Influence of a Hydrocarbon Biodestructor on the Growth and Content of Phytohormones in Secale cereale L. Plants under Petroleum Pollution of the Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions
2.2. Soil Microorganism Evaluation
2.3. Hormone Extraction, Purification, and Immunoassay
2.4. Detection of Lignin and Suberin Localization
2.5. Root Morphology Assessment
2.6. Statistics
3. Results and Discussion
3.1. Composition of Microorganisms in the Rhizosphere of Rye Roots
3.2. Morphometric Parameters of Shoots and Roots of Rye Plants under Soil Pollution and “Lenoil” Treatment
3.3. Concentration of Hormones in Rye Plants under Soil Pollution and Treatment with “Lenoil”
3.4. Root Structure of Rye Plants under Soil Pollution and Treatment with “Lenoil”
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oluremi, J.R.; Adewuyi, A.P.; Sanni, A.A. Compaction characteristics of oil contaminated residual soil. J. Eng. Technol. 2015, 6, 75–87. [Google Scholar]
- Korshunova, T.Y.; Chetverikov, S.P.; Bakaeva, M.D.; Kuzina, E.V.; Rafikova, G.F.; Chetverikova, D.V.; Loginov, O.N. Microorganisms in the elimination of oil pollution consequences (Review). Appl. Biochem. Microbiol. 2019, 55, 344–354. [Google Scholar] [CrossRef]
- Asemoloye, M.D.; Jonathan, S.G.; Ahmad, R. Synergistic plant-microbes interactions in the rhizosphere: A potential headway for the remediation of hydrocarbon polluted soils. Int. J. Phytoremediation 2019, 21, 71–83. [Google Scholar] [CrossRef]
- Rungwa, G.; Arpa, H.; Sakulas, A.; Harakuwe, T.D. Phytoremediation Potential of Fast-Growing Energy Plants: Challenges and Perspectives. Pol. J. Environ. Stud. 2020, 29, 505–516. [Google Scholar] [CrossRef]
- Numan, M.; Bashir, S.; Khan, Y.; Mumtaz, R.; Shinwari, K.Z.K.; Khan, A.L.; Khan, A.; Ahmed, A.H. Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: A review. Microbiol. Res. 2018, 209, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I.C. Phytohormone Mediation of Interactions Between Plants and Non-Symbiotic Growth Promoting Bacteria Under Edaphic Stresses. Front. Plant Sci. 2019, 10, 1368. [Google Scholar] [CrossRef]
- Bakaeva, M.; Kuzina, E.; Vysotskaya, L.; Kudoyarova, G.; Arkhipova, T.; Rafikova, G.; Chetverikov, S.; Korshunova, T.; Chetverikova, D.; Loginov, O. Capacity of Pseudomonas strains to degrade hydrocarbons, produce auxins and maintain plant growth under normal conditions and in the presence of petroleum contaminants. Plants 2020, 9, 379. [Google Scholar] [CrossRef]
