Growth Performance and Osmolyte Regulation of Drought-Stressed Walnut Plants Are Improved by Mycorrhiza
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Culture and Experimental Design
2.2. Determination of Plant Growth, Mycorrhizal Status, and Leaf Physiological Index
2.3. Determination of Sugar Concentrations in Leaves
2.4. Determination of Proline, Soluble Protein, and Hydrogen Peroxide Concentrations in Leaves
2.5. Determination of Betaine Concentrations in Leaves
2.6. Statistical Analysis
3. Results
3.1. Mycorrhizae in Roots and Soil
3.2. Aboveground Part Growth Response
3.3. Underground Part Growth Response
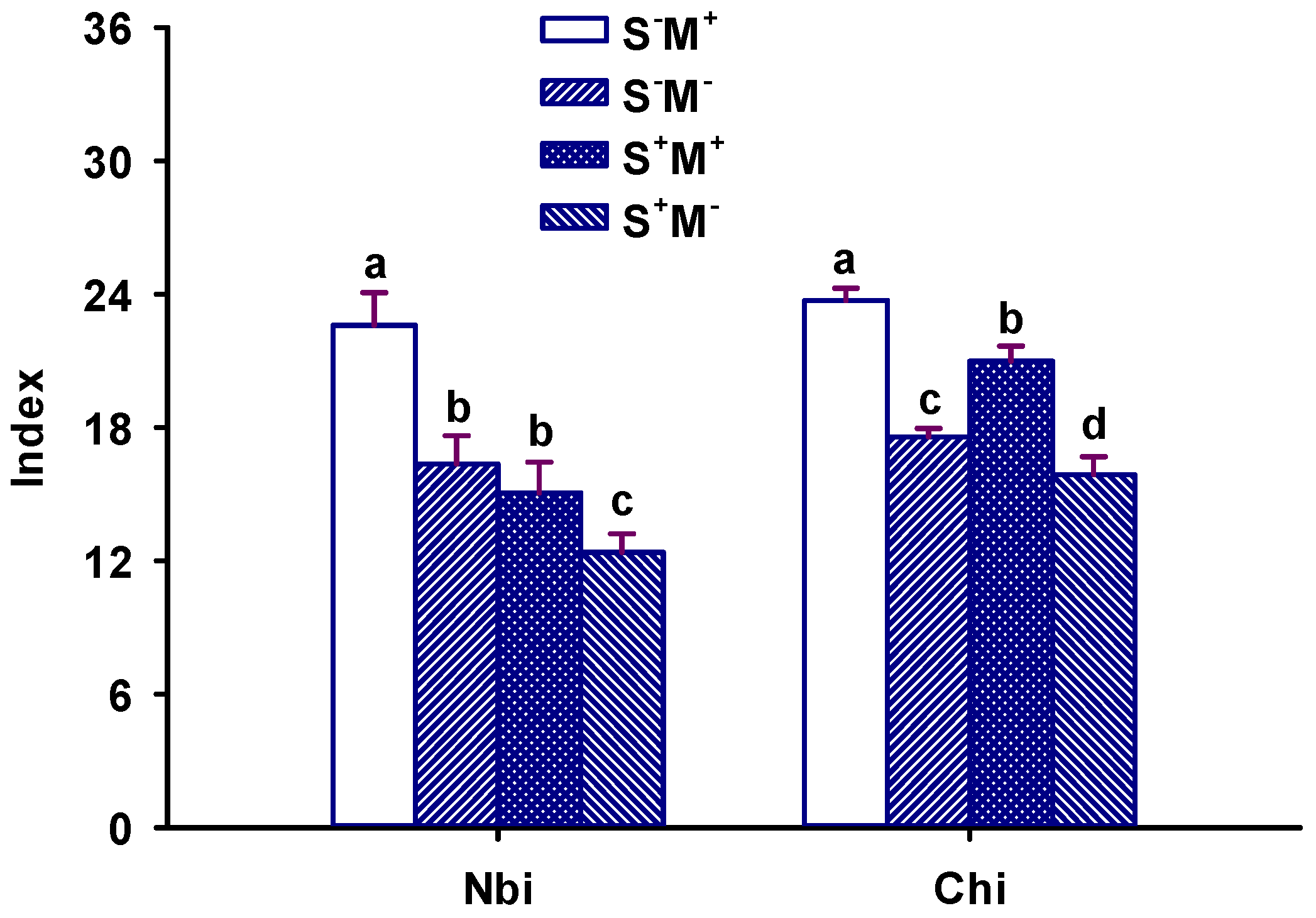
3.4. Leaf Physiological Index Response
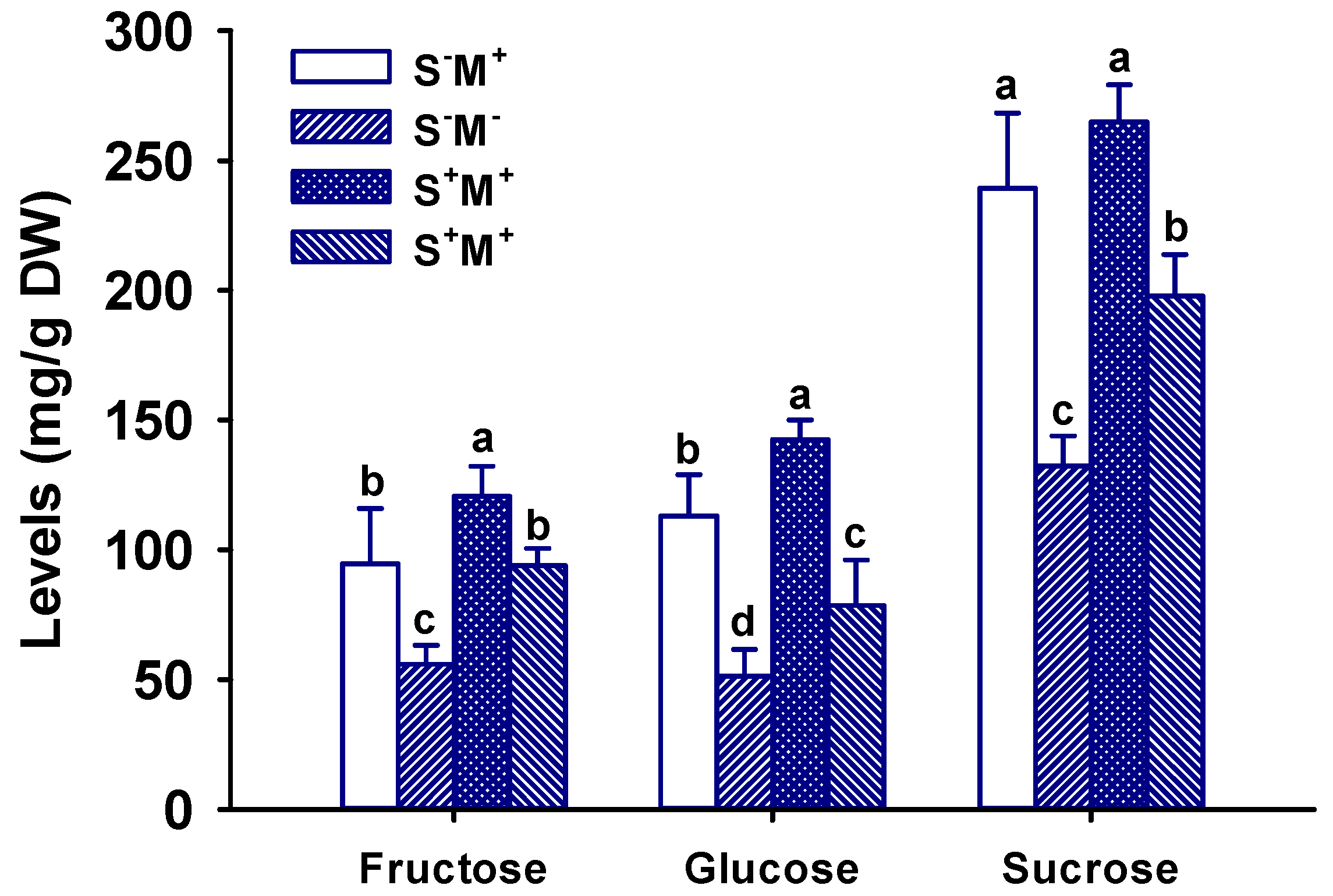
3.5. Leaf Sugar Concentration Response
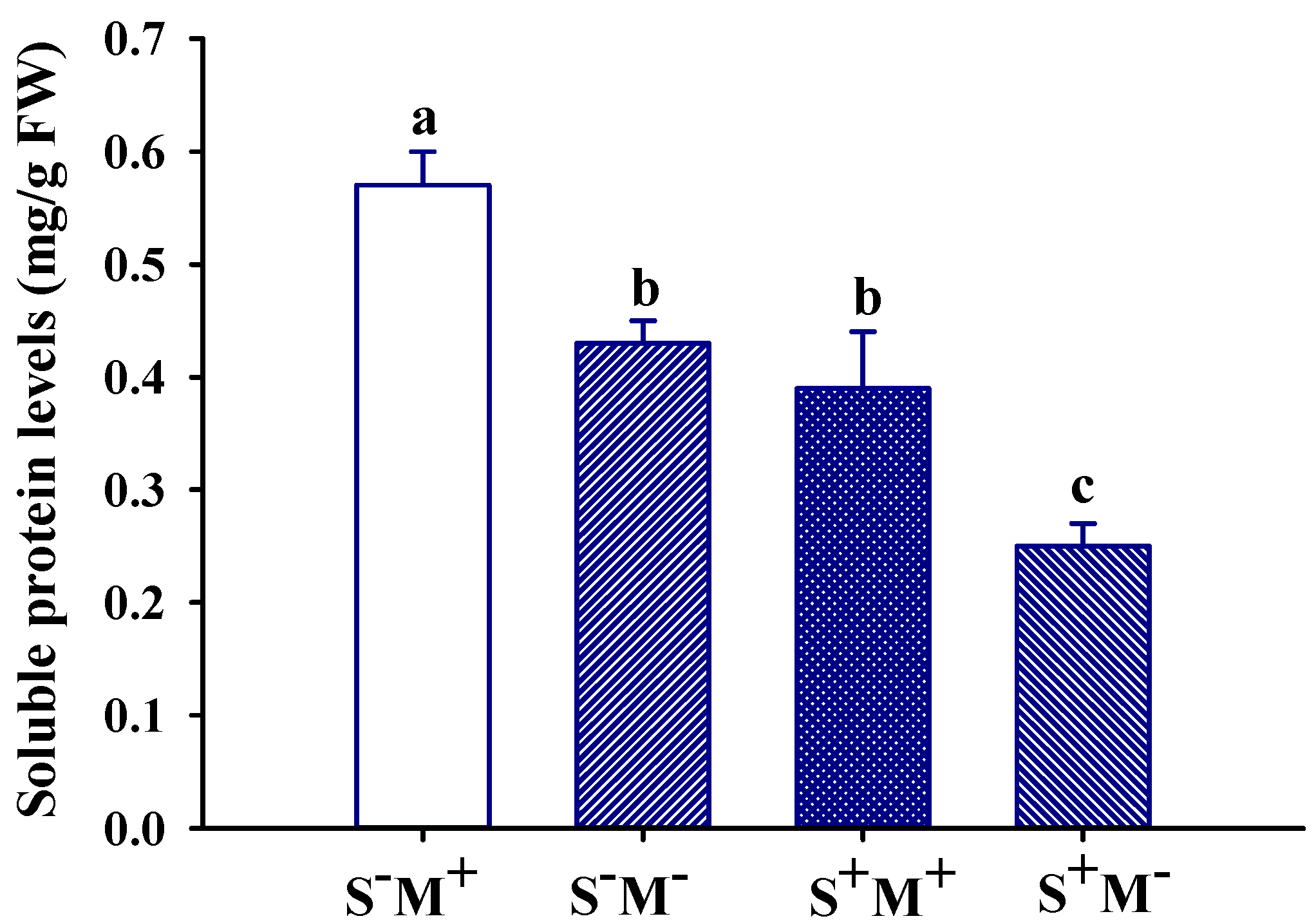
3.6. Leaf Soluble Protein Level Response
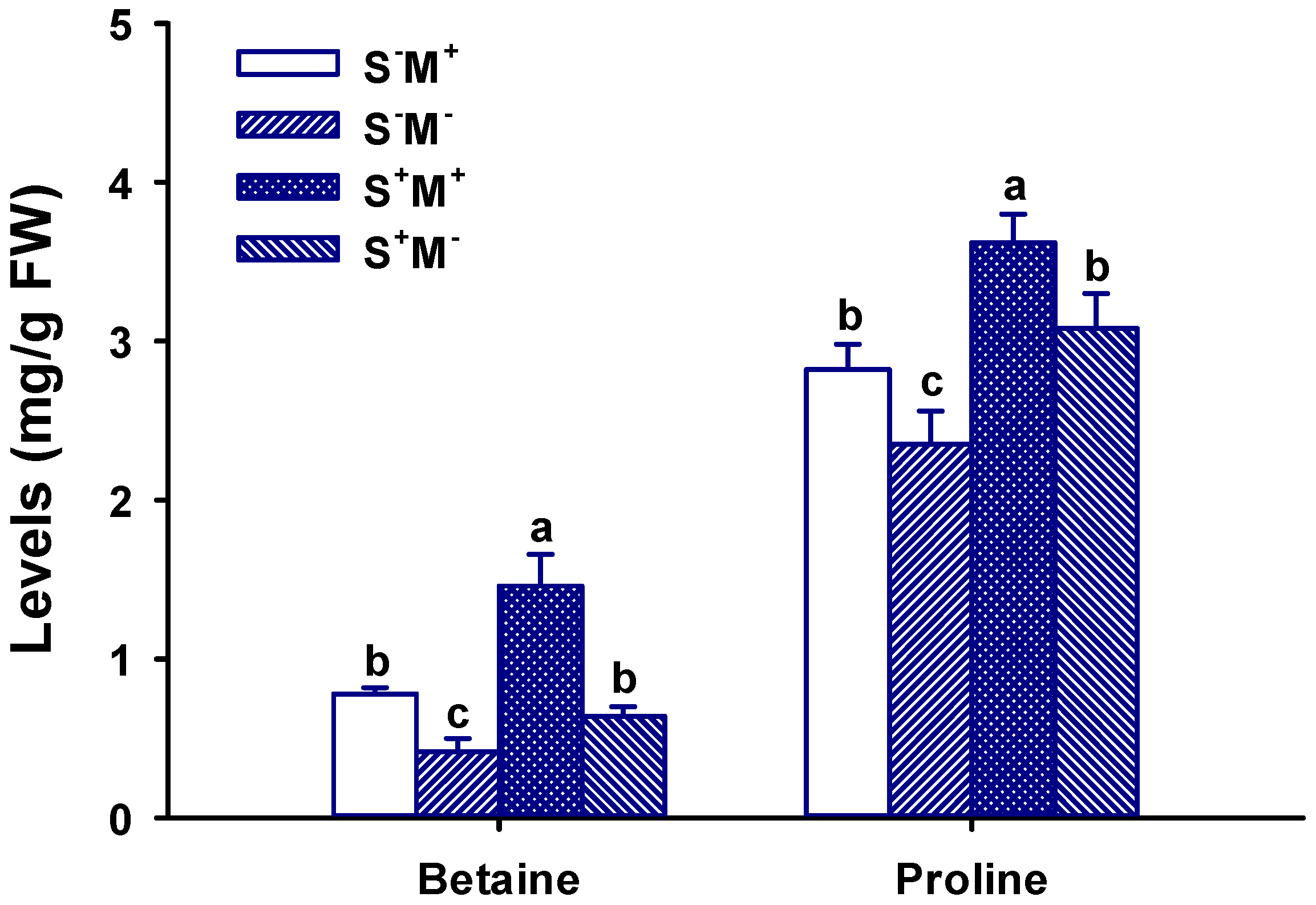
3.7. Leaf Proline and Betaine Concentration Response
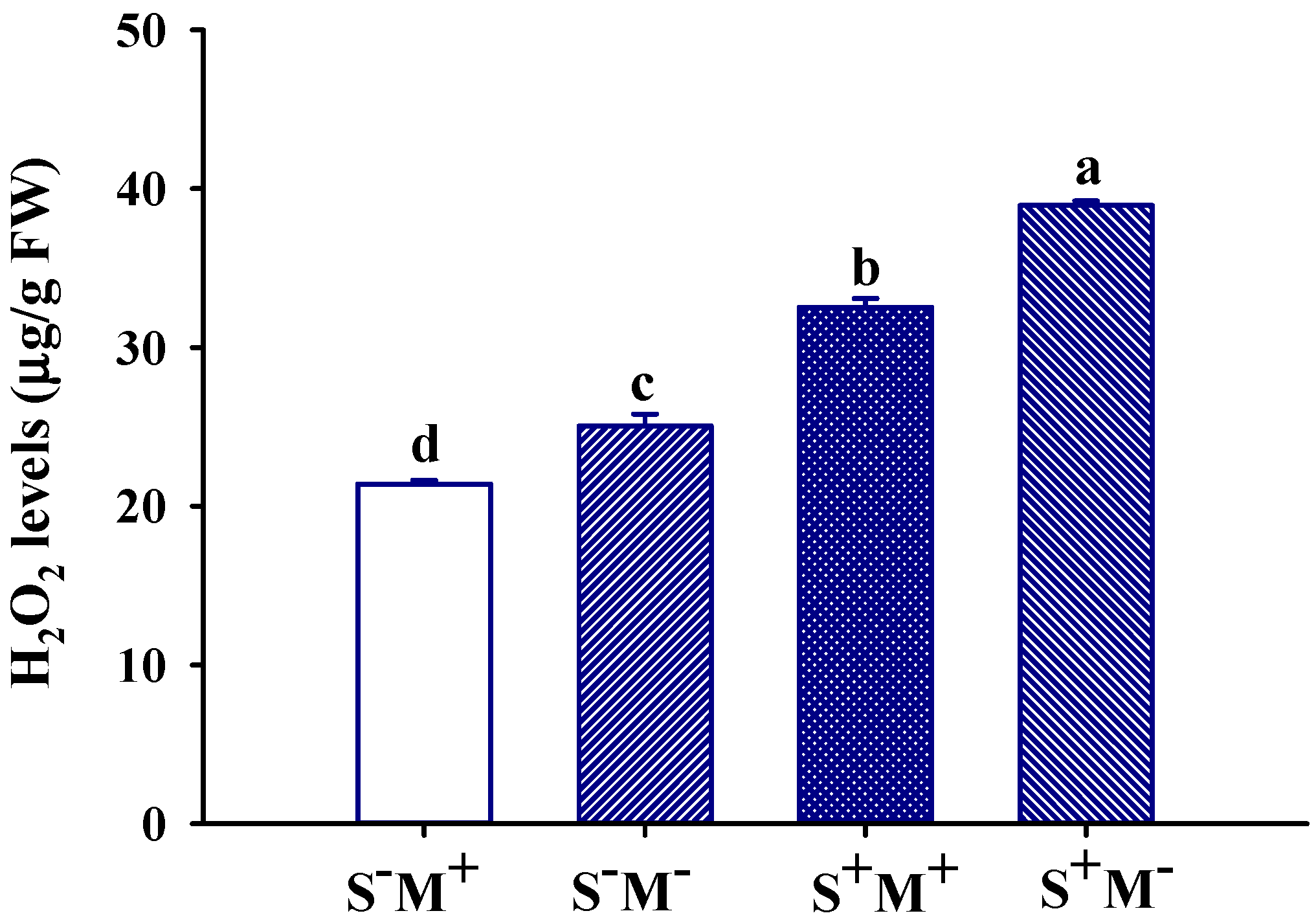
3.8. Leaf H2O2 Level Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kafkas, E.; Burgut, A.; Ozcan, H.; Ozcan, A.; Sutyemez, M.; Kafkas, S.; Türemis, N. Fatty acid, total phenol and tocopherol profiles of some walnut cultivars: A comparative study. Food Nutr. Sci. 2017, 8, 1074–1084. [Google Scholar] [CrossRef]
- Turek, K.; Wszołek, M. Comparative study of walnut and Camelina sativa oil as a functional components for the unsaturated fatty acids and conjugated linoleic acid enrichment of kefir. LWT 2021, 147, 111681. [Google Scholar] [CrossRef]
