Non-Destructive Evaluation of Physicochemical Properties for Egg Freshness: A Review
Abstract
1. Introduction
2. Physicochemical Changes in Eggs with Storage Condition and Time
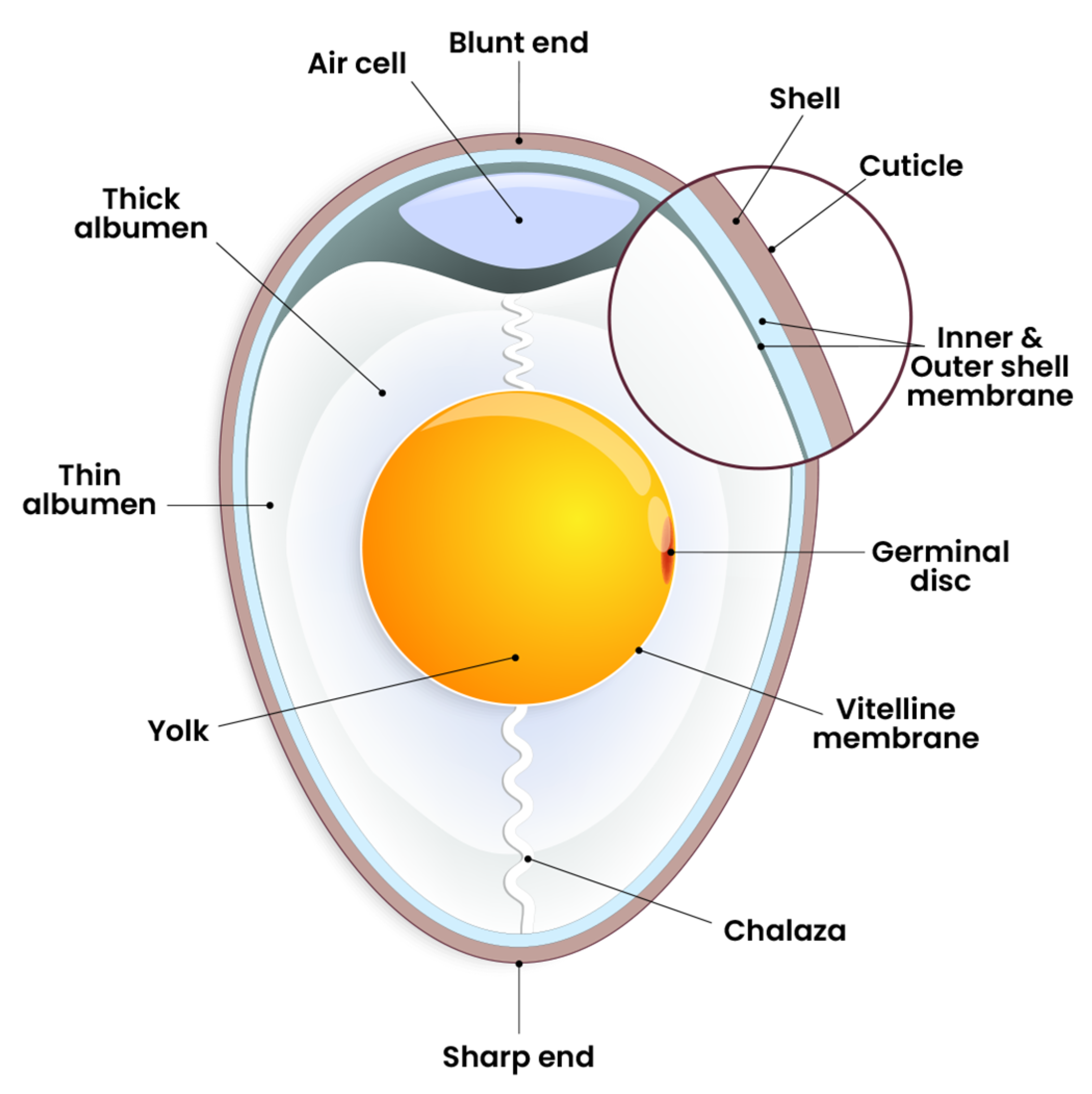
2.1. Egg White (Albumen) and Yolk
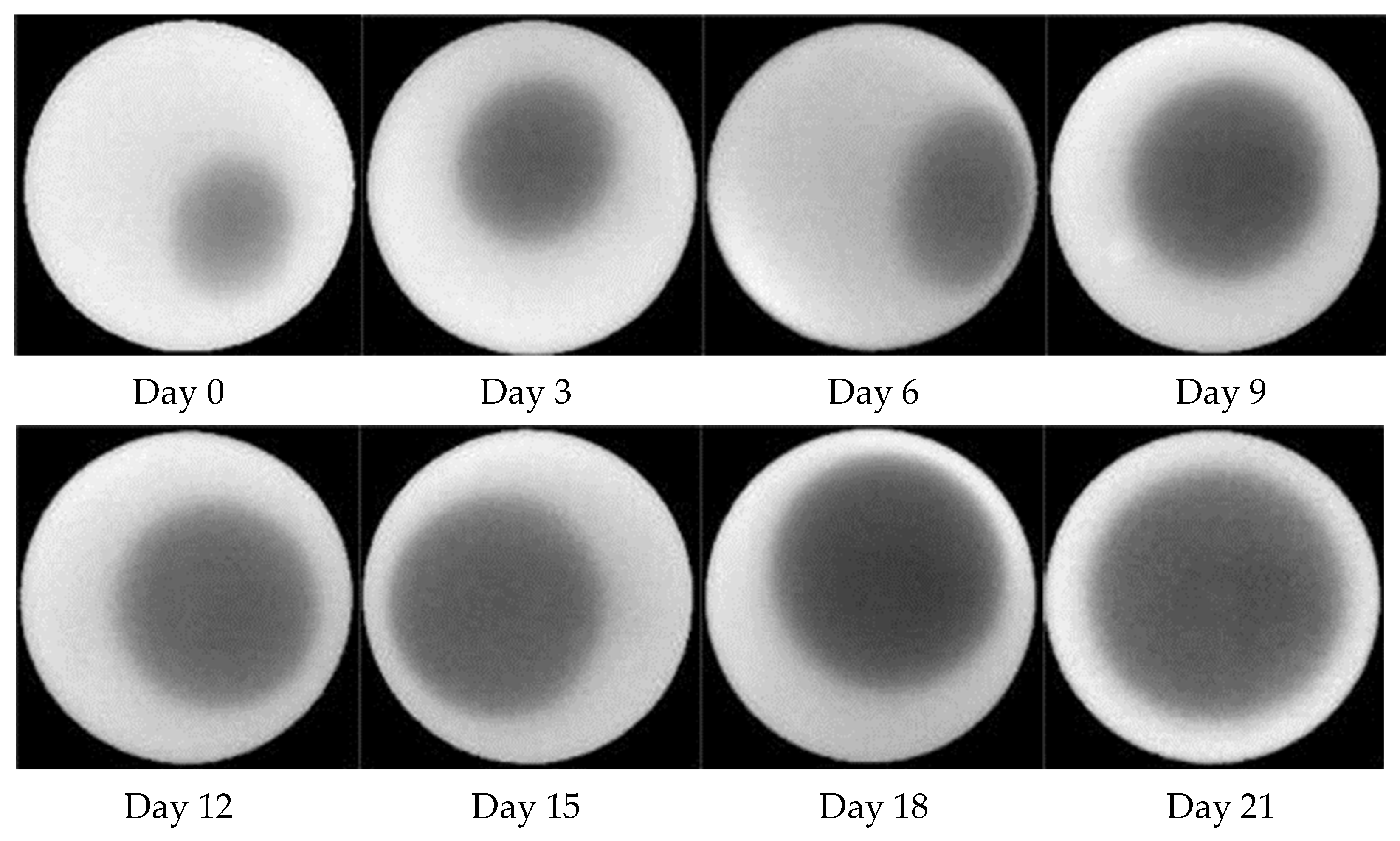
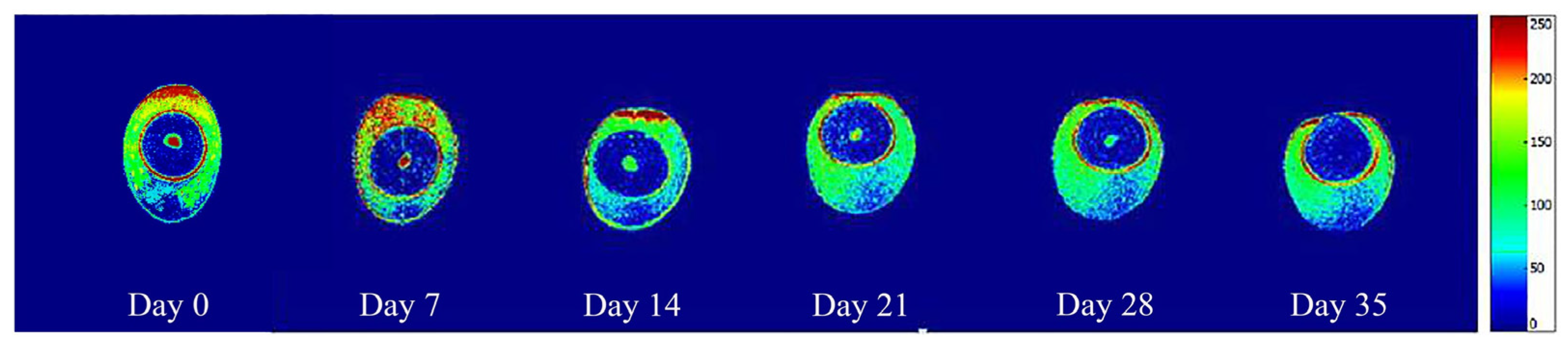
2.2. Air Cell
3. Current Methods for Assessing Egg Freshness
3.1. Candling
3.2. Haugh Unit (HU) Measurement
- HU = Haugh Unit
- H = Height of thick albumen (mm)
- W = Egg weight (g)
4. Non-Destructive Techniques for Assessing Egg Freshness
4.1. Vis-NIR Spectroscopy
4.2. Ultrasonic
4.3. Machine Vision
4.4. Thermal Imaging
4.5. Hyperspectral Imaging
4.6. Raman Spectroscopy
4.7. Fluorescence Spectroscopy
4.8. Low-Field NMR
4.9. MRI
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. FAO Statistical Yearbook—World Food and Agriculture; FAO: Rome, Italy, 2023; pp. 1–384. [Google Scholar] [CrossRef]
- OECD. Egg Projections: Production and Food Consumption; OECD: Paris, France, 2022. [CrossRef]
- OECD. OECD-FAO Agricultural Outlook 2023–2032; OECD: Paris, France, 2023. [CrossRef]
- Cardello, A.V.; Schutz, H.G. The Concept of Food Freshness: Uncovering its Meaning and Importance to Consumers; ACS Publications: Washington, DC, USA, 2003. [Google Scholar]
