Do Conservative Agricultural Practices Improve the Functional Biological State of Legume-Based Cropping Systems?
Abstract
1. Introduction
2. Materials and Methods
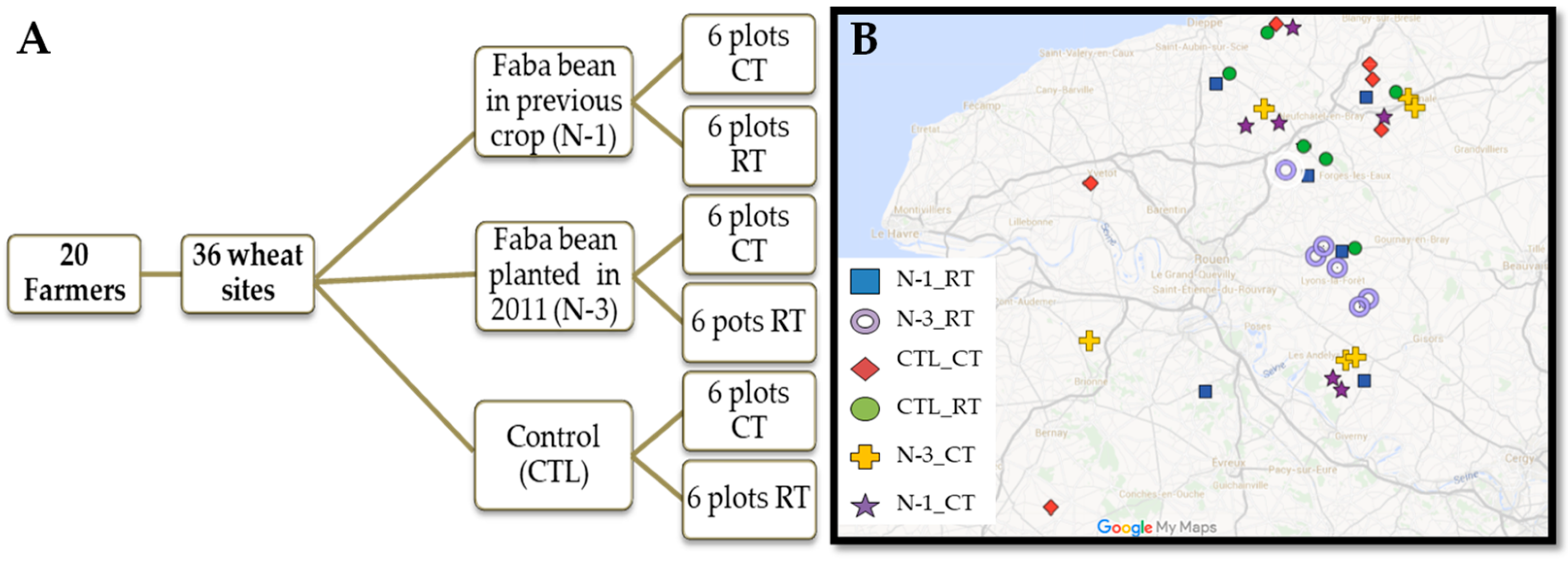
2.1. Field Experimental Design and Soil Sampling
2.2. Farmer Surveys of the Studied Plots
2.3. Chemical and Physical Characterization
2.4. Measurement of Enzyme Activities
2.5. Measurement of Gross N Fluxes
2.6. Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties
3.2. Soil Enzyme Activities
3.3. Nitrogen Fluxes
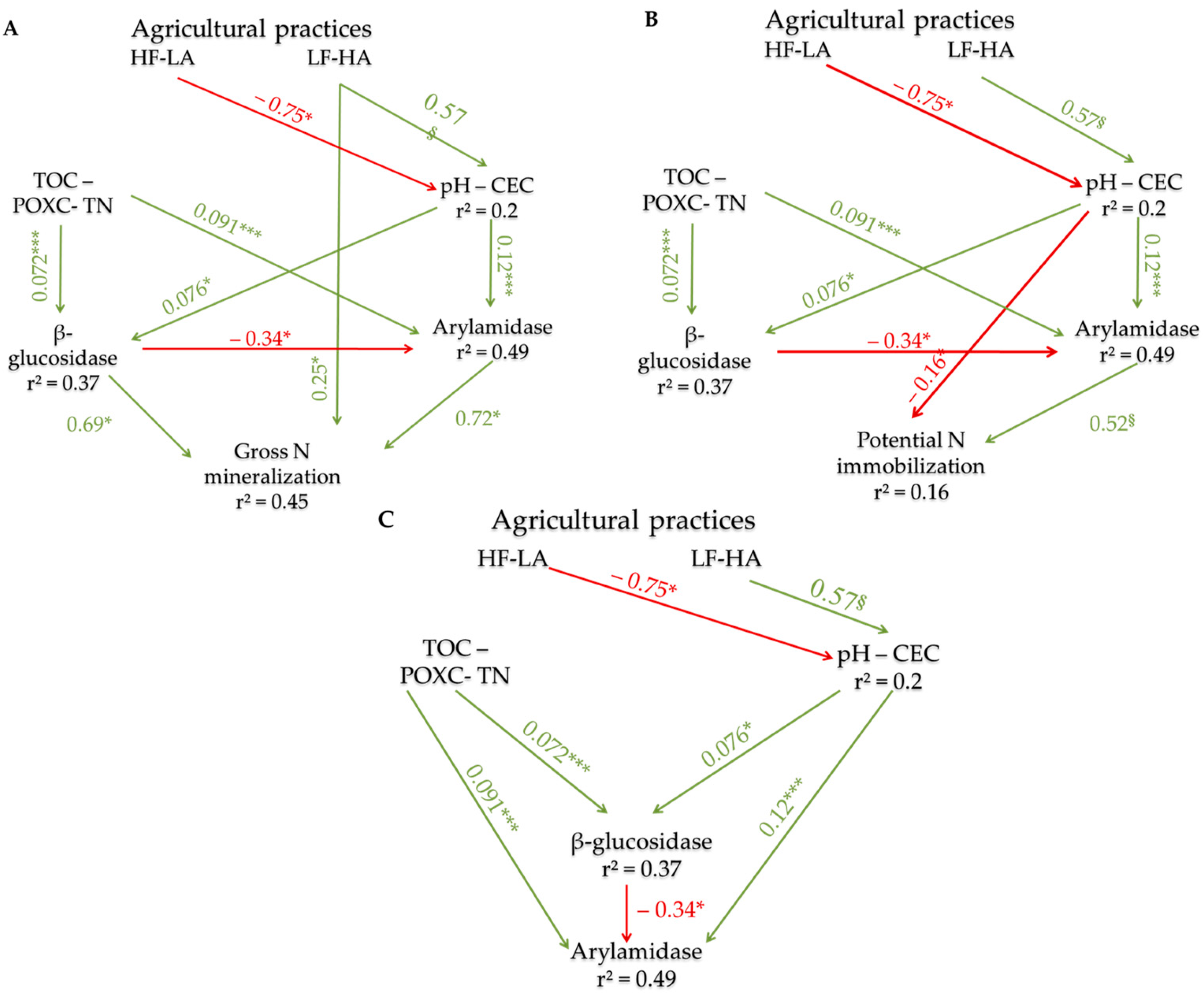
3.4. Direct and Indirect Effects of Agricultural Practices on N Fluxes
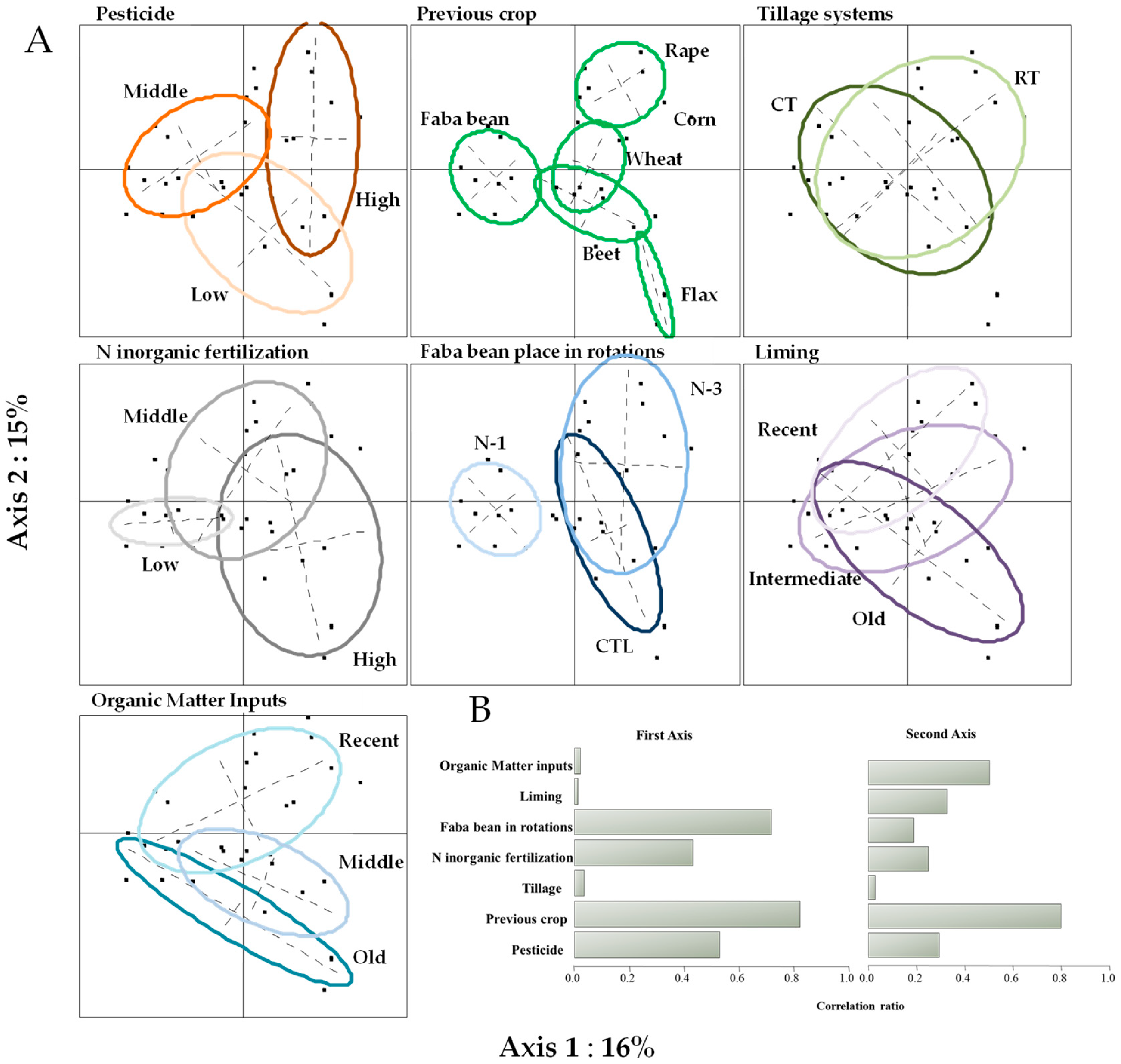
3.4.1. Farmer Survey Data Analysis
3.4.2. The Effects of Farming Practices
4. Discussion
4.1. Disentangling the Effects of Conservative Practices from the Effects of Crop Itineraries
4.2. The Effects of the Conservative Practices
4.3. The Direct and Indirect Effects of Crop Itineraries on Gross N Fluxes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tilman, D. Global Environmental Impacts of Agricultural Expansion: The Need for Sustainable and Efficient Practices. Proc. Natl. Acad. Sci. USA 1999, 96, 5995–6000. [Google Scholar] [CrossRef]
- Wezel, A.; Casagrande, M.; Celette, F.; Vian, J.-F.; Ferrer, A.; Peigné, J. Agroecological Practices for Sustainable Agriculture. A Review. Agron. Sustain. Dev. 2014, 34, 1–20. [Google Scholar] [CrossRef]
- Kremen, C.; Miles, A. Ecosystem Services in Biologically Diversified versus Conventional Farming Systems: Benefits, Externalities, and Trade-Offs. Ecol. Soc. 2012, 17, 40. [Google Scholar] [CrossRef]
- Produire Plus Avec Moins: Guide à L’intention des Décideurs sur L’intensification Durable de L’agriculture Paysane; FAO: Rome, Italy, 2011; ISBN 978-92-5-206871-6.
