OsHSP 17.9, a Small Heat Shock Protein, Confers Improved Productivity and Tolerance to High Temperature and Salinity in a Natural Paddy Field in Transgenic Rice Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Stress Treatments
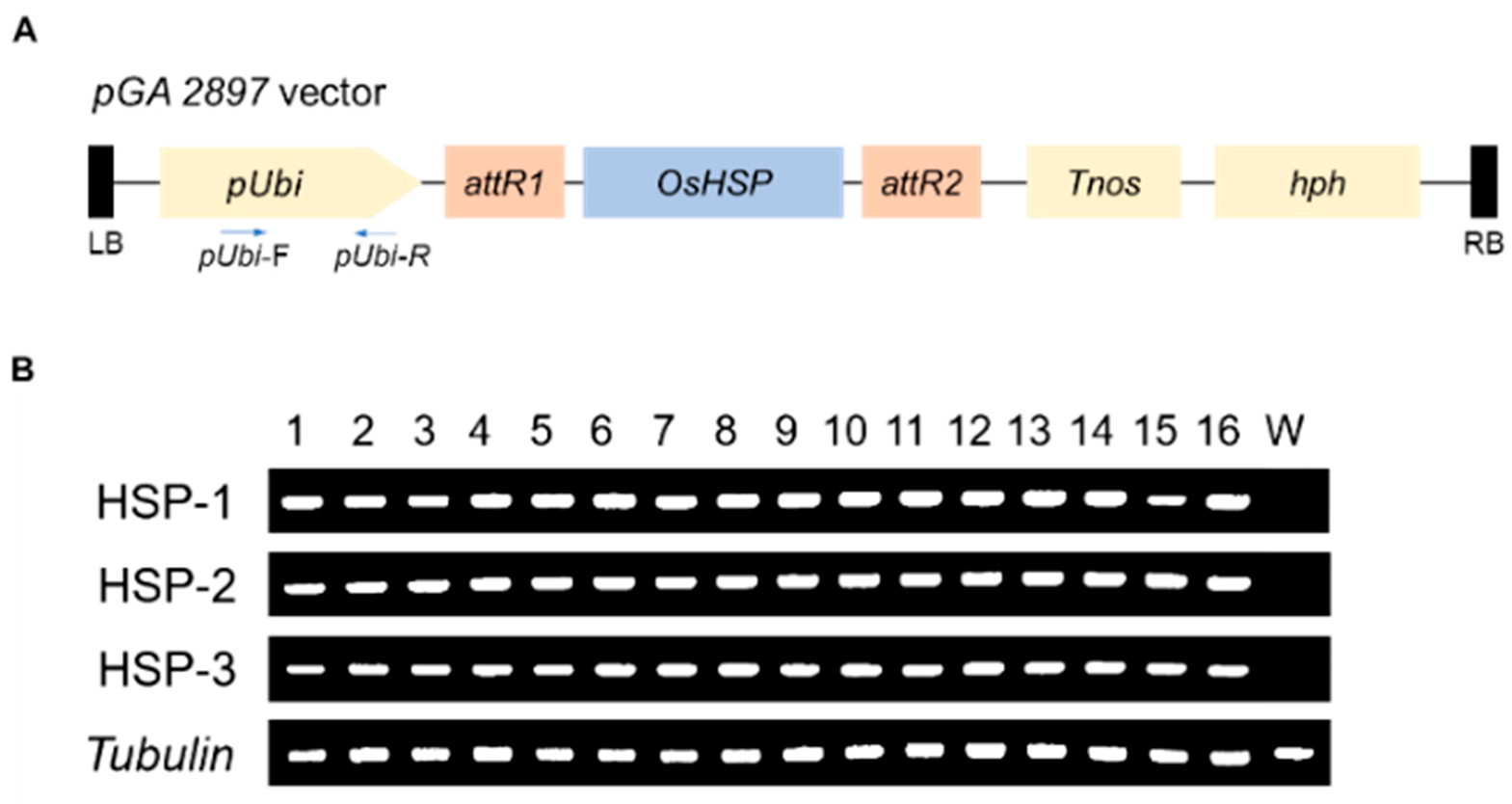
2.2. Genomic DNA Isolation and PCR Analysis
2.3. Analysis of Semi-Quantitative RT-PCR
2.4. Measurement of Accumulative ROS Content
2.5. Evaluation of Early Growth in a Natural Paddy Field and Ion Leakage in Response to Methyl Viologen (MV)
2.6. Antioxidant Enzyme Assay
2.7. Evaluation of Early Growth and Agronomic Traits in a Natural Paddy Field
2.8. Statistical Analysis
3. Results
3.1. Production of OsHSP 17.9 Gene Transgenic Plants
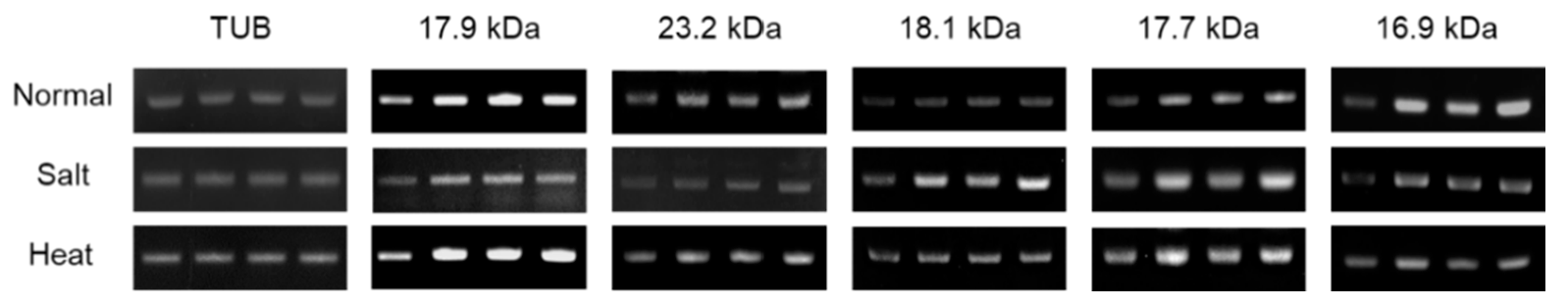
3.2. Comparison of Phenotype and OsHSP 17.9 Gene Expression Levels in Salt and High-Temperature Stress
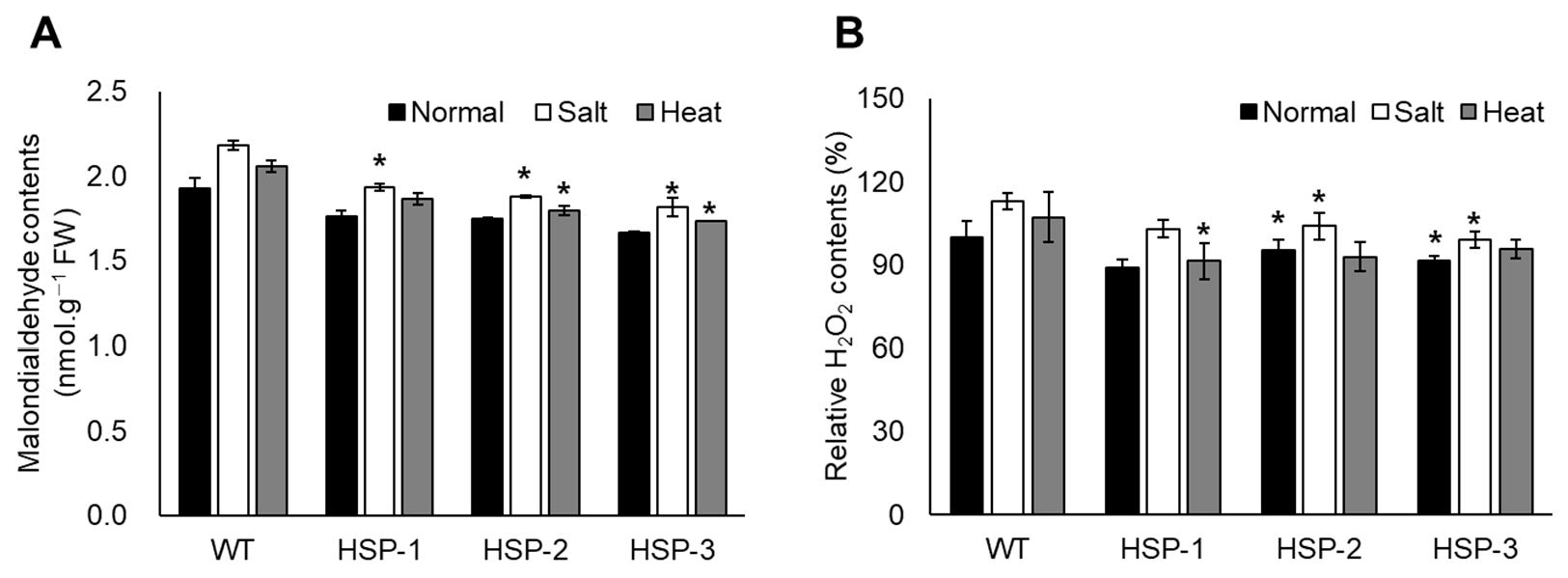
3.3. Analysis of Oxidative Stress Tolerance through Accumulated MDA and H2O2 Content
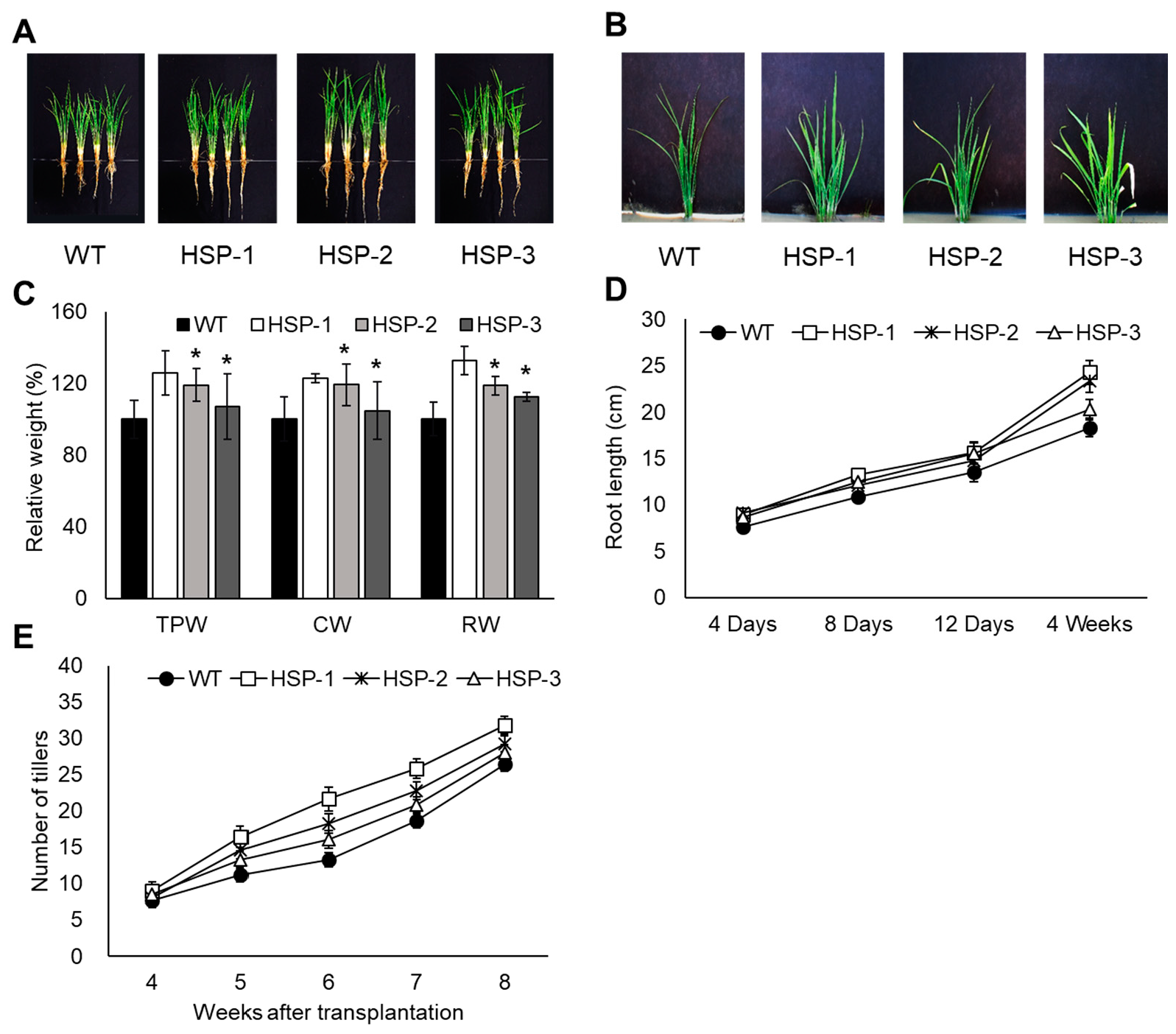
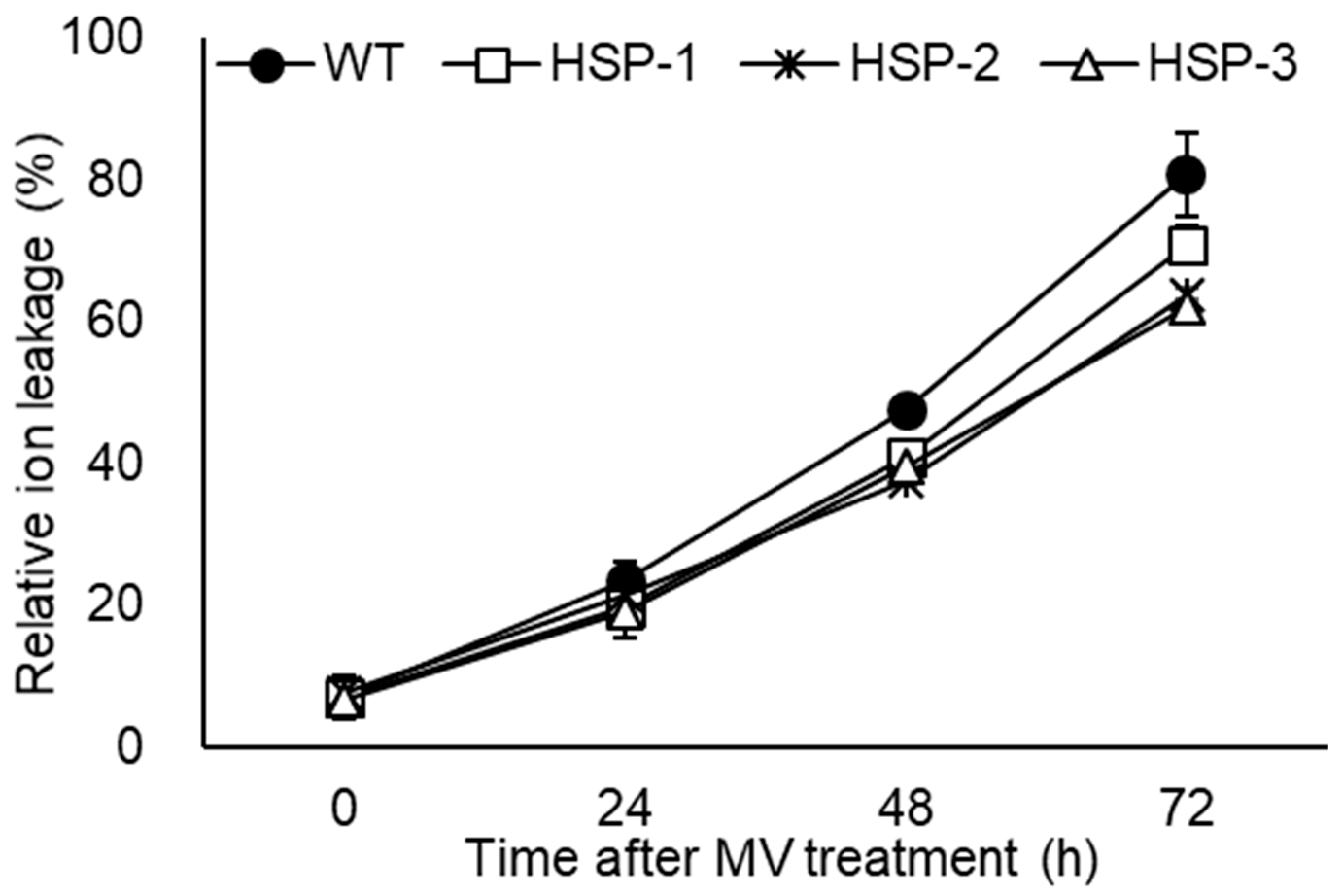
3.4. Early Growth and Environmental Adaptability in a Natural Paddy Field and Ion Leakage in Response to MV
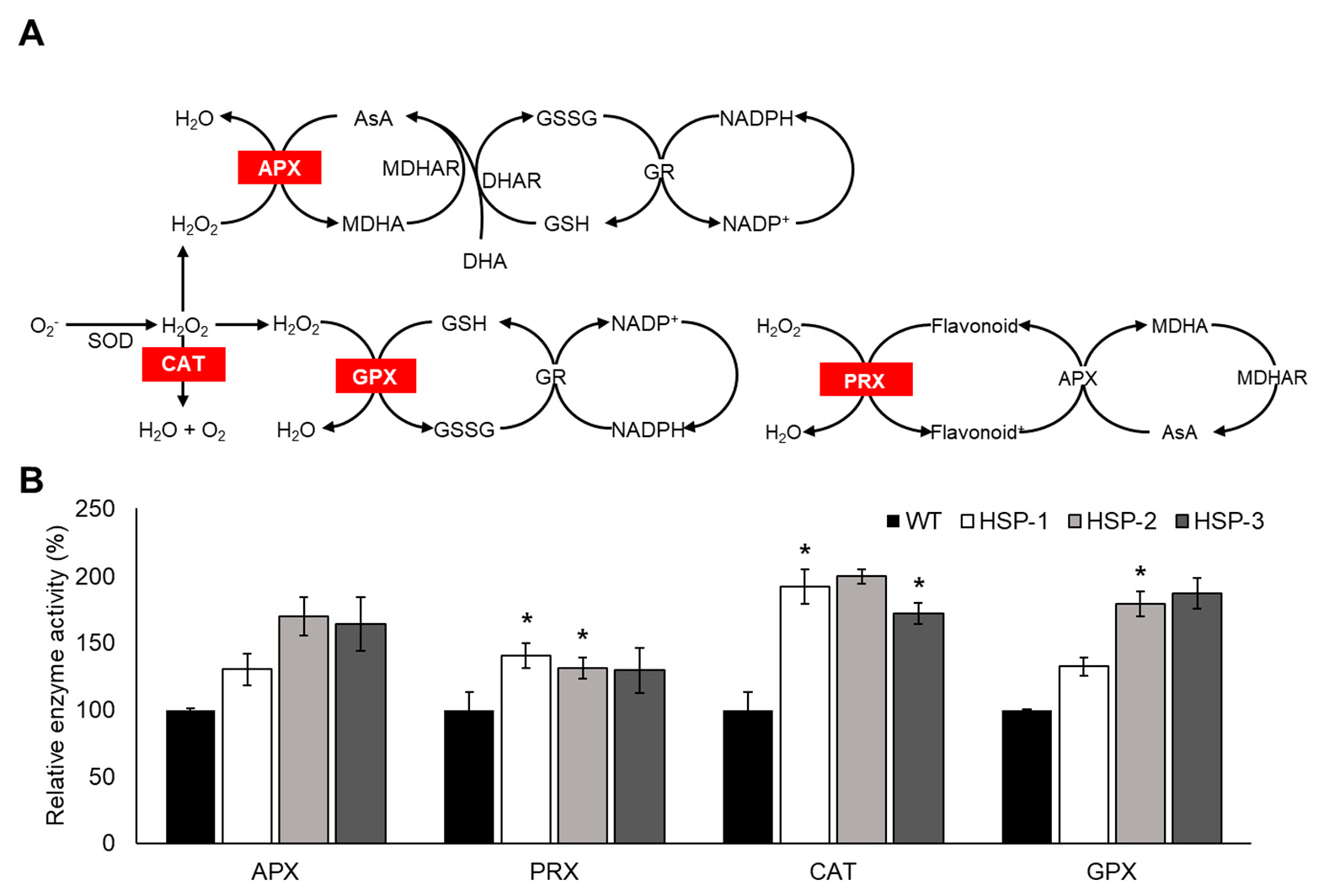
3.5. Enhanced Antioxidant Enzyme Activity of Transgenic Plants Grown in a Natural Paddy Field
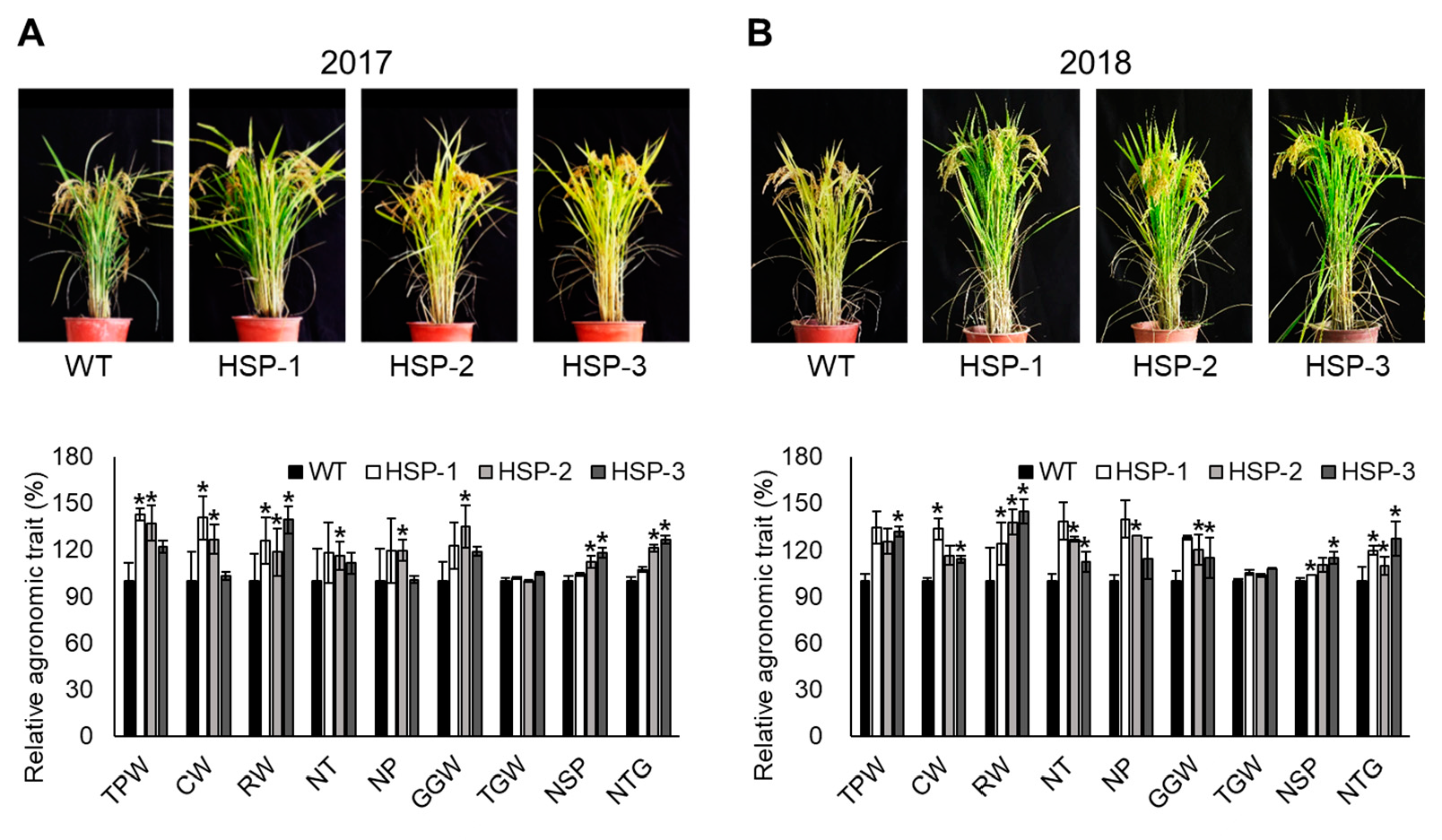
