The Synergistic Effect of Plant Growth-Promoting Rhizobacteria and Spent Mushroom Substrate Improves Ginseng Quality and Rhizosphere Nutrients
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Preparation of PGPR Bacterial Suspension
2.3. Pot Experimental Design
2.4. Sample Collection
2.5. Determination of Soil Physical and Chemical Properties and Soil Enzymes
2.6. Determination of Physiological and Biochemical Indexes of Ginseng Plants
2.7. Total DNA Extraction and PCR Amplification from Ginseng Rhizosphere Soil
2.8. High-Throughput Sequencing of Ginseng Rhizosphere Soil Samples
2.9. Data Analysis and Processing
3. Results
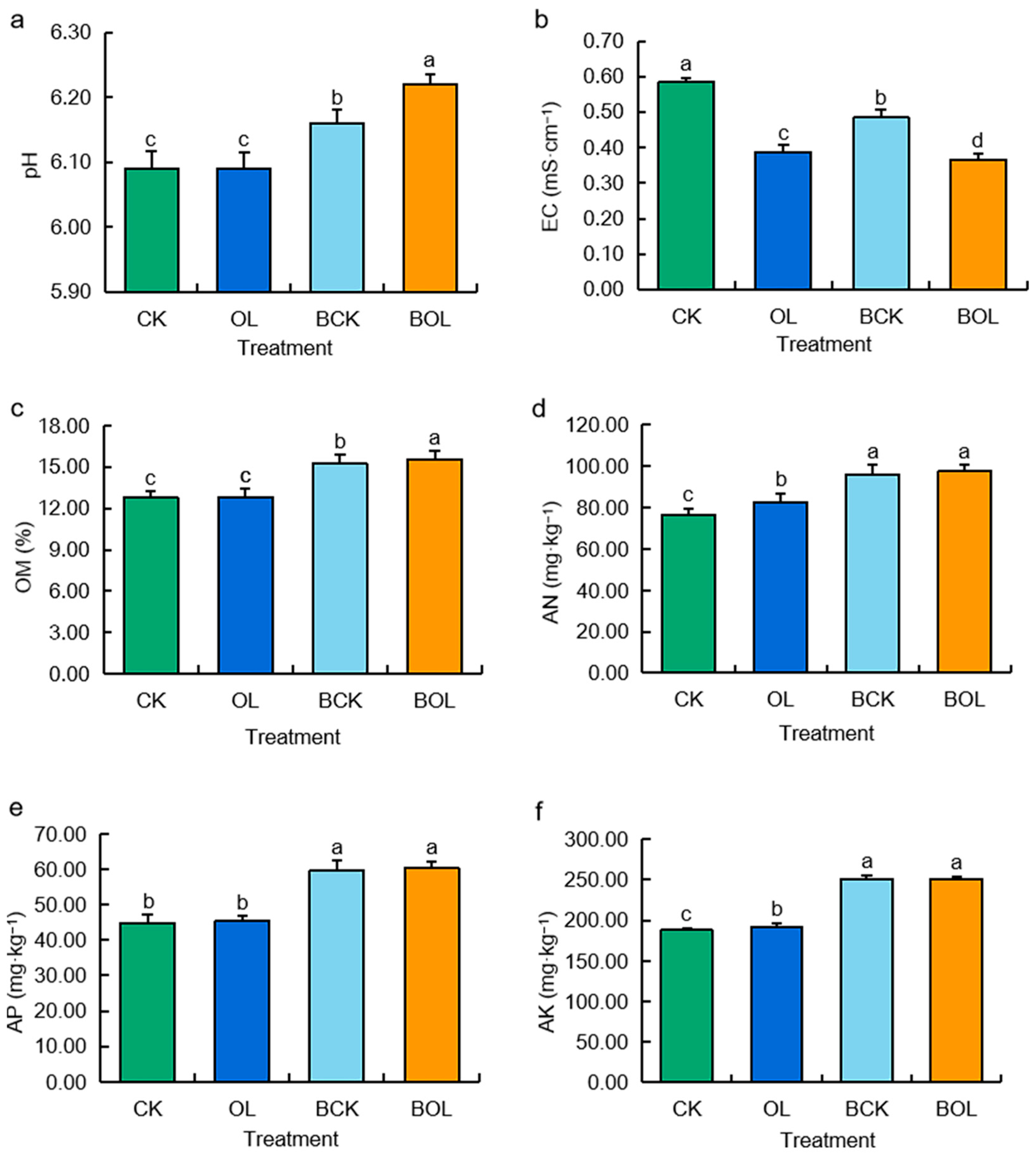
3.1. Effects of Physicochemical Characteristics of Ginseng Rhizosphere Soil Under Different Treatments
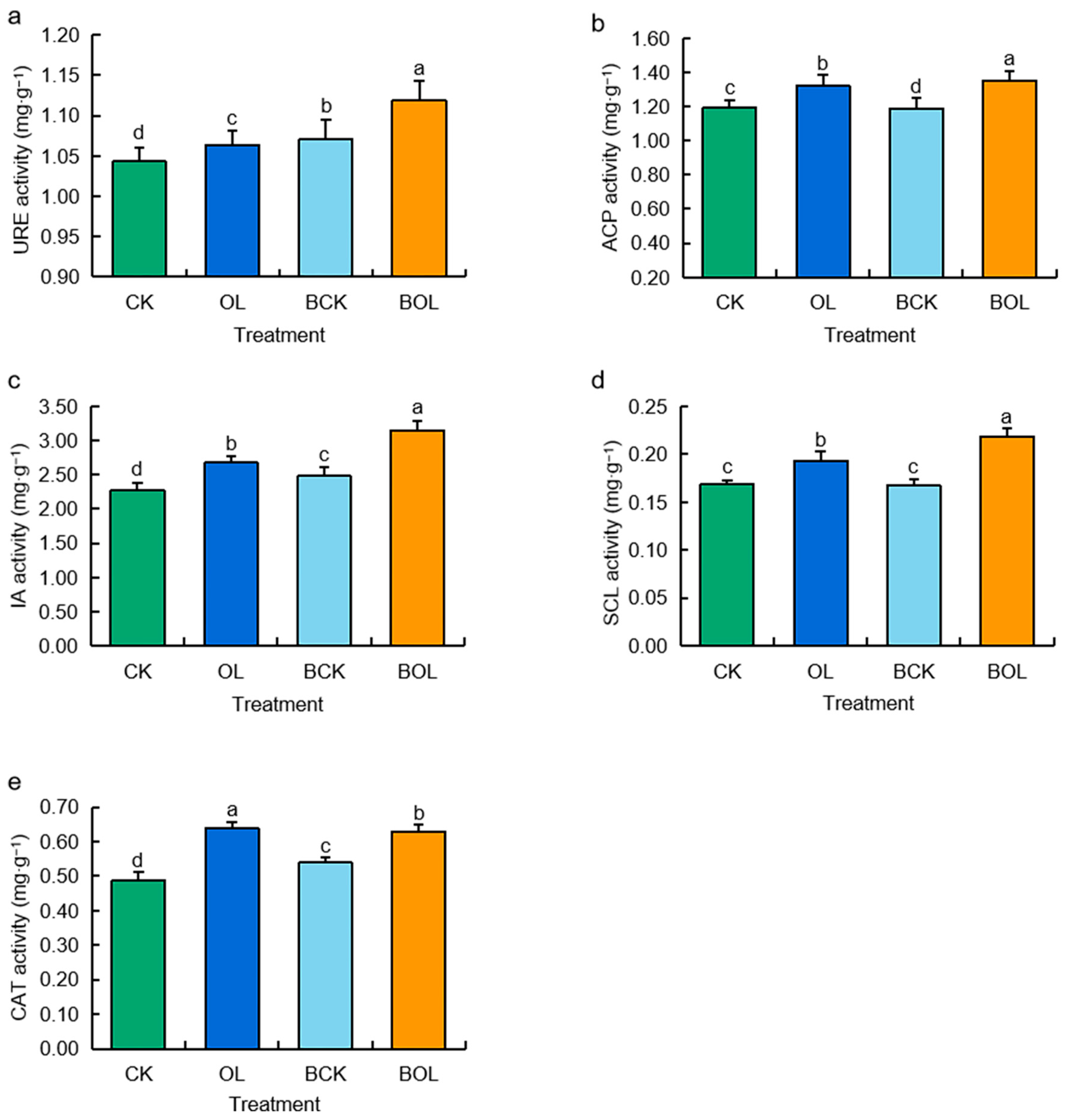
3.2. Effects of Enzyme Activity of Ginseng Rhizosphere Soil Under Different Treatments
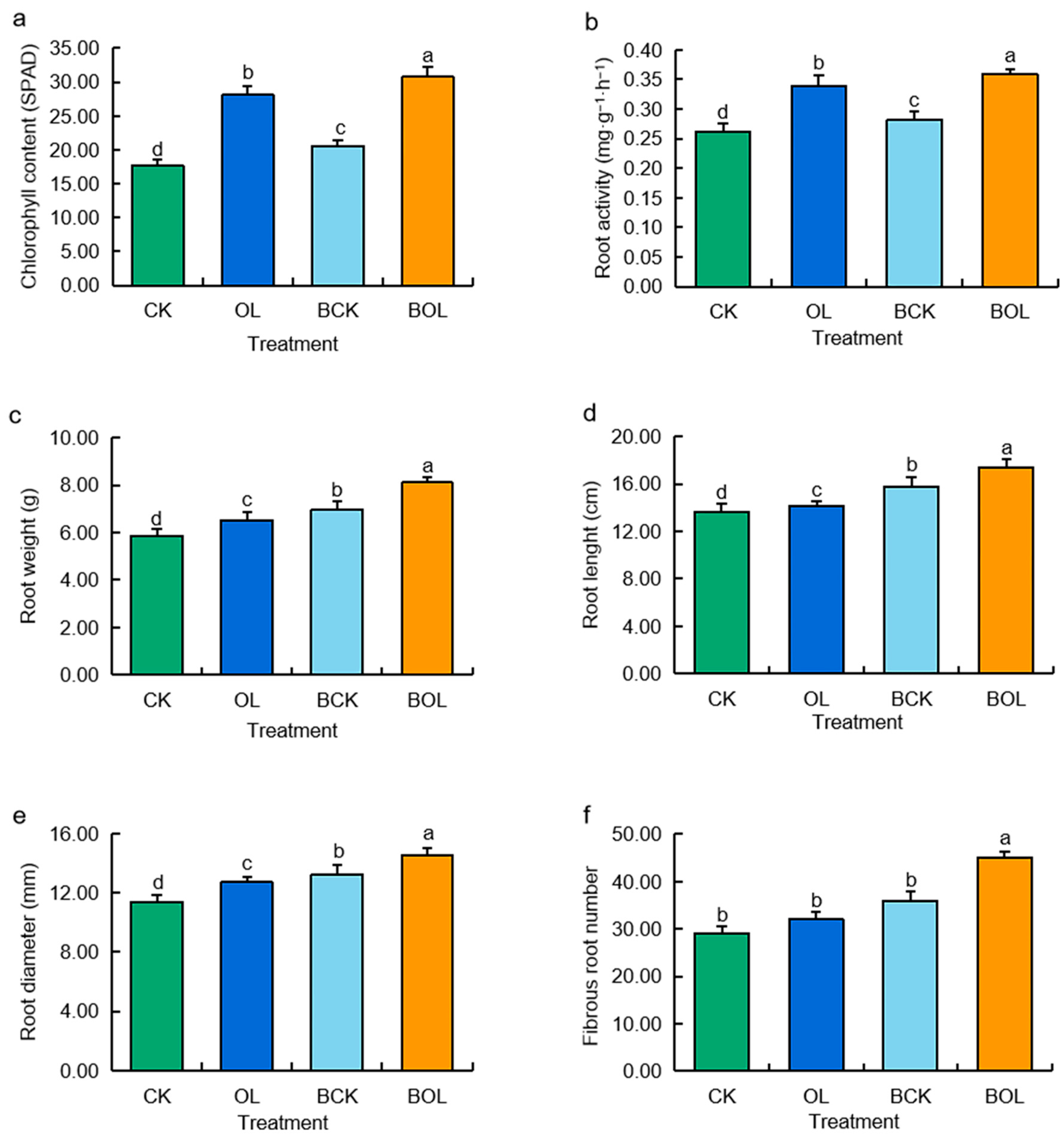
3.3. Effects of Various Inoculants on the Agronomic Characters of Ginseng Plants
3.3.1. Chlorophyll Content in Ginseng
3.3.2. Root Indexes of Ginseng Plants
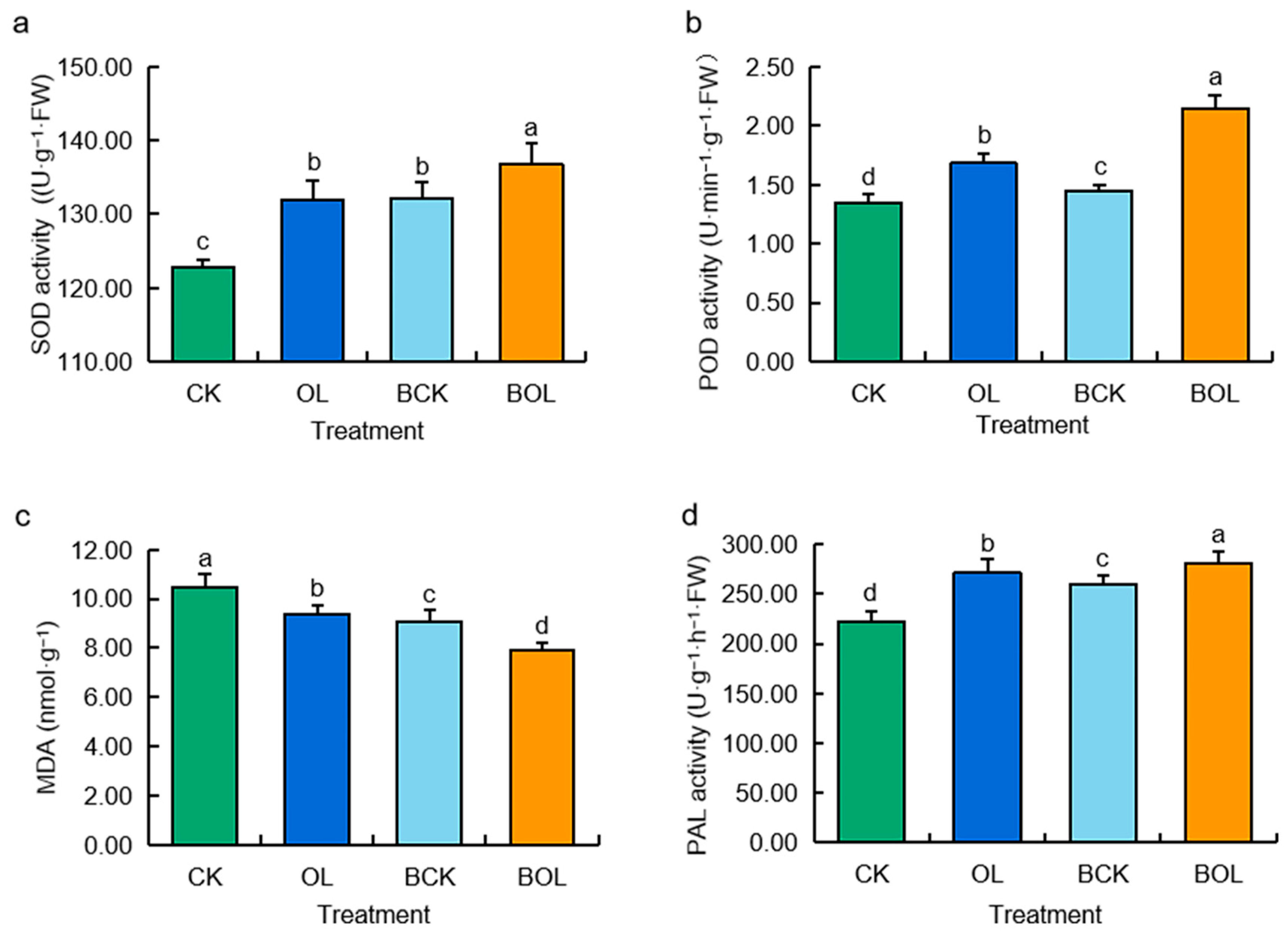
3.4. Antioxidant Enzyme Activity of Ginseng Plants in Response to Various Treatments
3.5. Effects of the Abundance and Diversity of Ginseng Rhizosphere Soil Under Different Treatments
3.6. Variations in the Bacterial and Fungal Community Composition in Ginseng Rhizosphere Soil Under Different Treatments
3.7. Correlation Investigation of Environmental Parameters of Microbial Populations in Ginseng Rhizosphere Soil
3.8. Analysis of β-Diversity for the Bacteria and Fungi in Ginseng Rhizosphere Soil Under Different Treatment Conditions
3.9. Correlation Network Analysis of Bacterial and Fungal Microbial Communities in Ginseng Rhizosphere Soil
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Potenza, M.; Montagnani, M.; Santacroce, L.; Charitos, I.A.; Bottalico, L. Ancient herbal therapy: A brief history of Panax ginseng. J. Ginseng Res. 2023, 47, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Shahrajabian, M.H.; Kuang, Y.; Cui, H.; Fu, L.; Sun, W. Metabolic changes of active components of important medicinal plants on the basis of traditional Chinese medicine under different environmental stresses. Curr. Org. Chem. 2023, 27, 782–806. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Wang, L. Effects of different cultivation methods on the growth and quality of Panax ginseng: A comparative study. J. Ginseng Res. 2019, 43, 431–437. [Google Scholar]