- Prabha, J.; Kumar, M.; Tripathi, R. Opportunities and challenges of utilizing energy crops in phytoremediation of environmental pollutants: A review. In Bioremediation for Environmental Sustainability; Kumar, V., Saxena, G., Shah, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 17, pp. 383–396. [Google Scholar] [CrossRef]
- Pinchuk, I.P.; Polyanskaya, L.M.; Kirillova, N.P.; Stepanov, A.L. Specific features of the microbial community development in soddy-podzolic soil in the course of barley (Hordeum vulgare L.) Growing. Eurasian Soil Sc. 2018, 51, 1480–1486. [Google Scholar] [CrossRef]
- Sotnikova, Y.M.; Fedyaev, V.V.; Grigoriadi, A.S.; Garipova, M.I.; Makhmutov, A.R.; Galin, I.R.; Novoselova, E.I.; Yamaleeva, A.A.; Farkhutdinov, R.G. Assessment of the agricultural plants’ phytoremediation potential under oil pollution of the soil. Univ. Proc. Volga Reg. Nat. Sci. 2021, 3, 99–109. (In Russian) [Google Scholar] [CrossRef]
- McGill, W.W.; Rowell, M.J. Determination of oil content of oil contaminated soil. Sci. Tot. Environ. 1980, 14, 245–253. [Google Scholar] [CrossRef]
- Sugiyama, A.; Ueda, Y.; Zushi, T.; Takase, H.; Yazaki, K. Changes in the bacterial community of soybean rhizospheres during growth in the field. PLoS ONE 2014, 9, 100709. [Google Scholar] [CrossRef] [PubMed]
- Netrusov, A.I. Practical Work on Microbiology; Academy: Moscow, Russia, 2005; p. 608. (In Russian) [Google Scholar]
- Kudoyarova, G.R.; Vysotskaya, L.B.; Arkhipova, T.N.; Kuzmina, L.Y.; Galimsyanova, N.F.; Sidorova, L.V.; Gabbasova, I.M.; Melentiev, A.I.; Veselov, S.Y. Effect of auxin producing and phosphate solubilizing bacteria on mobility of soil phosphorus, growth rate, and pacquisition by wheat plants. Acta Physiol. Plant 2017, 39, 253. [Google Scholar] [CrossRef]
- Sharipova, G.; Ivanov, R.; Veselov, D.; Akhiyarova, G.; Seldimirova, O.; Galin, I.; Fricke, W.; Vysotskaya, L.; Kudoyarova, G. Effect of salinity on stomatal conductance, leaf hydraulic conductance, HvPIP2 aquaporin, and abscisic acid abundance in barley leaf cells. Int. J. Mol. Sci. 2022, 23, 14282. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Melentiev, A.I.; Martynenko, E.V.; Timergalina, L.N.; Arkhipova, T.N.; Shendel, G.V.; Kuzmina, L.Y.; Dodd, I.C.; Veselov, S.Y. Cytokinin producing bacteria stimulate amino acid deposition by wheat roots. Plant Physiol. Bioch. 2014, 83, 285–291. [Google Scholar] [CrossRef]
