Abstract
Low-temperature semen storage technologies are already being used in poultry conservation programs, but the quality of reproductive material stored in cryobanks varies greatly and cannot always be successfully used for practical purposes. Therefore, it is necessary to improve the compositions of cryoprotective media to improve their quality. This study aimed to investigate the composition of membrane lipids and carbohydrates in the cytosol of rooster spermatozoa, to explain the dose-dependent effect of a combination of trehalose and fructose in cryoprotective media on the preservation of their morphological and kinetic parameters during freezing/thawing, and to determine the most effective diluent composition. Ejaculates were collected from Rhode Island Red roosters (n = 10). The effectiveness of three diluents containing trehalose was evaluated: LCM-control (0 mM), Treh20 (9.5 mM), and Treh30 (13.4 mM). Chromatographic analysis of membrane lipids, carbohydrates, and polyols of the spermatozoa cytosol was performed. A decrease in the content of glycolipids in the plasma membranes of spermatozoa from 2.0% in native spermatozoa to 1.1–1.4% (frozen/thawed) and phospholipids from 71.2% (native) to 70.5% (frozen/thawed) reduced the progressive sperm motility from 65.7% in native spermatozoa to 12.6–27.6% (frozen/thawed). The same dynamics were observed for the viability parameter of 90.4% (native) and 27.0–41.2% (frozen/thawed). The Treh20 diluent, using a combination of fructose (36 mM) and trehalose (9.5 mM) saccharides, maximally preserved the lipid profile of plasma membranes and the composition of the cytosol of frozen/thawed rooster spermatozoa, which positively affected the indicators of general and progressive mobility and viability.
1. Introduction
Cryopreservation of sperm in poultry farming is still in the field of scientific research since the level of fertilizing ability of frozen-thawed sperm for use in industrial poultry farming has not been reached. Nevertheless, it should be noted that these technologies are already used in programs for the conservation of poultry genetic resources in many countries; however, the quality of reproductive material stored in cryobanks varies greatly and cannot always be successfully used for practical purposes [,,,,]. The reproductive cells of male birds are very sensitive to various kinds of manipulations, especially at low temperatures. Researchers are currently working to increase the sperm fertility of all poultry species. Stable repeated results of fertilization of eggs from frozen/thawed rooster sperm, close to production standards (70–80%), were obtained and reproduced only by separate groups of researchers [,].
When solving problems of improving the quality of thawed domestic fowl semen, it is necessary to rely not only on the effectiveness of cryoprotective media but also to determine the “vulnerabilities”, the weakest points in the structure of the spermatozoa apparatus for their low-temperature preservation. In the process of cryopreservation and thawing of the semen, a chain reaction occurs that destabilizes the molecular bonds of the plasma membranes of spermatozoa and disrupts the functionality of the cell. An osmotic imbalance occurs due to changes in the composition of the cytosol, increased lipid peroxidation, and changes in the chromatic state.
The resulting complex of negative factors reduces the fertilization ability of spermatozoa. In many studies, the solution to this set of problems is aimed at finding the optimal composition of the cryoprotective medium. When selecting the components of a cryoprotective medium, preference is currently given to the use of organic substances that exhibit stabilizing and antioxidant properties in spermatozoa, which are more physiological for them [,]. Saccharides of various chemical groups (monosaccharides, disaccharides, oligosaccharides, and polysaccharides) can be used as part of diluents for cryopreservation, as they are often natural organic compounds, participate in the construction of various cellular structures, and their role in the regulation of cellular mechanisms is multifaceted. In published studies on cryoprotective media, saccharides such as fructose, lactose, sucrose, maltose, trehalose, and hyaluronic acid have been used. Saccharides in cryoprotective media play a critical role in low-temperature semen storage protocols due to their ability to interact with membrane phospholipids under conditions of dehydration caused by the cryopreservation process. Sugars can reduce the temperature of the membrane phase transition of dehydrated lipids, slowing down this phase transition, thereby reducing the destruction of the lipids themselves and stabilizing the membrane fluidity []. In addition, monosaccharides are used by spermatozoa as a source of available energy to maintain and preserve their functionality during the freezing process [].
