1. Introduction
Tomato (
Solanum lycopersicum L.) stands as a pivotal and economically advantageous commercial crop, predominantly owing to its substantial nutritional value. Global annual tomato production is estimated to reach 252 million tons. In 2020, China asserted its dominance as the leading producer, yielding 64.8 million tons, followed by India in second place with 20.5 million tons, and Türkiye securing the third position with 13.2 million tons [
1].
However, plant pathogens, particularly plant pathogenic viruses, cause significant losses in both yield and quality within tomato production. The tobacco mosaic virus (TMV) has been a focal point in plant virology research since its discovery, alongside the tomato mosaic virus (ToMV), which belongs to the tobamovirus. Thanks to the identification and utilization of resistance genes such as
Tm1,
Tm2, and
Tm22 in cultivation, these pathogens have been effectively managed [
2,
3]. However, in 2014, a newly emerged Tobamovirus, Tomato brown rugose fruit virus (ToBRFV), was reported, and it has been shown that this virus can overcome the previously identified resistance genes in tomatoes [
4,
5,
6,
7].
ToBRFV has emerged as a widespread viral disease in both tomato greenhouses and fields, achieving global significance in a short time after its emergence [
8]. Research indicates that ToBRFV is seed-borne and mechanically transmissible, like many Tobamoviruses, in addition to their high stability [
9]. Therefore, the sanitation of the virus from seeds and the prevention of contamination are integral aspects of controlling ToBRFV.
Prior research has highlighted the efficacy of various control methods, including the application of different formulations like 3% Virkon (LANXESS, Cologne, Germany) in managing the spread of ToBRFV [
10]. While prior investigations have delved into the effects of seed treatment in mitigating the spread of ToBRFV [
11], we have aimed to extend approaches to manage ToBRFV by comparing different formulations and combining heat treatment, UV light, and chemical disinfectants in this study. Furthermore, the duration of the virus activity on materials commonly used during production has been systematically monitored and determined, alongside the exploration of novel application methods for the management of ToBRFV.
2. Materials and Methods
2.1. Plant Materials
The HK-1314 tomato genotype was employed to assess the efficiencies of various applications, while Nicotiana benthamiana served as the indicator plant for monitoring ToBRFV symptoms, aiding in the determination of the virus activity duration. HK-1314 plants were cultivated with inter-row distances of 40 cm and interplant distances of 70 cm in greenhouses at Akdeniz University, Faculty of Agriculture, Türkiye. Seeds obtained from infected tomato fruit were stored in plastic bags and are currently preserved in a refrigerator at 4 °C.
2.2. Formulations
Tsunami 100 (30–60% Acetic acid, 15.2% Peroxyacetic acid, and 11.2% Hydrogen peroxide) from Ecolab (Saint Paul, MA, USA), BioconA (50% potassium peroxymonosulfate with buffer and organic acid) from KMK Laboratories (Istanbul, Türkiye), Desyclean (Hydrogen peroxide, sorbic acid, peracetic acid, and sodium benzoate) from Sojall (Izmır, Türkiye), Bioxi (Ozone O3) from Biotem (Ankara, Türkiye), Hydrochloric Acid (18% HCl) (CAS:7647-01-0) from Sigma (Burlington, MN, USA), and Incidine (10.0 g of 2-phenoxiethanol, 8.0 g of n-3-Aminopropile, n-Dodecylpropane,1.3 diamine, and 7.5 g of benzalkonium chloride) from Ecolab (Saint Paul, MA, USA) were used in the sanitation assay against ToBRFV. Their concentrations were adjusted according to manufacturers with slightly lower and higher concentrations; 0.5%, 0.75%, and 1.0% for Tsunami 100; 0.5%, 1.0%, and 1.5% for BioconA; 2.0%, 2.5%, and 3.0% for Bioxi; 1.0%, 1.5%, and 2.0% for Incidine; 0.1%, 0.5%, and 1.0% for HCl.
2.3. Heat and UV Treatment
A portable UV sterilization device manufactured by Kechaoda (Shenzhen, China) was used for the UV treatment of the seeds.