3.6. Grain Yield and Agronomic Analysis in Natural Paddy-Field Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Radhakrishnan, R. Magnetic Field Regulates Plant Functions, Growth and Enhances Tolerance against Environmental Stresses. Physiol. Mol. Biol. Plants 2019, 25, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, V.; Kapoor, D.; Kumar, S.; Singh, S.; Dhanjal, D.S.; Datta, S.; Samuel, J.; Dey, P.; Wang, S.; et al. Revealing on Hydrogen Sulfide and Nitric Oxide Signals Co-Ordination for Plant Growth under Stress Conditions. Physiol. Plant. 2020, 168, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.; Zandalinas, S.I.; Huck, C.; Fritschi, F.B.; Mittler, R. Meta-Analysis of Drought and Heat Stress Combination Impact on Crop Yield and Yield Components. Physiol. Plant. 2021, 171, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.; Wu, W. Soil and Crop Management Strategies to Ensure Higher Crop Productivity within Sustainable Environments. Sustainability 2019, 11, 1485. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alayafi, A.A. Overexpression of Rice Rab7 Gene Improves Drought and Heat Tolerance and Increases Grain Yield in Rice (Oryza sativa L.). Genes 2019, 10, 56. [Google Scholar] [CrossRef]
- Verma, S.; Dubey, R.S. Lead Toxicity Induces Lipid Peroxidation and Alters the Activities of Antioxidant Enzymes in Growing Rice Plants. Plant Sci. 2003, 164, 645–655. [Google Scholar] [CrossRef]
- Gechev, T.; Petrov, V. Reactive Oxygen Species and Abiotic Stress in Plants. Int. J. Mol. Sci. 2020, 21, 7433. [Google Scholar] [CrossRef]
- Devireddy, A.R.; Tschaplinski, T.J.; Tuskan, G.A.; Muchero, W.; Chen, J.G. Role of Reactive Oxygen Species and Hormones in Plant Responses to Temperature Changes. Int. J. Mol. Sci. 2021, 22, 8843. [Google Scholar] [CrossRef]
