Abstract
This research explores the potential of using common duckweed (Spyrodela polyrhiza) as a feeding substrate and supplement for yellow mealworm production. Duckweed is known for its high content of protein (20–35%) and essential amino acids. This study aims to assess the effect of the combination of semolina and duckweed as a feeding substrate for yellow mealworm larvae (Tenebrio molitor). The experiment involved different combinations of semolina and duckweed in varying proportions. The treatments included 100% semolina (S), 75% semolina + 25% duckweed (S75D25), 50% semolina + 50% duckweed (S50D50), 25% semolina + 75% duckweed (S25D75), and 100% duckweed (D). Over a six-week period, the production parameters, nutrient composition, amino acid composition, and fatty acid composition of the yellow mealworms were measured and analyzed. The results demonstrate that S75D25 and S50D50 feed combinations were recommended due to their positive effects on production parameters and nutrient composition. Although the D substrate exhibited the highest crude protein content, yellow mealworm larvae did not grow on this substrate. The inclusion of duckweed in the feed had no significant effect on the fatty acid composition of the mealworms, while substrates S25D75 and D induced an improved amino acid composition. In conclusion, incorporating duckweed into the feeding substrate can enhance the production parameters and nutrient composition of yellow mealworms.
1. Introduction
Yellow mealworm (Tenebrio molitor L.) (Coleoptera: Tenebrionidae) has gained significant popularity in the European Union (EU) for breeding and trade, making it the most widely utilized species in the region [1]. With its promising potential for industrial utilization and commercial-scale production, yellow mealworm has attracted considerable interest as a valuable source of food and feed [2]. This expansion in commercial production is primarily driven by the approval of yellow mealworms for use in pet, fish, poultry, and swine feed, as well as for human consumption [3,4].
Insects such as yellow mealworm larvae have garnered attention as an alternative protein source due to their economic viability, environmental benefits, and high nutritional value. Compared with traditional terrestrial livestock, the production of yellow mealworms offers advantages such as a higher feed conversion efficiency, the utilization of agri-food byproducts and wastes as insect diet, complete biomass consumption, a lower risk of biological and environmental hazards, reduced water consumption and land use, and decreased greenhouse gas emissions per unit of protein produced [5]. Despite these benefits, the insect production industry faces a major challenge in finding suitable, efficient, cost-effective, and sustainable diets [6,7,8].
The composition of diets fed to yellow mealworms has a significant impact on their nutrient composition and production parameters, as demonstrated by numerous studies [9,10,11,12,13,14,15]. Recent research indicates that diets rich in yeast-derived protein show favorable outcomes compared with those used by commercial breeders. These experimental diets utilizing organic byproducts from beer brewing, bread or cookie baking, potato processing, and bioethanol production have been associated with a shorter larval development time, reduced mortality, and increased weight gain [11]. Although diet composition does not influence larval protein content, it does alter larval fat composition to some extent [11]. Furthermore, yellow mealworms reared on a high-protein/high-fat diet (HPHF) demonstrate a feed conversion efficiency comparable to that of poultry when corrected for edible portions [10].
In pursuit of improving the nutritional composition of commercially viable insects, such as black soldier fly, lesser mealworm, and house cricket, several studies have focused on diet enrichment to enhance omega-3 fatty acids, calcium, and calcium/phosphorus ratios [16,17]. Similarly, efforts have been made to enhance different components of yellow mealworms, such as calcium and calcium/phosphorus ratios [18], zinc [19], and fatty acids [20].
Duckweed species exhibit rapid growth, simple morphology, and a high protein content, making them an attractive research subject [21,22]. Their potential for removing mineral contaminants from wastewaters generated by sewage works, intensive animal industries, and intensive irrigated crop production has garnered significant interest [21,22,23]. However, natural environmental conditions, including drought, inadequate nutrient supply, and insect and fungal infestation and contamination can limit duckweed growth [24]. The nutritional composition of duckweed is influenced by species and growth conditions. Protein content ranges from 16.0% (Lemna sp.) to 41.7% (Landoltia gibba), starch from 17.6% (Landoltia gibba) to 35.0% (Lemna sp.), lipid from 3.4% (Landoltia minor) to 9.0% (Lemna sp.), crude fiber from 8.8% (Spirodela polyrhiza) to 29.7% (Lemna minor), and ash from 3.5% (Landoltia gibba) to 26.0% (Lemna sp.) [25].
Although duckweed has been explored as a partial replacement for fish meal, soybean meal, and alfalfa leaf meal in animal feed [26], there is a lack of research on its use as a feeding substrate for yellow mealworm production. Species such as Lemna minor and Spirodela polyrhiza have been utilized in animal feed for fish, poultry, and pigs [27]. This study aims to investigate the feasibility of using common duckweed (Spirodela polyrhiza) in yellow mealworm farming. This research focuses on evaluating the impact of common duckweed on the growth, nutrient composition, amino acid profile, and fatty acid profile of yellow mealworm larvae.
2. Materials and Methods
2.1. Materials and Research Environment
Yellow mealworm larvae used for this experiment were obtained from a commercial farm and transported to the Aquaculture Laboratory of the University of Debrecen. The mealworm larvae were 6 weeks old (~7th instars). The experiment was set up in a biological chamber (Memmert HPP110ECO, Büchenbach, Germany) in the laboratory at 25 °C, 67% humidity, and 0% light intensity.
2.2. Substrate Composition
The duckweed used was harvested from the pond at the Aquaculture Laboratory of the University of Debrecen. The duckweed was dried at a temperature range between 40 °C and 43 °C for 3 days. After drying the duckweed, we grinded it to a particle size of 0.4 ± 2.01 mm. The semolina used in this study was obtained from the market, it was a durum wheat semolina with particle size 0.5 ± 1.98 mm.
Table 1 shows the nutrient composition of duckweed and semolina on a dry matter basis.

Table 1.
Nutrient composition of semolina and duckweed (% DM).
