Abstract
The research work was carried out with the progeny of two soybean cultivars, Richy and Izidor, from the years 2019 and 2020. Plants were grown from seeds pretreated with low temperature (2–5 °C) before sowing for two periods of treatment: 12 days marked as “treated control” (tr. K) and 22 days marked as “treated” (tr.); and “non-treated” (K0) used as a control. Transcriptional profiles of the gene encoding a stress protein kinase were evaluated after the application of abiotic stresses caused by the following: 150 mM NaCl solution/salinity stress/for 24 h; 350 mM mannitol solution/drought stress/for 24 h; and low temperature (4 °C) for 72 h. Transcript levels were established by real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) in leaf tissue collected from seedlings of the 2019 and 2020 progeny of “tr. K”, “tr.”, and “K0” samples. Analyses determining the quantity of malondialdehyde (MDA) and total antioxidant capacity (TAC) were performed. The expression of investigated stress kinase was highly upregulated after the application of abiotic stress caused by 150 mM solution of NaCl and to a lesser extent by 350 mM solution of mannitol. Detected transcript levels depend on the type of sample out of “tr. K”, “tr.”, and “K0”; the progeny; and the genotype.
1. Introduction
One of the most important crops from the Fabaceae family is soybean (Glycine max (L.) Merill.), and it is cultivated worldwide. This crop is very rich in protein and high-quality edible oil, and its products are included in the diet of humans and animals [1]. Soybean is an environmentally friendly crop, which influences soil fertility via symbiotic nitrogen fixation with bacteria from the genus Rhizobium [2]. The agriculture of the 21st century along with increased climate changes and world population require novel soybean varieties, which possess high and stable yields and high quality of vegetable protein and oil [3,4]. The cultivation of this important legume crop is affected mostly by environmental conditions and above all by drought and soil salinity [5]. Soybean breeding is focused on the establishment of local cultivars suitable for the climate specificity of each country [6,7,8]. Bulgaria belongs to the Danube region and possesses all the environmental requirements for intense soybean production. Severe summer drought is the main limiting factor for sustainable crop yield in Bulgaria [9]. Modern agricultural research is focused on the interaction between plant genotype and environment. This kind of multidisciplinary research is complicated and includes fundamental and applied studies and experimental work which is based on genetics, genomics, transcriptomics, proteomics, and metabolomics [10]. The investigations of research teams around the globe are concentrated on increasing the yield and quality and quantity of plant proteins and oil.
Current agriculture, in the condition of climate changes and abiotic stressors like drought, soil salinity, heat, and cold, is limited in reaching sustainable yields [11]. The effects of drought and heat cause severe yield damage in cereal and non-cereal crops, which is five times higher than that caused by cold [12]. Extreme salt stress can even cause plant death. Salt stress alters the photosynthesis process that results in oxidative stress with the massive production of reactive oxygen species (ROS) [13].
In this investigation, the experimental work was concentrated on the functional genomics study of one stress protein kinase. This gene was selected by us in the year 2019 from the data of highly expressed genes in the R5–R7 stages of the development of soybean published in a set of transcriptomics data (www.soybean.org accessed on 15 April 2019). In the study of [14], the authors performed genome-wide comparative analysis of abscisic acid (ABA) signaling components. The same stress protein kinase is determined as sucrose non-fermentation (snf) related protein kinase subfamily 2 (SnRK2), which mediates phosphorylation of transcription factors and subsequently promotes the expression of ABA-responsive genes. In plants, ABA controls the plant’s adaptive response to abiotic stress conditions, which mainly are drought and salinity [14,15].
Additionally, analyses of antioxidant activity were performed with seedlings from the progeny from 2019 and 2020 subjected to the abiotic stress of drought and salinity. Abiotic stress is characterized by enhanced production of reactive oxygen species (ROS). In plants, ROS generate free radicals, which cause serious oxidative damage to cellular lipids, membranes, proteins, and DNA. The plants also possess some ROS-scavenging mechanisms, an antioxidant defense system [16]. The free radicals are scavenged by antioxidants to diminish cellular oxidative stress via enzymatic and non-enzymatic mechanisms. The members of antioxidant enzyme systems are catalases, peroxidases, and superoxide dismutases. Tocopherols, carotenes, and vitamin A function as lipid-soluble antioxidants; glutathione and ascorbate are water-soluble antioxidants [17]. The establishment of the level of total non-enzymatic antioxidant capacity (TAC) of plant samples is an indicator of their ability to counteract against cell damage caused by oxidative stress [18,19,20]. Lipid peroxidation is the degradation of lipids that occurs as a result of oxidative damage and is a useful marker for oxidative stress. Polyunsaturated lipids are susceptible to oxidative attack, typically by reactive oxygen species, resulting in the production of end products such as malondialdehyde (MDA). The purpose of MDA analysis is to estimate the amount of malondialdehyde, which is the end product of lipid peroxidation. It is widely used as an indicator for determining the oxidative stress in plants exposed to abiotic stress. Establishment of a lower value of MDA in plant tissue indicates a lower level of oxidative stress [21].
The aim of this study was to unravel the function of a gene encoding a stress protein kinase after treatment with abiotic stressors as follows: drought caused by 350 mM solution of mannitol for 24 h; salinity caused by 150 mM solution of NaCl for 24 h; and treatment with low temperature (4 °C) for 72 h. As a plant material, we used the progeny of soybean plants harvested in the years 2019 and 2020. These plants were grown from low-temperature-pretreated seeds before sowing. Additionally, total antioxidant capacity and the level of lipid peroxidation were established in different samples from the progeny of 2019 and 2020.
