Abstract
The Canary Islands lead banana (Musa acuminata) production in the EU. Different fungal pathogens affect this crop in subtropical areas, with Fusarium oxysporum f. sp. cubense subtropical race 4 (Foc-STR4) being the most important in the Canary Islands. With the aim of developing environmentally sustainable techniques for disease control, this study presents the results of the evaluation of the antifungal capacity of a native Trichoderma collection (12 species, 109 isolates) obtained from banana soils. The results demonstrate the diversity of biocontrol genes and the in vitro antagonistic potential of different native Trichoderma species/isolates against two Foc-STR4 strains obtained from plants with Panama disease symptoms. Trichoderma virens (TF18), a dominant species in banana soils in the Canary Islands, showed a high capacity to inhibit the growth of Foc-STR4 in different in vitro assays. Trichoderma atrobrunneum (TF01) showed mycoparasitism capacity through the spiral coil around the hyphae of the pathogen. In addition, the genome analysis of T. atrobrunneum (TF03) showed 69 putative biosynthetic gene clusters, with the notable presence of the trichothecene tri5 gene. Finally, our work demonstrates that the soils of the Canary Islands banana crops are a potential source of environmentally adapted biological control agents to control or reduce the incidence of Foc-STR4.
1. Introduction
Bananas (Musa acuminata) are the most relevant fruit in the world and rank among the top ten food products for Southeast Asia, Africa, and Latin America [1]. The total worldwide area under banana production is approximately 5.2 million hectares and its consumption is widespread in almost all countries of the world [1,2]. In Europe, Spain is the primary producer of this crop, with the Canary Islands offering the most suitable subtropical climate for its cultivation (outdoor and in greenhouse) [3,4].
Different diseases affect the banana production. Among these, the major threat to banana crops is the Fusarium wilt or Panama disease, caused by the soil fungal Fusarium oxysporum f. sp. cubense (Foc) [5]. Based on the pathogenicity to reference banana cultivars, three races of the pathogen are recognized: Foc race 1 (R1) affects principally ‘Gros Michel’ (AAA) and Manzano/Apple/Latundan (Silk, AAB) cultivars, Foc race 2 (R2) affects cooking bananas of the Bluggoe (ABB) subgroup, and Foc race 4 (R4) affects all cultivars in the Cavendish (AAA) subgroup as well as those susceptible to R1 and R2 [6,7]. Furthermore, R4 has two divisions: subtropical Race 4 (STR4), restricted to subtropical areas with cold temperature stress, and tropical Race 4 (TR4), which does not require low temperatures and other predisposing factors to cause the disease [8]. In the Canary Islands, the causal agent of the disease is Foc-STR4 [9].
The management of the disease is not straightforward as a consequence of different factors: (1) the potential of the pathogen to spread through contaminated materials (plant tissue, tools, water, etc.), to resist adverse conditions, and to remain latent in the soil for a long period of time; (2) the endophytic properties of the pathogen that increase its protection against external fungicidal agents; and (3) the lack of crop rotations (monoculture) during banana production allows the pathogen to reproduce in a continuous cycle [2]. Based on these factors, the control of the pathogen has been a continuous and important challenge for farmers and health authorities. As a result, different strategies have been used, including soil treatments, fungicide application, and the use of resistant cultivars and contingency plans, to prevent the spread of the pathogen to non-contaminated areas. A successful example was the replacement of the “Gros Michel” cultivar (susceptible to Foc-R1 and R2) by Cavendish in the 1950s. However, this strategy was efficient until new variants of the pathogen (race 4) appeared, which affected the cultivars of the Cavendish subgroup very aggressively [10]. Therefore, progress in creating fully Foc-TR4-resistant bananas, either through classical breeding or genetic engineering, has been limited [11]. In this context, considering the complexity of the disease and the new regulations on sustainable crop management, the use of biological control agents (BCAs) offers a useful alternative for the management of Fusarium wilt of banana [12]. Mycorrhizal fungi, Trichoderma spp., Pseudomonas spp., and Bacillus spp. are the most studied BCAs against Foc [10]. In this regard, different studies highlight the potential of Trichoderma spp. to act as a BCA based on the activation of multiple mechanisms, such as competition for space and nutrients, modification of environmental conditions, promotion of plant growth and plant defense mechanisms, antibiosis, and mycoparasitism. The latter is based on the production of fungal cell wall-degrading enzymes (CWDEs) (chitinases, glucanases, and proteases, produced alone or in combination) [13,14], which play an important role during the antibiosis and mycoparasitism processes through the degradation of the pathogen’s cell wall [15,16]. The genus Trichoderma shows a wide genetic diversity with more than 400 species [17] and is able to produce more than 100 different secondary metabolites with antimicrobial activities [18]. Likewise, serine proteases, like tvps1 in T. virens [19] or prb1 in T. harzianum [20], are also believed to facilitate the penetration into the host tissue by degrading the protein linkages in the host’s outer layers (fungal cell wall) and/or use them for their own nutrition [19].
Several authors have demonstrated the ability of certain Trichoderma species to reduce Foc growth (mainly Foc-TR4) under in vitro conditions and, in some cases, the severity of the disease under controlled greenhouse conditions [10,21,22,23]. However, most of the studies evaluate the biocontrol capacity of Trichoderma using isolates from other crops, environments, or culture collection reservoirs, while few studies evaluate isolates obtained from the soil or banana plants affected by the disease [22,24,25,26,27,28]. This aspect is important as the success of a BCA under field conditions depends not only on the inhibitory capacity of the pathogen, but also on the ability to adapt and preserve a high level of inoculum under the environmental and agricultural conditions of the crop where it is to be applied. Taking this into account, probably the best method to obtain a potential biocontrol agent is to isolate the candidate microorganism from a similar environment (soil or plant) to the pathogen in which the disease is to be controlled. In this way, the potential biocontrol agent would be naturally adapted to the environmental, biological, and nutritional conditions of the environment in which it is to be applied.
The aim of this study was to evaluate the biocontrol capacity of Trichoderma against Foc-STR4 and to detect the presence of CWDE-encoding genes. For this purpose, a Trichoderma spp. native collection from the Canary Islands obtained from the rhizosphere of banana plants with Panama disease symptoms was used. Oligonucleotides related to the biocontrol genes were designed and different in vitro assay methods were evaluated for the control of two Foc-STR4 strains obtained from the same bioclimatic zone as the Trichoderma isolates. Additionally, the genome of T. atrobrunneum carrying the tri5 gene, which has been described as being involved in the biosynthesis of the trichothecene mycotoxins, was also sequenced, and its analysis revealed the presence of 69 biosynthetic genes clusters (BGCs) encoding secondary metabolite genes. The importance of this study is to provide an initial knowledge about the potential capacity of a Trichoderma native collection to control the most important pathogen of the banana crops of the Canary Island. This is the first step for the selection of field-applicable Trichoderma isolates to control the disease using environmentally friendly and cost-effective alternatives.
2. Materials and Methods
2.1. Isolates Used and Growth Conditions
The Trichoderma isolates were obtained from the rhizosphere of soils of banana crops affected by Foc-STR4, following the procedures described in a previous study by Correa-Delgado et al. [29]. Briefly, fourteen banana farms located in different bioclimatic areas and production zones of the northern (colder and wetter) and southern (warmer and drier) slopes of Tenerife island (Canary Islands, Spain) were selected considering the following factors: a) agroclimatic characteristics, b) distribution of the production zones, and c) distribution of the affected banana crops (Foc-STR4). A total of 84 soil samples and 109 native Trichoderma isolates were obtained and identified as: T. aff. harzianum, T. aff. hortense, T. atrobrunneum, T. afroharzianum, T. asperellum, T. gamsii, T. guizhouense, T. harzianum, T. hamatum, T. hirsutum, T. longibrachiatum, and T. virens. Summarized information of each isolate is shown in Table S1 (Supplementary Materials) [29].
Two virulent strains (code numbers 9 and 62) of Foc-STR4 [identified as Fusarium phialophorum by molecular methods [30]] were isolated from banana plants with severe wilt disease symptoms and used in the antifungal assays. These strains were obtained from samples of plant tissues with vascular necrosis from farms situated in various production areas of Tenerife and are part of an extensive Fusarium culture collection of the Department of Plant Protection at the “Instituto Canario de Investigaciones Agrarias” (ICIA, Spain). The criteria for selecting these strains from the collection were: (a) the macroscopic morphotypes diversity in PDA; (b) the intraspecific genotypic diversity detected by inter simple sequence repeat (ISSR) (CAG5) and the random amplification of polymorphic DNA (RADP) (CRL-9, 5′-CAGCCGCCCC-3′) analyses; (c) the biogeographical origin of the pathogen (north and south slope of the island); and (d) the severity of the disease in banana plants (Figure 1). The isolation and identification methods were as previously described by Correa-Delgado et al. [29].
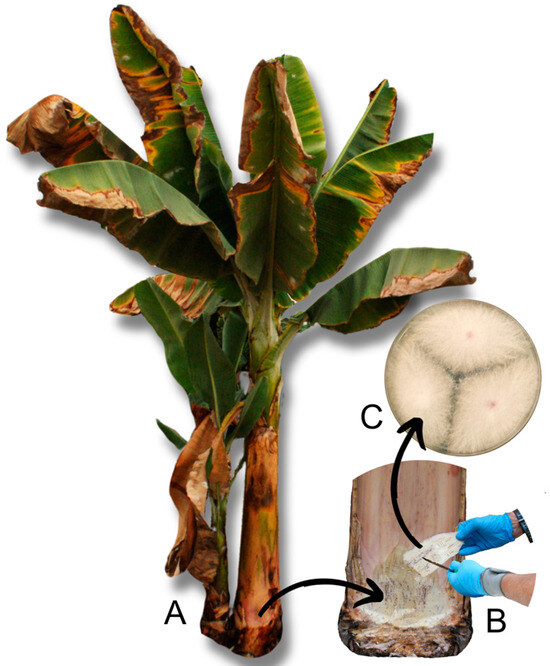
Figure 1.
Sampling and pathogen isolation process. (A) Banana plant with symptoms of Panama disease. (B) Selection of a corm sample with xylem necrosis. (C) Foc-STR4 colonies isolated in PDA medium (25 °C, 7 days).
All Trichoderma and Fusarium isolates were cultured in potato dextrose agar (PDA, Condalab, Laboratory Conda S.A. Madrid, Spain) for 7–10 days at 25 °C and stored at −80 °C in a 30% glycerol solution. One additional growth media, Spezieller Nährstoffarmer Agar (SNA), was used to grow one Trichoderma isolate (T. atrobrunneum TF03) for macroscopic and microscopic observations with differential interference contrast (DIC) in a Nikon Eclipse 80i optical microscope.