- Marandola, D.; Belliggiano, A.; Romagnoli, L.; Ievoli, C. The Spread of No-till in Conservation Agriculture Systems in Italy: Indications for Rural Development Policy-Making. Agric. Food Econ. 2019, 7, 7. [Google Scholar] [CrossRef]
- Cordeau, S. Conservation Agriculture and Agroecological Weed Management. Agronomy 2022, 12, 867. [Google Scholar] [CrossRef]
- Jangid, K.; Williams, M.A.; Franzluebbers, A.J.; Sanderlin, J.S.; Reeves, J.H.; Jenkins, M.B.; Endale, D.M.; Coleman, D.C.; Whitman, W.B. Relative Impacts of Land-Use, Management Intensity and Fertilization upon Soil Microbial Community Structure in Agricultural Systems. Soil Biol. Biochem. 2008, 40, 2843–2853. [Google Scholar] [CrossRef]
- Van Capelle, C.; Schrader, S.; Brunotte, J. Tillage-Induced Changes in the Functional Diversity of Soil Biota–A Review with a Focus on German Data. Eur. J. Soil Biol. 2012, 50, 165–181. [Google Scholar] [CrossRef]
- Paré, M.C.; Lafond, J.; Pageau, D. Best Management Practices in Northern Agriculture: A Twelve-Year Rotation and Soil Tillage Study in Saguenay–Lac-Saint-Jean. Soil Tillage Res. 2015, 150, 83–92. [Google Scholar] [CrossRef]
- Kumar, S.; Meena, R.S.; Datta, R.; Verma, S.; Yadav, G.; Pradhan, G.; Molaei, A.; Rahman, G.K.M.M.; Mashuk, H. Legumes for Carbon and Nitrogen Cycling: An Organic Approach. In Carbon and Nitrogen Cycling in Soil; Springer: Berlin/Heidelberg, Germany, 2020; pp. 337–375. ISBN 9789811372636. [Google Scholar]
- Mooshammer, M.; Grandy, A.S.; Calderón, F.; Culman, S.; Deen, B.; Drijber, R.A.; Dunfield, K.; Jin, V.L.; Lehman, R.M.; Osborne, S.L. Microbial Feedbacks on Soil Organic Matter Dynamics Underlying the Legacy Effect of Diversified Cropping Systems. Soil Biol. Biochem. 2022, 167, 108584. [Google Scholar] [CrossRef]
- Gil, S.V.; Meriles, J.; Conforto, C.; Basanta, M.; Radl, V.; Hagn, A.; Schloter, M.; March, G.J. Response of Soil Microbial Communities to Different Management Practices in Surface Soils of a Soybean Agroecosystem in Argentina. Eur. J. Soil Biol. 2011, 47, 55–60. [Google Scholar]
- Lauber, C.L.; Ramirez, K.S.; Aanderud, Z.; Lennon, J.; Fierer, N. Temporal Variability in Soil Microbial Communities across Land-Use Types. ISME J. 2013, 7, 1641–1650. [Google Scholar] [CrossRef]
- Dunn, L.; Lang, C.; Marilleau, N.; Terrat, S.; Biju-Duval, L.; Lelièvre, M.; Perrin, S.; Prévost-Bouré, N.C. Soil Microbial Communities in the Face of Changing Farming Practices: A Case Study in an Agricultural Landscape in France. PLoS ONE 2021, 16, e0252216. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil Enzymes in a Changing Environment: Current Knowledge and Future Directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Gleixner, G. Soil organic matter dynamics: A biological perspective derived from the use of compound-specific isotopes studies. Ecol. Res. 2013, 28, 683–695. [Google Scholar] [CrossRef]
- Riah-Anglet, W.; Trinsoutrot-Gattin, I.; Martin-Laurent, F.; Laroche-Ajzenberg, E.; Norini, M.-P.; Latour, X.; Laval, K. Soil Microbial Community Structure and Function Relationships: A Heat Stress Experiment. Appl. Soil Ecol. 2015, 86, 121–130. [Google Scholar] [CrossRef]
- Babujia, L.C.; Hungria, M.; Franchini, J.C.; Brookes, P.C. Microbial Biomass and Activity at Various Soil Depths in a Brazilian Oxisol after Two Decades of No-Tillage and Conventional Tillage. Soil Biol. Biochem. 2010, 42, 2174–2181. [Google Scholar] [CrossRef]
- Busari, M.A.; Kukal, S.S.; Kaur, A.; Bhatt, R.; Dulazi, A.A. Conservation Tillage Impacts on Soil, Crop and the Environment. Int. Soil Water Conserv. Res. 2015, 3, 119–129. [Google Scholar] [CrossRef]
- Garofalo, P.; Paolo, E.D.; Rinaldi, M.; Garofalo, P.; Paolo, E.D.; Rinaldi, M. Durum Wheat (Triticum Durum Desf.) in Rotation with Faba Bean (Vicia faba Var. Minor L.): Long-Term Simulation Case Study. Crop Pasture Sci. 2009, 60, 240–250. [Google Scholar] [CrossRef]
- Köpke, U.; Nemecek, T. Ecological Services of Faba Bean. Field Crops Res. 2010, 115, 217–233. [Google Scholar] [CrossRef]
- Recous, S.; Lashermes, G.; Bertrand, I.; Duru, M.; Pellerin, S. C-N-P Decoupling Processes Linked to Arable Cropping Management Systems in Relation with Intensification of Production. In Agrosystem Diversity; Agroecosystem Diversity Reconciling Contemporary Agriculture and Environmental Quality; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Li, W.; Li, L.; Sun, J.; Zhang, F.; Christie, P. Effects of Nitrogen and Phosphorus Fertilizers and Intercropping on Uptake of Nitrogen and Phosphorus by Wheat, Maize, and Faba Bean. J. Plant Nutr. 2003, 26, 629–642. [Google Scholar] [CrossRef]
- Schneider, K.D.; Thiessen Martens, J.R.; Zvomuya, F.; Reid, D.K.; Fraser, T.D.; Lynch, D.H.; O’Halloran, I.P.; Wilson, H.F. Options for Improved Phosphorus Cycling and Use in Agriculture at the Field and Regional Scales. J. Environ. Qual. 2019, 48, 1247–1264. [Google Scholar] [CrossRef]
- De Mastro, F.; Traversa, A.; Brunetti, G.; Debiase, G.; Cocozza, C.; Nigro, F. Soil Culturable Microorganisms as Affected by Different Soil Managements in a Two Year Wheat-Faba Bean Rotation. Appl. Soil Ecol. 2020, 149, 103533. [Google Scholar] [CrossRef]
