Role of Chitosan in the Coloring of Berries and Phytochemical Changes in Physalis angulata L. During Harvest Maturity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Materials and Instruments
2.3. pH, Titratable Acidity (TA), Total Soluble Solids Content (TSS), and TSS/TA Assessment
2.4. Weight Loss Assessment
2.5. Preparation of Berry Powder to Measure Phenol, Flavonoids, and Antioxidant Properties
2.5.1. Total Phenol Content
2.5.2. Flavonoid Content
2.5.3. Total Antioxidant Capacity
2.6. Berry Color Assessment
2.7. Total Chlorophyll and Carotenoid Content
2.8. Statistical Analysis
3. Results
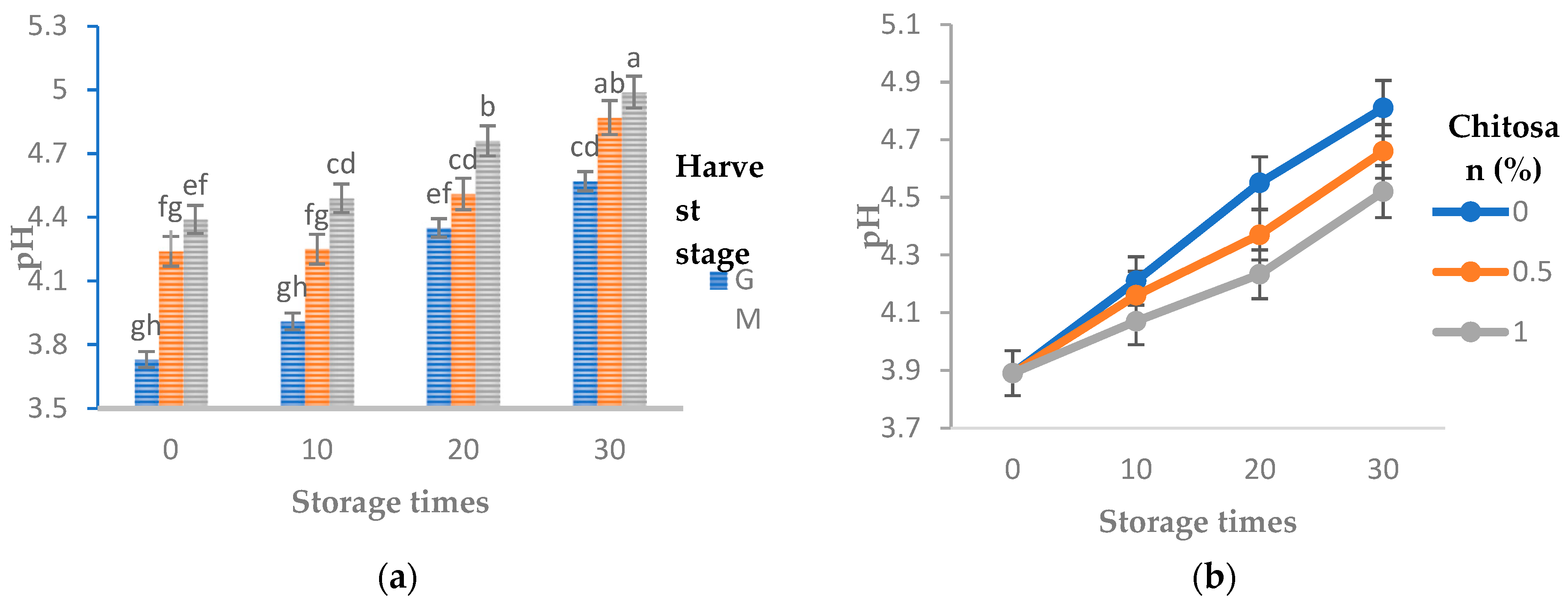
3.1. pH
3.2. Weight Loss Analysis
3.3. Titratable Acidity (TA), TSS, and TSS/TA
3.4. Total Phenolic (TPC) and Flavonoid Content (TFC)
3.5. Effect of Treatment on Antioxidant Capacity
3.6. Effect of Different Treatment Chlorophyll Content (TChl)
3.7. Carotenoid Content
3.8. Effective Treatments Berry Coloring
3.8.1. Chroma Index
3.8.2. Hue Angle
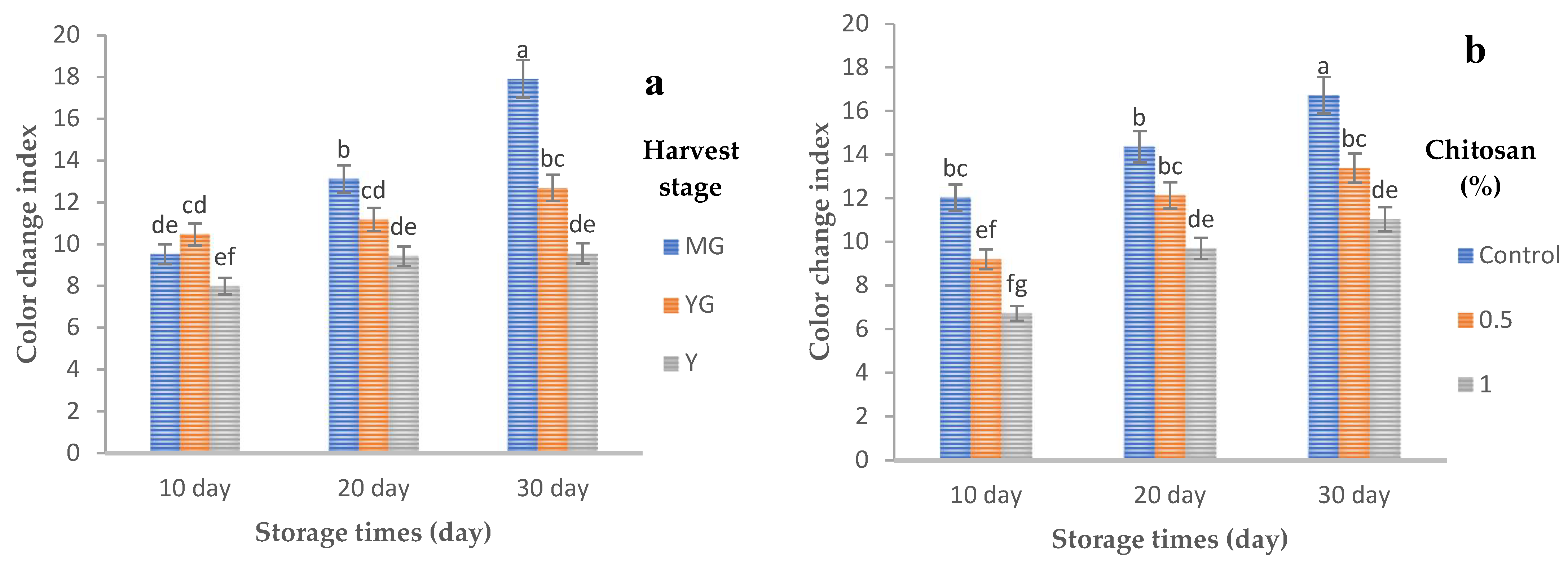
3.8.3. Color Change Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Souza, C.L.M.d.; Souza, M.O.d.; Oliveira, M.F.d.; Oliveira, L.M.d.; Pelacani, C.R. Morfologia de sementes e desenvolvimento pós-seminal de Physalis angulata L. Acta Bot. Bras. 2010, 24, 1082–1085. [Google Scholar] [CrossRef]
- Shang, D.; Zhang, L.; Han, S.; Wang, G. Adjuvant effect of a novel water-soluble polysaccharide isolated from the stem of Physalis alkekengi L. var. francheti (Mast.) Makino. J. Med. Plants Res. 2011, 5, 3814–3818. [Google Scholar]
- Jin, T.; Gurtler, J. Inactivation of Salmonella on tomato stem scars by edible chitosan and organic acid coatings. J. Food Prot. 2012, 75, 1368–1372. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Tenorio, M.-L.; Dekker, M.; Verkerk, R.; van Boekel, M.A. Health-promoting compounds in cape gooseberry (Physalis peruviana L.): Review from a supply chain perspective. Trends Food Sci. Technol. 2016, 57, 83–92. [Google Scholar] [CrossRef]
- Ávila, J.A.; Moreno, P.; Fischer, G.; Miranda, D. Influencia de la madurez del fruto y del secado del cáliz en uchuva (Physalis peruviana L.), almacenada a 18 C. Acta Agronómica 2006, 55, 29–37. [Google Scholar]
- Rodrigues, F.A.; Penoni, E.d.S.; Soares, J.D.R.; Pasqual, M. Caracterização do ponto de colheita de Physalis peruviana L. na região de Lavras, MG. Biosci. J. 2012, 28, 862–867. [Google Scholar]
- Singh, V.; Jawandha, S.; Gill, P.; Gill, M. Suppression of fruit softening and extension of shelf life of pear by putrescine application. Sci. Hortic. 2019, 256, 108623. [Google Scholar] [CrossRef]