2.2. Design of Oligonucleotides for PCR Amplification of Internal Fragments of Biocontrol Genes
In order to analyze the presence of genes associated to the biocontrol activity of Trichoderma in the banana rhizosphere culture collection, oligonucleotides corresponding to 9 biocontrol-related genes were designed based on CWDEs, such as chitinases (tv-ech1, 42-kDa, chit36Y, glyc), glucanases (bgn13, lam1.3, egl1), proteases (p6281, tvps1), and one trichothecene biosynthetic gene [sesquiterpenoid epoxides formed from the parent compound trichodiene through the isomerization and cyclization of farnesyl pyrophosphate [31]] (tri5) (Table S2).
2.3. PCR Amplifications and Screening of Biocontrol Genes
Each Trichoderma isolate (109) was grown in PDA at 25 °C for 7–10 days, and subsequently, the mycelium was collected and transferred to a microtube (approximately 50–70 mg). The DNA was extracted and purified by a conventional protocol using SDS lysis buffer (Sigma-Aldrich, Darmstadt, Germany) combined with chloroform and ethanol for precipitation [29]. The DNA was quantified using a NanoDrop 2000c (ThermoFisher Scientific Inc., Wilmington, NC, USA).
Genomic DNA was used as a template in the PCR amplifications. Briefly, PCR reactions were performed in a 50 µL volume with the following final concentration: 1.6 ng/μL DNA, 1× buffer (20 mM Tris-HCl pH 8.0, 100 mM KCl, 0.1 mM EDTA, 1 mM dithiothreitol), 1.75 mM MgCl2, 0.2 mM dNTP, 1 μM of each primer, and 1.25 U/μL Taq DNA polymerase (EURX E2500 Taq DNA polymerase, Molecular Biology Products, Gdansk, Poland). Amplifications were carried out in an Eppendorf Mastercycler X50s (Eppendorf Ibérica S. L. U., San Sebastián de los Reyes, Madrid, Spain). The PCR conditions were optimized for each oligonucleotide pair (Table S2) and are shown in Table S3. PCR product were visualized by horizontal electrophoresis on agarose gel.
To confirm the specificity of the PCR products, nine amplicons corresponding to each biocontrol gene were purified using exonuclease I (M0293S, USA, New England BioLabs, Ipswich, MA, USA) and shrimp alkaline phosphatase (M0371S, BioLabs), following the manufacturer’s instructions (Shrimp Alkaline Phosphatase (rSAP), BioLabs). The purified products were sequenced using Sanger sequencing at Macrogen (Macrogen Inc., Madrid, Spain). The partial sequences of each gene were deposited in Genbank (PP842884, PP919098–PP919106) (Table S4).
2.4. Kluyveromyces Marxianus CECT 1018 Assay
The isolates positive in trichothecene biosynthetic gene (tri5) were tested by a biological method using K. marxianus CECT 1018 as a sensitive trichothecenes indicator. For this purpose, the yeast was grown on a malt extract agar (MEA) medium (2% malt extract, 2% glucose, 0.1% peptone, and 2% agar) at 30 °C for 2–3 days, and the antibiograms were carried out as previously described by Cardoza et al. [31].
2.5. In Vitro Antifungal Assays Methodology
The Trichoderma isolates with the best performance in the analysis biocontrol genes were evaluated for their antagonistic ability against Foc-STR4 (strains 9 and 62) using three different assays. For each of them, 6 mm diameter plugs picked from the edge of growing fungal colonies were used to inoculate the PDA medium into sterile 9 cm diameter Petri dishes. The plates were incubated in the dark at 25 °C for 7 days and subsequently used for the different antifungal assays:
- Membrane antifungal assay. This procedure was used to quantify the ability of the Trichoderma isolates to produce in a solid medium metabolites and/or enzymes with inhibitory activity against Foc-STR4 strains. The antifungal assay was performed as previously described by Mayo et al. [32] with some modifications. Briefly, a sterile cellophane membrane was placed on the surface of the PDA medium, and Trichoderma plugs were incubated on this membrane for 48 h at 25 °C. After the removal of the membrane with the Trichoderma mycelia, Fusarium plugs were placed on the same plates to assess growth inhibition. In parallel, a Fusarium control was grown on PDA plates (without Trichoderma spp.) under the same conditions. The growth of the pathogen was monitored every day until the mycelium covered the entire surface of the Petri dish. Assays were performed in triplicate with each Fusarium strain. Photographs were taken after 7 days.
- Broth antifungal assay. This procedure was used to evaluate the ability of each Trichoderma isolate to produce in a liquid medium metabolites and enzymes with antifungal activity. PDA-sporulated plates were used to obtain a Trichoderma inoculum of 1 × 106 spores/mL of each isolate. Subsequently, the suspensions were inoculated in sterile 250 mL flaks containing 150 mL of Blakeeslee’s malt extract broth (MEBbl [33]) and incubated at 25 °C and 220 rpm in the dark for 24 h. Five milliliters of broth suspension (broth and mycelium) were filtered through a 0.22 µm pore sterile filter (Minisart, Sartorius Stedim, Misuri, USA, Biotech) and stored at −20 °C until use.
Once the broths of each isolate were obtained, a 6 mm diameter hole was made in a Petri plate (9 cm diameter) with 25 mL of PDA (1% agar) and 60 µL of each Trichoderma-processed broth were loaded. Plates were incubated at 4 °C overnight to allow diffusion. After this time, 6 mm diameter plugs picked from the edge of Foc-STR4 colony growth on PDA were used to inoculate each plate at the same position as the broth was added. The plates were incubated at 25 °C and Foc-STR4 growth was monitored daily. Control plates were prepared under the same conditions, but the broth was replaced by sterile water. The broths of each Trichoderma isolate were tested in triplicate against the two Foc-STR4 strains (9 and 62).
- 3.
- Direct confrontation antifungal assay. This assay was used to verify the ability of Trichoderma isolates to overgrow the pathogen. The process was performed as previously described by Mayo et al. [32] with some modifications. Each Trichoderma isolate was grown in a dual culture with Foc-STR4 strains 9 and 62. Both isolates (Trichoderma vs. Foc-STR4) were placed 5.5 cm apart on the same plate with PDA medium and incubated at 25 °C for 5 days. The behavior of Trichoderma against the pathogen was examined visually each day until Trichoderma had overgrown or surrounded the pathogen colony. Assays were performed in triplicate and single cultures of Fusarium were used as a control. Colony photographic documentation was conducted after 5 days. Likewise, in order to determine the mycoparasitism capacity (spirally coiled hyphae) of the different Trichoderma isolates, the mycelium was observed in the intersection areas of both colonies using an optical microscope Nikon Eclipse 80i with DIC (Tokyo, Japan).
The following formula was used to calculate the percentage inhibition of the above assays: % I = [C − T/C] × 100 [32].
For the membrane (% IM) and broth (% IB) antifungal assay, “C” represents the diameter (mm) of Fusarium control plates, and “T” is the diameter (mm) of Fusarium after being exposed to the metabolites of the Trichoderma isolates. For the direct confrontation assay (% ID), “C” represents the radius (mm) of Fusarium control plates, and “T” is the radius (mm) of Fusarium on the Trichoderma confrontation plates.
2.6. Fungal DNA Extraction and Genome Sequencing
Genomic DNA from T. atrobrunneum TF03 was extracted with the DNeasy Plant Mini Kit (QIAGEN, Hilden, Germany), according to the manufacturer’s instructions, and subsequently, the purity and concentration were determined using a Nanodrop ND-1000 spectrophotometer (ThermoFisher Scientific Inc., Wilmington, NC, USA). The genome sequence was developed by Macrogen, Inc. (Seoul, Republic of Korea; https://dna.macrogen.com, [Accessed on 20 October 2023]) using an Illumina platform, and sequence assemblies were created using the SPAdes (v3.15.0) assembler software [34]. The processed sequence was submitted to the Sequence Read Archive (SRA) database at the National Center for Biotechnology Information (NCBI) as accession SRR29211434 (Bioproject PRJNA1117644). Assembled genome was analyzed to identify secondary metabolite gene clusters with the prediction and annotation tool Augustus version 3.4.0 [35,36] and Blast2GO software [37]. Then, the predicted genes/proteins were analyzed by Blast software using the OmicsBox v. 2.1.14 package (BioBam Informatics, Cambridge, MA, USA, https://www.biobam.com/omicsbox (accessed on 18 March 2024)). At the end, the Augustus annotated genome was analyzed with the AntiSMASH software version 3.0.5 to screen putative secondary metabolite gene clusters [38].
2.7. Phylogenetic Analysis
Nucleotide and amino acid sequences from 20 Trichoderma housekeeping (HK) genes (Table S5) retrieved from the genome of 35 Trichoderma species [39] were used to infer a Trichoderma species tree. The analysis was carried out following the two methods proposed by Gutiérrez et al. [39]. Thus, nucleotide sequences of the open reading frames from 20 housekeeping genes were aligned using the software MUSCLE 3.8 as implemented in MEGA X [40], and then, the alignments were concatenated using the Sequence Matrix software [41]. The resulting concatenated alignment was subjected to maximum likelihood (ML) analysis using IQ-Tree software, version 1.6.7 [42]. Branch support was evaluated through a bootstrap analysis with 1000 pseudoreplicates. Additionally, to assess tree consistency (deduced from the 20 housekeeping genes), a gene concordance factor (gCF) analysis was conducted as described by [43]. The percentage of individual nucleotide trees that contained each branch was calculated and incorporated into the species tree. For the gCF analysis, a second concatenated–partitioned tree was generated by selecting the best-fit evolutionary model for each nucleotide sequence alignment of each housekeeping gene (HK), as determined from the prior IQ-Tree analysis, following the methodology of Gutiérrez et al. [39]. This concatenated–partitioned alignment was also subjected to a ML analysis as implemented in IQ-Tree.v2. The resulting IQ-tree file was used for gCF calculation based on Minh et al. [43]. In addition, three other trees were deduced using partial amino acid sequences determined from coding sequences of three housekeeping genes [acl1 (ATP citrate lyase), rpb2 (RNA polymerase 2nd major subunit), and tef1 (translation elongation factor 1-alpha)] from TF03, and those partial sequences retrieved from the GenBank databases, which were selected from those previously reported [44]. These sequences were aligned using the MUSCLE software implemented in MEGA X, and trees were generated using the IQ-TREE software version 1.6.12. Branch support was assessed using a bootstrap analysis based on 1000 pseudoreplicates.