- Mishra, D.; Shekhar, S.; Singh, D.; Chakraborty, S.; Chakraborty, N. Heat Shock Proteins and Abiotic Stress Tolerance in Plants; Springer: New York, NY, USA, 2018; pp. 41–69. [Google Scholar] [CrossRef]
- Balchin, D.; Hayer-Hartl, M.; Hartl, F.U. In Vivo Aspects of Protein Folding and Quality Control. Science 2016, 353, aac4354. [Google Scholar] [CrossRef]
- Khan, S.; Jabeen, R.; Deeba, F.; Waheed, U.; Khanum, P.; Iqbal, N. Heat Shock Proteins: Classification, Functions and Expressions in Plants during Environmental Stresses. J. Bioresour. Manag. 2021, 8, 85–97. [Google Scholar] [CrossRef]
- Singh, L.P.; Gill, S.S.; Tuteja, N. Unraveling the Role of Fungal Symbionts in Plant Abiotic Stress Tolerance. Plant Signal. Behav. 2011, 6, 175–191. [Google Scholar] [CrossRef]
- Vermeulen, S.J.; Aggarwal, P.K.; Ainslie, A.; Angelone, C.; Campbell, B.M.; Challinor, A.J.; Hansen, J.W.; Ingram, J.S.I.; Jarvis, A.; Kristjanson, P.; et al. Options for Support to Agriculture and Food Security under Climate Change. Environ. Sci. Policy 2012, 15, 136–144. [Google Scholar] [CrossRef]
- Bakthisaran, R.; Tangirala, R.; Rao, C.M. Small Heat Shock Proteins: Role in Cellular Functions and Pathology. Biochim. Biophys. Acta Proteins Proteom. 2015, 1854, 291–319. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, J.; Li, X.; Li, Z.; Han, L.; Luo, H. Heat Shock Protein Integrates Different Signaling Pathways to Modulate Plant Abiotic Stress Response. BMC Plant Biol. 2020, 20, 184. [Google Scholar] [CrossRef]