2.4. ToBRFV Inoculation and Scoring of Symptoms
ToBRFV (NCBI GenBank Number: MT107885)-infected tomato leaves were freshly collected and ground after the addition of 0.02 M phosphate buffer (pH: 7.0) + 0.1% 2-mercaptoethanol (Burlington, MN, USA) at a 1:10 ratio onto the leaves. Inoculation of ToBRFV was performed onto HK-1314 leaves at the true two-leaf stage, following previously described methods [
12]. The inoculation process was replicated three times for each group. As a positive control, a phosphate buffer solution containing ToBRFV-infected leaves was utilized without any treatment, whereas mock-inoculated plants served as the negative control. Plants were systematically monitored and assessed using a 0–3 scale: 0 denoting asymptomatic, 1 indicating mild mosaic and chlorosis on leaves, 2 signifying severe mosaic and blistering on leaf surfaces, and 3 representing severe symptoms.
2.5. Detection of ToBRFV Using Double Antibody Sandwich Enzyme-Linked Immunosorbent Assay (DAS-ELISA)
For the detection of ToBRFV, a commercial LOEWE reagent set (Cat No. 07175) was utilized, incorporating a positive control (No: 02029001) in adherence to the kit protocol. Results were acquired through ERBA LisaScan-EM (Mannheim, Germany).
2.6. RNA Extraction and RT-PCR Test Analysis
For the detection of ToBRFV using RT-PCR, total nucleic acid was isolated following the Dellaporta method [
13]. The reaction mix was prepared using the Verso 1-step RT-PCR kit (Thermo, Waltham, MA, USA) according to the kit protocol, incorporating ToBRFV1-F (5′CTTCCAAACGTGTACGCACC 3′) and ToBRFV1-R (5′ATGCATCTTCCATTGCGCTG 3′). RT-PCR was conducted with an initial cycle of cDNA synthesis at 50 °C for 15 min, followed by inactivation at 95 °C for 2 min, denaturation at 95 °C for 20 s, annealing at 59 °C for 30 s, extension at 72 °C for 45 s (repeated for 35 cycles), and a final extension at 72 °C for 15 min [
14].
2.7. Greenhouse Planting and Disinfection Procedures
A 500 m
2 polyethylene-covered greenhouse was prepared for cultivation, and three hundred and six (306) tomato seedlings were planted on 30 October 2021. Standard cultural practices were applied, and a comprehensive plant nutrition and fertilization program ensured optimal levels of both vegetative and generative development throughout plant growth. The planting scheme in the field and the sequence of disinfectants applied are outlined in this study. Each row in the greenhouse accommodated 51 plants, totaling 306 seedlings across six rows. Disinfectants were sprayed with indicated concentrations, consistent with the randomized complete block design (RCBD). One plant in each row served as the inoculum source and was inoculated with ToBRFV. Consequently, there were 17 different treatments (12 disinfectants + 1 water treatment + 1 ToBRFV positive control) in the experiment. Six rows were designated in the greenhouse, with 3 plants for each treatment in each row. To ensure pollination for homogeneous fruit formation,
Bombus terrestris, known for carrying the primary ToBRFV inoculum, was introduced, contributing to the disease’s spread in tomatoes [
15]. The initial disinfectant application, coupled with mechanical contamination, was performed in the greenhouse and repeated at 15-day intervals for the second and third applications, resulting in a total of four applications.
2.8. Determination of ToBRFV Transmission
Approximately 1000 seeds obtained from 50 ToBRFV-infected tomato fruits were individually assessed. The seeds were immersed in a 10% Trisodium Phosphate (TSP) solution for 3 h and subsequently washed with sterile distilled water for 5 min. Subsequently, the surface-sterilized seeds were transferred to 1.5 mL centrifuge tubes and individually tested with ELISA.
2.9. Detection of the ToBRFV-Infection Site on Seed and Transmission Mechanisms
For the detection of ToBRFV transmission mechanisms, different parts of the seeds were evaluated separately: embryo, endosperm, and seed coat. A total of 2000 seeds were harvested from ToBRFV-infected tomato plants exhibiting symptomatic fruits. Subsequently, 1000 seeds underwent surface sterilization treatment to impede virus transmission to the embryo during the separation of the seed coat and the embryo. This involved immersing the seeds in a 10% Trisodium Phosphate (TSP) solution for 3 h and subsequently washing them with sterile distilled water for 5 min. Due to the challenge of extracting embryos from matured fruits (32 days or more) caused by endosperm hardening, which may result in embryo damage during separation, it has been reported that the optimal period for embryo rescue is between 28–32 days after pollination [
16]. To ascertain transmission rates in the embryo and endosperm, 1000 embryos and 1000 endosperms were isolated and individually subjected to RT-PCR testing. Additionally, seed coats were removed from 100 seeds that were not surface sterilized, followed by RT-PCR testing.