2.8. Statistical Analysis
The inhibition values of Fusarium growth by the Trichoderma isolates in the different in vitro assays were compared by an analysis of variance (ANOVA), pairwise t-test, and post hoc test (Fisher’s LSD) applied when appropriate, using the statistical software R (R version 4.0.3, R Foundation for Statistical Computing, Vienna, Austria) and the integrated development environment RStudio (RStudio version 1.4.1103, RStudio Team 2016, Boston, MA, USA).
3. Results
3.1. Screening of Biocontrol Genes in the Collection of Trichoderma Isolates
A total of 109 Trichoderma native isolates were screened by PCR with the designed oligonucleotide pairs based in conserved sequences of nine biocontrol genes. Table 1 shows the number of positive genes and the percentage of positive isolates for each Trichoderma species. All the genes analyzed were detected; nevertheless, none of the species studied were positive for all the genes tested. Trichoderma aff. harzianum, T. harzianum, T. virens, and T. asperellum were the species with the highest number of PCR-positive genes (7/10), while T. afroharzianum, T. hirsutum, T. hamatum, and T. gamsii had the lowest number of PCR-positive genes (4/10). Regarding the percentage of PCR-positive isolates on the different genes, differences between species were observed. For example, T. aff. harzianum had more than 90% of the isolates PCR-positive for the egl1 and tvps1 genes, while T. harzianum had more than 70% of the isolates PCR-positive for the tv-ech1, bgn13, and tvps1 genes. Moreover, it is noteworthy that the only species that showed PCR-positive results for the tri5 gene was T. atrobrunneum (54.55% of the isolates) and, in addition, this species had 100% of PCR-positive isolates for the bgn13 and tvps1 genes. The gene with the highest percentage of PCR-positive species was bgn13 (11 positive species out of 12 tested: 91.7%) and the lowest was tri5 (one positive species: T. atrobrunneum: 8.3%).

Table 1.
Biocontrol gene screening in the native collection of Trichoderma.
All PCR-positive T. atrobrunneum tri5 isolates (TF01, TF02, TF03, TF04, TF05, TF07) were evaluated by assays with K. marxianus CECT 1018 (trichothecenes sensitive yeast). TF03 isolate showed a positive result, while the rest of the isolates were negative (Figure S1).
3.2. In Vitro Antifungal Assays
Based on the biocontrol gene analysis results, the isolates from each Trichoderma clade that showed the highest number of biocontrol genes (PCR-positive) were selected (Table S6). The antagonistic capacity of each of the selected isolates (23) was evaluated against two Foc-STR4 strains (9 and 62) using three different assays.
3.2.1. Membrane Antifungal Assay
Trichoderma virens (isolate TF18) showed the highest percentage of inhibition (30.72% IM) against Foc-STR4 strain 9, followed by T. atrobrunneum (TF02: 28.23% IM) and T. gamsii (TF20: 21.81% IM) (Figure 2A). T. hamatum (TF16) showed the lowest inhibition rate (4.24% IM). On the other hand, T. virens (TF18) showed the highest inhibition percentage (30.55% IM) against the Foc-STR4 strain 62, followed by T. atrobrunneum (isolates TF01: 27.5% and TF02: 26.87% IM) and T. gamsii (TF20: 22.72% IM). Two isolates of T. aff. harzianum (TF13 and TF11) and T. atrobrunneum (TF05) showed the lowest inhibition percentages (2.78%, 4.11%, and 4.37% IM, respectively) (Figure 2B). Regarding the difference of the percentage inhibition between the Foc-STR4 strains (% IM strain 9 vs. % IM strain 62) in each of the Trichoderma isolates, it was observed that 13.04% of the Trichoderma isolates showed significant differences (p < 0.05) of the % IM between each pathogen strain. Accordingly, in 86.96% of the isolates, no significant differences (p < 0.05) were observed between the Foc-STR4 strains. In addition, the average percentage inhibition for each Foc-STR4 strain was not significantly different (p < 0.05): 12.77% IM for Foc-STR4 strain 9 and 12.88% IM for strain 62 (Figure 2A,B). At the species level, i.e., considering the average of the % IM of Foc with all the isolates of each Trichoderma species, T. gamsii showed the highest % IM in both strains of Foc-STR4 (Figure 2C), while T. hamatum was the species with the lowest inhibitory capacity against Foc-STR4 strain 9 (6.53% IM) and T. harzianum (6.25% IM) against Foc-STR4 strain 62. Regarding the percentage inhibition between Foc-STR4 strains (strain 9 vs. strain 62), significant differences (p < 0.05) were observed at the species level in T. hamatum and T. aff. harzianum (Figure 2C).
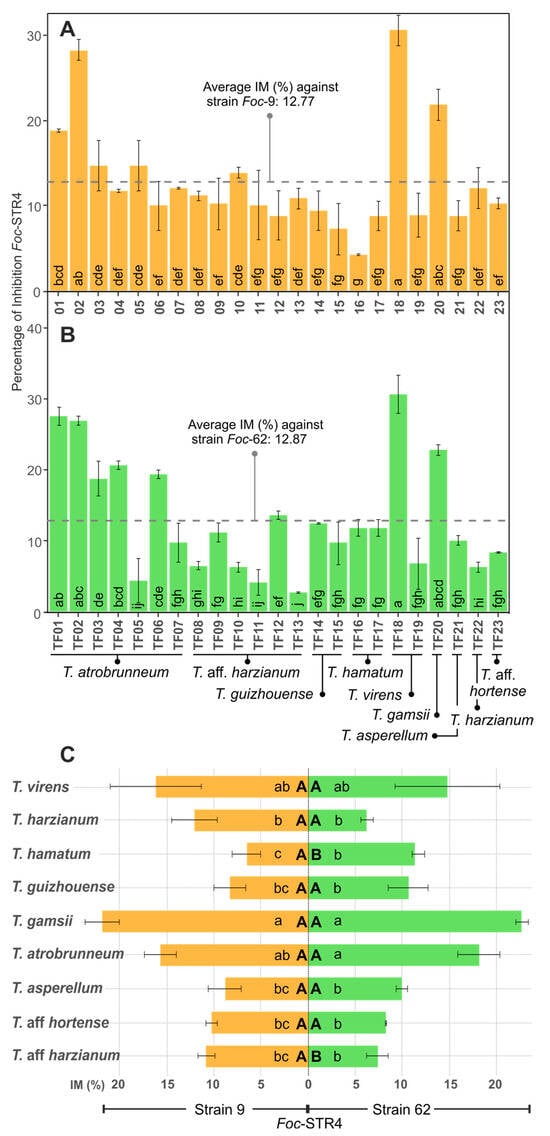
Figure 2.
In vitro antifungal activity of Trichoderma spp. against Foc-STR4 in the cellophane membrane assay (IM). (A,B): Percentage of inhibition (% IM) of Foc-STR4 strain 9 (A) and 62 (B) with each Trichoderma isolate. (C): % IM of Foc-STR4 with each Trichoderma species (average of the % IM of the isolates of each species). Different lower-case letters in bars indicate significant differences (Fisher’s LSD, p < 0.05) between each Trichoderma isolates (A,B) or species (C) against each Foc-STR4 strain. Different capital letters in bars (C) indicate significant differences (p < 0.05), according to the t test) between Foc-STR4 strains in the same Trichoderma species. Error bars represent the standard error of the mean.
3.2.2. Broth Antifungal Assay
To test the metabolite/enzyme production capacity of Trichoderma in a liquid medium, the 23 isolates were grown at 25 °C 220 rpm for 24 h in a liquid medium in the dark. Strains 9 and 62 of Foc-STR4 were tested with this broth to determine the percentage inhibition (% IB). Trichoderma guizhouense (TF14) and T. asperellum (TF21) showed the highest percentage inhibition against both Foc-STR4 strains (Figure 3A,B). While T. atrobrunneum (TF07), T. aff. harzianum (TF09), and T. virens (TF19) showed the lowest inhibition rates (0.01% IB) against Foc-STR4 strain 9 (Figure 3A), and T. aff. harzianum (TF08 and TF12; 1.76 and 1.92% IB, respectively), T. atrobrunneum (TF07: 1.76% IB), and T. aff. hortense (TF23: 1.76% IB) against Foc-STR4 strain 62 (Figure 3B). In addition, no significant effect was observed in the inhibition capacity of each Trichoderma isolate between the Foc-STR4 strains (p < 0.05). Likewise, no significant differences (p < 0.05) were observed between the average inhibition percentages of the two strains of Foc (5.48% and 6.75% for strains 9 and 62, respectively) (Figure 3A,B). At the species level, T. asperellum showed the highest percentage inhibition of both strains of Foc-STR4 (15.83% and 15.78% for strains 9 and 62, respectively), and T. aff. hortense showed the lowest percentage (3.32% and 1.76% for strains 9 and 62, respectively) (Figure 3C). Regarding the differences between the inhibition percentages between each Foc strain, no significant differences (p < 0.05) were observed in the species tested. Therefore, in this assay, the inhibition results did not depend on the Foc strain used.
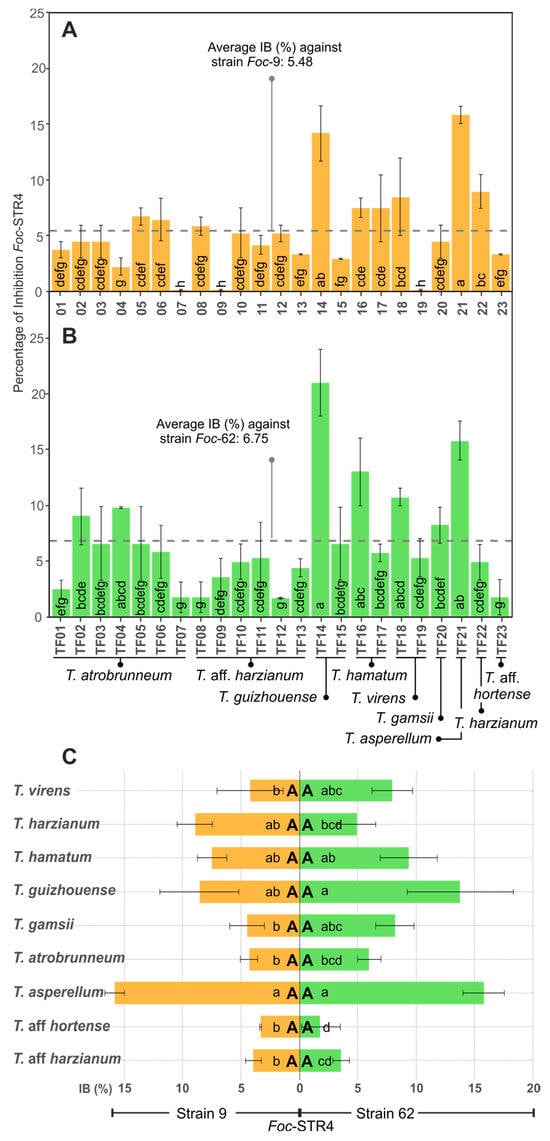
Figure 3.
