In Vitro Propagation of Commercially Used Krymsk 5® (Prunus fruticosa × Prunus lannesiana) Cherry Rootstock: Impact of Sugar Types and pH Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Explant Source, Plant Material, Culture Conditions
2.2. Shoot Proliferation Experiments
2.2.1. Experiment 1: Effect of Different Types of Sugar
2.2.2. Experiment 2: Effect of Different Pre-Autoclaving pH Levels on Post-Autoclaving pH and Shoot Proliferation
2.2.3. Experimental Design and Statistical Analysis
2.3. Rooting Experiments
2.3.1. Experiment 3: Effect of Different Types of Sugars on Rooting in the Absence of Auxins
2.3.2. Experiment 4: Effect of Different Prior Autoclaving pH Levels on Post-Autoclaving pH and Rooting in the Absence of Auxins
2.3.3. Experimental Design and Statistical Analysis
3. Results
3.1. Effect of Different Types of Sugar in the Proliferation Stage
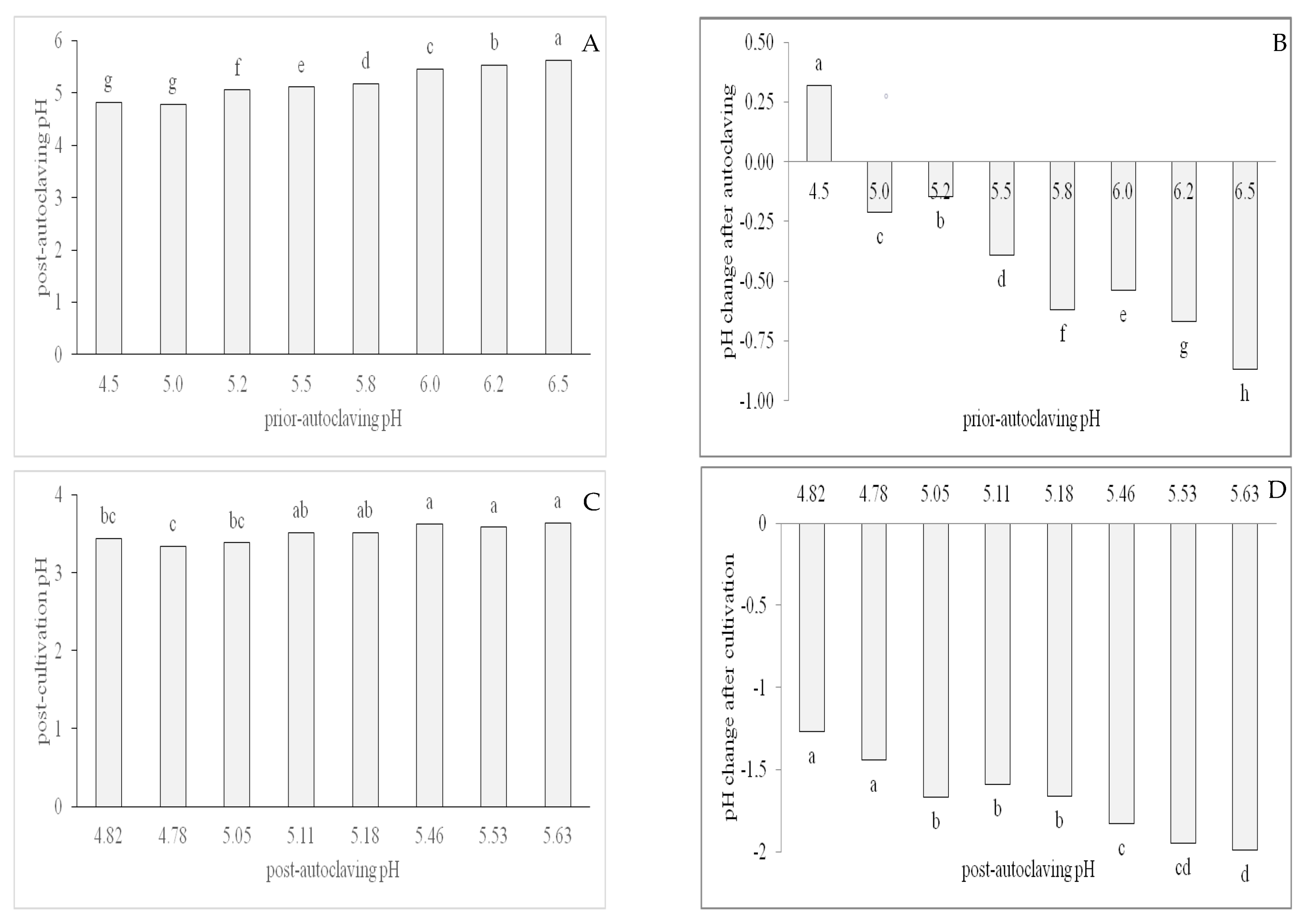
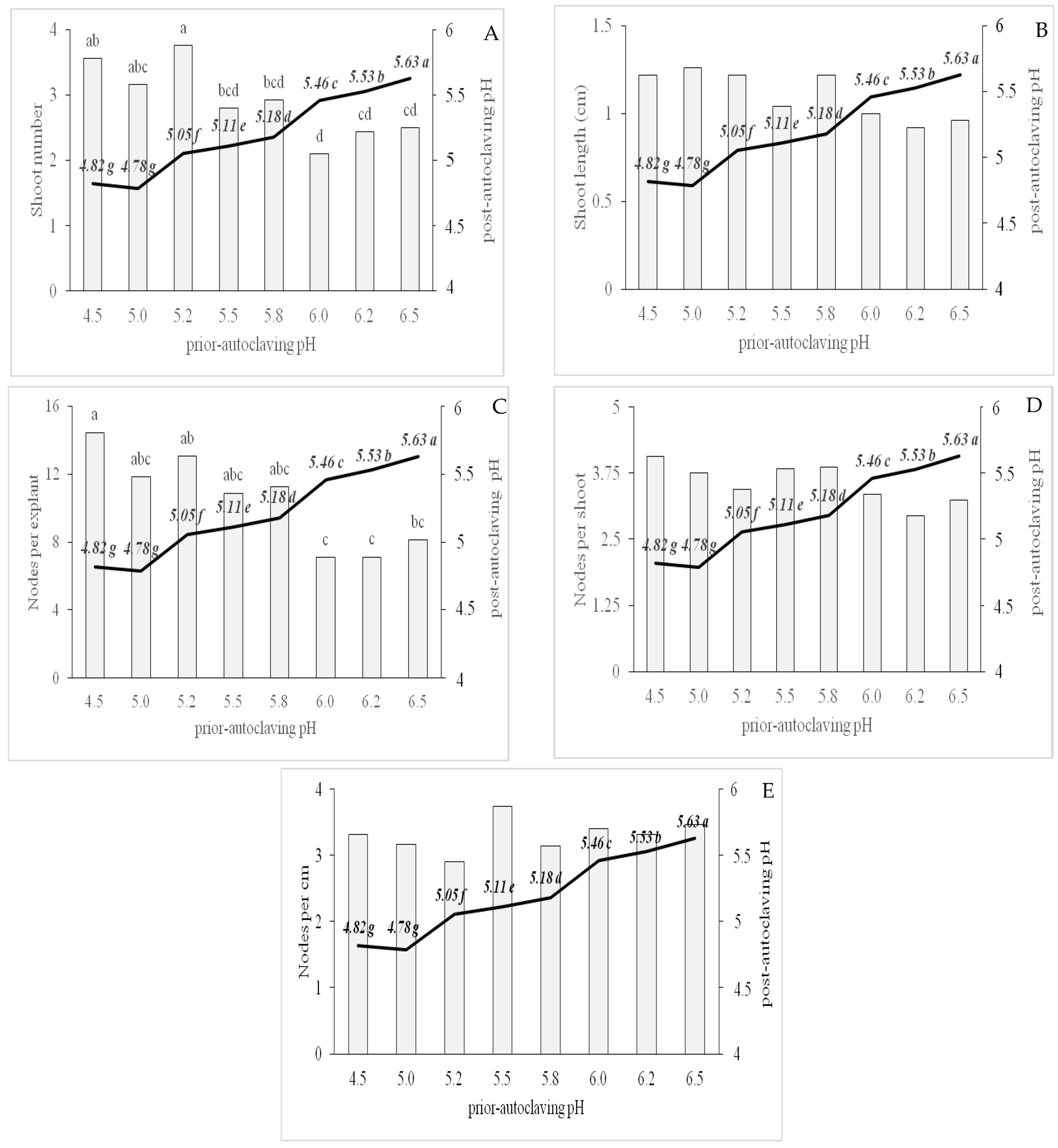
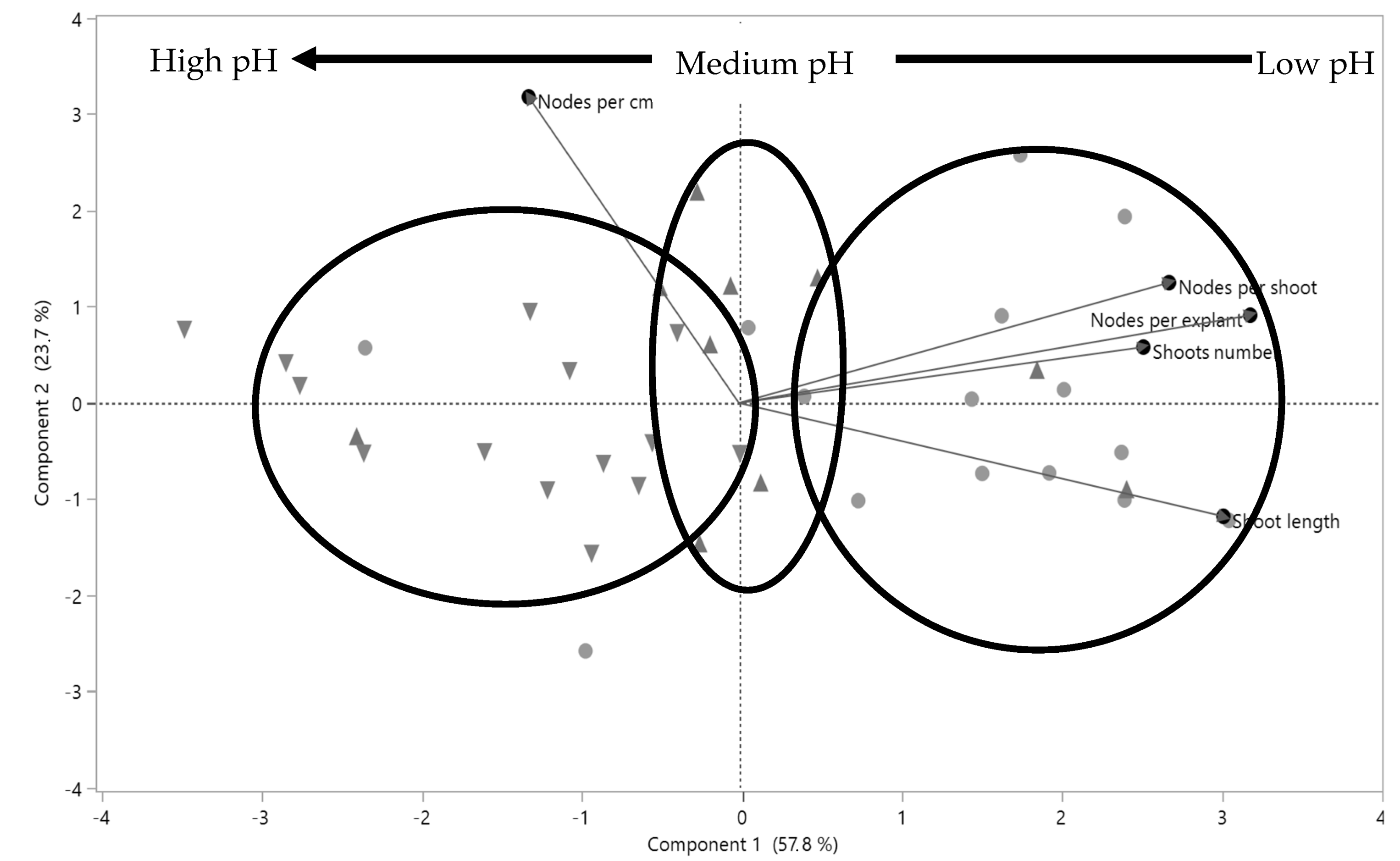
3.2. Effect of Different Prior Autoclaving pH Levels on Post-Autoclaving pH and Shoot Proliferation Variables
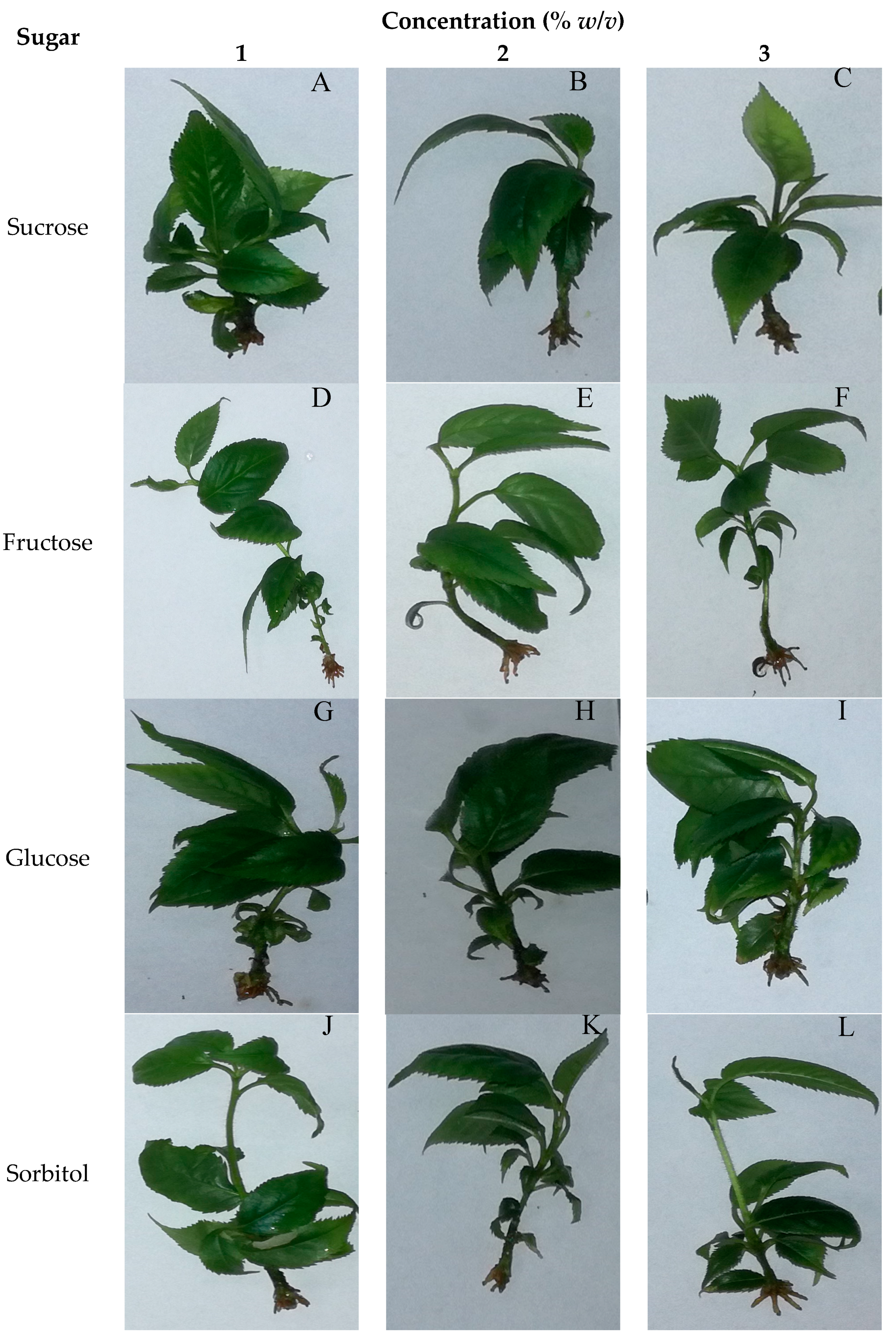
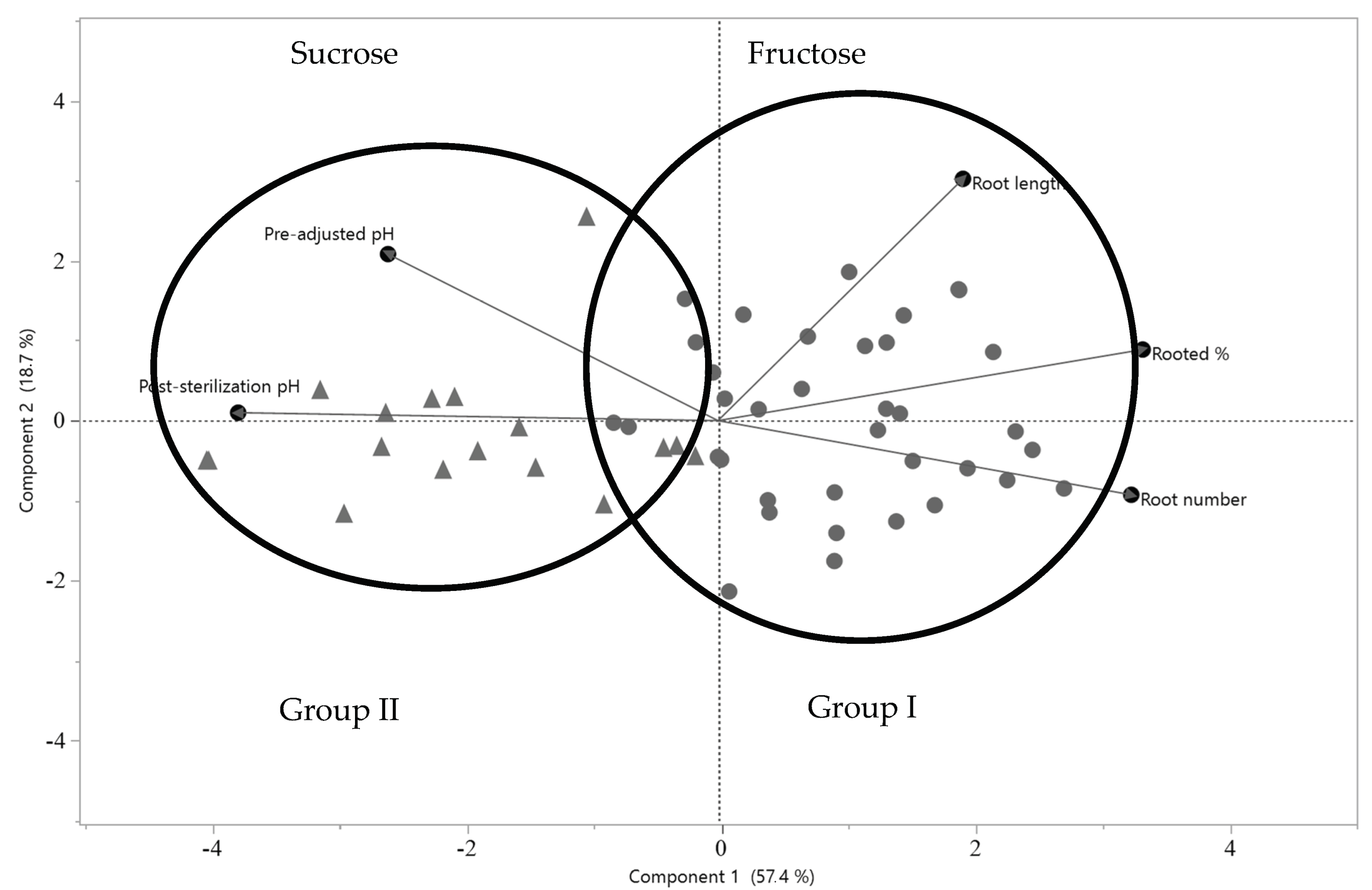
3.3. Effect of Sugars and Their Concentrations on Rooting Variables
3.4. Effect of Sugar and Prior Autoclaving pH Levels on Rooting Variables and Post-Autoclaving pH Levels
4. Discussion
4.1. Effects of Sugars in Krymsk’s 5 In Vitro Propagation
4.2. Effects of Sugars and Prior Autoclaving pH on Post-Autoclaving pH Levels
4.3. Effects of pH in “Krymsk 5®” In Vitro Propagation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsafouros, A.; Roussos, P.A. First report of Krymsk® 5 (cv. VSL 2) cherry rootstock in vitro propagation: Studying the effect of cytokinins, auxins and endogenous sugars. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 152–161. [Google Scholar] [CrossRef]
- Maas, F.M.; Balkhoven-Baart, J.; van der Steeg, P.A.H. Evaluation of Krymsk®5 (VSL-2) and Krymsk®6 (LC-52) as rootstocks for sweet cherry “Kordia”. Acta Hortic. 2014, 1058, 531–536. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; Klerk, G.J.D. Plant Propagation by Tissue Culture, 3rd ed.; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Dobranski, J.; da Silva, J.A.T. Micropropagation of apple—A review. Biotechnol. Adv. 2010, 28, 462–488. [Google Scholar] [CrossRef] [PubMed]
- Roussos, P.; Pontikis, C. In vitro propagation of olive (Olea europaea L.) cv. Koroneiki. Plant Growth Regul. 2022, 37, 295–304. [Google Scholar] [CrossRef]
- Fotopoulos, S.; Sotiropoulos, T.E. In vitro propagation of the peach rootstock: The effect of different carbonsources and types of sealing material on rooting. Biol. Plant. 2004, 48, 629–631. [Google Scholar] [CrossRef]
