The Importance of the Targeted Design of Biochar Physicochemical Properties in Microbial Inoculation for Improved Agricultural Productivity—A Review
Abstract
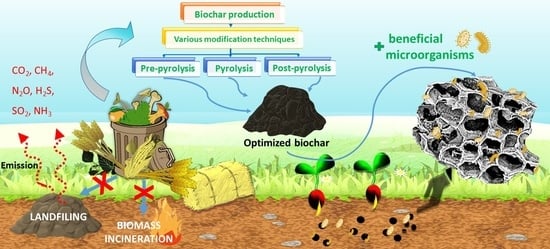
1. Introduction
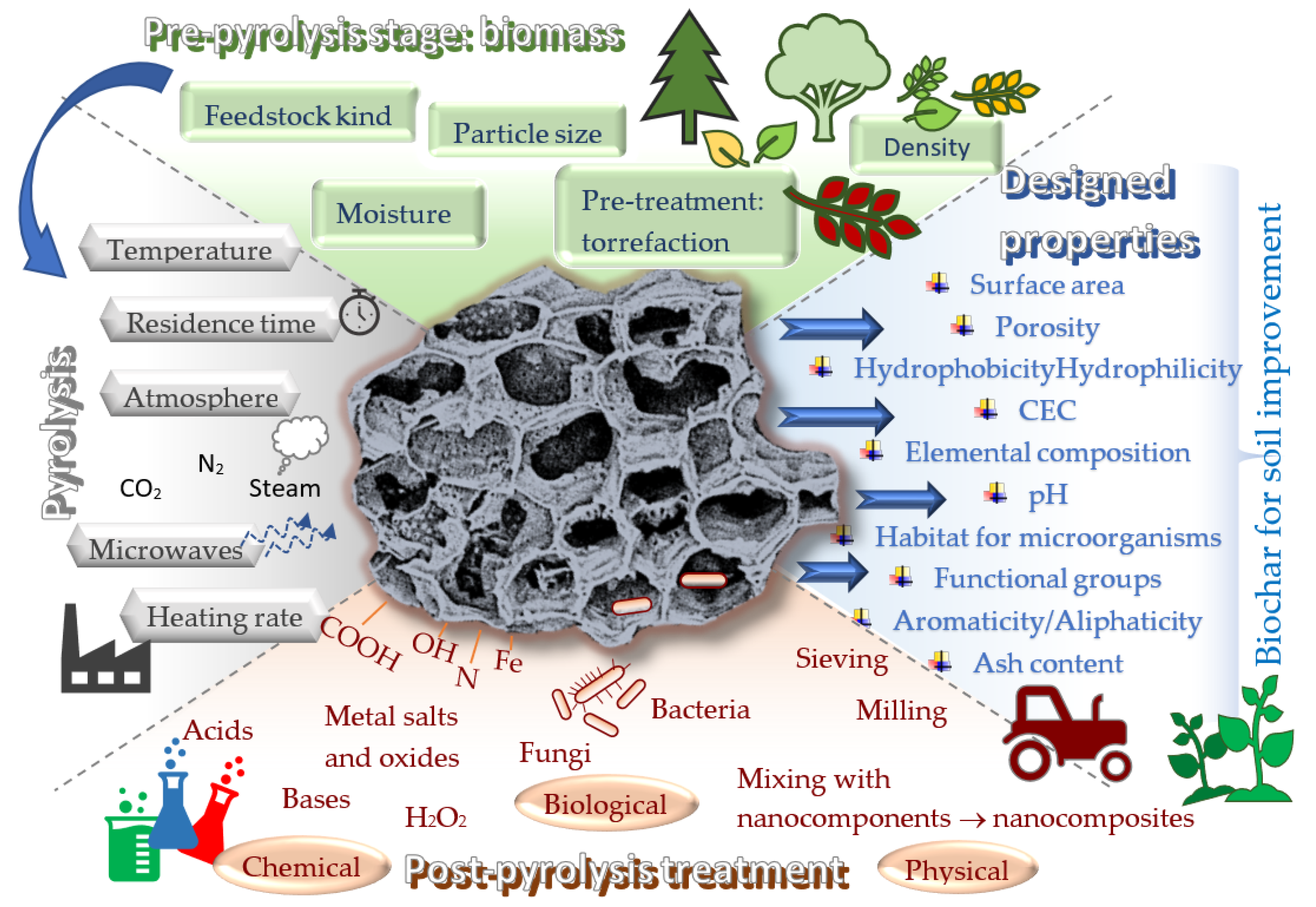
2. Effect of Various Modification Strategies and Major Factors on Biochar Properties
2.1. Prospects for Designing Biochar Properties at the Pre-Pyrolysis Stage
2.2. Designing Biochar Properties by Controlling Pyrolysis Conditions
2.3. Modifications of Biochar Properties through Post-Pyrolysis Processes
3. Optimal Properties of Biochar as a Carrier and Habitat for Microorganisms
3.1. Valuable Features of Microbiological Carriers
3.2. Physical Properties of Biochar
3.3. Chemical Properties of Biochar
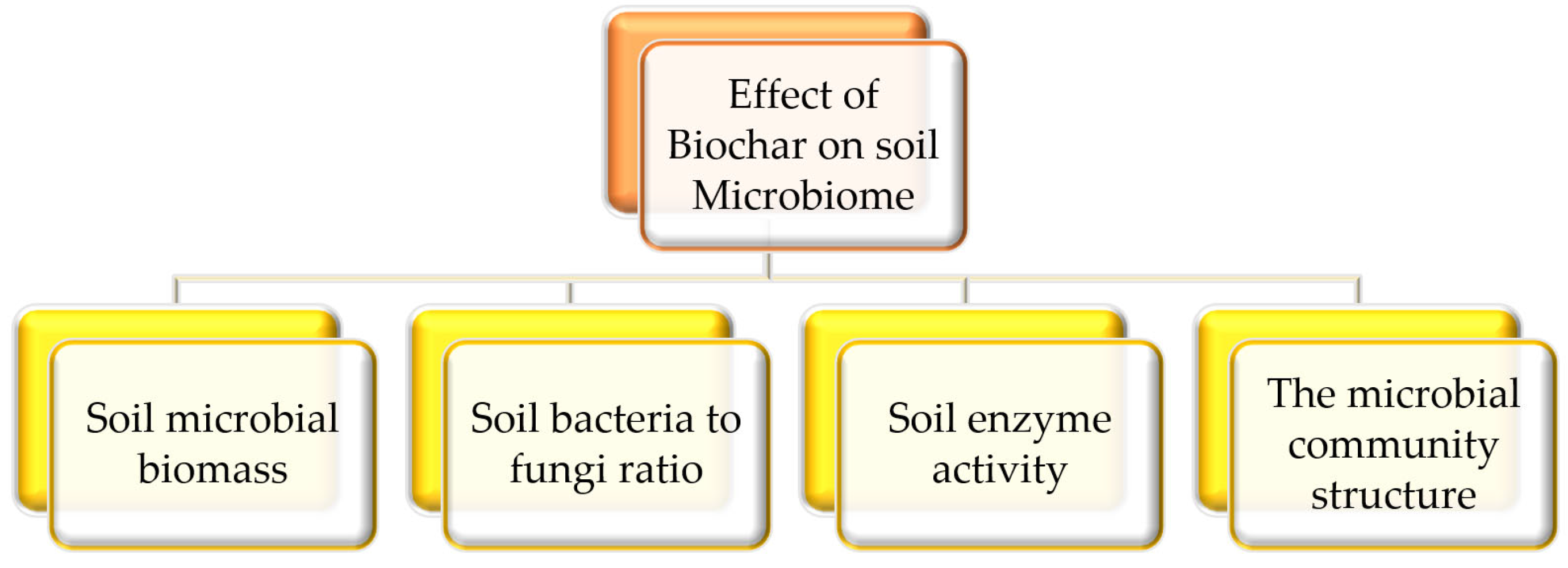
4. Microbiological Indicators for Assessing the Impact of Biochar in Soil
4.1. Microbial Biomass
4.2. Microbial Abundance
4.3. Bacteria to Fungi Ratio
4.4. Enzyme Activity
5. Implications of Using Biochar and Biochar-Based Products for the Environment and Agriculture
5.1. Limitations in the Use of Biochar and Biochar-Based Products
5.2. Environmental Risk Resulting from the Use of Biochar
5.3. Environmental Benefits from the Use of Biochar
5.4. Future Research
6. Conclusions
- The selection of appropriate biomass should primarily consider the relationship between the content of lignin, cellulose, and hemicellulose and the temperature at which the pyrolysis will be carried out. Biomass with high lignin content favors the formation of biochar with higher carbon content, larger specific surface area, and lower nitrogen and ash contents. However, lignin substrates may require higher temperatures to be decomposed. Pyrolysis of biomass with excessive moisture content may negatively affect the economic balance of the process.
- The achievement of desired biochar properties at the stage of pyrolysis may depend on several different factors regulating this process simultaneously, among which the temperature generates clear, strong, and predictable effects. An increase in pyrolysis temperature increases porosity, specific surface area, pH, and ash content and decreases the negative surface charge, the contents of volatile matter, and the oxygen-containing functional groups.
- Post-pyrolysis modifications can lead to remarkable improvements in porosity, specific surface area, or reactivity (e.g., treatment with acids, bases, or hydrogen peroxide) but also to the generation of completely new properties (e.g., treatment with nanoparticles of metal salts and oxides). Detailed research is still needed in this area because the effectiveness of modification does not always increase with the concentration of the modifying agent. Moreover, modification with aggressive chemicals generates the problem of waste, while the production of nanocomposite or magnetic biochars meets issues of the appropriate durability of the product.
- Biochar’s physical properties, such as high surface area and porosity, are primary factors providing a suitable habitat for various microorganisms. Given that the porosity and surface area of biochar increase with the temperature of the pyrolysis, biochar can be designed specifically for particular microbes and various soil conditions. The highly porous nature of biochars increases their capacity for cell adhesion as well as for water and nutrient retention and prefers the survival of microorganisms under storage conditions and after application to the soil, improving the protection and proliferation of microorganisms in the soil and plant rhizosphere.
- The chemical properties of biochar depend on the selection of raw materials, pyrolysis temperature, residence time, and pyrolytic gaseous environment, and it is obvious that they are not the same for different biochars. Depending on the CEC, buffering properties, kind of surface functional groups, and mineral content, biochar can be a source of nutrients with different accessibilities for the microbial community, affecting the metabolism and colonization of functional microorganisms. Some biochars can have an inhibitory effect on microorganisms by releasing residual toxic chemicals, hindering nutrient availability, and inhibiting the biofilm formation process. The quality and quantity of surface functional groups influence the immobilization process of microorganisms on the biochar surface through electrostatic, ionic, and hydrophobic interactions.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Our World in Data. Available online: https://ourworldindata.org/population-growth?utm_source=Histmag.org&utm_medium=article-24834 (accessed on 27 October 2023).