In vitro antifungal activity of Trichoderma spp. against Foc-STR4 in broth assays (IB). (A,B): Percentage of inhibition (% IB) of Foc-STR4 strain 9 (A) and 62 (B) with each Trichoderma isolate. (C): % IB of Foc-STR4 with each Trichoderma species (average of the % IB of the isolates of each species). Different lower-case letters in bars indicate significant differences (Fisher’s LSD, p < 0.05) between each Trichoderma isolates (A,B) or species (C) against each Foc-STR4 strain. Different capital letters in bars (C) indicate significant differences (p < 0.05, according to the t test) between Foc-STR4 strains in the same Trichoderma species. Error bars represent the standard error of the mean.
3.2.3. Direct Confrontation Antifungal Assay
A total of 86.95% of the Trichoderma isolates showed an % ID higher than 30% and 30.43% of the isolates showed a percentage over 40% against Foc-STR4 strain 9 (Figure 4A). Trichoderma atrobrunneum (TF01: 46.66%; TF02, TF04, TF06: 43.33% ID), and T. guizhouense (TF14: 43.33% ID) showed the highest % ID. T. aff. harzianum (TF12) showed the lowest percentage inhibition (25.26% ID). Regarding Foc-STR4 strain 62, all the isolates (except TF13 and TF19) presented an inhibition percentage higher than 30%, and the 56.52% of the isolates showed an % ID higher than 40% (Figure 4B). Thus, T. gamsii (TF20: 54.92% ID) and T. atrobrunneum (TF01: 52.1% ID) showed the highest percentage of inhibition, followed by T. guizhouense (TF14: 50.71% ID), T. atrobrunneum (TF02: 50.71% ID), and T. hamatum (TF16: 49.29% ID). T. virens (TF19) showed the lowest percentage inhibition (14.05% ID) (Figure 4B). Regarding the inhibition percentages between Foc strains, a significant difference (p < 0.05) in the % ID was observed in 26.08% of the isolates. Consequently, 73.92% of the isolates remained with the same biocontrol activity against each Foc strain. On the other hand, no significant difference (p < 0.05) was observed between the average of the % ID of each Foc strain: 36.52% (Foc-STR4 strain 9) and 40.71% (Foc-STR4 strain 62) (Figure 4A,B). At the species level (Figure 4C), the highest % ID against Foc-STR4 strain 9 was observed in T. atrobrunneum (39.68%); nevertheless, the differences between species were not statistically significant (p < 0.05), with the exception of T. aff. harzianum, which had a significantly lower % ID than that of T. atrobrunneum. Moreover, in the case of Foc-STR4 strain 62, T. gamsii showed the highest % ID (54.92%), followed by T. guizhouense (47.18% ID) and T. atrobrunneum (44.65% ID). Regarding the comparison of the inhibition percentages between the Foc strains for each Trichoderma species, no significant difference (p < 0.05) was observed between Foc strains in all the Trichoderma species analyzed (with the exception of T. gamsii and T. atrobrunneum) (Figure 4C).
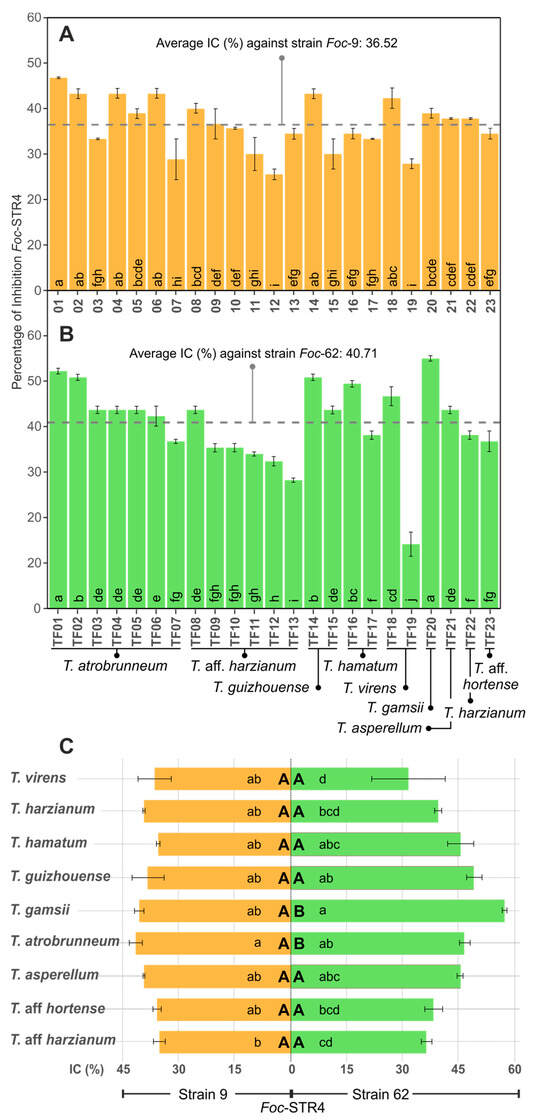
Figure 4.
In vitro antifungal activity of Trichoderma spp. against Foc-STR4 in direct confrontation assays (ID). (A,B): Percentage of inhibition (% ID) of Foc-STR4 strain 9 (A) and 62 (B) with each Trichoderma isolate. (C): % ID of Foc-STR4 with each Trichoderma species (average of the % ID of the isolates of each species). Different lower-case letters in bars indicate significant differences (Fisher’s LSD, p < 0.05) between each Trichoderma isolates (A,B) or species (C) against each Foc-STR4 strain. Different capital letters in bars (C) indicate significant differences (p < 0.05, according to the t test) between Foc-STR4 strains in the same Trichoderma species. Error bars represent the standard error of the mean.
To summarize the results of the different antifungal assays and to know the efficiency of the different Trichoderma isolates for the in vitro control of the two tested Foc-STR4 strains, the data of each assay were analyzed as a whole. For this purpose, the data of each assay (membrane, broth, and direct confrontation) were transformed independently, where “0” represents the lowest percentage value and “1” the highest percentage in each assay. Once the values were transformed, a unified database was generated for the three assays with both Foc-STR4 isolates. The results show that the isolates with the highest inhibitory capacity were T. virens (TF18), T. guizhouense (TF14), T. atrobrunneum (TF02 and TF01), T. gamsii (TF20), and T. asperellum (TF21) (Figure 5A,B). Consequently, taking into account the results of the three assays, these species/isolates showed the highest inhibitory capacity against both Foc-STR4 strains.
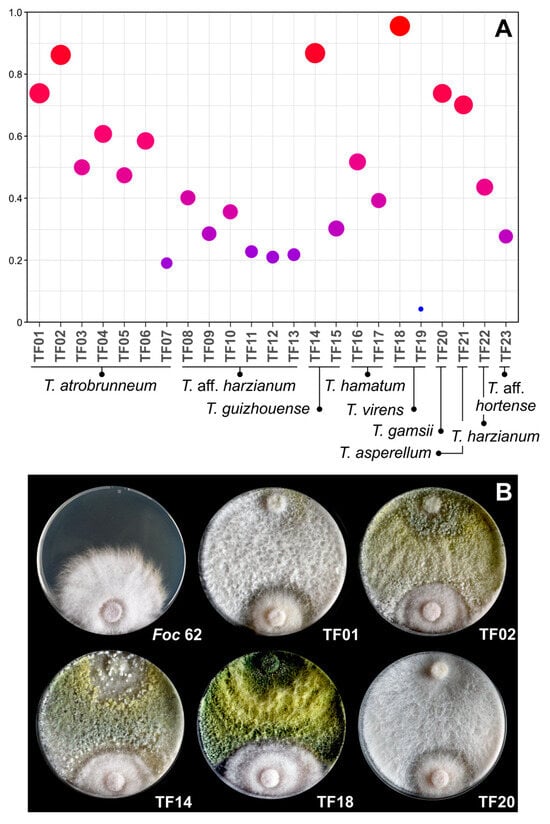
Figure 5.
In vitro antifungal activity of Trichoderma spp. against Foc-STR4 considering the dataset from all assays. (A) represents the transformed (normalized) dataset of the three assays (membrane % IM; broth % IB; direct confrontation % ID), where “0” represents the lowest percentage of inhibition in the set of assays and “1” the highest percentage. The color and diameter of the circles within the graph represent the level of the values (blue and small circle = 0; red and large circle = 1). (B) shows the direct confrontation (dual culture) assay on Petri dishes (9 cm diameter) with PDA between Trichoderma isolates and Foc-STR4 strain 62 at 25 °C for 5 days. Foc-62: Foc-STR4 strain 62 control plate.
3.3. Analysis of the T. atrobrunneum TF03 Genome Sequence
A summary overview of the main features found in the genome sequences of the TF03 is shown in Table 2. AntiSMASH software analysis was used to predict the secondary metabolite biosynthetic gene clusters. As result, 69 BGCs were detected, which include one or more of the so-called “core” genes, which are distributed as follows: 16 polyketide synthase (PKS), 11 non-ribosomal peptide synthetase (NRPS), 16 hybrid PKS-NRPS, and 10 terpene synthase (TS) (Table 3 and Table S7). Within the classification of terpenes, cluster 23 (location: 2,817,761–2,818,946) included the tri5 gene (TATRO_4483) (total: 1035 nt, excluding introns), which encodes the TS that catalyzes the first committed step in trichothecene biosynthesis [45]. No regions classified as “other” or regions without biosynthetic gene clusters were detected.

Table 2.
Summary of genome sequence assembly statistics for T. atrobrunneum TF03 sequenced in this study.

Table 3.
Number of biosynthetic gene clusters (BGCs), including PKS, NRPS, hybrid PKS-NRPS, and terpene synthase-encoding genes, identified in the genome of the T. atrobrunneum TF03 isolate in this study, as determined by Augustus and antiSMASH analyses.
3.4. Phylogenetic Analyses
The twenty-five Trichoderma species used in the phylogenetic analysis belong to 11 previously identified lineages [39,46]. To locate the TF03 isolate, a species tree was constructed using a maximum likelihood analysis of concatenated alignments of twenty housekeeping genes obtained from the genomic sequences of type Trichoderma strains and TF03 [39]. In the resulting tree (Figure 6), TF03 appears as a single branch within the Harzianum/Virens section, with a bootstrap value of 100 and a gCF of 90. The macroscopic and microscopic morphological observations of isolate TF03 are shown in Figure 7.