Many authors have noted the exceptional properties of trehalose disaccharide as a non-toxic organic cryoprotectant due to its high hydration ability compared to other sugars, which explains the excellent effectiveness of trehalose as a bioprotector []. The spatial organization of water molecules around trehalose molecules prevents the formation of ice crystals. During cell dehydration, which occurs during freezing, trehalose acts as a stabilizer for proteins and phospholipids in the lipid bilayer of cells. Slowing down the rate of protein aggregation with the participation of trehalose prevents their denaturation. The regulation of osmotic pressure and hydration balance via the formation of long-lived hydrogen bonds between trehalose and water promotes cell survival under various thermal stresses []. A particularly significant effect of trehalose on the stabilization and modulation of cell enzymes was noted, which is based on the ability of the disaccharide to form hydrogen bonds []. Upon cooling or dehydration, the lipids of the plasma membranes of cells become more vulnerable to the peroxidation processes, but trehalose effectively prevents their irreversible changes, thereby neutralizing the formation of harmful free radicals. Therefore, the use of this disaccharide is promising for increasing the functionality of frozen/thawed birds’ spermatozoa. But the most significant results in assessing the functionality of frozen/thawed rooster semen were obtained using a combination of saccharides [,,]. The features of the biochemical composition of the plasma membrane of spermatozoa are an important aspect of spermatozoa physiology when studying the effect of cryopreservation. The lipid ratio of the plasma membranes can be postulated as a general basis for the predicted cryodamage of spermatozoa. Spermatozoa of both domestic fowl and other animal species have plasma membranes highly specialized in their lipid composition, with a high content of polyunsaturated fatty acids (PUFAs), sterols, phosphatidylcholines (PC), and sphingomyelins (SM), which play an important role in the interactions between spermatozoa and oocyte and fertilization ability [,].
The aim of this study was to investigate the composition of membrane lipids and soluble carbohydrates in the cytosol of rooster spermatozoa to provide a biological explanation for the dose-dependent effect of a combination of non-reducing trehalose disaccharide and fructose monosaccharide in synthetic cryoprotective media on the preservation of their morphological and kinetic parameters during freezing/thawing, and to determine the most effective diluent composition.
2. Materials and Methods
2.1. Animals
Rhode Island Red roosters from the Central Collective Use Center “Genetic Collection of Rare and Endangered Breeds of Chickens” (https://vniigen.ru/ckp-geneticheskaya-kollekciya-redkix-i-ischezayushhix-porod-kur/ (accessed on 6 June 2023) RRIFAGB, St. Petersburg, Russia) were used for the experiments. The experimental flock was kept in individual cages, with refreshing and feeding regimes corresponding to the technology used in the collection.
2.2. Sperm Collection, Evaluation, and Dilution
Ejaculates were collected from roosters (n = 10) at the age of 52–56 weeks, and semen was collected twice a week by abdominal massage (Burrows & Quinn 1935) [].
All ejaculates were evaluated individually according to the following criteria: volume (with a graduated pipette, mL); concentration (photometer Accuread® IMV Technologies, L’Aigle, France, 2019, billion/mL); total and progressive sperm motility (CASA, Motic BA410E, Fujian, China, 2019, ArgusSoft software-1, St. Petersburg, Russia, 2020, %). Sperm were pooled, divided into 3 parts, and diluted with the cryoprotective medium in a ratio of 1:1: LCM-control (trehalose 0 mM) [] and the experimental medium Treh20 (trehalose 9.5 mM) and Treh30 (trehalose 13.4 mM) (Table 1); the composition of the cryoprotective medium was developed and published earlier []. The osmolarity of each diluent was assessed using an OSCR-1M cryoscopic medical osmometer (KIWI Osmometry, Saint-Petersburg, Russia, 2019). Semen freezing was carried out in pellets according to the protocol described by Stanishevskaya et al., 2023 []. The pellets were stored for one month, and thawing was performed on a heated metal plate at 60 °C (in-house developed equipment, RRIFAGB, Leningrag, USSR, 1989).

Table 1.
Composition of cryoprotective media for rooster sperm.
Microscopy, image collection, and processing: Experiments to assess the viability of spermatozoa and the integrity of their acrosomes were performed by bright-field and phase-contrast microscopy using a Motic BA410E microscope (Fujian, China, 2019).
2.3. Sperm Evaluation after Thawing
Motility: Total and progressive sperm motility was assessed using CASA (Motic BA410E, China, 2019, ArgusSoft software, St. Petersburg, Russia, 2020, %). Sperm motility was assessed on thawed samples by applying a drop of semen to a warm (37 °C) glass slide using the CASA software. The semen concentration for assessing motility parameters was 3.0 × 107 pcs/mL. Approximately 2000 cells were randomly selected and analyzed for each seed sample. The following parameters of spermatozoa movement were assessed: total motility (TM, %) and progressive motility (PM, %) [].
Viability: The viability of spermatozoa (1:20 dilution) was studied on the basis of histological smears prepared from freshly obtained semen. The prepared smears were stained with nigrosine-eosin (aqueous solution: eosin-3% and nigrosine-10%) and evaluated under oil immersion magnification ×1000 for the presence of color. Pink cells were considered dead, and unstained (white) cells were considered alive. The results were expressed as a percentage of individual categories of spermatozoa (each sample was evaluated with at least 200 cells, which was taken as 100%) []. The experiments were carried out in triplicates.