- Gerland, P.; Raftery, A.E.; Ševčíková, H.; Li, N.; Gu, D.; Spoorenberg, T.; Alkema, L.; Fosdick, B.K.; Chunn, J.; Lalic, N.; et al. World population stabilization unlikely this century. Science 2014, 346, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.; Hills, C.D.; Singh, R.S.; Atkinson, C.J. Biomass waste utilisation in low-carbon products: Harnessing a major potential resource. Clim. Atmos. Sci. 2019, 2, 35. [Google Scholar] [CrossRef]
- Wang, K.; Tester, J.W. Sustainable management of unavoidable biomass wastes. Green Energy Resour. 2023, 1, 100005. [Google Scholar] [CrossRef]
- European Environment Agency. Bio-Waste in Europe—Turning Challenges into Opportunities; EEA Report No. 04/2020; Publications Office of the European Union: Luxembourg, 2020; ISSN 1977-8449. [Google Scholar] [CrossRef]
- Borzecki, K.; Pudelko, R.; Kozak, M.; Borzecka, M.; Faber, A. Przestrzenne rozmieszczenie odpadów drzewnych w Europie. Sylwan 2018, 162, 563–571. [Google Scholar]
- Donner, M.; Verniquet, A.; Broeze, J.; Kayser, K.; de Vries, H. Critical success and risk factors for circular business models valorizing agricultural waste and by-products. Resour. Conserv. Recycl. 2021, 165, 105236. [Google Scholar] [CrossRef]
- Dunnigan, L.; Morton, B.J.; Ashman, P.J.; Zhang, X.P.; Kwong, C.W. Emission characteristics of a pyrolysis-combustion system for the co-production of biochar and bioenergy from agricultural wastes. Waste Manag. 2018, 77, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Rex, P.; Mohammed Ismail, K.R.; Meenakshisundaram, N.; Barmavatu, P.; Sai Bharadwaj, A.V.S.L. Agricultural biomass waste to biochar: A Review on biochar applications using machine learning approach and circular economy. Chem. Eng. 2023, 7, 50. [Google Scholar] [CrossRef]
- Janiszewska, D.; Ossowska, L. The role of agricultural biomass as a renewable energy source in European Union countries. Energies 2022, 15, 6756. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y. Recent progress in the conversion of biomass wastes into functional materials for value-added applications. Sci. Technol. Adv. Mater. 2020, 21, 787–804. [Google Scholar] [CrossRef]
- Mishra, S.; Kharkar, P.S.; Pethe, A.M. Biomass and waste materials as potential sources of nanocrystalline cellulose: Comparative review of preparation methods (2016–Till date). Carbohydr. Polym. 2019, 207, 418–427. [Google Scholar] [CrossRef]
- Bello, O.S.; Adegoke, K.A.; Olaniyan, A.A.; Olaniyan, A.A.; Abdulazeez, H. Dye adsorption using biomass wastes and natural adsorbents: Overview and future prospects. Desalin Water Treat. 2015, 53, 1292–1315. [Google Scholar] [CrossRef]
- Junginger, M.; Järvinen, M.; Olsson, O.; Hennig, C.; Dadhich, P. Transboundary flows of woody biomass waste streams in Europe. In IEA Bioenergy: Task 40; IEA Bioenergy: Paris, France, 2018; Volume 12, pp. 1–65. [Google Scholar]
- Andersen, S.P.; Allen, B.; Domingo, G.C. Biomass in the EU Green Deal: Towards Consensus on the Use of Biomass for EU Bioenergy: Policy Report; Institute for European Environmental Policy (IEEP): Brussels, Belgium, 2021. [Google Scholar]
- Xiao, Y.; Igalavithana, A.D.; Oh, S.-E.; Nam, H.; Zhang, M.; Wang, C.-H.; Kwon, E.E.; Tsang, D.C.W.; Ok, Y.S. Characterization of bioenergy biochar and its utilization for metal/metalloid immobilization in contaminated soil. Sci. Total Environ. 2018, 640–641, 704–713. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Lin, K.Y.; Jung, S.; Kwon, E.E. Abatement of odor emissions from wastewater treatment plants using biochar. Environ. Pollut. 2023, 336, 122426. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Qin, S.; Verma, S.; Sar, T.; Sarsaiya, S.; Ravindran, B.; Liu, T.; Sindhu, R.; Patel, A.K.; Binod, P.; et al. Production and beneficial impact of biochar for environmental application: A comprehensive review. Bioresour. Technol. 2021, 337, 125451. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Dutta, S.; You, S.; Luo, G.; Zhang, S.; Show, P.L.; Sawarkar, A.D.; Singh, L.; Tsang, D.C. A critical review on biochar for enhancing biogas production from anaerobic digestion of food waste and sludge. J. Clean. Prod. 2021, 305, 127143. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Changes in physical, chemical, and microbiological properties during the two-stage co-composting of green waste with spent mushroom compost and biochar. Bioresour. Technol. 2014, 171, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Matovic, D. Biochar as a viable carbon sequestration option: Global and Canadian perspective. Energy 2016, 36, 2011–2016. [Google Scholar] [CrossRef]
- Cybulak, M.; Sokołowska, Z.; Boguta, P. Impact of biochar on physicochemical properties of Haplic Luvisol soil under different land use: A plot experiment. Agronomy 2019, 9, 531. [Google Scholar] [CrossRef]
- Kalina, M.; Sovova, S.; Svec, J.; Trudicova, M.; Hajzler, J.; Kubikova, L.; Enev, V. The effect of pyrolysis temperature and the source biomass on the properties of biochar produced for the agronomical applications as the soil conditioner. Materials 2022, 15, 8855. [Google Scholar] [CrossRef]
- Yang, W.; Feng, G.; Miles, D.; Gao, L.; Jia, Y.; Li, C.; Qu, Z. Impact of biochar on greenhouse gas emissions and soil carbon sequestration in corn grown under drip irrigation with mulching. Sci. Total Environ. 2020, 729, 138752. [Google Scholar] [CrossRef]
- Sopena, F.; Semple, K.; Sohi, S.; Bending, G. Assessing the chemical and biological accessibility of the herbicide isoproturon in soil amended with biochar. Chemosphere 2012, 88, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Cybulak, M.; Sokołowska, Z.; Boguta, P. The influence of biochar on the content of carbon and the chemical transformations of fallow and grassland humic acids. Sci. Rep. 2021, 11, 5698. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, W.; Yue, S.; Adeel, M.; Qiao, Y. Role of biochar and Eisenia fetida on metal bioavailability and biochar effects on earthworm fitness. Environ. Pollut. 2020, 263, 114586. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhou, W.; Jiang, B.; Wang, L.; Ma, Y.; Guo, H.; Schulin, R.; Ji, R.; Evangelou, M.W.H. Effects of biochar on the transformation and earthworm bioaccumulation of organic pollutants in soil. Chemosphere 2016, 145, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Brewer, C.E.; Chuang, V.J.; Masiello, C.A.; Gonnermann, H.; Gao, X.; Dugan, B.; Driver, L.E.; Panzacchi, P.; Zygourakis, K.; Davies, C.A. New approaches to measuring biochar density and porosity. Biomass Bioenergy 2014, 66, 176–185. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Qi, X.; Gou, J.; Chen, X.; Xiao, S.; Ali, I.; Shang, R.; Wang, D.; Wu, Y.; Han, M.; Luo, X. Application of mixed bacteria-loaded biochar to enhance uranium and cadmium immobilization in a co-contaminated soil. J. Hazard. Mater. 2021, 401, 123823. [Google Scholar] [CrossRef]
- Bolan, S.; Hou, D.; Wang, L.; Hale, L.; Egamberdieva, D.; Tammeorg, P.; Li, R.; Wang, B.; Xu, J.; Wang, T. The potential of biochar as a microbial carrier for agricultural and environmental applications. Sci. Total Environ. 2023, 886, 163968. [Google Scholar] [CrossRef]
- Wang, L.; Olsen, M.N.P.; Moni, C.; Dieguez-Alonso, A.; de la Rosa, J.M.; Stenrød, M.; Liu, X.; Mao, L. Comparison of properties of biochar produced from different types of lignocellulosic biomass by slow pyrolysis at 600 °C. Appl. Energy Combust. Sci. 2022, 12, 100090. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Byrne, C.E.; Nagle, D.C. Carbonization of wood for advanced materials applications. Carbon 1997, 35, 259–266. [Google Scholar] [CrossRef]
- Li, L.; Long, A.; Fossum, B.; Kaiser, M. Effects of pyrolysis temperature and feedstock type on biochar characteristics pertinent to soil carbon and soil health: A meta-analysis. Soil Use Manag. 2023, 39, 43–52. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the composition and application of biomass ash. Part 1. Phase-mineral and chemical composition and classification. Fuel 2013, 105, 40–76. [Google Scholar] [CrossRef]
- Gezahegn, S.; Sain, M.; Thomas, S.C. Variation in feedstock wood chemistry strongly influences biochar liming potential. Soil Syst. 2019, 3, 26. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels production through biomass pyrolysis—A technological review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Abbas, Q.; Liu, G.; Yousaf, B.; Ali, M.U.; Ullah, H.; Munir, M.A.M.; Liu, R. Contrasting effects of operating conditions and biomass particle size on bulk characteristics and surface chemistry of rice husk derived-biochars. J. Anal. Appl. Pyrolysis 2018, 134, 281–292. [Google Scholar] [CrossRef]
- Titova, J.; Baltrėnaitė, E. Physical and chemical properties of biochar produced from sewage sludge compost and plants biomass, fertilized with that compost, important for soil improvement. Waste Biomass Valor. 2021, 12, 3781–3800. [Google Scholar] [CrossRef]
- Jafri, N.; Wong, W.Y.; Doshi, V.; Yoon, L.W.; Cheah, K.H. A review on production and characterization of biochars for application in direct carbon fuel cells. Process. Saf. Environ. 2018, 118, 152–166. [Google Scholar] [CrossRef]
- Minkova, V.; Razvigorova, M.; Bjornbom, E.; Zanzi, R.; Budinova, T.; Petrov, N. Effect of water vapour and biomass nature on the yield and quality of the pyrolysis products from biomass. Fuel Proc. Technol. 2001, 70, 53–61. [Google Scholar] [CrossRef]
- Darmstadt, H.; Pantea, D.; Sümmchen, L.; Roland, U.; Kaliaguine, S.; Roy, C. Surface and bulk chemistry of charcoal obtained by vacuum pyrolysis of bark: Influence of feedstock moisture content. J. Anal. Appl. Pyrolysis 2000, 53, 1–17. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Q.; Hu, H.; Li, A.; Yao, H. Influence of residual moisture on deep dewatered sludge pyrolysis. Int. J. Hydrogen Energy 2014, 39, 1253–1261. [Google Scholar] [CrossRef]
- Boguta, P.; Sokołowska, Z.; Skic, K. Use of thermal analysis coupled with differential scanning calorimetry, quadrupole mass spectrometry and infrared spectroscopy (TG-DSC-QMS-FTIR) to monitor chemical properties and thermal stability of fulvic and humic acids. PLoS ONE 2017, 12, e0189653. [Google Scholar] [CrossRef] [PubMed]
- Boguta, P.; Cybulak, M.; Sokołowska, Z.; Zarzycki, R.; Kacprzak, A.; Kobyłecki, R. Quality and quantity of humic-like and fulvic-like acids entrapped in biochars—The effect of various forestry feedstock and pyrolysis temperature of biochars. Fuel 2023, 333, 126405. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P.; Szewczuk-Karpisz, K. Comparison of monovalent and divalent ions removal from aqueous solutions using agricultural waste biochars prepared at different temperatures-experimental and model study. Int. J. Mol. Sci. 2020, 21, 5851. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.K.; Lima, H.V.; Costa, A.N.; Rodrigues, S.; Pedroso, A.J.S.; de Freitas Maia, C.M.B. Biochar from acai agroindustry waste: Study of pyrolysis conditions. Waste. Manag. 2019, 96, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Ronsse, F.; van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. Glob. Chang. Biol. Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Lee, J.W.; Kidder, M.; Evans, B.R.; Paik, S.; Buchanan Iii, A.C.; Garten, C.T.; Brown, R.C. Characterization of biochars produced from cornstovers for soil amendment. Environ. Sci. Technol. 2010, 44, 7970–7974. [Google Scholar] [CrossRef]
- Marshall, J.; Muhlack, R.; Morton, B.J.; Dunnigan, L.; Chittleborough, D.; Kwong, C.W. Pyrolysis temperature effects on biochar–water interactions and application for improved water holding capacity in vineyard soils. Soil Syst. 2019, 3, 27. [Google Scholar] [CrossRef]
- Wu, L.; Zhao, X.; Bi, E. Predicting the effect of dissolved humic acid on sorption of benzotriazole to biochar. Biochar 2022, 4, 15. [Google Scholar] [CrossRef]
- Zhu, X.; Li, X.; Shen, B.; Zhang, Z.; Wang, J.; Shang, X. Bioremediation of lead-contaminated soil by inorganic phosphate-solubilizing bacteria immobilized on biochar. Ecotoxicol. Environ. Saf. 2022, 237, 113524. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, Y.; Zhao, X.; Wang, S.; Xing, G. Comparisons of biochar properties from wood material and crop residues at different temperatures and residence times. Energy Fuels 2013, 27, 5890–5899. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sust. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Angın, D. Effect of pyrolysis temperature and heating rate on biochar obtained from pyrolysis of safflower seed press cake. Bioresour. Technol. 2013, 128, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ban, Y.; Wen, Y.; Zhu, J.; Wang, Y.; Hu, H.; Jin, L. Removal of ash in biochar from carbonization by CO2-enhanced water leaching and its mechanism. J. Fuel Chem. Technol. 2023, 51, 544–551. [Google Scholar] [CrossRef]
