Advancements and Innovations in Harnessing Microbial Processes for Enhanced Biogas Production from Waste Materials
Abstract
1. Introduction
| Microorganism | Substrate | Major Events during Substrate Degradation | Biogas Yield (mL/g VS) | Condition | Ref. |
|---|---|---|---|---|---|
| Acetobacteroides hydrogenigenes | Corn straw | Hydrogen gas production | 258.1 | Laboratory scale | [33] |
| Methanospirillum hungatei | Nonfat dry milk | Increase of methanogenic activity | 32 | Laboratory scale | [34] |
| Pseudobutyrivibrio xylanivorans | Brewery spent grain | Hydrolysis | 261.3 | Piolot scale | [35] |
| C. saccharolyticus | Pig slurry and sweet sorghum | Hydrogen gas production | 62.5 | Laboratory scale | [36] |
| Clostridium cellulolyticum | Wheat straw | Hydrolysis | 342.5 | Laboratory scale | [37] |
| Clostridium sp. | Cellulosic waste | Hydrolysis | 168 | Laboratory scale | [38] |
| Enterobacter cloacae | Maize silage | Hydrolysis | 718.5 | Laboratory scale | [39] |
| T. hermosaccharolyticum Caldanaerobacter subterraneus Thermoanaerobacter pseudethanolicus C. cellulolyticum | Corn stover and cellulose | Hydrolysis | 165 | Laboratory scale | [40] |
| Lignocellulose degrading microbial consortium | Swine manure | Hydrolysis | 180 | Laboratory scale | [41] |
| Gelria, Anaerovorax, Dethiobacter, Clostridia | Grass siliage | Hydrolysis and fatty acid metabolism | - | Laboratory scale | [42] |
2. Methods
3. Microbial Diversity and Dynamics Involved in Biogas Production
4. Effect of Feedstock Substrate on Microbial Community and Biogas Production in an Anaerobic Digester
4.1. Bioaugmentation with Microbial Additives to the Feedstock
4.2. Co-Digestion Strategy for Biogas Production from Agricultural Waste and Animal Waste
4.3. Biological Pretreatments of Organic Waste for Enhanced Biogas Production
4.4. Advantages and Disadvantages of Biogas Production Enhancement Strategies
5. Anaerobic Metabolic Pathways and Genes Involved in Biogas Production
6. Microbial Metagenomics, High-Throughput Sequencing, and Its Relevance to Biogas Production
6.1. Applications of Meta-Transcriptomics in Biogas Production
6.2. Integration of Omics Approaches with Molecular Probing Techniques
7. Biogas Production and Circular Economy
8. Microbe-Based Large-Scale Commercial Biogas Plants
9. Future Prospects
9.1. Genetic Engineering of Microorganisms for Enhanced Biogas Production
9.2. Microbial Conversion of CH4 and CO2 into Other Renewable Energy
9.3. Policy Support and Market Incentives from Government
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adnan, A.I.; Ong, M.Y.; Nomanbhay, S.; Chew, K.W.; Show, P.L. Technologies for biogas upgrading to biomethane: A review. Bioengineering 2019, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.; Kumar, D.; Singh, B.; Sachan, P.K. Biofuels and their sources of production: A review on cleaner sustainable alternative against conventional fuel, in the framework of the food and energy nexus. Energy Nexus 2021, 4, 100036. [Google Scholar] [CrossRef]
- Hossain, M.; Haque, M. Stability analyses of municipal solid waste landfills with decomposition. Geotech. Geol. Eng. 2009, 27, 659–666. [Google Scholar] [CrossRef]
- Fajrina, N.; Yusof, N.; Ismail, A.F.; Aziz, F.; Bilad, M.R.; Alkahtani, M. A Crucial Review on the Challenges and Recent Gas Membrane Development for Biogas Upgrading. J. Environ. Chem. Eng. 2023, 11, 110235. [Google Scholar] [CrossRef]
- Theuerl, S.; Klang, J.; Prochnow, A. Process disturbances in agricultural biogas production—Causes, mechanisms and effects on the biogas microbiome: A review. Energies 2019, 12, 365. [Google Scholar] [CrossRef]
- Atelge, M.; Krisa, D.; Kumar, G.; Eskicioglu, C.; Nguyen, D.D.; Chang, S.W.; Atabani, A.; Al-Muhtaseb, A.H.; Unalan, S. Biogas production from organic waste: Recent progress and perspectives. Waste Biomass Valorization 2020, 11, 1019–1040. [Google Scholar] [CrossRef]
- Nwokolo, N.; Mukumba, P.; Obileke, K.; Enebe, M. Waste to energy: A focus on the impact of substrate type in biogas production. Processes 2020, 8, 1224. [Google Scholar] [CrossRef]
- Anukam, A.; Mohammadi, A.; Naqvi, M.; Granström, K. A review of the chemistry of anaerobic digestion: Methods of accelerating and optimizing process efficiency. Processes 2019, 7, 504. [Google Scholar] [CrossRef]
- Abanades, S.; Abbaspour, H.; Ahmadi, A.; Das, B.; Ehyaei, M.; Esmaeilion, F.; El Haj Assad, M.; Hajilounezhad, T.; Jamali, D.; Hmida, A. A critical review of biogas production and usage with legislations framework across the globe. Int. J. Environ. Sci. Technol. 2021, 19, 3377–3400. [Google Scholar] [CrossRef]
- Patel, S.K.; Gupta, R.K.; Das, D.; Lee, J.-K.; Kalia, V.C. Continuous biohydrogen production from poplar biomass hydrolysate by a defined bacterial mixture immobilized on lignocellulosic materials under non-sterile conditions. J. Clean. Prod. 2021, 287, 125037. [Google Scholar] [CrossRef]
- Kasinath, A.; Fudala-Ksiazek, S.; Szopinska, M.; Bylinski, H.; Artichowicz, W.; Remiszewska-Skwarek, A.; Luczkiewicz, A. Biomass in biogas production: Pretreatment and codigestion. Renew. Sustain. Energy Rev. 2021, 150, 111509. [Google Scholar] [CrossRef]
- Aziz, M.M.A.; Kassim, K.A.; ElSergany, M.; Anuar, S.; Jorat, M.E.; Yaacob, H.; Ahsan, A.; Imteaz, M.A. Recent advances on palm oil mill effluent (POME) pretreatment and anaerobic reactor for sustainable biogas production. Renew. Sustain. Energy Rev. 2020, 119, 109603. [Google Scholar] [CrossRef]
- Rajeswari, G.; Jacob, S.; Chandel, A.K.; Kumar, V. Unlocking the potential of insect and ruminant host symbionts for recycling of lignocellulosic carbon with a biorefinery approach: A review. Microb. Cell Factories 2021, 20, 107. [Google Scholar] [CrossRef]
- Sidhu, C.; Vikram, S.; Pinnaka, A.K. Unraveling the microbial interactions and metabolic potentials in pre-and post-treated sludge from a wastewater treatment plant using metagenomic studies. Front. Microbiol. 2017, 8, 1382. [Google Scholar] [CrossRef]
- Hashemi, S.; Hashemi, S.E.; Lien, K.M.; Lamb, J.J. Molecular microbial community analysis as an analysis tool for optimal biogas production. Microorganisms 2021, 9, 1162. [Google Scholar] [CrossRef]
- Tian, G.; Zhang, W.; Dong, M.; Yang, B.; Zhu, R.; Yin, F.; Zhao, X.; Wang, Y.; Xiao, W.; Wang, Q. Metabolic pathway analysis based on high-throughput sequencing in a batch biogas production process. Energy 2017, 139, 571–579. [Google Scholar] [CrossRef]
- Hao, L.; Lü, F.; Mazéas, L.; Desmond-Le Quéméner, E.; Madigou, C.; Guenne, A.; Shao, L.; Bouchez, T.; He, P. Stable isotope probing of acetate fed anaerobic batch incubations shows a partial resistance of acetoclastic methanogenesis catalyzed by Methanosarcina to sudden increase of ammonia level. Water Res. 2015, 69, 90–99. [Google Scholar] [CrossRef]
- Maus, I.; Bremges, A.; Stolze, Y.; Hahnke, S.; Cibis, K.G.; Koeck, D.E.; Kim, Y.S.; Kreubel, J.; Hassa, J.; Wibberg, D. Genomics and prevalence of bacterial and archaeal isolates from biogas-producing microbiomes. Biotechnol. Biofuels 2017, 10, 264. [Google Scholar] [CrossRef]
- Jagadevan, S.; Banerjee, A.; Banerjee, C.; Guria, C.; Tiwari, R.; Baweja, M.; Shukla, P. Recent developments in synthetic biology and metabolic engineering in microalgae towards biofuel production. Biotechnol. Biofuels 2018, 11, 185. [Google Scholar] [CrossRef]
- Pöschl, M.; Ward, S.; Owende, P. Evaluation of energy efficiency of various biogas production and utilization pathways. Appl. Energy 2010, 87, 3305–3321. [Google Scholar] [CrossRef]
- Achinas, S.; Achinas, V.; Euverink, G.J.W. A technological overview of biogas production from biowaste. Engineering 2017, 3, 299–307. [Google Scholar] [CrossRef]
- Divya, D.; Gopinath, L.; Christy, P.M. A review on current aspects and diverse prospects for enhancing biogas production in sustainable means. Renew. Sustain. Energy Rev. 2015, 42, 690–699. [Google Scholar] [CrossRef]
- Mishra, A.; Kumar, M.; Bolan, N.S.; Kapley, A.; Kumar, R.; Singh, L. Multidimensional approaches of biogas production and up-gradation: Opportunities and challenges. Bioresour. Technol. 2021, 338, 125514. [Google Scholar] [CrossRef]
- Deena, S.R.; Vickram, A.; Manikandan, S.; Subbaiya, R.; Karmegam, N.; Ravindran, B.; Chang, S.W.; Awasthi, M.K. Enhanced biogas production from food waste and activated sludge using advanced techniques—A review. Bioresour. Technol. 2022, 355, 127234. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, T.; Chowdhury, H.; Hossain, N.; Ahmed, A.; Hossen, M.S.; Chowdhury, P.; Thirugnanasambandam, M.; Saidur, R. Latest advancements on livestock waste management and biogas production: Bangladesh’s perspective. J. Clean. Prod. 2020, 272, 122818. [Google Scholar] [CrossRef]
