Promising Strains of Hydrocarbon-Oxidizing Pseudomonads with Herbicide Resistance and Plant Growth-Stimulating Properties for Bioremediation of Oil-Contaminated Agricultural Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Hydrocarbon-Degrading Strains
2.2. Identification of the Isolates
2.3. Hydrocarbon-Oxidizing Activity of Strains
2.4. Resistance of Strains to Herbicides
2.5. Resistance of Strains to Heavy Metals
2.6. Production of Hydrolytic Enzymes of Strains
2.7. PGP Properties of Strains
2.7.1. Phosphate Solubilization
2.7.2. Nitrogen Fixation
2.7.3. IAA Production
2.8. Growth Stimulating Activity
2.8.1. The Influence of Strains on the Growth and Development of Plants in Oil-Contaminated Soil
2.8.2. Influence of Strains on Plant Growth and Development under Herbicide Contamination
2.9. Influence of Strains and Plants on the Content of Petroleum Hydrocarbons in the Soil
2.10. Statistical Analysis
3. Results
3.1. Isolation and Identification of Strains
3.2. Hydrocarbon-Oxidizing Activity of Strains
3.3. Resistance of Strains to Herbicides
3.4. Resistance of Strains to Heavy Metals
3.5. Production of Hydrolytic Enzymes
3.6. PGP Properties of Strains
3.6.1. Phosphate Mobilization
3.6.2. Nitrogen-Fixing Ability
3.6.3. IAA Products
3.7. Growth-Stimulating Activity
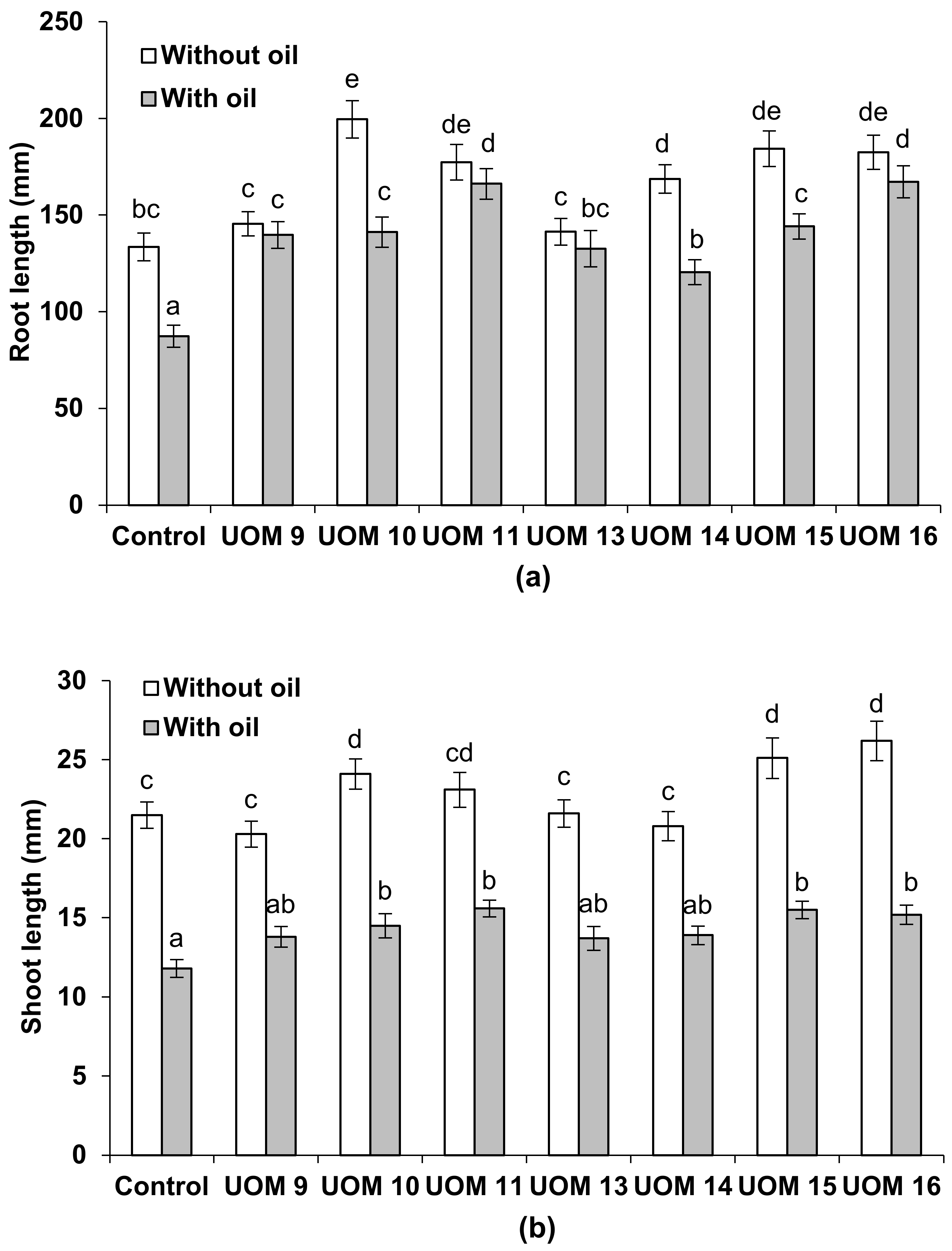
3.7.1. Influence of Bacterization on the Growth and Development of Plants in Oil-Contaminated Soil
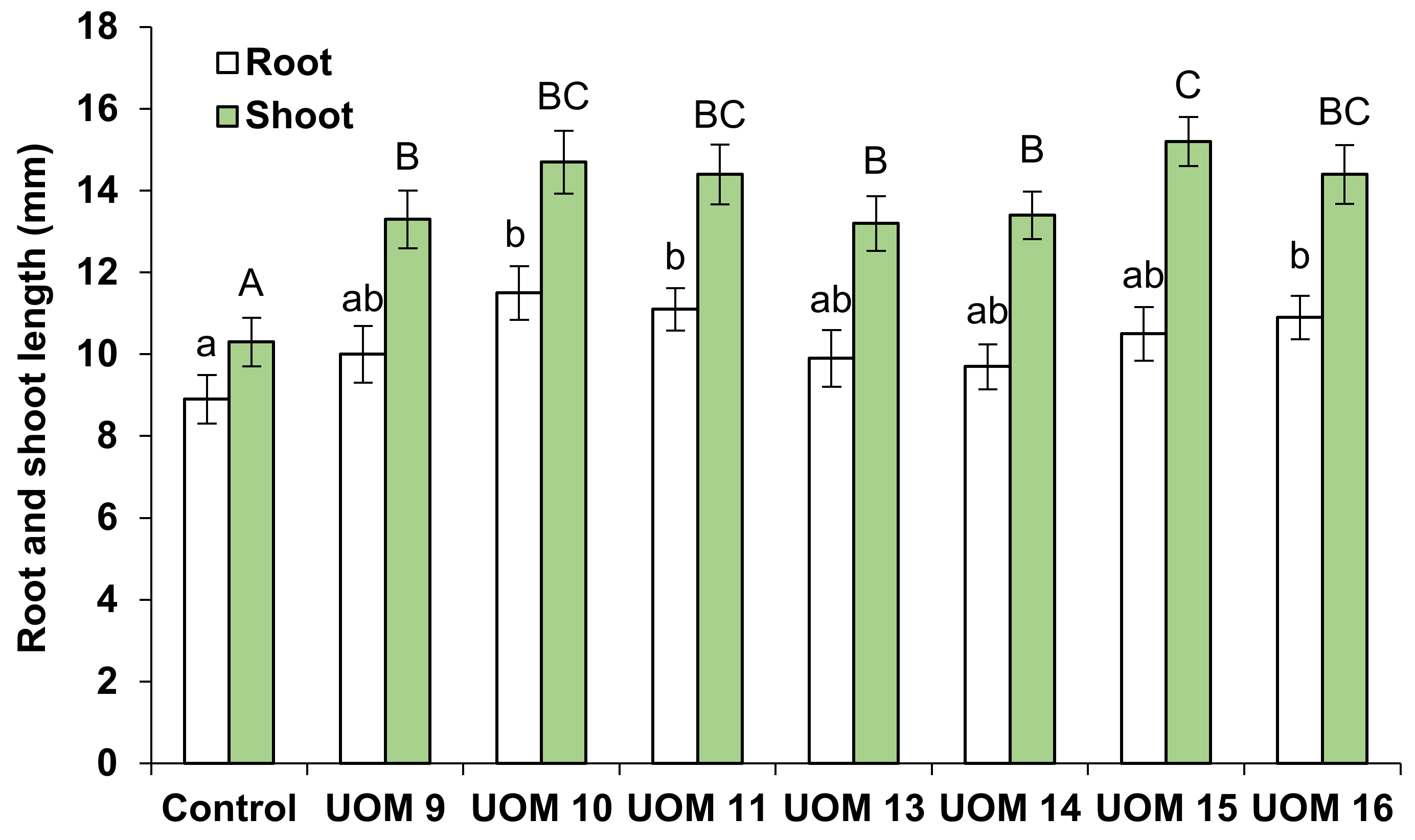
3.7.2. Influence of Strains on Plant Growth and Development under Herbicide Contamination
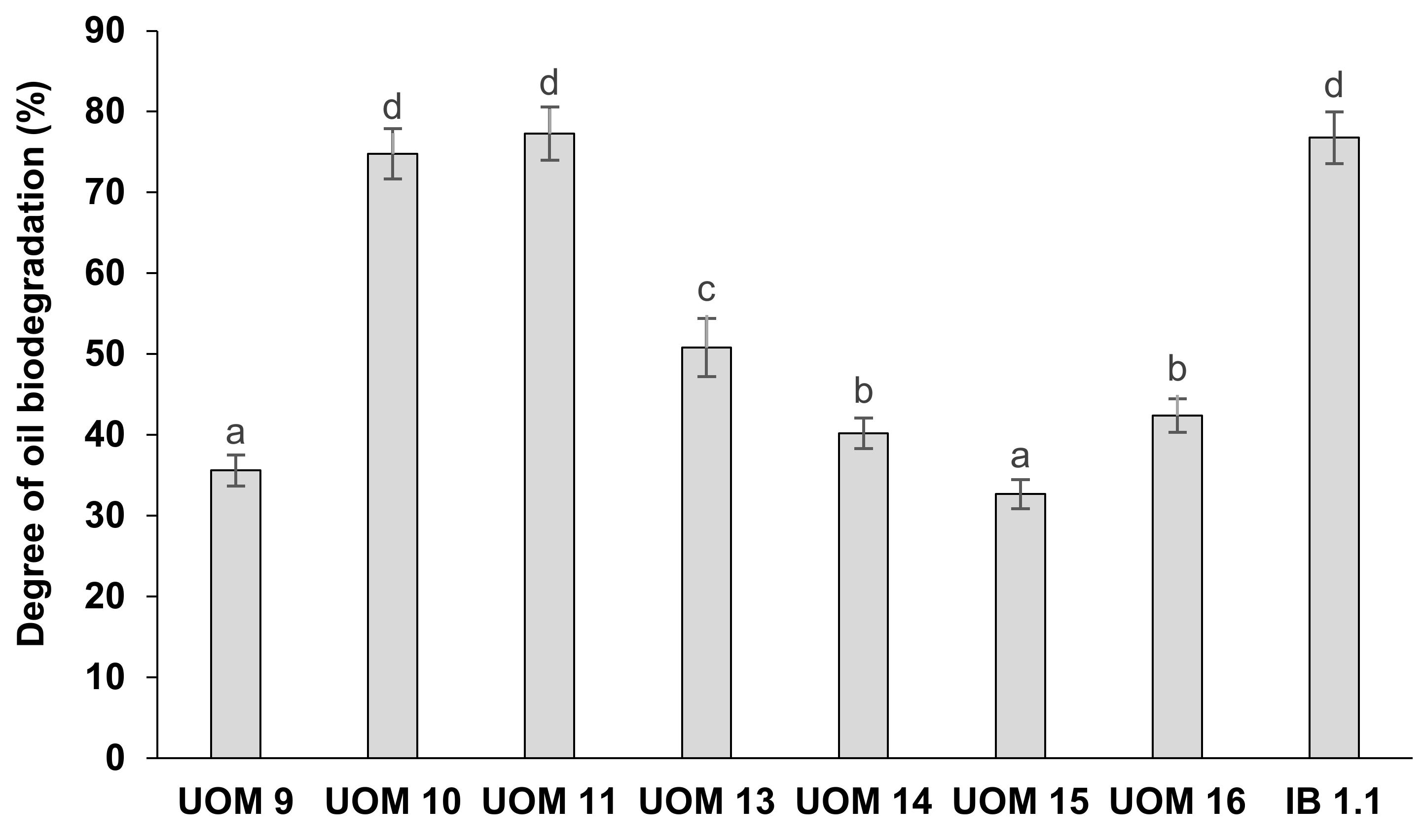
3.8. Biodegradation of Hydrocarbons in the Soil
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Polyak, Y.M.; Bakina, L.G.; Chugunova, M.V.; Mayachkina, N.V.; Gerasimov, A.O.; Bure, V.M. Effect of remediation strategies on biological activity of oil-contaminated soil—A field study. Int. Biodeterior. Biodegrad. 2018, 126, 57–68. [Google Scholar] [CrossRef]
- Nardeli, S.M.; Saad, C.F.; de Barros Rossetto, P.; Caetano, V.S.; Ribeiro-Alves, M.; Paes, J.E.S.; Danielowski, R.; da Maia, L.S.; Costa de Oliveira, A.; Peixoto, R.S.; et al. Transcriptional responses of Arabidopsis thaliana to oil contamination. Environ. Exp. Bot. 2016, 127, 63–72. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Kucharski, J. The resistance of Lolium perenne L.× hybridum, Poa pratensis, Festuca rubra, F. arundinacea, Phleum pratense and Dactylis glomerata to soil pollution by diesel oil and petroleum. Plant. Soil Environ. 2019, 65, 307–312. [Google Scholar] [CrossRef]
- Sui, X.; Wang, X.; Li, Y.; Ji, H. Remediation of petroleum-contaminated soils with microbial and microbial combined methods: Advances, mechanisms, and challenges. Sustainability 2021, 13, 9267. [Google Scholar] [CrossRef]
- Guo, M.; Gong, Z.; Allinson, G.; Tai, P.; Miao, R.; Li, X.; Jia, C.; Zhuang, J. Variations in the bioavailability of polycyclic aromatic hydrocarbons in industrial and agricultural soils after bioremediation. Chemosphere 2016, 144, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Martínez, B.C.S.; Benavides, L.M.; Santoyo, G.; Sánchez-Yáñez, J.M. Biorecovery of agricultural soil impacted by waste motor oil with Phaseolus vulgaris and Xanthobacter autotrophicus. Plants 2022, 11, 1419. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K. Herbicides and fungicides. In Reproductive and Developmental Toxicology; Academic Press: Cambridge, MA, USA, 2017; pp. 657–679. [Google Scholar]
