Influence and Role of Fungi, Bacteria, and Mixed Microbial Populations on Phosphorus Acquisition in Plants
Abstract
1. Introduction
2. Mechanism of Fungal-Mediated Phosphorus Transport and Effects on Plant Growth
2.1. Phosphate Transporters Play a Key Role in Plant–Fungal Interactions
| Name | Species | References |
|---|---|---|
| Hcpt1/Hcpt2 | Hebeloma cylindrosporum | [30] |
| GiPT | Glomus intraradices | [33] |
| GmosPT | Glomus mosseae | [34] |
| GigmPT | Gigaspora margarita | [35] |
| RiPT7 | Rhizophagus irregularis | [36] |
| LjPT3 | Lotus japonicus | [38] |
| PT4 | Medicago truncatula | [39] |
| HVPT8 | Triticum aestivum | [40] |
| StPT4 | Lycopersicon esculentum | [43] |
| ZmPT6 | Zea mays | [44] |
| PhPT3/PhPT5 | Solanum lycopersicum | [45] |
| GmPT10/GmPT11 | Glysin max | [42] |
| VvPht1-1/VvPht1-2 | Funneliformis mosseae | [46] |
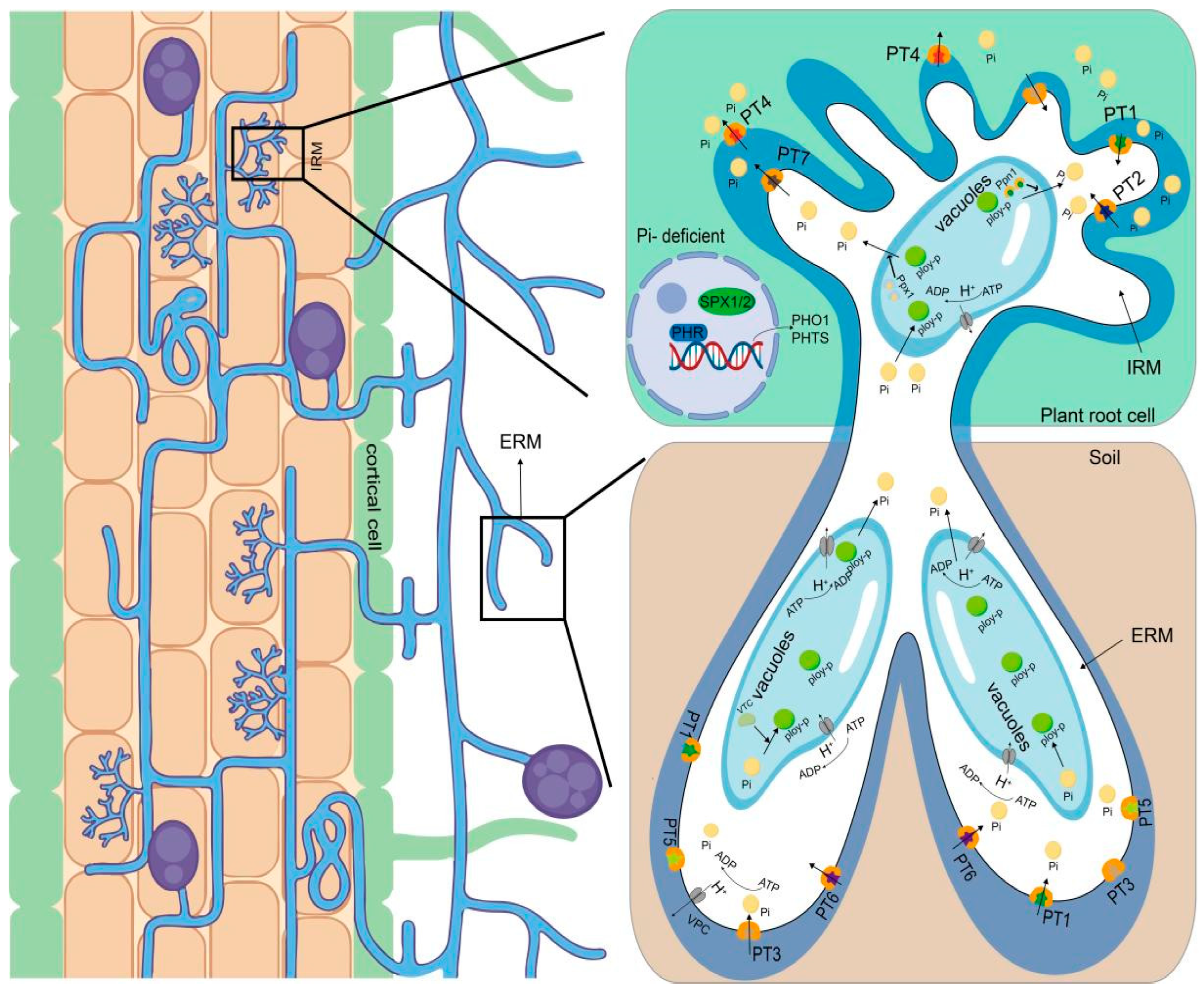
2.2. Fungi Affect Plant Physiological Traits and Reduce Phosphorus Fertilizer Application
2.3. Fungi Are Involved in Plant Phosphorus Signal Transduction and Hormone Synthesis
2.4. Fungal Secretions Contribute to the Transport of Phosphorus
2.5. Phosphorus Transport and Uptake by Fungi Is Not limited to Mycorrhizal Plants
3. Bacteria-Mediated Phosphorus Transport System and Its Effects on Plant Growth
3.1. Phosphate Transporters Play an Important Role in the Interaction between Bacteria and Plants
3.2. Bacteria Convert Soil Phosphorus, Which Helps Plants to Effectively Uptake Phosphorus
3.3. Beneficial Bacteria Regulate Plants’ Growth and Development and Reduce Phosphorus Fertilizer Application
3.4. Colonization of Bacterial Communities in Plants Is influenced by Phosphorus
3.5. Bacterial Hormones Indirectly Affect Phosphorus Uptake by Plants
4. Effect of a Combination of Mixed Microbial Populations on Plant Phosphorus Acquisition
4.1. Co-Application of Fungi and Bacteria Improves Crop Yields and Reduces Phosphorus Fertilizer
4.2. Co-Application of Two or More Bacteria Is Effective for Plant Phosphorus Uptake
5. Conclusions
6. Application Prospects of Composite Microbiological Fertilizer and Suggestions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Malhotra, H.; Vandana; Sharma, S.; Pandey, R. Phosphorus Nutrition: Plant Growth in Response to Deficiency and Excess. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp. 171–190. [Google Scholar]
- Chen, S.; Zhang, S.; Yan, Z.; Peng, Y.; Chen, Q. Differences in main processes to transform phosphorus influenced by ammonium nitrogen in flooded intensive agricultural and steppe soils. Chemosphere 2019, 226, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Azam, H.M.; Alam, S.T.; Hasan, M.M.; Yameogo, D.D.S.; Kannan, A.D.; Rahman, A.; Kwon, M.J. Phosphorous in the environment: Characteristics with distribution and effects, removal mechanisms, treatment technologies, and factors affecting recovery as minerals in natural and engineered systems. Environ. Sci. Pollut. Res. 2019, 26, 20183–20207. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Xu, J.; Wang, L.; Tang, H.; Wu, M.; Xu, P. Influence of Different Single Factors on the Spatial-Temporal Distribution Law of Phosphorus in the Generalized River. Sustainability 2022, 14, 2070. [Google Scholar] [CrossRef]
- Zhang, C.; Tian, H.; Liu, J.; Wang, S.; Liu, M.; Pan, S.; Shi, X. Pools and distributions of soil phosphorous in China. Glob. Biogeochem. Cycles 2005, 19, B1020. [Google Scholar] [CrossRef]
- Astiko, W.; Ernawati, N.; Silawibawa, I. Nutrient concentration of nitrogen and phosphorus on intercropping of several varieties maize and soybean in dryland North Lombok, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 824, 12001. [Google Scholar] [CrossRef]
- Nowaki, R.H.D.; Parent, S.; Cecílio Filho, A.B.; Rozane, D.E.; Meneses, N.B.; Da Silva, J.S.; Natale, W.; Parent, L.E. Phosphorus Over-Fertilization and Nutrient Misbalance of Irrigated Tomato Crops in Brazil. Front. Plant Sci. 2017, 8, 825. [Google Scholar] [CrossRef]
- Haygarth, P.M.; Rufino, M.C. Local solutions to global phosphorus imbalances. Nat. Food 2021, 2, 459–460. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Qiang, R.; Lu, E.; Cuilan, L.; Zhang, J.; Gao, Q. Response of Soil Microbial Community Structure to Phosphate Fertilizer Reduction and Combinations of Microbial Fertilizer. Front. Environ. Sci. 2022, 10, 899727. [Google Scholar] [CrossRef]
- Dinca, L.; Grenni, P.; Onet, A.; Onet, C. Fertilization and Soil Microbial Community: A Review. Appl. Sci. 2022, 12, 1198. [Google Scholar] [CrossRef]
- Wang, X.; Xiong, J.; He, Z. Activated dolomite phosphate rock fertilizers to reduce leaching of phosphorus and trace metals as compared to superphosphate. J. Environ. Manag. 2019, 255, 109872. [Google Scholar] [CrossRef]
- Kianpoor Kalkhajeh, Y.; Huang, B.; Sørensen, H.; Holm, P.; Hansen, H. Phosphorus accumulation and leaching risk of greenhouse vegetable soils in Southeast China. Pedosphere 2021, 31, 683–693. [Google Scholar] [CrossRef]
- Kaminsky, L.; Thompson, G.; Trexler, R.; Bell, T.; Kao-Kniffin, J. Medicago sativa has Reduced Biomass and Nodulation When Grown with Soil Microbiomes Conditioned to High Phosphorus Inputs. Phytobiomes J. 2018, 2, 237–248. [Google Scholar] [CrossRef]
- Schipper, L.A.; Sparling, G.P.; Fisk, L.M.; Dodd, M.B.; Power, I.L.; Littler, R.A. Rates of accumulation of cadmium and uranium in a New Zealand hill farm soil as a result of long-term use of phosphate fertilizer. Agric. Ecosyst. Environ. 2011, 144, 95–101. [Google Scholar] [CrossRef]
- Cheraghi, M.; Lorestani, B.; Merrikhpour, H. Investigation of the Effects of Phosphate Fertilizer Application on the Heavy Metal Content in Agricultural Soils with Different Cultivation Patterns. Biol. Trace Elem. Res. 2012, 145, 87–92. [Google Scholar] [CrossRef]
- Coleman, D.; Wall, D. Fauna: The engine for microbial activity and transport. In Soil Microbiology, Ecology and Biochemistry, 3rd ed.; Academic Press: Cambridge, MA, USA, 2006; pp. 163–191. [Google Scholar] [CrossRef]
- Sosa, O.A. Phosphorus redox reactions as pinch hitters in microbial metabolism. Proc. Natl. Acad. Sci. USA 2018, 115, 7–8. [Google Scholar] [CrossRef]
- Pierret, A.; Doussan, C.; Capowiez, Y.; Bastardie, F.; Pagès, L. Root Functional Architecture: A Framework for Modeling the Interplay between Roots and Soil. Vadose Zone J. 2007, 6, 269–281. [Google Scholar] [CrossRef]
- Haney, C.H.; Samuel, B.S.; Bush, J.; Ausubel, F.M. Associations with rhizosphere bacteria can confer an adaptive advantage to plants. Nat. Plants 2015, 1, 15051. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.; Gaur, R. 9—Bioactivity of soil microorganisms for agriculture development. In Microbes in Land Use Change Management; Singh, J.S., Tiwari, S., Singh, C., Singh, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 197–220. [Google Scholar]
- Choi, K.; Khan, R.; Lee, S. Dissection of plant microbiota and plant-microbiome interactions. J. Microbiol. 2021, 59, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Brettell, L.E.; Qiu, Z.; Singh, B.K. Microbiome-Mediated Stress Resistance in Plants. Trends Plant Sci. 2020, 25, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Khodaverdiloo, H.; Sepehri, M.; Rasouli-Sadaghiani, M. Arbuscular mycorrhizal fungi and heavy metal contaminated soils. Afr. J. Microbiol. Res. 2011, 5, 1571–1576. [Google Scholar] [CrossRef]
