Abstract
The purpose of this paper is to summarize the available important information on local pig breeds maintained in Europe. Genetic erosion has prompted national and international bodies to take organized action aimed at the minimization of further losses of biodiversity. Since the genetic resources of livestock ensure both food security and the sustainable development of rural areas, genetic diversity is indispensable for sufficient flexibility of future animal husbandry to adapt to changing consumer demands and climatic conditions. Therefore, the problem of biodiversity has recently become an essential part of the comprehensive international program of the World Conservation Strategy and the global idea of the so-called sustainable development. The issue of biodiversity protection occupies an important place in the provisions of the EU Common Agricultural Policy. The successive financial perspectives assume an increase in relevant expenditure from the EU and national budgets. With regard to the protection of native pig breeds, a particularly large increase in funding was recorded in 2014–2020.
1. Introduction
Pigs (Sus scrofa domestica L.) are one of the world’s most important meat-producing species. They also play a fundamental role in many cultures in areas ranging from Southern Europe to the Pacific islands [1]. The occurrence range of this species extends from Western Europe to Eastern Siberia. It is limited by the 55th parallel in the north and expands to the Indian subcontinent and the Indonesian islands in the south. Pigs originate from populations of the European and Asian wild boar [2]. As indicated in the most recent studies [3], these animals were domesticated at different times and independently in different regions of China and East Anatolia, and then in Europe. Pigs were first domesticated around 8500 BC in the Middle East (Asia) [4] and 6600 years ago in Central Europe [5].
Human activities such as isolation and selective breeding, as well as adaptation, mutations, and genetic drift, have contributed to the extensive phenotypic changes in a number of traits of domestic pigs, e.g., behavior, body composition, resistance to diseases, reproduction, and color [6,7]. This has yielded a huge variety of local pig populations characterized by genetic and production performance diversity [2,8,9,10,11]. Unfortunately, the market demand for lean fatteners and changes in European agriculture observed over the last few decades have impeded the sustenance of diversity. There was a tendency towards the standardization of genotypes and mass rearing of high-yielding breeds. Currently, the global pork production is dominated by only five breeds [12]. As a result of these changes, the number of pig breeds has drastically declined, with some of them becoming threatened with extinction. Additionally, the intensification of animal production has led to the emergence of multiple problems that have a negative effect not only on the environment but also on the performance and health of pigs. Equally alarming are serious threats such as disease epidemics and crisis situations (e.g., droughts, floods, armed conflicts) [13]. The Food and Agriculture Organization (FAO), dealing with the problem of conservation of genetic resources, compiled and published the World Watch List for Domestic Animal Diversity in 1993. Data were collected from national reports. Currently, an inventory of pig breeds and genetic variation studies are being conducted for the quantitative estimation of species diversity. Given the detailed information system on genetic resources, including information on the size, distribution, and phenotype of the population, it is possible to assess the status of endangered breeds and take action to prevent their extinction. The protection and enhancement of biodiversity in agriculture is one of the main political challenges addressed by the Common Agricultural Policy (CAP) in the EU as well [14].
The aim of this review is to present the history, phenotypic traits, and available basic information on the performance, genetics, and current status of endangered local pig breeds reared in Europe. The economic aspect of the protection of local breeds is also mentioned. This review is based on available literature data presenting the key characteristics of these breeds.
2. Rationale behind Undertaking the Research
This review is based on the analysis of literature data on the conservation of genetic resources of various European pig breeds, with consideration for the expenditure from the EU budget for the preservation of animals covered by the conservation program. It presents the basic morphological, functional, and production traits of local pig breeds, which are important for the maintenance of the genetic variability of this species. The reproduction performance analysis included the number and body weight of piglets on the day of birth. The fattening value was characterized on the basis of daily gains, and the slaughter value was evaluated based on the lean meat content and the backfat thickness. The meat quality (longissimus lumborum muscle) was assessed via the determination of the intramuscular fat content, color and pH. The diversity of breeds is critical for environmental integrity and food security. The most important achievements and applications resulting from the genetic and genome characteristics of pig breeds are summarized as well.
2.1. Bibliographic Databases and Phrases Used in Data Search
The review of the traits of the domestic pig breeds reared in Europe and covered by the program for protection of native breeds was compiled using 12 specialized digital scientific databases: EBSCO, Google Scholar, Medline, ProQuest Central, ProQuest SciTech Collection, ScienceDirect, Scopus, Springer, Taylor & Francis, Web of Knowledge, Web of Science, and Wiley Online Library. The publications covered mainly biological, natural, agricultural, social, and veterinary sciences. The databases of the UN Food and Agriculture Organization DAD-IS were also used.
Original scientific publications closely related to the subject of the review were searched using the following keywords: pigs, European local/endangered/protected breeds, genetic resources, history of the breed, domestication, coat color, reproductive performance, fertility, milk yield, fattening and carcass value, meat quality characteristics, longevity, resistance, genetic tests, genetic variability, level of inbreeding, population structure, phenotypic diversity, mutation, genome, selection signature, and single nucleotide polymorphism.
2.2. Number and Timeline of Literature Data
In this review, 197 thematically consistent scientific reports were cited in total. This number includes 131 original scientific publications, 24 chapters in monographs, 7 books, 7 doctoral theses, and 28 publications from other sources (conference reports, FAO data, EU regulations). The literature sources cover the period from 1993 to 2023 and one publication originates from 1961. Literature data published in the last 10 years constitute 64%, and papers from the last 5 years account for 45% of the total number of publications cited in the review. This paper is a mainly descriptive analysis. The results of this research on the economic aspect of endangered native pig breeds are summarized and presented in graphs.
3. Meat in the Human Diet
Meat and meat products are a very important element of the diet associated with the traditions and eating habits of the majority of the population. Pork currently ranks first among the most important types of meat in the EU, with a production of 22.1 million tons per year. The European pig population represents approximately 18% (140 million animals) of the global swine population [15]. Therefore, the EU is the second-largest producer of pork after China and the largest exporter of this meat [16]. Germany, Spain, France, Poland, and the Netherlands are the largest pork consumers in Europe [17]. Pork meat comes mainly from selected pigs characterized by optimal feed conversion rates, effective weight gains, and a high meat content. Unfortunately, the rapid weight gains in these animals and the high lean meat percentage are not always associated with the good quality and taste of their meat. Due to the excessive popularity of commercial breeds, almost all pork products available in shops have a similar flavor. In terms of the production system, the majority of pigs in the EU are reared in intensive systems that exert substantial pressure on the environment. In many European countries, there is a large group of consumers who are guided by health, social, ethical, and animal welfare considerations when buying meat and its products [18,19,20]. For several years, there has been a constantly growing consumer demand for products derived from animals kept in natural conditions and produced in a traditional way [21,22]. Sustainable development also plays an important role in shaping the EU meat market [19,20].
4. Conscious Management of Global Biodiversity in Agriculture
The importance of biodiversity has long been emphasized. However, it was not until June 1992 that 167 UN countries signed the Convention on Biological Diversity at the United Nations Conference on Environment and Development (UNCED) in Rio de Janeiro. The experts emphasized that the protection of biodiversity is a “common concern of humanity” and an integral part of the development process at the global level [23]. The Global Strategy for the Conservation of Livestock Genetic Resources [24] was developed the following year. Its aim was to establish an organizational structure (National and Regional Coordination Centers) for the inventorization, characterization (diagnostic description), and monitoring of the global livestock genetic resources in the Domestic Animal Diversity Information System (DAD-IS) and the Early Warning System monitoring endangered livestock populations. The next step was to support activities aimed at the development and implementation of programs aimed to protect endangered breeds and to promote their wider use in production. The DAD-IS IT systems and the Red Book identify breeds that are threatened with extinction and have a critical population size [25]. They also serve as a global data repository and an information-sharing hub to support countries in the management of their animal genetic resources (AnGR) data and information and in the fulfillment of their obligations to report the status of their national biodiversity [26]. Native breeds have been included in programs for the protection of genetic resources aimed at, e.g., the development of populations to a size that ensures their biological durability with the stabilization of the size of the active population, preservation of the existing intra-breed variability and genetic distinctiveness, consolidation of breed traits, and stabilization of the exterior pattern and the particularly valuable and desirable performance traits of breeds.
5. Methods for Protection of Endangered Pig Breeds
Pork breeds at risk are protected by in situ and ex situ (cryogenic) methods. Conservation of the breeding stock in the natural production system is an in situ conservation method. It is also called on-farm protection and covers entire agro-ecosystems [27]. In turn, ex situ conservation involves the relocation of a breed from its native area to other localities. The ex situ method also consists of the preservation of genetic material, i.e., sperm cells, egg cells, embryos, or tissues. It is a very important tool for the prevention of the irreversible loss of breeds or genes that can be used for the potential reproduction of the breed, protection of genetic resources against sanitary threats, support of breeding small populations, and preservation of genetic variability in selection programs. In turn, in vitro protection programs should only be implemented in emergency situations as a future effective conservation strategy to support the maintenance of live animals [28].
A basic tool for the effective protection of livestock breeds is the assessment of their threat status mainly based on the population size and structure. However, the FAO emphasizes the need for individual solutions in this regard taking into account the situation of each country. Therefore, the protection program has covered pig breeds that are threatened with extinction (populations with less than 1000 females and less than 20 males) and breeds with a critical population size (less than 100 females and less than 5 males) [29,30]. The applicable Regulation of the European Parliament and of the Council (EU) 2016/1012 of 8 June 2016 [31] defined endangered breeds as “local breeds recognized by the Member States as threatened with extinction, genetically adapted to one or more national production systems, with the endangered status confirmed scientifically by an authority with the required skills and knowledge in the field of endangered breeds” [32].
In the area of Europe and the Caucasus, the Food and Agriculture Organization (FAO) has identified 48 extinct pig breeds, representing approximately 20% of the global number of swine breeds. In this number, there are 16 critical-status, 2 critical-maintained, 42 endangered, 13 endangered-maintained breeds, 4 vulnerable, 104 unknown, 51 extinct, and only 2 cryo-conserved breeds; 16 breeds have a not-at-risk status [33]. This represents over 25% of the European local pig populations, which is alarming.
6. Importance of Biodiversity
Biodiversity is referred to as genetic variability, i.e., the presence of the largest possible number of different genes and their combinations in a given population as well as the largest possible number of species, races, and diverse ecological systems (e.g., meadows, forests, etc.) occurring locally or globally. Although the pig industry is currently based on the use of several cosmopolitan breeds and lines, there are still a large number of local breeds in many regions of Europe. They are characterized by a variety of coat color phenotypes and specific morphological traits. They also have special traits, i.e., resistance to diseases, longevity, and high levels of maternal traits. Compared to conventional breeds, native breeds exhibit a slower growth rate [23,34]. The mean daily protein deposition was reported to range between 41 and 105 g/d in most local breeds assessed by Brossard [35] and from 113 to 116 g/d in the Krškopolje breed. These pigs were found to digest fiber more efficiently than commercial breeds due to the greater richness of their cellulolytic and hemicellulolytic flora [36]. Native breeds are characterized by a higher adiposity, higher slaughter weight [2,37], and high meat quality [38,39]. They are perfectly adapted to local environmental conditions, e.g., climate, soil, and available feed resources [35,40]. They can be reared with poor feed resources available in permanent grassland, which contributes to the development and protection of areas of high landscape value. Native breeds are usually reared in extensive or semi-extensive systems linked to local and traditional niche markets [2]. Their meat is most frequently used to produce unique quality products with a centuries-old tradition of production, which are regarded as premium products in some countries [38]. The raw material from native breeds is also used for the production of high-quality regional and traditional products characterized by a strong taste and aroma, and a high nutritional value [22,38,41,42].
An argument supporting the need to preserve the genetic variation between breeds is their potential future usefulness, as some of them have genes and unique combinations of alleles that are absent or rare in other breeds [42,43,44]. They, therefore, constitute a genetic reserve. The most recent forecasts for agriculture reported by the Organization for Economic Co-operation and Development (OECD) and the UN Food and Agriculture Organization (FAO) predict an increase in global meat production from 345.2 million tons to 377.2 million tons in 2022–2031, with 8.1 million tons (25.2%) of pork [33,45]. Therefore, local breeds can contribute to the efficient and sustainable production of animal protein and a secure food supply for the growing world population (8.5 billion in 2031) [12,27].
The preservation of the genetic diversity of native breeds may have key evolutionary applications, e.g., in the adaptation to climate change and promotion of sustainable agriculture [40]. Sustainable systems are more environmentally friendly and ensure animal welfare, in addition to allowing farmers to become independent from large multinational corporations. Local swine breeds contribute to the preservation of the cultural landscape associated with animal habitats and production systems [46] as well as the development of rural areas. This, in turn, may help to preserve the cultural and ethnological characteristics of European rural communities associated with agricultural activities [46,47]. Local breeds are part of the heritage of specific communities [43]. The biodiversity of pig populations is essential for efficient and sustainable food production, sustainable improvement of food and nutrition security, and satisfaction of the highly diverse needs of human societies in the future.
7. Characteristics of Selected European Native Pig Breeds
Native breeds are animal populations originating from local primitive breeds in a specific region or country. Breeders and their associations maintain the purity of the breed by enforcing the maintenance of certain conformational and performance traits, including the pedigree of each registered animal [48].
The best-known representative local pig breed is the Iberian pig reared in the south-western part of the Iberian Peninsula [23,49]. At present, Iberian pigs are not at risk of extinction [50]. In this breed, there are many varieties divided into black and colored. The black varieties are mainly represented by Entrepelado, Lampiño, and Porc Negre Mallorquí (Majorcan Black), whereas Retinto, Manchado de Jabugo, Torbiscal, Chato Murciano, Euskal Txerria, Gochu Asturcelta, and Porco Celta are the main colored varieties. Iberian pigs are characterized by a medium-sized harmoniously structured body. Their daily weight gains range from 400 to 550 g. Their meat is characterized by high adipogenic potential. The intramuscular fat (IMF) content in the longissimus dorsi muscle in Iberian pigs ranges from 3.27% to 29.21%, the pH24 from 5.61 to 5.75, and the L* value from 34 to 54 [51]. On average, sows of the Iberian pig breed have 2.2 litters per year with 14 piglets whose weight at birth varies from 1.1 to 1.4 kg [50]. These animals are reared in the extensive system based on native agricultural, forest, and pasture resources [52]. Fatteners are most often slaughtered upon reaching the body weight of approximately 150 kg. The slaughter weight in breeds reared in semi-extensive conditions is substantially higher, ranging from 160 to 190 kg [53]. These breeds are perfectly adapted to the continental and semi-arid Mediterranean climate. Iberian ham (jamón ibérico) as well as Tuscan, Basque, and Corsican prisuttu, coppa, and pancetta are typical products in this geographical area [53,54]. These products are legally protected and certified. Hams produced from the meat of Iberian pigs are divided into three categories according to the nutritional regime (Bellota, Cebo, and Recebo). In the case of the most highly valued Bellota hams, animals in the final fattening phase graze on oak plantations. Cebo hams are produced from pigs that receive mainly grains, while Recebo hams are produced from the meat of fatteners fed grain- and legume-based feed [55]. The Ibérico breed is particularly well adapted to grazing in the dehesa areas. The Entrepelado, Lampiño, Retinto, and Torbiscal varieties are used for the exploitation of the montanera natural resources, as they feed on acorns of cork oak (Quercus suber), holm oak (Quercus ilex), and grass [23].
The Entrepelado variety was created by crossing Retinto and Lampiño pigs. Their carcass cuts are characterized by a lower fat content than that in Lampiño but not as high as that in Retinto. They have a sparse hair coat and their skin is dark in juveniles but becomes dull in adult individuals. They are almost hairless animals with short limbs and characteristic skin folds in the front parts of the body [50]. The Torbiscal is the youngest variety of the Iberian race. It was created by crossing two breeds from Extremadura (Campanario, Puebla) and two breeds from Portugal (Ervideira, Caldeira) in the 1940s. The pigs have an elongated snout, lop ears, and light or dark skin. At present, Entrepelado, Retinto and Torbiscal pigs are not at risk of extinction [50].
The following varieties are threatened with extinction: Manchado de Jabugo, Gochu Asturcelta, Porc Negre Mallorquí, Euskal Txerria, Chato Murciano, and Porco Celta (white or white with black, sometimes spotted coat) [55]. The animals of this breed are very well adapted to semi-extensive and extensive rearing systems. Gochu Asturcelta is a primitive breed with a rough body build and a strong structure. The animals have an elongated trunk, flat ribs, a sloping rump, and poorly filled hams. They are resistant and adapt to the environment easily. Gochu Asturcelta pigs have been traditionally reared in the extensive system in Ibero-Atlantic deciduous forests [56,57]. The origin of the Majorcan Black breed is uncertain, but the existence of pig livestock in Mallorca dates back to the period of the first settlers, approximately 3500 BC. This breed has black or grey skin color, pendulous ears, a concave nose profile, and tassels in the neck. Sows have 2.0 litters per year with 6.8–7.5 piglets weighing 0.9 kg. The growth rate for the whole fattening stage is 410 g/day. The IMF content ranges from 6.0 to 9.7%, the pH45 is 6.17, the pH24 is 5.67, the L* value is 46, and the drip loss has an average value of 1.65% [58]. The Majorcan Black breed is characterized by the absence of a mutation in the halothane gene (RYR1 gene). This breed is free from classical and African swine fever [50]. They are traditionally fed on pasture, cereals (mainly barley), figs, almonds, legumes, and carob [52]. The typical product obtained from the meat of this pig is the sobrassada (Spanish Sobrasada de Porc Negre Mallorquí), i.e., a dry-cured spicy high-fat sausage spread. This product received the Protected Geographical Indication (PGI) mark in 1994 [52]. Euskal Txerria pigs have a piebald black and white hair coat, typical of saddleback breeds. They have a characteristic very wide belt covering the middle part of the body, a dark-colored head, and a sloping dark rump. These animals have a mild temperament [50]. This breed was very popular in the 1920s [59].