- Lee, J.; Yang, X.; Cho, S.-H.; Kim, J.K.; Lee, S.S.; Tsang, D.C.W.; Sik Ok, Y.; Kwon, E.E. Pyrolysis process of agricultural waste using CO2 for waste management, energy recovery, and biochar fabrication. Appl. Energy 2017, 185, 214–222. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A Review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fr€oling, M.; Antal, M.J., Jr.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub-and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Prathiba, R.; Shruthi, M.; Miranda, L.R. Pyrolysis of polystyrene waste in the presence of activated carbon in conventional and microwave heating using modified thermocouple. Waste Manag. 2018, 76, 528–536. [Google Scholar] [CrossRef]
- Hafeez, A.; Pan, T.; Tian, J.; Cai, K. Modified biochars and their effects on soil quality: A review. Environments 2022, 9, 60. [Google Scholar] [CrossRef]
- Lievens, C.; Mourant, D.; Gunawan, R.; Hu, X.; Wang, Y. Organic compounds leached from fast pyrolysis mallee leaf and bark biochars. Chemosphere 2015, 139, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Cybulak, M.; Sokołowska, Z.; Boguta, P.; Tomczyk, A. Influence of pH and grain size on physicochemical properties of biochar and released humic substances. Fuel 2019, 240, 334–338. [Google Scholar] [CrossRef]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Rouissi, T.; Verma, M.; Surampalli, R.Y.; Valero, J.R. A green method for production of nanobiochar by ball milling-optimization and characterization. J. Clean. Prod. 2017, 164, 1394–1405. [Google Scholar] [CrossRef]
- Wei, X.; Wang, X.; Gao, B.; Zou, W.; Dong, L. Facile ball-milling synthesis of CuO/biochar nanocomposites for efficient removal of reactive red 120. ACS Omega 2020, 5, 5748–5755. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, Z.; Xu, L.; Buyong, F.; Chay, T.C.; Li, Z.; Wang, X. Modified biochar: Synthesis and mechanism for removal of environmental heavy metals. Carbon Res. 2022, 1, 8. [Google Scholar] [CrossRef]
- Amusat, S.O.; Kebede, T.G.; Dube, S.; Nindi, M.M. Ball-milling synthesis of biochar and biochar-based nanocomposites and prospects for removal of emerging contaminants: A review. J. Water Process Eng. 2021, 41, 101993. [Google Scholar] [CrossRef]
- Boguta, P.; Sokołowska, Z.; Skic, K.; Tomczyk, A. Chemically engineered biochar—Effect of concentration and type of modifier on sorption and structural properties of biochar from wood waste. Fuel 2019, 256, 115893. [Google Scholar] [CrossRef]
- Yakout, S.M.; Daifullah, A.E.H.M.; El-Reefy, S.A. Pore structure characterization of chemically modified biochar derived from rice straw. Environ. Eng. Manag. J. 2015, 14, 473–480. [Google Scholar] [CrossRef]
- Huff, M.D.; Lee, J.W. Biochar-surface oxygenation with hydrogen peroxide. J. Environ. Manag. 2016, 165, 17–21. [Google Scholar] [CrossRef]
- Xiong, Z.; Shihong, Z.; Haiping, Y. Influence of NH3/CO2 modification on the characteristic of biochar and the CO2 capture. BioEnergy Res. 2013, 6, 1147–1153. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Bąk, J.; Kozioł, M.; Pylychuk, L.V. Investigations of heavy metal ion sorption using nanocomposites of iron-modified biochar. Nanoscale Res. Lett. 2017, 12, 433. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Wang, J.; Pan, Y.; Shen, B.; Wu, C. Review of biochar for the management of contaminated soil: Preparation, application and prospect. Sci. Total Environ. 2019, 659, 473–490. [Google Scholar] [CrossRef] [PubMed]
- Rajapaksha, A.U.; Vithanage, M.; Ahmad, M.; Seo, D.C.; Cho, J.S.; Lee, S.S.; Lee, S.O.; Ok, Y.S. Enhanced sulfamethazine removal by steam-activated invasive plant-derived biochar. J. Hazard. Mater. 2015, 290, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Mašek, O.; Budarin, V.; Gronnow, M.; Crombie, K.; Brownsort, P.; Fitzpatrick, E.; Hurst, P. Microwave and slow pyrolysis biochar-Comparison of physical and functional properties, J. Anal. Appl. Pyrolysis 2013, 100, 41–48. [Google Scholar] [CrossRef]
- Jing, X.R.; Wang, Y.Y.; Liu, W.J.; Wang, Y.K.; Jiang, H. Enhanced adsorption performance of tetracycline in aqueous solutions by methanol-modified biochar. Chem. Eng. J. 2014, 248, 168–174. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, F.; Xue, J. Enhanced removal of heavy metal ions from aqueous solution using manganese dioxide-loaded biochar: Behavior and mechanism. Sci. Rep. 2020, 10, 6067. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Microbial inoculants: Reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express 2019, 9, 205. [Google Scholar] [CrossRef]
- Alori, E.T.; Dare, M.O.; Babalola, O.O. Microbial Inoculants for Soil Quality and Plant Health. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer: Cham, Switzerland, 2017; Volume 22, pp. 281–308. [Google Scholar] [CrossRef]
- Alori, E.T.; Babalola, O.O. Microbial inoculants for improving crop quality and human health in Africa. Front. Microbiol. 2018, 9, 2213. [Google Scholar] [CrossRef]
- Barbu, L.D.N.; Boiu-Sicuia, O.A. Plant-beneficial microbial inoculants and their formulation—A review. Rom. J. Plant Prot. 2021, 14, 32–43. [Google Scholar] [CrossRef]
- Nanjani, S.; Soni, R.; Paul, D.; Keharia, H. Genome analysis uncovers the prolific antagonistic and plant growth-promoting potential of endophyte Bacillus velezensis K1. Gene 2022, 836, 146671. [Google Scholar] [CrossRef]
- O’Callaghan, M.; Ballard, R.A.; Wright, D. Soil microbial inoculants for sustainable agriculture: Limitations and opportunities. Soil Use Manag. 2022, 38, 1340–1369. [Google Scholar] [CrossRef]
- Ajeng, A.A.; Abdullah, R.; Ling, T.C.; Ismail, S.; Lau, B.F.; Ong, H.C.; Chew, K.W.; Show, P.L.; Chang, J.-S. Bioformulation of biochar as a potential inoculant carrier for sustainable agriculture. Environ. Technol. Innov. 2020, 20, 101168. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Berninger, T.; González López, Ó.; Bejarano, A.; Preininger, C.; Sessitsch, A. Maintenance and assessment of cell viability in formulation of non-sporulating bacterial inoculants. Microb. Biotechnol. 2018, 11, 277–301. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ma, J.; Wei, J.; Gong, X.; Yu, X.; Guo, H.; Zhao, Y. Biochar increases plant growth and alters microbial communities via regulating the moisture and temperature of green roof substrates. Sci. Total Environ. 2018, 635, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Vanek, S.J.; Thies, J. Pore-size and water activity effects on survival of rhizobium tropici in biochar inoculant carriers. J. Microb. Biochem. Technol. 2016, 8, 296–306. [Google Scholar] [CrossRef]
- Bouabidi, Z.B.; El-Naas, M.H.; Zhang, Z. Immobilization of microbial cells for the biotreatment of wastewater: A review. Environ. Chem. Lett. 2019, 17, 241–257. [Google Scholar] [CrossRef]
- Valdivia-Rivera, S.; Ayora-Talavera, T.; Lizardi-Jiménez, M.A.; García-Cruz, U.; Cuevas-Bernardino, J.C.; Pacheco, N. Encapsulation of microorganisms for bioremediation: Techniques and carriers. Rev. Environ. Sci. Biotechnol. 2021, 20, 815–838. [Google Scholar] [CrossRef]
- Wu, P.; Wang, Z.; Bhatnagar, A.; Jeyakumar, P.; Wang, H.; Wang, Y.; Li, X. Microorganisms-carbonaceous materials immobilized complexes: Synthesis, adaptability and environmental applications. J. Hazard. Mater. 2021, 416, 125915. [Google Scholar] [CrossRef]
- Malusá, E.; Sas-Paszt, L.; Ciesielska, J. Technologies for beneficial microorganisms inocula used as biofertilizers. Sci. World J. 2012, 2012, 491206. [Google Scholar] [CrossRef]
- Hale, L.; Luth, M.; Crowley, D. Biochar characteristics relate to its utility as an alternative soil inoculum carrier to peat and vermiculite. Soil Biol. Biochem. 2015, 81, 228–235. [Google Scholar] [CrossRef]
- Khavazi, K.; Rejali, F.; Seguin, P.; Miransari, M. Effects of carrier, sterilisation method, and incubation on survival of Bradyrhizobium japonicum in soybean (Glycine max L.) inoculants. Enzyme Microb. Technol. 2007, 41, 780–784. [Google Scholar] [CrossRef]
- Sun, D.; Hale, L.; Crowley, D. Nutrient supplementation of pinewood biochar for use as a bacterial inoculum carrier. Biol. Fertil. Soils 2016, 52, 515–522. [Google Scholar] [CrossRef]
- Zhang, M.; Xia, H.; Riaz, M.; Liu, B.; El-Desouki, Z.; Jiang, C. Various beneficial microorganisms colonizing on the surface of biochar primarily originated from the storage environment rather than soil environment. Appl. Soil Ecology 2023, 182, 104700. [Google Scholar] [CrossRef]
- Ngan, N.M.; Riddech, N. Use of spent mushroom substrate as an inoculant carrier and an organic fertilizer and their impacts on roselle growth (Hibiscus sabdariffa L.) and soil quality. Waste Biomass Valorization 2021, 12, 3801–3811. [Google Scholar] [CrossRef]
- Sahu, P.K.; Brahmaprakash, G.P. Formulations of biofertilizers—Approaches and advances. In Microbial Inoculants in Sustainable Agricultural Productivity: Functional Applications; Singh, D.P., Singh, H.B., Prabha, R., Eds.; Springer: New Delhi, India, 2016; Volume 2, pp. 179–198. [Google Scholar] [CrossRef]
- Husna, N.; Budianta, D.; Napoleon, A. Evaluation of several biochar types as inoculant carrier for indigenous phosphate solubilizing microoorganism from acid sulphate soil. J. Ecol. Eng. 2019, 20, 1–8. [Google Scholar] [CrossRef]
- Tripti; Kumar, A.; Usmani, Z.; Kumar, V.; Anshumali. Biochar and flyash inoculated with plant growth promoting rhizobacteria act as potential biofertilizer for luxuriant growth and yield of tomato plant. J. Environ. Manag. 2017, 190, 20–27. [Google Scholar] [CrossRef]
- Zheng, B.X.; Ding, K.; Yang, X.R.; Wadaan, M.A.M.; Hozzein, W.N.; Peñuelas, J.; Zhu, Y.G. Straw biochar increases the abundance of inorganic phosphate solubilizing bacterial community for better rape (Brassica napus) growth and phosphate uptake. Sci. Total Environ. 2019, 647, 1113–1120. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Hua, M.; Reckling, M.; Wirth, S.; Bellingrath-Kimura, S.D. Potential effects of biochar-based microbial inoculants in agriculture. Environ. Sustain. 2018, 1, 19–24. [Google Scholar] [CrossRef]
- Qi, X.; Zhu, M.; Yuan, Y.; Dang, Z.; Yin, H. Bioremediation of PBDEs and heavy metals co-contaminated soil in e-waste dismantling sites by Pseudomonas plecoglossicida assisted with biochar. J. Hazard. Mater. 2023, 460, 132408. [Google Scholar] [CrossRef]
- Chuaphasuk, C.; Prapagdee, B. Effects of biochar-immobilized bacteria on phytoremediation of cadmium-polluted soil. Environ. Sci. Pollut. Res. 2019, 26, 23679–23688. [Google Scholar] [CrossRef] [PubMed]
- Thies, J.E.; Rillig, M.C. Characteristics of biochar: Biological properties. In Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S., Eds.; Science and Technology Earthscan: London, UK, 2009; pp. 85–105. ISBN 9781849770552. [Google Scholar]
- Tripti; Kumar, A.; Kumar, V.; Anshumali; Bruno, L.B.; Rajkumar, M. Synergism of industrial and agricultural waste as a suitable carrier material for developing potential biofertilizer for sustainable agricultural production of eggplant. Horticulturae 2022, 8, 444. [Google Scholar] [CrossRef]
- Azeem, M.; Hassan, T.U.; Tahir, M.I.; Ali, A.; Jeyasundar, P.G.S.A.; Hussain, Q.; Bashir, S.; Mehmood, S.; Zhang, Z. Tea leaves biochar as a carrier of Bacillus cereus improves the soil function and crop productivity. Appl. Soil Ecol. 2021, 157, 103732. [Google Scholar] [CrossRef]
- Mankar, M.K.; Sharma, U.S.; Sahay, S. Lantana charcoal as potent carrier material for Azotobacter chroococcum. Die Bodenkult. J. Land Manag. Food Environ. 2020, 72, 83–91. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, H.; Zhu, Z.; Xing, S.; Wang, S.; Chen, B. Low-temperature straw biochar: Sustainable approach for sustaining higher survival of B. megaterium and managing phosphorus deficiency in the soil. Sci. Total Environ. 2022, 830, 154790. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, N.M.; Clode, P.L.; Abbott, L.K. Soil microbial responses to biochars varying in particle size, surface and pore properties. Pedosphere 2015, 25, 770–780. [Google Scholar] [CrossRef]
- Tao, S.; Wu, Z.; He, X.; Ye, B.C.; Li, C. Characterization of biochar prepared from cotton stalks as efficient inoculum carriers for Bacillus subtilis SL-13. BioResources 2018, 13, 1773–1786. [Google Scholar] [CrossRef]
- Hammer, E.C.; Balogh-Brunstad, Z.; Jakobsen, I.; Olsson, P.A.; Stipp, S.L.S.; Rillig, M.C. A mycorrhizal fungus grows on biochar and captures phosphorus from its surfaces. Soil Biol. Biochem. 2014, 77, 252–260. [Google Scholar] [CrossRef]
- Saranya, K.; Santhana, K.P.; Kumutha, K.; John, F. Potential for biochar as an alternate carrier to lignite for the preparation of biofertilizers in India. Int. J. Agric. Environ. Biotechnol. 2011, 4, 167–172. [Google Scholar]
- Tao, Y.; Han, S.; Zhang, Q.; Yang, Y.; Shi, H.; Akindolie, M.S.; Jiao, Y.; Qu, J.; Jiang, Z.; Han, W.; et al. Application of biochar with functional microorganisms for enhanced atrazine removal and phosphorus utilization. J. Clean. Prod. 2020, 257, 120535. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Dzionek, A.; Wojcieszyńska, D.; Guzik, U. Natural carriers in bioremediation: A review. Electron. J. Biotechnol. 2016, 23, 28–36. [Google Scholar] [CrossRef]
- Głodowska, M.; Schwinghamer, T.; Husk, B.; Smith, D. Biochar based inoculants improve soybean growth and nodulation. Agric. Sci. 2017, 08, 1048–1064. [Google Scholar] [CrossRef]
