Abstract
The purpose of our research is to evaluate the agroecological condition of soils under long-term irrigation (over 50 years) and to improve existing measures to slow down degradation processes by introducing phosphogypsum. The possibility of simultaneously addressing the ecological issue of using large amounts of phosphogypsum waste and the agronomic characteristics of slowing or eliminating salinisation processes in irrigated soils has been studied and justified. The research methodology was based on the comparison of different meliorative doses of phosphogypsum under the following conditions: by the amount of exchangeable sodium that should displace calcium in the calculated soil layer; by the coagulation limit; and by the absorption norm and the corresponding amount of sodium coming with irrigation water. To determine water-soluble salts (anions, cations) and pH level, a water extract was utilized. Multi-year studies to determine the impact of phosphogypsum on irrigation-salinised soils with and without irrigation showed positive changes in the anion–cation composition of water extraction, resulting in a reduction in the degree of the salinity of these soils. It was established that in chernozem soils under irrigation, the sodium adsorption ratio decreases by 74.5% compared to the control indicators, and without irrigation, by 23%. The best results in the displacement of exchangeable sodium were observed when phosphogypsum was applied at a dose calculated by the coagulation limit without irrigation and with irrigation—by the absorption norm.
1. Introduction
The constant population growth of the planet contributes to the increased anthropogenic pressure on natural resources. In the forecast perspective [1], by 2050, this will lead to an expansion of degraded lands and a 10% reduction in the productivity of agricultural crops. The annual loss of ecosystem services from soils will contribute to a 10–17% reduction in global gross domestic product. Global climate change and the associated risks to food security prompt the expansion of irrigated agriculture in the Middle East, Africa, Central Asia [2,3], and Europe [4]. Over the past 20 years, the global arable land area has increased by 16%. Increasing the proportion of irrigated agriculture allows for a 25–35% increase in the productivity of agricultural land on average.
On a planet-scale, 23% of arable land is salinised, and 37% undergo the salinisation process [5], with a total degraded land area of 11,737 million km2. Most of them are located in arid [6] or semi-arid climates [7]. Based on information from FAO [8], nearly 830 million hectares faced potential salinisation in 2015, experiencing elevated salt levels resulting from irrigation with water of poor quality containing high mineral contents (exceeding 1 g/L).
The presence of Haplic Chernozems in the southern part of Ukraine (the Steppe zone) and the construction of the Kakhovka Reservoir on the Dnieper River in the mid-20th century allowed for the development of irrigated agriculture. The maximum area of irrigated land was reached in 1990—2598 thousand hectares, or 6.2% of the total agricultural land. With the gain of independence in 1991, political transformation and economic restructuring in Ukraine led to the decline of the agricultural sector. Due to the lack of funding, proper maintenance, and the physical wear of reclamation infrastructure, in 2018, the actual irrigated area in Ukraine was approximately 460 thousand hectares [9], and due to wartime actions in 2022, it decreased to 300 thousand hectares and continues to decrease.
One of the key factors in the cessation of irrigation was the high cost and deterioration of water quality due to the excessive anthropogenic load and climate changes in the last 70 years [10,11,12]. According to Ukrainian standards [13], irrigation water is classified into three quality classes: I—suitable, II—limited suitability, and III—unsuitable. The water-quality class [10] is determined by the risk of irrigation salinisation, waterlogging, soil salinisation, and toxic impact on plants. According to this classification, the current structure of irrigated agricultural land with water of different qualities is 13.1% (class I), 84.2% (class II), and 2.7% (class III). This structure shows that the largest areas are irrigated with water of limited suitability for irrigation. It is worth noting that among these 388 thousand hectares (as of 2018), the main negative impacts of irrigation water on soils are as follows: 342 thousand hectares are at risk of waterlogging and 46 thousand hectares are at risk of toxic effects on plants and secondary salinisation. About 13 thousand hectares are irrigated with water of high mineralisation, which is not suitable for irrigation [14]. This requires the implementation of measures to prevent or slow the processes of salinisation and alkalinisation when irrigating with poor-quality water [15,16].
Despite numerous scientific studies in the field of the chemical reclamation of irrigated soils and water, this problem remains relevant. The extensive negative impact of irrigation in southern Ukraine on the soil’s agroameliorative condition is supported by numerous scientific studies [17,18]. This necessitates the adoption of novel strategic approaches to land management in these areas [19] and the restoration of fertility to chernozem soils [20].
Questions about the expediency and effectiveness of gypsum application to Haplic Chernozems with a low degree of salinisation remain unresolved.
In the conditions of irrigated agriculture in Ukraine, agroameliorative measures [10] with the use of chemical amelioration are widespread, involving the introduction of substances of natural or technogenic origin into the soil [21]. Gypsum is given preference in this regard [22,23,24]. Gypsum application has been established to increase the content of exchangeable calcium and significantly reduce the amount of sodium absorbed. However, even with high doses of gypsum, it is not possible to achieve the required level of saturation of the soil solution with calcium in the absence of irrigation [25,26]. By displacing sodium from the soil-absorptive complex with calcium or other divalent or trivalent cations, the mobility of soil colloids decreases, alkalinity decreases, and the availability of nitrogen, phosphorus, potassium, and calcium for plants increases, while microbiological processes are activated [27,28,29,30,31]. At the same time, the application of gypsum limits or weakens alkalinisation processes only, but does not eliminate them completely [32,33].
There are also debates about approaches to calculating the doses of ameliorants and the peculiarities of the interaction of gypsum with soil and water. Compliance with ecological aspects of ameliorant application is also relevant, which necessitates the search for new, more effective measures in terms of resource and energy conservation and environmental safety [32,34,35,36,37].
The increase in the area of degraded soils is exacerbated by the military aggression of the Russian Federation on the territory of Ukraine. The war has already led and continues to lead to catastrophic consequences for the environment, including water and soil pollution [38]. According to the authors of [39], the undermining of the Kakhovska Hydroelectric Station has resulted in the interruption of water supply for 31 irrigation systems in the Dnipropetrovsk (30%), Zaporizhzhia (74%), and Kherson (94%) regions. Currently, farms are forced to use mineralised soil and mine waters, leading to an annual increase in the risk of further salinisation [40].
The use of phosphogypsum can become one of the important directions for the reclamation and amelioration of soils contaminated due to direct and indirect military actions. The incorporation of phosphogypsum into the soil improves aeration, porosity, infiltration, oxygen supply, and the intake of silicon-containing substances with a strong potential for coagulation with organic soil compounds. The practice of using phosphogypsum as an ameliorant is gaining significance [41]. The formation of organo-mineral complexes with phosphogypsum in the soil occurs due to the binding of labile organic substances into stable aggregate formations with colloid micro-particles of phosphogypsum.
It is also possible to highlight the features of phosphogypsum that allow it to be used for the development of ecosystem services:
- -
- Phosphogypsum serves as a source of macro- and microelements for the development of various ecotrophic groups of microorganisms;
- -
- The acidic reaction of phosphogypsum creates favourable conditions for the breakdown of organic compounds, such as surfactants, hydrocarbons, and other substances, allowing it to be composted with waste containing such substances as sewage sludge, straw, manure, and bird droppings;
- -
- Composting various types of organic waste together with phosphogypsum, as well as its use together with digestate, significantly improves the sanitary-epidemiological situation and can find practical applications in environmental remediation.
