Abstract
Fragrant rice has high market value and is popular among consumers because of its pleasant fragrance. Plant growth regulators and trace elements can influence the yield and physiological indices of fragrant rice. Riboflavin, a vital component of plant vitamins, plays a crucial role in plant growth and development, and it is a water-soluble vitamin that is essential for sustaining normal photosynthesis and metabolic processes. However, the effects of riboflavin on nutrient accumulation and yield formation in fragrant rice have rarely been reported. Therefore, to further increase the yield and quality of fragrant rice, this study investigated the impact of the foliar application of riboflavin at different growth stages on nutrient accumulation and yield formation in two different genotypes of fragrant rice via a pot experiment. The experimental design consisted of three treatment groups, i.e., the T0 treatment group (control), which was sprayed with water; the T1 treatment group, which was sprayed at the booting stage; and the T2 treatment group, which was sprayed at the flowering stage; in all of these groups, 20 mg·L−1 riboflavin solution was used. The results revealed that the yields of the T1 and T2 treatments increased by 6.56–7.25% and 10.52–13.80%, respectively, compared with those of T0, which was attributed mainly to the increase in 1000-grain weight and grain filling. Furthermore, foliar spraying of riboflavin significantly increased the chlorophyll content, which resulted in the increased production of more total starch, soluble sugars, and sucrose and facilitated their transportation to the seeds. Moreover, applying riboflavin directly to the leaves increased the activity levels of the sucrose synthase (SS) and sucrose phosphate synthase (SPS) enzymes. Among the three treatments, the T2 treatment had the most pronounced effect. The results revealed that the foliar application of riboflavin could increase photosynthesis and promote the production of nonstructural carbohydrates, thereby increasing the total aboveground biomass of fragrant rice by 17.00–20.91% and 21.07–72.91% under the T1 and T2 treatments, respectively. Additionally, spraying riboflavin promoted rapid increases in the weight of fragrant rice seeds. In conclusion, foliar spraying of riboflavin during the flowering stage of aromatic rice can effectively enhance photosynthetic accumulation and yield, which is a promising physiological regulation strategy and provides new theoretical guidance for high-yield cultivation practices.
1. Introduction
Fragrant rice is a valuable rice variety that is highly popular among consumers on the market because of its unique rich fragrance, excellent taste, and rich nutritional value [1,2]. However, with the global population expected to reach 9.7 billion in 2050, the demand for food is expected to double [3], which calls for a significant increase in crop yields to meet this challenge. In addition, as the standard of living improves, people’s dietary habits shift, with an increasing tendency to choose foods that are richer in protein [4]. In this context, fragrant rice is not only favored for its superior eating quality, but also as an important criterion for consumer choice because of its potential health and nutritional benefits. Therefore, enhancing the yield, safety, and health of fragrant rice has become a critical issue that needs to be urgently addressed in the rice industry, which is important for meeting future food demands and enhancing consumer well-being.
Extensive research has consistently demonstrated that the ultimate grain yield and quality of rice are primarily influenced by the grain-filling process, particularly the biosynthetic pathways and accumulation mechanisms of starch [5]. The products of rice photosynthesis are transported from the source organs to the seed grain in the form of sucrose and undergo a series of enzymatic reactions to form starch [6]. In rice grains, sucrose is initially degraded by sucrose–phosphate synthase (SPS) and sucrose synthase (SuSy) into glucose, fructose, and uridine diphosphate glucose (UDP-glucose). These sugars are subsequently converted into glucose 1-phosphate by the action of hexokinase, fructokinase, and phosphoglucose mutase, which then enter the amyloplast. There, adenosine diphosphate glucose pyrophosphorylase (ADP-glucose PPase) catalyzes the formation of ADP-glucose as a donor substrate. Finally, under the influence of starch synthase, starch-branching enzymes, etc., amylose and amylopectin are synthesized, culminating in the formation of starch granules [5,7,8]. The strengths and weaknesses of these substances and enzyme activities all play a role in regulating the rate of grain filling, the amount of starch accumulation, the grain weight, and the rice quality, thus affecting the final grain yield of rice [9,10]. Moreover, poor development of amyloplasts and proteins in the rice endosperm can lead to an increase in the gap between starch grains, resulting in an increase in chalkiness and an overall decrease in rice quality [11,12].
Furthermore, the increased carbohydrate content in leaves facilitates the transport of soluble sugars from the leaves to the grains, which reduces the soluble sugar content, increases the total starch content, accelerates the development of the length and width of the grains, and increases the water content within the grains, thereby effectively mitigating the source–sink imbalance [13]. Vitamins are essential dietary components in the diets of humans and animals, are synthesized by many photosynthesizing plants and bacteria, and act as indispensable cofactors in numerous metabolic pathways within plants. The synthesis of vitamins by plants and their production by microorganisms in the rhizosphere suggest that vitamins mediate interactions between plant growth and the rhizosphere environment [14,15]. Riboflavin (IUPAC name: 7,8-dimethyl-10-ribitylisoalloxazine, molecular formula: C17H20N4O6), also referred to as vitamin B2 (vitamin B2), which can be biosynthesized by many plants, microorganisms, and yeasts, is a hydrosoluble and thermostable vitamin [16,17]. Riboflavin has attracted considerable research interest because it is a key component in the formation of flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD). These are essential cofactors that participate in the fundamental energy metabolism of cells, particularly within the electron transport chain [18]. Riboflavin also acts as a photosensitizer, playing a significant role in electron transfer processes during photosynthesis. It aids in the transfer of electrons during light-dependent reactions, leading to the production of ATP and NADPH, which are crucial energy carriers for the dark reactions of photosynthesis. Furthermore, riboflavin is a vital component in the synthesis of photosynthetic pigments, such as chlorophyll and carotenoids, which are responsible for capturing light energy and promoting photosynthesis. Enhanced photosynthesis, in turn, can lead to increased crop yields and improved rice quality [19]. Research has demonstrated that riboflavin has strong antioxidant capabilities and is capable of scavenging free radicals and reducing oxidative stress in rice plants, thereby increasing their stress and pathogen resistance [20]. Cheng et al. demonstrated that exogenous riboflavin application sustains enzymatic activity, regulates metabolic pathways, and increases the content of 2-acyl-1-pyridinium (2-AP) in fragrant rice to improve its yield [17]. Riboflavin is critically essential for in sustaining the equilibrium of reactive oxygen species (ROS) and in nurturing chloroplast development within rice, and it also plays a key role in the regulation of grain filling in maize, a process which is harmonized with mitochondrial energy metabolism and cell cycle control [21,22]. In addition to its role in metabolism, riboflavin, recognized as a vital coenzyme, acts as a bioactivator that regulates the expression of various genes involved in plant growth and developmental pathways, thereby triggering a series of molecular reactions in plant growth signal transduction pathways and significantly enhancing plant growth and development [23].
However, few studies have reported the effects of riboflavin on yield and photosynthate accumulation in fragrant rice. In this study, two representative fragrant rice varieties (Nanjingxiangzhan and Meixiangzhan 2) were selected to analyze the yield, yield composition, chlorophyll content, related enzyme activities, and nutrient accumulation of fragrant rice after two different periods of foliar spraying with riboflavin at a concentration of 20 mg·L−1. Aims: (1) to determine the effects of riboflavin on the yield and yield-related enzyme activities of fragrant rice; (2) to assess the effects of the photosynthesis rate, nutrient synthesis, and transport in fragrant rice; and (3) to determine the optimal spraying period to promote nutrient accumulation and improve yield, provide practical technical guidance for agricultural production, and provide a new theoretical basis for the cultivation of high-quality and high-yield fragrant rice.
2. Materials and Methods
Research was conducted on rice (Oryza sativa L.) at Yulin Normal University in Yulin, Guangxi, China, from July to November 2023. The region has a subtropical monsoon climate, and the average monthly temperature and precipitation for rice growth are shown in Figure 1. The experimental soil was a sandy loam, and the characteristics of the soil before the experiment were as follows: pH 6.45; total nitrogen 1.84 g·kg−1; total phosphorus 1.16 g·kg−1; total potassium 22.54 g·kg−1; and organic matter 21.27 g·kg−1.
