Monthly and Pregnancy-Related Concentration of Cu and Zn in Serum of Mares in an Equine Breeding Herd
Abstract
:1. Introduction
2. Materials and Methods
2.1. Breeding Mares’ Housing
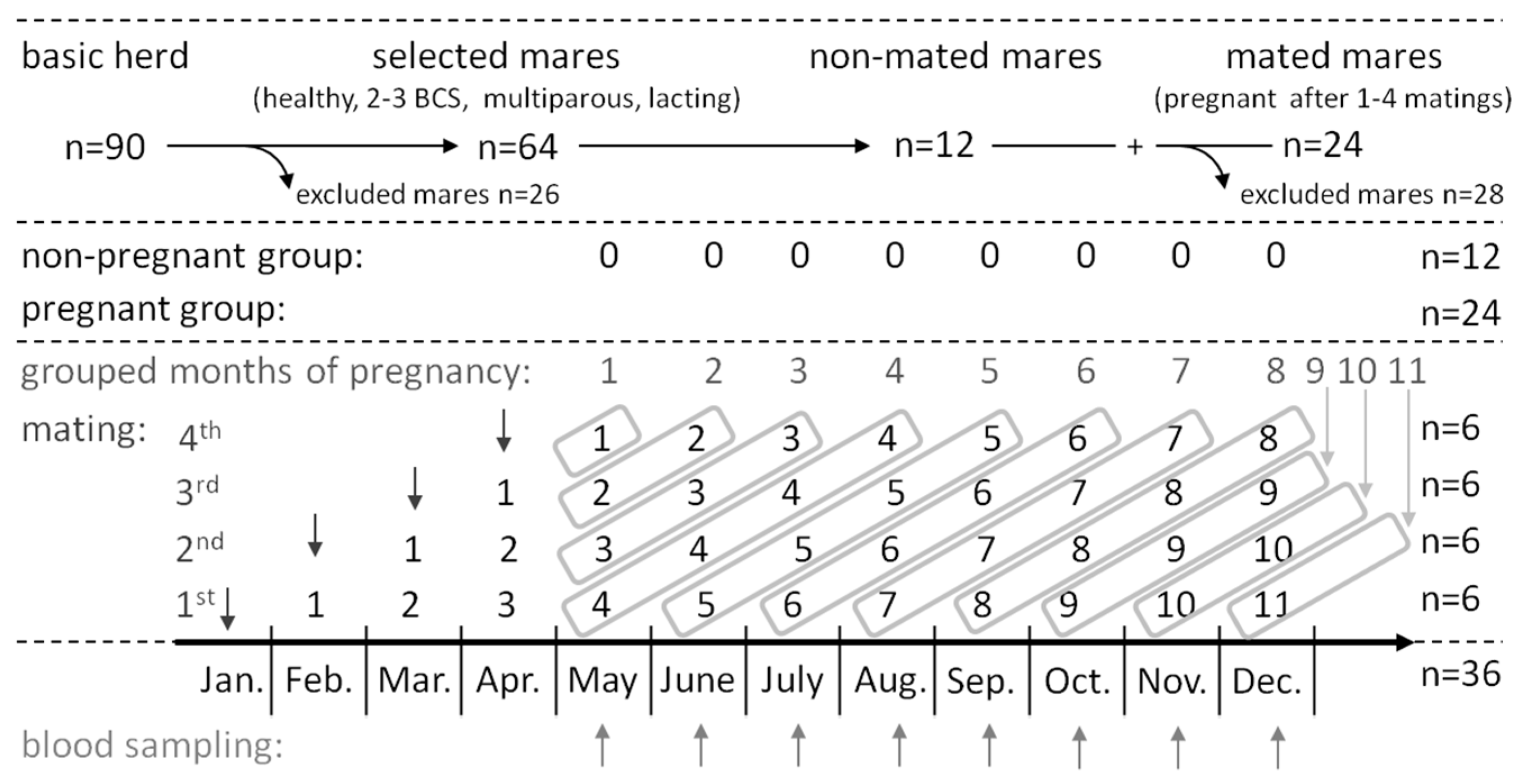
2.2. Breeding Mare Grouping
2.3. Sample Collection
2.4. Sample Grouping
2.5. Cu and Zn Concentrations
2.6. Statistical Analyses
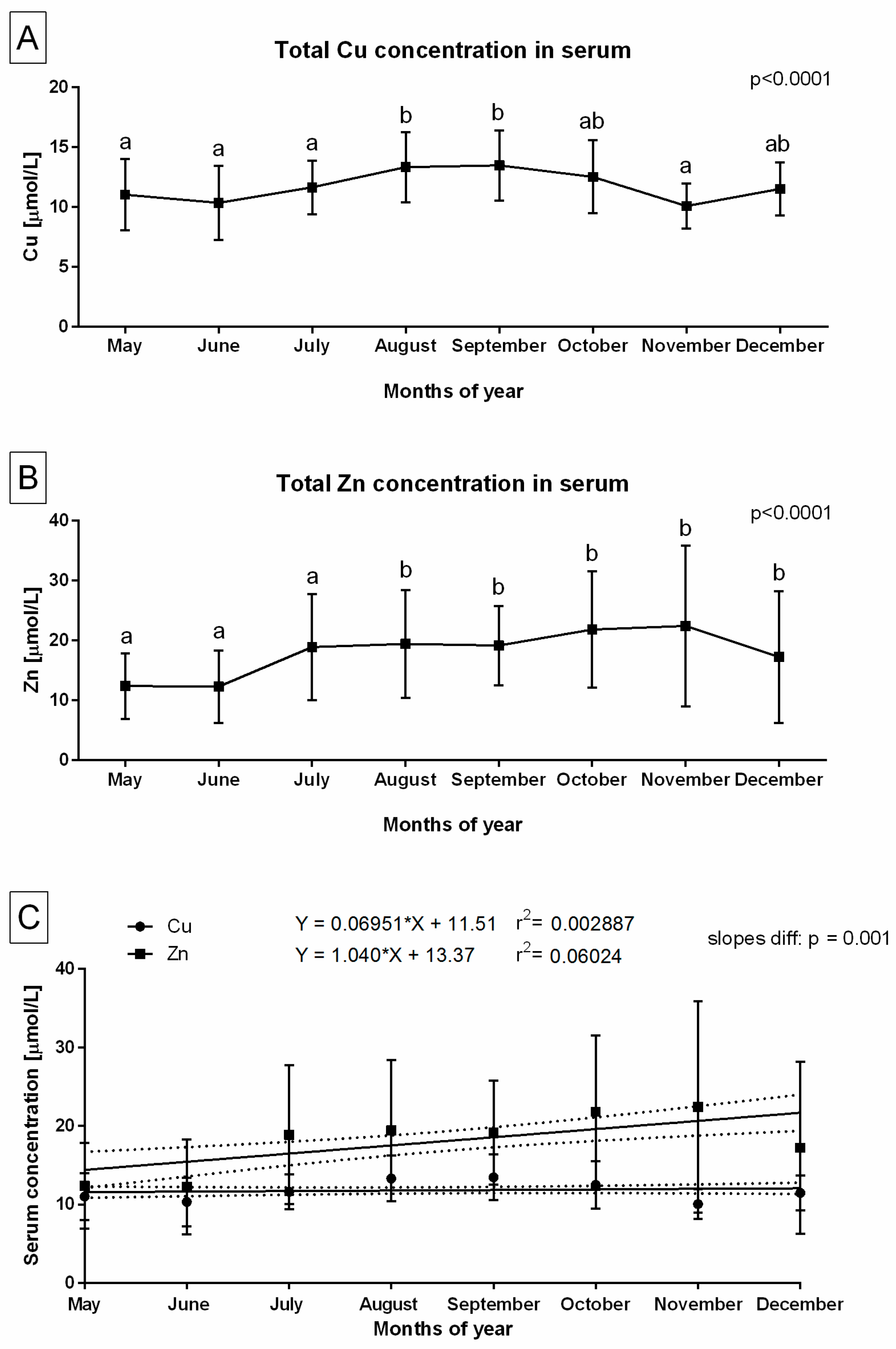
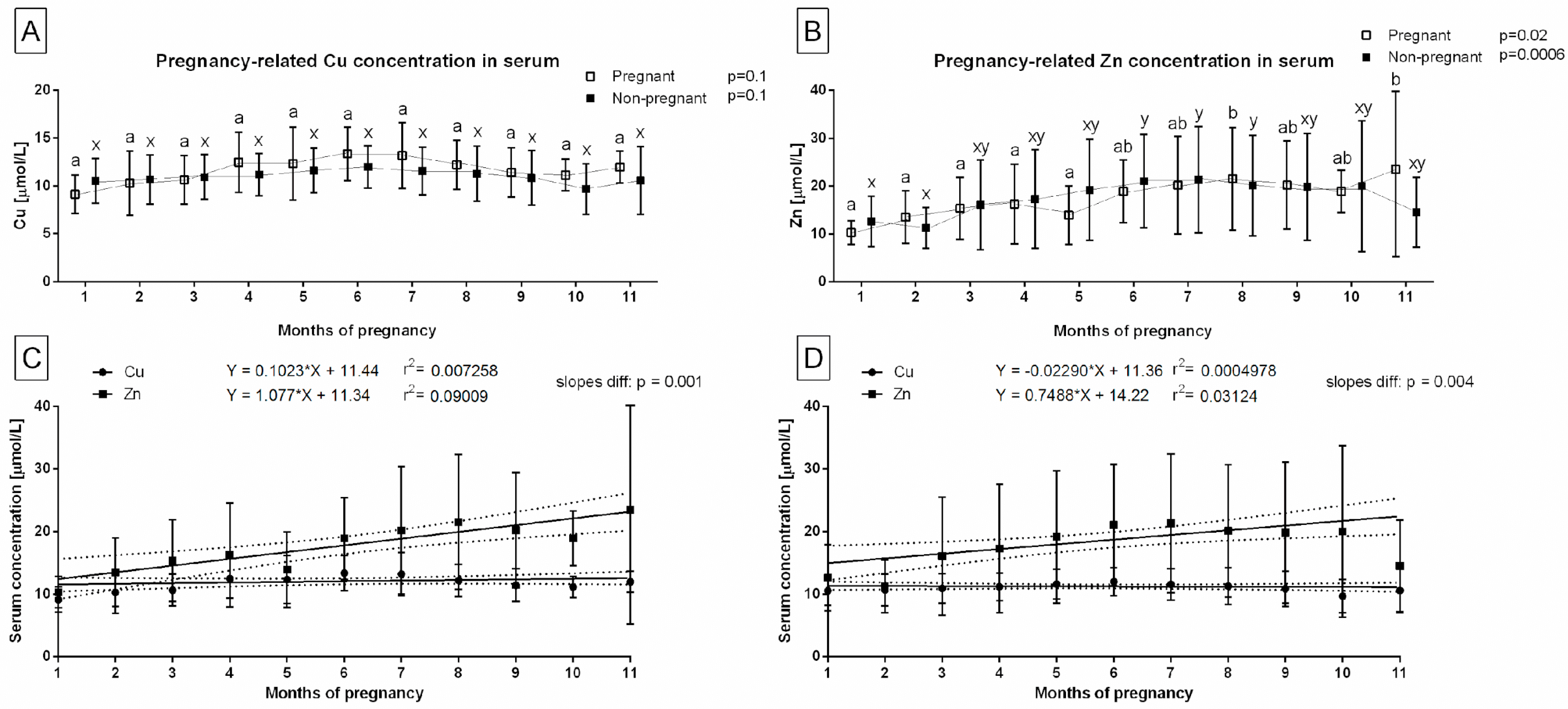
3. Results
4. Discussion
4.1. The Most Relevant Results
4.2. Cu and Zn Concentrations in Mares’ Serum
4.3. Limitations
4.4. Further Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coenen, M. Macro and Trace Elements in Equine Nutrition. In Equine Applied and Clinical Nutrition: Health, Welfare and Performance; Geor, R.J., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 190–228. [Google Scholar]
- Bazzano, M.; Giannetto, C.; Fazio, F.; Arfuso, F.; Giudice, E.; Piccione, G. Metabolic Profile of Broodmares During Late Pregnancy and Early Post-Partum. Reprod. Domest. Anim. 2014, 49, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, B.; Rich, L.J.; Wu, J. Hematological and Serum Biochemical Profile Values in Pregnant and Non-Pregnant Mares. Can. J. Vet. Res. 2018, 82, 287–293. [Google Scholar] [PubMed]
- Miraglia, N.; Salimei, E.; Fantuz, F. Equine milk production and valorization of marginal areas—A review. Animals 2020, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Dezzutto, D.; Barbero, R.; Valle, E.; Giribaldi, M.; Raspa, F.; Biasato, I.; Cavallarin, L.; Bergagna, S.; McLean, A.; Gennero, M.S. Observations of the hematological, hematochemical, and electrophoretic parameters in lactating donkeys (Equus asinus). J. Equine Vet. Sci. 2018, 65, 1–5. [Google Scholar] [CrossRef]
- Peugnet, P.; Robles, M.; Wimel, L.; Tarrade, A.; Chavatte-Palmer, P. Management of the Pregnant Mare and Long-Term Consequences on the Offspring. Theriogenology 2016, 86, 99–109. [Google Scholar] [CrossRef]