In Italy, eight breeds, i.e., Apulo-Calabrese, Casertana, Cinta Senese (black with white stripe, lop ears), Mora Romagnola, Nero Siciliano, Nero di Lomellina, Nero di Parma, and Sarda, are reared and have survived the changes occurring in agriculture [50]. The Cinta Senese breed is reared mainly in the Tuscany region. Its origins date back to the 13th–14th century, as immortalized in the famous painting by Ambrogio Lorenzetti exhibited in the Palazzo Comunale in Siena [60]. The animals of this breed have black skin with a white stripe over the shoulders and front legs. Their elongated body on long and strong limbs and their elongated and narrow head are other distinguishing traits of this breed. The pigs are well adapted to mountainous terrain. Sows produce an average of 6.82 piglets per litter [54] with approximately 1.2 kg of live body weight. Lactation lasts for 60 days [61]. Fatteners are characterized by the deposition of large amounts of subcutaneous fat (32 to 58 mm at the last rib level) and their meat is regarded as flawless. Cinta Senese fatteners have an IMF from 2.5 to 6.0%. Studies reporting the meat quality of Cinta Senese pigs have shown pH45 and pH24 of 6.4 and 5.7, respectively, and an L* value ranging from 45 to 50. The main cured meats produced from the Cinta Senese breed are dry-cured ham, Tuscan salami, Lardo, Capocollo and Pancetta [61]. This breed is most often kept in a semi-wild state and is always reared on pastures and/or forests [53]. Today, there are 392 breeding sows and 65 boars [50].
The Apulo-Calabrese breed comes from the region of Calabria. It is a medium- to small-sized breed with plain black coat color, long and thin snout with a straight head profile, and droopy ears projected forwards. On average, sows of the Apulo-Calabrese pig breed have 1.7 litters per year with 6.9 piglets of approximately 1.0 kg live body weight. The average daily gain in the overall fattening stage ranges from 300 to 706 g/day. This breed is characterized by a low meat content (44.8%) and backfat thickness of 48 mm at the level of the last rib. The pH45 and pH24 of the longissimus muscle are 6.30 and 5.85, respectively [62]. The quality of its meat is suitable for the production of salami and pancetta with the Protected Denomination of Origin (PDO) [60,62], which is highly appreciated both in Italy and abroad. This breed adapts well to various production systems, but is often kept outdoors [63,64]. In 2021, there were 1003 breeding sows and 145 boars [50].
The ancestors of the Casertana breed were representatives of Romanesque and Asian breeds. The animals are perfectly adapted to mountainous regions and poor pastures. They are small-sized pigs with a short, narrow, laterally flattened body and slim hams. A distinctive feature of this breed is the presence of two skin wattles, called bells, on both sides of the neck. Their body color ranges from gray to black with a purple hue. They are almost devoid of bristles, with only sparse tufts growing on their head, neck, and tail tip. Sows give birth to 4–6 piglets (weighing 1.0 kg) per litter. Fatteners achieve weight gains in the range of 400–450 g, which are accompanied by a high feed consumption per kg of gain. The pigs of this breed are characterized by a thick layer of backfat (often exceeding 40 mm) [65]. They are slaughtered after reaching 150 kg body weight [66]. This breed has the following parameters: lean meat content 42.1%, pH45 6.23, pH24 5.7, IMF 2.0–4.7%, and L* 42 [67]. Presently, there are 1403 breeding sows and 172 boars [50].
The Nero Siciliano breed is mainly found on Mount Nebrodi in the province of Messina in eastern Sicily. It probably has an ancient origin (7–8th century BC) [59]. It is a medium-sized pig with thick black skin and thick black bristles reaching an approximate length of 10 cm on the back. Their head is large and elongated with a strong snout, and their limbs are long and strong [67]. Most of the pigs are kept outdoors and fed barley and field beans. Only a small number of these pigs are kept in a semi-wild state with nutrition from natural undergrowth vegetation. Sows give birth to 8.97 piglets per litter (of 1.4 kg body weight) [67] and raise 7.54 piglets [53], whereas 2–3 piglets are born in a semi-wild state. By the age of 1 year, the pigs have gained only 50–60 kg of weight [59]. Their average daily weight gain is 328–600 g/day. The lean meat content ranges from 39.7 to 59.0%, the IMF content from 2.7 to 10.0%, pH45 6.24, and pH24 5.58. The color of their meat is very variable (L* 46–61) [68]. Today, there are 1332 breeding sows and 252 boars [55].
The distinctive traits of the Mora Romagnola breed are the black and navy blue hair coat with long red-tipped hair [53] and the pink color of the belly, inner parts of the legs, and throat. The breed standard was established in 1942 [69]. Mora Romagnola pigs are widespread throughout Romagna and in the provinces of Forli and Ravenna. They are characterized by a long, narrow, and poorly muscled trunk, long and strong limbs, poorly filled hams, and strongly overgrown tusks [69]. The Nero di Parma is a swine reared in the North of Italy, which was officially recognized as a breed in 2016. Its ancestor was the “Nera Parmigiana” breed, whose main characteristics were a slate-colored coat, the presence of wattles, ears hanging and facing forward, and a strong conformation. Today, the live population comprises 1603 pigs. The fertility rates in Mora Romagnola and Nero di Parma are estimated at 7.4 and 8.31, respectively. Sows of this breed are characterized by low milk yields; they raise 5–8 piglets weighing 0.9 kg, which are weaned at the age of 60 days [53]. Their meat content is approximately 39.2%, backfat thickness is 52 cm, pH45 is 6.57, pH24 is 5.97, IMF content is 6.8%, and their color is relatively dark (43 for CIE L*). A characteristic trait is the relatively dark color (43 for CIE L*) of their meat [69]. Nero di Parma and Mora Romagnola pigs are kept in semi-extensive (fattening up to 190 kg body weight) and extensive (fattening to up to 150 kg) systems [53]. The most popular raw-ripened Parma ham (Prosciutto di Parma) with the PDO mark is a national Italian delicacy. In the DAD-IS databases, 414 breeding sows and 70 boars are recorded [50].
Sarda pigs originate from Sardinia. This breed was recognized in 2006. These small-sized pigs with black, grey, tawny or spotted coat color resembling wild boars, have short and strong limbs and long, thick, and rough bristles forming a mane along the dorsal line [59]. Sows have 1.2–2.0 litters per year with 5.6–9.4 piglets of only 0.3 kg live body weight. The daily gain is between 300 and 529 g/day. The other parameters are as follows: pH45 6.07, pH24 5.98, and L* 48 [70]. Their meat is used to produce traditional Sardinian sausages. Presently, there are 184 breeding sows and 41 boars [50].
Alentejano, Bísaro, and Malhado de Alcobaça are indigenous breeds in Portugal. Alentejano pigs are medium-sized animals with a light bone structure [71], while Bísaro pigs are characterized by a tall, long, and narrow body, long legs, and a substantial share of skin and bones [44,72]. The Alentejano breed, representing the Iberian type, is reared mainly in the south of Portugal. It is characterized by a low growth rate (except for the “montanheira” regime) and prematurely high adipogenicity [49,73,74]. Their carcass cuts are characterized by a low content of lean meat (from 37.5 to 51%) [75,76]. Sows give birth to 6.7 to 9.4 piglets weighing 1–1.3 kg, which are raised by the mothers for 35–60 days [34]. Bísaro is a Celtic type breed from the north of Portugal [49,74,75]. On average, Bísaro sows have 1.9 litters per year with 9.3 piglets born per litter [77]. It has lower growth rates (although higher than those of the Alentejano), and its carcass cuts have higher amounts of muscle (lean mean content ranging from 46 to 51%) [76,77] and a lower content of fat [72]. The backfat thickness at the last rib ranges from 19 to 42 mm [77]. Before slaughter, fatteners feed on acorns and grass. In the carcasses of Alentejano pigs, the reported pH24 values are slightly higher than those from modern breeds, suggesting the existence of lower glycogen stores before slaughter and higher oxidative muscle metabolism. The intramuscular fat content (IMF) is highly variable among studies (3.1–7.5%) [75]. The IMF content in the Bísaro fatteners was in ranged 2.6 to 2.7% and the pH measured in longissimus muscle at 45 min and 24 h postmortem varied between 5.95 and 6.34 and 5.32 and 5.56, respectively [77]. In 2017 [50], there were 6582 (Alentejano) and 6095 (Bísaro) breeding sows and 462 and 496 boars, respectively. The meat of these breeds is used to produce high-quality traditional fermented and dried meat products awarded the European PDO certificate [44,76]. The meat of Alentejano pigs is used to produce dried hams and shoulders (Presunto do Alentejo and Paleta do Alentejo), and the Bísaro breed provides meat for the production of Presunto de Vinhais.
Pigs of the Malhado de Alcobaça breed, which is historically known as Porco Sintrão, Torrejano, da Granja, or da terra, have a black and white coat or black and white spots [78]. Their medium-sized head has a concave profile. Their long and wide lop ears cover the eyes. The trunk is long, broad, and muscular with well-developed shoulders and a narrow and slightly elongated sloping rump. The pigs have high, straight, and muscular limbs and their bodies are covered abundantly by large and strong bristles. This breed was initially refined via crossing with the Berkshire breed and then with the Yorkshire and Tamworth breeds. The pigs are reared in 11 herds mainly in the central-western region (from Sintra to Leiria) in extensive and intensive systems [50]. Today, there are 211 breeding sows and 12 boars. On average, 9.1 ± 2.6 and 8.3 ± 2.2 piglets are born alive and weaned, respectively [79]. Fatteners have a thick backfat layer (2.5 cm). Their daily weight gains are in the range of 450–500 g [59]. The pigs of this breed are reared in semi-extensive and intensive systems.
There are three indigenous pig breeds in Croatia, Banijska šara (spotted), Black Slavonian pig, and Turopolje, that are adapted to outdoor rearing systems. The Banija spotted breed is characterized by a white coat with black spots and high fertility [80]. The Black Slavonian pig (Crna Slavonska) is an autochthonous breed from the eastern region of Croatia [81] created by crossing Mangalitza, Berkshire, Poland China, and Large Black (Cornwall) pigs [82,83,84]. The most important characteristic of this breed is its black coat color. The Black Slavonian pig is a medium-sized pig; its head is medium long with medium-sized and semi-circular drooping ears. The chest is deep and wide. The body and legs are relatively short, whereas the hips are wide. Sows have 1.1 to 2.2 litters per year with 5.0 to 7.4 piglets of approximately 1.3 kg live body weight [83]. This fat–meat type of pig (meat content ranging from 27.9 to 47.2%) [83] is characterized by longevity and hardiness [20]. It is kept on grasslands or in forest–pasture systems until approximately 18 months of age or the achievement of 130–150 kg of weight [81,83,85]. The average daily gain (ADG) is from 206 to 567 g/day [83]. It is usually used to produce lard and bacon as well as traditional high-quality meat products. A characteristic trait of the breed is the high content of IMF (5–12.3%), appropriate meat acidity (pH45 6.11–6.75; pH24 5.57–5.91), and the meat color brightness parameter L* 49 [83]. The meat of this breed has received the PDO mark [20]. The Black Slavonian pork is produced exclusively within the administrative borders of Pannonia and northern Croatia. Presently, there are 607 registered breeding sows and 62 boars of the Black Slavonian pig breed [50].
In turn, the Turopolje breed originates from Šiška, Krškopolje, and Berkshire pigs [86]. It is characterized by a medium-length head with a convex profile, a medium-length muzzle, and medium-length semi-lop ears. The body is covered with thick, white-yellow, curly hair with hand-sized black patches scattered on the entire trunk. The pigs have unpigmented skin and a pink snout. The back and the legs are weakly muscled. This typical fat-type pig [87] was recognized as a breed in 1911. Sows have 10 to 12 teats and give birth to an average of 7 to 8 piglets weighing 1.2 kg. After weaning, two-month-old piglets weigh between 10 and 15 kg. Pigs in the intensive fattening system achieve a weight that ranges from 20 to 100 kg with a daily gain of 550 g. The lean meat content is around 40.0%, the IMF content is 3.7%, pH45 is 6.1, and pH24 is 5.68 [88]. A characteristic trait of the breed is their high tolerance of extremely high and low temperatures [88], the tasty and juicy pink meat, and the firm backfat [50]. These animals have low nutritional demands. They are usually reared in extensive (swampy pastures in oak and beech forests) or semi-intensive systems [87,89]. In the DAD-IS databases, 240 breeding sows and 18 boars are recorded [50].
The Krškopolje pig is an indigenous breed reared in Slovenia. It originates from the south-eastern part of the country. The pigs have a black coat with a characteristic white stripe on the shoulders and forelegs, and a white nose [90]. The back is long, wide and straight, and the hams are wide, full and long. Adult Krškopolje sows have a body length of 155–175 cm, a height at the withers of 80–85 cm, and a height at the rump of 85–90 cm. The coat is strong, straight, and dark on the pigmented parts of the body. The pigs of the breed exhibit a mild temperament and a big appetite and are characterized by high feed conversion efficiency. Sows are characterized by moderate fertility (8.1–10.5 piglets of approximately 1.2 kg live body weight) and a high growth rate. The backfat thickness measured at the level of the last rib ranges from 22 to 49 mm, and the lean meat content is around 44% [91]. The IMF content ranges from 2.0 to 4.3% [10,22,91], the pH values measured in longissimus muscle at 45 min and 24 h postmortem are 6.08 and 5.47, respectively, and the L* value is ranges from 48 to 52 [91]. This breed is reared in highly diverse conditions, i.e., in intensive and extensive (organic or agritourism farm) systems [92,93,94]. The animals are often kept in combined indoor and outdoor systems [95]. The Krškopolje breed is intended for the production of high-quality meat and lard. With its high fat content, the meat is especially suitable for processing into traditional dry-cured meat products [23,90,92], e.g., dried salami-type sausages [41]. In 1992, the breed was represented by less than 30 sows and only 3 boars. The current population consists of 355 registered sows and 68 boars [50]. In Slovenia, Prekmurska ham, Kranjska sausage, Prleška tünka, Zgornjesavinjski želodec, Šebreljski želodec, Kraški pršut, Kraški zanik, and Kraška pancetta have received the Protected Geographical Indication (PGI) mark [55]. In the DAD-IS databases, there are 355 breeding sows and 68 boars [50].
The Mangalica breed is believed to have originated in the Balkans. Interestingly, woolly pigs are thought to have existed for thousands of years, as they have been depicted as early as in Roman paintings [96]. Mangalica (also referred to as Mangalitsa or Mangalitza) is a Hungarian breed of pigs created via the crossing of the Szalonta and Bakony varieties bred in the country. This breed was officially recognized in 1927. Mangalica pigs have a thick curly (woolly) coat. Some varieties differ only in the coat color: Black, Blond, Red, and Swallow Bellied [50]. The animals have a compact build and a short and massive back. The head is small with ears drooping forward. The snout is darkly pigmented and the limbs are short and strong. The pigs are well adapted to mountainous terrain [59]. Sows give birth to 5–9 live piglets. Boars are characterized by a mild temperament and stay together with the sows and piglets. Mangalica pigs have low nutritional demands. They provide high-quality fat. Bacon dried and smoked with the use of beech wood is one of the very popular products from their meat [59].
