Proteomic and Metabolomic Profiling Elucidate the Impact of PEDV on Yorkshire Piglets and Reveal the Underlying Molecular Mechanism of PEDV Response
Abstract
:1. Introduction
2. Materials and Methods
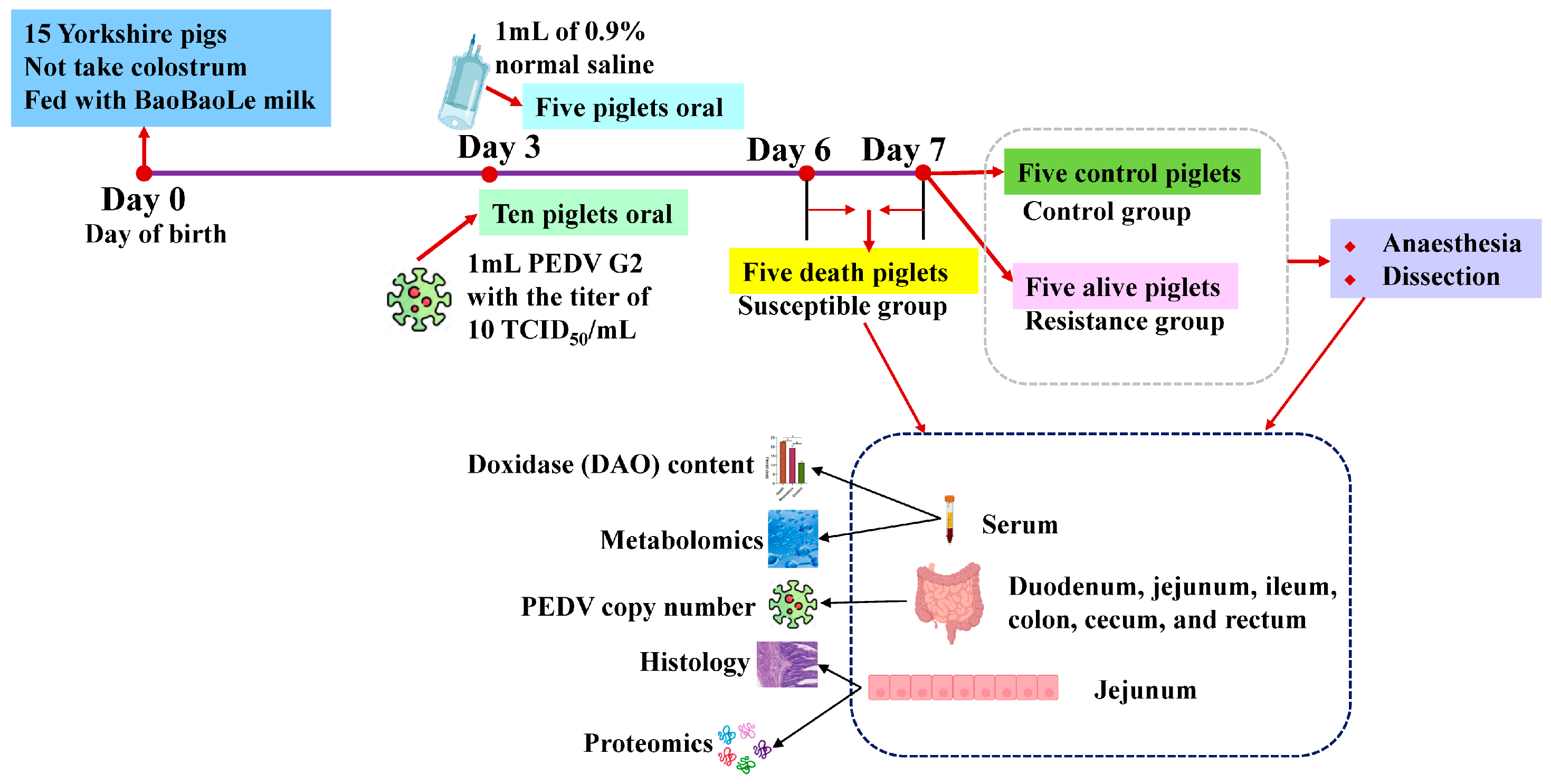
2.1. Animals and Samples
2.2. Measurements of PEDV and Degree of Jejunal Injury
2.3. Jejunum Tissue Histology
2.4. Protein Extraction of Jejunum and Four-Dimensional (4D) Data-Independent Acquisition (DIA)-Based Proteomics
2.5. Metabolite Extraction of Serum and Ultrahigh-Performance Liquid Chromatography-MS/MS (UHPLC–MS/MS) Analysis
2.6. Identification of Proteins and Metabolites
2.7. Data Analysis and Functional Annotation
3. Results
3.1. Determinations of PEDV Copy Numbers, Degree of Jejunum Damage and Weight Loss
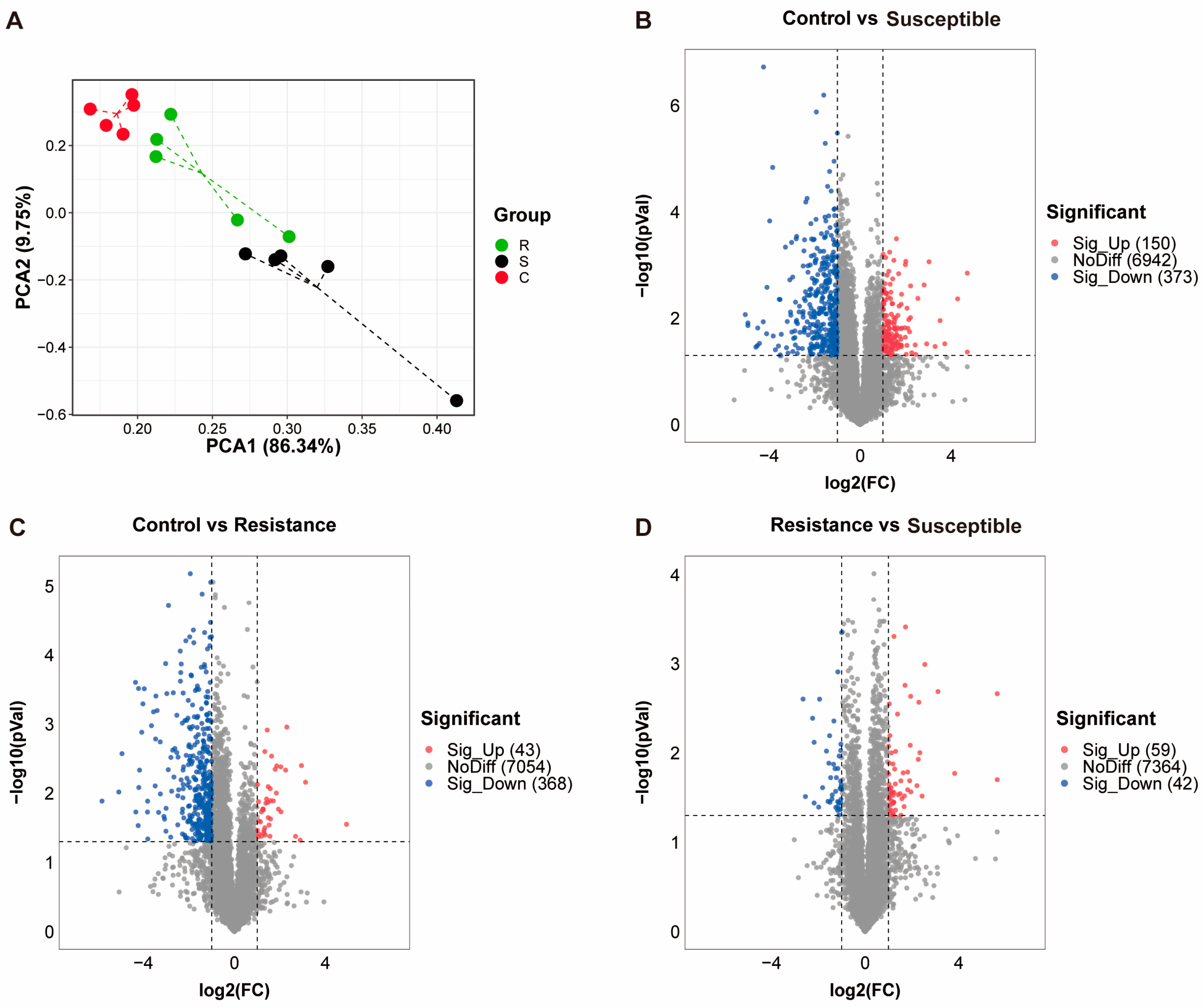
3.2. Protein Identification from the Jejunum of Piglets Involved in PEDV Model
3.3. Differential Expression Analysis and Functional Annotation of Protein
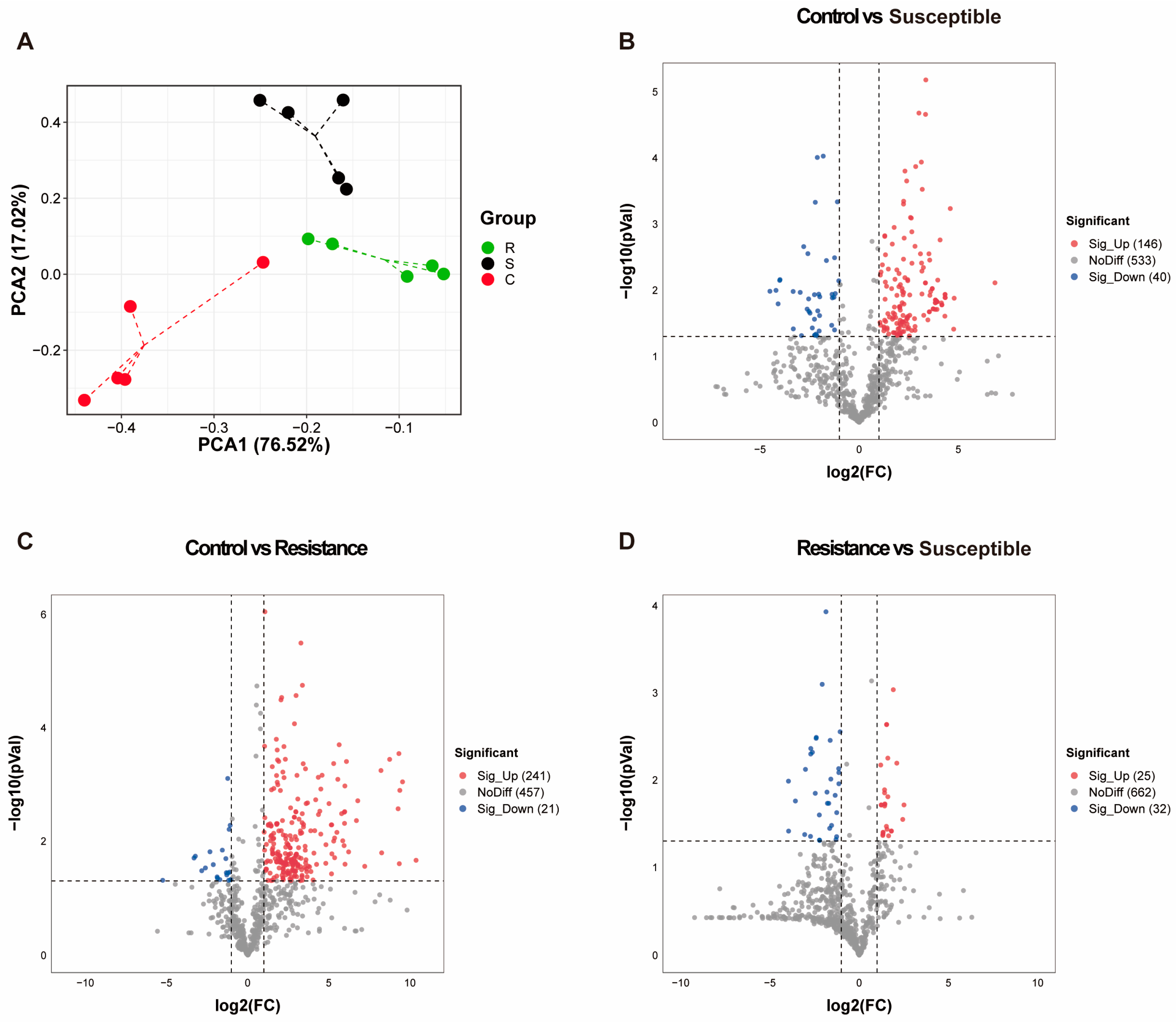
3.4. Metabolomic Profiling of Jejunum from Piglets Involved in PEDV Model
3.5. Biomarker Metabolites Identification and Functional Annotation
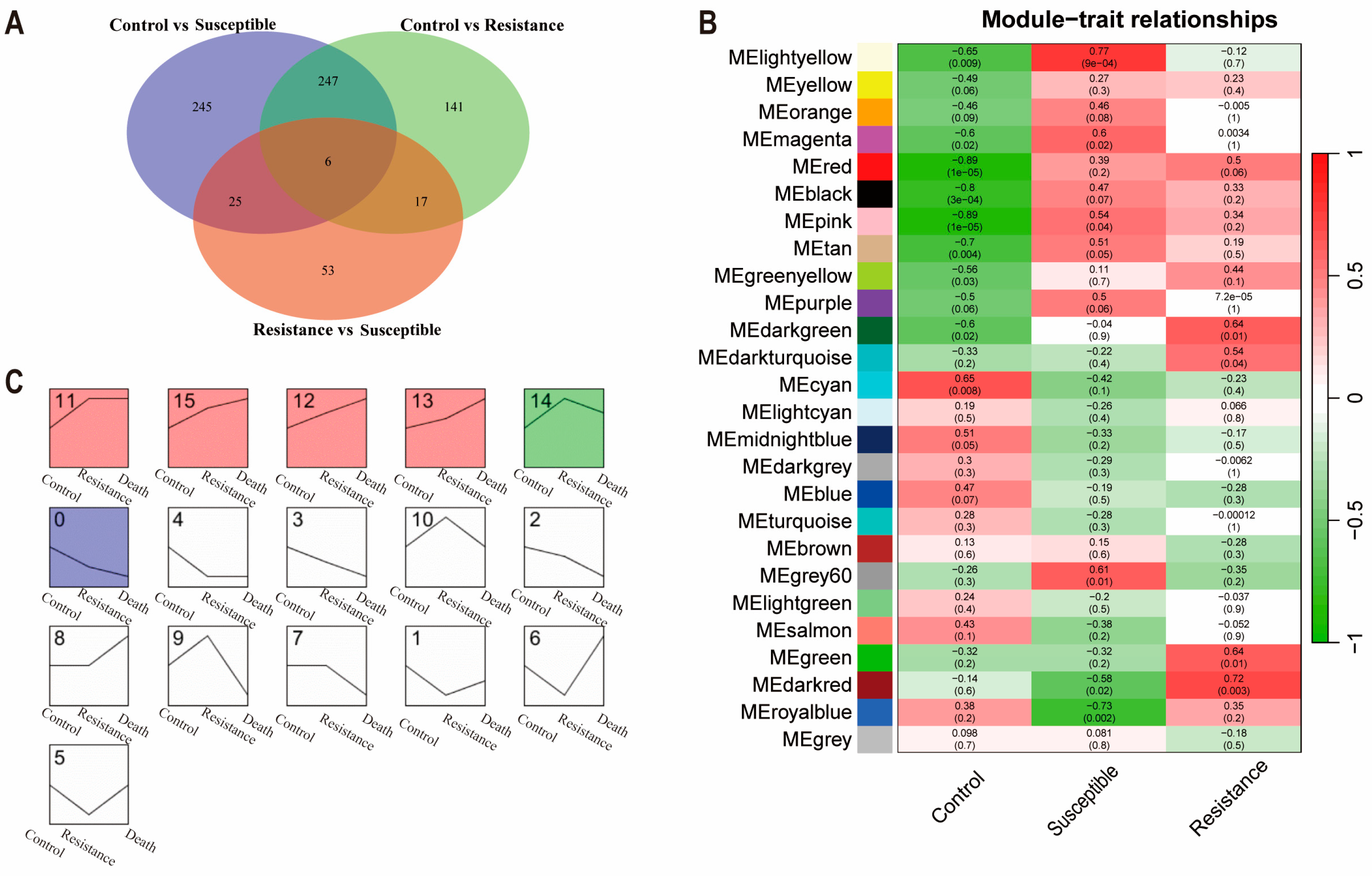
3.6. Weighted Gene Co-Expression Network Analysis (WGCNA) and Short Timeseries Expression Miner (STEM) Analysis
3.7. Detection of Characteristic Biomarkers for Susceptible and Resistance
3.8. Random Forest Analysis of the Key Metabolites
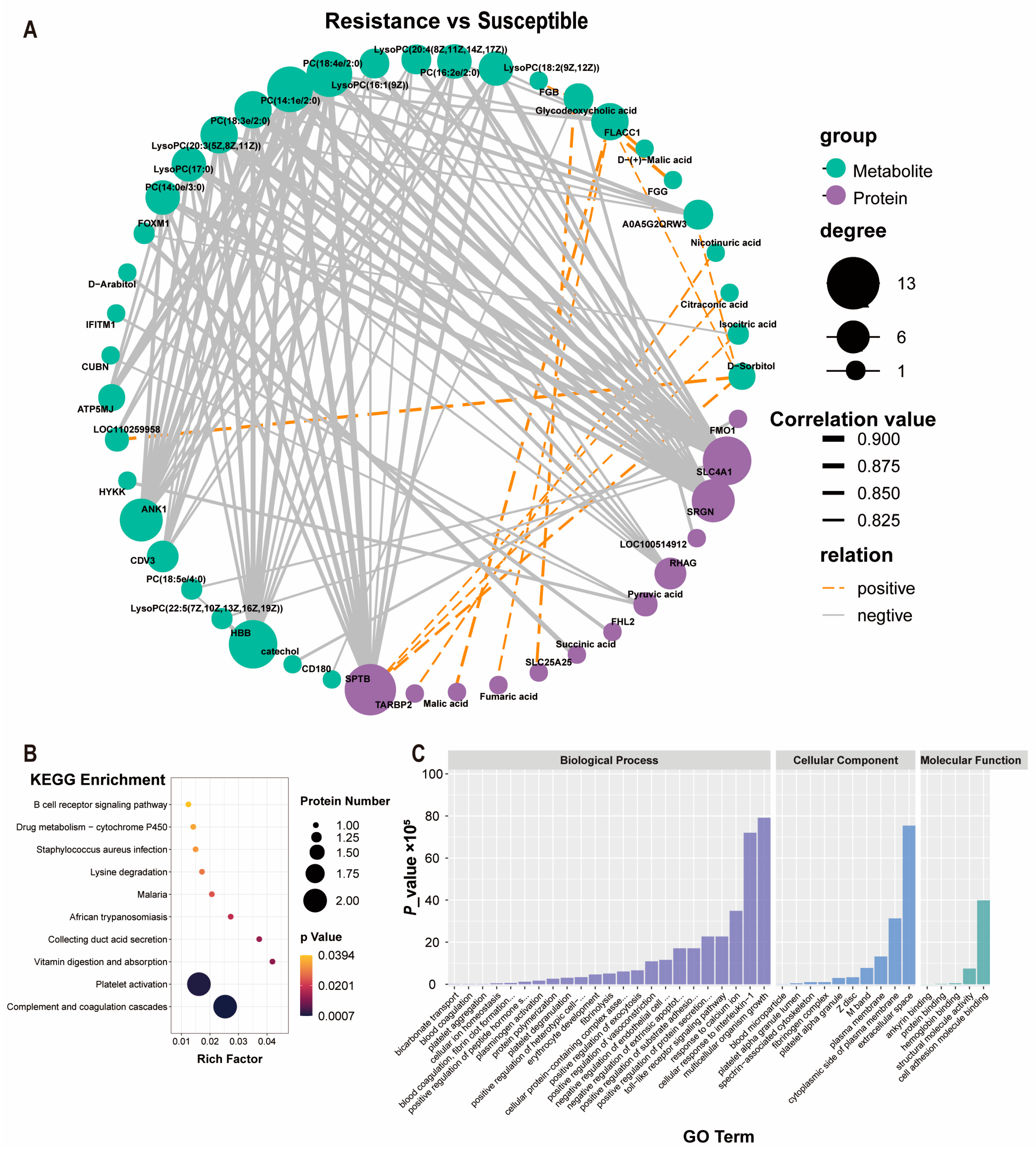
3.9. Features of the Protein–Metabolite Interactome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.L.; Zhang, W.D.; Su, L.J.; Huang, Z.Y.; Zhang, W.C.; Ma, L.L.; Sun, J.S.; Guo, J.Y.; Wen, F.; Mei, K.; et al. Difference analysis of intestinal microbiota and metabolites in piglets of different breeds exposed to porcine epidemic diarrhea virus infection. Front. Microbiol. 2022, 13, 990642. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Q.X.; Huang, L.L.; Yuan, C.; Wang, J.L.; Yang, Q. An alternative pathway of enteric PEDV dissemination from nasal cavity to intestinal mucosa in swine. Nat. Commun. 2018, 9, 3811. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Saif, L.J.; Wang, Q. Porcine epidemic diarrhea virus (PEDV): An update on etiology, transmission, pathogenesis, and prevention and control. Virus Res. 2020, 286, 198045. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Zhang, H.Y.; Li, L.Q.; Yang, Y.; Zou, X.D.; Chen, J.H.; Tang, X.C. PEDV: Insights and Advances into Types, Function, Structure, and Receptor Recognition. Viruses 2022, 14, 1744. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Z.; Li, Y.; Gao, S.; Xiao, S. Porcine epidemic diarrhea virus: Molecular mechanisms of attenuation and vaccines. Microb. Pathog. 2020, 149, 104553. [Google Scholar] [CrossRef]
