The Application of Melatonin and Organic Waste Derived from Vitamin C Industry Effectively Promotes Seed Germination and Seedling Growth of Cotton in Saline–Alkali Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Treatments
2.2.1. Seed Disinfection and Soaking
2.2.2. Experimental Design and Treatments
2.3. Determination of Germination Rate, Seedling Establishment Rate, and Seed Vigor
2.4. Determination of Seedling Physiological Parameters and Soil Characteristics
2.4.1. Determination of Seedling Physiological Parameters
2.4.2. Analysis of Soil Characteristics
2.5. Statistical Analysis
3. Results
3.1. Seed Germination and Seedling Establishment
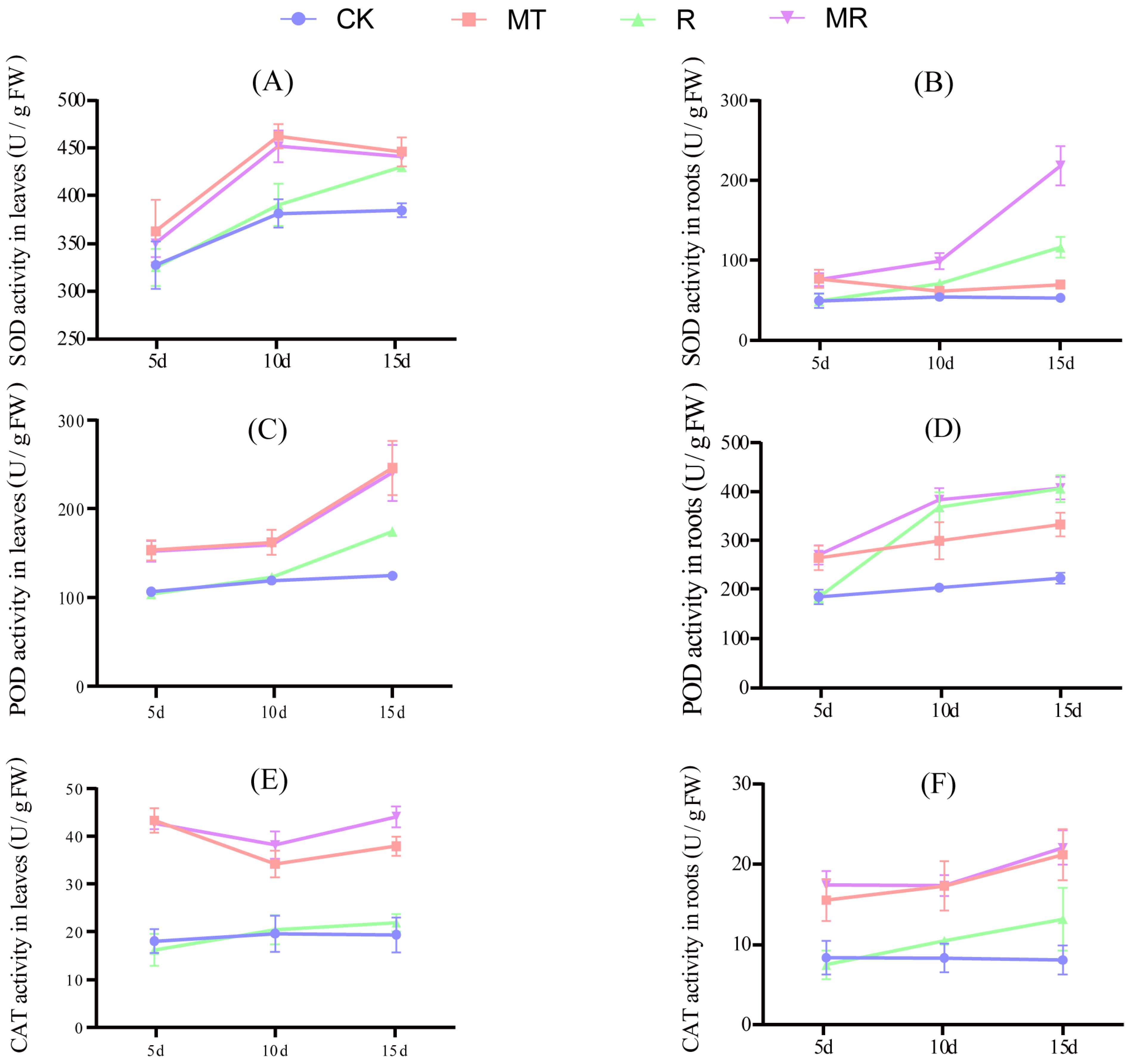
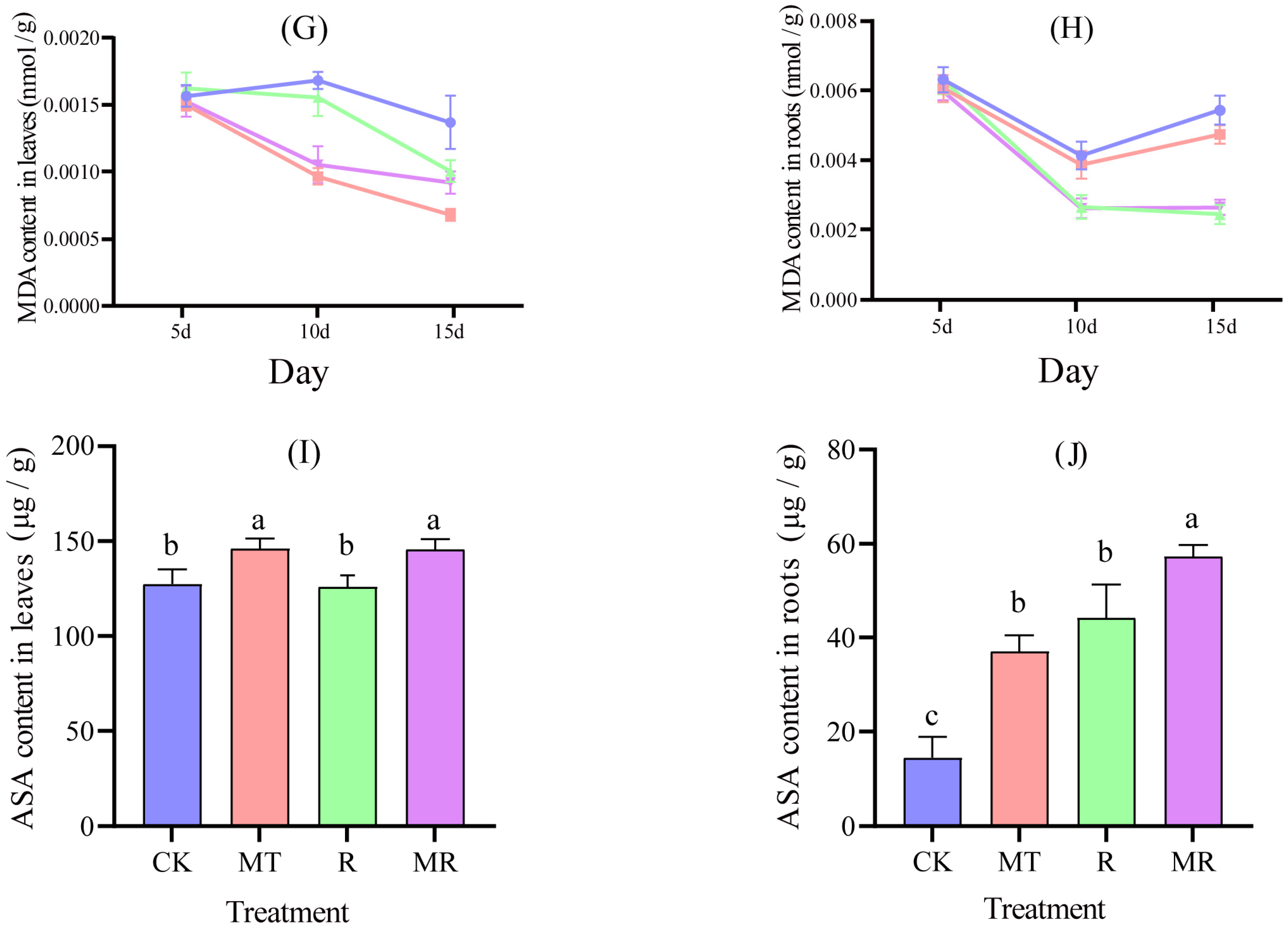
3.2. Effects of Exogenous MT and RAE on the Antioxidase Activities, and Contents of MDA and ASA
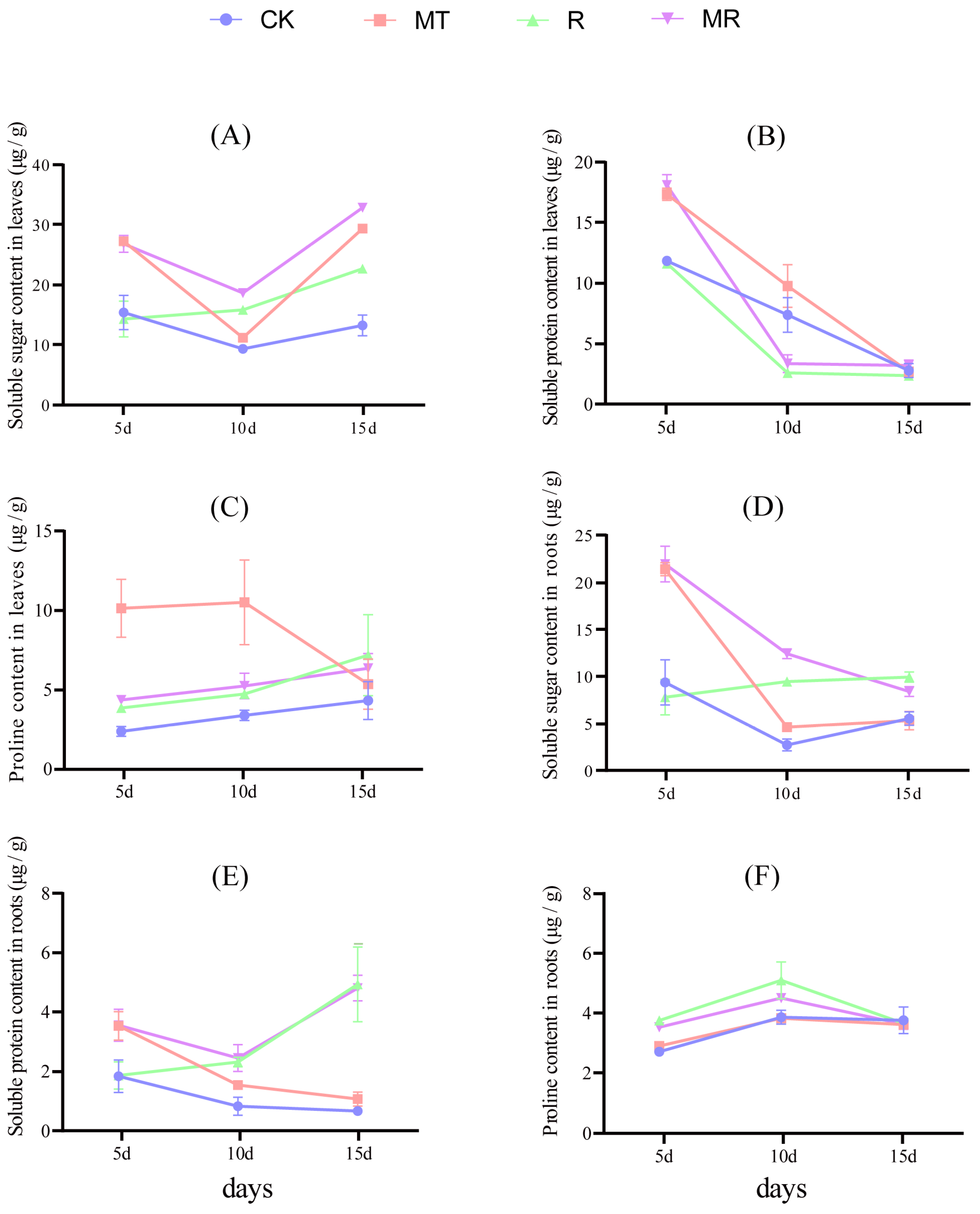
3.3. Effects of Exogenous MT and RAE on the Contents of Soluble Sugar, Soluble Protein and Proline
3.4. Effects of Exogenous MT and RAE on the Root Characteristics
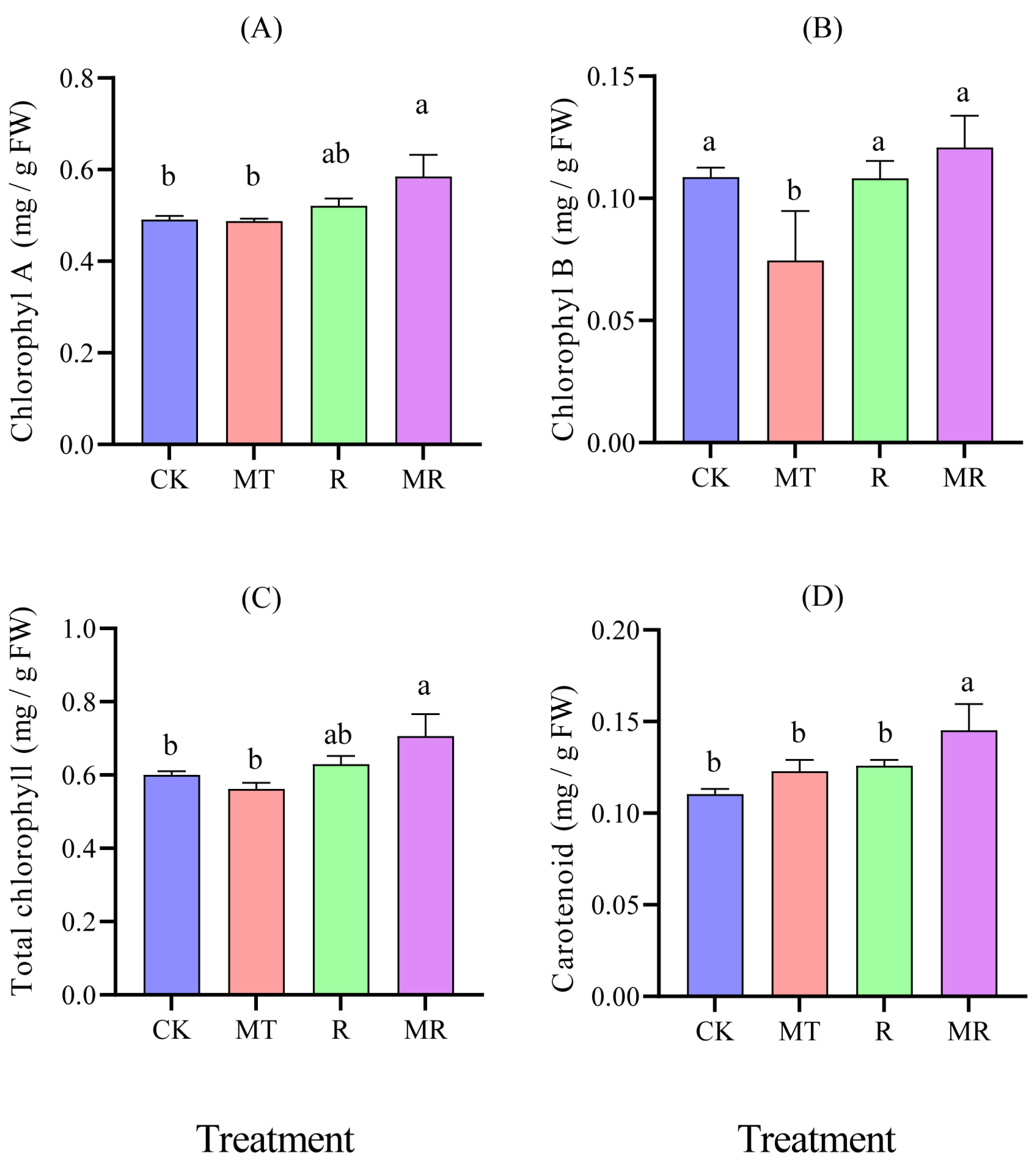
3.5. Effects of Exogenous MT and RAE on the Content of Photosynthetic Pigments
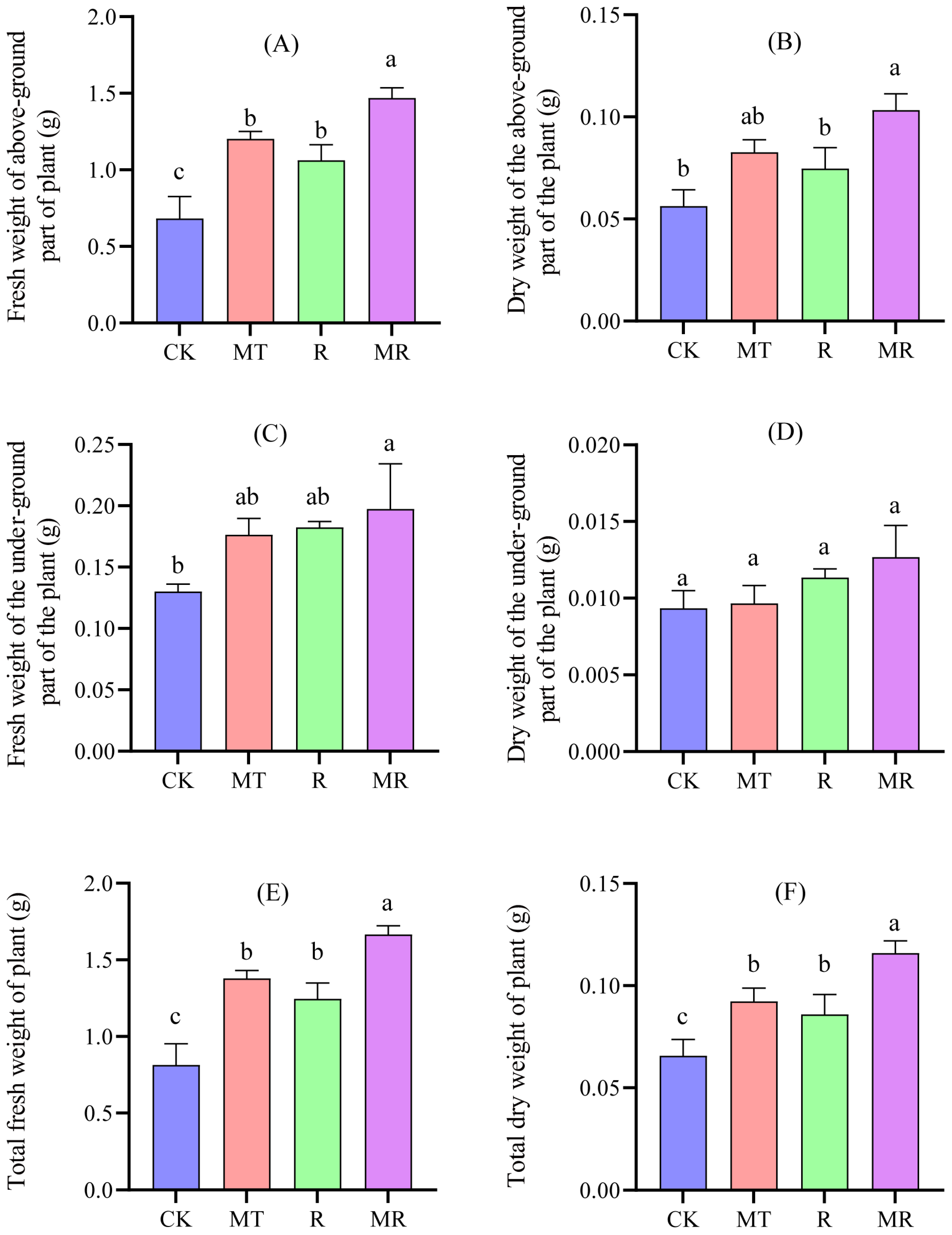
3.6. Effects of Exogenous MT and RAE on Biomass of Cotton Seedlings
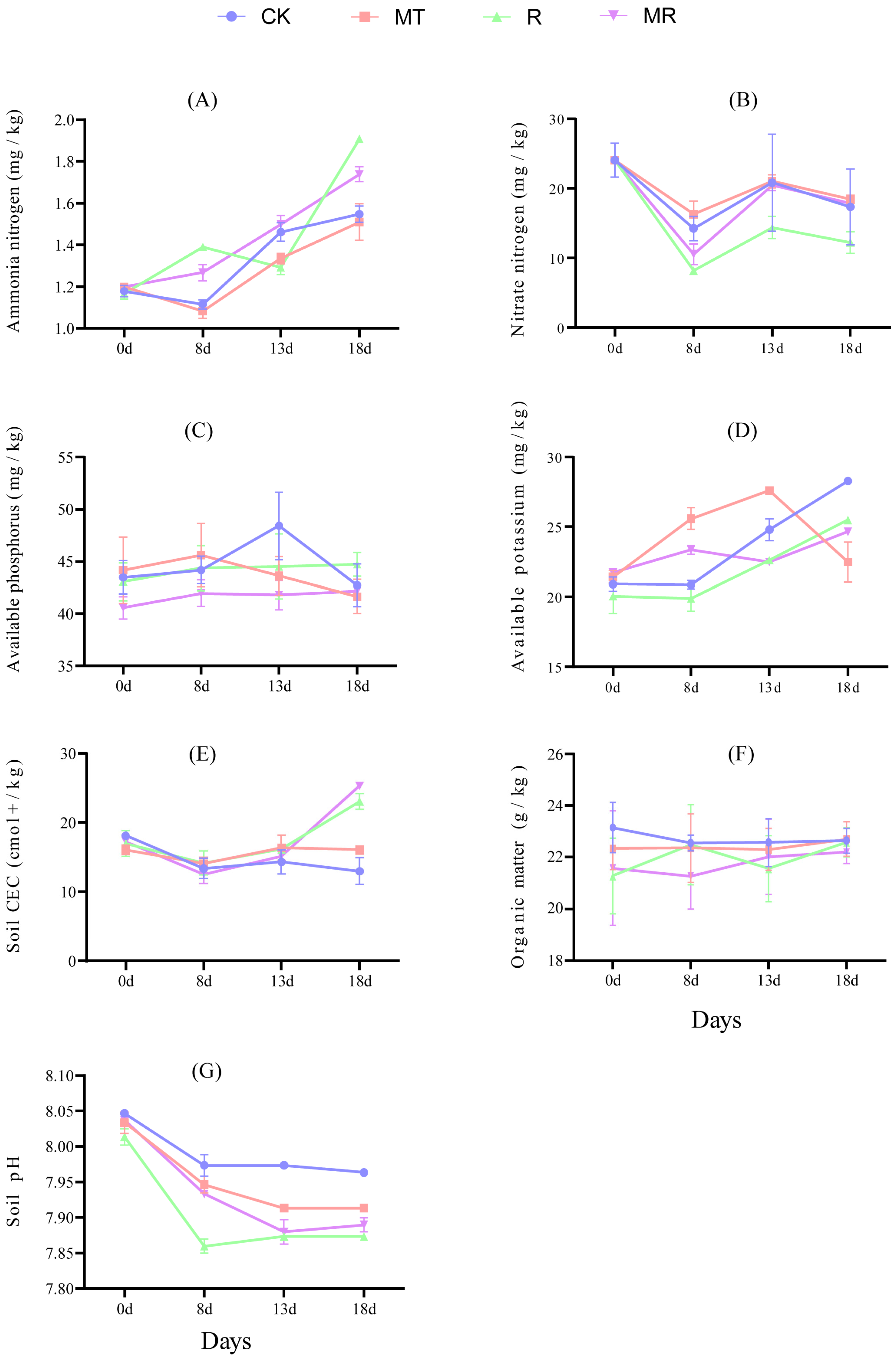
3.7. Effects of Exogenous MT and RAE on the Soil Nutrition
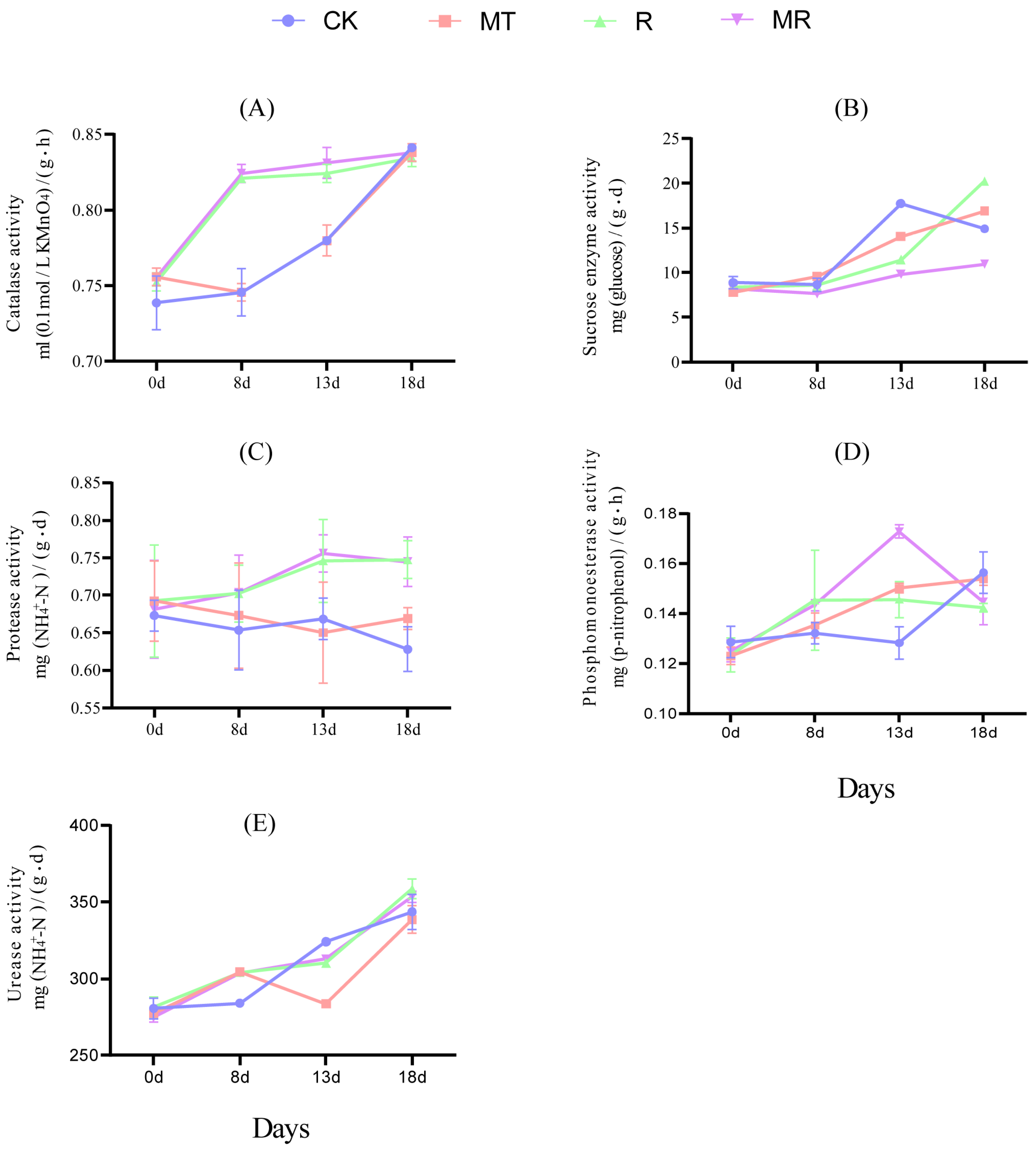
3.8. Effects of Exogenous MT and RAE to the Activity of Soil Enzyme
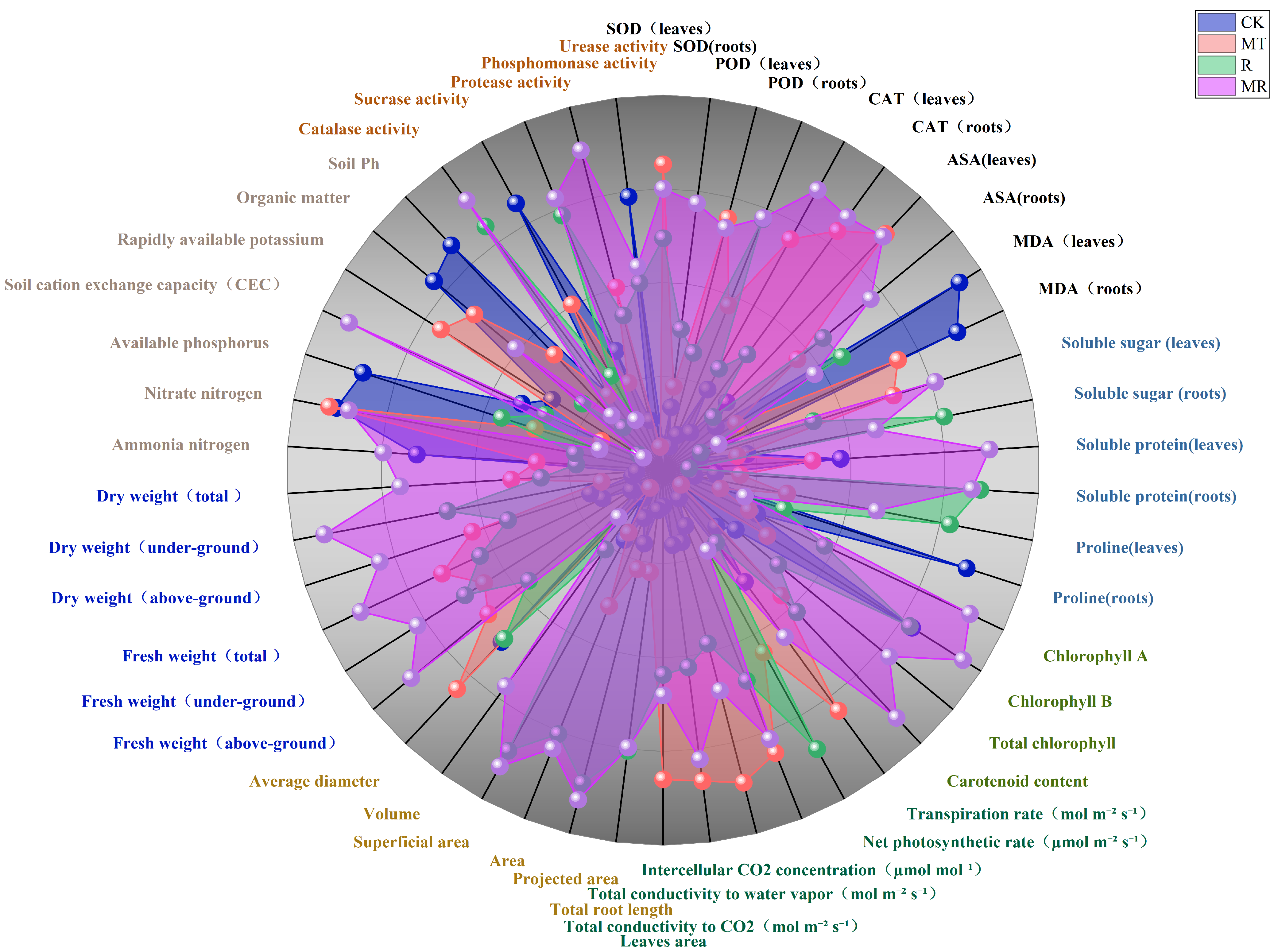
3.9. Correlation Analysis Between Physiological and Agronomic Indexes of Cotton Seedling and Physicochemical Properties of Rhizosphere Soil
4. Discussion
4.1. Effects of Melatonin Application on Seed Germination and Seedling Growth of Cotton
4.2. The Effects of RAE Application on Seed Germination and Seedling Growth of Cotton
4.3. Synergistic Effects of Combined Application of Melatonin and RAE on Seed Germination and Seedling Growth of Cotton
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, G.; Nimir, N.; Lu, S.; Zhai, F.; Wang, Y. Gibberellic Acid and Salinity Affected Growth and Antioxidant Enzyme Activities in Castor Bean Plants at Early Growth Stage. Agron. J. 2014, 106, 1340–1348. [Google Scholar] [CrossRef]
- Zhou, G.; Ma, B.L.; Li, J.; Feng, C.; Lu, J.; Qin, P. Determining Salinity Threshold Level for Castor Bean Emergence and Stand Establishment. Crop Sci. 2010, 50, 2030–2036. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, X.; Wang, X.; Shao, H.; Yang, J.; Wang, X. Soil enzymes as indicators of saline soil fertility under various soil amendments. Agric. Ecosyst. Environ. 2017, 237, 274–279. [Google Scholar]