2.10. Heat Treatment of ToBRFV Infected Seeds
For each seed group obtained from ToBRFV-infected tomato fruits, three sets of 1000 seeds underwent different durations of heat treatment. The seed batches were categorized into three groups based on the duration (24 h, 48 h, and 72 h) of the heat treatment. The initial group of 1000 seeds experienced a gradual temperature increase from 20 °C to 72 °C for 24 h in a modified thermal machine (Delta, Antalya, Türkiye). The second group was kept at 72 °C for an additional 24 h, while the third group underwent an additional 48 h of heat treatment at 72 °C, in addition to the previous treatment received by the first group. A control set comprising 400 healthy tomato seeds was employed during this study. Post-treatment, seeds from each set underwent testing for the presence or absence of ToBRFV using RT-PCR.
2.11. UV Treatment
The efficacy of UV light treatment in eradicating ToBRFV spread through seeds was evaluated. UV-C radiation with a wavelength of 254 nm was applied to ToBRFV-infected seeds for a duration of 30 min. A total of 1000 seed pieces were subjected to UV light treatment and organized into four groups, each comprising 250 seeds. To determine the presence of the virus in the seeds, the DAS-ELISA (Double Antibody Sandwich Enzyme-Linked Immunosorbent Assay) test was conducted, following the established protocols [
17,
18]. Seed samples were prepared in accordance with the International Seed Testing Association (ISTA) method, allowing simultaneous testing of multiple samples. Two repetitions of 500 seeds were taken and tested using the DAS-ELISA method [
19,
20,
21]. This testing procedure was repeated three times, and the average of the three repetitions was reported in the results.
2.12. Disinfectant Treatment of Seeds
Approximately 1000 seeds were used as the initial material for each treatment, and six distinct disinfectants were individually applied to the seeds after dilution with water at specified concentrations of formulations. Each set of 1000 seeds treated with a disinfectant was further subdivided into four groups (three different concentrations and control), each containing 250 seeds.
2.13. Combination of Treatment
Various combinations of seed treatments were administered to groups, each comprising 300 seeds. In the first set, heat treatment was initially applied to 300 infected seeds, followed by a combination of heat treatment and disinfectant treatment. In another set of seeds, a combination of heat treatment and UV treatment was applied. Furthermore, all three methods (heat treatment + disinfectant + UV) were simultaneously employed. This approach allowed for the assessment of the effectiveness of the applied methods in eradicating ToBRFV.
2.14. Determination of ToBRFV Survival on Farm Tools and Equipment
The active survival time of ToBRFV is contingent upon the farm tools and equipment utilized in greenhouse production, including gloves, pruning shears, harvesting crates, staking ropes, plant pots, and clothing employed during spraying and farm labor. These materials, integral to daily cultural practices, play a pivotal role in the direct transmission of the disease from one plant to another. Various materials, including tools and equipment, metal pieces, cotton fabric, glass, string, and plastic, were selected for assessment as well. These chosen materials were immersed and withdrawn from a phosphate-buffered solution containing ToBRFV. Subsequently, swab samples were collected from each material at 1, 8, 24, 48, and 72-h intervals. These swab samples were then applied to separate, numbered tomato and tobacco plants for inoculation.
2.15. Statistical Analysis and Data Visualization
An analysis of variance (ANOVA) was employed to identify variations between the treatments and their concentrations. The Least Significant Difference (LSD) test was subsequently applied to elucidate differences among the disinfectants following the variance analysis. Additionally, the experimental data were analyzed using Python (v3.9) with the assistance of the “pandas” and “NumPy” libraries, while data visualization was conducted using the “matplotlib” library.
3. Results
3.1. ToBRFV Is Localized on Seed-Coat and Endosperm, Not in Embryo
Upon scrutinizing the infection rates of ToBRFV in the endosperm, the first trial revealed a 0.7% infection rate, followed by 0.8% in the second trial, 0.9% in the third, and 0.8% in the fourth trial. Averaging the results from the four trials, it was established that ToBRFV was detected in 8 out of 1000 seeds, constituting 0.8% of the endosperm.