Alongside its positive qualities, this ameliorant also possesses negative characteristics. Like any other ameliorant, it does not reduce the content of toxic salts in the root-containing layer of the soil, and therefore does not prevent the potential irrigation salinisation of the soil. It is not sufficiently soluble in water to completely eliminate the danger of irrigation salting in soils irrigated with third- and second-class waters at risk of salinisation. Even with the application of very high doses of this ameliorant, the residual salting of the soil to a weak and moderate extent is possible [42]. It is also necessary to take into account harmful impurities (heavy metals, radionuclides, etc.) that can be present in phosphogypsum as waste from the chemical industry should be considered, depending on the raw materials and technological processes of mineral-fertiliser production [42].
In places where phosphogypsum accumulates, heavy metals undergo horizontal and vertical redistribution in the soil profile due to leaching from dumps and precipitation, which can lead to their subsequent migration to aquifers [43,44]. Therefore, expanding the possibilities of using phosphogypsum in an environmentally friendly manner is an urgent necessity not only in Ukraine but also worldwide [45].
For the application of ameliorants to the soil, their doses are calculated individually for each specific case. The appropriateness of using a particular calculation method is determined by the properties and genesis of solonetzic soils. Three types of ameliorant doses are distinguished as ameliorative, ecological, and agronomic. The ameliorative doses are based on the difference between the total amount of exchangeable sodium (Na, meq per 100 g of soil) and its permissible content (K) from the total exchange capacity of the absorption layer (T, meq per 100 g of soil), determining the amount of exchangeable sodium that must be replaced with calcium in 1 g of soil [46]:
To remove excess exchangeable sodium from the soil’s calculation layer N with a volume mass of d, it is necessary to introduce calcium ameliorants [46]:
where 0.086 is the equivalent molar mass of gypsum, meq; H is the depth of the soil layer, cm; Na is the total content of exchangeable sodium, meq per 100 g of soil; T is the layer’s cation-exchange capacity, meq per 100 g of soil; K is the permissible content of exchangeable sodium in the soil, a fraction of T; and d is the volume mass of the soil layer, g/cm3.
Da = 0.086·(Na − K·T)·H·d,
For soils with a low sodium content, of less than 5%, the calculated dose of ameliorant is based on the coagulation limit of the colloidal fraction of the soil [46]:
where KCa is the amount of calcium in gypsum necessary for the coagulation of colloids in the soil, meq/100 g of soil.
Da = 0.086·KCa·H·d,
The purpose of our research is to assess the agroecological condition of soils that have been irrigated for a long time, as well as to improve existing measures to slow the degradation processes associated with the salinisation of irrigated soils by introducing phosphogypsum.
The practical experience of using phosphogypsum under the conditions of the Steppe zone of Ukraine (Haplic Chernozems) is represented by a small number of scientific publications [14,16,32]. Therefore, we hope that this study can be useful in expanding scientific knowledge and approaches in the fight for the ecological improvement of soil cover in similar climatic zones and conditions of irrigated agriculture.
2. Materials and Methods
2.1. Materials of Inverstigation
Long-term research on detecting changes in the salt composition of soil water extract, which exhibits signs of salinity, has been initiated in the village of Oleksandrivka in the Dniprovsky district of the Dnipropetrovsk region, Ukraine (48°31.656′ N, 35°13.431′ E—48°31.665′ N, 35°13.428′ E).
The obtained data from experimental research were processed to identify certain regularities in the nature of relationships between levels of factors and response functions. For this purpose, calculations were carried out based on the results of experimental studies, including mean values; variances; mean square deviations; absolute errors; relative errors; coefficients of variation; correlation coefficients; regression coefficients; confidence intervals for the mean value; and least significant difference (LSD). To perform these calculations, the software–information complex “Agrostat (v01)” [47] was used as an add-on to the Microsoft Office Excel 2003 program.
2.1.1. Soil Properties and Composition
Perennial studies were conducted on Haplic Chernozems (Loamic, Aric), which occupy 42.3% of arable land in the Dnipropetrovsk region. Our research has determined that the soil in the study area has low humus content, with 2.5% humus in the ploughed layer of soil and 0.3% at a depth of 10 m.
According to M.O. Kachinsky, the granulometric composition of the research soil was determined by the ratio of fractions of physical clay (all mechanical elements <0.01 mm) and physical sand (particles ranging in size from 1 mm to 0.01 mm) [48]. In the research soils, the physical sand content was 72.5%, and physical clay was 27.4%. A significant indicator of agricultural soil is its density. Density or volumetric weight of the soil was determined as the mass per unit volume of absolutely dry soil taken in its natural state (with undisturbed structure) in g/cm3 [49]. The highest compaction, reaching 2.04 g/cm3, was observed in the soil profile at a depth of 15–30 cm.
The research plot has been irrigated with mineralized water for over 50 years, which has negatively affected the soil’s salt composition. Based on the presence of toxic salts (0.48%), pH of 7.4–7.8, and SAR of 8.8, the soils are classified as moderately saline [50,51]. Examining a meter-deep soil profile, the highest salt concentration was observed in the top layer (0–30 cm), decreasing gradually below this depth. According to the respective ratios (Ca2+/Mg 2+, Na1+/Mg 2+, Na1+/Ca2+), the prevailing salinity type in the research areas is sulphate and sodium. The determination of cation-exchange capacity was based on the methods used in [52]. The exchangeable sodium content exceeds 3% of the soil-absorption complex capacity of 20.1–26.4 meq per 100 g of soil, indicating the development of salinisation processes in the upper soil layer (0–30 cm). The groundwater mineralization at the research site, at a depth of 5 m, is 15 g/L.
Therefore, the research plots are representative of typical soils in this region and the chernozem zone of irrigated agriculture in Ukraine. However, they show signs of physical and physicochemical salinity in the soil layer. Visually, when moist, the soil is highly plastic, sticky, and swells significantly. It easily forms aggregates due to the displacement of calcium by sodium in the soil-absorbing complex. Upon drying, the soil mass is compressed, resulting in low water permeability.
For irrigation of the research plots, water from the Samara River reservoir was utilized. Mineralization was determined as the sum of major cations (potassium + sodium, magnesium, and calcium) and anions (chlorides, sulphates, carbonates, and bicarbonates). The mineralization of irrigation water ranged from 2.3 to 3.1 g/L [12,53] and varied significantly throughout the irrigation period (see Table 1). According to agronomic criteria, the chemical composition of water remained chloride–sulphate and sodium–magnesium throughout the research period. An Irtec reel-type machine was employed for sprinkler irrigation, and the irrigation rate during the research years ranged from 1150 to 1700 m3/ha.

Table 1.
Results of the analysis of irrigation water from the reservoir on the Samara River for the study years.
2.1.2. Phosphogypsum Composition
Phosphogypsum was supplied from the dumps of the Dnipro Mineral Fertiliser Plant (Kamianske, Dnipropetrovsk region, Ukraine) [54], whose reserves reach almost 15 million tons. The characteristics of phosphogypsum are provided in Table 2, and they meet the criteria for agricultural use.

Table 2.
Characteristics of phosphogypsum.
The radiation background of phosphogypsum, soil, and the experimental site was determined using a radiometer that measures the flux of gamma radiation within the range of 0 to 10,000 s−1; and the power of exposure dose of gamma radiation within the range of 0 to 3000 microroentgens per hour (μR/h).