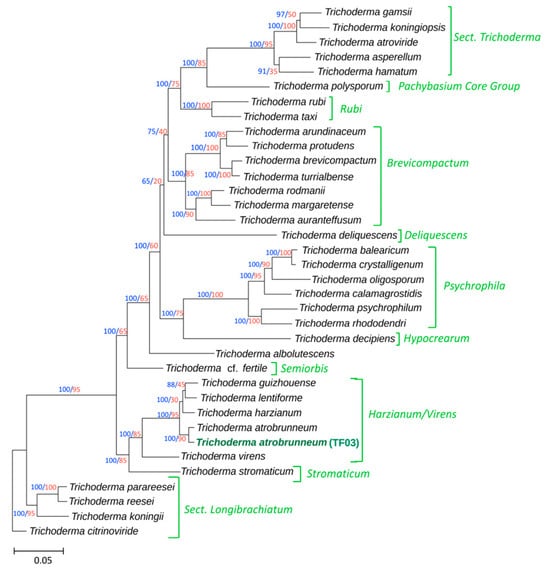
Figure 6.
The phylogenetic tree was inferred from full-length exon sequences of 20 housekeeping genes (totaling 54,000 sites). T. atrobrunneum isolate TF03 (genome sequenced in this work) is indicated in green boldface type. Bootstrap values, calculated from 1000 pseudoreplicates, are shown in blue on each branch, while gene concordance factors (gCFs) are displayed in red. Previously identified subgeneric lineages [39,46] are indicated to the right of the tree.
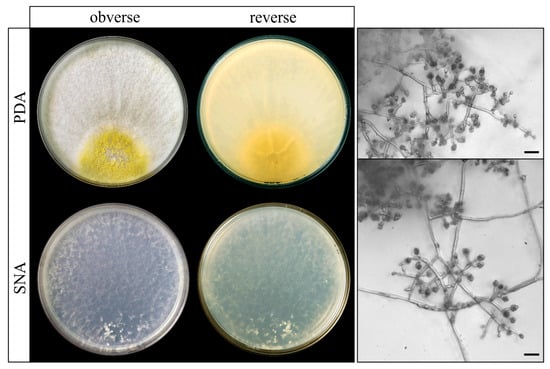
Figure 7.
Trichoderma atrobrunneum (isolate TF03) on SNA and PDA after 7 days at 25 °C. Scale bars: 10 μm.
Three of the genetic markers used in the above study (acl1, tef1, and rpb2) were analyzed separately. The phylogenetic analyses of the partial genes acl1, tef1, and rpb2 from a broader representation of isolates produced individual trees that align consistently (Figures S2–S4) with the main tree of the 20 housekeeping genes (Figure 6). In the individual trees, bootstrap values obtained on the TF03 branch were 100%.
4. Discussion
For decades, it has been widely recognized that the main biocontrol mechanisms used by Trichoderma as BCAs are mycoparasitism [47], antibiosis, and nutrient competition [48]. However, currently, this fungus is considered a popular player and a key component of plant biostimulants, bioprotectants, biofertilizers, soil amendments, soil integrators, biodegraders, and bioremediators [49,50]. An evidence of this is the record increase in Trichoderma-containing products in the international market since 2014, with 21 registrations increasing to 144 in 2022 [14,51]. Different methods are used for the selection of Trichoderma BCAs. In this respect, it is well known that in vitro tests are only an approximation to the much more complex natural contexts and need to be complemented and validated by subsequent tests in vivo and under culture conditions [32,52]. Nevertheless, in vitro assays are the first approach to finding BCAs and understanding their mechanisms of action. In this study, potential BCAs belonging to the genus Trichoderma were selected against the causal agent of Panama disease in banana crops of the Canary Islands (Foc-STR4). For this purpose, a collection of native Trichoderma isolates obtained from the rhizosphere soil of banana plants affected with Panama disease in different bioclimatic and agronomic conditions was used. The selection was carried out using molecular techniques to screen genes involved in biocontrol mechanisms (specific oligonucleotides) and three different types of antifungal assays: membrane antifungal assay, broth assay, and direct confrontation assay. These assays were carried out against two different strains of Foc-STR4, obtained from banana plants with symptoms of Panama disease located in the same areas from where the Trichoderma collection was obtained.
The analysis of nine biocontrol-related genes in the Trichoderma collection led to determining their distribution in the different species of Trichoderma and, curiously, the two majority species (T. aff. harzianum and T. virens) in the banana soils of Tenerife island (Canary Islands) (Correa-Delgado et al. [29]) were those showing a higher number of biocontrol genes, along with T. harzianum and T. asperellum (Table 1). Taking into account the PCR screening, 23 isolates were selected to carry out the second stage of the work: the in vitro antifungal assays.
The antifungal membrane assay method was used to measure the ability of the Trichoderma isolates to produce metabolites and/or enzymes with inhibitory activity against the pathogen [32]. Many studies have used this assay to test the inhibitory capacity of Trichoderma against different pathogens, such as Rosellinia [53], Rhizoctonia [32,39,54,55], Sclerotinia [56], Phaeoacremonium [57], Botrytis [45], Verticillium [58], and Fusarium [59,60], among others. In the case of F. oxysporum, this type of assay was tested with different formae speciales: F. oxysporum f. sp. phaseoli, F. oxysporum f. sp. lycopercisi, etc. [52,61]. To our knowledge, this is the first work using a membrane antifungal assay to determine the ability of Trichoderma spp. against Foc-STR4. In this study, T. virens (TF18), T. atrobrunneum (TF02), and T. gamsii (TF20) were the isolates that showed the highest inhibitory effect against the two tested Foc-STR4 strains (over 20% IM) (Figure 2A,B). These results are in agreement with previous reports, showing a high percentage of inhibitory activity of T. gamsii T057 against F. oxysporum isolated from bean (Phaseolus vulgaris) [52] or the inhibitory activity of T. virens against F. oxysporum f. sp. lycopersici [61].
The broth assay was used to test the ability of Trichoderma to produce metabolites and enzymes in a liquid culture medium. This type of assay has been widely used to characterize compounds of microbial origin with activity against fungi, bacteria, or nematodes [62,63,64,65,66]. However, to our knowledge, this is the first work to use this assay against Foc-STR4. Our results show that T. asperellum (TF21) and T. guizhouense (TF14) have the highest percentage of inhibition against the two Foc-STR4 strains (Figure 3A,B). Nevertheless, this assay showed a remarkable variability in data between Trichoderma isolates. These results are in agreement with the studies reported by Olowe et al., who detected different inhibitory capacities of Trichoderma culture filtrates against rice and oak leaf pathogens of the genus Fusarium: F. proliferatum (Fus 294 and Fus 296) and F. tricinctum [67].
The direct confrontation assays (also known as “dual/collision method”) were used to test the ability of Trichoderma to overgrow the pathogen. The usefulness of this assay is mainly attributed to the antibiosis effect of several antimicrobial/antagonistic compounds and to the production of extracellular hydrolytic enzymes that are able to degrade antagonistic cell wall polymers. Likewise, the capacity for aggressive growth and the physiology of the biocontrol agent, including mycoparasitism (via hydrolytic enzyme secretion) and competition in the culture medium, are factors that significantly affect the results of the assay [68,69]. Furthermore, the literature is very extensive in relation to studies aimed to control Fusarium using Trichoderma isolates, specifically Fusarium oxysporum f. sp. cubense (R1, R2 and R4) from banana crops [10,22,24,27,67,70]. In our work, all Trichoderma isolates (except TF19) were able to reduce the growth of Foc-STR4 with a percentage of inhibition raising values up to 25% ID, with the most effective being T. gamsii and T. atrobrunneum (average % ID: 46.9% and 42.2%, respectively) (Figure 4C). This agrees with results from Taribuka et al., where T. gamsii presented the highest percentage of growth inhibition (60.61%) against Foc-A-13 [27]. Likewise, Natsiopoulos et al. showed a significant reduction in the mycelium growth of F. oxysporum f. sp. lycopersici (42.57%) with T. atrobrunneum [71]. In the same trend, the isolate VKPMF-1434 of T. atrobrunneum showed 39.10% and 47.50% of inhibition against FocБ/14 and MOS509 strains, respectively [72]. This agrees with the results obtained in our study with the T. atrobrunneum isolates that showed a high rate of growth inhibition of both strains of Foc-STR4.
Other species that showed a high percentage inhibition against Foc-STR4 were T. guizhouense and T. asperellum (average of 41.95 and 40.75% ID, respectively). Trichoderma guizhouense has been reported to be a powerful antagonist of Foc-TR4 in in vitro assays and in situ in the banana rhizosphere [73,74], which emphasizes our result obtained with isolates TF14 and TF15 (higher than 30% ID). On the other hand, Thangavelu and Gopi observed that T. asperellum NRCB2 showed a 33.4–82.5% inhibition in the mycelial growth of Foc-R1 [24].
In addition, several studies have demonstrated the mycoparasitism capacity of Trichoderma against different pathogens [14,75]. During this mechanism, Trichoderma hyphae recognize the pathogen and grow in a spiral shape. Subsequently, cell wall-degrading enzymes, mainly chitinases, glucanases, and proteases, are secreted and penetrate the hyphae of the pathogen, causing nutrient uptake and the dissolution and death of the parasitized fungus [18,76,77]. Considering the importance of this mechanism during the biocontrol process, we performed a microscopic observation of the confrontation plates in the zone where both fungi interact physically (Trichoderma and Fusarium). As shown in Figure 8, T. atrobrunneum (TF01) hypha was spirally coiled around the hypha of Foc-STR4 strain 62. These structures were not observed in other isolates because the mycoparasitic potential of Trichoderma spp. probably varies depending on the strains confronted [78]. This behavior was reported by Damodaran et al. and Thangavelu et al., who observed the mycoparasitism of T. reesei and T. asperellum against Foc-TR4 [22,79].
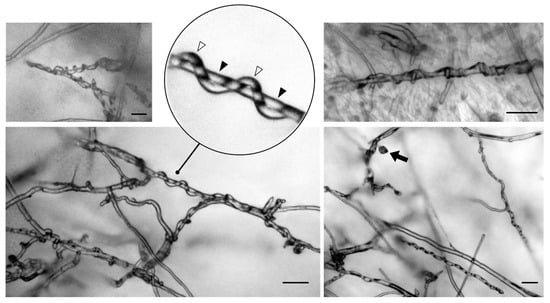
Figure 8.
Mycoparasitism exhibited by Trichoderma atrobrunneum against Fusarium oxysporum f. sp. cubense STR4 on PDA medium at 25 °C in the direct confrontation antifungal assays. The microphotographs show the coiling and encircling of T. atrobrunneum hyphae (empty triangles) on Foc hyphae (filled triangles) after 5 days of confrontation. The filled arrow shows a Foc false head on a short lateral conidiophore. Scale bars: 50 μm.