- Wang, D.; Lv, S.; Jiang, P.; Li, Y. Roles, Regulation, and Agricultural Application of Plant Phosphate Transporters. Front. Plant Sci. 2017, 8, 817. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Dai, Z.; Lin, J.; Li, D.; Ye, H.; Dahlgren, R.A.; Xu, J. Labile carbon facilitated phosphorus solubilization as regulated by bacterial and fungal communities in Zea mays. Soil Biol. Biochem. 2021, 163, 108465. [Google Scholar] [CrossRef]
- Saia, S.; Aissa, E.; Luziatelli, F.; Ruzzi, M.; Colla, G.; Ficca, A.G.; Cardarelli, M.; Rouphael, Y. Growth-promoting bacteria and arbuscular mycorrhizal fungi differentially benefit tomato and corn depending upon the supplied form of phosphorus. Mycorrhiza 2020, 30, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Zhu, Q.; Zhang, F.; Zhang, W.; Yuan, J.; Sun, K.; Xu, F.; Dai, C. Enhanced nitrogen and phosphorus activation with an optimized bacterial community by endophytic fungus Phomopsis liquidambari in paddy soil. Microbiol. Res. 2019, 221, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, J.; Cheng, Z.; Li, M.; Liu, Z.; Wang, J.; Lin, X. Can phosphorus (P)-releasing bacteria and earthworm (Eisenia fetida L.) co-enhance soil P mobilization and mycorrhizal P uptake by maize (Zea mays L.)? J. Soil Sediments 2021, 21, 842–852. [Google Scholar] [CrossRef]
- Tatry, M.; El Kassis, E.; Lambilliotte, R.; Corratgé, C.; Van Aarle, I.; Amenc, L.K.; Alary, R.; Zimmermann, S.; Sentenac, H.; Plassard, C. Two differentially regulated phosphate transporters from the symbiotic fungus Hebeloma cylindrosporum and phosphorus acquisition by ectomycorrhizal Pinus pinaster. Plant J. 2009, 57, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, X.; Wang, E. Mycorrhizal Symbiosis in Plant Growth and Stress Adaptation: From Genes to Ecosystems. Annu. Rev. Plant Biol. 2023, 74, 569–607. [Google Scholar] [CrossRef]
- Hijikata, N.; Murase, M.; Tani, C.; Ohtomo, R.; Osaki, M.; Ezawa, T. Polyphosphate has a central role in the rapid and massive accumulation of phosphorus in extraradical mycelium of an arbuscular mycorrhizal fungus. New Phytol. 2010, 186, 285–289. [Google Scholar] [CrossRef]
- Maldonado-Mendoza, I.E.; Dewbre, G.R.; Harrison, M.J. A Phosphate Transporter Gene from the Extra-Radical Mycelium of an Arbuscular Mycorrhizal Fungus Glomus intraradices Is Regulated in Response to Phosphate in the Environment. Mol. Plant-Microbe Interact. 2001, 14, 1140–1148. [Google Scholar] [CrossRef]
- Benedetto, A.; Magurno, F.; Bonfante, P.; Lanfranco, L. Expression profiles of a phosphate transporter gene (GmosPT) from the endomycorrhizal fungus Glomus mosseae. Mycorrhiza 2005, 15, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Lin, H.; Peng, X.; Xu, C.; Sun, Z.; Jiang, K.; Huang, A.; Wu, X.; Tang, N.; Salvioli, A.; et al. Arbuscular Mycorrhizal Symbiosis Requires a Phosphate Transceptor in the Gigaspora margarita Fungal Symbiont. Mol. Plant 2016, 9, 1583–1608. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Lai, W.; Che, X.; Wang, S.; Ren, Y.; Hu, W.; Chen, H.; Tang, M. A SPX domain-containing phosphate transporter from Rhizophagus irregularis handles phosphate homeostasis at symbiotic interface of arbuscular mycorrhizas. New Phytol. 2022, 234, 650–671. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Huang, W.; Liu, F.; Tang, N.; Liu, Y.; Lin, H.; Zhao, B. Functional analysis of the novel mycorrhiza-specific phosphate transporter AsPT1 and PHT1 family from Astragalus sinicus during the arbuscular mycorrhizal symbiosis. New Phytol. 2013, 198, 836–852. [Google Scholar] [CrossRef]
- Maeda, D.; Ashida, K.; Iguchi, K.; Chechetka, S.A.; Hijikata, A.; Okusako, Y.; Deguchi, Y.; Izui, K.; Hata, S. Knockdown of an Arbuscular Mycorrhiza-inducible Phosphate Transporter Gene of Lotus japonicus Suppresses Mutualistic Symbiosis. Plant Cell Physiol. 2006, 47, 807–817. [Google Scholar] [CrossRef]
- Harrison, M.J.; Dewbre, G.R.; Liu, J. A Phosphate Transporter from Medicago truncatula Involved in the Acquisition of Phosphate Released by Arbuscular Mycorrhizal Fungi. Plant Cell 2002, 14, 2413–2429. [Google Scholar] [CrossRef]
- Grace, E.J.; Cotsaftis, O.; Tester, M.; Smith, F.A.; Smith, S.E. Arbuscular mycorrhizal inhibition of growth in barley cannot be attributed to extent of colonization, fungal phosphorus uptake or effects on expression of plant phosphate transporter genes. New Phytol. 2009, 181, 938–949. [Google Scholar] [CrossRef]
- Wegmüller, S.; Svistoonoff, S.; Reinhardt, D.; Stuurman, J.; Amrhein, N.; Bucher, M. A transgenic dTph1 insertional mutagenesis system for forward genetics in mycorrhizal phosphate transport of Petunia. Plant J. 2008, 54, 1115–1127. [Google Scholar] [CrossRef]
- Tamura, Y.; Kobae, Y.; Mizuno, T.; Hata, S. Identification and Expression Analysis of Arbuscular Mycorrhiza-Inducible Phosphate Transporter Genes of Soybean. Biosci. Biotechnol. Biochem. Biotechnol. Biochem. 2012, 76, 309–313. [Google Scholar] [CrossRef][Green Version]
- Nagy, R.; Karandashov, V.; Chague, V.; Kalinkevich, K.; Tamasloukht, M.; Xu, G.; Jakobsen, I.; Levy, A.A.; Amrhein, N.; Bucher, M. The characterization of novel mycorrhiza-specific phosphate transporters from Lycopersicon esculentum and Solanum tuberosum uncovers functional redundancy in symbiotic phosphate transport in solanaceous species. Plant J. 2005, 42, 236–250. [Google Scholar] [CrossRef]
- Willmann, M.; Zellerhoff, N.; Buer, B.; Polatajko, A.; Nagy, R.; Koebke, E.; Jansa, J.; Flisch, R.; Bucher, M. Mycorrhizal phosphate uptake pathway in maize: Vital for growth and cob development on nutrient poor agricultural and greenhouse soils. Front. Plant Sci. 2013, 4, 533. [Google Scholar] [CrossRef]
- Molinero-Rosales, N.; Martín-Rodríguez, J.Á.; Ho-Plágaro, T.; García-Garrido, J.M. Identification and expression analysis of the arbuscular mycorrhiza-inducible Rieske non-heme oxygenase Ptc52 gene from tomato. J. Plant Physiol. 2019, 237, 95–103. [Google Scholar] [CrossRef]
- Valat, L.; Deglène-Benbrahim, L.; Kendel, M.; Hussenet, R.; Le Jeune, C.; Schellenbaum, P.; Maillot, P. Transcriptional induction of two phosphate transporter 1 genes and enhanced root branching in grape plants inoculated with Funneliformis mosseae. Mycorrhiza 2018, 28, 179–185. [Google Scholar] [CrossRef]
