Region and Crop Type Influenced Fungal Diversity and Community Structure in Agricultural Areas in Qinghai Province
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Sampling of Soils from Multiple Locations and Physicochemical Analysis
2.2. Microbial DNA Extraction and Illumina High-Throughput Sequencing
2.3. Data Processing and Statistical Analysis
3. Results
3.1. Regions and Crop Types Influenced the α-Diversity of Fungi
3.2. Regions and Crop Types Influenced the β-Diversity of Fungal Communities
3.3. OTUs of Fungi
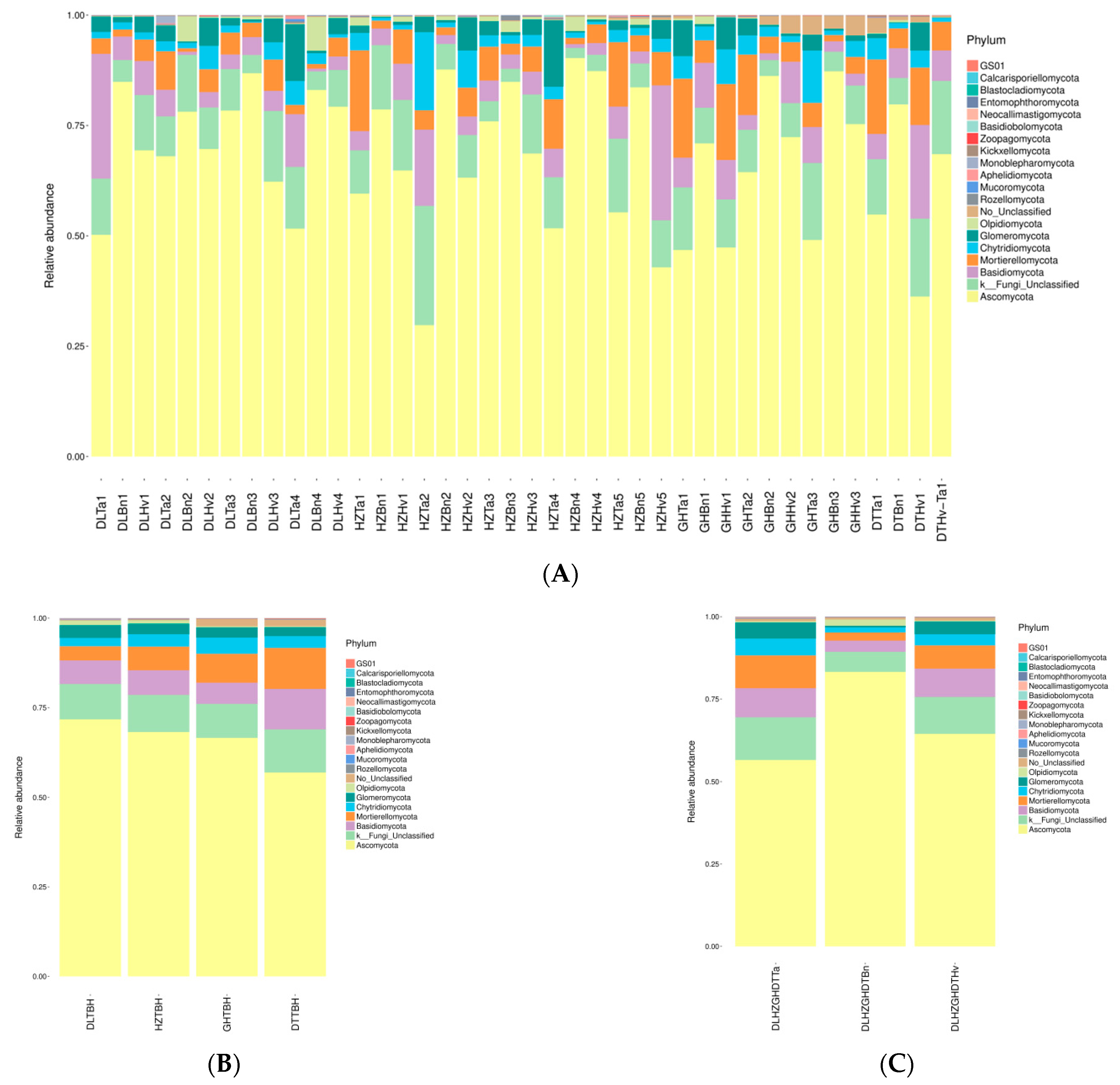
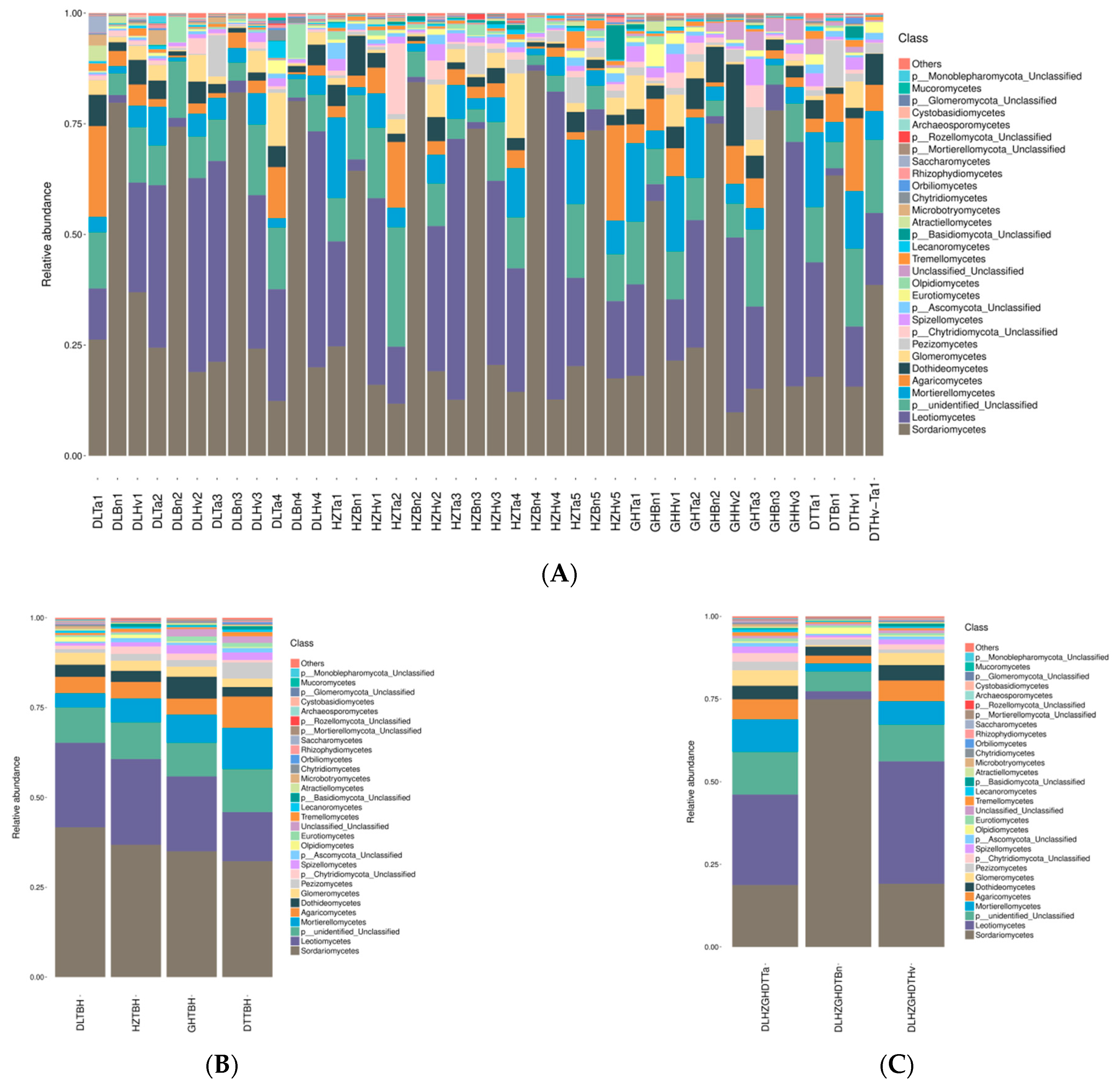
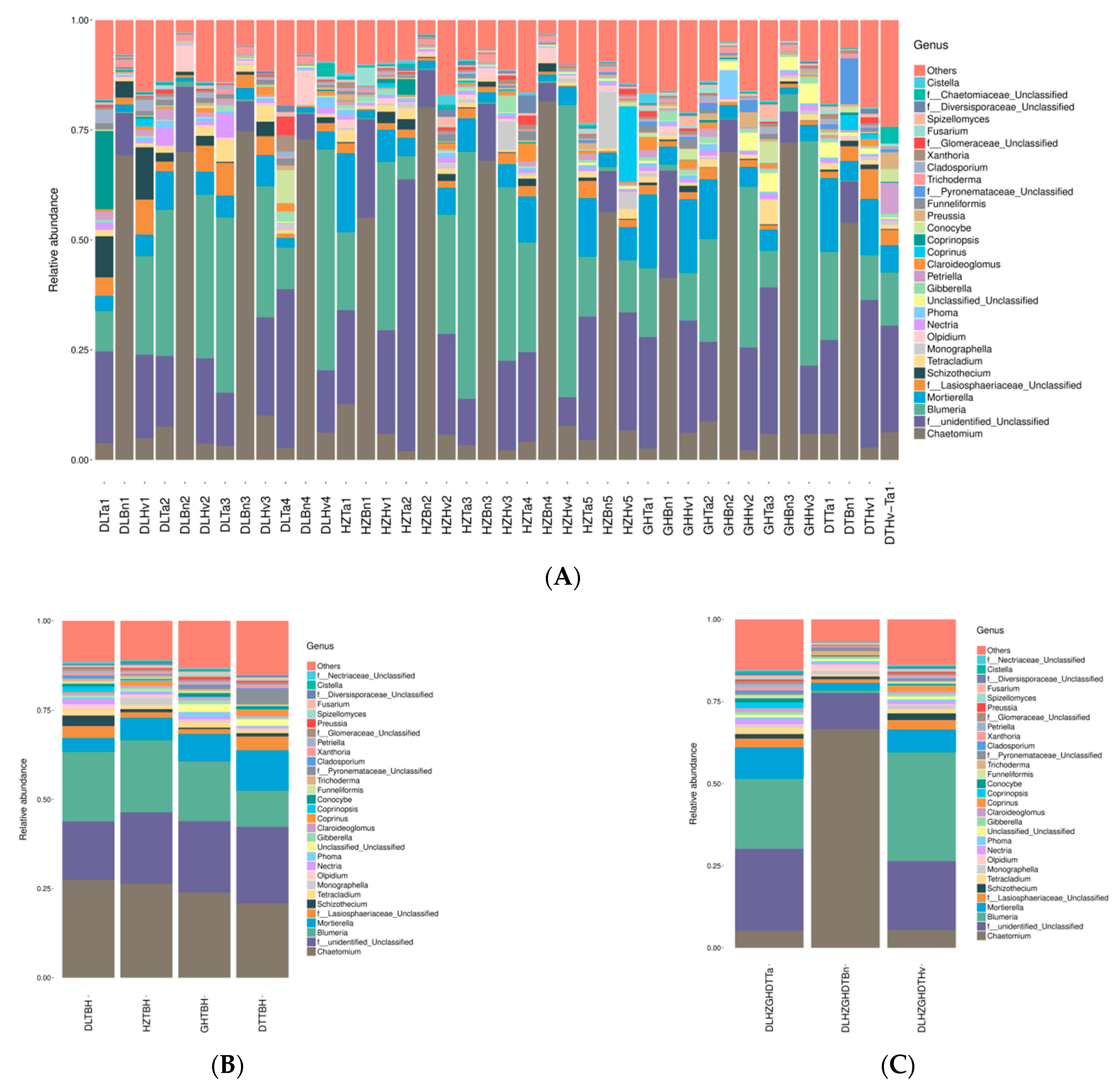
3.4. Regions and Crop Types Influenced Fungal Community Compositions
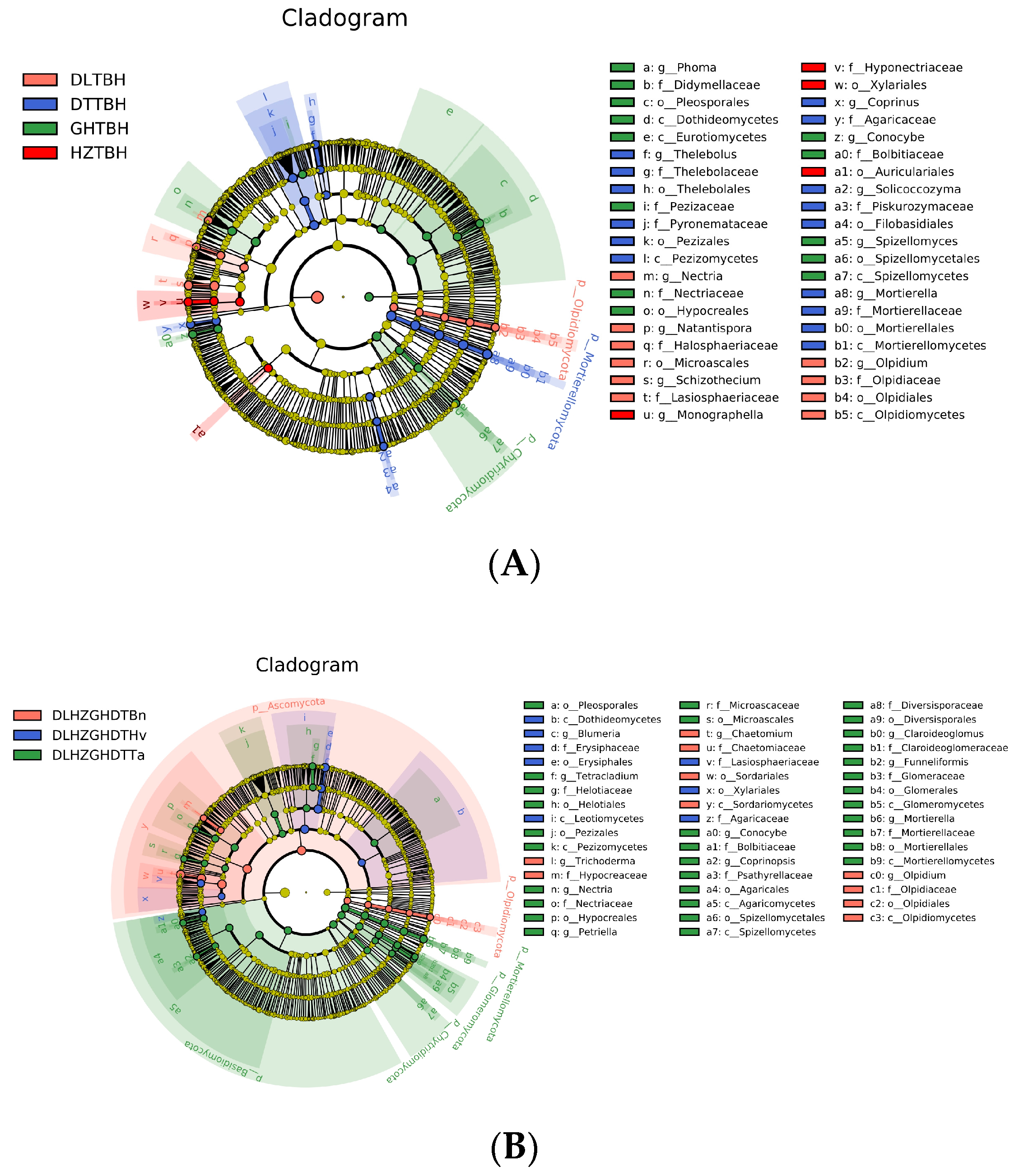
3.5. Regions and Crop Types Influenced Biomarker Taxa
3.6. Regions and Crop Types Influenced Soil Properties and Fungal Genera
3.7. Relationships among Selected Fungal Genera, Measured Diversity Indices, and Soil Properties
4. Discussion
4.1. Variation in Soil Fungal Diversity among Regions and Crop Types
4.2. Variations in Fungal Community Compositions among Regions and Crop Types
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, M.G.A.; Wagg, C. Soil microbial diversity and agro-ecosystem functioning. Plant Soil 2013, 363, 1–5. [Google Scholar] [CrossRef]
- Avidano, L.; Gamalero, E.; Cossa, G.P.; Carraro, E. Characterization of soil health in an Italian polluted site by using microorganisms as bioindicators. Appl. Soil Ecol. 2005, 30, 21–33. [Google Scholar] [CrossRef]
- Vukicevich, E.; Lowery, T.; Bowen, P.; Úrbez-Torres, J.R.; Hart, M. Cover crops to increase soil microbial diversity and mitigate decline in perennial agriculture. A review. Agron. Sustain. Dev. 2016, 36, 48. [Google Scholar] [CrossRef]
- Maguire, V.G.; Bordenave, C.D.; Nieva, A.S.; Llames, M.E.; Colavolpe, M.B.; Gárriz, A.; Ruiz, O.A. Soil bacterial and fungal community structure of a rice monoculture and rice-pasture rotation systems. Appl. Soil Ecol. 2020, 151, 103535. [Google Scholar] [CrossRef]