- Moretto, J.A.S.; Altarugio, L.M.; Andrade, P.A.; Fachin, A.L.; Andreote, F.D.; Stehling, E.G. Changes in bacterial community after application of three different herbicides. FEMS Microbiol. Lett. 2017, 364, 13. [Google Scholar] [CrossRef]
- Zaller, J.G.; Cantelmo, C.; Santos, G.D.; Muther, S.; Gruber, E.; Pallua, P.; Mandl, K.; Friedrich, B.; Hofstetter, I.; Schmuckenschlager, B.; et al. Herbicides in vineyards reduce grapevine root mycorrhization and alter soil microorganisms and the nutrient composition in grapevine roots, leaves, xylem sap and grape juice. Environ. Sci. Pollut. Res. 2018, 25, 23215–23226. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.S.; Kumar, S.; Datta, R.; Lal, R.; Vijayakumar, V.; Brtnicky, M.; Sharma, M.P.; Yadav, G.S.; Jhariya, M.K.; Jangir, C.K.; et al. Impact of Agrochemicals on Soil Microbiota and Management: A Review. Land 2020, 9, 34. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Rousk, J.; Bååth, E.; Bollmann, U.E.; Bester, K.; Brandt, K.K. Short-term toxicity assessment of a triazine herbicide (terbutryn) underestimates the sensitivity of soil microorganisms. Soil Biol. Biochem. 2021, 154, 108130. [Google Scholar] [CrossRef]
- Choudhury, P.P. Transformation of herbicides in the environment. In Herbicide Residue Research in India; Springer: Singapore, 2019; pp. 415–442. [Google Scholar]
- Mahapatra, K.; De, S.; Banerjee, S.; Roy, S. Pesticide mediated oxidative stress induces genotoxicity and disrupts chromatin structure in fenugreek (Trigonella foenum—Graecum, L.) seedlings. J. Hazard. Mater. 2019, 369, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Renoux, A.Y.; Zajdlik, B.; Stephenson, G.L.; Moulins, L.J. Risk-based management of site soils contaminated with a mixture of hazardous substances: Methodological approach and case study. Hum. Ecol. Risk Assess. 2013, 19, 1127–1146. [Google Scholar] [CrossRef]
- Ye, S.; Zeng, G.; Wu, H.; Zhang, C.; Liang, J.; Dai, J.; Liu, Z.; Xiong, W.; Wan, J.; Xu, P.; et al. Co-occurrence and interactions of pollutants, and their impacts on soil remediation—A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1528–1553. [Google Scholar] [CrossRef]
- Sun, S.; Sidhu, V.; Rong, Y.; Zheng, Y. Pesticide pollution in agricultural soils and sustainable remediation methods: A review. Curr. Pollut. Rep. 2018, 4, 240–250. [Google Scholar] [CrossRef]
- Pileggi, M.; Pileggi, S.A.V.; Sadowsky, M.J. Herbicide bioremediation: From strains to bacterial communities. Heliyon 2020, 6, e05767. [Google Scholar] [CrossRef] [PubMed]
- Adedeji, J.A.; Tetteh, E.K.; Opoku Amankwa, M.; Asante-Sackey, D.; Ofori-Frimpong, S.; Armah, E.K.; Rathilal, S.; Mohammadi, A.H.; Chetty, M. Microbial bioremediation and biodegradation of petroleum products—A mini review. Appl. Sci. 2022, 12, 12212. [Google Scholar] [CrossRef]
- Michael-Igolima, U.; Abbey, S.J.; Ifelebuegu, A.O. A systematic review on the effectiveness of remediation methods for oil contaminated soils. Environ. Adv. 2022, 9, 100319. [Google Scholar] [CrossRef]
- Muter, O. Current trends in bioaugmentation tools for bioremediation: A critical review of advances and knowledge gaps. Microorganisms 2023, 11, 710. [Google Scholar] [CrossRef] [PubMed]
- Islas-García, A.; Vega-Loyo, L.; Aguilar-López, R.; Xoconostle-Cázares, B.; Rodríguez-Vázquez, R. Evaluation of hydrocarbons and organochlorine pesticides and their tolerant microorganisms from an agricultural soil to define its bioremediation feasibility. J. Environ. Sci. Health B. 2015, 50, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of soil and water contaminated with petroleum hydrocarbon: A review. Environ. Technol. Innov. 2020, 17, 100526. [Google Scholar] [CrossRef]
