Biochar, Halloysite, and Alginite Improve the Quality of Soil Contaminated with Petroleum Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Characteristics
2.1.1. Soil
2.1.2. Plant
2.1.3. Petroleum-Derived Products
2.1.4. Sorbents
2.2. Research Design
- (1)
- Preparation of 2.5 kg of LS or SL soils whose characteristics are presented in Table 1.
- (2)
- Soil amendment with:
- N, P, K and Mg in the following amounts (mg kg−1 dm of soil): 112, 39, 112 and 15, respectively. Nitrogen was applied as N2H4CO, phosphorus as—KH2PO4, potassium as—KH2PO4 and KCl, and magnesium as—MgSO4 × 7H2O;
- petroleum-derived products: diesel oil (DO) and petrol (P) at a rate of 0 and 7 cm3 kg−1 dm of soil;
- sorbents: biochar (B), halloysite (H) or alginite (A) at a rate of 0 and 10 g kg−1 dm of soil.
- (3)
- Mixing the soils was conducted with mineral fertilizers and, in appropriate treatments, with petroleum-derived products and sorbents. The soil was packed into polyethylene pots with a capacity of 3.0 dm3 (upper base diameter: 18.5 cm; lower base diameter: 14 cm; height: 15 cm) and the soil moisture was adjusted to 60% of the maximum water-holding capacity. This moisture level was maintained throughout the entire plant growth period (60 days).
- (4)
- Sowing 8 Zea mays seeds and leaving 5 plants in each pot after germination.
- (5)
- Harvesting the aerial and root parts of Zea mays at the Biologische Bundesanstalt, Bundessortenamt and Chemical (BBCH) stage 59. On the same day as the plant harvest, soil samples were collected for biochemical analyses. These samples were sieved through a 2.0 mm mesh sieve.
2.3. Methodology for Physico-Chemical and Chemical Determinations
2.4. Methodology for Determining Soil Enzyme Activities
2.5. Calculations
- IF—impact index of DO or P,
- DO—biomass of Zea mays or the activity value of the tested enzyme in DO-contaminated soil or P-contaminated soil without Ad,
- C2—biomass of Zea mays or the activity value of the tested enzyme in the control soil uncontaminated by DO or P without Ad.
- IF—impact index of sorbents: B, H or A,
- Ad_C2—biomass of Zea mays or the activity value of the tested enzyme in the control soil uncontaminated by DO or P with the addition of Ad (B, H, A),
- C2—the explanation is provided in formula number 1.
- IFAd_DO—impact index of sorbents: B, H lub A in soil contaminated DO,
- Ad_DO—biomass of Zea mays or the activity value of the tested enzyme in DO-contaminated soil with the addition of Ad (B, H, or A),