Mangalica (Mangulica), Moravka, and Resavka are three indigenous pig breeds reared in the Republic of Serbia, but the first breed mentioned is also found in other European countries. Mangulica originates from the ancient Šumadinka breed. The Black or Swallow Belly variety emerged as a result of planned selection in the Srem area [97]. The color of the Mangalica coat varies; it can be white (blonde), reddish brown, or black-brown. The curly hair and lop ears are distinctive traits [10]. Sows have 1.2–2.0 litters per year with around five piglets weighing 1.1–1.6 kg. The average daily gain in the fattening period is 257–830 g/day. The backfat thickness ranges from 42 to 81 mm (at the level of the last rib) [97]. The Swallow Belly Mangulica is considered a fat-type breed, with a high fat content (65–70%) in the carcass and less than 40% lean meat in the half-carcass [97,98,99]. The IMF content is highly variable and ranges from 2.9 to 18.2% [97,99], pH45 is 6.1, and pH24 is 5.6 [97]. The meat is intensely red (L* value 45) and has a firm texture. It is used for the production of ham [99]. This breed is reared in a semi-intensive system. The feed conversion rate is estimated at 5.9 kg/kg body weight gain [50]. Moravka was created by crossing the extinct native Šumadinka breed with the Berkshire and probably Yorkshire breeds [84,99,100]. The pigs have black-pigmented skin and straight and smooth dark hair [10]. Their shoulder and ham are weakly developed and poorly muscled. The high limbs have thin bones [50]. Moravka pigs have only 4–6 pairs of teats. Sows give birth to 4–16 piglets. The average piglet birth weight is 1.3 kg. Lactation is prolonged to 60 days [100]. The average daily weight gain in the traditional fattening scheme is 385 g [59]. In comparison with Mangulica, this breed is characterized by higher meat content and a longer carcass with less fat (about 6.7%) [99,101]. The meat of Moravka fatteners has a pH45 ranging from 6.0 to 6.5, pH24 5.7–5.9, and an L* value of 52 [100]. Moravka pigs are known for their hardiness and longevity. They are reared in an extensive farming system, and their feed conversion rate is estimated at 4.95 kg per 1 kg of weight gain [50]. Presently, in the DAD-IS database, 3824 breeding sows and 237 boars of the Moravka pig breed are recorded [100].
Rasava was created by crossing Shumadia and Mangulica with Berkshire pigs. The animals have a white, yellow, and black coat. Their head is relatively long with lop ears. Their limbs are moderately long or short. This breed has valuable traits such as adaptability, vitality, hardiness, and longevity [50]. The average number of piglets per litter is 7. These pigs have similar fattening characteristics to those of the Moravka breed. They feed efficiently on pastures. In Serbia, fatteners are slaughtered after reaching a body weight of 150, 180, and 200 kg. Their meat is used for the production of “black” ham, smoked and dried sirloin, and salami [59]. The Rasavka breed is threatened with extinction. The current population consists of 204 registered sows and 20 boars [50].
The Greek pig is the only traditional native pig breed bred in the northern regions of Greece [102]. The dominant color in this breed is black, but the animals can be black and brown, brown with white stripes, and black with white spots [53]. Sows are characterized by the seasonality of their reproductive activity and produce two litters per year with an average of 8.48 ± 1.94 piglets in each. The weight of the piglets at weaning is approximately 7.69 ± 0.69 kg [102]. Greek pigs are usually reared outdoors and grazed in oak forests [23]. Finishers are usually slaughtered after 240–300 days after achieving a carcass weight of approximately 60 kg, and provide meat with excellent quality [102]. In the DAD-IS databases, there were 3647 breeding sows and 291 boars in 2021 [50].
Blanc de l’Ouest (white coat), Cul Noir Limousin (black with white saddle, erect ears), Porc de Bayeux (black and white), Gasconne (black), and Nustrale (multicolored: black, red, blonde) are the main native breeds in France [50]. Blanc de l’Ouest is one of the largest pig breeds. It was created by crossing three groups of native pigs: Craonnaise, Norman, and Flemish breeds. In the late 1960s, the breed was refined through a combination with genes of the German Landrace breed. The pigs have a strong elongated trunk and strong limbs. They have a deep chest and a wide and slightly sloping rump. The snout has a clearly concave profile. Large ears cover the eyes and the snout. Sows give birth to 8–9 piglets weighing approximately 2 kg. They are excellent mothers, producing large amounts of milk. Fatteners achieve daily weight gains of 700–800 g [59].
The Nustrale breed (formerly known as Porc Corse) [23] is a local pig breed in Corsica. It originates from native Iberian-type pigs. For centuries, it has been reared on the island, with acorns and chestnuts used as basic nutrition. Despite the long tradition of rearing, it was only recognized as a breed in 2007. These primitive pigs are small and have long limbs. Sows give birth to six piglets per litter. At the age of 400 days, Nustrale pigs reach a body weight of 140 kg and have a 3 cm layer of backfat. They are reared in an extensive system based on the use of local resources such as pastures, chestnuts, and acorns. The IMF content in their meat is 6%. The meat is processed into regional specialties such as coppa, lonzu, and prisutta [59]. These products are traditionally processed and often sold by farmers [53]. The Blanc de l’Ouest and Nustrale breeds are of critical-maintained population size [50].
The Basque Black Pied breed (Pie noir du Pays Basque) originates from Basco-Béarnaise pigs. Originally, the pigs were reared in the Pyrenees and provinces of northern Spain. The pigs are piebald, black and white with a black head and rump, with a slightly convex back and a sloping croup. They have rare and fine bristles. Their chest is large and the hams have an elongated shape. Their limbs are large and strong, well-suited for outdoor rearing in extensive zones characterized by hills. On average, sows of the Basque pig breed have 1.6 litters per year with 7.5 piglets born alive. Fatteners achieve weight gains of 342–537 g/day. The backfat thickness value measured at the level of the last rib ranging from 26 to 51 mm is a consequence of the wide range of the final live weight of pigs and different feeding regimes. The loin meat from Basque pigs are characterized by a high intramuscular fat content (3.3–5.7%), a dark color (L* value 43–52), and a pH24 between 5.54 and 5.76 [103]. Their meat has contributed to the good reputation of Bayonne ham in recent centuries. This breed was restored in 1981 with the use of 40 Bigourdan and 10 Basque pigs and bred in an extensive system in mountainous areas. The meat from these pigs is used in the manufacturing of two products: “Kintoa” (Meat and Abas) and “Kintoa ham”, both of which have the PDO certificate [59,103]. The latest update of information about the breed in the Domestic Animal Diversity Information System [50] was introduced on 1 March 2006; only 59 breeding sows were recorded. According to Mercat [103], 580 sows were registered in the herd book in 2017.
Gascon pigs are considered the oldest French breed of pigs reared in the foothills of the Pyrenees. A pointed snout with narrow ears slightly covering the eyes, and thin and tough limbs are characteristic traits of these pigs. They have black skin, a black hair coat [104], and a well-muscled body. They tolerate strong sunlight and high temperatures well. Sows give birth to 6–7 piglets per litter. The animals are reared outdoors in a local production system (Noir the Bigorre pork chain). These extensively reared animals (20 pigs per 1 ha) achieve weight gains of 342–537 g/day and are slaughtered at the age of 12–24 months [34]. The average IMF content in the longissimus lumborum muscle of Gascon pigs is 2.6% [42] and the meat content is approximately 43% [59]. Their meat exhibits good quality (pH45 6.34 and 6.76, pH24 5.55 and 5.92, and the L* value of 38–48 in the longissimus lumborum muscle) [104]. The main products of processing the meat from the local breeds include Jambon Noir de Bigorre, Porc Noir de Bigorre, Boudin noir, Pâté, Rillettes, and Andouille [104]. Jambon sec de Corse is produced from the meat of Nustrale pigs [55]. “Noir de Bigorre” fresh loin and “Noir de Bigorre” dry-cured hams produced from Gascon pigs obtained AOC (Appellation d’Origine Contrôlée) and PDO official quality labels [104]. There are 826 sows of Gasgon pig and 100 breeding males in the Domestic Animal Diversity Information System [50].
There are two indigenous breeds in Lithuania, as shown by current data: Native Lithuanian (multicolored: white, black, brownish, but with different-shaped patches in most cases) kept in two herds, and Lithuanian White (Latvijas Baltā) with white color and erect or semi-erect ears, which is reared in one herd [50]. These breeds are threatened with extinction. Lithuanian White pigs were refined by crossing with the following breeds: Large White, Middle White, Edelsweine, Berkshire, and local Danish pigs [105]. These animals are known for their strong constitution and low stress susceptibility [106]. In this breed, sows give birth to 9.6–10.4 piglets per litter weighing 1.3 kg. They are known for their low susceptibility to stress. Lithuanian Indigenous Wattle pigs have characteristic wattles under the neck and are insensitive to sunlight [106,107]. They have a friendly temperament. Sows give birth to 7.2–9.7 piglets weighing 1.3 kg [106]. The lactation period lasts 60 days. The Lithuanian White and Native Lithuanian breeds are characterized by daily weight gains of 743.3 and 619.2 g, the IMF content in longissimus lumborum is 2.14 and 2.26%, and the value of the L* parameter of their meat is 54.48 and 55.53, respectively [107]. The lean meat content in the carcass is 50% and 47%, respectively [105,106]. Pigs of the Lithuanian White breed are endangered and Native Lithuanian pigs have a critical status. In the DAD-IS databases, there are 109 and 90 breeding sows and 31 and 17 boars, respectively [50].
In Sweden, the Linderöd Swine (Linderödssvin) breed, characterized by a light gray or brown coat with black spots and abundant bristles, is reared in the southern part of the country. The body of the pigs is slightly rounded with a slightly convex backline, and the medium-sized ears droop slightly forward. The pigs have a straight snout [108] and short, straight, and strong legs [109]. This breed is related to the old forest pig, which is mentioned as the only pig breed in Sweden before the 18th century. In 1952, the Skånes Djurpark Zoo took care of a pregnant sow that was believed to be related to the forest pig and gave rise to the current Linderöd population. The sows of this breed usually farrow once a year; the average total litter size is 8.5 piglets, and 6.9 juveniles are weaned [108]. The body weight of the piglets of this breed is approximately 1.5 kg after a week and 25 kg at the age of 12 weeks. The lactation period usually lasts 11 weeks, and the offspring mortality in the rearing period is estimated at 16% [110]. Linderöd pigs have a special property of accumulation of subcutaneous fat. Most pigs are kept outdoors for the entire year and are fed mainly on grains, hay, root vegetables, forage and food waste [108]. Fatteners are slaughtered upon reaching 104 kg of body weight at the age of 300 days. The average daily weight gain from birth to slaughter is 333 g/day. The slaughter yield reaches 72% [111]. Their meat is healthy and has an excellent flavor quality. This breed has been registered in the Ark of Taste, and the cooperation with Slow Food is targeted at selling Linderöd pig meat to restaurants [108]. In 2021, 464 breeding sows and 151 boars were kept [50].
The Danube White is a local pig breed reared in Bulgaria. These animals typically have a white hair coat, but black spots covered by white hair are accepted in the breed standard as well. It is reared in an intensive system in only two herds [50]. Another native breed is the East Balkan Swine with a black coat and grey-brown skin. It has a long head with a straight profile, a short neck, and small erect ears. A characteristic trait is the presence of long hair on the neck. The animals are reared in an extensive system in two herds, and the products from their meat are referred to as organic [50]. The size of the population of these breeds indicates a critical status. The lard and meat of the breed are used for the production of the traditional Bulgarian sausage Smyadovska lukanka. In Bulgaria, there is also one herd of the Polish Big White pig, i.e., a breed created in the 20th century by crossing German Noble and Large White pigs of English and Swedish origin. The white coat and the pale pink skin are characteristic traits of this breed.
The Czech Black-Spotted Prestice (Presticke cernostrakate) breed was created from the Old Czech Bristly Spotted and Black and Prestice Spotted breeds. German and English saddle pig boars were used for the refinement of the breed in 1945, and Angeln Saddlebacks were imported from Germany and the United Kingdom in 1970. The pigs of this breed have a black coat with a white saddle and lop ears [50]. They are characterized by longevity, strong body build, and good adaptability [112,113,114,115]. Black-Spotted Prestice sows have been shown to have moderate fertility (8.16 piglets born alive) [112], a very calm temperament, a high milk yield, and strong maternal instincts [116,117]. These pigs are reared in an extensive farming system. Fatteners achieve weight gains of 622 g/day [112] and very high meat quality parameters [115]. Boar and gilt carcasses have a meat content of 52.74 and 56.35%, a backfat thickness of 32.11 and 26.85 mm, a pH45 of 56.23 and 6.35, a pH24 of 5.68 and 5.63, an IMF of 2.38 and 2.20%, and a meat color parameter L* of 51.45 and 49.64, respectively [118]. In the DAD-IS databases, there were 500 breeding sows and 85 boars in 2018 [50].
In Germany, there are several local breeds among the native pigs listed by the FAO [50]: Angler Sattelschwein, Bunte Bentheimer, Schwäbisch-Hällisches Schwein, Rassegruppe der Sattelschweine, Deutsches Sattelschwein, Rotbuntes Husumer Schwein, and Leicoma. The Angler Sattelschwein breed is the maternal breed reared in the north of Germany. It was created in the early 20th century by crossing the local black and white Landrace with Tamworth and Berkshire, and then with the British Wessex Saddleback breed. It was recognized as a breed in 1937 [119]. The animals have a black-pigmented coat with a white stripe along the forelegs and shoulders. They have a strong build, a broad and deep body, well-muscled shoulders and hams, and long shiny bristles. Fatteners achieve daily weight gains of 700–800 g and provide high-quality meat [120]. In 2019, 26 boars and 82 sows were registered [120].
The history of Bunten Bentheimer pigs was presented comprehensively by Hermeling in 1957. In the early 1950s, the black and white Bentheim breed was first registered in the herd books of Bentheim and Cloppenburg districts in Lower Saxony [121]. It was created from the Marschschwein, Berkshire, and Cornwall breeds from England, the Poland China, and the Landrace breeds and then crossed with the Angler-Sattel and Schwäbisch-Hällischen breeds, and animals with impeccable conformation were entered into the register in 1950 [121]. These animals were officially entered in the herd book in 1955 [122]. This local breed and its name originate from the area of Bad Bentheim. The 1950s, i.e., a period of high demand for high-fat foods, was the heyday of the colored Bentheim breed. However, the changing consumer interests noted soon after recognition of the breed resulted in its disappearance from the official records in 1964. The Bunte Bentheimer pig breed was revived with 4 boars and 23 sows in Lower Saxony in 1988 [123]. The animals have lop ears and irregularly scattered black spots on a white or light gray background [122,124]. They have a long and muscular back, a stocky body, strong bones, a broad forehead, and an elongated snout. In addition to animals kept by breeders, Bunte Bentheimer pigs were kept in 13 zoos in 2001–2003. They are characterized by longevity and good health. Their advantages are high fertility (10 piglets per litter), a quiet temperament, and high resistance to adverse weather conditions [125]. The population was restored thanks to the German breeders’ premiums and the BLE project “Development of an economy-oriented breeding program for the endangered Bunte Bentheimer pig breed” funded by the Landwirtschaftliche Rentenbank [120]. The Association for the Preservation of Bunte Bentheimer Pigs was established in March 2003. In 2004, the Association of Bunte Bentheimer Pig Producers patented the “Bunte Bentheimer Schwein” product as a graphic and wordmark logo. The entries include meat, meat products, cold cuts, live pigs, company administration, and advice on breeding and inbreeding [121]. The Slow Food Deutschland Association registered the Bunte Bentheimer breed in the Ark of Taste in 2004. This international project, which is aimed at the protection of local and regional food products, dishes, and species of farm animals and plants, recorded a significant increase in the number of Bentheim pigs since 2003. In 2019, there were 129 boars and 542 sows of this breed [120]. In the national program “Animal Genetic Resources”, Sattelschweine pigs [126] are kept on organic farms.
The Deutsches Sattelschwein breed is classified into the Sattelschweine breed group. These floppy-eared pigs are dark with a white stripe (saddle) extending from the shoulder blades and forelegs to the tail and sometimes to the hind legs. This breed was created from Angler Sattelschwein and Schwäbisch-Hällisches Schwein pigs. It was recognized as a breed in 1937 [126]. After the war, it was the most common pig breed reared in Schleswig-Holstein [122]. This pig has a medium-length head, lop ears, a long and wide shoulder blade, a deep and wide chest, large hams, strong legs, and at least 14 teats. They are stress-resistant, long-lived, undemanding animals. The sows of the breed provide high levels of maternal care and high milk yields. They give birth to 9.3–11 piglets per litter [127] and are able to raise the offspring even in unfavorable conditions in outdoor systems [120]. Adult sows reach a body weight of 250 kg [126]. This breed is characterized by high adiposity [126]. It is suitable for intensive farming. Fatteners achieve daily weight gains in the range of 800–900 g. This breed is reared mainly on small and organic farms. In Saxony, only 26 pigs of the breed were registered by the Association in 2020 [128]. In 2019, the population comprised 55 boars and 209 sows [120].