- Wang, G.; Bei, S.; Li, J.; Bao, X.; Zhang, J.; Schultz, P.A.; Li, H.; Li, L.; Zhang, F.; Bever, J.D. Soil Microbial Legacy Drives Crop Diversity Advantage: Linking Ecological Plant–Soil Feedback with Agricultural Intercropping. J. Appl. Ecol. 2021, 58, 496–506. [Google Scholar] [CrossRef]
- Aschi, A.; Aubert, M.; Riah-Anglet, W.; Nélieu, S.; Dubois, C.; Akpa-Vinceslas, M.; Trinsoutrot-Gattin, I. Introduction of Faba Bean in Crop Rotation: Impacts on Soil Chemical and Biological Characteristics. Appl. Soil Ecol. 2017, 120, 219–228. [Google Scholar] [CrossRef]
- Thioye, B.; Legras, M.; Castel, L.; Hirissou, F.; Chaftar, N.; Trinsoutrot-Gattin, I. Understanding Arbuscular Mycorrhizal Colonization in Walnut Plantations: The Contribution of Cover Crops and Soil Microbial Communities. Agriculture 2022, 12, 1. [Google Scholar] [CrossRef]
- Yang, Z.X.; Tang, L.; Zheng, Y.; Dong, Y.; Dong, K. Effects of Different Wheat Cultivars Intercropped with Faba Bean on Faba Bean Fusarium Wilt, Root Exudates and Rhizosphere Microbial Community Functional Diversity. Plant Nutr. Fertil. Sci. 2014, 20, 570–579. [Google Scholar]
- Xu, Y.; Lei, B.; Tang, Y. Effects of Wheat-Faba Bean Intercropping on Soil Microbial Community Structure in the Rhizosphere. Agric. Sci. 2018, 9, 1389–1400. [Google Scholar] [CrossRef]
- McDaniel, M.D.; Tiemann, L.K.; Grandy, A.S. Does Agricultural Crop Diversity Enhance Soil Microbial Biomass and Organic Matter Dynamics? A Meta-Analysis. Ecol. Appl. 2014, 24, 560–570. [Google Scholar] [CrossRef]
- Sun, B.; Jia, S.; Zhang, S.; McLaughlin, N.B.; Zhang, X.; Liang, A.; Chen, X.; Wei, S.; Liu, S. Tillage, Seasonal and Depths Effects on Soil Microbial Properties in Black Soil of Northeast China. Soil Tillage Res. 2016, 155, 421–428. [Google Scholar] [CrossRef]
- Drijber, R.A.; Doran, J.W.; Parkhurst, A.M.; Lyon, D.J. Changes in soil microbial community structure with tillage under long-term wheat-fallow management. soil biology and biochemistry. Soil Biol. Biochem. 2000, 32, 1419–1430. [Google Scholar] [CrossRef]
- Lechenet, M.; Bretagnolle, V.; Bockstaller, C.; Boissinot, F.; Petit, M.-S.; Petit, S.; Munier-Jolain, N.M. Reconciling Pesticide Reduction with Economic and Environmental Sustainability in Arable Farming. PLoS ONE 2014, 9, e97922. [Google Scholar] [CrossRef] [PubMed]
- Culman, S.W.; Snapp, S.S.; Freeman, M.A.; Schipanski, M.E.; Beniston, J.; Lal, R.; Drinkwater, L.E.; Franzluebbers, A.J.; Glover, J.D.; Grandy, A.S.; et al. Permanganate Oxidizable Carbon Reflects a Processed Soil Fraction That Is Sensitive to Management. Soil Sci. Soc. Am. J. 2012, 76, 494–504. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Tabatabai, M.A. Arylamidase Activity of Soils. Soil Sci. Soc. Am. J. 2000, 64, 215–221. [Google Scholar] [CrossRef]
- Trap, J.; Riah, W.; Akpa-Vinceslas, M.; Bailleul, C.; Laval, K.; Trinsoutrot-Gattin, I. Improved Effectiveness and Efficiency in Measuring Soil Enzymes as Universal Soil Quality Indicators Using Microplate Fluorimetry. Soil Biol. Biochem. 2012, 45, 98–101. [Google Scholar] [CrossRef]
- Barraclough, D. The Use of Mean Pool Abundances to Interpret 15N Tracer Experiments. Plant Soil 1991, 131, 89–96. [Google Scholar] [CrossRef]
- Brooks, P.D.; Stark, J.M.; McInteer, B.B.; Preston, T. Diffusion Method to Prepare Soil Extracts for Automated Nitrogen-15 Analysis. Soil Sci. Soc. Am. J. 1989, 53, 1707–1711. [Google Scholar] [CrossRef]
- Anderson, M.J. A New Method for Non-Parametric Multivariate Analysis of Variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A Protocol for Data Exploration to Avoid Common Statistical Problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Roger-Estrade, J.; Anger, C.; Bertrand, M.; Richard, G. Tillage and Soil Ecology: Partners for Sustainable Agriculture. Soil Tillage Res. 2010, 111, 33–40. [Google Scholar] [CrossRef]
- Tsiafouli, M.A.; Thébault, E.; Sgardelis, S.P.; de Ruiter, P.C.; van der Putten, W.H.; Birkhofer, K.; Hemerik, L.; de Vries, F.T.; Bardgett, R.D.; Brady, M.V.; et al. Intensive Agriculture Reduces Soil Biodiversity across Europe. Glob. Change Biol. 2015, 21, 973–985. [Google Scholar] [CrossRef]
- Yang, T.; Siddique, K.H.; Liu, K. Cropping Systems in Agriculture and Their Impact on Soil Health—A Review. Glob. Ecol. Conserv. 2020, 23, e01118. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Kennedy, A.C. Grain Legumes in Northern Great Plains: Impacts on Selected Biological Soil Processes. Agron. J. 2007, 99, 1700–1709. [Google Scholar] [CrossRef]
- Hungria, M.; Franchini, J.C.; Brandao-Junior, O.; Kaschuk, G.; Souza, R.A. Soil Microbial Activity and Crop Sustainability in a Long-Term Experiment with Three Soil-Tillage and Two Crop-Rotation Systems. Appl. Soil Ecol. 2009, 42, 288–296. [Google Scholar] [CrossRef]
- Jensen, E.S.; Peoples, M.B.; Hauggaard-Nielsen, H. Faba Bean in Cropping Systems. Field Crops Res. 2010, 115, 203–216. [Google Scholar] [CrossRef]
- Labreuche, J.; Closset, M. Crop Yields after 41 Years of No Tillage and Minimum Tillage Farming in a Wheat–Corn Cropping System in France. Available online: https://www.researchgate.net/publication/319328807_Crop_yields_after_41_years_of_no_tillage_and_minimum_tillage_farming_in_a_wheat-corn_cropping_system_in_France (accessed on 24 September 2012).