- Yaseen, M.; Touqeer, A.; Tahani, A.; Nadeem, A.; Ishfaq, H. In vitro shoot proliferation competence of apple rootstocks M. 9 and M. 26 on different carbon sources. Pak. J. Bot. 2009, 41, 1781–1795. [Google Scholar]
- Yaseen, M.; Tahani, A.; Nadeem, A.; Ishfaq, H. Assessment of apple rootstocks M 9 and M 26 for in vitro rooting potential using different carbon sources. Pak. J. Bot. 2009, 41, 769–781. [Google Scholar]
- Tsafouros, A.; Roussos, P.A. ‘Krymsk 86’ stone fruit rootstock: High in vitro rooting potential even in absence of auxins. Acta Hortic. 2021, 1322, 187–192. [Google Scholar] [CrossRef]
- Kadota, M.; Niimi, Y. Influences of carbon sources and their concentrations on shoot proliferation and rooting of ‘Hosui’ Japanese pear. HortScience 2004, 39, 1681–1683. [Google Scholar] [CrossRef]
- Marino, G.; Bertazza, G.; Magnanini, E.; Doro Altan, A. Comparative effects of sorbitol and sucrose as main carbon energy sources in micropropagation of apricot. Plant Cell Tissue Organ Cult. 1993, 34, 235–244. [Google Scholar] [CrossRef]
- Cheong, E.J.; An, C. Effect of carbohydrates on in vitro shoot growth of various Prunus species. Korean J. Plant Res. 2015, 28, 357–362. [Google Scholar] [CrossRef]
- Ibrahim, K.; Alromaihi, K.; Elmeer, K. The combined role of sucrose with IBA and NAA in rooting of date palm somatic embryos cv. Khanaizi. Plant Tissue Cult. Biotechnol. 2009, 19, 127–132. [Google Scholar] [CrossRef][Green Version]
- Owen, H.R.; Wengerd, D.; Miller, A.R. Culture medium pH is influenced by basal medium, carbohydrate source, gelling agent, activated charcoal, and medium storage method. Plant Cell Rep. 1991, 10, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, T.; Stasolla, C.; Yeung, E.; de Klerk, G.-J.; Roberts, A.; George, E.F. The components of plant tissue culture media II. Organic additions, osmotic and pH effects, and support systems. In Plant Propagation by Tissue Culture Vol. 1: The Background. 1, 3rd ed.; George, E.F., Hall, M., de Klerk, G.J., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 115–173. [Google Scholar]
- Skirvin, R.M.; Chu, M.C.; Mann, M.L.; Young, H.; Sullivan, J.; Fermanian, T. Stability of tissue culture medium pH as a function of autoclaving, time, and cultured plant material. Plant Cell Rep. 1986, 5, 292–294. [Google Scholar] [CrossRef]
- Pasqua, G.; Manes, F.; Monacelli, B.; Natale, L.; Anselmi, S. Effects of the culture medium pH and ion uptake in in vitro vegetative organogenesis in thin cell layers of tobacco. Plant Sci. 2002, 162, 947–955. [Google Scholar] [CrossRef]
- Harbage, J.F.; Stimart, D.P.; Auer, C. pH affects 1 H-indole-3-butyric acid uptake but not metabolism during the initiation phase of adventitious root induction in apple microcuttings. J. Am. Soc. Hortic. Sci. 1998, 123, 6–10. [Google Scholar] [CrossRef]