- Akhtyamova, Z.; Martynenko, E.; Arkhipova, T.; Seldimirova, O.; Galin, I.; Belimov, A.; Vysotskaya, L.; Kudoyarova, G. Influence of Plant Growth-Promoting Rhizobacteria on the Formation of Apoplastic Barriers and Uptake of Water and Potassium by Wheat Plants. Microorganisms 2023, 11, 1227. [Google Scholar] [CrossRef]
- Bezrukova, M.; Lubyanova, A.; Shakirova, F. Effect of WGA on 24-epibrassinolide-induced resistance of wheat plants and cell walls reinforcement under the influence of cadmium acetate. Biol. Life Sci. Forum. 2021, 4, 80. [Google Scholar] [CrossRef]
- Kitamura, R.S.A.; Maranho, L.T. Phytoremediation of petroleum hydrocarbons-contaminated soil using Desmodium incanum DC., Fabaceae. Rev. Latinoam. De Biotecnol. Ambient. Y Algal 2016, 7, 1–15. [Google Scholar] [CrossRef]
- Kuzina, E.V.; Rafikova, G.F.; Stolyarova, E.A.; Loginov, O.N. Efficiency of associations of legume plants and growth-stimulating bacteria for restoration of oil-contaminated soils. Agrochemistry 2021, 4, 87–96. (In Russian) [Google Scholar]
- Koshlaf, E.; Ball, A.S. Soil bioremediation approaches for petroleum hydrocarbon polluted environments. AIMS Microbiol. 2017, 3, 25–49. [Google Scholar] [CrossRef]
- Liao, J.Q.; Wang, J.; Huang, Y. Bacterial community features are shaped by geographic location, physicochemical properties, and oil contamination of soil in main oil fields of China. Microb. Ecol. 2015, 70, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Romero-Hernández, L.; Velez, P.; Betanzo-Gutiérrez, I.; Camacho-López, M.D.; Vázquez-Duhalt, R.; Riquelme, M. Extra-heavy crude oil degradation by Alternaria sp. isolated from deep-sea sediments of the Gulf of Mexico. Appl. Sci. 2021, 11, 6090. [Google Scholar] [CrossRef]
- Khabibullina, F.M.; Panyukov, A.N. Microbiota transformation under the influence of agriculture in tundra zone. Theor. Appl. Ecol. 2010, 3, 52–58. (In Russian) [Google Scholar]
- Isakova, E.A. Features of influence of oil and oil products on soil biota. Colloq. J. 2019, 12, 7–10. (In Russian) [Google Scholar]