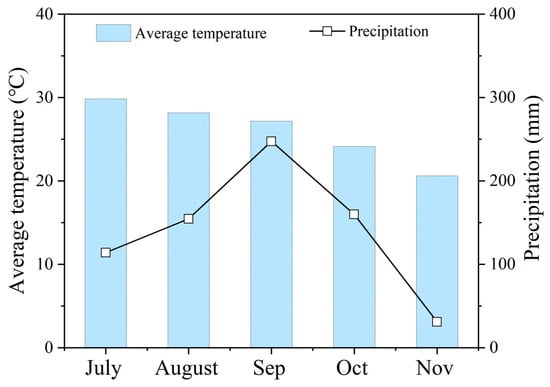
Figure 1.
Average monthly temperature and precipitation in 2023.
2.1. Experimental Design and Management
Two fragrant rice varieties, Nanjingxiangzhan and Meixiangzhan 2, which are widely cultivated in southern China, were selected. These varieties, developed by the Institute of Agricultural Science of Zhaoqing City, Guangdong, China, have mean fertility periods of 117 and 113 days in late-season rice, respectively. The seeds were subjected to sterilization via a 20% hydrogen peroxide solution (H2O2) for 20 min, after which they were carefully washed three times with ultrapure water. These seeds were subsequently soaked for 24 h before sowing. The plants were then transferred to an artificial incubator for a 12 h germination period. After successful germination, the seeds were sown in polyvinyl chloride (PVC) pots for further cultivation. At 15 days of age, the rice seedlings were transplanted into plastic pots containing 12 kg of dry soil and planted in 4 hills per pot. In this study, rice seeds were sown on 15 July and transplanted into pots on 30 July; the rice plants reached the booting and flowering stages on 20 September and 10 October, respectively; and they reached maturity and were harvested on 5 November.
In this study, foliar spraying of riboflavin at 20 mg·L−1 was carried out at both the pregnant booting and flowering stages according to the results of Cheng et al. [18]. The pot experiment was designed with a completely randomized layout. Three treatments were designed, i.e., (T0) spraying equal amounts of clear water; (T1) spraying riboflavin at the booting stage; and (T2) spraying riboflavin at the flowering stage. Each treatment was repeated four times for a total of 20 pots per treatment. To ensure the accuracy and reliability of the study results, the pots for each treatment were arranged in 5 columns with 4 pots each, maintaining a 50 cm interval between columns. Notably, foliar spraying was applied at nightfall on sunny days in order to ensure adequate uptake of riboflavin and to prevent photosensitization reactions. Other agronomic measures, such as water management and pest control, were carried out according to local provincial integrated management practices.
2.2. Sampling and Measurement
During the early, mid-grain filling, and maturity stages of rice growth, fresh leaves and seeds of rice plants in 2 hills per pot were collected from 4 pots of each treatment and stored at −80 °C to be used for physiological and biochemical analyses. These samples were used to determine physiological indices such as soluble sugars, sucrose content, total starch content, and the activity of sucrose–phosphate synthase (SPS) as well as sucrose synthase (SS).
2.2.1. Determination of Dry Matter Accumulation and the Grain Filling Rate
During the early grain filling, mid-grain filling, and maturity stages of rice growth, eight representative rice plants were selected from four pots for each treatment. Two hills were selected from each pot to determine dry matter accumulation, and the remaining two hills were used for physiological index determination. All the plant samples were dried at 105 °C for 30 min and then dried to a constant weight at 70 °C, after which the total dry matter of the aboveground parts was weighed.
Post riboflavin spray treatment, from the beginning of flowering to the maturation phase, ten uniformly vigorous panicles from each treatment were randomly sampled every four days. These panicles were dried at 70 °C for 72 h until they reached a constant weight, after which their dry weight was recorded. The panicles were then dehusked and reweighed to track the progress of grain weight gain. In accordance with the methods of Zhu et al. [24], Richard’s method was used to fit the grain-filling process and calculate the corresponding seed-filling characteristic parameters. The dynamic simulation diagram of seed weight gain after flowering was synthesized by comparing the difference in seed weight at different days after flowering with the number of days of seed filling. With seed weight W as the dependent variable and the number of days after flowering as the independent variable (the day of flowering was set as 0), the Richards equation was fitted via the nonlinear least squares method:
where W represents the weight of the seed grain (mg) in each sampling period; t represents the number of days after flowering (d); A, B, N, and k are equation parameters; A represents the maximum grain weight; and k is closely related to the grain filling rate.
2.2.2. Determination of Chlorophyll and Carotenoid Contents
Fresh leaves (0.2 g) were ground into powder and placed into a 25 mL graduated test tube, 10 mL of 80% acetone was added, the mixture was shaken well, and the mixture was extracted for 24 h away from light until all the leaves lost their green color. Afterwards, 200 μm of the extract was removed, and the absorbance values were measured at 645 nm, 652 nm, and 663 nm, with 80% acetone as the control, to calculate the contents of chlorophyll a, chlorophyll b, and carotenoids [25].
2.2.3. Determination of Related Enzymatic Activities
The enzyme activities of sucrose synthase (SS) and sucrose phosphate synthase (SPS) were determined according to the methods of Nakamura et al. [26].
2.2.4. Determination of Grain Yield and Its Components
The yield and yield components were determined according to the methods of Dai et al. [27]. At maturity, the number of panicles per hill was counted and averaged for 30 representative rice plants from each treatment. From each treatment, the rice grains were collected by hand from six pots and sun-dried to a constant weight (with a moisture content of approximately 14%), and the yield was calculated as grams per pot. Furthermore, the number of grains per panicle, the weight of 1000 grains, and the grain filling rate were determined. The moisture content of the rice was determined via a grain moisture meter (LDS-1H, Zhejiang Top Cloud Agriculture Technology Co., Ltd., Hangzhou, China).
2.2.5. Determination of Carbohydrate Contents
The samples were crushed and ground after the weight of dry matter was determined, and each treatment was bagged separately. The soluble sugar, sucrose, and starch contents were subsequently determined. The soluble sugar and sucrose contents were determined via anthrone colorimetry and resorcinol colorimetry [28]. The seeds of the yield samples were dried at room temperature to 14% moisture and then hulled, and the content of straight-chain starch was determined according to the method of [25].
2.2.6. Statistical Analysis
In this study, the collection and analysis of experimental data were conducted via Microsoft Excel 2010 and SPSS 26 software. Two-way analysis of variance (ANOVA) was performed on the treatment groups for each rice variety to assess the impact of different treatments on rice growth. The least significant difference (LSD) test was applied at a significance level of p < 0.05 to compare differences between groups. Additionally, detailed charts and graphs were created via Origin 2023 and SigmaPlot 12.5 software to provide an intuitive presentation of the experimental results, ensuring the accuracy and visual clarity of the statistical outcomes.
3. Results
3.1. Yield and Its Components
The grain yields and components of fragrant rice subjected to different treatments are shown in Table 1. Foliar spraying of riboflavin at the booting and flowering stages increased the grain yield of both rice varieties by increasing the grain filling and 1000-grain weight of both varieties; in particular, spraying riboflavin at the flowering stage significantly increased the yield of both varieties. Compared with the T0 treatment, Meixiangzhan 2 resulted in increases of 4.33%, 0.06%, and 7.26% in terms of grain filling, 1000-grain weight and grain yield, respectively, under the T1 treatment. Among them, the increases in grain filling, 1000-grain weight, and yield were more significant, increasing significantly, by 4.97%, 2.79%, and 13.80%, respectively, under the T2 treatment. Under the T1 treatment, the grain filling, 1000-grain weight. and grain yield of Nanjingxiangzhan increased by 4.70%, 0.25%, and 6.57%, respectively, compared with those under the T0 treatment, whereas the increase in grain filling was significant and significantly increased by 4.86%, 3.84%, and 10.52%, respectively, under the T2 treatment. However, spraying riboflavin did not significantly affect the number of panicle per hills and grains number per panicle in either rice variety.

Table 1.
