Insecticide Monitoring in Cattle Dip with an E-Nose System and Room Temperature Screen-Printed ZnO Gas Sensors
Abstract
1. Introduction
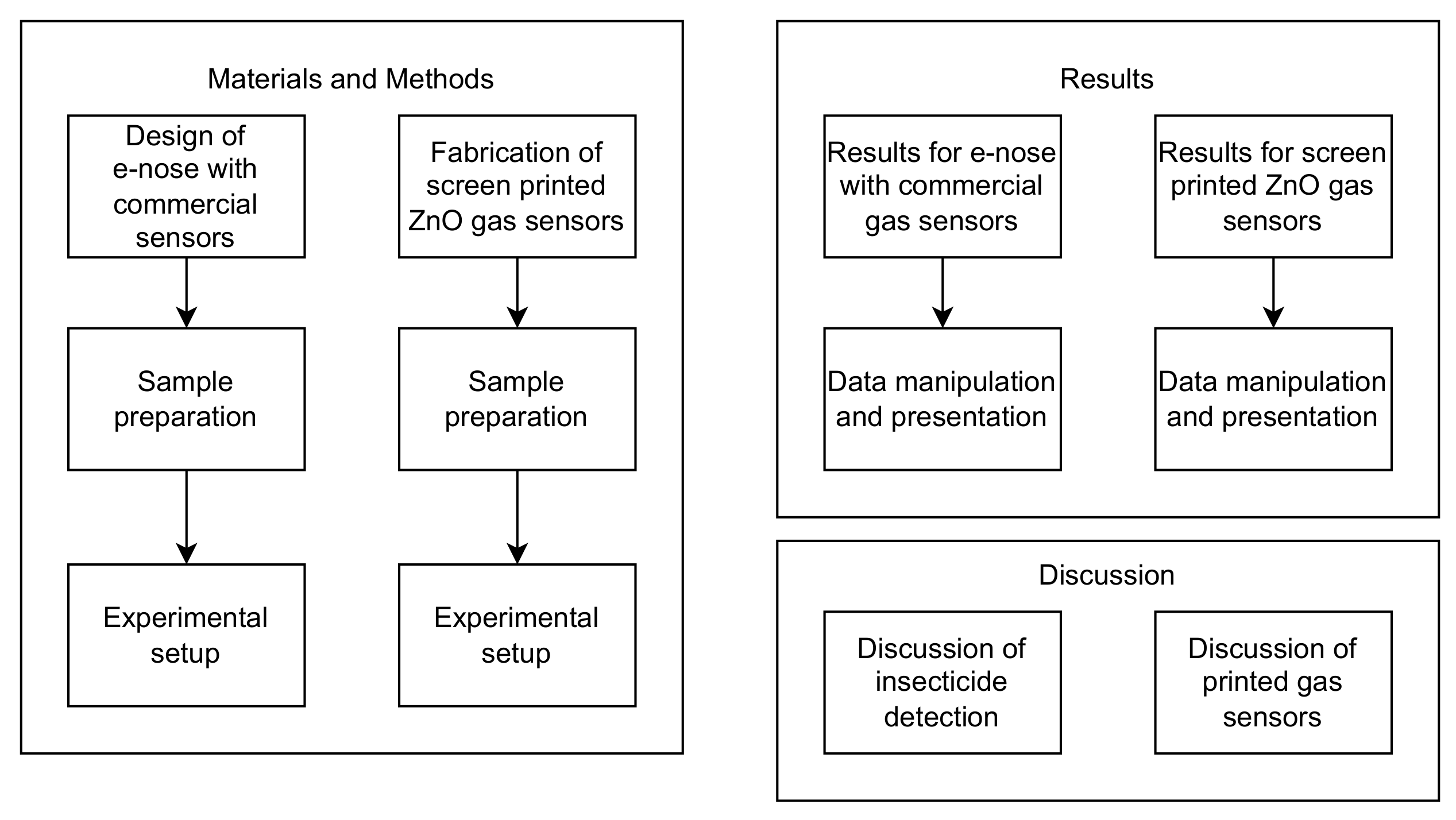
2. Materials and Methods
2.1. Materials and Methods for an E-Nose That Is Made with Commercial Gas Sensors
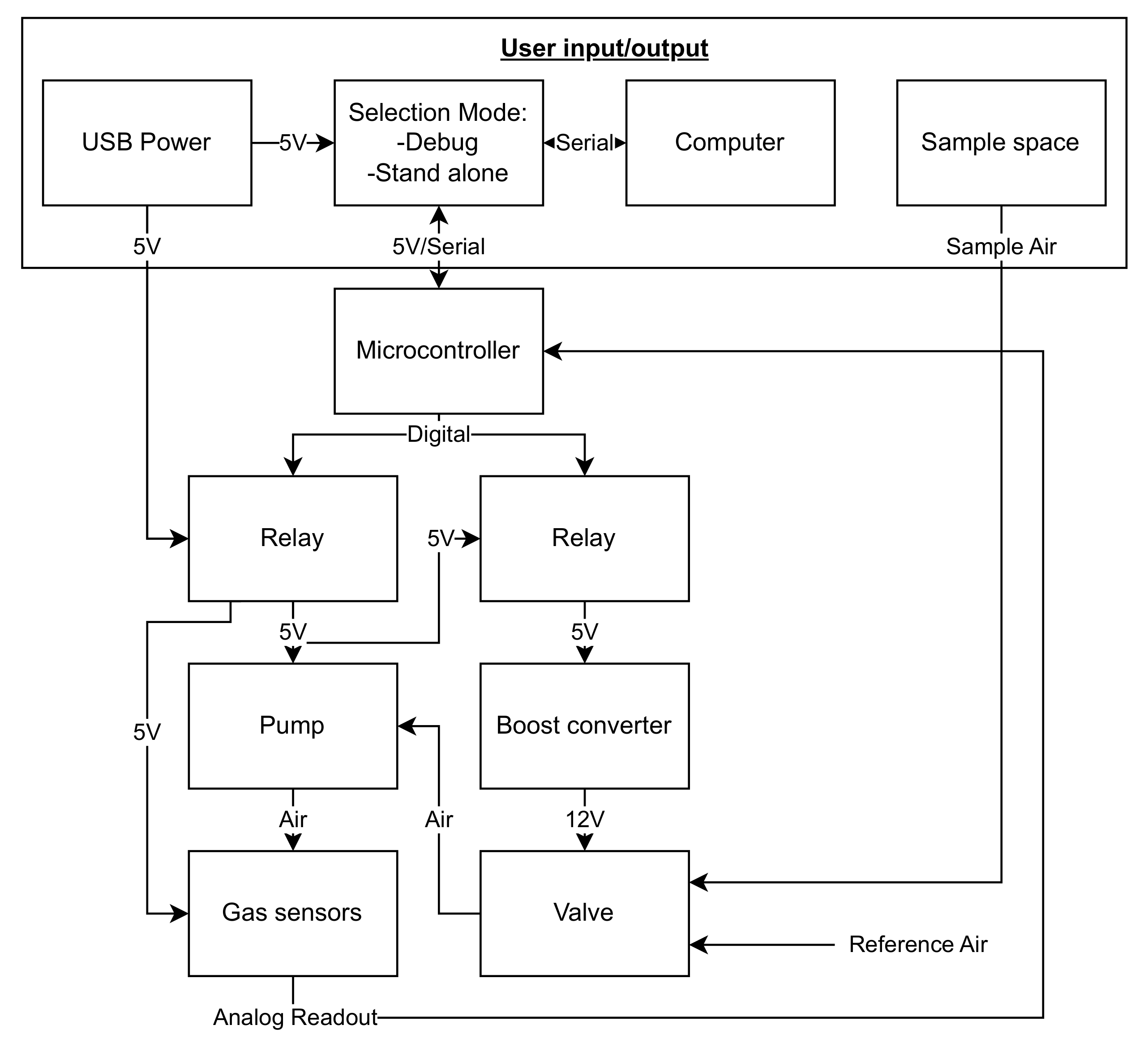
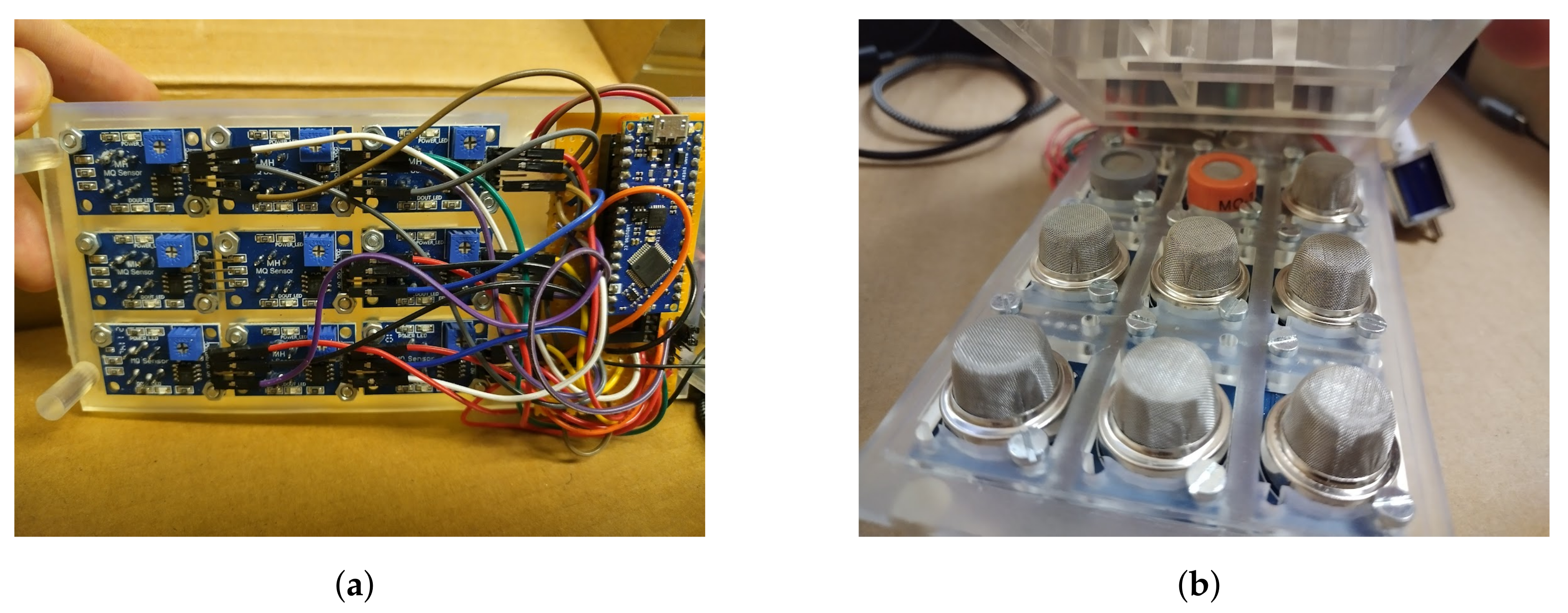
2.1.1. Design of E-Nose with Commercial Sensors
2.1.2. Sample Preparation for E-Nose with Commercial Sensors
2.1.3. Experimental Setup for E-Nose with Commercial Sensors
2.2. Materials and Methods for Screen-Printed ZnO Gas Sensors
2.2.1. Fabrication of Screen Printed ZnO Gas Sensors
- The first ZnO solution was prepared by dissolving zinc acetate dihydrate (Saarchem UnivAR, Mumbai, India) in isopropanol (ACE Platinum line (AR), Johannesburg, South Africa) [45]. Monoethanolamine (MEA) (ACE Gold line (CP), Johannesburg, South Africa) was added to the solution, maintaining a molar ratio of MEA to zinc acetate dihydrate of 1.0 and the concentration of zinc acetate of 0.5 M. All precursors were used as received, without further processing. The solution was continuously stirred for 2 h at 60 °C to obtain a homogeneous and clear solution. This solution was aged for 48 h before use;
- The second ZnO solution was also made with the sol-gel process, but substituted isopropanol with ethanol (Merck/Sigma-Aldrich, Darmstadt, Germany) (as in [46]). For this solution, the concentration of the zinc acetate dihydrate (Merck/Sigma-Aldrich, Darmstadt, Germany) was 1.5 M;
- As a validation metric, the final solution is an off-the-shelf ZnO nanoparticle ink. Avantama N-10, Nanograde N-10, ZnO suspension was purchased from Merck/Sigma-Aldrich, Darmstadt, Germany, and was used without further processing.
