Antifungal Activity of Bacillus velezensis X3-2 Against Plant Pathogens and Biocontrol Effect on Potato Late Blight
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture Media, Sample Source, and Test Strains
2.2. Isolation of Rhizosphere Bacteria
2.3. Screening of P. infestans Antagonist Bacteria
2.4. The Effect of X3-2 on the Growth of Other Plant Pathogens In Vitro
2.5. Taxonomic Identification of Strain X3-2
2.6. Strain X3-2 Biocontrol Assays on Potato Tissues In Vitro
2.7. Talc-Based Formulation of X3-2 Bioagents
2.8. Effect of Formulated X3-2 Bioagents on Potato Late Blight in a Pot Experiment
2.9. Antagonistic Effects of X3-2 Against P. infestans
2.10. Determination of Extracellular Enzymes and Traits of Plant Growth Promoting (PGP) of X3-2
2.11. Data Analysis
3. Results
3.1. Isolation and Screening of P. infestans Antagonist Bacteria
3.2. Identification of Strain X3-2
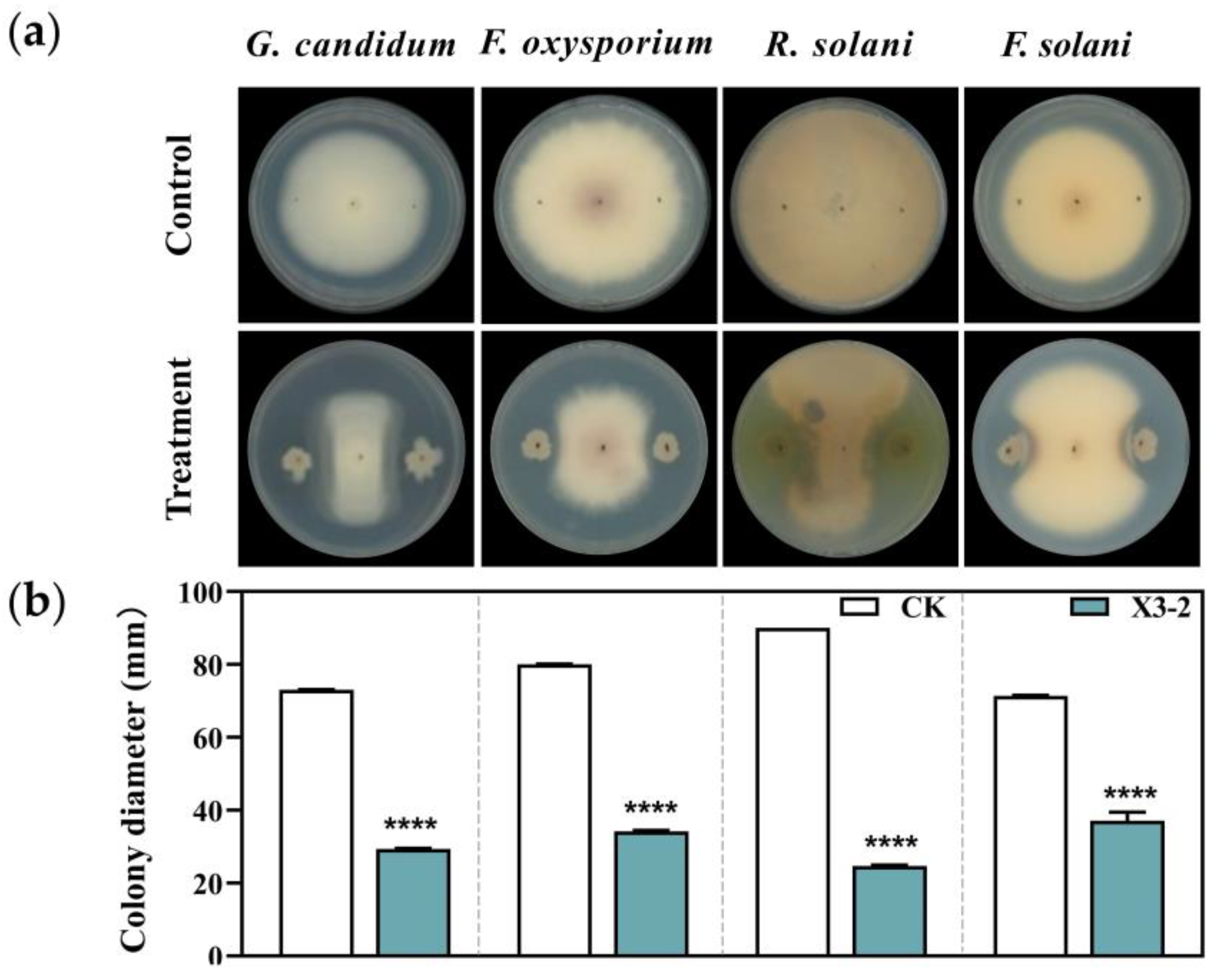
3.3. The Effect of X3-2 Against Other Plant Pathogens In Vitro
3.4. Effect of X3-2 on Inducing Potato Tissue Resistance to Late Blight
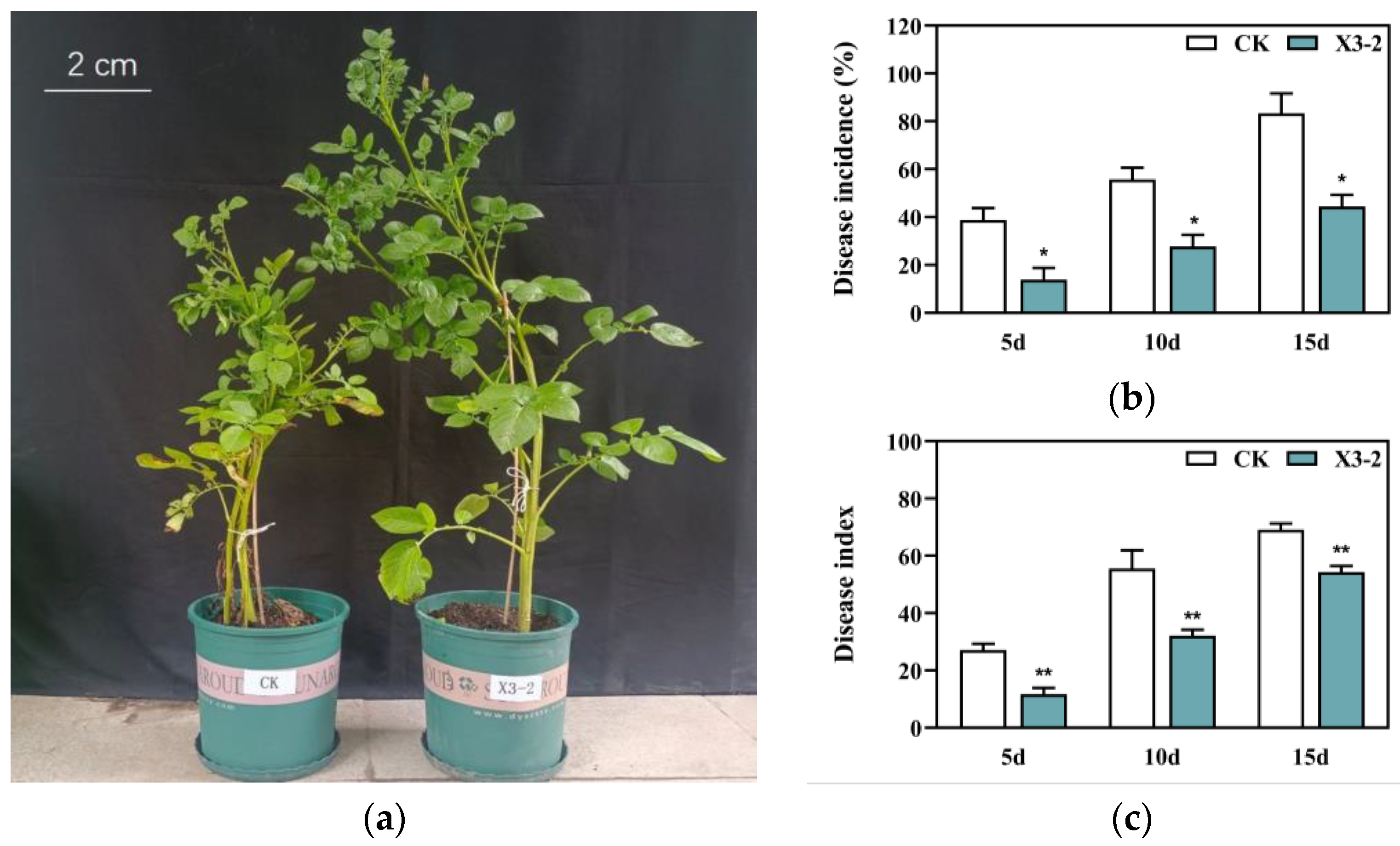
3.5. Effect of Formulated X3-2 Bioagents on Potato Plants in a Pot Experiment
3.6. Evaluation of the Antagonistic Effects of X3-2 Against P. infestans
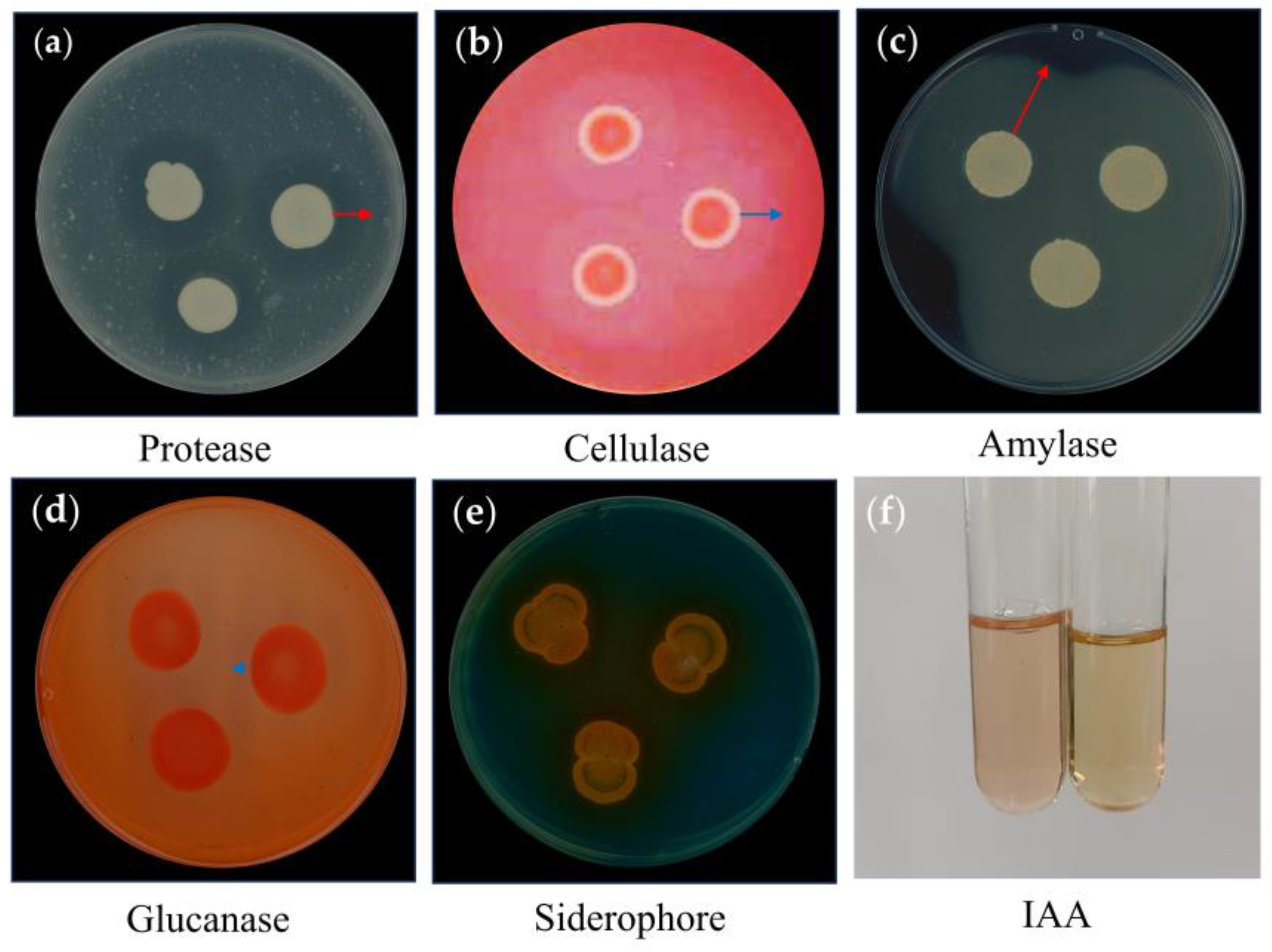
3.7. Detection of Extracellular Enzymes and Traits of Plant Growth Promoting (PGP) of X3-2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bvenura, C.; Witbooi, H.; Kambizi, L. Pigmented Potatoes: A Potential Panacea for Food and Nutrition Security and Health? Foods 2022, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Fry, W. Phytophthora infestans: The Plant (and R Gene) Destroyer. Mol. Plant Pathol. 2008, 9, 385–402. [Google Scholar] [CrossRef] [PubMed]
- Fry, W.; Birch, P.; Judelson, H.; Grünwald, N.; Danies, G.; Everts, K.; Gevens, A.; Gugino, B.; Johnson, D.; Johnson, S.; et al. Five Reasons to Consider Phytophthora infestans a Reemerging Pathogen. Phytopathology 2015, 105, 966–981. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, S.; Ru, J.; Tang, B.; Zhai, Y.; Wang, Z.; Wang, L. Biological Control of Potato Late Blight by Streptomyces sp. FXP04 and Potential Role of Secondary Metabolites. Biol. Control 2022, 169, 104891. [Google Scholar] [CrossRef]
