Zinc Absorption through Leaves and Subsequent Translocation to the Grains of Bread Wheat after Foliar Spray
Abstract
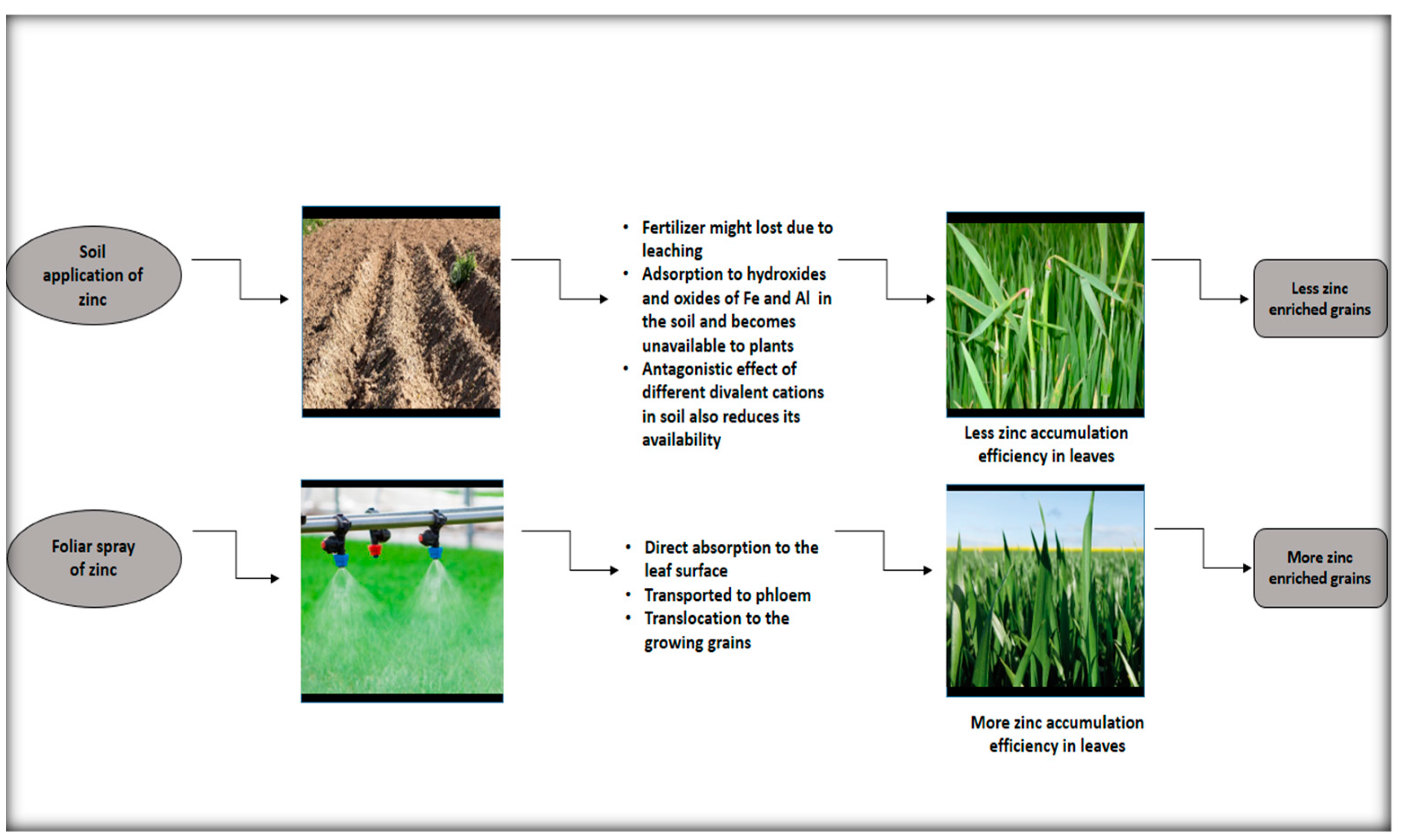
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Layout
2.2. Zinc Treatments and Morphological Data
2.3. Chemical Analysis
2.3.1. Leaf and Grain Analysis
2.3.2. Protocol for Zinc Analysis
2.3.3. Protocol for Grain Protein Analysis
2.4. Statistical Analysis
3. Results
3.1. Amount of Foliar Zn Applied and Absorbed in Leaves
3.2. Amount of Zn Translocated into Grains and Cultivars Efficiency
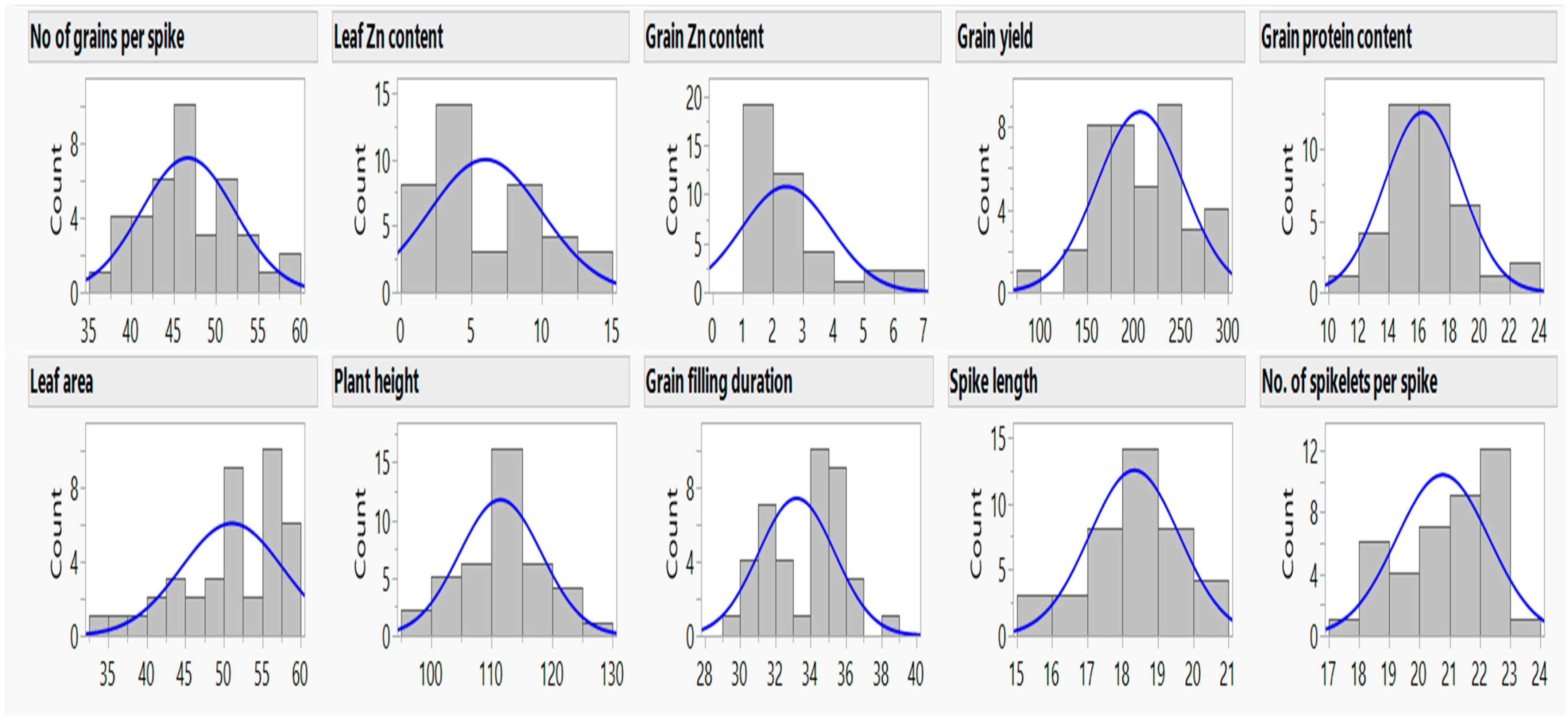
3.3. Vegetative and Reproductive Parameters
3.3.1. Leaf Area, Plant Height, and Grain Filling Duration
3.3.2. Spike Length, Number of Spikelets Spike−1, and Number of Grains Spike−1
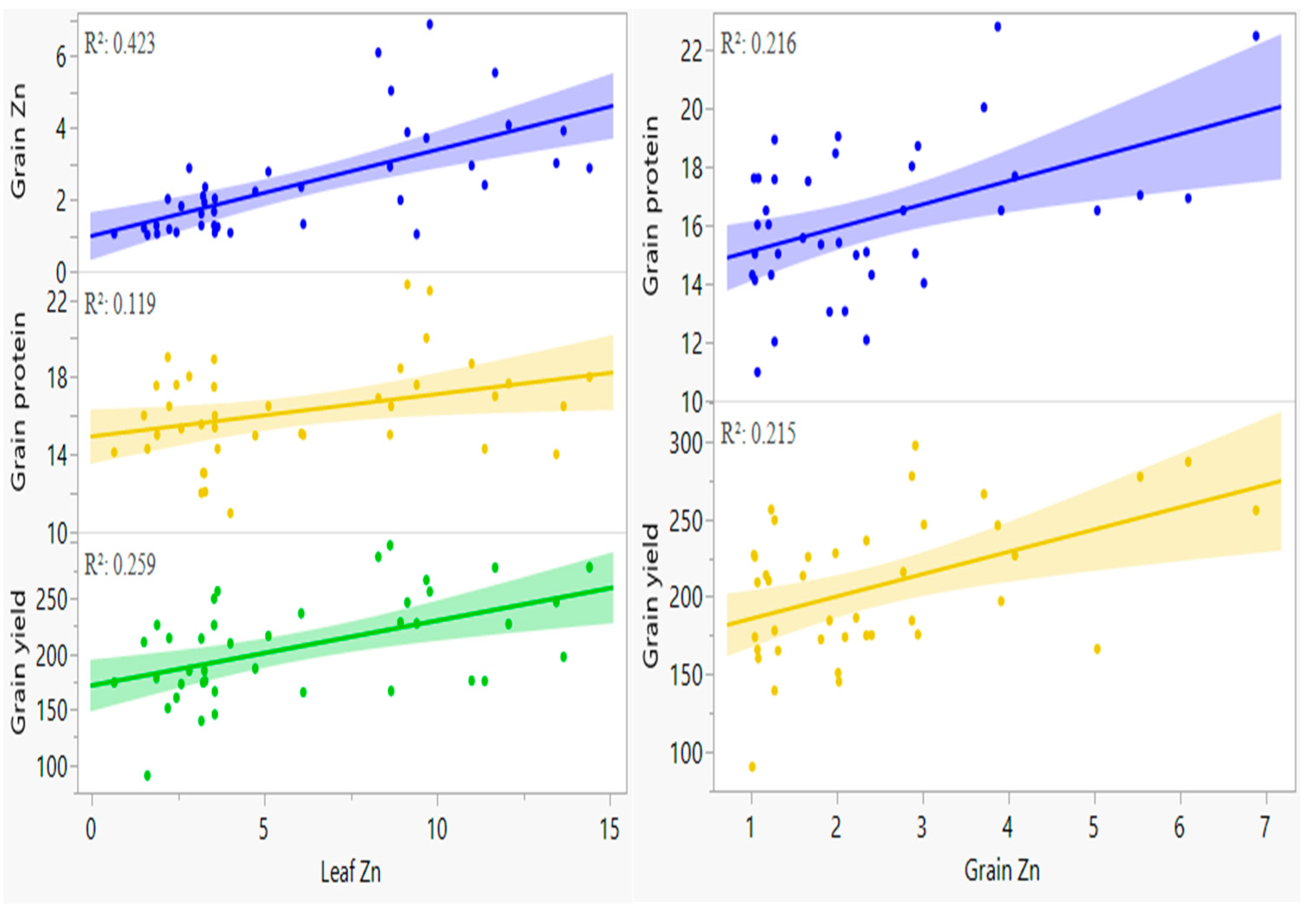
3.3.3. Leaf and Grain Zinc Content
3.3.4. Grain Protein Content
3.3.5. Grain Yield
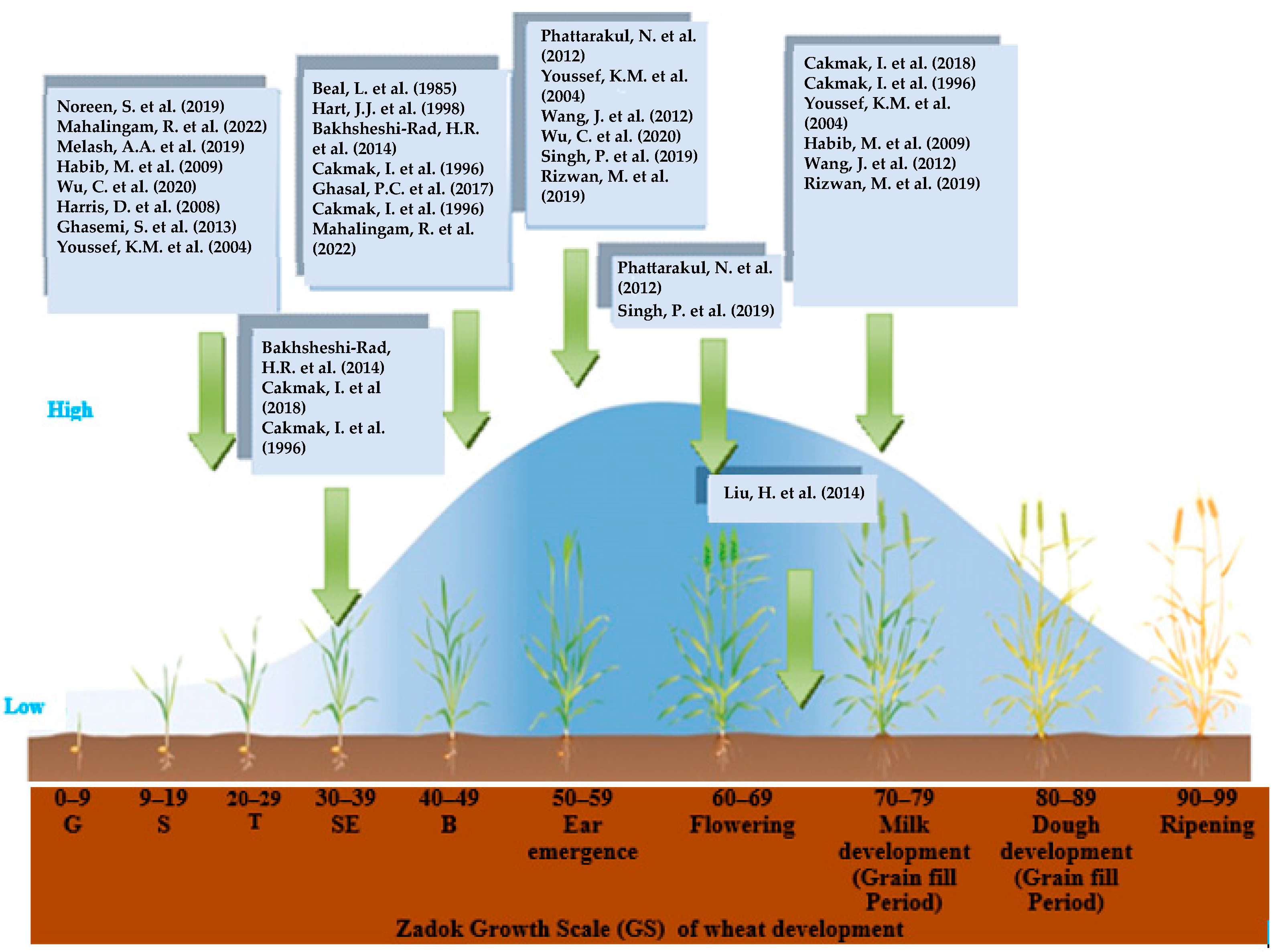
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification. Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Sekhon, B. Chelates for micronutrient nutrition among crops. Resonance 2003, 8, 46–53. [Google Scholar] [CrossRef]
- Bharti, K.; Pandey, N.; Shankhdhar, D.; Srivastava, P.; Shankhdhar, S. Improving nutritional quality of wheat through soil and foliar zinc application. Plant Soil Environ. 2013, 59, 348–352. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.Á.; Afzal, S.; Singh, T.; Hussain, I. Zinc oxide nanoparticles: A review of their biological synthesis, antimicrobial activity, uptake, translocation and biotransformation in plants. J. Mater. Sci. 2018, 53, 185–201. [Google Scholar] [CrossRef]
- Alloway, B. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem. Health 2009, 31, 537–548. [Google Scholar] [CrossRef]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Zinc through the three domains of life. J. Proteome Res. 2006, 5, 3173–3178. [Google Scholar] [CrossRef]