- Li, B.X.; Ge, J.W.; Li, Y.J. Porcine aminopeptidase N is a functional receptor for the PEDV coronavirus. Virology 2007, 365, 166–172. [Google Scholar] [CrossRef]
- Dubin, R.F.; Rhee, E.P. Proteomics and Metabolomics in Kidney Disease, including Insights into Etiology, Treatment, and Prevention. Clin. J. Am. Soc. Nephrol. 2020, 15, 404–411. [Google Scholar] [CrossRef]
- Christians, U.; Klawitter, J.; Klawitter, J. Biomarkers in Transplantation-Proteomics and Metabolomics. Ther. Drug Monit. 2016, 38, S70–S74. [Google Scholar] [CrossRef]
- Lo, D.J.; Kaplan, B.; Kirk, A.D. Biomarkers for kidney transplant rejection. Nat. Rev. Nephrol. 2014, 10, 215–225. [Google Scholar] [CrossRef]
- Huang, H.M.; Li, Y.T.; Wang, L.; Song, Y.P.; Zhang, G.P. Membrane proteomic analysis identifies the polarity protein PARD3 as a novel antiviral protein against PEDV infection. J. Proteom. 2022, 253, 104462. [Google Scholar] [CrossRef]
- Zeng, S.L.; Zhang, H.; Ding, Z.; Luo, R.; An, K.; Liu, L.Z.; Bi, J.; Chen, H.C.; Xiao, S.B.; Fang, L.R. Proteome analysis of porcine epidemic diarrhea virus (PEDV)-infected Vero cells. Proteomics 2015, 15, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.B.; Shi, H.Y.; Guo, D.H.; Chen, J.F.; Shi, D.; Zhu, Q.H.; Zhang, X.; Feng, L. Analysis of protein expression changes of the Vero E6 cells infected with classic PEDV strain CV777 by using quantitative proteomic technique. J. Virol. Methods 2015, 218, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Chen, F.Z.; Ye, S.Y.; Guo, X.Z.; Memon, A.M.; Wu, M.Z.; He, Q.G. Comparative Proteome Analysis of Porcine Jejunum Tissues in Response to a Virulent Strain of Porcine Epidemic Diarrhea Virus and Its Attenuated Strain. Viruses 2016, 8, 323. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.X.; Li, B.; Chen, L.; Ma, Z.; He, K.W.; Fan, H.J. Differential Protein Analysis of IPEC-J2 Cells Infected with Porcine Epidemic Diarrhea Virus Pandemic and Classical Strains Elucidates the Pathogenesis of Infection. J. Proteome Res. 2017, 16, 2113–2120. [Google Scholar] [CrossRef] [PubMed]