- Ivanova, A.A.; Vetrova, A.A.; Filonov, A.E.; Boronin, A.M. Oil biodegradation by microbial–plant associations. Appl. Biochem. Microbiol. 2015, 51, 196–201. [Google Scholar] [CrossRef]
- Massa, F.; Defez, R.; Bianco, C. Exploitation of plant growth promoting bacteria for sustainable agriculture: Hierarchical approach to link laboratory and field experiments. Microorganisms 2022, 10, 865. [Google Scholar] [CrossRef]
- Vysotskaya, L.B.; Kudoyarova, G.R.; Arkhipova, T.N.; Kuzina, E.V.; Rafikova, G.F.; Akhtyamova, Z.A.; Ivanov, R.S.; Chetverikov, S.P.; Chetverikova, D.V.; Bakaeva, M.D.; et al. The influence of the association of barley plants with petroleum degrading bacteria on the hormone content, growth and photosynthesis of barley plants grown in the oil contaminated soil. Acta Physiol. Plant. 2021, 43, 67. [Google Scholar] [CrossRef]
- Maksimov, I.V.; Veselova, S.V.; Nuzhnaya, T.V.; Sarvarova, E.R.; Khairullin, R.M. Plant growth-promoting bacteria in regulation of plant resistance to stress factors. Russ. J. Plant Physiol. 2015, 62, 715–726. [Google Scholar] [CrossRef]
- Rafikova, G.F.; Kuzina, E.V.; Vysotskaya, L.B.; Arkhipova, T.N.; Korshunova, T.Y.; Chetverikova, D.V.; Bakaeva, M.D.; Kudoyarova, G.R.; Chetverikov, S.P. The influence of hydrocarbon-oxidizing auxin-producing bacteria on the growth, biochemical parameters, and hormonal status of barley plants in the process of bioremediation of oil-contaminated soil. J. Sib. Fed. Univ. Biol. 2022, 15, 314–332. (In Russian) [Google Scholar] [CrossRef]
- Mearaji, H.S.; Ansari, A.; Igdelou, N.K.M.; Lajayer, B.A.; Pessarakli, M. Phytohormones and abiotic stresses: Roles of phytohormones in plants under abiotic stresses. In Handbook Plant and Crop Physiology, 4th ed.; Pessarakli, M., Ed.; CRC Press: New York, NY, USA, 2021; pp. 175–213. [Google Scholar] [CrossRef]
- Trekozova, A.V.; Akhiyarova, G.R.; Vysotskaya, L.B.; Veselov, S.Y.; Kudoyarova, G.R. Effect of phosphorus deficit on root to shoot mass ratio, root elongation, branching and hormones content in Arabidorsis plants. Agrochemistry 2015, 8, 32–38. (In Russian) [Google Scholar]
- Arkhipova, T.; Martynenko, E.; Sharipova, G.; Kuzmina, L.; Ivanov, I.; Garipova, M.; Kudoyarova, G. Effects of plant growth promoting rhizobacteria on the content of abscisic acid and salt resistance of wheat plants. Plants 2020, 9, 1429. [Google Scholar] [CrossRef]