- Yimenu, S.M.; Kim, J.; Kim, B. Prediction of egg freshness during storage using electronic nose. Poult. Sci. 2017, 96, 3733–3746. [Google Scholar] [CrossRef] [PubMed]
- Çiftçi, H.G.; Bilge, G.; Aytaç, E. Monitoring the quality changes and freshness assessment of eggs based on chemometrics method. J. Food Process Eng. 2024, 47, e14591. [Google Scholar] [CrossRef]
- Jacob, J.P.; Miles, R.D.; Mather, F.B. Egg Quality; Cooperative Extension Service, Institute of Food and Agricultural Sciences (IFAS); University of Florida: Gainesville, FL, USA, 2000; Volume 24. [Google Scholar]
- Roberts, J.R. Factors affecting egg internal quality and egg shell quality in laying hens. J. Poult. Sci. 2004, 41, 161–177. [Google Scholar] [CrossRef]
- Travel, A.; Nys, Y.; Bain, M. Effect of hen age, moult, laying environment and egg storage on egg quality. In Improving the Safety and Quality of Eggs and Egg Products; Elsevier: Amsterdam, The Netherlands, 2011; pp. 300–329. [Google Scholar]
- Dedousi, A.; Stojčić, M.Đ.; Sossidou, E. Effects of housing systems on keel bone damage and egg quality of laying hens. In Proceedings of the Veterinary Research Forum, Urmia, Iran, 15 December 2020; p. 299. [Google Scholar]