- Novak, J.M.; Lima, I.; Xing, B.; Gaskin, J.W.; Steiner, C.; Das, K.; Ahmedna, M.; Rehrah, D.; Watts, D.W.; Busscher, W.J.; et al. Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Ann. Environ. Sci. 2009, 3, 195–206. [Google Scholar]
- Enders, A.; Hanley, K.; Whitman, T.; Joseph, S.; Lehmann, J. Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour. Technol. 2012, 114, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Kloss, S.; Zehetner, F.; Dellantonio, A.; Hamid, R.; Ottner, F.; Liedtke, V.; Schwanninger, M.; Gerzabek, M.H.; Soja, G. Characterization of slow pyrolysis biochars: Effects of feedstocks and pyrolysis temperature on biochar properties. J. Environ. Qual. 2012, 41, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon(biochar)-supporting information. Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.; Sheng, G.; Chiou, G.T.; Xing, B. Compositions and sorptive properties of crop residue-derived chars. Environ. Sci. Technol. 2004, 38, 4649–4655. [Google Scholar] [CrossRef]
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Riling, M.C. Mycorrhizal responses to biochar in soil—Concepts and mechanisms. Plant Soil 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Biochar and soil physical properties. Soil Sci. Soc. Am. J. 2017, 81, 687–711. [Google Scholar] [CrossRef]
- Sun, F.; Lu, S. Biochars improve aggregate stability, water retention, and pore-space properties of clayey soil. J. Plant Nutr. Soil Sci. 2014, 177, 26–33. [Google Scholar] [CrossRef]
- Xue, P.; Fu, Q.; Li, T.; Liu, D.; Hou, R.; Li, Q.; Li, M.; Meng, F. Effects of biochar and straw application on the soil structure and water-holding and gas transport capacities in seasonally frozen soil areas. J. Environ. Manag. 2022, 301, 113943. [Google Scholar] [CrossRef] [PubMed]
- Głąb, T.; Palmowska, J.; Zaleski, T.; Gondek, K. Effect of biochar application on soil hydrological properties and physical quality of sandy soil. Geoderma 2016, 281, 11–20. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sarkar, B.; Aralappanavar, V.K.; Mukhopadhyay, R.; Basak, B.B.; Srivastava, P.; Marchut-Mikołajczyk, O.; Bhatnagar, A.; Semple, K.T.; Bolan, N. Biochar-microorganism interactions for organic pollutant remediation: Challenges and perspectives. Environ. Pollut. 2022, 308, 119609. [Google Scholar] [CrossRef] [PubMed]
- Schimel, J.; Balser, T.; Wallenstein, M. Microbial stress-response physiology and its implications. Ecology 2007, 88, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, T.; Yang, F.; Cen, R.; Liao, H.; Qu, Z. Effects of biochar on soil evaporation and moisture content and the associated mechanisms. Environ. Sci. Eur. 2023, 35, 66. [Google Scholar] [CrossRef]
- Ascough, P.L.; Sturrock, C.J.; Bird, M.I. Investigation of growth responses in saprophytic fungi to charred biomass. Isotopes Environ. Health Stud. 2010, 46, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.; Huang, Q.; Lou, Y.; Lu, J.; Hu, B.; Lin, Q. Adsorption and degradation in the removal of nonylphenol from water by cells immobilized on biochar. Chemosphere 2019, 228, 676–684. [Google Scholar] [CrossRef]
- Hill, R.A.; Hunt, J.; Sanders, E.; Tran, M.; Burk, G.A.; Mlsna, T.E.; Fitzkee, N.C. Effect of biochar on microbial growth: A metabolomics and bacteriological investigation in E. coli. Environ. Sci. Technol. 2019, 53, 2635–2646. [Google Scholar] [CrossRef]
- Novosad, N.; Kay, B.D. Water-filled microbially habitable pores: Relation to denitrification. Can. J. Soil Sci. 2007, 87, 269–280. [Google Scholar] [CrossRef][Green Version]
- Quilliam, R.S.; Glanville, H.C.; Wade, S.C.; Jones, D.L. Life in the “charosphere”—Does biochar in agricultural soil provide a significant habitat for microorganisms? Soil Biol. Biochem. 2013, 65, 287–293. [Google Scholar] [CrossRef]
- Samonin, V.V.; Elikova, E.E. A study of the adsorption of bacterial cells on porous materials. Microbiology 2004, 73, 696–701. [Google Scholar] [CrossRef]
- Gorovtsov, A.V.; Minkina, T.M.; Mandzhieva, S.S.; Perelomov, L.V.; Soja, G.; Zamulina, I.V.; Rajput, V.D.; Sushkova, S.N.; Mohan, D.; Yao, J. The mechanisms of biochar interactions with microorganisms in soil. Environ. Geochem. Health 2020, 42, 2495–2518. [Google Scholar] [CrossRef] [PubMed]
- Kharel, G.; Sacko, O.; Feng, X.; Morris, J.R.; Phillips, C.L.; Trippe, K.; Kumar, S.; Lee, J.W. Biochar surface oxygenation by ozonization for super high cation exchange capacity. ACS Sustain. Chem. Eng. 2019, 7, 16410–16418. [Google Scholar] [CrossRef]
- Mia, S.; Singh, B.; Dijkstra, F.A. Aged biochar affects gross nitrogen mineralization and recovery: A 15N study in two contrasting soils. GCB Bioenergy 2017, 9, 1196–1206. [Google Scholar] [CrossRef]
- Gaskin, J.W.; Steiner, C.; Harris, K.; Das, K.C.; Bibens, B. Effect of low-temperature pyrolysis conditions on biochar for agriculture use. Am. Soc. Agric. Biol. Eng. 2008, 51, 2061–2069. [Google Scholar] [CrossRef]
- Purakayastha, T.J.; Bera, T.; Bhaduri, D.; Sarkar, B.; Mandal, S.; Wade, P.; Kumari, S.; Biswas, S.; Menon, M.; Pathak, H.; et al. A review on biochar modulated soil condition improvements and nutrient dynamics concerning crop yields: Pathways to climate change mitigation and global food security. Chemosphere 2019, 227, 345–365. [Google Scholar] [CrossRef]
- Khadem, A.; Raiesi, F.; Besharati, H.; Khalaj, M.A. The effects of biochar on soil nutrients status, microbial activity and carbon sequestration potential in two calcareous soils. Biochar 2021, 3, 105–116. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Novak, J.M.; Busscher, W.J.; Ahmedna, M.; Rehrah, D.; Watts, D.W. Switchgrass Biochar Affects Two Aridisols. J. Environ. Qual. 2012, 41, 1123–1130. [Google Scholar] [CrossRef]
- Bilias, F.; Kalderis, D.; Richardson, C.; Barbayiannis, N.; Gasparatos, D. Biochar application as a soil potassium management strategy: A review. Sci. Total Environ. 2023, 858, 159782. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, B.; Chen, Z.; Zhu, L.; Schnoor, J.L. insight into multiple and multilevel structures of biochars and their potential environmental applications: A critical review. Environ. Sci. Technol. 2018, 52, 5027–5047. [Google Scholar] [CrossRef]
- Glaser, B.; Lehr, V.I. Biochar effects on phosphorus availability in agricultural soils: A meta-analysis. Sci. Rep. 2019, 9, 9338. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.; Wu, Y.; Zhang, C.; Dilinuer, Y.; Pasang, L.; Lu, Y.; Wang, Y.; Chen, H.; Li, Z. Combination of biochar and phosphorus solubilizing bacteria to improve the stable form of toxic metal minerals and microbial abundance in lead/cadmium-contaminated soil. Agronomy 2022, 12, 1003. [Google Scholar] [CrossRef]
- Liu, S.; Meng, J.; Jiang, L.; Yang, X.; Lan, Y.; Cheng, X.; Chen, W. Rice husk biochar impacts soil phosphorous availability, phosphatase activities and bacterial community characteristics in three different soil types. Appl. Soil Ecol. 2017, 116, 12–22. [Google Scholar] [CrossRef]
- Vanek, S.J.; Lehmann, J. Phosphorus availability to beans via interactions between mycorrhizas and biochar. Plant Soil 2015, 395, 105–123. [Google Scholar] [CrossRef]
- Anderson, C.R.; Condron, L.M.; Clough, T.J.; Fiers, M.; Stewart, A.; Hill, R.A.; Sherlock, R.R. Biochar induced soil microbial community change: Implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia 2011, 54, 309–320. [Google Scholar] [CrossRef]
- Gomez, J.D.; Denef, K.; Stewart, C.E.; Zheng, J.; Cotrufo, M.F. Biochar addition rate influences soil microbial abundance and activity in temperate soils. Eur. J. Soil Sci. 2014, 65, 28–39. [Google Scholar] [CrossRef]
- Zimmerman, A.R. Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.; Sohi, S.P. The priming potential of biochar products in relation to labile carbon contents and soil organic matter status. Soil Biol. Biochem. 2011, 43, 2127–2134. [Google Scholar] [CrossRef]
- Farrell, M.; Kuhn, T.K.; Macdonald, L.M.; Maddern, T.M.; Murphy, D.V.; Hall, P.A.; Singh, B.P.; Baumann, K.; Krull, E.S.; Baldock, J.A. Microbial utilisation of biochar-derived carbon. Sci. Total Environ. 2013, 465, 288–297. [Google Scholar] [CrossRef]
- Bruun, E.W.; Hauggaard-Nielsen, H.; Ibrahim, N.; Egsgaard, H.; Ambus, P.; Jensen, P.A.; Dam-Johansen, K. Influence of fast pyrolysis temperature on biochar labile fraction and short-term carbon loss in a loamy soil. Biomass Bioenergy 2011, 35, 1182–1189. [Google Scholar] [CrossRef]
- Spokas, K.A.; Novak, J.M.; Stewart, C.E.; Cantrell, K.B.; Uchimiya, M.; DuSaire, M.G.; Ro, K.S. Qualitative analysis of volatile organic compounds on biochar. Chemosphere 2011, 85, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Haas, T.J.; Nimlos, M.R.; Donohoe, B.S. Real-time and post-reaction microscopic structural analysis of biomass undergoing pyrolysis. Energy Fuels 2009, 23, 3810–3817. [Google Scholar] [CrossRef]
- Warnock, D.D.; Mummey, D.L.; McBride, B.; Major, J.; Lehmann, J.; Rillig, M.C. Influences of non-herbaceous biochar on arbuscular mycorrhizal fungal abundances in roots and soils: Results from growth-chamber and field experiments. Appl. Soil Ecol. 2010, 46, 450–456. [Google Scholar] [CrossRef]
- Gaur, A.; Adholeya, A. Effects of the particle size of soil-less substrates upon AM fungus inoculum production. Mycorrhiza 2000, 10, 43–48. [Google Scholar] [CrossRef]
- Rondon, M.A.; Lehmann, J.; Ramírez, J.; Hurtado, M. Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol. Fertil. Soils 2007, 43, 699–708. [Google Scholar] [CrossRef]
- Wang, C.; Lv, H.; Yang, W.; Li, T.; Fang, T.; Lv, G.; Han, Q.; Dong, L.; Jiang, T.; Jiang, B. SVCT-2 determines the sensitivity to ascorbate-induced cell death in cholangiocarcinoma cell lines and patient derived xenografts. Cancer Lett. 2017, 398, 1–11. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, B.; Liu, G.; Cai, Z.; Zhang, C. Potential Toxic Compounds in Biochar: Knowledge Gaps between Biochar Research and Safety; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128117293. [Google Scholar]
- Dutta, T.; Kwon, E.; Bhattacharya, S.S.; Jeon, B.H.; Deep, A.; Uchimiya, M.; Kim, K.H. Polycyclic aromatic hydrocarbons and volatile organic compounds in biochar and biochar-amended soil: A review. GCB Bioenergy 2017, 9, 990–1004. [Google Scholar] [CrossRef]
- Smith, C.R.; Hatcher, P.G.; Kumar, S.; Lee, J.W. investigation into the sources of biochar water-soluble organic compounds and their potential toxicity on aquatic microorganisms. ACS Sustain. Chem. Eng. 2016, 4, 2550–2558. [Google Scholar] [CrossRef]
- Freddo, A.; Cai, C.; Reid, B.J. Environmental contextualisation of potential toxic elements and polycyclic aromatic hydrocarbons in biochar. Environ. Pollut. 2012, 171, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, J.L. Effects of biochar on soil microbial diversity and community structure in clay soil. Ann. Microbiol. 2022, 72, 35. [Google Scholar] [CrossRef]
- Nkoh, J.N.; Al Baquy, M.A.; Mia, S.; Shi, R.; Kamran, M.A.; Mehmood, K.; Xu, R. A critical-systematic review of the interactions of biochar with soils and the observable outcomes. Sustainability 2021, 13, 13726. [Google Scholar] [CrossRef]
- Banik, C.; Lawrinenko, M.; Bakshi, S.; Laird, D.A. Impact of pyrolysis temperature and feedstock on surface charge and functional group chemistry of biochars. J. Environ. Qual. 2018, 47, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Li, J.; Ni, N.; Xu, R. Understanding the biochar’s role in ameliorating soil acidity. J. Integr. Agric. 2019, 18, 1508–1517. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, D.; Zhu, L. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef]
- Gray, M.; Johnson, M.G.; Dragila, M.I.; Kleber, M. Water uptake in biochars: The roles of porosity and hydrophobicity. Biomass Bioenergy 2014, 61, 196–205. [Google Scholar] [CrossRef]
- He, P.; Zhang, H.; Duan, H.; Shao, L.; Lü, F. Continuity of biochar-associated biofilm in anaerobic digestion. Chem. Eng. J. 2020, 390, 124605. [Google Scholar] [CrossRef]
- Elzobair, K.A.; Stromberger, M.E.; Ippolito, J.A. Stabilizing effect of biochar on soil extracellular enzymes after a denaturing stress. Chemosphere 2016, 142, 114–119. [Google Scholar] [CrossRef]
- Keiblinger, K.M.; Liu, D.; Mentler, A.; Zehetner, F.; Zechmeister-Boltenstern, S. Biochar application reduces protein sorption in soil. Org. Geochem. 2015, 87, 21–24. [Google Scholar] [CrossRef]
- Senbayram, M.; Saygan, E.P.; Chen, R.; Aydemir, S.; Kaya, C.; Wu, D.; Bladogatskaya, E. Effect of biochar origin and soil type on the greenhouse gas emission and the bacterial community structure in N fertilised acidic sandy and alkaline clay soil. Sci. Total Environ. 2019, 660, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Whitman, W.B.; Gundale, M.J.; Chien, C.C.; Chiu, C.Y. Functional response of the soil microbial community to biochar applications. GCB Bioenergy 2021, 13, 269–281. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xiong, Y.; Cai, L. Effects of biochar on the soil carbon cycle in agroecosystems: A promising way to increase the carbon pool in dryland. In IOP Conference Series: Earth and Environmental Science, Proceedings of the 8th Annual International Conference on Geo-Spatial Knowledge and Intelligence, Xi’an, China, 18–19 December 2020; IOP Publishing: Bristol, UK, 2021; Volume 693, p. 012082. [Google Scholar] [CrossRef]