After the evaluation of the different feeding material, we prepared a substrate by combining semolina and duckweed to obtain five experiment treatments, as shown in the following table (Table 2). We made a homogenous mixture from the semolina and the dried duckweed in each box according to different ratios described in Table 2. The moisture content of both semolina and duckweed was ~13%.

Table 2.
Substrate composition.
2.3. Experimental Set-Up
Five different treatments were applied in the experiment (S, S75D25, S50D50, S25D75, and D), and each were repeated four times. The experiment was set up in a transparent 0.15 L box. Forty mealworms were stocked per box; the biomass was 2.6 g (as an average, body weight was 0.065 g).
The experiment lasted for 6 weeks; the content of the box was separated on a 2 mm diameter mesh filter, which allowed the substrate to pass through while the worms stayed on the filter. We measured the growth and feed consumption of the larvae to calculate the FCR. To measure the length, we used a centimeter rule, while the weight of individual mealworms was measured using an analytical balance (Kern ABT 100-5NM, Kern & Shon, Stuttgart–Balingen, Germany). Also, we calculated the survival rate every week throughout the duration of the experiment. The weight of the mealworms was measured weekly, while the lengths and widths measurements were performed at the end of the experiment. The larvae were then stored at −20 °C until further analysis.
2.4. Feed Conversion Ratio
To calculate the FCR during the experimental period, the weight gain during the 6-week period of the experiment was calculated:
Also, the total feed consumption per treatment was calculated by weighing the feed left at the end of the experiment.
The feed conversion ratio (FCR) was calculated as
2.5. Proximate Analysis
Whole yellow mealworm larvae were used for the analysis. Gravimetric measurement after drying according to ISO 6496:1999 was used to determine the dry matter content, where the weight was determined as an average of the two measurements [28]. The Kjeldahl method, according to ISO 5983-2:2009, was used to determine the crude protein content [29]. Total crude fat was determined gravimetrically after the acid hydrolysis and solvent extraction. The crude fiber was determined using the Fibertec method (FOSS, Hilleroed, Denmark) (ISO 6865:2000) [30].
The method described by Hahn et al. (2018) was used to determine the chitin content by subtracting the acid detergent lignin (ADL) content from the acid detergent fiber (ADF) [31]. The ADL and ADF content were measured gravimetrically according to the ISO 13906:2008 standard method [32]. Initial preparatory steps before ADF and ADL measurements were carried out as follows: samples were defatted using a hexane solvent extraction and grinding into a <0.5 mm particle size. Then, 1 g of the defatted sample was suspended in 100 mL of 0.5 mol/L H2SO4 and 20 g/L of cetyltrimethylammonium bromide (CTAB) and was boiled under reflux for 1 h. The suspension was transferred to a fritted disc crucible and filtered under a vacuum. The retentate was then suspended in 50 mL of 80 °C demineralized water for 5 min. The suspension was filtered under a vacuum and the washing step was repeated twice.
An additional washing step with 50 mL of acetone was also conducted twice. The sample was dried and weighed. The ADF content was expressed as the percentage of the mass fraction of the dry defatted biomass relative to the applied dry mass before the process of the lipid extraction. After the determination of the ADF, the residuum was treated further with 12 mol/L of H2SO4 for 3 h, and then it was filtered under a vacuum and washed with water. After drying and weighting the residuum, it was cremated at 525 ± 15 °C in an incineration furnace. The ADL content was subtracted from the calculated ADF value and expressed as the chitin of the dry weight of the insect larvae [31,32].
The crude ash was determined gravimetrically [28].
2.6. Calculation of the Crude Protein Content of Individual Yellow Mealworms
Using the obtained average weight data and applying results of proximate analysis, we calculated the average crude protein content of individual yellow mealworms fed the different diet compositions.
ABW = average body weight.
DM = dry matter.
CP = crude protein.
2.7. Amino Acid Analysis
The protein content of the samples was determined using the Kjeldahl method [33]. First, the nitrogen content of the sample was converted into ammonium salt by boiling it in concentrated sulfuric acid. Fourteen milliliters of concentrated sulfuric acid and two catalyst tablets (VWR International Ltd., Lutterworth, Leicestershire.) containing selenium were added. The sample was destructed at 420 °C when placed on a destructive block (VELP DKL Kjeldahl). After cooling the sample, it was distilled on a VELP UDK-149 distiller. An automatic titrator (VELP TITROLINE 5000, Velp Scientifica, Usmate Velate, Italy) was applied, and the nitrogen content was calculated. The protein content of the samples was calculated from the nitrogen content using a conversion factor (6.38). Measurements were repeated four times with CV% < 10%.
For protein hydrolysis, the same amount of protein was measured into a hydrolysis tube with a Teflon top with 5N HCl and was reacted at 105℃ for 5 h in an oven (Memmert UN55, Buechenbach, Germany). Amino acid analysis of the samples was performed after cooling them down and filtering through a regenerated cellulose filter (0.2 µm, Whatman 10463040 Spartan syringe filter).
For total amino acid analysis, an AAA500 amino acid analyzer (INGOS Ltd., Praha, Czech Republic) with low-pressure ion exchange chromatography as well as postcolumn derivatization with ninhydrin (INGOS Ltd., Praha, Czech Republic) and photometric detection at 210 and 254 nm were used. Amino acid standard mixture (INGOS Ltd., Praha, Czech Republic) was applied as a reference. The recovery was higher than 95%. Amino acids were expressed as a percentage of the original sample weight.
2.8. Fatty Acid Composition
The fatty acid composition was determined as a fatty acid methyl ester as follows. The fat content (200–300 mg) was solved in 6 mL of hexane and 4 mL of 0.5M NaOH: MeOH. The solution was treated at 80 ± 1 °C for 10 min in an oven. After saponification, the sample was diluted with 5–10 mL of distilled water and the unsaponified materials were extracted with 2 × 2 mL of hexane. After the extraction, the solution was acidified with 0.5 mL of 6 M H2SO4, and the saponified fatty acids were extracted with 2 mL of hexane. The purified fraction was treated with 2 mL of 14% BF3: MeOH at 80 ± 1 °C for 30 min in an oven. In total, 2 mL of saturated NaCl solution was used, and the supernatant hexane phase was applied into a GC vial with dry Na–sulphate and applied into GC-FID (Varian GC 3800) (Table 3). The Supelco 37 component FAME mix (Sigma-Aldrich, St. Louis, MO, USA) was used as a reference. Measurements were repeated four times with CV% < 5%. The calculated results were expressed as a percentage of the total fat content.