- Wu, J.; Gao, T.; Hu, J.; Zhao, L.; Yu, C.; Ma, F. Research Advances in Function and Regulation Mechanisms of Plant Small Heat Shock Proteins (sHSPs) under Environmental Stresses. Sci. Total Environ. 2022, 825, 154054. [Google Scholar] [CrossRef]
- Collier, M.P.; Benesch, J.L.P. Small Heat-Shock Proteins and Their Role in Mechanical Stress. Cell Stress Chaperones 2020, 25, 601–613. [Google Scholar] [CrossRef]
- Waters, E.R.; Vierling, E. Plant Small Heat Shock Proteins—Evolutionary and Functional Diversity. New Phytol. 2020, 227, 24–37. [Google Scholar] [CrossRef]
- Park, C.J.; Seo, Y.S. Heat Shock Proteins: A Review of the Molecular Chaperones for Plant Immunity. Plant Pathol. J. 2015, 31, 323–333. [Google Scholar] [CrossRef]
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Liu, Q. Heat Shock Proteins: Biological Functions, Pathological Roles, and Therapeutic Opportunities. MedComm 2022, 3, e161. [Google Scholar] [CrossRef]
- Ul Haq, S.; Khan, A.; Ali, M.; Khattak, A.M.; Gai, W.X.; Zhang, H.X.; Wei, A.M.; Gong, Z.H. Heat Shock Proteins: Dynamic Biomolecules to Counter Plant Biotic and Abiotic Stresses. Int. J. Mol. Sci. 2019, 20, 5321. [Google Scholar] [CrossRef]
- Firmansyah, F.; Argosubekti, N. A Review of Heat Stress Signaling in Plants. IOP Conf. Ser. Earth Environ. Sci. 2020, 484, 012041. [Google Scholar] [CrossRef]
- Guo, L.M.; Li, J.; He, J.; Liu, H.; Zhang, H.M. A Class I Cytosolic HSP20 of Rice Enhances Heat and Salt Tolerance in Different Organisms. Sci. Rep. 2020, 10, 1383. [Google Scholar] [CrossRef]
