Abstract
Maize is among the world’s three most important cereals because it is used for human consumption and agricultural feed. The embryo in monocotyledons contains a cotyledon that is the scutellum, which in Chalqueño maize constitutes approximately 80% of the embryo’s mass. The activation of metabolism during germination is accompanied by the production of reactive oxygen species, which must be maintained at a low level to avoid damage. Little is known about the oxidative state of the scutellum, but it is important to understand the control of oxidative stress during the final phase of germination and the embryo–seedling transition. Among the enzymes involved are class III peroxidase (POX), catalase (CAT), and superoxide dismutase (SOD), which were observed in the scutella of isolated imbibed embryos between 0 and 36 h. The activity of SOD fluctuated over a baseline value. The activity of class III POX was greater than that of CAT, showing differences between them in germination and postgermination. The activities of CAT and POX increased during germination (0 to 18 h), stabilized towards the final phase of germination (18 to 24 h), and then increased again in postgermination (24 to 36 h). The POX activity is a biochemical marker of the scutellum metabolism and marks the transition from germination to the embryo–seedling transition.
1. Introduction
Regarding the diversity of crops used for food, maize, wheat, and rice are major cereal species according to production volume [1] and constitute 94% of human consumption [2]. Due to its nutritional value, maize grain is used in various foods and industrial products, including starches, sweeteners, oils, beverages, gums, industrial alcohol, and fuel ethanol [3,4,5,6,7,8,9]. In Mexico, maize production is the largest among these three cereal products. In 2022, according to data extracted from the FAO [10], it was the third producer in America and represented 7.63% of the maize produced in the USA. Mexico is a producer of white maize, used for human nutrition, and while it is estimated that the average consumption of maize as a food source worldwide is 50 g person−1 day−1, in Mexico, it is an estimated 267 g person−1 day−1, which is the highest in the entire North American continent [2].
The conservation and study of native breeds adapted to various climatic conditions, soil types, and resistances to abiotic or biotic stresses provides genetic resources and strategic investment opportunities [11], allowing for future plant breeding strategies. Mexico is the center of origin and genetic diversification of maize (Zea mays L.) [12,13,14,15]. The genetic groups of greatest interest are those of the Mexican Pyramidal Complex from central Mexico [16]; these groups are cultivated in the Central High Valleys of Mexico at an altitude greater than 1800 m above sea level in volcanic soils that retain moisture from rainfall, and various native varieties are planted in this region, particularly Zea mays subspecies mays landrace Chalqueño [17,18,19,20], one of the seven breeds with the highest cultivation and production in the region. In the central region of Mexico, Chalqueño maize is generally used in the spring–summer agricultural cycle; it is a tall annual plant with a long life cycle and a vegetative period of 100 to 107 days. During the growth stage, the plant is moderately resistant to drought and produces large conical ears approximately 15 to 16 cm long with high yields per row, in which the grains are highly vigorous and have high germination capacity [20,21]. Chalqueño maize is a polymorphic breed due to its texture variant (floury to semiserrated to semicrystalline), with a coloration that varies from white to yellow to pink to red to blue. In Valle de Chalco-Amecameca, varieties of the creamy type are common, with semicrystalline grains with light yellow to white coloration [13,19,21,22,23,24,25]. Chalqueño maize is used for human consumption and has played an important role in the economy and culture of Mexico since the colonial era, when this variety was created [18,21].
Seed germination is the initial step in the life cycle of plants; it involves cellular and metabolic activation in a specific and highly regulated sequence. As germination progresses and mitochondrial functionality is reestablished, the greater mitochondrial activity coincides when the seed increases oxygen capture. The increased activation of oxidative phosphorylation can lead to oxidative stress [26]. For cereals, the energy required from germination until seedling establishment is initially derived from the metabolism of reserve substances within the embryo, initially of the embryonic axis, and subsequently, the reserves stored in the scutellum. Later, the scutellum captures and transfers various hydrolysable products from the endosperm to the growing seedling. The major storage components stored in the scutellum and endosperm are lipids, proteins, and various macro- and micronutrients [27,28,29,30]. The seed germination process is fundamental for plant establishment, and the lack of uniform germination can affect crop yield [31].
The scutellum of Chalqueño maize contributes approximately 80% of the embryo’s mass. The scutellum is important because during germination and the embryo–seedling transition stage it provides nutrients to the growing embryonic axis, mainly to the embryonic root. Later, the scutellum captures and transfers from the endosperm various nutritional molecules to the mesocotyl when the nutriment demand is increased due to growth through the soil, until the coleptile reaches the soil surface and the primordium foliar are exposed to light; simultaneously, the establishment of the first true roots occurs. Therefore, the scutellum is a fundamental organ for seedling establishment [32].
The activation of metabolism in the scutellum is accompanied by the production of reactive oxygen species (ROS). Several species of ROS are present in plants, including superoxide radicals (O2·−), oxygen singlets (1O2), hydroxyl radicals (°OH), and hydrogen peroxide (H2O2) [33]. In adequate concentrations, ROS play a positive role in dormancy release, seed germination signaling, the weakening and decay of the endosperm, and protection against pathogens [34]. Among ROS, H2O2 is an important redox molecule, is a stable compound with a longer half-life, and is relatively less toxic than the other species; however, it reacts with different molecules and is a substrate for different enzymes, and because of this, under certain circumstances it is capable of being a substrate to produce hydroxyl radicals, which are harmful at high concentrations [33,35,36,37]. ROS function in signaling pathways and are related to mechanisms that are specifically finely regulated both temporally and spatially. These species are involved in signal transduction cascades that regulate many physiological, structural, and developmental processes at different stages of the plant life cycle [38]. The uncontrolled accumulation of ROS due to the imbalance between ROS production and/or removal/inactivation generates oxidative stress. Avoiding structural and functional damage during and after germination is essential to keep the physiological concentration of ROS low [39], causing lipid peroxidation, defective proteins, mitochondrial degradation, and DNA and RNA damage [34,40,41], generating cellular damage, and even inducing programmed cell death [33,42].
Plants have an antioxidant system that involves both enzymatic and nonenzymatic mechanisms. The enzymes responsible for ROS detoxification are of particular interest during and after germination. The degree of germination success depends on the efficiency of free radical scavenging and impacts the vigor of the seed [38,43,44,45,46]. Enzymatic antioxidant mechanisms include superoxide dismutase (SOD), catalase (CAT), and class I and III peroxidases. The former two enzymes were previously considered the most important in the removal of ROS during vegetative growth [33,47,48,49]. Plants also possess two types of peroxidases, ascorbate peroxidase and glutathione peroxidase, which are class I peroxidases, and a second group which includes the large family of class III peroxidases (POX; EC: 1.11.1.7), in which the latter group presents high activity during germination. In addition to their antioxidant activity, under certain conditions, some isoforms of the class III peroxidase family possess pro-oxidant activity that generates ROS through the peroxidative and hydroxylic cycles and are important in cell wall modification during radicle expansion [50] and the permeation of the fibrous layer of the maize [51].
Although the importance of the participation of the scutellum during germination has been established for different cereals, most studies about the scutellum have been carried out on rice and wheat and have mainly been related to the mobilization and transfer of reserve substances from the endosperm [52,53,54,55,56,57,58,59,60,61]. Fewer studies have been performed on maize, for example, its development during embryogenesis [62]. During germination, processes related to the description of morphological and metabolic changes in the epidermis have been described in [32,63,64], such as the uptake of glutamine [65], amino acids, and peptides [59]. During postgermination, the production of amylases and their effect on the endosperm have been studied [66], and the contributions of the scutellum and the endosperm of the maize to the growth of the axis were reported [67]. Compared with rice and wheat, maize has a larger scutellum with a greater capacity to store reserve substances, and with a high metabolism during and after germination coupled with ROS production. Considering these, it is important to identify the antioxidant mechanisms of the scutellum that participate in homeostasis and control ROS fluctuation, as well as to identify the structural and functional changes of the scutellum associated with germination and the beginning of seed growth. These processes are essential for understanding the progress of germination and the correct establishment of seedlings
Therefore, in the present work, changes in the activity and location of class III POX, catalase, and superoxide dismutase during germination and the start of seedling growth, were investigated; the results revealed that POX activity is the highest and that it can act as a component of the oxidative stress control mechanism and allow for the transition between embryonic and seedling growth (the transition from germination to skotomorphogenesis).
2. Materials and Methods
2.1. Biological Material, Storage Conditions, and Reagents Used
The source of grains was Chalqueño maize cultivated in Valle de Chalco, Mexico.
Dry mature grains were acquired from producers in the local market. Once in the laboratory, the grains were cleaned to remove debris, placed in airtight plastic containers, and stored in the dark in a refrigerator at 7 °C and 40% relative humidity [51]. The chemical reagents, substrates, inhibitors, solvents, and antibodies used in the study were all purchased from Sigma-Aldrich, Merck, Baker or Abcam from the United States of America.
2.2. Germination Conditions and Obtaining the Scutellum
Embryos dissected from grains were surface disinfected with 3% commercial NaClO for 5 min and washed three times with sterile distilled water. They were then incubated in moist germinators in the dark at 25 to 27 °C for 0, 18, 24, or 36 h in a Riossa Bacteriological Incubator (Elmhurst, NY, USA). Once the embryo germinated, the fresh weight of the embryos was determined. The scutellum of the germinated embryo was dissected, and its fresh weight was determined.
2.3. Histochemical and Immunochemical Analysis
2.3.1. Fixation, Infiltration, and Cutting
Embryos from dry grains were germinated for different times as described before, and then they were fixed in 4% paraformaldehyde (pH 6.9) for 1 h or 70% ethanol for 1 day. The material fixed was infiltrated with 300 mM saccharose and cut with a cryostat to obtain 8 µm sections [68] or it was infiltrated and included in paraplast, where we obtained sections of 8 µm thickness using a rotary microtome American Optical [69]. The histological sections were adhered to slides coated with 1% gelatine [70,71]. Prior to use, from the sections obtained from the paraplast blocks, the inclusion medium was removed with xylene, followed by ethanol in decreasing concentrations [69]. The tissue sections were used for anatomical staining to detect reserve substances or for in situ enzyme immunolocalization.
2.3.2. Anatomical Staining
Histological sections were equilibrated in 70% ethanol and were stained by the regressive safranine and fast green technique of Johansen [69].
2.3.3. Lipid Detection
The histological sections were immersed in 60% ethanol for 10 min and stained with a 0.5% Oil Red O solution in propanol–water (6:4, v:v) for 15 min, after which the excess dye was washed with distilled water [51,72,73]. The preparations were mounted in aqueous medium and observed via Nomarski differential interference contrast microscopy using a Zeiss Axioskop microscope (Zeiss, Jena, Germany).
2.3.4. Starch Detection
Histological sections of germinated embryos were hydrated for 10 min. Iodine–potassium iodide was then added and was left to react for 5 min until the starch grains acquired an intense blue color [69]. The preparations were mounted in an aqueous medium and observed via Nomarski differential interference contrast microscopy using a Zeiss Axioskop microscope (Zeiss, Jena, Germany).
2.3.5. Immunolocalization
The in situ locations of the antioxidant enzymes catalase (CAT), superoxide dismutase (SOD), and class III peroxidase (PPOX) were determined using specific antibodies via two alternative methods. The first method used 8 µm sections of tissue samples fixed in 70% ethanol. The sections were pretreated with a solution to permit the antibody to permeate and avoid nonspecific immunoglobulin binding. The solution consisted of 2.5% nonfat skimmed milk with 1:60 rat preimmune serum in blocking PBS (PBSbloc: 10 mM phosphate buffer, pH 7.4; 150 mM NaCl; 2% cellulase; 5 mM MgSO4; 0.01% Triton X-100; 5 mM EDTA; and 1% polyvinylpolypyrrolidone). The samples were subsequently washed three times for 5 min each with a PBS incubation buffer (PBSinc: 10 mM phosphate buffer, pH 7.4; 150 mM NaCl; 2 mM KCl). To continue with infiltration and incubation, the tissue was incubated with specific primary antibodies for each enzyme in independent sections: (a) rabbit polyclonal anti-catalase antibody peroxisome marker diluted 1:200 (Abcam 1877, Santa Cruz, Califormia, USA); (b) rabbit anti-superoxide dismutase affinity-isolated antibody (Mn-SOD, DD-17) diluted 1:30 (Sigma S5069, Sigma-Aldrich, St. Louis, MO, USA); and (c) rabbit anti-horseradish peroxidase diluted 1:15 (Sigma, P7899). Each specific immune serum was diluted in PBSinc and incubated for 72 h at 7–8 °C. Afterwards, the sample was washed three times for 5 min with PBSinc, and finally incubated for 1 h at room temperature with goat anti-rabbit IgG-H and L secondary polyclonal fluorescent antibody (Abcam 96896, Santa Cruz, CA, USA) diluted to 1:50 in PBSinc.