Effect of riboflavin spraying for different periods on yield and yield components (n = 4).
3.2. Total Aboveground Biomass Accumulation
The total aboveground biomass (TAB) of the two fragrant rice varieties, Meixiangzhan 2 and Nanjingxiangzhan, under different treatment conditions is shown in Figure 2. Foliar spraying of riboflavin at both the booting and flowering stages affected the total aboveground biomass of the two varieties, especially spraying at the flowering stage (T2 treatment), which had the greatest effect. Compared with that in the T0 treatment, the biomass accumulation of Meixiangzhan 2 increased by 20.91%, 17.00%, and 18.52% in the three periods under the T1 treatment and 41.82%, 48.89%, and 72.91% under the T2 treatment, respectively, with the T2 treatment resulting in the most significant increase in biomass accumulation in the MG and MS periods. The biomass accumulation of Nanjingxiangzhan also increased by 21.07%, 30.16%, and 30.48% under the T2 treatment compared with that under the T0 treatment, with the exception of the MS period. No significant effect was observed in any of the three periods except for the T0 treatment, which improved by 30.48 compared with the T0 treatment.
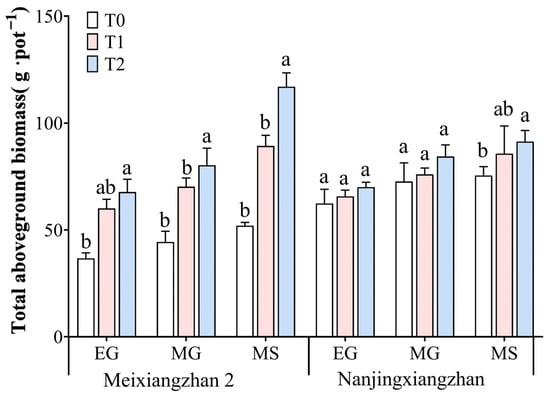
Figure 2.
Effect of spraying riboflavin on the total aboveground biomass of fragrant rice at different periods. Different lowercase letters above the bars denote statistically significant differences among treatments at the level of p < 0.05 (n = 4). In each column, means followed by the same letters are not significantly different according to LSD. EG: early grain filling stage; MG: mid-grain filling stage; MS: maturity stage.
3.3. Photosynthetic Pigment Content
The chlorophyll content in rice leaves under different treatments is depicted in Figure 3. As grain filling progresses, the levels of chlorophyll a, chlorophyll b, and carotenoids decrease gradually. The application of riboflavin during the booting and flowering stages increased the chlorophyll a, chlorophyll b, and carotenoid contents in both rice varieties, with particularly significant increases during the flowering stage. Compared with that in the T0 treatment, the chlorophyll a content in the Meixiangzhan 2 treatment increased by 12.18%, 15.73%, and 7.07% during the early and middle grain filling and maturity stages, respectively, under the T1 treatment and significantly increased by 33.33%, 34.83%, and 14.14%, respectively, under the T2 treatment. The chlorophyll a content of Nanjingxiangzhan increased by 10.20%, 16.49%, and 10.81% under the T1 treatment and significantly increased by 31.29%, 29.90%, and 36.49% under the T2 treatment. The chlorophyll b content in the Meixiangzhan 2 variety increased by 9.37%, 9.37%, and 0.00% during the early, middle, and mature stages of grain filling, respectively, under the T1 treatment and significantly increased by 53.13%, 28.13%, and 31.25%, respectively, under the T2 treatment. The Nanjingxiangzhan variety presented increases of 23.81%, 47.83%, and 23.08% under the T1 treatment and significantly increased by 50.00%, 95.65%, and 76.92% under the T2 treatment. The carotenoid content in the Meixiangzhan 2 variety decreased by 14.29%, 9.52%, and 0.00% during the early, middle, and mature stages of grain filling, respectively, under the T1 treatment and significantly increased by 25.00%, 23.81%, and 12.50%, respectively, under the T2 treatment. The Nanjingxiangzhan variety decreased by 17.24%, 14.29%, and 0.00% under the T1 treatment and significantly increased by 31.03%, 33.33%, and 41.67% under the T2 treatment.
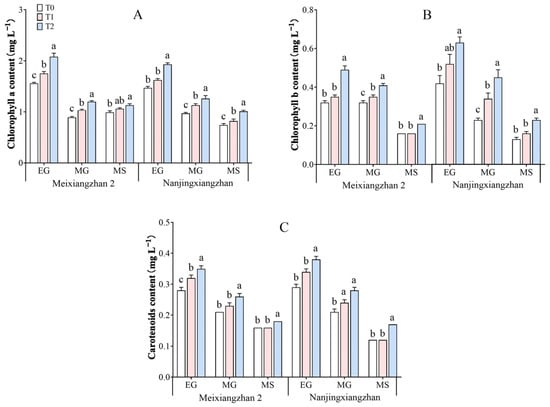
Figure 3.
Effects of spraying riboflavin on the chlorophyll a (A), chlorophyll b (B), and carotenoid (C) contents of fragrant rice at different periods. Different lowercase letters above the bars denote statistically significant differences among treatments at the level of p < 0.05 (n = 4). In each column, means followed by the same letters are not significantly different according to LSD. EG: early grain-filling stage; MG: mid-grain filling stage; MS: maturity stage.
3.4. Soluble Sugar Content
Figure 4 illustrates the soluble sugar content in both rice leaves and grains under different treatments. As the grain-filling process progresses, the soluble sugar content tends to decrease gradually. The application of riboflavin during the booting and flowering stages increased the soluble sugar content in the leaves and grains of both rice varieties, with the highest content observed during the flowering stage. Compared with those in the T0 treatment, across the three periods in the leaves, the soluble sugar content in Meixiangzhan 2 increased by 16.43–26.55% and 12.14–43.04% under the T1 and T2 treatments, respectively, and in the grains, it increased by 4.64–49.10% and 7.83–71.31%, respectively. Compared with that in the T0 treatment, across the three periods, the soluble sugar content in the leaves of Nanjingxiangzhan increased by 8.35–14.88% and 11.48–24.66% under the T1 and T2 treatments, respectively, and in the grains, it increased by 8.88–32.36% and 10.71–21.16%, respectively.
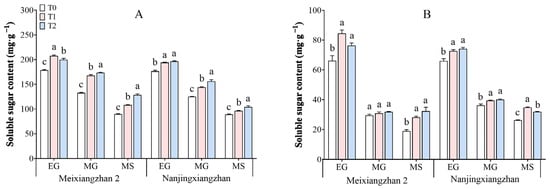
Figure 4.
Effects of spraying riboflavin at different periods on the soluble sugar content of the leaves (A) and seeds (B) of fragrant rice. Different lowercase letters above the bars denote statistically significant differences among treatments at the level of p < 0.05 (n = 4). EG: early grain filling stage; MG: mid-grain filling stage; MS: maturity stage.
3.5. Sucrose Content
As shown in detail in Figure 5, the sucrose content in the rice leaves and grains under the different treatments exhibited a pattern of initial increase and subsequent decrease in the leaves and an inverse trend in the grains. Spraying riboflavin at the booting and flowering stages increased the sucrose content of the leaves and grains of both varieties, especially at the flowering stage, when the sucrose content of sprayed riboflavin was the highest. Except for a tendency toward a decrease in the sucrose content of leaves under the T1 treatment at the early stage of grain filling, the sucrose content of Meixiangzhan 2 increased by 8.73–21.83% under T1 and by 6.57–21.67% under T2 across the three periods in the leaves, whereas it increased by 41.28–71.09% under T1 and 43.24–93.74% under T2 in the grains compared with those under T0. Compared with T0, Nanjingxiangzhan presented a similar trend, with an increase in the leaf sucrose content from 9.23–36.32% under T1 to 5.89–44.08% under T2 and from 14.52–65.75% to 5.53–41.31% in the grains.
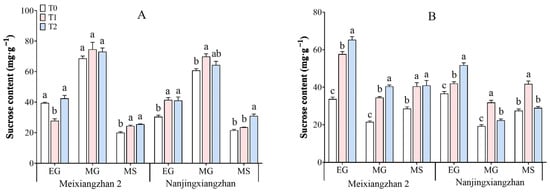
Figure 5.