- Sharma, S.; Sundaresha, S.; Bhardwaj, V. Biotechnological Approaches in Management of Oomycetes Diseases. 3 Biotech 2021, 11, 274. [Google Scholar] [CrossRef]
- Wharton, P.S.; Kirk, W.W.; Schafer, R.L.; Tumbalam, P. Evaluation of Biological Seed Treatments in Combination with Management Practices for the Control of Seed-Borne Late Blight in Potato. Biol. Control 2012, 63, 326–332. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Zhang, Y.; Chen, Y.; Tan, X.; Su, P.; Zhang, D.; Liu, Y. Integrated Control of Potato Late Blight with a Combination of the Photosynthetic Bacterium Rhodopseudomonas palustris Strain GJ-22 and Fungicides. BioControl 2020, 65, 635–645. [Google Scholar] [CrossRef]
- Cohen, Y.; Rubin, A.E.; Galperin, M. Effective Control of Two Genotypes of Phytophthora infestans in the Field by Three Oxathiapiprolin Fungicidal Mixtures. PLoS ONE 2021, 16, e0258280. [Google Scholar] [CrossRef]
- Axel, C.; Zannini, E.; Coffey, A.; Guo, J.; Waters, D.M.; Arendt, E.K. Ecofriendly Control of Potato Late Blight Causative Agent and the Potential Role of Lactic Acid Bacteria: A Review. Appl. Microbiol. Biot. 2012, 96, 37–48. [Google Scholar] [CrossRef]
- Ivanov, A.A.; Ukladov, E.O.; Golubeva, T.S. Phytophthora infestans: An Overview of Methods and Attempts to Combat Late Blight. J. Fungi 2021, 7, 1071. [Google Scholar] [CrossRef]
- Sun, X.; Xu, Z.; Xie, J.; Hesselberg-Thomsen, V.; Tan, T.; Zheng, D.; Strube, M.L.; Dragoš, A.; Shen, Q.; Zhang, R.; et al. Bacillus velezensis Stimulates Resident Rhizosphere Pseudomonas stutzeri for Plant Health through Metabolic Interactions. ISME J. 2022, 16, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.B.; Aleklett, K.; Digdarshika, G.; Lankinen, Å.; Grenville-Briggs, L.J. Pythium oligandrum Induces Growth Promotion in Starch Potato without Significantly Altering the Rhizosphere Microbiome. Appl. Soil Ecol. 2024, 199, 105423. [Google Scholar] [CrossRef]
- Wei, D.; Zhu, D.; Zhang, Y.; Yang, Z.; Hu, Y.; Song, C.; Yang, W.; Chang, X. Pseudomonas chlororaphis IRHB3 Assemblies Beneficial Microbes and Activates JA-Mediated Resistance to Promote Nutrient Utilization and Inhibit Pathogen Attack. Front. Microbiol. 2024, 15, 1328863. [Google Scholar] [CrossRef] [PubMed]