- Singh, B.; Natesan, S.K.A.; Singh, B.; Usha, K. Improving zinc efficiency of cereals under zinc deficiency. Curr. Sci. 2005, 88, 36–44. [Google Scholar]
- Black, R.E.; Allen, L.H.; Bhutta, Z.A.; Caulfield, L.E.; De Onis, M.; Ezzati, M.; Juan, R.; Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet 2008, 371, 243–260. [Google Scholar] [CrossRef]
- Lowe, N.M.; Zaman, M.; Moran, V.H.; Ohly, H.; Sinclair, J.; Fatima, S.; Lark, R.M. Biofortification of wheat with zinc for eliminating deficiency in Pakistan: Study protocol for a cluster-randomised, double-blind, controlled effectiveness study (BIZIFED2). BMJ Open 2020, 10, e039231. [Google Scholar] [CrossRef]
- Singh, P.; Shukla, A.K.; Behera, S.K.; Tiwari, P.K. Zinc application enhances superoxide dismutase and carbonic anhydrase activities in zinc-efficient and zinc-inefficient wheat genotypes. J. Soil Sci. Plant Nutr. 2019, 19, 477–487. [Google Scholar] [CrossRef]
- Putnam, J.; Allshouse, J.; Kantor, L.S. US per capita food supply trends: More calories, refined carbohydrates, and fats. Food Rev. 2002, 25, 2–15. [Google Scholar]
- Iqra, L.; Rashid, M.S.; Ali, Q.; Latif, I.; Mailk, A. Evaluation for Na+/K+ ratio under salt stress condition in wheat. Life Sci. J. 2020, 17, 43–47. [Google Scholar]
- Ramzan, Y.; Hafeez, M.B.; Khan, S.; Nadeem, M.; Batool, S.; Ahmad, J. Biofortification with zinc and iron improves the grain quality and yield of wheat crop. Int. J. Plant Prod. 2020, 14, 501–510. [Google Scholar] [CrossRef]
- Cakmak, I.; Kutman, U.Á. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef]
- Wakeel, A.; Farooq, M.; Bashir, K.; Ozturk, L. Micronutrient Malnutrition and Biofortification: Recent advances and future perspectives. In Plant Micronutrient Use Efficiency; Elsevier: Amsterdam, The Netherlands, 2018; pp. 225–243. [Google Scholar]