- Ostrolucká, M.; Gajdošová, A.; Ondrušková, E.; Latečková, M.; Libiaková, G. Effect of medium pH on axillary shoot proliferation of selected Vaccinium vitis-idaea L. cultivars. Acta Biol. Crac. Ser. Bot. 2010, 52, 92–96. [Google Scholar] [CrossRef]
- Driver, J.A.; Kuniyuki, A.H. In vitro propagation of Paradox walnut rootstock. HortScience 1984, 19, 507–509. [Google Scholar] [CrossRef]
- Mc Granahan, G.H.; Driver, J.A.; Tulecke, W. Tissue Culture of Juglans. In Cell and Tissue Culture in Forestry: Case Histories: Gymnosperms, Angiosperms and Palms; Springer: Dordrecht, The Netherlands, 1987; pp. 261–271. [Google Scholar]
- Nacheva, L.; Gercheva, P. Micropropagation of Gisela 5 (cherry dwarf rootstock): The effect of the type and the concentration of the carbohydrates in the nutrient medium. Acta Hortic. 2009, 825, 261–268. [Google Scholar] [CrossRef]
- Yaseen, M.; Ahmad, T.; Sablok, G.; Standardi, A.; Hafiz, I.A. Review: Role of carbon sources for in vitro plant growth and development. Mol. Biol. Rep. 2013, 40, 2837–2849. [Google Scholar] [CrossRef]
- Ritterbusch, C.W.; Lucho, S.R.; Radmann, E.B.; Bianchi, V.J. Effect of cytokinins, carbohydrate source and auxins on in vitro Propagation of the “G × N-9” peach rootstock. Int. J. Fruit Sci. 2020, 20, S1607–S1619. [Google Scholar] [CrossRef]
- Borkowska, B.; Szczerba, J. Influence of different carbon sources on invertase activity and growth of sour cherry (Prunus cerasus L.) shoot cultures. J. Exp. Bot. 1991, 42, 911–915. [Google Scholar]
- Hammatt, N. Micropropagation of fastigiate bird cherry (Prunus padus L.) and adventitious shoot formation from leaves. J. Hortic. Sci. 1993, 68, 975–981. [Google Scholar] [CrossRef]
- Kadota, M.; Imizu, K.; Hirano, T. Double-phase in vitro culture using sorbitol increases shoot proliferation and reduces hyperhydricity in Japanese pear. Sci. Hortic. 2001, 89, 207–215. [Google Scholar] [CrossRef]
- Moncousin, C.; Ribaux, M.; o’Rourke, J.O.; Gavillet, S. Effects of type of carbohydrate during proliferation and rooting of microcuttings of Malus ‘Jork 9′. Agronomie 1992, 12, 775–781. [Google Scholar] [CrossRef]
- Ahmad, T.; Abbasi, N.A.; Hafiz, I.A.; Ali, A. Comparison of sucrose and sorbitol as main carbon energy source in morphogenesis of peach rootstock GF-677. Pak. J. Bot. 2007, 39, 1264–1275. [Google Scholar]
- lo Bianco, R. Carbohydrate metabolism and source-sink relationshipsin peach. In Tree Growth: Influences, Layers and Types; Karam, W.P., Ed.; Nova Science Publishers: New York, NY, USA, 2009; pp. 79–91. [Google Scholar]
- Gürel, S.; Gülsen, Y. The effects of different sucrose, agar and pH levels on in vitro shoot production of almond Amygdalus communis L.). Turk. J. Bot. 1998, 22, 363–373. [Google Scholar]