- Cheng, Z.; Chen, Y.; Zhang, F. Effect of reclamation of abandoned salinized farmland on soil bacterial communities in arid northwest China. Sci. Total. Environ. 2018, 630, 799–808. [Google Scholar] [CrossRef]
- Li, J.; Pu, L.; Han, M.; Zhu, M.; Zhang, R.; Xiang, Y. Soil salinization research in China: Advances and prospects. J. Geogr. Sci. 2014, 24, 943–960. [Google Scholar] [CrossRef]
- Yang, S.; Hao, X.; Xu, Y.; Yang, J.; Su, D. Meta-Analysis of the Effect of Saline-Alkali Land Improvement and Utilization on Soil Organic Carbon. Life 2022, 12, 1870. [Google Scholar] [CrossRef]
- Chaudhary, M.T.; Majeed, S.; Rana, I.A.; Ali, Z.; Jia, Y.; Du, X.; Hinze, L.; Azhar, M.T. Impact of salinity stress on cotton and opportunities for improvement through conventional and biotechnological approaches. BMC Plant Biol. 2024, 24, 20. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Lu, B.; Liu, L.; Meng, Y.; Ma, X.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H.; et al. Effects of Exogenous Melatonin on Root Physiology, Transcriptome and Metabolome of Cotton Seedlings under Salt Stress. Int. J. Mol. Sci. 2022, 23, 9456. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Zhang, Y.; Bi, Y.; Sun, Z. Responses of Saline Soil Properties and Cotton Growth to Different Organic Amendments. Pedosphere 2018, 28, 521–529. [Google Scholar] [CrossRef]
- Abdelraheem, A.; Esmaeili, N.; O’connell, M.; Zhang, J. Progress and perspective on drought and salt stress tolerance in cotton. Ind. Crops Prod. 2018, 130, 118–129. [Google Scholar] [CrossRef]
- Sharif, I.; Aleem, S.; Farooq, J.; Rizwan, M.; Younas, A.; Sarwar, G.; Chohan, S.M. Salinity stress in cotton: Effects, mechanism of tolerance and its management strategies. Physiol. Mol. Biol. Plants 2019, 25, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, X.; Shi, J.; Liu, X.; Xu, Q.; Zhou, H.; Song, M.; Yan, G. Mepiquat chloride-priming induced salt tolerance during seed germination of cotton (Gossypium hirsutum L.) through regulating water transport and K+/Na+ homeostasis. Environ. Exp. Bot. 2019, 159, 168–178. [Google Scholar] [CrossRef]
- Wang, R.; Wan, S.; Sun, J.; Xiao, H. Soil salinity, sodicity and cotton yield parameters under different drip irrigation regimes during saline wasteland reclamation. Agric. Water Manag. 2018, 209, 20–31. [Google Scholar] [CrossRef]