2. Materials and Methods
2.1. Plant Material and Growing Conditions
The research work of our team in a period of three years, 2019–2021, was focused on the development of an innovative approach with long-lasting cold pretreatment of soybean seeds before sowing [22,23]. Each year, a low-temperature (2–5 °C) pretreatment of soybean seeds before sowing was performed. The plant material used in the current study was seedlings from the progeny of 2019 and 2020 of soybean plants grown from low-temperature-pretreated seeds and untreated seeds from cvs. Richy and Izidor. Richy is a Bulgarian soybean cultivar with high yield. Izidor is a French cultivar used as a standard. This plant material was used in the experiments for evaluation of transcript level and analyses of antioxidant activity. The cold pretreatment of the seeds sown in 2019 and 2020 was performed with low temperature (2–5 °C), for two periods of treatment: 12 days, called “treated control” (tr. K), and 22 days, called “treated” (tr.). As a control, soybean plants grown from “non-treated” seeds (K0) were used. The progeny of plants grown from seeds in years 2019 and 2020 are called “treated control” (tr. K), “treated” (tr.), and “non-treated” control (K0).
2.2. Abiotic Stress Treatment of Seedlings
Treatment of seedlings was performed with 150 mM NaCl solution (salinity stress) for 24 h, 350 mM mannitol solution (drought stress) for 24 h, and 4 °C (low temperature) for 72 h. Concentrations of stressors and duration of treatments were selected by our previous studies with the model species M.truncatula and A.thaliana. The germination of soybean seeds tr. K, tr., and K0 from cvs. Richy and Izidor of progeny of 2019 and 2020 was performed in Petri dishes on moistened filter paper for 12 days. Later on, seedlings were placed in Magenta culture boxes on filter paper bridges, with the root part submerged in liquid Murashige and Skoog (MS) basal medium or in one of the stress agents. The application of the stress agents was performed on 20-day-old seedlings, when the first true leaves were well developed. The Magenta boxes were placed in a growth camera with the following parameters: temperature 20–22 °C, relative humidity 60–70%, photoperiod 16/8 h (day/night), light intensity 150–300 mol m−2 s−1. Each treatment was repeated three times with three replicates. In total, 108 seedlings from each cultivar and progeny were used in the experiments.
2.3. Gene Expression Analyses
For this study we selected the gene coding protein kinase GM01G41260, which was found to be highly expressed in the R5–R7 stages of soybean development (www.soybean.org). This gene is an ortholog of A.thaliana AT4G33950 which encodes calcium-independent ABA-activated protein kinase, a member of the SNF1-related protein kinases (SnRK2), and is activated by ionic (salt) and non-ionic (mannitol) osmotic stress. For the expression analyses, the total RNA was extracted from one of the first two true leaves of the seedlings subjected to abiotic stress and controls. The samples were taken immediately, 24 h after salinity and drought stress application, and 72 h after cold stress. For RNA isolation, the RNA Plant Kit (EURx Ltd., Gdansk, Poland) was used. One microgram of total RNA was reverse transcribed with the First Strand cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). Relative expression levels were determined with the 7300 Real-Time qPCR System (Applied Biosystems, Foster city, CA, USA, http://www.appliedbiosystems.com, accessed on 16 April 2019). The qRT-PCR analyses were carried out in a total volume of 20 µL containing 5 µL of cDNA, 0.5 µL gene-specific primers (10 µmol/L), 10 µL SYBR Green Mix (EURx Ltd., Gdansk, Poland), and 4 µL of RNase-free ddH2O. The PCR conditions were: 95 °C for 5 min, followed by 60 cycles of 95 °C for 15 s and 60 °C for 30 s. Two different reference genes (ACTIN and UBIQUITIN10) were used for Ct value normalization. The obtained values of the studied gene were tracked and averaged. The data were processed with a specialized program, qBASE v 1.3.5 (Center for Medical Genetics, Ghent University Hospital, http://medgen.ugent.be/qbase accessed on 10 February 2012). The sequences of the primers used for evaluation of expression are given in Table S1. For each set of samples, three biological repeats, each with three technical repeats, were used for transcript data. The transcript profiles displayed in figures are the mean value of three biological repeats.
2.4. Analyses of Antioxidant Activity TAC and MDA
For the antioxidant analyses, 100 mg of plant tissue from one of the first two true leaves of the seedlings, after application of abiotic stresses and controls, was used. For preparation of each sample, the leaf tissue was weighed on an electronic balance. The samples were taken immediately after 24 h application of salinity and drought stress.
Lipid peroxidation was measured colorimetrically following the procedure of the “Lipid Peroxidation (MDA) Assay Kit” (Sigma-Aldrich, Burligton, MA, USA) and the samples and standards were prepared according to the instruction of the supplier. In this kit, the lipid peroxidation is determined by the reaction between malondialdehyde (MDA) and thiobarbituric acid (TBA) to form a colorimetric product, measured at 532 nm, proportional to the MDA present. Briefly, 100 mg leaf tissue was homogenized on ice in 300 µL “MDA lysis buffer” supplemented with 3 µL butylated hydroxytoluene (BHT). The samples were centrifuged at 13,000 × g for 10 min and 200 µL supernatant of each sample was used for the assay. MDA standards were prepared by dilution of “MDA standard solution” to final concentrations of 0.1 M and 2 mM MDA. The standard curve had the following points: 0, 2, 4, 8, 12, 16, and 20 nmol. The MDA–TBA complex was formed by adding 600 µL of the TBA solution in each vial containing standard or sample. Samples were incubated at 95 °C for 60 min and cooled in an ice bath for 10 min. Then, 200 µL from the reaction mixture was pipetted into 96-well reader plates. The absorbance was measured at 532 nm on the reader (Biotek, Sinergy HTX multi-mode reader, Agilent, Santa Clara, CA, USA). The concentration of MDA was calculated by the formula described in the “Lipid Peroxidation (MDA) Assay Kit”:
C = (Sa/Sv) × D, where Sa = amount of MDA in unknown sample (nmol) from the standard curve; Sv = sample volume (µL) added into the wells; D = sample dilution factor; C = concentration of MDA in sample in nmol/µL. The amount of MDA in nmol/µL−1 was recalculated for 100 mg leaf tissue.
Total antioxidant capacity (TAC) assay is electron-transfer-based procedure, measuring the capacity of an antioxidant in the reduction of an oxidant, which changes color when reduced. The degree of color change is correlated with the sample’s antioxidant concentrations. Total antioxidant capacity assay uses a Cu(II) complex as an oxidant. Samples are compared to known concentrations of Trolox standards within a 96-well microtiter plate format.
