Abstract
To examine the mechanisms of organic carbon transformation and sequestration by biochar in citrus orchard soil, a 100-day organic carbon mineralization test was conducted using citrus orchard soil from a 5-year-old forest. Calcium-modified citrus peel biochar (OBC-Ca) was applied at rates of 0%, 1%, 2%, and 4%. The results indicated that different percentages of OBC-Ca significantly influenced the mineralization processes in citrus orchards. Specifically, the cumulative mineralization of soil organic carbon was notably reduced by 8.68% and 17.00% with the application of 2% and 4% OBC-Ca, respectively, compared to the control group. Random forest analysis revealed that microbial biomass carbon (MBC), readily oxidizable carbon (ROC), and dissolved organic carbon (DOC) were critical indicators for predicting the cumulative mineralization of soil organic carbon. MBC and ROC were found to inhibit the cumulative mineralization, while DOC promoted it. As the proportion of OBC-Ca applications increased, MBC rose by 2.63% to 10.46%, ROC increased by 16.41% to 108.59%, and DOC increased by 0.48% to 11.67%. Correlation analysis demonstrated a significant negative correlation between the cumulative mineralization rate of soil organic carbon and soil enzyme activity, with soil sucrase content increasing significantly by 216.42% to 393.44% compared to the control. The application of calcium-modified biochar effectively reduces carbon dioxide emissions from citrus orchard soils, with a 4% application yielding the most favorable outcomes for enhancing soil carbon sinks, thereby positively impacting the carbon sequestration potential of citrus orchard soil.
1. Introduction
Guangxi is the leading region in China for both citrus planting area and production. In 2023, the total citrus production in China reached 60.039 million tons, with 18.080 million tons produced in the Guangxi Zhuang Autonomous Region, accounting for 30.11% of the national total. Research indicates that the soil in citrus orchards in Guangxi suffers from significant acidification and low levels of effective phosphorus, quick-acting potassium, and exchangeable calcium [1,2]. Biochar, recognized as an effective soil additive, can enhance soil fertility and increase carbon storage [3,4]. Modifying biochar can considerably improve its activity and augment its carbon sequestration potential in the soil [5]. Calcium(Ca) is a low-cost, ecologically non-toxic, and abundant natural resource that can effectively ameliorate soil acidity, making it an ideal material for modifying biochar to enhance its structural properties and adsorption capacity [6]. Currently, calcium-modified biochar is predominantly utilized for the adsorption of phosphorus, lead, and tetracycline in contaminated water bodies [7,8,9,10,11,12]. Additionally, it has been employed as a soil conditioner [12,13]. However, the application of calcium-modified biochar for soil carbon sequestration remains relatively limited. Nan [14] created calcium-enriched biochar with CaCl2 and discovered that the nitimate sequestration rate of calcium-enriched biochar was enhanced to 27–80%, which is superior to virgin biochar. Nan [15] prepared biochar from cow dung at various temperatures and found that the highest carbon sequestration rate (56.3%) was achieved at 600 °C, with the addition of exogenous calcium. Additionally, the calcium-enriched biochar increased soil pH, enriched soil cations, and enhanced the cation exchange capacity. Ren [16] prepared biochar using sewage sludge as feedstock at various temperatures using sewage sludge as feedstock and discovered that adding Ca(OH)2 increased the surface area and alkalinity of the biochar, increasing the potential application of the biochar product and producing biochar with high carbon retention and stability. Cheng [17] prepared calcium-based biochar (Ca-BC) using CaCl₂, and the results indicated that soil water content, total organic carbon (TOC), and pH increased by 45.48%, 47.19%, and 7.96%, respectively. Furthermore, the mineralization rate decreased to 0.451 mg CO2·g−1·d−1, following the application of Ca-BC. The increased carbon sequestration rate, by 21.70%, was attributed to the formation of unstable carbon structures and a higher proportion of conjugated structures in the Ca-BC.
In this study, biochar was prepared from citrus fruit peels, calcium-modified, and put into citrus orchard soil in different proportions to complete a 100-day constant-temperature incubation experiment. We investigated the characteristics of calcium-modified biochar on the mineralization of organic carbon in citrus orchard soil and the changes in its different carbon fractions and enzyme activities, and we explored the effects and mechanisms of calcium-modified biochar on organic carbon sequestration in citrus orchard soil.
2. Materials and Methods
2.1. Materials
The experimental soil was gathered using the random sample method from the 0–20 cm surface soil of the core demonstration base for citrus planting in Yulong River, Guilin, Guangxi. Plant and animal debris, as well as stones, were removed from the soil samples, which were then air-dried naturally and sieved through a 10-mesh sieve. The basic properties of the experimental soil are shown in Table S1.
The citrus peels utilized in the experiment came from the citrus cultivation demonstration base in Yu Long River, Guilin, Guangxi. The citrus peels were dried in an oven at 60 °C for 24 h. After drying, the peels were pulverized using a grinder, and the resulting powder was passed through a 60-mesh sieve. It was then combined with 1 mol·L−1 CaCl2 in a 1:10 ratio for 24 h, followed by filtration and drying at 60 °C for another 24 h. The dry powder was carbonized at 500 °C for 2 h to produce calcium-modified biochar (OBC-Ca) (Figure 1). The basic elemental composition, pH, and yield of OBC-Ca are shown in Table S2.
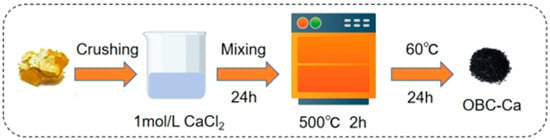
Figure 1.
Flow chart of calcium-modified biochar preparation.
2.2. Experimental Design
Based on the results of the preliminary experiments, four treatments were applied to the soil with OBC-Ca additions of 1%, 2%, 4%, and a control (no OBC-Ca). Each treatment was replicated three times. The experimental design is presented in Table S3.
Soil culture test: A total of 1000 g of experimental soil was placed into a culture box. According to Table S3, various quantities of OBC-Ca were used to ensure that it mixed well with the soil. Ultrapure water was added, and the soil’s field water-holding capacity was maintained at approximately 60% using the weighing method. At predetermined intervals (days 1, 3, 5, 10, 15, 20, 30, 40, 60, 80, and 100), soil samples were taken for analysis while the prepared samples were incubated for 100 days at 25 °C in a thermostatic incubator.
Mineralization test: In total, 50 g of the experimental soil was deposited in 500 mL polyethylene bottles. The soil received the same treatment as for the incubation test. A 500 mL polyethylene bottle was filled with a 10 mL beaker filled with 0.1 mol·L−1 NaOH, and the bottle was securely closed. Samples were taken at the same time as the incubation test to determine soil organic carbon mineralization.
2.3. Measurement Method and Statistical Analysis
The biochar and soil determination methods are shown in SII. As indicated in SIII, the CO2 emission rate was calculated.