The controls were treated as follows: the first antibody was omitted and replaced with preimmune serum or the second antibody was eliminated and replaced with preimmune serum [71,74]. The tissue sections were analyzed using a Carl Zeiss Confocal Spectral Microscopy System (model LSM 780 NLO; Germany) with two independent tracks. To provide anatomical context, tissue autofluorescence was used, with an excitation wavelength of 405 nm and emissions of 410 to 516 nm. The fluorescent secondary antibody was irradiated at 562 nm for excitation, and emission was detected at 578–599 nm (following the supplier’s instructions). These wavelengths were selected based on the Dylight 550 fluorescent antibody to avoid the intrinsic autofluorescence of the tissue at 512 nm and 626–635 nm Figure S2 in [51].
A second method was used to localize CAT, in which the tissue samples were fixed in 4% paraformaldehyde. In the hydrated sections, the endogenous POX enzymatic activity was removed from the tissue sections by treatment with 2.5% H2O2 and 1% periodic acid for 1 h or by incubation in the presence of 0.3% H2O2 in methanol [68,75]. Next, the tissue sample was permeabilized, and the nonspecific binding of immunoglobulin was blocked with 2.5% nonfat skimmed milk containing 1:60 rat serum in blocking PBSbloc. The immunolocalization of the enzyme was carried out using the primary antibody: rabbit polyclonal anti-catalase antibody peroxisome marker (Abcam 1877, Santa Cruz, CA, USA) diluted to 1:200 in PBSinc and incubated for 72 h at 7–8 °C. After three washes for 5 min with PBSinc, the secondary antibody conjugated to horseradish peroxidase (Sigma P 9209, Sigma-Aldrich, St. Louis, MO, USA) diluted to 1:50 in PBS was added and incubated for 1 h at room temperature, after which three 10 min washes were performed. The peroxidase reaction was developed with 3-amino-9-ethylcarbazole and H2O2 following the supplier’s instructions (Sigma A 6926; Sigma-Aldrich, St. Louis, MO, USA). The samples were washed three times for 10 min and directly mounted in water for observation [48]. A Zeiss Axioskop microscope (Zeiss, Jena, Germany) was utilized for visualization and photography via Nomarski differential interference contrast microscopy.
2.4. Enzyme Activity Assays
2.4.1. Enzymatic Extraction
The embryos were imbibed for 0, 18, 24, or 36 h, and the scutella were dissected from the embryonic axes on an ice bed and weighed. The embryonic axes were discarded to eliminate the high peroxidase activity present in the areas of embryonic root growth [76]. For each assay, four scutella were homogenized in 3 mL of 100 mM phosphate buffer (pH 6.8) and centrifuged at 10,000× g for 30 min at 4 °C in a RC-5 Superspeed Refrigerated Centrifuge Sorvall, (Waltham, MA, USA). The resulting supernatants were used as enzyme extracts and were maintained at 4 °C until analysis [77]. The protein concentrations were determined via the method described by Lowry et al. [78] with bovine serum albumin as the standard.
2.4.2. Quantification of Catalase Activity
CAT activity was determined using a modified version of the method of Bailly and Kranner [79]. The reaction mixture consisted of 50 to 100 µL of enzyme extract and 43 µL of 3% H2O2 to a final concentration of 37.5 mM, and the final volume was adjusted to 1 mL with 50 mM phosphate buffer (pH 6.8). The decrease in absorption at 240 nm was determined every 10 s for 2 min. For the spectrophotometric assessments, a Mecasys Optizen Pop UV/Vis spectrophotometer (Daejeon, Republic of Korea) was used. The results were expressed as Δ DO/min scutellum or Δ mmol/min scutellum, in which the molar extinction coefficient ε = 39.4 mM−1 cm−1 [80] was used. Enzyme activity was confirmed by the addition of inhibitors to the reaction mixture: 10 µL of 0.1 M KCN or 10 µL of 0.1 M 3-amino-1,2,4-triazole [81].
2.4.3. Quantification of Superoxide Dismutase Activity
SOD activity was determined via the method of inhibition of nitrotetrazolium blue chloride (NBT) photoreduction. The reaction mixture consisted of 2960 µL of 50 mM phosphate buffer at pH 6.8 with 5.8 mg of methionine, to which 10 µL of NBT was added to a concentration of 63 mM and 30 µL of 1.3 mM riboflavin; the reaction was started with the addition of 100 µL of enzyme extract. As a control to quantify the total production of superoxide in the test, a reaction mixture with a final volume of 3060 µL was used with a composition like that of the enzyme assay, in which the volume of the enzyme extract was replaced by an identical volume of phosphate buffer [79]. The reaction mixture was incubated at 25 °C and irradiated with a fluorescent lamp (75 watt Phillips lamp (Naucalpan, Mexico); 760 lm, 54 lm/W, l = 195 mA) for 2, 4, 6, 8, or 10 min, and the absorbance at 560 nm was determined. For the spectrophotometric assessments, a Mecasys Optizen Pop UV/Vis spectrophotometer (Daejeon, Republic of Korea) was used. Tubes with identical mixtures that were not irradiated were used as blanks. To determine the type of enzyme involved, KCN, at a final concentration of 0.33 or 0.66 µM in the assay [76,82], or H2O2, at a final concentration of 0.26 or 0.52 mM [83], was used as an inhibitor. An enzymatic unit of SOD is defined as the enzyme activity that results in a 50% reduction in the absorption of NBT [51,79].
2.4.4. Quantification of Peroxidase Activity
The POD activity was determined via the method described by Corona-Carrillo et al. [51]. Briefly, activity was determined via the following two reaction mixtures: (a) Guaiacol peroxidase activity (guPOX) was determined using a reaction mixture consisting of 870 μL of 50 mM phosphate buffer (pH 6.8), 10 μL of enzyme extract, and 10 μL of 1 M guaiacol, and 9 μL of 3% H2O2 was added to begin the reaction; the absorbance at 475 nm was recorded every 20 s for 2 min. As a control, in the reaction mixture the H2O2 was substituted by reaction buffer and (b) peroxidase activity with catechin (caPOX) was evaluated using a reaction mixture consisting of 440 μL of 50 mM phosphate buffer (pH 6.8), 20 μL of enzyme extract, 440 μL of 20 mM (+)-catechin, and 4 μL of 3% H2O2, and the absorbance at 475 nm was recorded every minute for 5 min. As a control, H2O2 was replaced with the same volume of reaction buffer. Inhibitors of POX activity, including 10 μL of 0.3 M salicylhydroxamic acid (SHAM) or 10 μL of 0.1 M KCN [76], were added to the reaction mixture described above, and the activity levels were quantified as described. For the spectrophotometric assessments, a Mecasys Optizen Pop UV/Vis spectrophotometer was used. The results of the activity assay were expressed as ΔOD/min scutellum or Δmmol/min scutellum using a molar extinction coefficient for guaiacol ε = 26.6 mM−1 cm−1 [84,85].
2.5. Statistical Analysis
The quantitative results are expressed as the average and standard error of 4–12 values or independent tests, as specified in each figure. A one-way ANOVA was performed, followed by a Tukey–Kramer multiple-comparison test, with a significance level of p < 0.05. Analyses were performed with NCSS and PASS 2000 software.
3. Results
3.1. Morphometry During Germination
During germination, the fresh weight of the scutellum remained stable, with a value that fluctuated around 84% of the total weight of the embryo between 0 and 18 h after the start of imbibition. As time progressed, the contribution of the scutellum to the total fresh weight of the embryo decreased to a minimum value at 36 h, and this change was statistically significant (Figure 1A). The amount of soluble protein is relative to fresh weight, which decreased from 0 h to 18 h, followed by a greater decrease between 18 and 24 h. Later from this time, the amount of protein remained steady until 36 h, although the differences were not statistically significant (Figure 1B).
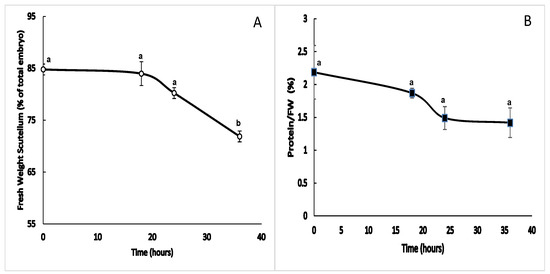
Figure 1.
Morphometric parameters during germination. Changes in the fresh weight and amount of soluble protein in the scutellum between 0 and 36 h after the start of imbibition. (A) Proportion of the change in the fresh weight of the scutellum in relation to the total weight of the embryo during imbibition. (B) Change in the amount of soluble protein in relation to the fresh weight during the study period. The results are shown as the means ± SEs of n = 4, and each experiment consisted of four scutella. ANOVA and Tukey–Kramer multiple-comparison tests were performed, with p < 0.05. Symbols: data groups with different letters are significantly different.
3.2. Anatomical Changes in the Scutellum Epidermis and the Accumulation of Reserve Substances
During the transition from germination to the start of seedling growth, the morphology of the scutellar epidermis undergoes changes. At 22 h after imbibition, the epidermis of the scutellum consists of a monostratified layer of polyhedral cells (Figure 2A), which begins to expand and undergoes structural modification (Figure 2B). The cells of the parenchyma in contact with the epidermis are smaller than the cells most internal to the organ (Figure 2C,D). The region of the endosperm exposed to the scutellum consists of dead cells that are compact with variable thicknesses and constitute the fibrous layer (Figure 2B,D) and show that the edge of the domain of the starchy endosperm is in contact with the embryo (Figure 3A); these confirmed the previous findings [51]. At 24 h, the cells of the scutellum epidermis elongate asymmetrically and asynchronously, and the basal region of the scutellum near the radicle presents the largest number of elongated cells (Figure 2E–J). At this time, the smallest cells of the parenchyma were located under to epidermis, whereas the largest cells were located internally and close to the vascular bundles (Figure 2G,H).
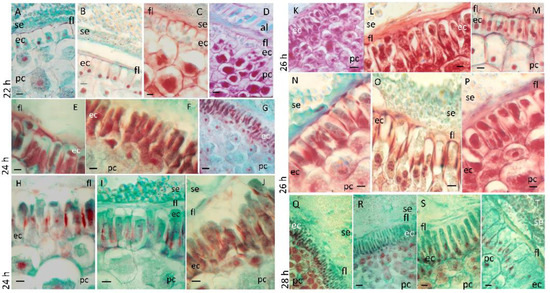
Figure 2.
Morphological changes in the scutellum at the end of germination and at the start of seedling growth. Scutella obtained from embryos that had germinated between 22 and 28 h were processed to obtain sections that were subjected to safranin–fast green regressive staining; the incubation time for each dye was standardized at 18 h of imbibition and maintained for the remainder of the germination times. Scutellum at different imbibition times: (A–D), 22 h; (E–J), 24 h; (K–P), 26 h; and ((Q–T), 28 h. Symbols: ec, epidermal cell; fl, fibrous layer; pc, parenchyma cell; se, starchy endosperm. Bar, 50 µm: (A–C,E,F,H–J,L–P,R–T); 125 µm: (D,G,K,Q).
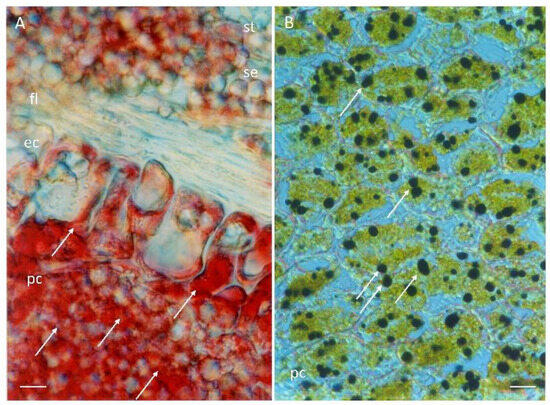
Figure 3.
Reserve substances in the scutellum. Histochemical tests of germinated embryo scutellum samples at 24 h of imbibition were stained for the following: (A), lipids were detected via Oil Red O reagent; (B), starch grains were detected using an iodine–potassium iodide solution. Symbols: ec, epidermal cell; fl, fibrous layer; pc, parenchyma cell; se, starchy endosperm; st, starch grain; →, reserve substance. Bar 50 µm: (A,B).
The cells of the epidermis and parenchyma contain a considerable amount of reserve substances consisting of lipids (Figure 3A) and starch, which was colocalized via histochemical techniques (Figure 3B). At 26 h after the start of imbibition, different degrees of expansion were observed in the cells of the epidermis (Figure 2K–P), with the cells widening at the base and moving between different planes as they increased in size (Figure 2O), with a greater affinity for safranin. The fibrous layer was evident in some domains (Figure 2K,L) with a decrease in thickness, presenting diffuse staining through it (Figure 2N–P). After 28 h of imbibition, the epidermis was composed of a monolayer of very elongated cells, which moved each other in the same plane and acquired finger-like or columnar shapes, characteristic of an epithelium (Figure 2Q–S), although the apical region of the scutellum still contained cells in the process of expansion (Figure 2T). The parenchyma at this time presented the same arrangement in terms of cell size. Depending on the position in the scutellum, the fibrous layer present was associated with the expanding cells (Figure 2S,T) or was partially present (Figure 2R) or absent (Figure 2Q).