The standard curve was prepared by dilution of the Trolox with water following the protocol of the “Total Antioxidant Assay Kit”, Sigma-Aldrich. The standard points were: 0, 4, 8, 12, 16, and 20 nmol/well. Leaf tissue (100 mg) samples were washed with ice-cold PBS and resuspended in 500 µL PBS buffer. After incubation on ice for 10 min, the samples were centrifuged for 5 min at 4 °C. The supernatant was collected and kept on ice.
All samples were diluted with protein mask 1:1 prior to addition to the well. Then, 100 µL of Cu2+ working solution was added to all standard and sample wells. The plate was incubated for 90 min at room temperature in the microplate reader (Biotek, Sinergy HTX multi-mode reader) protected from light. The output was measured at OD = 570 nm.
Concentration of Trolox nmol/µL was calculated using the following formula:
Sample total antioxidant capacity = (Ts/Sv) × D, where: Ts = TAC amount in the sample well calculated from standard curve (nmol); Sv = sample volume in the sample wells (µL); D = sample dilution factor. The amount of TAC in nmol/µL−1 was recalculated for 100 mg leaf tissue.
2.5. Statistical Analyses
Data represent the mean ± SE or SD and on average were analyzed with n ≥ 3. The one-way analysis of variance (ANOVA) (Holm–Sidak) and Student (t-test) statistical tests (Microsoft, Excel 2010 software) were applied to estimate the difference between all the variants (differences were considered statistically significant at the p < 0.05 level).
3. Results
The mean values of relative transcript level, TAC activity, and quantity of MDA with and without application of abiotic stress for three types of the samples from the two progenies of both cultivars are summarized in Table S3.
3.1. Expression Profiles of Protein Kinase in the Stressed and Control Samples of Progeny of 2019 and 2020 of Treated Control, Treated, and Non-Treated Groups
All performed analyses with the expression data are displayed in Figure 1, Figure 2, Figure 3 and Figure 4. Figure 1, panel “a” presents the expression data of Izidor samples “tr. K”, “tr.”, and “K0” from the progeny of 2019 and 2020 without application of abiotic stressors—cold, salinity, and drought. The level of detected relative expression in 2019 varied from 1 in the samples “K0” and “tr. K” and dropped down to 0.034 in “tr.”. In the samples of progeny of 2020, the opposite tendency was observed. In the “K0” samples, 0.14 was the lowest expression detected and slowly rises in “tr. K” to 0.67, to reach the highest level in “tr.”, 1.4. In cv. Richy (Figure 1b), samples “tr. K”, “tr.”, and “K0” from the progeny of 2019 reacted like in cv. Izidor with detected level of expression from 1 to 0.04. In cv. Richy samples of progeny of 2020, the level of detected expression was absolutely different from those of cv. Izidor. The level of expression established in the “K0” samples was 5.0, while in the “tr. K” samples it was 4.23 and dropped down to 0.69 in “tr.”.
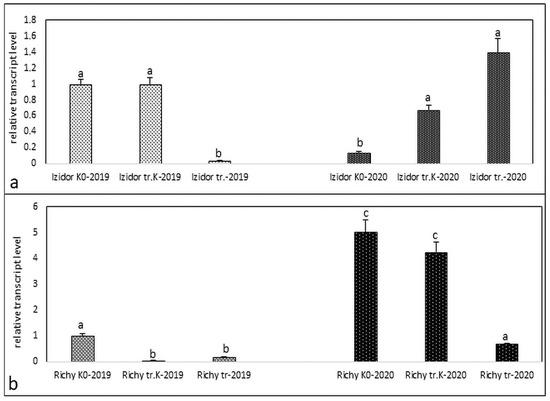
Figure 1.
Transcript level of protein kinase in samples of “tr. K”, “tr.”, and “K0” from the progeny of 2019 and 2020 without abiotic stress. (a) cv. Izidor; (b) cv. Richy. Data represent the mean ± SE. Different letters denote statistically significant differences.
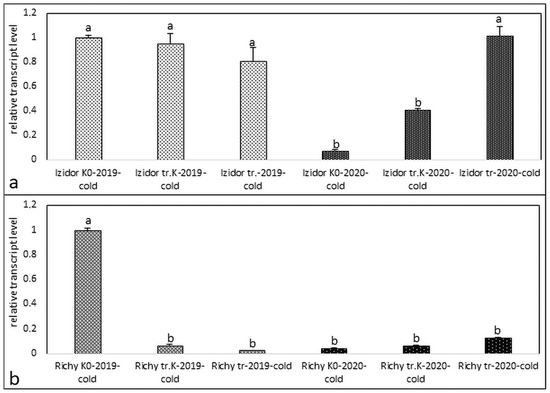
Figure 2.
Transcript level of protein kinase in samples of “tr. K”, “tr.”, and “K0” from the progeny of 2019 and 2020 after cold stress. (a) cv. Izidor; (b) cv. Richy. Data represent the mean ± SE. Different letters denote statistically significant differences.
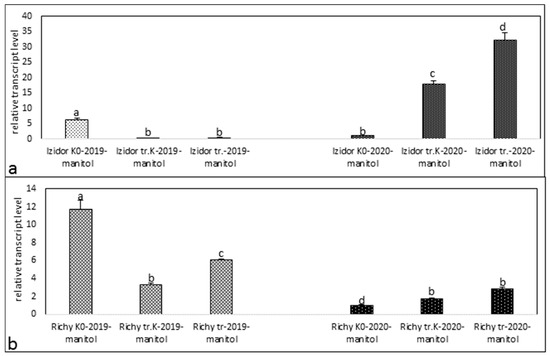
Figure 3.
Transcript level of protein kinase in samples of “tr. K”, “tr.”, and “K0” from the progeny of 2019 and 2020 after drought stress. (a) cv. Izidor; (b) cv. Richy. Data represent the mean ± SE. Different letters denote statistically significant differences.