- Aguilar-Trigueros, C.A.; Hempel, S.; Powell, J.R.; Anderson, I.C.; Antonovics, J.; Bergmann, J.; Cavagnaro, T.R.; Chen, B.; Hart, M.M.; Klironomos, J.; et al. Branching out, towards a trait-based understanding of fungal ecology. Fungal Biol. Rev. 2015, 29, 34–41. [Google Scholar] [CrossRef]
- Ehrmann, J.; Ritz, K. Plant: Soil interactions in temperate multi-cropping production systems. Plant Soil 2014, 376, 1–29. [Google Scholar] [CrossRef]
- Finlay, B.J. Global dispersal of free-living microbial eukaryote species. Science 2002, 296, 1061–1063. [Google Scholar] [CrossRef]
- Fontaine, S.; Henault, C.; Aamor, A.; Bdioui, N.; Bloor, J.M.G.; Maire, V.; Mary, B.; Revaillot, S.; Maron, P.A. Fungi mediate long term sequestration of carbon and nitrogen in soil through their priming effect. Soil Biol. Biochem. 2011, 43, 86–96. [Google Scholar] [CrossRef]
- Tautges, N.E.; Sullivan, T.S.; Reardon, C.L.; Burke, I.C. Soil microbial diversity and activity linked to crop yield and quality in a dryland organic wheat production system. Appl. Soil Ecol. 2016, 108, 258–268. [Google Scholar] [CrossRef]
- Liu, C.; Ding, N.; Fu, Q.; Brookes, P.C.; Xu, J.; Guo, B.; Lin, Y.; Li, H.; Li, N. The influence of soil properties on the size and structure of bacterial and fungal communities along a paddy soil chronosequence. Eur. J. Soil Biol. 2016, 76, 9–18. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.H.; Li, X.; Li, X.Y.; Zhang, H.W. Distribution characteristics of fungal communities with depth in paddy fields of three soil types in China. J. Microb. 2020, 58, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Moll, J.; Hoppe, B.; König, S.; Wubet, T.; Buscot, F.; Krüger, D. Spatial distribution of fungal communities in an arable soil. PLoS ONE 2016, 11, e0148130. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.Y.; He, H.; Zhu, F.X.; Liu, X.B.; Ma, Y.; Xie, W.M.; Meng, H.; Zhang, L.M. Community structure and co-occurrence network analysis of bacteria and fungi in wheat fields vs fruit orchards. Arch. Microbiol. 2022, 204, 453. [Google Scholar] [CrossRef] [PubMed]