Following three days of dry air application, only 3 out of 1000 seeds were identified as ToBRFV positive. To assess the pathogenicity of the virus, 1000 seeds were sown and monitored in the application greenhouses. Molecular tests were conducted on the 7th, 14th, 21st, 28th, and 45th days. The experiment’s results revealed that the virus lost its infection capability post-heat treatment, rendering it non-infectious. Although a 0.3% contamination rate was detected, heat treatment led to the inactivation of the virus. Moreover, the impact of heat treatment on seed germination rate was evaluated using 400 randomly selected seeds. The germination rate was determined to be 95.8%, with 384 of the seeds successfully germinating and only 16 seeds failing to do so.
3.2. UV Treatment Is Not Sufficient for Inactivation of ToBRFV
ToBRFV-contaminated seeds underwent UV radiation treatment at a wavelength of 254 nm for 30 min. Subsequent analysis revealed that 4 out of 1000 treated seeds remained infected with ToBRFV. The rate of virus transmission in seeds was reduced to 0.4% after UV treatment. Post UV-C radiation at 254 nm for 30 min, the treated seeds were planted in the greenhouse, and disease observations were conducted. Leaf samples were collected from the greenhouse plants after 45 days and subjected to testing using the DAS-ELISA method. Contrary to the efficacy observed with dry air blowing, serological tests confirmed that the disease agent retained its infectivity post UV treatment.
3.3. The Efficiency of Disinfectants Varies Depending on the Dose against ToBRFV
Upon assessing the plants germinated from seeds treated with disinfectants, a notable reduction in ToBRFV symptoms on the leaves was observed. While most applications exhibited effectiveness at varying doses, Desyclean, even at its highest dose (3%), proved less successful in preventing ToBRFV infection and reducing symptoms com-pared to other disinfectants. Increasing the dose of Tsunami, Bioxi, Biocon, Incidin, and HCl correlated with fewer symptoms and complete prevention of ToBRFV, as evidenced by scoring the inoculated plants. In the Desyclean treatment group, although a dose-dependent reduction in ToBRFV symptoms was observed, no symptomless plants were detected (
Figure 1). However, in the second dose applications were most effective, further emphasizing the influence of dosage on the efficacy of disinfectants in preventing and mitigating ToBRFV infection.
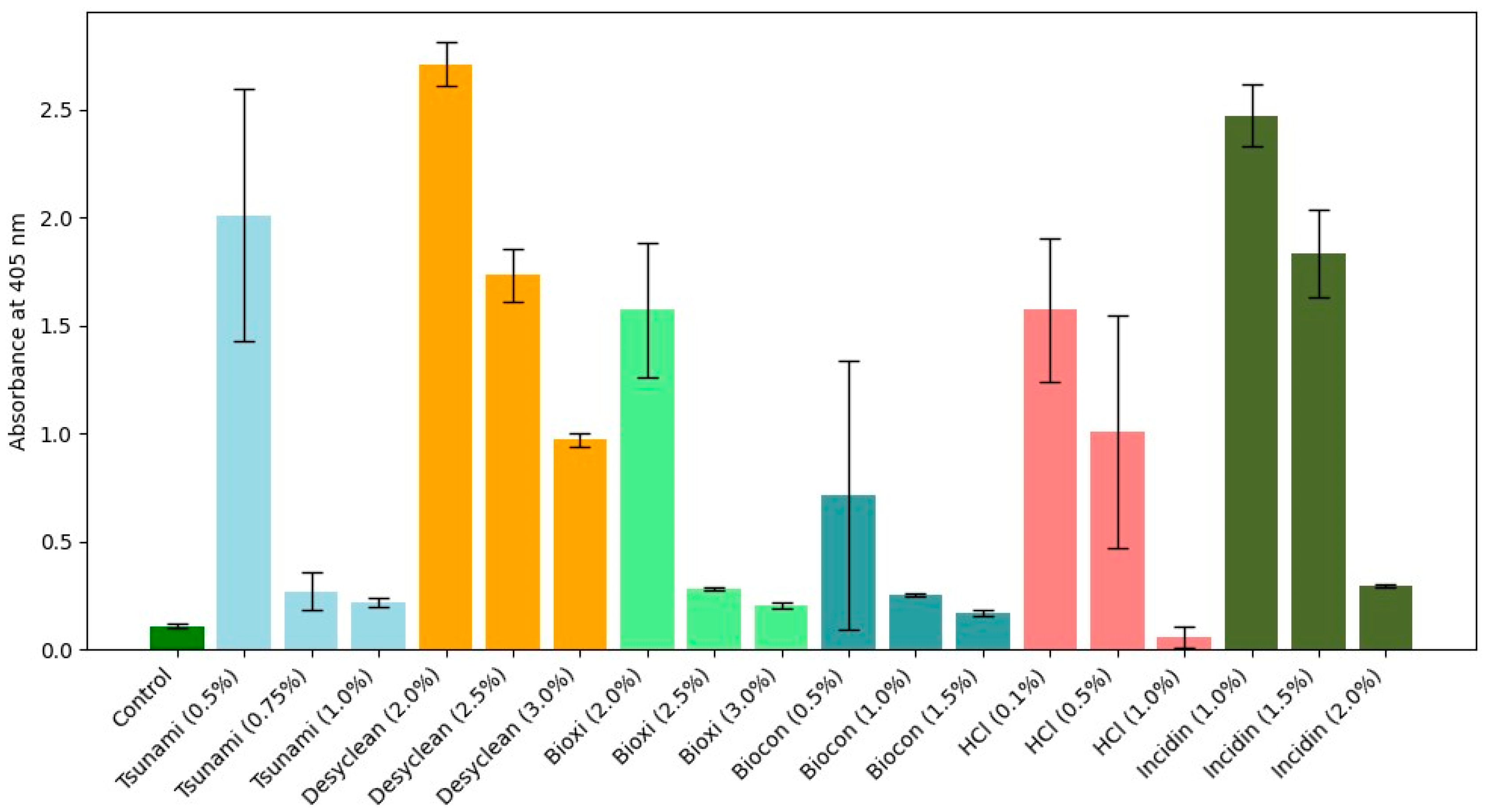