- Fang, J.; Xu, Z.F.; Zhang, T.; Chen, C.B.; Liu, C.S.; Liu, R.; Chen, Y.Q. Effects of soil microbial ecology on ginsenoside accumulation in Panax ginseng across different cultivation years. Ind. Crop. Prod. 2024, 215, 118637. [Google Scholar] [CrossRef]
- Sun, H.; Shao, C.; Liang, H.; Qian, J.Q.; Jin, Q.; Zhu, J.P.; Zhang, G.; Lv, B.; Zhang, Y. Bacterial community response in ginseng rhizosphere soil after Pseudomonas P1 inoculation integrating intracellular non-targeted metabolomics analysis. Environ. Technol. Innov. 2024, 35, 103633. [Google Scholar] [CrossRef]
- Zhang, W.T.; Mao, G.H.; Zhuang, J.Y.; Yang, H. The co-inoculation of Pseudomonas chlororaphis H1 and Bacillus altitudinis Y1 promoted soybean [Glycine max (L.) Merrill] growth and increased the relative abundance of beneficial microorganisms in rhizosphere and root. Front. Microbiol. 2022, 13, 1079348. [Google Scholar] [CrossRef]
- Leong, W.H.; The, S.Y.; Hossain, M.M.; Nadarajaw, T.; Zabidi-Hussin, Z.; Chin, S.Y.; Lai, K.S.; Lim, S.H.E. Application, monitoring and adverse effects in pesticide use: The importance of reinforcement of good agricultural practices (GAPs). J. Environ. Manag. 2020, 260, 109987. [Google Scholar] [CrossRef]
- Shi, Z.T.; Yang, M.L.; Li, K.X.; Yang, L.; Yang, L.M. Influence of cultivation duration on microbial taxa aggregation in Panax ginseng soils across ecological niches. Front. Microbiol. 2024, 14, 1284191. [Google Scholar] [CrossRef]
- Iván, P.; Marine, B.; Ezequiel, Z.L.; Anaïs, G.; Jeanne, G.; Florence, V. Decomposition rates of fine roots from three herbaceous perennial species: Combined effect of root mixture composition and living plant community. Plant Soil 2017, 415, 359–372. [Google Scholar]
- Speir, T.W.; Cowling, J.C. Phosphatase activities of pasture plants and soils: Relationship with plant productivity and soil P fertility indices. Biol. Fertil. Soils 1991, 12, 189–194. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.P.; Xie, B.K.; Wan, C.; Song, R.F.; Zhong, W.R.; Xin, S.Q.; Song, K. Enhancing soil health and plant growth through microbial fertilizers: Mechanisms, benefits, and sustainable agricultural practices. Agronomy 2024, 14, 609. [Google Scholar] [CrossRef]
