Abstract
The national regulation on animal welfare measure under the Rural Development Programme 2014–2022 identified grazing and helminth control as important measures to improve the welfare of cattle in Slovenia. The aim of the study was to evaluate these measures in terms of improving animal welfare and helminth control. Compositional fecal samples for coprological analysis were collected in the region of central Slovenia. Samples were qualitatively analysed for the presence of endoparasites using the flotation and sedimentation methods. During a seven-year period, 4480 compositional fecal samples were collected from cattle herds in the central Slovenia. In all seven years, the most prevalent helminths at the cattle herd level were Strongylida (ranging from 45.49% to 74.22%) and Paramphistomum sp. (ranging from 21.12% to 28.46%). After the treatments against helminths in grazing cattle, the prevalence of positive herds decreased from 83.63% to 63.64%. The calculated cross-correlation values showed significant positive association of the percentage of helminth-positive cattle herds with the prevalence of Fasciola hepatica (0.975), Nematodirus sp. (0.859), Strongylida (0.986), Strongyloides sp. (0.879) and Trichuris sp. (0.835). Hence, the efficient helminth control and improved animal welfare, as well as financial support of 53.40 EUR per livestock unit, contributed to a positive outcome of the programme.
1. Introduction
Helminth infections are widespread in livestock production and negatively affect feed intake, growth, productivity, reproductive performance, health status, and welfare. They also increase greenhouse gas emissions associated with ruminant production [1]. Nematode infection has been reported to be the second leading cause of health care costs among dairy farmers after mastitis, costing in the Netherlands, for instance, an estimated 19 million EUR annually [2]. In addition, the annual costs due to helminth infections in dairy and livestock farming in 18 European countries are estimated to be 941 million EUR and 1.8 billion EUR, respectively [3]. The detrimental effects of helminth infections on livestock production efficiency and animal health necessitate effective control of parasites to achieve goals such as future protein requirements, more efficient and sustainable production, improved health status, and animal welfare [1,4,5].
All grazing animals are exposed to helminth infections on pasture, and any future intensification of pasture-based systems will likely increase the risk of helminth disease. Gastrointestinal nematodes (GIN) and liver flukes are the two major causes of productivity loss in ruminants, with lungworms also playing a role in some cases [6]. The liver fluke Fasciola hepatica is a known disruptor of productivity and immune responses in cattle [7]. F. hepatica occurs worldwide, but its prevalence is often regionally clustered because it requires suitable environmental conditions for its snail intermediate host [8].
Although there have been and continue to be a number of different approaches to controlling helminth infections in livestock, including nutritional, environmental, immunological, and biological measures [9], effective control currently relies almost exclusively on effective anthelmintics [6]. However, due to their genetic diversity and resistance to anthelmintics, helminth infections have consistently evolved to circumvent existing control measures [10]. In addition, the appearance of anthelmintic resistance is increasing due to the development of very effective nematode control programs that have significantly improved productivity in livestock production, but, at the same time, have exerted high selection pressure on the parasite genome [11,12]. For decades, benzimidazoles such as albendazole, imidothiazoles-tetrahydropyrimidines, and avermectin have been the major anthelmintic classes used for treating gastrointestinal nematodes of small ruminants and cattle [13,14].
Agriculture and animal husbandry in Slovenia strive for higher productivity of cultivated land. However, given the resources available for agriculture, Slovenia cannot keep up with most European countries. The main reason for this is the fact that most of the agricultural land used for animal husbandry is located in less favoured areas (LFA). As a result, traditional forms of agriculture disappeared, and much agricultural land, especially in LFAs, was abandoned and covered with woody vegetation, partly due to intensive land use in lowlands and technologies that developed a system for keeping dairy cattle indoors. As a result, the majority of Slovenian cattle are kept indoors, with 75% in tie stall barns. Recently, EU countries have implemented national and regional rural development programmes co-financed by the European Agricultural Fund for Rural Development (EAFRD) and national budgets. The national regulation on animal welfare measures under the Rural Development Programme 2014–2022 includes grazing and helminth control as important measures to improve the welfare of cattle in Slovenia [15,16]. One of the objectives of the regulation was to encourage and financially support farmers to use continuous grazing of cattle (grazing is not common in Slovenia) and helminth control as measures of animal welfare. The aim of this retrospective study is to evaluate these measures in terms of improving the welfare of the cattle.