- Ahmad, I.; Zhu, G.; Zhou, G.; Song, X.; Ibrahim, M.E.H.; Salih, E.G.I.; Hussain, S.; Younas, M.U. Pivotal Role of Phytohormones and Their Responsive Genes in Plant Growth and Their Signaling and Transduction Pathway under Salt Stress in Cotton. Int. J. Mol. Sci. 2022, 23, 7339. [Google Scholar] [CrossRef]
- Ahmad, I.; Song, X.; Ibrahim, M.E.H.; Jamal, Y.; Younas, M.U.; Zhu, G.; Zhou, G.; Ali, A.Y.A. The role of melatonin in plant growth and metabolism, and its interplay with nitric oxide and auxin in plants under different types of abiotic stress. Front. Plant Sci. 2023, 14, 1108507. [Google Scholar] [CrossRef]
- Lv, Y.; Pan, J.; Wang, H.; Reiter, R.J.; Li, X.; Mou, Z.; Zhang, J.; Yao, Z.; Zhao, D.; Yu, D. Melatonin inhibits seed germination by crosstalk with abscisic acid, gibberellin, and auxin in Arabidopsis. J. Pineal Res. 2021, 70, e12736. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Lu, B.; Ma, T.; Jiang, D.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Bai, Z.; et al. Exogenous melatonin promotes seed germination and osmotic regulation under salt stress in cotton (Gossypium hirsutum L.). PLoS ONE 2020, 15, e0228241. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Wang, X.; Gao, Y. Effect of Plant Growth Regulators on Cotton Seedling Root Growth Parameters and Enzyme Activity. Plants 2022, 11, 2964. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Y.; Dong, G.; Zhu, G.; Zhou, G. Progress of Research on the Physiology and Molecular Regulation of Sorghum Growth under Salt Stress by Gibberellin. Int. J. Mol. Sci. 2023, 24, 6777. [Google Scholar] [CrossRef]
- Lei, K.; Sun, S.; Zhong, K.; Li, S.; Hu, H.; Sun, C.; Zheng, Q.; Tian, Z.; Dai, T.; Sun, J. Seed soaking with melatonin promotes seed germination under chromium stress via enhancing reserve mobilization and antioxidant metabolism in wheat. Ecotoxicol. Environ. Saf. 2021, 220, 112241. [Google Scholar] [CrossRef]
- Jiang, D.; Lu, B.; Liu, L.; Duan, W.; Meng, Y.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H.; et al. Exogenous melatonin improves the salt tolerance of cotton by removing active oxygen and protecting photosynthetic organs. BMC Plant Biol. 2021, 21, 331. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Han, X.; Yang, W.; Sun, H.; Zhang, L.; Xu, H. A strategy for improving saline-alkali soil properties and cotton stress tolerance using vitamin C industrial wastes: A “prebiotics-probiotics” interaction between organic acids and Bacillus endo-phyticus. Ind. Crops Prod. 2024, 220, 119187. [Google Scholar] [CrossRef]