- Qi, Y.Q.; Liu, H.L.; Zhang, B.P.; Geng, M.X.; Cai, X.X.; Wang, J.H.; Wang, Y. Investigating the effect of microbial inoculants Frankia F1 on growth-promotion, rhizosphere soil physicochemical properties, and bacterial community of ginseng. Appl. Soil Ecol. 2022, 172, 104369. [Google Scholar] [CrossRef]
- Shi, L.; Du, N.S.; Shu, S.; Sun, J.; Li, S.Z.; Guo, S.R. Paenibacillus polymyxa NSY50 suppresses Fusarium wilt in cucumbers by regulating the rhizospheric microbial community. Sci. Rep. 2017, 7, 41234. [Google Scholar] [CrossRef]
- Mohamed, I.; Eid, K.E.; Abbas, M.H.H.; Salem, A.A.; Ahmed, N.; Ali, M.; Shah, G.M.; Fang, C. Use of plant growth promoting rhizobacteria (PGPR) and mycorrhizae to improve the growth and nutrient utilization of common bean in a soil infected with white rot fungi. Ecotoxicol. Environ. Safe 2019, 171, 539–548. [Google Scholar] [CrossRef]
- Ling, N.; Zhang, W.W.; Tan, S.Y.; Huang, Q.W.; Shen, Q.R. Effect of the nursery application of bioorganic fertilizer on spatial distribution of Fusarium oxysporum f. sp. niveum and its antagonistic bacterium in the rhizosphere of watermelon. Appl. Soil Ecol. 2012, 59, 13–19. [Google Scholar]
- Wang, J.W.; Deng, Z.H.; Gao, X.Z.; Long, J.J.; Wang, Y.W.; Wang, W.Y.; Li, C.; He, Y.; Wu, Z. Combined control of plant diseases by Bacillus subtilis SL44 and Enterobacter hormaechei Wu15. Sci. Total. Environ. 2024, 934, 173297. [Google Scholar] [CrossRef]
- Malik, K.A.; Bilal, R.; Mehnaz, S.; Rasul, G.; Mirza, M.S.; Ali, S. Association of nitrogen-fixing, plant-growth-promoting rhizobacteria (PGPR) with kallar grass and rice. In Opportunities for Biological Nitrogen Fixation in Rice and Other Non-Legumes, Proceedings of the Second Working Group Meeting of the Frontier Project on Nitrogen Fixation in Rice Held at the National Institute for Biotechnology and Genetic Engineering (NIBGE), Faisalabad, Pakistan, 13–15 October 1996; Springer: Dordrecht, The Netherlands, 1997; pp. 37–44. [Google Scholar]
- Yang, G.T.; Ma, Y.; Ma, X.C.; Wang, X.Q.; Lu, C.; Xu, W.Y. Changes in soil organic carbon components and microbial community following spent mushroom substrate application. Front. Microbiol. 2024, 15, 1351921. [Google Scholar] [CrossRef]
- Pan, X.; Deng, T.F.; Zhang, L.; Ge, L.J.; Li, L.Q.; Yang, L.S.; Gao, M.; Cao, J.F.; Wei, F.X.; Liu, X.L.; et al. Epimedium herbal residue as a bulking agent for lignite and spent mushroom substrate co-composting. Waste Biomass Valorization 2023, 14, 2547–2555. [Google Scholar] [CrossRef]