- Yaqoob, H.; Teoh, Y.H.; Din, Z.U.; Sabah, N.U.; Jamil, M.A.; Mujtaba, M.; Abid, A. The potential of sustainable biogas production from biomass waste for power generation in Pakistan. J. Clean. Prod. 2021, 307, 127250. [Google Scholar] [CrossRef]
- Devi, M.K.; Manikandan, S.; Oviyapriya, M.; Selvaraj, M.; Assiri, M.A.; Vickram, S.; Subbaiya, R.; Karmegam, N.; Ravindran, B.; Chang, S. Recent advances in biogas production using Agro-Industrial Waste: A comprehensive review outlook of Techno-Economic analysis. Bioresour. Technol. 2022, 363, 127871. [Google Scholar] [CrossRef]
- Gao, Z.; Alshehri, K.; Li, Y.; Qian, H.; Sapsford, D.; Cleall, P.; Harbottle, M. Advances in biological techniques for sustainable lignocellulosic waste utilization in biogas production. Renew. Sustain. Energy Rev. 2022, 170, 112995. [Google Scholar] [CrossRef]
- Ekwebelem, O.C.; Ofielu, E.S.; Dike, O.V.N.; Aleke, J.C.; Nwachukwu, G.A. Towards Sustainable Energy: The Requisite Role of Microorganisms in the Production of Biogas and Bioethanol. J. Energy Res. Rev. 2020, 6, 20–32. [Google Scholar] [CrossRef]
- Akinsemolu, A.A. The role of microorganisms in achieving the sustainable development goals. J. Clean. Prod. 2018, 182, 139–155. [Google Scholar] [CrossRef]
- Ferdeș, M.; Dincă, M.N.; Moiceanu, G.; Zăbavă, B.Ș.; Paraschiv, G. Microorganisms and enzymes used in the biological pretreatment of the substrate to enhance biogas production: A review. Sustainability 2020, 12, 7205. [Google Scholar] [CrossRef]
- Nwokolo, N.L.; Enebe, M.C. An insight on the contributions of microbial communities and process parameters in enhancing biogas production. Biomass Convers. Biorefinery 2022, 1–17. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, R.-B.; Qiu, Y.-L.; Qiao, J.-T.; Yuan, X.-Z.; Shi, X.-S.; Wang, C.-S. Bioaugmentation with an acetate-type fermentation bacterium Acetobacteroides hydrogenigenes improves methane production from corn straw. Bioresour. Technol. 2015, 179, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Tale, V.P.; Maki, J.; Zitomer, D. Bioaugmentation of overloaded anaerobic digesters restores function and archaeal community. Water Res. 2015, 70, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Čater, M.; Fanedl, L.; Malovrh, Š.; Logar, R.M. Biogas production from brewery spent grain enhanced by bioaugmentation with hydrolytic anaerobic bacteria. Bioresour. Technol. 2015, 186, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Kovács, K.L.; Ács, N.; Kovács, E.; Wirth, R.; Rákhely, G.; Strang, O.; Herbel, Z.; Bagi, Z. Improvement of biogas production by bioaugmentation. BioMed Res. Int. 2013, 2013, 482653. [Google Scholar] [CrossRef]
- Peng, X.; Börner, R.A.; Nges, I.A.; Liu, J. Impact of bioaugmentation on biochemical methane potential for wheat straw with addition of Clostridium cellulolyticum. Bioresour. Technol. 2014, 152, 567–571. [Google Scholar] [CrossRef]
- Martin-Ryals, A.; Schideman, L.; Li, P.; Wilkinson, H.; Wagner, R. Improving anaerobic digestion of a cellulosic waste via routine bioaugmentation with cellulolytic microorganisms. Bioresour. Technol. 2015, 189, 62–70. [Google Scholar] [CrossRef]
- Ács, N.; Bagi, Z.; Rákhely, G.; Minárovics, J.; Nagy, K.; Kovács, K.L. Bioaugmentation of biogas production by a hydrogen-producing bacterium. Bioresour. Technol. 2015, 186, 286–293. [Google Scholar] [CrossRef]
- Strang, O.; Ács, N.; Wirth, R.; Maróti, G.; Bagi, Z.; Rákhely, G.; Kovács, K.L. Bioaugmentation of the thermophilic anaerobic biodegradation of cellulose and corn stover. Anaerobe 2017, 46, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Tuesorn, S.; Wongwilaiwalin, S.; Champreda, V.; Leethochawalit, M.; Nopharatana, A.; Techkarnjanaruk, S.; Chaiprasert, P. Enhancement of biogas production from swine manure by a lignocellulolytic microbial consortium. Bioresour. Technol. 2013, 144, 579–586. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, J.A.; Wall, D.M.; Jackson, S.A.; Murphy, J.D.; Dobson, A.D. Trace element supplementation is associated with increases in fermenting bacteria in biogas mono-digestion of grass silage. Renew. Energy 2019, 138, 980–986. [Google Scholar] [CrossRef]
- Aryal, N.; Kvist, T.; Ammam, F.; Pant, D.; Ottosen, L.D. An overview of microbial biogas enrichment. Bioresour. Technol. 2018, 264, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Kazda, M.; Langer, S.; Bengelsdorf, F.R. Fungi open new possibilities for anaerobic fermentation of organic residues. Energy Sustain. Soc. 2014, 4, 6. [Google Scholar] [CrossRef]
- Ghosh, P.; Kumar, M.; Kapoor, R.; Kumar, S.S.; Singh, L.; Vijay, V.; Vijay, V.K.; Kumar, V.; Thakur, I.S. Enhanced biogas production from municipal solid waste via co-digestion with sewage sludge and metabolic pathway analysis. Bioresour. Technol. 2020, 296, 122275. [Google Scholar] [CrossRef]
- Heffernan, J.K.; Lai, C.-Y.; Gonzalez-Garcia, R.A.; Nielsen, L.K.; Guo, J.; Marcellin, E. Biogas upgrading using Clostridium autoethanogenum for value-added products. Chem. Eng. J. 2023, 452, 138950. [Google Scholar] [CrossRef]
- Westerholm, M.; Crauwels, S.; Houtmeyers, S.; Meerbergen, K.; Van Geel, M.; Lievens, B.; Appels, L. Microbial community dynamics linked to enhanced substrate availability and biogas production of electrokinetically pre-treated waste activated sludge. Bioresour. Technol. 2016, 218, 761–770. [Google Scholar] [CrossRef]
- Ozbayram, E.G.; Kleinsteuber, S.; Nikolausz, M. Biotechnological utilization of animal gut microbiota for valorization of lignocellulosic biomass. Appl. Microbiol. Biotechnol. 2020, 104, 489–508. [Google Scholar] [CrossRef]
- Boscaro, M.E.; Marin, D.F.C.; da Silva, D.C.; Maintinguer, S.I. Effect of Fe3O4 nanoparticles on microbial diversity and biogas production in anaerobic digestion of crude glycerol. Biomass Bioenergy 2022, 160, 106439. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, R.; Xiang, Y.; Lu, Y.; Jia, M.; Huang, J.; Xu, Z.; Cao, J.; Xiong, W.; Yang, Z. Addition of nanoparticles increases the abundance of mobile genetic elements and changes microbial community in the sludge anaerobic digestion system. J. Hazard. Mater. 2021, 405, 124206. [Google Scholar] [CrossRef]
- Govarthanan, M.; Manikandan, S.; Subbaiya, R.; Krishnan, R.Y.; Srinivasan, S.; Karmegam, N.; Kim, W. Emerging trends and nanotechnology advances for sustainable biogas production from lignocellulosic waste biomass: A critical review. Fuel 2022, 312, 122928. [Google Scholar] [CrossRef]
- Treu, L.; Kougias, P.G.; Campanaro, S.; Bassani, I.; Angelidaki, I. Deeper insight into the structure of the anaerobic digestion microbial community; the biogas microbiome database is expanded with 157 new genomes. Bioresour. Technol. 2016, 216, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Bareither, C.A.; Wolfe, G.L.; McMahon, K.D.; Benson, C.H. Microbial diversity and dynamics during methane production from municipal solid waste. Waste Manag. 2013, 33, 1982–1992. [Google Scholar] [CrossRef]
- Kröber, M.; Bekel, T.; Diaz, N.N.; Goesmann, A.; Jaenicke, S.; Krause, L.; Miller, D.; Runte, K.J.; Viehöver, P.; Pühler, A. Phylogenetic characterization of a biogas plant microbial community integrating clone library 16S-rDNA sequences and metagenome sequence data obtained by 454-pyrosequencing. J. Biotechnol. 2009, 142, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, A.; Bekel, T.; Diaz, N.N.; Dondrup, M.; Eichenlaub, R.; Gartemann, K.-H.; Krahn, I.; Krause, L.; Krömeke, H.; Kruse, O. The metagenome of a biogas-producing microbial community of a production-scale biogas plant fermenter analysed by the 454-pyrosequencing technology. J. Biotechnol. 2008, 136, 77–90. [Google Scholar] [CrossRef] [PubMed]
- De Francisci, D.; Kougias, P.G.; Treu, L.; Campanaro, S.; Angelidaki, I. Microbial diversity and dynamicity of biogas reactors due to radical changes of feedstock composition. Bioresour. Technol. 2015, 176, 56–64. [Google Scholar] [CrossRef]
- Luo, G.; De Francisci, D.; Kougias, P.G.; Laura, T.; Zhu, X.; Angelidaki, I. New steady-state microbial community compositions and process performances in biogas reactors induced by temperature disturbances. Biotechnol. Biofuels 2015, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-J.; Lee, S.-H.; Lim, T.-G.; Park, H.-D. Effect of microbial community structure in inoculum on the stimulation of direct interspecies electron transfer for methanogenesis. Bioresour. Technol. 2021, 332, 125100. [Google Scholar] [CrossRef]