- Beauregard, M.S.; Hamel, C.; Atul-Nayyar St-Arnaud, M. Long-term phosphorus fertilization impacts soil fungal and bacterial diversity but not AM fungal community in Alfalfa. Microb. Ecol. 2010, 59, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Ai, C.; Ding, W.; Jiang, R.; Zhao, S.; Zhang, J.; Zhou, W.; Hou, Y.; He, P. The response of soil fungal diversity and community composition to long-term fertilization. Appl. Soil Ecol. 2019, 140, 35–41. [Google Scholar] [CrossRef]
- Schmidt, R.; Mitchell, J.; Scow, K. Cover cropping and notill increase diversity and symbiotroph: Saprotroph ratios of soil fungal communities. Soil Biol. Biochem. 2019, 129, 99–109. [Google Scholar] [CrossRef]
- Wang, J.C.; Rhodes, G.; Huang, Q.W.; Shen, Q.R. Plant growth stages and fertilization regimes drive soil fungal community compositions in a wheat-rice rotation system. Biol. Fertil. Soils 2018, 54, 731–742. [Google Scholar] [CrossRef]
- Jiang, Y.; Liang, Y.; Li, C.; Wang, F.; Sui, Y.; Suvannange, N.; Zhou, J.; Sun, B. Crop rotations alter bacterial and fungal diversity in paddy soils across East Asia. Soil Biol. Biochem. 2016, 95, 250–261. [Google Scholar] [CrossRef]
- Yuan, C.L.; Zhang, L.M.; Wang, J.T.; Teng, W.K.; Hu, H.W.; Shen, J.P.; He, J.Z. Limited effects of depth (0–80 cm) on communities of archaea, bacteria and fungi in paddy soil profiles. Eur. J. Soil Sci. 2020, 71, 955–966. [Google Scholar] [CrossRef]
- Liu, J.J.; Sui, Y.Y.; Yu, Z.H.; Shi, Y.; Chu, H.Y.; Jin, J.; Liu, X.B.; Wang, G.H. Soil carbon content drives the biogeographical distribution of fungal communities in the black soil zone of northeast China. Soil Biol. Biochem. 2015, 83, 29–39. [Google Scholar] [CrossRef]
- Hannula, S.E.; de Boer, W.; van Veen, J.A. In situ dynamics of soil fungal communities under different genotypes of potato, including a genetically modified cultivar. Soil Biol. Biochem. 2010, 42, 2211–2223. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, C.G.; Kang, H. Temporal dynamics of bacterial and fungal communities in a genetically modified (GM) Rice Ecosystem. Microb. Ecol. 2011, 61, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zheng, Z.C.; Li, T.X.; Wang, Y.D.; Huang, H.G.; Yu, H.Y.; Ye, D.H.; Liu, T.; Yao, T.Y.; Zhang, X.Z. Variations of fungal communities within the soils of different tea varieties (Camellia sinensis L.) following long-term plantation. Plant Soil 2022, 477, 665–677. [Google Scholar] [CrossRef]