- DO—biomass of Zea mays or the activity value of the tested enzyme in DO-contaminated soil without Ad.
- IFAd_P—impact index of sorbents: B, H lub A in soil contaminated P,
- Ad_P—biomass of Zea mays or the activity value of the tested enzyme in P-contaminated soil with the addition of Ad (B, H, or A),
- P—biomass of Zea mays or the activity value of the tested enzyme in P-contaminated soil without Ad.
2.6. Statistical Analyses
3. Results
3.1. The Biomass Yield of Zea mays under the Influence of Diesel Oil, Petrol, Biochar, Halloysite, and Alginite
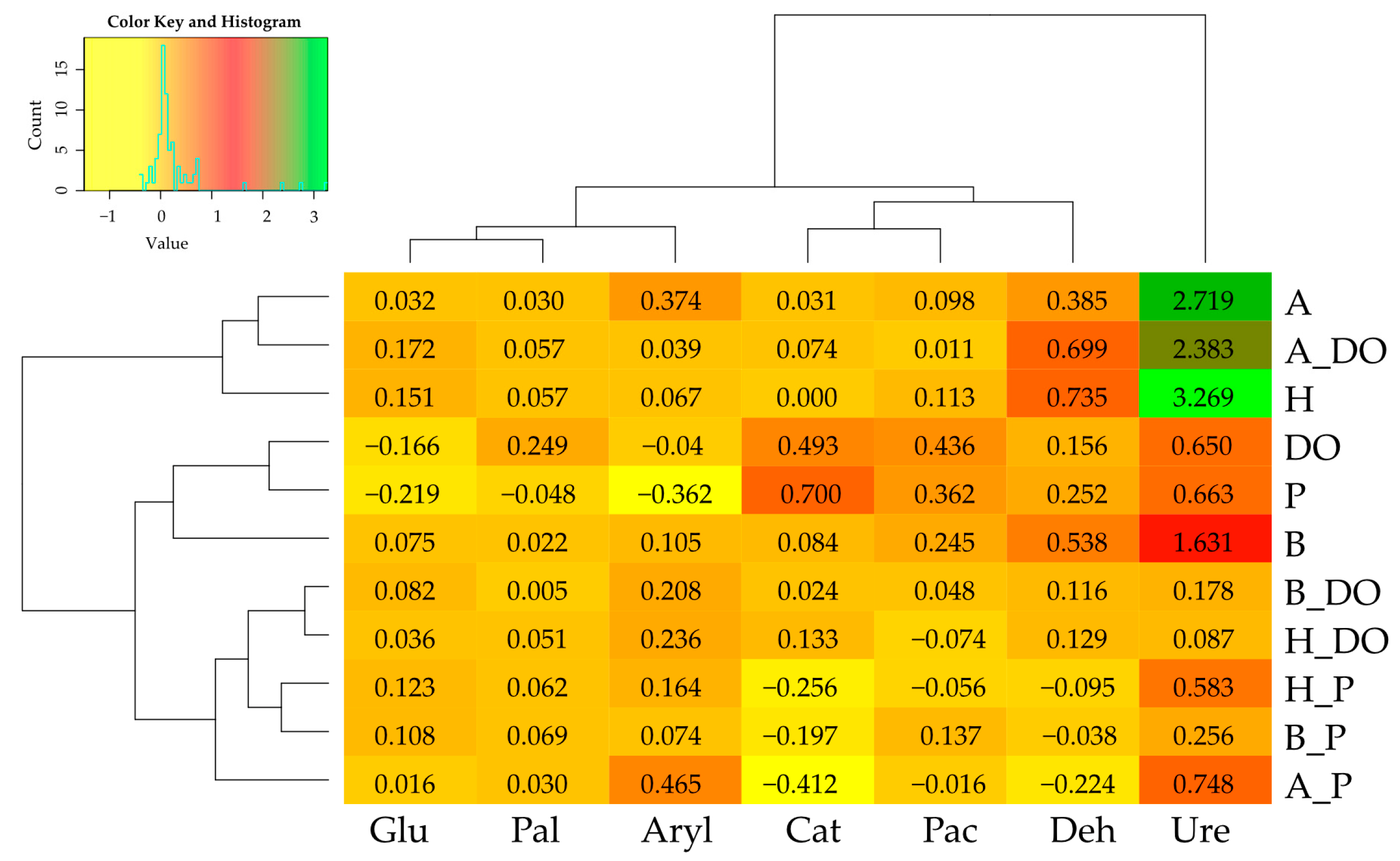
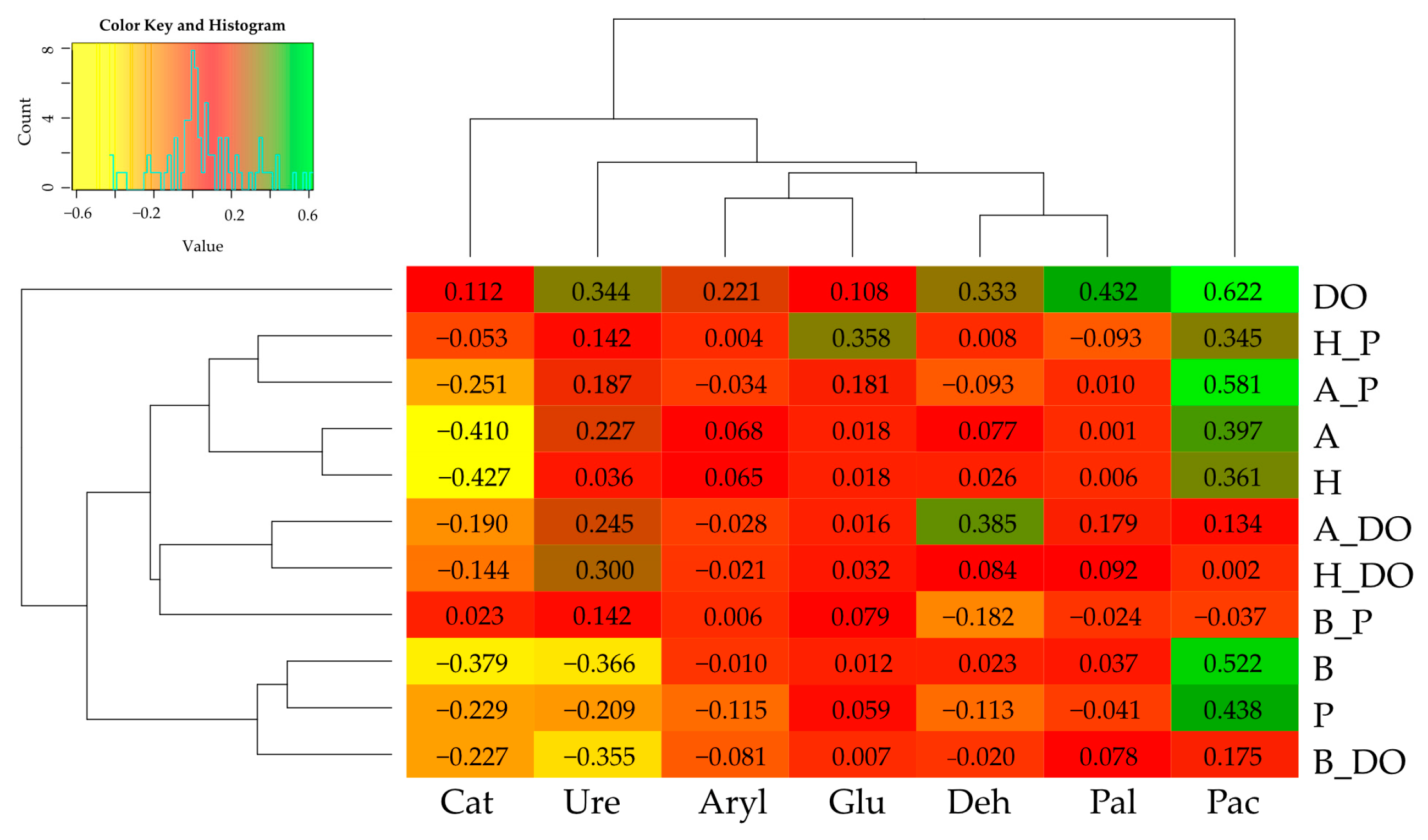
3.2. Changes in Soil Enzyme Activity under the Influence of Diesel Oil, Petrol, Biochar, Halloysite, and Alginite
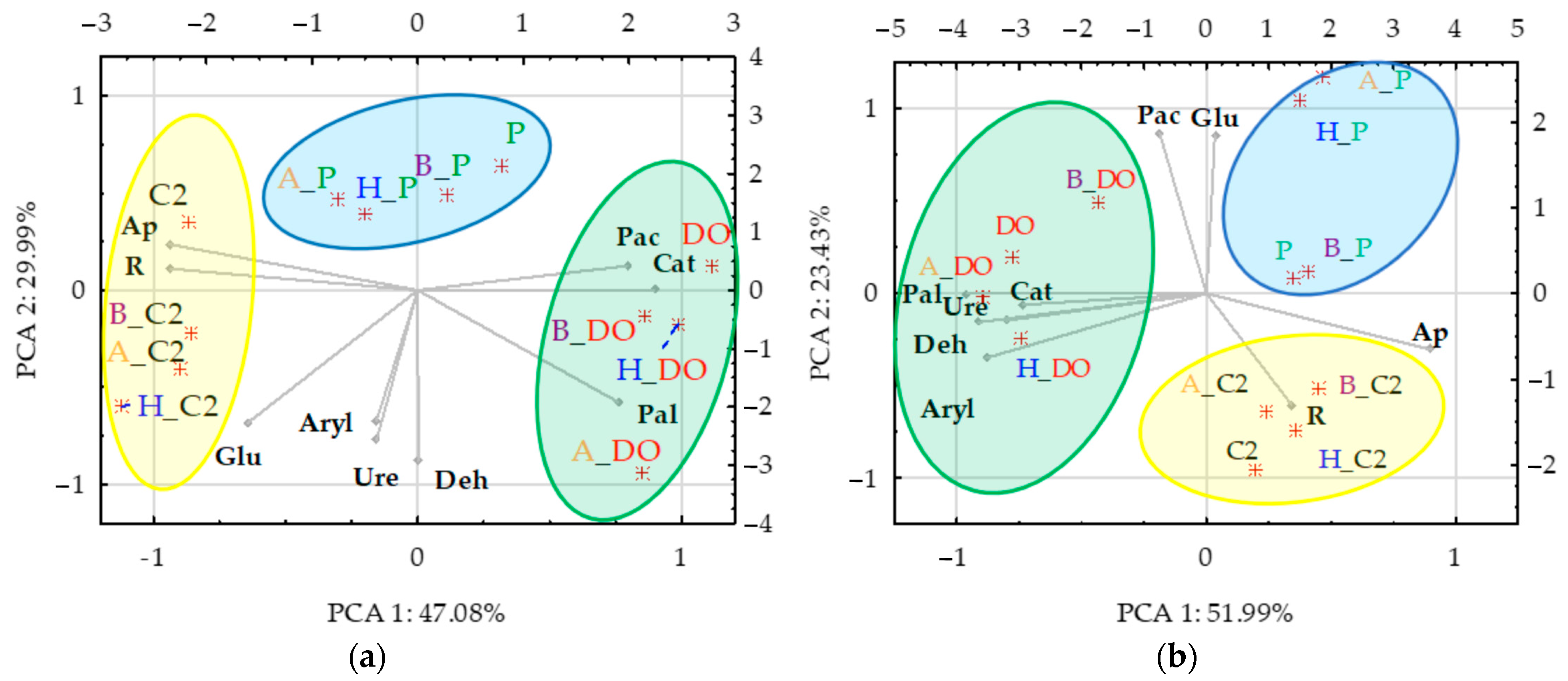
3.3. Correlations between Zea mays Biomass and Soil Enzyme Activity—Independent Variable Analysis and PCA
4. Discussion
4.1. Response of Zea mays and Soil Enzymes to Soil Contamination with Petroleum-Derived Products
4.2. The Role of Sorbents in Improving the Quality of Soil Contaminated with Petroleum-Derived Products
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ambaye, T.G.; Chebbi, A.; Formicola, F.; Prasad, S.; Gomez, F.H.; Franzetti, A.; Vaccari, M. Remediation of Soil Polluted with Petroleum Hydrocarbons and its Reuse for Agriculture: Recent Progress, Challenges, and Perspectives. Chemosphere 2022, 293, 133572. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wei, M.; Liao, K.; Qianli, M.; Shao, M.; Gu, F.; Fan, Y.; Longjie, L.; Yanfeng, H. Application of Environmentally Stimuli-Responsive Materials in the Development of Oil and Gas Field. J. Pet. Sci. Eng. 2022, 219, 111088. [Google Scholar] [CrossRef]
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of Soil and Water Contaminated with Petroleum Hydrocarbon: A Review. Environ. Technol. Innov. 2020, 17, 100526. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Kucharski, M.; Kucharski, J. Implications of Soil Pollution with Diesel Oil and BP Petroleum with ACTIVE Technology for Soil Health. Int. J. Environ. Res. Public. Health 2019, 16, 2474. [Google Scholar] [CrossRef]
- Directive 2012/18/EU of the European Parliament and of the Council of 4 July 2012 on the Control of Major-Accident Hazards Involving Dangerous Substances, Amending and Subsequently Repealing Council Directive 96/82/EC Text with EEA Relevance. Available online: https://eur-lex.europa.eu/eli/dir/2012/18/oj (accessed on 13 July 2023).