- Ghasemi, S.; Khoshgoftarmanesh, A.H.; Afyuni, M.; Hadadzadeh, H. The effectiveness of foliar applications of synthesized zinc-amino acid chelates in comparison with zinc sulfate to increase yield and grain nutritional quality of wheat. Eur. J. Agron. 2013, 45, 68–74. [Google Scholar] [CrossRef]
- Johnson, S.; Lauren, J.; Welch, R.; Duxbury, J. A comparison of the effects of micronutrient seed priming and soil fertilization on the mineral nutrition of chickpea (Cicer arietinum), lentil (Lens culinaris), rice (Oryza sativa) and wheat (Triticum aestivum) in Nepal. Exp. Agric. 2005, 41, 427–448. [Google Scholar] [CrossRef]
- Toor, M.D.; Adnan, M.; Shozib, M.; Ahmad, R. Foliar application of Zn: Best way to mitigate drought stress in plants; A review. IJAR 2020, 6, 16–20. [Google Scholar]
- Fageria, N.; Filho, M.B.; Moreira, A.; Guimarães, C. Foliar fertilization of crop plants. J. Plant Nutr. 2009, 32, 1044–1064. [Google Scholar] [CrossRef]
- Noreen, S.; Kamran, A. Foliar application of zinc sulphate to improve yield and grain zinc content in wheat (Triticum aestivum L.). Afr. J. Agricul. Res. 2019, 14, 867–876. [Google Scholar]
- Mahalingam, R.; Duhan, N.; Kaundal, R.; Smertenko, A.; Nazarov, T.; Bregitzer, P. Heat and drought induced transcriptomic changes in barley varieties with contrasting stress response phenotypes. Front. Plant Sci. 2022, 13, 1066421. [Google Scholar] [CrossRef]
- Melash, A.A.; Mengistu, D.K.; Aberra, D.A.; Tsegay, A. The influence of seeding rate and micronutrients foliar application on grain yield and quality traits and micronutrients of durum wheat. J. Cereal Sci. 2019, 85, 221–227. [Google Scholar] [CrossRef]
- Habib, M. Effect of foliar application of Zn and Fe on wheat yield and quality. Afr. J. Biotechnol. 2009, 8, 6795–6798. [Google Scholar]