The local Schwäbisch-Hällisches Schwein breed originally comes from the Hohenlohe region of Baden-Württemberg, which is still the major breeding area. This breed was created almost 200 years ago, i.e., in 1821 [119,129], by crossing local pigs with Chinese Old Saddlebacks. It was crossed with the Berkshire breed in the second half of the century and with the Angler Sattelschwein breed after World War II. This breed probably originates from the Celtic–Germanic large-eared farm pig. The herd book for the breed was established in 1925 [124]. A black color with a white saddle and long strong legs are characteristic traits of the breed. These visually lighter and slightly longer pigs produce slightly more meat than the other indigenous Sattelschweine pig breeds. The sows of this breed are characterized by excellent fertility (11 piglets per litter weighing 1.6 kg), especially in comparison with that of the other traditional pig breeds [129]. Finishers exhibit good daily weight gains (from 800 to 900 g). The lean meat content is approximately 52%, IMF 1.8–2.3%, pH45 6.48, and pH24 5.55 [129]. In 1986, the Schwäbisch-Hallisches Pig Breeders’ Association was founded. The rearing of the Schwäbisch-Hällisches Schwein breed was supported by the project “Fattening of pigs with acorns” [120]. Relatively large numbers of Schwäbisch Hällisch pigs are reared on organic farms, and some are sold under the Ecoland organic brand. Their fresh meat is used to produce high-quality regional products (Schwäbisch Hall quality pork, SHQ), e.g., ham and sausages. In 1998, the meat received the PGI mark [129]. Due to its close genetic relationship with other populations, the Rotbuntes Husumer Schwein breed, which is characterized by a red color with a white saddle, was included in the Sattelschweine group in 2011. Red Angler Sattelschwein pigs, as well as Jutland and piebald wandering pigs crossed with the Tamworth breed, are considered to be the progenitors of Rotbuntes Husumer Schwein [59]. These strong animals are resistant to the cold. This breed is represented by 4 boars and 101 sows [120].
The name Leicoma combines the initials of the former GDR districts of Leipzig, Cottbus, and Magdeburg. This synthetic line was created by crossing Deutsches Sattelschwein, Niederländische Landrasse, and Estonian Baconrasse pigs in the first breeding stage (1971–1975). In order to improve the intensity of growth and the amounts of produced meat, the Duroc and German Landrace breeds were included in the second breeding stage (1976). A white color, lop ears, and good quality meat are distinguishing traits of the breed. The meat of these pigs is suitable for the production of high-quality pork with a very good flavor. The sows of the breed give birth to 11 piglets. These pigs are reared mainly in the eastern part of Germany. Leicoma pigs achieve daily weight gains of over 900 g. In 2012, there was only one farm with fewer than 30 Leicoma pigs. In 2020, 83 sows and 21 boars were entered into the herd book. This pig breed is still commonly reared mainly in central Germany [128]. According to data from the DAD-IS database, this breeds is at risk of extinction [50].
Three breeds are reared in Austria: Mangalica, Schwäbisch-Hällisches Schwein, and Turopolje. Mangalica, which is characterized by a very high quality of lard and meat, was imported from Hungary. The meat of this breed is used to produce special air-dried Vulcano hams, a specialty of southern Styria. Schwäbisch-Hällisches Schwein pigs were imported from Germany. This 100% stress-resistant breed is reared in intensive and semi-intensive systems in six herds. Turopolje pigs originate from Croatia. This typical pasture breed is well adapted to wetlands. It produces very high quality lard and meat used for the manufacturing of air-dried ham [50]. The number of animals of these breeds indicates a risk of extinction [50].
In Poland, three native breeds, Puławska, Złotnicka Spotted, and Złotnicka White, which are protected by genetic resources, are used for breeding and rearing. The Puławska breed is one of the oldest pig breeds, as it has been reared for almost 100 years in the country. It was created by crossing local pigs with Berkshire boars and refined with the English Large White breed. The typical conformation of the pig is a black and white coat with irregular black spots on a white background. These pigs have medium-length trunk and strong limbs. The well-developed hind part of the body has prominent medium-length hams. The sows of the breed exhibit a high level of reproductive performance indices [130,131], high intra-familial variability [132], specific chemical composition of milk, and good maternal care [133,134,135]. Neonates have a high level of energy reserves. This breed is characterized by resistance to stress and adverse environmental conditions (including diseases) and longevity [136,137]. Fatteners achieve daily weight gains of 400–590 g [131,138,139] and have meat content at the level of 48.70–56.45% [131,139]. They are reared mainly on family and organic farms until they reach a body weight of 125–130 kg. Their meat exhibits good quality with a pH45 6.15–6.19, pH24 5.59–5.61 in the longissimus lumborum muscle [39,140], a low level of drip loss of 3.82–4.2%, a thermal drip loss of 26.27–27.46%, and a good water holding capacity WHC of 20.02% [39,139]. Their meat is dark (L* lightness 48.75), which results from the presence of a large number and % proportion of red fibers (17.15%) [39,141]. It has a high protein content (22.54%), low levels of cholesterol (56.90 mg/100 g−1) [140], and an IMF value ranging from 2.2 to 3.7% [139,142]. The percentage content of SFA and PUFA is 37.79% and 10.77%, respectively [140]. Traditional products made of the meat of this breed include “Puławska pig” and “Nadwieprzańskie cold cuts” such as gammon, ham, and tenderloin [143]. Assessment of the polymorphism (insertion/deletion) in the prolactin (PRL) locus revealed that Del/Del homozygous sows were characterized by significantly higher fertility, whereas better maternal traits were exhibited by sows with the PRL Ins/Ins genotype [144]. Today, there are 2260 breeding sows and 159 boars [50].
“Złotnicka” pigs were derived from primitive long-eared and short-eared pigs. The Złotnicka White breed was selected to increase their meat content by crossing with the Swedish Landrace breed. These pigs have a small head, a medium-length straight snout, and ears drooping forward. They have a long, trapezoidal, and slightly narrowing trunk, a well-filled rump, and long legs. The pigs are reared mainly in central Poland. The sows of this breed give birth to 9–10 piglets and raise 8–9 juveniles [59]. They are caring mothers characterized by strong maternal instincts and the production of large amounts of milk. Złotnicka White fatteners have daily weight gains of 500–700 g. The average thickness of their backfat is 2 cm. Their meat content ranges from 46.33% to 50% [145]. The meat of this breed contains 21.7 to 24.5% of protein [39,139,140,141,145] and 1.87% of fat, and the levels of natural and thermal drip are 3.36% and 27.62%, respectively [145]. Their red meat has the following parameters: L* 43.88, pH45 6.38–6.6, and pH24 5.53 [146]. It is an excellent raw material for the production of traditional and regional cold cuts. Their meat has been entered into the National List of Traditional Products. Presently, there are 903 breeding sows and 46 boars [50].
The medium-sized Złotnicka Spotted pigs are black- and white-spotted animals with lop ears and a relatively large head. Their trunk is long and slightly flattened. They are characterized by a massive front part of the body with relatively weak musculature of the hams and the back. These pigs have strong and thick legs. They represent the meat–fat type. This breed is reared in several herds in north-western and central Poland. The pigs are characterized by high feed conversion rates. Sows give birth to 8–9 piglets per litter and are caring mothers, producing large amounts of milk. Low daily weight gains (400–600 g) [38], high adiposity (3.44–4.22 cm), and 48–51% meat content are characteristic traits of this breed [18,38]. Fatteners are resistant to stress. They are reared until they reach a body weight of 130 kg, and their meat is used to produce boiled and baked hams and tenderloin [54]. The pH45 value in the longissimus dorsi muscle ranges from 6.15 to 6.39 and the pH24 value is 5.5 [18,38,147]. Wielkopolska Złotnicka pork, jarred white sausage paté, roast leg from Złotnicka White meat, and nowotomyska sausage are examples of regional and traditional products made from the meat of the Złotnicka breeds [148]. A “Rasy Rodzime” (Native Breeds) system of certification of indigenous breeds and products obtained from their raw materials has been developed for the Polish breeds [149]. Since 2019, this brand has been involved in the local promotion of products from specific native Polish breeds reared in traditional (natural) management and nutrition systems. Today, there are 861 breeding sows and 31 boars [50].
The native English breeds include Berkshire (black coat and a white snout, feet, and tail tip), British Lop (fine and soft white skin, white long and silky hair), British Saddleback (black and white coat with a white stripe around the shoulders and forelegs; a white nose, tail tip, and hind legs are accepted as well), Gloucestershire Old Spots, Large Black (fine and soft blue-black skin covered by moderately silky hair), Middle White (white coat without spots, erect ears), Oxford Sandy and Black (sandy coat, black markings in random blotches rather than small spots, with sandy as the predominant color, and characteristic pale feet, blaze, and tassel), and Tamworth (golden-red coat with abundant fine straight hair) [50]. In the UK, such traditional breeds as Gloucester Old Spot, Berkshire, Saddleback, and Tamworth are more resistant to disease and better adapted to outdoor conditions. They have small litters and are generally good mothers, showing maternal care [150].
Tamworth is one of the oldest pig breeds. It comes from the original varieties reared in Great Britain in the Middle Ages, i.e., in a period when breeds were not yet officially distinguished. There is evidence of pure breeding dating back over 140 years. The name of the breed comes from the town of Tamworth on the River Tame in Staffordshire [151]. The Tamworth breed reportedly evaded (to some extent) the 19th century refinement practices and is currently believed to have remained in its more or less original state [74,96]. The pigs of the breed are medium-sized and quite slender, and have a long body and legs. They have a light-to-dark red coat, erect ears, and a long snout. On average, adult males weigh 280 kg and females weigh 250 kg. Tamworth sows give birth to 7–8 piglets. These hardy pigs are adapted to various climatic conditions and can be kept even on poor pastures [30].
The Berkshire breed originates from Cantonese and Old English breeds with some contribution from Neapolitan breeds. These medium-sized pigs have regular body proportions, small erect ears, and a short convex snout [151]. They are black with white on the snout, feet, and tail-tip [30]. Their shoulders and shoulder blades are sometimes white as well. Their head is small with a concave profile. Their hams are well filled. Berkshire pigs are very hardy and mature quickly. Sows give birth to nine piglets per litter and raise the offspring well. They have a mild temperament. Fatteners are slaughtered after reaching 50–80 kg body weight. They are well known for their juicy, marbled, and aromatic meat.
The Large Black breed originates from the extinct Old English Black Dorset, the Large Black (Devon/Cornwall), and the Small Suffolk breeds. The pigs have a black coat, a stocky body, and lop ears. The large ears protect the eyes from dirt and debris during the search for food. In the early 20th century, Large Black pigs were described as a fast growing breed, as the sows weighed 227 to 272 kg and boars weighed 318 to 363 kg [152], with an average height at the withers of 100 cm and 90 cm, respectively [30]. Females give birth to nine piglets per litter on average. They are characterized by a mild temperament and high weight gains (800 g) [59]. British Lop pigs are reared in South West England. The Oxford Sandy and Black breed became extinct around 1969 but was restored in the 1970s. The animals have a sand-colored coat with black patches and lop ears [30]. The Saddleback breed is a hybrid of the local Essex and Wessex Saddleback breeds. The pigs are black in color with a white saddle and lop ears. Saddleback sows have a strong territorial instinct and farrow outdoors. Gloucestershire Old Spot pigs are large and mostly white with a few black spots. They have a long slightly arched back and huge lop ears. Gloucestershire Old Spot sows are characterized by good maternal care [50]. This breed is very hardy, gentle, and easy to handle. These pigs produce sweet, well-marbled, extremely aromatic, slightly nutty pork. In terms of their origin, the Large Black, Berkshire, Gloucester Old Spot, and British Saddleback breeds are believed to be part of the Southern English and Midlands ancestry, while the British Lop and Large White/Yorkshire breeds originate from the Northern English population. Furthermore, all the South/Midlands English groups are believed to be largely influenced by crossbreeding with Neapolitan and/or Asian pigs in the 18th century [96].
The Middle White breed was created in 1850 as a result of crossing the Large White and Small White breeds. The animals are white and have erect ears. Their short and broad head has a concave profile and an upturned snout. The pigs have a barrel-shaped trunk with straight short legs and a drooping belly. The hams are wide and well filled. This breed represents the fat–meat type. On average, adult males weigh 275 kg and females weigh 225 kg, and the average height at the withers is 90 cm and 80 cm, respectively [30]. The sows in this breed are caring mothers producing large amounts of milk; they give birth to 8–10 piglets per litter. They are characterized by extraordinary gentleness [59]. All the breeds cited are at risk of extinction [50].
8. Genetic Studies of Native Breeds
Genetic variability is of great importance for the quality and suitability of the gene pool of conservation populations [7]. Genetic diversity is associated with the diversity of alleles (i.e., differences in DNA sequences in genes coding for certain traits) between many populations of the same species [49]. It is ensured by natural selection, genetic drift, and limited gene flow [153]. Mainly due to their highly polymorphic nature and high informativeness, in the early 2000s, microsatellites became one of the most widely used markers for the estimation of genetic variation and the identification of individuals [10,154,155]. In recent years, techniques based on single nucleotide polymorphism (SNP) arrays with whole-genome sequencing data are the most widely used approaches. The level of inbreeding of local breeds has been estimated with these techniques [10,96,156,157,158,159,160,161]. The genetic uniqueness of the European local breeds compared to major international breeds has been confirmed [154,162]. Genetic studies can also show differences in the history of the domestication and emergence of breeds [96]. To date, several genome-wide studies of some European indigenous pig breeds have been carried out [2,10,163,164,165] to provide preliminary information on their population structure. Ancestor-associated selection signatures have been detected in the entire genomes of several indigenous breeds, which may partly explain their adaptations to various environments, greater rusticity, and usually lower productivity than that of commercial breeds [2]. Research has provided information on well-known genes that determine these morphological traits (i.e., KIT, NCAPG-LCORL, CASP10 [166,167] MC1R, and OCA2) and explain the phenotypic diversity [10,49,60,168]. Additionally, these data can be used for the identification of breeds and confirmation of the authenticity of single-breed products [59]. Silio [169] and [49,84,156,157,160,170,171] presented results on the genetic variation, population structure, genetic distances, linkage disequilibrium, and effective population size. Subsequent studies identified genome regions and mutations associated with morphological, production, meat, and carcass traits [10,169,170,172,173,174,175]. In the Swallow-bellied Mangalitsa breed, genomic regions containing genes involved in cholesterol biosynthesis, fatty acid metabolism, and daily weight gains were identified, and candidate regions containing genes with a potential role in reproductive traits and disease resistance were revealed [10]. Various candidate genes, mainly involved in lipogenesis, have been proposed to explain the variability in fatty acid content in animal tissues [49]. In some genome regions, candidate genes responsible for the differences in phenotypic characteristics and adaptation to specific environments and production systems have been identified [84]. Candidate genes involved in female and male fertility [176], growth rate variability, daily gains, backfat thickness [164,177], higher adiposity, feed intake, meat and fat quality, and resistance to diseases have been identified to date [10,49,168]. Studies have shown that local pigs have different genomic variants that may serve as a valuable genetic reservoir for the future livestock industry.
9. Economic Aspects of the Protection of Native Swine Breed Biodiversity
The problem of biodiversity preservation is inseparable from the preservation of environmental resources and from the pollution of the environment. The loss of biodiversity is currently being observed at many levels: local, regional, and national. It is not only a serious environmental problem but also a social, cultural, and economic issue.
Activities related to the protection of native livestock breeds are specified in the Rural Development Program (RDP) under Priority 4 covering the restoration, protection, and enrichment of agriculture and forestry ecosystems. In 2014–2020, the total amount of funds planned for RDP measures for the 27 Member States amounted to EUR 157.1 billion, with the largest share, amounting to 44.2%, being allocated to measures under priority 4 (Figure 1).
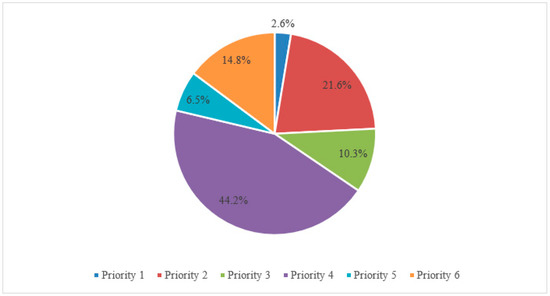
Figure 1.
Structure of the total planned allocation of EAFRD funds and national funds under RDP in 2014–2020 (%).
In total, EUR 148.4 billion (94.5%) of the total EUR 157.1 billion allocation of the European Agricultural Fund for Rural Development (EAFRD) and national funds planned for the 27 EU Member States for 2014–2020 under the six RDP priorities were contracted by individual EU Member States. The amount of payments from the RDP program at the end of 2021 amounted to EUR 109.0 billion (69.4%) of the allocated funds. The use of the funds under the individual priorities varied, with the lowest amounts being used under priority 1 (26.0%) and the highest expenditure under priority 4 (86.9%). Under priority 4, which covers the restoration, protection, and enrichment of agriculture and forestry ecosystems, i.e., activities related to the protection of native livestock breeds, the planned allocation amounted to EUR 72.7 billion, the amount of contracts concluded—EUR 72.3 billion—and the amount of payments made—EUR 62.8 billion—which comprised 86.9% in total (Figure 2).
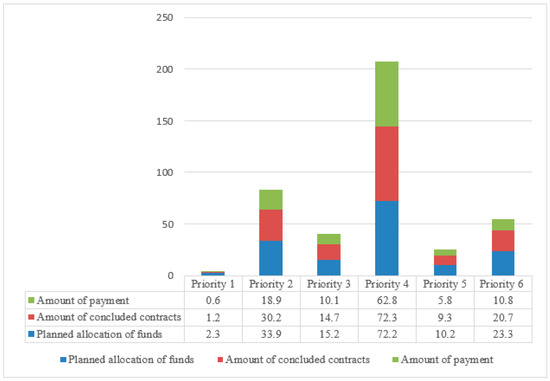
Figure 2.