- Lupwayi, N.Z.; Lafond, G.P.; May, W.E.; Holzapfel, C.B.; Lemke, R.L. Intensification of Field Pea Production: Impact on Soil Microbiology. Agron. J. 2012, 104, 1189–1196. [Google Scholar] [CrossRef]
- Stagnari, F.; Maggio, A.; Galieni, A.; Pisante, M. Multiple Benefits of Legumes for Agriculture Sustainability: An Overview. Chem. Biol. Technol. Agric. 2017, 4, 2. [Google Scholar] [CrossRef]
- Seitz, S.; Goebes, P.; Puerta, V.L.; Pereira, E.I.P.; Wittwer, R.; Six, J.; Van Der Heijden, M.G.; Scholten, T. Conservation tillage and organic farming reduce soil erosion. Agron. Sustain. Dev. 2019, 39, 4. [Google Scholar] [CrossRef]
- Veloso, M.G.; Cecagno, D.; Bayer, C. Legume Cover Crops under No-Tillage Favor Organomineral Association in Microaggregates and Soil C Accumulation. Soil Tillage Res. 2019, 190, 139–146. [Google Scholar] [CrossRef]
- Jian, J.; Du, X.; Reiter, M.S.; Stewart, R.D. A Meta-Analysis of Global Cropland Soil Carbon Changes Due to Cover Cropping. Soil Biol. Biochem. 2020, 143, 107735. [Google Scholar] [CrossRef]
- Rezgui, C.; Riah-Anglet, W.; Benoit, M.; Bernard, P.Y.; Laval, K.; Trinsoutrot-Gattin, I. Impacts of the Winter Pea Crop (Instead of Rapeseed) on Soil Microbial Communities, Nitrogen Balance and Wheat Yield. Agriculture 2020, 10, 548. [Google Scholar] [CrossRef]
- Kanté, M.; Riah-Anglet, W.; Cliquet, J.B.; Trinsoutrot-Gattin, I. Soil enzyme activity and stoichiometry: Linking soil microorganism resource requirement and legume carbon rhizodeposition. Agronomy 2021, 11, 2131. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Mikha, M.M.; Vigil, M.F. Microbial Communities and Enzyme Activities in Soils under Alternative Crop Rotations Compared to Wheat–Fallow for the Central Great Plains. Appl. Soil Ecol. 2007, 37, 41–52. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Tabatabai, M. Tillage and Residue Management Effects on Arylamidase Activity in Soils. Biol. Fertil. Soils 2001, 34, 21–24. [Google Scholar]
- Acosta-Martínez, V.; Tabatabai, M.A. Arylamidase Activity in Soils: Effect of Trace Elements and Relationships to Soil Properties and Activities of Amidohydrolases. Soil Biol. Biochem. 2001, 33, 17–23. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, S.; Ma, S.; Zheng, X.; Wang, Z.; Lu, C. Effects of Commonly Used Nitrification Inhibitors—Dicyandiamide (DCD), 3, 4-Dimethylpyrazole Phosphate (DMPP), and Nitrapyrin—On Soil Nitrogen Dynamics and Nitrifiers in Three Typical Paddy Soils. Geoderma 2020, 380, 114637. [Google Scholar] [CrossRef]
- Cui, L.; Li, D.; Wu, Z.; Xue, Y.; Song, Y.; Xiao, F.; Zhang, L.; Gong, P.; Zhang, K. Effects of Nitrification Inhibitors on Nitrogen Dynamics and Ammonia Oxidizers in Three Black Agricultural Soils. Agronomy 2022, 12, 294. [Google Scholar] [CrossRef]
- Karmarkar, S.V.; Tabatabai, M.A. Effects of Biotechnology Byproducts and Organic Acids on Nitrification in Soils. Biol. Fertil. Soils 1991, 12, 165–169. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Tabatabai, M.A. Enzyme Activities in a Limed Agricultural Soil. Biol. Fertil. Soils 2000, 31, 85–91. [Google Scholar] [CrossRef]
- Ajwa, H.A.; Tabatabai, M.A. Decomposition of Different Organic Materials in Soils. Biol. Fertil. Soils 1994, 18, 175–182. [Google Scholar] [CrossRef]
- Alhameid, A.; Singh, J.; Sekaran, U.; Kumar, S.; Singh, S. Soil Biological Health: Influence of Crop Rotational Diversity and Tillage on Soil Microbial Properties. Soil Sci. Soc. Am. J. 2019, 83, 1431–1442. [Google Scholar] [CrossRef]
- Dodor, D.E.; Tabatabai, M.A. Arylamidase Activity as an Index of Nitrogen Mineralization in Soils. Commun. Soil Sci. Plant Anal. 2007, 38, 2197–2207. [Google Scholar] [CrossRef]
- Acosta-Martinez, V.; Mikha, M.M.; Sistani, K.R.; Stahlman, P.W.; Benjamin, J.G.; Vigil, M.F.; Erickson, R. Multi-location study of soil enzyme activities as affected by types and rates of manure application and tillage practices. Agriculture 2011, 1, 4–21. [Google Scholar] [CrossRef]