- Rhodes, A.H.; Riding, M.J.; McAllister, L.E.; Lee, K.; Semple, K.T. Influence of activated charcoal on desorption kinetics and biodegradation of phenanthrene in soil. Environ. Sci. Technol. 2022, 46, 12445–12451. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wang, L.; Ma, F.; Wang, Y.; Bai, S. A bio-functions integration microcosm: Self-immobilized biochar-pellets combined with two strains of bacteria to remove atrazine in water and mechanisms. J. Hazard. Mater. 2020, 384, 121326. [Google Scholar] [CrossRef] [PubMed]
- Mierzwa-Hersztek, M.; Wolny-Koładka, K.; Gondek, K.; Gałązka, A.; Gawryjołek, K. Effect of coapplication of biochar and nutrients on microbiocenotic composition, dehydrogenase activity index and chemical properties of sandy soil. Waste Biomass Valorization 2020, 11, 3911–3923. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Chang, S.X.; Jiang, X.; Song, Y. Biochar increases soil microbial biomass but has variable effects on microbial diversity: A meta-analysis. Sci. Total Environ. 2020, 749, 141593. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, Y.; Xiang, Y.; Zhang, R.; Lu, H. Responses of soil microbial community structure changes and activities to biochar addition: A meta-analysis. Sci. Total Environ. 2018, 643, 926–935. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Pathy, A.; Ray, J.; Paramasivan, B. Biochar amendments and its impact on soil biota for sustainable agriculture. Biochar 2020, 2, 287–305. [Google Scholar] [CrossRef]
- Brookes, P. The soil microbial biomass: Concept, measurement and applications in soil ecosystem research. Microbes Environ. 2001, 16, 131–140. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H.Y. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Tian, J.; Wang, J.; Dippold, M.; Gao, Y.; Blagodatskaya, E.; Kuzyakov, Y. Biochar affects soil organic matter cycling and microbial functions but does not alter microbial community structure in a paddy soil. Sci. Total Environ. 2016, 556, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Ajema, L. Effects of biochar application on beneficial soil organism. Int. J. Res. Stud. Sci. Eng. Technol. 2018, 5, 9–18. [Google Scholar]
- Domene, X.; Mattana, S.; Hanley, K.; Enders, A.; Lehmann, J. Medium-term effects of corn biochar addition on soil biota activities and functions in a temperate soil cropped to corn. Soil Biol. Biochem. 2014, 72, 152–162. [Google Scholar] [CrossRef]
- Jones, D.; Rousk, J.; Edwards-Jones, G.; DeLuca, T.; Murphy, D. Biochar-mediated changes in soil quality and plant growth in a three year field trial. Soil Biol. Biochem. 2012, 45, 113–124. [Google Scholar] [CrossRef]
- Murphy, D.V.; Cookson, W.R.; Braimbridge, M.; Marschner, P.; Jones, D.L.; Stockdale, E.A.; Abbott, L.K. Relationships between soil organic matter and the soil microbial biomass (size, functional diversity, and community structure) in crop and pasture systems in a semi-arid environment. Soil Res. 2011, 49, 582–594. [Google Scholar] [CrossRef]
- Domene, X.; Hanley, K.; Enders, A.; Lehmann, J. Short-term mesofauna responses to soil additions of corn stover biochar and the role of microbial biomass. Appl. Soil Ecol. 2015, 89, 10–17. [Google Scholar] [CrossRef]
- Liao, N.; Li, Q.; Zhang, W.; Zhou, G.; Ma, L.; Min, W.; Hou, Z. Effects of biochar on soil microbial community composition and activity in drip-irrigated desert soil. Eur. J. Soil Biol. 2016, 72, 27–34. [Google Scholar] [CrossRef]
- Plaza, C.; Pawlett, M.; Fernandez, J.M.; Mendez, E.; Gasco, G.; Ritz, K. Does biochar interfere with standard methods for determining soil microbial biomass and phenotypic community structure. Soil Biol. Biochem. 2015, 81, 143–146. [Google Scholar] [CrossRef]
- Mitchell, P.J.; Simpson, A.J.; Soong, R.; Simpson, M.J. Shifts in microbial community and water-extractable organic matter composition with biochar amendment in a temperate forest soil. Soil Biol. Biochem. 2015, 81, 244–254. [Google Scholar] [CrossRef]
- Quilliam, R.S.; Marsden, K.A.; Gertler, C.; Rousk, J.; DeLuca, T.H.; Jones, D.L. Nutrient dynamics, microbial growth and weed emergence in biochar amended soil are influenced by time since application and reapplication rate. Agric. Ecosyst. Environ. 2012, 158, 192–199. [Google Scholar] [CrossRef]
- Rutigliano, F.A.; Romano, M.; Marzaioli, R.; Baglivo, I.; Baronti, S.; Miglietta, F.; Castaldi, S. Effect of biochar addition on soil microbial community in a wheat crop. Eur. J. Soil Biol. 2014, 60, 9–15. [Google Scholar] [CrossRef]
- Imparato, V.; Hansen, V.; Santos, S.S.; Nielsen, T.K.; Giagnoni, L.; Hauggaard-Nielsen, H.; Johansen, A.; Renella, G.; Winding, A. Gasification biochar has limited effects on functional and structural diversity of soil microbial communities in a temperate agroecosystem. Soil Biol. Biochem. 2016, 99, 128–136. [Google Scholar] [CrossRef]
- Luo, S.; Wang, S.; Tian, L.; Li, S.; Li, X.; Shen, Y.; Tian, C. Long-term biochar application influences soil microbial community and its potential roles in semiarid farmland. Appl. Soil Ecol. 2017, 117, 10–15. [Google Scholar] [CrossRef]
- Khodadad, C.L.; Zimmerman, A.R.; Green, S.J.; Uthandi, S.; Foster, J.S. Taxa-specific changes in soil microbial community composition induced by pyrogenic carbon amendments. Soil Biol. Biochem. 2011, 43, 385–392. [Google Scholar] [CrossRef]
- Abujabhah, I.S.; Doyle, R.B.; Bound, S.A.; Bowman, J.P. Assessment of bacterial community composition, methanotrophic and nitrogen-cycling bacteria in three soils with different biochar application rates. J. Soils Sediments 2018, 18, 148–158. [Google Scholar] [CrossRef]
- Hu, L.; Cao, L.; Zhang, R. Bacterial and fungal taxon changes in soil microbial community composition induced by short-term biochar amendment in red oxidized loam soil. World J. Microbiol. Biotechnol. 2014, 30, 1085–1092. [Google Scholar] [CrossRef]
- Li, Q.; Lei, Z.; Song, X.; Zhang, Z.; Ying, Y.; Peng, C. Biochar amendment decreases soil microbial biomass and increases bacterial diversity in Moso bamboo (Phyllostachys edulis) plantations under simulated nitrogen deposition. Environ. Res. Lett. 2018, 13, 044029. [Google Scholar] [CrossRef]
- Song, Y.; Li, X.; Xu, M.; Jiao, W.; Bian, Y.; Yang, X.; Gu, C.; Wang, F.; Jiang, X. Does biochar induce similar successions of microbial community structures among different soils? Bull. Environ. Contam. Toxicol. 2019, 103, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Kolton, M.; Meller Harel, Y.; Pasternak, Z.; Graber, E.R.; Elad, Y.; Cytryn, E. Impact of biochar application to soil on the root-associated bacterial community structure of fully developed greenhouse pepper plants. Appl. Environ. Microbiol. 2011, 77, 4924–4930. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.J.; Wang, X.H.; Li, H.; Yao, H.Y.; Su, J.Q.; Zhu, Y.G. Biochar impacts soil microbial community composition and nitrogen cycling in an acidic soil planted with rape. Environ. Sci. Technol. 2014, 48, 9391–9399. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, X.; Li, L.; Zheng, J.; Qu, J.; Zheng, J.; Pan, G. Consistent increase in abundance and diversity but variable change in community composition of bacteria in topsoil of rice paddy under short term biochar treatment across three sites from South China. Appl. Soil Ecol. 2015, 91, 68–79. [Google Scholar] [CrossRef]
- Cheng, J.; Li, Y.; Gao, W.; Chen, Y.; Pan, W.; Lee, X.; Tang, Y. Effects of biochar on Cd and Pb mobility and microbial community composition in a calcareous soil planted with tobacco. Biol. Fertil. Soils 2018, 54, 373–383. [Google Scholar] [CrossRef]
- Anderson, C.R.; Hamonts, K.; Clough, T.J.; Condron, L.M. Biochar does not affect soil N-transformations or microbial community structure under ruminant urine patches but does alter relative proportions of nitrogen cycling bacteria. Agric. Ecosyst. Environ. 2014, 191, 63–72. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, J.; Yu, Z.; Li, Y.; Jin, J.; Liu, X.; Wang, G. Changes of bacterial community compositions after three years of biochar application in a black soil of northeast China. Appl. Soil Ecol. 2017, 113, 11–21. [Google Scholar] [CrossRef]
- Liu, X.R.; Li, J.; Yu, L.; Pan, H.; Liu, H.Y.; Liu, Y.M.; Di, H.J.; Li, Y.; Xu, J.M. Simultaneous measurement of bacterial abundance and composition in response to biochar in soybean field soil using 16S rRNA gene sequencing. Land Degrad. Dev. 2018, 29, 2172–2182. [Google Scholar] [CrossRef]
- Cheng, J.; Lee, X.; Tang, Y.; Zhang, Q. Long-term effects of biochar amendment on rhizosphere and bulk soil microbial communities in a karst region, southwest China. Appl. Soil Ecol. 2019, 140, 126–134. [Google Scholar] [CrossRef]
- Hockaday, W.C.; Grannas, A.M.; Kim, S.; Hatcher, P.G. The transformation and mobility of charcoal in a fireimpacted watershed. Geochim. Cosmochim. Acta 2007, 71, 3432–3445. [Google Scholar] [CrossRef]
- Ascough, P.L.; Bird, M.I.; Scott, A.C.; Collinson, M.E.; Cohen-Ofri, I. Charcoal reflectance measurements: Implications for structural characterization and assessment of diagenetic alteration. J. Archaeol. Sci. 2010, 37, 1590–1599. [Google Scholar] [CrossRef]
- Jaafar, N.M.; Clode, P.L.; Abbott, L.K. Microscopy observations of habitable space in biochar for colonization by fungal hyphae from soil. J. Integr. Agric. 2014, 13, 483–490. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Zheng, J.; Zhang, B.; Lu, H.; Chi, Z.; Pan, G.; Li, L.; Zheng, J.; Zhang, X.; et al. Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Appl. Soil Ecol. 2013, 71, 33–44. [Google Scholar] [CrossRef]
- Bamminger, C.; Zaiser, N.; Zinsser, P.; Lamers, M.; Kammann, C.; Marhan, S. Effects of biochar, earthworms, and litter addition on soil microbial activity and abundance in a temperate agricultural soil. Biol. Fertil. Soils 2014, 50, 1189–1200. [Google Scholar] [CrossRef]
- Thies, J.E.; Rillig, M.C.; Graber, E.R. Biochar effects on the abundance, activity and diversity of the soil biota. In Biochar for Environmental Management: Science, Technology and Implementation; Lehmann, J., Joseph, S., Eds.; Routledge: New York, NY, USA, 2015; pp. 327–390. [Google Scholar]
- Alkorta, I.; Aizpurua, A.; Riga, P.; Albizu, I.; Amézaga, I.; Garbisu, C. Soil enzyme activities as biological indicators of soil health. Rev. Environ. Health. 2003, 18, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Masto, R.E.; Kumar, S.; Rout, T.K.; Sarkar, P.; George, J.; Ram, L.C. Biochar from water hyacinth (Eichornia crassipes) and its impact on soil biological activity. Catena 2013, 111, 64–71. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Gascó, G.; Gutiérrez, B.; Méndez, A. Soil biochemical activities and the geometric mean of enzyme activities after application of sewage sludge and sewage sludge biochar to soil. Biol. Fertil. Soils 2012, 48, 511–517. [Google Scholar] [CrossRef]
- Gascó, G.; Paz-Ferreiro, J.; Cely, P.; Plaza, C.; Méndez, A. Influence of pig manure and its biochar on soil CO2 emissions and soil enzymes. Ecol. Eng. 2016, 95, 19–24. [Google Scholar] [CrossRef]
- Munir, M.A.; Yousaf, B.; Ali, M.U.; Dan, C.; Abbas, Q.; Arif, M.; Yang, X. In situ synthesis of micro-plastics embedded sewage-sludge co-pyrolyzed biochar: Implications for the remediation of Cr and Pb availability and enzymatic activities from the contaminated soil. J. Clean. Prod. 2021, 302, 127005. [Google Scholar] [CrossRef]
- Raiesi, F.; Khadem, A. Short-term effects of maize residue biochar on kinetic and thermodynamic parameters of soil β-glucosidase. Biochar 2019, 1, 213–227. [Google Scholar] [CrossRef]
- Sandhu, S.; Sekaran, U.; Ozlu, E.; Hoilett, N.O.; Kumar, S. Short-term impacts of biochar and manure application on soil labile carbon fractions, enzyme activity, and microbial community structure. Biochar 2019, 1, 271–282. [Google Scholar] [CrossRef]
- Zhang, L.; Xiang, Y.; Jing, Y.; Zhang, R. Biochar amendment effects on the activities of soil carbon, nitrogen, and phosphorus hydrolytic enzymes: A meta-analysis. Environ. Sci. Pollut. Res. 2019, 26, 22990–23001. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, P.; Ma, Z.; Chang, S.X. Biochar increases soil microbial biomass with changes in extra-and intracellular enzyme activities: A global meta-analysis. Biochar 2020, 2, 65–79. [Google Scholar] [CrossRef]
- Dai, Z.; Xiong, X.; Zhu, H.; Xu, H.; Leng, P.; Li, J.; Xu, J. Association of biochar properties with changes in soil bacterial, fungal and fauna communities and nutrient cycling processes. Biochar 2021, 3, 239–254. [Google Scholar] [CrossRef]
- Guo, K.; Zhao, Y.; Liu, Y.; Chen, J.; Wu, Q.; Ruan, Y.; Li, S.; Shi, J.; Zhao, L.; Sun, X.; et al. Pyrolysis temperature of biochar affects ecoenzymatic stoichiometry and microbial nutrient-use efficiency in a bamboo forest soil. Geoderma 2020, 363, 114162. [Google Scholar] [CrossRef]