- Wu, C.; Dun, Y.; Zhang, Z.; Li, M.; Wu, G. Foliar application of selenium and zinc to alleviate wheat (Triticum aestivum L.) cadmium toxicity and uptake from cadmium-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 190, 110091. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.; Rashid, A.; Miraj, G.; Arif, M.; Yunas, M. ‘On-farm’ seed priming with zinc in chickpea and wheat in Pakistan. Plant Soil 2008, 306, 3–10. [Google Scholar] [CrossRef]
- Youssef, K.M.; Koch, C.C.; Fedkiw, P.S. Influence of additives and pulse electrodeposition parameters on production of nanocrystalline zinc from zinc chloride electrolytes. J. Electrochem. Soc. 2004, 151, C103. [Google Scholar] [CrossRef]
- Cakmak, I.; Sari, N.; Marschner, H.; Ekiz, H.; Kalayci, M.; Yilmaz, A.; Braun, H.J. Phytosiderophore release in bread and durum wheat genotypes differing in zinc efficiency. Plant. Soil. 1996, 180, 183–189. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Idris, M.H.; Abdul-Kadir, M.R.; Ourdjini, A.; Medraj, M.; Daroonparvar, M.; Hamzah, E. Mechanical and bio-corrosion properties of quaternary Mg–Ca–Mn–Zn alloys compared with binary Mg–Ca alloys. Mater. Des. 2014, 53, 283–292. [Google Scholar] [CrossRef]
- Wang, J.; Mao, H.; Zhao, H.; Huang, D.; Wang, Z. Different increases in maize and wheat grain zinc concentrations caused by soil and foliar applications of zinc in Loess Plateau, China. Field Crop. Res. 2012, 135, 89–96. [Google Scholar] [CrossRef]
- Phattarakul, N.; Rerkasem, B.; Li, L.J.; Wu, L.H.; Zou, C.Q.; Ram, H.; Cakmak, I. Biofortification of rice grain with zinc through zinc fertilization in different countries. Plant Soil. 2012, 361, 131–141. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; Waris, A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.H.; Li, F.; Li, K.; Yang, N.; Yang, Y.; Qiu, W. Grain iron and zinc concentrations of wheat and their relationships to yield in major wheat production areas in China. Field Crop. Res. 2014, 156, 151–160. [Google Scholar] [CrossRef]