- Seekram, P.; Thammasittirong, A.; Thammasittirong, N.R. Evaluation of spent mushroom substrate after cultivation of Pleurotus ostreatus as a new raw material for xylooligosaccharides production using crude xylanases from Aspergillus flavus KUB2. 3 Biotech 2021, 11, 176. [Google Scholar] [CrossRef]
- Li, H.Y.; Qiu, Y.Z.; Yao, T.; Ma, Y.C.; Zhang, H.R.; Yang, X.L. Effects of PGPR microbial inoculants on the growth and soil properties of Avena sativa, Medicago sativa, and Cucumis sativus seedlings. Soil Tillage Res. 2020, 199, 104577. [Google Scholar] [CrossRef]
- Libutti, A.; Mucci, M.; Francavilla, M.; Monteleone, M. Effect of biochar amendment on nitrate retention in a silty clay loam soil. Ital. J. Agron. 2016, 11, 273–276. [Google Scholar] [CrossRef]
- Drescher, G.L.; DA Silva, L.S.; Sarfaraz, Q.; Molin, G.; Marzari, L.B.; Lopes, A.F.; Cella, C.; Facco, D.B.; Hammerschmitt, R.K. Alkaline hydrolyzable nitrogen and properties that dictate its distribution in paddy soil profiles. Pedosphere 2020, 30, 326–335. [Google Scholar] [CrossRef]
- Ghani, M.I.; Ahanger, M.A.; Sial, T.A.; Haider, S.; Siddique, J.A.; Fan, R.D.; Liu, Y.; Ali, E.F.; Kumar, M.; Yang, X.; et al. Almond shell-derived biochar decreased toxic metals bioavailability and uptake by tomato and enhanced the antioxidant system and microbial community. Sci. Total. Environ. 2024, 929, 172632. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Yuan, C.Y.; Zhang, Y.; Wu, J.J.; Chen, G.C.; Chen, S.Y.; Wu, H.; Zhu, H.; Ye, Y. Dredging wastewater discharge from shrimp ponds affects mangrove soil physical-chemical properties and enzyme activities. Sci. Total. Environ. 2024, 926, 171916. [Google Scholar] [CrossRef]
- Chu, H.Y.; Su, W.H.; Zhou, Y.Q.; Wang, Z.Y.; Long, Y.M.; Sun, Y.T.; Fan, S. Enzyme activity stoichiometry suggests that fertilization, especially nitrogen fertilization, alleviates nutrient limitation of soil microorganisms in moso bamboo forests. Forests 2024, 15, 1040. [Google Scholar] [CrossRef]
- Yang, Z.D.; Qiu, D.Y.; Jiang, K.; Du, M.X.; Li, H.Y. Abatement effects of different soil amendments on continuous cropping of Codonopsis pilosula. Front. Plant Sci. 2024, 15, 1376362. [Google Scholar] [CrossRef]