- Das, D.; Paries, M.; Hobecker, K.; Gigl, M.; Dawid, C.; Lam, H.; Zhang, J.; Chen, M.; Gutjahr, C. PHOSPHATE STARVATION RESPONSE transcription factors enable arbuscular mycorrhiza symbiosis. Nat. Commun. 2022, 13, 477. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Gu, M.; Sun, S.; Zhu, L.; Hong, S.; Xu, G. Identification of two conserved cis-acting elements, MYCS and P1BS, involved in the regulation of mycorrhiza-activated phosphate transporters in eudicot species. New Phytol. 2011, 189, 1157–1169. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Limpens, E.; Yao, R. Orchestrating plant direct and indirect phosphate uptake pathways. Trends Plant Sci. 2022, 27, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Jia, X.; Yu, N.; Murray, J.D.; Yi, K.; Wang, E. Microbe-dependent and independent nitrogen and phosphate acquisition and regulation in plants. New Phytol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, S.; Xie, Q.; Xia, Y.; Lu, L.; Wang, M.; Wang, G.; Long, S.; Cai, Y.; Xu, L.; et al. Control of arbuscule development by a transcriptional negative feedback loop in Medicago. Nat. Commun. 2023, 14, 5743. [Google Scholar] [CrossRef] [PubMed]
- George, T.S.; Simpson, R.J.; Hadobas, P.A.; Richardson, A.E. Expression of a fungal phytase gene in Nicotiana tabacum improves phosphorus nutrition of plants grown in amended soils. Plant Biotechnol. J. 2005, 3, 129–140. [Google Scholar] [CrossRef]
- Giovannini, L.; Sbrana, C.; Giovannetti, M.; Avio, L.; Lanubile, A.; Marocco, A.; Turrini, A. Diverse mycorrhizal maize inbred lines differentially modulate mycelial traits and the expression of plant and fungal phosphate transporters. Sci. Rep. 2022, 12, 21279. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Hijikata, N.; Yokoyama, K.; Ohtomo, R.; Handa, Y.; Kawaguchi, M.; Saito, K.; Ezawa, T. Polyphosphate accumulation is driven by transcriptome alterations that lead to near-synchronous and near-equivalent uptake of inorganic cations in an arbuscular mycorrhizal fungus. New Phytol. 2014, 204, 638–649. [Google Scholar] [CrossRef]
- Uetake, Y.; Kojima, T.; Ezawa, T.; Saito, M. Extensive tubular vacuole system in an arbuscular mycorrhizal fungus, Gigaspora margarita. New Phytol. 2002, 154, 761–768. [Google Scholar] [CrossRef]
- Andreeva, N.; Ledova, L.; Ryazanova, L.; Tomashevsky, A.; Kulakovskaya, T.; Eldarov, M. Ppn2 endopolyphosphatase overexpressed in Saccharomyces cerevisiae: Comparison with Ppn1, Ppx1, and Ddp1 polyphosphatases. Biochimie 2019, 163, 101–107. [Google Scholar] [CrossRef]
- Wipf, D.; Krajinski, F.; van Tuinen, D.; Recorbet, G.; Courty, P. Trading on the arbuscular mycorrhiza market: From arbuscules to common mycorrhizal networks. New Phytol. 2019, 223, 1127–1142. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, G.; Garstecka, Z.; Trejgell, A.; Dąbrowski, H.; Konieczna, W.; Szyp-Borowska, I. The Impact of Forest Fungi on Promoting Growth and Development of Brassica napus L. Agronomy 2021, 11, 2475. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.; Raza, S.; Khan, M.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Rigobelo, E.; Baron Cozentino, N. Endophytic fungi: A tool for plant growth promotion and sustainable agriculture. Mycology 2021, 13, 39–55. [Google Scholar] [CrossRef]
- Silva, J.; Montaldo, Y.; Almeida, A.; Dalbon, V.; Acevedo, J.; Santos, T.M.; Lima, G.S.D. Rhizospheric Fungi to Plant Growth Promotion: A Review. J. Agric. Stud. 2021, 9, 411. [Google Scholar] [CrossRef]
- Gao, X.; Guo, H.; Zhang, Q.; Guo, H.; Zhang, L.; Zhang, C.; Gou, Z.; Liu, Y.; Wei, J.; Chen, A.; et al. Arbuscular mycorrhizal fungi (AMF) enhanced the growth, yield, fiber quality and phosphorus regulation in upland cotton (Gossypium hirsutum L.). Sci. Rep. 2020, 10, 2084. [Google Scholar] [CrossRef]
- Ceballos, I.; Ruiz, M.; Fernández, C.; Peña, R.; Rodríguez, A.; Sanders, I.R. The In Vitro Mass-Produced Model Mycorrhizal Fungus, Rhizophagus irregularis, Significantly Increases Yields of the Globally Important Food Security Crop Cassava. PLoS ONE 2013, 8, e70633. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbeny, T.M.S.; Mousa, A.M.; El-Sayed, E.R. Use of mycorrhizal fungi and phosphorus fertilization to improve the yield of onion (Allium cepa L.) plant. Saudi J. Biol. Sci. 2022, 29, 331–338. [Google Scholar] [CrossRef]
- Mai, W.; Xue, X.; Feng, G.; Yang, R.; Tian, C. Arbuscular mycorrhizal fungi—15-Fold enlargement of the soil volume of cotton roots for phosphorus uptake in intensive planting conditions. Eur. J. Soil Biol. 2019, 90, 31–35. [Google Scholar] [CrossRef]
- Hiruma, K.; Kobae, Y.; Toju, H. Beneficial associations between Brassicaceae plants and fungal endophytes under nutrient-limiting conditions: Evolutionary origins and host–symbiont molecular mechanisms. Curr. Opin. Plant Biol. 2018, 44, 145–154. [Google Scholar] [CrossRef]
- Merlin, E.; Melato, E.; Lourenço, E.L.B.; Jacomassi, E.; Junior, A.G.; Da Cruz, R.M.S.; Otênio, J.K.; Da Silva, C.; Alberton, O. Inoculation of arbuscular mycorrhizal fungi and phosphorus addition increase coarse mint (Plectranthus amboinicus Lour.) plant growth and essential oil content. Rhizosphere 2020, 15, 100217. [Google Scholar] [CrossRef]
- Yadav, A.; Suri, V.K.; Kumar, A.; Choudhary, A.K. Effect of AM fungi and phosphorus fertilization on P-use efficiency, nutrient acquisition and root morphology in pea (Pisum sativum L.) in an acid Alfisol. J. Plant Nutr. 2018, 41, 689–701. [Google Scholar] [CrossRef]
- Cao, M.; Liu, R.; Xiao, Z.; Hashem, A.; Abd Allah, E.F.; Alsayed, M.F.; Harsonowati, W.; Wu, Q. Symbiotic Fungi Alter the Acquisition of Phosphorus in Camellia oleifera through Regulating Root Architecture, Plant Phosphate Transporter Gene Expressions and Soil Phosphatase Activities. J. Fungi 2022, 8, 800. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, J.; Zhu, H.; Xu, P.; Chen, J.; Yao, Q. The differential and interactive effects of arbuscular mycorrhizal fungus and phosphorus on the lateral root formation in Poncirus trifoliata (L.). Sci. Hortic.-Amst. 2017, 217, 258–265. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H.; Reich, P.B. Global-scale latitudinal patterns of plant fine-root nitrogen and phosphorus. Nat. Commun. 2011, 2, 344. [Google Scholar] [CrossRef] [PubMed]
- Kirchgesser, J.; Hazarika, M.; Bachmann-Pfabe, S.; Dehmer, K.J.; Kavka, M.; Uptmoor, R. Phenotypic variation of root-system architecture under high P and low P conditions in potato (Solanum tuberosum L.). BMC Plant Biol. 2023, 23, 68. [Google Scholar] [CrossRef]
- Liu, C.; Yan, H.; Wang, W.; Han, R.; Li, Z.; Lin, X.; Wang, D. Layered application of phosphate fertilizer increased winter wheat yield by promoting root proliferation and phosphorus accumulation. Soil Tillage Res. 2023, 225, 105546. [Google Scholar] [CrossRef]
- Pongrac, P.; Castillo-Michel, H.; Reyes-Herrera, J.; Hancock, R.D.; Fischer, S.; Kelemen, M.; Thompson, J.A.; Wright, G.; Likar, M.; Broadley, M.R.; et al. Effect of phosphorus supply on root traits of two Brassica oleracea L. genotypes. BMC Plant Biol. 2020, 20, 368. [Google Scholar] [CrossRef] [PubMed]
- Liu, D. Root developmental responses to phosphorus nutrition. J. Integr. Plant Biol. 2021, 63, 1065–1090. [Google Scholar] [CrossRef] [PubMed]
- Frew, A. Arbuscular mycorrhizal fungal diversity increases growth and phosphorus uptake in C3 and C4 crop plants. Soil Biol. Biochem. 2019, 135, 248–250. [Google Scholar] [CrossRef]