- Kong, X.; Han, Z.F.; Tai, X.; Jin, D.C.; Ai, S.; Zheng, X.X.; Bai, Z.H. Maize (Zea mays L. Sp.) varieties significantly influence bacterial and fungal community in bulk soil, rhizosphere soil and phyllosphere. FEMS Microbiol. Ecol. 2020, 96, fiaa020. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Yuan, C.L.; Zhang, L.M.; Hu, H.W.; Wang, J.T.; Shen, J.P.; He, J.Z. The biogeography of fungal communities in paddy soils is mainly driven by geographic distance. J. Soil Sediment. 2018, 18, 1795–1805. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Huang, X.P.; Mo, C.H.; Yu, J.; Zhao, H.M.; Meng, C.; Li, Y.W.; Li, H.; Cai, Q.Y.; Wong, M.H. Variations in microbial community and ciprofloxacin removal in rhizospheric soils between two cultivars of Brassica parachinensis L. Sci. Total Environ. 2017, 603–604, 66–76. [Google Scholar] [CrossRef]
- Granzow, S.; Kaiser, K.; Wemheuer, B.; Pfeiffer, B.; Daniel, R.; Vidal, S.; Wemheuer, F. The effects of cropping regimes on fungal and bacterial communities of wheat and faba bean in a greenhouse pot experiment differ between plant species and compartment. Front. Microbiol. 2017, 8, 902. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, B.; Liu, Y.; Guo, Y.; Shi, P.; Wei, G. Distinct large-scale biogeographic patterns of fungal communities in bulk soil and soybean rhizosphere in China. Sci. Total Environ. 2018, 644, 791–800. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.N.; Zhang, J.C.; Liu, H.; Wang, M.; Pan, L.J.; Chen, N.; Wang, T.; Jing, Y.; Chi, X.Y.; Du, B.H. Long-term continuously monocropped peanut significantly disturbed the balance of soil fungal communities. J. Microbiol. 2020, 58, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.J.; Gu, H.D.; Liu, J.J.; Zhou, B.K.; Wei, D.; Chen, X.L.; Wang, G.H. High variation of fungal communities and associated potential plant pathogens induced by long-term addition of N fertilizers rather than P, K fertilizers: A case study in a Mollisol field. Soil Ecol. Lett. 2022, 4, 348–361. [Google Scholar] [CrossRef]