Notably, the highest concentration of HCl (1.0%) resulted in deformed seeds with an 80% germination rate. The elevated acidity of HCl is posited to have a potentially detrimental impact on the seed coat post-application. Conversely, seeds treated with Tsunami 100 (1%) and Biocon (1%) exhibited germination rates between 96% and 98%. A subset of symptomless plants (randomly selected from 28 plants) underwent RT-PCR testing for the presence of ToBRFV. Surprisingly, 12 of these plants tested positive despite the absence of typical symptoms. Subsequently, DAS-ELISA was performed for each treatment to assess ToBRFV load on leaves. Plants with absorbance values ranging from 0.3956 to 1.7109 exhibited mild to moderate mosaic symptoms, while those with absorbance values between 1.3697 and 2.7989 displayed severe mosaic and leaf deformation symptoms (
Figure 2). The reduction in viral load directly correlated with disinfectant treatment on the seeds, aligning with the evaluation scale for symptoms. Tsunami (0.75% and 1%), Bioxi (2.5% and 3%), Biocon (1.5%), HCl (1%), and Incidin (2%) demonstrated high efficacy in reducing ToBRFV load on the leaves.
Figure 2.
Assessment of absorbance values through DAS-ELISA for the detection of ToBRFV in inoculated plants treated with various formulations. A positive absorbance value at 405 nm was recorded as 2.7989. Same colors indicate the same disinfectant and their formulations with different concentrations. Statistical analyses revealed no significant differences between Incidin, HCl, Tsunami, and Bioxi, ordered from the lowest to the highest effect value, respectively. Biocon A exhibited the highest efficacy (
Table 1 and
Table 2).
Figure 2.
Assessment of absorbance values through DAS-ELISA for the detection of ToBRFV in inoculated plants treated with various formulations. A positive absorbance value at 405 nm was recorded as 2.7989. Same colors indicate the same disinfectant and their formulations with different concentrations. Statistical analyses revealed no significant differences between Incidin, HCl, Tsunami, and Bioxi, ordered from the lowest to the highest effect value, respectively. Biocon A exhibited the highest efficacy (
Table 1 and
Table 2).
3.4. Integrated Seed Treatment Methods for ToBRFV Eradication
Sequential treatments were applied to ToBRFV-infected seeds, beginning with heat treatment, followed by a combination of heat treatment + disinfectants, then heat treatment and UV applications, and finally a comprehensive approach involving heat treatment, disinfectant application, and UV light exposure. Among these treatments, the most successful method in eradicating ToBRFV from seeds was the combination of heat treatment and disinfectant. In this study, seeds soaked in a 10% Trisodium Phos-phate (TSP) solution for 3 h, followed by heat treatment at 72 °C for 72 h, exhibited the lowest viral load compared to seeds subjected to different treatments.