2.2. Methodology of Field Research
The research plan (Figure 1) envisions the application of phosphogypsum in the reserve for three years with (i index) and without irrigation at various calculated rates [46]:
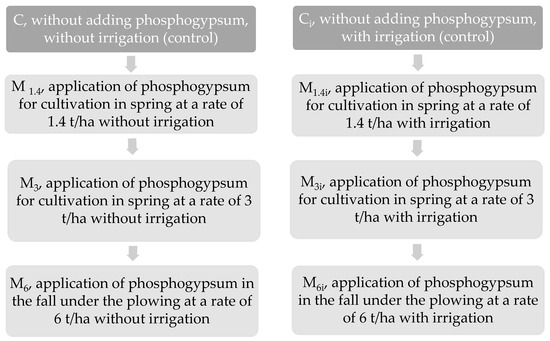
Figure 1.
Scheme of the field experiment.
- -
- 1.4 t/ha—ameliorative dose for displacing exchangeable sodium in saline-sodic soils with malonic reactions;
- -
- 3 t/ha—dose of soil calcium supplementation;
- -
- 6 t/ha—dose calculated by the coagulation-peptisation method.
Phosphogypsum at rates of 1.4 and 3 t/ha was applied during spring cultivation, while 6 t/ha was applied during autumn ploughing. The area of one experimental plot is 25.2 m2. The experiment was repeated four times using systematic plot placement.
The impact of chemical amelioration on soil quality was determined by changes in the soil’s water-extraction indicators based on anionic and cationic values. To determine water-soluble salts (anions, cations), and pH level, a water extract was utilized. This extract consisted of distilled water and air-dried soil with particle sizes ranging from 1 to 2 mm, with a soil-to-water ratio of 1:5. The soil was in an air-dry state. The ionic composition (anions, cations) of the water extract was determined using the titration technique. The type of salinity was established by corresponding ratios of major anions (Cl1−/SO42−, HCO31−/Cl1, HCO31−/SO42−) and cations (Ca2+/Mg2+, Na1+/Mg2+, Na1+/Ca2+) (Table 3 and Table 4).

Table 3.
Type of soil salinisation by anionic composition based on [55].

Table 4.
Type of soil salinisation by cation composition based on [55].
The degree of salinity was determined by calculations of the percentage content of toxic ions (SO4 tox., HCO3 tox., Ca tox.) considering active calcium (Ca1) and the sum of toxic salts (Stox. salts) at various ratios of Ca2+, HCO31− and SO42− in the water extract [55]:
- (1)
- For HCO31− less than Ca2+,
- Ca1 = Ca2+ − HCO31−
- SO4 tox. = SO42− − Ca1
- Stox. salts = (Na1+ + Mg 2++ Cl1− + SO4 tox.)
- (2)
- For HCO31− more than Ca2+,
- HCO3 tox. = HCO31− − Ca2+
- Stox. salts = (Na1+ + Mg 2++ Cl1−+ SO42− + HCO3 tox.)
- (3)
- For SO42− less than Ca2+,
- Ca1 = Ca2+− HCO31−
- Ca tox. = Ca1 − SO42−
- Stox. salts = (Ca tox. + Na1+ + Mg 2++ Cl1−).
The degree of soil salinity was determined taking into account the “cumulative effect” of toxic salts. Since salt mixtures are less toxic than their pure accumulations, the “cumulative effect” of mixtures is considered, which is less toxic than individual ions. The “cumulative effect” of toxic ions is expressed in equivalents of chlorine (eCl, meq per 100 g of soil) based on the following relation: 1Cl1− = 0.1CO32− = (2.5–3.0)HCO31− = (5.6–6.0)SO42−. Table 5 shows the toxicity of major salts (the most harmful ones are located below the line) and the sequence of their combinations (corresponding to the numbers in the middle of the table).

Table 5.
Scheme of ion binding in toxic salts based on [55].
Soils with a salt content of less than 0.3% are considered non-saline, and those with a weak salinity have a content of 0.3–1.5%.
Another indicator for determining soil salinity was the sodium adsorption ratio (SAR) according to the Gapon formula [56]
SAR = Na1+/[(Ca2+ + Mg2+)/2]1/2,
The dose of ameliorant by the coagulation–precipitation method was determined by the method used in [46]: a sample (100 g of dry soil) taken from the corresponding horizon was mixed with various amounts of phosphogypsum (20, 50, 100, 150, etc., mg) and then poured into cylinders, into which 100 mL of water was poured. After that, the mixture was carefully stirred and left for a day to sediment the soil colloids. The minimum weight of phosphogypsum at which complete the sedimentation of the suspension occurred is the required amount of calcium.
The particular distinction of our research lies in the use of technogenetic phosphogypsum as a waste product of the technological production of mineral fertilisers, more precisely, the product of phosphoric acid production. In connection with this, we established an environmentally safe dose of its application using the following formula:
where MPC is the maximum permissible concentration of chemical elements in the soil, mg/kg; C1 and C2—the content of the chemical element in the ameliorant and soil, respectively, mg/kg; H—the depth of the plough layer of soil, cm; —the content of gypsum (CaSO4 · 2H2O) in the ameliorant, %; and W—the moisture content of the ameliorant, %.
Calculating the dose of phosphogypsum by the specified formulas leads to pure gypsum, and in the presence of impurities, adjustments are made for ballast. The amount of gypsum to remove sodium from the soil sorption complex with weak soil salinity usually ranges from 2 to 4 tons per hectare.
3. Results
The dynamics of the content of toxic salts in the soil during years of research and the influence of phosphogypsum dose were described.
The results of our own research on the chemical composition of water extract in meq/100 g of soil for the years of observation are presented in Table 6. The type of salinity based on the anion composition is sulphate for all experimental variants and for all years of observation is presented. An exception is the variant Ci in the fifth year of the study with chloride–sulphate salinity. This is explained by an increase in the indicator of toxic ions equivalent to chlorine (2.08 meq) (Figure 2).

Table 6.
The chemical composition of the water extract of the soil during the years of research, meq/100 g of soil.
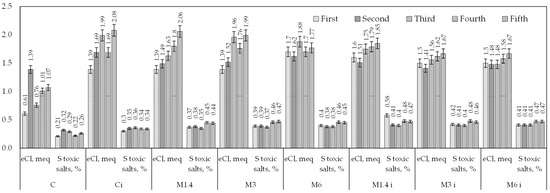
Figure 2.
The content of toxic salts in the soil (for five years of research).
The most dangerous compounds for the growth and development of plants are precisely those containing chlorine. It has been proven that an excessive amount of salts in the root zone of the soil leads to an increase in osmotic pressure, reducing the water absorption capacity of plants and the accumulation of specific toxic ions in them [57,58,59]. All chlorine salts are toxic to agricultural crops. The increase in chloride ions is explained by their influx with irrigation water. We observed a clear tendency for an increase in chlorine ions in irrigated experimental variants over the years of research. The control irrigated plots had 33% more chloride ions compared to non-irrigated variants. The application of phosphogypsum during irrigation reduced the concentration of Cl by 13–34% compared to the irrigated control variant.
The concentration of chlorine in the soil with the application of phosphogypsum without irrigation did not change significantly: for doses of 1.4 t/ha of phosphogypsum, the average indicators during the years of the study were at the level of 1.14 meq/100 g of soil; for doses of 3 and 6 t/ha, the concentration of chlorine decreased to 0.9 meq/100 g of soil.
The absence of ameliorants led to a decrease in the levels of SO42− ions in the control variants (C and Ci). This trend is explained by the leaching of sulphates with rainwater and irrigation water, corresponding to previous research [60]. In the non-irrigated variants (C), the amount of sulphates gradually decreased in the control area.