- Rincón Soledad, M.C.; Buitrago Guacaneme, C.M.; Ligarreto Moreno, G.A.; Torres Aponte, W.S.; Balaguera López, H.E. Comportamiento del fruto de agraz (Vaccinium meridionale Swartz) cosechado en diferentes estados de madurez y almacenado en refrigeración. Rev. Fac. Nac. Agron. Medellín 2012, 65, 6615–6625. [Google Scholar]
- Rufato, L.; Rufato, A.d.R.; Schlemper, C.; Lima, C.; Kretzschmar, A. Aspectos técnicos da cultura da Physalis. Lages CAV/Udesc 2008, 34, 23–31. [Google Scholar]
- Campbell, J.; Sarkhosh, A.; Habibi, F.; Gajjar, P.; Ismail, A.; Tsolova, V.; El-Sharkawy, I. Evaluation of biochemical juice attributes and color-related traits in muscadine grape population. Foods 2021, 10, 1101. [Google Scholar] [CrossRef]
- Rolle, L.; Giacosa, S.; Gerbi, V.; Bertolino, M.; Novello, V. Varietal comparison of the chemical, physical, and mechanical properties of five colored table grapes. Int. J. Food Prop. 2013, 16, 598–612. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Heydarnajad Giglou, R.; Torabi Giglou, M.; Hatami, M.; Ghorbanpour, M. Potential of natural stimulants and spirulina algae extracts on Cape gooseberry plant: A study on functional properties and enzymatic activity. Food Sci. Nutr. 2024. [Google Scholar] [CrossRef]
- Martinez, L.A.; Chen, Y.; Fischer, S.M.; Conti, C.J. Coordinated changes in cell cycle machinery occur during keratinocyte terminal differentiation. Oncogene 1999, 18, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.G. Calidad y madurez de la uchuva (Physalis peruviana L.) en relacion con la coloracion del fruto. Agron. Colomb. 1999, 16, 35–39. [Google Scholar]
- Liato, V.; Hammami, R.; Aïder, M. Influence of electro-activated solutions of weak organic acid salts on microbial quality and overall appearance of blueberries during storage. Food Microbiol. 2017, 64, 56–64. [Google Scholar] [CrossRef]
- Sobhanizadeh, A.; Yadegari, H.; Fazeli-nasab, B.; Fakheri, B.; Shahpesandi, S. Introduction on application of herbal medicine. In Proceedings of the 1st Annual Iranian Agricultural Research Conference, Kharazmi Higher Institute of Science and Technology, Shiraz, Iran, 22 July 2015. [Google Scholar]
- de Oliveira, C.E.V.; Magnani, M.; de Sales, C.V.; de Souza Pontes, A.L.; Campos-Takaki, G.M.; Stamford, T.C.M.; de Souza, E.L. Effects of chitosan from Cunninghamella elegans on virulence of post-harvest pathogenic fungi in table grapes (Vitis labrusca L.). Int. J. Food Microbiol. 2014, 171, 54–61. [Google Scholar] [CrossRef]
- Fazeli-Nasab, B.; Solouki, M.; Sobhanizadeh, A. Green synthesis of silver nanoparticles using an ephedra sinica herb extract with antibacterial properties. J. Med. Bacteriol. 2021, 10, 30–47. [Google Scholar]
