide-production and low Km value are the three elements for highly effective removal of aflatoxin oxidase. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2015, 76, 125–131. [Google Scholar] [CrossRef]
- Sadiq, F.A.; Yan, B.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Lactic Acid Bacteria as Antifungal and Anti-Mycotoxigenic Agents: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1403–1436. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, Y.; Yang, Q. Antifungal properties and AFB1 removal activity of a new strain of Lactobacillus plantarum. J. Hazard Mater. 2021, 414, 125569. [Google Scholar] [CrossRef]
- Ragoubi, C.; Quintieri, L.; Greco, D.; Mehrez, A.; Maatouk, I.; D’Ascanio, V.; Landoulsi, A.; Avantaggiato, G. Mycotoxin Removal by Lactobacillus spp. and Their Application in Animal Liquid Feed. Toxins 2021, 13, 185. [Google Scholar] [CrossRef]
- Chelule, P.K.; Mbongwa, H.P.; Carries, S.; Gqaleni, N. Lactic acid fermentation improves the quality of amahewu, a traditional South African maize-based porridge. Food Chem. 2010, 122, 656–661. [Google Scholar] [CrossRef]
- Nazareth, T.d.M.; Luz, C.; Torrijos, R.; Quiles, J.M.; Luciano, F.B.; Mañes, J.; Meca, G. Potential Application of Lactic Acid Bacteria to Reduce Aflatoxin B1 and Fumonisin B1 Occurrence on Corn Kernels and Corn Ears. Toxins 2019, 12, 21. [Google Scholar] [CrossRef]
- Kim, H.S.; Han, O.K.; Kim, S.C.; Kim, M.J.; Kwak, Y.S. Screening and investigation Lactobacillius spp. to improve Secale cereale silage quality. Anim. Sci. J. Nihon Chikusan Gakkaiho 2017, 88, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, Q. Isolation of Antibacterial, Nitrosylmyoglobin Forming Lactic Acid Bacteria and Their Potential Use in Meat Processing. Front. Microbiol. 2020, 11, 1315. [Google Scholar] [CrossRef]
- Hazan, R.; Que, Y.A.; Maura, D.; Rahme, L.G. A method for high throughput determination of viable bacteria cell counts in 96-well plates. BMC Microbiol. 2012, 12, 259. [Google Scholar] [CrossRef]
- Xiong, D.; Wen, J.; Lu, G.; Li, T.; Long, M. Isolation, Purification, and Characterization of a Laccase-Degrading Aflatoxin B1 from Bacillus amyloliquefaciens B10. Toxins 2022, 14, 250. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, S.J.; Haskard, C.A.; Ouwehand, A.C.; Salminen, S.J.; Ahokas, J.T. Binding of aflatoxin B1 to cell wall components of Lactobacillus rhamnosus strain GG. Food Addit. Contam. 2004, 21, 158–164. [Google Scholar] [CrossRef]
- Hernandez-Mendoza, A.; Guzman-de-Pena, D.; Garcia, H.S. Key role of teichoic acids on aflatoxin B binding by probiotic bacteria. J. Appl. Microbiol. 2009, 107, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Muaz, K.; Riaz, M.; Rosim, R.E.; Akhtar, S.; Corassin, C.H.; Gonçalves, B.L.; Oliveira, C.A.F. In vitro ability of nonviable cells of lactic acid bacteria strains in combination with sorbitan monostearate to bind to aflatoxin M1 in skimmed milk. LWT 2021, 147, 111666. [Google Scholar] [CrossRef]
- Michael, G.; Peter, K.; Hans, B.; Martha, E.; Trujillo, K.S.; Wolfgang, L.; William, B. Bergey’s Manual of Systematic Bacteriology; Williams & Wilkins: New York, NY, USA, 1984; pp. 7–10. [Google Scholar]
- Martha, E.T. International Journal of Systematic and Evolutionary Microbiology; Microbiology Society: London, UK, 2000; pp. 25–36. [Google Scholar]
- Abdelazez, A.; Abdelmotaal, H.; Evivie, S.E.; Melak, S.; Jia, F.F.; Khoso, M.H.; Zhu, Z.T.; Zhang, L.J.; Sami, R.; Meng, X.C. Screening Potential Probiotic Characteristics of Lactobacillus brevis Strains In Vitro and Intervention Effect on Type I Diabetes In Vivo. BioMed Res. Int. 2018, 2018, 7356173. [Google Scholar] [CrossRef]
- Alfano, A.; Perillo, F.; Fusco, A.; Savio, V.; Corsaro, M.M.; Donnarumma, G.; Schiraldi, C.; Cimini, D. Lactobacillus brevis CD2: Fermentation Strategies and Extracellular Metabolites Characterization. Probiotics Antimicrob. Proteins 2020, 12, 1542–1554. [Google Scholar] [CrossRef]