- Samli, H.; Agma, A.; Senkoylu, N. Effects of storage time and temperature on egg quality in old laying hens. J. Appl. Poult. Res. 2005, 14, 548–553. [Google Scholar] [CrossRef]
- Kumari, A.; Tripathi, U.K.; Maurya, V.; Kumar, M. Internal quality changes in eggs during storage. Int. J. Sci. Environ. Technol. 2020, 9, 615–624. [Google Scholar]
- Yamak, U.S.; Sarica, M.; Erensoy, K.; Ayhan, V. The effects of storage conditions on quality changes of table eggs. J. Consum. Prot. Food Saf. 2021, 16, 71–81. [Google Scholar] [CrossRef]
- Okubo, T.; Akachi, S.; Hatta, H. Structure of hen eggs and physiology of egg laying. In Hen Eggs; CRC Press: Boca Raton, FL, USA, 2018; pp. 1–12. [Google Scholar]
- Ministry of Agriculture, Food and Rural Affairs (MAFRA). Detailed Standards for Livestock Product Grading; Ministry of Agriculture, Food and Rural Affairs: Sejong, Republic of Korea, 2018; p. 40.
- United Nations Economic Commission for Europe (UNECE). Egg-1 UNECE Standard Eggs-In-Shell; United Nations: New York, NY, USA, 2017.
- United States Department of Agriculture (USDA). USDA Egg-Grading Manual; United States Department of Agriculture: Washington, DC, USA, 2020.