- Luce, M.S.; Whalen, J.K.; Ziadi, N.; Zebarth, B.J. Nitrogen dynamics and indices to predict soil nitrogen supply in humid temperate soils. Adv. Agron. 2011, 112, 55–102. [Google Scholar]
- Talbot, J.M.; Bruns, T.D.; Taylor, J.W.; Smith, D.P.; Branco, S.; Glassman, S.I.; Erlandson, S.; Vilgalys, R.; Liao, H.-L.; Smith, M.E.; et al. Endemism and Functional Convergence across the North American Soil Mycobiome. Proc. Natl. Acad. Sci. USA 2014, 111, 6341–6346. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, N.B.; Creamer, R.E.; Stone, D.; Winding, A. Soil Exo-Enzyme Activities across Europe—The Influence of Climate, Land-Use and Soil Properties. Appl. Soil Ecol. 2016, 97, 44–48. [Google Scholar] [CrossRef]
- Colbach, N.; Vacher, C. Travail Du Sol et Gestion de La Flore Adventice. In Faut-il Travailler le Sol? Acquis et Innovations Pour une Agriculture Durable; Labreuche, J., Laurent, F., Roger-estrade, J., Eds.; Éditions Quae: Versailles, France, 2014; pp. 113–125. [Google Scholar]
- Preissel, S.; Reckling, M.; Schläfke, N.; Zander, P. Magnitude and Farm-Economic Value of Grain Legume Pre-Crop Benefits in Europe: A Review. Field Crops Res. 2015, 175, 64–79. [Google Scholar] [CrossRef]
- Oliveira, M.; Barre, P.; Trindade, H.; Virto, I. Different Efficiencies of Grain Legumes in Crop Rotations to Improve Soil Aggregation and Organic Carbon in the Short-Term in a Sandy Cambisol. Soil Tillage Res. 2019, 186, 23–35. [Google Scholar] [CrossRef]
- Rodriguez, C.; Carlsson, G.; Englund, J.-E.; Flöhr, A.; Pelzer, E.; Jeuffroy, M.-H.; Makowski, D.; Jensen, E.S. Grain Legume-Cereal Intercropping Enhances the Use of Soil-Derived and Biologically Fixed Nitrogen in Temperate Agroecosystems. A Meta-Analysis. Eur. J. Agron. 2020, 118, 126077. [Google Scholar] [CrossRef]
- Iturri, L.A.; Buschiazzo, D.E.; Díaz-Zorita, M. Acidification Evidences of No-Tilled Soils of the Central Region of Argentina. Cienc. Suelo 2011, 29, 13–19. [Google Scholar]
- Asadi, H.; Raeisvandi, A.; Rabiei, B.; Ghadiri, H. Effect of Land Use and Topography on Soil Properties and Agronomic Productivity on Calcareous Soils of a Semiarid Region, Iran. Land Degrad. Dev. 2012, 23, 496–504. [Google Scholar] [CrossRef]
- Ozgoz, E.; Gunal, H.; Acir, N.; Gokmen, F.; Birol, M.; Budak, M. Soil Quality and Spatial Variability Assessment of Land Use Effects in a Typic Haplustoll. Land Degrad. Dev. 2013, 24, 277–286. [Google Scholar] [CrossRef]
- Iturri, L.A.; Buschiazzo, D.E. Light Acidification in N-Fertilized Loess Soils along a Climosequence Affected Chemical and Mineralogical Properties in the Short-Term. Catena 2016, 139, 92–98. [Google Scholar] [CrossRef]
- Schroder, J.L.; Zhang, H.; Girma, K.; Raun, W.R.; Penn, C.J.; Payton, M.E. Soil Acidification from Long-Term Use of Nitrogen Fertilizers on Winter Wheat. Soil Sci. Soc. Am. J. 2011, 75, 957–964. [Google Scholar] [CrossRef]
- Yan, F.; Schubert, S. Soil PH Changes after Application of Plant Shoot Materials of Faba Bean and Wheat. Plant Soil 2000, 220, 279–287. [Google Scholar] [CrossRef]
- Butterly, C.; Baldock, J.; Tang, C. Chemical Mechanisms of Soil PH Change by Agricultural Residues. In Proceedings of the Dalam: 19th World Congress of Soil Science, Soil Solutions for a Changing World. Brisbane 2010, 4, 43–46. [Google Scholar]
- Tang, C.; Barton, L.; McLay, C.D.A. A Comparison of Proton Excretion of Twelve Pasture Legumes Grown in Nutrient Solution. Aust. J. Exp. Agric. 1997, 37, 563–570. [Google Scholar] [CrossRef]
- Bessho, T.; Bell, L.C. Soil Solid and Solution Phase Changes and Mung Bean Response during Amelioration of Aluminium Toxicity with Organic Matter. Plant Soil 1992, 140, 183–196. [Google Scholar] [CrossRef]
- Vanzolini, J.I.; Galantini, J.A.; Martínez, J.M.; Suñer, L. Changes in Soil PH and Phosphorus Availability during Decomposition of Cover Crop Residues. Arch. Agron. Soil Sci. 2017, 63, 1864–1874. [Google Scholar] [CrossRef]