- Kuramae, E.; Hillekens, R.; de Hollander, M.; van der Heijden, M.; van den Berg, M.; van Straalen, N.; Kowalchuk, G. Structural and functional variation in soil fungal communities associated with litter bags containing maize leaf. FEMS Microbiol. Ecol. 2013, 84, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, M.; Tamaki, H.; Manome, A.; Koyama, O.; Kamagata, Y. Isolation and characterization of antagonistic fungi against potato scab pathogens from potato field soils. FEMS Microb. Lett. 2010, 305, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Li, R.; Ren, Y.; Liu, C.; Zhao, Q.; Wu, H.; Jousset, A.; Shen, Q. Distinct roles for soil fungal and bacterial communities associated with the suppression of vanilla Fusarium wilt disease. Soil Biol. Biochem. 2017, 107, 198–207. [Google Scholar] [CrossRef]
- Johnson, J.M.; Ludwig, A.; Furch, A.; Mithöfer, A.; Scholz, S.S.; Reichelt, M.; Oelmüller, R. The beneficial root-colonizing fungus Mortierella hyalina promotes the aerial growth of Arabidopsis and activates calcium-dependent responses which restrict Alternaria brassicae-induced disease development in roots. Mol. Plant Microbe Interact. 2019, 32, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Osorio Vega, N.W.; Habte, M.; León Peláez, J.D. Effectiveness of a rock phosphate solubilizing fungus to increase soil solution phosphate impaired by the soil phosphate sorption capacity. Rev. Fac. Nac. Agron. 2015, 68, 7627–7636. [Google Scholar] [CrossRef]
- Tamayo-Velez, A.; Osorio, N.W. Co-inoculation with an arbuscular mycorrhizal fungus and a phosphate-solubilizing fungus promotes the plant growth and phosphate uptake of avocado plantlets in a nursery. Botany 2017, 95, 539–545. [Google Scholar] [CrossRef]