Table 3.
Technical data of GC-FID.
2.9. Statistical Analysis
The results were analyzed using IBM SPSS 22.0 (Armonk, NY, USA). A one-way analysis of variance (ANOVA) and Tukey’s test (p < 0.05) were used to evaluate the results.
3. Results
3.1. Production Parameters
3.1.1. Growth Performance of Yellow Mealworm at Different Substrate Compositions
Table 4 shows the average body weight, length, and width of yellow mealworms in the different treatments using substrates S, S75D25, S50D50, S25, D75, and D. The results show that for the mealworms fed the S75D25 substrate composition, they reached the highest body weight. Also, the greatest length was achieved using S75D25 and S50D50. The greatest width was observed in these substrate compositions as well. However, as the semolina composition falls below 50%, the body weight, length, and width get smaller. Feeding with duckweed alone resulted in the lowest average body weight of the larvae.

Table 4.
Average weight, length, and width of yellow mealworm at different substrate compositions.
3.1.2. Feed Conversion Ratio
Figure 1 shows the FCR of yellow mealworms reared on different substrates. Feeding on S, S75D25, and S50D50 resulted in the lowest FCR, while S25D75 had the highest FCR. Substrate D induced a negative FCR.

Figure 1.
FCR of yellow mealworms at different substrate compositions: Bars with different alphabets differ significantly (p < 0.05). S: 100% semolina; S75D25: 75% semolina + 25% duckweed; S50D50: 50% semolina + 50% duckweed; S25D75: 25% semolina + 75% duckweed; D: 100% duckweed.
3.1.3. Survival Rate
Figure 2 shows the survival rates of the mealworms under the different treatments. The survival rate was the highest when feeding with S75D25, reaching a 90% survival rate, followed by S at 89%, D at 88%, and S50D50 at 87%. The lowest survival rate is even lower than that, with a value of 77%, which was observed when using substrate S25D75.
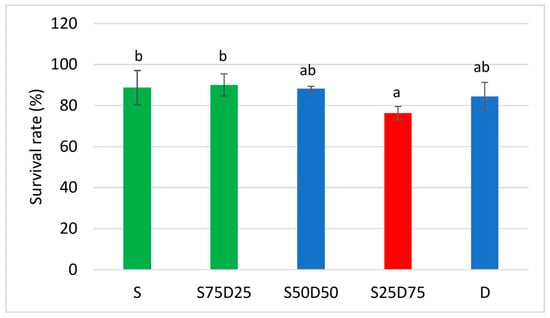
Figure 2.
Survival rates of yellow mealworms at different substrate compositions: Bars with different alphabets differ significantly (p < 0.05). S: 100% semolina; S75D25: 75% semolina + 25% duckweed; S50D50: 50% semolina + 50% duckweed; S25D75: 25% semolina + 75% duckweed; D: 100% duckweed.
3.2. Nutrient Composition
Table 5 shows that the nutrient composition of the yellow mealworm, crude protein, crude fat (CFa), crude fiber (CF), and crude ash were significantly affected by every 25% inclusion of duckweed. The CP, CF, and ash content followed the same trend; they increased with every 25% increase in the duckweed inclusion, while the CFa decreased with every 25% increase in the duckweed content in the substrate.

Table 5.
Average nutrient composition of yellow mealworm at different substrate compositions (% DM).
Table 6 shows the average protein content of individual yellow mealworms reared on each different substrate. The protein content of yellow mealworms reared on a diet of S75D25, S50D50, and S25D75 was significantly higher than that of S. The average weight of S75D25 is higher than S50D50, but the crude protein content per individual of these two treatments are statistically the same.

Table 6.
Average crude protein content of individual yellow mealworms at each different substrate compositions (DM %).
3.3. Amino Acid Composition of Yellow Mealworm at Different Substrate Compositions
Table 7 shows the amino acid (AA) composition of yellow mealworms reared on different substrate compositions. A composition of the yellow mealworms reared on different substrate compositions shows a statistically significant difference. The tendency observed in most of the amino acids is that the groups reared on substrates S and S75D25 show no statistical difference; this also occurred in the same vein on S25D75, and the use of substrate D did not result in any statistical differences. At the same time, the values of yellow mealworms fed substrate S50D50 are either statistically the same as those of the group reared on substrate S or with those of the groups reared on S and S75D25 or S25D75 and D, or, in some cases, they are statistically different from all. In yellow mealworms fed substrate S, the most dominant amino acids are glutamine, alanine, phenylalanine + tyrosine, and isoleucine, ranked in order. However, for the yellow mealworms fed diets containing duckweed, phenylalanine + tyrosine is the second most abundant amino acid.

Table 7.
Average amino acid content of yellow mealworm at different substrate compositions (%).
3.4. Fatty Acid Composition of Yellow Mealworm at Different Substrate Compositions
Table 8 shows the fatty acid (FA) composition of yellow mealworms fed substrates of different compositions. The FA compositions of yellow mealworms fed substrates of different compositions shows statistically significant differences regarding the p-value (0.05); no particular trend was identified for differences in the FA compositions. The yellow mealworms fed substrate S25D75 had the highest saturated fatty acid (SFA) content of 31.98% and the lowest content of MUFA at 49.21%. Yellow mealworms fed substrate S50D50 had the highest MUFA of 54.70% and the lowest PUFA n-6 and PUFA n-3 contents of 16.88% and 0.63%, respectively. The lowest ratio of PUFA n-6 to PUFA n-3 was observed in the yellow mealworms fed substrate D at 15.63%.