- Martínez, M.L.; Labuckas, D.O.; Lamarque, A.L.; Maestri, D.M. Walnut (Juglans regia L.): Genetic resources, chemistry, by-products. J. Sci. Food Agric. 2010, 90, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Peng, S.; Chen, S.; Li, Z.; He, Y.; Ren, B.; Yang, G. Identification and characterization of 5 walnut MYB genes in response to drought stress involved in ABA signaling. Physiol. Mol. Biol. Plants 2021, 27, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.; Pandey, G.K.; Kaur, T.; Pericak, O.; Olson, C.; Mohan, R.; Akansha, K.; Yadav, A.; Devi, R.; Kour, D.; et al. Arbuscular mycorrhizal fungi as a potential biofertilizers for agricultural sustainability. J. Appl. Biol. Biotechnol. 2022, 10, 90–107. [Google Scholar] [CrossRef]
- Chaudhary, V.B.; Holland, E.P.; Charman-Anderson, S.; Guzman, A.; Bell-Dereske, L.; Cheeke, T.E.; Corrales, A.; Duchicela, J.; Egan, C.; Gupta, M.M.; et al. What are mycorrhizal traits? Trends Ecol. Evol. 2022, 37, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zou, Y.N.; Wu, Q.S. Quantitative estimation of water uptake by mycorrhizal extraradical hyphae in citrus under drought stress. Sci. Hortic. 2018, 229, 132–136. [Google Scholar] [CrossRef]
- Basyal, B.; Emery, S.M. An arbuscular mycorrhizal fungus alters switchgrass growth, root architecture, and cell wall chemistry across a soil moisture gradient. Mycorrhiza 2021, 31, 251–258. [Google Scholar] [CrossRef]
- Zou, Y.N.; Wu, Q.S.; Kuča, K. Unravelling the role of arbuscular mycorrhizal fungi in mitigating the oxidative burst of plants under drought stress. Plant Biol. 2021, 23, 50–57. [Google Scholar] [CrossRef]