Effects of spraying riboflavin at different periods on the sucrose content of leaves (A) and seeds (B) of fragrant rice. Different lowercase letters above the bars denote statistically significant differences among treatments at the level of p < 0.05 (n = 4). EG: early grain filling stage; MG: mid-grain filling stage; MS: maturity stage.
3.6. Sucrose–Phosphate Synthase (SPS) Activity
The activity of sucrose–phosphate synthase (SPS) in rice leaves and grains under different treatments is depicted in Figure 6. As the grain-filling process progresses, the activity of SPS initially increases but then decreases. The application of riboflavin during the booting stage and flowering stage increased the SPS activity in the leaves and grains of both rice varieties, with the highest activity observed during the flowering stage, which was particularly effective during the mid-grain filling stage. Compared with that in the T0 treatment, across the three periods in the leaves, the SPS activity in Meixiangzhan 2 increased by 4.75–22.34% and 8.75–28.28% under the T1 and T2 treatments, respectively, and in the grains across the three periods, it increased by 4.85–13.76% and 7.62–20.86%, respectively. Compared with that in the T0 treatment, across the three periods in the leaves, the SPS activity in Nanjingxiangzhan increased by 10.08–19.41% and 2.40–21.02% under the T1 and T2 treatments, respectively, and in the grains, it increased by 0.60–17.97% and 2.19–17.48%, respectively.
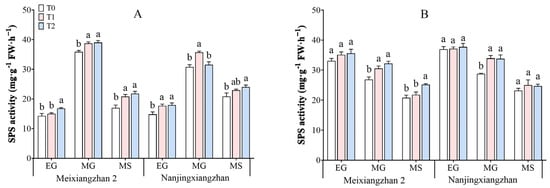
Figure 6.
Effects of spraying riboflavin on leaf (A) and seed (B) sucrose–phosphate synthase (SPS) activity in fragrant rice at different periods. Different lowercase letters above the bars denote statistically significant differences among treatments at the level of p < 0.05 (n = 4). EG: early grain filling stage; MG: mid-grain filling stage; MS: maturity stage.
3.7. Sucrose Synthase (SS) Activity
The activity of SS (sucrose synthase) in rice leaves and grains under different treatments is shown in Figure 7. As the grain-filling process progresses, the SS activity tends to first increase but then decreases. The application of riboflavin during the booting and flowering stages increased the SS activity in the leaves and grains of both rice varieties, with the greatest increase observed during the middle grain-filling stage. Compared with that in the T0 treatment, across the three periods in the leaves, the SS activity in Meixiangzhan 2 increased by 18.48–23.43% and 7.07–21.96% under the T1 and T2 treatments, respectively, and in the grains, it increased by 2.81–22.51% and 5.12–24.94%, respectively. Compared with that in the T0 treatment, across the three periods in the leaves, the SS activity in Nanjingxiangzhan increased by 6.16–30.98% and 7.06–36.30% under the T1 and T2 treatments, respectively, and in the grains, it increased by 7.57–26.68% and 13.50–17.71%, respectively.
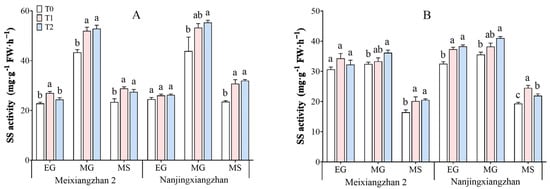
Figure 7.
Effects of spraying riboflavin on leaf (A) and seed (B) sucrose synthase (SS) activity in fragrant rice at different periods. Different lowercase letters above the bars denote statistically significant differences among treatments at the level of p < 0.05 (n = 4). EG: early grain filling stage; MG: mid-grain filling stage; MS: maturity stage.
3.8. Amylose Content
The amylose contents of the rice grains subjected to different treatments are shown in Figure 8, which continued to increase with increasing irrigation. The spraying of riboflavin at the booting and flowering stages reduced the straight-chain starch content of the two varieties, especially the lowest straight-chain starch content of the two varieties at the flowering stage. Compared with that in the T0 treatment, across the three periods, the amylose content in Meixiangzhan 2 decreased by 18.07%, 6.63%, and 4.25% during the early and middle grain filling and maturity stages, respectively, under the T1 treatment, with significant decreases in the early grain filling and maturity stages. Compared with those under T0, the amylose contents under T2 significantly decreased by 31.25%, 7.51%, and 5.57%, respectively. For Nanjingxiangzhan, under the T1 treatment, the amylose content decreased by 15.83%, 8.24%, and 2.68% during the early and middle grain filling and mature stages, respectively, with significant decreases in the early and middle grain filling stages. Under the T2 treatment, the amylose content decreased by 19.00%, 11.17%, and 3.09%, respectively, with significant decreases in the early and middle grain filling stages.
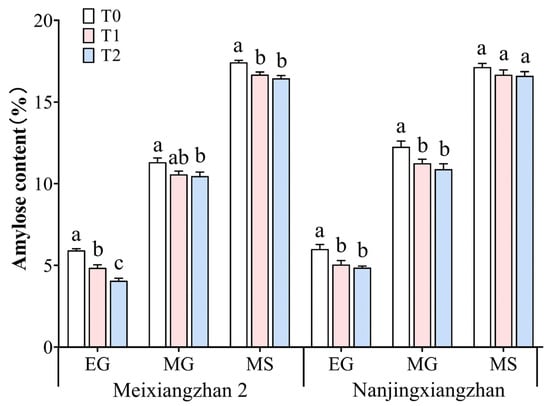
Figure 8.
Effects of spraying riboflavin on the amylose content of fragrant rice at different periods. Different lowercase letters above the bars denote statistically significant differences among treatments at the level of p < 0.05 (n = 4). EG: early grain filling stage; MG: mid-grain filling stage; MS: maturity stage.
3.9. Total Starch Content
The total starch contents of the rice leaves and grains under the different treatments are presented in Figure 9. As the grain-filling process progresses, the total starch content in leaves tends to decrease, whereas that in grains tends to increase. The application of riboflavin during the panicle development stage and flowering stage increased the total starch content in both the leaves and grains of the two rice varieties, especially at the flowering stage, when the highest total starch content was sprayed with riboflavin. Compared with that in the T0 treatment, across the three periods in the leaves, the total starch content in Meixiangzhan 2 increased by 0.36–11.98% under T1 and 12.49–22.93% under T2, and in the grains, it significantly increased by 9.62–17.08% under T1 and 11.21–17.11% under T2. Compared with the T0 treatment, during the three periods, Nanjingxiangzhan resulted in an increase in the total starch content of 6.17–12.89% under T1 and 10.85–13.19% under T2, and in the grains, the total starch content increased by 4.11–31.20% under T1 and 4.77–11.02% under T2.
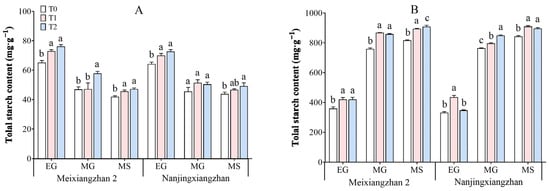
Figure 9.
Effects of spraying riboflavin on the leaf (A) and seed (B) total starch content of fragrant rice at different periods. Different lowercase letters above the bars denote statistically significant differences among treatments at the level of p < 0.05 (n = 4). EG: early grain filling stage; MG: mid-grain filling stage; MS: maturity stage.
The impact of exogenous riboflavin application at various growth stages on the yield and nutrient accumulation indices of fragrant rice was assessed using analysis of variance (ANOVA), and the significant results are presented in Table 2. The analysis revealed that both the rice varieties (V) and the treatment (T) significantly influenced the grain yield, whereas the number of productive panicles was affected primarily by the variety. The grain filling rate was significantly impacted by the treatment (T), and the 1000-grain weight was markedly influenced by both variety and treatment (V × T). Furthermore, photosynthetic characteristics, aboveground total biomass, starch content, amylose content, sucrose content, and the activities of the sucrose synthase (SS) and sucrose phosphate synthase (SPS) enzymes, which are closely related to nutrient accumulation, were also affected to varying degrees. These findings suggest that exogenous riboflavin not only affects the yield of fragrant rice but may also influence nutrient accumulation through the modulation of related physiological and biochemical processes.