- Hoeksema, J.D.; Chaudhary, V.B.; Gehring, C.A.; Johnson, N.C.; Karst, J.; Koide, R.T.; Pringle, A.; Zabinski, C.; Bever, J.D.; Moore, J.C.; et al. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol. Lett. 2010, 13, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Jin, Z.; George, T.S.; Feng, G.; Zhang, L. Arbuscular mycorrhizal fungi enhance plant phosphorus uptake through stimulating hyphosphere soil microbiome functional profiles for phosphorus turnover. New Phytol. 2023, 238, 2578–2593. [Google Scholar] [CrossRef] [PubMed]
- Lagunas, B.; Richards, L.; Sergaki, C.; Burgess, J.; Pardal, A.J.; Hussain, R.M.F.; Richmond, B.L.; Baxter, L.; Roy, P.; Pakidi, A.; et al. Rhizobial nitrogen fixation efficiency shapes endosphere bacterial communities and Medicago truncatula host growth. Microbiome 2023, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Glassop, D.; Smith, S.E.; Smith, F.W. Cereal phosphate transporters associated with the mycorrhizal pathway of phosphate uptake into roots. Planta 2005, 222, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Rui, W.; Ma, J.; Wei, N.; Zhu, X.; Li, Z. Genome-Wide Analysis of the PHT Gene Family and Its Response to Mycorrhizal Symbiosis in Tomatoes under Phosphate Starvation Conditions. Int. J. Mol. Sci. 2023, 24, 10246. [Google Scholar] [CrossRef]
- Binbin, C.; Vancov, T.; Si, H.; Yang, W.; Tong, K.; Chen, W.; Fang, Y. Isolation and Characterization of Endomycorrhizal Fungi Associated with Growth Promotion of Blueberry Plants. J. Fungi 2021, 7, 584. [Google Scholar] [CrossRef]
- Javed, S.; Chai, X.; Wang, X.; Xu, S. The phytohormones underlying the plant lateral root development in fluctuated soil environments. Plant Soil 2023, 1–14. [Google Scholar] [CrossRef]
- Du, Y.; Scheres, B. Lateral root formation and the multiple roles of auxin. J. Exp. Bot. 2017, 69, 155–167. [Google Scholar] [CrossRef]
- Ivanchenko, M.G.; Muday, G.K.; Dubrovsky, J.G. Ethylene–auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. Plant J. 2008, 55, 335–347. [Google Scholar] [CrossRef]
- Sun, L.; Tian, J.; Zhang, H.; Liao, H. Phytohormone regulation of root growth triggered by P deficiency or Al toxicity. J. Exp. Bot. 2016, 67, 3655–3664. [Google Scholar] [CrossRef]
- Liu, R.; Yang, L.; Zou, Y.; Wu, Q. Root-associated endophytic fungi modulate endogenous auxin and cytokinin levels to improve plant biomass and root morphology of trifoliate orange. Hortic. Plant J. 2023, 9, 463–472. [Google Scholar] [CrossRef]
- Wu, F.; Li, Y.; Tian, W.; Sun, Y.; Chen, F.; Zhang, Y.; Zhai, Y.; Zhang, J.; Su, H.; Wang, L. A novel dark septate fungal endophyte positively affected blueberry growth and changed the expression of plant genes involved in phytohormone and flavonoid biosynthesis. Tree Physiol. 2020, 40, 1080–1094. [Google Scholar] [CrossRef]
- Saha, A.; Mandal, P.; Dasgupta, S.; Saha, D. Influence of culture media and environmental factors on mycelial growth and sporulation of Lasiodiplodia theobromae (Pat.) Griffon and Maubl. J. Environ. Biol./Acad. Environ. Biol. India 2008, 29, 407–410. [Google Scholar] [CrossRef]
- Cunguo, W.; Zong, S.; Li, M. The Contrasting Responses of Mycorrhizal Fungal Mycelium Associated with Woody Plants to Multiple Environmental Factors. Forests 2019, 10, 973. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, J.; George, T.S.; Limpens, E.; Feng, G. Arbuscular mycorrhizal fungi conducting the hyphosphere bacterial orchestra. Trends Plant Sci. 2022, 27, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, G.; Luo, X.; Hou, E.; Zheng, M.; Zhang, L.; He, X.; Shen, W.; Wen, D. Mycorrhizal fungi and phosphatase involvement in rhizosphere phosphorus transformations improves plant nutrition during subtropical forest succession. Soil Biol. Biochem. 2021, 153, 108099. [Google Scholar] [CrossRef]
- Khan, M.R.; Khan, S.M. Effects of root-dip treatment with certain phosphate solubilizing microorganisms on the fusarial wilt of tomato. Bioresour. Technol. 2002, 85, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Hachiya, S.; Inamura, N.; Ezawa, T.; Cheng, W.; Tawaraya, K. Secretion of acid phosphatase from extraradical hyphae of the arbuscular mycorrhizal fungus Rhizophagus clarus is regulated in response to phosphate availability. Mycorrhiza 2019, 29, 599–605. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, G.; Declerck, S. Signal beyond nutrient, fructose, exuded by an arbuscular mycorrhizal fungus triggers phytate mineralization by a phosphate solubilizing bacterium. ISME J. 2018, 12, 2339–2351. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Kong, X.; Long, Y.; Liu, S.; Zhang, H.; Jia, J.; Cui, W.; Zhang, Z.; Song, X.; Qiu, L.; et al. Integrated single-nucleus and spatial transcriptomics captures transitional states in soybean nodule maturation. Nat. Plants 2023, 9, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xiao, A.; Wu, J.; Li, H.; Duan, Y.; Chen, Q.; Zhu, H.; Cao, Y. GmNAC039 and GmNAC018 activate the expression of cysteine protease genes to promote soybean nodule senescence. Plant Cell 2023, 35, 2929–2951. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, R.; Zhang, C.; Yang, J.; Lyu, L.; Shi, Z.; Man, Y.B.; Wu, F. Selenium uptake and accumulation in winter wheat as affected by level of phosphate application and arbuscular mycorrhizal fungi. J. Hazard. Mater. 2022, 433, 128762. [Google Scholar] [CrossRef]
- Zhang, F.; Hou, Y.; Zed, R.; Mauchline, T.H.; Shen, J.; Zhang, F.; Jin, K. Root exudation of organic acid anions and recruitment of beneficial actinobacteria facilitate phosphorus uptake by maize in compacted silt loam soil. Soil Biol. Biochem. 2023, 184, 109074. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Khashi U Rahman, M.; Gao, D.; Wei, Z.; Wu, F.; Dini-Andreote, F. Interspecific plant interaction via root exudates structures the disease suppressiveness of rhizosphere microbiomes. Mol. Plant 2023, 16, 849–864. [Google Scholar] [CrossRef]
- Ho-Plágaro, T.; Morcillo, R.J.L.; Tamayo-Navarrete, M.I.; Huertas, R.; Molinero-Rosales, N.; López-Ráez, J.A.; Macho, A.P.; García-Garrido, J.M. DLK2 regulates arbuscule hyphal branching during arbuscular mycorrhizal symbiosis. New Phytol. 2021, 229, 548–562. [Google Scholar] [CrossRef]
- Almario, J.; Jeena, G.; Wunder, J.; Langen, G.; Zuccaro, A.; Coupland, G.; Bucher, M. Root-associated fungal microbiota of nonmycorrhizal Arabis alpina and its contribution to plant phosphorus nutrition. Proc. Natl. Acad. Sci. USA 2017, 114, E9403–E9412. [Google Scholar] [CrossRef]
- Glynou, K.; Nam, B.; Thines, M.; Maciá-Vicente, J.G. Facultative root-colonizing fungi dominate endophytic assemblages in roots of nonmycorrhizal Microthlaspi species. New Phytol. 2018, 217, 1190–1202. [Google Scholar] [CrossRef]
- Bai, B.; Liu, W.; Qiu, X.; Zhang, J.; Zhang, J.; Bai, Y. The root microbiome: Community assembly and its contributions to plant fitness. J. Integr. Plant Biol. 2022, 64, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Mahala, D.M.; Maheshwari, H.S.; Yadav, R.K.; Prabina, B.J.; Bharti, A.; Reddy, K.K.; Kumawat, C.; Ramesh, A. Microbial Transformation of Nutrients in Soil: An Overview. In Rhizosphere Microbes: Soil and Plant Functions; Sharma, S.K., Singh, U.B., Sahu, P.K., Singh, H.V., Sharma, P.K., Eds.; Springer: Singapore, 2020; pp. 175–211. [Google Scholar]