- Patil, S.M.; Kurade, M.B.; Basak, B.; Saha, S.; Jang, M.; Kim, S.-H.; Jeon, B.-H. Anaerobic co-digester microbiome during food waste valorization reveals Methanosaeta mediated methanogenesis with improved carbohydrate and lipid metabolism. Bioresour. Technol. 2021, 332, 125123. [Google Scholar] [CrossRef]
- Mazurkiewicz, J. Energy and economic balance between manure stored and used as a substrate for biogas production. Energies 2022, 15, 413. [Google Scholar] [CrossRef]
- Lv, Z.; Chen, Z.; Chen, X.; Liang, J.; Jiang, J.; Loake, G.J. Effects of various feedstocks on isotope fractionation of biogas and microbial community structure during anaerobic digestion. Waste Manag. 2019, 84, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Li, D.; Choi, O.; Sang, B.-I.; Chiang, P.C.; Kim, H. Effects of supplement additives on anaerobic biogas production. Korean J. Chem. Eng. 2017, 34, 2678–2685. [Google Scholar] [CrossRef]
- Leca, E.; Zennaro, B.; Hamelin, J.; Carrère, H.; Sambusiti, C. Use of additives to improve collective biogas plant performances: A comprehensive review. Biotechnol. Adv. 2023, 65, 108129. [Google Scholar] [CrossRef]
- Yan, M.; Treu, L.; Campanaro, S.; Tian, H.; Zhu, X.; Khoshnevisan, B.; Tsapekos, P.; Angelidaki, I.; Fotidis, I.A. Effect of ammonia on anaerobic digestion of municipal solid waste: Inhibitory performance, bioaugmentation and microbiome functional reconstruction. Chem. Eng. J. 2020, 401, 126159. [Google Scholar] [CrossRef]
- Aydin, S. Enhancement of microbial diversity and methane yield by bacterial bioaugmentation through the anaerobic digestion of Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 2016, 100, 5631–5637. [Google Scholar] [CrossRef] [PubMed]
- Nkemka, V.N.; Gilroyed, B.; Yanke, J.; Gruninger, R.; Vedres, D.; McAllister, T.; Hao, X. Bioaugmentation with an anaerobic fungus in a two-stage process for biohydrogen and biogas production using corn silage and cattail. Bioresour. Technol. 2015, 185, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Fonoll, X.; Shrestha, S.; Khanal, S.K.; Dosta, J.; Mata-Alvarez, J.; Raskin, L. Understanding the anaerobic digestibility of lignocellulosic substrates using rumen content as a cosubstrate and an inoculum. ACS EST Eng. 2021, 1, 424–435. [Google Scholar] [CrossRef]
- Dang, Y.; Holmes, D.E.; Zhao, Z.; Woodard, T.L.; Zhang, Y.; Sun, D.; Wang, L.-Y.; Nevin, K.P.; Lovley, D.R. Enhancing anaerobic digestion of complex organic waste with carbon-based conductive materials. Bioresour. Technol. 2016, 220, 516–522. [Google Scholar] [CrossRef]
- Wang, H.; Fotidis, I.A.; Angelidaki, I. Ammonia effect on hydrogenotrophic methanogens and syntrophic acetate-oxidizing bacteria. FEMS Microbiol. Ecol. 2015, 91, fiv130. [Google Scholar] [CrossRef]
- Westerholm, M.; Müller, B.; Singh, A.; Karlsson Lindsjö, O.; Schnürer, A. Detection of novel syntrophic acetate-oxidizing bacteria from biogas processes by continuous acetate enrichment approaches. Microb. Biotechnol. 2018, 11, 680–693. [Google Scholar] [CrossRef]
- Xu, N.; Liu, S.; Xin, F.; Zhou, J.; Jia, H.; Xu, J.; Jiang, M.; Dong, W. Biomethane production from lignocellulose: Biomass recalcitrance and its impacts on anaerobic digestion. Front. Bioeng. Biotechnol. 2019, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, R.; Chen, C.; Liu, G.; He, Y.; Liu, X. Biogas production from co-digestion of corn stover and chicken manure under anaerobic wet, hemi-solid, and solid state conditions. Bioresour. Technol. 2013, 149, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Triolo, J.M.; Pedersen, L.; Qu, H.; Sommer, S.G. Biochemical methane potential and anaerobic biodegradability of non-herbaceous and herbaceous phytomass in biogas production. Bioresour. Technol. 2012, 125, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, Y.; Hua, D.; Mu, H.; Zhao, Y.; Chen, G. Methane production from the anaerobic digestion of substrates from corn stover: Differences between the stem bark, stem pith, and leaves. Sci. Total Environ. 2019, 694, 133641. [Google Scholar] [CrossRef] [PubMed]
- Rasit, N.; Idris, A.; Harun, R.; Ghani, W.A.W.A.K. Effects of lipid inhibition on biogas production of anaerobic digestion from oily effluents and sludges: An overview. Renew. Sustain. Energy Rev. 2015, 45, 351–358. [Google Scholar] [CrossRef]
- Kalamaras, S.D.; Vasileiadis, S.; Karas, P.; Angelidaki, I.; Kotsopoulos, T.A. Microbial adaptation to high ammonia concentrations during anaerobic digestion of manure-based feedstock: Biomethanation and 16S rRNA gene sequencing. J. Chem. Technol. Biotechnol. 2020, 95, 1970–1979. [Google Scholar] [CrossRef]
- Westerholm, M.; Moestedt, J.; Schnürer, A. Biogas production through syntrophic acetate oxidation and deliberate operating strategies for improved digester performance. Appl. Energy 2016, 179, 124–135. [Google Scholar] [CrossRef]
- Shaibur, M.R.; Husain, H.; Arpon, S.H. Utilization of cow dung residues of biogas plant for sustainable development of a rural community. Curr. Res. Environ. Sustain. 2021, 3, 100026. [Google Scholar] [CrossRef]
- Siddiki, S.Y.A.; Uddin, M.; Mofijur, M.; Fattah, I.; Ong, H.C.; Lam, S.S.; Kumar, P.S.; Ahmed, S. Theoretical calculation of biogas production and greenhouse gas emission reduction potential of livestock, poultry and slaughterhouse waste in Bangladesh. J. Environ. Chem. Eng. 2021, 9, 105204. [Google Scholar] [CrossRef]
- Ning, J.; Zhou, M.; Pan, X.; Li, C.; Lv, N.; Wang, T.; Cai, G.; Wang, R.; Li, J.; Zhu, G. Simultaneous biogas and biogas slurry production from co-digestion of pig manure and corn straw: Performance optimization and microbial community shift. Bioresour. Technol. 2019, 282, 37–47. [Google Scholar] [CrossRef]
- Orlando, M.-Q.; Borja, V.-M. Pretreatment of animal manure biomass to improve biogas production: A review. Energies 2020, 13, 3573. [Google Scholar] [CrossRef]
- Tian, H.; Fotidis, I.A.; Mancini, E.; Treu, L.; Mahdy, A.; Ballesteros, M.; González-Fernández, C.; Angelidaki, I. Acclimation to extremely high ammonia levels in continuous biomethanation process and the associated microbial community dynamics. Bioresour. Technol. 2018, 247, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, L.; Song, Z.; Ren, G.; Feng, Y.; Han, X.; Yang, G. Biogas production by co-digestion of goat manure with three crop residues. PLoS ONE 2013, 8, e66845. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Verma, Y.P.; Chauhan, S. Effect of chemical pretreatment of sugarcane bagasse on biogas production. Mater. Today Proc. 2020, 21, 1937–1942. [Google Scholar] [CrossRef]
- Buivydas, E.; Navickas, K.; Venslauskas, K.; Žalys, B.; Župerka, V.; Rubežius, M. Biogas Production Enhancement through Chicken Manure Co-Digestion with Pig Fat. Appl. Sci. 2022, 12, 4652. [Google Scholar] [CrossRef]
- Orfanoudaki, A.; Makridakis, G.; Maragkaki, A.; Fountoulakis, M.; Kallithrakas-Kontos, N.; Manios, T. Anaerobic co-digestion of pig manure and spent coffee grounds for enhanced biogas production. Waste Biomass Valorization 2020, 11, 4613–4620. [Google Scholar] [CrossRef]
- Adelodun, A.A.; Olajire, T.M.; Ojo, O.M. Biogas Generation from Co-Digestion Waste Systems: The Role of Water Hyacinth. In Sustainable Rural Development Perspective and Global Challenges; IntechOpen: London, UK, 2022. [Google Scholar]
- Song, Z.; Zhang, C. Anaerobic codigestion of pretreated wheat straw with cattle manure and analysis of the microbial community. Bioresour. Technol. 2015, 186, 128–135. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.R. Enhancing the production of biogas through anaerobic co-digestion of agricultural waste and chemical pre-treatments. Chemosphere 2020, 255, 126805. [Google Scholar]
- Angelidaki, I.; Ahring, B.K. Methods for increasing the biogas potential from the recalcitrant organic matter contained in manure. Water Sci. Technol. 2000, 41, 189–194. [Google Scholar] [CrossRef]
- Liu, M.; Ni, H.; Yang, L.; Chen, G.; Yan, X.; Leng, X.; Liu, P.; Li, X. Pretreatment of swine manure containing β-lactam antibiotics with whole-cell biocatalyst to improve biogas production. J. Clean. Prod. 2019, 240, 118070. [Google Scholar] [CrossRef]
- Costa, J.; Barbosa, S.; Alves, M.; Sousa, D. Thermochemical pre-and biological co-treatments to improve hydrolysis and methane production from poultry litter. Bioresour. Technol. 2012, 111, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Patinvoh, R.J.; Feuk-Lagerstedt, E.; Lundin, M.; Sárvári Horváth, I.; Taherzadeh, M.J. Biological pretreatment of chicken feather and biogas production from total broth. Appl. Biochem. Biotechnol. 2016, 180, 1401–1415. [Google Scholar] [CrossRef] [PubMed]
- Rouches, E.; Zhou, S.; Steyer, J.-P.; Carrère, H. White-Rot Fungi pretreatment of lignocellulosic biomass for anaerobic digestion: Impact of glucose supplementation. Process Biochem. 2016, 51, 1784–1792. [Google Scholar] [CrossRef]