- Mertens, K.; Kemps, B.; Perianu, C.; De Baerdemaeker, J.; Decuypere, E.; De Ketelaere, B.; Bain, M. Advances in egg defect detection, quality assessment and automated sorting and grading. Improv. Saf. Qual. Eggs Egg Prod. 2011, Volume 1, 209–241. [Google Scholar]
- Stadelman, W.J. Quality identification of shell eggs. In Egg Science and Technology; CRC Press: Boca Raton, FL, USA, 2017; pp. 39–66. [Google Scholar]
- Kim, J.; Semyalo, D.; Rho, T.-G.; Bae, H.; Cho, B.-K. Non-Destructive Detection of Abnormal Chicken Eggs by Using an Optimized Spectral Analysis System. Sensors 2022, 22, 9826. [Google Scholar] [CrossRef]
- Bell, D. Egg Shell Quality: Its Impact on Production, Processing and Marketing Economics; University of California: Riverside, CA, USA, 1998. [Google Scholar]
- Chukwuka, O.; Okoli, I.; Okeudo, N.; Udedibie, A.; Ogbuewu, I.; Aladi, N.; Iheshiulor, O.; Omede, A. Egg quality defects in poultry management and food safety. Asian J. Agric. Res. 2011, 5, 1–16. [Google Scholar] [CrossRef]
- So, J.-H.; Joe, S.Y.; Hwang, S.H.; Hong, S.J.; Lee, S.H. Current advances in detection of abnormal egg: A review. J. Anim. Sci. Technol. 2022, 64, 813. [Google Scholar] [CrossRef]
- Sun, L.; Bi, X.-K.; Lin, H.; Zhao, J.-W.; Cai, J.-R. On-line detection of eggshell crack based on acoustic resonance analysis. J. Food Eng. 2013, 116, 240–245. [Google Scholar] [CrossRef]
- Feddern, V.; Prá, M.C.D.; Mores, R.; Nicoloso, R.d.S.; Coldebella, A.; Abreu, P.G.d. Egg quality assessment at different storage conditions, seasons and laying hen strains. Ciência Agrotecnologia 2017, 41, 322–333. [Google Scholar] [CrossRef]
- Stadelman, W.J.; Newkirk, D.; Newby, L. Egg Science and Technology; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Li-Chan, E.C.; Powrie, W.D.; Nakai, S. The chemistry of eggs and egg products. In Egg Science and Technology; CRC Press: Boca Raton, FL, USA, 2017; pp. 105–175. [Google Scholar]
- Solomon, S. The eggshell: Strength, structure and function. Br. Poult. Sci. 2010, 51, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Eke, M.; Olaitan, N.; Ochefu, J. Effect of storage conditions on the quality attributes of shell (table) eggs. Niger. Food J. 2013, 31, 18–24. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Tu, Y.; Zhao, Y.; Luo, X.; Wang, J.; Wang, M. Changes in gel characteristics of egg white under strong alkali treatment. Food Hydrocoll. 2015, 45, 1–8. [Google Scholar] [CrossRef]
- Qi, L.; Zhao, M.-C.; Li, Z.; Shen, D.-H.; Lu, J. Non-destructive testing technology for raw eggs freshness: A review. SN Appl. Sci. 2020, 2, 1113. [Google Scholar] [CrossRef]
- Min, B.; Nam, K.C.; Jo, C.; Ahn, D.U. Irradiation of shell egg on the physicochemical and functional properties of liquid egg white. Poult. Sci. 2012, 91, 2649–2657. [Google Scholar] [CrossRef]
- Scott, T.; Silversides, F.G. The effect of storage and strain of hen on egg quality. Poult. Sci. 2000, 79, 1725–1729. [Google Scholar] [CrossRef]
- Jin, Y.; Lee, K.; Lee, W.; Han, Y. Effects of storage temperature and time on the quality of eggs from laying hens at peak production. Asian-Australas. J. Anim. Sci. 2011, 24, 279–284. [Google Scholar] [CrossRef]
- Sharp, P.F.; Powell, C.K. Increase in the pH of the white and yolk of hens’ eggs. Ind. Eng. Chem. 1931, 23, 196–199. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, L.; Wu, Y.; Huang, X.; Wang, G.; Song, H.; Geng, F.; Luo, P. Mechanism of differences in characteristics of thick/thin egg whites during storage: Physicochemical, functional and molecular structure characteristics analysis. Food Chem. 2022, 369, 130828. [Google Scholar] [CrossRef] [PubMed]
- Raji, A.; Aliyu, J.; Igwebuike, J.; Chiroma, S. Effect of storage methods and time on egg quality traits of laying hens in a hot dry climate. ARPN J. Agric. Biol. Sci. 2009, 4, 1–7. [Google Scholar]
- LANG, E.R.; RHA, C. Apparent shear viscosity of native egg white. Int. J. Food Sci. Technol. 1982, 17, 595–606. [Google Scholar] [CrossRef]