2. Materials and Methods
2.1. National Regulation on Animal Welfare Measure Protocol
Every head of a cattle establishment in Slovenia could apply for the National Regulation on Animal Welfare Measures call under the Rural Development Programme 2014–2022 [16] if they met the following requirements:
- Mandatory continuing education course on animal welfare measures for 4 h.
- Minimum number of cattle owning being 2 livestock units (LSU). For the calculation, a coefficient of 0.4 applies to animals up to 6 months old, a coefficient of 0.6 applies to animals 6 months to 2 years old, and a coefficient of 1.0 applies to animals 2 years old and over.
- Cattle must be grazed continuously for at least 120 days yearly between 1 April and 15 November (“Cattle Grazing Period”).
- The cattle may stay in the stables during the night.
- Dairy cows must be continuously grazed daily between milking times.
- Helminth control must be based on qualitative preliminary coprological analysis.
- Fecal samples for coprological analysis must be collected once before the start of each grazing season; one composite sample is collected from 1–20 cattle.
- Treatment of animals with anthelmintics is based on positive results of coprological analysis and the professional judgement of the veterinarian, which must be recorded in the farm veterinary intervention book. Dairy cows may be treated during the dry period.
If the requirements are met, the head of a cattle establishment is entitled to a payment of 53.40 EUR annually per LSU from The Animal Welfare Measures.
2.2. Feces Sampling and Coprological Analysis
In the Central Slovenia region (Figure 1), compositional fecal samples were collected for coprological analysis, one composite sample from 1–20 cattle, regardless of animal category or age, and sent to the Institute of Microbiology and Parasitology, Veterinary Faculty, University of Ljubljana. Samplers were instructed to form a fresh composite sample by combining at least 5 individual samples of 20 grammes (two large spoons) collected from different animals and send to a laboratory within 24 h. The composite fecal samples were collected before cattle went out to pasture (before 1 April), which allowed veterinarians and farmers to determine the treatment strategy.
Figure 1.
Density of cattle establishments in Central Slovenia region.
In total, 4480 compositional fecal samples were examined in the 7-year program. Thus, in 2016, 673 composite samples were examined in 281 herds; in 2017, 711 composite samples were examined in 286 herds; in 2018, 730 composite samples were examined in 301 herds; in 2019, 619 composite samples were examined in 303 herds; in 2020, 671 composite samples were examined in 337 herds; in 2021, 567 composite samples were examined in 288 herds; and in 2022, 509 composite samples were examined in 252 herds. 90% of all herds included in the study had less than 50 animals.
The fecal samples were examined for the presence of endoparasites using the flotation and the sedimentation methods. A saturated salt solution (density 1.20 at 20 °C) was used to flotate nematode and cestode eggs, and tap water was used for sedimentation [17]. Taxon determination was based on the different egg morphotype.
2.3. Treatment
The anthelmintics used to treat helminths were macrocyclic lactones, benzimidazoles, levamisole, and oxyclozanide, as indicated by coprological results and records in the veterinary intervention books of the farms. The decisions on the need to treat animals in the herd and the categories to be treated, the anthelmintics to be used, and the most appropriate time for treatment were made in consultation between the bovine veterinary practitioner and the animal owner. On Slovenian farms, it is usually decided to treat the animals before they go out to pasture. If necessary, the additional use of anthelmintics is decided mainly on the basis of the available pasture area and microclimatic conditions (moisture of the pasture, sun exposure to the pasture, botanical composition of the sward, etc.). Production also plays an important role in the choice of active ingredient in terms of withdrawal periods.