2.2.2. Sample Preparation for Screen-Printed ZnO Gas Sensors
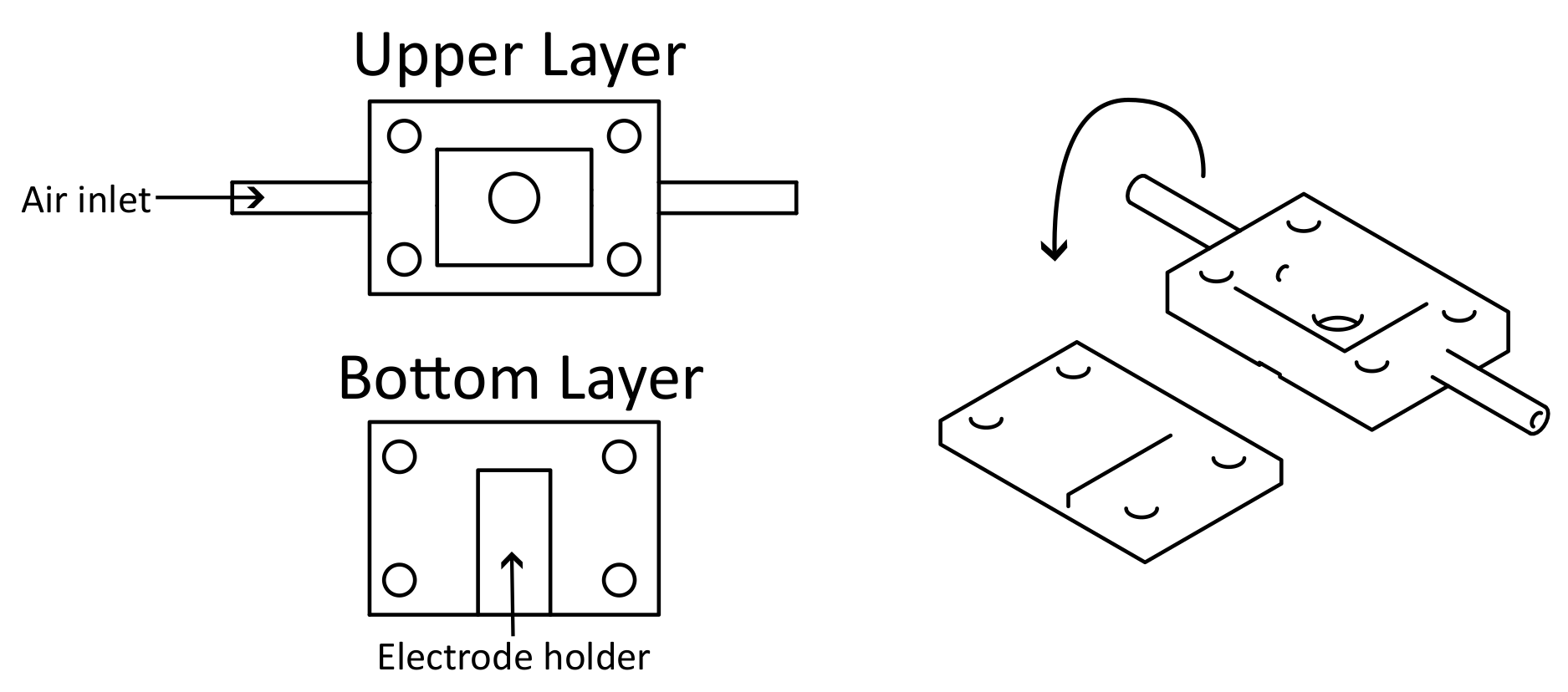
2.2.3. Experimental Setup for Screen Printed ZnO Gas Sensors
3. Results
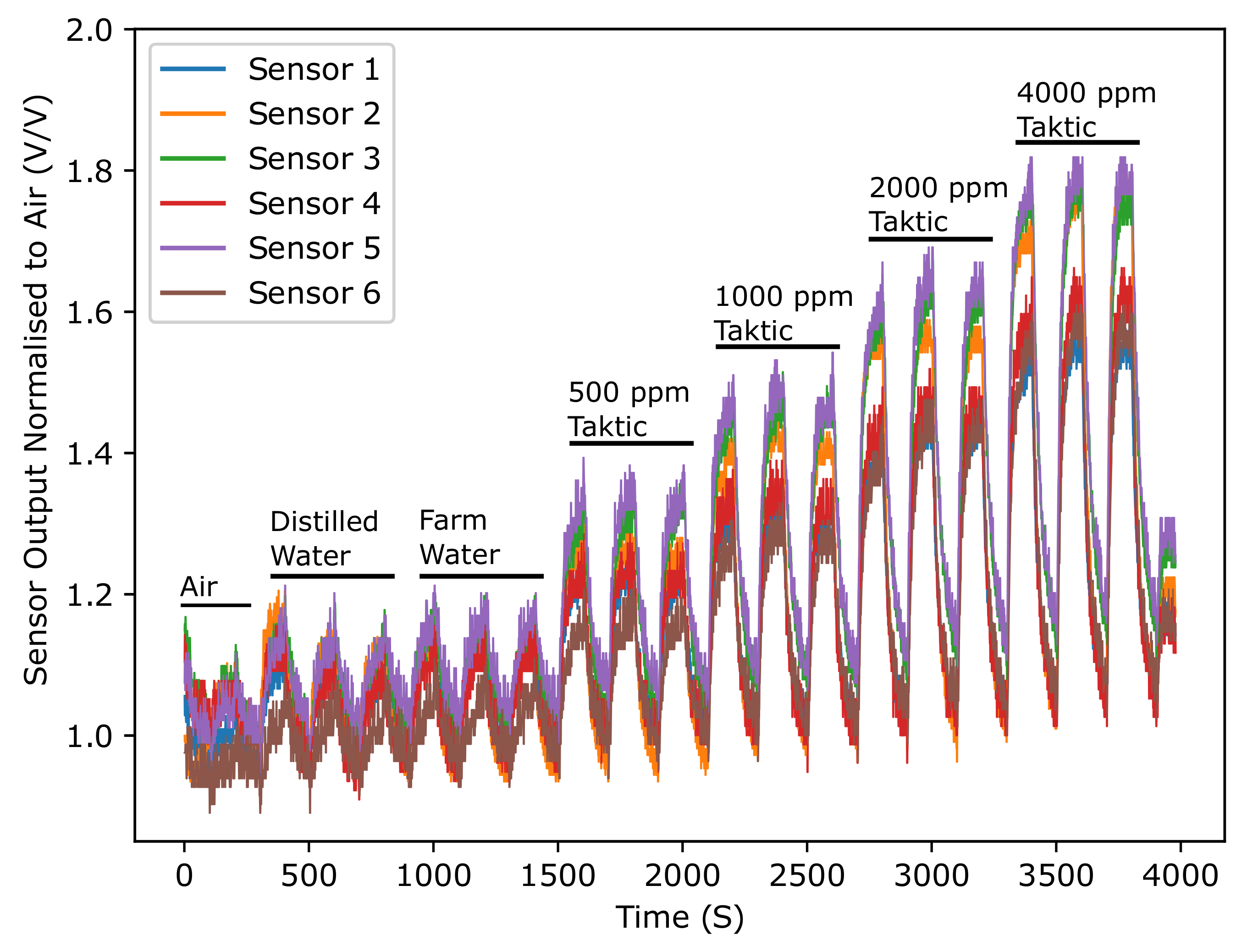
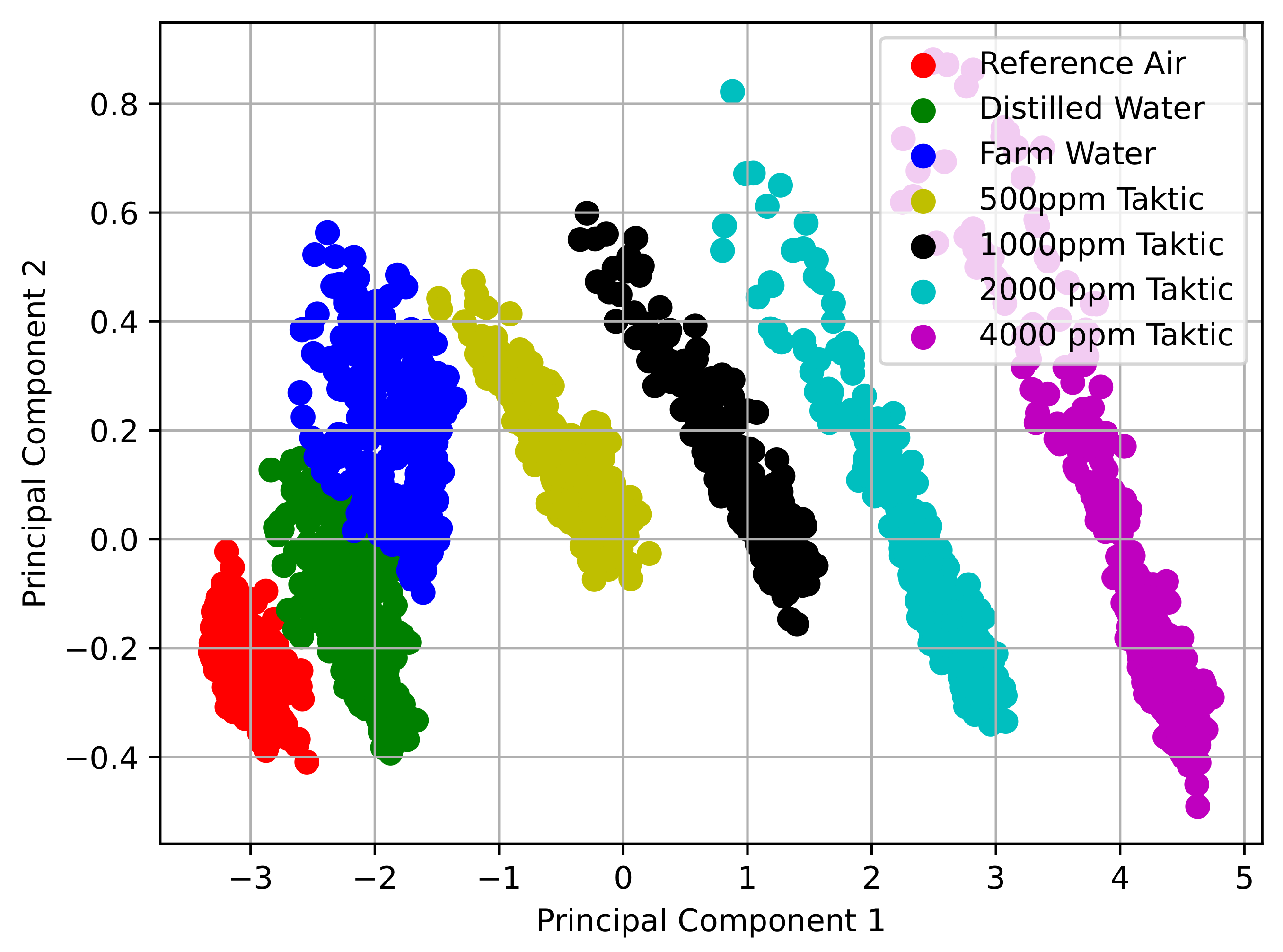
3.1. Results for E-Nose with Commercial Gas Sensors
3.2. Results for Screen Printed ZnO Gas Sensors
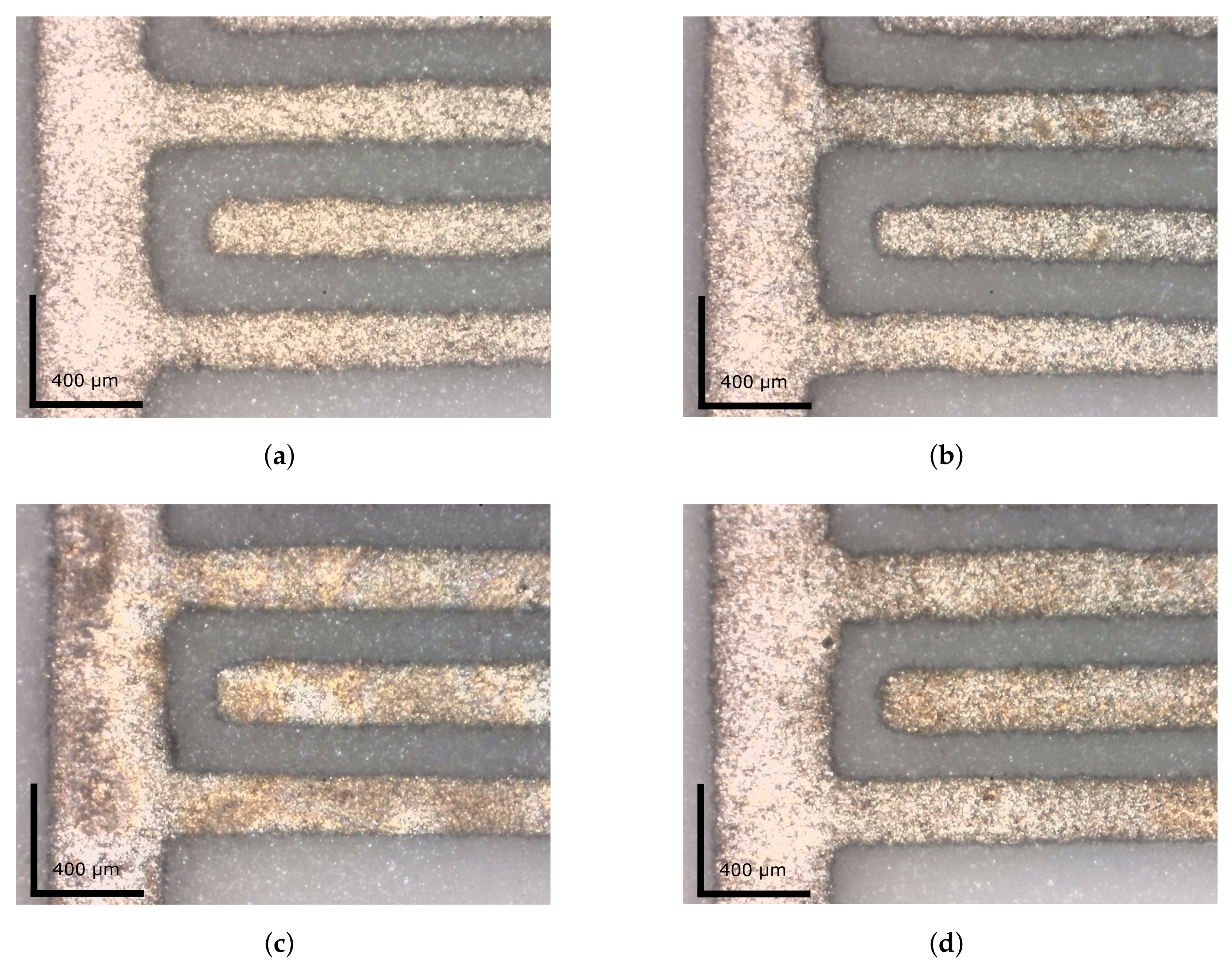
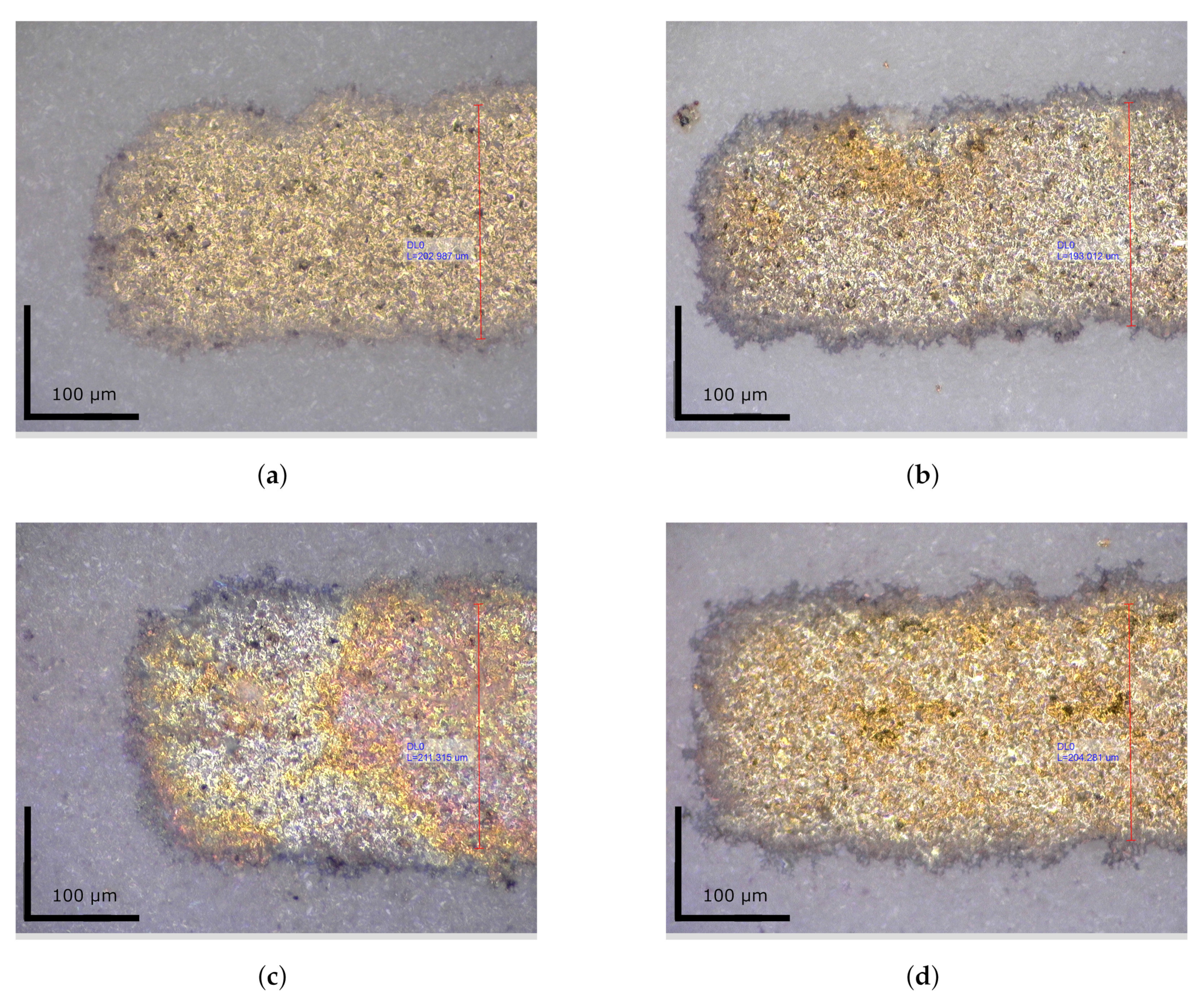
3.2.1. Microscopy
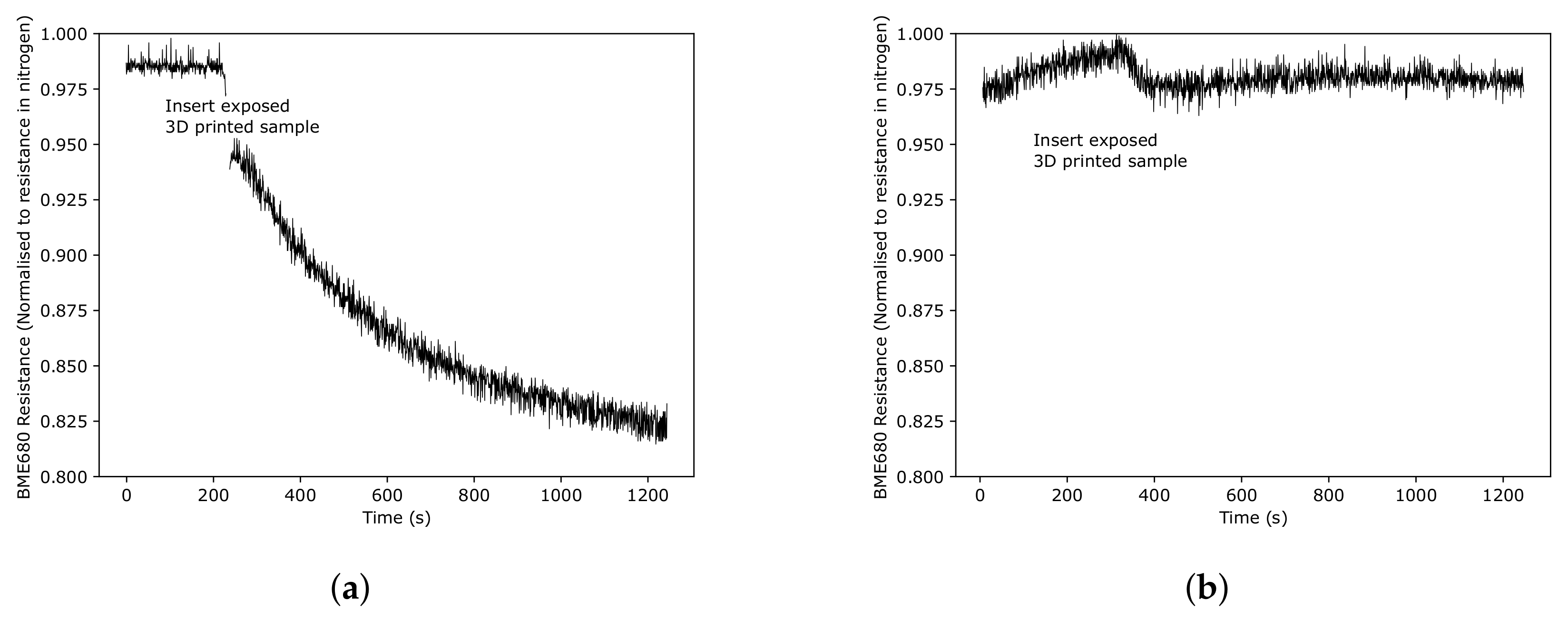
3.2.2. Response of ZnO Gas Sensors to Pure Taktic Fumes
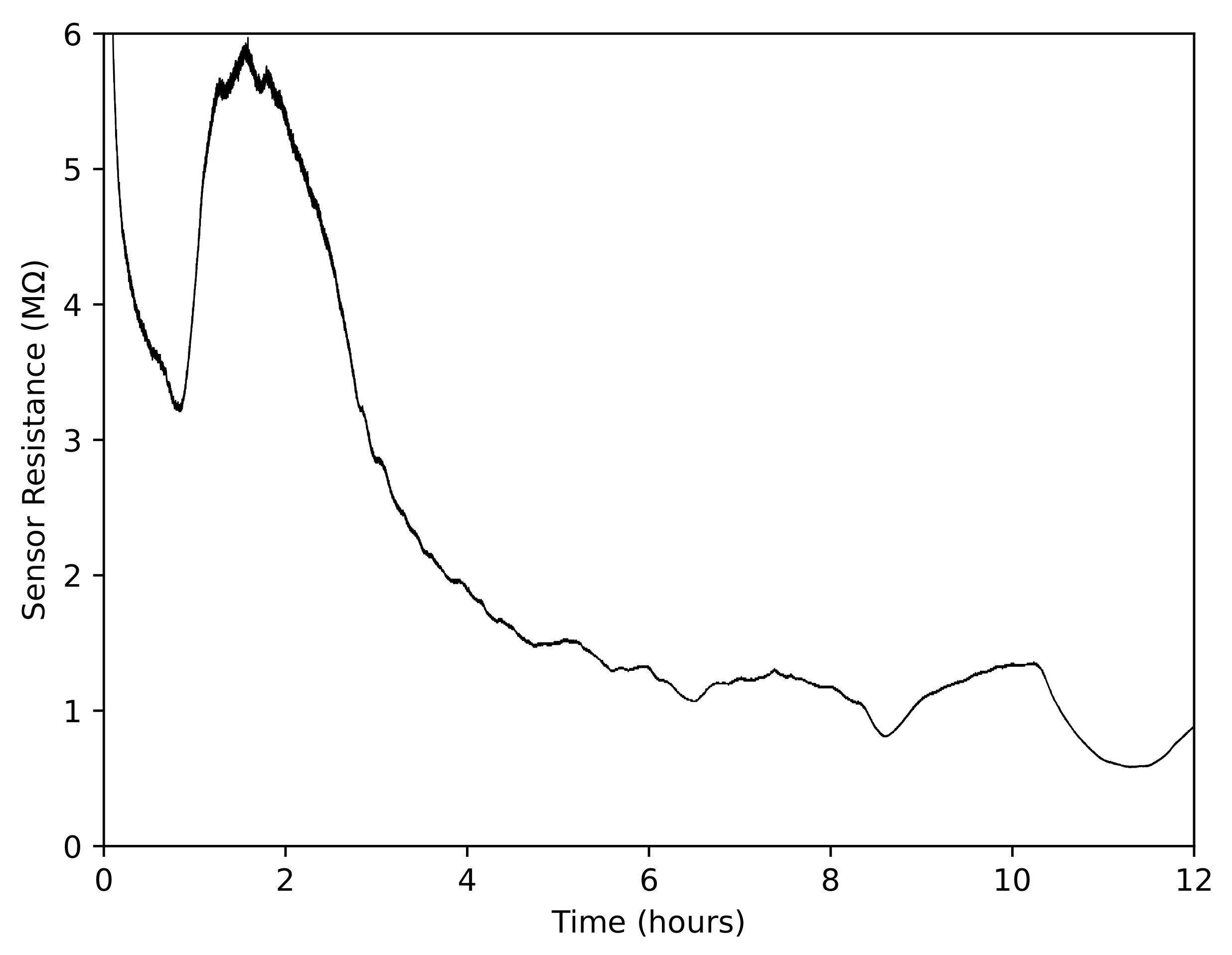
3.2.3. Gas Sensor Response over Long Periods of Time
3.2.4. UV Light Intensity
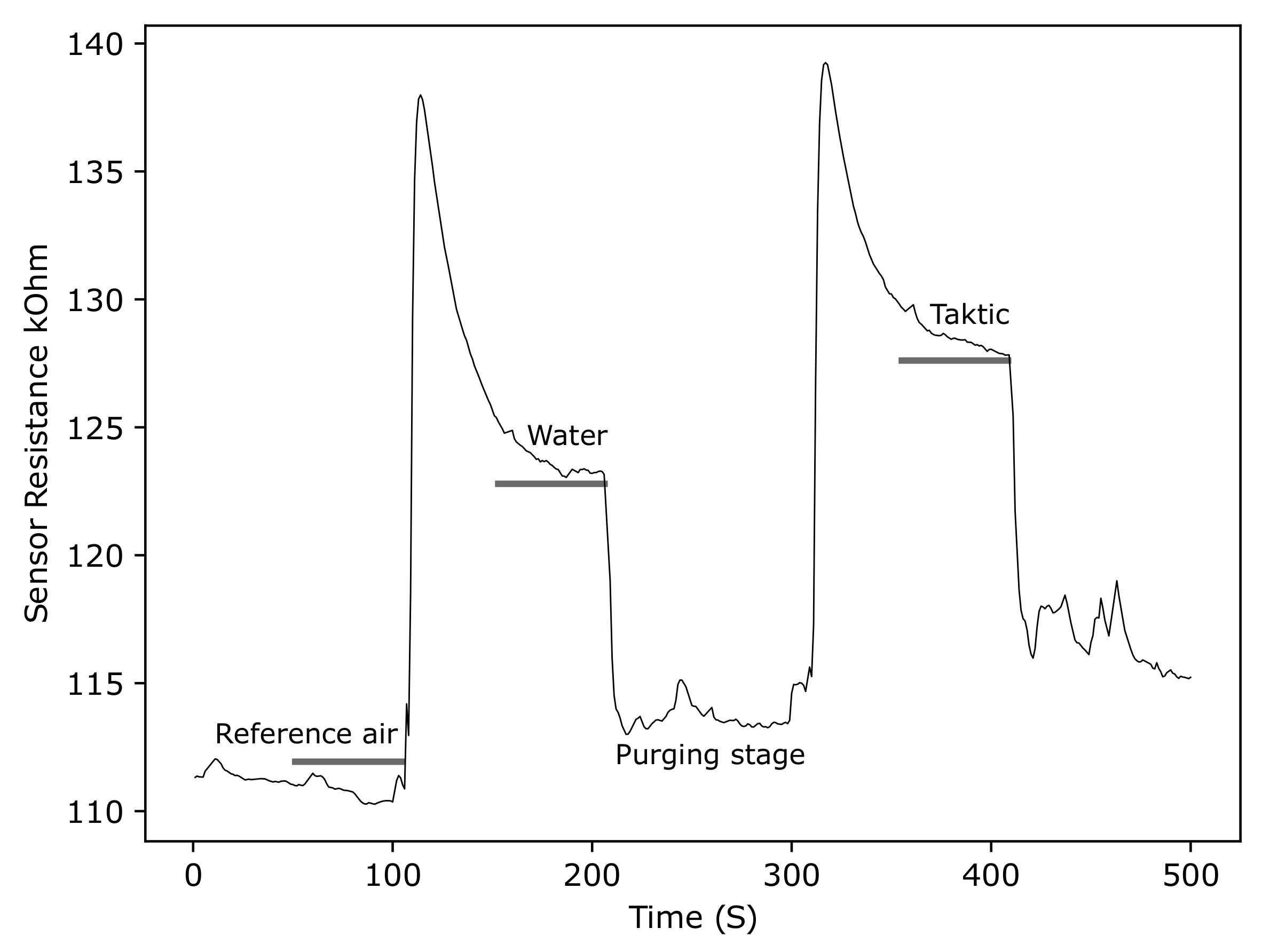
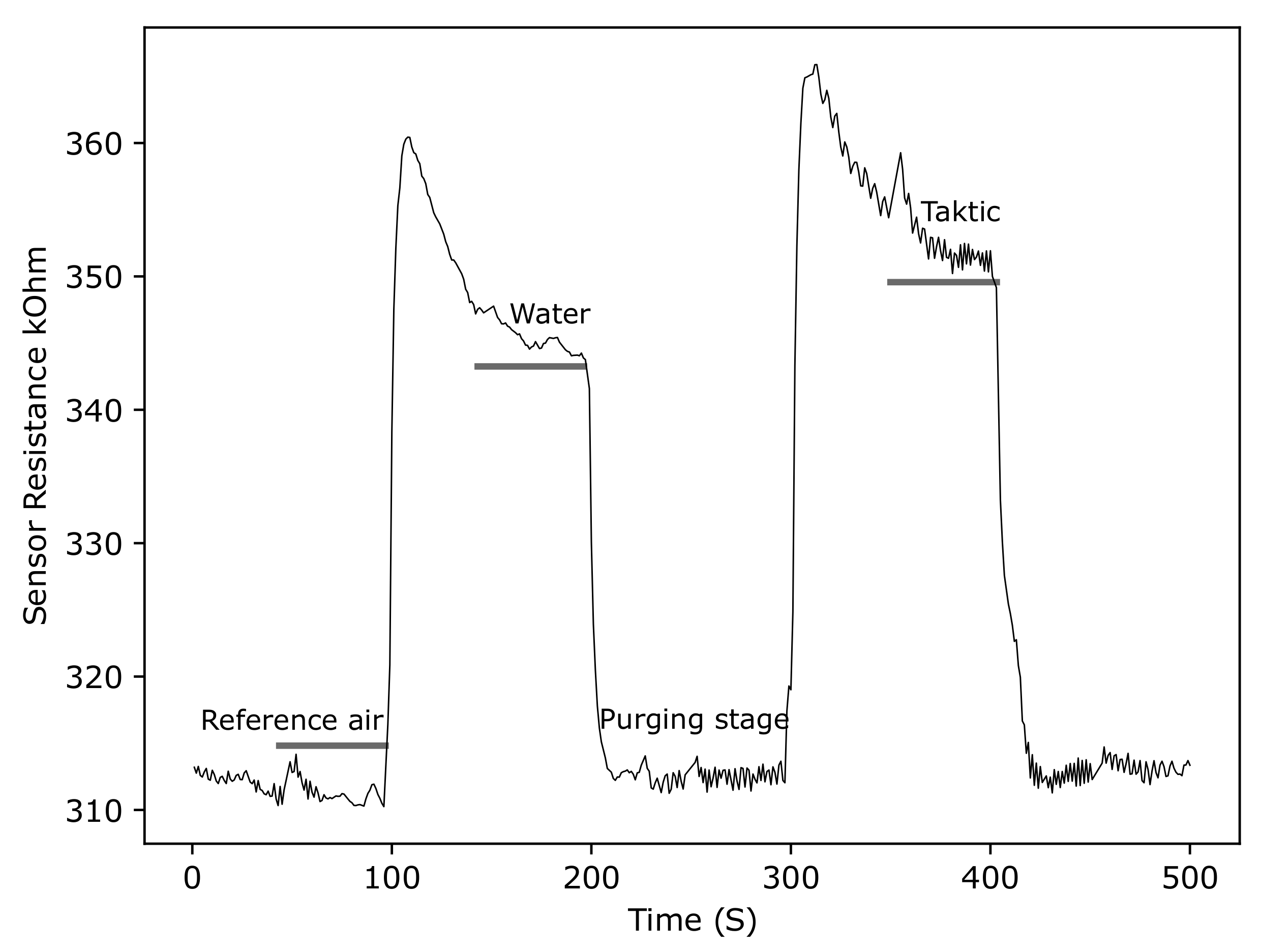
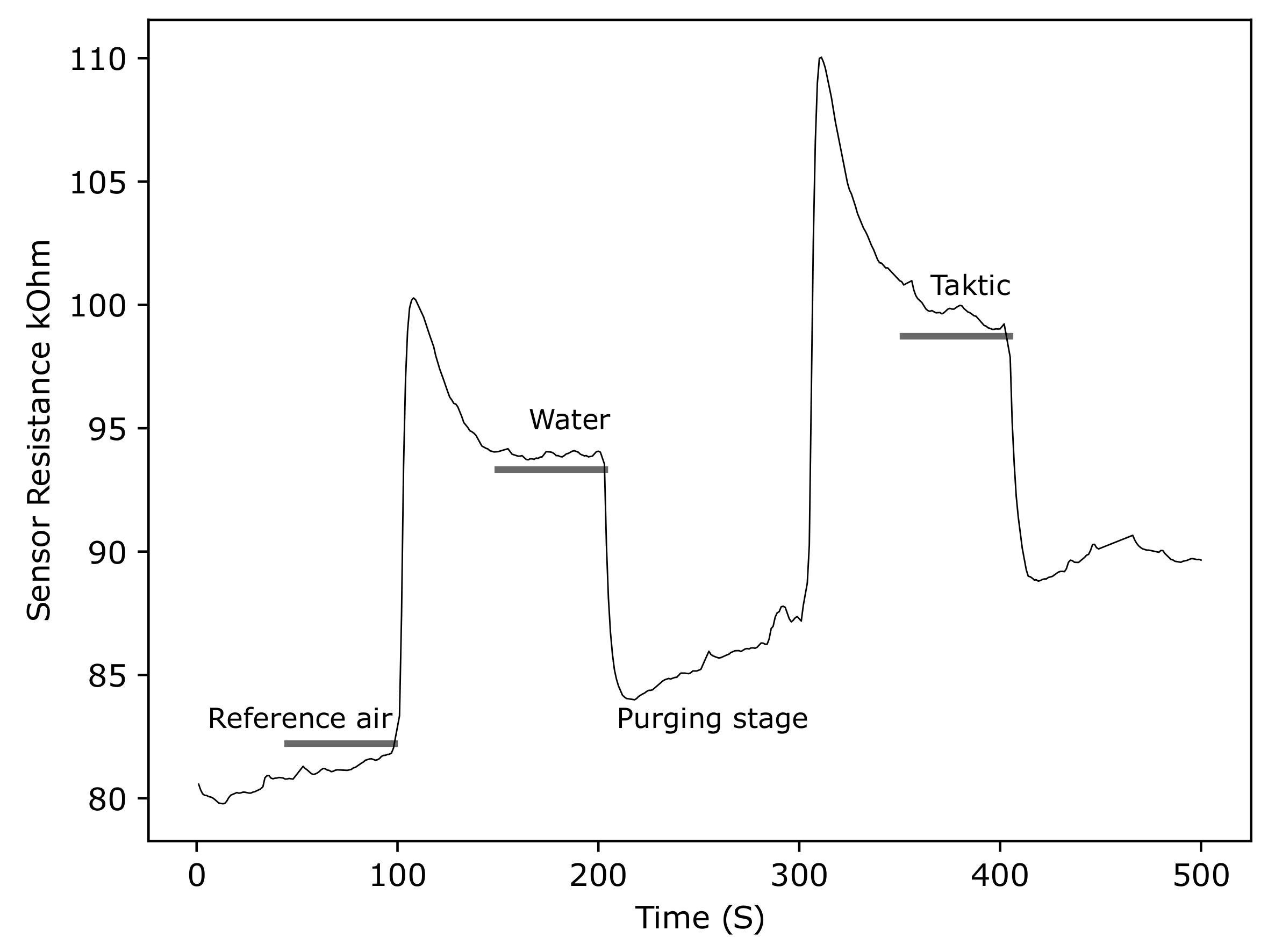
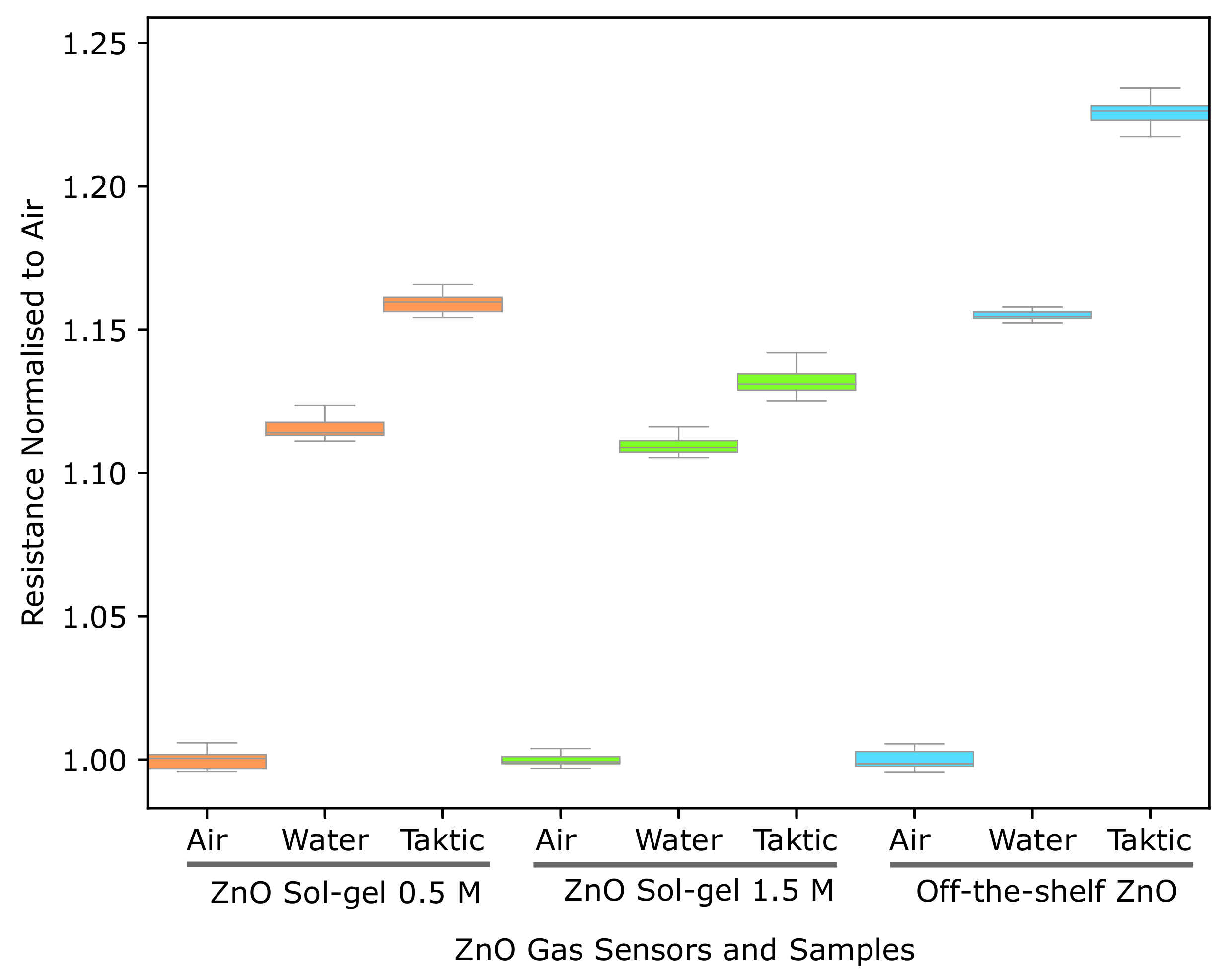
3.2.5. ZnO Gas Sensor Response to Taktic in Water
4. Discussion
4.1. Insecticide Monitoring with an E-Nose and Commercial Gas Sensors
4.2. Room Temperature Screen-Printed ZnO Gas Sensors for Insecticide Detection
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| E-nose | Electronic nose |
| ADC | Analogue-to-digital converter |
| ANN | Artificial neural network |
| UV | Ultra violet |
| EHD | Electrohydrodynamic |
| EMF | Electromagnetic fields |
| ppm | Parts per million |
| MEA | Monoethanolamine |
| IDE | Interdigitated electrode |
| PCA | Principle component analysis |
References
- Moyo, B.; Masika, P. Tick control methods used by resource-limited farmers and the effect of ticks on cattle in rural areas of the Eastern Cape Province, South Africa. Trop. Anim. Health Prod. 2009, 41, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Murigu, M.M.; Waruiru, R.M. Comparative Efficacy of Metarhizium anisopliae and Amitraz in Control of Rhipicephalus decoloratus on Cattle under Field Conditions in Kenya. Alex. J. Vet. Sci. 2020, 66, 52–59. [Google Scholar]
- De Meneghi, D.; Stachurski, F.; Adakal, H. Experiences in tick control by acaricide in the traditional cattle sector in Zambia and Burkina Faso: Possible environmental and public health implications. Front. Public Health 2016, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R. Molecular markers and their application in the monitoring of acaricide resistance in Rhipicephalus microplus. Exp. Appl. Acarol. 2019, 78, 149–172. [Google Scholar] [CrossRef] [PubMed]
- Baron, S.; Barrero, R.A.; Black, M.; Bellgard, M.I.; van Dalen, E.M.; Fourie, J.; Maritz-Olivier, C. Differentially expressed genes in response to amitraz treatment suggests a proposed model of resistance to amitraz in R. decoloratus ticks. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 361–371. [Google Scholar] [CrossRef]