- Fagundes, C.; Palou, L.; Monteiro, A.R.; Pérez-Gago, M.B. Effect of antifungal hydroxypropyl methylcellulose-beeswax edible coatings on gray mold development and quality attributes of cold-stored cherry tomato fruit. Postharvest Biol. Technol. 2014, 92, 1–8. [Google Scholar] [CrossRef]
- Basit, A.; Ayaz, S.; Rab, A.; Ullah, I.; Shah, S.T.; Ahmad, I.; Ullah, I.; Khalid, M.A. 43. Effect of stevia (Stevia Rebaudiana L.) leaf extract on the quality and shelf life of lemon (Citrus limon L.). Pure Appl. Biol. (PAB) 2019, 8, 1456–1468. [Google Scholar]
- Romanazzi, G.; Feliziani, E.; Baños, S.B.; Sivakumar, D. Shelf life extension of fresh fruit and vegetables by chitosan treatment. Crit. Rev. Food Sci. Nutr. 2017, 57, 579–601. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yuan, Y.; Liu, Y.; Li, X.; Wu, S. Application of chitosan in fruit preservation: A review. Food Chem. X 2024, 101589. [Google Scholar] [CrossRef]
- Morgado, J.; Pereira, A.; Bragança, A.; Ferreira, Q.; Fernandes, S.C.; Freire, C.; Silvestre, A.; Neto, C.P.; Alcácer, L. Self-standing chitosan films as dielectrics in organic thin-film transistors. Express Polym. Lett 2013, 7, 960–965. [Google Scholar] [CrossRef]
- Tang, Y.; Xie, L.; Sai, M.; Xu, N.; Ding, D. Preparation and antibacterial activity of quaternized chitosan with iodine. Mater. Sci. Eng. C 2015, 48, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yuan, C.; Chen, Y.; Li, H.; Liu, J. Combined effects of ascorbic acid and chitosan on the quality maintenance and shelf life of plums. Sci. Hortic. 2014, 176, 45–53. [Google Scholar] [CrossRef]
- Kou, X.-h.; Wang, S.; Zhang, Y.; Guo, R.-z.; Wu, M.-s.; Chen, Q.; Xue, Z.-h. Effects of chitosan and calcium chloride treatments on malic acid-metabolizing enzymes and the related gene expression in post-harvest pear cv.‘Huang guan’. Sci. Hortic. 2014, 165, 252–259. [Google Scholar] [CrossRef]
- Silva, G.M.C.; Silva, W.B.; Medeiros, D.B.; Salvador, A.R.; Cordeiro, M.H.M.; da Silva, N.M.; Santana, D.B.; Mizobutsi, G.P. The chitosan affects severely the carbon metabolism in mango (Mangifera indica L. cv. Palmer) fruit during storage. Food Chem. 2017, 237, 372–378. [Google Scholar] [CrossRef]
- Yang, Z.; Zou, X.; Li, Z.; Huang, X.; Zhai, X.; Zhang, W.; Shi, J.; Tahir, H.E. Improved postharvest quality of cold stored blueberry by edible coating based on composite gum arabic/roselle extract. Food Bioprocess Technol. 2019, 12, 1537–1547. [Google Scholar] [CrossRef]
- Hosseini, Z. Methods in Food Analysis; University of Shiraz Publishing: Shiraz, Iran, 2003; p. 220. [Google Scholar]
- Ayala-Zavala, J.F.; Wang, S.Y.; Wang, C.Y.; González-Aguilar, G.A. Effect of storage temperatures on antioxidant capacity and aroma compounds in strawberry fruit. LWT-Food Sci. Technol. 2004, 37, 687–695. [Google Scholar] [CrossRef]