- Tao, J.; Dong, F.; Wang, Y.; Chen, H.; Tang, M. Arbuscular mycorrhizal fungi enhance photosynthesis and drought tolerance by regulating MAPK genes expressions of Populus simonii × P. nigra. Physiol. Plant. 2022, 174, e13829. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, Y.N.; Shu, B.; Wu, Q.S. Deciphering molecular mechanisms regarding enhanced drought tolerance in plants by arbuscular mycorrhizal fungi. Sci. Hortic. 2023, 308, 111591. [Google Scholar] [CrossRef]
- Seutra Kaba, J.; Abunyewa, A.A.; Kugbe, J.; Kwashie, G.K.; Owusu Ansah, E.; Andoh, H. Arbuscular mycorrhizal fungi and potassium fertilizer as plant biostimulants and alternative research for enhancing plants adaptation to drought stress: Opportunities for enhancing drought tolerance in cocoa (Theobroma cacao L.). Sustain. Environ. 2021, 7, 1963927. [Google Scholar] [CrossRef]
- Mortier, E.; Lamotte, O.; Martin-Laurent, F.; Recorbet, G. Forty years of study on interactions between walnut tree and arbuscular mycorrhizal fungi. A review. Agron. Sustain. Dev. 2020, 40, 1–21. [Google Scholar] [CrossRef]
- Huang, G.M.; Zou, Y.N.; Wu, Q.S.; Xu, Y.J.; Kuča, K. Mycorrhizal roles in plant growth, gas exchange, root morphology, and nutrient uptake of walnuts. Plant Soil Environ. 2020, 66, 295–302. [Google Scholar] [CrossRef]
- Zou, Y.N.; Xu, Y.J.; Liu, R.C.; Huang, G.M.; Kuča, K.; Srivastava, A.K.; Hashem, A.; Abd-Allah, E.F.; Wu, Q.S. Two different strategies of Diversispora spurca-inoculated walnut seedlings to improve leaf P acquisition at low and moderate P levels. Front. Plant Sci. 2023, 14, 1140467. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.N.; Qin, Q.Y.; Ma, W.Y.; Zhou, L.J.; Wu, Q.S.; Xu, Y.J.; Kuča, K.; Hashem, A.; Al-Arjani, A.B.F.; Almutairi, K.F.; et al. Metabolomics reveals arbuscular mycorrhizal fungi-mediated tolerance of walnut to soil drought. BMC Plant Biol. 2023, 23, 118. [Google Scholar] [CrossRef] [PubMed]