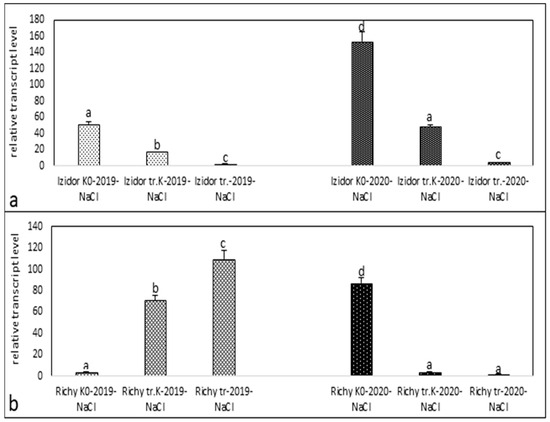
Figure 4.
Relative transcript level of stress protein kinase in samples of “tr. K”, “tr.”, and “K0” from the progeny of 2019 and 2020 after salinity stress. (a) cv. Izidor; (b) cv. Richy. Data represent the mean ± SE. Different letters denote statistically significant differences.
The treatment with abiotic cold stress (4 °C for 72 h) is presented in Figure 2a,b for the samples of the two investigated cvs. The established expression profiles for cv. Izidor (Figure 2a) drop down slowly after cold treatment of the samples of 2019 for “tr. K”, “tr.”, and “K0”, from 1 to 0.8. In contrast, for the samples of progeny of 2020, a slow increase in the relative expression levels was observed, which varied in the range from 0.07 to 1.01 from “K0” followed by “tr. K” and “tr.”. The treatment with abiotic cold stress for cv. Richy led to similar expression models for both progenies of 2019 and 2020. The cold-treated seedlings from the progeny of 2019 possessed expression levels from 1.0 to 0.02 in the order “K0”—“tr. K”—“tr”. The expression models for the samples of progeny of 2020 possess a very weak tendency of increase from “K0” to “tr. K” and “tr.” from 0.04 to 0.12. Obviously, the cold stress factor was not able to provoke activation of the investigated protein kinase. For this reason, further antioxidant activity analyses were not performed after applying cold stress.
Treatment with stress agent mannitol in cv. Izidor (Figure 3a) for the seedling samples of the progeny of 2019 showed a tendency of decreased expression in the order “K0”—“tr. K”—“tr.” from 6.26 to 0.3. For the progeny of 2020, an activation of expression level was detected from 1, in “K0”, to 17.89 in “tr. K”, to 32.19 in “tr.”. In cv. Richy (Figure 3b), the expression profiles of the seedlings from progeny of 2019 treated with stress factor mannitol resembled those of cv. Izidor 2019. Detected expression level drops down from 11.67 in the “K0” samples to 3.3 in “tr. K” and again slowly rises to 6.04 in “tr.” samples. The tendency of expression levels in seedling samples from progeny of 2020 in cv. Richy was similar to that in cv. Izidor. The lowest expression was detected in “K0” at 1 and slowly rises to 1.76 in “tr. K” and to 2.8 in “tr.”.
After NaCl solution treatment of the seedling samples of Izidor progeny of 2019 (Figure 4a), the tendency to decrease expression levels from 51.58 in “K0” to 17.12 in “tr. K” and 2.5, which was the lowest, in “tr.” was observed. This tendency was much more clearly detected in the seedling samples of the progeny of 2020, where the expression level was 153.7 in “K0”, 48.77 in “tr. K”, and 4.19 in “tr.”. The seedling samples of cv. Richy from the progeny of 2019 treated with NaCl (Figure 4b) showed different profiles of expression from those observed in cv. Izidor. Detected expression levels rise strongly from 3.48 in “K0” to 71.10 in “tr. K” and to 109.39 in “tr.”. In the NaCl-treated seedling samples of the progeny of 2020, the expression profile drops down sharply from 86.84 in “K0” to 3.94 in “tr. K” and to the lowest value of 2 in “tr.”.
3.2. Evaluation of TAC in the Stressed and Control Samples of Progeny of 2019 and 2020 of “Treated Control”, “Treated”, and “Non-Treated” Groups
The antioxidant activity analyses were performed with seedling samples without application and after application of abiotic agents NaCl and mannitol.
The results obtained from TAC analyses for cv. Richy and cv. Izidor are presented in Figure 5 and Figure 6. The established values of TAC were close and no significant differences were found among samples. Red-colored bars reflect the highest TAC values. In the samples of the 2019 and 2020 progeny, non-treated samples and samples treated with abiotic stress agents NaCl and mannitol showed the highest values of TAC recorded in both varieties tested. In cv. Richy, the highest value of TAC was detected for “tr. K” samples without applied abiotic stress (Figure 5a). After application of stress agents NaCl and mannitol, the highest values were detected in the “tr.” samples from both 2019 and 2020 progeny (Figure 5b,c). In cv. Izidor, the highest value of TAC varied among “tr. K” and “tr.” samples of the two progenies, with and without applied abiotic stress (Figure 6a–c).
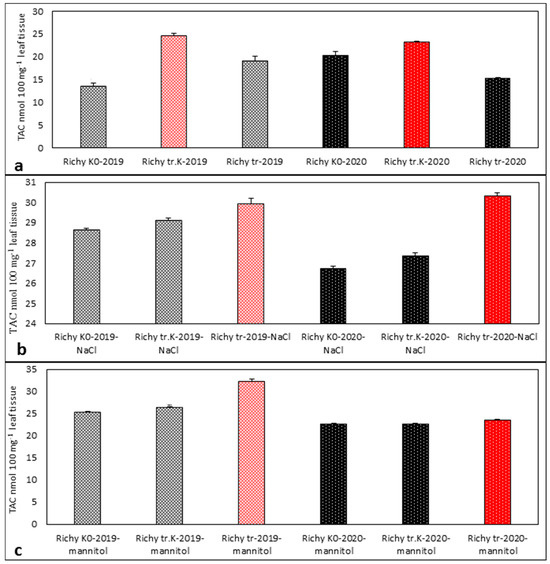
Figure 5.