Table 2.
Analysis of variance (ANOVA) of the F values of the grain yield and metrics.
3.10. Panicle and Grain Weight Dynamics
The effects of spraying riboflavin at different periods of time on the panicle and grain weight dynamics of the two varieties are shown in Figure 10. Both seed grain weight dynamics and panicle dry weight showed an increasing trend with the progression of grain filling. Compared with T0, both the T1 and T2 treatments promoted an increase in seed grain weight, which was in a rapid growth trend during the pre-filling period and then tended to level off. In addition, panicle weight was significantly higher under the riboflavin spray treatment than the T0 treatment for the same sampling time, while the variety Meixiangzhan 2 exhibited a similar pattern of dry weight increase in both T1 and T2 treatments. The overall growth trends of the three treatments in panicle and grain weight dynamics were T2 > T1 > T0.
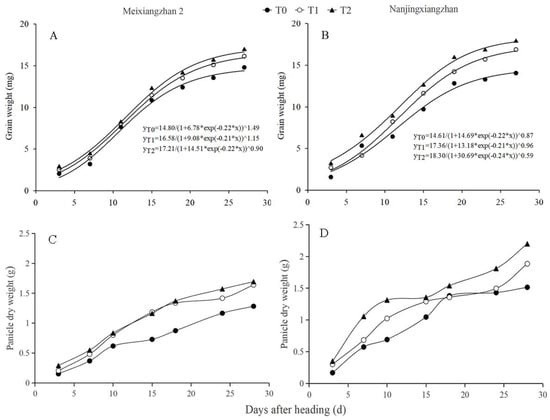
Figure 10.
Effect of spraying with riboflavin at different periods on the panicle dry weight and grain weight dynamics in fragrant rice. (A) Grain weight dynamics of Meixiangzhan 2; (B) Grain weight dynamics of Nanjingxiangzhan; (C) Panicle dry weight dynamics of Meixiangzhan 2; (D) Panicle dry weight dynamics of Nanjingxiangzhan.
3.11. Regression Analysis and Heatmaps for Various Indexes
To discern any potential variations in the study parameters across treatments, a correlation analysis was conducted, as depicted in Figure 11. This analysis revealed that grain yield was correlated positively with the grain filling, 1000-grain weight, TAB-EG, SPS-EG-L, SPS-MS-L, SPS-EG-G, SS-EG-G, SS-MG-G, Soluble Sugar—MG-G, Chl-b-EG, GDW-7d, GDW-11d, GDW-23d, and GDW-27d. Furthermore, the grain filling rate was correlated with the TAB-MG, SPS-EG-L, SPS-MG-L, SPS-MG-G, SS-MG-L, SS-MS-L, SS-MS-G, Soluble Sugar—MG-G, Total Starch—EG-L, Total Starch—MD-G, Chl-a-MG, Caro-EG, Caro-MG, GDW-3d, GDW-11d, GDW-15d, GDW-27d, 1000-grain weight—3d, 1000-grain weight—15d, 1000-grain weight—19d, 1000-grain weight—23d, and 1000-grain weight—27d.
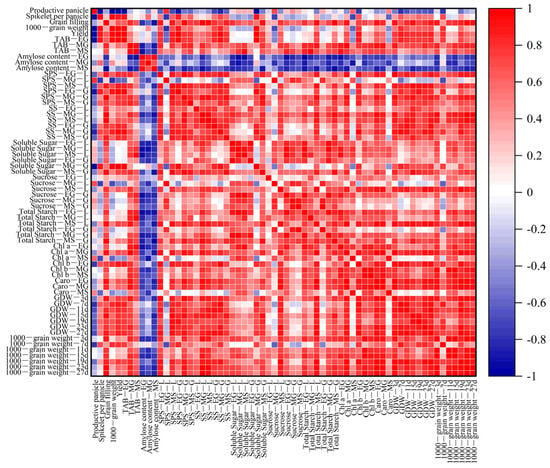
Figure 11.
Correlation analysis of the indicators. The data used for the correlation analysis were from a late-season pot trial in 2022. Red indicates a significant positive correlation, and blue indicates a significant negative correlation between two parameters, with both determined at the p < 0.05 level. L, leaves; G, seeds; EG, early grain filling stage; MG, mid-grain filling stage; MS: maturity stage. TAB, total aboveground biomass; SPS, sucrose–phosphate synthase; SS, sucrose synthase; Chl—a, chlorophyll a content; Chl—b, chlorophyll b content; Caro, carotenoid content; GDW, grain dry weight.
4. Discussion
Riboflavin, also known as vitamin B2, is named for its brassy appearance because of the functional structure of ribose [29,30]. The application of exogenous riboflavin to plants can mitigate disease development and promote many metabolic processes to improve plant disease resistance. Riboflavin can be used as a regulatory mechanism to modulate plant growth and development and the stress response by increasing the riboflavin content in plants [31,32]. Fragrant rice is a rice variety with high economic benefits, and synergistic improvement of its yield and fragrance has been the focus of fragrant rice production [33]. Previous studies have shown that exogenous spraying of riboflavin at the onset of tasseling can increase aroma and yield while increasing the resistance of aromatic rice, and the optimum concentration is approximately 20 mg·L−1 [17]. In this study, 20 mg·L−1 riboflavin was sprayed onto Meixiangzhan 2 and Nanjingxiangzhan at both the booting and flowering stages to determine the optimum period for exogenous spraying of riboflavin. The results of the present study revealed that spraying riboflavin during both periods significantly increased the yield of both varieties, which was attributed mainly to the increase in fruit set and thousand-grain weight of aromatic rice. Tian et al. [22] reported that riboflavin can increase maize yield and quality by regulating endosperm development and harmonizing mitochondrial energy metabolism and the cell cycle. In this study, exogenous spraying of riboflavin increased the fruiting rate and 1000-grain weight, mainly by increasing the level of grain filling, which is fundamentally consistent with findings from previous investigations. Moreover, since the periods of exogenous spraying of riboflavin were the booting and flowering stages, in which the differentiation of rice ears had essentially been completed, exogenous riboflavin had almost no significant effect on the effective number of productive panicles or spikelets per panicle.
As one of the key substances for plant growth and development, the chlorophyll content can affect not only the ability of plants to photosynthesize but also the strength of plant stress tolerance [34]. Previous studies have demonstrated that salt stress leads to a reduction in plant chlorophyll content, with a significant decrease observed as salt concentration increases [35,36,37]. Flooding also leads to a decrease in chlorophyll synthesis capacity, resulting in a decrease in chlorophyll content. In addition, flooding stress leads to accelerated leaf senescence and accelerated chlorophyll degradation [38]. In this study, we found that the spraying of riboflavin at both the booting and flowering stages effectively increased the chlorophyll content, and compared with the other treatments, the spraying of riboflavin at the flowering stage led to a greater chlorophyll content in the plant, and this effect continued until the maturity stage. As a substance that can increase plant stress tolerance, riboflavin can effectively maintain the balance of reactive oxygen species (ROS), which not only promotes the development of rice chloroplasts but also alleviates the degradation of chlorophyll through increased stress tolerance under adverse conditions [21], which explains why exogenous spraying of riboflavin increased the chlorophyll content in the present study. Many previous studies have demonstrated a significant positive correlation between chlorophyll content and grain yield in rice, and the contents of chlorophyll and carotenoids directly affect the net photosynthetic rate [39,40], which is consistent with our study. In addition, maintaining a high chlorophyll content during the grain filling period is the basis for strong photosynthetic capacity, which results in the production of more photosynthetic products, such as starch and sucrose, as well as improved grain filling efficiency.