- George, J.; Davey, R.; Ahrens, E.; Pound, J.; Drummond, R. Efficacy of amitraz (Taktic® 12.5% EC) as a dip for the control of Boophilus microplus (Canestrini) (Acari: Ixodidae) on cattle. Prev. Vet. Med. 1998, 37, 55–67. [Google Scholar] [CrossRef]
- Muvhuringi, P.B.; Murisa, R.; Sylvester, D.; Chigede, N.; Mafunga, K. Factors worsening tick borne diseases occurrence in rural communities. A case of Bindura district, Zimbabwe. Cogent Food Agric. 2022, 8, 2082058. [Google Scholar] [CrossRef]
- Msimang, V.; Rostal, M.K.; Cordel, C.; Machalaba, C.; Tempia, S.; Bagge, W.; Burt, F.J.; Karesh, W.B.; Paweska, J.T.; Thompson, P.N. Factors affecting the use of biosecurity measures for the protection of ruminant livestock and farm workers against infectious diseases in central South Africa. Transbound. Emerg. Dis. 2022, 69, e1899–e1912. [Google Scholar] [CrossRef]
- Delaire, C.; Peletz, R.; Kumpel, E.; Kisiangani, J.; Bain, R.; Khush, R. How Much Will It Cost to Monitor Microbial Drinking Water Quality in Sub-Saharan Africa? Environ. Sci. Technol. 2017, 51, 5869–5878. [Google Scholar] [CrossRef]
- Sungirai, M.; Baron, S.; Moyo, D.Z.; De Clercq, P.; Maritz-Olivier, C.; Madder, M. Genotyping acaricide resistance profiles of Rhipicephalus microplus tick populations from communal land areas of Zimbabwe. Ticks Tick-Borne Dis. 2018, 9, 2–9. [Google Scholar] [CrossRef]
- Cheng, L.; Meng, Q.H.; Lilienthal, A.J.; Qi, P.F. Development of compact electronic noses: A review. Meas. Sci. Technol. 2021, 32, 062002. [Google Scholar] [CrossRef]
- Jia, W.; Liang, G.; Tian, H.; Sun, J.; Wan, C. Electronic nose-based technique for rapid detection and recognition of moldy apples. Sensors 2019, 19, 1526. [Google Scholar] [CrossRef]
- Snitz, K.; Andelman-Gur, M.; Pinchover, L.; Weissgross, R.; Weissbrod, A.; Mishor, E.; Zoller, R.; Linetsky, V.; Medhanie, A.; Shushan, S.; et al. Proof of concept for real-time detection of SARS CoV-2 infection with an electronic nose. PLoS ONE 2021, 16, e0252121. [Google Scholar] [CrossRef] [PubMed]
- Lee-Rangel, H.A.; Mendoza-Martinez, G.D.; Diaz de León-Martínez, L.; Relling, A.E.; Vazquez-Valladolid, A.; Palacios-Martínez, M.; Hernández-García, P.A.; Chay-Canul, A.J.; Flores-Ramirez, R.; Roque-Jiménez, J.A. Application of an electronic nose and HS-SPME/GC-MS to determine volatile organic compounds in fresh mexican cheese. Foods 2022, 11, 1887. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, K.; Zhao, B.; Zhang, M.; Gong, C.; Wan, H.; Wang, Y.; Yang, Z. A novel electronic nose for the detection and classification of pesticide residue on apples. RSC Adv. 2021, 11, 20874–20883. [Google Scholar] [CrossRef]