- Lopes-Caitar, V.S.; de Carvalho, M.C.C.G.; Darben, L.M.; Kuwahara, M.K.; Nepomuceno, A.L.; Dias, W.P.; Abdelnoor, R.V.; Marcelino-Guimarães, F.C. Genome-Wide Analysis of the Hsp20 Gene Family in Soybean: Comprehensive Sequence, Genomic Organization and Expression Profile Analysis under Abiotic and Biotic Stresses. BMC Genom. 2013, 14, 577. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.T.; Cheng, G.X.; Huang, L.J.; Liu, S.; Ali, M.; Khan, A.; Yu, Q.H.; Yang, S.B.; Luo, D.X.; Gong, Z.H. Modified Expression of a Heat Shock Protein Gene, CaHSP22.0, Results in High Sensitivity to Heat and Salt Stress in Pepper (Capsicum annuum L.). Sci. Hortic. 2019, 249, 364–373. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S.; Chunduri, V.; Kaur, A.; Kaur, S.; Malhotra, N.; Kumar, A.; Kapoor, P.; Kumari, A.; Kaur, J.; et al. Genome-Wide Identification and Characterization of Heat Shock Protein Family Reveals Role in Development and Stress Conditions in Triticum aestivum L. Sci. Rep. 2020, 10, 7858. [Google Scholar] [CrossRef]
- Zou, J.; Liu, C.; Liu, A.; Zou, D.; Chen, X. Overexpression of OsHsp17.0 and OsHsp23.7 Enhances Drought and Salt Tolerance in Rice. J. Plant Physiol. 2012, 169, 628–635. [Google Scholar] [CrossRef]
- Sato, Y.; Yokoya, S. Enhanced Tolerance to Drought Stress in Transgenic Rice Plants Overexpressing a Small Heat-Shock Protein, SHSP17.7. Plant Cell Rep. 2008, 27, 329–334. [Google Scholar] [CrossRef]
- Murakami, T.; Matsuba, S.; Funatsuki, H.; Kawaguchi, K.; Saruyama, H.; Tanida, M.; Sato, Y. Over-Expression of a Small Heat Shock Protein, SHSP17.7, Confers Both Heat Tolerance and UV-B Resistance to Rice Plants. Mol. Breed. 2004, 13, 165–175. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Shao, L.Y.; Yan, X.; Peng, H.; Ouyang, J.X.; Li, S.B. Expression and Function Analysis of a Rice OsHSP40 Gene under Salt Stress. Genes Genom. 2019, 41, 175–182. [Google Scholar] [CrossRef]