- Majeed, A.; Muhammad, Z.; Ullah, Z.; Ullah, R.; Ahmad, H. Late Blight of Potato (Phytophthora infestans) I: Fungicides Application and Associated Challenges. Turk. J. Agric.-Food Sci. Technol. 2017, 5, 261–266. [Google Scholar] [CrossRef]
- Wu, Z.; Cui, H.; Sun, Z.; Liu, H. Biocontrol Mechanism of Myxococcus xanthus B25-I-1 against Phytophthora infestans. Pestic. Biochem. Physiol. 2021, 175, 104832. [Google Scholar] [CrossRef]
- Yan, H.; Qiu, Y.; Yang, S.; Wang, Y.; Wang, K.; Jiang, L.; Wang, H. Antagonistic Activity of Bacillus velezensis SDTB038 against Phytophthora infestans in Potato. Plant Dis. 2021, 105, 1738–1747. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, J.; Zhang, C.; Wang, L.; Gao, W.; Jiang, J. Bacillus megaterium WL-3 Lipopeptides Collaborate against Phytophthora infestans to Control Potato Late Blight and Promote Potato Plant Growth. Front. Microbiol. 2020, 11, 542112. [Google Scholar] [CrossRef]
- Yao, Y.; Li, Y.; Chen, Z.; Zheng, B.; Zhang, L.; Niu, B.; Meng, J.; Li, A.; Zhang, J.; Wang, Q. Biological Control of Potato Late Blight Using Isolates of Trichoderma. Am. J. Potato Res. 2016, 93, 33–42. [Google Scholar] [CrossRef]
- Kadiri, M.; Sevugapperumal, N.; Nallusamy, S.; Ragunathan, J.; Ganesan, M.V.; Alfarraj, S.; Ansari, M.J.; Sayyed, R.Z.; Lim, H.R.; Show, P.L. Pan-Genome Analysis and Molecular Docking Unveil the Biocontrol Potential of Bacillus velezensis VB7 against Phytophthora infestans. Microbiol. Res. 2023, 268, 127277. [Google Scholar] [CrossRef]
- Kim, M.J.; Shim, C.K.; Park, J.-H. Control Efficacy of Bacillus velezensis AFB2-2 against Potato Late Blight Caused by Phytophthora infestans in Organic Potato Cultivation. Plant Pathol. J. 2021, 37, 580. [Google Scholar] [CrossRef]
- Nanjani, S.; Soni, R.; Paul, D.; Keharia, H. Genome Analysis Uncovers the Prolific Antagonistic and Plant Growth-Promoting Potential of Endophyte Bacillus velezensis K1. Gene 2022, 836, 146671. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, P.N.; Jha, D.K. Plant Growth-Promoting Rhizobacteria (PGPR): Emergence in Agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef] [PubMed]