- Chivenge, P.; Vanlauwe, B.; Gentile, R.; Wangechi, H.; Mugendi, D.; van Kessel, C.; Six, J. Organic and Mineral Input Management to Enhance Crop Productivity in Central Kenya. Agron. J. 2009, 101, 1266–1275. [Google Scholar] [CrossRef]
- Muema, E.K.; Cadisch, G.; Röhl, C.; Vanlauwe, B.; Rasche, F. Response of Ammonia-Oxidizing Bacteria and Archaea to Biochemical Quality of Organic Inputs Combined with Mineral Nitrogen Fertilizer in an Arable Soil. Appl. Soil Ecol. 2015, 95, 128–139. [Google Scholar] [CrossRef]
- Muema, E.K.; Cadisch, G.; Musyoki, M.K.; Rasche, F. Dynamics of Bacterial and Archaeal AmoA Gene Abundance after Additions of Organic Inputs Combined with Mineral Nitrogen to an Agricultural Soil. Nutr. Cycl. Agroecosyst. 2016, 104, 143–158. [Google Scholar] [CrossRef]
- Ekenler, M.; Tabatabai, M.A. Arylamidase and Amidohydrolases in Soils as Affected by Liming and Tillage Systems. Soil Tillage Res. 2004, 77, 157–168. [Google Scholar] [CrossRef]
- Whalen, J.K.; Chang, C.; Clayton, G.W.; Carefoot, J.P. Cattle Manure Amendments Can Increase the PH of Acid Soils. Soil Sci. Soc. Am. J. 2000, 64, 962–966. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, J.; Vogt, R.D.; Mulder, J.; Wang, Y.; Qian, C.; Wang, J.; Zhang, X. Soil Acidification as an Additional Driver to Organic Carbon Accumulation in Major Chinese Croplands. Geoderma 2020, 366, 114234. [Google Scholar] [CrossRef]
- Agegnehu, G.; Nelson, P.N.; Bird, M.I. Crop Yield, Plant Nutrient Uptake and Soil Physicochemical Properties under Organic Soil Amendments and Nitrogen Fertilization on Nitisols. Soil Tillage Res. 2016, 160, 1–13. [Google Scholar] [CrossRef]

| Faba Bean in Rotations | Tillage | pHwater | CEC (cmol+.kg−1 DWsoil) | Moisture (%) | Total N (g.kg−1 DWsoil) | TOC (g.kg−1 DWsoil) | POXC (mg.kg−1 DWsoil) | Gross N Mineralization (mg kg−1 Dry Soil Day) | Potential N Immobilization (mg kg−1 Dry Soil Day) | Potential N Nitrification (mg kg−1dry Soil Day) | ARYL (U) | GLU (U) | CEL (U) | NAG (U) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Permanova | f | p | f | p | f | p | f | p | f | p | f | p | f | p | f | p | f | p | f | p | f | p | f | p | f | p | |
| Tillage | 4.82 | § | 0.15 | ns | 0.15 | ns | 3.61 | * | 7.57 | ** | 4.56 | * | 1.44 | ns | 0.40 | ns | 2.14 | ns | 0.34 | ns | 0.13 | ns | 0.68 | ns | 0.70 | ns | |
| Faba bean in rotations | 0.66 | ns | 1.10 | ns | 0.39 | ns | 0.60 | ns | 0.52 | ns | 1.72 | ns | 1.74 | ns | 0.38 | ns | 0.10 | ns | 3.20 | § | 0.20 | ns | 0.80 | ns | 0.66 | ns | |
| Tillage x faba bean in rotations | 1.68 | ns | 1.51 | ns | 0.67 | ns | 1.99 | ns | 1.81 | ns | 3.87 | * | 0.80 | ns | 1.50 | ns | 2.20 | ns | 2.02 | ns | 2.39 | ns | 0.70 | ns | 0.01 | ns | |
| Tukey test interaction effect | |||||||||||||||||||||||||||
| CTL | RT | 6.99 ± 0.62 | 10.06 ± 1.50 | 20.99 ± 2.08 | 1.54 ± 0.58 | 13.27 ± 4.31 | 525.87 ± 84.11 ab | 2.35 ± 1.97 | 1.73 ± 0.91 | 2.85 ± 1.92 | 0.44 ± 0.26 | 5.48 ± 2.57 | 0.46 ± 0.28 | 1.53 ± 0.49 | |||||||||||||
| CT | 7.05 ± 0.46 | 9.44 ± 1.91 | 19.38 ± 2.62 | 1.19 ± 0.14 | 10.48 ± 1.43 | 450.06 ± 47.86 b | 2.06 ± 0.83 | 2.15 ± 1.08 | 3.48 ± 2.48 | 0.34 ± 0.13 | 4.13 ± 0.93 | 0.38 ± 0.07 | 1.44 ± 0.21 | ||||||||||||||
| N-1 | RT | 6.89 ± 0.66 | 9.94 ± 2.07 | 19.28 ± 2.95 | 1.25 ± 0.54 | 11.64 ± 5.37 | 483.90 ± 73.81 ab | 1.52 ± 0.58 | 2.09 ± 1.04 | 2.23 ± 0.65 | 0.46 ± 0.12 | 4.59 ± 1.45 | 0.65 ± 0.45 | 1.70 ± 0.95 | |||||||||||||
| CT | 7.47 ± 0.43 | 11.87 ± 2.78 | 19.34 ± 1.76 | 1.31 ± 0.39 | 11.09 ± 2.08 | 525.08 ± 116.12 ab | 2.66 ± 1.80 | 1.29 ± 0.48 | 5.45 ± 2.76 | 0.73 ± 0.44 | 4.32 ± 0.73 | 0.44 ± 0.17 | 1.51 ± 0.28 | ||||||||||||||
| N-3 | RT | 6.47 ± 0.94 | 10.77 ± 1.58 | 19.55 ± 3.44 | 1.62 ± 0.27 | 15.52 ± 2.98 | 609.00 ± 55.96 a | 2.66 ± 1.29 | 2.06 ± 0.51 | 3.01 ± 0.94 | 0.63 ± 0.21 | 4.15 ± 0.41 | 0.41 ± 0.07 | 1.41 ± 0.24 | |||||||||||||