3.3. Activity and Location of Antioxidant Enzymes in the Scutellum
3.3.1. Catalase Activity
At 0 h of imbibition, the maize scutellum presented low and basal catalase (CAT) activity, which increased 1.6-fold at 18 h and remained unchanged until 24 h. As the imbibition time increased, the activity increased 1.4-fold at 36 h, compared with that at 24 h (Figure 4A). Enzymatic activity in the extracts at 24 h was inhibited by 90.8% in the presence of KCN and by 73.13% in the presence of amino-triazole (AT); the decrease in activity in the presence of both inhibitors was significant with respect to the control, confirming that the activity from the scutellum was due to catalase (Figure 4B).
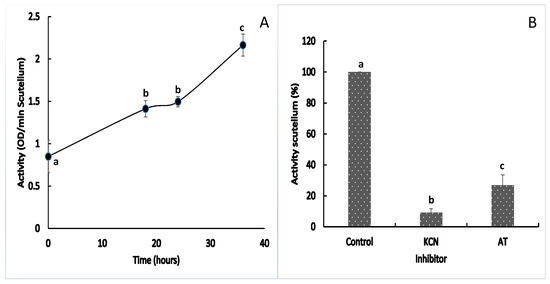
Figure 4.
Catalase activity in the maize scutellum. (A) Enzymatic activity in scutellum between 0 and 36 h after the start of imbibition; (B) Effects of the inhibition of KCN and 3-amino-1,2,4-triazole in extracts at 24 h. The values represent the mean ± SE of n = 8 independent assays, and in each test, four scutella were used. ANOVA and Tukey–Kramer multiple-comparison tests were performed, with p < 0.05. Data groups with different letters are significantly different.
3.3.2. Location of the Catalase
At 24 h of imbibition, catalase was differentially expressed in the scutellum. In elongated epidermal cells, the immunomarker for CAT was present at a lower intensity with respect to the internal parenchyma (Figure 5A,F). CAT in the epidermis was immunolocalized in the basal part of the scutellum that adjoins the aleurone layer (Figure 5D). In the middle region of the scutellum, CAT was situated in the epidermis but not in the starchy endosperm (Figure 5E). The strongest immunomarker of CAT was found in the larger cells of the internal parenchyma of the scutellum, whereas the weakest immunomarker was adjacent to the epidermis with the smallest cells of the parenchyma (Figure 5A,B). The localization of CAT is intracellular and was observed in domains associated with small vesicles (Figure 5C,G,H); the use of a specific antibody against peroxisomal CAT indicated that this enzyme was associated to the glyoxysomes, a peroxisome specialized in germination, and that its cellular location colocalizes with lipid deposits in the scutellum (Figure 3A).
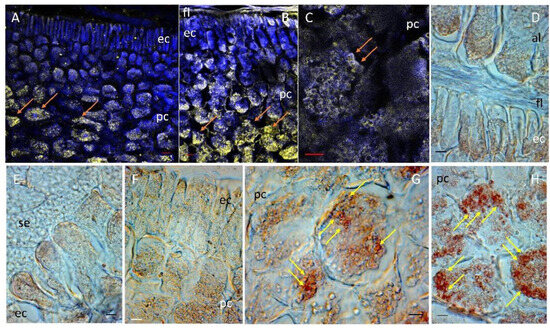
Figure 5.
Location of catalase (CAT) in maize scutellum. CAT was detected in the scutellum after 24 h of imbibition using a specific antibody. (A–C) Show the location of CAT by immunofluorescence in yellow. To provide anatomical context, tissue autofluorescence in blue was used. (D–H) Location of CAT by immunohistochemistry in brown. Symbols; al, aleurone layer; ec, epidermal cells; fl, fibrous layer; pc, parenchymal cells; se, starchy endosperm; →, specific location of CAT in yellow or brown depending on the technique used. Bar, (D,E,G). (H): 50 µm; (A,B): 100 µM; (F): 125 µm; (C): 200 µm.
3.3.3. Superoxide Dismutase Activity
The activity of superoxide dismutase (SOD) in the scutellum fluctuated at a baseline value from the beginning of imbibition, with an oscillation that varied between 0.8- and 1.3-fold during the study period (Figure 6A). The addition of 0.3 or 0.6 mM KCN to the enzymatic extract reaction mixtures at 24 h reduced the enzymatic activity to 84.7% or 78.6%, respectively, with respect to the control; this proportion of inhibition indicates the presence of a Cu/Zn-SOD in the extract (Figure 6B). Adding hydrogen peroxide to the reaction mixture did not cause an inhibitory effect on the original activity, confirming the presence of Cu/Zn-SOD in the enzyme extract. In addition, the proportion of the detected activity was attributed to the Mn-SOD fraction (Figure 6B), as confirmed by immunolocalization with a specific antibody (Figure 7). The possibility that another component of the enzyme extract affects superoxide production cannot be ruled out, but the concentrations of the components in the reaction mixture used to produce the substrate were high for its formation and not limiting for enzymatic reaction. On the other hand, the decrease in absorbance of the reaction time was due to enzymatic activity in the extract, because it was partially inhibited by KCN; however, the possible effect of a nonenzymatic component cannot be discarded.
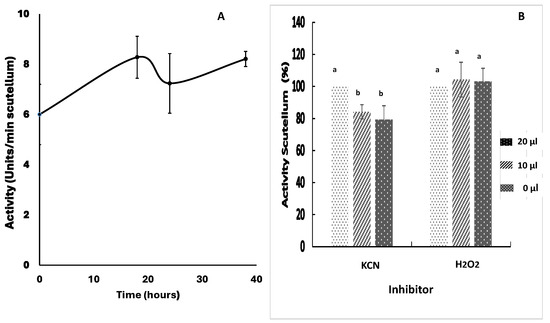
Figure 6.
Superoxide dismutase (SOD) activity in maize scutellum. SOD activity was determined in scutellum from embryos germinated from 0 to 36 h. (A) Enzymatic activity in the period of 0 to 36 h of imbibition. (B) Effect of the KCN and H2O2 inhibitors on the enzymatic extract reaction mixtures after 24 h; 10 or 20 µL of a concentrated KCN solution (indicators on the right of the graph) was added to yield a final concentration of 0.3 or 0.6 mM; 10 or 20 µL of H2O2 was added to achieve a final concentration of 0.26 or 0.52 mM. The values represent the mean ± SE of n = 6 independent assays, and in each test, four scutella were used. ANOVA and Tukey–Kramer multiple-comparison tests were performed, with p < 0.05. Data groups with different letters are significantly different.
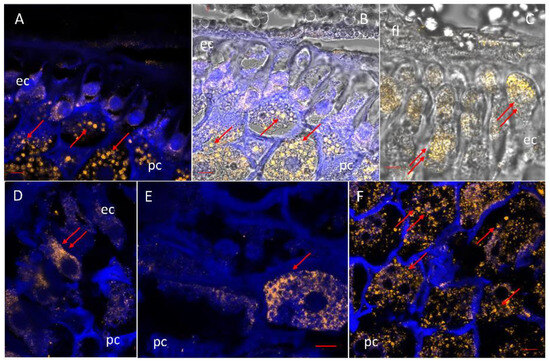
Figure 7.
Localization of Mn-superoxide dismutase in maize scutellum. SOD was detected by immunofluorescence in scutella at 24 h of imbibition using a specific antibody against mitochondrial SOD. (A,D–F) Immunofluorescence in yellow for SOD-specific antibody, merged on tissue autofluorescence in blue. (B,C) Immunofluorescence in yellow for SOD-specific antibody merged on tissue autofluorescence in blue and the phase contrast microscopy image. Symbols: fl, fibrous layer; ep, epidermal cell; fl, fibrous layer; pc, parenchyma cell; → critical points of the location of the SOD. Bar: (A–D), (F): 100 µm; (E): 200 µm.
3.3.4. Location of Superoxide Dismutase
SOD was located mainly in the parenchyma of the scutellum in embryos at 24 h of imbibition (Figure 7A,B). The level of the immunomarker for SOD is lower in the epidermis than in the parenchyma and is localized within the expanding cells that form the future epithelium (Figure 7C). In the parenchyma, the immunofluorescence revealed the existence of a gradient that increased towards the innermost part of the parenchyma, containing larger cells. The enzyme was detected in small agglomerates corresponding to organelles (Figure 7A,B,F). The commercial antibody used was specific to Mn-SOD and establishes the presence of this enzyme in the scutella in the parenchyma and epidermis and reveals the high density of mitochondria in this organ, which implies that a high metabolism is maintained. There was also a lower immunomarker intensity located diffusely around circular structures in the cytoplasm (Figure 7E).
3.3.5. Class III Peroxidase Activity
The enzymatic activity of class III peroxidase (POX) was detected between 0 and 36 h of imbibition using two substrate mixtures in the presence of H2O2, guaiacol and catechin. When hydrogen peroxide was eliminated from the reaction mixture containing either guaiacol or catechin, no activity was detected in the enzymatic extract in the scutellum of Chalqueño maize, indicating that during this period, only peroxidase activity occurred. The peroxidase activity results revealed that the use of guaiacol + H2O2 (Figure 8B) was superior to the use of catechin + H2O2 (Figure 8A). At 0 h of imbibition, basal activity was observed in the scutellum, which increased towards the end phase of germination (18 h), that is, a 12.4- or 3.8-fold increase with catechin + H2O2 or guaiacol + H2O2, respectively (boxes in Figure 8A,B). The activity at 24 h was similar to that found at 18 h for both reaction mixtures. After 36 h of imbibition, the activity increased, with a proportional 21-fold increase with catechin + H2O2 or 3.1-fold with guaiacol + H2O2 with respect to the value observed at 24 h (Figure 8A,B). The use of inhibitors in extracts at 24 h confirmed that the activity observed was that of class III peroxidase (Figure 8C). In the presence of KCN, the activity decreased by 96.6% with both substrate mixtures, whereas the use of SHAM inhibited 82.6% of the activity with catechin + H2O2 or 92.2% with guaiacol + H2O2.

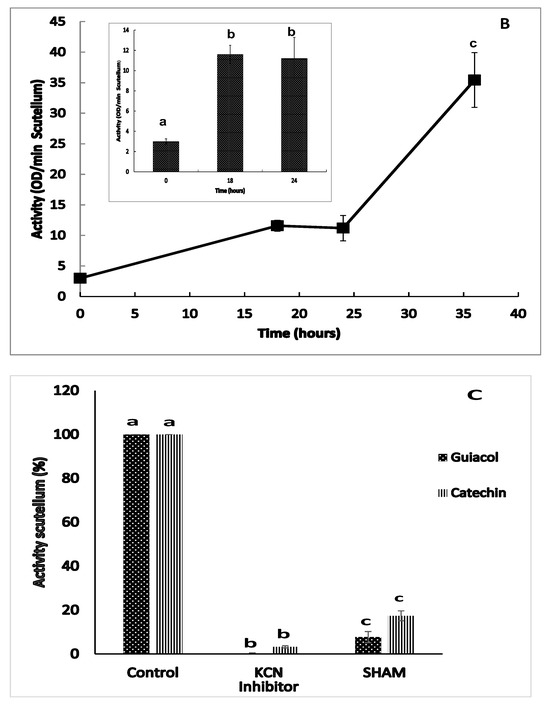
Figure 8.
Class III peroxidase activity (POX) in the scutellum during germination and early postgermination. The enzymatic activity from scutellum after 0 to 36 h of imbibition, in which the activity was quantified as follows: (A) catechin + H2O2 (▲); (B) guaiacol + H2O2 (■); and (C) effect of SHAM and KCN as inhibitors on enzyme activity in extracts at 24 h. The results are shown as the means ± SEs of n = 8–12 independent assays, and in each test four scutella were used. ANOVA and Tukey–Kramer multiple-comparison tests were performed, with p < 0.05. Data groups with different letters are significantly different.
3.3.6. Location of Class III Peroxidase
POX was highly enriched at 24 h in both the epidermis and the parenchyma of the scutellum. The expansion of epidermal cells after germination causes them to move towards each other in different planes, and in some of them POX is localized in the middle part and at the apex of those cells (Figure 9A). In the middle of the epidermal cells, fluorescence was detected in small invaginations of the cell surface (Figure 9B). The displacement between the cells caused an alternation in the immunolocalization of POX in the epidermal cells, which allowed us to locate the immunological markers on the cell surface corresponding to the plasma membrane (Figure 9C,D). In addition, POX was also located outside the plasma membrane (Figure 9E), confirming previous findings by our group [51]. The parenchyma adjacent to the epidermis showed an immunolocalization of POX near the periphery of the cells (Figure 9E,F).
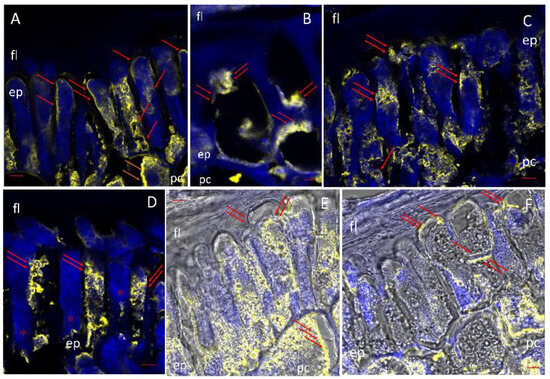
Figure 9.