Data were processed using Excel 2021 and SPSS 25.0. Experimental data from different incubation times were analyzed by one-way ANOVA in SPSS, and significance was tested using LSD (p < 0.05). The relationships between soil physicochemical properties, enzyme activities, and mineralization were assessed using Pearson’s correlation coefficient. Plotting was performed using Origin2023. Correlation heatmaps were plotted with the Correlation Plot App in Origin2023. The Random forest model was plotted in R 4.4.0.
3. Results
3.1. Structural Characteristics of Calcium-Modified Biochar
After calcium alteration, the surface of biochar became rough, resulting in fluffy precipitation (Figure 2). According to the EDS analysis results, the elemental Carbon concentration on the OBC-Ca surface fell from 78.59% to 27.41%, while elemental Ca increased from 1.09% to 20.75% [18]. The drop in C content could be attributed to Ca absorption into carbon’s surface [19], which resulted in a decrease in the C content and an increase in Ca content in response. The elemental mapping investigation confirms that calcium was successfully loaded into the OBC-Ca surface. In addition, the EDS analysis identified the elements Al, Si, Cl, and K. The EDS analysis further revealed that the OBC-Ca surface was successfully loaded with Ca.
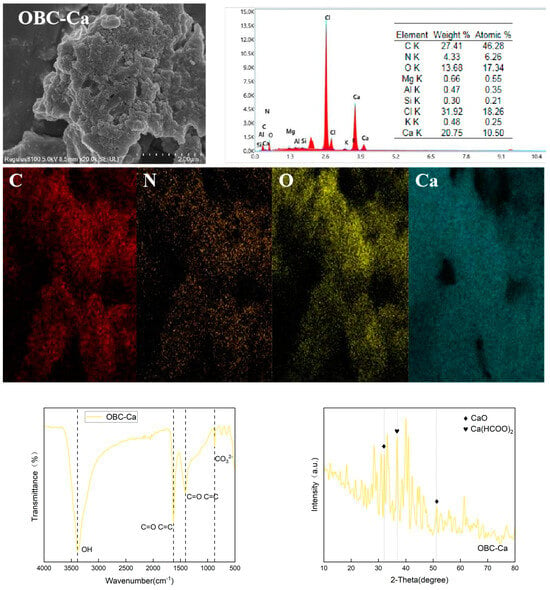
Figure 2.
SEM-EDS, FT-IR, and XRD analysis of calcium-modified biochar.
The broad absorption peak of OBC-Ca at 3387 cm−1 corresponds to the -OH stretching vibration, while C=C and C=O vibrations are responsible for the peaks at 1626 cm−1 and 1406 cm−1, respectively [20]. The peak at 873 cm−1 is primarily associated with the out-of-plane bending mode of CO₃2− (Figure 2). Loading calcium onto citrus peel biochar enhanced the intensity of the -OH peaks and created additional adsorption sites. The peak observed at 36.84° for OBC-Ca is related to Ca(HCOO)₂, while those at 32.1° and 51.2° correspond to CaO [21] (Figure 2). These findings indicate that calcium is incorporated into citrus peel biochar as both CaO and Ca(HCOO)₂.
3.2. Soil Nutrients
Figure 3 illustrates how OBC-Ca affects soil nutrients. Throughout the incubation period, no significant change in the pH of the control (CK) soil was observed. The application of OBC-Ca considerably elevated soil pH, with a 4%, 2%, and 1% rise in CK. The overall trend of soil CEC decreased as the incubation time increased. On the 15th day of incubation, CK soil exhibited the highest cation exchange capacity (CEC) at 27.31 cmol/kg, while it reached the lowest value of 7.22 cmol/kg by the end of the experiment. The application of OBC-Ca led each treatment’s CEC to be lower than that of CK in the later phases and greater than that in the late phase, and at 100 days, 1%, 2%, and 4% OBC-Ca soil CECs increased, respectively, by 32.61%, 12.33%, and 55.99%, respectively. The treatment of OBC-Ca considerably enhanced the soil AP concentration, which peaked at 120.03 mg/kg (1% OBC-Ca), 108.20 mg/kg (2% OBC-Ca), and 111.84 mg/kg (4% OBC-Ca); it dropped and stabilized between the 30th and 100th day of culture. At the conclusion of incubation, the soil AP concentration of 1%, 2%, and 4% OBC-Ca increased by 85.85%, 65.18%, and 79.49%, respectively, compared to CK. The application of OBC-Ca led to a decrease in the amount of soil-extractable potassium (AK). Only the 1% OBC-Ca treatment briefly exceeded the CK level during the incubation period. By the end of the incubation, the soil AK content of citrus orchards applying OBC-Ca (1%, 2%, 4%) was lower than that of CK. Compared with CK, the soil AK concentration at 100 days was reduced from 48.30% to 67.38%.
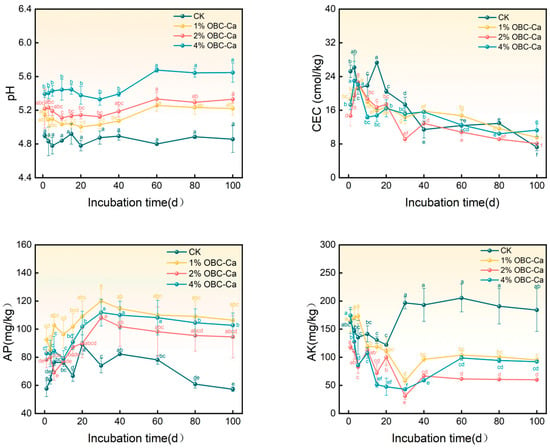
Figure 3.
Changes in soil nutrients imposed by calcium-modified biochar. Different lowercase letters represent significant differences (p < 0.05).
3.3. Soil Mineralization
Figure 4a shows how OBC-Ca affects the rate of soil organic carbon mineralization. The rate of organic carbon mineralization in the soil is significantly affected by the application of OBC-Ca. The CO2 emission rate declined in every therapy from the 1st to the 10th day of incubation, then increased between days 10 and 20, and decreased as the incubation duration increased after 20 days in all treatments. The CO2 emission rate dropped by 9.20%, 20.69%, and 52.30% after applying 1%, 2%, and 4% OBC-Ca, respectively.
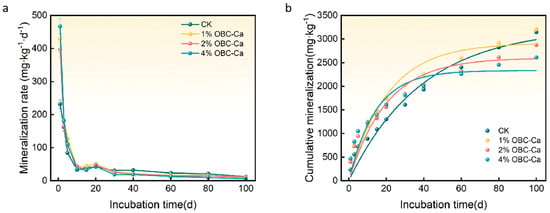
Figure 4.
Changes in soil organic carbon mineralization rate (a) and cumulative mineralization (b) after calcium-modified biochar application.
