3.1. Physical, Chemical and Biological Properties of Soil
Under ARD conditions, soil productivity deteriorates. The previous way of using the soil analysed in the experiment changed some of its physical and chemical properties. The volumetric weight of the soil, its salinity and the content of phenolic compounds did not significantly differ (
Table 2). The level of acidity and the amount of organic matter varied. The pH value of the replanted soil was significantly lower than that of the agricultural soil (
Table 2). The above confirms other researchers’ conclusions on the replanted soil’s high acidity [
5]. The organic matter content in the agricultural soil (1.87%) was higher than that in the replanted soil (1.63%).
Supplementing the replanted soil with the two types of organic additives, regardless of their amount, significantly reduced the volumetric weight of the soil and the level of its salinity. Carbomat Eco was more effective than biocarbon in that respect. In the treatments with the addition of Carbomat Eco, in particular in the amount of 30% of the substrate volume, compared to the replanted soil with no additives, the salinity of the soil decreased more than three times (from 0.47 to 0.15 g NaCl dm
−3) (
Table 2). High soil salinity, causing its alkalisation, reduces the assimilation by plants of macro- and microelements and limits its enzymatic activity. The experiment showed significant differences in the content of phenolic compounds in the soil depending on the treatment. Their content was higher in the replanted soil with added carbon than in the replanted soil with no additives. Biocarbon was a particular case. In the treatments with biocarbon, an increase in phenolic compounds content by approximately 90% (from 14.79 to 27.83 mg g
−1 dm) was observed (
Table 2). In the treatments with Carbomat Eco, the increase in the content of the abovementioned compounds was a dozen or so per cent (volume in the substrate 30 % and 45%) or insignificant (volume 15%).
The content of the five macronutrients under study in replanted and agricultural soil did not significantly differ. This is in contrast to the experimental results obtained by the authors of earlier studies [
8], when the macronutrients content in replanted soil was considerably lower than that in agricultural soil. In the treatments, where replanted soil was supplemented with organic additives, N-NO
3 content decreased. In the soil with the addition of biocarbon at 20% of the substrate volume, the content of that element (48 mg dm
−1) was even 3.5 times lower than in the control treatment (agricultural soil) (180 mg kg
−1) (
Table 3). The decrease in N-NO
3 content of the soil was equally significant after adding Carbomat Eco, especially at 45% of the substrate volume. Furthermore, compared to replanted and agricultural soil, a lower content of P and K was also noticed in the treatments with its use.
The decrease in N, P and K content in the soil with the addition of Carbomat Eco may have resulted from a more intensive uptake of those elements by plants. However, in the replanted soil with the addition of biocarbon, especially at 10 and 20%, P, K and Mg content increased compared to their content in the replanted soil with no additives (
Table 3).
In the experiment, the number of micronutrients in the soil was less diverse than that of macronutrients. The Cu, Mn and Fe content in the replanted soil did not significantly differ from their content in the agricultural soil. The difference was only noticed in the case of Zn, the content of which in treatments with the application of biocarbon significantly decreased (several times compared to the control). The reduction was greater with an increase in its content in the soil (
Table 4). Even though both organic additives used in the experiment contained macro- and microelements, their effect on the content of nutrients in the soil was relatively small. After adding biocarbon to the replanted soil, regardless of its amount, a significant decrease in the content of Mn was observed. The number of other micronutrients in the soil did not significantly change. A slightly different effect was caused by adding Carbomat Eco to the replanted soil. In the treatment with the addition of Carbomat Eco lignite, irrespective of its amount, there was a significant increase in the content of Zn and Fe in the soil. The content of Mn and Cu did not significantly change (
Table 4).
The level of biological activity of soil is measured by its enzymatic activity. The higher the activity of soil enzymes, the higher the rate of mineralisation of organic compounds, and thus, the amount of macro- and microelements available to the plants. Some of the most important soil enzymes are dehydrogenases and proteases. These enzymes are involved in the soil’s biochemical cycle of carbon, nitrogen and phosphorus [
28]. Dehydrogenases are particularly important as they are the source of information regarding the activity of the soil microflora [
29]. In the experiment, the biological properties of the replanted soil significantly differed from those of the agricultural soil. For example, dehydrogenase activity was more than three times lower (0.22 and 0.78 cm
3 H
2 24 h
−1 kg
−1 dm of soil) (
Table 5). The level of respiration of replanted and agricultural soil was less differentiated.