Table 8.
The average fatty acid profile of yellow mealworms at different substrate compositions (%).
4. Discussion
4.1. Production Parameters
The highest average body weight was achieved with the S75D25 substrate composition. Also, the average body weight achieved when feeding S50D50 was higher than the one reached when feeding semolina alone. However, including less than 50% semolina in the feed resulted in an average body weight which was lower than that obtained when feeding substrate S. This is understandable because the yellow mealworm is a pest of stored grains and flour [1,2], which are rich in carbohydrates, whereas semolina is richer in carbohydrates compared with duckweed.
The average body weight of yellow mealworm larvae reared on substrate S75D25 increased significantly to reach approximately 0.16 g (wet weight basis). Over the six weeks, the average weight, length, and width of the mealworm larvae increased, and the usage of duckweed substrate supplementation improved the growth of the mealworms. The width of yellow mealworms reared on different diets was not significantly different, but there were significant differences between the lengths of the yellow mealworms reared on different substrates. Length is directly proportional to weight; the yellow mealworms with the longest length also had the highest weight.
In our study, the feed conversion ratio (FCR) of yellow mealworms showed significantly high values; when applying substrate S25D75, the FCR was approximately 6.9 g/g. The literature indicates FCR values of 1.98 g/g for larvae harvested on a fresh matter basis, as reported by Thévenot et al. (2018) [34].
Feeding duckweed alone resulted in a negative FCR, as this substrate contained only duckweed, and the mealworms were unable to effectively utilize the substrate and convert it into absorbable nutrients and eventually into body mass. This can be clearly seen when the average body weight data are analyzed together with the FCR. The body weight of the mealworms fed the duckweed substrate did not increase; rather, their body weight reduced from what was measured at the beginning of the experiment. This shows that the energy content of duckweed was not sufficient to build an appropriate amount of protein to increase body mass. The FCR 2.58 obtained in S75D25 is higher than the 1.57 to 2.08 rate obtained in the studies of Bordiean et al. (2020) in mealworms fed chicken feed and wheat bran, but it is lower than the 4.42 obtained in mealworms fed willowleaf sunflower. The FCRs obtained when applying semolina and S50D50 are also lower than those obtained from mealworms fed willowleaf sunflower. The FCR achieved when feeding S75D25 (2.58) is similar to the FCR in chicken (expressed in Kg of feed/Kg of live weight), but the FCRs observed when feeding semolina and S50D50 are higher than that. All the FCRs in our experiment are lower than the FCR in swine (4.5) and cattle (10) [35].
There was no significant difference in the survival rate of yellow mealworms reared on substrate S, S75D25, S50D50, and D. Similarly, there were no significant differences between yellow mealworms reared on substrates S50D50, S25D75, and D. The survival rate of yellow mealworms fed S75D25 are higher, reaching 90%, followed by values of 89%, 88%, and 87%, which were reached when feeding S, D, and S50D50, respectively. These values are quite similar to the larval survival rates of mealworms fed the control diet (wheat bran only), and diets supplemented with red cabbage, carrot, and orange were 91.7%, 92.5%, 89.3%, and 89.7%, respectively, as reported by Liu et al. (2020) [9]. Although the yellow mealworm had the lowest weight when applying the D substrate, it appears that the diet provided enough energy to keep the yellow mealworms alive as the survival rate of yellow mealworms reared on the D diets was not significantly different from those observed in those reared on S50D50 and S25D75.
The survival rates observed in the different treatments did not follow any particular trends, but the highest survival rate was achieved when applying S75D25, while the lowest was obtained when feeding S25D75. This shows that out of all the feeding substrates, the S75D25 had the best production parameters.
4.2. Nutrient Composition
The CP, CF, and ash content followed the same trend, showing an increase with every 25% increase in duckweed inclusion, while the CFa decreased with every 25% increase in the duckweed content in the substrate. The increasing CP, CF, and ash content with the increasing amount of duckweed in the feeding substrate is due to the CP, CF, and crude ash content of duckweed being approximately 2 times, 5 times, and 20 times higher than those of semolina, respectively.
The CP and crude ash contents observed in yellow mealworms fed the S diet in this study are similar to those reported in the study of Ravzanaadii et al. (2012) [36], while the CP and crude ash contents of yellow mealworms on all duckweed diets are higher than those reported in the same studies. When wheat bran was supplemented with fresh carrot, orange peel, and red cabbage in the diet of yellow mealworm larvae, there were no significant differences in the nutrient composition of yellow mealworms fed any of these substrates. The CP (49.1%) and CFa (38.8%) reported are within the range observed in the S75D25 diet in our study, while the crude ash (4.1) is similar to what was observed in yellow mealworms fed the S50D50 diet in our study [9]. A study performed by Toviho and Bársony in 2022 [37] reported the nutrient composition of yellow mealworm larvae. We found that CP values of mealworms reared on the S diet in this present study fell within the range (43–45%) reported by that previous study. This is probably due to the fact that the feeding substrate used in that previous study was a mixture of semolina, flour, and oat flakes. With regard to CFa, in our current study, the values observed in yellow mealworms reared on substrate S75D25 were within the range (37.8–39.6%) reported in the above study, while in regard to CF, the values observed in yellow mealworm larvae fed D are similar to those reported in the previous study [37].
The best protein content of the larvae was achieved when feeding substrate D, but the application of this substrate resulted in the worst production parameters. The decrease in CFa can be explained as a result of the difference in the nutrient composition of duckweed and semolina, where semolina contains approximately two times more carbohydrates than duckweed. As a result of this, yellow mealworms fed substrates with higher semolina contents have more energy available for conversion into fat. These results support what has been reported in other studies, namely, that the nutrient composition of yellow mealworms can be enhanced using high nutritional value feedstuff [20,35,38]. The ash content was in a range between 1.11 and 1.88 for the different substrate compositions, which is similar to or higher than the value reported by Liu et al. (2020) [9]. This difference could have resulted from different environmental conditions and feeding. The protein content per unit of yellow mealworm produce is higher in mealworms reared on substrates S75D25 and S50D50 than those fed any of the other substrates. Although the weight of yellow mealworms fed S75D25 is significantly higher than that of those reared on S50D50, the protein contents per individual yellow mealworm were not statistically different. Similarly, while the use of feeding substrates S25D75 and S resulted in statistically similar weights, with S, the protein contents achieved when feeding S25D75 were statistically higher than those observed when feeding substrate S.