Figure 4b depicts the influence of OBC-Ca on cumulative CO2 emissions. After applying OBC-Ca, soil mineralization gradually increased with increasing incubation times. At 100 days, cumulative mineralization increased considerably before stabilizing. At 100 days, the cumulative CO2 emissions of 1% OBC-Ca increased by 2.07%, whereas the cumulative mineralization of 2% OBC-Ca and 4% OBC-Ca decreased by 8.68% and 17.00%, respectively, when compared to CK. According to the findings, citrus orchard soil treated with 2% and 4% OBC-Ca may be able to effectively lower soil CO2 emissions to a certain amount.
The dynamics of soil organic carbon mineralization and accumulation in citrus orchards following OBC-Ca application were fitted to the kinetic equation. The model fit correlation coefficient R2 ≥ 0.873 was good according to soil carbon mineralization kinetics (Table 1). As the proportion of calcium-modified biochar applied was larger, the C0/SOC values were larger. As a result, OBC-Ca (1%, 2%, 4%) decreased by 16.81–39.28% in contrast to CK.

Table 1.
Kinetics parameters of soil carbon mineralization.
3.4. Soil Organic Carbon (SOC) and Active Organic Carbon Fractions
Differences between treatments became significant (p < 0.05) as incubation time increased, with the greatest increase in SOC content observed at higher percentages of biochar application (Figure 5). At the conclusion of the incubation times, 1% OBC-Ca was 1.08-fold, 2% was 1.09-fold, and 4% was 1.32-fold CK. The addition of OBC-Ca to citrus crop soils led to significant (p < 0.05) alterations in MBC levels. MBC content in soil grew continuously from the 20th to the 100th day of incubation after OBC-Ca was sprayed, reaching the summit on the 100th day. At 100 days, 1% OBC-Ca climbed to 10.46%, 2% grew by 2.63%, and 4% reduced by 1.56% when contrasted with CK. Putting OBC-Ca to citrus orchard soils resulted in significant changes (p < 0.05) in soil DOC levels. The administration of OBC-Ca resulted in a general rise in DOC content from day 1 to day 15 of incubation, followed by an overall declining trend from day 15 to day 100 of incubation, with DOC at the conclusion of incubation greater than CK at each application rate. In comparison to CK, the DOC content at 100 days increased by 0.48% (1% OBC-Ca) and 9.84% (2% OBC-Ca), respectively, as opposed to CK, 11.67% (4% OBC-Ca). The use of OBC-Ca in the soil considerably changed the ROC content (p < 0.05). After applying OBC-Ca to the land, the ROC steadily declined from days 1 to 5, climbed from day 10 to day 20 of incubation, and then decreased again from day 20 to day 60 of incubation. At the conclusion of the incubation period, 1% OBC-Ca grew by 16.41%, 2% OBC-Ca climbed by 28.91%, and 4% OBC-Ca showed a remarkable increase of 108.59% versus the control group (CK).
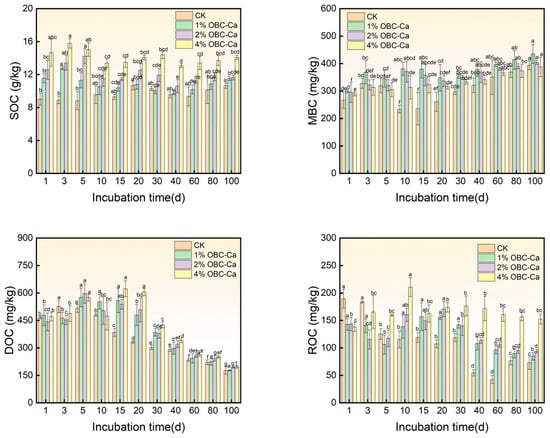
Figure 5.
Changes in soil organic carbon fractions after calcium-modified biochar application. Different lowercase letters represent significant differences (p < 0.05).
3.5. Soil Enzyme Activity
OBC-Ca application significantly improved soil catalase activity (p < 0.05), with an overall increasing trend from days 1 to 60 of incubation and increasing after decreasing from days 60 to 80 of incubation (Figure 6). At the conclusion of the incubation period, 1%, 2%, and 4% OBC-Ca levels had grown by 53.33%, 96.67%, and 116.67%, respectively. Furthermore, a higher application rate of OBC-Ca to the soil correlated with greater catalase activity. Applying OBC-Ca to the soil was considered to improve urease activity (p < 0.05). Urease activity fluctuated during the first 40 days of incubation, dropped sharply after the 40th day, and was lowest on the 60th day of incubation, following which it began to rise (Figure 5). At the final stage of incubation, the 1%, 2%, and 4% OBC-Ca treatments had 2.24 to 2.56 times the soil urease activity of CK. Applying OBC-Ca to the soil also significantly improved sucrase activity (p < 0.05). Sucrase activity in every treatment increased steadily over time after OBC-Ca was applied to the soil (Figure 6). At the finale of the incubation phase, sucrase activity stood out than that of the control group (CK) in all treatment percentages, with increases ranging from 216.42% to 393.44% for the 1%, 2%, and 4% OBC-Ca treatments against CK.
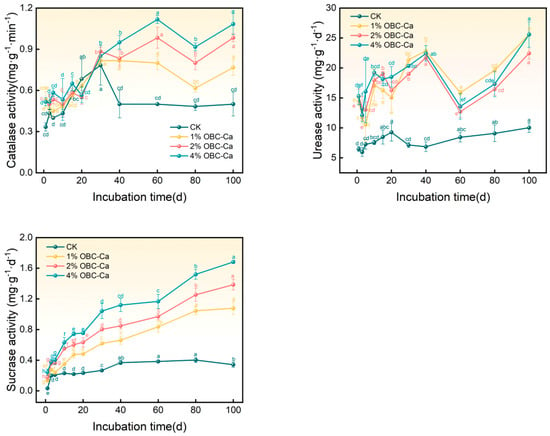
Figure 6.
Changes in soil enzyme activities after application of calcium-modified biochar. Different lowercase letters represent significant differences (p < 0.05).
3.6. Random Forest Modeling and Correlation Analysis
A randomized forest experiment was conducted to investigate the effects of physicochemical properties, active fractions of organic carbon, and enzyme activities on soil organic carbon mineralization. The results showed that MBC, ROC, DOC, and sucrase activities were important factors affecting the amount of loamy organic carbon mineralization (Figure 7a).
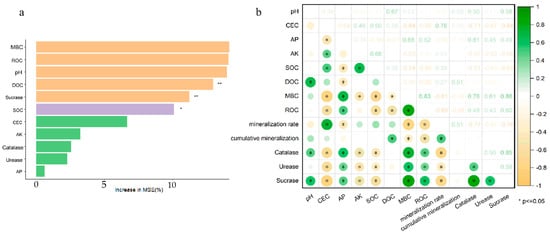
Figure 7.
Random forest model (a) and correlation analysis (b). MSE, mean square error. * p < 0.05, ** p < 0.01.