- Khalilova, H.K. The impact of oil contamination on soil ecosystem. Biol. Chem. Res. 2015, 2015, 133–139. [Google Scholar]
- Mohan, S.V.; Kisa, T.; Ohkuma, T.; Kanaly, R.A.; Shimizu, Y. Bioremediation technologies for treatment of PAH-contaminated soil and strategies to enhance process efficiency. Rev. Environ. Sci. Biotechnol. 2006, 5, 347–374. [Google Scholar] [CrossRef]
- Nasehi, S.A.; Uromeihy, A.; Nikudel, M.R.; Morsali, A. Influence of gas oil contamination on geotechnical properties of fine and coarse-grained soils. Geotech. Geol. Eng. 2016, 34, 333–345. [Google Scholar] [CrossRef]
- Kucharski, J.; Jastrzębska, E. Effects of heating oil on the count of microorganisms and physico-chemical properties of soil. Pol. J. Environ. Stud. 2005, 14, 189–198. [Google Scholar]
- Bolan, N.; Sarkar, B.; Yan, Y.; Li, Q.; Wijesekara, H.; Kannan, K.; Tsang, D.C.W.; Schauerte, M.; Bosch, J.; Noll, H.; et al. Remediation of poly- and perfluoroalkyl substances (PFAS) contaminated soils—To mobilize or to immobilize or to degrade? J. Hazard. Mater. 2021, 401, 123892. [Google Scholar] [CrossRef]
- Haghollahi, A.; Fazaelipoor, M.H.; Schaffie, M. The effect of soil type on the bioremediation of petroleum contaminated soils. J. Environ. Manag. 2016, 180, 197–201. [Google Scholar] [CrossRef]
- Yuan, L.; Wu, Y.; Fan, Q.; Li, P.; Liang, J.; Liu, Y.; Ma, R.; Li, R.; Shi, L. Remediating petroleum hydrocarbons in highly saline–alkali soils using three native plant species. J. Environ. Manag. 2023, 339, 117928. [Google Scholar] [CrossRef] [PubMed]
- Borowik, A.; Wyszkowska, J. Response of Avena sativa L. and the soil microbiota to the contamination of soil with Shell diesel oil. Plant Soil. Environ. 2018, 64, 102–107. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Kucharski, J. The Resistance of Lolium Perenne L. × Hybridum, Poa Pratensis, Festuca Rubra, F. Arundinacea, Phleum Pratense and Dactylis Glomerata to Soil Pollution by Diesel Oil and Petroleum. Plant Soil. Environ. 2019, 65, 307–312. [Google Scholar] [CrossRef]
- Yu, J.; Feng, H.; Tang, L.; Pang, Y.; Wang, J.; Zou, J.; Xie, Q.; Liu, Y.; Feng, C.; Wang, J. Insight into the Key Factors in Fast Adsorption of Organic Pollutants by Hierarchical Porous Biochar. J. Hazard. Mater. 2021, 403, 123610. [Google Scholar] [CrossRef] [PubMed]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. The Usability of Sorbents in Restoring Enzymatic Activity in Soils Polluted with Petroleum-Derived Products. Materials 2023, 16, 3738. [Google Scholar] [CrossRef]
- da Silva, L.J.; Alves, F.C.; de França, F.P. A Review of the Technological Solutions for the Treatment of Oily Sludges from Petroleum Refineries. Waste Manag. Res. 2012, 30, 1016–1030. [Google Scholar] [CrossRef]
- Bandura, L.; Woszuk, A.; Kołodyńska, D.; Franus, W. Application of Mineral Sorbents for Removal of Petroleum Substances: A Review. Minerals 2017, 7, 37. [Google Scholar] [CrossRef]
- Padhye, L.P.; Srivastava, P.; Jasemizad, T.; Bolan, S.; Hou, D.; Shaheen, S.M.; Rinklebe, J.; O’Connor, D.; Lamb, D.; Wang, H. Contaminant Containment for Sustainable Remediation of Persistent Contaminants in Soil and Groundwater. J. Hazard. Mater. 2023, 455, 131575. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Kordala, N. Role of Different Material Amendments in Shaping the Content of Heavy Metals in Maize (Zea mays L.) on Soil Polluted with Petrol. Materials 2022, 15, 2623. [Google Scholar] [CrossRef]
- Valdiviezo Gonzales, L.G.; Castañeda-Olivera, C.A.; Cabello-Torres, R.J.; García Ávila, F.F.; Cerrón, R.V.M.; Alfaro Paredes, E.A. Scientometric Study of Treatment Technologies of Soil Pollution: Present and Future Challenges. Appl. Soil. Ecol. 2023, 182, 104695. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Wyszkowska, J.; Kordala, N.; Borowik, A. Effects of Coal and Sewage Sludge Ashes on Macronutrient Content in Maize (Zea mays L.) Grown on Soil Contaminated with Eco-Diesel Oil. Materials 2022, 15, 525. [Google Scholar] [CrossRef] [PubMed]
- Borowik, A.; Wyszkowska, J. Remediation of Soil Contaminated with Diesel Oil. J. Elementol. 2018, 23, 767–788. [Google Scholar] [CrossRef]
- Stepanova, A.Y.; Gladkov, E.A.; Osipova, E.S.; Gladkova, O.V.; Tereshonok, D.V. Bioremediation of Soil from Petroleum Contamination. Processes 2022, 10, 1224. [Google Scholar] [CrossRef]
- Kisić, I.; Hrenović, J.; Zgorelec, Ž.; Durn, G.; Brkić, V.; Delač, D. Bioremediation of Agriculture Soil Contaminated by Organic Pollutants. Energies 2022, 15, 1561. [Google Scholar] [CrossRef]
- Hamidzadeh, Z.; Ghorbannezhad, P.; Ketabchi, M.R.; Yeganeh, B. Biomass-Derived Biochar and Its Application in Agriculture. Fuel 2023, 341, 127701. [Google Scholar] [CrossRef]
- Jabborova, D.; Annapurna, K.; Paul, S.; Kumar, S.; Saad, H.A.; Desouky, S.; Ibrahim, M.F.M.; Elkelish, A. Beneficial Features of Biochar and Arbuscular Mycorrhiza for Improving Spinach Plant Growth, Root Morphological Traits, Physiological Properties, and Soil Enzymatic Activities. J. Fungi 2021, 7, 571. [Google Scholar] [CrossRef]
- Song, X.; Li, H.; Song, J.; Chen, W.; Shi, L. Biochar/Vermicompost Promotes Hybrid Pennisetum Plant Growth and Soil Enzyme Activity in Saline Soils. Plant Physiol. Biochem. 2022, 183, 96–110. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Z.; Wang, X.; Sun, Q.; Dong, H.; Wang, G.; Chen, X.; Yin, C.; Han, Z.; Mao, Z. Effects of Biochar on the Growth of Apple Seedlings, Soil Enzyme Activities and Fungal Communities in Replant Disease Soil. Sci. Hortic. 2019, 256, 108641. [Google Scholar] [CrossRef]
- Ajeng, A.A.; Abdullah, R.; Ling, T.C.; Ismail, S.; Lau, B.F.; Ong, H.C.; Chew, K.W.; Show, P.L.; Chang, J.-S. Bioformulation of Biochar as a Potential Inoculant Carrier for Sustainable Agriculture. Environ. Technol. Innov. 2020, 20, 101168. [Google Scholar] [CrossRef]
- Li, C.; Ahmed, W.; Li, D.; Yu, L.; Xu, L.; Xu, T.; Zhao, Z. Biochar Suppresses Bacterial Wilt Disease of Flue-Cured Tobacco by Improving Soil Health and Functional Diversity of Rhizosphere Microorganisms. Appl. Soil. Ecol. 2022, 171, 104314. [Google Scholar] [CrossRef]
- Selvarajoo, A.; Wong, Y.L.; Khoo, K.S.; Chen, W.-H.; Show, P.L. Biochar Production via Pyrolysis of Citrus Peel Fruit Waste as a Potential Usage as Solid Biofuel. Chemosphere 2022, 294, 133671. [Google Scholar] [CrossRef]
- Ren, H.-Y.; Wei, Z.-J.; Wang, Y.; Deng, Y.-P.; Li, M.-Y.; Wang, B. Effects of Biochar Properties on the Bioremediation of the Petroleum-Contaminated Soil from a Shale-Gas Field. Environ. Sci. Pollut. Res. 2020, 27, 36427–36438. [Google Scholar] [CrossRef]
- Guo, M.; Song, W.; Tian, J. Biochar-Facilitated Soil Remediation: Mechanisms and Efficacy Variations. Front. Environ. Sci. 2020, 8, 521512. [Google Scholar] [CrossRef]
- Fedeli, R.; Alexandrov, D.; Celletti, S.; Nafikova, E.; Loppi, S. Biochar Improves the Performance of Avena Sativa L. Grown in Gasoline-Polluted Soils. Environ. Sci. Pollut. Res. 2023, 30, 28791–28802. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wei, Y.; Liu, Y.; Niu, S.; Xu, Y.; Park, J.-H.; Wang, J.J. Biochar-Based Materials as Remediation Strategy in Petroleum Hydrocarbon-Contaminated Soil and Water: Performances, Mechanisms, and Environmental Impact. J. Environ. Sci. 2024, 138, 350–372. [Google Scholar] [CrossRef]