| CT | 7.31 ± 0.44 | 10.39 ± 1.31 | 20.16 ± 1.00 | 1.15 ± 0.08 | 9.92 ± 0.92 | 480.03 ± 61.49 ab | 3.64 ± 2.02 | 1.88 ± 0.95 | 5.77 ± 3.18 | 0.60 ± 0.13 | 5.26 ± 1.09 | 0.46 ± 0.23 | 1.28 ± 0.69 | ||||||||||||||
| Tukey test Main effect | |||||||||||||||||||||||||||
| Faba bean in rotations | CTL | 7.02 ± 0.52 | 9.75 ± 1.67 | 20.19 ± 2.41 | 1.37 ± 0.44 | 11.87 ± 3.39 | 487.97 ± 76.32 | 2.20 ± 1.45 | 1.94 ± 0.98 | 4.23 ± 4.16 | 0.39 ± 0.20 B | 4.80 ± 1.97 | 0.42 ± 0.20 | 1.46 ± 0.37 | |||||||||||||
| N-1 | 7.18 ± 0.61 | 10.91 ± 2.55 | 19.32 ± 2.32 | 1.28 ± 0.45 | 11.37 ± 3.89 | 504.49 ± 95.23 | 2.09 ± 1.40 | 1.69 ± 0.87 | 3.84 ± 2.54 | 0.60 ± 0.34 AB | 4.46 ± 1.10 | 0.53 ± 0.28 | 1.61 ± 0.68 | ||||||||||||||
| N-3 | 6.89 ± 0.83 | 10.58 ± 1.40 | 19.86 ± 2.44 | 1.38 ± 0.31 | 12.72 ± 3.60 | 544.51 ± 87.62 | 3.14 ± 1.69 | 1.96 ± 0.73 | 4.39 ± 2.66 | 0.61 ± 0.17 A | 4.71 ± 0.97 | 0.43 ± 0.16 | 1.35 ± 0.50 | ||||||||||||||
| Tillage | CT | 7.26 ± 0.52 A | 10.57 ± 2.22 | 19.63 ± 1.84 | 1.22 ± 0.24 B | 10.49 ± 1.54 B | 485.06 ± 82.21 | 2.79 ± 1.67 | 1.77 ± 0.90 | 4.90 ± 2.85 | 0.56 ± 0.31 | 4.57 ± 1.01 | 0.43 ± 0.16 | 1.40 ± 0.43 | |||||||||||||
| RT | 6.81 ± 0.72 B | 10.26 ± 1.67 | 19.95 ± 2.81 | 1.47 ± 0.48 A | 13.48 ± 4.38 A | 539.59 ± 86.40 | 2.18 ± 1.41 | 1.96 ± 0.81 | 3.41 ± 3.28 | 0.51 ± 0.21 | 4.74 ± 1.71 | 0.49 ± 0.27 | 1.55 ± 0.61 | ||||||||||||||
| TOC | POXC | TN | pH water | CEC | GLU | CEL | NAG | ARYL | Gross N Mineralization | Potential N Immobilization | Potential N Nitrification | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TOC | |||||||||||||||||||||||
| POXC | 0.70 | *** | |||||||||||||||||||||
| TN | 0.96 | *** | 0.74 | *** | |||||||||||||||||||
| pH Water | −0.09 | ns | 0.19 | ns | −0.06 | ns | |||||||||||||||||
| CEC | 0.38 | * | 0.66 | *** | 0.47 | ** | 0.67 | *** | |||||||||||||||
| GLU | 0.24 | ns | 0.35 | * | 0.33 | * | 0.54 | *** | 0.54 | *** | |||||||||||||
| CEL | 0.17 | ns | 0.35 | * | 0.20 | ns | 0.18 | ns | 0.36 | * | 0.38 | * | |||||||||||
| NAG | 0.52 | *** | 0.32 | * | 0.48 | ** | 0.27 | ns | 0.39 | * | 0.46 | ** | 0.29 | ns | |||||||||
| ARYL | 0.18 | ns | 0.58 | *** | 0.27 | ns | 0.39 | * | 0.56 | *** | 0.18 | ns | 0.31 | ns | −0.13 | ns | |||||||
| Gross N Mineralization | 0.29 | ns | 0.47 | ** | 0.40 | * | 0.42 | ** | 0.50 | ** | 0.33 | * | 0.16 | ns | 0.20 | ns | 0.59 | *** | |||||
| Potential N Immobilization | 0.23 | ns | 0.09 | ns | 0.25 | ns | −0.19 | ns | −0.09 | ns | −0.25 | ns | 0.17 | ns | −0.07 | ns | 0.21 | ns | 0.33 | * | |||
| Potential N Nitrification | 0.01 | ns | 0.36 | * | 0.14 | ns | 0.63 | *** | 0.61 | *** | 0.30 | ns | 0.10 | ns | 0.01 | ns | 0.65 | *** | 0.64 | *** | 0.13 | ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aschi, A.; Riah-Anglet, W.; Recous, S.; Bailleul, C.; Aubert, M.; Trinsoutrot-Gattin, I. Do Conservative Agricultural Practices Improve the Functional Biological State of Legume-Based Cropping Systems? Agriculture 2023, 13, 1223. https://doi.org/10.3390/agriculture13061223
Aschi A, Riah-Anglet W, Recous S, Bailleul C, Aubert M, Trinsoutrot-Gattin I. Do Conservative Agricultural Practices Improve the Functional Biological State of Legume-Based Cropping Systems? Agriculture. 2023; 13(6):1223. https://doi.org/10.3390/agriculture13061223
Chicago/Turabian StyleAschi, Amira, Wassila Riah-Anglet, Sylvie Recous, Caroline Bailleul, Michaël Aubert, and Isabelle Trinsoutrot-Gattin. 2023. "Do Conservative Agricultural Practices Improve the Functional Biological State of Legume-Based Cropping Systems?" Agriculture 13, no. 6: 1223. https://doi.org/10.3390/agriculture13061223
APA StyleAschi, A., Riah-Anglet, W., Recous, S., Bailleul, C., Aubert, M., & Trinsoutrot-Gattin, I. (2023). Do Conservative Agricultural Practices Improve the Functional Biological State of Legume-Based Cropping Systems? Agriculture, 13(6), 1223. https://doi.org/10.3390/agriculture13061223