From Figure 7b, pH correlated positively with DOC, catalase, and sucrase after applying OBC-Ca. The three enzyme activities of MBC, ROC, and catalase showed strong correlations with both AP and AK, whereas SOC had a significantly negative connection with urease, catalase, and sucrase activity. MBC and ROC demonstrated a substantial positive association with the three enzyme activities and with organic carbon mineralization. While the rate of organic carbon mineralization and the total amount of mineralized organic carbon exhibited a negative association with MBC and ROC, the activities of the three enzymes demonstrated a positive correlation. The three enzymes’ activity was positively correlated with MBC and ROC, whereas the CO2 emission rate and the cumulative CO2 emission were negatively correlated. Furthermore, there was a favorable association between catalase, sucrase, and urease.
4. Discussion
4.1. Effects of Calcium-Modified Biochar on Soil Nutrient Dynamics in Citrus Orchards
The pH of citrus orchard soil was significantly raised by applying OBC-Ca (p < 0.05). As the amount applied grew, the influence on pH gradually grew as well, corresponding with the findings of Zou [22]. The presence of soluble organic and inorganic bases in biochar, which contribute to its high alkalinity, is responsible for the pH increase that occurs after applying OBC-Ca [23,24]. The CEC of the soil was lowered by applying OBC-Ca, and a similar result was observed [25]. The loss of O and H from the surface of biochar reduces the CEC of the biochar itself, and the generation of minus charges on the surface of biochar is pH [26]. In this investigation, OBC-Ca had a lower pH and a lower H content, so OBC-Ca itself had a low CEC, which in turn caused the CEC to decrease.
The use of OBC-Ca considerably raised the level of quick-acting phosphorus (p < 0.05), supporting the findings of Liu [27] and Zhang [28]. The increase in readily available phosphorus can be attributed to three factors: the presence of phosphorus in biochar itself, which directly enhances soil effective phosphorus levels [29]. Biochar also increases soil pH and alters cation activity, which can reduce phosphorus adsorption or promote phosphorus desorption, thereby increasing phosphorus availability [30]. Furthermore, the application of biochar to the soil modifies the microbial environment, enhancing the soil’s phosphorus fixation capacity [31]. The decrease in readily available phosphorus content with prolonged incubation time was primarily attributed to the reduction in effective phosphorus resulting from soil phosphorus fixation [32]. The application of alkaline biochar to acidic soils resulted in improved phosphorus bioavailability [33], and differences in the effect of OBC-Ca application on quick-acting phosphorus content in soils could be attributed to inconsistencies in the chemical formula and surface features of the biochar loads.
According to the majority of research, applying biochar raises the amount of AK in the soil. Dong [34] discovered a notable rise in the amount of AK in the rhizosphere soil of southern pine following biochar application. Yao [35] also found that AK in the soil rose with the quantity of biochar material added. The interaction between biochar and soil minerals alters nutrient release from the soil, increasing quick-acting potassium [36]. The reduction in quick-acting potassium in citrus orchard soils following the usage of OBC-Ca, combined with the EDS analysis of the biochar, could be attributed to the calcium-modified biochar’s decreased K content. Correlation analysis revealed that the quick-acting potassium content was extremely significant and positively linked with CEC following the application of OBC-Ca, and that as CEC fell, so did the quick-acting potassium content.
4.2. Effects of Calcium-Modified Biochar on the Mineralization of Citrus Orchard Soil
The application of different proportions of OBC-Ca boosted soil carbon dioxide emission during the preincubation period, which dropped and subsequently stabilized with prolonged incubation time, corresponding with Ameloot [37]. Because biochar was applied to the soil prior to incubation, the native carbon pool was perturbed, and soil organisms were stimulated, resulting in the biodegradation of biochar components and increased CO2 release [38]. The gradual decrease in CO2 emission in the later phases of incubation was related to the addition of biochar, which increased the carbon levels and aromatic structure, increasing capacity for biodegradation and culminating in a possibly adversely stimulating impact. The mineralization rate increased when OBC-Ca was added to citrus orchard soil; however, this could be explained by the fact that soil microbes broke down the water-soluble organic matter in the biochar more quickly. More carbon dioxide is released as a result of this process, which also increases microbial activity [39]. A correlation study revealed that the CO2 emission rate in all therapies was significantly adversely associated with the concentration of quick-acting phosphorus, indicating that the CO2 emission rate decreased as quick-acting phosphorus content increased, and that increasing soil quick-acting phosphorus had an inhibitory effect on organic carbon mineralization.
The use of 1% OBC-Ca increased the cumulative mineralization in citrus orchard soils, which might be because the low application of biochar had no inhibitory effect on soil mineralization. The application of 2% and 4% OBC-Ca decreased the cumulative mineralization. Khan [40] had a study that found that the cumulative mineralization of organic carbon of rice straw modified using magnesium chloride decreased by 5% after a 3-month incubation experiment, which suggests that magnesium-modified biochar can reduce CO2 emission to a certain extent, which is consistent with the results of the present study. Other investigations have reported comparable effective decreases in cumulative mineralization of soil organic carbon due to biochar [41,42]. While increasing soil humus content, biochar also promotes the synthesis of organic macromolecules that are difficult for microorganisms to consume, reducing microorganisms’ use of organic carbon and hence reducing soil organic carbon mineralization [43].
The C0/SOC value can be used as an indicator for evaluating the carbon sequestration capacity of soil, and if the value is lower, it indicates a stronger capacity for soil carbon sequestration [44]. The C0/SOC value of CK soil was the highest in this study, indicating that the its carbon sequestration potential was the lowest. The application of biochar enhanced the carbon sequestration capacity of citrus orchard soils. Soil carbon sequestration capacity showed an increasing trend with the increase in OBC-Ca application, which indicated that the application of calcium-modified biochar improved the carbon sequestration capacity of the soil.
4.3. Effects of Calcium-Modified Biochar on Soil Organic Carbon and Its Fractions in Citrus Orchards
Soil SOC content increased with higher OBC-Ca application rates, similar to Novak [45]. The rise in SOC could be due to the high level of carbon of biochar itself, and adding it to the soil is equivalent to applying a new source of carbon [46]. Additionally, biochar can remain in the soil due to its slow rate of degradation, further contributing to the rise in organic carbon levels [47,48]. Similarly, Dong [49] observed that adding varying quantities of rice husk and cottonseed husk charcoal to the soil significantly enhanced the SOC (p < 0.05), and the amount of biochar applied was positively associated.
The addition of OBC-Ca modified the microbial biomass carbon (MBC) content in citrus orchard soil. The initial decrease in MBC during incubation was primarily attributed to the depletion and mineralization of unstable organic carbon fractions, leading to a reduction in MBC levels [50]. The progressive rise in MBC in the latter phases can be attributed to the fact that in the later stages, the soil environment was more stable and less disturbed, and the increase in microbial activity contributed to the increase in MBC. The increase in soil MBC content in citrus orchards with 1% OBC-Ca and 2% OBC-Ca applied at 100 days, as well as the decrease in soil MBC content in citrus orchards with 4% OBC-Ca applied, indicated that applying a tiny bit of biochar had a favorable effect on soil MBC. This study found a strong negative connection between MBC and cumulative mineralization of organic carbon following the application of OBC-Ca, implying that MBC can either promote or inhibit the mineralization of soil organic carbon [51].