- Bae, M.J.; Kim, Y.S.; Kim, I.S.; Choe, Y.H.; Lee, E.J.; Kim, Y.H.; Park, H.M.; Yoon, H.S. Transgenic Rice Overexpressing the Brassica Juncea Gamma-Glutamylcysteine Synthetase Gene Enhances Tolerance to Abiotic Stress and Improves Grain Yield under Paddy Field Conditions. Mol. Breed. 2013, 31, 931–945. [Google Scholar] [CrossRef]
- Gueta-Dahan, Y.; Yaniv, Z.; Zilinskas, B.A.; Ben-Hayyim, G. Salt and Oxidative Stress: Similar and Specific Responses and Their Relation to Salt Tolerance in Citrus. Planta 1997, 203, 460–469. [Google Scholar] [CrossRef]
- Park, S.I.; Kim, Y.S.; Kim, J.J.; Mok, J.E.; Kim, Y.H.; Park, H.M.; Kim, I.S.; Yoon, H.S. Improved Stress Tolerance and Productivity in Transgenic Rice Plants Constitutively Expressing the Oryza Sativa Glutathione Synthetase OsGS under Paddy Field Conditions. J. Plant Physiol. 2017, 215, 39–47. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, Y.S.; Lee, C.B. The Inductive Responses of the Antioxidant Enzymes by Salt Stress in the Rice (Oryza sativa L.). J. Plant Physiol. 2001, 158, 737–745. [Google Scholar] [CrossRef]
- Jebara, S.; Jebara, M.; Limam, F.; Aouani, M.E. Changes in Ascorbate Peroxidase, Catalase, Guaiacol Peroxidase and Superoxide Dismutase Activities in Common Bean (Phaseolus vulgaris) Nodules under Salt Stress. J. Plant Physiol. 2005, 162, 929–936. [Google Scholar] [CrossRef]