- Aranyi, N.R.; Varga, I.; Poczai, P.; Cernák, I.; Vida, G.; Molnár-Láng, M.; Hoffmann, B. What types of powdery mildew can infect wheat-barley introgression lines? Eur. J. Plant Pathol. 2014, 139, 19–25. [Google Scholar] [CrossRef]
- Zhao, J.L.; Xu, C.; Xu, J.P.; Huang, L.S.; Zhang, D.Y.; Liang, D. Forecasting the wheat powdery mildew (Blumeria graminis f. Sp. tritici) using a remote sensing-based decision-tree classification at a provincial scale. Australas. Plant Pathol. 2018, 47, 53–61. [Google Scholar]
- Wu, Q.; Sun, R.Y.; Ni, M.; Yu, J.; Li, Y.Q.; Yu, C.J.; Dou, K.; Ren, J.H.; Chen, J. Identification of a novel fungus, Trichoderma asperellum GDFS1009, and comprehensive evaluation of its biocontrol efficacy. PLoS ONE 2017, 12, e0179957. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.T.; Wang, Y.F.; Liu, C.L.; Zhao, M.; Chang, Y.; Zhang, R.S. Trichoderma asperellum ACCC30536 inoculation improves soil nutrition and leaf artemisinin production in Artemisia annua. Acta Physiol. Plant. 2019, 41, 46. [Google Scholar] [CrossRef]
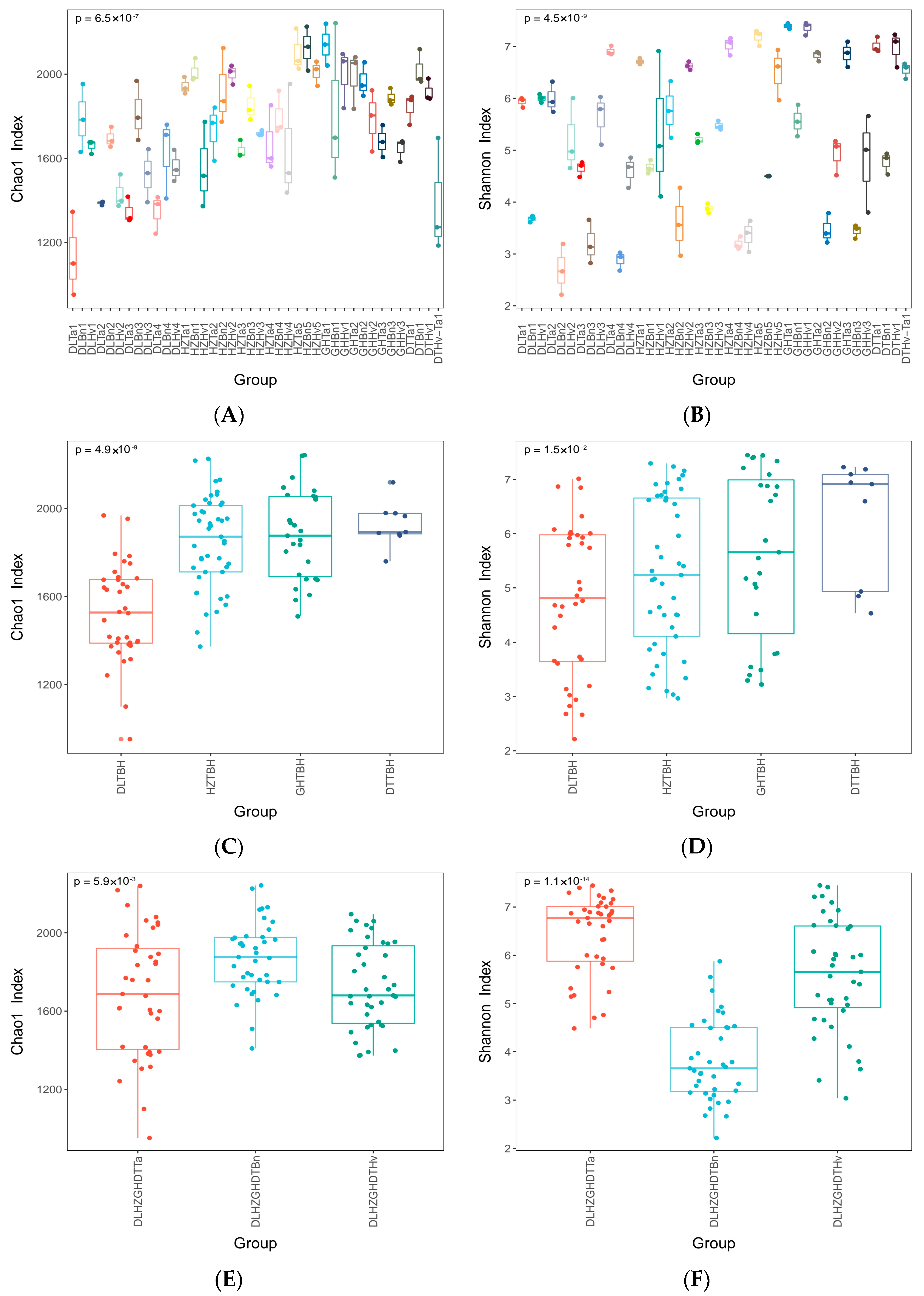
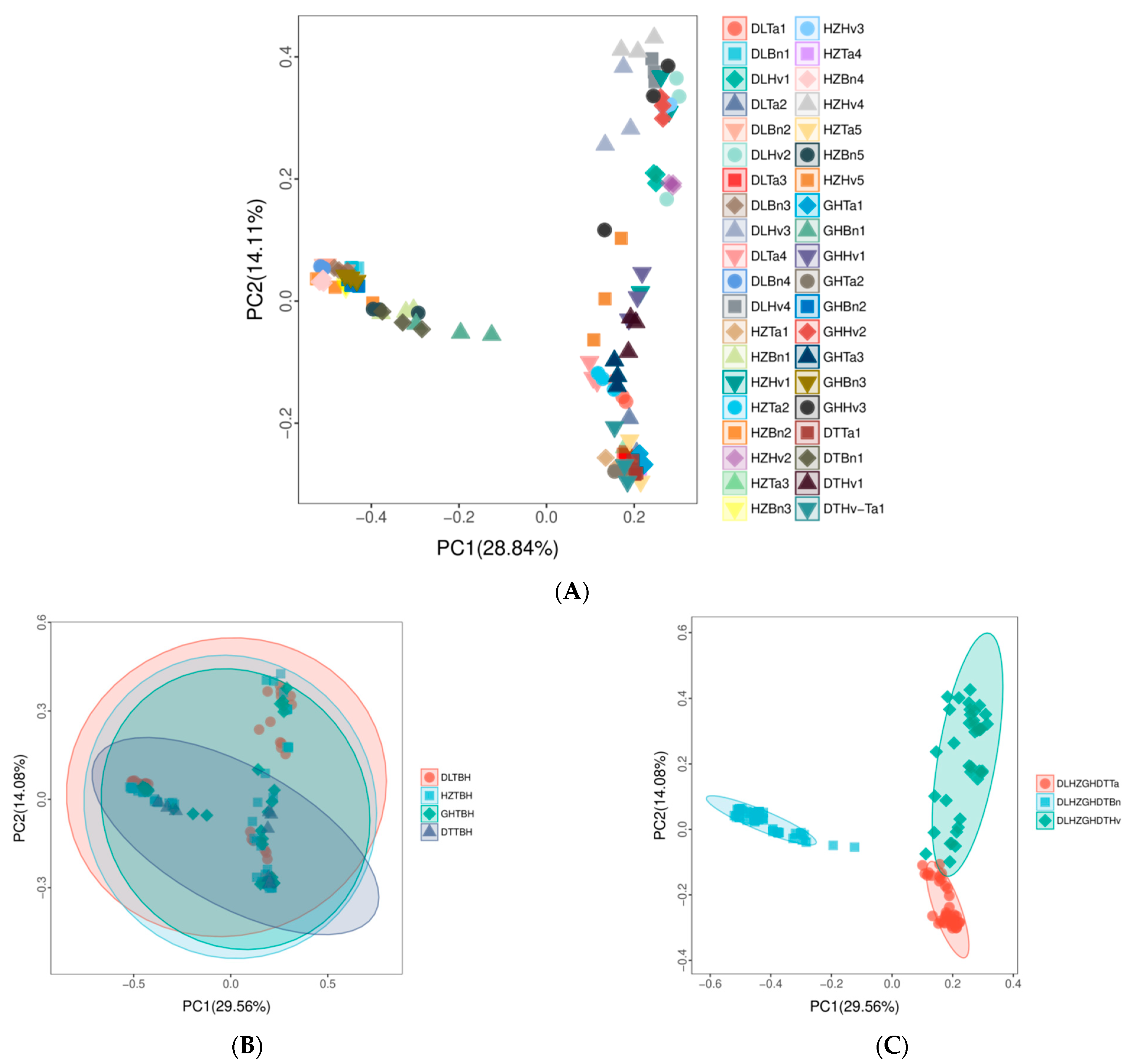

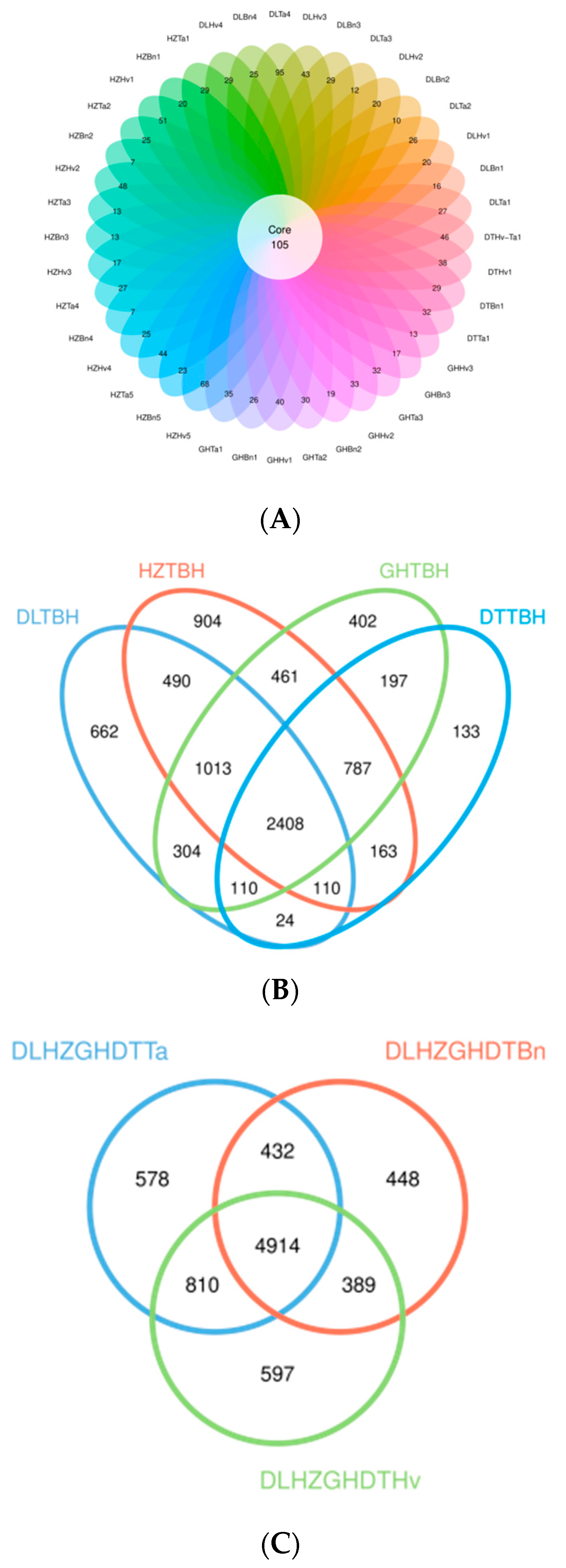
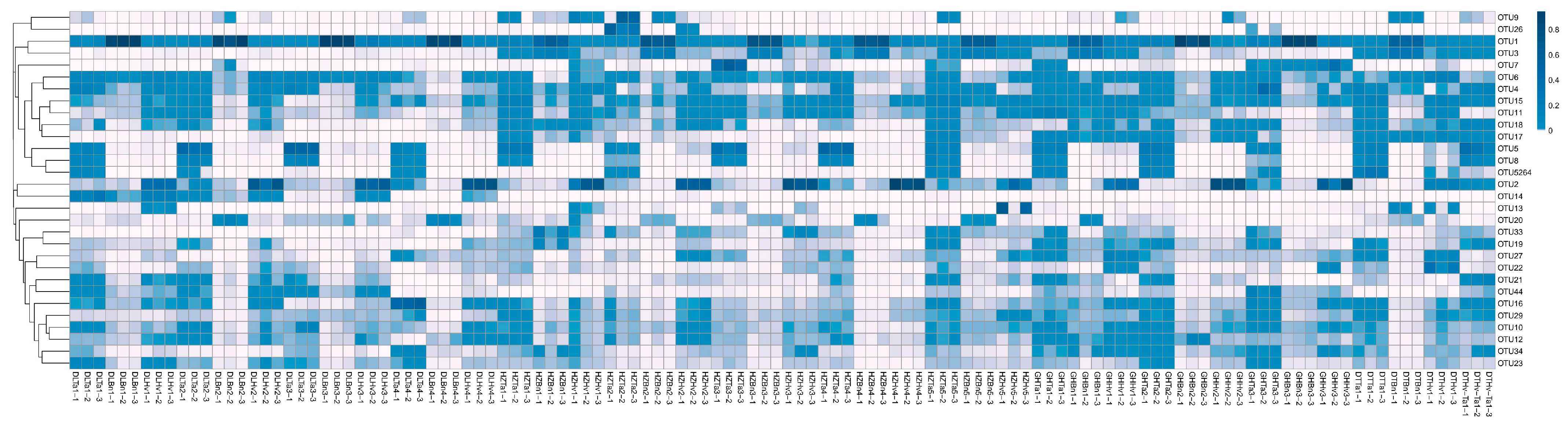
| Fungal Genera or Soil Physicochemical Properties | Region | Crop | Region × Crop | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Ace | 31.795 | 0.000 | 10.270 | 0.000 | 3.325 | 0.005 |
| Chao1 | 31.137 | 0.000 | 6.859 | 0.002 | 3.734 | 0.002 |
| Shannon | 8.984 | 0.000 | 61.972 | 0.000 | 0.881 | 0.512 |
| Simpson | 6.791 | 0.000 | 49.290 | 0.000 | 4.194 | 0.001 |
| Goods coverage | 11.917 | 0.000 | 22.613 | 0.000 | 2.713 | 0.017 |
| pH | 25.543 | 0.000 | 1.658 | 0.196 | 6.147 | 0.000 |
| Organic matter | 58.721 | 0.000 | 2.509 | 0.086 | 3.482 | 0.004 |
| Ammonium nitrogen | 8.414 | 0.000 | 1.813 | 0.168 | 1.335 | 0.248 |
| Nitrate nitrogen | 16.850 | 0.000 | 1.495 | 0.229 | 3.614 | 0.003 |
| Total phosphate | 31.092 | 0.000 | 7.782 | 0.001 | 4.513 | 0.000 |
| Effective phosphate | 5.349 | 0.002 | 8.127 | 0.001 | 5.479 | 0.000 |
| Total sulfur | 50.497 | 0.000 | 1.831 | 0.165 | 26.452 | 0.000 |
| Effective sulfur | 16.768 | 0.000 | 3.064 | 0.051 | 7.126 | 0.000 |