The use of OBC-Ca in citrus orchard soils raised DOC concentration, although the overall trend decreased with increasing treatment. One possible explanation for the rise in DOC content following the use of biochar is that the introduction of active organic carbon fractions into the soil caused the stable carbon fractions to change, which in turn raised the DOC content of the soil [52]. While the stable organic carbon in biochar increases soil SOC later in the incubation process, the active organic carbon degrades and results in a fall in DOC content [53]. Correlation analysis revealed that soil DOC was favorably and positively linked with cumulative CO2 emission, implying that DOC plays an important role in soil organic carbon mineralization.
Compared with CK, OBC-Ca markedly added ROC in soil (p < 0.05), and the effect on ROC increased with the increase in the amount of OBC-Ca applied. ROC and DOC are the same as reactive organic carbon, and the rationale for the shift is similar to that of DOC. The addition of biochar to the citrus orchard soil boosted soil microbial activity, which subsequently increased ROC levels [54]. As active organic carbon in biochar increased, its degradation led to a decrease in the concentration of ROC. After 100 days, the ROC content increased with higher OBC-Ca application rates. Correlation analysis revealed a significant negative correlation between ROC content and cumulative organic carbon mineralization, suggesting that the increase in ROC positively influenced the reduction in organic carbon mineralization. Meanwhile, the 4% OBC-Ca application rate was found to have the most pronounced effect on reducing mineralization.
4.4. Effects of Calcium-Modified Biochar on Enzyme Activities in Citrus Orchard Soil
As the OBC-Ca ratio increased, catalase activity rose as well, which is similar to Masto [55]. The significant increase in catalase activity by OBC-Ca was due to the fact that catalase is not affected by environmental conditions; however, the surface structure and properties of the calcium-modified biochar changed considerably, altering the physical structure of the soil and thus increasing the effect on the soil environment [56]. Properties changed considerably, altering the physical structure of the soil and increasing the effect on the soil environment [56], which led to an increase in catalase activity. The correlation analysis also clearly indicates that an increase in catalase activity inhibits the rate of mineralization.
The increase in urease activity might be attributed to the application of OBC-Ca, which improved the soil’s physicochemical qualities and provided a suitable environment for microorganisms, hence raising soil urease activity [24,57]. Jing [58] found that the application of wheat straw biochar, rice straw biochar, and corn stover biochar increased soil urease activity by up to 94.2%. Similarly, Yang [59] found that urease activity was significantly enhanced by applying 5% rice straw biochar. Soil sucrase catalyzes the decomposition of soil organic matter into glucose and sucrose, affecting soil carbohydrate turnover. We made important predictions in R language regarding the effects of nutrient indicators, carbon fractions, and enzyme activities on cumulative mineralization, from which we can see that sucrase activity was the most sensitive to changes in this study, which better reflects the importance of the effects on cumulative mineralization of organic carbon. Sucrase activity increased with the OBC-Ca application because biochar accelerates sugar hydrolysis in the soil, boosting soil enzymatic processes involving sucrase [60]. The activities of sucrase and urease showed a substantial positive correlation with MBC, and as the content of MBC increased, so did soil urease and sucrase activities, indicating that MBC promoted urease and sucrase activities. MBC is an important indicator affecting the activity of soil enzymes, and MBC and soil urease and sucrase activity showed a strong positive association, suggesting that urease and sucrase in the process of participating in soil carbon mineralization are closely related to MBC decomposition and transformation, which is consistent with the findings of Wang [61].
Furthermore, the three enzyme activities showed a significant positive correlation with MBC and ROC, which, as active carbon components, can directly affect the number of microorganisms in the soil. The stronger the enzyme activities, the better the metabolic activities of microorganisms in the soil. Catalase and sucrase activities were highest in the high-applied amount (4%) condition, indicating that soil microorganisms had substantial metabolic activities. The strength of the metabolic activities of microorganisms in the soil was connected to the dark fixation of CO2 [62], showing that more carbon was fixed in the soil at the high application rate (4%).
5. Conclusions
The application of calcium-modified biochar in citrus orchard soils reduced the cumulative carbon emissions of soil organic carbon, especially in the 4% OBC-Ca treatment, which reduced the cumulative mineralization of organic carbon by 17.00% compared to CK. Additionally, the addition of calcium-modified biochar altered the content of various carbon fractions in the soil, with the highest increase in ROC (108.59%) observed following the application of 4% OBC-Ca. Furthermore, applying varying proportions of calcium-modified biochar to citrus orchard soils improved the soil’s organic carbon content, which rose with the proportion applied. Therefore, the application of calcium-modified biochar in citrus orchard soil could not only reduce the amount of soil organic carbon mineralization but also improve soil organic carbon sequestration capacity, with readily oxidized organic carbon being the primary influencing component. Since this experiment was a one-time application of modified biochar to simulate a field base fertilizer, the adverse effects of repeated applications of modified biochar on plants and soils were not considered in this experiment for the time being, in addition to the fact that our study was only conducted on citrus orchard soils, and we plan to conduct further studies on them in the future.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agriculture14122222/s1, Table S1: Basic properties of experimental soil; Table S2: Basic properties and elemental content of modified biochar; Table S3: Experiment design. References [18,43,63,64,65,66,67,68,69,70,71,72,73] are cited in supplementary materials.
Author Contributions
Conceptualization, X.L. and Q.F.; data curation, R.H.; funding acquisition, S.L. (Shengqiu Liu); investigation, S.L. (Shu Li); software, Y.B. and R.H.; validation, R.H.; writing—original draft, Y.B.; writing—review & editing, L.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Guangxi Key Research and Development Project (No. GuiKe AB22080097) and the Guangxi Natural Science Foundation project of China (2022GXNSFAA035555).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Materials).