- Hallman, I.; Karikoski, N.; Kareskoski, M. The Effects of Obesity and Insulin Dysregulation on Mare Reproduction, Pregnancy, and Foal Health: A Review. Front. Vet. Sci. 2023, 10, 1180622. [Google Scholar] [CrossRef]
- Coverdale, J.A.; Hammer, C.J.; Walter, K.W. Horse species symposium: Nutritional programming and the impact on mare and foal performance. J. Anim. Sci. 2015, 93, 3261–3267. [Google Scholar] [CrossRef]
- Pearce, S.G.; Firth, E.C.; Grace, N.D.; Fennesy, P.F. Effect of Copper Supplementation on the Evidence of Developmental Orthopaedic Disease in Pasture-Fed New Zealand Thoroughbreds. Equine Vet. J. 1998, 30, 211–218. [Google Scholar] [CrossRef]
- Ali, F.; Lodhi, L.; Qureshi, Z.; Ahmad, I.; Hussain, R. Serum Mineral Profile in Various Reproductive Phases of Mares. Pak. Vet. J. 2013, 33, 296–299. [Google Scholar]
- Biricik, H.; Ocal, N.; Gucus, A.I.; Ediz, B.; Uzman, M. Seasonal Changes of Some Mineral Status in Mares. J. Equine Vet. Sci. 2005, 25, 346–348. [Google Scholar] [CrossRef]
- van Bömmel-Wegmann, S.; Gehlen, H.; Barton, A.-K.; Büttner, K.; Zentek, J.; Paßlack, N. Zinc Status of Horses and Ponies: Relevance of Health, Horse Type, Sex, Age, and Test Material. Vet. Sci. 2023, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Stublely, R.F.; Fletcher, J.M. Copper and Zinc Levels in the Blood of Thoroughbreds in Training in the United Kingdom. Equine Vet. J. 1983, 15, 99–103. [Google Scholar]
- Murase, H.; Sakai, S.; Kusano, K.; Hobo, S.; Nambo, Y. Serum Zinc Levels and Their Relationship with Diseases in Racehorses. J. Vet. Med. Sci. 2013, 75, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Humann-Ziehank, E. Selenium, Copper and Iron in Veterinary Medicine—From Clinical Implications to Scientific Models. J. Trace Elem. Med. Biol. 2016, 37, 96–103. [Google Scholar] [CrossRef]
- Górski, K.; Jania, B.; Andraszek, K. Changes in the Level of Calcium, Zinc and Copper in the Serum of Horses in Relation to the Feeding Season. Folia Pomer. Univ. Technol. Stetin. Agric. Aliment. Pisc. Zootech. 2017, 334, 35–42. [Google Scholar] [CrossRef]