- Gao, J.; Han, H.; Gao, C.; Wang, Y.; Dong, B.; Xu, Z. Organic Amendments for in Situ Immobilization of Heavy Metals in Soil: A Review. Chemosphere 2023, 335, 139088. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Evaluation of the Usefulness of Sorbents in the Remediation of Soil Exposed to the Pressure of Cadmium and Cobalt. Materials 2022, 15, 5738. [Google Scholar] [CrossRef] [PubMed]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Mitigation of the Adverse Impact of Copper, Nickel, and Zinc on Soil Microorganisms and Enzymes by Mineral Sorbents. Materials 2022, 15, 5198. [Google Scholar] [CrossRef]
- Tamsu Selli, N.; Basaran, N. Controlling the Hardness and Wear Resistance of Opaque White Glaze by Addition of Halloysite Clay in the Composition. Bol. Soc. Esp. Ceram. Vidr. 2023, 62, 243–256. [Google Scholar] [CrossRef]
- Maj, I.; Matus, K. Aluminosilicate Clay Minerals: Kaolin, Bentonite, and Halloysite as Fuel Additives for Thermal Conversion of Biomass and Waste. Energies 2023, 16, 4359. [Google Scholar] [CrossRef]
- Masoudniaragh, A.; Oraei, M.; Gohari, G.; Akbari, A.; Faramarzi, A. Using Halloysite Nanotubes as Carrier for Proline to Alleviate Salt Stress Effects in Sweet Basil (Ocimum Basilicum L.). Sci. Hortic. 2021, 285, 110202. [Google Scholar] [CrossRef]
- Gömöryová, E.; Vass, D.; Pichler, V.; Gömöry, D. Effect of Alginite Amendment on Microbial Activity and Soil Water Content in Forest Soils. Biologia 2009, 64, 585–588. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zimmerman, A.R.; Harris, W. Surface Chemistry Variations among a Series of Laboratory-Produced Biochars. Geoderma 2011, 163, 247–255. [Google Scholar] [CrossRef]
- Hippmann, S.; Ahmed, S.S.; Fröhlich, P.; Bertau, M. Demulsification of Water/Crude Oil Emulsion Using Natural Rock Alginite. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 71–79. [Google Scholar] [CrossRef]
- Wang, B.; Gao, B.; Zimmerman, A.R.; Zheng, Y.; Lyu, H. Novel Biochar-Impregnated Calcium Alginate Beads with Improved Water Holding and Nutrient Retention Properties. J. Environ. Manag. 2018, 209, 105–111. [Google Scholar] [CrossRef]
- Strachel, R.; Wyszkowska, J.; Baćmaga, M. An Evaluation of the Effectiveness of Sorbents in the Remediation of Soil Contaminated with Zinc. Water Air Soil. Pollut. 2018, 229, 235. [Google Scholar] [CrossRef]
- Matvieieva, N.; Duplij, V.; Vozár, Ľ.; Kovár, P.; Hric, P. Stimulation Effect of Alginite on Rhodiola Rosea L. in Vitro Growth. Agrobiodivers Improv. 2023, 7, 7–16. [Google Scholar]
- Pour, M.M.; Riseh, R.S.; Ranjbar-Karimi, R.; Hassanisaadi, M.; Rahdar, A.; Baino, F. Microencapsulation of Bacillus Velezensis Using Alginate-Gum Polymers Enriched with TiO2 and SiO2 Nanoparticles. Micromachines 2022, 13, 1423. [Google Scholar] [CrossRef]
- Zhao, L.; Angel Hernandez-Viezcas, J.; Peralta-Videa, J.R.; Bandyopadhyay, S.; Peng, B.; Munoz, B.; Keller, A.A.; Gardea-Torresdey, J.L. ZnO Nanoparticle Fate in Soil and Zinc Bioaccumulation in Corn Plants (Zea mays) Influenced by Alginate. Environ. Sci. Process. Impacts 2013, 15, 260–266. [Google Scholar] [CrossRef]
- Szopa, D.; Mielczarek, M.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Chojnacka, K.; Witek-Krowiak, A. Encapsulation Efficiency and Survival of Plant Growth-Promoting Microorganisms in an Alginate-Based Matrix—A Systematic Review and Protocol for a Practical Approach. Ind. Crops Prod. 2022, 181, 114846. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Hassanisaadi, M.; Vatankhah, M.; Soroush, F.; Varma, R.S. Nano/Microencapsulation of Plant Biocontrol Agents by Chitosan, Alginate, and Other Important Biopolymers as a Novel Strategy for Alleviating Plant Biotic Stresses. Int. J. Biol. Macromol. 2022, 222, 1589–1604. [Google Scholar] [CrossRef]
- Song, Y.; Bechtel, A.; Sachsenhofer, R.F.; Groß, D.; Liu, Z.; Meng, Q. Depositional Environment of the Lower Cretaceous Muling Formation of the Laoheishan Basin (NE China): Implications from Geochemical and Petrological Analyses. Org. Geochem. 2017, 104, 19–34. [Google Scholar] [CrossRef]
- OECD-FAO. OECD-FAO Agricultural Outlook 2021–2030; OECD Publishing: Paris, France, 2021; ISBN 978-92-64-43607-7. [Google Scholar]
- Erenstein, O.; Chamberlin, J.; Sonder, K. Estimating the Global Number and Distribution of Maize and Wheat Farms. Glob. Food Secur. 2021, 30, 100558. [Google Scholar] [CrossRef]
- Yi, L.; Shenjiao, Y.; Shiqing, L.; Xinping, C.; Fang, C. Growth and Development of Maize (Zea mays L.) in Response to Different Field Water Management Practices: Resource Capture and Use Efficiency. Agric. Meteorol. 2010, 150, 606–613. [Google Scholar] [CrossRef]
- Aghili, S.; Golzary, A. Greening the Earth, Healing the Soil: A Comprehensive Life Cycle Assessment of Phytoremediation for Heavy Metal Contamination. Environ. Technol. Innov. 2023, 32, 103241. [Google Scholar] [CrossRef]
- Usowicz, B.; Lipiec, J. Spatial Variability of Soil Properties and Cereal Yield in a Cultivated Field on Sandy Soil. Soil Tillage Res. 2017, 174, 241–250. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106.; FAO: Rome, Italy, 2015. [Google Scholar]
- Morales-Máximo, C.N.; López-Sosa, L.B.; Rutiaga-Quiñones, J.G.; Corral-Huacuz, J.C.; Aguilera-Mandujano, A.; Pintor-Ibarra, L.F.; López-Miranda, A.; Delgado-Domínguez, S.N.; Rodríguez-Magallón, M.D.C.; Morales-Máximo, M. Characterization of Agricultural Residues of Zea mays for Their Application as Solid Biofuel: Case Study in San Francisco Pichátaro, Michoacán, Mexico. Energies 2022, 15, 6870. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Calorific Value of Zea mays Biomass Derived from Soil Contaminated with Chromium (VI) Disrupting the Soil’s Biochemical Properties. Energies 2023, 16, 3788. [Google Scholar] [CrossRef]
- Liao, C.; Xu, W.; Lu, G.; Liang, X.; Guo, C.; Yang, C.; Dang, Z. Accumulation of Hydrocarbons by Maize (Zea mays L.) in Remediation of Soils Contaminated with Crude Oil. Int. J. Phytoremediation 2015, 17, 693–700. [Google Scholar] [CrossRef]
- Murtaza, G.; Ahmed, Z.; Eldin, S.M.; Ali, B.; Bawazeer, S.; Usman, M.; Iqbal, R.; Neupane, D.; Ullah, A.; Khan, A.; et al. Biochar-Soil-Plant Interactions: A Cross Talk for Sustainable Agriculture under Changing Climate. Front. Environ. Sci. 2023, 11, 1059449. [Google Scholar] [CrossRef]
- Ayaz, M.; Feizienė, D.; Tilvikienė, V.; Akhtar, K.; Stulpinaitė, U.; Iqbal, R. Biochar Role in the Sustainability of Agriculture and Environment. Sustainability 2021, 13, 1330. [Google Scholar] [CrossRef]
- Radziemska, M.; Gusiatin, Z.M.; Kumar, V.; Brtnicky, M. Co-Application of Nanosized Halloysite and Biochar as Soil Amendments in Aided Phytostabilization of Metal(-Oid)s-Contaminated Soil under Different Temperature Conditions. Chemosphere 2022, 288, 132452. [Google Scholar] [CrossRef] [PubMed]
- Zaborowska, M.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Effect of Separate and Combined Toxicity of Bisphenol A and Zinc on the Soil Microbiome. Int. J. Mol. Sci. 2022, 23, 5937. [Google Scholar] [CrossRef] [PubMed]
- Borowik, A.; Wyszkowska, J.; Wyszkowski, M. Resistance of Aerobic Microorganisms and Soil Enzyme Response to Soil Contamination with Ekodiesel Ultra Fuel. Environ. Sci. Pollut. Res. 2017, 24, 24346–24363. [Google Scholar] [CrossRef]
- RStudio Team RStudio Team. RStudio: Integrated Development for R. RStudio Version 2023.06.0, PBC, Boston, MA, USA. 2020. Available online: http://www.rstudio.com/ (accessed on 10 July 2023).