- Son, S.H.; Jeon, H.L.; Yang, S.J.; Lee, N.K.; Paik, H.D. In vitro characterization of Lactobacillus brevis KU15006, an isolate from kimchi, reveals anti-adhesion activity against foodborne pathogens and antidiabetic properties. Microb. Pathog. 2017, 112, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Yakabe, T.; Shimohata, T.; Takahashi, A. Lactobacillus brevis KB290 enhances IL-8 secretion by Vibrio parahaemolyticus-infected Caco-2 cells. J. Microbiol. Biotechnol. 2013, 23, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Soundharrajan, I.; Kim, D.; Kuppusamy, P.; Muthusamy, K.; Lee, H.J.; Choi, K.C. Probiotic and Triticale Silage Fermentation Potential of Pediococcus pentosaceus and Lactobacillus brevis and Their Impacts on Pathogenic Bacteria. Microorganisms 2019, 7, 318. [Google Scholar] [CrossRef] [PubMed]
- Ondiek, W.; Wang, Y.; Sun, L.; Zhou, L.; On, S.L.W.; Zheng, H.; Ravi, G. Removal of aflatoxin b1 and t-2 toxin by bacteria isolated from commercially available probiotic dairy foods. Food Sci. Technol. Int. 2021, 28, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Taheur, F.B.; Fedhila, K.; Chaieb, K.; Kouidhi, B.; Bakhrouf, A.; Abrunhosa, L. Adsorption of aflatoxin B1, zearalenone and ochratoxin A by microorganisms isolated from Kefir grains. Int. J. Food Microbiol. 2017, 251, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, R.; Zahid, B.; Alyas, S.; Akhtar, R.; Zahra, N.; Kouser, S.; Hashmi, A.S.; Athar, M.; Tayyab, M.; Anjum, A.A. Ameliorative effects of Lactobacillus against Aflatoxin B1. Braz. J. Biol. 2021, 84, e250517. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.-R.; Thuy Ho, V.T.; Lo, R.; Bansal, N.; Turner, M.S. A genetic diversity study of antifungal Lactobacillus plantarum isolates. Food Control 2017, 72, 83–89. [Google Scholar] [CrossRef]
- Gomaa, E.Z.; Abdelall, M.F.; El-Mahdy, O.M. Removal of Aflatoxin B1 by Antifungal Compounds from Lactobacillus brevis and Lactobacillus paracasei, Isolated from Dairy Products. Probiotics Antimicrob. Proteins 2018, 10, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.; Venancio, A.; Abrunhosa, L. Antifungal effect of organic acids from lactic acid bacteria on Penicillium nordicum. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 2018, 35, 1803–1818. [Google Scholar] [CrossRef]
- Liew, W.-P.-P.; Nurul-Adilah, Z.; Than, L.T.L.; Mohd-Redzwan, S. The Binding Efficiency and Interaction of Lactobacillus casei Shirota Toward Aflatoxin B1. Front. Microbiol. 2018, 9, 1503. [Google Scholar] [CrossRef]
- Peltonen, K.; el-Nezami, H.; Haskard, C.; Ahokas, J.; Salminen, S. Aflatoxin B1 binding by dairy strains of lactic acid bacteria and bifidobacteria. J. Dairy Sci. 2001, 84, 2152–2156. [Google Scholar] [CrossRef] [PubMed]
- Adácsi, C.; Kovács, S.; Pócsi, I.; Pusztahelyi, T. Elimination of Deoxynivalenol, Aflatoxin B1, and Zearalenone by Gram-Positive Microbes (Firmicutes). Toxins 2022, 14, 591. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Zheng, Y.; Liu, L.; Chen, S.; He, L.; Ao, X.; Yang, Y.; Liu, S. Decontamination of Aflatoxins by Lactic Acid Bacteria. Curr. Microbiol. 2020, 77, 3821–3830. [Google Scholar] [CrossRef] [PubMed]
- Sezer, Ç.; GÜVen, A.; BİLge Oral, N.; Vatansever, L. Removal of aflatoxin B1 by bacteriocins and bacteriocinogenic lactic acid bacteria. Turk. J. Vet. Anim. Sci. 2013, 37, 594–601. [Google Scholar] [CrossRef]
- Russo, P.; Arena, M.P.; Fiocco, D.; Capozzi, V.; Drider, D.; Spano, G. Lactobacillus plantarum with broad antifungal activity: A promising approach to increase safety and shelf-life of cereal-based products. Int. J. Food Microbiol. 2017, 247, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Mokoena, M.P.; Chelule, P.K.; Gqaleni, N. Reduction of fumonisin B1 and zearalenone by lactic acid bacteria in fermented maize meal. J. Food Prot. 2005, 68, 2095–2099. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).