The research results (Table 2) on the anion composition showed an increase in SO42− ions in all variants and a 15% increase in irrigated variants compared to the control (C). Additionally, an increase in sulphate ions was observed when phosphogypsum was applied in irrigated variants (M1,4 i, M3 i, M6 i) with an average value of 3.09 meq/100 g of soil. Conversely, when ameliorants (M1,4, M3, M6) were applied without irrigation, a decrease in the content of this ion was noted. This is explained by a greater influx of sulphates into the soil along with phosphogypsum application and a slight influx along with irrigation water.
No clear pattern of changes in bicarbonates was established over the years of study. A reduction in the quantity of HCO31− was observed when irrigated with the application of phosphogypsum. On average, the decrease ranged from 0.04 to 0.09 meq/100 g of soil compared to control plots, where only irrigation without ameliorants was carried out.
On control plots without irrigation and without the application of an improvement (C), an increase in hydrocarbonates by 0.04 meq/100 g of soil was observed in the fourth year of the study, with a gradual decrease in the fifth year. This trend persisted in variants with phosphogypsum and without irrigation, as in other studies [61].
Various doses of phospho-gypsum had no significant effect on the concentration of hydrocarbons in the soil. An increase in HCO31− was observed with a dose of 3 t/ha in the irrigated variant, reaching 20% compared to the norm of 1.4 t/ha, while in the absence of irrigation, this difference increased to 5%. A significant increase was noted in the fifth year of the study, reaching 0.4 meq/100 g of soil in the non-irrigated variant (M6).
Regarding cation composition, the highest amount was attributed to sodium ions in all study variants, resulting in a sodium salinity type. The calcium–sodium salinity type was observed in the fifth year of the study in the control variant without irrigation (C). The amount of sodium increased significantly in the irrigated plots, indicating a substantial flow of this ion with irrigation water. When comparing control plots, the amount of Na1+ increased by 34% in the irrigated variant on average (Table 2).
The application of phosphogypsum, as in many previous studies [14,60], led to a reduction in the sodium-ion content [22]. In this case, salinisation processes caused by an excessive sodium content in the soil absorption complex are slowed down, resulting in improved physical properties of the soil. There is a reduction in the crust on the soil surface, an improvement in the action of soil capillaries, and an enhancement in the movement of air and water in the soil.
In variants with the application of phosphogypsum and irrigation, the sodium content decreased by 20–43%. The application of phosphogypsum in non-irrigated plots led to a reduction in sodium ions by 15–32% compared to the non-irrigated control.
The most significant change in the sodium ion content (a decrease of 2.0 meq/100 g of soil) was observed in the variant with a dose of 6 t/ha (M6 i) in the fifth year of the study. The difference in values compared to the control irrigated variant with a dose of 1.4 t/ha was 1.8 meq/100 g of soil. At doses of 3 t/ha and 6 t/ha, this difference was 2.0 and 2.6 meq/100 g of soil, respectively. Variants with a dose of 6 t/ha reacted best to irrigation, with a difference of 2.6 meq/100 g of soil compared to the control in reducing Na1+ ions.
According to FAO standards, the degree of soil salinisation depends on the sodium-adsorption ratio (SAR). In our case, SAR values were obtained based on cationic indicators over the 5 years of the study (Figure 3).
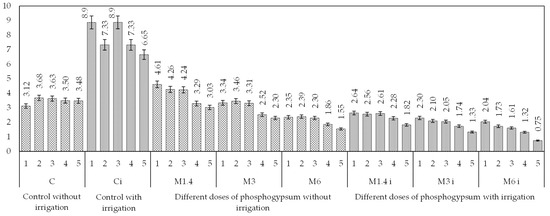
Figure 3.
Indicators of the sodium-adsorption ratio according to research options for five years of observation.
The sodium-adsorption ratio indicator (SAR) shows that the degree of soil salinity for all research variants is characterised as slightly saline, while for the second variant (C), salinity is moderate. The non-irrigated control variant almost did not change the SAR value during the observation period and averaged 3.54. The highest average value over the past three years, 7.62, was associated with the irrigated control variant (Ci).
The application of phosphogypsum led to a significant reduction in the sodium-adsorption ratio in the irrigated variants. The application of phosphogypsum at a dose of 1.4 t/ha resulted in a 68% reduction in the indicator compared to the control irrigated plot. At application doses of 3 and 6 t/ha, the SAR indicator decreased to 81%. In non-irrigated variants, there was also a tendency for a decrease in the sodium-adsorption ratio of values ranging from 3.52 to 1.9. The lowest indicators were observed in the experimental plots where phosphogypsum was applied at a dose of 6 t/ha [62].
Our research has shown that mineralised water (2.3–3.0 g/L) on ordinary chernozem soils exhibits signs of salinisation: an increase in the exchangeable sodium and magnesium content and a decrease in the percentage of exchangeable calcium. Although the chemical characteristics of the soil were not very high, significant signs of salinisation were visually observed: the soil was structureless, sticky and dense. This was observed in similar studies due to the accumulation of salts in the root zone of the soil, leading to the deterioration of physical–mechanical and hydro-physical properties [63].
In the control plots (C and Ci in Table 7), rapid salinisation processes were observed, especially in irrigated variants.

Table 7.
Change of exchangeable cations and pH under the influence of phosphogypsum in the arable layer of the soil (30 cm) over the years of research.
During 5 years of research on experimental plots (C), the exchangeable sodium content under irrigation conditions increased from 4.5% to 5.27% of the sum of exchangeable cations [52]. In the non-irrigated control (C), the average exchangeable sodium content was 4.28%, which was 15% lower than the corresponding value under irrigation. However, even in this case, secondary soil salinisation processes did occur. The calcium-to-magnesium exchange ratio in variant Si is 2.53, while in variant C, it is 2.76, indicating the displacement of exchangeable calcium by magnesium from the soil-exchange complex. The total exchangeable cations in the control variants remained significantly unchanged, ranging from 28.3 to 28.87 meq/100 g of soil.
When phosphogypsum was applied at different doses, a decrease in exchangeable sodium was observed compared to the control variant by 2.3% of the sum of exchangeable cations without irrigation and by 3.7% under irrigation. This factor indicates a more significant impact of phosphogypsum, especially under irrigation. Similar results were observed in studies on exchangeable aluminium [64].
A decrease in exchangeable sodium was observed with an increase in the application dose of phosphogypsum, confirming the results of previous research [65]. In variants without irrigation, the lowest exchangeable sodium values were recorded when phosphogypsum was applied in the fall, under ploughing, at a dose of 6 t/ha–1.25% in the fourth year and 1.01% of the sum of exchangeable cations in the fifth year. The exchangeable sodium values decreased from 4.48% to 1.01%, remaining within the range of weak salinisation.
Under irrigation, a decrease in exchangeable sodium was also observed with an increase in the application dose of phosphogypsum. In this case, the best option was the application of phosphogypsum under spring cultivation at a dose of 3 t/ha. The amount of exchangeable sodium in the soil-exchange complex decreased to 0.98% of the sum of exchangeable cations in the fourth year and to 0.89% in the fifth year of research. This indicates a slowdown in soil salinisation processes.
4. Discussion
The global experience in combating soil salinity and alkalinisation indicates a significant number of methods [66,67,68,69]: the prevention of water losses for filtration from irrigation canals; using modern means to prepare irrigation water; implementing operational measures in accordance with water norms and methods of irrigation; adhering to scientifically justified crop rotations with salt-tolerant crops; and applying chemical and biochemical improvements, etc. The most effective but costly and energy-intensive method is flushing the saline layer of soil with fresh water [63].