- Thaker, S.K.; Ch’ng, J.; Christofk, H.R. Viral hijacking of cellular metabolism. BMC Biol. 2019, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; He, R.; Fang, P.N.; Li, M.Q.; Yu, H.S.; Wang, Q.M.; Yu, Y.; Wang, F.B.; Zhang, Y.; Chen, A.D.; et al. Hepatitis B virus rigs the cellular metabolome to avoid innate immune recognition. Nat. Commun. 2021, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Liu, H.S.; Zhu, Z.X.; Yang, F.; Song, Y.Y.; Li, Z.Q.; Xue, Z.N.; Cao, W.J.; Liu, X.T.; Zheng, H.X. African Swine Fever Virus Regulates Host Energy and Amino Acid Metabolism To Promote Viral Replication. J. Virol. 2022, 96, e01919-21. [Google Scholar] [CrossRef]
- Wang, H.; Hui, P.; Uemoto, Y.; Ding, Y.; Yin, Z.; Bao, W. Metabolomic and Proteomic Profiling of Porcine Intestinal Epithelial Cells Infected with Porcine Epidemic Diarrhea Virus. Int. J. Mol. Sci. 2023, 24, 5071. [Google Scholar] [CrossRef]
- Li, H.; Zhou, C.; Zhang, M.; Yuan, N.; Huang, X.; Xiang, J.; Wang, L.; Shi, L. Transcriptomics yields valuable information regarding the response mechanisms of Chinese Min pigs infected with PEDV. Front. Vet. Sci. 2023, 10, 1295723. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3--new capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Schnedl, W.J.; Schenk, M.; Lackner, S.; Enko, D.; Mangge, H.; Forster, F. Diamine oxidase supplementation improves symptoms in patients with histamine intolerance. Food Sci. Biotechnol. 2019, 28, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.W.; Ou, Y.W.; Cai, W.W.; Zheng, Y.L.; Li, H.J.; Mao, Y.Y.; Zhou, S.A.; Tu, J.F. Advances in the Clinical Application of Histamine and Diamine Oxidase (DAO) Activity: A Review. Catalysts 2023, 13, 48. [Google Scholar] [CrossRef]
- Rieger, J.; Pelckmann, L.M.; Drewes, B. Preservation and Processing of Intestinal Tissue for the Assessment of Histopathology. Methods Mol. Biol. 2021, 2223, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhu, P.; Wan, Q.; Ruan, X.; Wu, P.; Hao, Y.; Zhang, Z.; Sun, J.; Nie, W.; Chen, S. High-Coverage Four-Dimensional Data-Independent Acquisition Proteomics and Phosphoproteomics Enabled by Deep Learning-Driven Multidimensional Predictions. Anal. Chem. 2023, 95, 7495–7502. [Google Scholar] [CrossRef] [PubMed]