- Harada, H.; Murai, Y. Micropropagation of Prunus mume. Plant Cell Tissue Organ Cult. 1996, 46, 265–267. [Google Scholar] [CrossRef]
- Rugini, E.; Tarini, P.; Rossodivita, M.E.; Costanzo, S. Control of shoot vitrification of almond and olive grown in vitro. Acta Hortic. 1987, 212, 177–184. [Google Scholar] [CrossRef]
- Bahmani, R.; Karami, O.; Gholami, M. Influence of carbon sources and their concentrations on rooting and hyperhydricity of apple rootstock MM.106. World Appl. Sci. J. 2009, 6, 1513–1517. [Google Scholar]
- Murai, Y.; Harada, H.; Yamashita, H. In vitro propagation of apricot (Prunus armeniaca L.) cv. ‘Bakuoh junkyou’. J. Jpn. Soc. Hortic. Sci. 1997, 66, 475–480. [Google Scholar] [CrossRef]
- Tsafouros, A.; Roussos, P.A. Dopamine, chlorogenic acid, and quinones as possible cofactors of increasing adventitious rooting potential of in vitro Krymsk 5 cherry rootstock explants. Agronomy 2022, 12, 1154. [Google Scholar] [CrossRef]
- Gibson, S.I. Control of plant development and gene expression by sugar signaling. Curr. Opin. Plant Biol. 2005, 8, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Formela-Luboińska, M.; Labudda, M.; Morkunas, I. The Role of sugars in plant responses to stress and their regulatory function during development. Int. J. Mol. Sci. 2022, 23, 5161. [Google Scholar] [CrossRef]
- Yu, W.; Peng, F.; Wang, W.; Liang, J.; Xiao, Y.; Yuan, X. SnRK1 phosphorylation of SDH positively regulates sorbitol metabolism and promotes sugar accumulation in peach fruit. Tree Physiol. 2021, 41, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Maurel, K.; Berenhauser Leite, G.; Bonhomme, M.; Guilliot, A.; Rageau, R.; Pétel, G.; Sakr, S. Trophic control of bud break in peach (Prunus persica) trees: A possible role of hexoses. Tree Physiol. 2004, 24, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Bieleski, R.L.; Redgwell, R.J. Sorbitol versus sucrose as photosynthesis and translocation products in developing apricot leaves. Aust. J. Plant Physiol. 1985, 12, 657–668. [Google Scholar] [CrossRef]
- Li, Y.; Sun, W.; Yao, Y.; Zhang, L.; Xu, S.; Zhang, Q.; Huang, T. FRUCTOSE INSENSITIVE1 regulates stem cell function in Arabidopsis in response to fructose signaling. J. Exp. Bot. 2023, 74, 3060–3073. [Google Scholar] [CrossRef]
- Yasodha, R.; Kamala, S.; Anand Kumar, S.P.; Durai Kumar, P.; Kalaiarasi, K. Effect of glucose on in vitro rooting of mature plants of Bambusa nutans. Sci. Hortic. 2008, 116, 113–116. [Google Scholar] [CrossRef]
- Corrêa, L.R.; Paim, D.C.; Schwambach, J.; Fett-Neto, A.G. Carbohydrates as regulatory factors on the rooting of Eucalyptus saligna Smith and Eucalyptus globulus Labill. Plant Growth Regul. 2005, 45, 63–73. [Google Scholar] [CrossRef]