Symptomatic observations under greenhouse and field conditions were solely based on symptom presence or absence, neglecting factors like plant density and environmental conditions. Graphs illustrating symptomatic observations were generated for 102 plants across seven different treatments, including disinfectant and control treatments (
Table 3 and
Table 4). DAS-ELISA results indicated that, post the last disinfectant treatment, 15 greenhouse samples were considered negative, displaying absorbance values below three times that of the negative control. Among these samples, ToBRFV was detected in 87 instances. The highest absorbance reading at a wavelength of 405 nm was 3.2022 in positive control plants directly infected with ToBRFV. In disinfectant-treated plants, the highest positive value was 2.1893 in HCl first dose-treated plants, while the lowest positive value was 0.2991 in Biocon A highest dose-treated plants. RT-PCR tests on 15 samples negative in DAS-ELISA tests showed no statistical difference between the replicates in the experiment (
Table 3). Disinfectant differences were evaluated using the LSD test (
Table 4). The positive control had the highest value. In analyzing DAS-ELISA absorbance readings of disinfectant-treated plants, HCl, Bioxi, and Desyclean exhibited a similar level of effectiveness, while Tsunami and Biocon A were in the group with the lowest contamination values. Regarding application doses, there seemed to be no statistical difference between lower and upper doses of the target dose. However, the highest doses of disinfectants effectively reduced the absorbance value in the DAS-ELISA test. It was observed that higher doses of HCl and Tsunami disinfectants had a negative impact on plant leaves.
3.5. Determination of the Stability of ToBRFV on Materials Frequently Used during Production
Swabbing tobacco and tomato plants at predetermined intervals on six different surfaces (cotton fabric, metal pieces, latex gloves, glass, tying ropes, and plastic) initiated symptom formation, observed two weeks post-inoculation. Symptomatic observation results revealed that 26 out of 72 plants did not exhibit any symptoms. RT-PCR tests on symptom-free plants confirmed their freedom from ToBRFV.
4. Discussion
The comprehensive investigation into the integrated management of ToBRFV unfolds a multifaceted approach encompassing seed treatments, disinfection methods, and field conditions. The elucidation of localized viral presence in seeds, coupled with the nuanced impacts of diverse treatments, navigates the path toward a more resilient and effective strategy for management of ToBRFV throughout the agricultural production cycle.
The results indicated that ToBRFV predominantly localizes in the seedcoat and endosperm of tomato seed, without presence in the embryo. This finding aligns with previous reports on the virus’s localization in the seed coat through various analytical methods [
22]. The observed transmission rate is consistent with similar studies on pepper seeds [
23]. The absence of ToBRFV in the embryo signifies the potential for seed treatments to target specific seed compartments, enhancing control measures without affecting seed germination.
The results obtained from seed treatments to eradicate ToBRFV were compared using UV, disinfectant, heat treatment, and their combinations. The experiment revealed that the virus lost its infection capability post-heat treatment, rendering it non-infectious. However, it was determined that UV applications had no discernible effect on the pathogenicity of ToBRFV. Therefore, the use of heat treatment can be considered an effective method to reduce ToBRFV-induced damage in tomato plants, as opposed to UV treatment. In addition to the previously reported efficiency of heat treatment (70 °C for 90 min) in reducing the occurrence of
Cladosporium cucumerinum,
Ascochyta citrullina, and
Colletotrichum orbiculare on cucumber seeds [
24] the methods pro-vided in our study indicate the efficiency of heat treatment in reducing ToBRFV infection without affecting seed germination rates. Furthermore, heat treatment can be optimized for other commonly cultivated plants such as pepper, cucumber, zucchini, or eggplant by gradually increasing the temperature while monitoring pathogen activity. Although UV-C was found successful in reducing the contamination rate of viruses carried on the seed coat, the study underscores the need for further exploration, especially on larger scales and with expanded UV applications, potentially involving large machines and greater volumes of seeds.