- Severa, L.; Nedomová, Š.; Křivánek, I.; Buchar, J. Rheological properties of ageing egg yolk. Acta Univ. Agric. Silv. Mendel. Brun 2005, 53, 127–138. [Google Scholar] [CrossRef]
- Heiman, V.; Carver, J. The albumen index as a physical measurement of observed egg quality. Poult. Sci. 1936, 15, 141–148. [Google Scholar] [CrossRef]
- Silversides, F.; Scott, T.A. Effect of storage and layer age on quality of eggs from two lines of hens. Poult. Sci. 2001, 80, 1240–1245. [Google Scholar] [CrossRef]
- Nys, Y.; Bain, M.; Van Immerseel, F. Egg chemistry, production and consumption. In Improving the Safety and Quality of Eggs and Egg Products; Elsevier: Amsterdam, The Netherlands, 2011; Volume 1. [Google Scholar]
- Kashimori, A. The Illustrated Egg Handbook; Context Publications: New York, NY, USA, 2017. [Google Scholar]
- Tabidi, M.H. Impact of storage period and quality on composition of table egg. Adv. Environ. Biol. 2011, 5, 856–861. [Google Scholar]
- Hester, P.Y. Effects of temperature and storage conditions on eggs. In Egg Innovations and Strategies for Improvements; Elsevier: Amsterdam, The Netherlands, 2017; pp. 125–134. [Google Scholar]
- Parkhurst, C.; Mountney, G.J. Poultry Meat and Egg Production; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Akter, Y.; Kasim, A.; Omar, H.; Sazili, A.Q. Effect of storage time and temperature on the quality characteristics of chicken eggs. J. Food Agric. Environ. 2014, 12, 87–92. [Google Scholar]
- Grashorn, M.; Juergens, A.; Bessei, W. Effects of storage conditions on egg quality. Lohmann Inf. 2016, 50, 22–27. [Google Scholar]
- Rho, T.-G.; Park, D.-S.; Kim, J.; Cho, B.-K. Non-Destructive Measurement of Egg Air Cell using Longwave Infrared Thermal Imaging. J. Korean Soc. Nondestruct. Test. 2023, 43, 145–153. [Google Scholar] [CrossRef]
- Brake, J.; Walsh, T.; Benton, C., Jr.; Petitte, J.; Meijerhof, R.; Penalva, G. Egg handling and storage. Poult. Sci. 1997, 76, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Hagan, J.K.; Eichie, F.O. Egg quality of two layer strains as influenced by extended storage periods and storage temperatures. Livest. Res. Rural Dev. 2019, 31, 145. [Google Scholar]
- Vaclavik, V.A.; Christian, E.W.; Vaclavik, V.A.; Christian, E.W. Eggs and egg products. Essent. Food Sci. 2008, 205–235. [Google Scholar]
- Ernst, R.; Bradley, F.; Delany, M.; Abbott, U.; Craig, R. Egg Candling and Breakout Analysis; UCANR Publications: Oakland, CA, USA, 2004. [Google Scholar]
- Haugh, R.R. The Haugh unit for measuring egg quality. U. S. Egg Poult. Mag. 1937, 43, 552–573. [Google Scholar]
- Jensen, L.S.; Sauter, E.; Stadelman, W. The detection and disintegration of blood spots as related to age of eggs. Poult. Sci. 1952, 31, 381–387. [Google Scholar] [CrossRef]
- Pennington, M.E.; Betts, H.M.P.; Jenkins, M.K. How to Candle Eggs; USA Department of Agriculture: Washington, DC, USA, 1918.
- Vasileva, A.; Gorbunova, E.; Vasilev, A.; Peretyagin, V.; Chertov, A.; Korotaev, V. Assessing exterior egg quality indicators using machine vision. Br. Poult. Sci. 2018, 59, 636–645. [Google Scholar] [CrossRef]
- Omid, M.; Soltani, M.; Dehrouyeh, M.H.; Mohtasebi, S.S.; Ahmadi, H. An expert egg grading system based on machine vision and artificial intelligence techniques. J. Food Eng. 2013, 118, 70–77. [Google Scholar] [CrossRef]
- Cozzolino, D.; Sanal, P.; Schreuder, J.; Williams, P.J.; Assadi Soumeh, E.; Dekkers, M.H.; Anderson, M.; Boisen, S.; Hoffman, L.C. Predicting Egg Storage Time with a Portable Near-Infrared Instrument: Effects of Temperature and Production System. Foods 2024, 13, 212. [Google Scholar] [CrossRef]
- Cruz-Tirado, J.; da Silva Medeiros, M.L.; Barbin, D.F. On-line monitoring of egg freshness using a portable NIR spectrometer in tandem with machine learning. J. Food Eng. 2021, 306, 110643. [Google Scholar] [CrossRef]