The lower activity of dehydrogenases in the replanted soil may have resulted from the insufficiently effective work of soil microorganisms, which decreases once the soil acidity is reduced [
30]. The replanted soil used in the experiment was more acidic than the agricultural soil (
Table 2). However, the authors did not show any significant differences in the activity of soil proteases depending on the previous way of using the soil.
Organic matter in soil is the source of nutrients and energy necessary for the functioning of soil microorganisms [
31]. When it decomposes, the microorganisms produce enzymes, thus determining the level of soil productivity [
32]. The indication of the level of activity of soil microorganisms is soil respiration [
31,
33]. Adding the two organic additives to the replanted soil significantly improved that parameter. In the treatments with the addition of biocarbon in the amounts of 10 and 20%, compared to the replanted soil with no additives, the respiration of the soil increased by 30 and 25%, respectively (
Table 5). A better result was obtained in the treatments by adding Carbomat Eco. With the increase in its amount in the substrate, the replanted soil respiration significantly increased—from approximately 50% (volume 15%) to almost 100% (volume 45%). The respiration level was much higher than that of the agricultural (control) soil.
Similar results were obtained when soil enzymatic activity was analysed. As a result of adding both biocarbon and Carbomat Eco lignite to the replanted soil, protease activity in the soil significantly increased. The addition of Carbomat Eco was more effective in this regard. In the replanted soil with Carbomat Eco added at 45% of the substrate volume, an over 2.5-fold increase in protease activity was obtained compared to the replanted soil with no additives (5.51 and 2.08 mg of tyrosine h
−1 kg
−1 dm, respectively) (
Table 5). In the treatment with biocarbon added in the amount of 20% of the substrate volume, an increase in the activity of proteases was found at approximately 75%. Once the amount of the additive was increased from 5 to 20% (biocarbon) and from 15 to 45% (Carbomat Eco), no significant increase in the activity of proteases was observed in the replanted soil.
The level of activity of soil dehydrogenases varied depending on the treatment. When biocarbon was added to the replanted soil in the amount of 5% of the volume of the substrate, a significant (more than twofold) increase in the activity of the abovementioned enzyme was found. As in the case of protease activity and soil respiration, the best results were obtained in the treatments with the addition of Carbomat Eco lignite. At 30% of its content in the replanted soil, the activity of dehydrogenases, compared to the replanted soil with no additives, increased almost three times, and at 45%, it increased more than four times (0.65 and 0.22 and 0.93 0 0.22 cm
3 H
2 24 h
−1 kg
−1 dm, respectively) (
Table 5). The obtained results confirm the conclusions of the previous studies and indicate the positive effect of humic acids on both the enzymatic activity and respiration of soil [
8,
34].
3.2. Apple Tree Leaf Parameters
The leaf minerals can be used as an indicator of plant nutrition levels. However, several factors can influence the diversity of mineral content in plant leaves. These include, inter alia, their content in the soil, weather conditions, the yield level and the age of trees, as well as the cultivation conditions before planting the trees. In the experiment, depending on the treatments, the content of macroelements in the leaves of the apple trees (in % dm) was: N—from 2.3 to 2.52; P—from 0.15 to 0.40; K—from 1.44 to 3.9; CaO—from 0.69 to 1.14; Mg—from 0.17 to 0.30. The content of macroelements in the leaves of the apple trees grown on the agricultural and replanted soil did not significantly differ (
Table 6).
Supplementing the replanted soil with organic additives had a varied effect on the content of the macroelements under study. In the leaves of the apple trees grown on the replanted soil with the addition of biocarbon in the amounts of 10 and 20% of the substrate volume, compared to the replanted soil with no additives, the content of N, Ca and Mg did not change (
Table 6). However, the content of P and K increased. In the treatments with the addition of Carbomat Eco, the content of Ca and Mg in the leaves did not change, the content of K and P significantly decreased (especially at 30 and 45% volume) and the content of N significantly increased. The increase in the macronutrient contents in the leaves of apple trees under the influence of humic acids can be explained by the fact that they increase the permeability of cell membranes, which accelerates the absorption of nutrients by the roots and their accumulation in plant tissues.