Estimated TAC activity in samples of “tr. K”, “tr.”, and “K0” from the progeny of 2019 and 2020 of cv. Richy. (a) Without abiotic stress; (b) after salinity stress; (c) after drought stress. Data represent the mean ± SD.
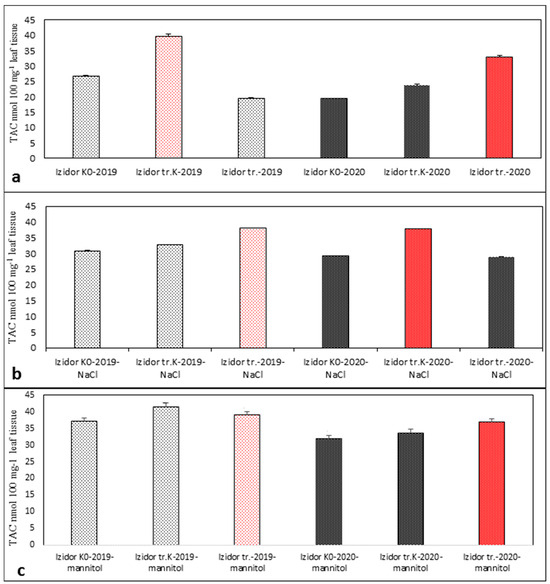
Figure 6.
Estimated TAC activity in samples of “tr. K”, “tr.”, and “K0” from the progeny of 2019 and 2020 of cv. Izidor. (a) Without abiotic stress; (b) after salinity stress; (c) after drought stress. Data represent the mean ± SD.
3.3. Evaluation of the Quantity of MDA in the Stressed and Control Samples of Progeny of 2019 and 2020 of “Treated Control”, “Treated”, and “Non-Treated” Groups
The lowest value of detected quantity of MDA characterized less oxidative stress among the samples treated with abiotic agents and control samples of 2019 and 2020 progeny. Red-colored bars reflect the lowest MDA values. In cv. Richy, the lowest value of MDA varied among “tr. K” and “tr.” samples of the two progenies, with and without applied abiotic stress (Figure 7a–c). In cv. Izidor, the lowest value of MDA was detected for “tr.” samples without applied abiotic stress (Figure 8a) and in the samples after application of stress agent NaCl (Figure 8b), while after mannitol treatment, the lowest value was detected for “tr. K” samples for both groups of offspring tested (Figure 8c).
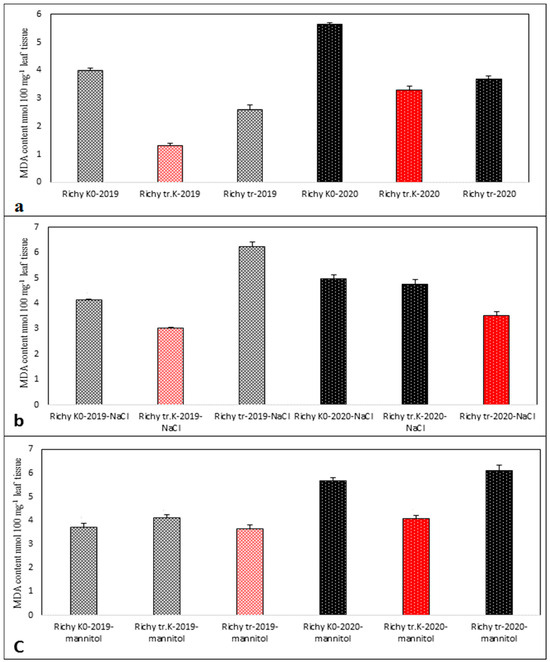
Figure 7.
Established MDA amount in samples of “tr. K”, “tr.”, and “K0” from the progeny of 2019 and 2020 of cv. Richy. (a) Without abiotic stress; (b) after salinity stress; (c) after drought stress. Data represent the mean ± SD.
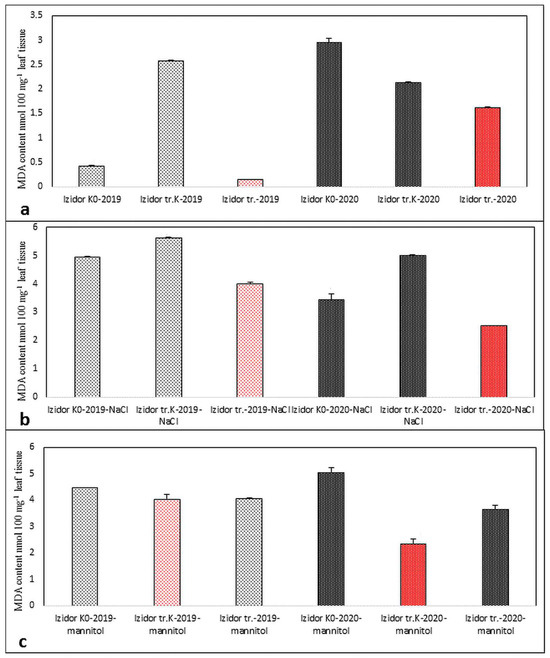
Figure 8.
Established MDA amount in samples of “tr. K”, “tr.”, and “K0” from the progeny of 2019 and 2020 of cv. Izidor. (a) Without abiotic stress; (b) after salinity stress; (c) after drought stress. Data represent the mean ± SD.