- Guo, Z.X.; Ye, W.H.; Wang, H.; He, W.; Tian, Y.L.; Hu, G.Q.; Lou, Y.; Pan, H.; Yang, Q.; Zhuge, Y. Straw and phosphorus applications promote maize (Zea mays L.) growth in saline soil through changing soil carbon and phosphorus fractions. Front. Plant Sci. 2024, 15, 1336300. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Han, F.L.; Huang, K.Q.; Zhang, J.L.; Qin, J.G.; Chen, L.; Li, E.C. Impact of imidacloprid exposure on the biochemical responses, transcriptome, gut microbiota and growth performance of the Pacific white shrimp Litopenaeus vannamei. J. Hazard. Mater. 2021, 424, 127513. [Google Scholar] [CrossRef]
- Sun, J.; Luo, H.M.; Yu, Q.; Kou, B.X.; Jiang, Y.X.; Weng, L.L.; Xiao, C. Optimal NPK fertilizer combination increases Panax ginseng yield and quality and affects diversity and structure of rhizosphere fungal communities. Front. Microbiol. 2022, 13, 919434. [Google Scholar] [CrossRef]
- Li, Y.B.; Han, L.B.; Wang, H.Y.; Zhang, J.; Sun, S.F.; Feng, D.Q.; Yang, C.L.; Sun, Y.D.; Zhong, N.Q.; Xia, G.X. The thioredoxin GbNRX1 plays a crucial role in homeostasis of apoplastic reactive oxygen species in response to verticillium dahliae infection in Cotton. Plant Physiol. 2016, 170, 2392–2406. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Ghorbani, J.; Motamedi, J.; Vahabzadeh, G.; Ent, A.V.; Edraki, M. Soil contamination around porphyry copper mines: An example from a semi-arid climate. Environ. Monit. Assess. 2024, 196, 204. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.C.; Deng, J.J.; Jiang, Y.; Wu, S.J.; Zhou, Y.B.; Zhu, W.X. Functional distribution of bacterial community under different land use patterns based on FaProTax function prediction. Pol. J. Environ. Stud. 2019, 29, 1245–1261. [Google Scholar] [CrossRef]
- Saha, S. Biological control of plant diseases by beneficial microorganisms: A review on Lysinibacillus spp. Biol. Control. 2018, 120, 52–62. [Google Scholar]
- Mishra, S.K.; Khan, M.H.; Misra, S.; Dixit, V.K.; Khare, P.; Srivastava, S.; Chauhan, P.S. Characterisation of Pseudomonas spp. and Ochrobactrum sp. isolated from volcanic soil. Antonie Van Leeuwenhoek 2017, 110, 253–270. [Google Scholar] [CrossRef]
- Jamal, Q.M.S.; Ahmad, V. Lysinibacilli: A biological factory intended for bio-insecticidal, bio-control, and bioremediation activities. J. Fungi. 2022, 12, 1288. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martín, A.; Hilton, S.L.; Bending, G.D.; Rodríguez-Cruz, M.S.; Sánchez-Martín, M.J. Changes in activity and structure of the soil microbial community after application of azoxystrobin or pirimicarb and an organic amendment to an agricultural soil. Appl. Soil Ecol. 2016, 106, 47–57. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y. Improving soil physical and chemical properties with sugarcane molasses in sandy soils. Agric. Res. 2020, 9, 295–302. [Google Scholar]