In the experiment, depending on the treatment, the content of micronutrients in the leaves of the apple trees (in mg kg dm
−1) was: Zn—from 11.3 to 14.2; Cu—from 3.96 to 5.31; Mn—from 57.7 to 337; Fe—from 139 to 178; B—from 17.35 to 29.05 (
Table 7).
The content was higher (Fe, Mn), comparable (Cu, B) or lower (Zn) than in the experiment performed by Sosna et al. [
35], presenting the micronutrient contents in the leaves of apple trees grafted on M.9 rootstock. Generally, supplementing the substrate with organic additives had little effect on the content of microelements in the leaves of apple trees. Regardless of the type of additive, there were no significant differences in the Zn and Fe content of the leaves. This result differs from the results obtained in the experiment conducted by Abourayya et al. [
36], which showed an increase in the Fe and Zn content in almond leaves treated with humic acid. In the leaves of the apple trees grown on the replanted soil with the addition of Carbomat Eco, compared to those grown on the replanted soil with no additives and agricultural soil, the content of B significantly increased. With the addition of biocarbon, the content of Cu also increased (
Table 7).
The experiment showed the significant influence of the previous method of using the soil on the parameters of the apple trees’ leaves, particularly in the case of the replanted soil, compared to the agricultural soil. For example, the leaf area of the apple trees grown on the replanted soil (28.41 cm
2) was approximately 70% smaller than those in the control variant (49.27 cm
2) (
Table 8). Furthermore, a significant difference (from 50% to 70%, depending on the number of organic additives) was also observed in other parameters, such as the length of the leaves and their mass. Thus, the earlier conclusion of the study’s authors was confirmed—the biometric parameters of the leaves of apple trees grown on replanted soil were adversely affected [
37].
Eisa et al. [
38] and Suppels et al. [
16] reported that treating apple trees with biostimulants containing humic acids increased the leaf area. The authors of the experiment confirm the above conclusion. In addition, supplementing the replanted soil with organic additives significantly improved the biometric parameters of the leaves of the apple trees. In that respect, Carbomat Eco was more effective than biocarbon. Even with its smallest amount in the substrate of 15%, the leaf area of the apple trees (55.01 cm
2) was almost twice as large as that in the replanted soil with no additive combination (28.41 cm
2) (
Table 8). In the variant with 30% of the substrate volume, the difference was over 2.5 times. A further increase in the amount of Carbomat Eco to 45% did not significantly impact the leaf area of the trees. Similar results were obtained when the leaf mass of the apple trees was analysed. Again, adding Carbomat Eco lignite to the replanted soil at 15% of the substrate volume increased the average leaf mass of the apple trees grown on the soil with no additives from 4.43 g to 7.23 g. A further significant increase in average leaf mass to 8.49 g was possible when the amount of the abovementioned additive in the substrate was increased to 45%. Once biocarbon was added to the replanted soil, the leaf area of the trees also increased. Compared to the replanted soil with no additives, the difference was less than 70% (28.41 and 47.0 cm
2 in the variant with 20% substrate volume) (
Table 8). Notably, the amount of biocarbon in the substrate had no significant effect on the leaf area.
Another parameter that positively responded to the addition of biocarbon to the soil was the length of the leaves. Compared to the replanted soil with no additive treatment, the difference in length ranged from approximately 30% (10% of the substrate volume) to about 20% (20% volume). The analysis of leaf width and leaf mass showed no significant difference between the treatments with the addition of biocarbon, regardless of its amount in the substrate, and the replanted soil with no additives (
Table 8).