- Millati, R.; Syamsiah, S.; Niklasson, C.; Cahyanto, M.N.; Ludquist, K.; Taherzadeh, M.J. Biological pretreatment of lignocelluloses with white-rot fungi and its applications: A review. BioResources 2011, 6, 5224–5259. [Google Scholar]
- Christy, P.M.; Gopinath, L.; Divya, D. A review on anaerobic decomposition and enhancement of biogas production through enzymes and microorganisms. Renew. Sustain. Energy Rev. 2014, 34, 167–173. [Google Scholar]
- Mustafa, A.M.; Poulsen, T.G.; Sheng, K. Fungal pretreatment of rice straw with Pleurotus ostreatus and Trichoderma reesei to enhance methane production under solid-state anaerobic digestion. Appl. Energy 2016, 180, 661–671. [Google Scholar]
- Yadav, M.; Vivekanand, V. Combined fungal and bacterial pretreatment of wheat and pearl millet straw for biogas production–A study from batch to continuous stirred tank reactors. Bioresour. Technol. 2021, 321, 124523. [Google Scholar]
- Lalak, J.; Kasprzycka, A.; Martyniak, D.; Tys, J. Effect of biological pretreatment of Agropyron elongatum ‘BAMAR’ on biogas production by anaerobic digestion. Bioresour. Technol. 2016, 200, 194–200. [Google Scholar]
- Shah, T.A.; Lee, C.C.; Orts, W.J.; Tabassum, R. Biological pretreatment of rice straw by ligninolytic Bacillus sp. strains for enhancing biogas production. Environ. Prog. Sustain. Energy 2019, 38, e13036. [Google Scholar]
- Ali, S.S.; Sun, J. Physico-chemical pretreatment and fungal biotreatment for park wastes and cattle dung for biogas production. SpringerPlus 2015, 4, 712. [Google Scholar]
- Amirta, R.; Tanabe, T.; Watanabe, T.; Honda, Y.; Kuwahara, M.; Watanabe, T. Methane fermentation of Japanese cedar wood pretreated with a white rot fungus, Ceriporiopsis subvermispora. J. Biotechnol. 2006, 123, 71–77. [Google Scholar] [PubMed]
- Ge, X.; Matsumoto, T.; Keith, L.; Li, Y. Fungal pretreatment of albizia chips for enhanced biogas production by solid-state anaerobic digestion. Energy Fuels 2015, 29, 200–204. [Google Scholar] [CrossRef]
- Liu, X.; Hiligsmann, S.; Gourdon, R.; Bayard, R. Anaerobic digestion of lignocellulosic biomasses pretreated with Ceriporiopsis subvermispora. J. Environ. Manag. 2017, 193, 154–162. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Wu, S.; He, J.; Pang, C.; Deng, Y.; Dong, R. Fungal pretreatment by Phanerochaete chrysosporium for enhancement of biogas production from corn stover silage. Appl. Biochem. Biotechnol. 2014, 174, 1907–1918. [Google Scholar]
- Phutela, U.G.; Sahni, N. Effect of Fusarium sp. on paddy straw digestibility and biogas production. J. Adv. Lab. Res. Biol. 2012, 3, 9–12. [Google Scholar]
- Zhao, J.; Zheng, Y.; Li, Y. Fungal pretreatment of yard trimmings for enhancement of methane yield from solid-state anaerobic digestion. Bioresour. Technol. 2014, 156, 176–181. [Google Scholar]
- Muthangya, M.; Manoni Mshandete, A.; Kajumulo Kivaisi, A. Two-stage fungal pre-treatment for improved biogas production from sisal leaf decortication residues. Int. J. Mol. Sci. 2009, 10, 4805–4815. [Google Scholar] [CrossRef] [PubMed]
- Mutschlechner, M.; Illmer, P.; Wagner, A.O. Biological pre-treatment: Enhancing biogas production using the highly cellulolytic fungus Trichoderma viride. Waste Manag. 2015, 43, 98–107. [Google Scholar] [PubMed]
- Venturin, B.; Bonatto, C.; Damaceno, F.M.; Mulinari, J.; Fongaro, G.; Treichel, H. Physical, chemical, and biological substrate pretreatments to enhance biogas yield. In Improving Biogas Production: Technological Challenges, Alternative Sources, Future Developments; Springer: Berlin/Heidelberg, Germany, 2019; pp. 25–44. [Google Scholar]
- Mustafa, A.M.; Poulsen, T.G.; Xia, Y.; Sheng, K. Combinations of fungal and milling pretreatments for enhancing rice straw biogas production during solid-state anaerobic digestion. Bioresour. Technol. 2017, 224, 174–182. [Google Scholar]
- Choudhury, S.P.; Panda, S.; Haq, I.; Kalamdhad, A.S. Microbial pretreatment using Kosakonia oryziphila IH3 to enhance biogas production and hydrocarbon depletion from petroleum refinery sludge. Renew. Energy 2022, 194, 1192–1203. [Google Scholar]
- Shah, T.; Ullah, R. Pretreatment of wheat straw with ligninolytic fungi for increased biogas productivity. Int. J. Environ. Sci. Technol. 2019, 16, 7497–7508. [Google Scholar]
- Ali, S.S.; Abomohra, A.E.-F.; Sun, J. Effective bio-pretreatment of sawdust waste with a novel microbial consortium for enhanced biomethanation. Bioresour. Technol. 2017, 238, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Phutela, U.G.; Dar, R.A. Role of lignocellulolytic thermophilic fungus Thermoascus aurantiacus MTCC 375 in paddy straw digestibility and its implication in biogas production. Afr. J. Microbiol. Res. 2014, 8, 1798–1802. [Google Scholar]
- Zhao, J. Enhancement of Methane Production from Solid-State Anaerobic Digestion of Yard Trimmings by Biological Pretreatment; The Ohio State University: Columbus, OH, USA, 2013. [Google Scholar]
- Yuan, X.; Ma, L.; Wen, B.; Zhou, D.; Kuang, M.; Yang, W.; Cui, Z. Enhancing anaerobic digestion of cotton stalk by pretreatment with a microbial consortium (MC1). Bioresour. Technol. 2016, 207, 293–301. [Google Scholar] [CrossRef]
- Wen, B.; Yuan, X.; Li, Q.X.; Liu, J.; Ren, J.; Wang, X.; Cui, Z. Comparison and evaluation of concurrent saccharification and anaerobic digestion of Napier grass after pretreatment by three microbial consortia. Bioresour. Technol. 2015, 175, 102–111. [Google Scholar] [CrossRef]
- Poszytek, K.; Ciezkowska, M.; Sklodowska, A.; Drewniak, L. Microbial consortium with high cellulolytic activity (MCHCA) for enhanced biogas production. Front. Microbiol. 2016, 7, 324. [Google Scholar]
- Alexandropoulou, M.; Antonopoulou, G.; Ntaikou, I.; Lyberatos, G. Fungal pretreatment of willow sawdust with Abortiporus biennis for anaerobic digestion: Impact of an external nitrogen source. Sustainability 2017, 9, 130. [Google Scholar] [CrossRef]
- Alexandropoulou, M.; Antonopoulou, G.; Fragkou, E.; Ntaikou, I.; Lyberatos, G. Fungal pretreatment of willow sawdust and its combination with alkaline treatment for enhancing biogas production. J. Environ. Manag. 2017, 203, 704–713. [Google Scholar] [CrossRef]
- Cai, J.; Mo, X.; Cheng, G.; Du, D. Pretreatment of piggery wastewater by a stable constructed microbial consortium for improving the methane production. Water Sci. Technol. 2015, 71, 769–775. [Google Scholar] [CrossRef][Green Version]
- Meng, Y.; Luan, F.; Yuan, H.; Chen, X.; Li, X. Enhancing anaerobic digestion performance of crude lipid in food waste by enzymatic pretreatment. Bioresour. Technol. 2017, 224, 48–55. [Google Scholar]
- Domingues, R.D.F.; Sanches, T.; Silva, G.D.S.; Bueno, B.E.; Ribeiro, R.; Kamimura, E.S.; Neto, R.F.; Tommaso, G. Effect of enzymatic pretreatment on the anaerobic digestion of milk fat for biogas production. Food Res. Int. 2015, 73, 26–30. [Google Scholar]
- Karray, R.; Hamza, M.; Sayadi, S. Evaluation of ultrasonic, acid, thermo-alkaline and enzymatic pre-treatments on anaerobic digestion of Ulva rigida for biogas production. Bioresour. Technol. 2015, 187, 205–213. [Google Scholar]
- Su, H.; Tan, F.; Xu, Y. Enhancement of biogas and methanization of citrus waste via biodegradation pretreatment and subsequent optimized fermentation. Fuel 2016, 181, 843–851. [Google Scholar]
- Yu, S.; Zhang, G.; Li, J.; Zhao, Z.; Kang, X. Effect of endogenous hydrolytic enzymes pretreatment on the anaerobic digestion of sludge. Bioresour. Technol. 2013, 146, 758–761. [Google Scholar] [PubMed]
- Kavitha, S.; Jayashree, C.; Kumar, S.A.; Yeom, I.T.; Banu, J.R. The enhancement of anaerobic biodegradability of waste activated sludge by surfactant mediated biological pretreatment. Bioresour. Technol. 2014, 168, 159–166. [Google Scholar] [CrossRef]
- Ge, S.; Liu, L.; Xue, Q.; Yuan, Z. Effects of exogenous aerobic bacteria on methane production and biodegradation of municipal solid waste in bioreactors. Waste Manag. 2016, 55, 93–98. [Google Scholar] [PubMed]
- Merrylin, J.; Kumar, S.A.; Kaliappan, S.; Yeom, I.-T.; Banu, J.R. Biological pretreatment of non-flocculated sludge augments the biogas production in the anaerobic digestion of the pretreated waste activated sludge. Environ. Technol. 2013, 34, 2113–2123. [Google Scholar]
- Bagher, A.M.; Fatemeh, G.; Saman, M.; Leili, M. Advantages and disadvantages of biogas energy. Bull. Adv. Sci. Res. 2015, 1, 132–135. [Google Scholar]
- Khayal, O. Advantages and Limitations of Biogas Technologies. Preprint. Available online: https://www.researchgate.net/publication/336414255_ADVANTAGES_AND_LIMITATIONS_OF_BIOGAS_TECHNOLOGIES2019 (accessed on 13 August 2023).