- Tang, X.; Xiao, W.; Shang, T.; Zhang, S.; Han, X.; Wang, Y.; Sun, H. An Electronic Nose Technology to Quantify Pyrethroid Pesticide Contamination in Tea. Chemosensors 2020, 8, 30. [Google Scholar] [CrossRef]
- Rohde, A.; Joubert, T.H. A Portable e-nose for Monitoring Amitraz Insecticide in Cattle Dip. In Proceedings of the 2021 IEEE AFRICON, Arusha, Tanzania, 13–15 September 2021; pp. 1–6. [Google Scholar]
- Figaro Gas Sensors & Modules. Available online: https://www.figarosensor.com/ (accessed on 12 June 2023).
- Popa, D.; Udrea, F. Towards Integrated Mid-Infrared Gas Sensors. Sensors 2019, 19, 2076. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.S.; Li, W.H.; Xu, G. Metal–organic frameworks and their derivatives for electrically-transduced gas sensors. Coord. Chem. Rev. 2021, 426, 213479. [Google Scholar] [CrossRef]
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced Micro- and Nano-Gas Sensor Technology: A Review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef]
- Kuchmenko, T.A.; Lvova, L.B. A Perspective on Recent Advances in Piezoelectric Chemical Sensors for Environmental Monitoring and Foodstuffs Analysis. Chemosensors 2019, 7, 39. [Google Scholar] [CrossRef]
- Chen, Y.; Li, M.; Yan, W.; Zhuang, X.; Ng, K.W.; Cheng, X. Sensitive and low-power metal oxide gas sensors with a low-cost microelectromechanical heater. ACS Omega 2021, 6, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Schönauer-Kamin, D.; Moos, R. Novel Operation Strategy to Obtain a Fast Gas Sensor for Continuous ppb-Level NO2 Detection at Room Temperature Using ZnO—A Concept Study with Experimental Proof. Sensors 2019, 19, 4104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bu, M.; Hu, N.; Zhao, L. An overview on room-temperature chemiresistor gas sensors based on 2D materials: Research status and challenge. Compos. Part B Eng. 2023, 248, 110378. [Google Scholar] [CrossRef]
- Ji, H.; Zeng, W.; Li, Y. Gas sensing mechanisms of metal oxide semiconductors: A focus review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yin, W.; Gao, S.; Sun, Y.; Xu, P.; Wu, S.; Kong, H.; Yang, G.; Wei, G. The Combination of Two-Dimensional Nanomaterials with Metal Oxide Nanoparticles for Gas Sensors: A Review. Nanomaterials 2022, 12, 982. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, H.; Ji, H.; Yuan, Z.; Meng, F. Novel combined waveform temperature modulation method of NiO-In2O3 based gas sensor for measuring and identifying VOC gases. J. Alloys Compd. 2022, 918, 165510. [Google Scholar] [CrossRef]