- Mambwe, M.; Kalebaila, K.K.; Johnson, T. Remediation technologies for oil contaminated soil. Glob. J. Environ. Sci. Manag. 2021, 7, 419–438. [Google Scholar] [CrossRef]
- Salari, M.; Rahmanian, V.; Hashemi, S.A.; Chiang, W.-H.; Lai, C.W.; Mousavi, S.M.; Gholami, A. Bioremediation treatment of polyaromatic hydrocarbons for environmental sustainability. Water 2022, 14, 3980. [Google Scholar] [CrossRef]
- Stepanova, A.Y.; Gladkov, E.A.; Osipova, E.S.; Gladkova, O.V.; Tereshonok, D.V. Bioremediation of soil from petroleum contamination. Processes 2022, 10, 1224. [Google Scholar] [CrossRef]
- Allamin, I.A.; Halmi, M.I.E.; Yasid, N.A.; Ahmad, S.A.; Abdullah, S.R.S.; Shukor, Y. Rhizodegradation of petroleum oily sludgecontaminated soil using Cajanus cajan increases the diversity of soil microbial community. Sci. Rep. 2020, 10, 4094. [Google Scholar] [CrossRef]
- Ancona, V.; Rascio, I.; Aimola, G.; Campanale, C.; Grenni, P.; di Lenola, M.; Garbini, G.L.; Uricchio, V.F.; Caracciolo, A.B. Poplar-assisted bioremediation for recovering a PCB and heavy-metal-contaminated area. Agriculture 2021, 11, 689. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, F.; Dodd, I.C.; Belimov, A.A. Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase growth and photosynthesis of pea plants under salt stress by limiting Na+ accumulation. Funct. Plant Biol. 2016, 43, 161–172. [Google Scholar] [CrossRef]
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I.C. Phytohormone mediation of interactions between plants and non-symbiotic growth promoting bacteria under edaphic stresses. Front. Plant Sci. 2019, 10, 1368. [Google Scholar] [CrossRef]
- Kuzina, E.; Rafikova, G.; Vysotskaya, L.; Arkhipova, T.; Bakaeva, M.; Chetverikova, D.; Kudoyarova, G.; Korshunova, T.; Chetverikov, S. Influence of hydrocarbon-oxidizing bacteria on the growth, biochemical characteristics, and hormonal status of barley plants and the content of petroleum hydrocarbons in the soil. Plants 2021, 10, 1745. [Google Scholar] [CrossRef]
- Ebadi, A.; Khoshkholgh Sima, N.A.; Olamaee, M.; Hashemi, M.; Ghorbani Nasrabadi, R. Remediation of saline soils contaminated with crude oil using the halophyte Salicornia persica in conjunction with hydrocarbon-degrading bacteria. J. Environ. Manag. 2018, 219, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Korshunova, T.Y.; Bakaeva, M.D.; Kuzina, E.V.; Rafikova, G.F.; Chetverikov, S.P.; Chetverikova, D.V.; Loginov, O.N. Role of bacteria of the genus Pseudomonas in the sustainable development of agricultural systems and environmental protection (review). Appl. Biochem. Microbiol. 2021, 3, 281–296. [Google Scholar] [CrossRef]
- Pandey, P.; Pathak, H.; Dave, S. Microbial ecology of hydrocarbon degradation in the soil: A review. Res. J. Environ. Toxicol. 2016, 10, 1–15. [Google Scholar] [CrossRef]
- Koshlaf, E.; Ball, A.S. Soil bioremediation approaches for petroleum hydrocarbon polluted environments. AIMS Microbiol. 2017, 3, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Pathak, H. Pseudomonas in biodegradation. Int. J. Pure App. Biosci. 2014, 2, 213–222. [Google Scholar]
- Kahlon, R.S. Biodegradation and bioremediation of organic chemical pollutants by Pseudomonas. In Pseudomonas: Molecular and Applied Biology; Springer: Cham, Switzerland, 2016; pp. 343–417. [Google Scholar]