Rice biomass synthesis and grain filling are pivotal in determining rice yield and quality. Photosynthesis, a crucial energy conversion process in plants, transforms light energy into chemical energy through the leaves, generating photosynthetic products, primarily sucrose, which provide the essential material basis for grain filling. These products are further converted into starch within the seed, constituting the dry matter that underpins plant growth and yield formation [41,42]. In our research, the foliar application of riboflavin led to an increase in rice photosynthetic efficiency, potentially through increasing the photosynthetic pigment content. This treatment facilitates the production of more photosynthetic products and promotes their transport from the source organ (leaves) to the sink organ (seeds), thereby increasing seed dry matter accumulation and yield. Importantly, the use of riboflavin at the flowering stage of rice notably increased the synthesis and accumulation of seed grain biomass, a response linked to increased photosynthesis, which aligns with previous findings [43,44]. The conversion efficiency of sucrose, the principal carrier of photosynthetic products, in the seed kernel during the filling process is critical for ensuring grain weight and rice quality [45]. Our study revealed that the photosynthetic efficiency of rice can be effectively enhanced by the foliar application of riboflavin, which promotes the efficient transport of photosynthetic products to the seed grain, thereby increasing dry matter accumulation and the filling rate of the seed grain. Previous studies have shown that drastic fluctuations in the filling rate may cause uneven filling of amyloplasts in the endosperm, resulting in more gaps between the seeds, thus affecting the quality of the rice and the grain weight of the seeds [46,47]. Moreover, suboptimal filling rates can increase the demand for nutrients and water, hindering efficient nutrient and water use and impeding rice growth and yield improvement [12]. Consequently, exposure to lower minimum temperatures during grain filling, likely intensified by the suboptimal stratification of canopy temperature in taller canopies, resulted in fewer grains being filled [48]. It is essential to recognize the significant effect of temperature on the grouting rate; a moderate increase can promote photosynthesis and starch formation [49], whereas excessively high temperatures may reduce photosynthetic efficiency and hinder grain filling [50]. In our study, the foliar application of riboflavin, especially at the flowering stage of rice, significantly increased the rate of seed filling, which was related to the ability of riboflavin to increase photosynthesis and promote starch synthesis. This treatment not only increased dry matter accumulation in the seeds but was also expected to increase rice yield and quality. Although the reduction in biomass during grouting may contribute to an increase in the grain-filling rate and the promotion of starch synthesis and transport [51], the precise relationship between biomass and yield needs to be further explored.
Nonstructural carbohydrates, such as soluble sugars (monosaccharides and oligosaccharides such as glucose, fructose, and sucrose) and starch, are the main products of plant photosynthesis and key components of material transport in plants [52,53]. Sucrose, the major disaccharide in plants, not only plays an important role in energy storage and transfer but is also an important osmoregulatory substance [54]. Fructose functions through providing the carbon skeleton and energy required for cell growth and protects the plant cell wall when plants are subjected to external stress [55]. Starch is a crucial metabolic substance for the growth and development of plants, serving as the primary energy source in rice seeds. In a previous study, the exogenous spraying of riboflavin on fragrant rice resulted in the transient accumulation of reactive oxygen species (ROS) and may cause transient increases in membrane lipid peroxidation and malondialdehyde (MDA) content [20]. However, these negative effects are effectively mitigated by the increased activities of antioxidant enzymes such as superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) [17]. Soluble sugars and sucrose are not only involved in plant growth and development but also respond to a variety of abiotic stresses [56]. Therefore, riboflavin, as an inducer of resistance in fragrant rice, can increase soluble sugar and sucrose contents while improving the defense of fragrant rice against oxidative stress damage, which is consistent with the results of the present study. In addition, the exogenous spraying of riboflavin led to an increase in the chlorophyll content, which increased photosynthesis, which could be one of the reasons for the increase in the content of photosynthesis products, such as soluble sugars and sucrose, during the grouting period.
Sucrose synthesis is affected by many forms of sucrose, and sucrose phosphate synthase (SPS) and sucrose synthase (SS) are enzymes necessary for starch conversion and accumulation; the former mainly promotes the synthesis of sucrose, and the latter facilitates its transportation to storage organs such as seeds [57]. In the present study, spraying riboflavin during both periods effectively increased the activities of these two enzymes in the leaves and seeds of fragrant rice. Notably, the T2 treatment resulted in greater SPS activity in leaves than did the T1 treatment, suggesting that the T2 treatment was more favorable for starch breakdown in leaves throughout the grain-filling period, leading to the synthesis of high concentrations of sucrose in the leaves of fragrant rice and facilitating its transport to the seeds for starch synthesis. In addition, compared with T1, T2 also increased the activity of SS in fragrant rice kernels more significantly, which laid the foundation for the further decomposition of sucrose transported to kernels into monosaccharides and for the synthesis of large amounts of starch in seeds during the final grain filling period. In this study, in both the leaves and the grains, the T2 treatment resulted in a greater starch content in the grains at all time points than did the T1 treatment. Compared with the T1 treatment, the chlorophyll content in the T2 treatment was greater, which indicated that this treatment resulted in greater photosynthesis and the production of more nonstructural carbohydrates in fragrant rice. In comparison with the T1 treatment, the T2 treatment resulted in greater leaf SPS activity and seed SS activity, which resulted in a greater amount of leaf sucrose being transferred to the seeds and being involved mostly in the synthesis of starch in the seeds. These findings explain the low leaf and seed sucrose contents of fragrant rice under the T2 treatment compared with those under the T1 treatment in this study, whereas the starch content at the maturity stage and the final grain yield significantly increased. Notably, although the exogenous spraying of riboflavin promoted the accumulation of total starch during the filling stage of fragrant rice, it also reduced the content of straight-chain starch. We hypothesized that riboflavin might have an inhibitory effect on GBSS (granular starch synthase), which reduces the synthesis of straight-chain starch, or that riboflavin promotes the activities of SSS (soluble starch synthase) and SBE (starch-branching enzyme), which facilitate the conversion of straight-chain starch into branched-chain starch. However, the reasons for the decrease in the content of straight-chain starch and the changes in quality caused by the decrease in the content of straight-chain starch need to be further determined and explained.
5. Conclusions
Our study revealed the effects of riboflavin on yield formation and nutrient accumulation in fragrant rice. The influence of foliar spraying with a riboflavin solution at a concentration of 20 mg·L−1 on yield components and photosynthate accumulation in two genetically distinct fragrant rice varieties, Meixiangzhan 2 and Nanjingxiangzhan, was assessed during the spike gestation and flowering stages. The results revealed that spraying with riboflavin significantly increased the grain filling, 1000-grain weight, and total starch content of fragrant rice. Riboflavin application also increased the accumulation of essential nutrients in fragrant rice, primarily because of the increase in total aboveground biomass accumulation as a result of elevated photosynthesis, as well as the concurrent increase in sucrose content, soluble sugar content, SS activity, and SPS activity. As riboflavin is vital for plant growth and development, riboflavin can stimulate nutrient accumulation and yield formation in fragrant rice. This contributes to providing a new technical tool for the high-yield and high-quality cultivation of this crop from the perspective of the sustainable development of agriculture.