- Ojo, A.A.; Adeyemo, A.J. The role of sugarcane molasses in enhancing soil fertility and its impact on the growth of cowpea (Vigna unguiculata). Int. J. Agric. Res. 2019, 14, 184–192. [Google Scholar]
- Nguyen, T.H.H.; IeSungShim, S.; Kobayashi, K.; Kenji, U. Accumulation of some nitrogen compounds in response to salt stress and their relationships with salt tolerance in rice (Oryza sativa L.) seedlings. Plant Growth Regul. 2003, 41, 159–164. [Google Scholar]
- Shi, H.M.; Lu, L.X.; Ye, J.R.; Shi, L.N. Effects of two bacillus velezensis microbial inoculants on the growth and rhizosphere soil environment of prunus davidiana. Int. J. Mol. Sci. 2022, 23, 13639. [Google Scholar] [CrossRef] [PubMed]
- Dumigan, C.R.; Deyholos, M.K. Soil and seed both influence bacterial diversity in the microbiome of the Cannabis sativa seedling endosphere. Front. Plant Sci. 2024, 15, 1326294. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.X.; Wang, T.T.; Sun, C.W.; Liu, P.; Chen, J.; Hou, X.; Yu, T.; Gao, Y.; Liu, Z.; Yang, L.; et al. Eugenol improves salt tolerance via enhancing antioxidant capacity and regulating ionic balance in tobacco seedlings. Front. Plant Sci. 2024, 14, 1284480. [Google Scholar] [CrossRef]
- Zhang, J.X.; Zhou, D.P.; Yuan, X.Q.; Xu, Y.H.; Chen, C.B.; Zhao, L. Soil microbiome and metabolome analysis reveals beneficial effects of ginseng-celandine rotation on the rhizosphere soil of ginseng-used fields. Rhizosphere 2022, 23, 100559. [Google Scholar] [CrossRef]
- Cakmak, I.; Horst, W.J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plantarum. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Nozari, R.M.; Ramos, L.M.; Luz, L.A.; Almeida, R.N.; Lucas, A.M.; Cassel, E.; de Oliveira, S.D.; Astarita, L.V.; Santarém, E.R. Halotolerant Streptomyces spp. induce salt tolerance in maize through systemic induction of the antioxidant system and accumulation of proline. Rhizosphere 2022, 24, 100623. [Google Scholar] [CrossRef]
- Afragan, F.; Kazemeini, S.A.; Alinia, M.; Mastinu, A. Glomus versiforme and Micrococcus yunnanensis reduce the negative effects of salinity stress by regulating the redox state and ion homeostasis in Brassica napus L. crops. Biologia 2023, 78, 3049–3061. [Google Scholar] [CrossRef]