- Abdel-Nour, N.; Ngadi, M.; Prasher, S.; Karimi, Y. Prediction of egg freshness and albumen quality using visible/near infrared spectroscopy. Food Bioprocess Technol. 2011, 4, 731–736. [Google Scholar] [CrossRef]
- Kemps, B.J.; Bamelis, F.R.; De Ketelaere, B.; Mertens, K.; Tona, K.; Decuypere, E.M.; De Baerdemaeker, J.G. Visible transmission spectroscopy for the assessment of egg freshness. J. Sci. Food Agric. 2006, 86, 1399–1406. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, L.; Xu, H. On-line detection of blood spot introduced into brown-shell eggs using visible absorbance spectroscopy. Biosyst. Eng. 2015, 131, 95–101. [Google Scholar] [CrossRef]
- Dong, X.; Tang, X.; Dong, J.; Shen, Z.; Li, Y.; Peng, Y.; Li, Y. Nondestructive egg freshness assessment of air chamber diameter by VIS-NIR spectroscopy. In Proceedings of the 2018 ASABE Annual International Meeting, Detroit, MI, USA, 29 July–1 August 2018; p. 1. [Google Scholar]
- Wang, F.; Lin, H.; Xu, P.; Bi, X.; Sun, L. Egg freshness evaluation using transmission and reflection of NIR spectroscopy coupled multivariate analysis. Foods 2021, 10, 2176. [Google Scholar] [CrossRef]
- Yao, K.; Sun, J.; Zhang, B.; Du, X.; Chen, C. On-line monitoring of egg freshness using a portable NIR spectrometer combined with deep learning algorithm. Infrared Phys. Technol. 2024, 138, 105207. [Google Scholar] [CrossRef]
- Aboonajmi, M.; Setarehdan, S.; Akram, A.; Nishizu, T.; Kondo, N. Prediction of poultry egg freshness using ultrasound. Int. J. Food Prop. 2014, 17, 1889–1899. [Google Scholar] [CrossRef]
- Aboonajmi, M.; Akram, A.; Nishizu, T.; Kondo, N.; Setarehdan, S.; Rajabipour, A. An ultrasound based technique for the determination of poultry egg quality. Res. Agric. Eng. 2010, 56, 26–32. [Google Scholar] [CrossRef]
- Qin, H.; Wang, W.; Chu, X.; Jiang, H.; Zhao, X.; Jia, B.; Yang, Y.; Kimuli, D.; Dong, A.; Wang, B. Research on the nondestructive detection of egg freshness based on image processing. In Proceedings of the 2018 ASABE Annual International Meeting, Detroit, MI, USA, 29 July–1 August 2018; p. 1. [Google Scholar]
- Wang, Q.; Deng, X.; Ren, Y.; Ding, Y.; Xiong, L.; Ping, Z.; Wang, k. Egg freshness detection based on digital image technology. Sci. Res. Essay 2009, 4, 1073–1079. [Google Scholar]
- Zhang, J.; Lu, W.; Jian, X.; Hu, Q.; Dai, D. Nondestructive Detection of Egg Freshness Based on Infrared Thermal Imaging. Sensors 2023, 23, 5530. [Google Scholar] [CrossRef]
- Freni, F.; Quattrocchi, A.; Piccolo, S.; Montanini, R. Quantitative evaluation of eggs freshness using flash thermography. Quant. InfraRed Thermogr. J. 2020, 17, 13–25. [Google Scholar] [CrossRef]
- Nakaguchi, V.M.; Ahamed, T. Fast and Non-Destructive Quail Egg Freshness Assessment Using a Thermal Camera and Deep Learning-Based Air Cell Detection Algorithms for the Revalidation of the Expiration Date of Eggs. Sensors 2022, 22, 7703. [Google Scholar] [CrossRef]
- Park, B.; Lu, R. Hyperspectral Imaging Technology in Food and Agriculture; Springer: Berlin/Heidelberg, Germany, 2015; Volume 1. [Google Scholar]
- Lu, B.; Dao, P.D.; Liu, J.; He, Y.; Shang, J. Recent advances of hyperspectral imaging technology and applications in agriculture. Remote Sens. 2020, 12, 2659. [Google Scholar] [CrossRef]
- Wu, D.; Sun, D.-W. Advanced applications of hyperspectral imaging technology for food quality and safety analysis and assessment: A review—Part II: Applications. Innov. Food Sci. Emerg. Technol. 2013, 19, 15–28. [Google Scholar] [CrossRef]
- ElMasry, G.; Sun, D.-W. Principles of hyperspectral imaging technology. In Hyperspectral Imaging for Food Quality Analysis and Control; Elsevier: Amsterdam, The Netherlands, 2010; pp. 3–43. [Google Scholar]
- Kamruzzaman, M.; Sun, D.-W. Introduction to hyperspectral imaging technology. In Computer Vision Technology for Food Quality Evaluation; Elsevier: Amsterdam, The Netherlands, 2016; pp. 111–139. [Google Scholar]