- Esikova, T.Z.; Anokhina, T.O.; Suzina, N.E.; Shushkova, T.V.; Wu, Y.; Solyanikova, I.P. Characterization of a new Pseudomonas putida s Ch2, a degrader of toxic anthropogenic compounds epsilon-caprolactam and glyphosate. Microorganisms 2023, 11, 650. [Google Scholar] [CrossRef]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional biomolecules of the 21st century. Int. J. Mol. Sci. 2016, 3, 401. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.J.; Silva, I.A.; Brasileiro, P.P.; Correa, P.F.; Almeida, D.G.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Treatment of oily effluent using a low-cost biosurfactant in a flotation system. Biodegradation 2019, 30, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Travaglia, C.; Masciarelli, O.; Fortuna, J.; Marchetti, G.; Cardozo, P.; Lucero, M.; Zorza, E.; Luna, V.; Reinoso, H. Towards sustainable maize production: Glyphosate detoxification by Azospirillum sp. and Pseudomonas sp. Crop Prot. 2015, 77, 102–109. [Google Scholar] [CrossRef]
- Raheem, A.; Shaposhnikov, A.; Belimov, A.A.; Dodd, A.C.; Ali, B. Auxin production by rhizobacteria was associated with improved yield of wheat (Triticum aestivum L.) under drought stress. Arch. Agron. Soil Sci. 2017, 64, 574–587. [Google Scholar] [CrossRef]
- Kumar, P.; Thakur, S.; Dhingra, G.K.; Singh, A.; Pal, M.K.; Harshvardhan, K.; Dubey, R.C.; Maheshwari, D.K. Inoculation of siderophore producing rhizobacteria and their consortium for growth enhancement of wheat plant. Biocat. Agricul. Biotechnol. 2018, 15, 264–269. [Google Scholar] [CrossRef]
- Arkhipova, T.; Sharipova, G.; Akhiyarova, G.; Kuzmina, L.; Galin, I.; Martynenko, E.; Seldimirova, O.; Nuzhnaya, T.; Feoktistova, A.; Timergalin, M.; et al. The effects of rhizosphere inoculation with Pseudomonas mandelii on formation of apoplast barriers, HVPIP2 aquaporins and hydraulic conductance of barley. Microorganisms 2022, 10, 935. [Google Scholar] [CrossRef]
- Kaskatepe, B.; Yildiz, S.; Gumustas, M.; Ozkan, S. Rhamnolipid production by Pseudomonas putida IBS036 and Pseudomonas pachastrellae LOS20 with using pulps. Curr. Pharm. Anal. 2017, 13, 138–144. [Google Scholar] [CrossRef]
- Hassen, W.; Neifar, M.; Cherif, H.; Najjari, A.; Chouchane, H.; Driouich, R.C.; Salah, A.; Naili, F.; Mosbah, A.; Souissi, Y.; et al. Pseudomonas rhizophila S211, a new plant growth-promoting rhizobacterium with potential in pesticide-bioremediation. Front. Microbiol. 2018, 9, 34. [Google Scholar] [CrossRef]
- Weimer, A.; Kohlstedt, M.; Volke, D.C.; Nikel, P.I.; Wittmann, C. Industrial biotechnology of Pseudomonas putida: Advances and prospects. Appl. Microbiol. Biotechnol. 2020, 104, 7745–7766. [Google Scholar] [CrossRef] [PubMed]
- Kanavaki, I.; Drakonaki, A.; Geladas, E.D.; Spyros, A.; Xie, H.; Tsiotis, G. Polyhydroxyalkanoate (PHA) production in Pseudomonas sp. phDV1 strain grown on phenol as carbon sources. Microorganisms 2021, 9, 1636. [Google Scholar] [CrossRef]
- Benincasa, M.; Accorsini, F.R. Pseudomonas aeruginosa LBI production as an integrated process using the wastes from sunflower-oil refining as a substrate. Biores. Technol. 2008, 99, 3843–3849. [Google Scholar] [CrossRef] [PubMed]
- Gamal, R.F.; Abdelhady, H.M.; Khodair, T.A.; El-Tayeb, T.S.; Hassan, E.A.; Aboutaleb, K.A. Semi-scale production of PHAs from waste frying oil by Pseudomonas fluorescens S48. Braz. J. Microbiol. 2013, 44, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Li, K.-T.; Peng, W.-F.; Zhou, J.; Wei, S.-J.; Cheng, X. Establishment of beet molasses as the fermentation substrate for industrial vitamin B12 production by Pseudomonas denitrificans. J. Chem. Technol. Biotechnol. 2013, 88, 1730–1735. [Google Scholar] [CrossRef]