- Bethlenfalvay, G.J.; Ames, R.N. Comparison of two methods for quantifying extraradical mycelium of vesicular-arbuscular mycorrhizal fungi. Soil Sci. Soc. Am. J. 1987, 51, 834–837. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Wu, Q.S.; Srivastava, A.K.; Li, Y. Effect of mycorrhizal symbiosis on growth behavior and carbohdyrate metabolism of trifoliate orange under different substrate P levels. J. Plant Growth Regul. 2015, 34, 495–508. [Google Scholar] [CrossRef]
- Zheng, F.L.; Liang, S.M.; Chu, X.N.; Yang, Y.L.; Wu, Q.S. Mycorrhizal fungi enhance flooding tolerance of peach through inducing proline accumulation and improving root architecture. Plant Soil Environ. 2020, 66, 624–631. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wu, Y.M.; Hu, X.H.; Ni, H.T. Determination of betaine content in beet root by colorimetric method. Sugar Crops China 2008, 30, 27–28+30. [Google Scholar]
- Wang, Y.J.; He, X.H.; Meng, L.L.; Zou, Y.N.; Wu, Q.S. Extraradical mycorrhizal hyphae promote soil carbon sequestration through difficultly extractable glomalin-related soil protein in response to soil water stress. Microb. Ecol. 2023, 86, 1023–1034. [Google Scholar] [CrossRef]
- Mo, Y.; Wang, Y.; Yang, R.; Zheng, J.; Liu, C.; Li, H.; Ma, J.; Zhang, Y.; Wei, C.; Zhang, X. Regulation of plant growth, Photosynthesis, antioxidation and osmosis by an arbuscular mycorrhizal fungus in watermelon seedlings under well-watered and drought conditions. Front. Plant Sci. 2016, 7, 644. [Google Scholar] [CrossRef]