- Bu, D.C.; Luo, H.T.; Huo, P.P.; Wang, Z.H.; Zhang, S.; He, Z.H.; Wu, Y.; Zhao, L.H.; Liu, J.J.; Guo, J.C.; et al. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, L.; Wu, Y.; Yuan, Z.; Zhou, J. Weighted gene co-expression network analysis to identify key modules and hub genes associated with atrial fibrillation. Int. J. Mol. Med. 2020, 45, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Ernst, J.; Bar-Joseph, Z. STEM: A tool for the analysis of short time series gene expression data. BMC Bioinform. 2006, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.G.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef]
- Hollander, M.; Wolfe, D.A. Nonparametric Statistical Methods; John Wiley & Sons: New York, NY, USA, 1973; pp. 185–194. [Google Scholar]
- Chen, Y.-M.; Gabler, N.K.; Burrough, E.R. Porcine epidemic diarrhea virus infection induces endoplasmic reticulum stress and unfolded protein response in jejunal epithelial cells of weaned pigs. Vet. Pathol. 2021, 59, 82–90. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Wang, X. Serum metabolomics as a novel diagnostic approach for disease: A systematic review. Anal. Bioanal. Chem. 2012, 404, 1239–1245. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Y.; Zhang, Q.; Yang, F.; Yin, Z.; Wang, L.; Li, Q. Porcine epidemic diarrhea virus infections induce apoptosis in Vero cells via a reactive oxygen species (ROS)/p53, but not p38 MAPK and SAPK/JNK signalling pathways. Vet. Microbiol. 2019, 232, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, J.; Yoo, D. Inhibition of NF-κB activity by the porcine epidemic diarrhea virus nonstructural protein 1 for innate immune evasion. Virology 2017, 510, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Wang, X.; Ju, N.; Wang, Z.; Sui, L.; Wang, L.; Qiao, X.; Cui, W.; Jiang, Y.; Zhou, H.; et al. Immune Responses in Pregnant Sows Induced by Recombinant Lactobacillus johnsonii Expressing the COE Protein of Porcine Epidemic Diarrhea Virus Provide Protection for Piglets against PEDV Infection. Viruses 2022, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Kloc, M.; Zearfoss, N.R.; Etkin, L.D. Mechanisms of subcellular mRNA localization. Cell 2002, 108, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Pathak, C.; Vaidya, F.U.; Waghela, B.N.; Jaiswara, P.K.; Gupta, V.K.; Kumar, A.; Rajendran, B.K.; Ranjan, K. Insights of Endocytosis Signaling in Health and Disease. Int. J. Mol. Sci. 2023, 24, 2971. [Google Scholar] [CrossRef] [PubMed]