- Gago, D.; Bernal, M.Á.; Sánchez, C.; Aldrey, A.; Cuenca, B.; Christie, C.B.; Vidal, N. Effect of sucrose on growth and stress status of Castanea sativa × C. crenata shoots cultured in liquid medium. Plants 2022, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Selby, C.; Lee, R.; Harvey, B.M.R. The effects of culture medium rigidity on adventitious bud production and tissue vitrification in needle cultures of Sitka spruce (Picea sitchensis (Boog.) Carr.). New Physiol. 1989, 113, 203–210. [Google Scholar] [CrossRef]
- Khan, A.; Chauhan, Y.S.; Roberts, L.W. In vitro studies on xylogenesis in Citrus fruit vesicles. 2. Effect of pH of the nutrient medium on the induction of cytodifferentiation. Plant Sci. 1986, 46, 213–216. [Google Scholar] [CrossRef]
- Cherve, A.M.; Gill, S.S.; Mouras, A.; Salesses, G. In vitro vegetative multiplication of chestnut. J. Hortic. Sci. 1983, 58, 23–29. [Google Scholar]
- Andersone, U.; Ievinsh, G. Medium pH affects regeneration capacity andoxidative enzyme activity of Pinus sylvestris in tissue culture. Acta Univ. Latv. Biol. 2008, 745, 25–35. [Google Scholar]
- De Klerk, G.J.; Hanecakova, J.; Jásik, J. Effect of medium-pH and MES on adventitious root formation from stem disks of apple. Plant Cell Tissue Organ Cult. 2008, 95, 285–292. [Google Scholar] [CrossRef]
- Leifert, C.; Pryce, S.; Lumsden, P.J.; Waites, W.M. Carbon sources and their osmotic potential in plant tissue culture: Does it matter? Plant Cell Tissue Organ Cult. 1992, 30, 171–179. [Google Scholar] [CrossRef]
- Sharma, A.K.; Prasad, R.N.; Chaturvedi, H.C. Clonal propagation of Bougainvillea glabra ‘Magnifica’ through shoot apex culture. Plant Cell Tissue Organ Cult. 1981, 1, 33–38. [Google Scholar] [CrossRef]
- Chen, Q.; Dai, X.; De-Paoli, H.; Cheng, Y.; Takebayashi, Y.; Kasahara, H.; Kamiya, Y.; Zhao, Y. Auxin overproduction in shoots cannot rescue auxin deficiencies in Arabidopsis roots. Plant Cell Physiol. 2014, 55, 1072–1079. [Google Scholar] [CrossRef]
- Korver, R.A.; Koevoets, I.T.; Testerink, C. Out of shape during stress: A key role for auxin. Trends Plant Sci. 2018, 23, 783–793. [Google Scholar] [CrossRef]
- Dang, X.; Chen, B.; Liu, F.; Ren, H.; Liu, X.; Zhou, J.; Qin, Y.; Lin, D. Auxin signaling-mediated apoplastic pH modification functions in petal conical cell shaping. Cell Rep. 2020, 30, 3904–3916. [Google Scholar] [CrossRef]
- Tsafouros, A.; Roussos, P.A. The possible bottleneck effect of polyamines’ catabolic enzymes in efficient adventitious rooting of two stone fruit rootstocks. J. Plant Physiol. 2020, 244, 152999. [Google Scholar] [CrossRef]
- Lv, G.; Qing, J.; Du, H.; Du, Q.; Meng, Y.; He, F.; Liu, P.; Du, L.; Wang, L. Comparing rooting ability and physiological changes of two Eucommia ulmoides improved varieties. Forests 2021, 12, 1267. [Google Scholar] [CrossRef]