- Mao, J.H.; Li, R.B.; Jing, Y.B.; Ning, D.L.; Li, Y.P.; Chen, H.Y. Arbuscular mycorrhizal fungi associated with walnut trees and their effect on seedling growth. J. For. Environ. 2022, 42, 71–80. [Google Scholar]
- Quiroga, G.; Erice, G.; Aroca, R.; Zamarreño, Á.M.; García-Mina, J.M.; Ruiz-Lozano, J.M. Radial water transport in arbuscular mycorrhizal maize plants under drought stress conditions is affected by indole-acetic acid (IAA) application. J. Plant Physiol. 2020, 246, 153115. [Google Scholar] [CrossRef]
- Begum, N.; Wang, L.; Ahmad, H.; Akhtar, K.; Roy, R.; Khan, M.I.; Zhao, T. Co-inoculation of arbuscular mycorrhizal fungi and the plant growth-promoting rhizobacteria improve growth and photosynthesis in tobacco under drought stress by up-regulating antioxidant and mineral nutrition metabolism. Microb. Ecol. 2022, 83, 971–988. [Google Scholar] [CrossRef]
- Wang, K.; Nan, L.L.; Guo, Q.E.; Yao, Y.H.; He, H.P.; Xia, J.; Ma, B. Effects of drought stress on root architecture of different root- type alfalfa. Acta Ecol. Sin. 2022, 42, 8365–8373. [Google Scholar]
- Lynch, J. Root architecture and plant productivity. Plant Physiol. 1995, 109, 7–13. [Google Scholar] [CrossRef]
- Ketipearachchi, K.W.; Tatsumi, J. Local fractal dimensions and multifractal analysis of the root system of legumes. Plant Prod. Sci. 2000, 3, 289–295. [Google Scholar] [CrossRef]
- Xie, M.; Deng, Y.; Lei, X.G.; Fan, L. Effects of inoculating arbuscular mycorrhizal fungi on root growth of strawberry under drought stress. J. Anhui Agric. Sci. 2017, 45, 10–12. [Google Scholar]