- Chen, J.; Sun, X.; Li, L.; Liu, X.; Zhang, B.; Zheng, J.; Pan, G. Change in active microbial community structure, abundance and carbon cycling in an acid rice paddy soil with the addition of biochar. Eur. J. Soil. Sci. 2016, 67, 857–867. [Google Scholar] [CrossRef]
- Chen, P.; Liu, Y.; Mo, C.; Jiang, Z.; Yang, J.; Lin, J. Microbial mechanism of biochar addition on nitrogen leaching and retention in tea soils from different plantation ages. Sci. Total. Environ. 2021, 757, 143817. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, G.; Feng, H.; Sun, B.; Zhao, Y.; Chen, H.; Chen, J.; Dyck, M.; Wang, X.; Zhang, J.; et al. Effects of straw and biochar amendments on aggregate stability, soil organic carbon, and enzyme activities in the Loess Plateau, China. Environ. Sci. Pollut. Res. 2017, 24, 10108–10120. [Google Scholar] [CrossRef]
- Wang, X.; Song, D.; Liang, G.; Zhang, Q.; Ai, C.; Zhou, W. Maize biochar addition rate influences soil enzyme activity and microbial community composition in a fluvo-aquic soil. Appl. Soil Ecol. 2015, 96, 265–272. [Google Scholar] [CrossRef]
- Gujre, N.; Soni, A.; Rangan, L.; Tsang, D.C.; Mitra, S. Sustainable improvement of soil health utilizing biochar and arbuscular mycorrhizal fungi: A review. Environ. Pollut. 2021, 268, 115549. [Google Scholar] [CrossRef]
- Yang, X.N.; Chen, Z.F.; Wu, Q.H.; Xu, M.Y. Enhanced phenanthrene degradation in river sediments using a combination of biochar and nitrate. Sci. Total Environ. 2018, 619, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Mierzwa-Hersztek, M.; Gondek, K.; Baran, A. Effect of poultry litter biochar on soil enzymatic activity, ecotoxicity and plant growth. Appl. Soil. Ecol. 2016, 105, 144–150. [Google Scholar] [CrossRef]
- Ameloot, N.; Graber, E.R.; Verheijen, F.G.; De Neve, S. Interactions between biochar stability and soil organisms: Review and research needs. Eur. J. Soil Sci. 2013, 64, 379–390. [Google Scholar] [CrossRef]
- Bhaduri, D.; Saha, A.; Desai, D.; Meena, H.N. Restoration of carbon and microbial activity in salt-induced soil by application of peanut shell biochar during short-term incubation study. Chemosphere 2016, 148, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Hussain, Q.; Khan, K.S.; Akmal, M.; Ijaz, S.S.; Hayat, R.; Khalid, A.; Azeem, M.; Rashid, M. Response of soil microbial biomass and enzymatic activity to biochar amendment in the organic carbon deficient arid soil: A 2-year field study. Arab J. Geosci. 2019, 12, 95. [Google Scholar] [CrossRef]
- Demisie, W.; Liu, Z.; Zhang, M. Effect of biochar on carbon fractions and enzyme activity of red soil. Catena 2014, 121, 214–221. [Google Scholar] [CrossRef]
- Awad, Y.M.; Ok, Y.S.; Abrigata, J.; Beiyuan, J.; Beckers, F.; Tsang, D.C.; Rinklebe, J. Pine sawdust biomass and biochars at different pyrolysis temperatures change soil redox processes. Sci. Total Environ. 2018, 625, 147–154. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Xiong, X.; Wang, L.; Hou, D.; Bolan, N.S.; Ok, Y.S.; Rinklebe, J.; Tsang, D.C. A critical review on performance indicators for evaluating soil biota and soil health of biochar-amended soils. J. Hazard. Mater. 2021, 414, 125378. [Google Scholar] [CrossRef]
- He, M.; Xu, Z.; Sun, Y.; Chan, P.S.; Lui, I.; Tsang, D.C. Critical impacts of pyrolysis conditions and activation methods on application-oriented production of wood waste-derived biochar. Bioresour. Technol. 2021, 341, 125811. [Google Scholar] [CrossRef]
- Yang, X.; Tsibart, A.; Nam, H.; Hur, J.; El-Naggar, A.; Tack, F.M.; Wang, C.H.; Lee, Y.H.; Tsang, D.C.; Ok, Y.S. Effect of gasification biochar application on soil quality: Trace metal behavior, microbial community, and soil dissolved organic matter. J. Hazard. Mater. 2019, 365, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Futa, B.; Oleszczuk, P.; Andruszczak, S.; Kwiecińska-Poppe, E.; Kraska, P. Effect of natural aging of biochar on soil enzymatic activity and physicochemical properties in long-term field experiment. Agronomy 2020, 10, 449. [Google Scholar] [CrossRef]
- Khadem, A.; Raiesi, F. Influence of biochar on potential enzyme activities in two calcareous soils of contrasting texture. Geoderma 2017, 308, 149–158. [Google Scholar] [CrossRef]
- Oladele, S.O.; Adetunji, A.T. Agro-residue biochar and N fertilizer addition mitigates CO2-C emission and stabilized soil organic carbon pools in a rain-fed agricultural cropland. Int. Soil Water Conserv. Res. 2021, 9, 76–86. [Google Scholar] [CrossRef]
- Xia, H.; Riaz, M.; Zhang, M.; Liu, B.; El-Desouki, Z.; Jiang, C. Biochar increases nitrogen use efficiency of maize by relieving aluminum toxicity and improving soil quality in acidic soil. Ecotoxicol. Environ. Saf. 2020, 196, 110531. [Google Scholar] [CrossRef] [PubMed]
- Teixido, M.; Hurtado, C.; Pignatello, J.J.; Beltran, J.L.; Granados, M.; Peccia, J. Predicting contaminant adsorption in black carbon (biochar)-amended soil for the veterinary antimicrobial sulfamethazine. Environ. Sci. Technol. 2013, 47, 6197–6205. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Peng, Y.; Song, G.; Fang, Q.; Wang, R.; Zhang, C.; Zeng, G.; Huang, D.; Lai, C.; Zhou, Y.; et al. Degradation of sulfamethazine by biochar-supported bimetallic oxide/persulfate system in natural water: Performance and reaction mechanism. J. Hazard. Mater. 2020, 398, 122816. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Liu, S.; Ye, S.; Yang, H.; Song, B.; Qin, F.; Shen, M.; Tan, C.; Zeng, G.; Tan, X. Potential hazards of biochar: The negative environmental impacts of biochar applications. J. Hazard. Mater. 2021, 420, 126611. [Google Scholar] [CrossRef]
- Sparrevik, M.; Lindhjem, H.; Andria, V.; Fet, A.M.; Cornelissen, G. Environmental and socioeconomic impacts of utilizing waste for biochar in rural areas in Indonesia–a systems perspective. Environ. Sci. Technol. 2014, 48, 4664–4671. [Google Scholar] [CrossRef]
- Yang, H.; Ye, S.; Zeng, Z.; Zeng, G.; Tan, X.; Xiao, R.; Wang, J.; Song, B.; Du, L.; Qin, M.; et al. Utilization of biochar for resource recovery from water: A review. J. Chem. Eng. 2020, 397, 125502. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharya, T.; Shaikh, W.A.; Roy, A.; Chakraborty, S.; Vithanage, M.; Biswas, J.K. Multifaceted applications of biochar in environmental management: A bibliometric profile. Biochar 2023, 5, 11. [Google Scholar] [CrossRef]
- Laghari, M.; Naidu, R.; Xiao, B.; Hu, Z.; Mirjat, M.S.; Hu, M.; Kandhro, M.N.; Chen, Z.; Guo, D.; Jogi, Q.; et al. Recent developments in biochar as an effective tool for agricultural soil management: A review. J. Sci. Food Agric. 2016, 96, 4840–4849. [Google Scholar] [CrossRef] [PubMed]
- Ro, K.S.; Cantrell, K.B.; Hunt, P.G. High-temperature pyrolysis of blended animal manures for producing renewable energy and value-added biochar. Ind. Eng. Chem. Res. 2010, 49, 10125–11013. [Google Scholar] [CrossRef]
- Zilberman, D.; Laird, D.; Rainey, C.; Song, J.; Kahn, G. Biochar supply-chain and challenges to commercialization. GCB Bioenergy 2023, 15, 7–23. [Google Scholar] [CrossRef]
- Kung, C.-C.; McCarl, B.A.; Cao, X. Economics of pyrolysis-based energy production and biochar utilization: A case study in Taiwan. Energy Policy 2013, 60, 317–323. [Google Scholar] [CrossRef]
- Kim, D.; Anderson, N.M.; Chung, W. Financial performance of a mobile pyrolysis system used to produce biochar from sawmill residues. For. Prod. J. 2015, 65, 189–197. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Ok, Y.S.; Awad, Y.M.; Lee, S.S.; Sung, J.K.; Koutsospyros, A.; Moon, D.H. Impacts of biochar application on upland agriculture: A review. J. Environ. Manag. 2019, 234, 52–64. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Thavamani, P.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Agronomic and remedial benefits and risks of applying biochar to soil: Current knowledge and future research directions. Environ. Int. 2016, 87, 1–12. [Google Scholar] [CrossRef]
- Yadav, S.P.S.; Bhandari, S.; Bhatta, D.; Poudel, A.; Bhattarai, S.; Yadav, P.; Netra Ghimire, N.; Paudel, P.; Shrestha, J.; Oli, B. Biochar application: A sustainable approach to improve soil health. J. Agric. Food Res. 2023, 11, 100498. [Google Scholar] [CrossRef]
- Major, J.; Lehmann, J.; Rondon, M.; Goodale, C. Fate of soil-applied black carbon: Downward migration, leaching and soil respiration. Glob. Chang. Biol. 2010, 16, 1366–1379. [Google Scholar] [CrossRef]
- Cheng, C.H.; Lehmann, J.; Thies, J.E.; Burton, S.D.; Engelhard, M.H. Oxidation of black carbon by biotic and abiotic processes. Org. Geochem. 2006, 37, 1477–1488. [Google Scholar] [CrossRef]
- Masek, O.; Brownsort, P.; Cross, A.; Sohi, S. Influence of production conditions on the yield and environmental stability of biochar. Fuel 2011, 103, 151–155. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Jin, H. Characterization of Microbial Life Colonizing Biochar and Biochar-Amended Soils. Ph.D. Dissertation, Cornell University, Ithaca, NY, USA, 2010. [Google Scholar]
- Noyce, G.L.; Winsborough, C.; Fulthorpe, R.; Basiliko, N. The microbiomes and metagenomes of forest biochars. Sci. Rep. 2016, 6, 26425. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Zimmerman, A.R.; Hamdan, R.; Cooper, W.T. Physicochemical changes in pyrogenic organic matter (biochar) after 15 months of field aging. Solid Earth 2014, 5, 693–704. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, L.; He, L.Y.; Sheng, X.F. Increased biomass and reduced heavy metal accumulation of edible tissues of vegetable crops in the presence of plant growth-promoting Neorhizobium huautlense T1-17 and biochar. Agric. Ecosyst. Environ. 2016, 228, 9–18. [Google Scholar] [CrossRef]
- Wang, T.; Sun, H.; Ren, X.; Li, B.; Mao, H. Adsorption of heavy metals from aqueous solution by UV-mutant Bacillus subtilis loaded on biochars derived from different stock materials. Ecotoxicol. Environ. Saf. 2018, 148, 285–292. [Google Scholar] [CrossRef]
- Baskar, A.V.; Bolan, N.; Hoang, S.A.; Sooriyakumar, P.; Kumar, M.; Singh, L.; Jasemizad, T.; Padhye, L.P.; Singh, G.; Vinu, A. Recovery, regeneration and sustainable management of spent adsorbents from wastewater treatment streams: A review. Sci. Total Environ. 2022, 822, 153555. [Google Scholar] [CrossRef]
- Żur, J.; Wojcieszyńska, D.; Guzik, U. Metabolic responses of bacterial cells to immobilization. Molecules 2016, 21, 958. [Google Scholar] [CrossRef]
- Chen, C.H.; Whang, L.M.; Pan, C.L.; Yang, C.L.; Liu, P.W.G. Immobilization of diesel-degrading consortia for bioremediation of diesel-contaminated groundwater and seawater. Int. Biodeter. Biodeg. 2017, 124, 62–72. [Google Scholar] [CrossRef]
- Youngwilai, A.; Kidkhunthod, P.; Jearanaikoon, N.; Chaiprapa, J.; Supanchaiyamat, N.; Hunt, A.J.; Ngernyen, Y.; Ratpukdi, T.; Khan, E.; Siripattanakul-Ratpukdi, S. Simultaneous manganese adsorption and biotransformation by Streptomyces violarus strain SBP1 cell-immobilized biochar. Sci. Total Environ. 2020, 713, 136708. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, H.; Ni, J.; Chen, W.; Yang, L.; Wei, R. Effect of cadmium on pyrene biodegradation in solution and soil using free and immobilized Escherichia sp. on biochar. Appl. Soil Ecology. 2020, 150, 103472. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, M.H.; Hur, J.; Lee, Y.H.; Igalavithana, A.D.; Shaheen, S.M.; Ryu, C.; Rinklebe, J.; Tsang, D.C.W.; Ok, Y.S. Biochar-induced metal immobilization and soil biogeochemical process: An integrated mechanistic approach. Sci. Total Environ. 2020, 698, 134112. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Li, D.; Liu, X.; Fan, Y.; Zhang, X.; Zhang, S.; Fang, G.; Zhou, J. Dry-wet and freeze-thaw aging activate endogenous copper and cadmium in biochar. J. Clean. Prod. 2021, 288, 125605. [Google Scholar] [CrossRef]
- Galbally, P.; Ryan, D.; Finnan, J.; Grant, J.; Fagan, C.C.; McDonnell, K. Biosolids and distillery effluent amendments to Irish Miscanthus plantations: Impacts on overland flow and surface water quality. Sustain. Water Qual. Ecol. 2014, 3, 77–89. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Jośko, I.; Kuśmierz, M. Biochar properties regarding to contaminants content and ecotoxicological assessment. J. Hazard. Mater. 2013, 260, 375–382. [Google Scholar] [CrossRef]
- Devi, P.; Saroha, A.K. Risk analysis of pyrolyzed biochar made from paper mill effluent treatment plant sludge for bioavailability and eco-toxicity of heavy metals. Bioresour. Technol. 2014, 162, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Y.; Herath, H.M.S.K. Polycyclic aromatic hydrocarbons (PAHs) in biochar-their formation, occurrence and analysis: A review. Org. Geochem. 2017, 114, 1–11. [Google Scholar] [CrossRef]
- Hussain, M.; Farooq, M.; Nawaz, A.; Al-Sadi, A.M.; Solaiman, Z.M.; Alghamdi, S.S.; Ammara, U.; Ok, Y.S.; Siddique, K.H. Biochar for crop production: Potential benefits and risks. J. Soil Sediments 2017, 17, 685–716. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal-a review. Biol. Fertil. Soils. 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Hammond, D.S.; Steege, H.T.; Van Der Borg, K. Upland soil charcoal in the wet tropical forests of central Guyana. Biotropica 2007, 39, 153–160. [Google Scholar] [CrossRef]