- Tao, H.; Feng, H.; Xu, L.; Miao, M.; Yang, G.; Yang, X.; Fan, L. Estimation of the yield and plant height of winter wheat using UAV-based hyperspectral images. Sensors 2020, 20, 1231. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Sun, J.; Zhou, X.; Nirere, A.; Tian, Y.; Wu, X. Nondestructive detection for egg freshness grade based on hyperspectral imaging technology. J. Food Process Eng. 2020, 43, e13422. [Google Scholar] [CrossRef]
- Suktanarak, S.; Teerachaichayut, S. Non-destructive quality assessment of hens’ eggs using hyperspectral images. J. Food Eng. 2017, 215, 97–103. [Google Scholar] [CrossRef]
- Davari, M.; Bahreini, M.; Sabzevari, Z. Developing a non-destructive method for the detection of egg quality and freshness using micro-Raman spectroscopy. Appl. Food Res. 2024, 4, 100453. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, X.; Yu, H.; Cheng, Y.; Guo, Y.; Yao, W.; Xie, Y. Non-destructive and online egg freshness assessment from the egg shell based on Raman spectroscopy. Food Control 2020, 118, 107426. [Google Scholar] [CrossRef]
- Karoui, R.; Schoonheydt, R.; Decuypere, E.; Nicolaï, B.; De Baerdemaeker, J. Front face fluorescence spectroscopy as a tool for the assessment of egg freshness during storage at a temperature of 12.2 C and 87% relative humidity. Anal. Chim. Acta 2007, 582, 83–91. [Google Scholar] [CrossRef]
- Karoui, R.; Kemps, B.; Bamelis, F.; De Ketelaere, B.; Merten, K.; Schoonheydt, R.; Decuypere, E.; De Baerdemaeker, J. Development of a rapid method based on front face fluorescence spectroscopy for the monitoring of egg freshness: 1—Evolution of thick and thin egg albumens. Eur. Food Res. Technol. 2006, 223, 303–312. [Google Scholar] [CrossRef]
- Karoui, R.; Kemps, B.; Bamelis, F.; De Ketelaere, B.; Merten, K.; Schoonheydt, R.; Decuypere, E.; De Baerdemaeker, J. Development of a rapid method based on front-face fluorescence spectroscopy for the monitoring of egg freshness: 2—Evolution of egg yolk. Eur. Food Res. Technol. 2006, 223, 180–188. [Google Scholar] [CrossRef]
- Karoui, R.; Nicolaï, B.; De Baerdemaeker, J. Monitoring the egg freshness during storage under modified atmosphere by fluorescence spectroscopy. Food Bioprocess Technol. 2008, 1, 346–356. [Google Scholar] [CrossRef]
- Hu, M.; Zhao, M.; Qi, L.; Li, D.; Wang, X.; Li, Z.; Zhao, S.; Fan, K. Non-destructive inspection method for egg freshness evaluation via low-field nuclear magnetic resonance technology. J. Food Meas. Charact. 2024, 18, 7295–7307. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, Q.; Yang, L.; Tian, L.; Jia, J.; Lu, M.; Liu, X.; Duan, X. Actual time determination of egg freshness: A centroid rate based approach. Food Packag. Shelf Life 2020, 26, 100574. [Google Scholar] [CrossRef]


| Storage Time (d) | Storage Temperature (°C) | n | Egg Weight | Albumen | Yolk | Specific Gravity (g/cm3) | Air Cell (mm) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh (g) | Loss (g) | Haugh Units | Height (mm) | pH | Weight (g) | Yolk Index | pH | |||||
| Fresh eggs | 35 | 62.38 | - | 91.37 a | 8.56 a | 7.47 h | 17.97 b | 44.09 b | 5.75 de | 1.086 a | 3.18 e | |
| 2 | 5 | 35 | 61.83 | 0.17 d | 80.11 b | 6.65 b | 7.99 g | 18.49 ab | 46.21 b | 5.90 c | 1.085 a | 3.66 e |
| 21 | 35 | 63.85 | 0.32 d | 72.82 c | 5.80 c | 5.52 d | 19.36 a | 44.07 b | 5.90 cd | 1.082 b | 4.28 d | |
| 29 | 35 | 62.88 | 0.41 cd | 64.84 d | 4.85 d | 8.70 c | 19.26 a | 41.11 cd | 5.99 bc | 1.082 b | 4.56 d | |
| 5 | 5 | 35 | 61.94 | 0.32 d | 76.20 bc | 6.16 bc | 8.44 e | 18.33 ab | 48.48 a | 6.20 a | 1.082 b | 4.00 d |
| 21 | 35 | 63.67 | 0.65 c | 60.09 de | 4.41 de | 9.17 a | 19.33 a | 43.13 bc | 5.69 e | 1.078 c | 4.69 c | |
| 29 | 35 | 61.49 | 1.30 b | 55.68 e | 3.89 e | 9.20 a | 18.80 ab | 38.25 e | 5.85 cd | 1.071 d | 5.81 b | |
| 10 | 5 | 35 | 62.78 | 0.42 cd | 76.27 bc | 6.18 bc | 8.26 f | 18.50 ab | 40.77 cd | 5.86 cd | 1.080 bc | 4.24 cd |
| 21 | 35 | 61.69 | 1.03 b | 53.74 e | 3.76 e | 8.94 b | 19.34 a | 39.02 de | 6.08 ab | 1.074 d | 5.69 b | |