- Chandrasekaran, M. Arbuscular mycorrhizal fungi mediated enhanced biomass, root morphological traits and nutrient uptake under drought stress: A meta-analysis. J. Fungi 2022, 8, 660. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Wang, H.; Li, H. Arbuscular mycorrhizal fungi improve growth, photosynthetic activity, and chlorophyll fluorescence of Vitis vinifera L. cv. Ecolly under drought stress. Agronomy 2022, 12, 1563. [Google Scholar] [CrossRef]
- Asrar, A.W.A.; Elhindi, K.M. Alleviation of drought stress of marigold (Tagetes erecta) plants by using arbuscular mycorrhizal fungi. Saudi J. Biol. Sci. 2011, 18, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, K.S.; Charest, C. Arbuscular mycorrhizae and nitrogen assimilation in maize after drought and recovery. Physiol. Plant. 1998, 102, 285–296. [Google Scholar] [CrossRef]
- Tang, H.Y.; Hassan, M.U.; Feng, L.; Nawaz, M.; Shah, A.N.; Qari, S.H.; Liu, Y.; Miao, J.Q. The critical role of arbuscular mycorrhizal fungi to improve drought tolerance and nitrogen use efficiency in crops. Front. Plant Sci. 2022, 13, 919166. [Google Scholar] [CrossRef]
- Mukarram, M.; Choudhary, S.; Kurjak, D.; Petek, A.; Khan, M.M.A. Drought: Sensing, signalling, effects and tolerance in higher plants. Physiol. Plant. 2021, 172, 1291–1300. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef]
- Pamungkas, S.S.T.; Farid, N. Drought stress: Responses and mechanism in plants. Rev. Agric. Sci. 2022, 10, 168–185. [Google Scholar] [CrossRef]