- Borriss, R. Use of Plant-Associated Bacillus Strains as Biofertilizers and Biocontrol Agents in Agriculture. Bact. Agrobiol. Plant Growth Res. 2011, 41–76. [Google Scholar]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–Microbiome Interactions: From Community Assembly to Plant Health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Anderson, A.J.; Kim, Y.C. Insights into Plant-Beneficial Traits of Probiotic Pseudomonas chlororaphis Isolates. J. Med. Microbiol. 2020, 69, 361–371. [Google Scholar] [CrossRef]
- Medina, M.V.; Platt, H. Comparison of Different Culture Media on the Mycelial Growth, Sporangia and Oospore Production of Phytophthora infestans. Am. J. Potato Res. 1999, 76, 121–125. [Google Scholar] [CrossRef]
- Feng, S.; Jin, L.; Tang, S.; Jian, Y.; Li, Z. Combination of Rhizosphere Bacteria Isolated from Resistant Potato Plants for Biocontrol of Potato Late Blight. Pest Manag. Sci. 2022, 78, 166–176. [Google Scholar] [CrossRef]
- Huang, X.; You, Z.; Luo, Y.; Yang, C.; Ren, J.; Liu, Y.; Wei, G.; Dong, P.; Ren, M. Antifungal Activity of Chitosan against Phytophthora infestans, the Pathogen of Potato Late Blight. Int. J. Biol. Macromol. 2021, 166, 1365–1376. [Google Scholar] [CrossRef]
- Toral, L.; Rodríguez, M.; Béjar, V.; Sampedro, I. Antifungal Activity of Lipopeptides from Bacillus XT1 CECT 8661 against Botrytis cinerea. Front. Microbiol. 2018, 9, 377923. [Google Scholar] [CrossRef]
- Xie, J.; Chen, Y.; Cai, G.; Cai, R.; Hu, Z.; Wang, H. Tree Visualization By One Table (tvBOT): A Web Application for Visualizing, Modifying and Annotating Phylogenetic Trees. Nucleic Acids Res. 2023, 51, W587–W592. [Google Scholar] [CrossRef]
- Buchanan, R.; Gibbens, N. Berger’s Bacterial Identification Manual; Science Press: Beijing, China, 1984. [Google Scholar]
- Dong, X.; Cai, M. Common Bacterial System Identification Manual; Science Press: Beijing, China, 2001. [Google Scholar]
- Jiang, J.; Wang, Y.; Wang, X.; Li, L.; Wan, A.; Li, M. Identification of SR13-2 Strain against Phytophthora infestans and Control of Late Blight on Detached Potato Tissues. Crops 2017, 3, 146–150. [Google Scholar]