- Giovagnetti, V.; Brunet, C.; Conversano, F.; Tramontano, F.; Obernosterer, I.; Ridame, C.; Guieu, C. Assessing the role of dust deposition on phytoplankton ecophysiology and succession in a low-nutrient low-chlorophyll ecosystem: A mesocosm experiment in the Mediterranean Sea. Biogeosciences 2012, 10, 2973–2991. [Google Scholar] [CrossRef]
- Piromyou, P.; Noisangiam, R.; Uchiyama, H.; Tittabutr, P.; Boonkerd, N. Indigenous microbial community structure in rhizosphere of chinese kale as affected by plant growth-promoting rhizobacteria inoculation. Pedosphere 2013, 23, 577–592. [Google Scholar] [CrossRef]
- Sun, W.L.; Shahrajabian, M.H.; Soleymani, A. The roles of plant-growth-promoting rhizobacteria (PGPR)-based biostimulants for agricultural production systems. Plants 2024, 13, 613. [Google Scholar] [CrossRef]
- Balazs, H.E.; Schmid, C.A.; Cruzeiro, C.; Podar, D.; Szatmari, P.M.; Buegger, F. Post-reclamation microbial diversity and functions in hexachlorocyclohexane (HCH) contaminated soil in relation to spontaneous HCH tolerant vegetation. Sci. Total. Environ. 2021, 767, 144653. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Zhu, Y.X.; Xu, M.; Cai, X.Y.; Tian, F. Co-application of spent mushroom substrate and PGPR alleviates tomato continuous cropping obstacle by regulating soil microbial properties. Rhizosphere 2022, 23, 100563. [Google Scholar] [CrossRef]
- Ding, M.J.; Dai, H.X.; He, Y.; Liang, T.B.; Zhai, Z.; Zhang, S.X.; Hu, B.; Cai, H.; Dai, B.; Xu, Y.; et al. Continuous cropping system altered soil microbial communities and nutrient cycles. Front. Microbiol. 2024, 15, 1374550. [Google Scholar] [CrossRef] [PubMed]
- Carver, T.L.W.; Ingerson, S.M. Responses of Erysiphe graminis germlings to contact with artificial and host surfaces. Physiol. Mol. Plant Pathol. 1987, 30, 359–372. [Google Scholar] [CrossRef]
- Gastélum, G.; Ángeles-Morales, A.; Arellano-Wattenbarger, G.; Coronado, Y.; Guevara-Hernandez, E.; Rocha, J. Biofilm formation and maize root-colonization of seed-endophytic Bacilli isolated from native maize landraces. Appl. Soil Ecol. 2024, 199, 105390. [Google Scholar] [CrossRef]
- Fountain, J.C.; Bajaj, P.; Nayak, S.N.; Yang, L.; Pandey, M.K.; Kumar, V.; Jayale, A.S.; Chitikineni, A.; Lee, R.D.; Kemerait, R.C.; et al. Responses of Aspergillus flavus to oxidative stress are related to fungal development regulator, antioxidant enzyme, and secondary metabolite biosynthetic gene expression. Front. Microbiol. 2016, 7, 2048. [Google Scholar] [CrossRef]