Acrosome integrity: Acrosomes integrity was determined according to the following protocol: a drop of pre-diluted 1:20 semen was air-dried on glass slides, fixed with 5% paraformaldehyde in phosphate-buffered saline (PBS; pH 7.4) for 15 min, and washed once with PBS. Slides were stained for 5 min with an aqueous solution of 0.25% Coomassie brilliant blue R-250 in 10% glacial acetic acid and 25% methanol, washed with distilled water, and air-dried. Intact spermatozoa acrosomes were stained blue, and unstained acrosomes were damaged. Five to six microscopic fields were evaluated, with at least 200 cells in total [].
2.4. Chromatographic Analysis of Membrane Lipids, Carbohydrates, and Polyols of the Spermatozoa Cytosol
2.4.1. Sample Preparation for Lipid Profile Sperm Analysis
For quantitative analysis of carbohydrates associated with the membrane of spermatozoa, semen was collected from 10 roosters under quality control to obtain repeatability of the result within 2 weeks. The volume of pooled sperm for each day of collection was at least 15 mL for each 5 mL sample (LCM control, Treh20, and Treh30). Pooled ejaculates were divided into 3 aliquots for dilution with media: LCM control, Treh20, and Treh30 in a 1:1 ratio and equilibrated for 40 min. Then, the sperm was centrifuged for 10 min at 3000 rpm at a temperature of 5 °C and washed with 0.9% sodium chloride solution. This procedure was repeated three times. The centrifuged and washed semen was collected in small portions on a nylon filter and precipitated for another 30 min. The prepared centrifugate and supernatant samples were frozen and stored at −25 °C.
2.4.2. Lipid Analysis of Plasma Membrane Spermatozoa
Membrane lipid analyses were carried out as previously described (Stanishevskaya et al., 2023) []. Briefly, lipids were extracted using the Nichols method [] with phospholipase-deactivating isopropanol, separated by two-dimensional (polar lipids) or one-dimensional (neutral lipids) thin-layer chromatography (TLC), and quantified using standard compounds with densitometry (DENS software). To study the composition of fatty acids, the polar lipid fraction was isolated using one-dimensional TLC in a neutral lipid system. The polar lipid spots remaining at the start were scraped out and eluted with a mixture of chloroform: methanol (1:1); the extract was then evaporated, and methanolysis was carried out using 2.5% H2SO4 in a methanol solution for 2 h at 80 °C.
2.4.3. Fatty Acid Composition Profiles of Membrane Lipids
A full description was presented earlier (Silyukova et al., 2022) []; in brief, to determine the composition of fatty acids, the methyl esters were analyzed by GLC. Soluble cytosol carbohydrates were extracted with boiling water, proteins and charged compounds were removed, and trimethylsilyl derivatives of sugars were obtained and analyzed by GLC using internal standards.
The degree of unsaturation of phospholipids (CH) was determined using the following formula []:
CH = 1.0 × (% monoene FA)/100 + 2.0 × (% diene FA)/100 + 3.0 × (% triene FA)/100 + 4.0 × (% tetraene FA)/100
2.4.4. Cytosol Carbohydrates of Spermatozoa
For analysis, the dry biomass of semen samples was determined gravimetrically. Extraction of soluble sugars (300–400 mg fresh weight) was carried out four times in 5 mL of water at 100 °C for 20 min. Proteins were removed from the combined extract []. Purification of the carbohydrate extract from the charged compounds was carried out on a combined column with ion exchange resins Dowex-1 (acetate form) and Dowex 50 W (H+). The composition of carbohydrates was determined by gas–liquid chromatography to obtain derivatives of trimethylsilyl sugar from a lyophilized extract []. A-methyl-D-mannozide (Merck, Darmstadt, Germany) was used as an internal standard. Chromatography was carried out on a Kristall 5000.1 gas–liquid chromatograph (Khromatek, Yoshkar-Ola, Russia), fitted with ZB-5 30 m capillary column (0.32 mm, 0.25 µm) (Phenomenex, Torrance, CA, USA), maintaining the temperature from 130 to 270 °C, and with a speed of 5–6 deg/min. Glycerol, fructose, glucose, inositol, trehalose, and mannit were used as markers (Sigma Aldrich, St. Louis, MO, USA). This evaluation was carried out twice.