- Cheng, H.; Gao, M.; Yang, W.; Sun, H.; Kong, T.; Xu, H. Combined application of organic wastes and Trichoderma longibraciatum to promote vegetation restoration and soil quality on mining waste dump sites. Plant Soil 2024, 1–22. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, Z.; Yang, W.; Sun, H.; Xu, H. Application of Organic Waste Derived from Vitamin C Industry Increases Yield and Bioactive Constituents of Medicinal Food Plant Purslane (Portulaca oleracea L.). Horticulturae 2024, 10, 683. [Google Scholar] [CrossRef]
- Jia, L.; Tian, J.; Wei, S.; Zhang, X.; Xu, X.; Shen, Z.; Shen, W.; Cui, J. Hydrogen gas mediates ascorbic acid accumulation and antioxidant system enhancement in soybean sprouts under UV-A irradiation. Sci. Rep. 2017, 7, 16366. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Jalili, I.; Ebadi, A.; KalatehJari, S.; Aazami, M.A. Foliar application of putrescine, salicylic acid, and ascorbic acid mitigates frost stress damage in Vitis vinifera cv. ‘Giziluzum’. BMC Plant Biol. 2023, 23, 135. [Google Scholar] [CrossRef]
- Lisko, K.; Aboobucker, S.; Torres, R.; Lorence, A. Engineering Elevated Vitamin C in Plants to Improve their Nutritional Content, Growth, and Tolerance to Abiotic Stress. In Phytochemicals—Biosynthesis, Function and Application; Springer: Cham, Switzerland, 2014; Volume 44, pp. 109–128. [Google Scholar] [CrossRef]
- Ali, S.; Nawaz, A.; Hussain, S.; Khan, S.M.; Ejaz, S.; Ahmad, S. Abiotic Stress Tolerance in Plants by Priming and Pretreatments with Ascorbic Acid. Priming Pretreat. Seeds Seedl. 2019, 459–493. [Google Scholar]
- Wang, B.; Sun, H.; Yang, W.; Gao, M.; Zhong, X.; Zhang, L.; Chen, Z.; Xu, H. Potential utilization of vitamin C industrial effluents in agriculture: Soil fertility and bacterial community composition. Sci. Total Environ. 2022, 851, 158253. [Google Scholar] [CrossRef]
- Wang, S.; Wu, M.; Zhong, S.; Sun, J.; Mao, X.; Qiu, N.; Zhou, F. A Rapid and Quantitative Method for Determining Seed Viability Using 2,3,5-Triphenyl Tetrazolium Chloride (TTC): With the Example of Wheat Seed. Molecules 2023, 28, 6828. [Google Scholar] [CrossRef]