Amount of planned allocation of RDP funds in 2014–2020, concluded contracts, and payments made at the end of 2021.
The EAFRD budget for the 2014–2020 programming period amounted to EUR 157.1 billion, but was provisionally extended to 2021 and 2022 by the Interim Regulation 2020/2220 of the European Parliament and of the Council (adopted on 23 December 2020) [178]. In this period, the RDP funds will be increased by EUR 26.9 billion from the 2021–2027 EAFRD budget and an additional EUR 8.1 billion from the budget of the EU’s Next Generation Recovery instrument. Hence, many of the RDP projects and programs will continue until the end of 2025. Since 2023, all new rural development measures have been included in the CAP National Strategic Plans. The strategic plans of individual countries are based on key social, environmental, and economic objectives for agriculture, forestry, and rural areas in the EU.
The 2023–2027 CAP Strategic Plan assumes 5-year commitments under agri-environment–climate interventions. These actions will result from the obligations intended to contribute to the protection of natural habitats and endangered species of birds, protection of genetic resources of crops and farm animals, protection of such beneficial organisms as pollinators, and landscape protection.
The interventions planned for 2023–2027 are largely a continuation of packages from the RDP 2014–2020 agri-environment–climate program.
The aim of the “preservation of the genetic resources of endangered animals in agriculture” intervention is to improve the economic use of animal genetic resources and to rear conservation breeds. In Poland, the intervention covers the following breeds:
- Cattle—Polish Red, White-Back, Polish Red-and-White, Polish Black-and-White;
- Horses—Huculskie, Małopolskie, Śląskie, Wielkopolskie, Sokółka- and Sztum-type cold-blooded horses, and Konik Polski breeds;
- Sheep—Wrzosówka, Świniarka, Olkuska, Polish Colored Mountain Sheep, Colored Merino, Uhruska, Wielkopolska, Żelaznieńska, Korideil, Kamieniecka, Pomeranian, Cakiel Podhalański, Polish Old-Type Merino, Black-headed, Foothill Sheep, Polish mountain sheep, and White-Headed Meat Sheep;
- Pigs—Puławska, Złotnicka White, and Złotnicka Spotted.
The maintenance of a minimum population size of local livestock breeds is a key objective in the conservation of animal genetic resources defined in national biodiversity conservation programs. Markets and livestock production systems tend to favor improved high-yielding breeds; therefore, the EU Member States implement a system of financial and other incentives to motivate farmers to maintain local breeds. The financial incentive for rearing native breeds established as a fixed payment has been defined within the RDP for each Member State. The high economic value of such ecosystem payments has been shown [179,180]. The costs of the maintenance of local animal breeds are borne by the farmer, and the benefits also have a regional, national, and even global dimension. The applied EU intervention mechanisms have an impact on pig producers’ decisions regarding the production system, which results in the maintenance of the desired level of biodiversity in agriculture [180]. In the process of biodiversity protection, there is a substantial difference between private costs and public benefits resulting from the protection of the biodiversity [181]. The Convention on Biological Diversity has explicitly recognized the need for incentive mechanisms for breeders [182]. There is a wide range of potential public and voluntary incentive mechanisms in the EU [183]. Payments for environmental services are one type of incentive mechanisms widely used to provide ecosystem services for public welfare [182,183,184,185,186].
The economic and social importance of the conservation and use of animal genetic resources in agriculture is widely recognized [187,188,189]. The development of related policies is a high priority under the EU Biodiversity Strategy [190] and the Farm to Fork Strategy [191] of the European Green Deal [192]. Conservation and sustainable management require international and national strategies as well as concrete policy actions targeted at the direct support and market valorization of breeds and their products, effective breeding programs, and raising breeders’ awareness.
Payment per head of registered breeding animal is the most common form of support for the animal genetic resources program in the EU Member States [193,194]. These payments are usually part of the agri-environmental programs of the Common Agricultural Policy. They are implemented under the RDP, and will become part of the CAP Strategic Plan after 2023 [14]. Since their introduction in 1992, agri-environment schemes have steadily gained political importance, as evidenced by the total amount of support available under the European Agricultural Fund for Rural Development [195].
International trade rules and commitments specify that agri-environment payments should only compensate for lower yields or higher production costs related to the participation of farmers in agri-environment schemes [196]. Under the current legal framework of the CAP [197], the maximum amount of support for farmers is limited to EUR/LU 200. The documents governing the post-2023 CAP [198] indicate that the current incentive mechanism has changed partly as a result of the significant reduction in the relevant section of the CAP budget [198] and partly due to the change in the operational logic of agri-environment payments, i.e., rewarding performance instead of compliance.
The introduction of incentive payments for local livestock breeds coincided with the first national RDP 2004–2006 [199]. In accordance with the previous legal provisions [200], the level of payment was based on the calculation of the income lost due to the higher costs per unit of production related to local breed rearing. In the first 2004–2006 RDP implementation period, the payments were based on a fixed amount of EUR 56.20 per animal, and certain mean numbers of animals specified for each country were included in the program [199]. In the second financial perspective of the support scheme for local breeds in 2007–2013 [201], the payments were established at an average level of EUR 152.10/LU, which corresponded to EUR 45.60 per pig, i.e., less than in the previous programming period. In turn, the payments for breeding native pig breeds in 2014–2020 increased to EUR 103.60/LU [202] (Figure 3).
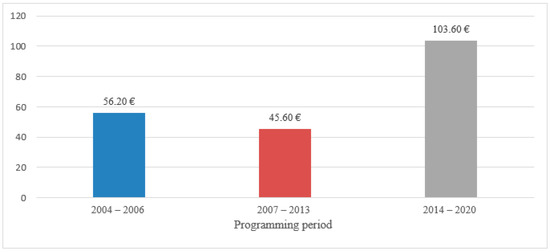
Figure 3.
Mean amount of financial support for breeding native pig breeds in the EU in 2004–2020 (EUR/LU).
The amount of support for breeding native breeds of pigs in 2004–2020 in the individual EU Member States varied in the subsequent financial perspectives. An analysis of the established RDP payments per head of native breed pig revealed the highest payments to be in Germany, Poland, and Sweden, and the lowest amounts to be in Bulgaria and Slovenia (Figure 4).
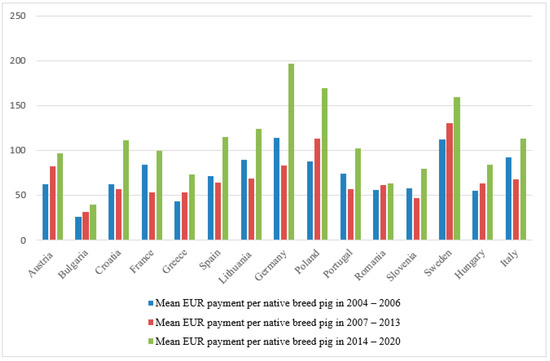
Figure 4.
Support for breeding native pig breeds under the 2004–2020 RDP in some EU Member States.
10. Conclusions
As shown by the available literature, the data on the population size and performance value of some local breeds are quite scarce. The information provided, even if limited, is very valuable as it may be the only available data on some of the most representative unique indigenous pig breeds in Europe. The raw material provided by these breeds has high quality and can be used for the manufacturing of meat products of the highest quality. The main objective of conservation programs is to keep old indigenous pig breeds as unaltered and original as possible for various reasons. The wide variety of pig breeds is the basis for sustainable animal husbandry. Local pig breeds are a valuable resource satisfying diverse consumers’ needs. They contribute to the improvement of specific economically important traits (longevity, health, resistance to diseases, and maternal traits) that have not been taken into account in selection to date. The conservation of old breeds as a gene reserve serves conventional animal breeding in terms of adaptation to the changing production and environmental conditions expected in the future. Considering climate change, there may be a need to create more resilient animals feeding on low-quality feed and poor pastures. The characteristic traits of local breeds indicate their ability to adapt to changing environmental conditions. Additionally, these breeds are part of the rural landscape of the region, a regional historical asset, and part of the cultural heritage deserving preservation akin to that afforded to old buildings and art objects. Pigs are an important part of the local community and culture. Rearing local pig breeds is highly important for local economic development and national cultural heritage. With the involvement of public and private financial resources, all efforts to preserve autochthonous breeds should be supported and the further impoverishment of their diversity and loss of potentially important genes should be prevented.
Author Contributions
Conceptualization, A.K.; data curation, A.K. and A.W.; formal analysis, A.K.; investigation, A.K. and A.W.; methodology, A.K.; project administration, A.K.; supervision, A.K.; visualization, A.K. and A.W.; writing—original draft, A.K. and A.W.; writing—review and editing, A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fanalli, S.L.; da Silva, B.P.M.; Gomes, J.D.; de Almeida, V.V.; Moreira, G.C.M.; Silva-Vignato, B.; Afonso, J.; Freitas, F.A.O.; Reecy, J.M.; Koltes, J.E.; et al. Transcriptome profile of skeletal muscle using different sources of dietary fatty acids in male pigs. Funct. Integr. Genom. 2023, 23, 73. [Google Scholar] [CrossRef]
- Bovo, S.; Ribani, A.; Muñoz, M.; Alves, E.; Araujo, J.P.; Bozzi, R.; Čandek-Potokar, M.; Charneca, R.; Di Palma, F.; Etherington, G.; et al. Whole-genome sequencing of European autochthonous and commercial pig breeds allows the detection of signatures of selection for adaptation of genetic resources to different breeding and production systems. Genet. Sel. Evol. 2020, 52, 33. [Google Scholar] [CrossRef]
- Amills, M. Biodiversity and origin of pig breeds. Bulletin Uasvm. Anim. Sci. Biotechnol. 2011, 68, 1–5. [Google Scholar]
- Larson, G.; Dobney, K.; Albarella, U.; Fang, M.; Matisoo-Smith, E.; Robins, J.; Lowden, S.; Finlayson, H.; Brand, T.; Willerslev, E.; et al. Worldwide phylogeography of wild boar reveals multiple centers of pig domestication. Science 2005, 307, 1618–1621. [Google Scholar] [CrossRef] [PubMed]
- Giuffra, E.; Kijas, J.; Amarger, V.; Carlborg, Ö.; Jeon, J.; Andersson, L. The origin of the domestic pig: Independent domestication and subsequent introgression. Genetics 2000, 154, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Larson, G.; Liu, R.; Zhao, X.; Yuan, J.; Fuller, D.; Barton, L.; Dobney, K.; Fan, Q.; Gu, Z.; Liu, X.H. Patterns of East Asian pig domestication, migration, and turnover revealed by modern and ancient DNA. Proc. Natl. Acad. Sci. USA 2010, 107, 7686–7691. [Google Scholar] [CrossRef] [PubMed]
- Groeneveld, L.F.; Lenstra, J.A.; Eding, H.; Toro, M.A.; Scherf, B.; Pilling, D.; Negrini, R.; Finlay, E.K.; Jianlin, H.; Groenveld, E. Genetic diversity in farm animals—A review. Anim. Genetics 2010, 41, 6–31. [Google Scholar] [CrossRef]
- Hall, S.J.G.; Bradley, D.G. Conserving livestock breed biodiversity. Trends Ecol. Evol. 1995, 10, 267–270. [Google Scholar] [CrossRef]
- Sponenberg, D.P.; Beranger, J.; Martin, A.M.; Couch, C.R. Conservation of rare and local breeds of livestock. Rev. Sci. Tech. L’oie 2018, 37, 259–267. [Google Scholar] [CrossRef]
- Zorc, M.; Škorput, D.; Gvozdanović, K.; Margeta, P.; Karolyi, D.; Luković, Z.; Salajpal, K.; Savić, R.; Muñoz, M.; Bovo, S.; et al. Genetic diversity and population structure of six autochthonous pig breeds from Croatia, Serbia, and Slovenia. Genet. Sel. Evol. 2022, 54, 30. [Google Scholar] [CrossRef]
- FAO. Genomic characterization of animal genetic resources. In FAO Animal Production and Health. Guidelines 32. Practical Guide; Food and Agriculture Organization of the United Nations: Rome, Italy, 2023. [Google Scholar]
- Sponenberg, D.P.; Martin, M.; Couch, C.; Beranger, J. Conservation strategies for local breed biodiversity. Diversity 2019, 11, 177. [Google Scholar] [CrossRef]
- FAO. The State of the World’s Animal Genetic Resources for Food and Agriculture—In Brief; Food and Agriculture Organization of the United Nations by the National Research Institute of Animal Production: Kraków, Poland, 2008. [Google Scholar]
- Lovec, M.; Šumrada, T.; Erjavec, E. New CAP Delivery Model, Old Issues. Intereconomics 2020, 55, 112–119. [Google Scholar] [CrossRef]
- Livestock Population in Numbers-Products Eurostat News. Available online: https://ec.europa.eu/eurostat (accessed on 6 May 2023).
- Kušec, G.; Dovč, P.; Karolyi, D.; Čandek Potokar, M. Local pig breeds and pork products in Croatia and Slovenia–unexploited treasure. Poljoprivreda 2015, 21, 16–21. [Google Scholar] [CrossRef]
- Dadousis, C.; Muñoz, M.; Óvilo, C.; Fabbri, M.C.; Araújo, J.P.; Bovo, S.; Potokar, M.; Charneca, R.; Crovetti, A.; Gallo, M.; et al. Admixture and breed traceability in European indigenous pig breeds and wild boar using genome-wide SNP data. Sci. Rep. 2022, 12, 7346. [Google Scholar] [CrossRef]
- Szulc, K.; Skrzypczak, E.; Buczyński, J.T.; Stanisławski, D.; Jankowska-Mąkosa, A.; Knecht, D. Evaluation of fattening and slaughter values and also the meat quality determination in Zlotnicka Spotted pigs and their crosses with duroc breed. Czech J. Anim. Sci. 2012, 57, 95–107. [Google Scholar] [CrossRef]
- Lefaucheur, L.; Lebret, B. The rearing system modulates biochemical and histological differences in loin and ham muscles between Basque and Large White pigs. Animal 2020, 14, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Milković, S.; Lončarić, R.; Kralik, I.; Kristić, J.; Crnčan, A.; Kušec, D.; Canavari, M. Consumers’ preference for the consumption of the fresh Black Slavonian pig’s meat. Foods 2023, 12, 1255. [Google Scholar] [CrossRef]
- Jeong, H.S.; Kim, D.W.; Chun, S.Y.; Sung, S.; Kim, H.J.; Cho, S. Native pig and chicken breed database: NPCDB. Asian-Australas. J. Anim. Sci. 2014, 27, 1394–1398. [Google Scholar] [CrossRef][Green Version]
- Poklukar, K.; Čandek-Potokar, M.; Batorek Lukač, N.; Škrlep, M. Biochemical and gene expression differences associated with higher fat deposition in Krškopolje pigs in comparison with lean hybrid pigs. Livest. Sci. 2023, 272, 105247. [Google Scholar] [CrossRef]
- Pugliese, C.; Sirtori, F. Quality of meat and meat products produced from southern European pig breeds. Meat Sci. 2012, 90, 511–518. [Google Scholar] [CrossRef]
- FAO. The Global Strategy for the Management of Farm Animal Genetic Resources; FAO: Rome, Italy, 1999. [Google Scholar]
- Krajowa Strategia Zrównoważonego Użytkowania i Ochrony Zasobów Genetycznych Zwierząt Gospodarskich; MRiRW, Zespół Wydawnictw i Poligrafii IZ PIB: Warsaw, Poland, 2014; pp. 1–169. (In Polish)
- FAO. Phenotypic Characterization of Animal Genetic Resources; FAO Animal Production and Health Guidelines: Rome, Italy, 2012. [Google Scholar]
- Magoro, A.M.; Mtileni, B.; Hadebe, K.; Zwane, A. Assessment of genetic diversity and conservation in South African indigenous goat ecotypes: A review. Animals 2022, 12, 3353. [Google Scholar] [CrossRef]
- Ajmone-Marsan, P. A global view of livestock biodiversity and conservation—Globaldiv. Anim. Genetics 2010, 41 (Suppl. 1), 1–5. [Google Scholar] [CrossRef]
- Loftus, R.; Scherf, B. World Watch List for Domestic Animal Diversity; Food and Agriculture Organization of the United Nations: Rome, Italy, 1993. [Google Scholar]
- Scherf, B.D. World Watch List for Animal Diversity, 3rd ed.; FAO: Rome, Italy, 2000. [Google Scholar]
- European Union. Regulation (EU) 2016/1012 of the European Parliament and of the Council, of 8 June 2016, on zootechnical and genealogical conditions for the breeding, trade in and entry into the Union of purebred breeding animals, hybrid breeding pigs and the germinal products thereof and amending Regulation (EU) No 652/2014, Council Directives 89/608/EEC and 90/425/EEC and repealing certain acts in the area of animal breeding (‘Animal Breeding Regulation’). Off. J. Eur. 2016, 171, 66–143. [Google Scholar]
- Polak, G.; Krupiński, J.; Martyniuk, E.; Calik, J.; Kawęcka, A.; Krawczyk, J.; Majewska, A.; Sikora, J.; Sosin-Bzducha, E.; Szyndler-Nędza, M.; et al. The risk status of Polish local breeds under conservation programmes—New approach. Ann. Anim. Sci. 2021, 21, 125–140. [Google Scholar] [CrossRef]
- Risk Status of Local Breeds by Regions and Species: Pig. Available online: fao.org/dad-is/risk-status-of-animal-genetic-resources/en/ (accessed on 6 May 2023).