It is really remarkable in the literature that there is a great variability between different in vitro assays [32,53,55]. In this sense, variability has been reported between species and between isolates of the same species, such as the results obtained in our work. Related to this, Anees et al. reported that the most efficient strain to control sugar beet disease caused by Rhizoctonia solani (AG 2-2 strain G6) was T. gamsii (strain T30); however, the different isolates of the same species presented different percentages of inhibition in a confrontation assay (19.29% to 46.31%) [80]. A similar observation was made by Anjum et al., where five different Trichoderma species tested showed disparities in the inhibition in F. oxysporum f. sp. capsica [81]. Likewise, Olowe et al. observed variability in the inhibitory capacity between different strains of T. virens (TCIV, TCVII, TMS1) and T. harzianum (TCVI, TCVIII) against F. oxysporum f. sp. lycopersici isolates [67].
Finally, variability against the pathogen used in in vitro assays has also been reported. In our work, variability was observed between Foc-STR4 strains 9 and 62 in membrane and broth assays, while the direct confrontation assay showed less variability. This situation was reported by Błaszczyk et al., who observed that the range of action of Trichoderma strains against different Fusarium species (F. avenaceum, F. cerealis, F. culmorum, F. graminearum, and F. temperatum) was variable [82]. In the same trend, the results obtained by Qualhato et al. indicated differences in the antagonistic abilities of Trichoderma spp. against S. sclerotiorum, F. solani, and R. solani [83].
In order to analyze the data from the different assays (% IM, % IB, % ID) as a whole, transformation and normalization were carried out. As shown in Figure 5, T. virens (TF18), T. guizhouense (TF14), T. atrobrunneum (TF01 and TF02), and T. gamsii (TF20) showed the best performance in the in vitro biological control of both Foc-STR4 strains. Taking into account the biocontrol potential of T. virens described in the literature and the results obtained in this work, this species could be of great interest for its application in field conditions, especially considering its wide distribution in different production areas and its dominant character over other Trichoderma species [29]. However, as demonstrated in this work, the selection of the most suitable isolates of Trichoderma species is very important, as there is a high variability in data between isolates of the same species.
In relation to the genome of T. atrobrunneum (TF03), the analysis revealed the detection of 69 putative BGCs (Table 2 and Table 3), and the number of TS, PKS, NRPS, hybrid PKS-NRPS, and terpene synthase genes were similar to those in the reference T. atrobrunneum ITEM 908 [84]. Taking into account the results of TF03 isolate obtained by the PCR tri5 test and the biological activity against K. marxianus, the tri5 gene (TATRO_4483) was predicted in cluster 23 based on the antiSMASH results (Table S7). However, this detection was not obtained by Fanelli et al., who identified a partial domain similar to tri5 (identity < 30%) in the genome T. atrobrunneum ITEM 908 [84]. Furthermore, the type and organization of genes located around tri5 gene in TF03 were similar to those found in other Trichoderma species [31,39].
5. Conclusions
Our findings demonstrate the diversity of biocontrol genes and the in vitro antagonistic potential of different native Trichoderma species/isolates against Foc-STR4 obtained from banana plants with Panama disease symptoms. The results suggest that T. virens (TF18), a dominant species in banana soils in the Canary Islands, could be an efficient BCA under field conditions due to its natural capacity for adaptation. However, other species/isolates less frequent in Canary Island soils should also be tested under field conditions. To summarize, this work demonstrates that Canary Island banana soils are a natural and potential source of BCAs for the control of Foc-STR4. This is an important aspect, as the use of indigenous isolates ensures to some extent their adaptability to the environment (temperature, humidity, nutrient availability, etc.) in which they are to be applied. Therefore, future research should build on these results and focus on different key areas. Firstly, confirming the efficacy of the most promising Trichoderma isolates, such as T. virens (TF18), under real agricultural conditions. Secondly, to evaluate and optimize different formulations and application methodologies to maintain a high and stable inoculum of the biocontrol agent under field conditions, e.g., direct soil inoculation, enrichment of organic substrates added to the soil (compost, biochar, etc.), and bioaugmentation of autochthonous soil BCAs by the addition of organic amendments, etc. In addition, new genomic studies on Trichoderma isolates, in particular those with a high inhibitory activity, may uncover new genes or pathways associated with biocontrol mechanisms, which could lead to the development of improved or genetically enhanced strains.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture14112016/s1. Figure S1: Biological activity of T. atrobrunneum TF03 broth against K. marxianus CECT 1018; Figure S2: Phylogenetic tree inferred by maximum likelihood (TN + F + I + G4) analysis of partial sequence of tef1; Figure S3: Phylogenetic tree inferred by maximum likelihood (TIMe + I + G4) analysis of partial sequence of rpb2; Figure S4: Phylogenetic tree inferred by maximum likelihood (TIMe + I + G4) analysis of partial sequence of acl1; Table S1: Trichoderma spp. isolates used in this study; Table S2: Oligonucleotide sequences designed for PCR analysis; Table S3: PCR conditions used for the amplification of an internal fragment from the selected genes; Table S4: GenBank accession of biocontrol genes; Table S5: 20 Trichoderma housekeeping (HK) genes retrieved from the genome of 35 Trichoderma species were used to infer a Trichoderma species tree; Table S6: Biocontrol genes detected by PCR in the selected Trichoderma isolates; Table S7: Trichoderma atrobrunneum TF03 gene clusters for SM biosynthesis detected by AntiSMASH analysis. Reference [39] has been cited in the Supplementary Materials.
Author Contributions
Conceptualization, S.G. and F.L.; methodology, S.G., F.L. and R.E.C.; software, R.C.-D.; validation, S.G., F.L. and R.E.C.; formal analysis, R.C.-D.; investigation, R.C.-D., S.G., F.L., P.B.-L. and R.E.C.; resources, F.L. and M.C.J.V.; data curation, R.C.-D.; writing—original draft preparation, R.C.-D. and F.L.; writing—review and editing, R.C.-D., S.G., F.L., P.B.-L. and R.E.C.; visualization, R.C.-D., S.G. and F.L.; supervision, F.L., M.C.J.V. and S.G.; project administration, M.C.J.V.; funding acquisition, M.C.J.V. and F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Consejería de Agricultura, Ganadería, Pesca y Aguas del Gobierno de Canarias (Project: CAIA 2023-001-04 “Estrategias agroecológicas para el manejo de sistemas plataneros”).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The datasets generated for this study can be found in the GenBank (https://www.ncbi.nlm.nih.gov/genbank/, [Accesed on 4 March 2024]).
Acknowledgments
The authors are thankful to Patricia C. Peréz Parrado for her technical assistance and dedication to this work. The authors would like to thank for the Grant PRE2019-1061 089319 funded by MCIN/AEI/10.13039/501100011033 and, as appropriate, by “ESF Investing in your future” or by “European Union Next Generation EU/PRTR” from the Spanish Government.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT Database. Available online: https://www.fao.org/faostat/en/#home (accessed on 3 January 2024).
- Ordonez, N.; Seidl, M.F.; Waalwijk, C.; Drenth, A.; Kilian, A.; Thomma, B.P.H.J.; Ploetz, R.C.; Kema, G.H.J. Worse Comes to Worst: Bananas and Panama Disease—When Plant and Pathogen Clones Meet. PLoS Pathog. 2015, 11, e1005197. [Google Scholar] [CrossRef] [PubMed]
- Instituto Canario de Estadística. ISTAC. Available online: https://www.gobiernodecanarias.org/istac/ (accessed on 22 March 2024).