- Hallmark, H.T.; Rashotte, A.M. Review—Cytokinin response factors: Responding to more than cytokinin. Plant Sci. 2019, 289, 110251. [Google Scholar] [CrossRef]
- Veselov, D.S.; Kudoyarova, G.R.; Kudryakova, N.V.; Kusnetsov, V.V. Role of cytokinins in stress resistance of plants. Russ. J. Plant Physiol. 2017, 64, 15–27. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Vysotskaya, L.B.; Martinenko, E.V.; Ivanov, I.I.; Kudoyarova, G.R. Participation of cytokinins in plant response to competitors. Russ. J. Plant Physiol. 2015, 62, 524–533. [Google Scholar] [CrossRef]
- Ilina, E.L.; Kiryushkin, A.S.; Tsyganov, V.E.; Pawlowski, K.; Demchenko, K.N. Molecular, genetic and hormonal outlook in root branching. Agric. Biol. 2017, 52, 856–868. (In Russian) [Google Scholar] [CrossRef]
- Sharipova, G.; Veselov, D.; Kudoyarova, G.; Fricke, W.; Dodd, I.; Katsuhara, M.; Furuichi, T.; Ivanov, I.; Veselov, S. Exogenous application of abscisic acid (ABA) increases root and cell hydraulic conductivity and abundance of some aquaporin isoforms in the ABA deficient barley mutant Az34. Ann. Bot. 2016, 118, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Akhiyarova, G.; Veselov, D.; Ivanov, R.; Sharipova, G.; Ivanov, I.; Dodd, I.C.; Kudoyarova, G. Root ABA accumulation delays lateral root emergence in osmotically stressed barley plants by decreasing root primordial IAA accumulation. Int. J. Plant Biol. 2023, 14, 77–90. [Google Scholar] [CrossRef]
- Belovezhets, L.A.; Tretyakova, M.S.; Markova, Y.A.; Oznobikhina, L.P. Physicochemical properties of biosurfactants produced by oil destructor microorganisms. Chem. Sustain. Dev. 2021, 29, 20–25. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Ngueagni, P.T. A review on new aspects of lipopeptide biosurfactant: Types, production, properties and its application in the bioremediation process. J. Hazard. Mater. 2021, 407, 124827. [Google Scholar] [CrossRef]
- Martynenko, E.; Arkhipova, T.; Safronova, V.; Seldimirova, O.; Galin, I.; Akhtyamova, Z.; Veselov, D.; Ivanov, R.; Kudoyarova, G. Effects of phytohormone-producing rhizobacteria on casparian band formation, ion homeostasis and salt tolerance of durum wheat. Biomolecules 2022, 12, 230. [Google Scholar] [CrossRef]


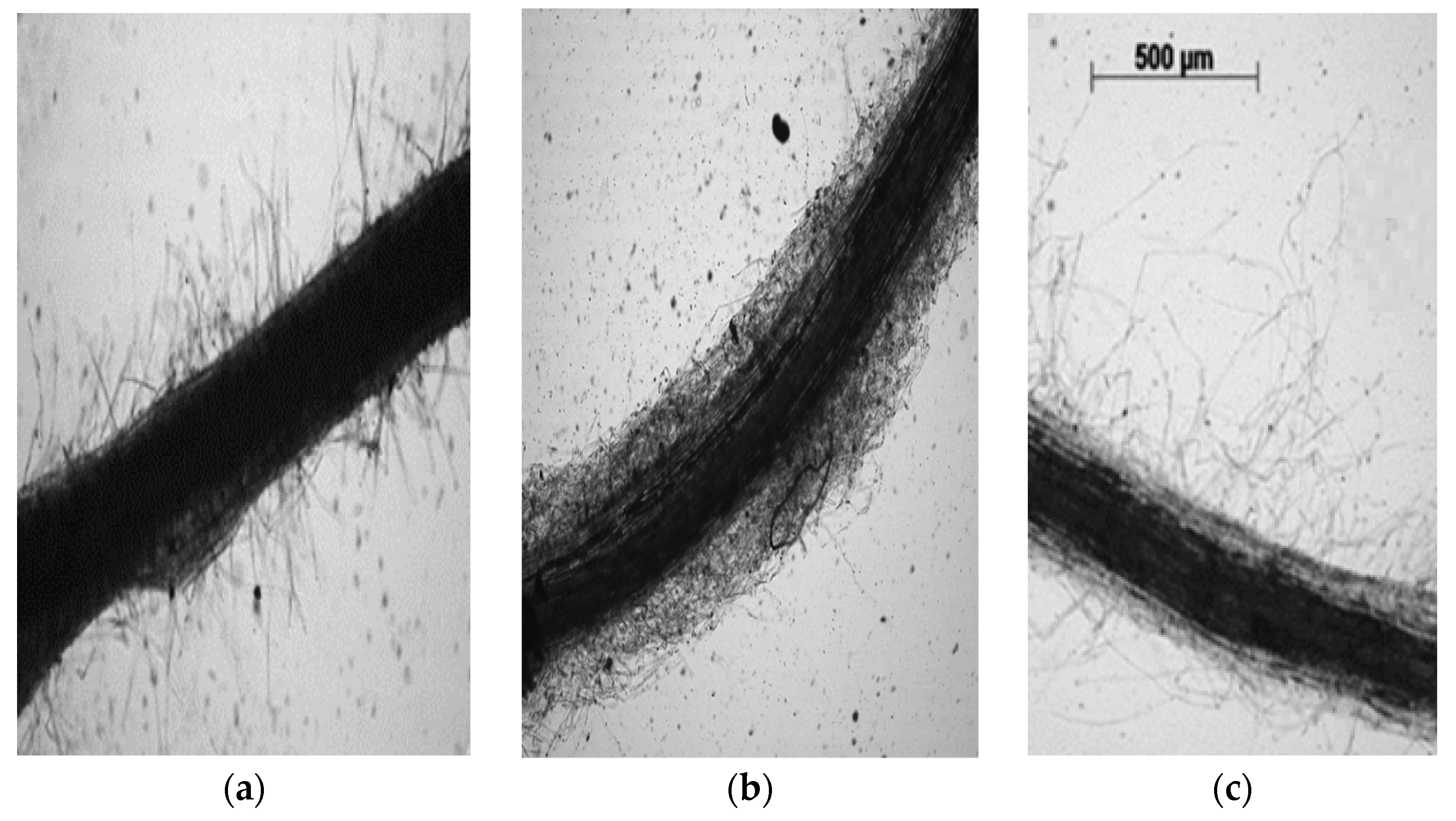
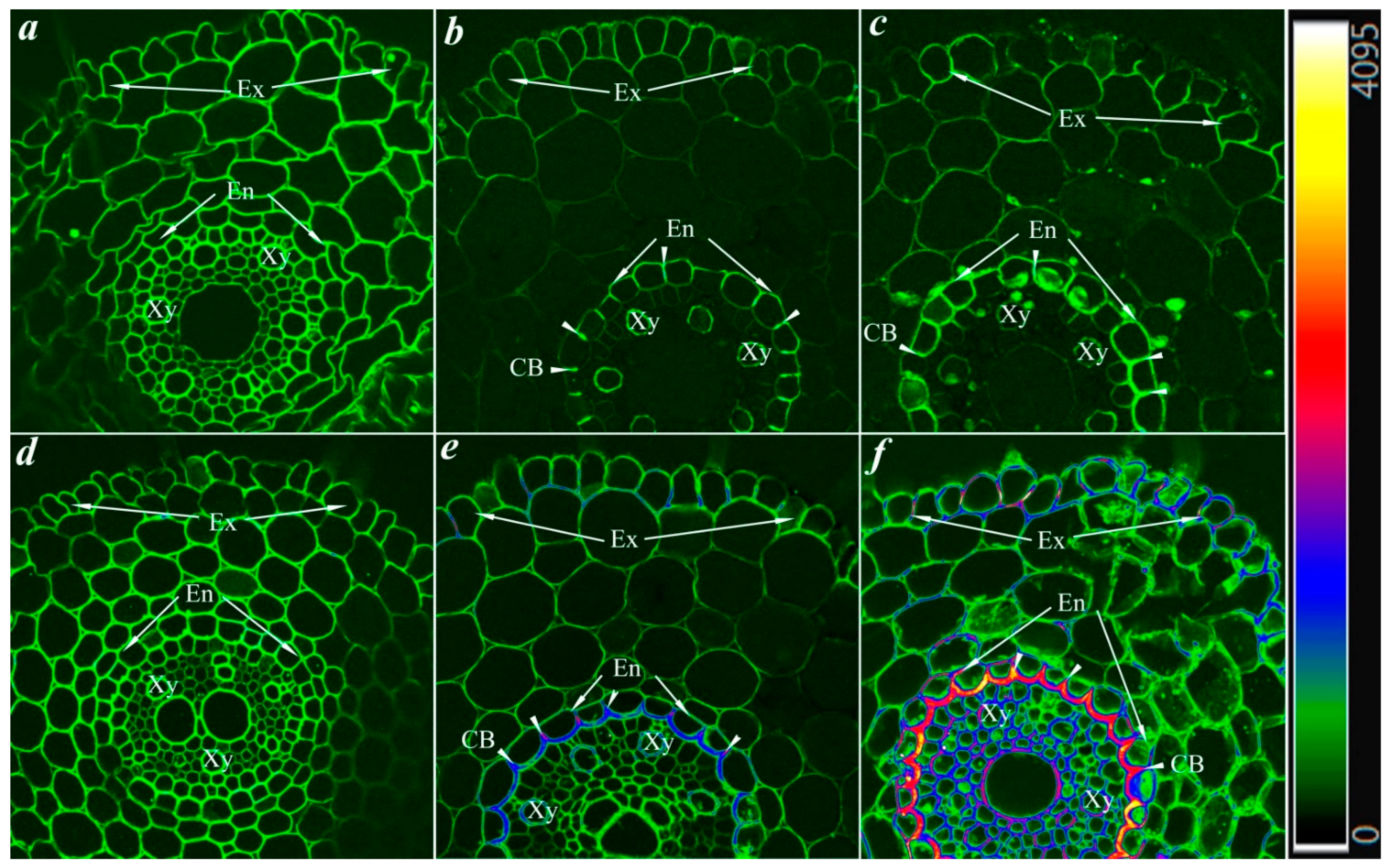