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Chang, J.-W.; Wu, W.-M.; Zhu, Y.-C.; Liu, G.-Q.; Fang, P.-L.; Chen, M.; Qin, H. Relationship between citrus growth and soil nutrient and microbial community. Chin. J. Soil Sci. 2022, 53, 1386–1394. [Google Scholar]
- Chen, G.-F.; Huang, Y.-Y.; Liu, B.; Xiong, L.-M. Investigation and analysis of calcium content in soil-plant leaves and roots of citrus orchards. J. Anhui Agric. Sci. 2011, 39, 15293–15294. [Google Scholar]
- Kuo, Y.-L.; Lee, C.-H.; Jien, S.-H. Reduction of Nutrient Leaching Potential in Coarse-Textured Soil by Using Biochar. Water 2020, 12, 2012. [Google Scholar] [CrossRef]
- Lyu, H.; Zhang, Q.; Shen, B. Application of biochar and its composites in catalysis. Chemosphere 2020, 240, 124842. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Mao, L.; Zhang, H.; Wang, P.; Wu, C.; Xie, J.; Yu, B.; Sial, M.U.; Zhang, L.; Zhang, Y.; et al. Modified Biochar as a More Promising Amendment Agent for Remediation of Pesticide-Contaminated Soils: Modification Methods, Mechanisms, Applications, and Future Perspectives. Appl. Sci. 2022, 12, 11544. [Google Scholar] [CrossRef]
- Xu, Y.; Liao, H.; Zhang, J.; Lu, H.; He, X.; Zhang, Y.; Wu, Z.; Wang, H.; Lu, M. A Novel Ca-Modified Biochar for Efficient Recovery of Phosphorus from Aqueous Solution and Its Application as a Phosphorus Biofertilizer. Nanomaterials 2022, 12, 2755. [Google Scholar] [CrossRef]
- Li, S.H.; Luo, C.; Yan, F.; Yang, Y.; Guo, B.; Wang, L.; Xu, S.Q.; Wu, F.; Ji, P.H. Remediation of Pb(II) and Cd(II) in polluted waters with calcium thioglycolate-modified straw biochar. Environ. Pollut. 2023, 338, 122638. [Google Scholar] [CrossRef]
- Liu, D.; Hao, Z.; Chen, D.; Jiang, L.; Li, T.; Tian, B.; Yan, C.; Luo, Y.; Chen, G.; Ai, H. Use of Eggshell-Catalyzed Biochar Adsorbents for Pb Removal from Aqueous Solution. ACS Omega 2022, 7, 21808–21819. [Google Scholar] [CrossRef]
- Wang, K.F.; Yao, R.L.; Zhang, D.Q.; Peng, N.; Zhao, P.; Zhong, Y.M.; Zhou, H.J.; Huang, J.H.; Liu, C. Tetracycline Adsorption Performance and Mechanism Using Calcium Hydroxide-Modified Biochars. Toxics 2023, 11, 841. [Google Scholar] [CrossRef]
- Wang, P.; Chen, W.; Zhang, R.; Xing, Y. Enhanced Removal of Malachite Green Using Calcium-Functionalized Magnetic Biochar. Int. J. Environ. Res. Public Health 2022, 19, 3247. [Google Scholar] [CrossRef]
- Zhuo, S.-N.; Dai, T.-C.; Ren, H.-Y.; Liu, B.-F. Simultaneous adsorption of phosphate and tetracycline by calcium modified corn stover biochar: Performance and mechanism. Bioresour. Technol. 2022, 359, 127477. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, B.; Siri, M.; Liu, C.; Feng, C.; Shao, X.; Liu, K. Calcium-modified biochar rather than original biochar decreases salinization indexes of saline-alkaline soil. Environ. Sci. Pollut. Res. 2023, 30, 74966–74976. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Yang, W.; Xu, Y.; Wang, L.; Liang, X.; Huang, Q.; Sun, Y. Effect of Ca-modified biochar coupling with low-Cd accumulation maize cultivars on remediation of Cd contaminated soils and microbial community composition. Soil Tillage Res. 2023, 232, 105765. [Google Scholar] [CrossRef]
- Nan, H.; Jiang, Y.; Zhou, W.; Zhao, L.; Yang, F. New Insights into the Enhancement Effect of Exogenous Calcium on Biochar Stability during Its Aging in Farmland Soil. Agronomy 2023, 13, 1676. [Google Scholar] [CrossRef]
- Nan, H.Y.; Yin, J.X.; Yang, F.; Luo, Y.; Zhao, L.; Cao, X.D. Pyrolysis temperature-dependent carbon retention and stability of biochar with participation of calcium: Implications to carbon sequestration. Environ. Pollut. 2021, 287, 117566. [Google Scholar] [CrossRef]
- Ren, N.; Tang, Y.; Li, M. Mineral additive enhanced carbon retention and stabilization in sewage sludge-derived biochar. Process Saf. Environ. Prot. 2018, 115, 70–78. [Google Scholar] [CrossRef]
- Ji, C.; Yang, S.; Cheng, Y.; Liu, L.; Wang, D.; Zhu, S.; Tao, E.; Li, Y. In situ formed CaSO4 on waste dander biochar to inhibit the mineralization of soil organic carbon. Sci. Total Environ. 2023, 854, 158776. [Google Scholar] [CrossRef]
- Hu, L.; Huang, R.; Zhou, L.; Qin, R.; He, X.; Deng, H.; Li, K. Effects of magnesium-modified biochar on soil organic carbon mineralization in citrus orchard. Front. Microbiol. 2023, 14, 1109272. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhou, B.; Awasthi, M.K.; Ali, A.; Zhang, Z.; Gaston, L.A.; Lahori, A.H.; Mahar, A. Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios. Sci. Total Environ. 2016, 559, 121–129. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, J.; Wang, H.; Lv, Q.; Xue, J. Enhanced removal of phosphate from aqueous solution using Mg/Fe modified biochar derived from excess activated sludge: Removal mechanism and environmental risk. Environ. Sci. Pollut. Res. 2021, 28, 16282–16297. [Google Scholar] [CrossRef]
- Liu, X.; Lv, J. Efficient Phosphate Removal from Wastewater by Ca-Laden Biochar Composites Prepared from Eggshell and Peanut Shells: A Comparison of Methods. Sustainability 2023, 15, 1778. [Google Scholar] [CrossRef]
- Zou, G.; Zhao, F.; Lan, X.; Nawaz, M.; Shohag, J.I. Role of Coconut Shell Biochar on Soil Properties, Microbial Diversity and Nitrogen Mineralization in Tropical Latosol. Pol. J. Environ. Stud. 2024, 33, 1487–1496. [Google Scholar] [CrossRef]
- Fidel, R.B.; Laird, D.A.; Thompson, M.L.; Lawrinenko, M. Characterization and quantification of biochar alkalinity. Chemosphere 2017, 167, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.-Y.; Li, J.-Y.; Ni, N.; Xu, R.-K. Understanding the biochar’s role in ameliorating soil acidity. J. Integr. Agric. 2019, 18, 1508–1517. [Google Scholar] [CrossRef]
- Kwon, S.; Pignatello, J.J. Effect of natural organic substances on the surface and adsorptive properties of environmental black carbon (char): Pseudo pore blockage by model lipid components and its implications for N2-probed surface properties of natural sorbents. Environ. Sci. Technol. 2005, 39, 7932–7939. [Google Scholar] [CrossRef]