- Kariman, K.; Scanlan, C.; Boitt, G.; Rengel, Z. Feremycorrhizal symbiosis confers growth and nutritional benefits to mycorrhizal and non-mycorrhizal crops. Soil Biol. Biochem. 2020, 151, 108060. [Google Scholar] [CrossRef]
- Jentschke, G.; Brandes, B.; Kuhn, A.J.; Schröder, W.H.; Godbold, D.L. Interdependence of phosphorus, nitrogen, potassium and magnesium translocation by the ectomycorrhizal fungus Paxillus involutus. New Phytol. 2001, 149, 327–337. [Google Scholar] [CrossRef]
- DiCenzo, G.C.; Sharthiya, H.; Nanda, A.; Zamani, M.; Finan, T.M. PhoU Allows Rapid Adaptation to High Phosphate Concentrations by Modulating PstSCAB Transport Rate in Sinorhizobium meliloti. J. Bacteriol. 2017, 199, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Robichon, D.; Arnaud, M.; Gardan, R.; Pragai, Z.; O’Reilly, M.; Rapoport, G.; Débarbouillé, M. Expression of a New Operon from Bacillus subtilis, ykzB-ykoL, under the Control of the TnrA and PhoP-PhoR Global Regulators. J. Bacteriol. 2000, 182, 1226–1231. [Google Scholar] [CrossRef]
- Santos-Beneit, F. The Pho regulon: A huge regulatory network in bacteria. Front. Microbiol. 2015, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Lamarche, M.G.; Wanner, B.L.; Crépin, S.; Harel, J. The phosphate regulon and bacterial virulence: A regulatory network connecting phosphate homeostasis and pathogenesis. FEMS Microbiol. Rev. 2008, 32, 461–473. [Google Scholar] [CrossRef]
- Siles, J.; Starke, R.; Martinovic, T.; Fernandes, M.; Orgiazzi, A.; Bastida, F. Distribution of phosphorus cycling genes across land uses and microbial taxonomic groups based on metagenome and genome mining. Soil Biol. Biochem. 2022, 174, 108826. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, R. Nitrogen and Phosphorus Signaling and Transport During Legume–Rhizobium Symbiosis. Front. Plant Sci. 2021, 12, 683601. [Google Scholar] [CrossRef]
- Qin, L.; Jiang, H.; Tian, J.; Zhao, J.; Liao, H. Rhizobia enhance acquisition of phosphorus from different sources by soybean plants. Plant Soil 2011, 349, 25–36. [Google Scholar] [CrossRef]
- Buernor, A.; Kabiru, M.; Bechtaoui, N.; Jibrin, J.M.; Asante, M.; Bouraqqadi, A.; Dahhani, S.; Ouhdouch, Y.; Hafidi, M.; Jemo, M. Grain Legume Yield Responses to Rhizobia Inoculants and Phosphorus Supplementation Under Ghana Soils: A Meta-Synthesis. Front. Plant Sci. 2022, 13, 877433. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qin, L.; Zhou, L.; Li, X.; Chen, Z.; Sun, L.; Wang, W.; Lin, Z.; Zhao, J.; Yamaji, N.; et al. A nodule-localized phosphate transporter GmPT7 plays an important role in enhancing symbiotic N2 fixation and yield in soybean. New Phytol. 2019, 221, 2013–2025. [Google Scholar] [CrossRef]
- Qin, L.; Zhao, J.; Tian, J.; Chen, L.; Sun, Z.; Guo, Y.; Lu, X.; Gu, M.; Xu, G.; Liao, H. The High-Affinity Phosphate Transporter GmPT5 Regulates Phosphate Transport to Nodules and Nodulation in Soybean. Plant Physiol. 2012, 159, 1634–1643. [Google Scholar] [CrossRef]
- Liu, J.; Yang, R.; Yan, J.; Li, C.; Lin, X.; Lin, L.; Cao, Y.; Xu, T.; Li, J.; Yuan, Y.; et al. VPT-like genes modulate Rhizobium–legume symbiosis and phosphorus adaptation. Plant J. 2023, 116, 112–127. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, J.; Li, Y.; Zhang, J.; Li, S.; An, Y.; Hu, T.; Yang, P. Functional Analysis of the Phosphate Transporter Gene MtPT6 From Medicago truncatula. Front. Plant Sci. 2021, 11, 620377. [Google Scholar] [CrossRef] [PubMed]
- Nussaume, L.; Kanno, S.; Javot, H.; Marin, E.; Pochon, N.; Ayadi Robert, A.; Nakanishi, T.; Thibaud, M. Phosphate Import in Plants: Focus on the PHT1 Transporters. Front. Plant Sci. 2011, 2, 83. [Google Scholar] [CrossRef] [PubMed]
- Puga, M.I.; Rojas-Triana, M.; de Lorenzo, L.; Leyva, A.; Rubio, V.; Paz-Ares, J. Novel signals in the regulation of Pi starvation responses in plants: Facts and promises. Curr. Opin. Plant Biol. 2017, 39, 40–49. [Google Scholar] [CrossRef]
- Barragán-Rosillo, A.C.; Peralta-Alvarez, C.A.; Ojeda-Rivera, J.O.; Arzate-Mejía, R.G.; Recillas-Targa, F.; Herrera-Estrella, L. Genome accessibility dynamics in response to phosphate limitation is controlled by the PHR1 family of transcription factors in Arabidopsis. Proc. Natl. Acad. Sci. USA 2021, 118, e2107558118. [Google Scholar] [CrossRef]
- Bari, R.; Datt Pant, B.; Stitt, M.; Scheible, W. PHO2, MicroRNA399, and PHR1 Define a Phosphate-Signaling Pathway in Plants. Plant Physiol. 2006, 141, 988–999. [Google Scholar] [CrossRef]
- Lu, M.; Cheng, Z.; Zhang, X.; Huang, P.; Fan, C.; Yu, G.; Chen, F.; Xu, K.; Chen, Q.; Miao, Y.; et al. Spatial Divergence of PHR-PHT1 Modules Maintains Phosphorus Homeostasis in Soybean Nodules. Plant Physiol. 2020, 184, 236–250. [Google Scholar] [CrossRef]
- Li, Y.; Ma, W.; Zhang, K.; Wang, X.; Liu, R.; Tian, Y.; Ma, N.; Zhao, Q.; Xu, R.; Zhong, Y.; et al. Overexpression of GmPHR1 Promotes Soybean Yield through Global Regulation of Nutrient Acquisition and Root Development. Int. J. Mol. Sci. 2022, 23, 15274. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Z.; Youbin, K.; Li, X.; Li, W.; Du, H.; Zhang, C. GmPAP12 Is Required for Nodule Development and Nitrogen Fixation Under Phosphorus Starvation in Soybean. Front. Plant Sci. 2020, 11, 450. [Google Scholar] [CrossRef] [PubMed]
- Smil, V. PHOSPHORUS IN THE ENVIRONMENT: Natural Flows and Human Interferences. Annu. Rev. Energy Environ. 2000, 25, 53–88. [Google Scholar] [CrossRef]
- Riskin, S.H.; Porder, S.; Neill, C.; Figueira, A.M.E.S.; Tubbesing, C.; Mahowald, N. The fate of phosphorus fertilizer in Amazon soya bean fields. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120154. [Google Scholar] [CrossRef]
- Alewell, C.; Ringeval, B.; Ballabio, C.; Robinson, D.A.; Panagos, P.; Borrelli, P. Global phosphorus shortage will be aggravated by soil erosion. Nat. Commun. 2020, 11, 4546. [Google Scholar] [CrossRef]
- Yu, Y.; Du, C. Leaching of phosphorus from phosphate tailings and extraction of calcium phosphates: Toward comprehensive utilization of tailing resources. J. Environ. Manag. 2023, 347, 119159. [Google Scholar] [CrossRef]
- Wei, Z.; Zuo, H.; Li, J.; Ding, G.; Zhan, Y.; Zhang, L.; Wu, W.; Su, L.; Wei, Y. Insight into the mechanisms of insoluble phosphate transformation driven by the interactions of compound microbes during composting. Environ. Sci. Pollut. Res. 2021, 28, 32844–32855. [Google Scholar] [CrossRef]
- Kalayu, G.; Clay, D. Phosphate Solubilizing Microorganisms: Promising Approach as Biofertilizers. Int. J. Agron. 2019, 2019, 4917256. [Google Scholar] [CrossRef]
- Wang, C.; Pan, G.; Lu, X.; Qi, W. Phosphorus solubilizing microorganisms: Potential promoters of agricultural and environmental engineering. Front. Bioeng. Biotechnol. 2023, 11, 1181078. [Google Scholar] [CrossRef]
- Sun, B.; Jing, R.; Wang, Z.; Tian, L.; Mao, F.; Liu, Y. Diversity and community structure of endophytic Bacillus with antagonistic and antioxidant activity in the fruits of Xisha Wild Noni (Morinda citrifolia L.). Microb. Pathog. 2021, 158, 105065. [Google Scholar] [CrossRef] [PubMed]