- R Core Team R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing, Version 4.2.2. Vienna, Austria. 2022. Available online: https://www.r-project.org/ (accessed on 10 July 2023).
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, M.; Moeller, S.; et al. Gplots: Various R Programming Tools for Plotting Data. Gplots 3.1.3. 2022. Available online: https://cran.r-project.org/web/packages/gplots/ (accessed on 10 July 2023).
- Wyszkowska, J.; Borowik, A.; Kucharski, M.; Borowik, A. Applicability of biochemical indices to quality assessment of soil polluted with heavy metal. J. Elementol. 2013, 18, 733–756. [Google Scholar] [CrossRef]
- Krzywinski, M.; Schein, J.; Birol, İ.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An Information Aesthetic for Comparative Genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef]
- TIBCO Software Inc Statistica, Version 13; Data Analysis Software System; Tibco Software Inc.: Palo Alto, CA, USA. 2021. Available online: http://statistica.io (accessed on 10 July 2023).
- Thacharodi, A.; Hassan, S.; Singh, T.; Mandal, R.; Chinnadurai, J.; Khan, H.A.; Hussain, M.A.; Brindhadevi, K.; Pugazhendhi, A. Bioremediation of Polycyclic Aromatic Hydrocarbons: An Updated Microbiological Review. Chemosphere 2023, 328, 138498. [Google Scholar] [CrossRef]
- Al-Rubaye, A.H.; Jasim, D.J.; Ameen, H.F.M.; Al-Robai, H.A.; Al-Assal, J.R. The Impacts of Petroleum on Environment. IOP Conf. Ser. Earth Environ. Sci. 2023, 1158, 032014. [Google Scholar] [CrossRef]
- Panchenko, L.; Muratova, A.; Dubrovskaya, E.; Golubev, S.; Turkovskaya, O. Natural and Technical Phytoremediation of Oil-Contaminated Soil. Life 2023, 13, 177. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J. Bioaugmentation of Soil Contaminated with Diesel Oil. J. Elementol. 2018, 23, 1161–1178. [Google Scholar] [CrossRef]
- Wang, W.; Gao, Y.; Du, J.; Zheng, L.; Kong, X.; Wang, H.; Yang, X.; Duan, L.; Zhao, Q.; Liu, Y.; et al. Dose–Effect of Nitrogen Regulation on the Bioremediation of Diesel Contaminated Soil. Environ. Technol. Innov. 2023, 32, 103245. [Google Scholar] [CrossRef]
- Pérez, A.P.; Eugenio, N.R. Status of Local Soil Contamination in Europe: Revision of the Indicator ‘Progress in the Management Contaminated Sites in EUROPE’. JRC. 2018. Available online: https://data.europa.eu/doi/10.2760/093804 (accessed on 15 July 2023).
- Bica, I. Contaminated Sites Investigation. Objectives and Methods. E3S Web Conf. 2020, 169, 02002. [Google Scholar] [CrossRef]
- Brombal, D.; Wang, H.; Pizzol, L.; Critto, A.; Giubilato, E.; Guo, G. Soil Environmental Management Systems for Contaminated Sites in China and the EU. Common Challenges and Perspectives for Lesson Drawing. Land Use Policy 2015, 48, 286–298. [Google Scholar] [CrossRef]
- Wanner, P.; Freis, M.; Peternell, M.; Kelm, V. Risk Classification of Contaminated Sites—Comparison of the Swedish and the German Method. J. Environ. Manag. 2023, 327, 116825. [Google Scholar] [CrossRef]
- Gao, J.; Faheem, M.; Yu, X. Global Research on Contaminated Soil Remediation: A Bibliometric Network Analysis. Land 2022, 11, 1581. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Wyszkowska, J.; Kordala, N.; Borowik, A. Applicability of Ash Wastes for Reducing Trace Element Content in Zea mays L. Grown in Eco-Diesel Contaminated Soil. Molecules 2022, 27, 897. [Google Scholar] [CrossRef]
- Rusin, M.; Gospodarek, J.; Barczyk, G.; Nadgórska-Socha, A. Antioxidant Responses of Triticum Aestivum Plants to Petroleum-Derived Substances. Ecotoxicology 2018, 27, 1353–1367. [Google Scholar] [CrossRef]
- Mishra, P.; Kiran, N.S.; Romanholo Ferreira, L.F.; Yadav, K.K.; Mulla, S.I. New Insights into the Bioremediation of Petroleum Contaminants: A Systematic Review. Chemosphere 2023, 326, 138391. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Kucharski, M.; Kucharski, J. The Role of Dactylis Glomerata and Diesel Oil in the Formation of Microbiome and Soil Enzyme Activity. Sensors 2020, 20, 3362. [Google Scholar] [CrossRef]
- Ptaszek, N.; Pacwa-Płociniczak, M.; Noszczyńska, M.; Płociniczak, T. Comparative Study on Multiway Enhanced Bio- and Phytoremediation of Aged Petroleum-Contaminated Soil. Agronomy 2020, 10, 947. [Google Scholar] [CrossRef]
- Mitter, E.K.; Kataoka, R.; de Freitas, J.R.; Germida, J.J. Potential Use of Endophytic Root Bacteria and Host Plants to Degrade Hydrocarbons. Int. J. Phytoremediation 2019, 21, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, Plant Selection and Enhancement by Natural and Synthetic Agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Meištininkas, R.; Vaškevičienė, I.; Dikšaitytė, A.; Pedišius, N.; Žaltauskaitė, J. Potential of Eight Species of Legumes for Heavy Fuel Oil-Contaminated Soil Phytoremediation. Sustainability 2023, 15, 4281. [Google Scholar] [CrossRef]
- Gawryluk, A.; Stępniowska, A.; Lipińska, H. Effect of Soil Contamination with Polycyclic Aromatic Hydrocarbons from Drilling Waste on Germination and Growth of Lawn Grasses. Ecotoxicol. Environ. Saf. 2022, 236, 113492. [Google Scholar] [CrossRef]
- Hou, J.; Wang, Q.; Liu, W.; Zhong, D.; Ge, Y.; Christie, P.; Luo, Y. Soil Microbial Community and Association Network Shift Induced by Several Tall Fescue Cultivars during the Phytoremediation of a Petroleum Hydrocarbon-Contaminated Soil. Sci. Total Environ. 2021, 792, 148411. [Google Scholar] [CrossRef]
- He, M.; Li, Z.; Chen, C.; Mei, P. Impact of Soil Types and Root Exudates on Cadmium and Petroleum Hydrocarbon Phytoremediation by Sorghum Sudanense, Festuca Arundinace, and Lolium Perenne. Front. Ecol. Evol. 2022, 10, 1036765. [Google Scholar] [CrossRef]
- Lin, M.-S.; Huang, C.-Y.; Lin, Y.-C.; Lin, S.-L.; Hsiao, Y.-H.; Tu, P.-C.; Cheng, P.-C.; Cheng, S.-F. Green Remediation Technology for Total Petroleum Hydrocarbon-Contaminated Soil. Agronomy 2022, 12, 2759. [Google Scholar] [CrossRef]
- Yavari, S.; Malakahmad, A.; Sapari, N.B. A Review on Phytoremediation of Crude Oil Spills. Water Air Soil. Pollut. 2015, 226, 279. [Google Scholar] [CrossRef]