| Plant Part | Parameter | Control | Petroleum | Petroleum + “Lenoil” |
|---|---|---|---|---|
| Shoot | Length, cm | 17.83 a ± 1.22 | 8.46 b ± 0.52 | 32.11 c ± 1.32 |
| Fresh weight, g | 8.36 a ± 0.66 | 2.38 b ± 0.26 | 10.46 a ± 0.96 | |
| Root | Length, cm | 12.24 a ± 1.04 | 7.34 b ± 0.56 | 18.41 c ± 1.26 |
| Fresh weight, g | 1.75 a ± 0.12 | 0.47 b ± 0.04 | 3.82 c ± 0.22 |
| Experiment Variant | Total Length of Roots, cm | Number of Primordia, Pixels/Root | Number of Lateral Roots, Pixels/Root |
|---|---|---|---|
| Control | 17.22 b ± 0.28 | 7.1 a ± 0.5 | 3.2 c ± 0.2 |
| Petroleum | 8.26 a ± 0.68 | 11.7 b ± 0.3 | 0.5 a ± 0.2 |
| Petroleum + Lenoil | 24.78 c ± 0.36 | 10.8 b ± 0.4 | 2.1 b ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotnikova, Y.; Grigoriadi, A.; Fedyaev, V.; Garipova, M.; Galin, I.; Sharipova, G.; Yamaleeva, A.; Chetverikov, S.; Veselov, D.; Kudoyarova, G.; et al. Influence of a Hydrocarbon Biodestructor on the Growth and Content of Phytohormones in Secale cereale L. Plants under Petroleum Pollution of the Soil. Agriculture 2023, 13, 1640. https://doi.org/10.3390/agriculture13081640
Sotnikova Y, Grigoriadi A, Fedyaev V, Garipova M, Galin I, Sharipova G, Yamaleeva A, Chetverikov S, Veselov D, Kudoyarova G, et al. Influence of a Hydrocarbon Biodestructor on the Growth and Content of Phytohormones in Secale cereale L. Plants under Petroleum Pollution of the Soil. Agriculture. 2023; 13(8):1640. https://doi.org/10.3390/agriculture13081640
Chicago/Turabian StyleSotnikova, Yulia, Anna Grigoriadi, Vadim Fedyaev, Margarita Garipova, Ilshat Galin, Guzal Sharipova, Anna Yamaleeva, Sergey Chetverikov, Dmitriy Veselov, Guzel Kudoyarova, and et al. 2023. "Influence of a Hydrocarbon Biodestructor on the Growth and Content of Phytohormones in Secale cereale L. Plants under Petroleum Pollution of the Soil" Agriculture 13, no. 8: 1640. https://doi.org/10.3390/agriculture13081640
APA StyleSotnikova, Y., Grigoriadi, A., Fedyaev, V., Garipova, M., Galin, I., Sharipova, G., Yamaleeva, A., Chetverikov, S., Veselov, D., Kudoyarova, G., & Farkhutdinov, R. (2023). Influence of a Hydrocarbon Biodestructor on the Growth and Content of Phytohormones in Secale cereale L. Plants under Petroleum Pollution of the Soil. Agriculture, 13(8), 1640. https://doi.org/10.3390/agriculture13081640