- Golic, M.; Walsh, K. Robustness of calibration models based on near infrared spectroscopy for the in-line grading of stonefruit for total soluble solids content. Anal. Chim. Acta 2006, 555, 286–291. [Google Scholar] [CrossRef]
- Dissa, A.; Desmorieux, H.; Bathiebo, J.; Koulidiati, J. Convective drying characteristics of Amelie mango (Mangifera Indica L. cv.‘Amelie’) with correction for shrinkage. J. Food Eng. 2008, 88, 429–437. [Google Scholar] [CrossRef]
- Pourmorad, F.; Hosseinimehr, S.; Shahabimajd, N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr. J. Biotechnol. 2006, 5, 11. [Google Scholar]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Mita, S.; Murano, N.; Akaike, M.; Nakamura, K. Mutants of Arabidopsis thaliana with pleiotropic effects on the expression of the gene for β-amylase and on the accumulation of anthocyanin that are inducible by sugars. Plant J. 1997, 11, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Miliauskas, G.; Venskutonis, P.; Van Beek, T. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Arnon, A. Method of extraction of chlorophyll in the plants. Agron. J. 1967, 23, 112–121. [Google Scholar]
- Sahin, S.; Sumnu, S.G. Physical Properties of Foods; Springer Science & Business Media: Berlin, Germany, 2006. [Google Scholar]
- Conforti, F.; Statti, G.A.; Menichini, F. Chemical and biological variability of hot pepper fruits (Capsicum annuum var. acuminatum L.) in relation to maturity stage. Food Chem. 2007, 102, 1096–1104. [Google Scholar] [CrossRef]
- Lin, Y.; Li, N.; Lin, H.; Lin, M.; Chen, Y.; Wang, H.; Ritenour, M.A.; Lin, Y. Effects of chitosan treatment on the storability and quality properties of longan fruit during storage. Food Chem. 2020, 306, 125627. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, H.; Wang, J.; Lin, Y.; Chen, Y. Effects of heat treatment on postharvest physiology and storage quality of longan fruits. J. Chin. Inst. Food Sci. Technol. 2014, 14, 124–133. [Google Scholar]
- Lin, Y.; Lin, Y.; Lin, H.; Chen, Y.; Wang, H.; Shi, J. Application of propyl gallate alleviates pericarp browning in harvested longan fruit by modulating metabolisms of respiration and energy. Food Chem. 2018, 240, 863–869. [Google Scholar] [CrossRef]
- Baini, R.; Langrish, T. Assessment of colour development in dried bananas–measurements and implications for modelling. J. Food Eng. 2009, 93, 177–182. [Google Scholar] [CrossRef]
- Shah, S.; Hashmi, M.S. Chitosan–aloe vera gel coating delays postharvest decay of mango fruit. Hortic. Environ. Biotechnol. 2020, 61, 279–289. [Google Scholar] [CrossRef]