- Tabatabaei, M.; Aghbashlo, M.; Valijanian, E.; Panahi, H.K.S.; Nizami, A.-S.; Ghanavati, H.; Sulaiman, A.; Mirmohamadsadeghi, S.; Karimi, K. A comprehensive review on recent biological innovations to improve biogas production, part 1: Upstream strategies. Renew. Energy 2020, 146, 1204–1220. [Google Scholar]
- Hagos, K.; Zong, J.; Li, D.; Liu, C.; Lu, X. Anaerobic co-digestion process for biogas production: Progress, challenges and perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1485–1496. [Google Scholar]
- Nielsen, H.B.; Angelidaki, I. Codigestion of manure and industrial organic waste at centralized biogas plants: Process imbalances and limitations. Water Sci. Technol. 2008, 58, 1521–1528. [Google Scholar] [CrossRef]
- Donkor, K.O.; Gottumukkala, L.D.; Lin, R.; Murphy, J.D. A perspective on the combination of alkali pre-treatment with bioaugmentation to improve biogas production from lignocellulose biomass. Bioresour. Technol. 2022, 351, 126950. [Google Scholar]
- Xue, S.; Wang, Y.; Lyu, X.; Zhao, N.; Song, J.; Wang, X.; Yang, G. Interactive effects of carbohydrate, lipid, protein composition and carbon/nitrogen ratio on biogas production of different food wastes. Bioresour. Technol. 2020, 312, 123566. [Google Scholar]
- Sivamani, S.; Saikat, B.; Naveen Prasad, B.; Baalawy, A.A.S.; Al-Mashali, S.M.A. A comprehensive review on microbial technology for biogas production. In Bioenergy Research: Revisiting Latest Development; Springer: Berlin/Heidelberg, Germany, 2021; pp. 53–78. [Google Scholar]
- Ma, J.; Frear, C.; Wang, Z.-w.; Yu, L.; Zhao, Q.; Li, X.; Chen, S. A simple methodology for rate-limiting step determination for anaerobic digestion of complex substrates and effect of microbial community ratio. Bioresour. Technol. 2013, 134, 391–395. [Google Scholar]
- Kanokratana, P.; Wongwilaiwalin, S.; Mhuantong, W.; Tangphatsornruang, S.; Eurwilaichitr, L.; Champreda, V. Characterization of cellulolytic microbial consortium enriched on Napier grass using metagenomic approaches. J. Biosci. Bioeng. 2018, 125, 439–447. [Google Scholar]
- Sarker, S.; Lamb, J.J.; Hjelme, D.R.; Lien, K.M. A review of the role of critical parameters in the design and operation of biogas production plants. Appl. Sci. 2019, 9, 1915. [Google Scholar]
- Ozbayram, E.G.; Ince, O.; Ince, B.; Harms, H.; Kleinsteuber, S. Comparison of rumen and manure microbiomes and implications for the inoculation of anaerobic digesters. Microorganisms 2018, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Razaviarani, V.; Buchanan, I.D. Anaerobic co-digestion of biodiesel waste glycerin with municipal wastewater sludge: Microbial community structure dynamics and reactor performance. Bioresour. Technol. 2015, 182, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Reichlen, M.J.; Vepachedu, V.R.; Murakami, K.S.; Ferry, J.G. MreA functions in the global regulation of methanogenic pathways in Methanosarcina acetivorans. MBio 2012, 3, e00189-12. [Google Scholar] [CrossRef] [PubMed]
- Lendormi, T.; Jaziri, K.; Béline, F.; Le Roux, S.; Bureau, C.; Midoux, C.; Barrington, S.; Dabert, P. Methane production and microbial community acclimation of five manure inocula during psychrophilic anaerobic digestion of swine manure. J. Clean. Prod. 2022, 340, 130772. [Google Scholar]
- Venkiteshwaran, K.; Bocher, B.; Maki, J.; Zitomer, D. Relating anaerobic digestion microbial community and process function: Supplementary issue: Water microbiology. Microbiol. Insights 2015, 8, 37–44. [Google Scholar] [CrossRef]
- Frank, J.; Arntzen, M.Ø.; Sun, L.; Hagen, L.H.; McHardy, A.; Horn, S.J.; Eijsink, V.G.; Schnürer, A.; Pope, P.B. Novel syntrophic populations dominate an ammonia-tolerant methanogenic microbiome. Msystems 2016, 1, e00092-16. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Tan, G.-Y.A.; Aslam, M.; Kim, J.; Lee, P.-H. Metatranscriptomic evidence for classical and RuBisCO-mediated CO2 reduction to methane facilitated by direct interspecies electron transfer in a methanogenic system. Sci. Rep. 2019, 9, 4116. [Google Scholar] [CrossRef]
- Zarraonaindia, I.; Smith, D.P.; Gilbert, J.A. Beyond the genome: Community-level analysis of the microbial world. Biol. Philos. 2013, 28, 261–282. [Google Scholar]
- Su, C.; Lei, L.; Duan, Y.; Zhang, K.-Q.; Yang, J. Culture-independent methods for studying environmental microorganisms: Methods, application, and perspective. Appl. Microbiol. Biotechnol. 2012, 93, 993–1003. [Google Scholar]
- Amani, T.; Nosrati, M.; Sreekrishnan, T. Anaerobic digestion from the viewpoint of microbiological, chemical, and operational aspects—A review. Environ. Rev. 2010, 18, 255–278. [Google Scholar]
- Ito, T.; Yoshiguchi, K.; Ariesyady, H.D.; Okabe, S. Identification and quantification of key microbial trophic groups of methanogenic glucose degradation in an anaerobic digester sludge. Bioresour. Technol. 2012, 123, 599–607. [Google Scholar]
- Pervin, H.M.; Dennis, P.G.; Lim, H.J.; Tyson, G.W.; Batstone, D.J.; Bond, P.L. Drivers of microbial community composition in mesophilic and thermophilic temperature-phased anaerobic digestion pre-treatment reactors. Water Res. 2013, 47, 7098–7108. [Google Scholar]
- Ho, D.P.; Jensen, P.D.; Batstone, D.J. Methanosarcinaceae and acetate-oxidizing pathways dominate in high-rate thermophilic anaerobic digestion of waste-activated sludge. Appl. Environ. Microbiol. 2013, 79, 6491–6500. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.C.; Morrison, M.; Yu, Z. A meta-analysis of the microbial diversity observed in anaerobic digesters. Bioresour. Technol. 2011, 102, 3730–3739. [Google Scholar] [PubMed]
- Sundberg, C.; Al-Soud, W.A.; Larsson, M.; Alm, E.; Yekta, S.S.; Svensson, B.H.; Sørensen, S.J.; Karlsson, A. 454 pyrosequencing analyses of bacterial and archaeal richness in 21 full-scale biogas digesters. FEMS Microbiol. Ecol. 2013, 85, 612–626. [Google Scholar] [PubMed]
- Vanwonterghem, I.; Jensen, P.D.; Ho, D.P.; Batstone, D.J.; Tyson, G.W. Linking microbial community structure, interactions and function in anaerobic digesters using new molecular techniques. Curr. Opin. Biotechnol. 2014, 27, 55–64. [Google Scholar]
- Stolze, Y.; Bremges, A.; Rumming, M.; Henke, C.; Maus, I.; Pühler, A.; Sczyrba, A.; Schlüter, A. Identification and genome reconstruction of abundant distinct taxa in microbiomes from one thermophilic and three mesophilic production-scale biogas plants. Biotechnol. Biofuels 2016, 9, 156. [Google Scholar] [PubMed]
- Fakruddin, M.; Mannan, K. Methods for analyzing diversity of microbial communities in natural environments. Ceylon J. Sci. 2013, 42, 19–33. [Google Scholar] [CrossRef]
- Yannarell, A.C.; Triplett, E.W. Geographic and environmental sources of variation in lake bacterial community composition. Appl. Environ. Microbiol. 2005, 71, 227–239. [Google Scholar] [CrossRef]
- Churko, J.M.; Mantalas, G.L.; Snyder, M.P.; Wu, J.C. Overview of high throughput sequencing technologies to elucidate molecular pathways in cardiovascular diseases. Circ. Res. 2013, 112, 1613–1623. [Google Scholar] [CrossRef]