Localization of class III peroxidase (POX) in the scutellum. After 24 h of imbibition, POX was detected by immunofluorescence with a primary antibody specific to POX and a secondary fluorescent antibody. (A–D): The POX immunolabel in yellow superimposed on the autofluorescence of the tissue in blue. (E,F) Immunolocalization of POX superimposed on the image in phase contrast. Symbols: ep, epidermal cell; fl, fibrous layer; pc, parenchyma cell; → POD location critical sites. Bar, (A,D), (F): 100 µm; (B,C,E): 200 µm.
3.4. Comparison of CAT and POX Activities
Among the three enzymes analyzed, POX and CAT showed changes during imbibition, whereas SOD activity oscillated at a baseline value during the period studied. By using the molar extinction coefficient reported in the literature for the enzymatic reaction product of guaiacol + H2O2 to determine the activity of POX, or using the extinction coefficient of H2O2 in the CAT assay, the activity could be expressed relative to the amount of product produced or substrate consumed by each of the enzymes in the scutellum (µmol/min scutellum), thus permitting a comparative analysis between the two enzymes, in which the activity of guaiacol POX (guPOX) was significantly greater than that of CAT (Figure 10). At 0 h of imbibition, the proportion was 5.1-fold greater for guPOX, and this difference increased to 11- or 12.1-fold at 18 and 24 h, respectively, and 24.2-fold at 36 h. Both enzymes were differentially inhibited with KCN, with a greater effect on POX; however, each of them was specifically inhibited, by SHAM for class III peroxidase or 3-amino-1,2,4-triazole for catalase (Figure 8C vs. Figure 4B). These results suggest that guPOX is a key enzyme in the development and antioxidant mechanism of the scutellum.
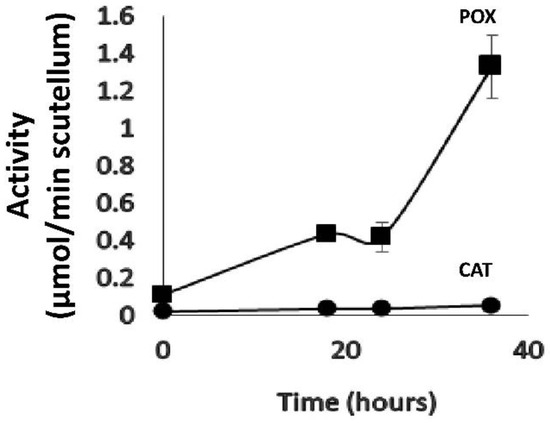
Figure 10.
Comparison of class III peroxidase and catalase activities in the scutellum. The enzymatic activity is expressed in mmol/min scutellum using the molar extinction coefficient for guaiacol of ε = 26.6 mM−1 cm−1 for guPOX activity and of ε = 39.4 mM−1 cm−1 for H2O2 consumption in the CAT activity. The values represent the mean ± SE of n = 8 independent assays, and in each test 4 scutella were used. ANOVA and Tukey–Kramer multiple-comparison tests were performed, with p < 0.05. The results indicate significant differences for each time point between the two enzymes (not shown in the figure).
4. Discussion
4.1. Functional Relevance of the Scutellum
Due to the changes that occur during and after germination and that depend on signals that are induced at the time of imbibition, it was decided to use isolated embryos in which the initial hydration time is controlled. The degree of hydration depends on the embryo itself and the competition of the other structures for water. The thickness and composition of the impermeable structures that cover the embryo play a fundamental role in determining the time at which germination begins. Therefore, by using isolated embryos and placing them directly in contact with a moist support in the germinator, the differences in hydration time between embryos from different grains are reduced, in such a way that hydration is given to the embryo and with it the beginning and induction of germination until the protrusion or expansion of the radicle, which is associated with the structural and metabolic transformations that occur in the scutellum in a shorth period, so it was decided to use the period from 0 a 30 h, in which previous studies have shown the germination to postgermination or seedling growth occur [27,51].
The size of the scutellum varies across cereals of economic importance, in species that have large scutella, which represent a large proportion relative to the complete embryo, such as those of the genera Sorghum and Zea, which implies a large volume of accumulation of reserves and interaction with the endosperm. In contrast, in embryos in which the scutellum is small, such as those of the genera Hordeum, Oryza, Secale, and Triticum, it only occupies a small basal portion of the caryopsis, for more details, see: [52]. The scutellum of grass species is an ephemeral structure but is highly important for the establishment of seedlings [53].
Germination involves a series of events that begin with the intake of water and oxygen and end with the emergence of the radicle. During radicle elongation, there is high metabolic activity, including the synthesis of macromolecules, and cellular and subcellular changes during which ROS are produced [54,55]. During this growth phase, the reserves stored in the radicle are used first, and later, the products of the catabolism of reserve substances in the scutellum are transferred to the embryonic axis, where the initial growth of the seedling is promoted until it is establishment. The reserves in the scutellum are mainly composed of phytate [86,87,88], various macro- and micronutrients, and lipids [27,89,90], among others. The reserve lipids are metabolized and transformed at least partially into starch stored temporarily in the scutellum, as demonstrated by the evidence of coexistence of both compounds in the parenchyma of the maize scutellum at 24 h in this study.
The results of this study during the study period of 0 to 36 h show that there is a decrease in the fresh weight of the scutellum and in the amount of total soluble protein, with a marked decrease in both parameters from 18 to 24 h after the start of the imbibition. This decrease is correlated with the transformation of the reserve substances and their translocation to the rest of the embryo and subsequent consumption that initially supports radicle elongation, corroborating what has been reported in the literature [27,91,92]. As the postgermination phase progresses, there is a greater demand for nutrients due to the increase in the growth rate of the radicle, and subsequently of the coleoptile; the growth is initially supported by the mobilization and transfer of the hydrolyzed reserves of the scutellum, and later those of the endosperm. The scutellum in the early postgermination stages exports phytohormones [93,94,95] and hydrolytic enzymes to the adjacent endosperm, which causes hydrolysis of the reserves in situ. In a later stage, the aleurone layer, which responds to the exported phytohormones, is responsible for the secretion of new degradative enzymes, and the products of hydrolysis in the starchy endosperm are incorporated into the scutellum and transferred to the growing seedling [56,57,66,96,97,98,99,100,101,102,103,104,105,106]. Among the reserves mobilized to the scutellum from the endosperm are catabolic starch products [54,107,108], reserve proteins [58,59,60,61,65,109], and macro- and micronutrients [110]. In this manner, the scutellum is important organ for seedling growth and establishment.
To fulfil the functions of the secretion and absorption of nutrients from the endosperm, the epidermis of the Chalqueño maize scutellum is transformed after germination. In mature kernels, the epidermis is made up of a monostratified layer of cells that are compact with few spaces between the cells [52]. In the case of Chalqueño maize, in the early stages of imbibition, the cells are isodiametric and are transformed and acquire nutrient secretion and absorption functions. During this period, they pass through a critical point after approximately the 24 h of imbibition that coincides with the protrusion of the radicle; after that, the cells begin elongation to become columnella cells or fingerlike cells, with the functions of papillary or epithelial cells, confirming our previous results [51]. An important difference in this study with isolated embryos is that most studies examine the function of the scutellum during the late postgermination phase in full grain, i.e., three/five to ten days after the start of imbibition.
The transformation process of the epidermis of the scutellum is asynchronous in small domains on the face adjacent to the endosperm (abaxial face of the scutellum); initially, the greatest changes occur in the basal region or portion of the scutellum near the apical region of the radicle and decrease towards the end of the scutellum close to the coleoptile, as confirmed in this study. In addition, the changes in the epidermis of the scutellum are related to the permeability of the fibrous layer (the border of the starchy endosperm in contact with the scutellum), in which the compartmentalization of the embryo is broken to allow for direct communication with the endosperm [51] with the free diffusion of substances between the two organs. This epithelium remains functional approximately 9 to 10 days after imbibition [63,111].
4.2. Relationships Between Metabolism with the Activity and Location of Antioxidant Enzymes
The elongation of the radicle and its metabolic and structural changes represent the end of germination [54,55] and are correlated with the synchronization of metabolism in the scutellum to contribute and transfer nutrients, which is an important source of ROS. The presence of H2O2 has been reported in the early phase of imbibition in different species [112,113,114] and is accompanied by the accumulation of other ROS, such as O2·− and °OH [113,115,116], as well as reactive nitrogen species [114,117,118]. Temporary patterns of the presence of H2O2 have been demonstrated in maize embryos, whose levels are high between 24 and 48 h after imbibition [119]. If the amount of ROS produced during seed germination exceeds a threshold, it is considered to have a negative effect and cause damage. For this reason, antioxidant mechanisms are considered vital for maintaining ROS concentrations within a certain range during germination; this is called the “oxidative window of germination” [112,120,121]. In this manner, when the water content in the embryo exceeds 50%, the mechanism of ROS generation changes from nonenzymatic to enzymatic, but before that occurs, the mitochondria is the main source of ROS production in the seed through the reduction of O2 to O2·− during electron transport in the respiratory chain [40,112,122,123,124].
In maize embryos, temporary accumulation of H2O2 followed by a subsequent decrease has been reported at the beginning of imbibition, which correlates with low catalase activity at the beginning of germination and a progressive increase as the imbibition time passes [119]. In this study, the presence of SOD and the increased activity of CAT and POX in the maize scutella between 0 and 18 h are consistent with findings reported in the germination of sunflower seeds, in that prior to the protrusion of the radicle, high concentrations of H2O2 are restricted to eliminate possible lipid peroxidation damage [112]. Lipid mobilization is essential for providing energy during the end of germination and seedling growth. The degradation of lipids stored in oil bodies occurs in glyoxysomes through β-oxidation and the glyoxylate cycle [125,126,127]. These pathways are of particular interest in germination because lipid catabolism results in the accumulation of H2O2 and, if not controlled, generates oxidative stress [128]. Correlating our results with those previously reported by Sánchez-Linares et al. [27], during the imbibition of isolated maize embryos, lipid degradation occurs slowly initially, which is related to a 1.6-fold increase in CAT activity in the scutellum between 0 and 18 h; when lipid catabolism accelerates in the period between 24 and 36 h, CAT activity increases 1.4-fold over the maximum reached at 18 h, and there is a correlation between lipid catabolism and the production of H2O2 and the control of ROS through CAT activity. On the other hand, CAT is intracellularly located in small structures that correspond to the glyoxysomes, which are specialized peroxisomes, according to experiments using a commercial peroxisome anti-catalase antibody, confirming previously published findings [129] and supporting results published on complete maize embryos [113,130] and those published in other species that metabolize lipids such as Arabidopsis [131] or soybean [132]. It is interesting to further investigate in the future the correlation between the production of H2O2 in lipid degradation, the key enzymes of the glyoxylate cycle, and the levels of triose phosphates formed, and relate this to the levels of sucrose and starch accumulated in the scutellum. The results obtained in this study imply that glyoxysome-associated catalase regulates stress and thus preserves the viability of the embryo during the transformation of reserve lipids, allowing for the reconversion and temporary accumulation of starch and the translocation of products derived from its metabolism to the embryo. The H+ ATPase [68] associated with the transport of sucrose [27] is involved in such translocation, thus allowing for the growth and later establishment of the seedling, as has been shown in different species [133].
At the end of the last century and at the beginning of this century, other antioxidant enzymes, such as SOD or class III peroxidase, were studied to a lesser extent during the imbibition and germination phases [113,121,134]. SOD (EC 1.15.1.1) is a metalloenzyme that catalyzes the dismutation of the O2·− to H2O2 and molecular oxygen [135] and can be classified into three groups, Mn-SOD, Fe-SOD, and Cu/Zn-SOD, depending on the metal in the active site. These enzymes are located in the cytosol, plastids, mitochondria, peroxisome, and the apoplast. In maize, there are nine isoenzymes: four Cu/Zn-SODs, four Mn-SODs associated with the mitochondria, and one chloroplastic Cu/Zn-SOD [136,137]. In the scutellum of Chalqueño maize imbibed from 0 to 36 h, SOD activity fluctuated around the baseline value. The use of inhibitors allowed us to confirm that the highest activity observed is due to Mn-SOD. The use of KCN as an inhibitor inactivates a small enzymatic fraction, but Mn-SOD and Fe-SOD are not affected; thus, there is a fraction of Cu/Zn-SOD in the enzyme extract [138,139,140]. On the other hand, the use of H2O2 did not affect the observed activity, confirming that most of the enzymatic fraction is due to the presence of Mn-SOD in the extract, since H2O2 affects only Cu/Zn-SOD and Fe-SOD [138,141,142]. The intracellular presence of Mn-SOD in maize scutellum is confirmed by immunolocalization using a specific antibody for Mn-SOD. The antibody used recognizes the mitochondrial Mn-SOD and relies on initial reports that identified four Mn-SODs in the mitochondria [143], although their presence in the peroxisomes, as has been demonstrated in other species [144], cannot be ruled out. SOD is localized intracellularly and is differentially distributed in the scutellum, with the greatest intensity in the parenchyma close to the vascular tissue and decreasing intensity nearer to the surface. In the epidermis, the immunolocalization results indicate punctate ubication in expanding fingerlike cells. The activity, inhibition, and immunolocalization results confirm the results described in the literature during germination and postgermination in the scutellum [141,143]. The increase in the energy demand required from the scutellum, associated with the protrusion of the radicle, is correlated with a high mitochondrial metabolism and increased oxygen consumption. An increase in mitochondrial metabolism is associated with an increase in the production of O2·− and its regulation through Mn-SOD [27,141,143]. The high intensity of the immunomarker is associated with cells of the parenchyma, mainly located near the vascular tissue, which has important functions in the mobilization of the scutellum reserves and involves high levels of metabolic activity. These results offer a new perspective in deepening the understanding of the correlation between the activity of Mn SOD and the change in mitochondrial activity in the scutellum, as part of the control of oxidative stress in the organ, and in turn correlate it to the growth of the mesocotylus and the radicle. Part of the metabolic activity and the energy supply in the epidermis is used for its transformation from isodiametric cells to fingerlike cells and the consequent acquisition of epithelium functions. With the establishment of this type of cell, the fundamental capacity to transfer nutrients from the endosperm through the scutellum to the growing seedling increases. In the present study, we quantified only the intracellular SOD in the scutellum, but in previous work, we reported the location of apoplastic O2·− during the transformation of the fibrous layer [51]. Therefore, it is important to establish whether the small fraction inhibited with KCN corresponds to a fraction of SOD that is subsequently secreted to the apoplast and that plays a role in germination and/or early postgermination via signaling and modification in the cell walls [115,116,145].