- Čandek-Potokar, M.; Linan, R.M.N. European Local Pig Breeds—Diversity and Performance; Intech Open: London, UK, 2019. [Google Scholar]
- Brossard, L.; Nieto, R.; Charneca, R.; Araujo, J.P.; Pugliese, C.; Radović, Č.; Čandek-Potokar, M. Modelling nutritional requirements of growing pigs from local breeds using InraPorc. Animals 2019, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.; Gispert, M.; Hortos, M.; Pérez, J.; Suárez, P.; Piñeiro, C. Evaluation of production performance and carcass quality characteristics of boars immunised against gonadotropin-releasing hormone (GnRH) compared with physically castrated male, entire male and female pigs. Span. J. Agric. Res. 2010, 8, 599–606. [Google Scholar] [CrossRef]
- Lebret, B.; Dourmad, J.Y.; Mourot, J.; Pollet, P.Y.; Gondret, F. Production performance, carcass composition, and adipose tissue traits of heavy pigs: Influence of breed and production system. J. Anim. Sci. 2014, 92, 3543–3556. [Google Scholar] [CrossRef]
- Szulc, K.; Knecht, D.; Jankowska-Mąkosa, A.; Skrzypczak, E. Results of the evaluation of meat quality of Zlotnicka Spotted pig. Zesz. Nauk. UP we Wrocławiu. Biol. Hod. Zwierząt 2012, 586, 51–60. [Google Scholar]
- Kasprzyk, A.; Bogucka, J. Meat quality of Pulawska breed pigs and image of longissimus lumborum muscle microstructure compared to commercial DanBred and Naima hybrids. Archives Anim. Breeding 2020, 63, 293–301. [Google Scholar] [CrossRef]
- Kristensen, T.N.; Hoffmann, A.A.; Pertoldi, C.; Stronen, A.V. What can livestock breeders learn from conservation genetics and vice versa? Front. Genet. Sec. Livest. Genom. 2015, 6, 38. [Google Scholar] [CrossRef]
- Škrlep, M.; Čandek-Potokar, M.; Batorek Lukač, N.; Tomažin, U.; Flores, M. Aromatic profile, physicochemical and sensory traits of dry-fermented sausages produced without nitrites using pork from Krškopolje pig reared in organic and conventional husbandry. Animals 2019, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Lebret, B.; Lenoir, H.; Daré, S.; Fonseca, A.; Fève, K.; Riquet, J.; Mercat, M.J. Finishing season and feeding resources influence the quality of products from extensive system Gascon pigs. Part 1: Carcass traits and quality of fresh loin. Animal 2021, 15, 100240. [Google Scholar] [CrossRef] [PubMed]
- Blasco, A. Breeds in danger of extinction and biodiversity. Rev. Bras. Zootec. 2008, 37, 101–109. [Google Scholar] [CrossRef]
- Martins, J.M.; Fialho, R.; Albuquerque, A.; Neves, J.; Freitas, A.; Nunes, J.T.; Charneca, R. Growth, blood, carcass and meat quality traits from local pig breeds and their crosses. Animal 2020, 14, 636–647. [Google Scholar] [CrossRef]
- OECD/FAO. OECD-FAO Agricultural Outlook 2022–2031; OECD Publishing: Paris, France, 2022. [Google Scholar]
- Kallas, Z.; Varela, E.; Čandek-Potokar, M.; Pugliese, C.; Cerjak, M.; Tomažin, U.; Karolyi, D.; Aquilani, C.; Vitale, M.; Gil, J.M. Can innovations in traditional pork products help thriving EU untapped pig breeds? A non-hypothetical discrete choice experiment with hedonic evaluation. Meat Sci. 2019, 154, 75–85. [Google Scholar] [CrossRef]
- Lauvie, A.; Couix, N.; Sorba, J.M. Farmers using local livestock biodiversity share more than animal genetic resources: Indications from a workshop with farmers who use local breeds. Genet. Res. 2022, 3, 15–21. [Google Scholar] [CrossRef]
- Vargas, J.C.; Bertolni, F.; Stalder, K.J.; Steibel, J.P.; Rothschild, M.F. Estimating breed composition for pigs: A case study focused on Mangalitsa pigs and two methods. Livest. Sci. 2021, 244, 104398. [Google Scholar] [CrossRef]
- Muñoz, M.; Bozzi, R.; García, F.; Núñez, Y.; Geraci, C.; Crovetti, A.; García-Casco, J.; Alves, E.; Škrlep, M.; Charneca, R.; et al. Diversity across major and candidate genes in European local pig breeds. PLoS ONE 2018, 13, e0207475. [Google Scholar] [CrossRef]
- Available online: https://www.fao.org/dad-is/browse-by-country-and-species/en/ (accessed on 6 May 2023).
- Fernàndez, A.; Garcìa-Gasco, J.; De Pedro, E.; Siliò, L.; Rodriguez, M.C. Genetic antagonism between intramuscular fat content and primal cuts in Iberian pigs. Option Mediterr. 2007, 76, 43–46. [Google Scholar]
- Varela, E.; Kallas, Z. Societal preferences for the conservation of traditional pig breeds and their agroecosystems: Addressing preference heterogeneity and protest responses through deterministic allocation and scale-extended models. J. Agric. Econ. 2022, 73, 761–788. [Google Scholar] [CrossRef]
- Bonanzinga, M.; Franci, O.; Cappè, F.; Sirtori, F.; Crovetti, A.; Esposito, S.; Pugliese, C. The breeding of the main local pig breeds in Mediterranean Europe. In Proceedings of the 7th International Symposium on the Mediterranean Pig; De Pedro, E.J., Cabezas, A.B., Eds.; Ciheam: Zaragoza, Spain, 2012; Volume 101, pp. 117–124. [Google Scholar]
- Węsierska, E.; Sobolewska-Zielińska, J.; Pasternak, M.; Niemczyńska-Wróbel, K.; Gąsior, R.; Wojtycza, K.; Pustkowiak, H.; Duda, I.; Migdał, W. Biochemical properties affecting the nutritional quality, safety, and aroma of dry-cured products manufactured from meat of rare native pig breeds. Foods 2021, 10, 1597. [Google Scholar] [CrossRef]
- Čandek-Potokar, M.; Tomažin, U.; Škrlep, M.; Prevolnik Povše, M.; Batorek Lukač, N. Tehnologija reje kot temelj za zaščito EU oznak in blagovnih znamk. In Pitanje Prašičev na Večjo Težo in Predelava Mesa v Izdelke Posebne Kakovosti; Prikazi in Informacije; Čandek-Potokar, M., Ed.; Publisher Kmetijski Inštitut Slovenije: Ljubljana, Slovenia, 2015; Volume 285, pp. 168–179. [Google Scholar]
- Argamentería, A.; Menéndez-Fernández, J. La recuperación del Gochu Asturcelta. In Manual del Gochu Asturcelta; Gutiérrez, A.A., Ed.; Serida: Villaviciosa, Spain, 2012; pp. 35–46. [Google Scholar]
- de la Roza-Delgado, B.; Feito, I.; Fuente-Maqueda, F.; Modroño, S.; Argamenteria, A.; Ciordia, M. Influence of production system and feeds on performance, carcass traits and estimated energy balance of autochthonous Gochu Asturcelta pigs. Span. J. Agric. Res. 2022, 20, e0604. [Google Scholar] [CrossRef]
- Tibau, J.; Torrentó, N.; Aguado, R.Q.; González, J.; Oliver, M.A.; Gil, M.; Jaume, J.; Batorek-Lukač, N. Negre Mallorquí (Majorcan Black) Pig. In European Local Pig Breeds—Diversity and Performance; Candek-Potokar, M., Linan, R.M.N., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Szulc, K.; Buczyński, J.T. Stare Europejskie Rasy Świń. Wielkopolskie Wyd; Rolnicze Spółka z.o.o.: Poznań, Poland, 2012. [Google Scholar]
- Fontanesi, L.; Scotti, E.; Gallo, M.; Nanni Costa, L.; Dall’Olio, S. Authentication of “mono-breed” pork products: Identification of a coat colour gene marker in Cinta Senese pigs useful to this purpose. Livest. Sci. 2016, 184, 71–77. [Google Scholar] [CrossRef]
- Pugliese, C.; Bozzi, R.; Gallo, M.; Geraci, C.; Fontanesi, L.; Batorek-Lukač, N. Cinta Senese Pig. In European Local Pig Breeds—Diversity and Performance; Candek-Potokar, M., Linan, R.M.N., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Bozzi, R.; Gallo, M.; Geraci, C.; Fontanesi, L.; Batorek-Lukač, N. Apulo-Calabrese Pig. In European Local Pig Breeds—Diversity and Performance; Candek-Potokar, M., Linan, R.M.N., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Micari, P.; Racinaro, L.; Sarullo, V.; Carpino, S.; Marzullo, A. Zoometric rates, reproductive and productive parameters of the Apulo-Calabrian swine, obtained in breeding certified by ANAS Calabria. Ital. J. Anim. Sci. 2009, 8, 519–521. [Google Scholar] [CrossRef]
- Aboagye, G.; Zappaterra, M.; Pasini, F.; Dall’Olio, S.; Davoli, R.; Costa, L.N. Fatty acid composition of the intramuscular fat in the longissimus thoracis muscle of Apulo-Calabrese and crossbreed pigs. Livest. Sci. 2020, 232, 232–103878. [Google Scholar] [CrossRef]
- Pietrolà, E.; Pilla, F.; Maiorano, G.; Matassino, D. Morphological traits, reproductive and productive performances of Casertana pigs reared outdoors. Ital. J. Anim. Sci. 2006, 5, 139–146. [Google Scholar] [CrossRef]
- Salvatori, G.; Filetti, F.; Di Cesare, C.; Maiorano, G.; Pilla, F.; Oriani, G. Lipid composition of meat and backfat from Casertana purebred and crossbred pigs reared outdoors. Meat Sci. 2008, 80, 623–631. [Google Scholar] [CrossRef]
- Bozzi, R.; Gallo, M.; Geraci, C.; Fontanesi, L.; Batorek-Lukač, N. Nero Casertana Pig. In European Local Pig Breeds—Diversity and Performance; Candek-Potokar, M., Linan, R.M.N., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Bozzi, B.; Gallo, M.; Geraci, C.; Fontanesi, L.; Batorek-Lukač, N. Nero Siciliano Pig. In European Local Pig Breeds—Diversity and Performance; Candek-Potokar, M., Linan, R.M.N., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Bozzi, R.; Gallo, M.; Geraci, C.; Fontanesi, L.; Batorek-Lukač, N. Mora Romagnola Pig. In European Local Pig Breeds—Diversity and Performance; Candek-Potokar, M., Linan, R.M.N., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Bozzi, R.; Gallo, M.; Geraci, C.; Fontanesi, L.; Batorek-Lukač, N. Sarda Pig. In European Local Pig Breeds—Diversity and Performance; Candek-Potokar, M., Linan, R.M.N., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Freitas, A.B. A raça suína Alentejana: Passado, presente e futuro. In Las Razas Porcinas Iberoamericanas: Un Enfoque Etnozootécnico; Silva Filha, O., Ed.; Instituto Federal Baiano: Salvador, Brasil, 2014; pp. 55–80. [Google Scholar]
- Silva, J.S.; Ferreira-Cardoso, J.; Bernardo, A.; de Costa, J.S.P. Conservation and development of the Bísaro pig. Characterization and zootechnical evaluation of the breed for production and genetic management. In Quality of Meat and Fat in Pigs as Affected by Genetics and Nutrition; Wenk, C., Fernández, A., Dupuis, M., Eds.; EEAP and Wageningen Pers: Zurich, Switzerland, 2000; pp. 85–92. [Google Scholar]
- Neves, J.A.; Sabio, E.; Freitas, A.; Almeida, J.A.A. Déposition des lipids intramusculaires dans le porc Alentejano. L’effet du niveau nutritif pendant la croissance et du régime alimentaire pendant l’engraissement. Prod. Anim. 1996, 9, 93–97. [Google Scholar]
- Porter, V. Pigs. In Handbook to the Breeds of the World; Helm Information Ltd.: Near Robertsbridge, UK, 1993. [Google Scholar]
- Charneca, R.; Martins, J.; Freitas, A.; Neves, J.; Nunes, J.; Paixim, H.; Bento, P.; Batorek-Lukač, N. Alentejano pig. In European Local Pig Breeds—Diversity and Performance; Candek-Potokar, M., Linan, R.M.N., Eds.; IntechOpen: London, UK, 2019; p. 24. [Google Scholar]
- Albuquerque, A.; Óvilo, C.; Núñez, Y.; Benítez, R.; López-Garcia, A.; García, F.; Félix, M.D.R.; Laranjo, M.; Charneca, R.; Martins, J.M. Transcriptomic Profiling of Skeletal Muscle Reveals Candidate Genes Influencing Muscle Growth and Associated Lipid Composition in Portuguese Local Pig Breeds. Animals 2021, 11, 1423. [Google Scholar] [CrossRef]
- Silva, J.S.; Araújo, J.P.; Cerqueira, J.O.; Pires, P.; Alves, C.; Batorek-Lukač, N. Bísaro Pig. In European Local Pig Breeds—Diversity and Performance; Candek-Potokar, M., Linan, R.M.N., Eds.; IntechOpen: London, UK, 2019; p. 13. [Google Scholar]
- Vicente, A.; Roque, A.; Bastos, J.; Carolino, N. Managing the herdbookof an endangered Portuguese swine population: The Malhadode Alcobaçapig. In Proceedings of the 71st Annual Meeting of EEAP European Federation of Animal Science, Virtual, 1–4 December 2020. [Google Scholar]
- Vicente, A. Caracterização do Porco Malhado de Alcobaça. Ph.D. Thesis, Universidade Tecnica de Lisboa, Lisboa, Portugal, 2006. [Google Scholar]
- Menčik, S.; Klišanić, V.; Špehar, M.; Mahnet, Ž.; Škorput, D.; Luković, Z.; Karolyi, D. Reproductive parameters in a Banija Spotted pig breed population during breed revitalization. Vet. Arh. 2019, 89, 183–199. [Google Scholar] [CrossRef]
- Djurkin Kušec, I.; Buha, I.; Margeta, V.; Gvozdanović, K.; Radišić, Ž.; Komlenić, M.; Kušec, G. Carcass composition and meat quality of Crna Slavonska pigs from two different housing conditions. Agric. Conspec. Sci. 2017, 82, 221–225. [Google Scholar]
- Bradić, M.; Uremović, M.; Uremović, Z.; Mioč, B.; Konjačić, M.; Luković, Z.; Safner, T. Microsatellite analysis of the genetic diversity in the Black Slavonian pig. Acta Vet. 2007, 57, 209–215. [Google Scholar]
- Margeta, V.; Gvozdanović, K.; Kušec, G.; Djurkin Kušec, I.; Batorek-Lukač, N. Black Slavonian (Crna slavonska) pig. In European Local Pig Breeds—Diversity and Performance; Candek-Potokar, M., Linan, R.M.N., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Muñoz, M.; Bozzi, R.; García-Casco, J.; Núñez, Y.; Ribani, A.; Franci, O.; García, F.; Škrlep, M.; Schiavo, G.; Bovo, S.; et al. Genomic diversity, linkage disequilibrium and selection signatures in European local pig breeds assessed with a high density SNP chip. Sci. Rep. 2019, 9, 13546. [Google Scholar] [CrossRef]
- Karolyi, D.; Luković, Z.; Salajpal, K. Production traits of black slavonian pigs. In Proceedings of the 6th International Symposium on the Mediterranean Pig, Messina—Capo d’Orlando, Bologna, Italy, 11–13 October 2007; pp. 207–213. [Google Scholar]
- Porter, V. Mason’s World Dictionary of Livestock Breeds, Types and Varieties; CABI Publishing: Wallingford, UK, 2002; pp. 1–380. [Google Scholar]
- Lukić, B.; Ferenčaković, M.; Šalamon, D.; Čačić, M.; Orehovački, V.; Iacolina, L.; Curik, I.; Cubric-Curik, V. Conservation genomic analysis of the Croatian indigenous Black Slavonian and Turopolje pig breeds. Front. Genet. 2020, 11, 261. [Google Scholar] [CrossRef] [PubMed]
- Karolyi, D.; Luković, Z.; Salajpal, K.; Škorput, D.; Vnučec, I.; Mahnet, Ž.; Klišanić, V.; Batorek-Lukać, N. Turopolje pig (Turopoljska svinja). In European Local Pig Breeds—Diversity and Performance; Candek-Potokar, M., Linan, R.M.N., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Margeta, V. Perspektive uzgoja crne slavonske svinje u Hrvatskoj u kontekstu pristupanja Europskoj uniji. In Proceedings of the 48th Croatian & 8th International Symposium on Agriculture, Dubrovnik, Hrvatska, 17–22 February 2013; pp. 22–29. [Google Scholar]
- Čandek-Potokar, M.; Nieto, R.; Pugliese, C.; Araujo, J.P.; Charneca, R.; Garcia Casco, J.M.; González Sánchez, E.; Hernandez-Garcia, F.I.; Izquierdo, M.; Karolyi, D.; et al. Local Pig Breeds: Nutritional requirements, innovative practices and local feeding resources as challenges, In Project Treasure. Agric. Conspectus Sci. 2017, 82, 127–131. [Google Scholar]
- Lukač, N.B.; Tomažin, U.; Škrlep, M.; Kastelic, A.; Poklukar, K.; Čandek-Potokar, M. Krškopoljski prašič (Krškopolje Pig). In European Local Pig Breeds—Diversity and Performance; Candek-Potokar, M., Linan, R.M.N., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Čandek-Potokar, M.; Žlender, B.; Kramar, Z.; Šegula, B.; Fazarinc, G.; Uršič, M. Evaluation of Slovene local pig breed Krškopolje for carcass and meat quality. Czech J. Anim. Sci. 2003, 48, 120–128. [Google Scholar]
- Kastelic, A.; Čandek-Potokar, M. Application of quality labels in support of conservation of local breeds—A challenge for Slovenian Krškopolje pig. Acta Agric. Slov. 2013, 4, 205–209. [Google Scholar]
- Tomažin, U.; Batorek-Lukač, N.; Škrlep, M.; Prevolnik-Povše, M.; Čandek-Potokar, M. Meat and fat quality of Krškopolje pigs reared in conventional and organic production systems. Animal 2019, 13, 1103–1110. [Google Scholar] [CrossRef]
- Škrlep, M.; Čandek-Potokar, M.; Tomažin, U.; Batorek Lukač, N.; Flores, M. Properties and aromatic profile of dry fermented sausages produced from Krškopolje pigs reared under organic and conventional rearing regime. Animal 2018, 12, 1316–1323. [Google Scholar] [CrossRef]
- Megens, H.J.; Crooijmans, R.; Cristobal, M.S.; Hui, X.; Li, N.; Groenen, M.A.M. Biodiversity of pig breeds from China and Europe estimated from pooled DNA samples: Differences in microsatellite variation between two areas of domestication. Genet. Sel. Evol. 2008, 40, 103–128. [Google Scholar]
- Radović, Č.; Savić, R.; Petrović, M.; Gogić, M.; Lukić, M.; Radojković, D.; Batorek-Lukač, N. Mangalitsa (Swallow-Belly Mangalitsa) Pig. In European Local Pig Breeds—Diversity and Performance; Candek-Potokar, M., Linan, R.M.N., Eds.; IntechOpen: London, UK, 2019; Available online: https://www.intechopen.com/books/european-localpig-breeds-diversity-and-performance-a-study-of-project (accessed on 6 May 2023).