- Li, Z.; Yu, J.; Dong, D.; Yao, G.; Wei, G.; He, A.; Wu, H.; Zhu, H.; Huang, Z.; Tang, Z. E-nose based on a high-integrated and low-power metal oxide gas sensor array. Sens. Actuators B Chem. 2023, 380, 133289. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A. An electronic nose based on carbon nanotube-titanium dioxide hybrid nanostructures for detection and discrimination of volatile organic compounds. Sens. Actuators B Chem. 2022, 357, 131418. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, W.; Hong, Y.; Lee, G.; Yoon, D.S. Recent advances in carbon material-based NO2 gas sensors. Sens. Actuators B Chem. 2018, 255, 1788–1804. [Google Scholar] [CrossRef]
- Lee, D.H.; Yoo, H. Recent Advances in Photo-Activated Chemical Sensors. Sensors 2022, 22, 9228. [Google Scholar] [CrossRef]
- Mishra, S.; Ghanshyam, C.; Ram, N.; Bajpai, R.; Bedi, R. Detection mechanism of metal oxide gas sensor under UV radiation. Sens. Actuators B Chem. 2004, 97, 387–390. [Google Scholar] [CrossRef]
- Chinh, N.D.; Hien, T.T.; Do Van, L.; Hieu, N.M.; Quang, N.D.; Lee, S.M.; Kim, C.; Kim, D. Adsorption/desorption kinetics of nitric oxide on zinc oxide nano film sensor enhanced by light irradiation and gold-nanoparticles decoration. Sens. Actuators B Chem. 2019, 281, 262–272. [Google Scholar] [CrossRef]
- Monereo, O.; Claramunt, S.; Vescio, G.; Lahlou, H.; Leghrib, R.; Prades, J.; Cornet, A.; Cirera, A. Carbon nanofiber flexible gas sensor modulated by UV light. In Proceedings of the 2013 Transducers & Eurosensors XXVII: The 17th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS & EUROSENSORS XXVII), Barcelona, Spain, 16–20 June 2013; pp. 1154–1157. [Google Scholar]
- Peña, Á.; Matatagui, D.; Ricciardella, F.; Sacco, L.; Vollebregt, S.; Otero, D.; López-Sánchez, J.; Marín, P.; Horrillo, M.C. Optimization of multilayer graphene-based gas sensors by ultraviolet photoactivation. Appl. Surf. Sci. 2023, 610, 155393. [Google Scholar] [CrossRef]
- Sik Choi, M.; Young Kim, M.; Mirzaei, A.; Kim, H.S.; il Kim, S.; Baek, S.H.; Won Chun, D.; Jin, C.; Hyoung Lee, K. Selective, sensitive, and stable NO2 gas sensor based on porous ZnO nanosheets. Appl. Surf. Sci. 2021, 568, 150910. [Google Scholar] [CrossRef]
- Reker, J.; Meyers, T.; Vidor, F.F.; Joubert, T.H.; Hilleringmann, U. Influence of electrode metallization on thin-film transistor performance. In Proceedings of the 2021 IEEE AFRICON, Arusha, Tanzania, 13–15 September 2021; pp. 1–5. [Google Scholar]
- Sahner, K.; Tuller, H. Novel deposition techniques for metal oxide: Prospects for gas sensing. J. Electroceram. 2010, 24, 177–199. [Google Scholar] [CrossRef]
- Simonenko, N.P.; Fisenko, N.A.; Fedorov, F.S.; Simonenko, T.L.; Mokrushin, A.S.; Simonenko, E.P.; Korotcenkov, G.; Sysoev, V.V.; Sevastyanov, V.G.; Kuznetsov, N.T. Printing Technologies as an Emerging Approach in Gas Sensors: Survey of Literature. Sensors 2022, 22, 3473. [Google Scholar] [CrossRef]