- Haghshenas, A.; Emam, Y. Accelerating leaf area measurement using a volumetric approach. Plant Methods 2022, 18, 61. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhang, B. Measurements of Proline and Malondialdehyde Content and Antioxidant Enzyme Activities in Leaves of Drought Stressed Cotton. Bio-Protocol 2016, 6, e1913. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, Z.; Zhang, G.; Chen, D.; Zang, L.; Liu, Q.; Guo, Y.; Xie, P.; Chen, H.; He, Y. Variability of soil enzyme activities and nutrients with forest gap renewal interacting with soil depths in degraded karst forests. Ecol. Indic. 2024, 166, 112332. [Google Scholar] [CrossRef]
- Frankeberger, W.T.; Johanson, J.B. Method of measuring invertase activity in soils. Plant Soil 1983, 74, 301–311. [Google Scholar] [CrossRef]
- Iwase, T.; Tajima, A.; Sugimoto, S.; Okuda, K.-I.; Hironaka, I.; Kamata, Y.; Takada, K.; Mizunoe, Y. A Simple Assay for Measuring Catalase Activity: A Visual Approach. Sci. Rep. 2013, 3, 3081. [Google Scholar] [CrossRef]
- Das, D.; Walvoort, M.T.; Lukose, V.; Imperiali, B. A Rapid and Efficient Luminescence-based Method for Assaying Phospho-glycosyltransferase Enzymes. Sci Rep. 2016, 6, 33412. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Guengerich, F.P. Dansylation of unactivated alcohols for improved mass spectral sensitivity and application to analysis of cytochrome P450 oxidation products in tissue extracts. Anal. Chem. 2010, 82, 7706–7712. [Google Scholar] [CrossRef]
- Dahlén, G.; Hassan, H.; Blomqvist, S.; Carlén, A. Rapid urease test (RUT) for evaluation of urease activity in oral bacteria in vitro and in supragingival dental plaque ex vivo. BMC Oral Health 2018, 18, 89. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Qin, S.; Yang, G.; Fang, K.; Zhu, B.; Kuzyakov, Y.; Chen, P.; Xu, Y.; Yang, Y. Regulation of priming effect by soil organic matter stability over a broad geographic scale. Nat. Commun. 2019, 10, 5112. [Google Scholar] [CrossRef]
- Hadi, J.; Tournassat, C.; Lerouge, C. Pitfalls in using the hexaamminecobalt method for cation exchange capacity measurements on clay minerals and clay-rocks: Redox interferences between the cationic dye and the sample. Appl. Clay Sci. 2016, 119, 393–400. [Google Scholar] [CrossRef]
- Del Egido, L.L.; Navarro-Miró, D.; Martinez-Heredia, V.; Toorop, P.E.; Iannetta, P.P.M. A Spectrophotometric Assay for Robust Viability Testing of Seed Batches Using 2,3,5-Triphenyl Tetrazolium Chloride: Using Hordeum vulgare L. as a Model. Front. Plant Sci. 2017, 8, 747. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant Growth Regulator and/or Biostimulant During Stress? Trends Plant Sci. 2014, 19, 1360–1385. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of Antioxidant Potential of Plants and its Relevance to Therapeutic Applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Cao, Y.; Song, H.; Zhang, L. New Insight into Plant Saline-Alkali Tolerance Mechanisms and Application to Breeding. Int. J. Mol. Sci. 2022, 23, 16048. [Google Scholar] [CrossRef] [PubMed]