Class III peroxidases (EC 1.11.1.7; donor: H2O2 oxidoreductases) are heme-containing enzymes that belong to the peroxidase–catalase superfamily [146,147]; there are at least 158 genes in the maize genome [148]. The large number of peroxidases in plant cells has allowed for the acquisition of a wide variety of functions during seedling/plant development and growth, such as the lignification, suberization, and crosslinking of cell wall components [149]. These enzymes function in both abiotic and biotic stress-related processes and in the oxidation of toxic substances [150,151,152,153,154,155], and thus play a relevant role during seed development and in the embryo during germination [155].
Fifty-three percent of class III peroxidases in maize are soluble enzymes, whereas forty-three percent are membrane-bound isoenzymes [156,157,158]. This implies that the enzymatic activity observed in the extracts used in the present study comes from free or soluble POX, along with a fraction contained in small membrane particles derived from tissue disruption or microsomes, and a part of the latter fraction corresponds to the enzyme located by immunolocalization. Peroxidase has been shown to exhibit a greater affinity for H2O2, approximately 1000 times greater than catalase, and plays a relevant role in ROS detoxification [159].
In the present study, guaiacol and catechin were used as substrates to determine the enzymatic activity in the maize scutellum because peroxidase could catalyze a wide variety of phenolic-type compounds [160,161]. Previous studies have characterized the peroxidation products of guaiacol [84,162,163,164]. In addition, López-Serrano and Barceló [165,166] characterized the products of the peroxidation vs. oxidation of catechin using various products with different degrees of polymerization. In the Chalqueño maize scutellum, during the study period, activity was only observed thought the use of guaiacol or catechin in the presence of H2O indicating that a peroxidase enzyme.
In this manner, between 0 h and 18 h, guPOX activity increases 3.8-fold, with the same level of activity at 24 h and further increased activity to 3.1-fold greater at 36 h than at 24 h. On the other hand, the activity of caPOX at the same pH is lower but presents the same pattern of increase, varying between 12.4-fold and 21.1-fold from 0 to 18 h and from 24 to 36 h, respectively, with similar activity at 18 and 24 h. Greater guPOX activity was observed in the scutellum under the same conditions as the test for the two substrate mixtures and detection of the products. The results show that the quantified activity is due to a class III peroxidase, since it is inhibited by 90% by SHAM, a specific POX inhibitor, with a greater effect on guPOX. Furthermore, KCN was confirmed to inhibit the enzymatic activity of POX by more than 95%, regardless of the substrate used.
POX activity has been reported in grains of various species, including rice, in which no activity has been detected on the first day of imbibition [167] and significantly increasing activity has been reported from the third to the fourth day of imbibition [168,169]. In the maize scutellum, low basal activity is detected at 0 h of imbibition, indicating that POX is a constitutive enzyme present since the final stage of embryo maturation. This phenomenon has been proposed for POX in the cotyledons of Jatropha curcas seeds, in which there is a gradual increase in POX activity, confirming the presence of a constitutive enzyme at the beginning of germination [170]. On the other hand, other seeds at the beginning of imbibition do not present POX activity, such as tomato [171], Chenopodium rubrum [46,172], and Picea omorika [133]; rather, the increase in activity occurs later during imbibition. Therefore, in these latter species, the main activity of POX can play an important role in the late stage of germination and during seedling growth.
Immunolocalization experiments indicated that POX is present in most of the cells of the parenchyma and in the epidermis. In epidermal cells, the immunolocalization marker is observed on the cell border corresponding to the plasma membrane, in regions where the membrane is invaginated or in the form of bands on the cell surface, and in the apoplast correlating with changes in the cell wall structure and the elongation of the scutellum epidermal cells during and after germination [51]. The presence of POX in epidermal cells can be associated with pro-oxidant processes [115] related to the relaxation of the wall, allowing for cell modification and expansion, which would support the mechanism for the acquisition of fingerlike cells reported in the literature [63,173].
Owing to the changes observed in this study, the increase in POX activity in the scutellum can be considered a marker of the germination stage itself (0 to 18 h), ending with stable POX activity (between 18 and 24 h), during which the intake and stabilization of oxygen consumption occurs (phases I and II), and the final portion of phase II (18 to 24 h) is critical in the beginning of the asynchronous transformation of the epidermis of the scutellum as described above, coinciding with radicle protrusion and maximum growth speed [27]. Therefore, POX in the scutellum can serve as an indicator of the transition of phases from germination to postgermination or the embryo–seedling transition (18 to 24 h). A later phase associated with a second increase in oxygen consumption, or phase III, occurs 24 h after the beginning of imbibition, which coincides with the growth of the mesocotyl and the continuation of radicle growth; in this phase, the highest rate degradation of the triacylglycerides stored in the scutellum occurs [27], along with the transformation of the epidermis and the permeability of the fibrous layer [51], which allows for free exchange between the endosperm and the scutellum, which supports seedling growth and establishment.
Among the three enzymes studied, only CAT and POX activity changed with increasing imbibition time. The peroxidase using guaiacol + H2O2 has the highest activity; thus, when guPOX is compared with CAT, with activity expressed as units of activity in µmol/min, the preponderance of POX activity during germination and early postgermination is clear. Therefore, we have highlighted the importance of class III peroxidases that constitute a group of enzymes that participate in a multitude of processes, including the regulation of ROS and pro-oxidant processes that may be associated with morphological changes in the epidermis, the permeabilization of the fibrous layer [51], or the establishment of functional vascular tissue, among other processes (for more information, consult the following reviews: [150,174,175]).
5. Conclusions
The scutellum is a fundamental organ in the germination and seedling growth and establishment of the plant. The function of the scutellum is related to two phases of development, the first during germination and the embryo–seedling transition, and the second during the growth of the seedling, in which it participates by providing nutrients initially by mobilizing reserves accumulated in the organ and subsequently transferring the hydrolysis products of the endosperm reserves to the seedling. At this stage, the metabolism used generates ROS that must be kept at a controlled level, so three enzymes can be fundamental: SOD, CAT, and POX. The first one maintains a stable activity, being mainly related. The SOD activity in the scutellum fluctuated at a baseline value, and the highest activity observed is due to Mn-SOD. POX and CAT showed changes during imbibition, in which the activity of guaiacol POX was significantly greater than that of CAT. Therefore, class III peroxidase activity can be considered a marker of the different phases of germination until the postgermination phase, in which activity stabilizes between 18 h and 24 h, indicating the final part of germination and the transition to embryo–seedling growth, with the sustained root growth and later growth of the mesocotyl/coleoptile. For this reason, this enzyme must be considered for the conservation and preservation strategies of genetic material.
Author Contributions
Conceptualization and Writing—Original Draft Preparation, Project Administration, D.D.-P.; Formal Analysis and Validation, D.D.-P. and J.I.C.-C.; Software, Supervision and Writing—Review and Editing, J.I.C.-C., Investigation and Methodology, J.I.C.-C., S.G. and G.C.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article, and further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Colin, W. Chapter 2—The Cereal Grains: Providing Our Food, Feed and Fuel Needs. In Cereal Grains, 2nd ed.; Wrigley, C., Batey, I., Miskelly, D., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2017; pp. 27–40. ISBN 9780081007198. Available online: https://www.sciencedirect.com/science/article/pii/B9780081007198000024 (accessed on 25 October 2024). [CrossRef]
- Ranum, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N. Global Maize Production, Utilization, and Consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef]
- Anderson, B.; Almeida, H. Corn Dry Milling: Processes, Products, and Applications. In Corn; Serna-Saldivar, S.O., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 405–433. [Google Scholar] [CrossRef]
- BeMiller, J.N. Corn Starch Modification. In Corn; Serna-Saldivar, S.O., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 537–549. [Google Scholar] [CrossRef]
- Helstad, S. Corn Sweeteners. In Corn; Serna-Saldivar, S.O., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 551–591. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, V. Bioethanol Production from Corn. In Corn; Serna-Saldivar, S.O., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 615–631. [Google Scholar] [CrossRef]
- Loy, D.D.; Lundy, E.L. Nutritional Properties and Feeding Value of Corn and Its Coproducts. In Corn; Serna-Saldivar, S.O., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 633–659. [Google Scholar] [CrossRef]
- Palacios-Rojas, N.; McCulley, L.; Kaeppler, M.; Titcomb, T.J.; Gunaratna, N.S.; Lopez-Ridaura, S.; Tanumihardjo, S.A. Mining Maize Diversity and Improving Its Nutritional Aspects within Agro-food Systems. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1809–1834. [Google Scholar] [CrossRef]
- Serna-Saldivar, S.O.; Chuck-Hernandez, C. Food Uses of Lime-Cooked Corn with Emphasis in Tortillas and Snacks. In Corn; Serna-Saldivar, S.O., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 469–500. [Google Scholar] [CrossRef]
- FAO. FAOSTAT. 2024. Available online: https://www.fao.org/faostat/en/#home (accessed on 5 October 2024).
- CONABIO. CONABIO, 25 Años de Evolución. 2017. Available online: https://www.gob.mx/cms/uploads/attachment/file/262393/25_an_os_Conabio_web.pdf (accessed on 11 October 2024).
- Arteaga, M.C.; Moreno-Letelier, A.; Mastretta-Yanes, A.; Vázquez-Lobo, A.; Breña-Ochoa, A.; Moreno-Estrada, A.; Eguiarte, L.E.; Piñero, D. Genomic Variation in Recently Collected Maize Landraces from Mexico. Genom. Data 2016, 7, 38–45. [Google Scholar] [CrossRef]
- Herrera-Cabrera, B.E.; Castillo-González, F.; Sánchez-González, J.J.; Hernández-Casillas, J.M.; Ortega-Pazkca, R.A.; Major-Goodman, M. Diversidad Del Maíz Chalqueño. Agrociencia 2004, 38, 191–206. [Google Scholar]
- Goodman, M.M.; Sanchez, G.J.J.; Stuber, C.W. Isozymatic and Morphological Diversity in the Races of Maize of Mexico. Econ. Bot. 2000, 54, 43–59. [Google Scholar] [CrossRef]
- Sánchez, G.J.J.; Stuber, C.W.; Goodman, M.M. Isozymatic Diversity of the Races of Maize of the Americas. Maydica 2000, 45, 185–203. [Google Scholar]
- Kuleshov, N.N. Maíces de México, Guatemala, Cuba, Panamá, y Colombia. In Las Plantas Cultivadas de México, Guatemala y Colombia; Bukasov, S.M., Ed.; CATIE: Turrialba, Costa Rica, 1981; pp. 40–53. [Google Scholar]
- CONABIO. Proyecto Global de maíces Nativos: Recopilación, Generación, Actualización y análisis de Información Acerca de la Diversidad Genética de Maíces y sus Parientes Silvestres en México. 2011. Available online: https://www.biodiversidad.gob.mx/media/1/genes/files/InformedeGestion_V1.pdf (accessed on 11 October 2024).
- Kato Yamakake, T.A.; Mapes Sánchez, C.; Mera Ovando, L.M.; Serratos Hernández, J.A.; Bye Boettler, R.A. Origen y Diversificaión del Maíz: Una Revisión Analìtica; Universidad Nacional Autónoma de México: México, Mexico, 2009. [Google Scholar]
- Wellhausen, E.J.; Roberts, L.M. Razas de Maíz En México: Su Origen, Características y Distribución; Oficina de Estudios Especiales, Secretaría de Agricultura y Ganadería de México: México, Mexico, 1951. [Google Scholar]
- Biodiversidad Mexicana. Visualización Interactiva: Diversidad y Distribución de Maíces. Proyecto Global de Maíces. 2023. Available online: https://conabio.shinyapps.io/conabio-pgmaices1/ (accessed on 11 October 2024).