- Beal, L.; Mehta, T. Zinc and phytate distribution in peas. Influence of heat treatment, germination, pH, substrate, and phosphorus on pea phytate and phytase. J. Food Sci. 1985, 50, 96–100. [Google Scholar] [CrossRef]
- Hart, J.J.; Norvell, W.A.; Welch, R.M.; Sullivan, L.A.; Kochian, L.V. Characterization of zinc uptake, binding, and translocation in intact seedlings of bread and durum wheat cultivars. Plant Physiol. 1998, 118, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Rawat, N.; Neelam, K.; Tiwari, V.K.; Dhaliwal, H.S. Biofortification of cereals to overcome hidden hunger. Plant Breed. 2013, 132, 437–445. [Google Scholar] [CrossRef]
- Rengel, Z.; Romheld, V. Root exudation and Fe uptake and transport in wheat genotypes differing in tolerance to Zn deficiency. Plant Soil 2000, 222, 25–34. [Google Scholar] [CrossRef]
- Ghasal, P.C.; Shivay, Y.S.; Pooniya, V.; Choudhary, M.; Verma, R.K. Response of wheat genotypes to zinc fertilization for improving productivity and quality. Arch. Agron. Soil Sci. 2017, 63, 1597–1612. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nishizawa, N.K. Iron uptake, translocation, and regulation in higher plants. Annu. Rev. Plant Biol. 2012, 63, 131–152. [Google Scholar] [CrossRef]
- Khan, M.; Fuller, M.; Baloch, F. Effect of soil applied zinc sulphate on wheat (Triticum aestivum L.) grown on a calcareous soil in Pakistan. Cereal Res. Commun. 2008, 36, 571–582. [Google Scholar] [CrossRef]
- Abdoli, M.; Esfandiari, E.; Mousavi, S.B.; Sadeghzadeh, B. Effects of foliar application of zinc sulfate at different phenological stages on yield formation and grain zinc content of bread wheat (cv. Kohdasht). Azarian J. Agric. 2014, 1, 11–16. [Google Scholar]