In the disinfectant treatment for seeds, an increase in the dose of Tsunami, Bioxi, Bio-con, Incidin, and HCl correlated with fewer symptoms and complete prevention of ToBRFV. However, Desyclean, even at its highest dose (3%), proved less successful in preventing ToBRFV infection and reducing symptoms compared to other disinfectants. Similar to our study, Virkon, tested at doses of 2% and 3% against CGMMV and To-BRFV, respectively, showed efficacy [
25,
26,
27]. Although disinfectants exhibit efficiency against ToBRFV in a dose-dependent manner, side effects on germination rate have been observed. Therefore, disinfectant concentration and dose must be carefully adjusted, maintaining the minimum level for reducing pathogen activity while maximizing germination rate. These concentrations and their efficiency on seed germination can vary for different plant seeds, necessitating a thorough examination of their efficacy.
The direct application of disinfectant trials under controlled conditions (greenhouse) was attributed to the chemical disruption of the virus’s coat protein structure and its impact on virulence within the disease triangle, isolated from environmental factors. It must be considered that we found Tsunami, Biocon, and Bioxi to be efficient disinfect-ants with their recommended concentrations under controlled conditions. However, it was noted that disinfectants were not as effective under field conditions. Direct applications cannot completely eradicate ToBRFV from the field, although they delayed symptom onset and spreading time in the field.
Research on the active survival time of viruses on surfaces has been conducted for both plant and animal viruses. Notably, studies on COVID-19 indicated survival times of up to 72 h in plastic and stainless steel, 4 h on copper, and 24 h on cardboard [
28]. Previous studies on Pepino mosaic virus (PepMV) reported that it retains pathogenicity on glass surfaces for 3 weeks at 15 °C and 72 h at 25 °C [
29]. In the present study, ToBRFV maintained its infection ability for 48 h under green-house conditions, as evidenced by swabs taken from glass surfaces. It underscores that the infection ability of different plant viruses varies on various surfaces, with ambient temperature being a significant factor in maintaining this ability.
In summary, the results highlight the efficacy of heat-treatment, involving gradual heating from 20 °C to 72° over 3 days, in effectively neutralizing ToBRFV without compromising seed germination rates. This method serves as a crucial initial step in preventing ToBRFV infections resulting from seed transmission. Additionally, the combination of disinfectant treatment with heat-treatment provides a safer approach for eradicating ToBRFV from seed stocks, although careful consideration is required due to potential disinfectant-induced reductions in germination rates. While direct disinfectant application proved effective in reducing ToBRFV transmission and infection rates, its efficiency was observed primarily under controlled conditions rather than in field settings. Field applications delayed the spread of ToBRFV among tomato plants but did not achieve complete eradication. Furthermore, commonly used equipment such as pots, plant containers, soil containers, garden scoops, shovels, and tray nurseries were identified as potential sources of ToBRFV spread within greenhouses or fields. Metal equipment retained the virus’s activity for more than 72 h, emphasizing the need for equipment replacement, sterilization, or disposal to prevent ToBRFV transmission. These precautions and treatments offer a viable strategy to minimize yield losses in tomato production attributed to ToBRFV. Moreover, the optimization of such treatments for other frequently cultivated plants against various pathogens holds promise for enhancing overall crop health and productivity.
5. Conclusions
In conclusion, this study addressed the pressing issue of Tomato brown rugose fruit virus (ToBRFV) in tomato production, a pathogen causing substantial economic losses worldwide. The tomato, being a significant commercial crop, faces constant threats from viral infections, notably ToBRFV. The prevalence of this virus, its stability, and the emergence of new strains necessitate a comprehensive approach to disease management. The subsequent sections explored various methods to manage ToBRFV, including chemical disinfectants, UV light treatment, and heat treatment. While these methods showed promise in reducing the spread of the virus, their efficacy varied. Notably, the study found that heat treatment combined with disinfectants exhibited the highest success rate in eradicating ToBRFV from seeds. The field experiments demonstrated the variability in the efficacy of disinfectants under greenhouse and field conditions, emphasizing the need for tailored approaches. Moreover, the research delved into the stability of ToBRFV on commonly used materials during production, revealing the potential risk of disease transmission through agricultural tools and equipment. The results underscored the importance of implementing effective sanitation measures to curtail the spread of the virus. In summary, this research contributes valuable insights into the management of ToBRFV in tomato production. The multifaceted approach, incorporating heat treatment, disinfectants, and sanitation measures, presents a promising strategy for mitigating the economic impact of ToBRFV. The findings underscore the importance of continuous research to stay ahead of evolving viral threats in agriculture.