| Chaetomium | 2.482 | 0.065 | 536.688 | 0.000 | 2.107 | 0.058 |
| Unclassified genus | 1.278 | 0.286 | 16.319 | 0.000 | 1.162 | 0.332 |
| Blumeria | 1.659 | 0.180 | 31.454 | 0.000 | 1.265 | 0.280 |
| Mortierella | 14.644 | 0.000 | 43.100 | 0.000 | 2.852 | 0.013 |
| Unclassified Lasiosphaeriaceae | 19.926 | 0.000 | 16.780 | 0.000 | 7.589 | 0.000 |
| Schizothecium | 8.861 | 0.000 | 1.217 | 0.300 | 1.516 | 0.180 |
| Tetracladium | 2.156 | 0.098 | 12.052 | 0.000 | 1.348 | 0.243 |
| Monographella | 3.165 | 0.028 | 0.217 | 0.806 | 0.617 | 0.716 |
| Olpidium | 2.773 | 0.045 | 6.956 | 0.001 | 2.813 | 0.014 |
| Nectria | 4.301 | 0.007 | 6.734 | 0.002 | 3.916 | 0.001 |
| α-Diversity Indices and Soil Properties | Chaetomium | Unclassified Genus | Blumeria | Mortierella | Unclassified Lasiosphaeriaceae | Schizothecium | Tetracladium | Monographella | Olpidium | Nectria |
|---|---|---|---|---|---|---|---|---|---|---|
| Ace | 0.329 * | −0.044 | −0.424 ** | 0.281 | −0.287 | −0.374* | −0.284 | 0.237 | −0.035 | −0.417 ** |
| Chao1 | 0.303 | −0.027 | −0.411 ** | 0.313 | −0.292 | −0.388* | −0.281 | 0.261 | −0.026 | −0.412 ** |
| Shannon | −0.780 ** | 0.667 ** | 0.143 | 0.770 ** | 0.317 * | 0.114 | 0.333 * | 0.011 | −0.510 ** | 0.156 |
| Simpson | −0.787 ** | 0.655 ** | 0.188 | 0.658 ** | 0.398 * | 0.171 | 0.383 * | 0.047 | −0.502 ** | 0.272 |
| Altitude | 0.028 | −0.073 | 0.059 | −0.293 | 0.144 | 0.150 | 0.280 | −0.079 | 0.032 | 0.367 * |
| pH | −0.132 | 0.088 | −0.002 | 0.144 | 0.042 | 0.046 | −0.011 | −0.001 | 0.010 | 0.049 |
| Water content | 0.345 * | −0.208 | −0.273 | −0.063 | −0.153 | −0.136 | −0.448 ** | 0.458 * | 0.184 | −0.410 * |
| Organic matter | 0.119 | −0.040 | −0.087 | 0.063 | −0.206 | −0.208 | −0.162 | 0.292 | 0.042 | −0.203 |
| Ammonium nitrogen | 0.119 | −0.061 | −0.144 | −0.120 | 0.326 * | −0.036 | 0.398 * | 0.120 | −0.038 | 0.301 |
| Nitrate nitrogen | −0.069 | 0.167 | 0.015 | −0.166 | −0.321 * | −0.096 | 0.169 | 0.339 * | 0.063 | −0.201 |
| Total phosphate | 0.144 | −0.077 | −0.084 | 0.113 | −0.319 * | −0.281 | −0.080 | 0.142 | −0.099 | −0.128 |