2.4. Statistical Analysis
Statistical analyses were performed using the software package R 4.1.0 [18]. Time series cross-correlation analysis has been used to determine the relationship between the yearly (from 2016 to 2022) percentage prevalence of different helminths in cattle herds on one side and the percentage of all positive cattle herds on the other side. In addition, the similarity of pairs of given time series as functions of displacement (lag of one or more years) was measured, which gives us the possibility to detect whether the increase/decrease in one series causes the increase/decrease in the other series in the same year or even some years later. Here, the cross-correlation values at lag zero (without displacement) have been determined as the Pearson correlation coefficient by pairing the data at identical time points. Using linear regression modelling accounting for the time-dependent nature of data, linear models explaining the yearly percentage of positive cattle herds in dependence of the percentage prevalence of helminths have been set up. The significance of the linear regression models at 0.05 (or 5%) level of significance has been further assessed using the residual standard error (i.e., a measure of variability of the residuals from a linear model), the F-statistic (i.e., a quotient of the variability of the fitted values to the variability of the residuals), and the p-value (i.e., an indicator of acceptance/rejection of the null hypothesis stating that the predictor variable has no effect on the response variable). Due to the relatively small sample of 7 yearly values for each helminth, no further time series analysis in terms of level, noise, trend, and seasonality have been performed [19]. Simple linear regression modelling accounting for the time-dependent nature of data has also been performed to detect possible linear trends in the yearly percentage prevalence of helminths. The significance of the linear regression models at 0.05 level of significance has been assessed using the residual standard error, the F-statistic, the coefficient of determination (i.e., the proportion of the variability of the dependent variable that is predictable from the independent variable), and the p-value.
3. Results
During a 7-year long period, 4480 compositional fecal samples were collected in total on cattle herds in the Central Slovenia region. Overall, among the 1558 positive herds, 894 (57.4%) had one helminth taxon, followed by 483 with two, 145 with three, and 36 with four or more taxa. Prevalence is shown in Table 1. Herd-level prevalence has been calculated for various helminths and presented in Table 2.

Table 1.
Yearly prevalence of single and multiple helminth infections on herd level.

Table 2.
Prevalence of different helminths in cattle herds included in the National Regulation on Animal Welfare Measures 2016–2022 programme.
The number of cattle herds included in the yearly animal welfare measures varied from 253 to 337 (Table 2).
All the cross-correlation values indicating the relationship between the yearly percentage prevalence of different helminths in cattle herds on one side and the percentage of all positive cattle herds on the other side (with 1 indicating perfect cross-correlation and −1 indicating perfect anti-cross-correlation of time series) are given in Table 3. The only statistically significant cross-correlation values at the 5% level of significance were found at lag zero. This means that the only direct association between the prevalence of any specific helminth in cattle herds and the percentage of all positive cattle herds has been found in the same year and not eventually a year earlier/later. The estimated cross-correlation values (at lag zero) reveal a significant positive association for 5 of the 10 helminths that have been considered in this research: Fasciola hepatica (0.975), Nematodirus sp. (0.859), Strongylida (0.986), Strongyloides sp. (0.879), and Trichuris sp. (0.835). Exclusively the linear regression models of the yearly percentage of positive cattle herds in dependence of the percent-age prevalence of any one of these 5 helminths have given a p-value less than 0.05.

Table 3.
Results of cross-correlation analysis of yearly percentage prevalence of helminths in cattle herds and the percentage of all positive cattle herds.
The results of linear regression analysis, which has been performed with the aim to detect/visualize possible linear trends in the yearly percentage prevalence of helminths, are given in Table 4. Statistically significant linear trends were found for 4 of the 10 helminths (on herd level) that have been considered in this research: Fasciola hepatica, Nematodirus sp., Strongylida, and Strongyloides sp. (Figure 2). Exclusively, the linear regression models of the yearly percentage prevalence of any one of these four helminths give a p-value less than 0.05. The corresponding coefficients of determination (R2) are 0.746, 0.833, 0. 868, and 0.665, respectively. Observe (Table 4) that the yearly percentage prevalence of Trichuris sp. does not show a statistically significant linear trend at the 5% level of significance. However, the p-value of 0.1036 is not large, which was expected because there is a positive association (see Table 3) between the yearly percentage prevalence of Trichuris sp. and the yearly percentage of positive herds (last row of Table 4), showing a statistically significant linear trend.

Table 4.
Linear regression analysis results of significant trends in helminth prevalence.
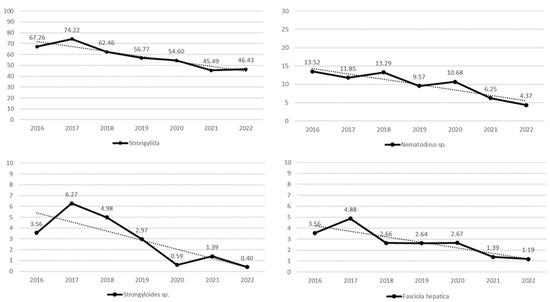
Figure 2.