Author Contributions
Conceptualization, Y.W., S.C. and H.T.; methodology, H.T.; validation, H.T.; formal analysis, Y.W., S.D. and X.L.; investigation, Y.W., X.D., X.L., S.D. and L.P.; data curation, Y.W. and L.P.; writing—original draft preparation, Y.W. and S.C.; writing—review and editing, H.T.; supervision, Y.W. and X.D.; funding acquisition, H.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Natural Science Foundation of Guangxi Province (2023GXNSFBA026307) and the Guangdong Provincial Rural Sci-tech Experts project (2024–2026, Dongping, Ruyuan, Shaoguan City).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data are presented in this article in the form of figures and tables.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sakthivel, K.; Sundaram, R.M.; Rani, N.S.; Balachandran, S.M.; Neeraja, C.N. Genetic and molecular basis of fragrance in rice. Biotechnol. Adv. 2009, 27, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Custodio, M.C.; Cuevas, R.P.; Ynion, A.G.; Velasco, M.L.; Demont, M. Rice quality: How is it defined by consumers, industry, food scientists, and geneticists? Trends Food Sci. Technol. 2019, 92, 122–137. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Department of economic and social affairs, population division. In World Population Prospects; United Nations: New York, NY, USA, 2019; Volume 2017. [Google Scholar]
- You, L.Z.; Sun, Z.L. Mapping global cropping system: Challenges, opportunities, and future perspectives. Crop Environ. 2022, 1, 68–73. [Google Scholar] [CrossRef]
- Liu, Q.H.; Cai, J.; Li, T. Key starch synthase in rice grain and its relation to grain filling and rice quality. Plant Physiol. Agric. Appl. 2006, 6, 1211–1216. (In Chinese) [Google Scholar]
- Doehlert, D.C.; Kuo, T.M.; Felker, F.C. Enzymes of sucrose and hexose metabolism in developing kernels of two inbreds of maize. Plant Physiol. 1988, 86, 1013–1019. [Google Scholar] [CrossRef]
- Ball, S.G.; van de Wal, M.H.B.J.; Visser, R.G.F. Progress in understanding the biosynthesis of amylose. Trends Plant Sci. 1998, 3, 462–467. [Google Scholar] [CrossRef]
- Seung, D. Amylose in starch: Toward an understanding of biosynthesis, structure and function. New Phytol. 2020, 228, 1490–1504. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zheng, F.Q.; Shen, G.Z.; Gao, J.P.; Snustad, D.P.; Li, M.G.; Zhang, J.L.; Hong, M.M. The amylose content in rice endosperm is related to the post-transcriptional regulation of the waxy gene. Plant J. 1995, 7, 613–622. [Google Scholar] [CrossRef]
- Liang, J.S.; Zhang, J.H.; Cao, X.Z. Grain sink strength may be related to the poor grain filling of indica-japonica (Oryza sativa) hybrids. Physiol. Plant. 2001, 112, 470–477. [Google Scholar] [CrossRef]
- Lisle, A.J.; Martin, M.; Fitzgerald, M.A. Chalky and translucent rice grains differ in starch composition and structure and cooking properties. Cereal Chem. 2000, 77, 627–632. [Google Scholar] [CrossRef]
- Ishimaru, T.; Horigane, A.K.; Ida, M.; Iwasawa, N.; Yoshida, M. Formation of grain chalkiness and changes in water distribution in developing rice caryopses grown under high-temperature stress. J. Cereal Sci. 2009, 50, 166–174. [Google Scholar] [CrossRef]
- Guo, C.C.; Yuan, X.J.; Yan, F.J.; Xiang, K.H.; Wu, Y.X.; Zhang, Q.; Wang, Z.L.; He, L.M.; Fan, P.; Yang, Z.Y.; et al. Nitrogen application rate affects the accumulation of carbohydrates in functional leaves and grains to improve grain filling and reduce the occurrence of chalkiness. Front. Plant Sci. 2022, 13, 921130. [Google Scholar] [CrossRef] [PubMed]
- Survase, S.A.; Bajaj, I.B.; Singhal, R.S. Biotechnological production of vitamins. Food Technol. Biotechnol. 2006, 44, 381–396. [Google Scholar]
- Asensi-Fabado, M.A.; Munné-Bosch, S. Vitamins in plants: Occurrence, biosynthesis and antioxidant function. Trends Plant Sci. 2010, 15, 582–592. [Google Scholar] [CrossRef]
- Palacios, O.A.; Bashan, Y.; De-Bashan, L.E. Proven and potential involvement of vitamins in interactions of plants with plant growth-promoting bacteria—An overview. Biol. Fertil. Soils 2014, 50, 415–432. [Google Scholar] [CrossRef]
- Cheng, S.R.; Liu, H.D.; Li, K.Q.; Zheng, L.K.; Su, M.L.; Lin, X.E.; Huang, G.B.; Ren, Y. Riboflavin improves grain yield, 2-acetyl-1-pyrroline accumulation, and antioxidative properties of fragrant rice. J. Sci. Food Agric. 2024, 104, 1178–1189. [Google Scholar] [CrossRef]
- Pinto, J.; Zempleni, J. Riboflavin. Adv. Nutr. 2016, 7, 973–975. [Google Scholar] [CrossRef]
- Whatley, F.R.; Allen, M.B.; Arnon, D.L. Photosynthesis by isolated chloroplasts. VII. Vitamin K and riboflavin phosphate as cofactors of cyclic photophosphorylation. BBA 1959, 32, 32–46. [Google Scholar]
- Deng, B.L.; Jin, X.H.; Yang, Y.; Lin, Z.W.; Zhang, Y.L. The regulatory role of riboflavin in the drought tolerance of tobacco plants depends on ROS production. Plant Growth Regul. 2014, 72, 269–277. [Google Scholar] [CrossRef]
- Hu, H.T.; Ren, D.Y.; Hu, J.; Jiang, H.Z.; Chen, P.; Zeng, D.L.; Qian, Q.; Guo, L.B. WHITE AND LESION-MIMIC LEAF1, encoding a lumazine synthase, affects reactive oxygen species balance and chloroplast development in rice. Plant J. 2021, 108, 1690–1703. [Google Scholar] [CrossRef]
- Tian, Q.Z.; Wang, G.; Ma, X.X.; Shen, Q.W.; Ding, M.L.; Yang, X.Y.; Luo, X.L.; Li, R.R.; Wang, Z.H.; Wang, X.H.; et al. Riboflavin integrates cellular energetics and cell cycle to regulate maize seed development. Plant Biotechnol. J. 2022, 20, 1487–1501. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.L.; Zhao, J.; Pan, X.M.L.; Zhao, J.F.; Dong, H.S.; Wang, J.S.; Liu, B.X.; Liu, G.Y.; Cheng, Y.J. Riboflavin activates growth signal transduction pathway in plants. J. Nanjing Agric. Univ. 2002, 4, 33–36. (In Chinese) [Google Scholar]
- Zhu, Q.; Cao, X.; Luo, Y. Growth analysis of rice grain filling. Acta Agron. Sin. 1988, 14, 182–193. (In Chinese) [Google Scholar]
- Tang, X. Crop Cultivation and Physiological Experiment Guidance; Guangdong Higher Education Press: Guangzhou, China, 2021. [Google Scholar]
- Nakamura, Y.; Yuki, K.; Park, S.Y.; Ohya, T. Carbohydrate metabolism in the developing endosperm of rice grains. Plant Cell Physiol. 1989, 30, 833–839. [Google Scholar] [CrossRef]
- Dai, L.; Ren, Y.; Ashraf, U.; Gui, R.F.; Wang, Z.M.; Tang, X.R.; Duan, M.Y.; Mo, Z.W. Optimized liquid fertilizer management increases 2-acetyl-1-pyrroline content and grain quality in aromatic rice. J. Food Compos. Anal. 2024, 133, 106433. [Google Scholar] [CrossRef]
- Jun, Z.; Peng, D.; Zhang, H.Z.; Meng, C.R.; Zhang, X.J.; Hou, J.W.; Wei, C.Z. Low soil temperature reducing the yield of drip irrigated rice in arid area by influencing anther development and pollination. J. Arid Land 2019, 11, 419–430. [Google Scholar]
- Powers, H.J. Riboflavin (vitaminB-2) and health. Am. J. Clin. Nutr. 2003, 77, 1352–1360. [Google Scholar] [CrossRef]
- Massey, V. The chemical and biological versatility of riboflavin. Biochem. Soc. Trans. 2000, 28, 283–296. [Google Scholar] [CrossRef]