- Netrusov, A.I.; Egorova, M.A.; Zakharchuk, L.M.; Dinarieva, T.Y. Workshop on Microbiology; Academy: Moscow, Russia, 2005; 608p. [Google Scholar]
- Raymond, R.L. Microbial oxidation of n-paraffinic hydrocarbons. Dev. Ind. Microbiol. 1961, 2, 23–32. [Google Scholar]
- Gerhardt, P. Manual of Methods for General Bacteriology; American Society of Microbiology: Washington, DC, USA, 1981; 524p. [Google Scholar]
- Holt, S.G.; Krieg, N.R.; Sneath, P.H.A.; Stanley, J.T.; Williams, S.T. Bergey’s Manual of Determinate Bacteriology; Williams and Wilkins: New York, NY, USA, 1998. [Google Scholar]
- Korshunova, T.Y.; Mukhamatdyarova, S.R.; Loginov, O.N. Taxonomic classification of the oil destructing bacterium using mass spectrometry methods by the results of analysis of cellular proteins and study of cellular fatty acids. Biol. Bull. 2015, 3, 220–225. [Google Scholar] [CrossRef]
- Wilson, K. Preparation of genomic DNA from bacteria. Curr. Protoc. Mol. Biol. 1997, 2, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematic; John Wiley and Sons: Chichester, UK, 1991; pp. 115–177. [Google Scholar]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Jukes, T.H.; Cantor, C.R. Evolution of Protein Molecules. In Mammalian Protein Metabolism; Academic Press: New York, NY, USA, 1969; pp. 21–132. [Google Scholar]
- Borzenkov, I.A.; Milekhina, E.I.; Gotoeva, M.T.; Rozanova, E.P.; Belyaev, S.S. The properties of hydrocarbon-oxidizing bacteria isolated from the oilfields of Tatarstan, Western Siberia, and Vietnam. Mikrobiologiya 2006, 1, 66–72. [Google Scholar] [CrossRef]
- Korshunova, T.Y.; Chetverikov, S.P.; Valiullin, E.G.; Loginov, O.N. Biotechnological potential of the bacterium Pseudomonas sp. IB-1.1 as bases of the multifunctional biological product. Proceed. Univ. Appl. Chem. Biotechnol. 2016, 1, 93–99. [Google Scholar]
- Rodea-Palomares, I.; Makowski, M.; Gonzalo, S.; González-Pleiter, M.; Leganés, F.; Fernández-Piñas, F. Effect of PFOA/PFOS pre-exposure on the toxicity of the herbicides 2,4-D, Atrazine, Diuron and Paraquat to a model aquatic photosynthetic microorganism. Chemosphere 2015, 139, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Pikovskaya, R.I. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 1948, 17, 362–370. [Google Scholar]
- Berraquero, F.R.; Baya, A.M.; Cormenzana, A.R. Establecimiento de índices para el estudio de la solubilización de fosfatos por bacterias del suelo. Ars Pharm. (Internet) 1976, 17, 399–406. [Google Scholar]
- Lisboa, P.H.G.; de Andrade, P.H.M.; Machado, P.C.; de Sousa, C.P.; Lacava, P.T. Isolation and in vitro screening of plant growth-promoting rhizobacteria from Solanum lycocarpum St. Hil., an endemic plant of the Brazilian tropical savannah. Afr. J. Microbiol. Res. 2021, 5, 253–261. [Google Scholar] [CrossRef]
- Starikov, S.N.; Chetverikov, S.P. The strain of Enterobacter sp. UOM-3 is capable of synchronous destruction of herbicides and synthesis of indole-3-acetic acid. Ecobiotech 2020, 4, 716–721. [Google Scholar] [CrossRef]
- Ertekin, O.; Erol, C.; Unlu, S.; Yildizhan, Y.; Pelitli, V.; Yuksel, B.; Memon, A. Aliphatic hydrocarbon fingerprints in Trifolium spp. Fresenius Environ. Bull. 2011, 20, 367–371. [Google Scholar]
- Karličić, V.M.; Radić, D.S.; Jovičić-Petrović, J.P.; Raičević, V.B. Red clover and plant growth promoting bacteria: The combination that can speed up soil remediation rate. J. Agric. Sci. 2020, 2. Available online: https://www.aseestant.ceon.rs/index.php/jas/issue/view/1002 (accessed on 1 April 2023).