4.3. Amino Acid Composition of Yellow Mealworm
Altogether, 17 different amino acids were detected in our mealworm samples, including 8 EAAs, plus cysteine and tyrosine. Jajic et al. (2020) reported the detection of all amino acids in the yellow mealworm samples involved in their experiment except for cysteine and tyrosine [39]. All amino acid values reported in the study of Jajic et al. (2020) were higher than the values obtained in our study, except for valine, where, compared with the 1.2–1.5% observed in our study, they reported a lower value of 0.65% [39]. The study of Jajic et al. (2022) reported the threonine, methionine, and lysine contents of yellow mealworms raised on six different diets (wheat bran, whole grain barley, wholegrain oat, whole oat and barley 1:1, and buckwheat oat and barley sprout 1:1), and the threonine, methionine, and lysine values reported were 3–7.5%, 1.6–2.5%, and 4.4–10.6%, respectively [40]. These values are higher than what we obtained in this study. All 17 amino acids detected in this study were also detected by Ravzanaadii et al. (2012). In their study, Ravzanaadii et al. reported the presence of the following EAAs in yellow mealworm larvae: isoleucine, leucine, lysine, cysteine + methionine, phenylalanine + tyrosine, threonine, valine, and histidine (3.6%,3.4%, 2.9%, 1.2%, 5.2%, 1.8%, 2.4%, and 1.5%, respectively). Compared with the values observed in our results, all amino acid values reported were higher [36]. The higher amino acid values reported in the study of Ravzanaadii et al. (2012) and Jajic et al. (2022) can be explained by the difference in the feeding substrates used in the two studies, where Ravzanaadii et al. used wheat bran and vegetables as feeding substrates, while Jajic et al. used six different substrates (wheat bran, whole grain barley, wholegrain oat, whole oat and barley 1:1, and buckwheat oat and barley sprout 1:1) [36,39]. Despite the various feeding substrates used in this experiment, tryptophan was not detected in any of the yellow mealworm samples, which is in line with the results obtained in the study of Ravzanaadii et al. (2012), Jajic et al. (2020), and Jajic et al. (2022) [36,39,40]. Nevertheless, the study of Yi et al. (2013) reported tryptophan in yellow mealworm larvae [41].
The levels of amino acids detected were significantly higher in yellow mealworms reared on substrates S25D75 and D for each individual amino acid. Although the levels of amino acids obtained in this study were lower than what was reported in other studies, there was an improvement in the values at 75% and 100% duckweed inclusion. This shows that duckweed can be used to increase the level of amino acids in yellow mealworm larvae. When comparing essential amino acids to the protein requirements of an adult human according to FAO/WHO/UN, the result shows that the leucine, cysteine + methionine, and histidine values are all below the requirements.
For every animal, an optimal level of EAA:NEAA is required for the highest protein utilization [42]. Therefore, the level of EAA:NEAA in yellow mealworm is an important aspect to be considered in yellow mealworm production. The EAA:NEAA ratios reported in our studies were between 0.86 and 1.05, where feeding 100% semolina resulted in an EAA:NEAA ratio of 0.86, which is below the ratio that produced the best weight gain in rainbow trout when they were fed six diets containing different EAA:NEAA levels between 0.3 and 1.9 in a six-week experiment. The best weight gain was observed in the diets containing 0.9 and 1.3 EAA:NEAA [42]; the ratios obtained in all diets containing duckweed in our experiment fall within this range. Schuhmacher et al. (1995) reported that the best gains were observed in rainbow trout that were fed diets with 0.67–1.5 EAA:NEAA [43]. The reported optimal EAA:NEAA for growth and protein retention in Nile tilapia was 0.67, which is lower than the ratio obtained in our study [44].
4.4. Fatty Acid Composition of Yellow Mealworm
The result obtained in our study shows that altogether, 20 FAs were detected in the yellow mealworm larvae samples, which is higher than the 14 FAs reported by Ravzanaadii et al. (2012). This shows that the inclusion of duckweed in the diet of yellow mealworms affected not just the fat content of the yellow mealworms but their fatty acid composition as well. There is no identifiable pattern regarding the changes in the individual FA values. The lack of a noticeable correlation of the changes in FA values with an increasing duckweed concentration in the feeding substrate indicates that this result is in line with the proposition that there is an internal regulation of lipids in insects [36].
Out of the fatty acids, oleic acid has the highest proportion, which is similar to the result reported by Dreassi et al. (2017) [38], where they analyzed the FA composition of yellow mealworm larvae reared on six different diets, although the 42–46% they reported is lower than the 48–53% observed in our study. At the same time, their study shows that the proportion of palmitic acid in yellow mealworm larvae is higher than that of linoleic acid, which contradicts the result observed in our own study, where the proportion of linoleic acid is higher than that of palmitic acid. Several other studies also show that oleic, linoleic, palmitic, stearic, myristic, palmitoleic, and αlinolenic FA are the most abundant FAs in yellow mealworm larvae, though the order from highest to lowest differed in proportion in the different studies [11,36,38]. The study of Siemianowska et al. (2013), however, reported elaidic acid to be the most abundant FA [45].