- Harvey, A.M.; Ramp, D.; Mellor, D.J. Review of the Foundational Knowledge Required for Assessing Horse Welfare. Animals 2022, 12, 3385. [Google Scholar] [CrossRef]
- Rueda-Carrillo, G.; Rosiles-Martínez, R.; Hernández-García, A.I.; Vargas-Bello-Pérez, E.; Trigo-Tavera, F.J. Preliminary Study on the Connection Between the Mineral Profile of Horse Hooves and Tensile Strength Based on Body Weight, Sex, Age, Sampling Location, and Riding Disciplines. Front. Vet. Sci. 2022, 8, 763935. [Google Scholar] [CrossRef]
- Hurtig, M.; Green, S.L.; Dobson, H.; Mikuni-Takagaki, Y.; Choi, J. Correlative Study of Defective Cartilage and Bone Growth in Foals Fed a Low-Copper Diet. Equine Vet. J. 1993, 16, 66–73. [Google Scholar] [CrossRef]
- Meyer, H.; Ahlswede, L. The Intrauterine Growth and Body Composition of Foals and the Nutrient Requirements of Pregnant Mares. Anim. Res. Dev. 1978, 8, 86–112. [Google Scholar]
- Robles, M.; Hammer, C.; Staniar, B.; Chavatte-Palmer, P. Nutrition of Broodmares. Vet. Clin. N. Am. 2021, 37, 177–205. [Google Scholar] [CrossRef]
- Sprinkle, J.E.; Baker, S.D.; Church, J.A.; Findlay, J.R.; Graf, S.M.; Jensen, K.S.; Williams, S.K.; Willmore, C.M.; Lamb, J.B.; Hansen, D.W. Case Study: Regional Assessment of Mineral Element Concentrations in Idaho Forage and Range Grasses. Prof. Anim. Sci. 2018, 34, 494–504. [Google Scholar] [CrossRef]
- Jones, G.B.; Tracy, B.F. Evaluating Seasonal Variation in Mineral Concentration of Cool-Season Pasture Herbage. Grass Forage Sci. 2015, 70, 94–101. [Google Scholar] [CrossRef]
- Domínguez-Vara, I.A.; Sánchez-Malváez, E.; Medina-Navarro, P.; de Oca-Jiménez, R.M.; Vieyra Alberto, R.; Morales-Almaraz, E.; de la Fuente, J.L.; Sánchez-Torres, J.E.; Bórquez-Gastelum, J.L.; Acosta-Dibarrat, J.; et al. Mineral Status and Interrelationship in Soil, Forage, and Blood Serum of Horses in the Rainy and Dry Seasons. JEVS 2017, 49, 101–107. [Google Scholar] [CrossRef]
- Wichert, B.; Frank, T.; Kienzle, E. Zinc, Copper and Selenium Intake and Status of Horses in Bavaria. J. Nutr. 2002, 132, 1776S–1777S. [Google Scholar] [CrossRef]