- Wang, Y.; Zhang, C.; Liang, J.; Wu, L.; Gao, W.; Jiang, J. Iturin A Extracted from Bacillus subtilis WL-2 Affects Phytophthora infestans via Cell Structure Disruption, Oxidative Stress, and Energy Supply Dysfunction. Front. Microbiol. 2020, 11, 536083. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Su, B.; Chen, X.; Xie, S.; Gu, S.; Wang, Q.; Huang, D.; Jiang, H. An Endophytic Bacterial Strain Isolated from Eucommia ulmoides Inhibits Southern Corn Leaf Blight. Front. Microbiol. 2017, 8, 903. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Yuan, C.; Luo, Y.; Chen, Y.; Lu, M.; Chen, G.; Ren, G.; Cui, C.; Zhang, J.; An, D. Biocontrol of Tobacco Black Shank Disease (Phytophthora nicotianae) by Bacillus velezensis Ba168. Pestic. Biochem. Physiol. 2020, 165, 104523. [Google Scholar] [CrossRef]
- Islam, M.H.; Masud, M.M.; Jannat, M.; Hossain, M.I.; Islam, S.; Alam, M.Z.; Serneels, F.J.; Islam, M.R. Potentiality of Formulated Bioagents from Lab to Field: A Sustainable Alternative for Minimizing the Use of Chemical Fungicide in Controlling Potato Late Blight. Sustainability 2022, 14, 4383. [Google Scholar] [CrossRef]
- Jeong, M.-H.; Lee, Y.-S.; Cho, J.-Y.; Ahn, Y.-S.; Moon, J.-H.; Hyun, H.-N.; Cha, G.-S.; Kim, K.-Y. Isolation and Characterization of Metabolites from Bacillus licheniformis MH48 with Antifungal Activity against Plant Pathogens. Microb. Pathog. 2017, 110, 645–653. [Google Scholar] [CrossRef]
- Aguilar, G.; Huitrón, C. Constitutive Exo-Pectinase Produced by Aspergillus sp. CH-Y-1043 on Different Carbon Source. Biotechnol. Lett. 1990, 12, 655–660. [Google Scholar] [CrossRef]
- Hankin, L.; Anagnostakis, S. The Use of Solid Media for Detection of Enzyme Production by Fungi. Mycologia 1975, 67, 597–607. [Google Scholar] [CrossRef]
- Teather, R.M.; Wood, P.J. Use of Congo Red-Polysaccharide Interactions in Enumeration and Characterization of Cellulolytic Bacteria from the Bovine Rumen. Appl. Environ. Microbiol. 1982, 43, 777–780. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhu, J.; Tan, T.; Xu, J.; Shen, A.; Yang, X.; Li, J.; Zeng, L.; Wei, L. Isolation and Characterization of Antagonistic Paenibacillus polymyxa HX-140 and Its Biocontrol Potential against Fusarium Wilt of Cucumber Seedlings. BMC Microbiol. 2021, 21, 75. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Hou, Y.; Xia, X.; Zhu, Z.; Huang, A.; Feng, S.; Li, P.; Shi, L.; Dong, P. Isolation and Screening of Antagonistic Endophytes against Phytophthora infestans and Preliminary Exploration on Anti-Oomycete Mechanism of Bacillus velezensis 6-5. Plants 2023, 12, 909. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Xi, Z.; Ma, J.; Li, Y.; Hao, C.; Lu, M.; Zhang, Z.-Z.; Deng, W.-W. Endophytic Bacteria from the Leaves of Two Types of Albino Tea Plants, Indicating the Plant Growth Promoting Properties. Plant Growth Regul. 2022, 96, 331–343. [Google Scholar] [CrossRef]