- Sarropoulou, V.; Dimassi-Theriou, K.; Therios, I.; Koukourikou-Petridou, M. Melatonin enhances root regeneration, photo-synthetic pigments, biomass, total carbohydrates and proline content in the cherry rootstock PHL-C (Prunus avium × Prunus cerasus). Plant Physiol. Biochem. 2012, 61, 162–168. [Google Scholar] [CrossRef]
- Hamayun, M.; Khan, S.A.; Khan, A.L.; Shin, J.H.; Ahmad, B.; Shin, D.H.; Lee, I.J. Exogenous gibberellic acid reprograms soybean to higher growth and salt stress tolerance. J. Agric. Food Chem. 2010, 58, 7226–7232. [Google Scholar] [CrossRef]
- Yabuta, Y.; Mieda, T.; Rapolu, M.; Nakamura, A.; Motoki, T.; Maruta, T.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. J. Exp. Bot. 2007, 58, 2661–2671. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- Zhang, J.; De-Oliveira-Ceciliato, P.; Takahashi, Y.; Schulze, S.; Dubeaux, G.; Hauser, F.; Azoulay-Shemer, T.; Tõldsepp, K.; Kollist, H.; Rappel, W.-J.; et al. Insights into the Molecular Mechanisms of CO2-Mediated Regulation of Stomatal Movements. Curr. Biol. 2018, 28, R1356–R1363. [Google Scholar] [CrossRef]
- Asensi-Fabado, M.A.; Munné-Bosch, S. Vitamins in plants: Occurrence, biosynthesis and antioxidant function. Trends Plant Sci. 2010, 15, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Khaledian, Y.; Brevik, E.C.; Pereira, P.; Cerdà, A.; Fattah, M.A.; Tazikeh, H. Modeling soil cation exchange capacity in multiple countries. Catena 2017, 158, 194–200. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Shen, R.F. Aluminum-Nitrogen Interactions in the Soil-Plant System. Front Plant Sci. 2018, 9, 807. [Google Scholar] [CrossRef]
- Margesin, R.; Schinner, F. Phosphomonoesterase, phosphodiesterase, phosphotriesterase, and inorganic pyrophosphatase activities in forest soils in an alpine area: Effect of pH on enzyme activity and extractability. Biol. Fertil. Soils 1994, 18, 320–326. [Google Scholar] [CrossRef]
- de Caire, G.Z.; de Cano, M.S.; Palma, R.M.; de Mule´, C.Z. Changes in soil enzyme activities following additions of cyanobacterial biomass and exopolysaccharide. Soil Biol. Biochem. 2000, 32, 1985–1987. [Google Scholar] [CrossRef]