- Egerszegi, I.; Rátky, J.; Solti, L.; Brüssow, K. Mangalica—An indigenous swine breed from Hungary (review). Arch. Tierz. 2003, 46, 245–256. [Google Scholar] [CrossRef]
- Núñez, Y.; Radović, Č.; Savić, R.; García-Casco, J.M.; Čandek-Potokar, M.; Benítez, R.; Radojković, D.; Lukić, M.; Gogić, M.; Muñoz, M.; et al. Muscle transcriptome analysis reveals molecular pathways related to oxidative phosphorylation, antioxidant defense, fatness and growth in Mangalitsa and Moravka pigs. Animals 2021, 11, 844. [Google Scholar] [CrossRef] [PubMed]
- Savić, R.; Radović, Č.; Petrović, M.; Gogić, M.; Radojković, D.; Batorek-Lukac, N. Moravka Pig. In European Local Pig Breeds—Diversity and Performance; Candek-Potokar, M., Linan, R.M.N., Eds.; IntechOpen: London, UK, 2019; Available online: https://www.intechopen.com/books/european-local-pig-breeds-diversity-and (accessed on 6 May 2023).
- Petrović, M.; Radovi, Č.; Parunović, N.; Mijatović, M.; Radojković, D.; Aleksić, S.; Stanišić, N.; Popovac, M. Quality traits of carcass sides and meat of moravka and mangalitsa pig breeds. Biotechnol. Anim. Hunsbandry 2010, 26, 21–27. [Google Scholar] [CrossRef][Green Version]
- Michailidou, S.; Kalivas, A.; Ganopoulos, I.; Stea, E.; Michailidis, G.; Tsaftaris, A.; Argiriou, A. A multi-farm assessment of Greek black pig genetic diversity using microsatellite molecular markers. Genet. Mol. Res. 2014, 13, 2752–2765. [Google Scholar] [CrossRef] [PubMed]
- Mercat, M.J.; Lebret, B.; Lenoir, H.; Batorek-Lukač, N. Basque Pig. In European Local Pig Breeds—Diversity and Performance; Candek-Potokar, M., Linan, R.M.N., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Mercat, M.J.; Lebret, B.; Lenoir, H.; Batorek-Lukač, N. Gascon pig. In European Local Pig Breeds—Diversity and Performance; Candek-Potokar, M., Linan, R.M.N., Eds.; IntechOpen: London, UK, 2019; pp. 101–114. [Google Scholar]
- Razmaitė, V.; Šveistienė, R.; Jatkauskienė, V.; Leikus, R.; Juška, R.; Batorek-Lukač, N. Lietuvos Baltosios SenojoTipo (Lithuanian White) Pigs. In European Local Pig Breeds—Diversity and Performance; Candek-Potokar, M., Linan, R.M.N., Eds.; IntechOpen: London, UK, 2019; pp. 165–172. [Google Scholar]
- Razmaitė, V.; Šveistienė, R.; Jatkauskienė, V.; Juška, R.; Leikus, R.; Batorek-Lukač, N. Lietuvos Vietinė (Lithuanian Indigenous Wattle) Pig. In European Local Pig Breeds—Diversity and Performance; Candek-Potokar, M., Linan, R.M.N., Eds.; IntechOpen: London, UK, 2019; pp. 155–163. [Google Scholar]
- Razmaitė, V.; Juška, R.; Leikus, R.; Jatkauskienė, V. Pork quality of two Lithuanian breeds: Effects of breed, gender and feeding regimen. Animals 2021, 11, 1103. [Google Scholar] [CrossRef]
- Hansson, M. The Linderöd Pig—An Investigation on Population Structure and Production Level. Ph.D. Thesis, Sveriges Lantbruksuniversitet, Uppsala, Sweden, 2008. [Google Scholar]
- Olsson, R. Rasbeskrivning-Linderödssvin; Föreningen Landtsvinet: Nässjö, Sweden, 2004; Volume 4, p. 5. [Google Scholar]
- Quality Genetics. 2008. Available online: www.qgenetics.se (accessed on 6 May 2023).
- Simonsson, A.; Andersson, K.; Andersson, P.; Dalin, A.M.; Jensen, P.; Johansson, E.; Jonasson, L.; Olsson, A.C.; Olosson, O. Svinboken; LTs förlag: Stockholm, Sweden, 1997; p. 135. [Google Scholar]
- Matoušek, V.; Kernerová, N.; Hyšplerová, K.; Jirotková, D.; Brzáková, M. Carcass traits and meat quality of Prestice black-Pied pig reed. Asian-Australas. J. Anim. Sci. 2016, 29, 1181–1187. [Google Scholar] [CrossRef][Green Version]
- Nevrkla, P.; Vclavková, E.; Hadaš, Z.; Horký, P. Evaluation of reproductive performance in sows of Prestice Black- Pied pig—Czech genetic resource. Indian J. Anim. Res. 2017, 51, 219–222. [Google Scholar]
- Nevrkla, P.; Václavková, E. Meat quality and fatty acid profile in M. longissimus lumborum et thoracis in Prestice Black-Pied pigs fed with linseed diet. Indian J. Anim. Sci. 2020, 90, 446–450. [Google Scholar] [CrossRef]
- Nevrkla, P.; Weisbauerová, E.; Horký, P.; Hadaš, Z.; Rozkot, M.; Čtvrtlíková Knitlová, D. Fatty acid and amino acid profiles in muscle longissimus lumborum et thoracis of the indigenous Prestice Black-Pied pig breed in comparison with a commercial pig hybrid. Ital. J. Anim. Sci. 2023, 22, 472–481. [Google Scholar] [CrossRef]
- Matoušek, V. Modernizovaný Šlechtitelský Program pro Přeštické Černostrakaté Prase—Genetický Živočišný Zdroj; Jihočeska Univerzita v Českych Budějovicich: Česke Budějovice, Czech Republic, 2013. [Google Scholar]
- Falkova, L.; Vrtkova, I.; Kratochvilova, L. Boar SNP variability in genetic resource Přeštice Black Pied pig. Reas. Pig Breed. 2014, 8, 4–7. [Google Scholar]
- Nevrkla, P.; Václavková, E.; Rozkot, M. The indigenous Prestice Black-Pied pig breed differs from a commercial hybrid in growth intensity, carcass value and meat quality. Agriculture 2021, 11, 331. [Google Scholar] [CrossRef]
- Probst, I. Genetische Diversität und Disposition des Hausschweins zur Atemwegsinfektion. Ph.D. Thesis, Gottfried Wilhelm Leibniz Universität, Hannover, Germany, 2011. [Google Scholar]
- BLE. Einheimische Nutztierrassen in Deutschland und Rote Liste gefährdeter Nutztierrassen; Bundesanstalt für Landwirtschaft und Ernährung (BLE) Informations- und Koordinationszentrum für Biologische Vielfalt (IBV): Bonn, Germany, 2021. [Google Scholar]
- Kolk, C. Das Bunte Bentheimer Schwein—Genetische Diversität und aktueller Status von Zucht, Haltung und Marktchancen. Ph.D. Thesis, Tierärztliche Hochschule, Hannover, Germany, 2006. [Google Scholar]
- Hörcher, U. Alte und Gefährdete Haustierrassen II, Schweine, Naturschutzverband Niedersachsen Biologische Schutzgemeinschaft Hunte Weser-Ems; Beilage zu Natur&Kosmos: München, Germany, 2002. [Google Scholar]
- Kolk, C.; Wrede, J.; Distl, O. Analyse der Populationsstruktur des Bunten Bentheimer Schweins. Arch. Tierz. 2006, 49, 447–461. [Google Scholar]
- Kühn, U. Vergleichende Anatomische Untersuchungen des Darmtraktes und des Darmassoziierten Lymphatischen Gewebes (GALT) bei alten Hausschweinrassen und Einer Modernen Fleischrasse. Ph.D. Thesis, Tierärztliche Hochschule, Hannover, Germany, 2001. [Google Scholar]
- Schröder, H. Das Bunte Bentheimer schwein. In Gefährdete Schweinerassen und Alternative Schweinezüchtung; Hörning, B., Ed.; NZH Verlag: Wetzlar, Germany, 1997; pp. 41–46. [Google Scholar]
- Klemm, R.; Walther, R.; Karwath, M.; Golze, M.; Gschwender, F.; Wehlitz, R. Gefährdete Einheimische Nutztierrassen in Sachsen, Basis Genetischer Vielfalt und Wertvolles Kulturgut; Sächsisches Landesamt für Umwelt, Landwirtschaft und Geologie Pillnitzer Platz 3, Dresden, dfd Dresdner Druckfabrik GmbH: Dresdner, Germany, 2011. [Google Scholar]
- Mathes, M. Sattelschweine in Deutschland—Genanteile, Verwandtschaft, Inzucht. Ph.D. Thesis, Tierärztliche Hochschule, Hannover, Germany, 1996. [Google Scholar]
- Welker, W.; Klemm, R.; Menzer, K.; Müller, U.; Förster, C.; Fischer, R.; Wehlitz, R. Vielfalt der Nutztiere erhalten, Gefährdete Rassen in Sachsen—Stand und Aktivitäten. 2020. Available online: https://nbn-resolving.org/urn:nbn:de:bsz:14-qucosa2-756365 (accessed on 7 May 2023).
- Petig, M.; Zimmer, C.; Bühlerand, R.; Batorek-Lukač, N. Schwäbisch-Hällisches Pig. In European Local Pig Breeds—Diversity and Performance; Candek-Potokar, M., Linan, R.M.N., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Walkiewicz, A.; Kasprzyk, A.; Babicz, M. Zmiany w użytkowości rozpłodowej loch ras należących do komponentu matecznego utrzymywanych na terenie Lubelszczyzny w latach 1994–2003. Ann. UMCS 2005, XXIII, 75–79. [Google Scholar]
- Stasiak, A.; Lechowski, J.; Kasprzyk, A. Ocena krzyżowania świń ras: Wbp, pbz i puławskiej. Rocz. Nauk. PTZ 2005, 1, 477–484. [Google Scholar]
- Walkiewicz, A.; Kasprzyk, A.; Babicz, M.; Kamyk, P. Analysis of family variability for reproductive traits of Pulawska sows. Ann. Anim. Sci. Suppl. 2015, 1, 75–77. [Google Scholar]
- Stasiak, A.; Kamyk-Kamieński, P.; Babicz, M.; Kasprzyk, A.; Lechowski, J. Płodność i behawior płciowy loszek mieszańców rasy puławskiej i wielkiej białej polskiej żywionych mieszankami z udziałem owsa nagoziarnistego. Prz. Hod. 2013, 3, 19–21. [Google Scholar]
- Babicz, M.; Rejduch, B.; Kozubska-Sobocińska, A.; Pastwa, K.A.; Stasiak, A.; Serafin-Kozak, M. Analysis of sexual activity in gilts in terms of their reproductive value. Ann. Anim. Sci. 2011, 11, 241–250. [Google Scholar]
- Lechowski, J.; Kasprzyk, A.; Tyra, M.; Trawińska, B. Effect of ascorbic acid as a feed additive on indicators of the reproductive performance of Pulawska breed gilts. Med. Wet. 2016, 72, 378–382. [Google Scholar] [CrossRef]
- Babicz, M.; Stasiak, A.; Kamyk, P.; Bajda, Z.; Kasprzyk, A.; Lechowski, J.; Pietrzak, K. Analiza Czynników Oddziaływujących na Długość Użytkowania Rozpłodowego Loch Rasy Puławskiej; LXXV Zjazd PTZ „Nauka dla praktyki hodowlanej”: Olsztyn, Poland, 2010. [Google Scholar]
- Stasiak, A.; Kasprzyk, A.; Sałyga, M. The effect of the season of farrowing on selected performance traits in Pulawska sows. Anim. Sci. Pap. Rep. 2006, 24, 87–91. [Google Scholar]
- Szyndler-Nędza, M.; Mirosław, T. Effect of fattening and slaughter value of Pulawska gilts on their lifetime piglet production. Anim. Sci. Genet. 2023, 19, 55–67. [Google Scholar] [CrossRef]
- Kasprzyk, A.; Babicz, M.; Kamyk-Kamieński, P.; Lechowski, J. Slaughter value and meat quality of Pulawska and Polish Landrace breeds fatteners. Ann. UMCS 2013, 3, 1–9. [Google Scholar]
- Kasprzyk, A.; Tyra, M.; Babicz, M. Fatty acid profile of pork from a local and a commercial breed. Arch. Anim. Breed. 2015, 58, 379–385. [Google Scholar] [CrossRef]
- Kasprzyk, A.; Stasiak, A.; Babicz, M. Meat quality and ultrastructure of muscle tissue from fatteners of Wild Boar, Pulawska and its crossbreed Pulawska × (Hamshire × Wild Boar). Archiv Tierz. 2010, 53, 184–193. [Google Scholar] [CrossRef][Green Version]
- Florowski, T.; Pisula, A.; Rola, M.; Adamczak, L. Influence of crossbreeding of Pulawska with PLW and PL breeds on culinary quality of meat. Rocz. Inst. Przem. Mięsn. Tłuszcz. 2007, 45, 25–34. (In Polish) [Google Scholar]
- Prasow, M.; Babicz, M.; Domaradzki, P.; Skałecki, P.; Litwińczuk, A.; Kaliniak, A. Slaughter value and quality of meat of Polish local breed pigs. J. Anim. Sci. Biol. Bioeconomy 2018, 36, 5–17. (In Polish) [Google Scholar] [CrossRef]
- Babicz, M.; Szyndler-Nędza, M.; Kasprzyk, A.; Kropiwiec, K. Analysis of maternal traits in native Puławska sows of known genotype (ins/del) at the PRL locus. Ann. Anim. Sci. 2017, 17, 131–142. [Google Scholar] [CrossRef]
- Grześkowiak, E.; Borys, A.; Borzuta, K.; Buczyński, J.; Lisiak, D. Slaughter value, meat quality and backfat fatty acid profile in Zlotnicka White and Zlotnicka Spotted fatteners. Anim. Sci. Pap. Rep. 2009, 27, 115–125. [Google Scholar]
- Szulc, K.; Skrzypczak, E. Meat quality of polish native pigs. Wiadomości Zoot. 2015, 53, 48–57. [Google Scholar]
- Szulc, K.; Wojtysiak, D.; Migdał, Ł.; Migdał, W. The muscle fibre characteristics and the meat quality of m. longissimus thoracis from Polish Native Złotnicka Spotted Pigs and the crossbreed fatteners from the crossing of Duroc and Polish Large White boars. Appl. Sci. 2022, 12, 3051. [Google Scholar] [CrossRef]
- Szyndler-Nędza, M.; Radomski, P. Zalety hodowli świń ras rodzimych znak RASY RODZIME. In Proceedings of the Kształtowanie Patriotyzmu Konsumenckiego a Hodowla Trzody Chlewnej, KPODR, Minikowo, Poland, 2 December 2020. [Google Scholar]
- Radomski, P.; Krupiński, J.; Krawczyk, W.; Moskała, P. Certyfikacja i promocja produktów szansą dla rozwoju gospodarstw utrzymujących rasy rodzime zwierząt gospodarskich. Zagadnienia Doradz. Rol. IZ–PIB 2019, 1, 72–87. [Google Scholar]
- Arey, D.; Brooke, P. Animal Welfare Aspects of Good Agricultural Practice: Pig Production. Compassion in World Farming. 2006. Available online: https://www.ciwf.org.uk/media/5492194/gap_pig_book_full.pdf (accessed on 6 May 2023).