- Auer, D.; Ng, J.; Seawright, A. Assessment of Copper and Zinc Status of Farm Horses and Training Thoroughbreds in South-East Queensland. Aust. Vet. J. 1988, 65, 317–320. [Google Scholar] [CrossRef]
- Preston, L. The Ultimate Guide to Horse Feed, Supplements, and Nutrition; Simon and Schuster: New York, NY, USA, 2016. [Google Scholar]
- Lawrence, L.M. Feeding Stallions and Broodmares. In Equine Applied and Clinical Nutrition; Geor, R.J., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 231–242. [Google Scholar]
- Gee, E.K.; Firth, E.C.; Morel, P.C.H.; Fennessy, P.F.; Grace, N.D.; Mogg, T.D. Articular/Epiphyseal Osteochondrosis in Thoroughbred Foals at 5 Months of Age: Influences of Growth of the Foal and Prenatal Copper Supplementation of the Dam. N. Z. Vet. J. 2005, 53, 448–456. [Google Scholar] [CrossRef]
- Ramin, A.G.; Asri-Rezaie, S.; Bukan, M. Evaluation of Serum Copper, Iron and Zinc Concentrations in Horse and Mule of Urmia. J. Anim. Sci. Res. 2017, 27, 101–112. [Google Scholar]
- Kappel, L.C.; Morgan, E.B.; Kilgore, L.; Ingraham, R.H.; Babcock, D.K. Seasonal changes of mineral content of southern forages. J. Dairy Sci. 1985, 68, 1822–1827. [Google Scholar] [CrossRef]
- López-Alonso, M. Trace minerals and livestock: Not too much not too little. ISRN Vet. Sci. 2012, 2012, 704825. [Google Scholar] [CrossRef]
- Grace, N.; Pearce, S.; Firth, E.; Fennessy, P. Content and Distribution of Macro- and Micro-Elements in the Body of Pasture-Fed Young Horses. Aust. Vet. J. 1999, 77, 172–176. [Google Scholar] [CrossRef]
- Massanyi, P.; Stawarz, R.; Halo, M.; Formicki, G.; Lukac, N.; Cupka, P.; Kovacik, J. Blood Concentration of Copper, Cadmium, Zinc, and Lead in Horses and Its Relation to Hematological and Biochemical Parameters. J. Environ. Sci. Health. 2014, 49, 973–979. [Google Scholar] [CrossRef]