- Oey, I.; Lille, M.; Van Loey, A.; Hendrickx, M. Effect of high-pressure processing on colour, texture and flavour of fruit-and vegetable-based food products: A review. Trends Food Sci. Technol. 2008, 19, 320–328. [Google Scholar] [CrossRef]
- Peretto, G.; Du, W.-X.; Avena-Bustillos, R.J.; Sarreal, S.B.L.; Hua, S.S.T.; Sambo, P.; McHugh, T.H. Increasing strawberry shelf-life with carvacrol and methyl cinnamate antimicrobial vapors released from edible films. Postharvest Biol. Technol. 2014, 89, 11–18. [Google Scholar] [CrossRef]
- Wills, R.; Ku, V. Use of 1-MCP to extend the time to ripen of green tomatoes and postharvest life of ripe tomatoes. Postharvest Biol. Technol. 2002, 26, 85–90. [Google Scholar] [CrossRef]
- Heydarnajad Giglou, R.; Torabi Giglou, M. Effects of calyx coating and storage temperature on antioxidant substances of cape gooseberry (Physalis peruviana L.). Int. J. Hortic. Sci. Technol. 2023, 10, 23–32. [Google Scholar]
- Anthon, G.E.; LeStrange, M.; Barrett, D.M. Changes in pH, acids, sugars and other quality parameters during extended vine holding of ripe processing tomatoes. J. Sci. Food Agric. 2011, 91, 1175–1181. [Google Scholar] [CrossRef]
- Oke, M.; Sobratee, N.; Workneh, T. Integrated pre-and postharvest management processes affecting fruit and vegetable quality. Stewart Postharvest Rev. 2013, 9, 1–8. [Google Scholar]
- Martínez, G.A.; Civello, P.M.; Chaves, A.R.; Añón, M.a.C. Characterization of peroxidase-mediated chlorophyll bleaching in strawberry fruit. Phytochemistry 2001, 58, 379–387. [Google Scholar] [CrossRef]
- Rapisarda, P.; Bianco, M.L.; Pannuzzo, P.; Timpanaro, N. Effect of cold storage on vitamin C, phenolics and antioxidant activity of five orange genotypes [Citrus sinensis (L.) Osbeck]. Postharvest Biol. Technol. 2008, 49, 348–354. [Google Scholar] [CrossRef]
- Rasouli, M.; Saba, M.K.; Ramezanian, A. Inhibitory effect of salicylic acid and Aloe vera gel edible coating on microbial load and chilling injury of orange fruit. Sci. Hortic. 2019, 247, 27–34. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, O.; Kohli, K. Post-harvest changes in functional and sensory properties of guava (Psidium guajava L. cv. Pant Prabhat) fruits as influenced by different edible coating treatments. J. Pharmacogn. Phytochem. 2017, 6, 1109–1116. [Google Scholar]
- Ding, C.-K.; Chachin, K.; Ueda, Y.; Imahori, Y.; Wang, C.Y. Modified atmosphere packaging maintains postharvest quality of loquat fruit. Postharvest Biol. Technol. 2002, 24, 341–348. [Google Scholar] [CrossRef]
- Heydarnejad, R.; Ghahremani, Z.; Barzegar, T.; Rabiei, V. The effect of harvesting stage and storage duration on fruit quality of physalis. J. Crops Improv. 2018, 20, 383–395. [Google Scholar]