- Sawicka, B.; Vambol, V.; Krochmal-Marczak, B.; Messaoudi, M.; Skiba, D.; Pszczółkowski, P.; Barbaś, P.; Farhan, A.K. Green technology as a way of cleaning the environment from petroleum substances in south-eastern Poland. Front. Biosci. 2022, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.W.; Lau, E.V.; Poh, P.E. A comprehensive guide of remediation technologies for oil contaminated soil—Present works and future directions. Mar. Poll. Bull. 2016, 109, 14–45. [Google Scholar] [CrossRef]
- Wasi, S.; Tabrez, S.; Ahmad, M. Use of Pseudomonas spp. for the bioremediation of environmental pollutants: A review. Environ. Monit. Assess. 2013, 185, 8147–8155. [Google Scholar] [CrossRef] [PubMed]
- Kaczorek, E.; Moszynska, S.; Olszanowski, A. Modification of cell surface properties of Pseudomonas alcaligenes S22 during hydrocarbon biodegradation. Biodegradation 2011, 22, 359–366. [Google Scholar] [CrossRef]
- Doszhanov, Y.O.; Mansurov, Z.A.; Ongarbaev, Y.K.; Tileuberdi, Y.; Zhubanova, A.A. The study of biodegradation of diesel fuels by different strains of Pseudomonas. Appl. Mech. Mater. 2013, 467, 12–15. [Google Scholar] [CrossRef]
- Gofar, N. Synergism of wild grass and hydrocarbonoclastic bacteria in petroleum biodegradation. J. Trop. Soils. 2013, 2, 161–168. [Google Scholar] [CrossRef]
- Adeleye, A.O.; Nkereuwem, M.E.; Omokhudu, G.I.; Amoo, A.O.; Shiaka, G.P.; Yerima, M.B. Effect of microorganisms in the bioremediation of spent engine oil and petroleum related environmental pollution. J. Appl. Sci. Environ. Manag. 2018, 2, 157–167. [Google Scholar] [CrossRef]
- Wang, R.; Wu, B.; Zheng, J.; Chen, H.; Rao, P.; Yan, L.; Chai, F. Biodegradation of total petroleum hydrocarbons in soil: Isolation and characterization of bacterial strains from oil contaminated soil. Appl. Sci. 2020, 10, 4173. [Google Scholar] [CrossRef]
- Ruiz, O.N.; Radwan, O.; Striebich, R.C. GC-MS hydrocarbon degradation profile data of Pseudomonas frederiksbergensis SI8, a bacterium capable of degrading aromatics at low temperatures. Data Brief. 2021, 35, 106864. [Google Scholar] [CrossRef]
- Kadri, N.; Magdouli, S.; Rouissi, T.; Brar, S.K. Ex-situ biodegradation of petroleum hydrocarbons using Alcanivorax borkumensis enzymes. Biochem. Engin. J. 2018, 132, 279–287. [Google Scholar] [CrossRef]
- Bamitale, O.M.; Ayomikun, A.M. Biodegradation potential of tropical hydrocarbon degrading Providencia stuartii. Trends Appl. Sci. Res. 2020, 15, 253–259. [Google Scholar] [CrossRef]
- Margesin, R.; Hämmerle, M.; Tscherko, D. Microbial activity and community composition during bioremediation of diesel-oil-contaminated soil: Effects of hydrocarbon concentration, fertilizers, and incubation time. Microb. Ecol. 2007, 2, 259–269. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, R.; Zhou, Y.; Li, N.; Hou, L.; Ma, Q.; Gao, B. Fire Phoenix facilitates phytoremediation of PAH-Cd co-contaminated soil through promotion of beneficial rhizosphere bacterial communities. Environ. Int. 2020, 136, 105421. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Sagasti, M.T.; Alkorta, I.; Becerril, J.M.; Epelde, L.; Anza, M.; Garbisu, C. Microbial monitoring of the recovery of soil quality during heavy metal phytoremediation. Water Air Soil Pollut. 2012, 6, 3249–3262. [Google Scholar] [CrossRef]
- Adesina, G.; Adelasoye, K. Effect of crude oil pollution on heavy metal contents, microbial population in soil, and maize and cowpea growth. Agric. Sci. 2014, 5, 43–50. [Google Scholar] [CrossRef]
- Masindi, V.; Muedi, K.L. Environmental contamination by heavy metals. In Heavy Metals; IntechOpen: London, UK, 2018; pp. 115–132. [Google Scholar]
- Li, Q.; Liu, J.; Gadd, G.M. Fungal bioremediation of soil co-contaminated with petroleum hydrocarbons and toxic metals. Appl. Microbiol. Biotechnol. 2020, 104, 8999–9008. [Google Scholar] [CrossRef]
- Etesami, H. Bacterial mediated alleviation of heavy metal stress and decreased accumulation of metals in plant tissues: Mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 147, 175–191. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Nan, Z.; Zang, F.; Sun, H.; Zhang, Q.; Huang, W.; Bao, L. Accumulation, fractionation and health risk assessment of fluoride and heavy metals in soil-crop systems in northwest China. Sci. Total Environ. 2019, 663, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, Ö.; Uğur, A. Bio-monitoring of heavy metal resistance in Pseudomonas and Pseudomonas related genus. J. Biol. Environ. Sci. 2012, 18, 233–242. [Google Scholar]
- Zhang, J.-K.; Wang, Z.-H.; Ye, Y. Heavy metal resistances and chromium removal of a novel cr(vi)-reducing pseudomonad strain isolated from circulating cooling water of iron and steel plant. Appl. Biochem. Biotechnol. 2016, 180, 1328–1344. [Google Scholar] [CrossRef]
- Bishnoi, U. Chapter four-PGPR interaction: An ecofriendly approach promoting the sustainable agriculture system. Adv. Bot. Res. 2015, 75, 81–113. [Google Scholar] [CrossRef]