- Wolde-meskel, E.; van Heerwaarden, J.; Abdulkadir, B.; Kassa, S.; Aliyi, I.; Degefu, T.; Wakweya, K.; Kanampiu, F.; Giller, K.E. Additive yield response of chickpea (Cicer arietinum L.) to Rhizobium inoculation and phosphorus fertilizer across smallholder farms in Ethiopia. Agric. Ecosyst. Environ. 2018, 261, 144–152. [Google Scholar] [CrossRef]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Song, J.; Du, Q.; Li, G.; Tarakina, N.V.; Antonietti, M. Synthetic Humic Acids Solubilize Otherwise Insoluble Phosphates to Improve Soil Fertility. Angew. Chem. Int. Ed. 2019, 58, 18813–18816. [Google Scholar] [CrossRef]
- Hu, H.; Tang, C.; Rengel, Z. Influence of phenolic acids on phosphorus mobilisation in acidic and calcareous soils. Plant Soil 2005, 268, 173–180. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; He, L.; Xu, X.; Wang, J.; Ren, C.; Guo, Y.; Zhao, F. Different mechanisms driving increasing abundance of microbial phosphorus cycling gene groups along an elevational gradient. iScience 2022, 25, 105170. [Google Scholar] [CrossRef]
- Tian, Y.; Shi, C.; Malo, C.U.; Kwatcho Kengdo, S.; Heinzle, J.; Inselsbacher, E.; Ottner, F.; Borken, W.; Michel, K.; Schindlbacher, A.; et al. Long-term soil warming decreases microbial phosphorus utilization by increasing abiotic phosphorus sorption and phosphorus losses. Nat. Commun. 2023, 14, 864. [Google Scholar] [CrossRef]
- Yu, H.; Wu, X.; Zhang, G.; Zhou, F.; Harvey, P.; Wang, L.; Fan, S.; Xie, X.; Li, F.; Zhou, H.; et al. Identification of the Phosphorus-Solubilizing Bacteria Strain JP233 and Its Effects on Soil Phosphorus Leaching Loss and Crop Growth. Front. Microbiol. 2022, 13, 892533. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.S.; Naidu, R.; Mahimairaja, S.; Baskaran, S. Influence of low-molecular-weight organic acids on the solubilization of phosphates. Biol. Fert. Soils 1994, 18, 311–319. [Google Scholar] [CrossRef]
- Panhwar, Q.A.; Jusop, S.; Naher, U.A.; Othman, R.; Razi, M.I.; Andrade, P.; Zhou, D. Application of Potential Phosphate-Solubilizing Bacteria and Organic Acids on Phosphate Solubilization from Phosphate Rock in Aerobic Rice. Sci. World J. 2013, 2013, 272409. [Google Scholar] [CrossRef] [PubMed]
- Kwong, W.K.; Zheng, H.; Moran, N.A. Convergent evolution of a modified, acetate-driven TCA cycle in bacteria. Nat. Microbiol. 2017, 2, 17067. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qu, F.; Zhu, Z.; Zhao, Y.; Chen, X.; Shi, M.; Wei, Z. The important role of tricarboxylic acid cycle metabolism pathways and core bacterial communities in carbon sequestration during chicken manure composting. Waste Manag. 2022, 150, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qu, J.; Zhang, L.; Xu, X.; Wei, G.; Zhao, Z.; Ren, M.; Cao, M. Identification and characterization of the TCA cycle genes in maize. BMC Plant Biol. 2019, 19, 592. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Zhang, J.; Wang, X.; Hong, J.; Qiu, C.; Meng, H. Transcriptome Profiling Analysis of Phosphate-Solubilizing Mechanism of Pseudomonas Strain W134. Microorganisms 2022, 10, 1998. [Google Scholar] [CrossRef]
- Ludueña, L.; Anzuay, S.; Angelini, J.; McIntosh, M.; Becker, A.; Rupp, O.; Goesmann, A.; Blom, J.; Fabra, A.; Taurian, T. Strain Serratia sp. S119: A potential biofertilizer for peanut and maize and a model bacterium to study phosphate solubilization mechanisms. Appl. Soil Ecol. 2018, 126, 107–112. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, F.; Zhou, J.; Harvey, P.; Yu, H.; Zhang, G.; Zhang, X. Genomic Analysis of Pseudomonas asiatica JP233: An Efficient Phosphate-Solubilizing Bacterium. Genes 2022, 13, 2290. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, L.; Zhou, J.; George, T.S.; Feng, G. Arbuscular mycorrhizal fungi enhance mineralisation of organic phosphorus by carrying bacteria along their extraradical hyphae. New Phytol. 2021, 230, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 587. [Google Scholar] [CrossRef]
- Leite, R.D.A.; Martins, L.C.; Ferreira, L.V.D.S.; Barbosa, E.S.; Alves, B.J.R.; Zilli, J.E.; Araújo, A.P.; Jesus, E.D.C. Co-inoculation of Rhizobium and Bradyrhizobium promotes growth and yield of common beans. Appl. Soil Ecol. 2022, 172, 104356. [Google Scholar] [CrossRef]
- Zavaleta-Pastor, M.; Sohlenkamp, C.; Gao, J.; Guan, Z.; Zaheer, R.; Finan, T.M.; Raetz, C.R.H.; López-Lara, I.M.; Geiger, O. Sinorhizobium meliloti phospholipase C required for lipid remodeling during phosphorus limitation. Proc. Natl. Acad. Sci. USA 2010, 107, 302–307. [Google Scholar] [CrossRef]
- Alam, F.; Khan, A.; Fahad, S.; Nawaz, S.; Ahmed, N.; Arif Ali, M.; Adnan, M.; Dawar, K.; Saud, S.; Hassan, S.; et al. Phosphate solubilizing bacteria optimize wheat yield in mineral phosphorus applied alkaline soil. J. Saudi Soc. Agric. Sci. 2022, 21, 339–348. [Google Scholar] [CrossRef]
- Elhaissoufi, W.; Ghoulam, C.; Barakat, A.; Zeroual, Y.; Bargaz, A. Phosphate bacterial solubilization: A key rhizosphere driving force enabling higher P use efficiency and crop productivity. J. Adv. Res. 2022, 38, 13–28. [Google Scholar] [CrossRef]
- Pradhan, M.; Sahoo, R.; Pradhan, C.; Tuteja, N.; Mohanty, S. Contribution of native phosphorous-solubilizing bacteria of acid soils on phosphorous acquisition in peanut (Arachis hypogaea L.). Protoplasma 2017, 254, 2225–2236. [Google Scholar] [CrossRef]
- Six, J. Plant nutrition for sustainable development and global health. Plant Soil 2011, 339, 1–2. [Google Scholar] [CrossRef]
- Sharma, P.; Tripathi, S.; Sirohi, R.; Kim, S.H.; Ngo, H.H.; Pandey, A. Uptake and mobilization of heavy metals through phytoremediation process from native plants species growing on complex pollutants: Antioxidant enzymes and photosynthetic pigments response. Environ. Technol. Innov. 2021, 23, 101629. [Google Scholar] [CrossRef]
- Finkel, O.M.; Salas-González, I.; Castrillo, G.; Spaepen, S.; Law, T.F.; Teixeira, P.J.P.L.; Jones, C.D.; Dangl, J.L. The effects of soil phosphorus content on plant microbiota are driven by the plant phosphate starvation response. PLoS Biol. 2019, 17, e3000534. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Du, Q.; Yan, P.; Yu, B.; Li, W.; Zou, C. The auxin signaling pathway contributes to phosphorus-mediated zinc homeostasis in maize. BMC Plant Biol. 2023, 23, 20. [Google Scholar] [CrossRef]
- Palmieri, D.; Vitale, S.; Lima, G.; Di Pietro, A.; Turrà, D. A bacterial endophyte exploits chemotropism of a fungal pathogen for plant colonization. Nat. Commun. 2020, 11, 5264. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Zhang, M.; Huang, G.; Yuan, Y.; Fu, C.; Yu, L. Coculture with two Bacillus velezensis strains enhances the growth of Anoectochilus plants via promoting nutrient assimilation and regulating rhizosphere microbial community. Ind. Crop. Prod. 2020, 154, 112697. [Google Scholar] [CrossRef]
- Berlanga-Clavero, M.V.; Molina-Santiago, C.; Caraballo-Rodríguez, A.M.; Petras, D.; Díaz-Martínez, L.; Pérez-García, A.; de Vicente, A.; Carrión, V.J.; Dorrestein, P.C.; Romero, D. Bacillus subtilis biofilm matrix components target seed oil bodies to promote growth and anti-fungal resistance in melon. Nat. Microbiol. 2022, 7, 1001–1015. [Google Scholar] [CrossRef] [PubMed]