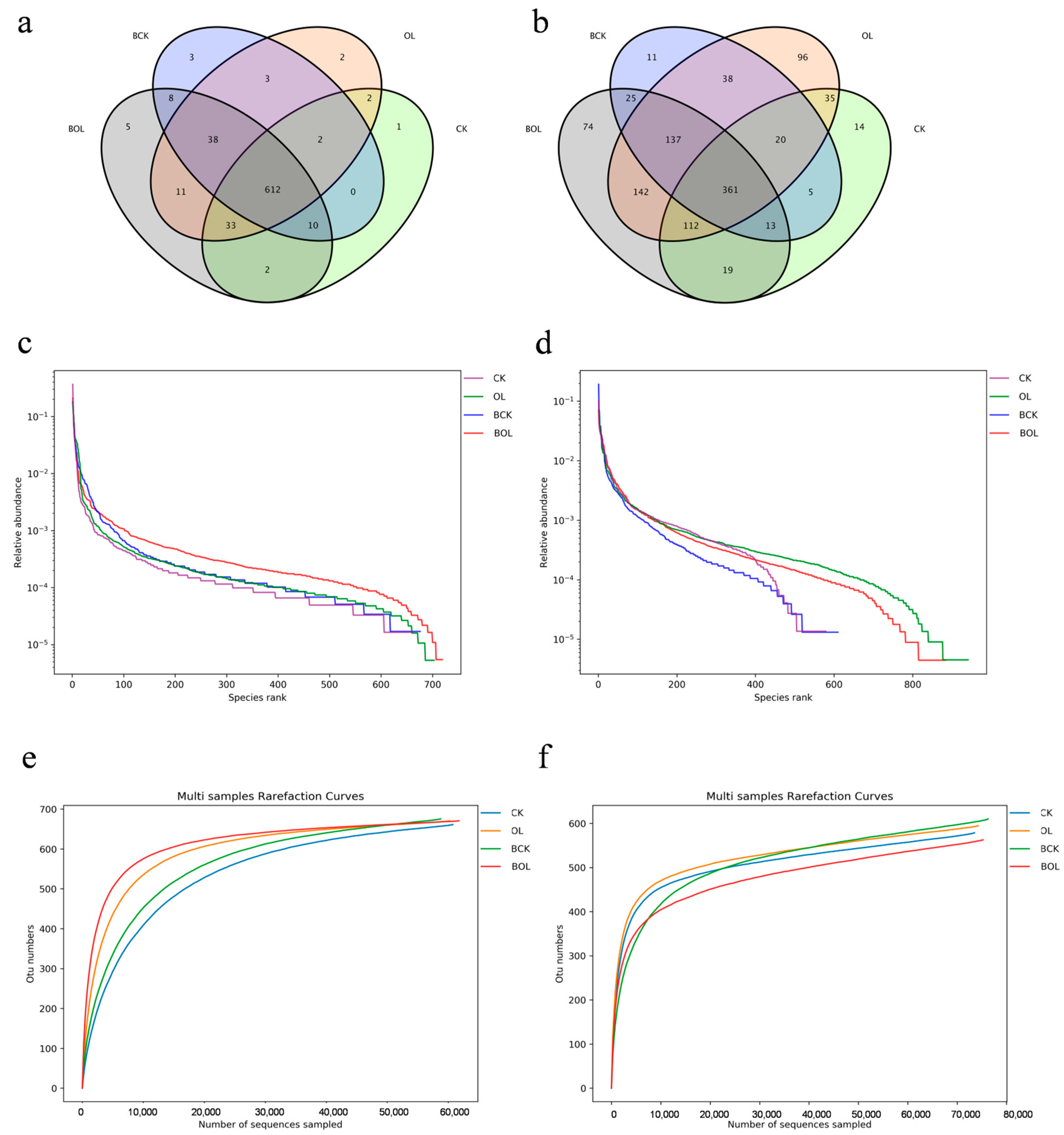
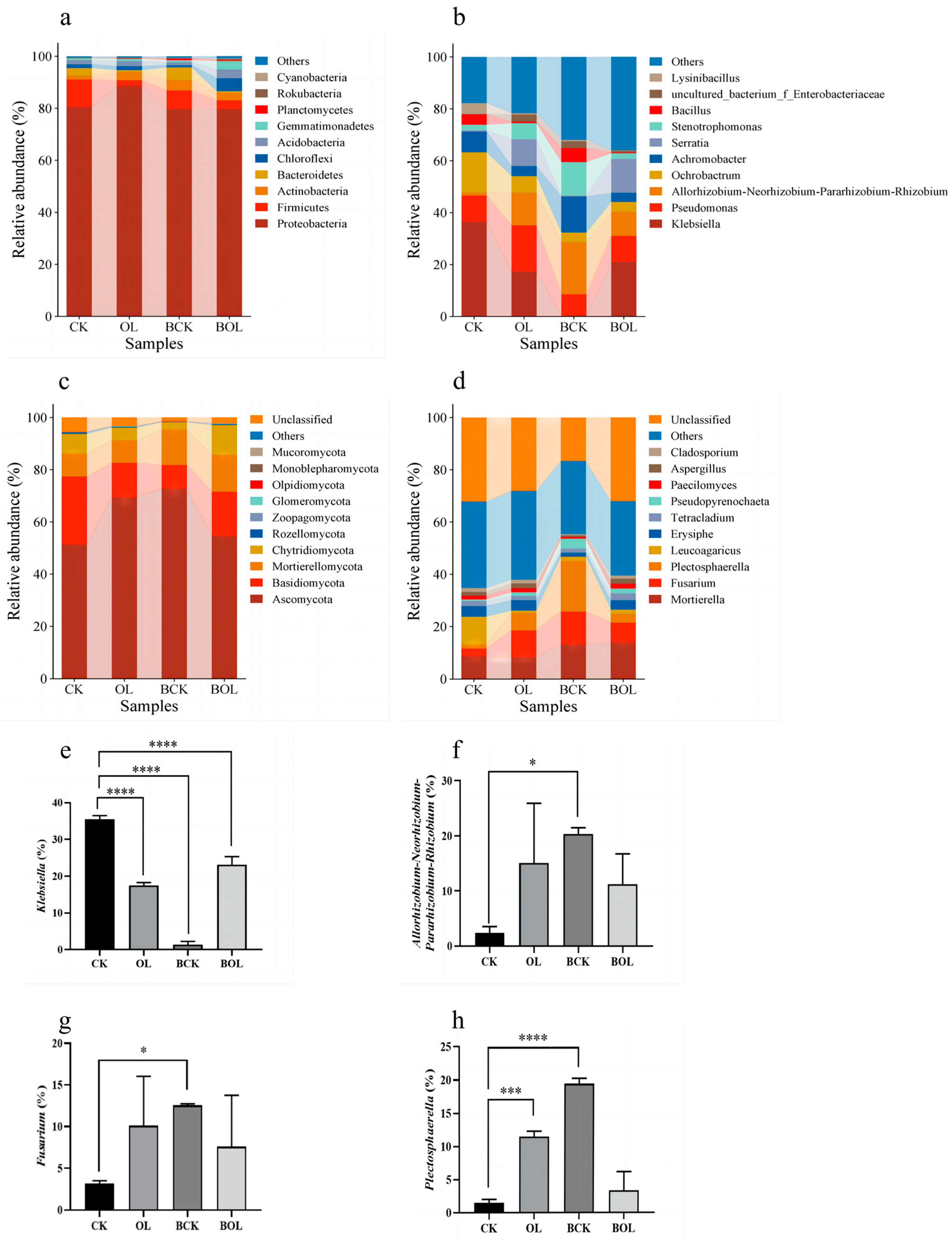
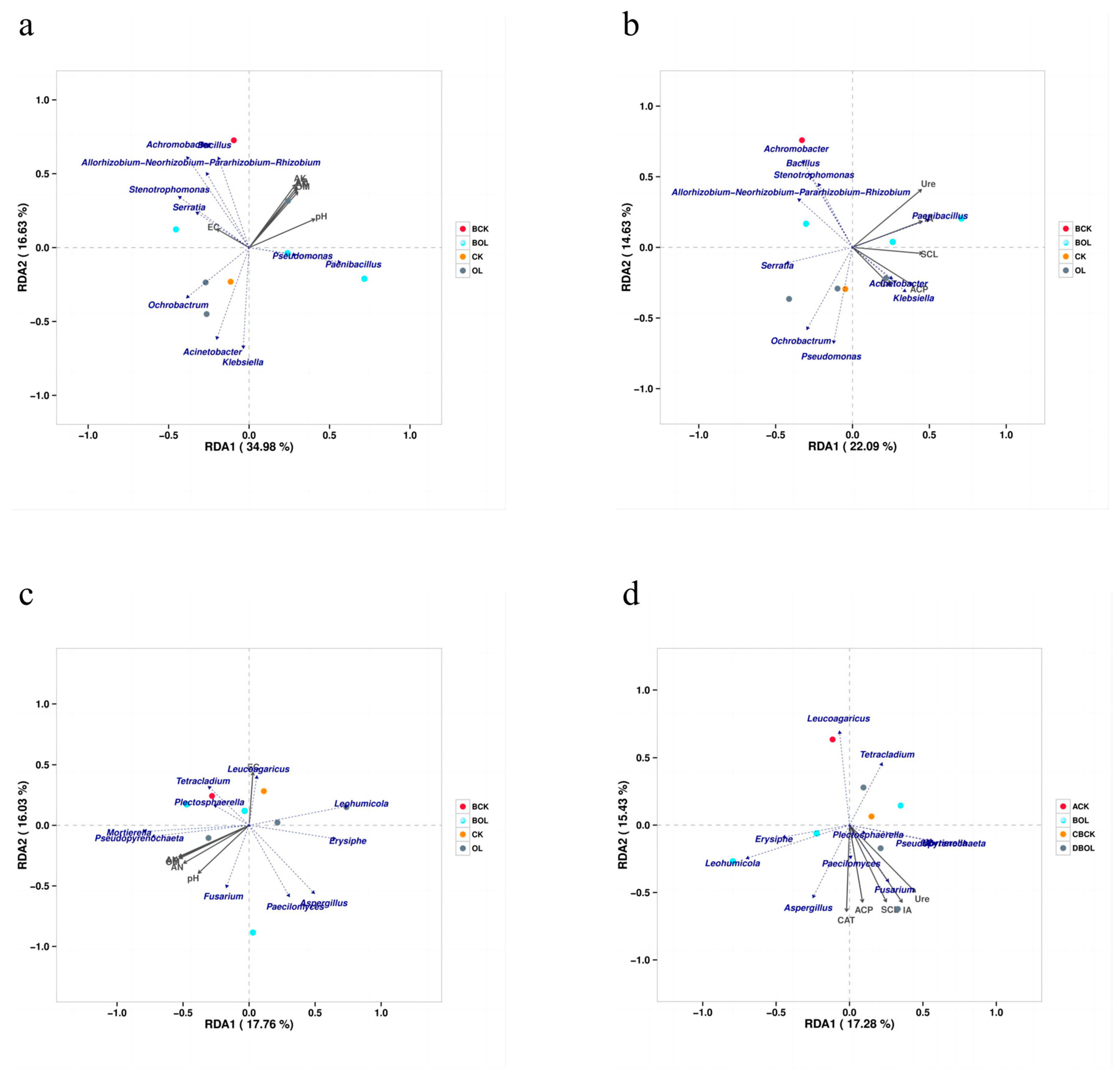
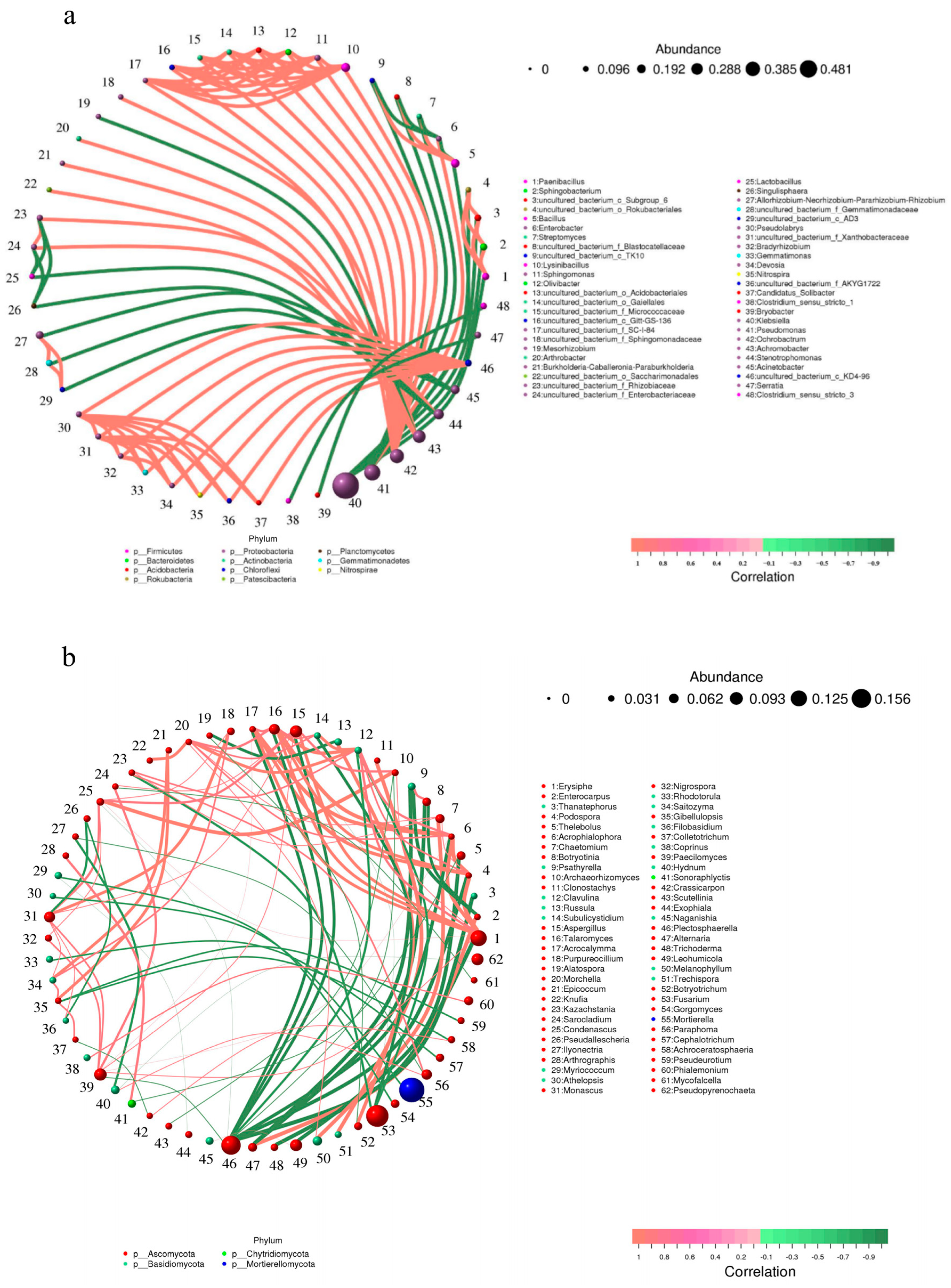
| Category | Treatment | ACE Index | Chao1 Index | Simpson Index | Shannon Index |
|---|---|---|---|---|---|
| bacterial | CK | 683.5732 ± 1.32 b | 688.1639 ± 2.31 b | 0.8266 ± 0.02 d | 4.1577 ± 0.13 d |
| OL | 639.7686 ± 2.61 d | 638.5988 ± 1.65 d | 0.8483 ± 0.06 c | 4.4643 ± 0.16 c | |
| BCK | 698.6491 ± 2.49 a | 708.9038 ± 2.54 a | 0.9336 ± 0.03 a | 5.5416 ± 0.11 a | |
| BOL | 677.1414 ± 1.69 c | 682.9095 ± 2.76 c | 0.8783 ± 0.04 b | 5.1309 ± 0.12 b | |
| fungal | CK | 751.3808 ± 2.31 b | 699.6522 ± 1.32 c | 0.9436 ± 0.04 c | 6.4715 ± 0.14 b |
| OL | 754.0475 ± 2.14 b | 742.2826 ± 1.45 b | 0.9557 ± 0.03 b | 5.9319 ± 0.11 d | |
| BCK | 783.2486 ± 3.15 a | 759.5000 ± 1.56 a | 0.9778 ± 0.08 a | 6.9699 ± 0.06 a | |
| BOL | 664.1192 ± 2.45 c | 657.6742 ± 2.31 d | 0.9487 ± 0.02 c | 6.2180 ± 0.12 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, S.; Hu, Q.; Liu, Q.; Xu, W.; Wang, Z.; Huang, Y.; Zhang, Y.; Ji, W.; Dong, W. The Synergistic Effect of Plant Growth-Promoting Rhizobacteria and Spent Mushroom Substrate Improves Ginseng Quality and Rhizosphere Nutrients. Agriculture 2024, 14, 1880. https://doi.org/10.3390/agriculture14111880
Fan S, Hu Q, Liu Q, Xu W, Wang Z, Huang Y, Zhang Y, Ji W, Dong W. The Synergistic Effect of Plant Growth-Promoting Rhizobacteria and Spent Mushroom Substrate Improves Ginseng Quality and Rhizosphere Nutrients. Agriculture. 2024; 14(11):1880. https://doi.org/10.3390/agriculture14111880
Chicago/Turabian StyleFan, Siyao, Qian Hu, Qi Liu, Wenman Xu, Zixin Wang, Yu Huang, Yang Zhang, Wenxiu Ji, and Weiwei Dong. 2024. "The Synergistic Effect of Plant Growth-Promoting Rhizobacteria and Spent Mushroom Substrate Improves Ginseng Quality and Rhizosphere Nutrients" Agriculture 14, no. 11: 1880. https://doi.org/10.3390/agriculture14111880
APA StyleFan, S., Hu, Q., Liu, Q., Xu, W., Wang, Z., Huang, Y., Zhang, Y., Ji, W., & Dong, W. (2024). The Synergistic Effect of Plant Growth-Promoting Rhizobacteria and Spent Mushroom Substrate Improves Ginseng Quality and Rhizosphere Nutrients. Agriculture, 14(11), 1880. https://doi.org/10.3390/agriculture14111880