| 29 | 35 | 61.96 | 1.94 a | 40.57 f | 2.81 f | 9.11 a | 19.25 a | 32.73 f | 6.07 ab | 1.063 e | 7.82 a | |
| SEM | 0.270 | 0.046 | 1.092 | 0.113 | 0.029 | 0.111 | 0.398 | 0.018 | 0.001 | 0.103 | ||
| Source of variation | ———————————————————— p ———————————————————— | |||||||||||
| Storage time | NS | <0.001 | <0.001 | <0.001 | <0.001 | 0.548 | <0.001 | 0.023 | <0.001 | <0.001 | ||
| Storage temperature | NS | <0.001 | <0.001 | <0.001 | <0.001 | 0.011 | <0.001 | 0.060 | <0.001 | <0.001 | ||
| Time × temperature | NS | <0.001 | <0.001 | <0.001 | <0.001 | 0.936 | <0.01 | <0.001 | <0.001 | <0.001 | ||
| R2 | RMSE | Correlation Coefficients | RPD | |||
|---|---|---|---|---|---|---|
| Validated | Calibrated | Validated | Calibrated | |||
| Albumen pH | 0.90 | 0.91 | 0.06 | 0.06 | 0.95 | 3.32 |
| Haugh unit | 0.79 | 0.79 | 5.05 | 4.90 | 0.89 | 2.16 |
| Number of storage days | 0.89 | 0.90 | 1.65 | 1.64 | 0.94 | 4.92 |
| Instrument Type | Chemometric Tool | Assessed Parameters | Reference |
|---|---|---|---|
| Portable NIR Spectrometer | PLSR, PCA | Storage time, Albumen pH | [59] |
| Portable NIR Spectrometer | Machine learning algorithms | Haugh unit | [60] |
| Benchtop NIR Spectrometer | LDA | Haugh unit, Yolk index, Weight loss | [65] |
| Portable NIR Spectrometer | Deep learning algorithms | Haugh unit | [66] |
| Method | Advantages | Disadvantages |
|---|---|---|
| Vis-NIR Spectroscopy | Quick data collection; can predict albumen pH and HU with high correlation coefficients. | Requires careful calibration; affected by shell color, thickness, and cleanliness. |
| Ultrasonic | Provides information on internal egg structure; effective for assessing albumen and yolk condition. | Contact measurement can risk contamination; may require complex setup and calibration. |
| Machine Vision | Automated inspection; high speed; can identify external and internal defects via digital imaging. | Dependent on lighting, camera quality, and algorithm robustness; orientation of eggs can affect accuracy. |
| Thermal Imaging | Can assess air cell size; non-contact; good for high-throughput operations. | Environmental factors, like temperature and humidity, can impact results; lower reliability for HU values. |
| Hyperspectral Imaging | Provides comprehensive chemical and physical information; can predict HU values. | High cost; large data volume; requires sophisticated data processing and calibration. |
| Raman Spectroscopy | High specificity; minimal sample preparation; can detect protein and lipid changes. | Expensive equipment; interpretation of complex spectra can be challenging. |
| Fluorescence Spectroscopy | Effective for assessing protein and vitamin changes; non-invasive. | Affected by environmental factors; requires robust data analysis algorithms. |
| Low-field NMR | Detects water mobility and distribution; non-destructive; correlated with albumen height and HU. | Accuracy can be influenced by egg size and shell thickness; calibration is essential. |
| MRI | Provides high-resolution internal images; can detect subtle changes and anomalies. | High cost; requires large space; slower than other methods; complex operation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rho, T.-G.; Cho, B.-K. Non-Destructive Evaluation of Physicochemical Properties for Egg Freshness: A Review. Agriculture 2024, 14, 2049. https://doi.org/10.3390/agriculture14112049
Rho T-G, Cho B-K. Non-Destructive Evaluation of Physicochemical Properties for Egg Freshness: A Review. Agriculture. 2024; 14(11):2049. https://doi.org/10.3390/agriculture14112049
Chicago/Turabian StyleRho, Tae-Gyun, and Byoung-Kwan Cho. 2024. "Non-Destructive Evaluation of Physicochemical Properties for Egg Freshness: A Review" Agriculture 14, no. 11: 2049. https://doi.org/10.3390/agriculture14112049
APA StyleRho, T.-G., & Cho, B.-K. (2024). Non-Destructive Evaluation of Physicochemical Properties for Egg Freshness: A Review. Agriculture, 14(11), 2049. https://doi.org/10.3390/agriculture14112049