- Baptista, J.P.; Teixeira, G.M.; de Jesus, M.L.A.; Bertê, R.; Higashi, A.; Mosela, M.; Da Silva, D.V.; de Oliveira, J.P.; Sanches, D.S.; Brancher, J.D.; et al. Antifungal activity and genomic characterization of the biocontrol agent Bacillus velezensis CMRP 4489. Sci. Rep. 2022, 12, 17401. [Google Scholar] [CrossRef]
- Liotti, R.G.; Da Silva Figueiredo, M.I.; Da Silva, G.F.; de Mendonça, E.A.F.; Soares, M.A. Diversity of cultivable bacterial endophytes in Paullinia cupana and their potential for plant growth promotion and phytopathogen control. Microbiol. Res. 2018, 207, 8–18. [Google Scholar] [CrossRef]
- Shen, Y.; Korkor, N.L.; Xiao, R.; Pu, Q.; Hu, M.; Zhang, S.; Kong, D.; Zeng, G.; Hu, X. Antagonistic activity of combined bacteria strains against southern blight pathogen of Dendrobium officinale. Biol. Control 2020, 151, 104291. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, S.; Shao, Y.; Zhang, K. Soil bacteria are more sensitive than fungi in response to nitrogen and phosphorus enrichment. Front. Microbiol. 2022, 13, 999385. [Google Scholar] [CrossRef]
- Ruibo Sun, W.Z.Y.L. Changes in phosphorus mobilization and community assembly of bacterial and fungal communities in rice rhizosphere under phosphate deficiency. Front. Microbiol. 2022, 13, 953340. [Google Scholar] [CrossRef]
- Kang, S.; Radhakrishnan, R.; Khan, A.L.; Kim, M.; Park, J.; Kim, B.; Shin, D.; Lee, I. Gibberellin secreting rhizobacterium, Pseudomonas putida H-2-3 modulates the hormonal and stress physiology of soybean to improve the plant growth under saline and drought conditions. Plant Physiol. Biochem. 2014, 84, 115–124. [Google Scholar] [CrossRef]
- Dao, G.; Wang, S.; Wang, X.; Chen, Z.; Wu, Y.; Wu, G.; Lu, Y.; Liu, S.; Hu, H. Enhanced Scenedesmus sp. growth in response to gibberellin secretion by symbiotic bacteria. Sci. Total Environ. 2020, 740, 140099. [Google Scholar] [CrossRef]
- Hu, L.; Li, D.; Sun, K.; Cao, W.; Fu, W.; Zhang, W.; Dai, C. Mutualistic fungus Phomopsis liquidambari increases root aerenchyma formation through auxin-mediated ethylene accumulation in rice (Oryza sativa L.). Plant Physiol. Biochem. 2018, 130, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Cosme, M.; Wurst, S. Interactions between arbuscular mycorrhizal fungi, rhizobacteria, soil phosphorus and plant cytokinin deficiency change the root morphology, yield and quality of tobacco. Soil Biol. Biochem. 2013, 57, 436–443. [Google Scholar] [CrossRef]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-Solubilizing Microorganisms: Mechanism and Their Role in Phosphate Solubilization and Uptake. J. Soil Sci. Plant Nutr. 2021, 21, 49–68. [Google Scholar] [CrossRef]
- Spring, A.; Rowan, K.S. Phosphorous Metabolism and Auxin Action. Nature 1966, 210, 1166–1167. [Google Scholar] [CrossRef]
- Huang, J.; Wu, Q.; Jing, H.K.; Shen, R.F.; Zhu, X.F. Auxin facilitates cell wall phosphorus reutilization in a nitric oxide-ethylene dependent manner in phosphorus deficient rice (Oryza sativa L.). Plant Sci. 2022, 322, 111371. [Google Scholar] [CrossRef]
- Xu, F.; Liao, H.; Yang, J.; Zhang, Y.; Yu, P.; Cao, Y.; Fang, J.; Chen, S.; Li, L.; Sun, L.; et al. Auxin-producing bacteria promote barley rhizosheath formation. Nat. Commun. 2023, 14, 5800. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, Y.; Hu, N.; Li, H.; Jiao, J. Interactions of bacterial-feeding nematodes and indole-3-acetic acid (IAA)-producing bacteria promotes growth of Arabidopsis thaliana by regulating soil auxin status. Appl. Soil Ecol. 2020, 147, 103447. [Google Scholar] [CrossRef]
- Tzipilevich, E.; Russ, D.; Dangl, J.L.; Benfey, P.N. Plant immune system activation is necessary for efficient root colonization by auxin-secreting beneficial bacteria. Cell Host Microbe 2021, 29, 1507–1520. [Google Scholar] [CrossRef]
- Arnold, A.E. Bacterial–fungal interactions: Bacteria take up residence in the house that Fungi built. Curr. Biol. 2022, 32, R327–R328. [Google Scholar] [CrossRef]
- Baudy, P.; Zubrod, J.P.; Konschak, M.; Kolbenschlag, S.; Pollitt, A.; Baschien, C.; Schulz, R.; Bundschuh, M. Fungal–fungal and fungal–bacterial interactions in aquatic decomposer communities: Bacteria promote fungal diversity. Ecology 2021, 102, e3471. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.J.; House, G.L.; Morales, D.P.; Kelliher, J.M.; Gallegos-Graves, L.V.; LeBrun, E.S.; Davenport, K.W.; Palmieri, F.; Lohberger, A.; Bregnard, D.; et al. Widespread bacterial diversity within the bacteriome of fungi. Commun. Biol. 2021, 4, 1168. [Google Scholar] [CrossRef] [PubMed]
- Nacoon, S.; Jogloy, S.; Riddech, N.; Mongkolthanaruk, W.; Ekprasert, J.; Cooper, J.; Boonlue, S. Combination of arbuscular mycorrhizal fungi and phosphate solubilizing bacteria on growth and production of Helianthus tuberosus under field condition. Sci. Rep. 2021, 11, 6501. [Google Scholar] [CrossRef] [PubMed]
- Nacoon, S.; Jogloy, S.; Riddech, N.; Mongkolthanaruk, W.; Kuyper, T.W.; Boonlue, S. Interaction between Phosphate Solubilizing Bacteria and Arbuscular Mycorrhizal Fungi on Growth Promotion and Tuber Inulin Content of Helianthus tuberosus L. Sci. Rep. 2020, 10, 4916. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Sarpong, C.K.; Zhang, X.; Wang, W.; Wang, L.; Gan, Y.; Yong, T.; Chang, X.; Wang, Y.; Yang, W. Mycorrhizosphere bacteria and plant-plant interactions facilitate maize P acquisition in an intercropping system. J. Clean. Prod. 2021, 314, 127993. [Google Scholar] [CrossRef]
- Cheng, X.; Xie, M.; Li, Y.; Liu, B.; Liu, C.; Wu, Q.; Kuča, K. Effects of field inoculation with arbuscular mycorrhizal fungi and endophytic fungi on fruit quality and soil properties of Newhall navel orange. Appl. Soil Ecol. 2022, 170, 104308. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, M.; Liu, Y.; Zhang, F.; Hodge, A.; Feng, G. Carbon and phosphorus exchange may enable cooperation between an arbuscular mycorrhizal fungus and a phosphate-solubilizing bacterium. New Phytol. 2016, 210, 1022–1032. [Google Scholar] [CrossRef]
- Godara, M.; Saxena, J.; Chandra, S.; Nain, L. Synergistic effect of phosphate solubilizing rhizobacteria and arbuscular mycorrhiza on growth and yield of wheat plants. J. Soil Sci. Plant Nutr. 2013, 13, 511–525. [Google Scholar] [CrossRef]
- Gupta, P.; Kumar, V.; Usmani, Z.; Rani, R.; Chandra, A.; Gupta, V.K. A comparative evaluation towards the potential of Klebsiella sp. and Enterobacter sp. in plant growth promotion, oxidative stress tolerance and chromium uptake in Helianthus annuus (L.). J. Hazard. Mater. 2019, 377, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, G.; Wei, Y.; Dong, Y.; Hou, L.; Jiao, R. Isolation and screening of multifunctional phosphate solubilizing bacteria and its growth-promoting effect on Chinese fir seedlings. Sci. Rep. 2021, 11, 9081. [Google Scholar] [CrossRef] [PubMed]
- George, D.M.; Vincent, A.S.; Mackey, H.R. An overview of anoxygenic phototrophic bacteria and their applications in environmental biotechnology for sustainable Resource recovery. Biotechnol. Rep. 2020, 28, e563. [Google Scholar] [CrossRef] [PubMed]