- Titiz, B.; Sanford, R.L., Jr. Soil charcoal in old-growth rain forests from sea level to the continental divide. Biotropica 2007, 39, 673–682. [Google Scholar] [CrossRef]
- Forbes, M.S.; Raison, R.J.; Skjemstad, J.O. Formation, transformation and transport of black carbon (charcoal) in terrestrial and aquatic ecosystems. Sci. Total Environ. 2006, 370, 190–206. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, G.; Traina, S.J.; Swanston, C.W. Black carbon’s properties and role in the environment: A comprehensive review. Sustainability 2010, 2, 294–320. [Google Scholar] [CrossRef]
- Nepal, J.; Ahmad, W.; Munsif, F.; Khan, A.; Zou, Z. Advances and prospects of biochar in improving soil fertility, biochemical quality, and environmental applications. Front. Environ. Sci. 2023, 11, 1114752. [Google Scholar] [CrossRef]
- Lai, W.Y.; Lai, C.M.; Ke, G.R.; Chung, R.S.; Chen, C.T.; Cheng, C.H. The effects of woodchip biochar application on crop yield, carbon sequestration and greenhouse gas emissions from soils planted with rice or leaf beet. J. Taiwan Inst. Chem. Eng. 2013, 44, 1039–1044. [Google Scholar] [CrossRef]
- Lehmann, J.; da Silva, J.P.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Zhou, L.; Cai, D.; He, L.; Zhong, N.; Yu, M.; Zhang, X. Fabrication of a highperformance fertilizer to control the loss of water and nutrient using micro/nano networks. ACS Sustain. Chem. Eng. 2015, 3, 645–653. [Google Scholar] [CrossRef]
- Sashidhar, P.; Kochar, M.; Singh, B.; Gupta, M.; Cahill, D.; Adholeya, A.; Dubey, M. Biochar for delivery of agri-inputs: Current status and future perspectives. Sci. Total Environ. 2020, 703, 134892. [Google Scholar] [CrossRef]
- Busscher, W.J.; Novak, J.M.; Evans, D.E.; Watts, D.W.; Niandou, M.A.S.; Ahmedna, M. Influence of pecan biochar on physical properties of a Norfolk loamy sand. Soil Sci. 2010, 175, 10–14. [Google Scholar] [CrossRef]
- Manyà, J.J. Pyrolysis for biochar purposes: A review to establish current knowledge gaps and research needs. Environ. Sci. Technol. 2012, 46, 7939–7954. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R. Biochar impacts on soil physical properties and greenhouse gas emissions. Agronomy 2013, 3, 313–339. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Does biochar application alleviate soil compaction? Review and data synthesis. Geoderma 2021, 404, 115317. [Google Scholar] [CrossRef]
- Omondi, M.O.; Xia, X.; Nahayo, A.; Liu, X.; Korai, P.K.; Pan, G. Quantification of biochar effects on soil hydrological properties using meta-analysis of literature data. Geoderma 2016, 274, 28–34. [Google Scholar] [CrossRef]
- Razzaghi, F.; Bilson Obour, P.; Emmanuel, A. Does biochar improve soil water retention? a systematic review and meta-analysis. Geoderma 2020, 361, 114055. [Google Scholar] [CrossRef]
- Carvalho, J.; Nascimento, L.; Soares, M.; Valério, N.; Ribeiro, A.; Faria, L.; Silva, A.; Pacheco, N.; Araújo, J.; Vilarinho, C. Life cycle assessment (lca) of biochar production from a circular economy perspective. Processes 2022, 10, 2684. [Google Scholar] [CrossRef]
- Azzi, E.S.; Karltun, E.; Sundberg, C. Assessing the diverse environmental effects of biochar systems: An evaluation framework. J. Environ. Manag. 2021, 286, 112154. [Google Scholar] [CrossRef]
- Ayaz, M.; Feizienė, D.; Tilvikienė, V.; Akhtar, K.; Stulpinaitė, U.; Iqbal, R. Biochar role in the sustainability of agriculture and environment. Sustainability 2021, 13, 1330. [Google Scholar] [CrossRef]
- Foong, S.Y.; Cheong, K.Y.; Kong, S.H.; Yiin, C.L.; Yek, P.N.Y.; Safdar, R.; Liew, R.K.; Loh, S.K.; Lam, S.S. Recent progress in the production and application of biochar and its composite in environmental biodegradation. Bioresour. Technol. 2023, 387, 129592. [Google Scholar] [CrossRef]


| Modification | Modulated Factor | Information on Biochar | Effect on Biochar Properties | Ref. |
|---|---|---|---|---|
| Pre-pyrolysis Modifications | ||||
| Feedstock composition | Different lignocellulosic biomass: spruce wood, corn stalk, corn cob | Pyrolysis: 600 °C, HR 10 °C min−1 for 1 h | The corn cob and corn stalk biochar showed higher concentrations of inorganic elements, P (4550 and 3230 mg/kg), and K (28,410 and 21,150 mg/kg). Ash content was the lowest for woody biochar (1.5 wt%) and the highest for corn cob biochar (12.4 wt%). The S (N2) was the highest for woody biochar (465 m2/g) and the lowest for corn cob (94 m2/g). | [33] |
| Feedstock moisture | Moisture: from 6 to 42 wt% | Feedstock: maple bark and softwood bark; vacuum pyrolysis: HR 10 K/min, 300–775 K. | With decreasing feedstock moisture content, oxygen concentration increased from 10 to 33% (stronger for softwood charcoal), the S decreased from 326 m2/g to 206 m2/g (softwood), and micropore volume decreased from 0.1 to 0.05 cm3/g (maple). | [44] |
| Feedstock moisture | Moisture: dry, wet, and soaked sludge | Feedstock: raw and dewatered sludge from MWTP, lignite; Pyrolysis: 60 min, HR 5–24 K/s (4–7 K/s at 873 K, 8–14 K/s at 1073 K, 14–22 K/s at 1273 K) | Higher moisture contributed to lower C content and increased O/C. No significant difference was found in the S between dry and soaked chars at 873 K. Bound water increased S from 114.09 m2/g to 128.98 m2/g at 1273 K. | [45] |
| Feedstock particle size | Biomass particle size: 10–50, 50–100, 100–200 mesh | Feedstock: rice husk; pyrolysis: 500 °C, RT 60 min, HR 10 C/min | Increase in particle size resulted in decrease in C content from 76.12 to 67.46% and in the S from 24.96 to 20.11 m2/g, as well as pore volume and pH of biochar, and in increase of biochar yield, N, O, and ash content. | [40] |
| Feedstock density | Feedstock density: tora willow: 314 kg/m3; artemisia dubia mugwort: 226 kg/m3; sewage sludge compost: 1043 kg/m3 | Feedstock: willow, mugwort, sewage sludge compost; pyrolysis: 2 h, 450 °C and 700 °C, HR 10 °C/min | The highest density (986 and 1149 kg/m3) was found for biochars produced from the feedstock of the highest density (1043 kg/m3). | [41] |
| Pyrolysis Modifications | ||||
| Pyrolysis temperature | PT: 300, 400, 500 °C | Feedstock: sunflower husks (SH), wood waste (WW); pyrolysis: 30 min. | Increasing PT of SH and WW caused increase in pH of biochars from 9.9 to 11.1 and from 8.1 to 10.1 and in the S from 71.7 to 85.6 m2/g and from 53.1 to 70.3 m2/g and decrease in carboxylic groups from 30 to 20 and from 40 to 30 cmol/kg. | [48] |
| Pyrolysis temperature and residence time | PT: 300, 450, 600, 750 °C RT: 10 and 60 min | Feedstock: wood, straw, green waste, dry algae | Increase in PT caused increase in ash, total C, and pH and decrease in volatile matter for biochars derived from all studied biomass and at two RT. Increase in RT caused decrease in volatile matter and increase in fixed and total C and pH for biochars derived from all studied biomasses. The S increased for all biomass at PT increase from 450 to 600 °C and decreased for woody biochar derived at PT 750 °C. | [51] |
| Heating rate | HR: 10, 30, 50 °C/min | Feedstock: safflower seed press cake; pyrolysis: 400, 450, 500, 550, and 600 °C | With increasing HR, the S and pore volume decreased from 3.41 to 2.47 m2/g at PT 600 °C and from 0.0064 to 0.0047 cm3/g. The yields of biochar changed from 34.18% to 29.70% at PT 400 °C. This effect was weaker for higher PT. | [58] |
| Pyrolysis atmosphere | CO2 and N2 atmosphere | Feedstock: red pepper stalk; pyrolysis: flow rate of agent gas: 500 mL min−1 | The S of biochar produced in CO2 increased from 32.46 to 109.15 m2g−1. Pore volume increased from 0.02 to 0.09 cm3/g. | [60] |
| Supporting process | Steam activation | Feedstock: burcucumber; pyrolysis: 300 and 700 °C, HR 7 °C min−1, RT 2 h. Steam activation after pyrolysis: flow rate 5 mL min−1 for 45 min | Steam-activated biochars produced at PT 300 and 700 °C showed increased ash content from 25.40 to 28.67% and from 43.72 to 70.66%, increased S from 0.85 to 1.22 m2/g and from 2.31 to 7.10 m2/g, and decreased yield from 51.83 to 50.21% and from 27.52 to 18.90%. | [77] |
| Supporting process | Microwaves | Feedstock: willow wood chips and mixed straw pellets consisting of wheat and rape straw; pyrolysis: HR 5 °C min−1, 200, 250, 300 and 350 °C, RT 10 min; microwave pyrolysis: MW power 1200 W, vacuum, 170 and 200 °C | Yield of char derived from willow wood and straw pellets was the lowest for microwave-assisted pyrolysis, reaching 27.3 and 33.7% as compared to 39.8% and 49.9% for conventional pyrolysis conducted at 350 °C. The S (BET) was higher for microwave-assisted biochars as compared to conventionally obtained biochars and reached 3.87 and 1.14 m2/g for biochars derived from willow wood and straw pellets as compared to 0.17 and 0.51 m2/g for biochars produced conventionally at 350 °C. | [78] |
| Post-pyrolysis Modification | ||||
| Chemical modification | KOH, HNO3, H2SO4, H2O2, and KMnO4 | Feedstock: rice straw; pyrolysis: 550, 650, 750 °C, HR 50 °C/10 min, N flow 300 mL/min | KOH treatment increased S (179.7 m2/g at 650 °C), followed by H2O2 and KMnO4, whereas H2SO4 treatment decreased S, followed by HNO3. HNO3 and H2SO4 treatment contributed to higher mesoporosity. H2O2 treatment contributed to the highest microporosity, while KOH increased both high micro- and mesoporosity. | [72] |
| Chemical modification | NaOH + methanol | Feedstock: rice husk; pyrolysis: fast, 723–773 K | Increase in the S from 51.86 m2 g−1 to 65.97 m2 g−1 for modified char, decrease in carbonyl groups, and increase in ester and hydroxyl groups after modification. | [79] |
| Chemical modification | H2O2 solution (1, 3, 10, 20, 30% w/w) | Feedstock: pinewood; pyrolysis: 400 °C | Biochar treated with H2O2 showed higher CEC (increase from 17.95 to 31.37 cmol/kg) and lower pH (decrease from 7.16 to 5.66). | [73] |
| Chemical modification | CO2 and NH3 at 600 °C | Feedstock: cotton stalk; pyrolysis: 600 °C | As compared to untreated biochar, modifications with NH3 and CO2 resulted in increase of the S of micropore from 224 m2/g to 252 and to 352 m2/g and N content change from 1.09 to 3.48 and to 1.02 wt (%). | [74] |
| Physical modification | Particle size reduction in ball milling process | Feedstock: pine wood, spruce and fir; pyrolysis: 525 °C | Milled biochar particles showed higher S (47.25 m2/g) compared to raw biochar (3.12 m2/g). Raw biochar adsorbed less than 14% of carbamazepine, milled biochar—more than 98% in 3 h. | [67] |
| Modification with nanoparticles | Loading MnO2 nanoparticles on biochar | Feedstock: water hyacinth; pyrolysis: 450 °C, HR 5 °C min−1, RT 3 h | Increasing loading of MnO2 from 0.6 to 18.4 wt% increased the S from 3.5 to 120.2 m2/g and decreased it at higher MnO2 loading. A 26.6% loading can show high capacity for heavy metals removal from electroplating wastewater. | [80] |
| Feedstock/Pyrolysis Conditions | Microorganism(s) | Most Important Effects of Using Biochar as a Carrier | Ref. |
|---|---|---|---|
| Maize silage, batch-wise hydrothermal carbonization at 210 °C Maize, pyrolysis at 600 °C Wood, pyrolysis at 800 °C | Bradyrhizobium sp. | The highest survival of bacteria was observed in hydrochar from maize silage. An effective carrier for inoculum, improving growth and nutrient uptake of lupins under drought conditions. | [105] |
| Agricultural waste (leaves of cabbage, spinach, cauliflower, and green gram leaves) Pyrolysis at 250 °C | Burkholderia sp. Bacillus sp. | Improving the physical, chemical, and biological properties of the soil Higher microbial viability was observed up to 270 days of storage. Increasing the parameters of the tested plant. | [109] |
| Agricultural wastes (discarded cabbage, cauliflower, leguminous plants, leafy vegetables) Pyrolysis at 600 °C | Burkholderia sp. Bacillus sp. | Increased the shelf life of bacteria. Increased seed germination and promoted tomato (Lycopersicon esculentum) growth and yield. Improved soil physical and chemical properties and enhanced dehydrogenase activity. | [103] |
| Cassava stem Pyrolysis at 300 °C | Arthrobacter sp. Micrococcus sp. | Enabling the survival and growth of bacteria in cadmium-contaminated soil. Promoting the efficiency of cadmium phytoextraction by Chlorophytum laxum R.Br. | [107] |
| Rice straw, rice husks, soybean straw, peanut shells, corn cobs, wood | Arthrobacter defluvii Burkholderia cepacia Bacillus megaterium Pseudomonas frederiksbergensis Rhodanobacter sp. Streptomyces prasinopilosus Variovorax paradoxus | Biochar affected survival and functioning of the bacterial community. Improved rape growth and phosphate uptake. The available P content of the biochar was recognized as dominant factor affecting bacterial community structure. | [104] |
| Tea leaves Pyrolysis at 350 °C and 600 °C | Bacillus cereus | Biochar produced at a higher temperature was a suitable carrier and revealed longer shelf life. Biochar enhanced community of Bacillus cereus as compared to peat alone. Improved soil properties, growth, and yield of mung bean. | [110] |