- Mikala, P.S.A.; Wasonga, D.O.; Solano Hernandez, A.; Santanen, A. Seedling growth and phosphorus uptake in response to different phosphorus sources. Agronomy 2020, 8, 1089. [Google Scholar] [CrossRef]
- Ando, K.; Yamaguchi, N.; Nakamura, Y.; Kasuya, M.; Taki, K. Speciation of phosphorus accumulated in fertilized cropland of Aichi prefecture in Japan with different soil properties by sequential chemical extraction and P K-edge XANES. Soil Sci. Plant Nutr. 2021, 2, 150–161. [Google Scholar] [CrossRef]
- Kalayu, G. Phosphate solubilizing microorganisms: Promising approach as biofertilizers. Int. J. Agron. 2019, 2019, 4917256. [Google Scholar] [CrossRef]
- Adam, W.; Heckel, F.; Saha-Möller, C.R.; Taupp, M.; Schreier, P. A highly enantioselective biocatalytic sulfoxidation by the topsoil bacterium Pseudomonas frederiksbergensis. Tetrahedron Asymmetry 2004, 15, 983–985. [Google Scholar] [CrossRef]
- Zeng, Q.W.; Wu, X.Q.; Wen, X.Y. Effects of soluble phosphate on phosphate-solubilizing characteristics and expression of gcd gene in Pseudomonas frederiksbergensis strain JW-SD2. Curr. Microbiol. 2016, 72, 198–206. [Google Scholar] [CrossRef]
- Devatha, C.P.; Vishnu Vishal, A.; Purna Chandra Rao, J. Investigation of physical and chemical characteristics on soil due to crude oil contamination and its remediation. Appl. Water Sci. 2019, 9, 89. [Google Scholar] [CrossRef]
- Bungau, S.; Behl, T.; Aleya, L.; Bourgeade, P.; Aloui-Sossé, B.; Purza, A.L.; Abid, A.; Samuel, A.D. Expatiating the impact of anthropogenic aspects and climatic factors on long term soil monitoring and management. Environ. Sci. Pollut. Res. 2021, 28, 30528–30550. [Google Scholar] [CrossRef] [PubMed]
- Rohrbacher, F.; St-Arnaud, M. Root exudation: The ecological driver of hydrocarbon rhizoremediation. Agronomy 2016, 6, 19. [Google Scholar] [CrossRef]
- Chetverikov, S.; Vysotskaya, L.; Kuzina, E.; Arkhipova, T.; Bakaeva, M.; Rafikova, G.; Korshunova, T.; Chetverikova, D.; Hkudaygulov, G.; Kudoyarova, G. Effects of association of barley plants with hydrocarbon-degrading bacteria on the content of soluble organic compounds in clean and oil-contaminated sand. Plants 2021, 10, 975. [Google Scholar] [CrossRef] [PubMed]
- Muratova, A.; Dubrovskaya, E.; Golubev, S.; Grinev, V.; Chernyshova, M.; Turkovskaya, O. The coupling of the plant and microbial catabolisms of phenanthrene in the rhizosphere of Medicago sativa. J. Plant Physiol. 2015, 188, 1–8. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.d.C.; Santoyo, G.; Glick, B.R. Recent advances in the bacterial phytohormone modulation of plant growth. Plants 2023, 12, 606. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010, 2, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Patten, C.L.; Blakney, A.J.C.; Coulson, T.J.D. Activity, distribution and function of indole-3-acetic acid biosynthetic pathways in bacteria. Crit. Rev. Microbiol. 2013, 39, 395–415. [Google Scholar] [CrossRef] [PubMed]
- Duca, D.; Lorv, J.; Patten, C.L.; Rose, D.; Glick, B.R. Indole-3-acetic acid in plant-microbe interactions. Antonie Van Leeuwenhoek 2014, 106, 85–125. [Google Scholar] [CrossRef] [PubMed]
- Duca, D.R.; Rose, D.R.; Glick, B.R. Indole acetic acid overproduction transformants of the rhizobacterium Pseudomonas sp. UW4. Antonie Van Leeuwenhoek 2018, 111, 1645–1660. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Spalding, E.P.; Gray, W.M. Rapid auxin-mediated cell expansion. Annu. Rev. Plant Biol. 2020, 71, 379–402. [Google Scholar] [CrossRef] [PubMed]
- Rubin, R.L.; van Groenigen, K.J.; Hungate, B.A. Plant growth promoting rhizo-bacteria are more effective under drought: A meta-analysis. Plant Soil. 2017, 416, 309–323. [Google Scholar] [CrossRef]
- Ali, B.; Sabri, A.N.; Ljung, K.; Hasnain, S. Auxin production by plant associated bacteria: Impact on endogenous IAA content and growth of Triticum aestivum L. Lett. Appl. Microbiol. 2009, 48, 542–547. [Google Scholar] [CrossRef]
- Dehnavi, S.M.; Ebrahimipour, G. Comparative remediation rate of biostimulation, bioaugmentation, and phytoremediation in hydrocarbon contaminants. Int. J. Environ. Sci. Technol. 2022, 19, 11561–11586. [Google Scholar] [CrossRef]
- Ladan, S.S. Phytotoxic follow-up imidazolinone on green manure and ways to reduce it. Plodorodie 2021, 6, 78–83. [Google Scholar] [CrossRef]
- Ptaszek, N.; Pacwa-Płociniczak, M.; Noszczyńska, M.; Płociniczak, T. Comparative study on multiway enhanced bio-and phytoremediation of aged petroleum-contaminated soil. Agronomy 2020, 10, 947. [Google Scholar] [CrossRef]
- Asghar, H.N.; Rafique, H.M.; Khan, M.Y.; Zahir, Z.A. Phytoremediation of light crude oil by maize (Zea mays L.) bio-augmented with plant growth promoting bacteria. Soil Sediment. Contam. 2017, 26, 749–763. [Google Scholar] [CrossRef]
- Liu, H.; Yang, G.; Jia, H.; Sun, B. Crude oil degradation by a novel strain Pseudomonas aeruginosa AQNU-1 isolated from an oil-contaminated lake wetland. Processes 2022, 10, 307. [Google Scholar] [CrossRef]