- Bahramisharif, A.; Rose, L.E. Efficacy of Biological Agents and Compost on Growth and Resistance of Tomatoes to Late Blight. Planta 2019, 249, 799–813. [Google Scholar] [CrossRef]
- Di Francesco, A.; Milella, F.; Mari, M.; Roberti, R. A Preliminary Investigation into Aureobasidium pullulans as a Potential Biocontrol Agent against Phytophthora infestans of Tomato. Biol. Control 2017, 114, 144–149. [Google Scholar] [CrossRef]
- Zegeye, E.D.; Santhanam, A.; Gorfu, D.; Tessera, M.; Kassa, B. Biocontrol Activity of Trichoderma viride and Pseudomonas fluorescens against Phytophthora infestans under Greenhouse Conditions. J. Agric. Technol. 2011, 7, 1589–1602. [Google Scholar]
- Zhang, J.; Islam, M.S.; Wang, J.; Zhao, Y.; Dong, W. Isolation of Potato Endophytes and Screening of Chaetomium globosum Antimicrobial Genes. Int. J. Mol. Sci. 2022, 23, 4611. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Liang, J.; Wang, L.; Gao, W.; Jiang, J.; Chang, R. Surfactin and Fengycin B Extracted from Bacillus pumilus W-7 Provide Protection against Potato Late Blight via Distinct and Synergistic Mechanisms. Appl. Microbiol. Biot. 2020, 104, 7467–7481. [Google Scholar] [CrossRef]
- Shemshura, O.; Alimzhanova, M.; Ismailova, E.; Molzhigitova, A.; Daugaliyeva, S.; Sadanov, A. Antagonistic Activity and Mechanism of a Novel Bacillus amyloliquefaciens MB40 Strain against Fire Blight. J. Plant Pathol. 2020, 102, 825–833. [Google Scholar] [CrossRef]
- Arrebola, E.; Tienda, S.; Vida, C.; de Vicente, A.; Cazorla, F.M. Fitness Features Involved in the Biocontrol Interaction of Pseudomonas chlororaphis with Host Plants: The Case Study of PcPCL1606. Front. Microbiol. 2019, 10, 439018. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, Y.; Yu, Z.; Zhuang, W.; Zeng, Z. Bacillus velezensis BV01 Has Broad-Spectrum Biocontrol Potential and the Ability to Promote Plant Growth. Microorganisms 2023, 11, 2627. [Google Scholar] [CrossRef]