- CONABIO. Proyecto Global de Maíces Nativos. Tabla Descriptiva de Razas de Maíz en México. 2010. Available online: https://www.biodiversidad.gob.mx/media/1/genes/files/Tabla_razas_marzo_2010.pdf (accessed on 10 October 2024).
- Antonio-Miguel, M.; Arellano-Vázquez, J.L.; García-de Los Santos, G.; Miranda-Colín, S.; Mejía-Contreras, J.A.; González-Cossío, F.V. Variedades Criollas De Maíz Azul Raza Chalqueño. Características Agronómicas y Calidad De Semilla. Rev. Fitotec. Mex. 2022, 27, 9–15. [Google Scholar] [CrossRef]
- Hernández, C.J.M. Proyecto FZ016 “Conocimiento de la Diversidad y Distribución Actual del Maíz Nativo y sus Parientes Silvestres en México. Segunda Etapa 2008–2009”; Informe Final del Estado de México y D. F. INIFAP, Campo Experimental Valle de México; INIFAP: Hidalgo, México, 2010; 17p. [Google Scholar]
- Hernández, C.J.M.; Díaz de la C, J.B.; Base de Datos de Colecciones de Maíces Nativos, Teocintle y Tripsacum de México. Informe Final de Actividades 2007–2010. Convenio Núm.FB1261/FY001/07 CONABIO/INIFAP. México, D.F. Informe final del Proyecto FY001 Base de Datos de Colecciones de Maíces Nativos, Teocintles y Tripsacum de México. Available online: http://www.conabio.gob.mx/institucion/proyectos/resultados/InfFY001.pdf (accessed on 14 October 2024).
- Rocandio-Rodríguez, M.; Santacruz-Varela, A.; Córdova-Téllez, L.; López-Sánchez, H.; Castillo-González, F.; Lobato-Ortiz, R.; García-Zavala, J.J.; Ortega-Paczka, R. Caracterización morfológica y agronómica de siete razas de maíz de los Valles Altos de México. Rev. Fitotec. Mex. 2014, 37, 351–361. [Google Scholar] [CrossRef]
- Dourmap, C.; Roque, S.; Morin, A.; Caubrière, D.; Kerdiles, M.; Béguin, K.; Perdoux, R.; Reynoud, N.; Bourdet, L.; Audebert, P.-A.; et al. Stress Signalling Dynamics of the Mitochondrial Electron Transport Chain and Oxidative Phosphorylation System in Higher Plants. Ann. Bot. 2020, 125, 721–736. [Google Scholar] [CrossRef]
- Sánchez-Linares, L.; Gavilanes-Ruíz, M.; Díaz-Pontones, D.; Guzmán-Chávez, F.; Calzada-Alejo, V.; Zurita-Villegas, V.; Luna-Loaiza, V.; Moreno-Sánchez, R.; Bernal-Lugo, I.; Sánchez-Nieto, S. Early Carbon Mobilization and Radicle Protrusion in Maize Germination. J. Exp. Bot. 2012, 63, 4513–4526. [Google Scholar] [CrossRef]
- Ali, A.S.; Elozeiri, A.A. Metabolic Processes During Seed Germination. In Advances in Seed Biology; Jimenez-Lopez, J.C., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Andrade, G.C.D.; Coelho, C.M.M.; Padilha, M.S. Seed Reserves Reduction Rate and Reserves Mobilization to the Seedling Explain the Vigour of Maize Seeds. J. Seed Sci. 2019, 41, 488–497. [Google Scholar] [CrossRef]
- Cao, H.; Duncan, O.; Millar, A.H. The Molecular Basis of Cereal Grain Proteostasis. Essays Biochem. 2022, 66, 243–253. [Google Scholar] [CrossRef]
- Makhaye, G.; Mofokeng, M.M.; Tesfay, S.; Aremu, A.O.; Van Staden, J.; Amoo, S.O. Influence of Plant Biostimulant Application on Seed Germination. In Biostimulants for Crops from Seed Germination to Plant Development; Gupta, S., Van Staden, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 109–135. [Google Scholar] [CrossRef]
- Tnani, H. The Structure and Function of Maize Scutellum during Early Stages of Germination. Doctoral Thesis, Universitat de Barcelona, Barcelona, Spain, 2012. Available online: https://diposit.ub.edu/dspace/handle/2445/36321 (accessed on 17 July 2024).
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Farooq, M.A.; Zhang, X.; Zafar, M.M.; Ma, W.; Zhao, J. Roles of Reactive Oxygen Species and Mitochondria in Seed Germination. Front. Plant Sci. 2021, 12, 781734. [Google Scholar] [CrossRef]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative Modifications to Cellular Components in Plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef]
- Akter, S.; Khan, M.S.; Smith, E.N.; Flashman, E. Measuring ROS and Redox Markers in Plant Cells. RSC Chem. Biol. 2021, 2, 1384–1401. [Google Scholar] [CrossRef]
- Mhamdi, A.; Van Breusegem, F. Reactive Oxygen Species in Plant Development. Development 2018, 145, dev164376. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From Intracellular Signaling Networks to Cell Death: The Dual Role of Reactive Oxygen Species in Seed Physiology. Comptes Rendus. Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef]
- Kurek, K.; Plitta-Michalak, B.; Ratajczak, E. Reactive Oxygen Species as Potential Drivers of the Seed Aging Process. Plants 2019, 8, 174. [Google Scholar] [CrossRef]
- Li, W.; Niu, Y.; Zheng, Y.; Wang, Z. Advances in the Understanding of Reactive Oxygen Species-Dependent Regulation on Seed Dormancy, Germination, and Deterioration in Crops. Front. Plant Sci. 2022, 13, 826809. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Priestley, D.A. Seed Aging: Implications for Seed Storage and Persistence in the Soil; Cornell University Press: Ithaca, NY, USA, 1986. [Google Scholar]
- Bailly, C.; Benamar, A.; Corbineau, F.; Côme, D. Changes in Superoxide Dismutase, Catalase and Glutathione Reductase Activities in Sunflower Seeds during Accelerated Ageing and Subsequent Priming. In Basic and Applied Aspects of Seed Biology; Ellis, R.H., Black, M., Murdoch, A.J., Hong, T.D., Eds.; Springer: Dordrecht, The Netherlands, 1997; Volume 30, pp. 665–672. [Google Scholar] [CrossRef]
- Bailly, C.; Audigier, C.; Ladonne, F.; Wagner, M.H.; Coste, F.; Corbineau, F.; Côme, D. Changes in Oligosaccharide Content and Antioxidant Enzyme Activities in Developing Bean Seeds as Related to Acquisition of Drying Tolerance and Seed Quality. J. Exp. Bot. 2001, 52, 701–708. [Google Scholar] [CrossRef]
- Dučić, T.; Lirić-Rajlić, I.; Mitrović, A.; Radotić, K. Activities of Antioxidant Systems During Germination of Chenopodium Rubrum Seeds. Biol. Plant 2003, 46, 527–533. [Google Scholar] [CrossRef]
- Blokhina, O. Antioxidants, Oxidative Damage and Oxygen Deprivation Stress: A Review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef]
- Devi, S.R.; Prasad, M.N.V. Antioxidant Capacity of Brassica Juncea Plants Exposed to Elevated Levels of Copper. Russ. J. Plant Physiol. 2005, 52, 205–208. [Google Scholar] [CrossRef]
- Scandalios, J.G. Oxygen Stress and Superoxide Dismutases. Plant Physiol. 1993, 101, 7–12. [Google Scholar] [CrossRef]
- Liszkay, A.; Kenk, B.; Schopfer, P. Evidence for the Involvement of Cell Wall Peroxidase in the Generation of Hydroxyl Radicals Mediating Extension Growth. Planta 2003, 217, 658–667. [Google Scholar] [CrossRef]
- Corona-Carrillo, J.I.; Flores-Ponce, M.; Chávez-Nájera, G.; Díaz-Pontones, D.M. Peroxidase Activity in Scutella of Maize in Association with Anatomical Changes during Germination and Grain Storage. SpringerPlus 2014, 3, 399. [Google Scholar] [CrossRef][Green Version]
- Negbi, M. The Structure and Function of the Scutellum of the Gramineae. Bot. J. Linn. Soc. 1984, 88, 205–222. [Google Scholar] [CrossRef]
- Domínguez, F.; Moreno, J.; Cejudo, F.J. The Scutellum of Germinated Wheat Grains Undergoes Programmed Cell Death: Identification of an Acidic Nuclease Involved in Nucleus Dismantling. J. Exp. Bot. 2012, 63, 5475–5485. [Google Scholar] [CrossRef][Green Version]
- Bewley, J.D. Seed Germination and Reserve Mobilization. In Encyclopedia of Life Sciences; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Miyata, S.; Okamoto, K.; Watanabe, A.; Akazawa, T. Enzymic Mechanism of Starch Breakdown in Germinating Rice Seeds: 10. In Vivo And In Vitro Synthesis Of A-Amylase in Rice Seed Scutellum. Plant Physiol. 1981, 68, 1314–1318. [Google Scholar] [CrossRef]
- Okamoto, K.; Kitano, H.; Akazawa, T. Biosynthesis and Excretion of Hydrolases in Germinating Cereal Seeds. Plant Cell Physiol. 1980, 21, 201–204. [Google Scholar] [CrossRef]
- Sopanen, T.; Burston, D.; Matthews, D.M. Uptake of Small Peptides by the Scutellum of Germinating Barley. FEBS Lett. 1977, 79, 4–7. [Google Scholar] [CrossRef][Green Version]
- Sopanen, T.; Burston, D.; Taylor, E.; Matthews, D.M. Uptake of Glycylglycine by the Scutellum of Germinating Barley Grain. Plant Physiol. 1978, 61, 630–633. [Google Scholar] [CrossRef]
- Salmenkallio, M.; Sopanen, T. Amino Acid and Peptide Uptake in the Scutella of Germinating Grains of Barley, Wheat, Rice, and Maize. Plant Physiol. 1989, 89, 1285–1291. [Google Scholar] [CrossRef]
- West, C.E.; Waterworth, W.M.; Stephens, S.M.; Smith, C.P.; Bray, C.M. Cloning and Functional Characterisation of a Peptide Transporter Expressed in the Scutellum of Barley Grain during the Early Stages of Germination. Plant J. 1998, 15, 221–229. [Google Scholar] [CrossRef]
- Feng, J.-H.; Xu, X.-B.; Liu, X.-D.; Zhang, C.-L.; Liang, X.-L.; Wu, W.-C. Embryogenesis, Germination, Structure and Cotyledon Dimorphism of Zea mays Embryo. J. Integr. Plant Biol. 2003, 45, 712–723. [Google Scholar]
- Tnani, H.; López, I.; Vicient, C.M. Control of the Scutellar Epithelial Cell Elongation during Germination in Maize (Zea mays L.). Seed Sci. Technol. 2011, 39, 253–258. [Google Scholar] [CrossRef]
- Tnani, H.; López-Ribera, I.; García-Muniz, N.; Vicient, C.M. ZmPTR1, a Maize Peptide Transporter Expressed in the Epithelial Cells of the Scutellum during Germination. Plant Sci. 2013, 207, 140–147. [Google Scholar] [CrossRef]
- Stewart, C.R. Some Characteristics of the Uptake of Glutamine by Corn Scutellum. Plant Physiol. 1971, 47, 157–161. [Google Scholar] [CrossRef]
- Subbarao, K.V.; Datta, R.; Sharma, R. Amylases Synthesis in Scutellum and Aleurone Layer of Maize Seeds. Phytochemistry 1998, 49, 657–666. [Google Scholar] [CrossRef]
- Dure, L.S. Gross Nutritional Contributions of Maize Endosperm and Scutellum to Germination Growth of Maize Axis. Plant Physiol. 1960, 35, 919–925. [Google Scholar] [CrossRef]
- Enríquez-Arredondo, C.; Sánchez-Nieto, S.; Rendón-Huerta, E.; González-Halphen, D.; Gavilanes-Ruíz, M.; Díaz-Pontones, D. The Plasma Membrane H+-ATPase of Maize Embryos Localizes in Regions That Are Critical during the Onset of Germination. Plant Sci. 2005, 169, 11–19. [Google Scholar] [CrossRef]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Osborn, M.; Brandfass, S. Immunocytochemistry of Frozen and Paraffin Tissue Sections. In Cell Biology: A Laboratory Handbook; Academic Press: New York, NY, USA, 1994; pp. 361–367. [Google Scholar]
- DeWitt, N.D.; Sussman, M.R. Immunocytological Localization of an Epitope-Tagged Plasma Membrane Proton Pump (H(+)-ATPase) in Phloem Companion Cells. Plant Cell 1995, 7, 2053–2067. [Google Scholar] [CrossRef]
- Krishnamurthy, K.V. Methods in Plant Histochemistry; S Viswanathan Printers & Publishers: Madras, India, 1988. [Google Scholar]