- Ziganshin, A.M.; Liebetrau, J.; Pröter, J.; Kleinsteuber, S. Microbial community structure and dynamics during anaerobic digestion of various agricultural waste materials. Appl. Microbiol. Biotechnol. 2013, 97, 5161–5174. [Google Scholar]
- Zhang, H.; Banaszak, J.E.; Parameswaran, P.; Alder, J.; Krajmalnik-Brown, R.; Rittmann, B.E. Focused-Pulsed sludge pre-treatment increases the bacterial diversity and relative abundance of acetoclastic methanogens in a full-scale anaerobic digester. Water Res. 2009, 43, 4517–4526. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.J.; Knights, D.; Garcia, M.L.; Scalfone, N.B.; Smith, S.; Yarasheski, K.; Cummings, T.A.; Beers, A.R.; Knight, R.; Angenent, L.T. Bacterial community structures are unique and resilient in full-scale bioenergy systems. Proc. Natl. Acad. Sci. USA 2011, 108, 4158–4163. [Google Scholar]
- Lee, S.-H.; Kang, H.-J.; Lee, Y.H.; Lee, T.J.; Han, K.; Choi, Y.; Park, H.-D. Monitoring bacterial community structure and variability in time scale in full-scale anaerobic digesters. J. Environ. Monit. 2012, 14, 1893–1905. [Google Scholar] [CrossRef]
- Ronaghi, M.; Karamohamed, S.; Pettersson, B.; Uhlén, M.; Nyrén, P. Real-time DNA sequencing using detection of pyrophosphate release. Anal. Biochem. 1996, 242, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Ari, Ş.; Arikan, M. Next-generation sequencing: Advantages, disadvantages, and future. In Plant Omics: Trends and Applications; Springer: Berlin/Heidelberg, Germany, 2016; pp. 109–135. [Google Scholar]
- Li, Z.; Bai, X.; Ruparel, H.; Kim, S.; Turro, N.J.; Ju, J. A photocleavable fluorescent nucleotide for DNA sequencing and analysis. Proc. Natl. Acad. Sci. USA 2003, 100, 414–419. [Google Scholar] [PubMed]
- Carballa, M.; Regueiro, L.; Lema, J.M. Microbial management of anaerobic digestion: Exploiting the microbiome-functionality nexus. Curr. Opin. Biotechnol. 2015, 33, 103–111. [Google Scholar]
- Koch, C.; Müller, S.; Harms, H.; Harnisch, F. Microbiomes in bioenergy production: From analysis to management. Curr. Opin. Biotechnol. 2014, 27, 65–72. [Google Scholar]
- Yang, B.; Wang, Y.; Qian, P.-Y. Sensitivity and correlation of hypervariable regions in 16S rRNA genes in phylogenetic analysis. BMC Bioinform. 2016, 17, 135. [Google Scholar]
- Kim, M.; Oh, H.-S.; Park, S.-C.; Chun, J. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int. J. Syst. Evol. Microbiol. 2014, 64 Pt 2, 346–351. [Google Scholar]
- Yarza, P.; Yilmaz, P.; Pruesse, E.; Glöckner, F.O.; Ludwig, W.; Schleifer, K.-H.; Whitman, W.B.; Euzéby, J.; Amann, R.; Rosselló-Móra, R. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat. Rev. Microbiol. 2014, 12, 635–645. [Google Scholar] [CrossRef]
- Moyer, C.L.; Dobbs, F.C.; Karl, D.M. Estimation of diversity and community structure through restriction fragment length polymorphism distribution analysis of bacterial 16S rRNA genes from a microbial mat at an active, hydrothermal vent system, Loihi Seamount, Hawaii. Appl. Environ. Microbiol. 1994, 60, 871–879. [Google Scholar] [CrossRef]
- Muyzer, G.; De Waal, E.C.; Uitterlinden, A. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef]
- Cabezas, A.; de Araujo, J.C.; Callejas, C.; Galès, A.; Hamelin, J.; Marone, A.; Sousa, D.Z.; Trably, E.; Etchebehere, C. How to use molecular biology tools for the study of the anaerobic digestion process? Rev. Environ. Sci. Bio/Technol. 2015, 14, 555–593. [Google Scholar]
- Wagner, J.; Coupland, P.; Browne, H.P.; Lawley, T.D.; Francis, S.C.; Parkhill, J. Evaluation of PacBio sequencing for full-length bacterial 16S rRNA gene classification. BMC Microbiol. 2016, 16, 274. [Google Scholar] [CrossRef] [PubMed]
- Hassa, J.; Maus, I.; Off, S.; Pühler, A.; Scherer, P.; Klocke, M.; Schlüter, A. Metagenome, metatranscriptome, and metaproteome approaches unraveled compositions and functional relationships of microbial communities residing in biogas plants. Appl. Microbiol. Biotechnol. 2018, 102, 5045–5063. [Google Scholar] [PubMed]
- Bassani, I.; Kougias, P.G.; Treu, L.; Angelidaki, I. Biogas upgrading via hydrogenotrophic methanogenesis in two-stage continuous stirred tank reactors at mesophilic and thermophilic conditions. Environ. Sci. Technol. 2015, 49, 12585–12593. [Google Scholar] [CrossRef]
- Angly, F.E.; Felts, B.; Breitbart, M.; Salamon, P.; Edwards, R.A.; Carlson, C.; Chan, A.M.; Haynes, M.; Kelley, S.; Liu, H. The marine viromes of four oceanic regions. PLoS Biol. 2006, 4, e368. [Google Scholar]
- Edwards, R.A.; Rodriguez-Brito, B.; Wegley, L.; Haynes, M.; Breitbart, M.; Peterson, D.M.; Saar, M.O.; Alexander, S.; Alexander, E.C.; Rohwer, F. Using pyrosequencing to shed light on deep mine microbial ecology. BMC Genom. 2006, 7, 57. [Google Scholar] [CrossRef]
- Gill, S.R.; Pop, M.; DeBoy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Raes, J.; Foerstner, K.U.; Bork, P. Get the most out of your metagenome: Computational analysis of environmental sequence data. Curr. Opin. Microbiol. 2007, 10, 490–498. [Google Scholar] [CrossRef]
- Krause, L.; Diaz, N.N.; Edwards, R.A.; Gartemann, K.-H.; Krömeke, H.; Neuweger, H.; Pühler, A.; Runte, K.J.; Schlüter, A.; Stoye, J. Taxonomic composition and gene content of a methane-producing microbial community isolated from a biogas reactor. J. Biotechnol. 2008, 136, 91–101. [Google Scholar]
- Bashiardes, S.; Zilberman-Schapira, G.; Elinav, E. Use of metatranscriptomics in microbiome research. Bioinform. Biol. Insights 2016, 10, BBI-S34610. [Google Scholar]
- Wei, Y.; Wu, Y.; Zhang, L.; Zhou, Z.; Zhou, H.; Yan, X. Genome recovery and metatranscriptomic confirmation of functional acetate-oxidizing bacteria from enriched anaerobic biogas digesters. Environ. Pollut. 2020, 265, 114843. [Google Scholar] [CrossRef] [PubMed]
- Stark, L.; Giersch, T.; Wünschiers, R. Efficiency of RNA extraction from selected bacteria in the context of biogas production and metatranscriptomics. Anaerobe 2014, 29, 85–90. [Google Scholar] [PubMed]
- Gilbert, J.A.; Hughes, M. Gene expression profiling: Metatranscriptomics. In High-Throughput Next Generation Sequencing: Methods and Applications; Springer: Berlin/Heidelberg, Germany, 2011; pp. 195–205. [Google Scholar]
- Jia, Y.; Ng, S.-K.; Lu, H.; Cai, M.; Lee, P.K. Genome-centric metatranscriptomes and ecological roles of the active microbial populations during cellulosic biomass anaerobic digestion. Biotechnol. Biofuels 2018, 11, 117. [Google Scholar]
- Nolla-Ardèvol, V.; Strous, M.; Tegetmeyer, H.E. Anaerobic digestion of the microalga Spirulina at extreme alkaline conditions: Biogas production, metagenome, and metatranscriptome. Front. Microbiol. 2015, 6, 597. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.; Kougias, P.G.; Treu, L.; Kovalovszki, A.; Valle, G.; Cappa, F.; Morelli, L.; Angelidaki, I.; Campanaro, S. Microbial activity response to hydrogen injection in thermophilic anaerobic digesters revealed by genome-centric metatranscriptomics. Microbiome 2018, 6, 194. [Google Scholar] [PubMed]