- Paßlack, N.; Mainzer, B.; Lahrssen-Wiederholt, M.; Schafft, H.; Palavinskas, R.; Breithaupt, A.; Zentek, J. Concentrations of Strontium, Barium, Cadmium, Copper, Zinc, Manganese, Chromium, Antimony, Selenium and Lead in the Equine Liver and Kidneys. SpringerPlus 2014, 3, 343. [Google Scholar] [CrossRef]
- Moinardeau, C.; Mesleard, F.; Ramone, H.; Dutoit, T. Grazing in temporary paddocks with hardy breed horses (Konik polski) improved species-rich grasslands restoration in artificial embankments of the Rhône river (Southern France). Glob. Ecol. Conserv. 2021, 31, e01874. [Google Scholar] [CrossRef]
- Moinardeau, C.; Mesleard, F.; Ramone, H.; Dutoit, T. Extensive horse grazing improves grassland vegetation diversity, seed bank and forage quality of artificial embankments (Rhône River-southern France): Influence of extensive horse grazing on artificial embankments. J. Nat. Conserv. 2020, 56, 125865. [Google Scholar] [CrossRef]
- Huntington, P.J.; Brown-Douglas, C.G.; Pagan, J.D. Growth and development of Thoroughbred horses. Anim. Prod. Sci. 2020, 60, 2093–2102. [Google Scholar] [CrossRef]
- Fradinho, M.J.; Bessa, R.J.; Martin-Rosset, W.; Ferreira-Dias, G.; Caldeira, R.M. Nutritional status of Lusitano broodmares on extensive feeding systems: Body condition, live weight and metabolic indicators. Ital. J. Anim. Sci. 2013, 12, e71. [Google Scholar] [CrossRef]
- Popova, M.; Malinova, R.; Nikolov, V.; Georgiev, B.; Taushanova, P.; Ivanova, M. Dietary mineral and vitamin supplementation improved the reproduction, the haematology, and some circulating biochemical’s of cyclic East-Bulgarian and Arabian mares. Ital. J. Anim. Sci. 2021, 20, 728–735. [Google Scholar] [CrossRef]
- Raspa, F.; Tarantola, M.; Muca, E.; Bergero, D.; Soglia, D.; Cavallini, D.; Vervuert, I.; Bordin, C.; De Palo, P.; Valle, E. Does feeding management make a difference to behavioural activities and welfare of horses reared for meat production? Animals 2022, 12, 1740. [Google Scholar] [CrossRef]
- Vinassa, M.; Cavallini, D.; Galaverna, D.; Baragli, P.; Raspa, F.; Nery, J.; Valle, E. Palatability assessment in horses in relation to lateralization and temperament. Appl. Anim. Behav. Sci. 2020, 232, 105110. [Google Scholar] [CrossRef]