- Pearse, A.G.E. Histochemistry: Theoretical and Applied; Churchill Livingstone: London, UK, 1968. [Google Scholar]
- Brown, R.C.; Lemmon, B.E. Chapter 7 Methods in Plant Immunolight Microscopy. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 1995; Volume 49, pp. 85–107. [Google Scholar] [CrossRef]
- Ingvardsen, C.; Veierskov, B.; Joshi, P.A. Immunohistochemical Localisation of Ubiquitin and the Proteasome in Sunflower (Helianthus annuus Cv. Giganteus). Planta 2001, 213, 333–341. [Google Scholar] [CrossRef]
- Liszkay, A.; Van Der Zalm, E.; Schopfer, P. Production of Reactive Oxygen Intermediates (O2−, H2O2, and ˙OH) by Maize Roots and Their Role in Wall Loosening and Elongation Growth. Plant Physiol. 2004, 136, 3114–3123. [Google Scholar] [CrossRef]
- García-Lara, S.; Arnason, J.T.; Díaz-Pontones, D.; Gonzalez, E.; Bergvinson, D.J. Soluble Peroxidase Activity in Maize Endosperm Associated with Maize Weevil Resistance. Crop Sci. 2007, 47, 1125–1130. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with The Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Bailly, C.; Kranner, I. Analyses of Reactive Oxygen Species and Antioxidants in Relation to Seed Longevity and Germination. In Seed Dormancy; Kermode, A.R., Ed.; Humana Press: Totowa, NJ, USA, 2011; Volume 773, pp. 343–367. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. In Methods in Enzymology; Packer, L., Ed.; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar] [CrossRef]
- Chandlee, J.M.; Tsaftaris, A.S.; Scandalios, J.G. Purification and Partial Characterization of Three Genetically Defined Catalases of Maize. Plant Sci. Lett. 1983, 29, 117–131. [Google Scholar] [CrossRef]
- Bolwell, G.P.; Davies, D.R.; Gerrish, C.; Auh, C.-K.; Murphy, T.M. Comparative Biochemistry of the Oxidative Burst Produced by Rose and French Bean Cells Reveals Two Distinct Mechanisms1. Plant Physiol. 1998, 116, 1379–1385. [Google Scholar] [CrossRef]
- Haddad, N.I.A.; Yuan, Q. Purification and Some Properties of Cu, Zn Superoxide Dismutase from Radix Lethospermi Seed, Kind of Chinese Traditional Medicine. J. Chromatogr. B 2005, 818, 123–131. [Google Scholar] [CrossRef]
- Maehly, A.C.; Chance, B. The Assay of Catalases and Peroxidases. In Methods of Biochemical Analysis; Glick, D., Ed.; Wiley: Hoboken, NJ, USA, 1954; Volume 1, pp. 357–424. [Google Scholar] [CrossRef]
- Dawson, R.M.C.; Elliott, D.C.; Dawson, R.M.C. Data for Biochemical Research, 3rd ed.; Clarendon Press: Oxford, UK, 1985; p. 352. [Google Scholar]
- Dragicevic, V.; Kovacevic, D.; Sredojevic, S.; Dumanovic, Z.; Mladenovic-Drinic, S. The Variation of Phytic and Inorganic Phosphorus in Leaves and Grain in Maize Populations. Genetika 2010, 42, 555–563. [Google Scholar] [CrossRef]
- Glass, R.L.; Geddes, W.F. Grain Storage Studies. XXVII. The Inorganic Phosphorus Content of Deteriorating Wheat. Cereal Chem. 1959, 36, 186–190. [Google Scholar]
- Raboy, V. Approaches and Challenges to Engineering Seed Phytate and Total Phosphorus. Plant Sci. 2009, 177, 281–296. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Wimer, L.T.; Huang, A.H.C. Lipase in the Lipid Bodies of Corn Scutella during Seedling Growth. Plant Physiol. 1983, 73, 460–463. [Google Scholar] [CrossRef]
- Qu, R.; Wang, S.M.; Lin, Y.H.; Vance, V.B.; Huang, A.H. Characteristics and Biosynthesis of Membrane Proteins of Lipid Bodies in the Scutella of Maize (Zea mays L.). Biochem. J. 1986, 235, 57–65. [Google Scholar] [CrossRef]
- Lombi, E.; Smith, E.; Hansen, T.H.; Paterson, D.; De Jonge, M.D.; Howard, D.L.; Persson, D.P.; Husted, S.; Ryan, C.; Schjoerring, J.K. Megapixel Imaging of (Micro)Nutrients in Mature Barley Grains. J. Exp. Bot. 2011, 62, 273–282. [Google Scholar] [CrossRef]
- Lu, L.; Tian, S.; Liao, H.; Zhang, J.; Yang, X.; Labavitch, J.M.; Chen, W. Analysis of Metal Element Distributions in Rice (Oryza sativa L.) Seeds and Relocation during Germination Based on X-Ray Fluorescence Imaging of Zn, Fe, K, Ca, and Mn. PLoS ONE 2013, 8, e57360. [Google Scholar] [CrossRef] [PubMed]
- Macleod, A.M.; Palmer, G.H. Gibberellin from Barley Embryos. Nature 1967, 216, 1342–1343. [Google Scholar] [CrossRef]
- Radley, M. Production of Gibberellin-like Substances in Barley Seed and Seedlings. In Plant Growth Regulators; S.C.I. Monographs: London, UK, 1968; Volume 31, pp. 53–69. [Google Scholar]
- Stoddart, J.L.; Thomas, H.; Robertson, A. Protein Synthesis Patterns in Barley Embryos during Germination. Planta 1973, 112, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Aoki, N.; Scofield, G.N.; Wang, X.-D.; Offler, C.E.; Patrick, J.W.; Furbank, R.T. Pathway of Sugar Transport in Germinating Wheat Seeds. Plant Physiol. 2006, 141, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Doll, N.M.; Ingram, G.C. Embryo–Endosperm Interactions. Annu. Rev. Plant Biol. 2022, 73, 293–321. [Google Scholar] [CrossRef] [PubMed]
- Fincher, G.B. Molecular and Cellular Biology Associated with Endosperm Mobilization in Germinating Cereal Grains. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 305–335. [Google Scholar] [CrossRef]
- Gibbons, G.C. On the Localisation and Transport of α-Amylase during Germination and Early Seedling Growth of Hordeum Vulgare. Carlsberg Res. Commun. 1979, 44, 353–366. [Google Scholar] [CrossRef]
- Gibbons, G.C. Immunohistochemical Determination of The Transport Pathways Of A-Amylase in Germinating Barley Seeds. Cereal Res. Commun. 1980, 8, 87–96. [Google Scholar]
- Gibbons, G.C. On the Sequential Determination of α-Amylase Transport and Cell Wall Breakdown in Germinating Seeds of Hordeum Vulgare. Carlsberg Res. Commun. 1980, 45, 177–184. [Google Scholar] [CrossRef]
- Gibbons, G.C. On the Relative Role of the Scutellum and Aleurone in the Production of Hydrolases during Germination of Barley. Carlsberg Res. Commun. 1981, 46, 215–225. [Google Scholar] [CrossRef]
- MacGregor, A.W.; Dushnicky, L. Starch Degradation in Endosperms of Developing Barley Kernels*. J. Inst. Brew. 1989, 95, 321–325. [Google Scholar] [CrossRef]
- Okamoto, K.; Akazawa, T. Enzymic Mechanisms of Starch Breakdown in Germinating Rice Seeds: 7. Amylase Formation in the Epithelium. Plant Physiol. 1979, 63, 336–340. [Google Scholar] [CrossRef]
- Palmer, G.H. The Morphology and Physiology of Malting Barley. In Cereals for Food and Beverages; Inglett, G.E., Munck, L., Eds.; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Ritchie, S.; Swanson, S.J.; Gilroy, S. Physiology of the Aleurone Layer and Starchy Endosperm during Grain Development and Early Seedling Growth: New Insights from Cell and Molecular Biology. Seed Sci. Res. 2000, 10, 193–212. [Google Scholar] [CrossRef]
- Bewley, J.D.; Black, M. Physiology and Biochemistry of Seeds in Relation to Germination; Springer: Berlin/Heidelberg, Germany, 1978. [Google Scholar] [CrossRef]
- MacNeill, G.J.; Mehrpouyan, S.; Minow, M.A.A.; Patterson, J.A.; Tetlow, I.J.; Emes, M.J. Starch as a Source, Starch as a Sink: The Bifunctional Role of Starch in Carbon Allocation. J. Exp. Bot. 2017, 68, 4433–4453. [Google Scholar] [CrossRef] [PubMed]
- Tan-Wilson, A.L.; Wilson, K.A. Mobilization of Seed Protein Reserves. Physiol. Plant 2012, 145, 140–153. [Google Scholar] [CrossRef]
- Chang, C.W. Study of Phytase and Fluoride Effects in Germinating Corn Seeds. Cereal Chem. 1967, 44, 129–142. [Google Scholar]
- Toole, E.H. The Transformations and Course of Development of Germinating Maize. Am. J. Bot. 1924, 11, 325–350. [Google Scholar] [CrossRef]
- Bailly, C. Active Oxygen Species and Antioxidants in Seed Biology. Seed Sci. Res. 2004, 14, 93–107. [Google Scholar] [CrossRef]
- Puntarulo, S.; Galleano, M.; Sanchez, R.A.; Boveris, A. Superoxide Anion and Hydrogen Peroxide Metabolism in Soybean Embryonic Axes during Germination. Biochim. Biophys. Acta 1991, 1074, 277–283. [Google Scholar] [CrossRef]
- Bykova, N.V.; Hu, J.; Ma, Z.; Igamberdiev, A.U. The Role of Reactive Oxygen and Nitrogen Species in Bioenergetics, Metabolism, and Signaling During Seed Germination. In Reactive Oxygen and Nitrogen Species Signaling and Communication in Plants; Gupta, K.J., Igamberdiev, A.U., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 23, pp. 177–195. [Google Scholar] [CrossRef]
- Schopfer, P. Hydroxyl Radical-induced Cell-wall Loosening in Vitro and in Vivo: Implications for the Control of Elongation Growth. Plant J. 2001, 28, 679–688. [Google Scholar] [CrossRef]
- Müller, K.; Linkies, A.; Vreeburg, R.A.M.; Fry, S.C.; Krieger-Liszkay, A.; Leubner-Metzger, G. In Vivo Cell Wall Loosening by Hydroxyl Radicals during Cress Seed Germination and Elongation Growth. Plant Physiol. 2009, 150, 1855–1865. [Google Scholar] [CrossRef]
- Caro, A.; Puntarulo, S. Nitric Oxide Generation by Soybean Embryonic Axes. Possible Effect on Mitochondrial Function. Free Radic. Res. 1999, 31 (Suppl. S1), 205–212. [Google Scholar] [CrossRef]
- Puglia, G.D. Reactive Oxygen and Nitrogen Species (RONS) Signalling in Seed Dormancy Release, Perception of Environmental Cues, and Heat Stress Response. Plant Growth Regul. 2024, 103, 9–32. [Google Scholar] [CrossRef]
- Hite, D.R.C.; Auh, C.; Scandalios, J.G. Catalase Activity and Hydrogen Peroxide Levels Are Inversely Correlated in Maize Scutella during Seed Germination. Redox Rep. 1999, 4, 29–34. [Google Scholar] [CrossRef] [PubMed]
- De Gara, L.; De Pinto, M.C.; Arrigoni, O. Ascorbate Synthesis and Ascorbate Peroxidase Activity during the Early Stage of Wheat Germination. Physiol. Plant 1997, 100, 894–900. [Google Scholar] [CrossRef]
- Tommasi, F.; Paciolla, C.; de Pinto, M.C.; De Gara, L. A Comparative Study of Glutathione and Ascorbate Metabolism during Germination of Pinus pinea L. Seeds. J. Exp. Bot. 2001, 52, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Bazin, J.; Langlade, N.; Vincourt, P.; Arribat, S.; Balzergue, S.; El-Maarouf-Bouteau, H.; Bailly, C. Targeted mRNA Oxidation Regulates Sunflower Seed Dormancy Alleviation during Dry After-Ripening. Plant Cell 2011, 23, 2196–2208. [Google Scholar] [CrossRef]
- Basbouss-Serhal, I.; Leymarie, J.; Bailly, C. Fluctuation of Arabidopsis Seed Dormancy with Relative Humidity and Temperature during Dry Storage. J. Exp. Bot. 2016, 67, 119–130. [Google Scholar] [CrossRef]
- Kibinza, S.; Vinel, D.; Côme, D.; Bailly, C.; Corbineau, F. Sunflower Seed Deterioration as Related to Moisture Content during Ageing, Energy Metabolism and Active Oxygen Species Scavenging. Physiol. Plant 2006, 128, 496–506. [Google Scholar] [CrossRef]
- Mhamdi, A.; Noctor, G.; Baker, A. Plant Catalases: Peroxisomal Redox Guardians. Arch. Biochem. Biophys. 2012, 525, 181–194. [Google Scholar] [CrossRef]