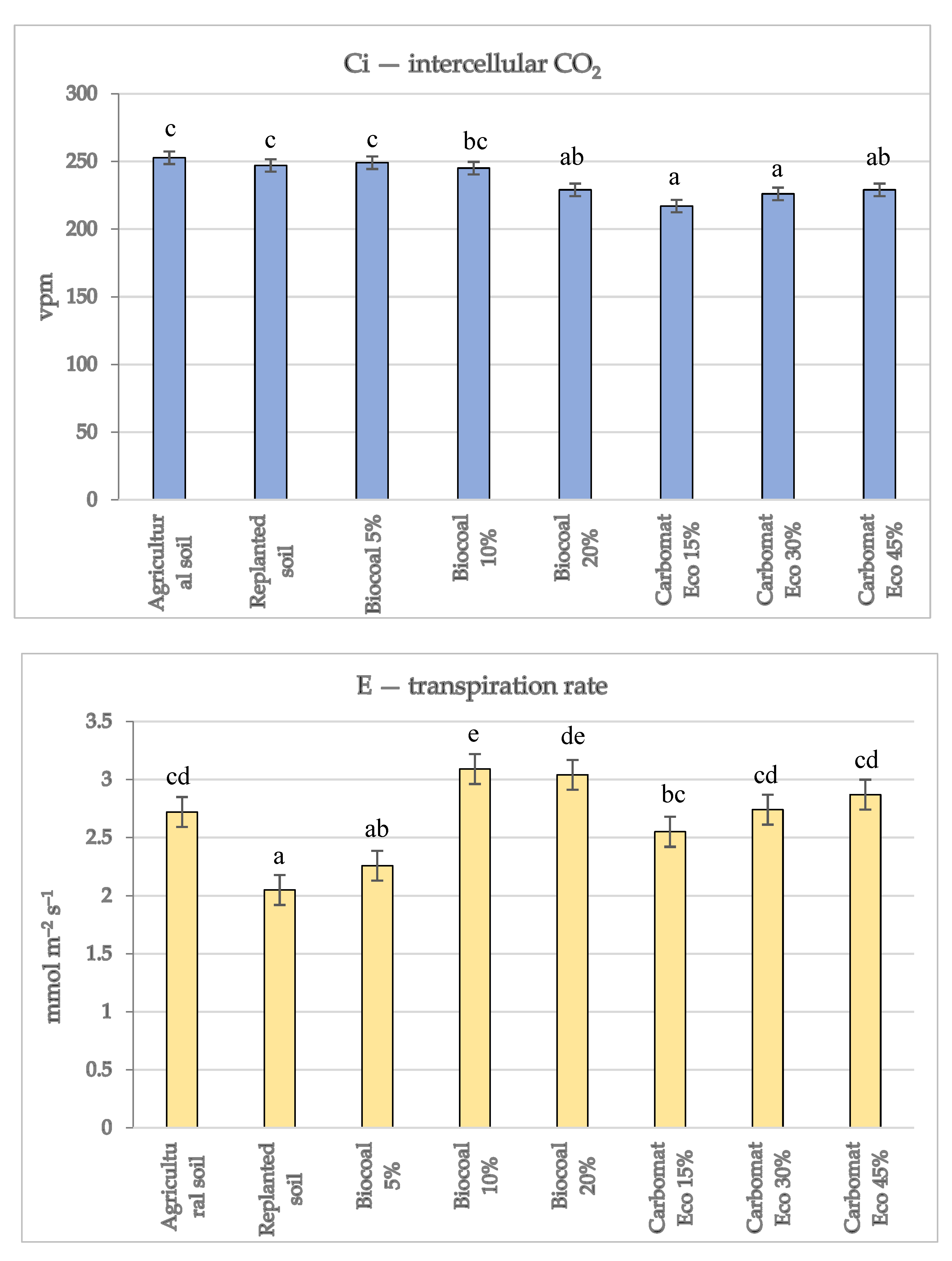
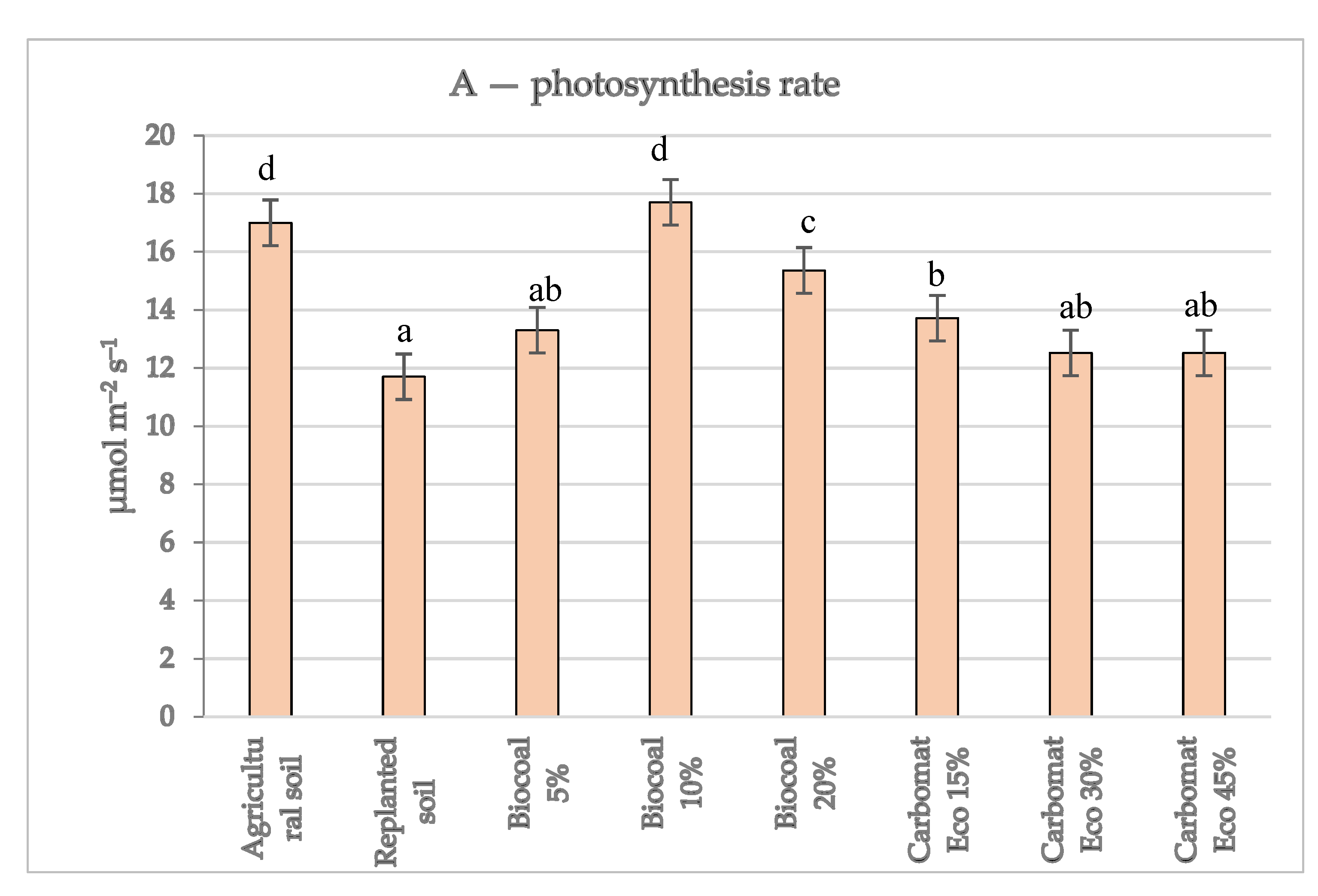
Well-developed leaves enable the proper course of photosynthesis, which translates into better plant growth. In the experiment, the intensity of gas exchange in the leaves of the apple trees was as follows: the amount of intercellular CO
2 (vpm) from 217.10 to 252.70; transpiration coefficient (mmol m
−2 s
−1) from 2.05 to 3.09; rate of photosynthesis (μmol m
−2 s
−1) from 11.70 to 17.00. The intensity of photosynthesis in the leaves of the apple trees was higher than in the leaves of pear trees—from 5.6 to 8.6 μmol m
−2 s
−1 [
39]. The previous method of using soil significantly affected the gas exchange intensity of the apple trees’s leaves. In the leaves of the trees grown on the replanted soil, the transpiration rate E (2.05 mmol m
−2 s
−1) was approximately 30% lower than in the case of the trees grown on agricultural soil (2.72 mmol m
−2 s
−1) (
Figure 1). Significant differences were also noticed when the rate of photosynthesis was analysed. That rate, in the agricultural soil variant, was approximately 45% higher than in the replanted soil (17.0 and 11.7 μmol m
−2 s
−1, respectively).