- Hasenstein, K.H.; Rayle, D. Cell wall pH and auxin transport velocity. Plant Physiol. 1984, 76, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Steinacher, A.; Leyser, O.; Clayton, R.H. A computational model of auxin and pH dynamics in a single plant cell. J. Theor. Biol. 2012, 296, 84–94. [Google Scholar] [CrossRef]
- Krupinski, P.; Jönsson, H. Modeling auxin-regulated development. Cold Spring Harb. Perspect Biol. 2010, 2, a001560. [Google Scholar] [CrossRef] [PubMed]
- Roitsch, T.; González, M.-C. Function and regulation of plant invertases: Sweet sensations. Trends Plant Sci. 2004, 9, 606–613. [Google Scholar] [CrossRef]
- Morris, D.A.; Arthur, E.D. Stimulation of acid invertase activity by indol-3yl-acetic acid in tissues undergoing cell expansion. Plant Growth Regul. 1986, 4, 259–271. [Google Scholar] [CrossRef]
- Ahkami, A.H.; Lischewski, S.; Haensch, K.-T.; Porfirova, S.; Hofmann, J.; Rolletschek, H.; Melzer, M.; Franken, P.; Hause, B.; Druege, U.; et al. Molecular physiology of adventitious root formation in Petunia hybrida cuttings: Involvement of wound response and primary metabolism. New Phytol. 2009, 181, 613–625. [Google Scholar] [CrossRef]
- Ahkami, A.H.; Scholz, U.; Steuernagel, B.; Strickert, M.; Haensch, K.-T.; Druege, U.; Reinhardt, D.; Nouri, E.; von Wirén, N.; Franken, P.; et al. Comprehensive transcriptome analysis unravels the existence of crucial genes regulating primary metabolism during adventitious root formation in Petunia hybrida. PLoS ONE 2014, 9, e100997. [Google Scholar] [CrossRef]
- Obata-Sasamoto, H.; Thorpe, T.A. Cell wall invertase activity in cultured tobacco tissues. Plant Cell Tissue Organ Cult. 1982, 2, 3–9. [Google Scholar] [CrossRef]
- Sturm, A. Invertases. Primary structures, functions, and roles in plant development and sucrose partitioning. Plant Physiol. 1999, 121, 1–8. [Google Scholar] [CrossRef] [PubMed]



| Treatment | pH | g L−1 | ||||
|---|---|---|---|---|---|---|
| Sucrose | Fructose | Glucose | Sorbitol | |||
| Experiment 1 | Treat 1 | 5.8 | 2.4 | 2.4 | 5.2 | 20 |
| Treat 2 | 7.5 | 7.5 | 7.5 | 7.5 | ||
| Treat 3 | 30 | |||||
| Treat 4 | 30 | |||||
| Treat 5 | 30 | |||||
| Treat 6 | 30 | |||||
| Experiment 2 | Treat 1 | 4.5 | 2.4 | 2.4 | 5.2 | 20 |
| Treat 2 | 5.0 | |||||
| Treat 3 | 5.2 | |||||
| Treat 4 | 5.5 | |||||
| Treat 5 | 5.8 | |||||
| Treat 6 | 6.0 | |||||
| Treat 7 | 6.2 | |||||
| Treat 8 | 6.5 | |||||
| Treatment | pH | Sugar Type | Sugar Concentration (% w/v) | Agar (g L−1) | |
|---|---|---|---|---|---|
| Experiment 3 | Treat 1 | 5.8 | Sucrose | 1 | 9 |
| Treat 2 | 2 | ||||
| Treat 3 | 3 | ||||
| Treat 4 | 5.8 | Fructose | 1 | 9 | |
| Treat 5 | 2 | ||||
| Treat 6 | 3 | ||||
| Treat 7 | 5.8 | Glucose | 1 | 9 | |