- Thangam, E.B.; Jemima, E.A.; Singh, H.; Baig, M.S.; Khan, M.; Mathias, C.B.; Church, M.K.; Saluja, R. The Role of Histamine and Histamine Receptors in Mast Cell-Mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets. Front. Immunol. 2018, 9, 1873. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, S.; Jutel, M.; Crameri, R.; O’Mahony, L. Histamine and gut mucosal immune regulation. Allergy 2014, 69, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Daschner, A.; González-Fernández, J.; Valls, A.; de Frutos, C.; Rodero, M.; Cuéllar, C. Diamine oxidase levels in different chronic urticaria phenotypes. Allergol. Immunopathol. 2015, 43, 593–600. [Google Scholar] [CrossRef]
- Kettner, L.; Seitl, I.; Fischer, L. Evaluation of porcine diamine oxidase for the conversion of histamine in food-relevant amounts. J. Food Sci. 2020, 85, 843–852. [Google Scholar] [CrossRef]
- van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Zou, H.; Qiu, L.; Zheng, Y.; Yang, D.; Wang, Y. Effects of light on secondary metabolite biosynthesis in medicinal plants. Front. Plant Sci. 2021, 12, 781236. [Google Scholar] [CrossRef] [PubMed]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed]
- Okita, M.; Gaudette, D.C.; Mills, G.B.; Holub, B.J. Elevated levels and altered fatty acid composition of plasma lysophosphatidylcholine(lysoPC) in ovarian cancer patients. Int. J. Cancer 1997, 71, 31–34. [Google Scholar] [CrossRef]
- Raynor, A.; Jantscheff, P.; Ross, T.; Schlesinger, M.; Wilde, M.; Haasis, S.; Dreckmann, T.; Bendas, G.; Massing, U. Saturated and mono-unsaturated lysophosphatidylcholine metabolism in tumour cells: A potential therapeutic target for preventing metastases. Lipids Health Dis. 2015, 14, 69. [Google Scholar] [CrossRef] [PubMed]
- Perrin-Cocon, L.; Agaugué, S.; Coutant, F.; Saint-Mézard, P.; Guironnet-Paquet, A.; Nicolas, J.F.; André, P.; Lotteau, V. Lysophosphatidylcholine is a natural adjuvant that initiates cellular immune responses. Vaccine 2006, 24, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Pan, T.; Qi, X.; Tan, R.; Wang, X.; Liu, Z.; Tao, Z.; Qu, H.; Zhang, Y.; Chen, H.; et al. Increased mortality of acute respiratory distress syndrome was associated with high levels of plasma phenylalanine. Respir. Res. 2020, 21, 99. [Google Scholar] [CrossRef] [PubMed]
- Demirkan, A.; van Duijn, C.M.; Ugocsai, P.; Isaacs, A.; Pramstaller, P.P.; Liebisch, G.; Wilson, J.F.; Johansson, Å.; Rudan, I.; Aulchenko, Y.S.; et al. Genome-wide association study identifies novel loci associated with circulating phospho- and sphingolipid concentrations. PLoS Genet. 2012, 8, e1002490. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Cai, M.; Weng, X.; Liu, Z.; Zhang, G. Porcine insulin receptor substrate 2: Molecular cloning, tissues distribution, and functions in hepatocyte and aortic endothelial cells. Pol. J. Vet. Sci. 2019, 22, 589–598. [Google Scholar] [CrossRef]