- Khan, S.; Briand, D. All-printed low-power metal oxide gas sensors on polymeric substrates. Flex. Print. Electron. 2019, 4, 015002. [Google Scholar] [CrossRef]
- Kwon, H.j.; Hong, J.; Nam, S.Y.; Choi, H.H.; Li, X.; Jeong, Y.J.; Kim, S.H. Overview of recent progress in electrohydrodynamic jet printing in practical printed electronics: Focus on the variety of printable materials for each component. Mater. Adv. 2021, 2, 5593–5615. [Google Scholar] [CrossRef]
- Mokrushin, A.S.; Simonenko, T.L.; Simonenko, N.P.; Gorobtsov, P.Y.; Bocharova, V.A.; Kozodaev, M.G.; Markeev, A.M.; Lizunova, A.A.; Volkov, I.A.; Simonenko, E.P.; et al. Microextrusion printing of gas-sensitive planar anisotropic NiO nanostructures and their surface modification in an H2S atmosphere. Appl. Surf. Sci. 2022, 578, 151984. [Google Scholar] [CrossRef]
- Jeong, H.; Noh, Y.; Lee, D. Highly stable and sensitive resistive flexible humidity sensors by means of roll-to-roll printed electrodes and flower-like TiO2 nanostructures. Ceram. Int. 2019, 45, 985–992. [Google Scholar] [CrossRef]
- Ahmed, M.; Taghizadeh, F.; Auret, F.; Meyer, W.; Nel, J. The effect of alpha particle irradiation on electrical properties and defects of ZnO thin films prepared by sol-gel spin coating. Mater. Sci. Semicond. Process. 2019, 101, 82–86. [Google Scholar] [CrossRef]
- Znaidi, L.; Touam, T.; Vrel, D.; Souded, N.; Ben Yahia, S.; Brinza, O.; Fischer, A.; Boudrioua, A. ZnO thin films synthesized by sol-gel process for photonic applications. Acta Phys. Pol. A 2012, 121, 165–168. [Google Scholar] [CrossRef]
- Dutronc, P.; Carbonne, B.; Menil, F.; Lucat, C. Influence of the nature of the screen-printed electrode metal on the transport and detection properties of thick-film semiconductor gas sensors. Sens. Actuators B Chem. 1992, 6, 279–284. [Google Scholar] [CrossRef]
- Barandun, G.; Gonzalez-Macia, L.; Lee, H.S.; Dincer, C.; Güder, F. Challenges and Opportunities for Printed Electrical Gas Sensors. ACS Sens. 2022, 7, 2804–2822. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Guo, J.; Zhao, J.; Xu, J.; Zhao, C.; Gao, Z.; Song, Y.Y. Engineering CuMOF in TiO2 Nanochannels as Flexible Gas Sensor for High-Performance NO Detection at Room Temperature. ACS Sens. 2022, 7, 2750–2758. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, W. Room-temperature gas sensing of ZnO-based gas sensor: A review. Sens. Actuators A Phys. 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Formlabs-Buy 3D Printing Materials. Available online: https://formlabs.com/store/materials/ (accessed on 29 April 2023).