- Hussein, Z.S.; Hamido, N.; Hegazy, A.K.; El-Dessouky, M.A.; Mohamed, N.H.; Safwat, G. Phytoremediation of Crude Petroleum Oil Pollution: A Review. EJBO 2022, 62, 611–640. [Google Scholar] [CrossRef]
- Franchi, E.; Cardaci, A.; Pietrini, I.; Fusini, D.; Conte, A.; De Folly D’Auris, A.; Grifoni, M.; Pedron, F.; Barbafieri, M.; Petruzzelli, G.; et al. Nature-Based Solutions for Restoring an Agricultural Area Contaminated by an Oil Spill. Plants 2022, 11, 2250. [Google Scholar] [CrossRef]
- Cook, R.L.; Hesterberg, D. Comparison of Trees and Grasses for Rhizoremediation of Petroleum Hydrocarbons. Int. J. Phytoremediation 2013, 15, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Aluvihara, S.; Weerawardena, T.E.; Gunaratne, H.D.A.K.; Deniyapahala, D.V.R.I.; Dissanayake, S.S.; Gunawardana, S.I. Advanced Review on Soil Microbiology and Fertility of Soils. Mech. Agric. Conserv. Resour. 2023, 67, 27–31. [Google Scholar]
- Singh, H.; Pant, G. Phytoremediation: Low Input-Based Ecological Approach for Sustainable Environment. Appl. Water Sci. 2023, 13, 85. [Google Scholar] [CrossRef]
- Tan, H.W.; Pang, Y.L.; Lim, S.; Chong, W.C. A State-of-the-Art of Phytoremediation Approach for Sustainable Management of Heavy Metals Recovery. Environ. Technol. Innov. 2023, 30, 103043. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Gałązka, A.; Kucharski, J. Role of Festuca Rubra and Festuca Arundinacea in Determinig the Functional and Genetic Diversity of Microorganisms and of the Enzymatic Activity in the Soil Polluted with Diesel Oil. Environ. Sci. Pollut. Res. 2019, 26, 27738–27751. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Han, G.; Cui, B.; Rennenberg, H. The Effects of Petroleum Contaminated Soils on the Growth, Gas Exchange and Antioxidative Level of Sea-Buckthorn. Plant Soil. 2023, 486, 535–550. [Google Scholar] [CrossRef]
- Houshani, M.; Salehi-Lisar, S.Y. Agronomic Crop Responses and Tolerance to Polycyclic Aromatic Hydrocarbon Toxicity. In Agronomic Crops: Volume 3: Stress Responses and Tolerance; Hasanuzzaman, M., Ed.; Springer: Singapore, 2020; pp. 265–283. ISBN 9789811500251. [Google Scholar]
- da Silva Correa, H.; Blum, C.T.; Galvão, F.; Maranho, L.T. Effects of Oil Contamination on Plant Growth and Development: A Review. Environ. Sci. Pollut. Res. 2022, 29, 43501–43515. [Google Scholar] [CrossRef]
- Meudec, A.; Poupart, N.; Dussauze, J.; Deslandes, E. Relationship between Heavy Fuel Oil Phytotoxicity and Polycyclic Aromatic Hydrocarbon Contamination in Salicornia Fragilis. Sci. Total Environ. 2007, 381, 146–156. [Google Scholar] [CrossRef]
- Yemashova, N.; Murygina, V.; Zhukov, D.; Zakharyantz, A.; Gladchenko, M.; Appanna, V.; Kalyuzhnyi, S. Biodeterioration of Crude Oil and Oil Derived Products: A Review. Rev. Environ. Sci. Biotechnol. 2007, 6, 315–337. [Google Scholar] [CrossRef]
- Koshlaf, E.; Ball, A.S. Soil Bioremediation Approaches for Petroleum Hydrocarbon Polluted Environments. AIMS Microbiol. 2017, 3, 25–49. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Kordala, N. Applicability of Compost and Mineral Materials for Reducing the Effect of Diesel Oil on Trace Element Content in Soil. Materials 2023, 16, 3655. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Kordala, N. Trace Element Contents in Petrol-Contaminated Soil Following the Application of Compost and Mineral Materials. Materials 2022, 15, 5233. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Kucharski, J. Microbiological Study in Petrol-Spiked Soil. Molecules 2021, 26, 2664. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Kucharski, J. Response of Avena Sativa, Microorganisms and Enzymes to Contamination of Soil with Diesel Oil. Plant Soil. Environ. 2015, 61, 483–488. [Google Scholar] [CrossRef]
- Dindar, E.; Şağban, F.O.T.; Başkaya, H.S. Variations of Soil Enzyme Activities in Petroleum-Hydrocarbon Contaminated Soil. Int. Biodeterior. Biodegrad. 2015, 105, 268–275. [Google Scholar] [CrossRef]
- Wu, B.; Lan, T.; Lu, D.; Liu, Z. Ecological and Enzymatic Responses to Petroleum Contamination. Environ. Sci. Process. Impacts 2014, 16, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, R.; Chen, G.; Yan, B.; Zhong, L.; Wang, Y.; Li, Y.; Li, J.; Zhang, Y. Bibliometric Analysis of Current Status on Bioremediation of Petroleum Contaminated Soils during 2000–2019. Int. J. Environ. Res. Public Health 2021, 18, 8859. [Google Scholar] [CrossRef]
- Fahimizadeh, M.; Pasbakhsh, P.; Mae, L.S.; Tan, J.B.L.; Raman, R.K.S. Sustained-Release of Nutrients by Yeast Extract-Loaded Halloysite Nanotubes Supports Bacterial Growth. Appl. Clay Sci. 2023, 240, 106979. [Google Scholar] [CrossRef]
- Teng, G.; Chen, C.; Jing, N.; Chen, C.; Duan, Y.; Zhang, L.; Wu, Z.; Zhang, J. Halloysite Nanotubes-Based Composite Material with Acid/Alkali Dual PH Response and Foliar Adhesion for Smart Delivery of Hydrophobic Pesticide. Chem. Eng. J. 2023, 451, 139052. [Google Scholar] [CrossRef]
- Wang, C.; Gu, L.; Ge, S.; Liu, X.; Zhang, X.; Chen, X. Remediation Potential of Immobilized Bacterial Consortium with Biochar as Carrier in Pyrene-Cr(VI) Co-Contaminated Soil. Environ. Technol. 2019, 40, 2345–2353. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, H.; Shao, J.; Chen, Y.; Zhang, S.; Chen, H. Multifunctional Carboxymethyl Cellulose Sodium Encapsulated Phosphorus-Enriched Biochar Composites: Multistage Adsorption of Heavy Metals and Controllable Release of Soil Fertilization. J. Chem. Eng. 2023, 453, 139809. [Google Scholar] [CrossRef]
- Khosravi, A.; Zheng, H.; Liu, Q.; Hashemi, M.; Tang, Y.; Xing, B. Production and Characterization of Hydrochars and Their Application in Soil Improvement and Environmental Remediation. Chem. Eng. J. 2022, 430, 133142. [Google Scholar] [CrossRef]
- European-BioChar. The European Biochar Certificate. Available online: http://www.european-biochar.org/en (accessed on 10 August 2023).
- International Biochar Initiative. About the IBI Biochar Certification Program. Available online: http://www.biochar-international.org/certification (accessed on 10 August 2023).