- Khuong, N.Q.; Huu, T.N.; Thuc, L.V.; Thu, L.T.M.; Xuan, D.T.; Quang, L.T.; Nhan, T.C.; Tran, H.N.; Tien, P.D.; Xuan, L.N.T.; et al. Two strains of Luteovulum sphaeroides (purple nonsulfur bacteria) promote rice cultivation in saline soils by increasing available phosphorus. Rhizosphere 2021, 20, 100456. [Google Scholar] [CrossRef]
- Liu, X.; Chen, C.; Wang, J.; Zou, S.; Long, X. Phosphorus solubilizing bacteria Bacillus thuringiensis and Pantoea ananatis simultaneously promote soil inorganic phosphate dissolution and soil Pb immobilization. Rhizosphere 2021, 20, 100448. [Google Scholar] [CrossRef]
- Qiu, F.; Liu, W.; Chen, L.; Wang, Y.; Ma, Y.; Lyu, Q.; Yi, S.; Xie, R.; Zheng, Y. Bacillus subtilis biofertilizer application reduces chemical fertilization and improves fruit quality in fertigated Tarocco blood orange groves. Sci. Hortic.-Amst. 2021, 281, 110004. [Google Scholar] [CrossRef]
- Estrada-Bonilla, G.A.; Durrer, A.; Cardoso, E.J.B.N. Use of compost and phosphate-solubilizing bacteria affect sugarcane mineral nutrition, phosphorus availability, and the soil bacterial community. Appl. Soil Ecol. 2021, 157, 103760. [Google Scholar] [CrossRef]
- Vidal, A.; Klöffel, T.; Guigue, J.; Angst, G.; Steffens, M.; Hoeschen, C.; Mueller, C.W. Visualizing the transfer of organic matter from decaying plant residues to soil mineral surfaces controlled by microorganisms. Soil Biol. Biochem. 2021, 160, 108347. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.; Ocampo Alvarez, H.; Santacruz-Ruvalcaba, F.; Sánchez-Hernández, C.; Casarrubias-Castillo, K.; Becerril, A.; Castañeda-Nava, J.; Hernández-Herrera, R. Physiological, Ecological, and Biochemical Implications in Tomato Plants of Two Plant Biostimulants: Arbuscular Mycorrhizal Fungi and Seaweed Extract. Front. Plant Sci. 2020, 11, 999. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, V.; Muhammad Zafar Iqbal, A.N.; Haseeb, M.; Khan, M.S. Antimicrobial potential of bacteriocin producing Lysinibacillus jx416856 against foodborne bacterial and fungal pathogens, isolated from fruits and vegetable waste. Anaerobe 2014, 27, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, H.A.; Issa, M.Y. In vitro and in vivo activity of Peganum harmala L. alkaloids against phytopathogenic bacteria. Sci. Hortic.-Amst. 2020, 264, 108940. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Bera, T.; Chakrabarty, A.M. Microbial siderophore—A boon to agricultural sciences. Biol. Control 2020, 144, 104214. [Google Scholar] [CrossRef]
- Rodríguez Chávez, J.L.; Juárez-Campusano, Y.; Delgado, G.; Pacheco Aguilar, J.R. Identification of lipopeptides from Bacillus strain Q11 with ability to inhibit the germination of Penicillium expansum, the etiological agent of postharvest blue mold disease. Postharvest Biol. Technol. 2019, 155, 72–79. [Google Scholar] [CrossRef]
- Kutschera, A.; Dawid, C.; Gisch, N.; Schmid, C.; Raasch, L.; Gerster, T.; Schäffer, M.; Smakowska-Luzan, E.; Belkhadir, Y.; Vlot, A.C.; et al. Bacterial medium-chain 3-hydroxy fatty acid metabolites trigger immunity in Arabidopsis plants. Science 2019, 364, 178–181. [Google Scholar] [CrossRef]
- Sehrawat, A.; Sindhu, S.; Glick, B. Hydrogen cyanide production by soil bacteria: Biological control of pests and promotion of plant growth in sustainable agriculture. Pedosphere 2022, 32, 15–38. [Google Scholar] [CrossRef]
- Dakora, F.D.; Phillips, D.A. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 2002, 245, 35–47. [Google Scholar] [CrossRef]
- Harbort, C.J.; Hashimoto, M.; Inoue, H.; Niu, Y.; Guan, R.; Rombolà, A.D.; Kopriva, S.; Voges, M.J.E.E.; Sattely, E.S.; Garrido-Oter, R.; et al. Root-Secreted Coumarins and the Microbiota Interact to Improve Iron Nutrition in Arabidopsis. Cell Host Microbe 2020, 28, 825–837. [Google Scholar] [CrossRef]
- Zhang, X.; Khadka, P.; Puchalski, P.; Leehan, J.D.; Rossi, F.R.; Okumoto, S.; Pilot, G.; Danna, C.H. MAMP-elicited changes in amino acid transport activity contribute to restricting bacterial growth. Plant Physiol. 2022, 189, 2315–2331. [Google Scholar] [CrossRef]
- Czaban, W.; Rasmussen, J.; Laursen, B.B.; Vidkjær, N.H.; Sapkota, R.; Nicolaisen, M.; Fomsgaard, I.S. Multiple effects of secondary metabolites on amino acid cycling in white clover rhizosphere. Soil Biol. Biochem. 2018, 123, 54–63. [Google Scholar] [CrossRef]
- Palacios, O.A.; Bashan, Y.; De-Bashan, L.E. Proven and potential involvement of vitamins in interactions of plants with plant growth-promoting bacteria—An overview. Biol. Fert Soils 2014, 50, 415–432. [Google Scholar] [CrossRef]
- Klee, H.J.; Hayford, M.B.; Kretzmer, K.A.; Barry, G.F.; Kishore, G.M. Control of ethylene synthesis by expression of a bacterial enzyme in transgenic tomato plants. Plant Cell 1991, 3, 1187–1193. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Singh, S.; Kumar, V.; Datta, S.; Singh, S.; Dhanjal, D.S.; Dhaka, V.; Sharma, K.; Singh, J. Chapter 12—Commercial production and formulation of microbial biocontrol agents. In Food Security and Plant Disease Management; Kumar, A., Droby, S., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 241–256. [Google Scholar]
- Liu, C.; Wu, J.; Chang, J. Diffusion characteristics and controlled release of bacterial fertilizers from modified calcium alginate capsules. Bioresour. Technol. 2008, 99, 1904–1910. [Google Scholar] [CrossRef] [PubMed]
- Wu, C. Controlled release evaluation of bacterial fertilizer using polymer composites as matrix. J. Control. Release 2008, 132, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef] [PubMed]
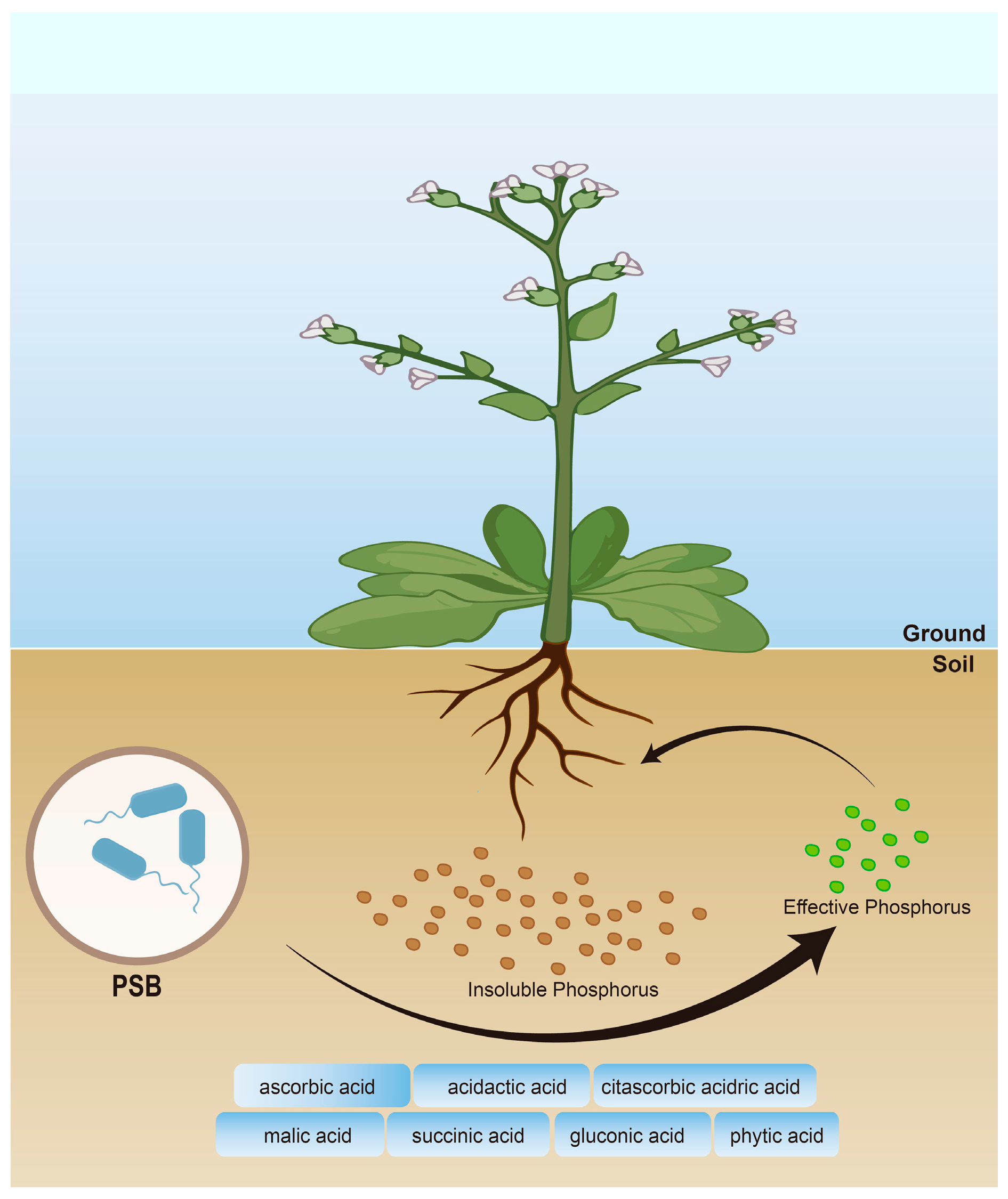
| Name | Species | References |
|---|---|---|
| PstA/PstB/PstC/PstS | Sinorhizobium meliloti | [108] |
| PhoU | Salmonella Typhimurium | [109] |
| PhoR/PhoB | Saccharomyces cerevisiae | [111] |
| YP6 | Bacillus amyloliquefaciens | [112] |
| PT7 | Glycine max | [116] |
| GmPT5 | Glycine max | [117] |
| VPT2/VPT3 | M. truncatula | [118] |
| MtPT6 | Arabidopsis | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Ma, L.; Feng, Q.; Luo, H.; Chen, C.; Wang, S.; Yuan, Y.; Liu, C.; Cao, X.; Li, N. Influence and Role of Fungi, Bacteria, and Mixed Microbial Populations on Phosphorus Acquisition in Plants. Agriculture 2024, 14, 358. https://doi.org/10.3390/agriculture14030358
Luo Y, Ma L, Feng Q, Luo H, Chen C, Wang S, Yuan Y, Liu C, Cao X, Li N. Influence and Role of Fungi, Bacteria, and Mixed Microbial Populations on Phosphorus Acquisition in Plants. Agriculture. 2024; 14(3):358. https://doi.org/10.3390/agriculture14030358
Chicago/Turabian StyleLuo, Yu, Lige Ma, Qirui Feng, Huan Luo, Chen Chen, Shuqi Wang, Yue Yuan, Can Liu, Xulv Cao, and Nannan Li. 2024. "Influence and Role of Fungi, Bacteria, and Mixed Microbial Populations on Phosphorus Acquisition in Plants" Agriculture 14, no. 3: 358. https://doi.org/10.3390/agriculture14030358
APA StyleLuo, Y., Ma, L., Feng, Q., Luo, H., Chen, C., Wang, S., Yuan, Y., Liu, C., Cao, X., & Li, N. (2024). Influence and Role of Fungi, Bacteria, and Mixed Microbial Populations on Phosphorus Acquisition in Plants. Agriculture, 14(3), 358. https://doi.org/10.3390/agriculture14030358