- Cavallini, D.; Penazzi, L.; Valle, E.; Raspa, F.; Bergero, D.; Formigoni, A.; Fusaro, I. When changing the hay makes a difference: A series of case reports. J. Equine Vet. Sci. 2022, 113, 103940. [Google Scholar] [CrossRef]
- Bordin, C.; Raspa, F.; Harris, P.; Ellis, A.D.; Roggero, A.; Palestrini, C.; Bergero, D.; Valle, E. Effect of pony morphology and hay feeding methods on back and neck postures. J. Anim. Physiol. Anim. Nutr. 2023, 1–12. [Google Scholar] [CrossRef]
- Raspa, F.; Roggero, A.; Palestrini, C.; Marten Canavesio, M.; Bergero, D.; Valle, E. Studying the shape variations of the back, the neck, and the mandibular angle of horses depending on specific feeding postures using geometric morphometrics. Animals 2021, 11, 763. [Google Scholar] [CrossRef]
- Raspa, F.; Dinardo, F.R.; Vervuert, I.; Bergero, D.; Bottero, M.T.; Pattono, D.; Dalmasso, A.; Vinassa, M.; Valvassori, E.; Bruno, E.; et al. A Fibre- vs. cereal grain-based diet: Which is better for horse welfare? Effects on intestinal permeability, muscle characteristics and oxidative status in horses reared for meat production. J. Anim. Physiol. Anim. Nutr. 2022, 106, 313–326. [Google Scholar] [CrossRef]
- Valle, E.; Raspa, F.; Giribaldi, M.; Barbero, R.; Bergagna, S.; Antoniazzi, S.; Mc Lean, A.K.; Minero, M.; Cavallarin, L. A functional approach to the body condition assessment of lactating donkeys as a tool for welfare evaluation. PeerJ 2017, 5, e3001. [Google Scholar] [CrossRef]
- Stowe, H.D. Effects of age and impending parturition upon serum copper of thoroughbred mares. J. Nutr. 1968, 95, 179–183. [Google Scholar] [CrossRef]
- Pourmohammad, R.; Mohri, M.; Seifi, H.; Sardari, K. Effect of exercise on some minerals, metabolites and enzyme activities in the serum of trained Arabian horses. Turk. J. Vet. Anim. Sci. 2019, 43, 791–799. [Google Scholar] [CrossRef]
- Gupta, A.R.; Bandyopadhyay, S.; Sultana, F.; Swarup, D. Heavy metal poisoning and its impact on livestock health and production system. Indian J. Anim. Health 2021, 60, 1–23. [Google Scholar] [CrossRef]