- Li, J.J.; Zhao, S.G.; An, X.H.; Tian, Y.; Wang, H.X.; Zhang, Z.H. Physiological adaptation of walnut under drought stress and screening of its resistance indexes. Chin. Fruits 2023, 3, 72–78. [Google Scholar] [CrossRef]
- Mirshad, P.P.; Puthur, J.T. Arbuscular mycorrhizal association enhances drought tolerance potential of promising bioenergy grass (Saccharum arundinaceum retz.). Environ. Monit. Assess. 2016, 188, 425. [Google Scholar] [CrossRef] [PubMed]
- Behrooz, A.; Vahdati, K.; Rejali, F.; Lotfi, M.; Sarikhani, S.; Leslie, C. Arbuscular mycorrhiza and plant growthpromoting bacteria alleviate drought stress in walnut. HortScience 2019, 4, 1087–1092. [Google Scholar] [CrossRef]
- Mariyam, S.; Bhardwaj, R.; Khan, N.A.; Sahi, S.V.; Seth, C.S. Review on nitric oxide at the forefront of rapid systemic signaling in mitigation of salinity stress in plants: Crosstalk with calcium and hydrogen peroxide. Plant Sci. 2023, 336, 111835. [Google Scholar] [CrossRef]
- Liu, X.Q.; Cheng, S.; Aroca, R.; Zou, Y.N.; Wu, Q.S. Arbuscular mycorrhizal fungi induce flavonoid synthesis for mitigating oxidative damage of trifoliate orange under water stress. Environ. Exp. Bot. 2022, 204, 105089. [Google Scholar] [CrossRef]
| Treatments | Soil Mycelium Length (cm/g) | Stem Diameter (mm) | Leaf Number | Height (cm) | Leaf Biomass (g/plant) |
|---|---|---|---|---|---|
| S-M+ | 38.39 ± 1.87 a | 7.35 ± 0.48 a | 44.4 ± 2.70 a | 30.4 ± 2.30 a | 9.61 ± 0.60 a |
| S-M- | 0 c | 6.68 ± 0.34 b | 43.8 ± 3.11 a | 26.0 ± 1.87 b | 8.82 ± 0.74 b |
| S+M+ | 13.81 ± 0.91 b | 5.54 ± 0.32 c | 25.4 ± 1.52 b | 20.4 ± 1.14 c | 4.80 ± 0.30 c |
| S+M- | 0 c | 4.72 ± 0.45 d | 20.2 ± 0.45 c | 19.4 ± 0.96 c | 2.75 ± 0.35 d |