- Cho, S.M.; Kang, B.R.; Han, S.H.; Anderson, A.J.; Park, J.-Y.; Lee, Y.-H.; Cho, B.H.; Yang, K.-Y.; Ryu, C.-M.; Kim, Y.C. 2R, 3R-Butanediol, a Bacterial Volatile Produced by Pseudomonas chlororaphis O6, Is Involved in Induction of Systemic Tolerance to Drought in Arabidopsis thaliana. Mol. Plant-Microbe Interact. 2008, 21, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, B.; Mahmood, R.; Nagesha, S.; Nagaraja, M.; Prashant, D.; Kerima, O.Z.; Karosiya, A.; Chavan, M. Field Application of Bacillus Subtilis Isolates for Controlling Late Blight Disease of Potato Caused by Phytophthora infestans. Biocatal. Agric. Biotechnol. 2019, 22, 101366. [Google Scholar] [CrossRef]
- Hunziker, L.; Bönisch, D.; Groenhagen, U.; Bailly, A.; Schulz, S.; Weisskopf, L. Pseudomonas Strains Naturally Associated with Potato Plants Produce Volatiles with High Potential for Inhibition of Phytophthora infestans. Appl. Environ. Microbiol. 2015, 81, 821–830. [Google Scholar] [CrossRef]
- Huang, X.; Ren, J.; Li, P.; Feng, S.; Dong, P.; Ren, M. Potential of Microbial Endophytes to Enhance the Resistance to Postharvest Diseases of Fruit and Vegetables. J. Sci. Food Agric. 2021, 101, 1744–1757. [Google Scholar] [CrossRef]
- Caulier, S.; Gillis, A.; Colau, G.; Licciardi, F.; Liépin, M.; Desoignies, N.; Modrie, P.; Legrève, A.; Mahillon, J.; Bragard, C. Versatile Antagonistic Activities of Soil-Borne Bacillus spp. and Pseudomonas spp. against Phytophthora infestans and Other Potato Pathogens. Front. Microbiol. 2018, 9, 143. [Google Scholar] [CrossRef]
- Hamaoka, K.; Aoki, Y.; Suzuki, S. Isolation and Characterization of Endophyte Bacillus velezensis KOF112 from Grapevine Shoot Xylem as Biological Control Agent for Fungal Diseases. Plants 2021, 10, 1815. [Google Scholar] [CrossRef]
- Han, X.; Shen, D.; Xiong, Q.; Bao, B.; Zhang, W.; Dai, T.; Zhao, Y.; Borriss, R.; Fan, B. The Plant-Beneficial Rhizobacterium Bacillus velezensis FZB42 Controls the Soybean Pathogen Phytophthora Sojae Due to Bacilysin Production. Appl. Environ. Microbiol. 2021, 87, e01601-21. [Google Scholar] [CrossRef]
- Keerthana, U.; Nagendran, K.; Raguchander, T.; Prabakar, K.; Rajendran, L.; Karthikeyan, G. Deciphering the Role of Bacillus subtilis var. amyloliquefaciens in the Management of Late Blight Pathogen of Potato, Phytophthora infestans. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 88, 1071–1080. [Google Scholar] [CrossRef]