Linear trends in yearly prevalence ( , %) of helminths on herd level (F. hepatica, p = 0.01224, Nematodirus sp., p = 0.004143, Strongylida, p = 0.002249, and Strongyloides sp., p = 0.02536) from 2016 to 2022.
, %) of helminths on herd level (F. hepatica, p = 0.01224, Nematodirus sp., p = 0.004143, Strongylida, p = 0.002249, and Strongyloides sp., p = 0.02536) from 2016 to 2022.
 , %) of helminths on herd level (F. hepatica, p = 0.01224, Nematodirus sp., p = 0.004143, Strongylida, p = 0.002249, and Strongyloides sp., p = 0.02536) from 2016 to 2022.
, %) of helminths on herd level (F. hepatica, p = 0.01224, Nematodirus sp., p = 0.004143, Strongylida, p = 0.002249, and Strongyloides sp., p = 0.02536) from 2016 to 2022.
4. Discussion
The national regulation on animal welfare measures under the Rural Development Programme 2014–2022 had several objectives. First and foremost, it seeks to encourage heads of cattle establishments to use grazing areas for cattle in LFAs that not only allow the animals to stay, move, and graze outside the barn (which improves their welfare) but also protects the landscape from undesirable afforestation. Further objectives are the improvement of the health status of animals through helminth control and treatment as well as providing financial support for carrying out measurements.
In Europe, there are similar voluntary programmes aimed at providing the livestock industry with the best available evidence-based information for sustainable parasite control through surveillance and treatment [20,21]. This underscores the recognized importance of helminth infections and suggests that surveillance campaigns generally contribute positively to parasite control [22]. However, if farmers also received direct financial payments (e.g., 53.40 euros per LSU), the impact of such programmes was even greater.
Of all 4272 cattle establishments in the Central Slovenia region, only 6–8% met the requirements or were motivated enough to participate in the national programme covered in this study. The mandatory continuing education course on animal welfare measures qualified the participants to take composite samples in cattle herds according to the protocol. One composite sample per 1–20 animals was considered to be sufficient to determine helminths at the herd level. According to George et al. [23], collecting fecal samples from a group of cattle (up to 20 animals) and then performing fecal egg counts on this composite sample yields very similar results to performing individual samples. The use of composite samples has been widely studied for endoparasites, with particular emphasis on gastrointestinal nematodes. Individual and composite sampling have their advantages, such as lower cost and labour for pools and a more complete representation of infection burden for individual samples [24]. Although sampling at a single time point in a cross-sectional study could limit the sensitivity of parasite detection, leading to an underestimation of true parasite prevalence, it provides an accessible and convenient source of up-to-date results when longitudinal or experimental studies are rarely available [25].
As shown in Table 1, the proportions of single and multiple helminth infections determined in composite samples in the present study showed that 57.4% had one endoparasite taxon, 31.0% had two, 9.3% had three, and 2.3% had four or more taxa. May et al. [26] reported similar results of 61.3%, 30.3%, 7.6%, and 0.8% for one, two, three, and four or more taxa, respectively, but at the individual level.
Nematodes of the order Strongylida are an important group of gastrointestinal helminths that significantly affect the health of ruminants. In general, Strongylida species cannot be distinguished by the examination of their eggs; instead, coproculture followed by identification of the third larval stage is required, which is time-consuming and requires an experienced investigator. Therefore, relatively rapid, uncomplicated, and inexpensive PCR methods are becoming very useful for identification and confirmation of morphological diagnoses [27]. The prevalence of positive herds in this 7-year-long study ranged from 89.2% to 63.64%, and of all the helminths detected, Strongylida appeared to be the most common endoparasite on herd level. Although many authors worldwide focus on the prevalence of helminths at the individual animal level [28,29,30,31] and few at the herd level [14], all these studies showed similar results to the present study.