- Maus, I.; Koeck, D.E.; Cibis, K.G.; Hahnke, S.; Kim, Y.S.; Langer, T.; Kreubel, J.; Erhard, M.; Bremges, A.; Off, S. Unraveling the microbiome of a thermophilic biogas plant by metagenome and metatranscriptome analysis complemented by characterization of bacterial and archaeal isolates. Biotechnol. Biofuels 2016, 9, 171. [Google Scholar]
- Zakrzewski, M.; Goesmann, A.; Jaenicke, S.; Jünemann, S.; Eikmeyer, F.; Szczepanowski, R.; Al-Soud, W.A.; Sørensen, S.; Pühler, A.; Schlüter, A. Profiling of the metabolically active community from a production-scale biogas plant by means of high-throughput metatranscriptome sequencing. J. Biotechnol. 2012, 158, 248–258. [Google Scholar]
- Güllert, S.; Fischer, M.A.; Turaev, D.; Noebauer, B.; Ilmberger, N.; Wemheuer, B.; Alawi, M.; Rattei, T.; Daniel, R.; Schmitz, R.A. Deep metagenome and metatranscriptome analyses of microbial communities affiliated with an industrial biogas fermenter, a cow rumen, and elephant feces reveal major differences in carbohydrate hydrolysis strategies. Biotechnol. Biofuels 2016, 9, 121. [Google Scholar]
- Dumont, M.G.; Murrell, J.C. Stable isotope probing—Linking microbial identity to function. Nat. Rev. Microbiol. 2005, 3, 499–504. [Google Scholar]
- Deng, Y.; Cui, X.; Dumont, M.G. Identification of active aerobic methanotrophs in plateau wetlands using DNA stable isotope probing. FEMS Microbiol. Lett. 2016, 363, fnw168. [Google Scholar]
- Buckley, D.H.; Huangyutitham, V.; Hsu, S.-F.; Nelson, T.A. Stable isotope probing with 15N2 reveals novel noncultivated diazotrophs in soil. Appl. Environ. Microbiol. 2007, 73, 3196–3204. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Murrell, J.C. When metagenomics meets stable-isotope probing: Progress and perspectives. Trends Microbiol. 2010, 18, 157–163. [Google Scholar] [PubMed]
- Mosbæk, F.; Kjeldal, H.; Mulat, D.G.; Albertsen, M.; Ward, A.J.; Feilberg, A.; Nielsen, J.L. Identification of syntrophic acetate-oxidizing bacteria in anaerobic digesters by combined protein-based stable isotope probing and metagenomics. ISME J. 2016, 10, 2405–2418. [Google Scholar]
- Zheng, D.; Wang, H.-Z.; Gou, M.; Nobu, M.K.; Narihiro, T.; Hu, B.; Nie, Y.; Tang, Y.-Q. Identification of novel potential acetate-oxidizing bacteria in thermophilic methanogenic chemostats by DNA stable isotope probing. Appl. Microbiol. Biotechnol. 2019, 103, 8631–8645. [Google Scholar] [PubMed]
- Wang, H.-Z.; Lv, X.-M.; Yi, Y.; Zheng, D.; Gou, M.; Nie, Y.; Hu, B.; Nobu, M.K.; Narihiro, T.; Tang, Y.-Q. Using DNA-based stable isotope probing to reveal novel propionate-and acetate-oxidizing bacteria in propionate-fed mesophilic anaerobic chemostats. Sci. Rep. 2019, 9, 17396. [Google Scholar]
- Ngo, V.Q.H.; Enault, F.; Midoux, C.; Mariadassou, M.; Chapleur, O.; Mazéas, L.; Loux, V.; Bouchez, T.; Krupovic, M.; Bize, A. Diversity of novel archaeal viruses infecting methanogens discovered through coupling of stable isotope probing and metagenomics. Environ. Microbiol. 2022, 24, 4853–4868. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J. Biomass to Renewable Energy Processes; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Xue, S.; Song, J.; Wang, X.; Shang, Z.; Sheng, C.; Li, C.; Zhu, Y.; Liu, J. A systematic comparison of biogas development and related policies between China and Europe and corresponding insights. Renew. Sustain. Energy Rev. 2020, 117, 109474. [Google Scholar]
- Meyer-Aurich, A.; Schattauer, A.; Hellebrand, H.J.; Klauss, H.; Plöchl, M.; Berg, W. Impact of uncertainties on greenhouse gas mitigation potential of biogas production from agricultural resources. Renew. Energy 2012, 37, 277–284. [Google Scholar]
- Leonard, L. Tackling Climate Change in the Global South: An Analysis of the Global Methane Initiative Multilateral Partnership. J. Soc. Dev. Sci. 2014, 5, 168–175. [Google Scholar] [CrossRef]
- Guilayn, F.; Rouez, M.; Crest, M.; Patureau, D.; Jimenez, J. Valorization of digestates from urban or centralized biogas plants: A critical review. Rev. Environ. Sci. Bio/Technol. 2020, 19, 419–462. [Google Scholar]
- Thema, M.; Bauer, F.; Sterner, M. Power-to-Gas: Electrolysis and methanation status review. Renew. Sustain. Energy Rev. 2019, 112, 775–787. [Google Scholar] [CrossRef]
- Jensen, M.B.; Kofoed, M.V.W.; Fischer, K.; Voigt, N.V.; Agneessens, L.M.; Batstone, D.J.; Ottosen, L.D.M. Venturi-type injection system as a potential H2 mass transfer technology for full-scale in situ biomethanation. Appl. Energy 2018, 222, 840–846. [Google Scholar] [CrossRef]
- Yang, D.; Park, S.Y.; Park, Y.S.; Eun, H.; Lee, S.Y. Metabolic engineering of Escherichia coli for natural product biosynthesis. Trends Biotechnol. 2020, 38, 745–765. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.R.; Jiao, S.; Lee, S.Y. Metabolic engineering strategies toward production of biofuels. Curr. Opin. Chem. Biol. 2020, 59, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Das, A.; Das, S.; Bhatawadekar, V.; Pandey, P.; Choure, K.; Damare, S.; Pandey, P. Petroleum Hydrocarbon Catabolic Pathways as Targets for Metabolic Engineering Strategies for Enhanced Bioremediation of Crude-Oil-Contaminated Environments. Fermentation 2023, 9, 196. [Google Scholar] [CrossRef]
- Rollin, J.A.; Martin del Campo, J.; Myung, S.; Sun, F.; You, C.; Bakovic, A.; Castro, R.; Chandrayan, S.K.; Wu, C.-H.; Adams, M.W. High-yield hydrogen production from biomass by in vitro metabolic engineering: Mixed sugars coutilization and kinetic modeling. Proc. Natl. Acad. Sci. USA 2015, 112, 4964–4969. [Google Scholar] [CrossRef]
- Sorathia, H.S.; Rathod, P.P.; Sorathiya, A.S. Biogas generation and factors affecting the Bio-gas generationea review study’. Int. J. Adv. Eng. Technol. 2012, 3, e78. [Google Scholar]
- Kosaka, F.; Liu, Y.; Chen, S.-Y.; Mochizuki, T.; Takagi, H.; Urakawa, A.; Kuramoto, K. Enhanced activity of integrated CO2 capture and reduction to CH4 under pressurized conditions toward atmospheric CO2 utilization. ACS Sustain. Chem. Eng. 2021, 9, 3452–3463. [Google Scholar] [CrossRef]
- Koytsoumpa, E.I.; Bergins, C.; Kakaras, E. The CO2 economy: Review of CO2 capture and reuse technologies. J. Supercrit. Fluids 2018, 132, 3–16. [Google Scholar] [CrossRef]
- dos Santos, R.G.; Alencar, A.C. Biomass-derived syngas production via gasification process and its catalytic conversion into fuels by Fischer Tropsch synthesis: A review. Int. J. Hydrog. Energy 2020, 45, 18114–18132. [Google Scholar] [CrossRef]
- Harahap, B.M.; Ahring, B.K. Acetate Production from Syngas Produced from Lignocellulosic Biomass Materials along with Gaseous Fermentation of the Syngas: A Review. Microorganisms 2023, 11, 995. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, M.; Busch, G. Methanation of hydrogen and carbon dioxide. Appl. Energy 2013, 111, 74–79. [Google Scholar] [CrossRef]
- Vogt, C.; Monai, M.; Kramer, G.J.; Weckhuysen, B.M. The renaissance of the Sabatier reaction and its applications on Earth and in space. Nat. Catal. 2019, 2, 188–197. [Google Scholar] [CrossRef]
- Zahed, M.A.; Movahed, E.; Khodayari, A.; Zanganeh, S.; Badamaki, M. Biotechnology for carbon capture and fixation: Critical review and future directions. J. Environ. Manag. 2021, 293, 112830. [Google Scholar] [CrossRef]