Supplementing the replanted soil with organic additives significantly affected the intensity of the gas exchange of apple trees leaves. Compared to the trees grown on the replanted soil with no additives, there was a several per cent decrease in the amount of intercellular CO
2 in the leaves of the trees grown on the soil supplemented with Carbomat Eco. Such a difference was not observed in the combination using biocarbon in the amounts of 5 and 10% of the substrate volume. The transpiration coefficient in the leaves of the trees grown on the replanted soil with the addition of Carbomat Eco significantly increased, regardless of its amount in the substrate. When biocarbon was used in 10% and 20% of the volume, the differences between those treatments and the replanted soil with no additives were more significant, reaching 50% (3.09 and 2.05 mmol m
−2 s
−1, respectively). The rate of photosynthesis in the leaves of the apple trees in the treatments with the addition of biocarbon to the soil in the amounts of 10% and 20% of the substrate volume was several per cent higher than the trees grown on the replanted soil with no additives (
Figure 1). A significant increase in the intensity of photosynthesis in the leaves was also observed for the treatment in which Carbomat Eco lignite was used. Tathermore, with its 15% content in the substrate, meant that the measurement results of that parameter (13.72 μmol m
−2 s
−1) were significantly higher than in the case of the replanted soil with no additives (11.70 μmol m
−2 s
−1). A further increase in the amount of Carbomat Eco in the substrate did not impact the rate of photosynthesis in the leaves of the apple trees. It should be noted that the intensity of photosynthesis in the leaves of the apple trees grown on the replanted soil, despite its supplementation with organic additives, was significantly lower than in the control variant with agricultural soil. The fact that the use of biostimulants increases the intensity of photosynthesis in the leaves of apple trees has also been confirmed by other researchers [
16,
17]. The change in the intensity of the photosynthesis in the leaves of the trees could have been influenced by the content of some macroelements in the replanted soil supplemented with organic additives responsible for the correct course of that process. An example of such a component is Mg; the content in the replanted soil with the addition of biocarbon was significantly higher than in the soil with no additives (
Table 3).
Another factor that influenced the intensity of gas exchange in the leaves of apple trees was the timing of the measurements. The amount of intercellular CO
2 (Ci), the transpiration rate (E) and the rate of photosynthesis (A) were significantly lower in the first period (middle of June) than in the second period (middle of August) (
Table 9).
3.3. The Growthing Power of Apple Trees
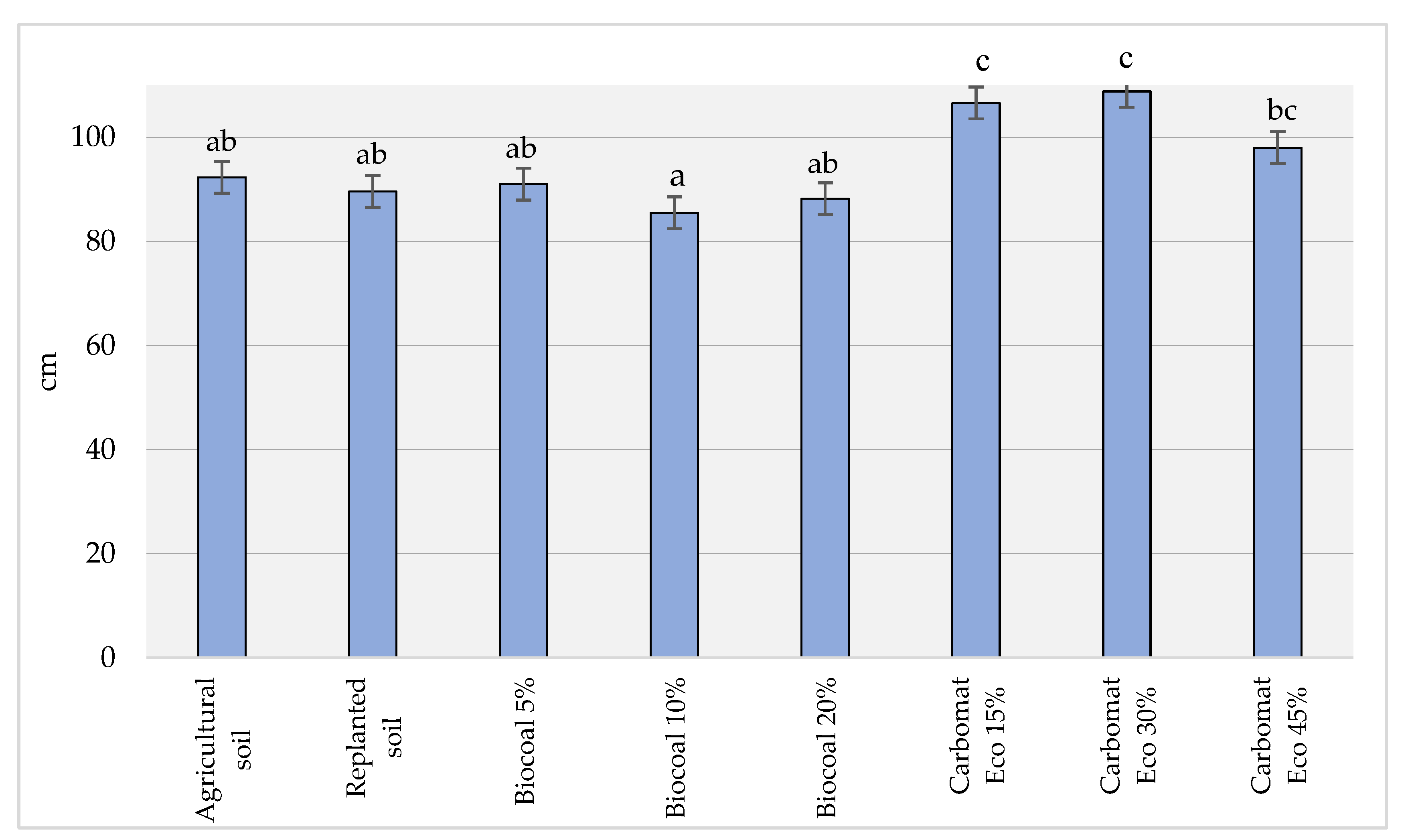