| Treatments | Total Length (cm) | Projected Area (cm2) | Surface Area (cm2) | Average Diameter (mm) | Volume (cm3) |
|---|---|---|---|---|---|
| S-M+ | 267.8 ± 4.2 a | 13.24 ± 0.17 a | 20.24 ± 0.57 a | 0.78 ± 0.01 a | 4.51 ± 1.80 a |
| S-M- | 197.9 ± 6.2 bc | 12.31 ± 0.43 b | 18.26 ± 0.21 b | 0.62 ± 0.01 b | 2.91 ± 0.64 ab |
| S+M+ | 210.4 ± 9.8 b | 11.78 ± 0.13 b | 16.18 ± 0.19 c | 0.63 ± 0.03 b | 2.43 ± 0.58 ab |
| S+M- | 182.1 ± 2.4 c | 10.32 ± 0.00 c | 14.49 ± 0.62 d | 0.52 ± 0.02 c | 1.37 ± 0.51 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Y.; Zhou, L.-J.; Xu, Y.-J.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.-S. Growth Performance and Osmolyte Regulation of Drought-Stressed Walnut Plants Are Improved by Mycorrhiza. Agriculture 2024, 14, 367. https://doi.org/10.3390/agriculture14030367
Wen Y, Zhou L-J, Xu Y-J, Hashem A, Abd_Allah EF, Wu Q-S. Growth Performance and Osmolyte Regulation of Drought-Stressed Walnut Plants Are Improved by Mycorrhiza. Agriculture. 2024; 14(3):367. https://doi.org/10.3390/agriculture14030367
Chicago/Turabian StyleWen, Yue, Li-Jun Zhou, Yong-Jie Xu, Abeer Hashem, Elsayed Fathi Abd_Allah, and Qiang-Sheng Wu. 2024. "Growth Performance and Osmolyte Regulation of Drought-Stressed Walnut Plants Are Improved by Mycorrhiza" Agriculture 14, no. 3: 367. https://doi.org/10.3390/agriculture14030367
APA StyleWen, Y., Zhou, L.-J., Xu, Y.-J., Hashem, A., Abd_Allah, E. F., & Wu, Q.-S. (2024). Growth Performance and Osmolyte Regulation of Drought-Stressed Walnut Plants Are Improved by Mycorrhiza. Agriculture, 14(3), 367. https://doi.org/10.3390/agriculture14030367