| Effective phosphate | −0.042 | −0.058 | −0.037 | 0.154 | 0.179 | −0.030 | −0.015 | 0.060 | −0.114 | 0.076 |
| Total sulfur | 0.087 | −0.094 | −0.091 | −0.127 | −0.078 | −0.109 | −0.152 | 0.387 * | 0.014 | −0.087 |
| Effective sulfur | 0.011 | −0.019 | −0.016 | −0.039 | −0.153 | −0.163 | −0.044 | 0.201 | 0.118 | −0.250 |
| Soil Physicochemical Properties | Ace | Chao1 | Shannon | Simpson |
|---|---|---|---|---|
| Altitude | –0.342 * | –0.351 * | –0.204 | –0.200 |
| pH | –0.114 | –0.102 | 0.200 | 0.104 |
| Water content | 0.420 ** | 0.438 ** | –0.174 | –0.208 |
| Organic matter | 0.429 ** | 0.426 ** | 0.008 | 0.085 |
| Ammonium nitrogen | –0.074 | –0.088 | –0.137 | –0.092 |
| Nitrate nitrogen | –0.028 | –0.011 | 0.080 | 0.080 |
| Total phosphate | 0.527 ** | 0.501 ** | –0.035 | 0.030 |
| Effective phosphate | 0.171 | 0.139 | 0.099 | 0.174 |
| Total sulfur | 0.133 | 0.139 | –0.050 | –0.004 |
| Effective sulfur | 0.249 | 0.264 | –0.006 | –0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Ma, X.; Wang, L.; Sun, W.; Liu, Y.; Ma, Y.; Xie, H.; Qiao, F. Region and Crop Type Influenced Fungal Diversity and Community Structure in Agricultural Areas in Qinghai Province. Agriculture 2024, 14, 6. https://doi.org/10.3390/agriculture14010006
Zhou L, Ma X, Wang L, Sun W, Liu Y, Ma Y, Xie H, Qiao F. Region and Crop Type Influenced Fungal Diversity and Community Structure in Agricultural Areas in Qinghai Province. Agriculture. 2024; 14(1):6. https://doi.org/10.3390/agriculture14010006
Chicago/Turabian StyleZhou, Lianyu, Xuelan Ma, Longrui Wang, Wenjuan Sun, Yu Liu, Yun Ma, Huichun Xie, and Feng Qiao. 2024. "Region and Crop Type Influenced Fungal Diversity and Community Structure in Agricultural Areas in Qinghai Province" Agriculture 14, no. 1: 6. https://doi.org/10.3390/agriculture14010006
APA StyleZhou, L., Ma, X., Wang, L., Sun, W., Liu, Y., Ma, Y., Xie, H., & Qiao, F. (2024). Region and Crop Type Influenced Fungal Diversity and Community Structure in Agricultural Areas in Qinghai Province. Agriculture, 14(1), 6. https://doi.org/10.3390/agriculture14010006