The previous method of using the soil had a varied effect on the power of vegetative growth of the apple trees. For example, trees of similar height (89.65 and 92.35 cm) grew on replanted and agricultural soil (
Figure 2). However, based on earlier studies [
37], the differences in apple tree growth depending on how the soil was used were much more pronounced.
In the experiment, the authors noted significant differences in the increase in the diameter of the tree trunks and the total increase in the growth of side shoots. The increase in the diameter of the trunks of the trees grown on the agricultural soil was 0.24 mm greater than that those grown on the replanted soil. On the other hand, the total growth of the side shoots of the trees grown on the replanted soil (56.98 cm) was several dozen cm smaller than of those grown on the agricultural soil (71.30 cm) (
Table 10). Weaker growth of plants under ARD conditions has also been reported by other researchers [
10,
11,
40,
41,
42,
43]. Weaker vegetative growth of trees grown on replanted soil may result from the deterioration of the growth parameters of the root system of plants grown under such conditions. It manifests in the formation of root necrosis, a reduction in the number of fine hair-like roots or slower growth, inter alia [
44,
45].
The addition of biocarbon to the replanted soil, regardless of its amount in the substrate, did not significantly affect the vegetative growing power of the apple trees. Their height, the increase in trunk diameter and the number and total growth of shoots of the trees grown on such a substrate did not significantly differ from the results of the measurements taken for the replanted soil with no additives treatments. However, a significant improvement in the vegetative growth of apple trees was observed when Carbomat Eco lignite was used. In such cases, each of the analysed parameters determining the growing power of the apple trees was significantly higher than in the replanted soil with no additives. For example, the trees were approximately 20% higher (30% of the volume of the substrate) (108.8 and 89.65 cm) (
Figure 2), and the total growth of their side shoots was approximately 60% (95.79 and 56.98 cm) (
Table 10). The trees grown in the soil supplemented with Carbomat Eco were even higher than those grown in the control combined with the agricultural soil. The best result was obtained when the mass of the side shoots of the apple trees was analysed. In the treatment with the addition of Carbomat Eco to the replanted soil in the amount of 30% of the substrate volume, the measurement result was almost three times better than in the case of the replanted soil with no additives (25.8 and 9.12 g, respectively) (
Table 10). Furthermore, further increasing the amount of Carbomat Eco in the substrate to 30% and 45% of the volume increased the total mass of the side shoots of the trees to 30.0 and 41.3 g, respectively.