- Marín, A.; Ferreres, F.; Tomás-Barberán, F.A.; Gil, M.I. Characterization and quantitation of antioxidant constituents of sweet pepper (Capsicum annuum L.). J. Agric. Food Chem. 2004, 52, 3861–3869. [Google Scholar] [CrossRef]
- Castrejón, A.D.R.; Eichholz, I.; Rohn, S.; Kroh, L.W.; Huyskens-Keil, S. Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum L.) during fruit maturation and ripening. Food Chem. 2008, 109, 564–572. [Google Scholar] [CrossRef]
- Gündüz, K.; Saraçoğlu, O.; Özgen, M.; Serce, S. Antioxidant, physical and chemical characteristics of cornelian cherry fruits (Cornus mas L.) at different stages of ripeness. Acta Sci. Pol. Hortorum Cultus 2013, 12, 59–66. [Google Scholar]
- Anton, D.; Bender, I.; Kaart, T.; Roasto, M.; Heinonen, M.; Luik, A.; Püssa, T. Changes in polyphenols contents and antioxidant capacities of organically and conventionally cultivated tomato (Solanum lycopersicum L.) fruits during ripening. Int. J. Anal. Chem. 2017, 2017, 2367453. [Google Scholar] [CrossRef]
- Witzell, J.; Gref, R.; Näsholm, T. Plant-part specific and temporal variation in phenolic compounds of boreal bilberry (Vaccinium myrtillus) plants. Biochem. Syst. Ecol. 2003, 31, 115–127. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y.; Wang, K.; Rui, H.; Tang, S. Effect of methyl jasmonate on cell wall modification of loquat fruit in relation to chilling injury after harvest. Food Chem. 2009, 118, 641–647. [Google Scholar]
- Sobhanizadeh, A.; Solouki, M.; Bahman Fazeli-Nasab, B. Optimization of callus induction and effects of biological and non-biological elicitors on content of phenol/flavonoid compounds in Nigella sativa under in-vitro conditions. Cell Tissue J. 2017, 8, 165–184. [Google Scholar]
- Díaz-Mula, H.; Zapata, P.; Guillén, F.; Valverde, J.; Valero, D.; Serrano, M. Modified atmosphere packaging of yellow and purple plum cultivars. 2. Effect on bioactive compounds and antioxidant activity. Postharvest Biol. Technol. 2011, 61, 110–116. [Google Scholar] [CrossRef]
- Loscalzo, J. L-arginine and atherothrombosis. J. Nutr. 2004, 134, 2798S–2800S. [Google Scholar] [CrossRef] [PubMed]

| TPC | Antioxidant Capacity (%) | TFC | TSS/TA | TSS | TA (%) | ST (Day) | Chitosan (%) | HS |
|---|---|---|---|---|---|---|---|---|
| (mg g−1 DW) | (mg g−1 DW) | |||||||
| 0.84 | 87.85 | 4.15 | 2.30 | 4.13 | 1.58 | - | MG | |
| 1.85 | 74.79 | 6.13 | 4.52 | 4.96 | 1.086 | 0 | - | YG |
| 2.41 | 69.14 | 8.19 | 6.16 | 5.8 | 0.941 | - | Y | |
| 1.61 hi | 74.52 b | 5.28 bc | 4.1 jk | 4.83 kl | 1.095 b | 10 | 0 | |
| 2.33 fg | 62.23 e | 4.16 e | 6.84 gh | 6.8 cd | 1.072 b | 20 | ||
| 2.76 de | 47.73 gh | 4.71 cd | 11.87 a | 7.833 a | 0.585 g | 30 | ||