- Food and Agriculture Organization of the United Nations (FAO). Market Review Preliminary Results 2023. Major Trop. Fruits 2023, 15, 4. [Google Scholar]
- Ploetz, R.C. Management of Fusarium Wilt of Banana: A Review with Special Reference to Tropical Race 4. Crop Prot. 2015, 73, 7–15. [Google Scholar] [CrossRef]
- Waite, B.H.; Stover, R.H. Studies on Fusarium Wilt of Bananas: VI. variability and the cultivar concept in Fusarium oxysporum f. cubense. Can. J. Bot. 1960, 38, 985–994. [Google Scholar] [CrossRef]
- Su, H.; Hwang, S.; Ko, W. Fusarial Wilt of Cavendish Bananas in Taiwan. Plant Dis. 1986, 70, 814. [Google Scholar]
- Ploetz, R.C. Fusarium Wilt of Banana. Phytopathology 2015, 105, 1512–1521. [Google Scholar] [CrossRef]
- Perera González, S.; Brito López, P.; Hernández, D.; Laich, F.; de Rosa, S.F. Study on Panama Disease Caused Fusarium oxysporum f. sp. cubense in Banana Crops of Tenerife; GMR Canarias: Canary Islands, Spain, 2023. [Google Scholar]
- Bubici, G.; Kaushal, M.; Prigigallo, M.I.; Cabanás, C.G.L.; Mercado-Blanco, J. Biological Control Agents against Fusarium Wilt of Banana. Front. Microbiol. 2019, 10, 616. [Google Scholar] [CrossRef]
- Butler, B.Y.D. Florida Forecasts. Nature 2013, 504, 195–196. [Google Scholar] [CrossRef]
- Guo, G.; Wang, B.; Ma, W.; Li, X.; Yang, X.; Zhu, C.; Ming, J.; Zeng, H. Biocontrol of Fusarium Wilt of Banana: Key Influence Factors and Strategies. Afr. J. Microbiol. Res. 2013, 7, 4835–4843. [Google Scholar] [CrossRef]
- Benítez, T.; Rincón, A.M.; Limón, M.C.; Codón, A.C. Biocontrol Mechanisms of Trichoderma Strains. Int. Microbiol. 2004, 7, 249–260. [Google Scholar]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A Multipurpose, Plant-Beneficial Microorganism for Eco-Sustainable Agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Reithner, B.; Brunner, K.; Schuhmacher, R.; Peissl, I.; Seidl, V.; Krska, R.; Zeilinger, S. The G Protein α Subunit Tga1 of Trichoderma Atroviride Is Involved in Chitinase Formation and Differential Production of Antifungal Metabolites. Fungal Genet. Biol. 2005, 42, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Mendoza-Mendoza, A.; Zeilinger, S.; Horwitz, B.A. Mycoparasitism as a Mechanism of Trichoderma-Mediated Suppression of Plant Diseases. Fungal Biol. Rev. 2022, 39, 15–33. [Google Scholar] [CrossRef]
- Cai, F.; Druzhinina, I.S. In Honor of John Bissett: Authoritative Guidelines on Molecular Identification of Trichoderma; Springer: Cham, Switzerland, 2021; Volume 107. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma Species-Opportunistic, Avirulent Plant Symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Pozo, M.J.; Baek, J.M.; García, J.M.; Kenerley, C.M. Functional Analysis of Tvsp1, a Serine Protease-Encoding Gene in the Biocontrol Agent Trichoderma virens. Fungal Genet. Biol. 2004, 41, 336–348. [Google Scholar] [CrossRef]
- Geremia, R.A.; Goldman, G.H.; Jacobs, D.; Ardrtes, W.; Vila, S.B.; Van Montagu, M.; Herrera-Estrella, A. Molecular Characterization of the Proteinase-encoding Gene, Prb1, Related to Mycoparasitism by Trichoderma harzianum. Mol. Microbiol. 1993, 8, 603–613. [Google Scholar] [CrossRef]
- Izzati, N.A.M.Z.; Maryam, S.S.S.A.R.; Azwady, N.A.A. Suppression of Fusarium Wilt of Banana with an Application of Trichoderma asperellum Inoculants. IOP Conf. Ser. Earth Environ. Sci. 2019, 260, 012119. [Google Scholar] [CrossRef]
- Damodaran, T.; Rajan, S.; Muthukumar, M.; Gopal, R.; Yadav, K.; Kumar, S.; Ahmad, I.; Kumari, N.; Mishra, V.K.; Jha, S.K. Biological Management of Banana Fusarium Wilt Caused by Fusarium oxysporum f. sp. cubense Tropical Race 4 Using Antagonistic Fungal Isolate CSR-T-3 (Trichoderma reesei). Front. Microbiol. 2020, 11, 595845. [Google Scholar] [CrossRef]
- Damodaran, T.; Mishra, M.; Muthukumar, M.; Rajan, S.; Yadav, K.; Kumar, A.; Debnath, P.; Kumari, S.; Bora, P.; Gopal, R.; et al. Secondary Metabolite Induced Tolerance to Fusarium oxysporum f. sp. cubense TR4 in Banana Cv. Grand Naine through in Vitro Bio-Immunization: A Prospective Research Translation from Induction to Field Tolerance. Front. Microbiol. 2023, 14, 1233469. [Google Scholar] [CrossRef]
- Thangavelu, R.; Gopi, M. Combined Application of Native Trichoderma Isolates Possessing Multiple Functions for the Control of Fusarium Wilt Disease in Banana Cv. Grand Naine. Biocontrol Sci. Technol. 2015, 25, 1147–1164. [Google Scholar] [CrossRef]
- Thangavelu, R.; Palaniswami, A.; Velazhahan, R. Mass Production of Trichoderma harzianum for Managing Fusarium Wilt of Banana. Agric. Ecosyst. Environ. 2004, 103, 259–263. [Google Scholar] [CrossRef]
- Galarza, L.; Akagi, Y.; Takao, K.; Kim, C.S.; Maekawa, N.; Itai, A.; Peralta, E.; Santos, E.; Kodama, M. Characterization of Trichoderma Species Isolated in Ecuador and Their Antagonistic Activities against Phytopathogenic Fungi from Ecuador and Japan. J. Gen. Plant Pathol. 2015, 81, 201–210. [Google Scholar] [CrossRef]
- Taribuka, J.; Wibowo, A.; M Widyastuti, S.; Sumardiyono, C. Potency of Six Isolates of Biocontrol Agents Endophytic Trichoderma against Fusarium Wilt on Banana. J. Degrad. Min. Lands Manag. 2017, 4, 723–731. [Google Scholar] [CrossRef]
- Long, W.; Chen, Y.; Wei, Y.; Feng, J.; Zhou, D.; Cai, B.; Qi, D.; Zhang, M.; Zhao, Y.; Li, K.; et al. A Newly Isolated Trichoderma parareesei N4-3 Exhibiting a Biocontrol Potential for Banana Fusarium Wilt by Hyperparasitism. Front. Plant Sci. 2023, 14, 1289959. [Google Scholar] [CrossRef] [PubMed]
- Correa-Delgado, R.; Brito-López, P.; Jaizme Vega, M.C.; Laich, F. Biodiversity of Trichoderma Species of Healthy and Fusarium Wilt-Infected Banana Rhizosphere Soils in Tenerife (Canary Islands, Spain). Front. Microbiol. 2024, 15, 1376602. [Google Scholar] [CrossRef]
- Maryani, N.; Lombard, L.; Poerba, Y.S.; Subandiyah, S.; Crous, P.W.; Kema, G.H.J. Phylogeny and Genetic Diversity of the Banana Fusarium Wilt Pathogen Fusarium oxysporum f. sp. cubense in the Indonesian Centre of Origin. Stud. Mycol. 2019, 92, 155–194. [Google Scholar] [CrossRef]
- Cardoza, R.E.; Malmierca, M.G.; Hermosa, M.R.; Alexander, N.J.; McCormick, S.P.; Proctor, R.H.; Tijerino, A.M.; Rumbero, A.; Monte, E.; Gutiérrez, S. Identification of Loci and Functional Characterization of Trichothecene Biosynthesis Genes in Filamentous Fungi of the Genus Trichoderma. Appl. Environ. Microbiol. 2011, 77, 4867–4877. [Google Scholar] [CrossRef]
- Mayo, S.; Gutiérrez, S.; Malmierca, M.G.; Lorenzana, A.; Campelo, M.P.; Hermosa, R.; Casquero, P.A. Influence of Rhizoctonia solani and Trichoderma spp. in Growth of Bean (Phaseolus Vulgaris L.) and in the Induction of Plant Defense-Related Genes. Front. Plant Sci. 2015, 6, 00685. [Google Scholar] [CrossRef]
- Blakeslee, A. Lindner’s Roll Tube Method of Separation Cultures. Phytopathology 1915, 5, 68–69. [Google Scholar]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Stanke, M.; Keller, O.; Gunduz, I.; Hayes, A.; Waack, S.; Morgenstern, B. AUGUSTUS: Ab Initio Prediction of Alternative Transcripts. Nucleic Acids Res. 2006, 34, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Hoff, K.J.; Stanke, M. WebAUGUSTUS—A Web Service for Training AUGUSTUS and Predicting Genes in Eukaryotes. Nucleic Acids Res. 2013, 41, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A Universal Tool for Annotation, Visualization and Analysis in Functional Genomics Research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Medema, M.H.; Blin, K.; Cimermancic, P.; De Jager, V.; Zakrzewski, P.; Fischbach, M.A.; Weber, T.; Takano, E.; Breitling, R. AntiSMASH: Rapid Identification, Annotation and Analysis of Secondary Metabolite Biosynthesis Gene Clusters in Bacterial and Fungal Genome Sequences. Nucleic Acids Res. 2011, 39, 339–346. [Google Scholar] [CrossRef]
- Gutiérrez, S.; McCormick, S.P.; Cardoza, R.E.; Kim, H.S.; Yugueros, L.L.; Vaughan, M.M.; Carro-Huerga, G.; Busman, M.; Sáenz de Miera, L.E.; Jaklitsch, W.M.; et al. Distribution, Function, and Evolution of a Gene Essential for Trichothecene Toxin Biosynthesis in Trichoderma. Front. Microbiol. 2021, 12, 791641. [Google Scholar] [CrossRef]
- Kumar, S.; Shukla, V.; Dubey, M.K.; Upadhyay, R.S. Activation of Defense Response in Common Bean against Stem Rot Disease Triggered by Trichoderma erinaceum and Trichoderma viride. J. Basic Microbiol. 2021, 61, 910–922. [Google Scholar] [CrossRef]
- Vaidya, G.; Lohman, D.; Rudolf, M. SequenceMatrix: Concatenation Software for the Fast Assembly of Multi-gene Datasets with Character Set and Codon Information. Cladistics 2011, 27, 171–180. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Minh, B.Q.; Hahn, M.W.; Lanfear, R. New Methods to Calculate Concordance Factors for Phylogenomic Datasets. Mol. Biol. Evol. 2020, 37, 2727–2733. [Google Scholar] [CrossRef]
- Jaklitsch, W.M.; Voglmayr, H. Biodiversity of Trichoderma (Hypocreaceae) in Southern Europe and Macaronesia. Stud. Mycol. 2015, 80, 1–87. [Google Scholar] [CrossRef]
- Malmierca, M.G.; Cardoza, R.E.; Alexander, N.J.; McCormick, S.P.; Collado, I.G.; Hermosa, R.; Monte, E.; Gutiérrez, S. Relevance of Trichothecenes in Fungal Physiology: Disruption of Tri5 in Trichoderma arundinaceum. Fungal Genet. Biol. 2013, 53, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Kubicek, C.P.; Steindorff, A.S.; Chenthamara, K.; Manganiello, G.; Henrissat, B.; Zhang, J.; Cai, F.; Kopchinskiy, A.G.; Kubicek, E.M.; Kuo, A.; et al. Evolution and Comparative Genomics of the Most Common Trichoderma Species. BMC Genom. 2019, 20, 485. [Google Scholar] [CrossRef] [PubMed]
- Papavizas, G.C. Trichoderma and Gliocladium: Biology, Ecology, and Potential for Biocontrol. Annu. Rev. Phytopathol. 1985, 23, 23–54. [Google Scholar] [CrossRef]