- van der Merwe, D.; van den Wollenberg, L.; van Hees-Valkenborg, J.; de Haan, T.; van der Drift, S.; Vandendriessche, V. Evaluation of hair analysis for determination of trace mineral status and exposure to toxic heavy metals in horses in the Netherlands. J. Vet. Diagn. 2022, 34, 1000–1005. [Google Scholar] [CrossRef]

| Data/Months | May | June | July | August | September | October | November | December |
|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 14.1 | 17.4 | 19.4 | 19.2 | 14.7 | 9.5 | 4.9 | 1.0 |
| Rainfall (mm) | 40 | 61 | 66 | 88 | 64 | 54 | 46 | 43 |
| Humidity (%) | 66% | 65% | 69% | 69% | 73% | 80% | 87% | 85% |
| Rainy days (day) | 8 | 8 | 9 | 8 | 7 | 7 | 7 | 8 |
| Sunny hours (h) | 10.5 | 11.0 | 10.9 | 10.2 | 7.5 | 5.0 | 3.0 | 2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maśko, M.; Chałabis-Mazurek, A.; Sikorska, U.; Ciesielska, A.; Zdrojkowski, Ł.; Domino, M. Monthly and Pregnancy-Related Concentration of Cu and Zn in Serum of Mares in an Equine Breeding Herd. Agriculture 2024, 14, 35. https://doi.org/10.3390/agriculture14010035
Maśko M, Chałabis-Mazurek A, Sikorska U, Ciesielska A, Zdrojkowski Ł, Domino M. Monthly and Pregnancy-Related Concentration of Cu and Zn in Serum of Mares in an Equine Breeding Herd. Agriculture. 2024; 14(1):35. https://doi.org/10.3390/agriculture14010035
Chicago/Turabian StyleMaśko, Małgorzata, Agnieszka Chałabis-Mazurek, Urszula Sikorska, Anna Ciesielska, Łukasz Zdrojkowski, and Małgorzata Domino. 2024. "Monthly and Pregnancy-Related Concentration of Cu and Zn in Serum of Mares in an Equine Breeding Herd" Agriculture 14, no. 1: 35. https://doi.org/10.3390/agriculture14010035
APA StyleMaśko, M., Chałabis-Mazurek, A., Sikorska, U., Ciesielska, A., Zdrojkowski, Ł., & Domino, M. (2024). Monthly and Pregnancy-Related Concentration of Cu and Zn in Serum of Mares in an Equine Breeding Herd. Agriculture, 14(1), 35. https://doi.org/10.3390/agriculture14010035