The treatment of animals with anthelmintics was based on positive results of compositional analysis and professional judgement of the bovine practitioner. Depending on clinical relevance, anthelmintics were mainly used against Strongylida and F. hepatica, which also affected some other helminths such as Strongyloides spp. and Nematodirus sp. in the case of multiple infections. In other cases, helminths were not treated causally because they were either of relatively low clinical importance [32,33,34] or low farm prevalence. However, we are aware that a part of the anthelmintics could still be used indiscriminately, either because parasite levels were too low to warrant treatment or because treatments were not programmed correctly. This could easily lead to under- or over-treatment [10,35] and anthelmintic resistance [1].
The endohelminth fauna in Slovenia is well known in domestic and wild ruminants. In the 1980s, systematic parasitological, faunal and epizootiological studies of the gastrointestinal tract of wild ruminants, sheep, goats, and cattle were carried out in Slovenia. Bidovec [36] found that Ostertagia circumcincta (O. circumcincta), Haemonchus contortus (H. contortus), Spiculopteragia spiculoptera, and Trichuris ovis were the most prevalent species. Otherwise, 36 different species of gastrointestinal helminths were identified by the above author. In studying endohelminths from the gastrointestinal tract of domestic ruminants in Slovenia, Kopitar [37] found that the following species were most frequent: O. circumcincta (52.47%), H. contortus (38.61%), Trichostrongylus axei (21.78%), Trichostrongylus vitrinus (21.45%), Trichostrongylus capricola (20.13%), Nematodirus filicollis (17.16%), Ostertagia trifurcata (16.83%), Strongyloides papillosus (16.50%) and Trichostrongylus colubriformis (10.89%). Based on his research, Brglez [38] states that the frequency of parasites in sheep in the next ten years has changed, with the prevalence of O. circumcincta (82%), T. axei (57%), Nematodirus spathiger (55%), N. filicollis (42%), T. vitrinus (22%), H. contortus (18%), and Chabertia ovina (25%). During the parasitological-faunal and epizootiological studies conducted in 1984–1987 on 257 samples of abomasum and intestine of cattle, among the 24 species of endohelminths detected in cattle in Slovenia, the following endoparasites were found: Ostertagia ostertagi, H. contortus, Ostertagia lyrata, Cooperia oncophora, Cooperia punctata, N. filicollis, N. spathiger, and some trichostrongylids of the genus Trichostrongylus were the most common. The prevalences found in these 257 animals were for Ostertagia spp. (86.77%), Nematodirus spp. (68.09%), Cooperia spp. (54.09%), C. ovina (54.47%), H. contortus (45.14%), Trichuris spp. (35.02%), Trichostrongylus spp. (19.07%), Oesophagostomum radiatum (19.07%), Moniezia spp. (14.01%), Capillaria spp. (5.84%), S. papillosus (5.06%), Neoascaris vitulorum (4.28%), and Bunostomum phlebotomum (2.72%) [39]. Some differences were noted depending on the age of the animals and geographic areas in Slovenia. In calves, Strongyloides papillosus was detected in 65.52% of the animals examined. In grazing cattle, the results showed that after housing, abdomina were rich in hypobiotic larvae of Ostertagia. Under our environmental and husbandry conditions, the “spring-rise phenomenon” and the “self-cure phenomenon” were predominant in cattle. In calves aged 3 months from the north-eastern part of Slovenia, nematodirosis was very common. Calves grazing near estates have also been found to be heavily infected [39]. Although some studies have been conducted since 1980, no reports or clinical problems related to antihelminth resistance in cattle in Slovenia have been identified to date. Comparing the nematode prevalence rates found in Slovenia in the 1980s [39] with those in the present study, all have declined sharply. The prevalence of Trichuris spp. decreased significantly from 35.02% to 0.04–2.44% in the present study (Table 2). The same is observed for Nematodirus spp., where the prevalence has decreased from 68.09% to between 4.35% and 13.52%. A significant decrease is also observed for Capillaria spp. It is evident that the management of parasitosis has improved considerably since the 1980s, and it is particularly noteworthy that, exceptionally, there are no reports of anthelmintic resistance in parasites in cattle.