Long-term studies [70,71] have shown that the application of phosphogypsum on irrigated saline chernozem soils has a positive effect on the anion–cation composition of water leaching and the degree of salinity of these soils [72,73]. In experimental plots, with the application of phosphogypsum during irrigation and without it, a sulphate type of salinity was observed in terms of the anion composition. For the cation composition, a sodium type of salinity was observed in all experimental variants.
Our research has shown that the salinity level in the experimental plots was initially characterised as moderately saline based on the sum of toxic ions and gradually shifted to a slightly saline type with the application of phosphogypsum at doses of 3 and 6 tons per hectare under irrigation. The sodium-adsorption ratio (SAR) decreased by 23% on non-irrigated plots with the addition of phosphogypsum, compared to the control. When phosphogypsum was applied with irrigation, the SAR indicator decreased by 74.5% compared to the control values. This confirms the theory of the increased ameliorative effect of phosphogypsum under irrigation conditions.
Improvements in SAR indicators were also observed with the application of calcium-containing ameliorants by other researchers [71]. Based on SAR indicators, the level of soil salinity in the experimental plots was characterised as slightly saline in the variants with phosphogypsum application and moderately saline in the control variants under irrigation. According to SAR values (the ratio of sodium cations to calcium–magnesium), the best results were obtained with the application of 6 tons per hectare of phosphogypsum under irrigation.
Under irrigation, more displacement of exchangeable sodium occurred with the application of phosphogypsum at the standard dose (3 tons per hectare). Without irrigation, a better substitution of exchangeable sodium with calcium was observed in variants with the application of phosphogypsum at a dose calculated beyond the coagulation limit (6 tons per hectare). This aligns with previous studies [22,46,74].
The implementation of chemical amelioration using phosphogypsum to prevent the salinisation of ordinary chernozem has led to improvements in the indicators of the soil-absorbing complex. It should be noted that this is an important factor in assessing the environmental safety of the impact of phosphogypsum on the development of plant communities, aligning with other studies [75]. In research by the authors of [76], one of the largest alluvial saline areas (Spain) was reclaimed by the application of phosphogypsum to reduce Na1+ saturation. The discontinuation of the application of phosphogypsum was justified because it did not support the vitality of the microbes. Stepwise discriminant analysis identified two physiologically distinct types of soil microflora: one less active, present in untreated soil, and the other more active, present in ameliorated soils [77].
In some studies [65], elevated radiation levels were observed in both the ameliorant (phosphogypsum) and the soil, whereas our studies showed optimal and even reduced radiation levels for the investigated phosphogypsum. The radiation background of the experimental site was 10.5 μR/h, the soil at the research site was 11.0–12.0 μR/h, and the phosphogypsum itself was 12.5 μR/h.
Summarising a series of ecological regularities of the impact of phosphogypsum on the soil complex, confirmed by previous studies in Ukraine [78,79], and consistent with results obtained by other researchers in this field [80,81,82], we can use the phosphogypsum to improve ecosystem services.
In this way, taking into account the volumes of already formed phosphogypsum and that which is being formed [83,84], the strategic direction is not only its removal, transportation, and storage in landfills and sludge repositories but also its effective utilization as a secondary raw material in an environmentally friendly manner. To reduce the environmental threat from phosphogypsum landfills, the development of a comprehensive approach is important, including, on the one hand, measures aimed at preventing soil and water pollution from existing phosphogypsum dumps and, on the other hand, the implementation of processing and utilisation technologies that minimise the entry of harmful components of phosphogypsum into the ecosystem. Accordingly, the integration of existing technological solutions have already been developed [85], and their improvement with a special focus on prospective directions for the application of phosphogypsum in soil remediation as a means of slowing down their degradation and post-war fertility restoration is necessary. In the future, further research in this direction is planned.
5. Conclusions
In the Ukrainian Steppe environment, significant improvements in sodium-adsorption ratio and exchangeable cations were observed by applying phosphogypsum at a rate of 6 t/ha during autumn mouldboard ploughing under non-irrigated conditions. Conversely, under irrigation, the application of phosphogypsum was found to be more effective during spring cultivation at a rate of 3 t/ha.
The tendency to increase the content of chloride ions on irrigated variants of the experiment was revealed. The application of phosphogypsum during irrigation was determined to reduce Cl1− concentration by 13–34% compared to the irrigated control.
When phosphogypsum was applied with irrigation, SAR decreased by 74.5% compared to the control. This confirms the theory of strengthening the ameliorative effect of phosphogypsum under irrigation conditions.
Further research is required to optimize phosphogypsum dosage for application to different soil types.
6. Patents
Onoprienko D. M., Makarova T. K., Pugach A. M. Method of melioration of irrigated solonetzic chernozems. Utility model patent of Ukraine. UA117577. 2017. 01A01B 79/00.
Author Contributions
Conceptualization, D.O. and T.M.; methodology, D.O. and T.M.; software, H.H.; validation, D.O., H.R. and Y.C.; formal analysis, H.H.; investigation, T.M.; resources, H.R.; data curation, D.O.; writing—original draft preparation, D.O., T.M., H.H., H.R. and Y.C.; writing—review and editing, D.O. and H.R.; visualization, T.M. and H.H.; supervision, D.O. and H.R.; project administration, D.O. and H.R.; funding acquisition, H.R. All authors have read and agreed to the published version of the manuscript.
Funding
The grant for the funding of scientific research of the Ministry of Foreign Affairs of the Czech Republic on the topic “Ensuring food security in the face of climate change on the principles of sustainable agricultural development and preventing soil degradation processes in irrigated lands through the application of chemical ameliorants” was awarded via AgriSciences Platform. Finally, by the Technology Agency of the Czech Republic (TA CR) [Grant Number: TH79020003], support for this project was offered under the coordination of the ERA-MIN3 action, which has received funding from the European Union under the Horizon 2020 Program [European Commission Grant Agreement No. 101003575].
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on request.
Acknowledgments
We are grateful for the funding of the scientific work by the Ministry of Foreign Affairs of the Czech Republic through Czech Development Cooperation via AgriSciences Platform. Yelizaveta Chernysh acknowledges the funding support through the MSCA4Ukraine project, which is funded by the European Union.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cherlet, M.; Hutchinson, C.; Reynolds, J.; Hill, J.; Sommer, S.; Von Maltitz, G. World Atlas of Desertification; Publications Office of the European Union: Luxembourg, 2018; ISBN 978-92-79-75349-7. [Google Scholar] [CrossRef]
- Zak, N.A.; Kløve, B.; Haghigh, A.T. Expanding the Irrigated Areas in the MENA and Central Asia: Challenges or Opportunities. Water 2022, 14, 2560. [Google Scholar] [CrossRef]
- Syed, A.; Sarwar, G.; Shah, S.H.; Muhammad, S. Soil Salinity Research in 21st Century in Pakistan: Its Impact on Availability of Plant Nutrients, Growth and Yield of Crops. Commun. Soil Sci. Plant Anal. 2020, 52, 183–200. [Google Scholar] [CrossRef]
- Zajac, Z.; Gomez, O.; Gelati, E.; van der Velde, M.; Bassu, S.; Ceglar, A.; Chukaliev, O.; Panarello, L.; Koeble, R.; van den Berg, M.; et al. Estimation of spatial distribution of irrigated crop areas in Europe for large-scale modelling applications. Agric. Water Manag. 2022, 266, 107527. [Google Scholar] [CrossRef]
- Jiang, Q.; Peng, J.; Biswas, A.; Hu, J.; Zhao, R.; He, K.; Shi, Z. Characterising dryland salinity in three dimensions. Sci. Total Environ. 2019, 682, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Roldán-Cañas, J.; Moreno-Pérez, M.F. Water and irrigation management in arid and semiarid zones. Water 2021, 13, 2446. [Google Scholar] [CrossRef]
- Hassani, A.; Azapagica, A.; Shokri, N. Predicting long-term dynamics of soil salinity and sodicity on a global scale. Proc. Natl. Acad. Sci. USA 2020, 117, 33017–33027. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Sonmez, O.; Saud, S.; Wang, D.; Wu, C.; Adnan, M.; Turan, V. (Eds.) Sustainable Soil and Land Management and Climate Change, 1st ed.; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- State Agency of Water Resources of Ukraine. Kyiv. Available online: https://davr.gov.ua/ (accessed on 30 October 2023).