| 1.156 jk | 78.42 a | 4.85 cd | 3.72 kl | 5.13 ij | 1.28 ab | 10 | 0.5 | MG |
| 2.13 gh | 67.35 cd | 3.52 f | 5.86 hi | 5.6 ij | 1.105 b | 20 | ||
| 2.94 cd | 53.25 f | 5.36 bc | 9.61 ab | 7.6 ab | 0.867 cd | 30 | ||
| 1.43 ij | 76.77 ab | 4.54 d | 3.07 kl | 4.73 kl | 1.37 a | 10 | 1 | |
| 1.92 hi | 67.32 cd | 4.90 cd | 4.9 jk | 7.6 ab | 1.201 ab | 20 | ||
| 3.28 ab | 51.92 f | 5.5 b | 8.25 cd | 6.6 de | 0.688 e | 30 | ||
| 2.66 de | 67.26 cd | 4.0067 ef | 5.62 hi | 6.066 ef | 1.109 b | 10 | 0 | |
| 3.28 ab | 57.35 ef | 4.14 e | 8.04 de | 6.4 de | 0.892 cd | 20 | ||
| 1.46 ij | 41.84 i | 6.33 a | 10.59 ab | 6.5 de | 0.545 h | 30 | ||
| 2.87 cd | 69.67 c | 4.34 de | 4.966 jk | 5.63 gh | 1.141 ab | 10 | 0.5 | YG |
| 3.38 a | 58.45 ef | 4.78 cd | 6.553 gh | 5.96 ef | 0.990 bc | 20 | ||
| 2.48 fg | 45.12 hi | 5.51 b | 7.976 de | 6.03 ef | 0.810 de | 30 | ||
| 3.38 a | 64.77 d | 4.82 cd | 4.726 jk | 5.4 ij | 1.171 ab | 10 | 1 | |
| 2.8 cd | 58.13 ef | 4.39 de | 5.61 hi | 5.8 gh | 1.002 bc | 20 | ||
| 1.58 hi | 44.41 hi | 4.33 de | 6.56 gh | 5.9 ef | 0.648 ef | 30 | ||
| 3.2 b | 62.56 e | 4.59 d | 8.12 cd | 7.26 bc | 0.987 bc | 10 | 0 | |
| 2.98 cd | 45.85 hi | 3.62 f | 8.9 bc | 6.96 cd | 0.825 d | 20 | ||
| 2.3 gh | 33.17 k | 3.22 g | 11.44 a | 6.93 cd | 0.507 i | 30 | ||
| 2.49 fg | 65.44 d | 5.97 ab | 7.33 ef | 6.06 ef | 1.01 bc | 10 | 0.5 | Y |
| 3.19 b | 49.57 g | 5.01 c | 7.94 de | 6.2 ef | 0.877 cd | 20 | ||
| 3.33 a | 37.21 j | 3.56 f | 9.27 bc | 5.73 gh | 0.618 f | 30 | ||
| 2.65 de | 49.70 g | 5.17 bc | 7.17 ef | 6.7 cd | 1.141 ab | 10 | 1 | |
| 2.97 cd | 46.07 h | 3.43 fg | 7.65 ef | 6.13 ef | 0.939 c | 20 | ||
| 2.04 gh | 37.14 j | 2.61 h | 8.47 cd | 5.86 gh | 0.612 f | 30 | ||
| ANOVA | ||||||||
| ns | * | * | * | * | * | ST | ||
| ns | * | ns | ** | ** | ns | C | ||
| * | ** | * | * | * | ** | HS | ||
| ** | * | ** | ** | ** | ** | ST × C | ||
| ns | ns | ns | * | ns | * | HS × ST | ||
| * | ** | * | * | * | ** | HS × C | ||
| ** | ** | ** | ** | ** | ** | HS × ST × C | ||
| Hue Angle (°) | Chroma Index | Chlorophyll Content (mg·100 g−1 FW) | Carotenoid Content | ST (Day) | C (%) | HS |
|---|---|---|---|---|---|---|
| (mg·100 g−1 FW) | ||||||
| 68.87 | 38.1 | 38.1 | 21.06 | - | MG | |
| 78.42 | 48.49 | 48.49 | 31.5 | 0 | - | YG |
| 81.89 | 49.41 | 49.41 | 39.26 | - | Y | |
| 83.45 c | 46.49 e | 46.49 e | 37.07 d | 10 | 0 | |
| 83.28 c | 51.90 cd | 51.90 cd | 16.120 ed | 20 | ||
| 85.62 b | 52.13 c | 52.13 c | 9.83 h | 30 | ||
| 81.22 cd | 43.48 f | 43.48 f | 37.67 d | 10 | 0.5 | |
| 80.55 cd | 47.84 e | 47.84 e | 37.09 d | 20 | ||
| 79.44 d | 45.26 ef | 45.26 ef | 9.49 h | 30 | MG | |
| 71.9 e | 43.78 f | 43.78 f | 37.87 d | 10 | 1 | |
| 77.6 d | 44.69 ef | 44.69 ef | 11.45 g | 20 | ||