- Zeller, J.H. Breeds of Swine; Agricultural Research Service; U.S. Government Printing Office: Washington, DC, USA, 1961. [Google Scholar]
- Sharp, K.G. Utilization of Frozen Thawed Semen in Large Black Pigs; Growth and Carcass Characteristics of Large Black Pigs Fed Diets Supplemented with or without Alfalfa. Ph.D. Thesis, Purdue University, West Lafayette, IN, USA, 2020. [Google Scholar]
- Hoban, S.; Archer, F.I.; Bertola, L.D.; Bragg, J.B.; Breed, M.F.; Bruford, M.W.; Coleman, M.A.; Ekblom, R.; Funk, W.C.; Grueber, C.E.; et al. Global genetic diversity status and trends: Towards a suite of Essential Biodiversity Variables (EBVs) for genetic composition. Biol. Rev. 2022, 97, 1511–1538. [Google Scholar] [CrossRef] [PubMed]
- SanCristobal, M.; Chevalet, C.; Haley, C.S.; Joosten, R.; Rattink, A.P.; Harlizius, B.; Groenen, M.A.M.; Amigues, Y.; Boscher, M.-Y.; Russell, G.; et al. Genetic diversity within and between European pig breeds using microsatellite markers.; International Society for Animal Genetics. Anim. Genet. 2006, 37, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, T.; Zhu, C.; Jiang, X.; Zhao, Y.; Xu, Z.; Yang, S.; Chen, A. Selection and use of microsatellite markers for individual identification and meat traceability of six swine breeds in the Chinese market. Food Sci. Technol. Int. 2018, 24, 292–300. [Google Scholar] [CrossRef]
- Biermann, A.D.M.; Pimentel, E.C.G.; Tietze, M.; Pinent, T.; Kőnig, S. Implementation of genetic evaluation and mating designs for the endangered local pig breed Bunte Bentheimer. J. Anim. Breed. Genet. 2014, 131, 36–45. [Google Scholar] [CrossRef]
- Herrero-Medrano, J.M.; Megens, H.J.; Groenen, M.; Boss, M.; Pérez-Enciso, M.; Crooijmans, R. Whole-genome sequence analysis reveals differences in population management and selection of European low-input pig breeds. BMC Genom. 2014, 15, 601. Available online: http://www.biomedcentral.com/1471-2164/15/601 (accessed on 6 May 2023). [CrossRef]
- Schiavo, G.; Bertolini, F.; Galimberti, G.; Bovo, S.; Dall’Olio, S.; Costa, L.N.; Gallo, M.; Fontanesi, L. A machine learning approach for the identification of population-informative markers from high-throughput genotyping data: Application to several pig breeds. Animal 2020, 14, 223–232. [Google Scholar] [CrossRef]
- Schiavo, G.; Bovo, S.; Bertolini, F.; Tinarelli, S.; Dall’Olio, S.; Costa, L.N.; Gallo, M.; Fontanesi, L. Comparative evaluation of genomic inbreeding parameters in seven commercial and autochthonous pig breeds. Animal 2020, 14, 910–920. [Google Scholar] [CrossRef]
- Szmatoła, T.; Jasielczuk, I.; Semik-Gurgul, E.; Szyndler-Nędza, M.; Blicharski, T.; Szulc, K.; Skrzypczak, E.; Gurgul, A. Detection of runs of homozygosity in conserved and commercial pig breeds in Poland. J. Anim. Breed. Genet. 2020, 137, 571–580. [Google Scholar] [CrossRef]
- Banos, G.; Talenti, A.; Chatziplis, D.; Sánchez-Molano, E. Genomic analysis of the rare British Lop pig and identification of distinctive genomic markers. PLoS ONE 2022, 17, e0271053. [Google Scholar] [CrossRef]
- Ollivier, L. European pig genetic diversity: A minireview. Animal 2009, 3, 915–924. [Google Scholar] [CrossRef]
- Wilkinson, S.; Lu, Z.H.; Megens, H.J.; Archibald, A.L.; Haley, C.; Jackson, I.J.; Groenen, M.A.M.; Crooijmans, R.P.M.A.; Ogden, R.; Wiener, P. Signatures of diversifying selection in European pig breeds. PLoS Genet. 2013, 9, e1003453. [Google Scholar] [CrossRef]
- Yang, B.; Cui, L.; Perez-Enciso, M.; Traspov, A.; Crooijmans, R.P.M.A.; Zinovieva, N.; Schook, L.B.; Archinald, A. Genome-wide SNP data unveils the globalization of domesticated pigs. Genet. Sel. Evol. 2017, 49, 71. [Google Scholar] [CrossRef]
- Gurgul, A.; Jasielczuk, I.; Ropka-Molik, K.; Semik-Gurgul, E.; Pawlina-Tyszko, K.; Szmatoła, T.; Szyndler-Nędza, M.; Bugno-Poniewierska, M.; Blicharski, T.; Szulc, K.; et al. A genome-wide detection of selection signatures in conserved and commercial pig breeds maintained in Poland. BMC Genet. 2018, 19, 95. [Google Scholar] [CrossRef] [PubMed]
- Giuffra, E.; Evans, G.; Törnsten, A.; Wales, R.; Day, A.; Looft, H.; Plastow, G.; Andersson, L. The Belt mutation in pigs is an allele at the dominant white (I/KIT) locus. Mamm. Genome 1999, 10, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Fontanesi, L.; D’Alessandro, E.; Scotti, E.; Liotta, L.; Crovetti, A.; Chiofalo, V.; Russo, V. Genetic heterogeneity and selection signature at the KIT gene in pigs showing different coat colours and patterns. Anim. Genet. 2010, 41, 478–492. [Google Scholar] [CrossRef] [PubMed]
- Ribani, A.; Utzeri, V.J.; Geraci, C.; Tinarelli, S.; Djan, M.; Veličković, N.; Doneva, R.; Dall’Olio, S. Signatures of de-domestication in autochthonous pig breeds and of domestication in wild boar populations from MC1R and NR6A1 allele distribution. Anim. Genet. 2019, 50, 166–171. [Google Scholar] [CrossRef]
- Silió, L.; Barragán, C.; Fernández, A.I.; García-Casco, J.; Rodríguez, M.C. Assessing effective population size, coancestry and inbreeding effects on litter size using the pedigree and SNP data in closed lines of the Iberian pig breed. J. Anim. Breed. Genet. 2016, 133, 145–154. [Google Scholar] [CrossRef]
- Wilkinson, S.; Haley, C.; Alderson, L.; Wiener, P. An empirical assessment of individual-based population genetic statistical techniques: Application to British pig breeds. Heredity 2011, 106, 261–269. [Google Scholar] [CrossRef]
- Jasielczuk, I.; Gurgul, A.; Szmatoła, T.; Semik-Gurgulb, E.; Pawlina-Tyszkob, K.; Szyndler-Nędza, M.; Blicharski, T.; Szulc, K.; Skrzypczak, E.; Bugno-Poniewierska, M. Comparison of linkage disequilibrium, effective population size and haplotype blocks in Polish Landrace and Polish native pig populations. Livest. Sci. 2020, 231, 103887. [Google Scholar] [CrossRef]
- Bertolini, F.; Schiavo, G.; Tinarelli, S.; Santoro, L.; Utzeri, V.J.; Dall’Olio, S.; Costa, L.N.; Gallo, M. Exploiting phenotype diversity in a local animal genetic resource. Identification of a single nucleotide polymorphism associated with the tail shape phenotype in the autochthonous Casertana pig breed. Livest. Sci. 2018, 216, 148–152. [Google Scholar] [CrossRef]
- Schiavo, G.; Bertolini, F.; Utzeri, V.J.; Ribani, A.; Geraci, C.; Santoro, L.; Óvilo, C.; Fernández, A.I.; Gallo, M.; Fontanesi, L. Taking advantage from phenotype variability in a local animal genetic resource: Identification of genomic regions associated with the hairless phenotype in Casertana pigs. Anim. Genet. 2018, 49, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, G.; Bovo, S.; Tinarelli, S.; Bertolini, F.; Dall’Olio, S.; Gallo, M.; Fontanesi, L. Genome-wide association analyses for several exterior traits in the autochthonous Casertana pig breed. Livest. Sci. 2019, 230, 103842. [Google Scholar] [CrossRef]
- Ovilo, C.; Clop, A.; Noguera, J.L.; Oliver, M.A.; Barragán, C.; Rodriguez, C.; Silió, L.; Toro, M.A.; Coll, A.; Folch, J.M.; et al. Quantitative trait locus mapping for meat quality traits in an Iberian × Landrace F2 pig population. J. Anim. Sci. 2002, 80, 2801–2808. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, D.C.; Day, A.E.; Nagy, A.; Wales, R.; Rothschild, M.F.; Plastow, G.S. Genetic variation in two conserved local Romanian pig breeds using type 1 DNA markers. Genet. Sel. Evol. 2001, 33, 417–432. [Google Scholar] [CrossRef][Green Version]
- Fontanesi, L.; Bertolini, F.; Dall’Olio, S.; Buttazzoni, L.; Gallo, M.; Russo, V. Analysis of association between the MUC4 g. 8227C> G polymorphism and production traits in Italian heavy pigs using a selective genotyping approach. Anim. Biotechnol. 2012, 23, 147–155. [Google Scholar] [CrossRef]
- European Union. Regulation (EU) 2020/2220 of the European Parliament and of the Council of 23 December 2020 laying down certain transitional provisions for support from the European Agricultural Fund for Rural Development (EAFRD) and from the European Agricultural Guarantee Fund (EAGF) in the years 2021 and 2022 and amending Regulations (EU) No 1305/2013, (EU) No 1306/2013 and (EU) No 1307/2013 as regards resources and application in the years 2021 and 2022 and Regulation (EU) No 1308/2013 as regards resources and the distribution of such support in respect of the years 2021 and 2022. Off. J. Eur. 2020, 437, 1. [Google Scholar]
- Costanza, R.; de Groot, R.; Sutton, P.; van der Ploeg, S.; Anderson, S.J.; Kubiszewski, I.; Farber, S.; Turner, R.K. Changes in the global value of ecosystem services. Glob. Environ. Chang. 2014, 26, 152–158. [Google Scholar] [CrossRef]
- Pallante, G.; Drucker, A.; Sthapit, S. Assessing the potential for niche market development to contribute to farmers’ livelihoods and agrobiodiversity conservation: Insights from the finger millet case study in Nepal. Ecol. Econ. 2016, 130, 92–105. [Google Scholar] [CrossRef]
- Nijkamp, P.; Vindigni, G.; Nunes, A.L.D.P. Economic Valuation of biodiversity: A comparative study. Ecol. Econ. 2008, 67, 217–231. [Google Scholar] [CrossRef]
- CBD (Convention of Biological Diversity) Aichi Biodiversity Targets. Available online: https://www.cbd.int/doc/strategic-plan/targets/compilation-quick-guide-en.pdf (accessed on 6 May 2023).
- Garrett, L.; Neves, B. Incentives for Ecosystem Services: Spectrum. Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/in-action/incentives-for-ecosystem-services/toolkit/sources-of-incentives/en/ (accessed on 6 May 2023).
- Wunder, S.; Brouwer, R.; Engel, S.; de Blas, D.E.; Muradian, R.; Pascual, U.; Pinto, R. From principles to practice in paying for nature’s services. Nat. Sustain. 2018, 1, 145–150. [Google Scholar] [CrossRef]
- Salzman, J.; Bennett, G.; Carroll, N.; Goldstein, A.; Jenkins, M. The Global Status and Trends of Payments for Ecosystem Services. Nat. Sustain. 2018, 1, 136–144. [Google Scholar] [CrossRef]
- Börner, J.; Baylis, K.; Corbera, E.; Ezzine-De-Blas, D.; Honey-Rose, J.; Persson, M.U.; Wunder, S. The effectiveness of payments for environmental services. World Dev. 2017, 96, 359–374. [Google Scholar] [CrossRef]
- Drucker, G.A.; Scarpa, R. Introduction and overview to the Special Issue on animal genetic resources. Ecol. Econ. 2003, 45, 315–318. [Google Scholar] [CrossRef]
- Mendelsohn, R. The challenge of conserving indigenous domesticated animals. Ecol. Econ. 2003, 45, 501–510. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). The Second Report on the State of the World’s Animal Genetic Resources for Food and Agriculture; Scherf, B.D., Pilling, D., Eds.; FAO Commission on Genetic Resources for Food and Agriculture Assessments: Rome, Italy, 2015. [Google Scholar]
- European Commission Biodiversity Strategy for 2030—Concrete Actions. Available online: https://ec.europa.eu/environment/strategy/biodiversity-strategy-2030_en (accessed on 6 May 2023).
- European Commission Factsheet: From Farm to Fork: Our Food, Our Health, Our Planet, Our Future. Available online: https://ec.europa.eu/commission/presscorner/detail/en/fs_20_908 (accessed on 6 May 2023).
- European Commission. A European Green Deal. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 6 May 2023).
- Soini, K.; Diaz, C. Developing a typology for local cattle breed farmers in Europe. J. Anim. Breed. Genet. 2012, 129, 436–447. [Google Scholar] [CrossRef]
- Bojkovski, D.; Simčič, M.; Kompan, D. Supports for local breeds in the European region—An overview. Poljoprivreda 2015, 21, 7–10. [Google Scholar] [CrossRef]
- European Commission; ECORYS; University of Wageningen; IEEP. Mapping and Analysis of the Implementation of the CAP Executive Summary; Publications Office of the EU: Brussels, Belgium, 2016. [Google Scholar]
- European Commission (Directorate General for Agriculture and Rural Development). Agri-Environment Measures: Overview on General Principles, Types of Measures and Application. Available online: https://ec.europa.eu/info/sites/info/files/food-farming-fisheries/sustainability_and_natural_resources/documents/report-agri-environ-measures-an-overview_2005_en.pdf (accessed on 6 May 2023).
- European Union. European Commission Commission Regulation (EC) 1305/2013 of 17 December 2013 on support for rural development by the European Agriculture Fund for Rural Development (EAFARD) and repealing Council Regulation (EC) 169. Off. J. Eur. Union. 2013, 347, 487–548. [Google Scholar]
- European Commission Impact Assessment. Commission Staff Working Document, Brussels, 1.6.2018 SWD (2018) 301 Final. Available online: https://ec.europa.eu/commission/sites/beta-political/files/budget-may2018-cap-swd-part1_en.pdf (accessed on 6 May 2023).
- Ministry of Agriculture Forestry and Food of Republic of Slovenia Rural Development Programme for Slovenia for the Period 2004–2006. (In Slovenian: Program Razvoja podeželja 2004–2006). Available online: http://www.pisrs.si/Pis.web/pregledPredpisa?id=DRUG1543 (accessed on 6 May 2023).
- Grima, N.; Singh, S.J.; Smetschka, B.; Ringhofer, L. Payment for Ecosystem Services (PES) in Latin America: Analysing the performance of 40 case studies. Ecosyst. Serv. 2016, 17, 24–32. [Google Scholar] [CrossRef]
- Ministry of Agriculture Forestry and Food of Republic of Slovenia Rural Development Programme for Slovenia for the Period 2007–2013. In Slovenian: Program Razvoja Podeželja 2007–2013. Available online: http://www.program-podezelja.si/images/phocadownload/Arhiv_PRP_2007-2013/prp_2007_2013_6_sprememba.pdf (accessed on 6 May 2023).
- Ministry of Agriculture Forestry and Food of Republic of Slovenia Rural Development Programme for Slovenia for the Period 2014–2020. (In Slovenian: Program Razvoja Podeželja 2014–2020). Available online: https://www.program-podezelja.si/en/rural-development-programme-2014-2020 (accessed on 6 May 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).