- DSTU 2730:2015; Quality of Natural Water for Irrigation. Agronomic Criteria. Derzhstandart Ukrayiny: Kyiv, Ukraine, 2015; p. 13. (In Ukrainian)
- Balyuk, S.A.; Romaschenko, M.I.; Truskavetskyi, R.S.; Grin, D.S. Soil Reclamation (Systematics, Perspectives, Innovations): Collective Monograph; Government of Ukraine: Kherson, Ukraine, 2015; pp. 363–373.
- Hapich, H.; Andrieiev, V.; Kovalenko, V.; Makarova, T. The analysis of spatial distribution of artificial reservoirs as anthropogenic fragmentation elements of rivers in the Dnipropetrovsk Region, Ukraine. J. Water Land Dev. 2022, 53, 80–85. [Google Scholar] [CrossRef]
- Onopriienko, D.M.; Makarova, T.K.; Hapich, H.V. Assessment of the hydrogeological and ameliorative state of the Kilchen irrigation system territory. IOP Conf. Ser. Earth Environ. Sci. 2023, 1254, 012087. [Google Scholar] [CrossRef]
- Onopriienko, D.M.; Makarova, T.K.; Tkachuk, A.V.; Hapich, H.V.; Roubik, H. The influence of phosphogypsumon the salt composition of salinated soil. Land Reclam. Water Manag. 2023, 301–350. [Google Scholar] [CrossRef]
- Yin, X.; Feng, Q.; Li, Y.; Liu, W.; Zhu, M.; Xu, G.; Zheng, X.; Sindikubwabo, C. Induced soil degradation risks and plant responses by salinity and sodicity in intensive irrigated agro-ecosystems of seasonally-frozen arid regions. J. Hydrol. 2021, 603, 127036. [Google Scholar] [CrossRef]
- Chushkina, I.; Hapich, H.; Matukhno, O.; Pavlychenko, A.; Kovalenko, V.; Sherstiuk, Y. Loss of small rivers across the steppe: Climate change or the hand of man? case study of the Chaplynka River. Int. J. Environ. Stud. 2024, 1–15. [Google Scholar] [CrossRef]
- Eltazarov, S. Soil Salinity Assessment in Syrdarya Province, Uzbekistan; Wageningen University and Research Centre: Wageningen, The Netherlands, 2016; 75p. [Google Scholar] [CrossRef]
- Chornyy, S.G.; Isaeva, V.V. Salinisation of chernozem soils by brackish irrigation water in southern Ukraine. Int. J. Environ. Stud. 2023, 80, 421–432. [Google Scholar] [CrossRef]
- Shevchenko, S.; Derevenets-Shevchenko, K.; Desyatnyk, L.; Shevchenko, M.; Sologub, I.; Shevchenko, O. Tillage effects on soil physical properties and maize phenology. Int. J. Environ. Stud. 2024, 1–10. [Google Scholar] [CrossRef]
- Baliuk, S.; Vorotyntseva, L.; Zakharova, M.; Panarin, R.; Kuts, O.; Mykhailyn, V. Changes in the properties of Chernozem soils under management and strategic approaches to restore their fertility. Int. J. Environ. Stud. 2023, 1–8. [Google Scholar] [CrossRef]
- Nosonenko, O.; Zakharova, M.; Vorotynseva, L.; Afanasiev, Y. Effect of differential of doses of chemical improver on the indicators of halogenesis of dark-chestnut alkaline soil. Bull. Agric. Sci. 2022, 110, 12–19. [Google Scholar] [CrossRef]
- Makarova, T.; Domaratskiy Ye Hapich, G.; Kozlova, O. Agromeliorative efficiency of phosphogypsum application on irrigation saline soils in the Northern Steppe of Ukraine. Indian J. Ecol. 2021, 48, 789–795. [Google Scholar]
- Brillante, L.; Singh, K. Use of Gypsum to Reclaim Salt Problems in Soils. Progressive Crop Consultant. 2022. Available online: https://surl.li/mylmx (accessed on 1 November 2023).
- Bello, S.K.; Alayafi, A.H.; Al-Solaimani, S.G.; Abo-Elyousr, K.A. Mitigating Soil Salinity Stress with Gypsum and Bio-Organic Amendments: A Review. Agronomy 2021, 11, 1735. [Google Scholar] [CrossRef]
- Seo, B.S.; Baek, N.; Park, S.W.; Shin, E.S.; Oh, Y.Y.; Kang, B.H.; Park, H.J.; Choi, W.J. Spatial Variations in Salinity and Sodicity of Reclaimed Tideland Paddy Soils in Coastal Areas of Southwestern Korea. Korean J. Soil 2023, 56, 217–225. [Google Scholar] [CrossRef]
- Baliuk, S.A.; Kucher, A.V.; Maksymenko, N.V. Soil resources of Ukraine: State, problems and strategy of sustainable management. Ukr. Geogr. J. 2021, 2, 3–11. [Google Scholar] [CrossRef]
- Dustnazarova, S.; Khasanov, A.; Khafizova, Z.; Davronov, K. The threat of saline lands, for example, in the Republic of Uzbekistan. E3S Web Conf. 2021, 284, 02002. [Google Scholar] [CrossRef]
- Hopmans, J.W.; Qureshi, A.S.; Kisekka, I.; Munns, R.; Grattan, S.R.; Rengasamy, P.; Ben-Gal, A.; Assouline, S.; Javaux, M.; Minhas, P.S.; et al. Taleisnik Chapter One—Critical knowledge gaps and research priorities in global soil salinity. Adv. Agron. 2021, 169, 1–191. [Google Scholar] [CrossRef]
- Kharytonov, M.; Martynova, N.; Babenko, M.; Rula, I.; Ungureanu, N.; Ștefan, V. Production of sweet sorghum bio-feedstock on technosol using municipal sewage sludge treated with flocculant, in Ukraine. Agriculture 2023, 13, 1129. [Google Scholar] [CrossRef]
- Arienzo, M.; Christen, E.; Jayawardane, N.S.; Quayle, W.C. The relative effects of sodium and potassium on soil hydraulic conductivity and implications for winery wastewater management. Geoderma 2012, 173–174, 303–310. [Google Scholar] [CrossRef]
- Degirmenci, N.; Okucu, A.; Turabi, A. Application of phosphogypsum in soil stabilization. Build. Environ. 2007, 42, 3393–3398. [Google Scholar] [CrossRef]
- Truskavetskyi, R.S.; Baliuk, S.A. Resource-Saving Technologies of Chemical Land Reclamation in the Conditions of Land Reform; Derzhavnyi Instytut Upravlinnya ta Ekonomiky Vodnykh Resursiv: Kyiv, Ukraine, 2000; 70p. [Google Scholar]