- Gams, W.; Bissett, J. Secondary Metabolism in Trichoderma and Gliocladium. In Trichoderma and Gliocladium; CRC Press: London, UK, 1998; Volume 1, pp. 153–206. [Google Scholar]
- Marra, R.; Gutiérrez, S.; Woo, S.L.; Bonanomi, G.; Vinale, F. Editorial: Designing Bio-Formulations Based on Organic Amendments, Beneficial Microbes and Their Metabolites. Front. Microbiol. 2022, 12, 832149. [Google Scholar] [CrossRef]
- Poveda, J. Trichoderma as Biocontrol Agent against Pests: New Uses for a Mycoparasite. Biol. Control 2021, 159, 104634. [Google Scholar] [CrossRef]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-Based Products and Their Widespread Use in Agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef]
- Álvarez-García, S.; Mayo-Prieto, S.; Gutiérrez, S.; Casquero, P.A. Self-Inhibitory Activity of Trichoderma Soluble Metabolites and Their Antifungal Effects on Fusarium oxysporum. J. Fungi 2020, 6, 176. [Google Scholar] [CrossRef]
- Ruano-Rosa, D.; del Moral-Navarrete, L.; Lopez-Herrera, C.J. Selección de Aislados de Trichoderma spp. Antagonistas a Rosellinia necatrix. Span. J. Agric. Res. 2010, 8, 1084–1097. [Google Scholar] [CrossRef]
- Cardoza, R.E.; Mayo-Prieto, S.; Martínez-Reyes, N.; McCormick, S.P.; Carro-Huerga, G.; Campelo, M.P.; Rodríguez-González, Á.; Lorenzana, A.; Proctor, R.H.; Casquero, P.A.; et al. Effects of Trichothecene Production by Trichoderma arundinaceum Isolates from Bean-Field Soils on the Defense Response, Growth and Development of Bean Plants (Phaseolus Vulgaris). Front. Plant Sci. 2022, 13, 1005906. [Google Scholar] [CrossRef]
- Mayo-Prieto, S.; Campelo, M.P.; Lorenzana, A.; Rodríguez-González, A.; Reinoso, B.; Gutiérrez, S.; Casquero, P.A. Antifungal Activity and Bean Growth Promotion of Trichoderma Strains Isolated from Seed vs Soil. Eur. J. Plant Pathol. 2020, 158, 817–828. [Google Scholar] [CrossRef]
- Cardoza, R.E.; McCormick, S.P.; Izquierdo-Bueno, I.; Martínez-Reyes, N.; Lindo, L.; Brown, D.W.; Collado, I.G.; Proctor, R.H.; Gutiérrez, S. Identification of Polyketide Synthase Genes Required for Aspinolide Biosynthesis in Trichoderma arundinaceum. Appl. Microbiol. Biotechnol. 2022, 106, 7153–7171. [Google Scholar] [CrossRef] [PubMed]
- Carro-Huerga, G.; Mayo-Prieto, S.; Rodríguez-González, Á.; Cardoza, R.E.; Gutiérrez, S.; Casquero, P.A. Vineyard Management and Physicochemical Parameters of Soil Affect Native Trichoderma Populations, Sources of Biocontrol Agents against Phaeoacremonium minimum. Plants 2023, 12, 887. [Google Scholar] [CrossRef] [PubMed]
- Carrero-Carrón, I.; Trapero-Casas, J.L.; Olivares-García, C.; Monte, E.; Hermosa, R.; Jiménez-Díaz, R.M. Trichoderma asperellum Is Effective for Biocontrol of Verticillium Wilt in Olive Caused by the Defoliating Pathotype of Verticillium dahliae. Crop Prot. 2016, 88, 45–52. [Google Scholar] [CrossRef]
- Porteous-Álvarez, A.J.; Fernández-Marcos, A.; Ramírez-Lozano, D.; Mayo-Prieto, S.; Cardoza, R.E.; Gutiérrez, S.; Casquero, P.A. Native Trichoderma Isolates from Soil and Rootstock to Fusarium spp. Control and Growth Promotion of Humulus lupulus L. Plantlets. Agriculture 2023, 13, 720. [Google Scholar] [CrossRef]
- Lindo, L.; McCormick, S.P.; Cardoza, R.E.; Brown, D.W.; Kim, H.S.; Alexander, N.J.; Proctor, R.H.; Gutiérrez, S. Effect of Deletion of a Trichothecene Toxin Regulatory Gene on the Secondary Metabolism Transcriptome of the Saprotrophic Fungus Trichoderma arundinaceum. Fungal Genet. Biol. 2018, 119, 29–46. [Google Scholar] [CrossRef]
- Taghdi, Y.; Hermosa, R.; Sara, D.; Rubio, M.B.; Essalmani, H.; Nicolás, C.; Monte, E. Effectiveness of Composts and Trichoderma Strains for Control of Fusarium Wilt of Tomato. Phytopathol. Mediterr. 2015, 54, 241–252. [Google Scholar] [CrossRef]
- Anand, S.; Jayarama, R. Biocontrol Potential of Trichoderma sp. against Plant Pathogens. Int. J. Agric. Sci. 2009, 1, 30–39. [Google Scholar] [CrossRef][Green Version]
- Ivonne González, D.I.; Martínez, B.; Arias, Y.; González, N.; Miranda, I.; Peteira, B. Induction of Chitinases and Glucanases in Trichoderma spp. Biotecnol. Apl. 2012, 29, 12–16. [Google Scholar]
- Kobori, N.N.; Mascarin, G.M.; Jackson, M.A.; Schisler, D.A. Liquid Culture Production of Microsclerotia and Submerged Conidia by Trichoderma harzianum Active against Damping-off Disease Caused by Rhizoctonia solani. Fungal Biol. 2015, 119, 179–190. [Google Scholar] [CrossRef]
- Nielsen, K.F.; Gräfenhan, T.; Zafari, D.; Thrane, U. Trichothecene Production by Trichoderma brevicompactum. J. Agric. Food Chem. 2005, 53, 8190–8196. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, B.; Zhang, J.; Gan, Y. Identification of the Antifungal Activity of Trichoderma longibrachiatum T6 and Assessment of Bioactive Substances in Controlling Phytopathgens. Pestic. Biochem. Physiol. 2018, 147, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Olowe, O.M.; Nicola, L.; Asemoloye, M.D.; Akanmu, A.O.; Sobowale, A.A.; Babalola, O.O. Characterization and Antagonistic Potentials of Selected Rhizosphere Trichoderma Species against Some Fusarium Species. Front. Microbiol. 2022, 13, 985874. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Brar, S.K.; Tyagi, R.D.; Surampalli, R.Y.; Valéro, J.R. Antagonistic Fungi, Trichoderma spp.: Panoply of Biological Control. Biochem. Eng. J. 2007, 37, 1–20. [Google Scholar] [CrossRef]
- Howell, C.R. Mechanisms Employed by Trichoderma Species in the Biological Control of Plant Diseases: The History and Evolution of Current Concepts. Plant Dis. 2003, 87, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Thangavelu, R.; Mustaffa, M. A Potential Isolate of Trichoderma viride NRCB1 and Its Mass Production for the Effective Management of Fusarium Wilt Disease in Banana. Tree For. Sci. Biotechnol. 2010, 4, 76–84. [Google Scholar]
- Natsiopoulos, D.; Tziolias, A.; Lagogiannis, I.; Mantzoukas, S.; Eliopoulos, P.A. Growth-Promoting and Protective Effect of Trichoderma atrobrunneum and T. Simmonsii on Tomato against Soil-Borne Fungal Pathogens. Crops 2022, 2, 202–217. [Google Scholar] [CrossRef]
- Pavlovskaya, N.; Gneusheva, I.; Solokhina, I.; Ageeva, N. The Biological Activity of Subspecies Trichoderma harzianum against Fusarium oxysporum, the Causative Agent of Fusarium Wilt Cucumber in Vitro. BIO Web Conf. 2020, 21, 00021. [Google Scholar] [CrossRef]
- Zhang, J.; Bayram Akcapinar, G.; Atanasova, L.; Rahimi, M.J.; Przylucka, A.; Yang, D.; Kubicek, C.P.; Zhang, R.; Shen, Q.; Druzhinina, I.S. The Neutral Metallopeptidase NMP1 of Trichoderma guizhouense Is Required for Mycotrophy and Self-Defence. Environ. Microbiol. 2016, 18, 580–597. [Google Scholar] [CrossRef]
- Pang, G.; Sun, T.; Yu, Z.; Yuan, T.; Liu, W.; Zhu, H.; Gao, Q.; Yang, D.; Kubicek, C.P.; Zhang, J.; et al. Azaphilones Biosynthesis Complements the Defence Mechanism of Trichoderma guizhouense against Oxidative Stress. Environ. Microbiol. 2020, 22, 4808–4824. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Seidl-Seiboth, V.; Herrera-Estrella, A.; Horwitz, B.A.; Kenerley, C.M.; Monte, E.; Mukherjee, P.K.; Zeilinger, S.; Grigoriev, I.V.; Kubicek, C.P. Trichoderma: The Genomics of Opportunistic Success. Nat. Rev. Microbiol. 2011, 9, 749–759. [Google Scholar] [CrossRef]
- Viterbo, A.; Montero, M.; Ramot, O.; Friesem, D.; Monte, E.; Llobell, A.; Chet, I. Expression Regulation of the Endochitinase Chit36 from Trichoderma asperellum (T. harzianum T-203). Curr. Genet. 2002, 42, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Sharon, E.; Chet, I.; Viterbo, A.; Bar-Eyal, M.; Nagan, H.; Samuels, G.J.; Spiegel, Y. Parasitism of Trichoderma on Meloidogyne javanica and Role of the Gelatinous Matrix. Eur. J. Plant Pathol. 2007, 118, 247–258. [Google Scholar] [CrossRef]
- Atanasova, L.; Le Crom, S.; Gruber, S.; Coulpier, F.; Seidl-Seiboth, V.; Kubicek, C.P.; Druzhinina, I.S. Comparative Transcriptomics Reveals Different Strategies of Trichoderma Mycoparasitism. BMC Genom. 2013, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Thangavelu, R.; Varun, G.; Ganga Devi, P. Identification of Differentially Expressed Genes from Fusarium oxysporum f. sp cubense and Trichoderma asperellum (Prr2) Interaction in the Susceptible Banana Cultivar Grand Naine. Turk. J. Bot. 2016, 40, 480–487. [Google Scholar] [CrossRef]
- Anees, M.; Tronsmo, A.; Edel-Hermann, V.; Hjeljord, L.G.; Héraud, C.; Steinberg, C. Characterization of Field Isolates of Trichoderma Antagonistic against Rhizoctonia solani. Fungal Biol. 2010, 114, 691–701. [Google Scholar] [CrossRef]
- Anjum, N.; Shahid, A.A.; Iftikhar, S.; Mubeen, M.; Ahmad, M.H.; Jamil, Y.; Nasrullah Rehan, M.K.; Aziz, A.; Iqbal, S.; Abbas, A. Evaluations of Trichoderma Isolates for Biological Control of Fusarium Wilt of Chili. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 42–57. [Google Scholar]
- Błaszczyk, L.; Basińska-Barczak, A.; Ćwiek-Kupczyńska, H.; Gromadzka, K.; Popiel, D.; Stępień, Ł. Supressive Effect of Trichoderma spp. on Toxigenic Fusarium Species. Pol. J. Microbiol. 2017, 66, 85–100. [Google Scholar] [CrossRef]
- Qualhato, T.F.; Lopes, F.A.C.; Steindorff, A.S.; Brandão, R.S.; Jesuino, R.S.A.; Ulhoa, C.J. Mycoparasitism Studies of Trichoderma Species against Three Phytopathogenic Fungi: Evaluation of Antagonism and Hydrolytic Enzyme Production. Biotechnol. Lett. 2013, 35, 1461–1468. [Google Scholar] [CrossRef]
- Fanelli, F.; Liuzzi, V.C.; Logrieco, A.F.; Altomare, C. Genomic Characterization of Trichoderma atrobrunneum (T. harzianum Species Complex) ITEM 908: Insight into the Genetic Endowment of a Multi-Target Biocontrol Strain. BMC Genom. 2018, 19, 662. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).