- Wu, Z.H.; Ma, Q.; Sun, Z.N.; Cui, H.C.; Liu, H.R. Biocontrol Mechanism of Myxococcus fulvus B25-I-3 against Phytophthora infestans and Its Control Efficiency on Potato Late Blight. Folia Microbiol. 2021, 66, 555–567. [Google Scholar] [CrossRef]
- Shanthiyaa, V.; Saravanakumar, D.; Rajendran, L.; Karthikeyan, G.; Prabakar, K.; Raguchander, T. Use of Chaetomium globosum for Biocontrol of Potato Late Blight Disease. Crop Prot. 2013, 52, 33–38. [Google Scholar] [CrossRef]
- Villa-Rivera, M.G.; Cano-Camacho, H.; López-Romero, E.; Zavala-Páramo, M.G. The Role of Arabinogalactan Type II Degradation in Plant-Microbe Interactions. Front. Microbiol. 2021, 12, 730543. [Google Scholar] [CrossRef] [PubMed]



| Treatments | Disease Index (DI) | |
|---|---|---|
| Tubers | Leaflets | |
| Sterile water | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Bacterial suspension only | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Bacterial suspension + P. infestans | 18.52 ± 3.23 b | 14.81 ± 2.67 b |
| P. infestans | 49.14 ± 2.26 c | 77.78 ± 5.34 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, P.; Gao, M.; Zhou, S.; Liu, G.; Wang, P.; Liu, C.; Yang, F.; Fu, H. Antifungal Activity of Bacillus velezensis X3-2 Against Plant Pathogens and Biocontrol Effect on Potato Late Blight. Agriculture 2024, 14, 2224. https://doi.org/10.3390/agriculture14122224
Wei P, Gao M, Zhou S, Liu G, Wang P, Liu C, Yang F, Fu H. Antifungal Activity of Bacillus velezensis X3-2 Against Plant Pathogens and Biocontrol Effect on Potato Late Blight. Agriculture. 2024; 14(12):2224. https://doi.org/10.3390/agriculture14122224
Chicago/Turabian StyleWei, Peixia, Mengying Gao, Shuang Zhou, Guohui Liu, Pan Wang, Chunguang Liu, Fengshan Yang, and Haiyan Fu. 2024. "Antifungal Activity of Bacillus velezensis X3-2 Against Plant Pathogens and Biocontrol Effect on Potato Late Blight" Agriculture 14, no. 12: 2224. https://doi.org/10.3390/agriculture14122224
APA StyleWei, P., Gao, M., Zhou, S., Liu, G., Wang, P., Liu, C., Yang, F., & Fu, H. (2024). Antifungal Activity of Bacillus velezensis X3-2 Against Plant Pathogens and Biocontrol Effect on Potato Late Blight. Agriculture, 14(12), 2224. https://doi.org/10.3390/agriculture14122224