| 77.74 d | 46.43 e | 46.43 e | 11.74 g | 30 | ||
| 82.57 c | 51.49 cd | 51.49 cd | 58.47 c | 10 | 0 | |
| 87.54 ab | 52.13 c | 52.13 c | 14.75 f | 20 | ||
| 88.02 a | 52.73 c | 52.73 c | 6.043 j | 30 | ||
| 84.55 bc | 36.13 gh | 36.13 gh | 68.013 ab | 10 | 0.5 | |
| 79.55 cd | 50.21 d | 50.21 d | 8.75 hi | 20 | YG | |
| 76.97 d | 43.40 fg | 43.40 fg | 6.12 j | 30 | ||
| 76.84 d | 39.53 g | 39.53 g | 65.77 b | 10 | 1 | |
| 87.94 a | 39.58 g | 39.58 g | 10.44 gh | 20 | ||
| 77.88 d | 27.58 h | 27.58 h | 8.54 hi | 30 | ||
| 85.52 b | 57.06 a | 57.06 a | 68.80 ab | 10 | 0 | |
| 84. 55 bc | 50.92 d | 50.92 d | 18.75 e | 20 | ||
| 82.37 c | 45.95 fg | 45.95 fg | 4.40 k | 30 | ||
| 87.20 ab | 51.42 cd | 51.42 cd | 73.39 a | 10 | 0.5 | Y |
| 86.74 ab | 54.62 bc | 54.62 bc | 8.76 hi | 20 | ||
| 84.77 bc | 42.95 gh | 42.95 gh | 7.10 i | 30 | ||
| 76.81 d | 50.77 d | 50.77 d | 74.87 a | 10 | 1 | |
| 87.61 ab | 53.98 bc | 53.98 bc | 8.21 hi | 20 | ||
| 83.13 c | 52.81 c | 52.81 c | 10.09 gh | 30 | ||
| ANOVA | ||||||
| ** | * | * | * | ST | ||
| ** | ** | * | ns | C | ||
| ** | ** | ** | ** | HS | ||
| ** | ** | * | ** | ST × C | ||
| ** | ns | ** | ** | HS × ST | ||
| ns | ** | * | ** | HS × C | ||
| ** | ** | ** | ** | HS × ST × C | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heydarnajad Giglou, R.; Ghahremani, Z.; Barzegar, T.; Rabiei, V.; Torabi Giglou, M.; Sobhanizadeh, A.; Nicola, S.; Adamski, M.; Wińska, K. Role of Chitosan in the Coloring of Berries and Phytochemical Changes in Physalis angulata L. During Harvest Maturity. Agriculture 2024, 14, 1924. https://doi.org/10.3390/agriculture14111924
Heydarnajad Giglou R, Ghahremani Z, Barzegar T, Rabiei V, Torabi Giglou M, Sobhanizadeh A, Nicola S, Adamski M, Wińska K. Role of Chitosan in the Coloring of Berries and Phytochemical Changes in Physalis angulata L. During Harvest Maturity. Agriculture. 2024; 14(11):1924. https://doi.org/10.3390/agriculture14111924
Chicago/Turabian StyleHeydarnajad Giglou, Rasoul, Zahra Ghahremani, Taher Barzegar, Vali Rabiei, Mousa Torabi Giglou, Ali Sobhanizadeh, Silvana Nicola, Maciej Adamski, and Katarzyna Wińska. 2024. "Role of Chitosan in the Coloring of Berries and Phytochemical Changes in Physalis angulata L. During Harvest Maturity" Agriculture 14, no. 11: 1924. https://doi.org/10.3390/agriculture14111924
APA StyleHeydarnajad Giglou, R., Ghahremani, Z., Barzegar, T., Rabiei, V., Torabi Giglou, M., Sobhanizadeh, A., Nicola, S., Adamski, M., & Wińska, K. (2024). Role of Chitosan in the Coloring of Berries and Phytochemical Changes in Physalis angulata L. During Harvest Maturity. Agriculture, 14(11), 1924. https://doi.org/10.3390/agriculture14111924