2.5. Statistical Methods
For statistical data processing, Excel 2013 software (Microsoft, Redmond, WA, USA) was used. All data were assessed using standard descriptive statistics and presented as the mean ± standard error of the mean. The significance of differences between the mean values for the motility, viability, and acrosome integrity semen evaluation criteria was determined using the nonparametric Mann–Whitney U-test. Differences were considered statistically significant at p < 0.05.
3. Results
No statistically significant differences in total motility and progressive motility of native (fresh) diluted sperm and fresh untreated sperm rates were observed between LCM-control, Treh20, and Treh30; the average levels are presented in Table 2. The sperm concentration was 3.6 ± 0.3 billion/mL.

Table 2.
The lipid composition of spermatozoa membranes of native and frozen/thawed rooster semen depending on the composition of the cryoprotective diluent.
3.1. Composition of Lipids in the Plasma Membranes of Spermatozoa
The composition of membrane lipids in native and frozen/thawed spermatozoa and their quantitative significance were determined. The following major (>9.0% of ∑ lipids) lipid fractions were determined: phosphatidylethanolamines, phosphatidylserines, phosphatidylcholines, sphingomyelins, and sterols (Table 2). The ratio of lipids in the plasma membranes of thawed spermatozoa varied depending on the composition of the cryoprotective medium used.
3.2. Composition and Degree of Unsaturation of Fatty Acids in Membrane Lipids of Spermatozoa
Fifteen fatty acids (Table 3) that make up the phospholipids of spermatozoa membranes were identified, of which polyunsaturated fatty acids were represented by Oleic, γ-Linoleic, Eicosatrienoic, Arachidonic, Erucic, and Docosatetraenoic fatty acids, which in total ranged from 47.4% to 50.3% in frozen/thawed spermatozoa, depending on the composition of the cryoprotective diluent. The most significant change in fatty acid content was recorded for the C22:4 fatty acid (Docosatetraenoic acid).

Table 3.
Fatty acid composition (% of ∑) of membrane lipids of native and frozen/thawed rooster spermatozoa.
A difference in the change in the degree of unsaturation of fatty acids in membrane lipids of frozen/thawed spermatozoa was established, taking into account the diluent used and in comparison with native sperm. The use of the cryoprotective medium LCM-control (0 mM trehalose) significantly increased the degree of unsaturation of fatty acids, and the use of trehalose as part of the diluent made it possible to stabilize this value at the level of native sperm values (Figure 1).
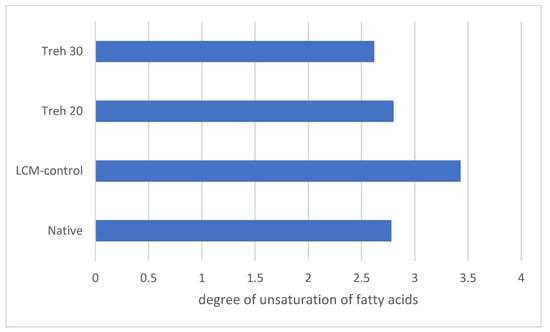
Figure 1.
The degree of unsaturation of fatty acids in membrane lipids of native and frozen/thawed rooster spermatozoa under the influence of different concentrations of trehalose.
The composition of the cytosol of native and frozen/thawed spermatozoa was studied based on the composition of cryoprotective diluents (Table 4). In native sperm, the main carbohydrate in the cytosol was fructose (mean 10.5% of Σ carbohydrates and polyols), and the main polyol in the cell was inositol (mean 83.0% of Σ). Under the influence of freezing and thawing, depending on the medium used, the ratio of the components of the spermatozoa cytosol underwent significant changes. The main changes were attributed to the percentage of polyols—a decrease in the percentage of inositol by up to eight times and an increase in the percentage of glycerol by up to eight times.

Table 4.
Composition of carbohydrates and polyols in the cytosol of native and frozen/thawed rooster spermatozoa.
A relationship was established between the dynamics of changes in the content (% of ∑) of membrane glycolipids and glycerol, as well as the percentage of Docosatetraenoic polyunsaturated fatty acid and phospholipids (Figure 2).
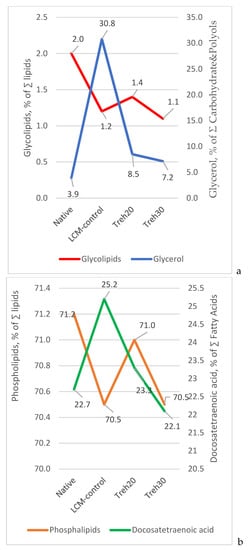
Figure 2.
Dynamics of changes in the content of glycolipids and phospholipids of plasma membranes, glycerol, and Docosatetraenoic acid in native and frozen/thawed spermatozoa depending on the composition of the cryoprotective medium: (a)—glycolipids/glycerol dependence; (b)—phospholipids/docosahexaenoic acid dependence.