| Product | Manufacturer | Active Substance | Class of Chemical Compounds | Crops | Object of Influence (Weeds) |
|---|---|---|---|---|---|
| Octapon extra | AHK-AGRO, LLC | 2,4-dichlorophenoxyacetic acid (2,4-D) | aryloxyalkanocarboxylic acids | cereals | annual and some perennial dicotyledons |
| Chistalan | AHK-AGRO, LLC | 2,4-D (2-ethylhexyl ether) and dicamba (sodium salt) | aryloxyalkanocarboxylic acids | cereals corn | annual and perennial dicotyledons |
| Tapir | Agro Expert Group, LLC | imazetapir | imidazolinones | soy peas | dicotyledons and cereal |
| Hermes | Shchelkovo Agrokhim, CJSC | imazamox and quizalofop-p-ethyl | aryloxyphenoxypropionates and imidazolinones | sunflower peas | annual dicotyledons, annual and perennial cereals |
| Fenizan | Shchelkovo Agrokhim, CJSC | dicamba and chlorsulfuron | sulfonylurea | cereals fiber flax | annual dicotyledons, including 2,4-D-resistant and some perennial dicotyledons |
| Isolate | MALDI-TOF MS Identification (Score; Consistency Category) | 16S rRNA Identification Closest Strain (% Similarity Score) | GenBank Accession Number |
|---|---|---|---|
| UOM 9 | Pseudomonas spp. (1.903; B) | P. silesiensis A3T (99.50) | OQ439800 |
| UOM 10 | Pseudomonas alcaligenes (2.122; A) | P. alcaligenes NBRC 14159T (99.15) | OP692728 |
| UOM 11 | Pseudomonas frederiksbergensis (2.073; A) | P. frederiksbergensis JAJ28T (99.72) | OP692729 |
| UOM 13 | Pseudomonas arsenicoxydans (2.186; A) | P. arsenicoxydans CECT 7543T (99.44) | OQ439801 |
| UOM 14 | Pseudomonas jessenii (2.201; A) | P. jessenii DSM 17150T (99.86) | OQ439802 |
| UOM 15 | Pseudomonas spp. (1.989; B) | P. zhaodongensis NEAU-ST5-21T (99.01) | OQ439803 |
| UOM 16 | Pseudomonas avellanae (2.154; A) | P. avellanae BPIC 631T (99.15) | OQ439804 |
| Property | Strain | |||||||
|---|---|---|---|---|---|---|---|---|
| UOM 9 | UOM 10 | UOM 11 | UOM 13 | UOM 14 | UOM 15 | UOM 16 | ||
| Maximum concentration of herbicide, mL/L | Oktapon extra | 10 | 10 | 10 | 10 | 5 | 10 | 10 |
| Chistalan | 5 | 5 | 1 | 5 | 1 | 1 | 5 | |
| Tapir | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| Hermes | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| Fenizan | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| Maximum concentration of heavy metals, mmol/L | Pb2+ | 5 | 5 | 5 | 5 | 6 | 5 | 5 |
| Zn2+ | 4 | 4 | 4 | 4 | 8 | 4 | 4 | |
| Cd2+ | 1 | - | 1 | 1 | 2 | - | 1 | |
| Co2+ | 3 | 3 | 4 | 3 | 3 | 2 | 4 | |
| Cu2+ | 2 | 3 | 3 | 2 | 4 | 3 | 2 | |
| Ni2+ | 4 | 4 | 4 | 4 | 4 | 4 | 4 | |
| Production of hydrolytic enzymes | lipase | − | + | + | + | + | + | − |
| amylase | − | − | − | − | − | + | − | |
| protease | − | + | − | − | + | − | − | |
| cellulase | − | − | − | − | − | + | − | |
| Solubilization index | 2.2 ± 0.2 | 1.8 ± 0.1 | 3.2 ± 0.2 | 2.3 ± 0.2 | 2.0 ± 0.1 | 1.8 ± 0.2 | 3.0 ± 0.2 | |
| Nitrogen fixation | + | + | + | + | + | + | + | |
| IAA production, ng/mL | 539 ± 29 | 1627 ± 75 | 898 ± 40 | 305 ± 22 | 1615 ± 69 | 975 ± 48 | 940 ± 53 | |
| Plant | Soil | Variant | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | UOM 9 | UOM 10 | UOM 11 | UOM 13 | UOM 14 | UOM 15 | UOM 16 | ||
| Barley | pure | 6.2 | 7.2 | 8.3 | 7.7 | 6.5 | 8.1 | 7.3 | 6.9 |
| with oil | 7.4 | 10.1 | 9.7 | 10.7 | 9.7 | 8.7 | 9.3 | 11.0 | |
| Clover | pure | 0.72 | 0.69 | 0.82 | 0.84 | 0.81 | 0.76 | 0.79 | 0.80 |
| with oil | 0.79 | 0.95 | 1.08 | 1.13 | 1.01 | 0.91 | 1.11 | 1.16 | |
| with Tapir | 0.86 | 0.75 | 0.78 | 0.77 | 0.75 | 0.72 | 0.69 | 0.76 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korshunova, T.; Kuzina, E.; Mukhamatdyarova, S.; Sharipova, Y.; Iskuzhina, M. Promising Strains of Hydrocarbon-Oxidizing Pseudomonads with Herbicide Resistance and Plant Growth-Stimulating Properties for Bioremediation of Oil-Contaminated Agricultural Soils. Agriculture 2023, 13, 1111. https://doi.org/10.3390/agriculture13061111
Korshunova T, Kuzina E, Mukhamatdyarova S, Sharipova Y, Iskuzhina M. Promising Strains of Hydrocarbon-Oxidizing Pseudomonads with Herbicide Resistance and Plant Growth-Stimulating Properties for Bioremediation of Oil-Contaminated Agricultural Soils. Agriculture. 2023; 13(6):1111. https://doi.org/10.3390/agriculture13061111
Chicago/Turabian StyleKorshunova, Tatyana, Elena Kuzina, Svetlana Mukhamatdyarova, Yuliyana Sharipova, and Milyausha Iskuzhina. 2023. "Promising Strains of Hydrocarbon-Oxidizing Pseudomonads with Herbicide Resistance and Plant Growth-Stimulating Properties for Bioremediation of Oil-Contaminated Agricultural Soils" Agriculture 13, no. 6: 1111. https://doi.org/10.3390/agriculture13061111
APA StyleKorshunova, T., Kuzina, E., Mukhamatdyarova, S., Sharipova, Y., & Iskuzhina, M. (2023). Promising Strains of Hydrocarbon-Oxidizing Pseudomonads with Herbicide Resistance and Plant Growth-Stimulating Properties for Bioremediation of Oil-Contaminated Agricultural Soils. Agriculture, 13(6), 1111. https://doi.org/10.3390/agriculture13061111