- Lyubimova, I.N.; Salpagarova, I.A. Possibility and Feasibility of Returning the Formerly Reclaimed Solonetz Lands to Agricultural Use: A Review. Eurasian Soil Sci. 2020, 53, 1270–1279. [Google Scholar] [CrossRef]
- El Zrelli, R.; Rabaoui, L.; Daghbouj, N.; Abda, H.; Castet, S.; Josse, C.; van Beek, P.; Souhaut, M.; Michel, S.; Bejaoui, N.; et al. Characterization of phosphate rock and phosphogypsum from. Gabes phosphate fertilizer factories (SE Tunisia): High mining potential and implications for environmental protection. Environ. Sci. Pollut. Res. 2018, 25, 14690–14702. [Google Scholar] [CrossRef]
- Manukyan, R.R. Development direction of the soil-formation processes for reclaimed soda solonetz-solonchak soils of the Ararat valley during their cultivation. Ann. Agrar. Sci. 2018, 16, 69–74. [Google Scholar] [CrossRef]
- Wei, Z.; Deng, Z. Research hotspots and trends of comprehensive utilization of phosphogypsum: Bibliometr. analysis. J. Environ. Radioact. 2022, 242, 106778. [Google Scholar] [CrossRef]
- Chernysh, Y.; Yakhnenko, O.; Chubur, V.; Roubík, H. Phosphogypsum Recycling. A Review of Environmental Issues, Current Trends, and Prospects. Appl. Sci. 2021, 11, 15–75. [Google Scholar] [CrossRef]
- Dmytruk, Y.; Cherlinka, V.; Cherlinka, L.; Dent, D. Soils in war and peace. Int. J. Environ. Stud. 2023, 80, 380–393. [Google Scholar] [CrossRef]
- Hapich, H.; Zahrytsenko, A.; Sudakov, A.; Pavlychenko, A.; Yurchenko, S.; Sudakova, D.; Chushkina, I. Prospects of alternative water supply for the population of Ukraine during wartime and post-war reconstruction. Int. J. Environ. Stud. 2024, 81, 1–12. [Google Scholar] [CrossRef]
- Ushkarenko, V.O.; Morozov, V.V.; Zhuzha, V.V.; Zhuzha, A.V. Evolution of the hydrogeological state under the influence of urbanization and land reclamation on the territory of the city of Kherson. Tavriysʹkyy Nauk. Visnyk. 2002, 21, 112–125. (In Ukrainian). Available online: http://surl.li/nelmt (accessed on 1 June 2023).
- Xu, X.; Guo, L.; Wang, S.; Wang, X.; Ren, M.; Zhao, P.; Huang, Z.; Jia, H.; Wang, J.; Lin, A. Effective strategies for reclamation of saline-alkali soil and response mechanisms of the soil-plant system. Sci. Total Environ. 2023, 905, 167179. [Google Scholar] [CrossRef]
- Bouargane, B.; Pérez-Moreno, S.M.; Barba-Lobo, A.; Bakiz, B.; Atbir, A.; Bolívar, J.P. Behavior of heavy metals and natural radionuclides along the Moroccan phosphogypsum carbonation process with several alkaline reagents. Chem. Eng. Sci. 2023, 280, 119013. [Google Scholar] [CrossRef]
- Pohrebennyk, V.; Dzhumelia, E. Methods of Soils Pollution Spread Analysis: Case Study of Mining and Chemical Enterprise in Lviv Region (Ukraine). Ecol. Eng. Environ. Technol. 2021, 4, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Jalali, J.; Gaudin, P.; Capiaux, H.; Ammar, E.; Lebeau, T. Fate and transport of metal trace elements from phosphogypsum piles in Tunisia and their impact on soil bacteria and wild plants. Ecotoxicol. Environ. Saf. 2019, 174, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Haneklaus, N.; Barbossa, S.; Basallote, M.D.; Bertau, M.; Bilal, E.; Chajduk, E.; Chernysh, Y.; Chubur, V.; Cruz, J.; Dziarczykowski, K.; et al. Closing the upcoming EU gypsum gap with phosphogypsum, Resources. Conserv. Recycl. 2022, 182, 106328. [Google Scholar] [CrossRef]
- Onopriienko, D.M.; Makarova, T.K. Analysis of degradation processes of irrigated soils of Dnipropetrovsk region. Taurian Sci. Bull. 2013, 86, 146–151. [Google Scholar]
- Ushkarenko, V.O.; Vozhegova, R.A.; Goloborodko, S.P.; Kokovikhin, S.V. Statistical Analysis of the Results of Field Experiments in Agriculture; Kherson: Ailant, Ukraine, 2013; p. 381. [Google Scholar]
- DSTU 4730:2007; Soil Quality. Determination of the Granulometric Composition by the Pipette Method in the Modification of N.A. Kaczynski. K. Derzhspozhyvstandart Ukrayiny: Kyiv, Ukraine, 2008; p. 18. (In Ukrainian)
- DSTU ISO 11508:2005; Soil Quality. Determination of Particle Density (ISO 11508:1998, IDT). Derzhspozhyvstandart Ukrayiny: Kyiv, Ukraine, 2008; p. 15. (In Ukrainian)
- DSTU ISO 112651; Modern Measures and Technologies of Land Reclamation of Naturally Saline and Secondary Saline Soils of Ukraine: Recommendations/NSC “Institute of Soil Science and Agrochemistry Named After O. N. Sokolovsky. Derzhspozhyvstandart Ukrayiny: Kyiv, Ukraine, 2011; p. 48. (In Ukrainian)
- DSTU 7537:2014; Soil Quality. Determination of Hydrolytic Acidity. Derzhspozhyvstandart Ukrayiny: Kyiv, Ukraine, 2015; p. 12. (In Ukrainian)
- DSTU 8345:2015; Soil Quality. Methods of Determining Cation Exchange Capacity. Derzhspozhyvstandart Ukrayiny: Kyiv, Ukraine, 2017; p. 10. (In Ukrainian)
- Regional Office of Water Resources in Dnipropetrovsk Region (Ukraine). Available online: http://dovr.gov.ua/ (accessed on 1 November 2023).
- Dnipro Mineral Fertilizer Plant. Product Certificates. Available online: https://dzmu.dp.ua/docs.php (accessed on 1 November 2023).
- Instructions for Soil and Salt Survey on Irrigated Lands of Ukraine: DND 33-5.5-11-2002, Valid from 20/08/2002; State Committee of Ukraine for Water Management: Kyiv, Ukraine, 2002; 31p.