Figure 2 illustrates the process of structural destabilization of spermatozoa membranes due to changes in the ratio of lipids included in their composition under the influence of low-temperature exposure and the composition of cryoprotective media. For example, when semen was frozen with an LCM-control diluent (0 mM trehalose), the destabilization of membrane glycolipids and phospholipids (in particular, phosphatidylserine) was recorded with the release of glycerol and Docosatetraenoic fatty acid molecules. It is known that an increase in the content of polyunsaturated fatty acids (including docosatetraenoic) in membrane phospholipids increases the vulnerability of spermatozoa membranes to oxidative stress []. Obviously, trehalose in the cryoprotective composition is a stabilizing component in the cryoprotective medium for the membrane lipids of spermatozoa.
3.3. Evaluation of Native and Frozen/Thawed Rooster Spermatozoa by Morphological and Kinetic Parameters
The use of the Treh20 diluent at a trehalose concentration of 9.5 mM gave better results in determining the total and progressive motility of frozen/thawed spermatozoa, which was higher in assessing the viability of spermatozoa (Table 5).

Table 5.
Results of evaluation of native and frozen/thawed rooster spermatozoa (rooster n = 10, number of measurements n = 3).
An increase in the concentration of trehalose to 13.4 mM in the composition of the cryoprotective diluent Treh30 significantly reduced the results of evaluating all these parameters. The exception was the acrosome integrity parameter.
4. Discussion
Trehalose is a naturally occurring, non-toxic compound found in bacteria, fungi, plants, and invertebrates, which has been shown to provide cellular protection under a variety of stresses [], including thermal, osmotic, and oxidative [,,,,]. Among the molecules that promote stability, trehalose provides the best membrane protection both in vivo and in vitro [,]. It is known that the response to stress caused by the freeze/thaw protocol is the rearrangement of membrane lipids in spermatozoa due to changes in the ratio of major lipids as well as changes in minor lipids. Such stress leads to a restructuring of the lipid organization of membranes and a change in the degree of unsaturation of fatty acids. It has been shown that trehalose, which has a significant inhibitory effect on the oxidation of unsaturated fatty acids due to the weak interaction with double bonds, reveals the molecular mechanism of the antioxidant function [].
A study of the effect of trehalose on the composition of a cryoprotective diluent at the subcellular level (the plasma membrane of spermatozoa and its biomolecules) showed that its concentration is of fundamental importance for preventing cell destruction and damage during cryopreservation [,]. A dose-dependent effect of the content of trehalose in the composition of the cryoprotective diluent on the safety of phospholipids and glycolipids as substructural units of the cell membrane was observed; as a result, the morphological integrity and kinetic parameters of spermatozoa were established. The best results were achieved using the Treh20 cryoprotective medium containing 9.5 mM trehalose and 36 mM fructose. The obtained results are consistent with the results of previous studies on the effect of the combination of saccharides (fructose–trehalose) on the fertility of frozen/thawed semen; when using the Treh20 diluent, egg fertility was the highest—86% versus 79% in the control (LCM-control) []. An increase in the content of trehalose to 13.4 mM (Treh30) led to a decrease in the estimated parameters, with the exception of the acrosome integrity parameter. The concentration of trehalose 0 mM (LCM-control) in the cryoprotective diluent reduced the parameters of acrosome integrity and viability of frozen/thawed spermatozoa compared to both the experimental diluent formulations. According to our assumption, the revealed destruction of glycolipids and phospholipids in membranes, associated with the separation of their carbohydrate residues (glycerol) and fatty acids (Docosatetraenoic acid), is explained by a change in the functioning of enzymes caused by a violation of cellular structures, including acrosomes. Enzymes (including phospholipase) are present in spermatozoa and are normally activated during the process of fertilization. The violation of the structural integrity of organoids during cryopreservation triggers the process of premature release of enzymes, which leads to the destruction of glycolipid and phospholipid molecules with the release of their components (glycerol and fatty acids); different localizations of cytosolic phospholipases in spermatozoa (heads, middle parts, and tails) have also been reported []. Trehalose protects intracellular enzymes under the influence of temperature factors [,,], acting as a stabilizer of cell enzymes and, under certain conditions, can inhibit their action by increasing the viscosity of the cytosol [], which, prevents premature activation of intracellular enzymes when using the Treh30 diluent.
In our study, structural changes in membrane lipids and changes in the degree of unsaturation of fatty acids are practical “mirror image” with morphokinetic parameters, i.e., the progressive motility and viability of frozen/thawed spermatozoa.