- Domingues, R.R.; Sanchez-Monedero, M.A.; Spokas, K.A.; Melo, L.C.A.; Trugilho, P.F.; Valenciano, M.N.; Silva, C.A. Enhancing Cation Exchange Capacity of Weathered Soils Using Biochar: Feedstock, Pyrolysis Conditions and Addition Rate. Agronomy 2020, 10, 824. [Google Scholar] [CrossRef]
- Liu, Z.; Yuan, D.; Qin, X.; He, P.; Fu, Y. Effect of Mg-Modified Waste Straw Biochar on the Chemical and Biological Properties of Acidic Soils. Molecules 2023, 28, 5225. [Google Scholar] [CrossRef]
- Zhang, Y.; Miao, S.; Song, Y.; Wang, X.; Jin, F. Biochar Application Reduces Saline-Alkali Stress by Improving Soil Functions and Regulating the Diversity and Abundance of Soil Bacterial Community in Highly Saline-Alkali Paddy Field. Sustainability 2024, 16, 1001. [Google Scholar] [CrossRef]
- Hong, C.; Lu, S. Does biochar affect the availability and chemical fractionation of phosphate in soils? Environ. Sci. Pollut. Res. 2018, 25, 8725–8734. [Google Scholar] [CrossRef]
- Yang, C.; Lu, S. Straw and straw biochar differently affect phosphorus availability, enzyme activity and microbial functional genes in an Ultisol. Sci. Total Environ. 2022, 805, 150325. [Google Scholar] [CrossRef]
- Tian, J.; Kuang, X.; Tang, M.; Chen, X.; Huang, F.; Cai, Y.; Cai, K. Biochar application under low phosphorus input promotes soil organic phosphorus mineralization by shifting bacterial phoD gene community composition. Sci. Total Environ. 2021, 779, 146556. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Fang, X.; Wang, L.; Xiang, W.; Alharbi, H.A.; Lei, P.; Kuzyakov, Y. Regulation of soil phosphorus availability and composition during forest succession in subtropics. For. Ecol. Manag. 2021, 502, 119706. [Google Scholar] [CrossRef]
- Bai, K.; Wang, W.; Zhang, J.; Yao, P.; Cai, C.; Xie, Z.; Luo, L.; Li, T.; Wang, Z. Effects of phosphorus-solubilizing bacteria and biochar application on phosphorus availability and tomato growth under phosphorus stress. BMC Biol. 2024, 22, 211. [Google Scholar] [CrossRef]
- Dong, P.; Zhang, Z.; Zhang, M. Combination of Phytoextraction and Biochar Improves Available Potassium and Alters Microbial Community Structure in Soils. Water 2024, 16, 118. [Google Scholar] [CrossRef]
- Yao, T.; Zhang, W.; Gulaqa, A.; Cui, Y.; Zhou, Y.; Weng, W.; Wang, X.; Liu, Q.; Jin, F. Effects of Peanut Shell Biochar on Soil Nutrients, Soil Enzyme Activity, and Rice Yield in Heavily Saline-Sodic Paddy Field. J. Soil Sci. Plant Nutr. 2021, 21, 655–664. [Google Scholar] [CrossRef]
- Buss, W.; Wurzer, C.; Manning, D.A.C.; Rohling, E.J.; Borevitz, J.; Masek, O. Mineral-enriched biochar delivers enhanced nutrient recovery and carbon dioxide removal. Commun. Earth Environ. 2022, 3, 67. [Google Scholar] [CrossRef]
- Ameloot, N.; Graber, E.R.; Verheijen, F.G.A.; De Neve, S. Interactions between biochar stability and soil organisms: Review and research needs. Eur. J. Soil Sci. 2013, 64, 379–390. [Google Scholar] [CrossRef]
- Singh Yadav, S.P.; Bhandari, S.; Bhatta, D.; Poudel, A.; Bhattarai, S.; Yadav, P.; Ghimire, N.; Paudel, P.; Paudel, P.; Shrestha, J.; et al. Biochar application: A sustainable approach to improve soil health. J. Agric. Food Res. 2023, 11, 100498. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, J.; Xue, J.; Zhang, L. Quantifying the Effects of Biochar Application on Greenhouse Gas Emissions from Agricultural Soils: A Global Meta-Analysis. Sustainability 2020, 12, 3436. [Google Scholar] [CrossRef]
- Khan, M.N.; Li, D.; Shah, A.; Huang, J.; Zhang, L.; Nunez-Delgado, A.; Han, T.; Du, J.; Ali, S.; Sial, T.A.; et al. The impact of pristine and modified rice straw biochar on the emission of greenhouse gases from a red acidic soil. Environ. Res. 2022, 208, 112676. [Google Scholar] [CrossRef]
- Kalu, S.; Seppanen, A.; Mganga, K.Z.; Sietio, O.-M.; Glaser, B.; Karhu, K. Biochar reduced the mineralization of native and added soil organic carbon: Evidence of negative priming and enhanced microbial carbon use efficiency. Biochar 2024, 6, 7. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, K.; Han, L.; Chen, Y.; Liu, J.; Xing, B. Biochar stability and impact on soil organic carbon mineralization depend on biochar processing, aging and soil clay content. Soil Biol. Biochem. 2022, 169, 108657. [Google Scholar] [CrossRef]
- Ke, Y.-J.; Hu, X.-Y.; Yi, Q.; Yu, Z. Impacts of Rice Straw Biochar on Organic Carbon and CO2 Release in Arable Soil. Environ. Sci. 2014, 35, 93–99. [Google Scholar]
- Li, S.-L. Effect and Mechanism of Potassium-Modified Bagasse Biochar on the Carbon Sequestration of Sugarcane Field and Manga-Nese-Contaminated Soil. Master’s Thesis, Guangxi Normal University, Guilin, China, 2021. [Google Scholar]
- Dong, L.; Yang, X.; Shi, L.; Shen, Y.; Wang, L.; Wang, J.; Li, C.; Zhang, H. Biochar and nitrogen fertilizer co-application changed SOC content and fraction composition in Huang-Huai-Hai plain, China. Chemosphere 2022, 291, 132925. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Chang, S.X.; Yang, Y.; Fu, S.; Jiang, P.; Luo, Y.; Yang, M.; Chen, Z.; Hu, S.; et al. Biochar reduces soil heterotrophic respiration in a subtropical plantation through increasing soil organic carbon recalcitrancy and decreasing carbon degrading microbial activity. Soil Biol. Biochem. 2018, 122, 173–185. [Google Scholar] [CrossRef]
- Rasul, M.; Cho, J.; Shin, H.-S.; Hur, J. Biochar-induced priming effects in soil via modifying the status of soil organic matter and microflora: A review. Sci. Total Environ. 2022, 805, 150304. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, Z.; Kuzyakov, Y. Biochar stability in soil: Meta-analysis of decomposition and priming effects. Glob. Change Biol. Bioenergy 2016, 8, 512–523. [Google Scholar] [CrossRef]
- Dong, X.; Singh, B.P.; Li, G.; Lin, Q.; Zhao, X. Biochar application constrained native soil organic carbon accumulation from wheat residue inputs in a long-term wheat-maize cropping system. Agric. Ecosyst. Environ. 2018, 252, 200–207. [Google Scholar] [CrossRef]