| Lantana camara biomass Pyrolysis at 300 °C | Azotobacter chroococcum | The biochar-based carrier revealed no contamination during storage. The highest moisture content and maintained microbial viability at the end of the storage. | [111] |
| Crop straws from cotton, peanut, maize, soybean, wheat Pyrolysis at 300 and 600 °C | Bacillus megaterium | Improved survival of bacteria by cotton straw biochar that acts as a carrier. Improved soil available phosphorus. | [112] |
| Wheat straw Pyrolysis at 700 °C | Acinetobacter calcoaceticus | Effective immobilization of bacteria was observed on biochar-based carriers due to the developed specific surface area and porous structure that supported the nutrients. Increased bioremediation of lead-contaminated soil. | [55] |
| Waste of Eichhornia crassipes Pyrolysis at 400, 500, and 600 °C | Pseudomonas plecoglossicida | Biochar alleviated the cytotoxicity towards microorganisms and promoted cell proliferation. Biochar stimulated extracellular substances’ secretion and sheltered microorganisms under unfavorable conditions. Biochar promoted effectiveness in reducing the availability of Cu and Pb and the degradation of 2,2′,4,4′-tetrabrominated diphenyl ether (BDE-47). | [106] |
| Pinewood Modified with Luria–Bertani broth, a worm-casting extract, or mixed with earthworm castings Pyrolysis at 600 °C | Pseudomonas putida | Biochar of neutral pH was near optimal for Pseudomonas putida. A high population abundance was sustained after five months of storage. Carrier supplementation does not promote increased shelf life or inoculum efficacy. | [98] |
| Four biochars types consisting: rice husk, coconut shell, oil palm empty bunch, corncob Pyrolysis at 500–600 °C | Paenibacillus alvei Burkholderia cepacian Acinetobacter baumannii Penicillium variabil | Optimal pH and higher nutrient content of biochar influenced higher cell density. Effective adhesion of microbes within pore space (2–4 µm) and at the surface was observed only for coconut shell biochar. Biochars were found to increase the survival of microbial inoculants up to six months. Due to limited moisture content, biochar does not present any storage problem. | [102] |
| Three biochar types prepared from Eucalyptus Marginata, Eucalyptus marginata and Eucalyptus wandoo, Acacia saligna Pyrolysis at 550–650 °C and 380 °C | NA | All biochars contributed potential habitats for soil microorganisms due to the high porosities and surface areas. Transient effects of particle size within each biochar source were observed at earlier stages of the incubation. | [113] |
| Cotton stalks Pyrolysis at 400 °C and 600 °C | Bacillus subtilis | Biochars stimulate the growth rate of B. subtilis in a liquid nutrient broth medium. Bacteria immobilization increased with biochar particle size decrease and with larger specific surface area. The contact area between the biochar particles and microorganisms was one of the most important factors in cell immobilization. Gradual release of bacteria from the biochar surface and tubular structure was observed. | [114] |
| Wood pellets biochar, a mixture of spruce and pine wood without bark (poor in ash and nutrients) Biochar from chicken manure (rich in ash and nutrients) Pyrolysis at 550 °C | Rhizophagus irregularis | Arbuscular mycorrhizal fungi (AMF) used biochar as a growth matrix and nutrient source. AMF were shown to grow on two contrasting types of biochar particles, strongly attaching to their inner and outer surfaces. Contact of hyphae with the biochar surface stimulated phosphorus uptake and its translocation to associated host roots. | [115] |
| Waste products (palm fronds, pine wood, coconut shells, pistachio nut shells, stone fruit pits) Pyrolysis at 300 °C and 600 °C | Enterobacter cloacae | Chemical properties of biochar, particularly nitrogen content and pH, were among the most important characteristics affecting initial inoculum survival and shelf life. Physical features, including surface area, pore diameters, and water-filled pore spaces, were more closely associated with inoculum survival after incorporation into the soil. | [96] |
| Acacia wood and coconut shell | Azospriliium lipoferum | Coconut shell-based biochar with the highest specific surface area, water holding capacity, and nutrient availability increased the survival of Azospirillum lipoferum up to 6 months. | [116] |
| Corn stalk Pyrolysis at 250 °C, 550 °C, and 850 °C | Acinetobacter lwoffii Bacillus megaterium Bacillus subtilis Pseudomonas aeruginosa Enterobacter sp. | Biochar produced at a temperature of 550 had the greatest effect on the growth, phosphorus solubilization, and acid phosphatase activity of phosphate-solubilizing bacteria. A reduction of residual atrazine and an increase in the content of total and available phosphorus were observed. | [117] |
| Type of Soil | Type of Biochar | Dose of Biochar | The Number of Microorganisms/Microbial Biomass and Enzymatic Activity | Type of Study | Ref. |
|---|---|---|---|---|---|
| Typical brown soil, Eutric Cambisol | Wheat straw biochar prepared at 300 °C | 0, 0.2, 0.5, 1, and 2% | The addition of biochar with nutrients increased the number and intensified the activity of soil microorganisms. | Laboratory experiment | [187] |
| Forest rhizospheric soil | Tea leaf biochar prepared at 350 °C (B1) and 600 °C (B2) | 1% (w/w) | B2 + BC (Bacillus cereus) significantly (p ≤ 0.05) enhanced the Proteobacteria (11%), Firmicutes (46%), Actinobacteria (20%), and Cyanobacteria (33%) community as compared to control and improved soil properties, i.e., enzyme activity urease (12%), dehydrogenase (40%), and phosphatase (49%). | Vase experiment | [110] |
| Drip-irrigated desert soil | Cotton straw biochar | 0, 2.25, or 4.5 t/ha | Rate: 4.5 t/ha: increased microbial biomass C (by 32%), microbial biomass N (by 58%), and basal respiration (by 13%); increased total PLFA (by 27%) compared with the control; shifted the microbial community toward bacteria and actinomycetes; and increased enzyme activities related to N cycling. Both rates of biochar increased the activities of three key enzymes related to C cycling. | Field study | [200] |
| Salt-induced soil (Salinity gradients (S0: control, S1: 2, S2: 4, S3: 6 EC iw)) | Peanut shell biochar | B0—control; B1—2.5%; B2—5%; B3—10% w/w | Soil enzyme activities depended on biochar rate, day of incubation, and type of enzyme. B1 showed highest dehydrogenase (20.5 mg TPF g−1 soil h−1), acid phosphatase (29.1 mg pnpg−1 soil h−1), and alkaline phosphatase (16.1 mg PNP g−1 soil h−1), and B2 increased the urease (5.51 mg urea-N g−1 soil h−1) and fluorescein diacetate hydrolyzing activities (3.95 mg fluorescein g−1 OD soil h−1) in soil. | Laboratory experiment | [245] |
| Three acidic soils: black clay loam (BCL) Vertosol, loamy red Dermosol (RL), and brown sandy loam (BSL) | Eucalypt green waste biochar prepared at 650–750 °C | 0, 2.5, 5, and 10% (w/w) | Biochar addition has a greater impact on the bacterial diversity in RL and BSL soils than in BCL soil. The abundance of nitrifying bacteria increased with increasing biochar rate, especially AOB (ammonia-oxidizing bacteria) in BCL soil. The abundance of Nitrospira and NOB (nitrite-oxidising bacteria) was greater than AOB in all biochar-amended soils. | Pot experiment | [208] |
| Silt loam soil | Biochar manufactured from Pinus radiata | 0, 15, and 30 t ha−1 | Comparing biochar-amended soils with controls, temporal changes in bacterial family abundances that were >5% included Bradyrhizobiaceae (~8%), Hyphomicrobiaceae (~14%), Streptosporangineae (~6%), and Thermomonosporaceae (~8%), where the biochar had a positive influence, either promoting an increase in abundance or reducing the magnitude of the loss, and Streptomycetaceae (~−11%) and Micromonosporaceae (~−7%), where biochar had a negative effect on bacterial family abundance. | Pot experiment | [154] |
| Calcaric cambisol | BC made from tobacco stalks | 0, 1, 2.5, and 5.0% (w/w) | Compared to the control, the BC addition increased the diversity and richness of bacteria; increased the relative abundance of Adhaeribacter, Rhodoplanes, Pseudoxanthomonas, and Candidatus Xiphinematobacter; and lowered the relative abundance of Lacibacter, Pirellula, and Kaistobacter. | Pot experiment | [215] |
| Calcareous soils in a karstic region of of southwestern China | BC produced from crop straws of maize and rapeseed at 550 °C | 0, 1, 5, and 10% (i.e., 0, 12.8, 64, and 128 t ha−1) | In rhizosphere soils with increasing BC amendment, the relative abundances of Gemmatimonadetes increased while those of the Bacteroidetes, Firmicutes, and Cyanobacteria decreased. In bulk soils with increasing BC application levels, the relative abundances of Proteobacteria and Chloroflexi increased while those of the Bacteroidetes and Verrucomicrobia decreased. | Field study | [219] |
| Haplic Podzol (pzha) originating from glaciofluvial fine-grained loamy sand | Biochar from wheat straw produced at temperature of 650 °C | Biochar at rates of 10 (BC10), 20 (BC20), and 30 (BC30) t ha−1 | Enzymatic activity of dehydrogenase, phosphatase, and urease were lower in successive years of the study, regardless of the biochar rate. | Field study | [252] |
| Sandy and clay soils | Three corn stalk biochars prepared at 200, 400, and 600 °C | 0.5 and 1% (w/w) | Compared with the control, the biochar addition stimulated the activities of catalase, dehydrogenase, cellulase, invertase, and protease, which varied with pyrolysis temperature biochar dose as well as soil texture. The positive effects of biochar addition on soil enzymes were greater for 1% than 0.5% biochar doses and greater for sandy soils than clayey soils for catalase, dehydrogenase, and invertase. | Laboratory experiment | [253] |
| An arable Typic Haplocalci | Biochar from maize residue produced at 600 °C | Two addition rates (low, 0.5%, and high, 1.0% w/w) | BG (β-glucosidase) showed an increase in potential enzymatic activity (81%) in the biochar-amended sandy loam soil but only at a higher biochar dose. In the clayey soil, biochar decreased potential BG activity (by 10–29%). | Laboratory experiment | [231] |
| BS—black soil; FS—fuvo-aquic soil; and RS—red soil | Biochar produced from wheat straw at 500 °C | 0, 1, and 2% of biochar addition | Biochar did not change the bacterial richness and diversity in BS, FS, and RS but shifted all the soil bacterial community structures. Biochar mainly increased the growth of low-abundance bacteria in FS and high-abundance bacteria in RS. The most abundant bacterial phylum in BS and FS, Proteobacteria, increased after biochar addition, while Chlorofexi, the most abundant phylum in RS, decreased. | Laboratory experiment | [211] |
| Soil from field in Bregentved Estate, Zealand, Denmark (type not specified) | Wheat straw biochar (GBC) | 6–8 and 0.8–1.4 t/ha of high and low doses, respectively | No significant effect of biochar was observed on microbial biomass content. In a higher dose of BGC, only a minor effect on the soil community composition was observed. An increase in phenol oxidase activity and decrease in cellulase activity were reported. In a lower dose of GBC, the relative abundance of the rare members, and, thus, diversity of soil microorganisms, increased. | Filed study | [205] |
| Silty loam texture soil from Pistoia | Wood-derived biochar | 30 and 60 t/ha | No significant effect of biochar was observed on microbial biomass-C, microbial quotient, or genetic diversity. Biochar stimulated soil microbial activity with no disruptions, but this positive effect was very short. | Filed study | [204] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gryta, A.; Skic, K.; Adamczuk, A.; Skic, A.; Marciniak, M.; Józefaciuk, G.; Boguta, P. The Importance of the Targeted Design of Biochar Physicochemical Properties in Microbial Inoculation for Improved Agricultural Productivity—A Review. Agriculture 2024, 14, 37. https://doi.org/10.3390/agriculture14010037
Gryta A, Skic K, Adamczuk A, Skic A, Marciniak M, Józefaciuk G, Boguta P. The Importance of the Targeted Design of Biochar Physicochemical Properties in Microbial Inoculation for Improved Agricultural Productivity—A Review. Agriculture. 2024; 14(1):37. https://doi.org/10.3390/agriculture14010037
Chicago/Turabian StyleGryta, Angelika, Kamil Skic, Agnieszka Adamczuk, Anna Skic, Magdalena Marciniak, Grzegorz Józefaciuk, and Patrycja Boguta. 2024. "The Importance of the Targeted Design of Biochar Physicochemical Properties in Microbial Inoculation for Improved Agricultural Productivity—A Review" Agriculture 14, no. 1: 37. https://doi.org/10.3390/agriculture14010037
APA StyleGryta, A., Skic, K., Adamczuk, A., Skic, A., Marciniak, M., Józefaciuk, G., & Boguta, P. (2024). The Importance of the Targeted Design of Biochar Physicochemical Properties in Microbial Inoculation for Improved Agricultural Productivity—A Review. Agriculture, 14(1), 37. https://doi.org/10.3390/agriculture14010037