- Zhao, M.; Pan, B.; He, Y.; Niu, B.; Gao, X. Elucidating the pharmacological mechanism by which Si-Wu-Tang induces cellular senescence in breast cancer via multilevel data integration. Aging 2022, 14, 5812–5837. [Google Scholar] [CrossRef]
- Song, L.; Chen, J.; Hao, P.; Jiang, Y.; Xu, W.; Li, L.; Chen, S.; Gao, Z.; Jin, N.; Ren, L.; et al. Differential Transcriptomics Analysis of IPEC-J2 Cells Single or Coinfected With Porcine Epidemic Diarrhea Virus and Transmissible Gastroenteritis Virus. Front. Immunol. 2022, 13, 844657. [Google Scholar] [CrossRef]
- Shalev, Z.; Duffy, S.P.; Adema, K.W.; Prasad, R.; Hussain, N.; Willett, B.J.; Tailor, C.S. Identification of a feline leukemia virus variant that can use THTR1, FLVCR1, and FLVCR2 for infection. J. Virol. 2009, 83, 6706–6716. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Gong, D.; Ren, K.; Zhou, G.; Huang, G.; Lei, J.; Zhou, Q.; Yan, N. Molecular basis for allosteric regulation of the type 2 ryanodine receptor channel gating by key modulators. Proc. Natl. Acad. Sci. USA 2019, 116, 25575–25582. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Chen, D.; Tian, G.; He, J.; Huang, Z.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; Luo, Y.; et al. All-Trans Retinoic Acid Attenuates Transmissible Gastroenteritis Virus-Induced Apoptosis in IPEC-J2 Cells via Inhibiting ROS-Mediated P(38)MAPK Signaling Pathway. Antioxidants 2022, 11, 345. [Google Scholar] [CrossRef]
- Wang, M.; Jia, J.; Cui, Y.; Peng, Y.; Jiang, Y. Molecular and Clinical Characterization of a Novel Prognostic and Immunologic Biomarker GPSM3 in Low-Grade Gliomas. Brain Sci. 2021, 11, 1529. [Google Scholar] [CrossRef] [PubMed]
- Rossard, L.; Favreau, F.; Demars, J.; Robert, R.; Nadeau, C.; Cau, J.; Thuillier, R.; Hauet, T. Evaluation of early regenerative processes in a preclinical pig model of acute kidney injury. Curr. Mol. Med. 2012, 12, 502–505. [Google Scholar] [PubMed]
- Lee, G.; Han, D.; Song, J.Y.; Lee, Y.S.; Kang, K.S.; Yoon, S. Genomic expression profiling in lymph nodes with lymphoid depletion from porcine circovirus 2-infected pigs. J. Gen. Virol. 2010, 91, 2585–2591. [Google Scholar] [CrossRef] [PubMed]