4. Discussion
4.1. Disclosure the Function of Investigated Stress Protein Kinase
Plants are characterized by their sessile life and are exposed to unfavorable environmental factors that affect their growth and development. Extreme abiotic conditions like drought, heat, soil salinity, and cold could seriously decrease the yield of crops in agricultural ecosystems [12]. Plants have developed different mechanisms to respond to adverse climatic conditions. One of the solutions to abiotic stress is ABA, a regulator of adaptive plant response. The aim of this study is to reveal the function of one protein kinase which is a part of ABA signaling components, a gene encoding sucrose non-fermentation (snf) related protein kinase subfamily 2 (SnRK2), responsible for the phosphorylation of transcription factors that promote the expression of ABA-responsive genes like PIL4, ABI2, MYB29, and DREB2A [24]. The investigated protein kinase appeared in a genome-wide comparative analysis of genes encoding core components of the ABA signaling pathway [14]. Also, this gene was selected by us in the year 2019 as a part of the data of highly expressed genes in the R5–R7 stages of soybean plant development, published in a set of transcriptomics data (www.soybean.org). A part of our investigation was focused on determining relative expression levels before and after application of abiotic stress caused by 150 mM NaCl, 350 mM mannitol, and cold (4 °C). Salt stress negatively affects plant growth, development, and crop production [25]. The abiotic stress caused by 150 mM NaCl was reported to delay the germination of soybean seeds. Post-germination growth of soybean seedlings was affected due to salinity stress. It was found that salt stress downregulates gibberellic acid (GA) and upregulates ABA [26]. The maximum rise of the expression level of stress kinase was detected after treatment with abiotic salinity stress, caused by a solution of NaCl. In cv. Izidor, samples from the two progenies react with the highest expression level in “K0” samples, followed by “tr. K” and “tr.”. In cv. Richy, the determined expression levels in samples from the progeny of 2019 rise in the order “K0”, “tr. K”, and “tr.”, which were in the opposite order from the samples of progeny of 2020. For both cultivars, the expression levels detected in the “tr. K” and “tr.” samples were lower than in “K0” in the progeny of 2020. These results confirm the idea that the “tr. K” and “tr.” samples possess higher adaptability to the applied stress agent NaCl and react with a lower level of stress protein kinase expression. Obviously, we could conclude that the studied protein kinase was highly upregulated by abiotic salinity stress and to a lesser extent by the applied drought stress. The applied abiotic cold stress (4 °C, 72 h) was not able to upregulate the expression of the studied protein kinase. The detected relative expression levels were even lower than in the samples without applied cold stress. The established transcript levels in the seedling samples “tr. K”, “tr.”, and “K0” from the two groups of offspring, after treatment with mannitol solution, showed at least double the expression levels compared to those not treated with mannitol, with the exception of Richy samples from the 2020 progeny. After mannitol treatment, the expression levels of the samples “tr. K” and “tr.” of 2020 progeny from both cultivars were increased compared to the “K0” samples.
4.2. Dependence of the Expression on Type of Sample, Genotype, and Environment
In order to better understand the results found in this study, it is important to mention, that the years of the two consecutive progenies (2019 and 2020) were characterized by different rainfall regimes during the vegetative and reproductive phases of soybean development. The year 2019 was very favorable in the periods of flowering and pod filling, compared with the year 2020, where severe drought took place during the stage of pod development. In our previous studies, which cover a three-year experimental work, we found that environmental factors and the factor of low-temperature treatment are both genotype-specific [22,23]. In this study, the transcript levels of the investigated protein kinase were also dependent on the type of the sample, i.e., “tr. K”, “tr.”, or “K0” seeds, year of the progeny, and the genotype. The role of genotype is clearly visible for the established transcript levels detected in samples without stress treatment from the 2020 progeny. They were completely different for both cvs. Izidor and Richy among tested samples of “tr. K”, “tr.”, and “K0”. As a plant material, we used soybean seedlings grown from long-lasting low-temperature-pretreated seeds before sowing in two consecutive years (2019 and 2020). This innovative approach has a positive effect on plants grown from seeds treated before sowing. There are not published studies with long-lasting low-temperature pretreatment of soybean seeds before sowing. An investigation of long-term cold treatment of lupine seedlings (7 °C) was published. The pretreated seeds germinated at 7 °C possess higher activity of α-amylase and enhanced levels of gibberellins, IAA, and kinetin [27]. In the investigation of Vieira and co-authors in 2010 [28], results of short cold pretreatment of soybean seeds at 10 °C for a 5-day period are presented. The seed germination ability observed varied from 84–94%. Other authors [29] studied the effect of fungicides, biostimulants, and proline pretreatment on seeds’ vigor and germination ability in the condition of low temperature in two crops—maize and soybean. Our published results confirmed the positive effect of low-temperature pretreatment on plant environmental adaptability, plant morphology, and quantity of free primary metabolites—amino acids, fatty acids, and sugars—in green and mature seeds collected from soybean plants grown from low-temperature-pretreated seeds [22,23]. Among published data for soybean crops, treatment of seeds before sowing with a magnetic field is the most popular technique. This approach aims to improve seed vigor, growth, and yield of soybean plants [30,31]. Also, treatments with atmospheric-pressure plasma and cold plasma are other methods recently published for the treatment of soybean seeds in order to improve seed germination, growth dynamics, and plant resistance [32,33]. In the last ten years, a lot of reports were published on short pretreatment of seeds before sowing, so-called seed priming, an approach that enhances seed performance by controlled hydration and dehydration cycles prior to sowing [34]. The advantageous effect of priming of soybean seeds is well known [35,36,37]. In future research our efforts will be concentrated on a more detailed study to understand the multifactorial relationship among the origin of the samples, environmental conditions of the year of the progeny, and genotype.
4.3. Enhanced Antioxidant Activity Depends on Type of the Sample
Determined values of TAC in the samples with applied abiotic stress caused by solutions of NaCl and mannitol were higher than those estimated for the samples without application of abiotic agents for both cvs. tested, in the two offspring. The “tr. K” and “tr.” samples had higher values of TAC. Established values of MDA were lower in the samples without application of abiotic agents. The detected amounts rise in the case of samples treated with NaCl and mannitol for both cvs. and both tested progenies. The “tr. K” and “tr.” samples possess lower values of MDA content, which indicate lower levels of oxidative stress. Recently published reports demonstrated that salt stress induced oxidative damage in soybean plants, but plants develop different mitigation strategies. Soybean seedlings exposed to salt stress (100 mM NaCl) react with 25% higher lipid peroxidation compared to the seedlings grown without stress [38]. Exposure of soybean plants to salinity stress could also increase MDA content by 56% [39]. Our results also confirmed the elevated level of detected content of MDA in salt-stress-treated samples.