- Chen, T.H.H.; Murata, N. Enhancement of Tolerance of Abiotic Stress by Metabolic Engineering of Betaines and Other Compatible Solutes. Curr. Opin. Plant Biol. 2002, 5, 250–257. [Google Scholar] [CrossRef]
- Choe, Y.H.; Kim, Y.S.; Kim, I.S.; Bae, M.J.; Lee, E.J.; Kim, Y.H.; Park, H.M.; Yoon, H.S. Homologous Expression of γ-Glutamylcysteine Synthetase Increases Grain Yield and Tolerance of Transgenic Rice Plants to Environmental Stresses. J. Plant Physiol. 2013, 170, 610–618. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Long, R.-C.; Zhang, T.-J.; Yang, Q.-C.; Kang, J.-M. Molecular Cloning and Characterization of the MsHSP17.7 Gene from Medicago sativa L. Mol. Biol. Rep. 2016, 43, 815–826. [Google Scholar] [CrossRef]
- Yang, R.; Yu, G.; Li, H.; Li, X.; Mu, C. Overexpression of Small Heat Shock Protein LimHSP16.45 in Arabidopsis hsp17.6II Mutant Enhances Tolerance to Abiotic Stresses. Russ. J. Plant Physiol. 2020, 67, 231–241. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Panchuk, I.I.; Volkov, R.A.; Schöffl, F. Heat Stress- and Heat Shock Transcription Factor-Dependent Expression and Activity of Ascorbate Peroxidase in Arabidopsis. Plant Physiol. 2002, 129, 838–853. [Google Scholar] [CrossRef] [PubMed]


| Primer Set | 5′-3′ |
|---|---|
| OsHSP-F2 | AGCATCTTCCCGTCCTTCC |
| OsHSP-R4 | TGACCTCCTCCTTCTTCAGC |
| OsHSP-26.7-F | CAGGAGAACAGGGACAACAC |
| OsHSP-26.7-R | CCATCGTGTCCAGCATCT |
| OsHSP-23.2-F | ATGGCGTCGATGAGAACT |
| OsHSP-23.2-R | AGGTCCTCCTTCCTCATCC |
| OsHSP-18.1-F | AAGGAGCAGGAGGAGAAGAC |
| OsHSP-18.1-R | TAACCTGGATGGACTTGACG |