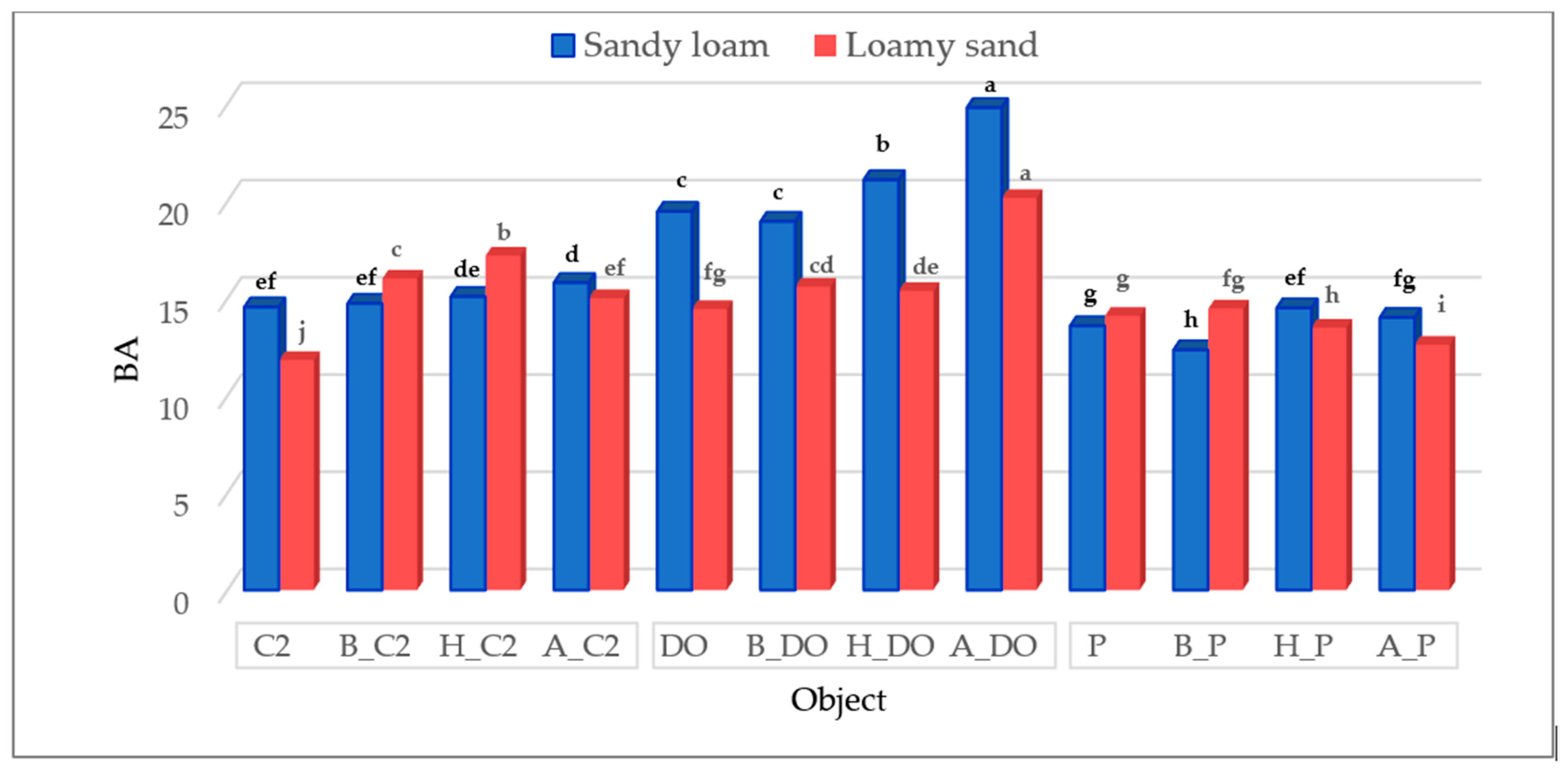

 —Sandy loam,
—Sandy loam,  —Loamy sand. Abbreviations are explained under the Table 2 and Table 3.
—Loamy sand. Abbreviations are explained under the Table 2 and Table 3.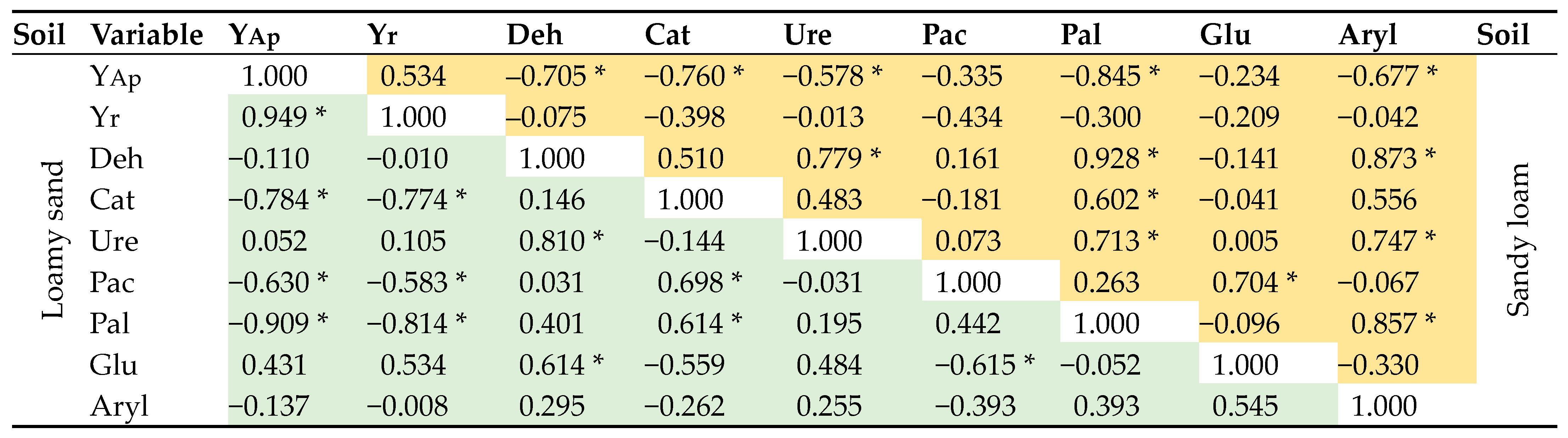
| Abbreviation | Properties | Unit | Soil | |
|---|---|---|---|---|
| Loamy Sand (LS) | Sandy Loam (SL) | |||
| Grain-size Composition | ||||
| Sand | 0.05–2.0 mm | % | 74.30 | 70.38 |
| Silt | 0.02–0.05 mm | 23.69 | 27.19 | |
| Clay | <0.002 mm | 2.01 | 2.43 | |
| Chemical and Physicochemical Properties | ||||
| Ntot | Total Nitrogen | g kg−1 dm | 0.98 | 1.01 |
| Corg | Organic Carbon | 11.20 | 11.50 | |
| Pavailable | Phosphorus Available | mg kg−1 dm | 164.05 | 172.73 |
| Kavailable | Potassium Available | 53.95 | 78.85 | |
| Mgavailable | Magnesium Available | 46.00 | 38.00 | |
| pH | Soil pHKCl Reaction | 1 mol KCl dm−3 | 6.98 | 7.13 |
| EBC | Sum of Exchangeable Base Cations | mM (+) kg−1 dm | 84.20 | 181.80 |
| HAC | Hydrolytic Acidity | 8.00 | 5.70 | |
| CEC | Cation Exchange Capacity | % | 92.20 | 187.50 |
| ACS | Alkaline Cation Saturation | 91.32 | 96.96 | |
| Enzymatic Activity per 1 kg dm h−1 | ||||
| Deh | Dehydrogenases | µM TFF | 5.426 | 8.007 |
| Cat | Catalase | M O2 | 0.211 | 0.369 |
| Ure | Urease | mM N-NH4 | 0.153 | 1.114 |
| Pac | Acid Phosphatase | mM PN | 2.291 | 0.954 |
| Pal | Alkaline Phosphatase | 2.037 | 1.572 | |
| Glu | β-glucosidase | 0.542 | 0.914 | |
| Aryl | Arylsulphatase | 0.386 | 0.721 | |
| Type of Sorbent | Objects | Loamy Sand (LS) | Sandy Loam (SL) | ||||
|---|---|---|---|---|---|---|---|
| Aerial Parts (Ap) | Roots (r) | Ap/r | Aerial Parts (Ap) | Roots (r) | Ap/r | ||
| g dm of pot−1 | g dm of pot−1 | ||||||
| Control (C1) | C2 | 40.712 b ±1.721 | 8.818 bc ±0.192 | 4.617 | 46.708 a ±1.031 | 8.533 ab ±0.293 | 5.474 |
| DO | 4.903 e ±0.690 | 1.656 f ±0.238 | 2.960 | 28.122 d ±2.060 | 4.545 e ±0.816 | 6.188 | |
| P | 33.378 d ±3.270 | 6.826 d ±0.131 | 4.890 | 42.422 b ±1.594 | 5.723 de ±0.468 | 7.412 | |
| Biochar (B) | C2 | 41.930 b ±0.408 | 11.022 a ±1.495 | 3.804 | 46.733 a ± 0.726 | 9.501 a ±0.206 | 4.919 |
| DO | 7.214 e ±0.148 | 4.953 e ±0.488 | 1.457 | 31.745 c ±1.011 | 5.302 de ±1.462 | 5.987 | |
| P | 35.805 d ±1.940 | 8.486 cd ±0.523 | 4.219 | 43.042 b ±0.489 | 6.588 cd ±0.363 | 6.534 | |
| Halloysite (H) | C2 | 47.858 a ±1.433 | 10.186 ab ±0.623 | 4.698 | 48.907 a ±0.893 | 9.212 a ± 0.629 | 5.309 |
| DO | 6.025 e ±0.022 | 3.885 e ±0.188 | 1.551 | 31.124 cd ±1.421 | 8.385 ab ±0.855 | 3.712 | |
| P | 32.799 d ± 1.070 | 8.492 cd ± 0.603 | 3.862 | 40.200 b ±1.270 | 8.181 abc ±0.819 | 4.914 | |
| Alginite (A) | C2 | 40.097 bc ± 1.850 | 9.234 bc ± 0.835 | 4.342 | 47.796 a ±2.553 | 8.404 ab ±0.418 | 5.687 |
| DO | 7.304 e ± 0.768 | 3.876 e ± 0.012 | 1.884 | 33.092 c ±0.699 | 7.031 bcd ±0.922 | 4.707 | |
| P | 36.641 cd ± 2.069 | 8.058 cd ± 1.098 | 4.547 | 46.807 a ±1.064 | 5.682 de ±0.367 | 8.237 | |
| Type of Sorbent | Object | Deh | Cat | Ure | Pac | Pal | Glu | Aryl |
|---|---|---|---|---|---|---|---|---|
| C1 | C2 | 5.901 h ±0.090 | 0.227 g ±00.008 | 0.160 g ±0.125 | 2.473 g ±0.004 | 2.086 de ±0.001 | 0.571 cd ±0.004 | 0.401 d ±0.002 |
| DO | 6.824 g ±0.087 | 0.339 c ±0.000 | 0.264 f ±0.027 | 3.551 bc ±0.012 | 2.606 b ±0.010 | 0.476 fg ±0.003 | 0.385 de ±0.003 | |
| P | 7.388 ef ±0.219 | 0.386 a ±0.011 | 0.266 f ±0.045 | 3.369 cd ±0.094 | 1.986 e ±0.005 | 0.446 h ±0.007 | 0.256 g ±0.007 | |
| B | C2 | 9.075 c ±0.001 | 0.246 f ±0.004 | 0.421 d ±0.025 | 3.079 e ±0.024 | 2.131 cd ±0.060 | 0.614 b ±0.006 | 0.443 c ±0.009 |