5. Conclusions
The results obtained in the current study clearly indicate that the expression of the investigated stress kinase was highly upregulated after the application of abiotic stress caused by 150 mM NaCl solution and to a lesser extent by 350 mM mannitol solution. The cold stress factor was not able to provoke activation of protein kinase expression. In this preliminary research it was found that the detected expression level depends on the type of the samples, i.e., “tr. K”, “tr.”, and “K0”; the progeny; and the genotype. The performed analyses on antioxidant defense confirmed a better response against the applied stress on the samples of plants grown from low-temperature-pretreated seeds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture14101731/s1, Table S1: Primer sequences used for the expression analyses; Table S2: Significant differences found in relative expression levels detected in the samples; Table S3: Summarized data of relative transcript level.
Author Contributions
M.R. (Mariana Radkova), M.R. (Miglena Revalska) and A.I. conceptualized the study. M.R. (Mariana Radkova), M.R. (Miglena Revalska) and A.I. collected and analyzed the data and A.I. wrote the manuscript. M.R. (Mariana Radkova) and M.R. (Miglena Revalska) provided a thorough review of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project “Legume Generation”, ID 101081329, EC and supported by project “Physiological, molecular and phytopathological study of Bulgarian soybean varieties”, GFTC-29 Agricultural Academy.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank technicians Kety Krastanova and Sonia Ivanova for their help in supporting experimental work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ge, L.; Yu, J.; Wang, H.; Luth, D.; Bai, G.; Wang, K.; Chen, R. Increasing seed size and quality by manipulating BIG SEEDS1 in legume species. Proc. Natl. Acad. Sci. USA 2016, 113, 12414–12419. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, M.; Feng, F.; Tian, Z. Toward a “green revolution” for soybean. Mol. Plant 2020, 3, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Staniak, M.; Szpunar-Krok, E.; Kocira, A. Responses of Soybean to Selected Abiotic Stresses—Photoperiod, Temperature and Water. Agriculture 2023, 13, 146. [Google Scholar] [CrossRef]
- Chaves, M.M.; Oliveira, M.M. Mechanisms underlying plant resilience to water deficits: Prospects for water-saving agriculture. J. Exp. Bot. 2004, 407, 2365–2379. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Ullah, I.; Ali, S.; Kang, S.M.; Lee, I.J. Alleviation of salt stress response in soybean plants with the endophytic bacterial isolate Curtobacterium sp. SAK1. Ann. Microbiol. 2019, 69, 797–808. [Google Scholar] [CrossRef]
- Grainger, C.M.; Rajcan, I. Characterization of the genetic changes in a multi-generational pedigree of an elite Canadian soybean cultivar. Theor. Appl. Genet. 2013, 127, 211–229. [Google Scholar] [CrossRef] [PubMed]
- Mandic, V.; Ðordevic, S.; Ðordevic, N.; Bijelic, Z.; Krnjaja, V.; Petricevic, M.; Brankov, M. Genotype and Sowing Time effects on Soybean Yield and Quality. Agriculture 2020, 10, 502. [Google Scholar] [CrossRef]
- Zając, T.; Oleksy, A.; Ślizowska, A.; Śliwa, J.; Klimek-Kopyra, A.; Kulig, B. Aboveground dry biomass partitioning and nitrogen accumulation in early maturing soybean ‘Merlin’. Acta Agrobot. 2017, 70, 1728. [Google Scholar] [CrossRef]
- Naydenova, G.; Radkova, M.; Iantcheva, A. Moldovan soybean varieties testing in the condition of North Bulgaria. Bulg. J. Agric. Sci. 2022, 28, 299–304. [Google Scholar]
- Deshmukh, R.; Sonah, H.; Patil, G.; Chen, W.; Prince, S.; Mutava, R.; Vuong, T.; Valliyodan, B.; Nguyen, H.T. Integrating omic approaches for abiotic stress tolerance in soybean. Front Plant Sci. 2014, 5, 244. [Google Scholar] [CrossRef]
- Bashir, K.; Matsui, A.; Rasheed, S.; Seki, M. Recent Advances in the Characterization of Plant Transcriptomes in Response to Drought, Salinity, Heat, and Cold Stress. F1000Research 2019, 8, 658. [Google Scholar] [CrossRef] [PubMed]
- Brás, T.A.; Seixas, J.; Carvalhais, N.; Jägermeyr, J. Severity of Drought and Heatwave Crop Losses Tripled over the Last Five Decades in Europe. Environ. Res. Lett. 2021, 16, 065012. [Google Scholar] [CrossRef]
- Alharby, H.F.; Hasanuzzaman, M.; Al-Zahrani, H.S.; Hakeem, K.R. Exogenous selenium mitigates salt stress in soybean by improving growth, physiology, glutathione homeostasis and antioxidant defense. Phyton-Int. J. Exp. Bot. 2021, 90, 373–388. [Google Scholar] [CrossRef]
- Inupakutika, M.; Devireddy, A.R.; Willmon, D.; Puppala, N.; Cho, Y. Genome-wide comparative analysis of genes encoding core components of ABA signaling pathway in legume family. Int. J. Comput. Bioinform. Silico Model. 2016, 5, 828–843. [Google Scholar]
- Shukla, V.; Mattoo, A.K. Sucrose non-fermenting 1-related protein kinase 2 (SnRK2): A family of protein kinases involved in hyperosmotic stress signaling. Physiol. Mol. Biol. Plants 2008, 14, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, H.; Wei, Y. Supplemental Silicon and Boron Alleviates Aluminum-Induced Oxidative Damage in Soybean Roots. Plants 2024, 13, 821. [Google Scholar] [CrossRef]