- Huang, R.; Lan, T.; Song, X.; Li, J.; Ling, J.; Deng, O.; Wang, C.; Gao, X.; Li, Q.; Tang, X.; et al. Soil labile organic carbon impacts C:N:P stoichiometry in urban park green spaces depending on vegetation types and time after planting. Appl. Soil Ecol. 2021, 163, 103926. [Google Scholar] [CrossRef]
- Fang, Y.; Singh, B.P.; Luo, Y.; Boersma, M.; Van Zwieten, L. Biochar carbon dynamics in physically separated fractions and microbial use efficiency in contrasting soils under temperate pastures. Soil Biol. Biochem. 2018, 116, 399–409. [Google Scholar] [CrossRef]
- Ye, R.; Horwath, W.R. Influence of rice straw on priming of soil C for dissolved organic C and CH4 production. Plant Soil 2017, 417, 231–241. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, X.; Shi, L.; Li, J.; Li, S.; Lu, J.; Li, Y. Efficient removal of lead from solution by celery-derived biochars rich in alkaline minerals. Bioresour. Technol. 2017, 235, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Abiven, S.; Hund, A.; Martinsen, V.; Cornelissen, G. Biochar amendment increases maize root surface areas and branching: A shovelomics study in Zambia. Plant Soil 2015, 395, 45–55. [Google Scholar] [CrossRef]
- Masto, R.E.; Kumar, S.; Rout, T.K.; Sarkar, P.; George, J.; Ram, L.C. Biochar from water hyacinth (Eichornia crassipes) and its impact on soil biological activity. Catena 2013, 111, 64–71. [Google Scholar] [CrossRef]
- Tang, B.; Xu, H.; Song, F.; Ge, H.; Chen, L.; Yue, S.; Yang, W. Effect of biochar on immobilization remediation of Cd-contaminated soil and environmental quality. Environ. Res. 2022, 204, 111840. [Google Scholar] [CrossRef]
- Wang, G.; Jin, Z.; Wang, X.; George, T.S.; Feng, G.; Zhang, L. Simulated root exudates stimulate the abundance of Saccharimonadales to improve the alkaline phosphatase activity in maize rhizosphere. Appl. Soil Ecol. 2022, 170, 104274. [Google Scholar] [CrossRef]
- Jing, Y.; Zhang, Y.; Han, I.; Wang, P.; Mei, Q.; Huang, Y. Effects of different straw biochars on soil organic carbon, nitrogen, available phosphorus, and enzyme activity in paddy soil. Sci. Rep. 2020, 10, 8837. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; McGrouther, K.; Huang, H.; Lu, K.; Guo, X.; He, L.; Lin, X.; Che, L.; Ye, Z.; et al. Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Environ. Sci. Pollut. Res. 2016, 23, 974–984. [Google Scholar] [CrossRef]
- Zheng, L.; Tong, C.; Gao, J.; Xiao, R. Effects of wetland plant biochars on heavy metal immobilization and enzyme activity in soils from the Yellow River estuary. Environ. Sci. Pollut. Res. 2022, 29, 40796–40811. [Google Scholar] [CrossRef]
- Wang, S.-N. The Characteristics and Influencing Factors of Soil Organic Carbon Source/Sink in Saline-alkaline Paddy Lands in Western Jilin Province. Ph.D. Thesis, Jilin University, Changchun, China, 2022. [Google Scholar]
- Wu, H.; Cui, H.; Fu, C.; Li, R.; Qi, F.; Liu, Z.; Yang, G.; Xiao, K.; Qiao, M. Unveiling the crucial role of soil microorganisms in carbon cycling: A review. Sci. Total Environ. 2024, 909, 168627. [Google Scholar] [CrossRef]
- Zhang, X.; Teng, Z.; Zhang, H.; Cai, D.; Zhang, J.; Meng, F.; Sun, G. Nitrogen application and intercropping change microbial community diversity and physicochemical characteristics in mulberry and alfalfa rhizosphere soil. J. For. Res. 2021, 32, 2121–2133. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Li, D.; Zhang, Z.-Y.; Liao, K.-J. Comparison of two methods for the determination of cation exchange in soil. Guizhou For. Sci. Technol. 2010, 38, 45–49. [Google Scholar]
- Wang, H.; Ren, T.; Mueller, K.; Van Zwieten, L.; Wang, H.; Feng, H.; Xu, C.; Yun, F.; Ji, X.; Yin, Q.; et al. Soil type regulates carbon and nitrogen stoichiometry and mineralization following biochar or nitrogen addition. Sci. Total Environ. 2021, 753, 141645. [Google Scholar] [CrossRef] [PubMed]
- Rong, G.; Zhang, X.; Wu, H.; Ge, N.; Yao, Y.; Wei, X. Changes in soil organic carbon and nitrogen mineralization and their temperature sensitivity in response to afforestation across China’s Loess Plateau. Catena 2021, 202, 105226. [Google Scholar] [CrossRef]
- Hu, L.; Li, S.; Li, K.; Huang, H.; Wan, W.; Huang, Q.; Li, Q.; Li, Y.; Deng, H.; He, T. Effects of Two Types of Straw Biochar on the Mineralization of Soil Organic Carbon in Farmland. Sustainability 2020, 12, 10586. [Google Scholar] [CrossRef]
- Gao, S.; Hoffman-Krull, K.; DeLuca, T.H. Soil biochemical properties and crop productivity following application of locally produced biochar at organic farms on Waldron Island, WA. Biogeochemistry 2017, 136, 31–46. [Google Scholar] [CrossRef]
- Huang, R.; Tian, D.; Liu, J.; Lu, S.; He, X.; Gao, M. Responses of soil carbon pool and soil aggregates associated organic carbon to straw and straw-derived biochar addition in a dryland cropping mesocosm system. Agric. Ecosyst. Environ. 2018, 265, 576–586. [Google Scholar] [CrossRef]
- Jien, S.-H.; Chen, W.-C.; Ok, Y.S.; Awad, Y.M.; Liao, C.-S. Short-term biochar application induced variations in C and N mineralization in a compost-amended tropical soil. Environ. Sci. Pollut. Res. 2018, 25, 25715–25725. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, C.; Li, F.; Gao, S.; Zhang, J. Effect of compost and inorganic fertilizer on organic carbon and activities of carbon cycle enzymes in aggregates of an intensively cultivated Vertisol. PLoS ONE 2020, 15, e0229644. [Google Scholar] [CrossRef]
- Sarma, B.; Borkotoki, B.; Narzari, R.; Kataki, R.; Gogoi, N. Organic amendments: Effect on carbon mineralization and crop productivity in acidic soil. J. Clean. Prod. 2017, 152, 157–166. [Google Scholar] [CrossRef]
- Xu, H.; Shao, H.; Lu, Y. Arbuscular mycorrhiza fungi and related soil microbial activity drive carbon mineralization in the maize rhizosphere. Ecotoxicol. Environ. Saf. 2019, 182, 109476. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).