A decrease in the content of glycolipids in the plasma membranes of spermatozoa from 2.0% in native spermatozoa to 1.1–1.4% in frozen/thawed spermatozoa and phospholipids from 71.2% (native) to 70.5% (frozen/thawed) reduced the progressive sperm motility from 65.7% in native spermatozoa to 12.6–27.6% in frozen/thawed spermatozoa. The same results were observed for the viability parameter of 90.4% (native) and 27.0–41.2% (frozen/thawed). Thus, the relationship between the cryoresistance of lipids in the plasma membranes of the spermatozoon, the integrity of the substructural units of the cell membrane, and kinetic parameters under the influence of the cryopreservation process has been proven.
This explains the sharp change in the content of phospholipids and glycolipids in the plasma membranes of frozen/thawed rooster sperm when using a cryoprotective medium that does not contain trehalose (LCM-control). The use of a combination of fructose monosaccharide (36 mM) and trehalose disaccharide (9.4 mM) as part of a cryoprotective medium made it possible to stabilize the lipid profile of the plasma membranes of frozen/thawed spermatozoa. However, an increase in the concentration of trehalose (13.4 mM) and a decrease in the concentration of fructose (31 mM) in the Treh30 diluent not only failed to stabilize the lipid profile of the membranes but also blocked the functional qualities (motility) of spermatozoa, despite an increase in the integrity of the acrosomes (p < 0.05).
5. Conclusions
Thus, the study of the lipid profile of membranes as an additional criterion for evaluating the functional disorders of spermatozoa and evaluating the effectiveness of cryoprotective diluents expanded the understanding of the mechanisms of action of trehalose and made it possible to determine the most effective combination of sugar concentrations to preserve the integrity of rooster spermatozoa membranes.
The Treh20 diluent, using a combination of fructose (36 mM) and trehalose (9.5 mM) saccharides, maximally preserves the lipid profile of plasma membranes and the composition of the cytosol of frozen/thawed rooster spermatozoa, which positively affects the indicators of general and progressive motility and viability.
Author Contributions
Conceptualization, O.S. and Y.S.; validation, O.S.; formal analysis, O.S., Y.S., V.T. and E.I.; investigation, O.S. and Y.S.; resources, Y.S., E.F., N.P. and A.K.; data curation, O.S., Y.S., V.T. and E.I.; writing—original draft preparation, O.S. and Y.S.; writing—review and editing, O.S. and Y.S.; visualization, Y.S., E.F., N.P., A.K., V.T. and E.I.; supervision, O.S.; project administration, O.S.; funding acquisition, O.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 19-16-00009 P.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the Russian Research Institute of Farm Animal Genetics and Breeding—Branch of the L.K. Ernst Federal Research Center for Animal Husbandry (RRIFAGB).
Data Availability Statement
Not applicable.
Acknowledgments
We thank the Centre for Collective Usage “Genetic collection of rare and endangered chicken breeds” and the Russian Research Institute of Farm Animal Genetics and Breeding—Branch of the L.K. Ernst Federal Research Center for Animal Husbandry (RRIFAGB).
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. The Second Report on the State of the World’s Animal Genetic Resources for Food and Agriculture; Scherf, B.D., Pilling, D., Eds.; FAO Commission on Genetic Resources for Food and Agriculture Assessments; FAO: Rome, Italy, 2015; Available online: http://www.fao.org/3/ai4787e/index.html (accessed on 16 September 2020).