- Aziz, N.; Anwar, S.; Kashmir, S.; Ahmad, J.; Saeed, B.; Khan, S. Response of wheat varieties to different zinc application methods. Pure Appl. Biol. 2019, 8, 489–495. [Google Scholar] [CrossRef]
- Esfandiari, E.; Abdoli, M.; Mousavi, S.-B.; Sadeghzadeh, B. Impact of foliar zinc application on agronomic traits and grain quality parameters of wheat grown in zinc deficient soil. Indian J. Plant Physiol. 2016, 21, 263–270. [Google Scholar] [CrossRef]
- Arafat, Y.; Shafi, M.; Khan, M.A.; Adnan, M.; Basir, A.; Arshad, M.; Rahman, Z. Yield response of wheat cultivars to zinc application rates and methods. Pure Appl. Biol. 2016, 5, 1. [Google Scholar] [CrossRef]
- Aslam, W.; Arfan, M.; Shahid, S.A.; Anwar, F.; Mahmood, Z.; Rashid, U. Effects of exogenously applied Zn on the growth, yield, chlorophyll. Int. J. Pharm. Chem. Biol. Sci. 2014, 5, 11–15. [Google Scholar]
- Zeidan, M.; Mohamed, M.F.; Hamouda, H. Effect of foliar fertilization of Fe, Mn and Zn on wheat yield and quality in low sandy soils fertility. World J. Agric. Sci. 2010, 6, 696–699. [Google Scholar]
- Majd, A.N.; Fazel, M.; Lak, S. The effect of foliar application of zinc (Zn) on yield and yield components of irrigated wheat cultivars in Ahvaz weather conditions. Int. J. Biosci. 2015, 6, 370–377. [Google Scholar]

| Texture | pH | E.C | SOM | N | P | K | Zn |
|---|---|---|---|---|---|---|---|
| (mS cm−1) | (%) | (mg kg−1) | |||||
| Loam | 7.0 | 0.8 | 0.79 | 50 | 4.9 | 112 | 0.68 |
| Months | Temperature (°C) | Rainfall (mm) | Humidity (%) | ||||
|---|---|---|---|---|---|---|---|
| Max | Min | Average | Max | Min | Average | ||
| November | 25.3 | 12.1 | 18.8 | 21.6 | 94 | 22 | 63 |
| December | 19.3 | 7.7 | 13.7 | 16.8 | 100 | 24 | 76 |
| January | 17.0 | 6.6 | 12.4 | 60.4 | 100 | 27 | 80 |