The main n-3 long-chain PUFAs involved in the prevention of cardiovascular diseases are eicosapentaeonic acid (EPA) and docosahexaenoic acid (DHA) [46]. One of these n-3 long-chain PUFAs (EPA) was detected in our yellow mealworm samples. Neither EPA nor DHA were found in the studies of Dreassi et al. (2017) and Ravzanaadii et al. (2012) [36,38]. The 0.74% reported in the study of Siemianowska et al. (2013) [45] is within the range observed in our result [45]. The studies of Jajic et al. (2020) and Van Broekhoven et al. (2015) reported a slightly lower value of 0.02% than what was observed in our result [11,39]. The studies of Jajic et al. (2022) reported the presence of both EPA and DHA, where EPA was detected in yellow mealworms raised on five of the six experimental diets, while DHA was detected in yellow mealworm samples from all the experimental diets. EPA values between 0.01% and 0.09% were reported in the study of Jajic et al. (2022) [40], which is close to the 0.03–0.08% observed in our study.
The values of capric, lauric, tricidelic, myristic, magaric, and myristoleic FAs observed in this study are lower than the values reported in the study of Dreassi et al. (2017) [38], while the values of arachidic and oleic FAs are higher in our study [38].
From the review of similar studies, it is evident that there is high variability in FA composition, but the major FAs found are similar [11,20,36,37,38,39,40,45].
The yellow mealworm larvae reared on 100% duckweed had the highest omega-3 fatty acid content, reaching 1.62%, which is significantly higher than the omega-3 FA contents of those fed a smaller composition of duckweed in their feeding substrate. Although the omega-3 FA of 100% duckweed is the highest, 100% semolina had higher n-3 PUFA and n-6 PUFA contents than those fed S75D25, S50D50, and S25D75, with no noticeable patterns. The ratio of n-6/n-3 in yellow mealworms fed 100% duckweed is the lowest of all the treatments in this study. This shows that duckweed can improve the n-6/n-3 in yellow mealworm. A lower n-6/n-3 ratio has been shown to improve fatty liver in teenagers and restore the liver fat content in 1/3 of the population involved in the experiment [47].
5. Conclusions
This study shows that duckweed is effective as a feeding substrate to improve the production parameters and nutrient composition of yellow mealworm. Considering both the production parameters and nutrient composition, the yellow mealworm diets most suitable for significantly improving both the production parameters and the nutrient composition are those in which the substrates include no more than 50% duckweed. With regard to both the production parameters and the nutrient composition, the combination of 25% duckweed and 75% semolina is the most suitable substrate to significantly improve the production parameters and nutrient composition. However, in an attempt to outperform the values of the nutrient composition and simultaneously achieve a significant improvement in the final body weights compared with the values achieved when feeding 25% duckweed and 75% semolina, exploring the potentials of rearing yellow mealworms on a substrate containing 50% duckweed and 50% semolina would be worth considering. The results show that the effect of the inclusion of duckweed on the fatty acid composition is not significant at an inclusion rate that is beneficial to the growth parameters of yellow mealworm. A 75% inclusion and 100% duckweed feeding substrate can be used to significantly improve the amino acid composition of yellow mealworm. The yellow mealworms fed 100% duckweed had the highest protein content, but the mealworms did not grow on this diet. Commercial yellow mealworm producers can take advantage of the inclusion of duckweed to improve growth, thus significantly improving the yield while maintaining a similar FCR value. This translates to the same feed quantity for a significantly higher yield. When using the conventional yellow mealworm feed (semolina), the yellow mealworm meal produced has a protein content similar to soy, but with the inclusion of duckweed substrate, a protein content of 69% was achieved, which is similar to the protein content of fishmeal. Although the total weight produced was lower, the yellow mealworm had a higher protein concentration.
Author Contributions
Conceptualization, B.P.; methodology, P.T.; software, O.A.T.; validation, B.P. and O.A.T.; formal analysis, O.A.T.; investigation, O.A.T., P.T. and M.I.; resources, B.P. and P.T.; data curation, B.P. and O.A.T.; writing—original draft preparation, O.A.T. and M.I.; writing—review and editing, O.A.T., B.P., M.I. and P.T.; visualization, B.P. and O.A.T.; supervision, B.P. and P.T.; project administration, B.P.; funding acquisition, B.P. All authors have read and agreed to the published version of the manuscript.
Funding
Project no. TKP2020-NKA-04 has been implemented with the support provided from the National Research, Development, and Innovation Fund of Hungary, financed under the 2020-4.1.1-TKP2020 funding scheme.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data from this experiment are contained in the article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| FCR | Feed conversion ratio |
| DM | Dry matter |
| CP | Crude protein |
| CFa | Crude fat |
| CF | Crude fiber |
| ADL | Acid detergent lignin |
| ADF | Acid detergent fiber |
| DHA | Docosahexaenoic acid |
| EPA | Eicosapentaenoic acid |
| PUFA | Polyunsaturated fatty acids |
| MUFA | Monounsaturated fatty acids |
| SFA | Saturated fatty acids |
| FA | Fatty acids |
| AA | Amino acid |
| NEAA | Non-essential amino acids |
| EAA | Essential amino acid |
| CTAB | Cetyltrimethylammonium bromide |
References
- Bordiean, A.; Krzyżaniak, M.; Stolarski, M.J.; Czachorowski, S.; Peni, D. Will yellow mealworm become a source of safe proteins for Europe? Agriculture 2020, 10, 233. [Google Scholar] [CrossRef]
- Hagstrum, D.W.; Klejdysz, T.; Subramanyam, B.; Nawrot, J. Atlas of Stored-Product Insects and Mites; AACC International Inc.: Eagan, Minnesota, USA, 2013; Volume 589. [Google Scholar]
- Commission Regulation (EU) 2021/1372 of 17 August 2021 Amending Annex IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council as Regards the Prohibition to Feed Non-Ruminant Farmed Animals, Other than fur Animals, with Protein Derived from Animals. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=OJ:L:2021:295:TOC (accessed on 15 March 2023).
- Commission Implementing Regulation (EU) 2022/169 of 8 February 2022 authorising the placing on the market of frozen, dried and powder forms of yellow mealworm (Tenebrio molitor larva) as a novel food under Regulation (EU) 2015/2283 of the European Parliament and of the Council and amending Commission Implementing Regulation (EU) 2017/2470. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32022R0169&qid=1678903371451 (accessed on 15 March 2023).