| Treat 8 | 2 | ||||
| Treat 9 | 3 | ||||
| Treat 10 | 5.8 | Sorbitol | 1 | 9 | |
| Treat 11 | 2 | ||||
| Treat 12 | 3 | ||||
| Experiment 4 | Treat 1 | 4.8 | Sucrose | 2 | 9 |
| Treat 2 | 5.2 | ||||
| Treat 3 | 5.5 | ||||
| Treat 4 | 5.8 | ||||
| Treat 5 | 6.2 | ||||
| Treat 6 | 6.5 | ||||
| Treat 7 | 4.8 | Fructose | 2 | 9 | |
| Treat 8 | 5.2 | ||||
| Treat 9 | 5.5 | ||||
| Treat 10 | 5.8 | ||||
| Treat 11 | 6.2 | ||||
| Treat 12 | 6.5 |
| Sugar Type | Shoots per Explant | Shoot Length (cm) | Nodes per Explant | Nodes per Shoot | Nodes per cm |
|---|---|---|---|---|---|
| Sucrose | 1.79 c | 1.15 b | 5.3 c | 2.3 a | 2.15 a |
| Glucose | 3.07 b | 1 b | 9.91 ab | 1.99 a | 2.07 a |
| Fructose | 4.08 a | 1.67 a | 13.68 a | 1.99 a | 1.22 a |
| Sorbitol | 2.38 bc | 1.15 b | 8.06 bc | 1.96 a | 1.77 a |
| Mixture 1 * | 2.34 bc | 1.29 ab | 7.9 bc | 2.4 a | 1.9 a |
| Mixture 2 ** | 1.65 c | 1.11 b | 5.18 c | 2.19 a | 1.73 a |
| r | p Value | ||
|---|---|---|---|
| Post-autoclaving pH | Prior autoclaving pH | 0.96 | *** |
| Shoot number | Prior autoclaving pH | −0.67 | *** |
| Shoot length | Prior autoclaving pH | −0.42 | ** |
| Nodes per shoot | Prior autoclaving pH | −0.41 | ** |
| Nodes per explant | Prior autoclaving pH | −0.68 | *** |
| Post-autoclaving pH | Shoot number | −0.65 | *** |
| Post-autoclaving pH | Shoot length | −0.46 | ** |
| Post-autoclaving pH | Nodes per shoot | −0.46 | ** |
| Post-autoclaving pH | Node per explant | −0.68 | *** |
| Difference post A/C- post culture pH | Shoot number | 0.59 | *** |
| Difference post A/C- post culture pH | Shoot length | 0.44 | *** |
| Difference post A/C- post culture pH | Nodes per shoot | 0.49 | *** |
| Difference post A/C- post culture pH | Nodes per explant | 0.57 | *** |
| Difference post A/C- post culture pH | Nodes per cm | −0.08 | ns |
| Rooted Explants (%) | Number of Roots | Root Length (cm) | |
|---|---|---|---|
| Sugar | |||
| Fructose (Fruc) | 88.3 a | 7.1 a | 0.48 a |
| Glucose (Gluc) | 74.3 ab | 5.4 b | 0.28 b |
| Sorbitol (Sor) | 70.8 b | 5.2 b | 0.24 b |
| Sucrose (Suc) | 54.9 c | 4.5 b | 0.18 b |
| Concentration (% w/v) | |||
| 1 | 58.2 b | 5.14 a | 0.25 b |
| 2 | 77.6 a | 6.07 a | 0.24 b |
| 3 | 80.5 a | 5.4 a | 0.40 a |
| Sugar × Concentration | |||
| Fruc × 1 | 75 abc | 6.54 ab | 0.32 bc |
| Fruc × 2 | 95 a | 7.97 a | 0.38 b |
| Fruc × 3 | 95 a | 6.85 ab | 0.73 a |
| Gluc × 1 | 69 abc | 4.3 b | 0.28 bc |
| Gluc × 2 | 71 abc | 6 ab | 0.2 bc |
| Gluc × 3 | 83.3 ab | 5.3 ab | 0.37 bc |
| Sor × 1 | 50 bc | 3.75 b | 0.23 bc |
| Sor × 2 | 81.2 abc | 5.05 ab | 0.2 bc |
| Sor × 3 | 81.2 abc | 4.58 b | 0.3 bc |
| Suc × 1 | 38.9 c | 6 ab | 0.17 bc |
| Suc × 2 | 63.2 bc | 5.28 ab | 0.16 c |
| Suc × 3 | 62.5 bc | 4.88 ab | 0.23 bc |