| February | 24.2 | 11.4 | 18.2 | 1.80 | 100 | 27 | 73 |
| March | 29.6 | 17.3 | 23.8 | 11.3 | 100 | 07 | 54 |
| April | 34.0 | 20.0 | 26.0 | 18.5 | 88 | 04 | 43 |
| Zinc Treatment | Cultivars | Amount Given (g) | Leaf Zn (mg kg−1) | % Absorbed | Grain Zn (mg kg−1) | % Translocated |
|---|---|---|---|---|---|---|
| Foliar application (zinc sulphate) | Faisalabad-08 | 1.2 | 8.47 * | 0.71% | 5.57 * | 66% |
| Gandum-1 | 1.2 | 10.72 * | 0.89% | 6.21 * | 58% | |
| Chakwal-50 | 1.2 | 9.40 * | 0.78% | 3.80 * | 40% | |
| Pakistan-13 | 1.2 | 12.85 * | 1.07% | 4.00 * | 31% | |
| Johar-16 | 1.2 | 12.69 * | 1.06% | 2.91 * | 23% | |
| Average | 10.83 | 0.90% | 4.50 | 43% | ||
| Foliar application (zinc amino acid chelates) | Faisalabad-08 | 1.2 | 12.73 * | 1.06% | 2.71 * | 21% |
| Gandum-1 | 1.2 | 6.87 * | 0.57% | 2.85 * | 41% | |
| Chakwal-50 | 1.2 | 4.87 * | 0.41% | 1.28 * | 26% | |
| Pakistan-13 | 1.2 | 9.33 * | 0.78% | 1.51 ns | 16% | |
| Johar-16 | 1.2 | 5.39 * | 0.45% | 2.29 * | 42% | |
| Average | 7.84 | 0.65% | 2.12 | 29.2% |
| Traits | Cultivar | Treatment | Cultivar × Treatment |
|---|---|---|---|
| Leaf area (cm)2 | 0.02 * | 0.00 ** | 0.00 ** |
| Plant height (cm) | 0.00 ** | 0.00 ** | 0.00 ** |
| Grain filling duration | 0.00 ** | 0.93 | 0.00 ** |
| Spike length (cm) | 0.00 ** | 0.00 ** | 0.00 ** |
| Number of spikelets spike−1 | 0.00 ** | 0.01 * | 0.00 ** |
| Number of grains spike−1 | 0.35 | 0.00 ** | 0.00 ** |
| Leaf zinc content (mg kg−1) | 0.40 | 0.00 ** | 0.00 ** |
| Grain zinc content (mg L−1) | 0.44 | 0.00 ** | 0.00 ** |
| Grain protein content (%) | 0.02 * | 0.00 ** | 0.00 ** |
| Grain yield (g) | 0.06 | 0.00 ** | 0.17 |
| Traits | Control | Foliar ZnSO4 | Foliar ZnAACh * |
|---|---|---|---|
| Leaf area (cm) | 41.53 b ± 1.92 | 54.08 a ± 1.50 | 55.31 a ± 1.61 |