- Van Huis, A.; Oonincx, D.G.A.B. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Karapanagiotidis, I.T.; Mente, E.; Psofakis, P.; Athanassiou, C.G. Evaluation of various commodities for the development of the yellow mealworm, Tenebrio molitor. Sci. Rep. 2020, 10, 11224. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.; Kristensen, T.N.; Heckmann, L.H.; Sørensen, J.G. Breeding and maintaining high-quality insects. In Insects as Food and Feed: From Production to Consumption; Van Huis, A., Tomberlin, J.K., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2017; pp. 175–198. [Google Scholar]
- Heckmann, L.H.; Andersen, J.L.; Gianotten, N.; Calis, M.; Fischer, C.H.; Calis, H. Sustainable mealworm production for feed and food. Edible Insects Sustain. Food Syst. 2018, 321–328. [Google Scholar] [CrossRef]
- Liu, C.; Masri, J.; Perez, V.; Maya, C.; Zhao, J. Growth performance and nutrient composition of mealworms (Tenebrio molitor) fed on fresh plant materials-supplemented diets. Foods 2020, 9, 151. [Google Scholar] [CrossRef]
- Oonincx, D.G.; Van Broekhoven, S.; Van Huis, A.; van Loon, J.J. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef]
- Van Broekhoven, S.; Oonincx, D.G.; Van Huis, A.; Van Loon, J.J. Growth performance and feed conversion efficiency of three edible mealworm species (Coleoptera: Tenebrionidae) on diets composed of organic by-products. J. Insect Physiol. 2015, 73, 1–10. [Google Scholar] [CrossRef]
- Morales-Ramos, J.; Rojas, M.; Shapiro-Ilan, D.; Tedders, W. Developmental plasticity in Tenebrio molitor (Coleoptera: Tenebrionidae): Analysis of instar variation in number and development time under different diets. J. Entomol. Sci. 2010, 45, 75–90. [Google Scholar] [CrossRef]
- Morales-Ramos, J.; Rojas, M.; Shapiro-Ilan, D.; Tedders, W. Self-selection of two diet components by Tenebrio molitor (Coleoptera: Tenebrionidae) larvae and its impact on fitness. Environ. Entomol. 2011, 40, 1285–1294. [Google Scholar] [CrossRef]
- Morales-Ramos, J.A.; Rojas, M.G.; Shapiro-llan, D.I.; Tedders, W.L. Use of nutrient self-selection as a diet refining tool in Tenebrio molitor (Coleoptera: Tenebrionidae). J. Entomol. Sci. 2013, 48, 206–221. [Google Scholar] [CrossRef]
- Dragojlović, D.; Đuragić, O.; Pezo, L.; Popović, L.; Rakita, S.; Tomičić, Z.; Spasevski, N. Comparison of Nutritional Profiles of Super Worm (Zophobas morio) and Yellow Mealworm (Tenebrio molitor) as Alternative Feeds Used in Animal Husbandry: Is Super Worm Superior? Animals 2022, 12, 1277. [Google Scholar] [CrossRef] [PubMed]
- Barroso, F.G.; Sánchez-Muros, M.J.; Segura, M.; Morote, E.; Torres, A.; Ramos, R.; Guil, J.L. Insects as food: Enrichment of larvae of Hermetia illucens with omega 3 fatty acids by means of dietary modifications. J. Food Compos. Anal. 2017, 62, 8–13. [Google Scholar] [CrossRef]
- Oonincx, D.G.; Laurent, S.; Veenenbos, M.E.; van Loon, J.J. Dietary enrichment of edible insects with omega 3 fatty acids. Insect Sci. 2020, 27, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.J. Increasing calcium levels in cultured insects. Zoo Biol.: Publ. Affil. Am. Zoo Aquar. Assoc. 2000, 19, 1–9. [Google Scholar] [CrossRef]
- Keil, C.; Maares, M.; Kröncke, N.; Benning, R.; Haase, H. Dietary zinc enrichment reduces the cadmium burden of mealworm beetle (Tenebrio molitor) larvae. Sci. Rep. 2020, 10, 20033. [Google Scholar] [CrossRef]
- Lawal, K.G.; Kavle, R.R.; Akanbi, T.O.; Mirosa, M.; Agyei, D. Enrichment in specific fatty acids profile of Tenebrio molitor and Hermetia illucens larvae through feeding. Future Foods 2021, 3, 100016. [Google Scholar] [CrossRef]
- Landolt, E.; Kandeler, R. Biosystematic investigations in the family of duckweeds (Lemnaceae). Veroff. Geobot. Inst. ETH Zur. 1987, 2, 42–43. [Google Scholar]
- Leng, R.A.; Stambolie, J.H.; Bell, R. Duckweed—A potential high-protein feed resource for domestic animals and fish. Livest. Res. Rural. Dev. 1995, 7, 36. [Google Scholar]
- Anderson, K.E.; Lowman, Z.; Stomp, A.M.; Chang, J. Duckweed as a feed ingredient in laying hen diets and its effect on egg production and composition. Int. J. Poult. Sci. 2011, 10, 4–7. [Google Scholar] [CrossRef]
- Xu, J.; Shen, Y.; Zheng, Y.; Smith, G.; Sun, X.S.; Wang, D.; Zhao, Y.; Zhang, W.; Li, Y. Duckweed (Lemnaceae) for potentially nutritious human food: A review. Food Rev. Int. 2022, 1–15. [Google Scholar] [CrossRef]
- Rusoff, L.L.; Blakeney, E.W.; Culley, D.D. Duckweeds (Lemnaceae Family): A Potential Source of Protein and Amino Acids. J. Agric. Food Chem. 1980, 28, 848–850. [Google Scholar] [CrossRef]
- Islam, K.M.S. Feasibility of Duckweed as Poultry Feed—A Review. Ind. J. Animal. Sci. 2002, 72, 486–491. [Google Scholar]
- Hasan, M.R.; Rina, C. Use of Algae and Aquatic Macrophytes as Feed in Small-scale Aquaculture: A Review (No. 531); Food and Agriculture Organization of the United Nations: Rome, Italy, 2009. [Google Scholar]
- ISO 6496:1999; Animal Feeding Stuffs—Determination of Moisture and Other Volatile Matter Content. International Organization for Standardization: Geneva, Switzerland, 1999.