The fast growth of the side shoots of the apple trees in the treatments with the addition of Carbomat Eco to the replanted soil could have been the result of a large number of such shoots on the trees. In the treatments with the addition of Carbomat Eco lignite in the amounts of 30% and 45% of the substrate volume, the average number of shoots (6.7 and 6.5) was significantly higher than in the control combination and in the variant with the addition of biocarbon (
Table 10). Mustafa and El-Shlazy [
46] and Fatma et al. [
47] also reported an increase in the number of side shoots under humic acid treatment.
Table 11 contains the results of the measurements of the growing power parameters of the aboveground and underground parts of apple trees. The researchers confirm the high efficiency of Carbomat Eco lignite as an additive in replanted soil. Both the weight of the main shoot and the length of the roots in combination with its use were significantly higher than in replanted soil with no additives. For example, the average root length of trees grown on soil with 30% Carbomat Eco (29.28 cm) was more than twice as long as in the combination without that additive (15.6 cm).
Figure 3 is a visual presentation of the growing power of the apple trees in different treatments. The well-developed root system of the trees allows the plant to be supplied with water and nutrients. The differences in the mass of the main shoot of the trees were smaller, ranging from approximately 20% (15% substrate volume in the soil) to 26% (30% volume). Increasing the amount of Carbomat Eco substrate in the soil from 15% to 45% did not significantly increase the mass of the main shoot of the apple trees or the length of their roots. However, both the mass of the main shoot of the apple trees and the length of their roots in the treatments with the addition of Carbomat Eco were significantly higher than those in the control variant with agricultural soil.
The high impact of Carbomat Eco lignite on the vegetative growth of apple trees can be confirmed by even a several-fold increase in the content of organic matter as a result of the supplementation of replanted soil with that additive, inter alia (
Table 2). Treatment with the increased activity of soil microorganisms in such a substrate, measured by, e.g., soil respiration (
Table 5), can significantly increase the number of available nutrients and improve plant nutrition. In the experiment, in the leaves of the apple trees grown on replanted soil with the addition of Carbomat Eco in the amounts of 30% and 45% of the substrate volume, a significant increase in the content of nitrogen was observed (
Table 6). As it is known, nitrogen is an element responsible for the vegetative growth of plants.
Information about the effect of humic acids on root growth is inconclusive. The Schoebitz et al. [
48] and Nunez et al. [
49] experiments show that it did not significantly affect blueberry root growth. According to other authors, humic acids present in the soil additives can stimulate root growth, making it easier for plants to absorb nutrients from the soil [
50,
51,
52]. After adding Carbomat Eco lignite to the replanted soil, regardless of its amount, the mass of the roots of the apple trees did not significantly change. In addition, adding biocarbon to the soil did not significantly change the growth parameters of the underground part of the apple trees. The length of the roots of the plants grown on such a substrate was similar to the length of the roots of the trees grown on the replanted soil with no additives, and the mass of the roots was even lower (
Table 11). In the opinion of the authors, this may be due to the high content of phenolic compounds in the replanted soil with the addition of biocarbon. Their amount in treatments with 10% and especially 20% content of biocarbon in the substrate was almost twice as high as in the agricultural and replanted soil with no additives (
Table 2). Phenolic compounds are formed from glycosides released as a result of decomposition by the microflora of root residues in the soil. The presence of remnants of apple tree roots in the soil increases the content of phenolic compounds in the soil up to seven times [
53]. They are considered the biological reason for replant disease [
42,
54,
55]. They slow down the development of soil microflora [
56], which can cause deficits of nutrients in the soil, and limit the growth of apple rootstocks [
57].