- Iaffaldano, N.; Di Iorio, M.; Rusco, G.; Antenucci, E.; Zaniboni, L.; Madeddu, M.; Marelli, S.; Schiavone, A.; Soglia, D.; Buccioni, A.; et al. Italian semen cryobank of autochthonous chicken and turkey breeds: A tool for preserving genetic biodiversity. Ital. J. Anim. Sci. 2021, 20, 2022–2033. [Google Scholar] [CrossRef]
- Thélie, A.; Bailliard, A.; Seigneurin, F.; Zerjal, T.; Tixier-Boichard, M.; Blesbois, E. Chicken semen cryopreservation and use for the restoration of rare genetic resources. Poult. Sci. 2019, 98, 447–455. [Google Scholar] [CrossRef]
- Partyka, A.; Niżański, W. Advances in storage of poultry semen. Anim. Reprod. Sci. 2022, 246, 106921. [Google Scholar] [CrossRef] [PubMed]
- Janosikova, M.; Petricakova, K.; Ptacek, M.; Savvulidi, F.G.; Rychtarova, J.; Fulka, J. New approaches for long-term conservation of rooster spermatozoa. Poult. Sci. 2023, 102, 102386. [Google Scholar] [CrossRef] [PubMed]
- Fulton, J.E. Avian genetic stock preservation: An industry perspective. Poult. Sci. 2006, 85, 227–231. [Google Scholar] [CrossRef]
- Zong, Y.; Li, Y.; Sun, Y.; Mehaisen, G.M.K.; Ma, T.; Chen, J. Chicken Sperm Cryopreservation: Review of Techniques, Freezing Damage, and Freezability Mechanisms. Agriculture 2023, 13, 445. [Google Scholar] [CrossRef]
- Silyukova, Y.I.; Stanishevskaya, O.I.; Pleshanov, N.V.; Kurochkin, A.A. Efficiency of using a combination of mono- and disaccharides in a diluent for freezing rooster semen. Sel’skokhozyaistvennaya Biol. 2020, 55, 1148–1158. [Google Scholar] [CrossRef]
- Thananurak, P.; Chuaychu-Noo, N.; Phasuk, Y.; Vongpralub, T. Comparison of TNC and standard extender on post-thaw quality and in vivo fertility of Thai native chicken sperm. Cryobiology 2020, 92, 197–202. [Google Scholar] [CrossRef]
- Najafi, A.; Zhandi, M.; Towhidi, A.; Sharafi, M.; Akbari Sharif, A.; Khodaei Motlagh, M.; Martinez-Pastor, F. Trehalose and glycerol have a dose-dependent synergistic effect on the post-thawing quality of ram semen cryopreserved in a soybean lecithin-based extender. Cryobiology 2013, 66, 275–282. [Google Scholar] [CrossRef]
- Gholami, D.; Sharafi, M.; Esmaeili, V.; Nadri, T.; Alaei, L.; Riazi, G.; Shahverdi, A. Beneficial effects of trehalose and gentiobiose on human sperm cryopreservation. PLoS ONE 2023, 18, 0271210. [Google Scholar] [CrossRef]
- Malferrari, M.; Nalepa, A.; Venturoli, G.; Francia, F.; Lubitz, W.; Möbius, K.; Savitsky, A. Structural and dynamical characteristics of trehalose and sucrose matrices at different hydration levels as probed by FTIR and high-field EPR. Phys. Chem. Chem. Phys. 2014, 16, 9831–9848. [Google Scholar] [CrossRef] [PubMed]
- Stanishevskaya, O.; Silyukova, Y.; Pleshanov, N.; Kurochkin, A. Role of Mono- and Disaccharide Combination in Cryoprotective Medium for Rooster Semen to Ensure Cryoresistance of Spermatozoa. Molecules 2021, 26, 5920. [Google Scholar] [CrossRef] [PubMed]
- Sola-Penna, M.; Meyer-Fernandes, J.R. Stabilization against thermal inactivation promoted by sugars on enzyme structure and function: Why is trehalose more effective than other sugars? Arch. Biochem. Biophys. 1998, 360, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Vireque, A.A.; Tata, A.; Silva, O.F.; LoTurco, E.G.; Azzolini, A.; Ferreira, C.R.; Dantas, M.H.; Ferriani, R.A.; Reis, R.M. Effects of n-6 and n-3 polyunsaturated acid-rich soybean phosphatidylcholine on membrane lipid profile and cryotolerance of human sperm. Fertil. Steril. 2016, 106, 273–283. [Google Scholar] [CrossRef]
- Stanishevskaya, O.I.; Silyukova, Y.; Fedorova, E.; Pleshanov, N.; Kurochkin, A.; Tereshina, V.M.; Ianutsevich, E. Effects of Trehalose Supplementation on Lipid Composition of Rooster Spermatozoa Membranes in a Freeze/Thaw Protocol. Animals 2023, 13, 1023. [Google Scholar] [CrossRef]
- Burrows, W.H.; Quinn, J.P. A method of obtaining spermatozoa from the domestic fowl. Poult. Sci. 1935, 14, 251–253. [Google Scholar] [CrossRef]
- Hamad, S.K.; Elomda, A.M.; Sun, Y.; Li, Y.; Zong, Y.; Chen, J.; Abbas, A.O.; Stino, F.K.R.; Nazmi, A.; Mehaisen, G.M.K. The In Vitro Evaluation of Rooster Semen Pellets Frozen with Dimethylacetamide. Animals 2023, 13, 1603. [Google Scholar] [CrossRef]