| OsHSP-17.7-F | AGGAGGAGAAGTCGGACAAG |
| OsHSP-17.7-R | AGATCTGGATGGACTTGACG |
| OsHSP-16.9-F | GAAGGAGGACAAGAACGACA |
| OsHSP-16.9-R | TTAACCGGAGATCTCAATGG |
| Tub-F | GAGTACCCTGACCGCATGAT |
| Tub-R | GTGGTCAGCTTGAGAGTCCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do, J.-M.; Kim, H.-J.; Shin, S.-Y.; Park, S.-I.; Kim, J.-J.; Yoon, H.-S. OsHSP 17.9, a Small Heat Shock Protein, Confers Improved Productivity and Tolerance to High Temperature and Salinity in a Natural Paddy Field in Transgenic Rice Plants. Agriculture 2023, 13, 931. https://doi.org/10.3390/agriculture13050931
Do J-M, Kim H-J, Shin S-Y, Park S-I, Kim J-J, Yoon H-S. OsHSP 17.9, a Small Heat Shock Protein, Confers Improved Productivity and Tolerance to High Temperature and Salinity in a Natural Paddy Field in Transgenic Rice Plants. Agriculture. 2023; 13(5):931. https://doi.org/10.3390/agriculture13050931
Chicago/Turabian StyleDo, Jeong-Mi, Hee-Jin Kim, Sun-Young Shin, Seong-Im Park, Jin-Ju Kim, and Ho-Sung Yoon. 2023. "OsHSP 17.9, a Small Heat Shock Protein, Confers Improved Productivity and Tolerance to High Temperature and Salinity in a Natural Paddy Field in Transgenic Rice Plants" Agriculture 13, no. 5: 931. https://doi.org/10.3390/agriculture13050931
APA StyleDo, J.-M., Kim, H.-J., Shin, S.-Y., Park, S.-I., Kim, J.-J., & Yoon, H.-S. (2023). OsHSP 17.9, a Small Heat Shock Protein, Confers Improved Productivity and Tolerance to High Temperature and Salinity in a Natural Paddy Field in Transgenic Rice Plants. Agriculture, 13(5), 931. https://doi.org/10.3390/agriculture13050931