- Fu, S.; Angelidaki, I.; Zhang, Y. In situ biogas upgrading by CO2-to-CH4 bioconversion. Trends Biotechnol. 2021, 39, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Szuhaj, M.; Ács, N.; Tengölics, R.; Bodor, A.; Rákhely, G.; Kovács, K.L.; Bagi, Z. Conversion of H2 and CO2 to CH4 and acetate in fed-batch biogas reactors by mixed biogas community: A novel route for the power-to-gas concept. Biotechnol. Biofuels 2016, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Angelidaki, I. Microbial electrolysis cells turning to be versatile technology: Recent advances and future challenges. Water Res. 2014, 56, 11–25. [Google Scholar] [CrossRef]
- Mendonça, M. Feed-in Tariffs: Accelerating the Deployment of Renewable Energy; Routledge: London, UK, 2009. [Google Scholar]
- Bong, C.P.C.; Ho, W.S.; Hashim, H.; Lim, J.S.; Ho, C.S.; Tan, W.S.P.; Lee, C.T. Review on the renewable energy and solid waste management policies towards biogas development in Malaysia. Renew. Sustain. Energy Rev. 2017, 70, 988–998. [Google Scholar] [CrossRef]
- Carrosio, G. Energy production from biogas in the Italian countryside: Policies and organizational models. Energy Policy 2013, 63, 3–9. [Google Scholar] [CrossRef]
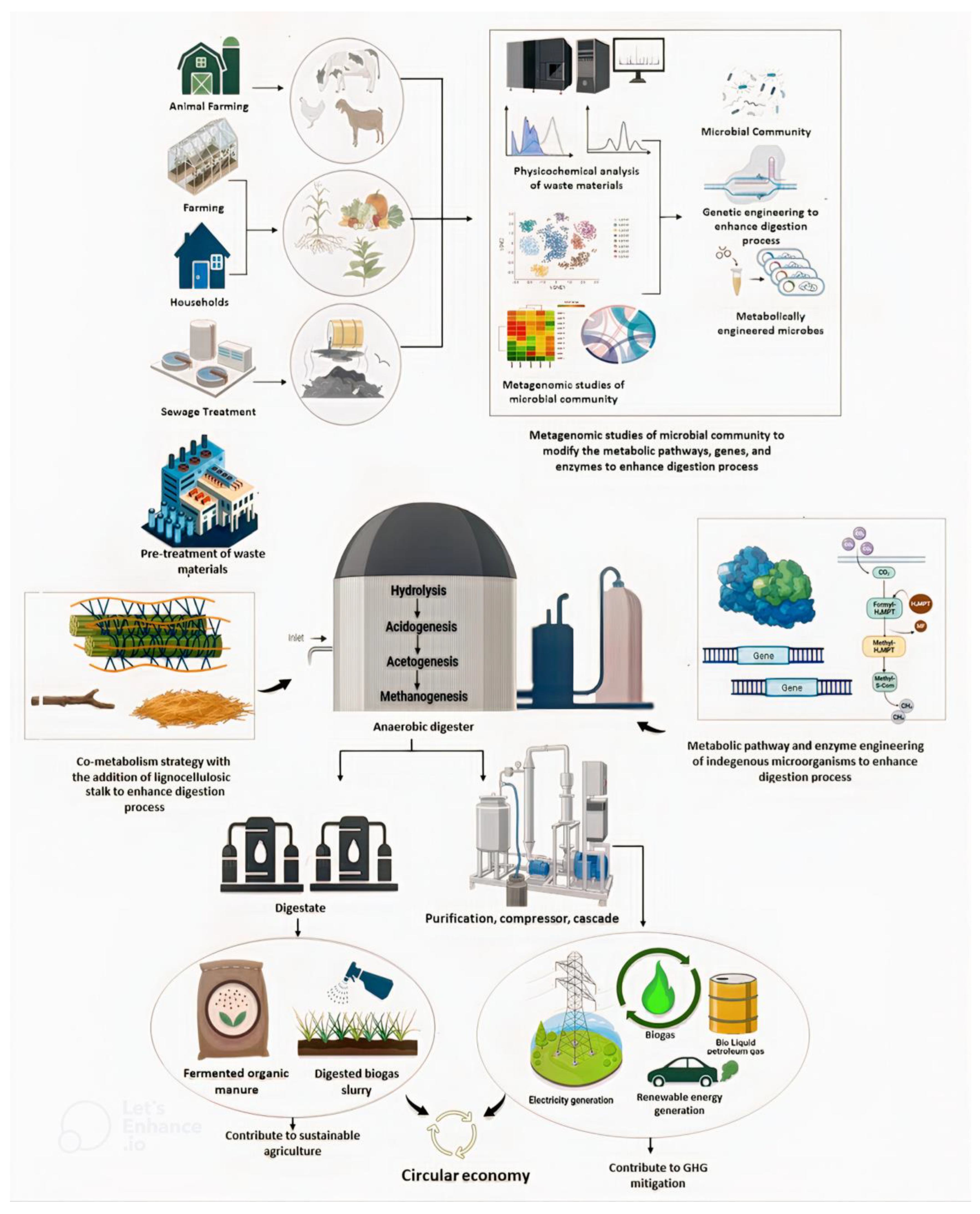
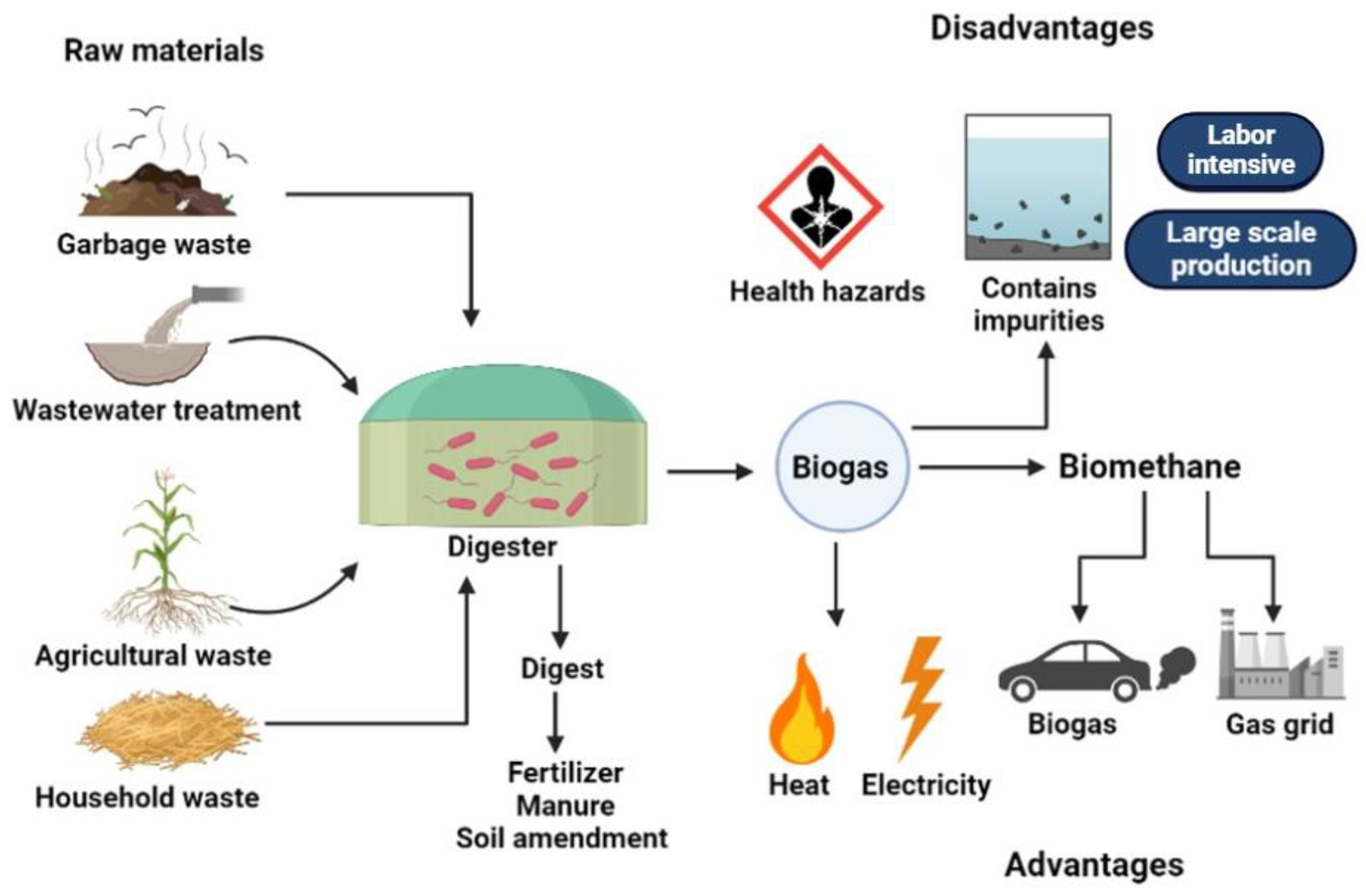
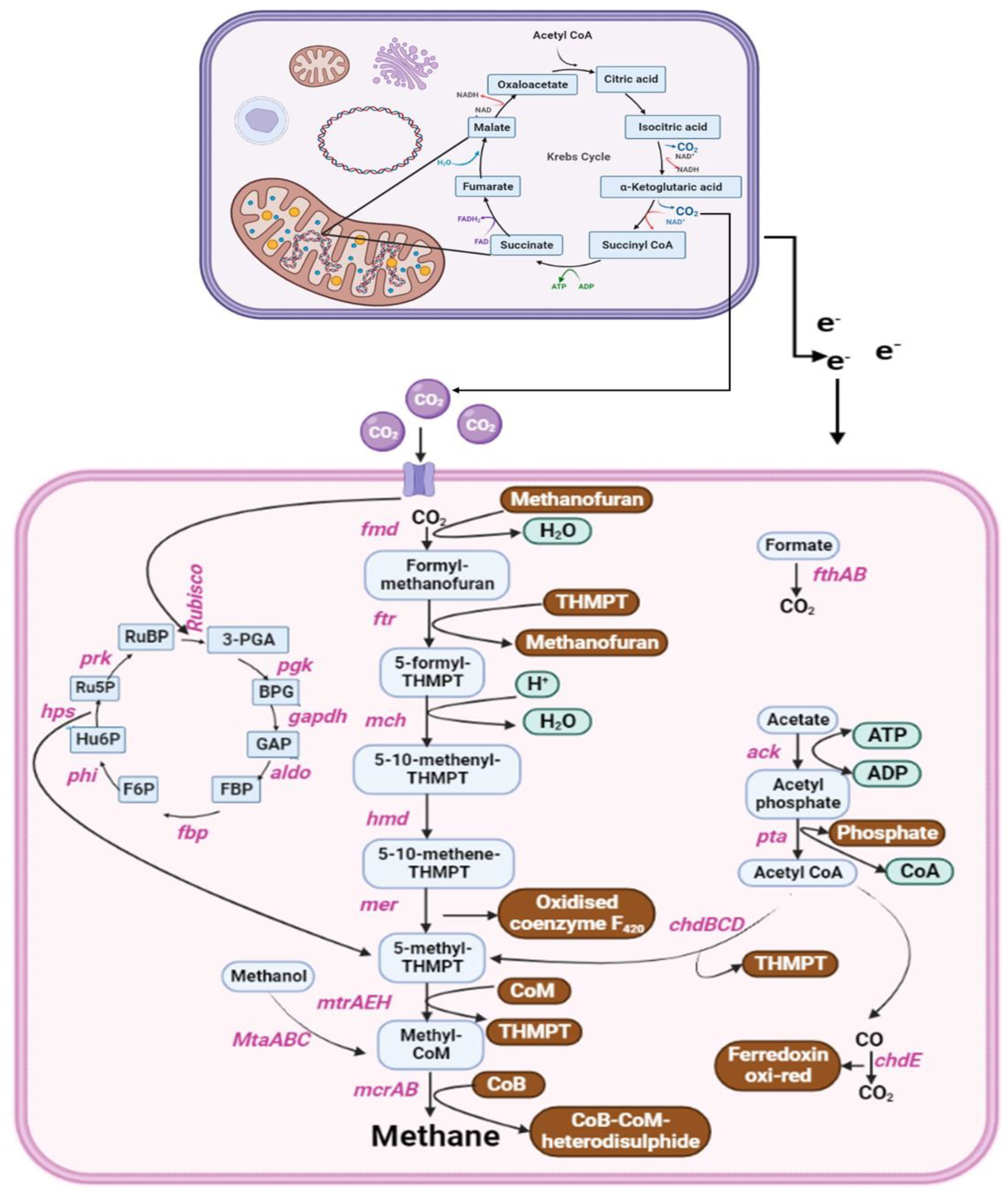
| Type of Waste Feed into Bioreactor | Pretreatment Strategy | Microorganism | Initial and Final Concentration of Biogas/Biomethane (mL/gVS) | Increase in Biogas/Biomethane Production (%) | Ref. |
|---|---|---|---|---|---|
| Tall Wheat Grass | Fungal | Agropyron elongatum | BG 120%; BM 134% | [99] | |
| Rice Straw | Bacteria | Ligninolytic Bacillus sp. Co-culture | BM Control: 270 Pretreated: 528.9 | BM 93.30% | [100] |
| Fresh leaves, dry leaves and cattle dung | Fungal | Aspergillus terreus and Trichoderma viride | BG Control: 102.6 Pretreated: 125.9 BM Control: 61.4 Pretreated: 79.8 | BG 22.71%; BM 29.97% | [101] |
| Japanese cedar wood | Fungal | Ceriporiopsis subvermispora | BG 35% | [102] | |
| Forestry waste | Fungal | Ceriporiopsis subvermispora | BG 270% | [103] | |
| Hazel branches | Fungal | Ceriporiopsis subvermispora | BM 60% | [104] | |
| Corn stover silage | Fungal | Phanerochaete chrysosporium | BM 19.6–32.6% | [105] | |
| Paddy straw | Fungal | Fusarium sp. | BG 53.8% | [106] | |
| Yard trimmings | Fungal | Ceriporiopsis subvermispora | BM Control: 20.5 Pretreated: 44.6 | BM 54% | [107] |
| Rice straw | Fungal | Pleurotus ostreatus | BM 20% | [97] | |
| Sisal leaf decortication residues | Fungal | Isolate CCHT-1 and Trichoderma reesei | BG Control: 292 Pretreated: 453 | BG 30–101% | [108] |
| Organic waste | Fungal | Trichoderma viride | BM 100% | [109] | |
| Agricultural Biomass | - | Pleurotus ostreatus | BM 120% | [110] | |
| Agricultural Biomass | - | Trichoderma reesei | BM 78.3% | [110] | |
| Rice straw | Fungal | Pleurotus ostreatus | BM Control: Pretreated: 258 | BM 165% | [111] |
| Petroleum refinery sludge | Bacterial | Kosakonia oryziphila | BG Control: 0.08 Pretreated: 5.15 | BG 56% | [112] |
| Wheat straw | Fungal | Lignin-degrading fungal culture from their natural habitat | BM 407.1% | [113] | |
| Sawdust waste | - | Gymnopilus pampeanus | BG Control: 232 Pretreated: 312 BM Control: 42.5 Pretreated: 155.2 | BG 25.6% BM 72.6% | [114] |
| Crop waste | Fungal | Polyporus brumalis | BM Control: 159.6 Pretreated: 280.5 | BM 75.75% | [94] |
| Crop waste | Fungal | Pl. ostreatus | BG Control: 270 Pretreated: 299 BM Control: 186 Pretreated: 212 | BG 10.74% BM 13.98% | [111] |
| Crop waste | Fungal | Thermoascus aurantiacus | BG Control: 390 Pretreated: 514.9 | BG 31.72% | [115] |
| Crop waste | Fungal | P. chrysosporium | BM 10.9% | [105] | |
| Crop waste | Fungal | C. subvermispora | BM Control: 36.1 Pretreated: 44.6 | BM 23.55% | [116] |
| Crop waste | MC | MC having Clostridium straminisolvens Pseudoxanthomonas Brevibacillus Bordetella Clostridium | BG Control: 173 Pretreated: 304 BM Control: 21 Pretreated: 79 | BG 75.72% BM 276.19% | [117] |
| Crop waste | MC | MC having Ochrobactrum sp. Coprinopsis cinereus | BM Control: 182.7 Pretreated: 279 | BM 49.04% | [118] |
| Crop waste | MC | MC having: Bacillus Providencia Ochrobactrum | BM Control: 249.3 Pretreated: 393.4 | BM 61.3% | [119] |
| Sawdust | Fungal | A. biennis | BM Control: 101.5 Pretreated: 145.3 | BM 47.88% | [120] |
| Sawdust | Fungal | L. menziesii A. biennis | BM Control: 101.3 Pretreated: 149.8 | BM 43.15% | [121] |
| Animal/Fish waste | Fungal | F. velutipe | BG Control: 330.2 Pretreated: 398.1 BM Control: 125.8 Pretreated: 169.2 | BG 20.56% BM 34.5% | [99] |
| Animal/Fish | MC | Bacilli Gammaproteobacteria Actinobacteria | BG Control: 107.9 Pretreated: 150.4 | BG 39.39% | [122] |
| Waste | Enzymatic | Aspergillus candida | BM Control: 68 Pretreated: 180 | BM 164.71% | [123] |
| Animal/Fish | Enzymatic | C. rugose G. candidum | BG Control: 219.4 Pretreated: 417.4 | BM 90.47% | [124] |
| Algae | Enzymatic | Geotrichum rugose | BG Control: 471 Pretreated: 626.5 | BG 33% | [125] |
| Fruit waste | Fungal | P. chrysosporium Aspergillus niger | BG Control: 145.2 Pretreated: 308.9 BM Control: 61 Pretreated: 176 | BG 112.74% BM 188.22% | [126] |
| Sludge | Enzymatic | B. subtilis A. hydrophila | BG Control: 207 Pretreated: 244.4 | BG 18.07% | [127] |
| Sludge | Enzymatic | Bacillus jerish | BG Control: 212 Pretreated: 467 | BG 120.28% | [128] |
| MSW | MC | MC having Bacillus cereus, B. subtilis, Staphylococcus saprophyticus Staphylococcus xylosus P. agglomerans P. chrysosporium | BM Control: 30.9 Pretreated: 81.8 | BM 190.61% | [129] |
| MSW | MC | B. licheniformis and others | BG Control: 24 Pretreated: 45.3 | BG 88.75% | [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, A.; Das, S.; Das, N.; Pandey, P.; Ingti, B.; Panchenko, V.; Bolshev, V.; Kovalev, A.; Pandey, P. Advancements and Innovations in Harnessing Microbial Processes for Enhanced Biogas Production from Waste Materials. Agriculture 2023, 13, 1689. https://doi.org/10.3390/agriculture13091689
Das A, Das S, Das N, Pandey P, Ingti B, Panchenko V, Bolshev V, Kovalev A, Pandey P. Advancements and Innovations in Harnessing Microbial Processes for Enhanced Biogas Production from Waste Materials. Agriculture. 2023; 13(9):1689. https://doi.org/10.3390/agriculture13091689
Chicago/Turabian StyleDas, Ankita, Sandeep Das, Nandita Das, Prisha Pandey, Birson Ingti, Vladimir Panchenko, Vadim Bolshev, Andrey Kovalev, and Piyush Pandey. 2023. "Advancements and Innovations in Harnessing Microbial Processes for Enhanced Biogas Production from Waste Materials" Agriculture 13, no. 9: 1689. https://doi.org/10.3390/agriculture13091689
APA StyleDas, A., Das, S., Das, N., Pandey, P., Ingti, B., Panchenko, V., Bolshev, V., Kovalev, A., & Pandey, P. (2023). Advancements and Innovations in Harnessing Microbial Processes for Enhanced Biogas Production from Waste Materials. Agriculture, 13(9), 1689. https://doi.org/10.3390/agriculture13091689