| DO | 7.614 e ±0.183 | 0.347 bc ±0.008 | 0.311 e ±0.027 | 3.722 ab ±0.190 | 2.619 b ±0.017 | 0.515 e ±0.004 | 0.009 | |
| P | 7.105 fg ±0.242 | 0.310 d ±0.008 | 0.334 e ±0.025 | 3.829 a ±0.113 | 2.123 cd ±0.026 | 0.494 ef ±0.024 | 0.275 g ±0.003 | |
| H | C2 | 10.240 b ±0.069 | 0.227 g ±0.008 | 0.683 b ±0.025 | 2.752 f ±0.010 | 2.204 c ±0.009 | 0.657 a ±0.002 | 0.428 c ±0.009 |
| DO | 7.707 e ±0.173 | 0.384 a ±0.004 | 0.287 e ±0.013 | 3.289 de ±0.020 | 2.738 a ±0.006 | 0.493 ef ±0.001 | 0.476 b ±0.007 | |
| P | 6.686 g ±0.090 | 0.287 e ±.0.000 | 0.421 d ±0.025 | 3.180 de ±0.002 | 2.110 cd ±0.010 | 0.501 ef ±0.004 | 0.298 f ±0.003 | |
| A | C2 | 8.173 d ±0.007 | 0.234 fg ±0.005 | 0.595 c ±0.025 | 2.715 f ±0.008 | 2.148 cd ±0.009 | 0.589 bc ±0.005 | 0.551 a ±0.005 |
| DO | 11.594 a ±0.256 | 0.364 b ±0.008 | 0.893 a ±0.013 | 3.591 b ±0.055 | 2.754 a ±0.026 | 0.558 d ±0.017 | 0.009 | |
| P | 5.732 h ±0.135 | 0.227 g ±0.000 | 0.465 d ±0.025 | 3.314 d ±0.015 | 2.046 de ±0.006 | 0.453 gh ±0.003 | 0.375 e ±0.003 |
| Type of Sorbent | Object | Deh | Cat | Ure | Pac | Pal | Glu | Aryl |
|---|---|---|---|---|---|---|---|---|
| C1 | C2 | 8.550 d ±0.327 | 0.393 b ±0.004 | 1.238 de ±0.066 | 1.011 h ±0.014 | 1.619 de ±0.013 | 0.986 e ±0.004 | 0.765 de ±0.014 |
| DO | 11.395 c ±0.116 | 0.437 a ±0.012 | 1.664 b ±0.069 | 1.640 d ±0.011 | 2.318 c ±0.018 | 1.092 cd ±0.020 | 0.934 a ±0.021 | |
| P | 7.588 e ±0.125 | 0.303 ef ±0.004 | 0.979 g ±0.020 | 1.454 f ±0.031 | 1.553 de ±0.044 | 1.044 cde ±0.041 | 0.677 f ±0.034 | |
| B | C2 | 8.746 d ±0.490 | 0.244 g ±0.004 | 0.785 h ±0.012 | 1.539 e ±0.025 | 1.679 d ±0.011 | 0.998 e ±0.009 | 0.757 e ±0.004 |
| DO | 11.168 c ±0.162 | 0.338 d ±0.008 | 1.074 fg ±0.069 | 1.927 b ±0.008 | 2.499 b ±0.128 | 1.100 c ±0.020 | 0.858 bc ±0.018 | |
| P | 6.206 f ±0.276 | 0.310 e ±0.008 | 1.118 ef ±0.025 | 1.400 g ±0.006 | 1.515 ef ±0.008 | 1.126 c ±0.004 | 0.681 f ±0.017 | |
| H | C2 | 8.769 d ±0.207 | 0.225 h ±0.007 | 1.282 d ±0.025 | 1.376 g ± 0.006 | 1.628 de ±0.011 | 1.004 de ±0.009 | 0.815 cde ±0.035 |
| DO | 12.351 b ±0.300 | 0.374 c ±0.004 | 2.163 a ±0.026 | 1.644 d ±0.003 | 2.531 b ±0.019 | 1.127 c ±0.012 | 0.914 ab ±0.017 | |
| P | 7.651 e ±0.139 | 0.287 f ±0.004 | 1.118 ef ±0.025 | 1.956 b ±0.012 | 1.408 f ±0.064 | 1.418 a ±0.086 | 0.680 f ±0.021 | |
| A | C2 | 9.210 d ±0.149 | 0.232 gh ±0.004 | 1.519 c ±0.045 | 1.412 fg ±0.012 | 1.621 de ±0.011 | 1.004 de ±0.005 | 0.817 cd ±0.008 |
| DO | 15.779 a ±0.466 | 0.354 d ±0.008 | 2.072 a ±0.026 | 1.859 c ±0.036 | 2.733 a ±0.021 | 1.109 c ±0.026 | 0.908 ab ±0.008 | |
| P | 6.884 ef ±0.344 | 0.227 gh ±0.004 | 1.162 def ±0.025 | 2.299 a ±0.015 | 1.568 de ±0.009 | 1.233 b ±0.020 | 0.654 f ±0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Biochar, Halloysite, and Alginite Improve the Quality of Soil Contaminated with Petroleum Products. Agriculture 2023, 13, 1669. https://doi.org/10.3390/agriculture13091669
Wyszkowska J, Borowik A, Zaborowska M, Kucharski J. Biochar, Halloysite, and Alginite Improve the Quality of Soil Contaminated with Petroleum Products. Agriculture. 2023; 13(9):1669. https://doi.org/10.3390/agriculture13091669
Chicago/Turabian StyleWyszkowska, Jadwiga, Agata Borowik, Magdalena Zaborowska, and Jan Kucharski. 2023. "Biochar, Halloysite, and Alginite Improve the Quality of Soil Contaminated with Petroleum Products" Agriculture 13, no. 9: 1669. https://doi.org/10.3390/agriculture13091669
APA StyleWyszkowska, J., Borowik, A., Zaborowska, M., & Kucharski, J. (2023). Biochar, Halloysite, and Alginite Improve the Quality of Soil Contaminated with Petroleum Products. Agriculture, 13(9), 1669. https://doi.org/10.3390/agriculture13091669