| Rooted Explants (%) | Number of Roots | Root Length (cm) | pH Post | |
|---|---|---|---|---|
| Sugar | ||||
| Sucrose (Suc) | 35.6 b | 4.2 b | 0.25 b | 5.24 a |
| Fructose (Fruc) | 86.0 a | 5.8 a | 0.41 a | 4.73 b |
| pH pre | ||||
| 4.8 | 66.2 a | 6.6 a | 0.35 ab | 4.78 e |
| 5.2 | 69.8 a | 5.8 ab | 0.49 ab | 4.71 f |
| 5.5 | 67.5 a | 4.9 ab | 0.28 ab | 4.90 d |
| 5.8 | 57.5 a | 5.2 ab | 0.47 a | 5.00 c |
| 6.2 | 55.0 a | 3.4 b | 0.22 b | 5.21 b |
| 6.5 | 49.1 a | 4.0 ab | 0.29 ab | 5.32 a |
| Sugar × pH pre | ||||
| Fruc × 4.8 | 95.0 a | 7.3 a | 0.36 a | 4.62 i |
| Fruc × 5.2 | 83.3 ab | 5.5 ab | 0.57 a | 4.62 i |
| Fruc × 5.5 | 85.0 ab | 5.3 ab | 0.57 a | 4.63 i |
| Fruc × 5.8 | 90.0 ab | 7.0 a | 0.54 a | 4.70 h |
| Fruc × 6.2 | 75.0 abc | 4.0 ab | 0.24 a | 4.9 f |
| Fruc × 6.5 | 88.2 ab | 5.6 ab | 0.44 a | 4.92 ef |
| Suc × 4.8 | 37.5 cde | 5.9 ab | 0.32 a | 4.94 e |
| Suc × 5.2 | 56.3 bcd | 6.2 ab | 0.22 a | 4.79 g |
| Suc × 5.5 | 50.0 b–e | 4.5 ab | 0.23 a | 5.16 d |
| Suc × 5.8 | 25.0 de | 3.5 ab | 0.40 a | 5.31 c |
| Suc × 6.2 | 35.0 de | 2.7 b | 0.20 a | 5.51 b |
| Suc × 6.5 | 10.0 e | 2.5 b | 0.15 a | 5.71 a |
| r | p Value | ||
|---|---|---|---|
| Post-autoclaving pH | Prior autoclaving pH | 0.59 | *** |
| Rooted explants % | Prior autoclaving pH | −0.23 | ns |
| Rooted explants % | Post-autoclaving pH | −0.79 | *** |
| Rooted explants % | Root number | 0.52 | *** |
| Rooted explants % | Root length | 0.31 | * |
| Root length | Prior autoclaving pH | −0.08 | ns |
| Root length | Post-autoclaving pH | −0.34 | * |
| Root length | Root number | 0.14 | ns |
| Root number | Prior autoclaving pH | −0.42 | *** |
| Root number | Post-autoclaving pH | −0.59 | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsafouros, A.; Roussos, P.A. In Vitro Propagation of Commercially Used Krymsk 5® (Prunus fruticosa × Prunus lannesiana) Cherry Rootstock: Impact of Sugar Types and pH Levels. Agriculture 2024, 14, 120. https://doi.org/10.3390/agriculture14010120
Tsafouros A, Roussos PA. In Vitro Propagation of Commercially Used Krymsk 5® (Prunus fruticosa × Prunus lannesiana) Cherry Rootstock: Impact of Sugar Types and pH Levels. Agriculture. 2024; 14(1):120. https://doi.org/10.3390/agriculture14010120
Chicago/Turabian StyleTsafouros, Athanasios, and Petros A. Roussos. 2024. "In Vitro Propagation of Commercially Used Krymsk 5® (Prunus fruticosa × Prunus lannesiana) Cherry Rootstock: Impact of Sugar Types and pH Levels" Agriculture 14, no. 1: 120. https://doi.org/10.3390/agriculture14010120
APA StyleTsafouros, A., & Roussos, P. A. (2024). In Vitro Propagation of Commercially Used Krymsk 5® (Prunus fruticosa × Prunus lannesiana) Cherry Rootstock: Impact of Sugar Types and pH Levels. Agriculture, 14(1), 120. https://doi.org/10.3390/agriculture14010120