- ISO 5983-2:2009; Animal Feeding Stuffs—Animal Feeding Stuffs—Determination of Nitrogen Content and Calculation of Crude Protein Content—Part 2: Block Digestion and Steam Distillation Method. International Organization for Standardization: Geneva, Switzerland, 1999.
- ISO 6865:2000; Animal Feeding Stuffs—Determination of Crude Fibre Content—Method with Intermediate Filtration. International Organization for Standardization: Geneva, Switzerland, 2000.
- Hahn, T.; Roth, A.; Febel, E.; Fijalkowska, M.; Schmitt, E.; Arsiwalla, T.; Zibek, S. New methods for high-accuracy insect chitin measurement. J. Sci. Food Agric. 2018, 98, 5069–5073. [Google Scholar] [CrossRef]
- ISO 13906:2008; Animal Feeding Stuffs—Determination of Acid Detergent Fibre (ADF) and Acid Detergent Lignin (ADL) Contents. International Organization for Standardization: Geneva, Switzerland, 2008.
- Lynch, J.M.; Barbano, D.M. Kjeldahl nitrogen analysis as a reference method for protein determination in dairy products. J. AOAC Int. 1999, 82, 1389–1398. [Google Scholar] [CrossRef]
- Thévenot, A.; Rivera, J.L.; Wilfart, A.; Maillard, F.; Hassouna, M.; Senga-Kiesse, T.; Le Féon, S.; Aubin, J. Mealworm meal for animal feed: Environmental assessment and sensitivity analysis to guide future prospects. J. Clean. Prod. 2018, 170, 1260–1267. [Google Scholar] [CrossRef]
- Bordiean, A.; Krzyżaniak, M.; Stolarski, M.J.; Peni, D. Growth Potential of Yellow Mealworm Reared on Industrial Residues. Agriculture 2020, 10, 599. [Google Scholar] [CrossRef]
- Ravzanaadii, N.; Kim, S.H.; Choi, W.H.; Hong, S.J.; Kim, N.J. Nutritional value of mealworm, Tenebrio Molitor as food source. Int. J. Ind. Entomol. 2012, 25, 93–9820. [Google Scholar] [CrossRef]
- Toviho, O.A.; Bársony, P. Nutrient Composition and Growth of Yellow Mealworm (Tenebrio molitor) at Different Ages and Stages of the Life Cycle. Agriculture 2022, 12, 1924. [Google Scholar] [CrossRef]
- Dreassi, E.; Cito, A.; Zanfini, A.; Materozzi, L.; Botta, M.; Francardi, V. Dietary fatty acids influence the growth and fatty acid composition of the yellow mealworm Tenebrio molitor (Coleoptera: Tenebrionidae). Lipids 2017, 52, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Jajić, I.; Popović, A.; Urošević, M.I.; Krstović, S.; Petrović, M.; Guljaš, D.; Samardžić, M. Fatty and amino acid profile of mealworm larvae (Tenebrio molitor L.). Biotechnol. Anim. Husb. 2020, 36, 167–180. [Google Scholar] [CrossRef]
- Jajić, I.; Krstović, S.; Petrović, M.; Urošević, M.; Glamočić, D.; Samardžić, M.; Popović, A.; Guljaš, D. Changes in the chemical composition of the yellow mealworm (Tenebrio molitor L.) reared on different feedstuffs. J. Anim. Feed. Sci. 2022, 31, 191–200. [Google Scholar] [CrossRef]
- Yi, L.; Lakemond, C.M.; Sagis, L.M.; Eisner-Schadler, V.; van Huis, A.; van Boekel, M.A. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef]
- Green, J.A.; Hardy, R.W.; Brannon, E.L. The optimum dietary essential: Nonessential amino acid ratio for rainbow trout (Oncorhynchus mykiss), which maximizes nitrogen retention and minimizes nitrogen excretion. Fish Physiol. Biochem. 2002, 27, 109–115. [Google Scholar] [CrossRef]
- Schuhmacher, A.; Münch, M.; Gropp, J.M. Non-essential amino acid sources in crystalline amino acid diets for trout (Oncorhynchus mykiss). J. Appl. Ichthyol. 1995, 11, 317–321. [Google Scholar] [CrossRef]
- Mambrini, M.; Kaushik, S.J. Partial replacement of dietary protein nitrogen with dispensable amino acids in diets of Nile tilapia, Oreochromis niloticus. Comp. Biochem. Physiol. 1994, 109, 469–477. [Google Scholar] [CrossRef]
- Siemianowska, E.; Kosewska, A.; Aljewicz, M.; Skibniewska, K.A.; Polak-Juszczak, L.; Jarocki, A.; Jedras, M. Larvae of mealworm (Tenebrio molitor L.) as European novel food. Agric. Sci. 2013, 4, 287–291. [Google Scholar]
- Kleber, M.E.; Delgado, G.E.; Lorkowski, S.; März, W.; von Schacky, C. Omega-3 fatty acids and mortality in patients referred for coronary angiography. The Ludwigshafen Risk and Cardiovascular Health Study. Atherosclerosis 2016, 252, 175–181. [Google Scholar] [CrossRef]
- Van Name, M.A.; Savoye, M.; Chick, J.M.; Galuppo, B.T.; Feldstein, A.E.; Pierpont, B.; Johnson, C.; Shabanova, V.; Ekong, U.; Valentino, P.L.; et al. A low ω-6 to ω-3 PUFA ratio (n–6: N–3 PUFA) diet to treat fatty liver disease in obese youth. J. Nutr. 2020, 150, 2314–2321. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).