- Pan, R.; Liu, J.; Wang, S.; Hu, J. Peroxisomes: Versatile Organelles with Diverse Roles in Plants. New Phytol. 2020, 225, 1410–1427. [Google Scholar] [CrossRef]
- Pinfield-Wells, H.; Rylott, E.L.; Gilday, A.D.; Graham, S.; Job, K.; Larson, T.R.; Graham, I.A. Sucrose Rescues Seedling Establishment but Not Germination of Arabidopsis Mutants Disrupted in Peroxisomal Fatty Acid Catabolism. Plant J. 2005, 43, 861–872. [Google Scholar] [CrossRef]
- Graham, I.A. Seed Storage Oil Mobilization. Annu. Rev. Plant Biol. 2008, 59, 115–142. [Google Scholar] [CrossRef] [PubMed]
- Scandalios, J.G. Control of Gene Expression and Enzyme Differentiation. In Physiological Genetics; Scandalios, J.G., Ed.; Elsevier: Amsterdam, The Netherlands, 1979; pp. 63–107. [Google Scholar] [CrossRef]
- Chiu, K.Y.; Chen, C.L.; Sung, J.M. Effect of Priming Temperature on Storability of Primed Sh-2 Sweet Corn Seed. Crop Sci. 2002, 42, 1996–2003. [Google Scholar] [CrossRef]
- Gallardo, K.; Job, C.; Groot, S.P.C.; Puype, M.; Demol, H.; Vandekerckhove, J.; Job, D. Proteomic Analysis of Arabidopsis Seed Germination and Priming. Plant Physiol. 2001, 126, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Posmyk, M.M.; Corbineau, F.; Vinel, D.; Bailly, C.; Côme, D. Osmoconditioning Reduces Physiological and Biochemical Damage Induced by Chilling in Soybean Seeds. Physiol. Plant 2001, 111, 473–482. [Google Scholar] [CrossRef]
- Prodanovic, O.; Prodanovic, R.; Bogdanovic, J.; Mitrovic, A.; Milosavic, N.; Radotic, K. Antioxidative Enzymes during Germination of Two Lines of Serbian Spruce [Picea Omorika (Panc.) Purkyně]. Arch. Biol. Sci. 2007, 59, 209–216. [Google Scholar] [CrossRef]
- De Tullio, M.C.; Arrigoni, O. The Ascorbic Acid System in Seeds: To Protect and to Serve. Seed Sci. Res. 2003, 13, 249–260. [Google Scholar] [CrossRef]
- Fridovich, I. The Biology of Oxygen Radicals: The Superoxide Radical Is an Agent of Oxygen Toxicity; Superoxide Dismutases Provide an Important Defense. Science 1978, 201, 875–880. [Google Scholar] [CrossRef]
- Baum, J.A.; Scandalios, J.G. Developmental Expression and Intracellular Localization of Superoxide Dismutases in Maize. Differentiation 1979, 13, 133–140. [Google Scholar] [CrossRef]
- Scandalios, J.G. Oxidative Stress and the Molecular Biology of Antioxidant Defenses; Scandalios, J.G., Ed.; Cold Spring Harbor Laboratory Press: Plainview, NY, USA, 1997. [Google Scholar]
- Bogdanovic, J.; Prodanovic, R.; Milosavic, N.; Prodanovic, O.; Radotic, K. Multiple Forms of Superoxide Dismutase in the Apoplast and Whole-Needle Extract of Serbian Spruce [Picea Omorika (Panc.) Purkyně]. Arch. Biol. Sci. 2006, 58, 211–214. [Google Scholar] [CrossRef]
- Geller, B.L.; Winge, D.R. Liver. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 105–114. [Google Scholar] [CrossRef]
- Van Camp, W.; Capiau, K.; Van Montagu, M.; Inze, D.; Slooten, L. Enhancement of Oxidative Stress Tolerance in Transgenic Tobacco Plants Overproducing Fe-Superoxide Dismutase in Chloroplasts. Plant Physiol. 1996, 112, 1703–1714. [Google Scholar] [CrossRef]
- Baum, J.A.; Scandalios, J.G. Isolation and Characterization of the Cytosolic and Mitochondrial Superoxide Dismutases of Maize. Arch. Biochem. Biophys. 1981, 206, 249–264. [Google Scholar] [CrossRef]
- Vyas, D.; Kumar, S. Purification and Partial Characterization of a Low Temperature Responsive Mn-SOD from Tea (Camellia sinensis (L.) O. Kuntze). Biochem. Biophys. Res. Commun. 2005, 329, 831–838. [Google Scholar] [CrossRef]
- Zhu, D.; Scandalios, J.G. Maize Mitochondrial Manganese Superoxide Dismutases Are Encoded by a Differentially Expressed Multigene Family. Proc. Natl. Acad. Sci. USA 1993, 90, 9310–9314. [Google Scholar] [CrossRef]
- Del Río, L.A.; Lyon, D.S.; Olah, I.; Glick, B.; Salin, M.L. Immunocytochemical Evidence for a Peroxisomal Localization of Manganese Superoxide Dismutase in Leaf Protoplasts from a Higher Plant. Planta 1983, 158, 216–224. [Google Scholar] [CrossRef]
- Oracz, K.; Voegele, A.; Tarkowská, D.; Jacquemoud, D.; Turečková, V.; Urbanová, T.; Strnad, M.; Sliwinska, E.; Leubner-Metzger, G. Myrigalone A Inhibits Lepidium Sativum Seed Germination by Interference with Gibberellin Metabolism and Apoplastic Superoxide Production Required for Embryo Extension Growth and Endosperm Rupture. Plant Cell Physiol. 2012, 53, 81–95. [Google Scholar] [CrossRef]
- Welinder, K.G. Superfamily of Plant, Fungal and Bacterial Peroxidases. Curr. Opin. Struct. Biol. 1992, 2, 388–393. [Google Scholar] [CrossRef]
- Zámocký, M.; Hofbauer, S.; Schaffner, I.; Gasselhuber, B.; Nicolussi, A.; Soudi, M.; Pirker, K.F.; Furtmüller, P.G.; Obinger, C. Independent Evolution of Four Heme Peroxidase Superfamilies. Arch. Biochem. Biophys. 2015, 574, 108–119. [Google Scholar] [CrossRef]
- Fawal, N.; Li, Q.; Savelli, B.; Brette, M.; Passaia, G.; Fabre, M.; Mathé, C.; Dunand, C. PeroxiBase: A Database for Large-Scale Evolutionary Analysis of Peroxidases. Nucleic Acids Res. 2012, 41, D441–D444. [Google Scholar] [CrossRef]
- Shigeto, J.; Tsutsumi, Y. Diverse Functions and Reactions of Class III Peroxidases. New Phytol. 2016, 209, 1395–1402. [Google Scholar] [CrossRef]
- Cosio, C.; Dunand, C. Specific Functions of Individual Class III Peroxidase Genes. J. Exp. Bot. 2009, 60, 391–408. [Google Scholar] [CrossRef]
- Daudi, A.; Cheng, Z.; O’Brien, J.A.; Mammarella, N.; Khan, S.; Ausubel, F.M.; Bolwell, G.P. The Apoplastic Oxidative Burst Peroxidase in Arabidopsis Is a Major Component of Pattern-Triggered Immunity. Plant Cell 2012, 24, 275–287. [Google Scholar] [CrossRef]
- Gabaldón, C.; López-Serrano, M.; Pedreño, M.A.; Barceló, A.R. Cloning and Molecular Characterization of the Basic Peroxidase Isoenzyme from Zinnia Elegans, an Enzyme Involved in Lignin Biosynthesis. Plant Physiol. 2005, 139, 1138–1154. [Google Scholar] [CrossRef]
- Pandey, V.P.; Awasthi, M.; Singh, S.; Tiwari, S.; Dwivedi, U.N. A Comprehensive Review on Function and Application of Plant Peroxidases. Biochem. Anal. Biochem. 2017, 6, 308. [Google Scholar] [CrossRef]
- Passardi, F.; Cosio, C.; Penel, C.; Dunand, C. Peroxidases Have More Functions than a Swiss Army Knife. Plant Cell Rep. 2005, 24, 255–265. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhao, Y.; Han, G.; Zhu, S. Systematic Analysis of Maize Class III Peroxidase Gene Family Reveals a Conserved Subfamily Involved in Abiotic Stress Response. Gene 2015, 566, 95–108. [Google Scholar] [CrossRef]
- Lüthje, S.; Meisrimler, C.-N.; Hopff, D.; Möller, B. Phylogeny, Topology, Structure and Functions of Membrane-Bound Class III Peroxidases in Vascular Plants. Phytochemistry 2011, 72, 1124–1135. [Google Scholar] [CrossRef]
- Möller, S.; Croning, M.D.R.; Apweiler, R. Evaluation of Methods for the Prediction of Membrane Spanning Regions. Bioinformatics 2001, 17, 646–653. [Google Scholar] [CrossRef]
- Sonnhammer, E.L.; von Heijne, G.; Krogh, A. A Hidden Markov Model for Predicting Transmembrane Helices in Protein Sequences. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1998, 6, 175–182. [Google Scholar]
- De Gara, L. Class III Peroxidases and Ascorbate Metabolism in Plants. Phytochem. Rev. 2004, 3, 195–205. [Google Scholar] [CrossRef]
- Fernandes, M.; Souza, D.H.; Henriques, R.O.; Alves, M.V.; Skoronski, E.; Junior, A.F. Obtaining Soybean Peroxidase from Soybean Hulls and Its Application for Detoxification of 2,4-Dichlorophenol Contaminated Water. J. Environ. Chem. Eng. 2020, 8, 103786. [Google Scholar] [CrossRef]
- Ramalho, R.P.R.S.; Scalize, P.S.; Caramori, S.S. Peroxidase de Gramínea de Cerrado como alternativa no tratamento de efluentes Agroindustrialis. Rev. Ambiente Água 2016, 11, 50–59. [Google Scholar] [CrossRef][Green Version]
- De Oliveira, F.K.; Santos, L.O.; Buffon, J.G. Mechanism of Action, Sources, and Application of Peroxidases. Food Res. Int. 2021, 143, 110266. [Google Scholar] [CrossRef]
- Oh, J.-H.; Kim, B.C.; Lee, J.-S. Colorimetric Detection of Acetylcholine with Plasmonic Nanomaterials Signaling. Anal. Bioanal. Chem. 2014, 406, 7591–7600. [Google Scholar] [CrossRef]
- Zeraik, A.E.; Souza, F.S.D.; Fatibello-Filho, O.; Leite, O.D. Desenvolvimento de Um Spot Test Para o Monitoramento Da Atividade Da Peroxidase Em Um Procedimento de Purificação. Química Nova 2008, 31, 731–734. [Google Scholar] [CrossRef]
- López-Serrano, M.; Ros Barceló, A. Reversed-Phase and Size-Exclusion Chromatography as Useful Tools in the Resolution of Peroxidase-Mediated (+)-Catechin Oxidation Products. J. Chromatogr. A 2001, 919, 267–273. [Google Scholar] [CrossRef]
- López-Serrano, M.; Ros Barceló, A. Comparative Study of the Products of the Peroxidase-Catalyzed and the Polyphenoloxidase-Catalyzed (+)-Catechin Oxidation. Their Possible Implications in Strawberry (Fragaria × ananassa) Browning Reactions. J. Agric. Food Chem. 2002, 50, 1218–1224. [Google Scholar] [CrossRef]
- Ito, S.; Hayashi, S. Effect of Limited Oxygen Supply on Activities of Catalase, Peroxidase and Cytochrome Oxidase in Germinating Rice Seeds. Jpn. J. Crop Sci. 1961, 30, 97–100. [Google Scholar] [CrossRef][Green Version]
- Palmiano, E.P. Studies on the Sequence of Biochemical Events in the Rice Grain during Germination. Master’s Thesis, University of the Philippines, Los Bafnos, Philippines, 1971. [Google Scholar]
- Palmiano, E.P.; Juliano, B.O. Changes in the Activity of Some Hydrolases, Peroxidase, and Catalase in the Rice Seed during Germination. Plant Physiol. 1973, 52, 274–277. [Google Scholar] [CrossRef]
- Cai, F.; Lan-Ju, M.; Xiao-Long, A.; Gao, S.H.U.N.; Tang, L.; Chen, F.A.N.G. Lipid Peroxidation and Antioxidant Response during Seed Germination of Jatropha Curcas. Int. J. Agric. Biol. 2011, 13, 25–30. [Google Scholar]
- Morohashi, Y. Peroxidase Activity Develops in the Micropylar Endosperm of Tomato Seeds Prior to Radicle Protrusion. J. Exp. Bot. 2002, 53, 1643–1650. [Google Scholar] [CrossRef]
- Mitrović, A.; Dučić, T.; Lirić-Rajlić, I.; Radotić, K.; Živanović, B. Changes in Chenopodium Rubrum Seeds with Aging. Ann. N. Y. Acad. Sci. 2005, 1048, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Szcziparev, S.M. Scutellum and Its Role in Germination. In Embryology of Flowering Plants: Terminology and Concepts; Volume 2, The Seed; Batygina, T.B., Ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Almagro, L.; Gómez Ros, L.V.; Belchi-Navarro, S.; Bru, R.; Ros Barceló, A.; Pedreño, M.A. Class III Peroxidases in Plant Defence Reactions. J. Exp. Bot. 2009, 60, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Francoz, E.; Ranocha, P.; Nguyen-Kim, H.; Jamet, E.; Burlat, V.; Dunand, C. Roles of Cell Wall Peroxidases in Plant Development. Phytochemistry 2015, 112, 15–21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).