- Peng, J.; Zhao, J.; Pan, X.; Zhao, J.; Dong, H. Preliminary study on the activation of plant growth signaling pathways by riboflavin. J. Nanjing Agric. Univ. 2002, 4, 33–36. (In Chinese) [Google Scholar]
- Namba, J.; Harada, M.; Shibata, R.; Toda, Y.; Maruta, T.; Ishikawa, T.; Shigeoka, S.; Yoshimura, K.; Ogawa, T. AtDREB2G is involved in the regulation of riboflavin biosynthesis in Arabidopsis thaliana. Plant Sci. 2024, 347, 112196. [Google Scholar] [CrossRef]
- Wilkie, K.; Wootton, M.; Paton, J.E. Sensory testing of Australian fragrant, imported fragrant, and non-fragrant rice aroma. Int. J. Food Prop. 2004, 7, 27–36. [Google Scholar] [CrossRef]
- Ben-Asher, J.; Tsuyuki, I.; Bravdo, B.A.; Sagih, M. Irrigation of grapevines with saline water: I. Leaf area index, stomatal conductance, transpiration and photosynthesis. Agric. Water Manag. 2006, 83, 13–21. [Google Scholar] [CrossRef]
- Hoshida, H.; Tanaka, Y.; Hibino, T.; Hayashi, Y.; Tanaka, A.; Takabe, T.; Takabe, T. Enhanced tolerance to salt stress in transgenic rice that overexpresses chloroplast glutamine synthetase. Plant Mol. Biol. 2000, 43, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Huang, Y.; Huang, J.; Xia, G.; Gong, N. Comparative study on the growth and physiological characteristics of plants with different salt tolerance under salt stress. J. Zhejiang Univ. (Agric. Life Sci.) 2006, 4, 420–427. (In Chinese) [Google Scholar]
- Li, X.; Liu, B.; Guo, Z.; Chang, Y.; He, L.; Chen, F.; Lu, B. Changes in photosynthetic characteristics and rapid chlorophyll fluorescence induction kinetics of Populus euphratica leaves under NaCl stress. Chin. J. Appl. Ecol. 2013, 24, 2479–2484. (In Chinese) [Google Scholar]
- Engelaar, W.M.H.G.; Matsumaru, T.; Yoneyama, T. Combined effects of soil water logging and compaction on rice (Oryza sativa L.) growth, soil aeration, soil N transformations and 15 N discrimination. Biol. Fertil. Soils 2000, 32, 484–493. [Google Scholar] [CrossRef]
- Luo, H.; He, L.; Du, B.; Wang, Z.; Zheng, A.; Lai, R.; Tang, X. Foliar application of selenium (Se) at heading stage induces regulation of photosynthesis, yield formation, and quality characteristics in fragrant rice. Photosynthetica 2019, 57, 1007–1014. [Google Scholar] [CrossRef]
- Wu, Q.X.; Du, B.; Jiang, S.C.; Zhang, H.W.; Zhu, J.Q. Side deep fertilizing of machine-transplanted rice to guarantee rice yield in conservation tillage. Agriculture 2022, 12, 528. [Google Scholar] [CrossRef]
- Hu, Q.; Jiang, W.Q.; Qiu, S.; Xing, Z.P.; Hu, Y.J.; Guo, B.W.; Liu, G.D.; Gao, H.; Zhang, H.C.; Wei, H.Y. Effect of wide-narrow row arrangement in mechanical pot-seedling transplanting and plant density on yield formation and grain quality of japonica rice. J. Integr. Agric. 2020, 19, 1197–1214. [Google Scholar] [CrossRef]
- An, N.; Zhang, L.; Liu, Y.X.; Shen, S.; Li, N.; Wu, Z.C.; Yang, J.F.; Han, W.; Han, X.R. Biochar application with reduced chemical fertilizers improves soil pore structure and rice productivity. Chemosphere 2022, 298, 134304. [Google Scholar] [CrossRef]
- Wie, H.Y.; Hu, L.; Zhu, Y.; Xu, D.; Zheng, L.M.; Chen, Z.F.; Hu, Y.J.; Cui, P.Y.; Guo, B.W.; Dai, Q.G.; et al. Different characteristics of nutrient absorption and utilization between inbred Japonica super rice and inter-sub-specific hybrid super rice. Field Crop. Res. 2018, 218, 88–96. [Google Scholar]
- Wu, H.; Xiang, J.; Zhang, Y.P.; Zhang, Y.K.; Peng, S.B.; Chen, H.Z.; Zhu, D.F. Effects of post-anthesis nitrogen uptake and translocation on photosynthetic production and rice yield. Sci. Rep. 2018, 8, 2045–2322. [Google Scholar] [CrossRef] [PubMed]
- You, C.C.; Chen, L.; He, H.B.; Wu, L.Q.; Wang, S.H.; Ding, Y.F.; Ma, C.X. iTRAQ-based proteome profile analysis of superior and inferior spikelets at early grain filling stage in japonica rice. BMC Plant Biol. 2017, 17, 100. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shon, J.; Lee, C.K.; Yang, W.; Yoon, Y.; Yang, W.H.; Kim, Y.G.; Lee, B.W. Relationship between grain filling duration and leaf senescence of temperate rice under high temperature. Field Crop. Res. 2011, 122, 207–213. [Google Scholar] [CrossRef]
- Ahmed, N.; Tetlow, I.; Nawaz, S.; Iqbal, A.; Mubin, M.; Shah, N.; Butt, A.; Lightfoot, D.; Maekawa, M. Effect of high temperature on grain filling period, yield, amylose content and activity of starch biosynthesis enzymes in endosperm of basmati rice. J. Sci. Food Agric. 2015, 95, 2237–2243. [Google Scholar] [CrossRef]
- Pasuquin, E.M.; Eberbach, P.L.; Hasegawa, T.; Lafarge, T.; Harnpichitvitaya, D.; Wade, L.J. Responses to elevated daytime air and canopy temperature during panicle development in four rice genotypes under paddy conditions in large field chambers. Crop Environ. 2023, 2, 147–156. [Google Scholar] [CrossRef]
- Taub, D.R.; Seemann, J.R.; Coleman, J.S. Growth in elevated CO2 protects photosynthesis against high-temperature damage. Plant Cell Environ. 2000, 23, 649–656. [Google Scholar] [CrossRef]
- Lu, G.H.; Wu, Y.F.; Bai, W.B.; Ma, B.; Wang, C.Y.; Song, J.Q. Influence of high temperature stress on net photosynthesis, dry matter partitioning and rice grain yield at flowering and grain filling stages. J. Integr. Agric. 2013, 12, 603–609. [Google Scholar] [CrossRef]
- Xu, H.F.; Wang, Z.X.; Xiao, F.; Yang, L.; Li, G.H.; Ding, Y.F.; Paul, M.J.; Li, W.W.; Liu, Z.H. Dynamics of dry matter accumulation in internodes indicates source and sink relations during grain-filling stage of japonica rice. Field Crop. Res. 2021, 263, 108009. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, F.; Hu, Y.; Kang, Z.; Zhang, Y.; Yang, J.; Yue, K.; Ni, X.; Yang, Y. Comparison of nonstructural carbohydrate contents in young and old leaves of 11 tree species in a subtropical homogeneous garden. Acta Ecol. Sin. 2021, 45, 771–779. (In Chinese) [Google Scholar]
- Sakr, S.; Wang, M.; Dédaldéchamp, F.; Pérez-Garcia, M.D.; Ogé, L.; Hamama, L.; Atanassova, R. The sugar-signaling hub: Overview of regulators and interaction with the hormonal and metabolic network. Int. J. Mol. Sci. 2018, 19, 2506. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yin, F.; Xu, K.; Jia, X.; Zhou, S.; Ma, C. The role of sucrose metabolism and signaling in plant development and stress responses. Agric. Sci. Technol. 2021, 35, 2044–2055. (In Chinese) [Google Scholar]
- Ye, X.Q.; Meng, J.L.; Zeng, B.; Ming, W. Improved flooding tolerance and carbohydrate status of flood-tolerant plant Arundinella anomala at lower water temperature. PLoS ONE 2018, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, H.P.; Batish, D.P.; Kaur, S.; Kohli, R.K. EMF radiations (1800 MHz)-inhibited early seedling growth of maize (Zea mays) involves alterations in starch and sucrose metabolism. Protoplasma 2016, 253, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Lee, J.; Kichey, T.; Gerentes, D.; Zivy, M.; Tatout, C.; Dubois, F.; Balliau, T.; Valot, B.; Davanture, M.; et al. Two cytosolic glutamine synthetase isoforms of maize are specifically involved in the control of grain production. Plant Cell 2006, 18, 3252–3274. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).