- Zofia; Wolińska, A.; Ziomek, J. Response of soil catalase activity to chromium contamination. J. Environ. Sci. 2009, 21, 1142–1147. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Shi, H.; Yang, Y.; Feng, X.; Chen, X.; Xiao, F.; Lin, H.; Guo, Y. Insights into plant salt stress signaling and tolerance. J. Genet. Genom. 2023, 51, 16–34. [Google Scholar] [CrossRef]
- Jeon, M.-W.; Ali, M.B.; Hahn, E.-J.; Paek, K.-Y. Photosynthetic pigments, morphology and leaf gas exchange during ex vitro acclimatization of micropropagated CAM Doritaenopsis plantlets under relative humidity and air temperature. Environ. Exp. Bot. 2004, 55, 183–194. [Google Scholar] [CrossRef]
- Peng, M.; He, H.; Jiang, M.; Wang, Z.; Li, G.; Zhuang, L. Morphological, physiological and metabolomic analysis to unravel the adaptive relationship between root growth of ephemeral plants and different soil habitats. Plant Physiol. Biochem. 2023, 202, 107986. [Google Scholar] [CrossRef]
- Cramer, M.D.; Hawkins, H.-J.; Verboom, G.A. The importance of nutritional regulation of plant water flux. Oecologia 2009, 161, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Ghestem, M.; Sidle, R.C.; Stokes, A. The Influence of Plant Root Systems on Subsurface Flow: Implications for Slope Stability. BioScience 2011, 61, 869–879. [Google Scholar] [CrossRef]
- Reich, M. The significance of nutrient interactions for crop yield and nutrient use efficiency. In Plant Macronutrient Use Efficiency; Elsevier: Amsterdam, The Netherlands, 2018; pp. 65–82. [Google Scholar] [CrossRef]
- Jin, K.; White, P.J.; Whalley, W.R.; Shen, J.; Shi, L. Shaping an Optimal Soil by Root–Soil Interaction. Trends Plant Sci. 2017, 22, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Gargallo-Garriga, A.; Preece, C.; Sardans, J.; Oravec, M.; Urban, O.; Peñuelas, J. Root exudate metabolomes change under drought and show limited capacity for recovery. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]


| Indices | Value | Indices | Value |
|---|---|---|---|
| pH | 0.29 | AP (mg·L−1) | 4.33 |
| COD (mg·L−1) | 1.18 × 106 | AK (mg·L−1) | 147.22 |
| TOC (g·L−1) | 177.52 | 2-KGA (g·L−1) | 201.83 |
| TN (g·L−1) | 4.69 | Oxalic acid (g·L−1) | 26.52 |
| TP (mg·L−1) | 0.18 | Formic acid (g·L−1) | 3.41 |
| TK (mg·L−1) | 2.11 | Valeric acid (g·L−1) | 0.42 |
| AN (mg·L−1) | 113.82 | Water content (%) | 55.69 |
| Treatment | Total Root Length (cm) | Projected Area (cm2) | Area (cm2) | Superficial Area (cm2) | Volume (cm3) | Average Diameter (mm) |