- Standard Datasheet-Formlabs. Available online: https://formlabs-media.formlabs.com/datasheets/Standard-DataSheet.pdf (accessed on 29 April 2023).
- Metrohm Dropsens Spectroelectrochemical Instruments. Available online: https://www.dropsens.com/ (accessed on 29 April 2023).
- Planet Nails-Rapidcure 9W UV/LED Lamp. Available online: https://www.nails-beauty.co.za/product/9w-uv-lamp-pni-white/ (accessed on 13 June 2023).
- Takealot-SUNR9. Available online: https://www.takealot.com/r9-uv-led-nail-lamp-110w/PLID71575494 (accessed on 13 June 2023).
- Hotelling, H. Analysis of a complex of statistical variables into principal components. J. Educ. Psychol. 1933, 24, 417. [Google Scholar] [CrossRef]
- Chen, H.; Huo, D.; Zhang, J. Gas Recognition in E-Nose System: A Review. IEEE Trans. Biomed. Circuits Syst. 2022, 16, 169–184. [Google Scholar] [CrossRef]
- Ahmed, M.E.I. Characterization of Electrical Properties and Defects in Er- and Yb-Doped ZnO Thin Films Grown by Sol-Gel Spin Coating. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2022. [Google Scholar]
- Myint, M.T.Z.; Kumar, N.S.; Hornyak, G.L.; Dutta, J. Hydrophobic/hydrophilic switching on zinc oxide micro-textured surface. Appl. Surf. Sci. 2013, 264, 344–348. [Google Scholar] [CrossRef]
- Olatinwo, S.O.; Joubert, T.H. Enabling Communication Networks for Water Quality Monitoring Applications: A Survey. IEEE Access 2019, 7, 100332–100362. [Google Scholar] [CrossRef]







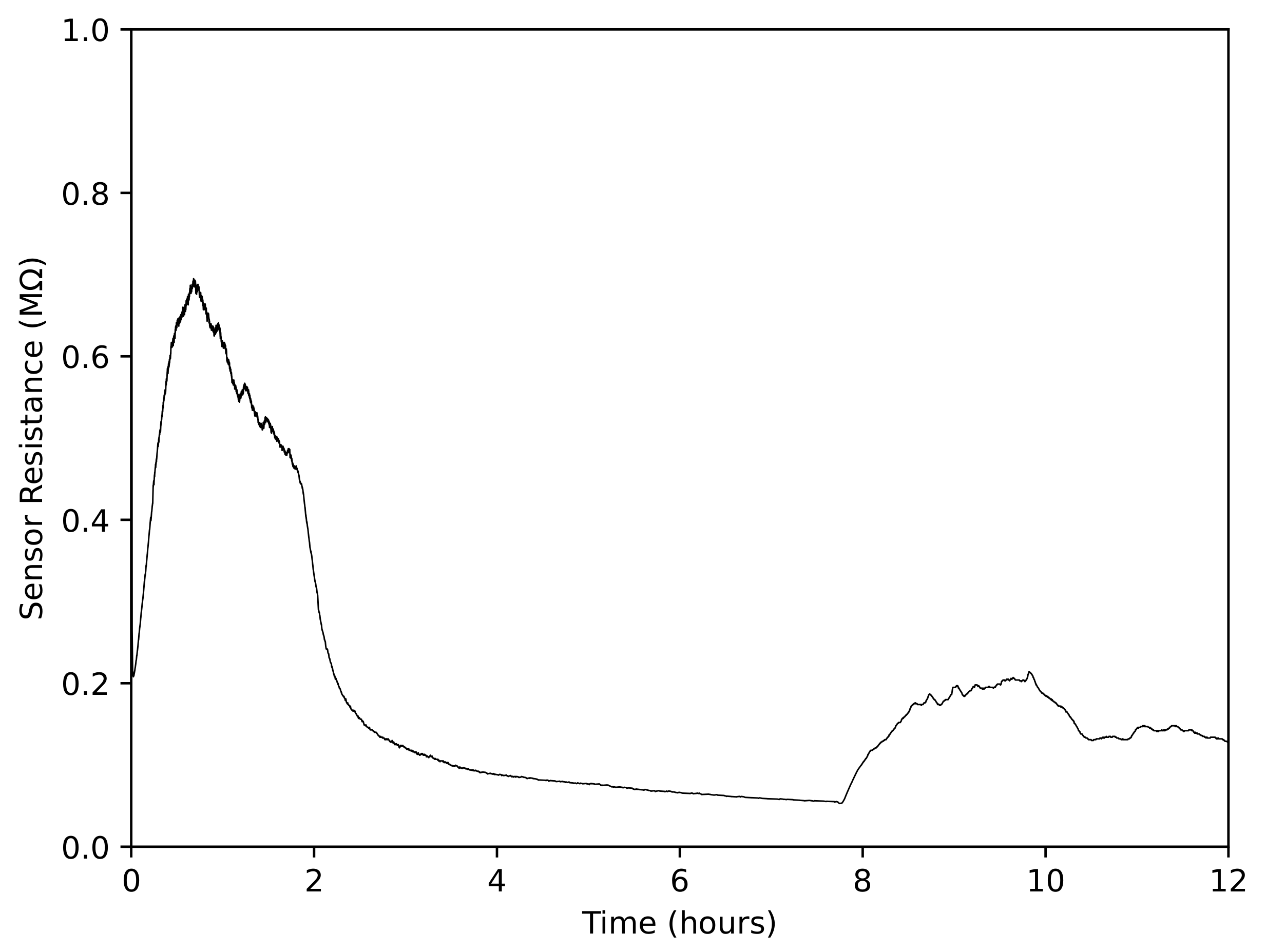
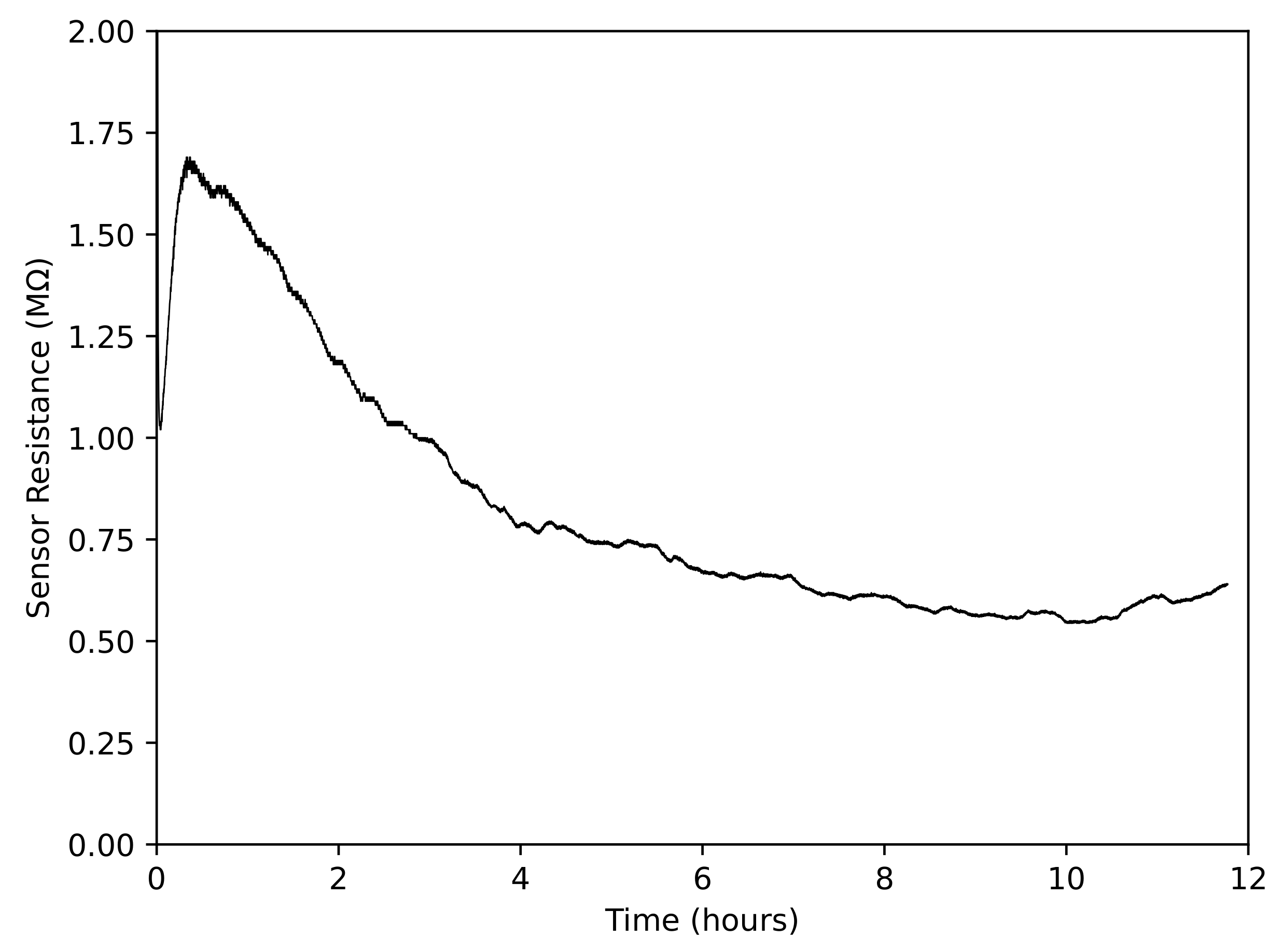
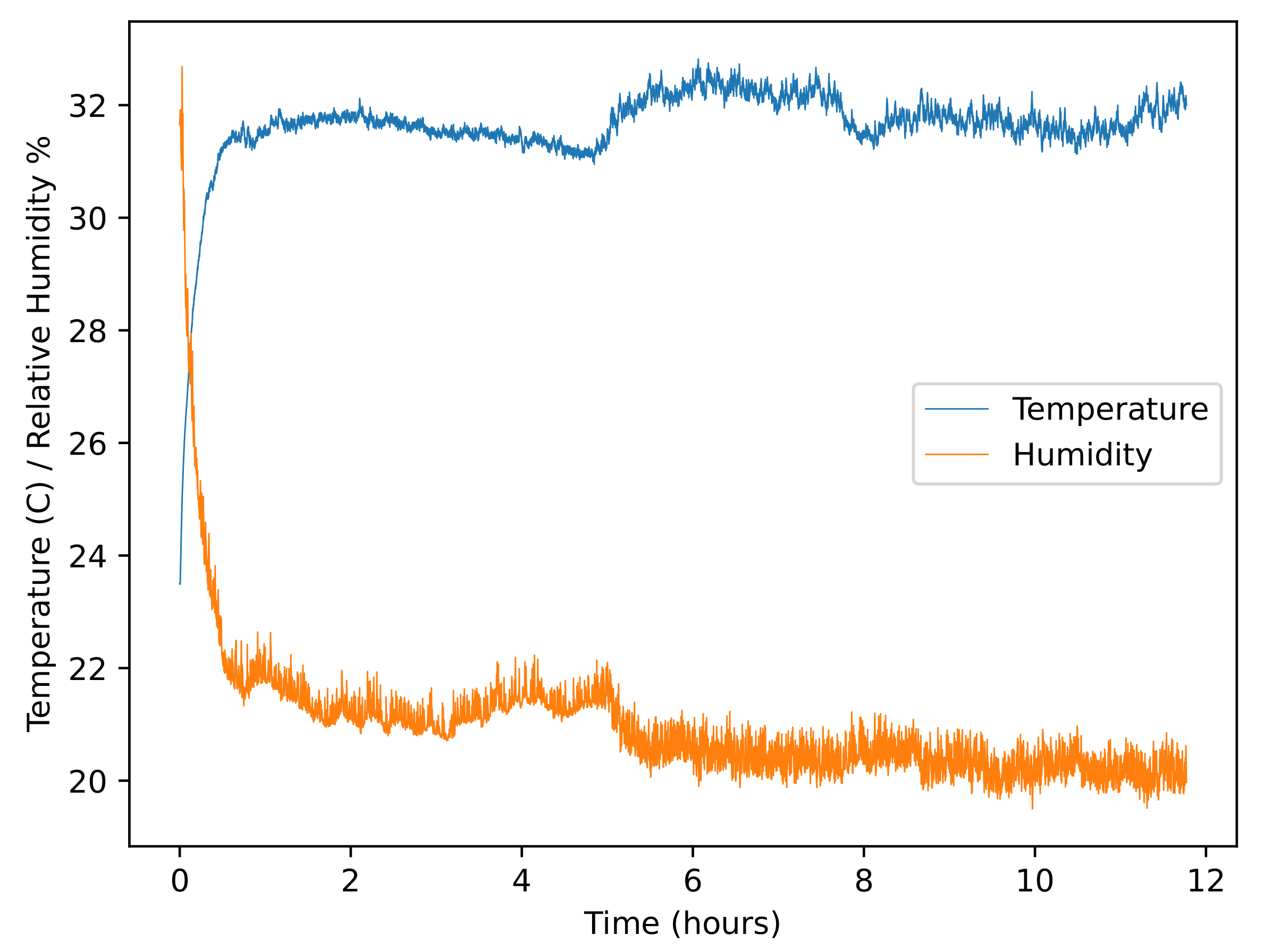

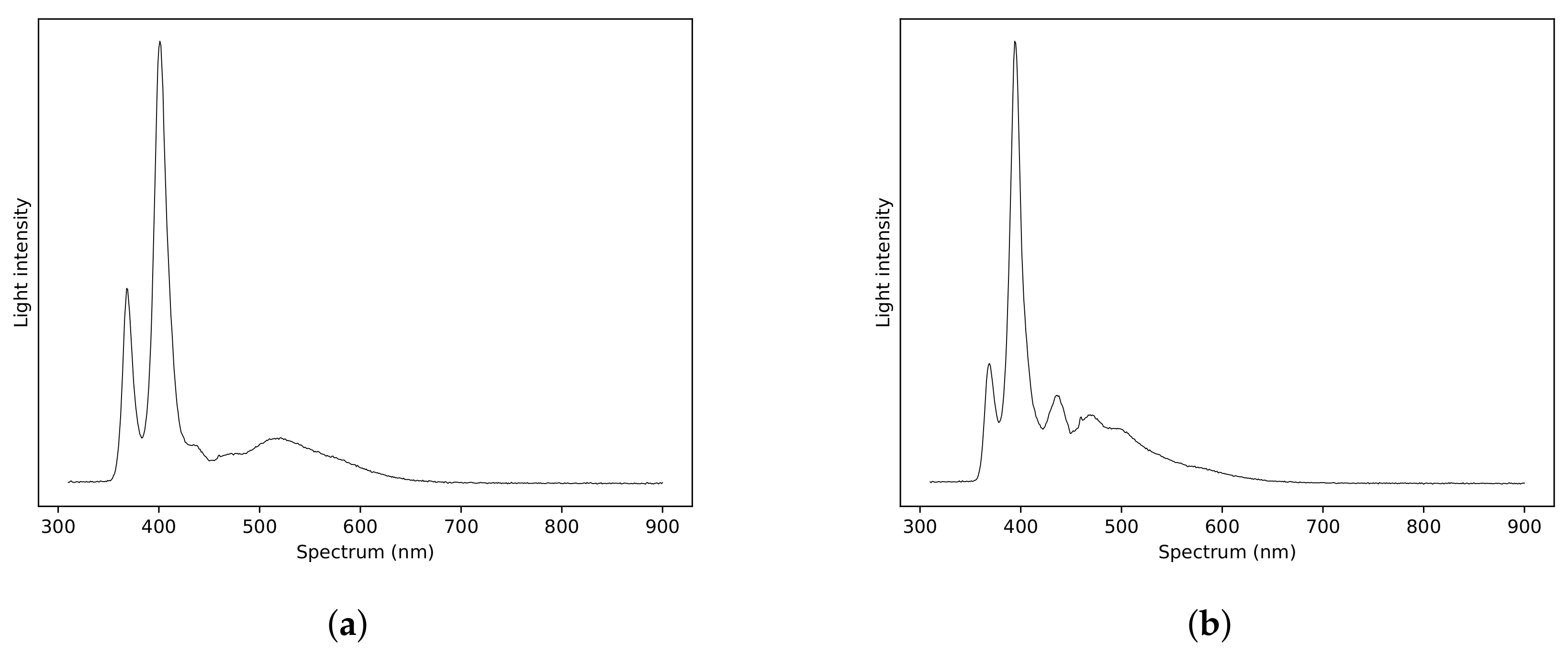
| % of Recommended Concentration | ppm Taktic in Farm Water | Taktic in 10 mL Water (L) |
|---|---|---|
| 0 | 0 | 0 |
| 25 | 500 | 5 |
| 50 | 1000 | 10 |
| 100 | 2000 | 20 |
| 200 | 4000 | 40 |
| Measurement 1 Resistance (k) | Measurement 2 Resistance (k) | Measurement 3 Resistance (k) | Average Resistance (k) | Variation % | ||
|---|---|---|---|---|---|---|
| 0.5 M | Resistance for Air | 66.66 | 57.813 | 51.233 | 58.57 | 26.35 |
| sol-gel ZnO | Resistance for Taktic | 42.25 | 37.45 | 34.04 | 37.91 | 21.66 |
| 1.5 M | Resistance for Air | 223.97 | 195.36 | 161.81 | 193.71 | 32.09 |
| sol-gel ZnO | Resistance for Taktic | 128.8 | 109.63 | 94.46 | 110.96 | 30.95 |