- Choi, Y.M.; Yoon, H.; Lee, S.; Ko, H.C.; Shin, M.J.; Lee, M.C.; Hur, O.S.; Ro, N.Y.; Desta, K.T. Isofavones, anthocyanins, phenolic content, and antioxidant activities of black soybeans (Glycine max (L.) Merrill) as afected by seed weight. Sci. Rep. 2020, 10, 19960. [Google Scholar] [CrossRef]
- Pinheiro, D.T.; dos Santos Dias, D.C.F.; da Silva, L.J.; Martins, M.S.; Finger, F.L. Oxidative stress, protein metabolism, and physiological potential of soybean seeds under weathering deterioration in the pre-harvest phase. Acta Sci. Agron. 2023, 45, e56910. [Google Scholar] [CrossRef]
- Porcel, R.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal influence on leaf water potential,solute accumulation, and oxidative stress in soybean plants subjected to drought stress. J. Exp. Bot. 2004, 55, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Iantcheva, A.; Dincheva, I.; Nedeva, R.; Naydenova, G.; Badjakov, I.; Radkova, M.; Revalska, M.; Apostolov, A. An innovative approach for assessment of Bulgarian soybean cultivars. Biotechnol. Biotechnol. Equip. 2021, 35, 1099–1117. [Google Scholar] [CrossRef]
- Naydenova, G.; Dincheva, I.; Badjakov, I.; Radkova, M.; Revalska, M.; Iantcheva, A. Long-lasting low temperature pretreatment of soybean seeds enhance plant field performance and content of free metabolites. Bulg. J. Agric. Sci. 2022, 28, 1063–1074. [Google Scholar]
- Liu, S.; Lv, Z.; Liu, Y.; Li, L.; Zhang, L. Network analysis of ABA-dependent and ABA-independent drought responsive genes in Arabidopsis thaliana. Genet. Mol. Biol. 2018, 41, 624–637. [Google Scholar] [CrossRef]
- Chourasia, K.N.; Lal, M.K.; Tiwari, R.K.; Dev, D.; Kardile, H.B.; Patil, V.U.; Kumar, A.; Vanishree, G.; Kumar, D.; Bhardwaj, V.; et al. Salinity Stress in Potato: Understanding Physiological, Biochemical and Molecular Responses. Life 2021, 11, 545. [Google Scholar] [CrossRef]
- Shu, K.; Qi, Y.; Chen, F.; Meng, Y.; Luo, X.; Shuai, H.; Zhou, W.; Ding, J.; Du, J.; Liu, J.; et al. Salt stress represses soybean seed germination by negatively regulating GA biosynthesis while positively mediating ABA biosynthesis. Front. Plant Sci. 2017, 8, 1372. [Google Scholar] [CrossRef]
- Płazek, A.; Dubert, F.; Kopec, P.; Dziurka, M.; Kalandyk, A.; Pastuszak, J.; Waligórski, P.; Wolko, B. Long-term effects of cold on growth, development and yield of narrow-leaf lupine may be alleviated by seed hydropriming or butenolide. Int. J. Mol. Sci. 2018, 19, 2416. [Google Scholar] [CrossRef]
- Vieira, B.G.T.L.; Vieira, R.D.; Krzyzanowski, F.C.; Franca-Neto, J.d.B. Alternative procedure for the cold test for soybean seeds. Sci. Agric. 2010, 67, 540–545. [Google Scholar] [CrossRef]
- Vinkovic, T.; Paradikovic, N.; Plavisic, H.; Guberac, V.; Lavai, L. Maize and soybean seed vigor under influence of seed age, seed treatment and temperature in cold stress test. Cereal Res. Commun. 2007, 35, 1213–1216. [Google Scholar] [CrossRef]
- Joshi-Paneri, J.; Sharma, S.; Guruprasad, K.N.; Kataria, S. Enhancing the Yield Potential of Soybean after Magneto-Priming: Detailed Study on Its Relation to Underlying Physiological Processes. Seeds 2023, 2, 60–84. [Google Scholar] [CrossRef]
- Radhakrishnan, R. Seed pretreatment with magnetic field alters the storage proteins and lipid profiles in harvested soybean seeds. Physiol. Mol. Biol. Plants 2018, 24, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Ďurčányová, S.; Slováková, L.; Klas, M.; Tomeková, J.; Ďurina, P.; Stupavská, M.; Kováčik, D.; Zahoranová, A. Efcacy Comparison of Three Atmospheric Pressure Plasma Sources for Soybean Seed Treatment: Plasma Characteristics, Seed Properties, Germination. Plasma Chem. Plasma Process. 2023, 43, 1863–1885. [Google Scholar] [CrossRef]
- Švubová, R.; Slováková, L.; Holubová, L.; Rovnanová, D.; Gálová, E.; Tomeková, J. Evaluation of the Impact of Cold Atmospheric Pressure Plasma on Soybean Seed Germination. Plants 2021, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Mazhar, K.; Ijaz, M.; Ali, Q.; Ahmad, S. Seedling pretreatment: Methods and protocols. In Priming and Pretreatment of Seeds and Seedlings; Hasanuzzaman, M., Fotopoulos, V., Eds.; Springer: Singapore, 2019; pp. 117–134. [Google Scholar]
- Agawane, R.B.; Parhe, S.D. Effect of seed priming on crop growth and seed yield of soybean [Glycine max (L.) MERILL]. Bioscan 2015, 10, 265–270. [Google Scholar]
- Lewandowska, S.; Łoziński, M.; Marczewski, K.; Kozak, M.; Schmidtke, K. Influence of priming on germination, development, and yield of soybean varieties. Open Agric. 2020, 5, 930–935. [Google Scholar] [CrossRef]
- Sadeghi, H.; Khazaei, F.; Yari, L.; Sheidaei, S. Effect of seed osmopriming on seed germination behavior and vigor of soybean (Glycine max L.). J. Agric. Biol. Sci. 2011, 6, 39–43. [Google Scholar]
- Elkelish, A.A.; Alnusaire, T.S.; Soliman, M.H.; Gowayed, S.; Senousy, H.H.; Fahad, S. Calcium availability regulates antioxidant system, physio-biochemical activities and alleviates salinity stress mediated oxidative damage in soybean seedlings. J. Appl. Bot. Food Qual. 2019, 92, 258–266. [Google Scholar] [CrossRef]
- Alharby, H.F.; Nahar, K.; Al-Zahrani, H.S.; Hakeem, K.R.; Hasanuzzaman, M. Enhancing salt tolerance in soybean by exogenous boron: Intrinsic study of the ascorbate-glutathione and glyoxalase pathways. Plants 2021, 10, 2085. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).