- Fook-Alves, V.L.; de Oliveira, M.B.; Zanatta, D.B.; Strauss, B.E.; Colleoni, G.W. TP53 Regulated Inhibitor of Apoptosis 1 (TRIAP1) stable silencing increases late apoptosis by upregulation of caspase 9 and APAF1 in RPMI8226 multiple myeloma cell line. Biochim. Biophys. Acta 2016, 1862, 1105–1110. [Google Scholar] [CrossRef]
- Zheng, J.; Bu, X.; Wei, X.; Ma, X.; Zhao, P. The role of FoxM1 in immune cells. Clin. Exp. Med. 2023, 23, 1973–1979. [Google Scholar] [CrossRef]
- Henriques-Pons, A.; Beghini, D.G.; Silva, V.D.S.; Iwao Horita, S.; da Silva, F.A.B. Pulmonary Mesenchymal Stem Cells in Mild Cases of COVID-19 Are Dedicated to Proliferation; In Severe Cases, They Control Inflammation, Make Cell Dispersion, and Tissue Regeneration. Front. Immunol. 2021, 12, 780900. [Google Scholar] [CrossRef]
- de Azevedo Muner Ferreira, B.; Fonseca, D.C.; Sala, P.; Alves, J.T.M.; Prudêncio, A.P.A.; Machado, N.M.; Marques, M.; Barcelos, S.; Ishida, R.K.; Guarda, I.; et al. Roux-en-Y gastric bypass affects the expression of genes related to the intestinal folate metabolism pathway in obese women. Nutrition 2023, 112, 112054. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.A.; Kim, Y.; Kim, J.; Hong, G.; Hong, J.; Choi, K.; Eom, C.S.; Baik, S.; Lee, M.K.; et al. Genetic Variants Associated with Elevated Plasma Ceramides in Individuals with Metabolic Syndrome. Genes 2022, 13, 1497. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Kanao, Y.; Yamanaka, M.; Sakuraba, H.; Mizutani, Y.; Igarashi, Y.; Kihara, A. Characterization of four mammalian 3-hydroxyacyl-CoA dehydratases involved in very long-chain fatty acid synthesis. FEBS Lett. 2008, 582, 2435–2440. [Google Scholar] [CrossRef] [PubMed]
- Lecluze, E.; Lettre, G. Association analyses of predicted loss-of-function variants prioritized 15 genes as blood pressure regulators. Can. J. Cardiol. 2023, 39, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Hu, Y.; Zhang, Z.; Chen, X.; Gao, J. Identification of metabolic biomarkers associated with nonalcoholic fatty liver disease. Lipids Health Dis. 2023, 22, 150. [Google Scholar] [CrossRef]
- Gong, X.; Hou, D.; Zhou, S.; Tan, J.; Zhong, G.; Yang, B.; Xie, L.; Han, F.; Zhong, L. FMO family may serve as novel marker and potential therapeutic target for the peritoneal metastasis in gastric cancer. Front. Oncol. 2023, 13, 1144775. [Google Scholar] [CrossRef]
- Hu, Z.; Li, Y.; Du, H.; Ren, J.; Zheng, X.; Wei, K.; Liu, J. Transcriptome analysis reveals modulation of the STAT family in PEDV-infected IPEC-J2 cells. BMC Genom. 2020, 21, 891. [Google Scholar] [CrossRef]