| Plant height (cm) | 106.48 c ± 2.77 | 116.62 a ± 2.27 | 112.85 b ± 1.84 |
| Grain filling duration | 33.10 a ± 1.19 | 33.00 a ± 1.03 | 33.10 a ± 1.01 |
| Spike length (cm) | 17.34 b ± 0.81 | 18.45 a ± 0.85 | 18.76 a ± 0.76 |
| Number of spikelets spike−1 | 19.89 b ± 0.92 | 21.26 a ± 0.96 | 20.76 ab ± 0.94 |
| Number of grains spike−1 | 40.40 c ± 1.39 | 51.90 a ± 1.80 | 48.36 b ± 1.58 |
| Leaf zinc content (mg kg−1) | 2.23 c ± 0.11 | 10.70 a ± 0.42 | 7.83 b ± 0.33 |
| Grain zinc content (mg L−1) | 1.27 c ± 0.32 | 4.50 a ± 1.34 | 2.13 b ± 0.71 |
| Grain protein content (%) | 15.04 b ± 1.05 | 18.65 a ± 1.35 | 15.52 b ± 1.49 |
| Grain yield (g) | 182.01 b ± 18.73 | 237.63 a ± 24.08 | 223.48 a ± 19.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamran, A.; Ghazanfar, M.; Khan, J.S.; Pervaiz, S.; Siddiqui, M.H.; Alamri, S. Zinc Absorption through Leaves and Subsequent Translocation to the Grains of Bread Wheat after Foliar Spray. Agriculture 2023, 13, 1775. https://doi.org/10.3390/agriculture13091775
Kamran A, Ghazanfar M, Khan JS, Pervaiz S, Siddiqui MH, Alamri S. Zinc Absorption through Leaves and Subsequent Translocation to the Grains of Bread Wheat after Foliar Spray. Agriculture. 2023; 13(9):1775. https://doi.org/10.3390/agriculture13091775
Chicago/Turabian StyleKamran, Atif, Munazza Ghazanfar, Jan Sher Khan, Sana Pervaiz, Manzer H. Siddiqui, and Saud Alamri. 2023. "Zinc Absorption through Leaves and Subsequent Translocation to the Grains of Bread Wheat after Foliar Spray" Agriculture 13, no. 9: 1775. https://doi.org/10.3390/agriculture13091775
APA StyleKamran, A., Ghazanfar, M., Khan, J. S., Pervaiz, S., Siddiqui, M. H., & Alamri, S. (2023). Zinc Absorption through Leaves and Subsequent Translocation to the Grains of Bread Wheat after Foliar Spray. Agriculture, 13(9), 1775. https://doi.org/10.3390/agriculture13091775