|---|---|---|---|---|---|---|
| CK | 68.65 ± 6.39 b | 2.90 ± 0.24 b | 2.77 ± 0.34 b | 10.06 ± 1.94 b | 0.17 ± 0.01 b | 0.36 ± 0.05 a |
| MT | 81.96 ± 3.21 b | 3.05 ± 0.41 b | 3.28 ± 0.20 b | 9.95 ± 0.76 b | 0.16 ± 0.01 b | 0.37 ± 0.02 a |
| R | 120.31 ± 3.45 a | 4.22 ± 0.11 a | 4.01 ± 0.07 a | 13.27 ± 0.33 a | 0.20 ± 0.01 b | 0.36 ± 0.02 a |
| MR | 119.61 ± 8.15 a | 4.31 ± 0.32 a | 4.10 ± 0.21 a | 13.51 ± 0.64 a | 0.29 ± 0.03 a | 0.33 ± 0.03 a |
| Treatment | Transpiration Rate (mol m−2 s−¹) | Net Photosynthetic Rate (µmol m−2 s−¹) | Intercellular CO2 Concentration (µmol mol−¹) | Leaves Area (cm2) | Total Conductivity to Water Vapor (mol m−2 s−¹) | Total Conductivity to CO2 (mol m−2 s−¹) |
|---|---|---|---|---|---|---|
| CK | 9.23 ± 0.92 bc | 134.71 ± 5.78 b | 38.00 ± 5.16 b | 7.48 ± 1.36 b | 179.77 ± 25.36 b | 29.04 ± 4.16 b |
| MT | 19.82 ± 2.73 a | 321.89 ± 73.80 ab | 70.56 ± 14.51 a | 10.94 ± 0.78 a | 279.00 ± 57.40 a | 41.70 ± 3.75 a |
| R | 5.99 ± 3.19 c | 439.58 ± 50.06 a | 60.17 ± 4.43 ab | 9.55 ± 0.96 ab | 221.55 ± 9.37 ab | 35.60 ± 1.67 ab |
| MR | 13.75 ± 1.01 b | 198.14 ± 55.54 b | 68.53 ± 10.54 a | 10.00 ± 0.48 a | 241.02 ± 13.19 ab | 40.54 ± 1.60 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Yang, W.; Sun, H.; Gao, M.; Wang, Y.; Xu, H. The Application of Melatonin and Organic Waste Derived from Vitamin C Industry Effectively Promotes Seed Germination and Seedling Growth of Cotton in Saline–Alkali Soil. Agriculture 2024, 14, 2135. https://doi.org/10.3390/agriculture14122135
Zhao X, Yang W, Sun H, Gao M, Wang Y, Xu H. The Application of Melatonin and Organic Waste Derived from Vitamin C Industry Effectively Promotes Seed Germination and Seedling Growth of Cotton in Saline–Alkali Soil. Agriculture. 2024; 14(12):2135. https://doi.org/10.3390/agriculture14122135
Chicago/Turabian StyleZhao, Xilai, Weichao Yang, Hao Sun, Mingfu Gao, Yushu Wang, and Hui Xu. 2024. "The Application of Melatonin and Organic Waste Derived from Vitamin C Industry Effectively Promotes Seed Germination and Seedling Growth of Cotton in Saline–Alkali Soil" Agriculture 14, no. 12: 2135. https://doi.org/10.3390/agriculture14122135
APA StyleZhao, X., Yang, W., Sun, H., Gao, M., Wang, Y., & Xu, H. (2024). The Application of Melatonin and Organic Waste Derived from Vitamin C Industry Effectively Promotes Seed Germination and Seedling Growth of Cotton in Saline–Alkali Soil. Agriculture, 14(12), 2135. https://doi.org/10.3390/agriculture14122135