- Gharaibeh, M.A.; Albalasmeh, A.A.; Pratt, C.; El Hanandeh, A. Estimation of exchangeable sodium percentage from sodium adsorption ratio of salt-affected soils using traditional and dilution extracts, saturation percentage, electrical conductivity, and generalized regression neural networks. CATENA 2021, 205, 105466. [Google Scholar] [CrossRef]
- Wang, R.; Wan, S.; Sun, J.; Xiao, H. Soil salinity, sodicity and cotton yield parameters under different drip irrigation regimes during saline wasteland reclamation. Agric. Water Manag 2018, 209, 20–31. [Google Scholar] [CrossRef]
- Mau, Y.; Porporato, A. A dynamical system approach to soil salinity and sodicity. Adv. Water Resour. 2015, 83, 68–76. [Google Scholar] [CrossRef]
- Geilfus, C.-M. Chloride in soil: From nutrient to soil pollutant. Environ. Exp. Bot. 2019, 157, 299–309. [Google Scholar] [CrossRef]
- Zakharchenko, E.; Tunguz, V. Effect of ammonium sulfate and phosphogypsum application on nutrients dynamics and acidity of black soil. Bull. Sumy Natl. Agrar. Univ. Ser. Agron. Biol. 2020, 42, 61–69. [Google Scholar] [CrossRef]
- Davydchuk, M.I.; Kisorets, P.F.; Hantsevska, N.A. Influence of calcium-containing chemical ameliorants on physicochemical and agrochemical properties of dark chestnut secondary saline soil. Ecology 2013, 220, 50–54. (In Ukrainian) [Google Scholar]
- Onopriienko, D.; Makarova, T.; Tkachuk, A.; Hapich, H.; Roubik, H. Prevention of degradation processes of soils irrigated with mineralized water through plastering. Ukr. Black Sea Reg. Agrar. Sci. 2023, 27, 9–20. [Google Scholar] [CrossRef]
- Beltrán, J.M. Irrigation with saline water: Benefits and environmental impact Agric. Water Manag. 1999, 98, 0378–3774. [Google Scholar] [CrossRef]
- Bouray, M.; Moir, J.; Condron, L.; Lehto, N. Impacts of Phosphogypsum, Soluble Fertilizer and Lime Amendment of Acid Soils on the Bioavailability of Phosphorus and Sulphur under Lucerne (Medicago sativa). Plants 2020, 9, 883. [Google Scholar] [CrossRef] [PubMed]
- Papastefanou, C.; Stoulos, S.; Ioannidou, A.; Manolopoulou, M. The application of phosphogypsum in agriculture and the radiological impact. J. Environ. Radioact. 2006, 89, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Minhas, P.S.; Ramos, T.B.; Ben-Gal, A.; Pereira, L.S. Coping with salinity in irrigated agriculture: Crop evapotranspiration and water management issues. Agric. Water Manag. 2020, 227, 105832. [Google Scholar] [CrossRef]
- Tariq, A.; Ullah, I.; Sardans, J.; Yaseen, A.; Peñuelas, J. Strigolactones can be a potential tool to fight environmental stresses in arid lands. Environ. Res. 2023, 229, 115966. [Google Scholar] [CrossRef]
- Kong, C.; Camps-Arbestain, M.; Clothier, B.; Bishop, P.; Vázquez, F.M. Reclamation of salt-affected soils using pumice and algal amendments: Impact on soil salinity and the growth of lucerne. Environ. Technol. Innov. 2021, 24, 101867. [Google Scholar] [CrossRef]
- Majumdar, S.; Barman, F.; Paul, A.; Kundu, R. Role of sulfur in protection against major environmental stress in plants. In Biology and Biotechnology of Environmental Stress Tolerance in Plants: Trace Elements in Environmental Stress Tolerance; Apple Academic Press: New York, NY, USA, 2023; pp. 473–529. [Google Scholar] [CrossRef]
- McKenna, B.A.; Kopittke, P.M.; Macfarlane, D.C.; Dalzell, S.A.; Menzies, N.W. Changes in soil chemistry after the application of gypsum and sulfur and irrigation with coal seam water. Geoderma 2019, 337, 782–791. [Google Scholar] [CrossRef]
- Bilal, E.; Bellefqih, H.; Bourgier, V.; Mazouz, H.; Dumitraş, D.G.; Bard, F.; Laborde, M.; Caspar, J.P.; Guilhot, B.; Iatan, L.; et al. Phosphogypsum circular economy considerations: A critical review from more than 65 storage sites worldwide. J. Clean. Prod. 2023, 414, 137561. [Google Scholar] [CrossRef]
- Gabsi, H.; Tallou, A.; Aziz, F.; Boukchina, R.; Karbout, N.; Caceres, L.A.; García-Tenorio, R.; Boudabbous, K.; Moussa, M. Application of Phosphogypsum and Organic Amendment for Bioremediation of Degraded Soil in Tunisia Oasis: Targeting Circular Economy. Sustainability 2023, 15, 47–69. [Google Scholar] [CrossRef]
- Matveeva, V.A.; Smirnov, Y.D.; Suchkov, D.V. Industrial processing of phosphogypsum into organomineral fertilizer. Environ. Geochem. Health 2022, 44, 1605–1618. [Google Scholar] [CrossRef] [PubMed]
- Onopriienko, D.; Shepel, A.; Makarova, T. Influence of phosphogypsum on the chemical composition of aqueous extract from soil. Agrology 2019, 2, 151–155. [Google Scholar] [CrossRef]
- Robinson, M.J.; Dhar, A.; Naeth, M.A.; Nichol, C.K. Phosphogypsum impacts on soil chemical properties and vegetation tissue following reclamation. Environ. Monit. Assess. 2023, 195, 769. [Google Scholar] [CrossRef] [PubMed]
- Laudicina, V.A.; Hurtado, M.D.; Badalucco, L.; Delgado, A.; Palazzolo, E.; Panno, M. Soil chemical and biochemical properties of a salt-marsh alluvial Spanish area after long-term reclamation. Biol. Fertil. Soils 2009, 45, 691–700. [Google Scholar] [CrossRef]
- Mahmoud, E.; Baroudy AEl El-Kader, N.A.; Othman, S.; Khamisy, R.E. Effects of Phosphogypsum and Biochar Addition on Soil Physical Properties and Nutrients Uptake by Maize yield in Vertic Torrifluvents. Int. J. Sci. Eng. Res. 2017, 8, 1–27. [Google Scholar]
- Plyatsuk, L.; Balintova, M.; Chernysh, Y.; Demcak, S.; Holub, M.; Yakhnenko, E. Influence of Phosphogypsum Dump on the Soil Ecosystem in the Sumy region (Ukraine). Appl. Sci 2019, 9, 5559. [Google Scholar] [CrossRef]
- Chernysh, Y.; Plyatsuk, L.; Dychenko, T. The Protective Functions Stimulation of the Soil Complex with the Use of Biogenic Composites Based on The Sewage Sludge and Phosphogypsum. J. Solid Waste Technol. Manag. 2019, 45, 226–233. [Google Scholar] [CrossRef]
- Sengupta, I.; Dhal, P.K. Impact of elevated phosphogypsum on soil fertility and its aerobic biotransformation through indigenous microorganisms (IMO’s) based technology. J. Environ. Manag. 2021, 297, 113195. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, D.; Nerebinski, M.; Kalaji, H.M.; Augustynowicz, J.; Predecka, A.; Russel, S. Efficiency of the photosynthetic apparatus in Cannabis sativa L. fertilized with sludge froma wastewater treatment plant and with phosphogypsum. Ecol. Quest. 2018, 28, 55–61. [Google Scholar] [CrossRef][Green Version]
- Ablieieva, I.; Berezhna, I.; Berezhnyi, D.; Enrich Prast, A.; Geletuha, G.; Lutsenko, S.; Yanchenko, I.; Carraro, G. Technologies for Environmental Safety Application of Digestate as Biofertilizer. Ecol. Eng. Environ. Technol. 2022, 23, 106–119. [Google Scholar] [CrossRef]
- Yelatontsev, D. Utilization of phosphogypsum in phenol removal from coking wastewater. J. Hazard. Mater. Lett. 2023, 4, 100089. [Google Scholar] [CrossRef]
- Tymoshchuk, V.; Rudakov, L.; Pikarenia, D.; Orlinska, O.; Hapich, H. Analyzing stability of protective structures as the elements of geotechnical tailing pond safety. Min. Miner. Depos. 2023, 17, 116–122. [Google Scholar] [CrossRef]
- Qi, J.; Zhu, H.; Zhou, P.; Wang, X.; Wang, Z.; Yang, S.; Yang, D.; Li, B. Application of phosphogypsum in soilization: A review. Int. J. Environ. Sci. Technol. 2023, 20, 10449–10464. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).