| Commercial | Resistance for Air | 457.47 | 457.88 | 441.4 | 452.25 | 3.64 |
| ZnO | Resistance for Taktic | 229.24 | 222.2 | 217.26 | 222.9 | 5.37 |
| Measurement 1 | Measurement 2 | Measurement 3 | Average | Variation % | ||
|---|---|---|---|---|---|---|
| 0.5 M | Recovery time (min) | 5.25 | 5.67 | 6.37 | 5.76 | 19.43 |
| sol-gel ZnO | Measurement time (min) | 4.78 | 4.35 | 4.82 | 4.65 | 10.11 |
| 1.5 M | Recovery time (min) | 6.98 | 5.93 | 6.5 | 6.47 | 16.23 |
| sol-gel ZnO | Measurement time (min) | 3.7 | 4.28 | 3.93 | 3.97 | 14.61 |
| Commercial | Recovery time (min) | 7.48 | 7.45 | 8.7 | 7.88 | 15.87 |
| ZnO | Measurement time (min) | 3.6 | 3.65 | 3.77 | 3.67 | 4.63 |
| 0.5 M Sol-Gel | 1.5 M Sol-Gel | Off-the-Shelf | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Air | Water | Taktic | Air | Water | Taktic | Air | Water | Taktic | |
| Standard Deviation | 0.0034 | 0.0037 | 0.0031 | 0.0026 | 0.0034 | 0.0062 | 0.0034 | 0.0013 | 0.0061 |
| Average | 1.0 | 1.1157 | 1.1590 | 1.0 | 1.1095 | 1.1327 | 1.0 | 1.1548 | 1.2261 |
| Minimum | 0.9957 | 1.1110 | 1.1542 | 0.9969 | 1.1053 | 1.1251 | 0.9955 | 1.1523 | 1.2174 |
| Maximum | 1.0058 | 1.1247 | 1.1657 | 1.0093 | 1.1208 | 1.1542 | 1.0110 | 1.1579 | 1.2463 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohde, A.W.; Nel, J.M.; Joubert, T.-H. Insecticide Monitoring in Cattle Dip with an E-Nose System and Room Temperature Screen-Printed ZnO Gas Sensors. Agriculture 2023, 13, 1483. https://doi.org/10.3390/agriculture13081483
Rohde AW, Nel JM, Joubert T-H. Insecticide Monitoring in Cattle Dip with an E-Nose System and Room Temperature Screen-Printed ZnO Gas Sensors. Agriculture. 2023; 13(8):1483. https://doi.org/10.3390/agriculture13081483
Chicago/Turabian StyleRohde, Archibald W., Jacqueline M. Nel, and Trudi-Heleen Joubert. 2023. "Insecticide Monitoring in Cattle Dip with an E-Nose System and Room Temperature Screen-Printed ZnO Gas Sensors" Agriculture 13, no. 8: 1483. https://doi.org/10.3390/agriculture13081483
APA StyleRohde, A. W., Nel, J. M., & Joubert, T.-H. (2023). Insecticide Monitoring in Cattle Dip with an E-Nose System and Room Temperature Screen-Printed ZnO Gas Sensors. Agriculture, 13(8), 1483. https://doi.org/10.3390/agriculture13081483