| Characteristic Biomarkers for Susceptibility | Characteristic Biomarkers for Resistance | |||
|---|---|---|---|---|
| Metabolites | Protein Descriptions | Proteins (Gene Name) | Protein Descriptions | Proteins (Gene Name) |
| Succinic acid | GB1/RHD3-type G domain-containing protein | LOC100523310 | TP53 regulated inhibitor of apoptosis 1 | |
| D-(+)-malic acid | Forkhead box M1 | FOXM1 | Abhydrolase domain containing 3, phospholipase | ABHD3 |
| Malic acid | Four and a half LIM domains 2 | FHL2 | Insulin receptor substrate 2 | IRS2 |
| Isocitric acid | 5-methyltetrahydrofolate-homocysteine methyltransferase reductase | MTRR | Interferon-induced transmembrane protein 1 | IFITM1 |
| 2,6-dihydroxypurine | Serine palmitoyltransferase 3 | SPTLC3 | Ryanodine receptor 2 | RYR2 |
| D-arabitol | Glutamyl aminopeptidase | ENPEP | Chromosome 2 C19orf25 homolog | C2H19orf25 |
| Adonitol | Very-long-chain (3R)-3-hydroxyacyl-CoA dehydratase | HACD1 | Mitotic spindle-organizing protein 2A isoform X4 | LOC100153925 |
| Propionic acid | Flavin-containing monooxygenase 1 | FMO1 | FLVCR heme transporter 1 | FLVCR1 |
| Fumaric acid | Glutathione peroxidase | GPX2 | ||
| 9(10)-DiHOME | G protein signaling modulator 3 | GPSM3 | ||
| (9Z)-(7S,8S)-Dihydroxyoctadecenoic acid | Translocator protein | TSPO | ||
| (9R,10R)-Dihydroxyoctadecanoic acid | CD180 antigen | CD180 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Li, H.; Zhou, C.; Wang, L. Proteomic and Metabolomic Profiling Elucidate the Impact of PEDV on Yorkshire Piglets and Reveal the Underlying Molecular Mechanism of PEDV Response. Agriculture 2024, 14, 43. https://doi.org/10.3390/agriculture14010043
Shi L, Li H, Zhou C, Wang L. Proteomic and Metabolomic Profiling Elucidate the Impact of PEDV on Yorkshire Piglets and Reveal the Underlying Molecular Mechanism of PEDV Response. Agriculture. 2024; 14(1):43. https://doi.org/10.3390/agriculture14010043
Chicago/Turabian StyleShi, Lijun, Huihui Li, Chunxiang Zhou, and Lixian Wang. 2024. "Proteomic and Metabolomic Profiling Elucidate the Impact of PEDV on Yorkshire Piglets and Reveal the Underlying Molecular Mechanism of PEDV Response" Agriculture 14, no. 1: 43. https://doi.org/10.3390/agriculture14010043
APA StyleShi, L., Li, H., Zhou, C., & Wang, L. (2024). Proteomic and Metabolomic Profiling Elucidate the Impact of PEDV on Yorkshire Piglets and Reveal the Underlying Molecular Mechanism of PEDV Response. Agriculture, 14(1), 43. https://doi.org/10.3390/agriculture14010043