- Łukaszewicz, E.; Jerysz, A.; Partyka, A.; Siudzińska, A. Efficacy of evaluation of rooster sperm morphology using different staining methods. Res. Vet. Sci. 2008, 85, 583–588. [Google Scholar] [CrossRef]
- Feng, H.L.; Han, Y.B.; Hershlag, A.; Zheng, L.J. Impact of Ca2+ Flux Inhibitors on Acrosome Reaction of Hamster Spermatozoa. J. Androl. 2007, 28, 561–564. [Google Scholar] [CrossRef]
- Nichols, B.W. Separation of the lipids of photosynthetic tissues: Improvements in analysis by thin-layer chromatography. Biochim. Biophys. Acta 1963, 70, 417–422. [Google Scholar] [CrossRef]
- Silyukova, Y.; Fedorova, E.; Stanishevskaya, O. Influence of Technological Stages of Preparation of Rooster Semen for Short-Term and Long-Term Storage on Its Quality Characteristics. Curr. Issues Mol. Biol. 2022, 44, 5531–5542. [Google Scholar] [CrossRef] [PubMed]
- Weete, J.D. Introduction to Fungal Lipids. In Fungal Lipid Biochemistry; Kritchevsky, D., Ed.; Springer: Berlin/Heidelberg, Germany, 1974; pp. 3–36. [Google Scholar]
- Somogui, M. Determination of blood sugar. J. Biol. Chem. 1945, 160, 69. [Google Scholar] [CrossRef]
- Brobst, K.M.; Scobell, H.D. Modern Chromatographic Methods for the Analysis of Carbohydrate Mixtures. Starch-Stärke 1982, 34, 117–121. [Google Scholar] [CrossRef]
- Solocinski, J.; Osgood, Q.; Wang, M.; Connolly, A.; Menze, M.A.; Chakraborty, N. Effect of trehalose as an additive to dimethyl sulfoxide solutions on ice formation, cellular viability, and metabolism. Cryobiology 2017, 75, 134–143. [Google Scholar] [CrossRef]
- Attfield, P.V. Trehalose accumulates in Saccharomyces cerevisiae during exposure to agents that induce heat shock response. FEBS Lett. 1987, 225, 259–263. [Google Scholar] [CrossRef]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17R–27R. [Google Scholar] [CrossRef]
- Hu, J.H.; Zan, L.S.; Zhao, X.L.; Li, Q.W.; Jiang, Z.L.; Li, Y.K.; Li, X. Effects of trehalose supplementation on semen quality and oxidative stress variables in frozen-thawed bovine semen. J. Anim. Sci. 2010, 88, 1657–1662. [Google Scholar] [CrossRef]
- Oku, K.; Watanabe, H.; Kubota, M.; Fukuda, S.; Kurimoto, M.; Tsujisaka, Y.; Komori, M.; Inoue, Y.; Sakurai, M. NMR and quantum chemical study on the OH...pi and CH...O interactions between trehalose and unsaturated fatty acids: Implication for the mechanism of antioxidant function of trehalose. J. Am. Chem. Soc. 2003, 125, 12739–12748. [Google Scholar] [CrossRef]
- Gulaya, N.M.; Margitich, V.M.; Govseeva, N.M.; Klimashevsky, V.M.; Gorpynchenko, I.I.; Boyko, M.I. Phospholipid composition of human sperm and seminal plasma in relation to sperm fertility. Arch. Androl. 2001, 46, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Xu, F.; Hirschfeld, M.; Brenig, B. Sperm Lipid Markers of Male Fertility in Mammals. Int. J. Mol. Sci. 2021, 22, 8767. [Google Scholar] [CrossRef] [PubMed]
- Stanishevskaya, O.; Silyukova, Y.; Pleshanov, N.; Kurochkin, A.; Fedorova, E.; Fedorova, Z.; Perinek, O.; Prituzhalova, A.; Meftakh, I. Effects of Saccharides Supplementation in the Extender of Cryopreserved Rooster (Gallus domesticus) Semen on the Fertility of Frozen/Thawed Spermatozoa. Animals 2021, 11, 189. [Google Scholar] [CrossRef] [PubMed]
- Collodel, G.; Castellini, C.; Iacoponi, F.; Noto, D.; Signorini, C. Cytosolic phospholipase A 2 and F 2 isoprostanes are involved in semen quality and human infertility—A study on leucocytospermia, varicocele and idiopathic infertility. Andrologia 2019, 52, e13465. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Miyazaki, T.; Toyonaga, M.; Tsutsui, T.; Taira, H.; Yamashita, T.; Suzuki, A. Characterisation of the carboxylesterase enzyme cauxin in the seminal fluid of the cat. Vet. J. 2011, 190, 378–382. [Google Scholar] [CrossRef]
- Sampedro, J.G.; Uribe, S. Trehalose-enzyme interactions result in structure stabilization and activity inhibition. The role of viscosity. Mol. Cell Biochem. 2004, 256–257, 319–327. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).