A decrease in the prevalence of Strongylida, including Nematodirus sp., and Strongyloides sp., as well as F. hepatica (Table 2 and Figure 2), is most likely the result of efficient helminth control and treatment, which could contribute to improved animal welfare, health status, and production performance. However, it is also true that the presence of helminths, which may be considered inevitable in grazing ruminants, does not always result in a decrease in animal welfare. Within a certain level of infection, it is considered that parasites may even have beneficial effects by stimulating the immune system and that their absence may make animals more vulnerable to new infections [40]. However, the decrease in the prevalence of helminth-positive herds from 83.63% to 63.64%. (Table 2) is promising and indicates the efficacy of helminth control and treatment strategy. It is also true that the financial support of the Animal Welfare Measures programme has encouraged cattle owners to allow their animals to be outdoor and graze, which would not have happened otherwise.
There was no decrease in the prevalence of clinically less significant helminths or helminths with low herd prevalence (Table 2). Such results were expected because cattle were not treated, partly to avoid unnecessary costs due to the use of anthelmintics and to avoid milk withdrawal costs.
5. Conclusions
Despite the relatively low percentage of participants, a constantly high number of cattle herds in the Central Slovenia region that continuously participated in the national programme over the period of seven years shows that the heads of cattle establishments were motivated enough to comply with the requirements of the programme and allow cattle to graze and treat them against helminths.
In the light of the experience of previous years, the measure should be upgraded with quantitative methods, at least for those parasites whose presence is detected by flotation. A uniform protocol should also be established for the use of active substances and for checking the effectiveness of treatment in order to detect the emergence of resistance at an early stage.
Although the financial support of 53.40 EUR per LSU was probably one of the most important incentives, the improved welfare and efficient helminth control contributed to a better overall outcome of the programme.
Author Contributions
Conceptualization, O.P. and J.P.; methodology, O.P., M.H. and J.P.; software, O.P., M.H. and J.P.; validation, O.P., M.H. and J.P.; formal analysis, M.H.; investigation, O.P. and J.P.; resources, J.P.; data curation, O.P., M.H. and J.P.; writing—original draft preparation, O.P.; writing—review and editing, O.P., M.H. and J.P.; visualization, O.P.; supervision, M.H. and J.P.; project administration, O.P.; funding acquisition, J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the Slovenian Research Agency: P2-0250 and P4-0053.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available and can be provided by the authors upon request.
Acknowledgments
The authors thank Jedrt Maurer Wernig, Administration of the Republic of Slovenia for Food Safety, Veterinary Service and Plant Protection, for the information on cattle establishments in the Central Slovenia region.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vineer, H.R.; Morgan, E.R.; Hertzberg, H.; Bartley, D.J.; Bosco, A.; Charlier, J.; Chartier, C.; Claerebout, E.; De Waal, T.; Hendrickx, G.; et al. Increasing importance of anthelmintic resistance in European livestock: Creation and meta-analysis of an Open Database. Parasite 2020, 27, 69. [Google Scholar]
- Coppieters, W.; Mes, T.H.M.; Druet, T.; Farnir, F.; Tamma, N.; Schrooten, C.; Cornelissen, A.W.; Georges, M.; Ploeger, H.W. Mapping QTL influencing gastrointestinal nematode burden in Dutch Holstein-Friesian dairy cattle. BMC Genom. 2009, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Charlier, J.; Rinaldi, L.; Musella, V.; Ploeger, H.W.; Chartier, C.; Vineer, H.R.; Hinney, B.; von Samson-Himmelstjerna, G.; Băcescu, B.; Mickiewicz, M.; et al. Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe. Prev. Vet. Med. 2020, 182, 105103. [Google Scholar] [CrossRef] [PubMed]
- Thornton, P.K. Livestock production: Recent trends, future prospects. Phil. Trans. R. Soc. B 2010, 365, 2853–2867. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, S.; Vitali, A.; Lacetera, N.; Amon, B.; Bannink, A.; Bartley, D.J.; Blanco-Penedo, I.; de Haas, Y.; Dufrasne, I.; Elliott, J.; et al. Challenges and priorities for modelling livestock health and pathogens in the context of climate change. Environ. Res. 2016, 151, 130–144. [Google Scholar] [CrossRef]
- Morgan, E.R.; Charlier, J.; Hendrickx, G.; Biggeri, A.; Catalan, D.; von Samson-Himmelstjerna, G.; Demeler, J.; Müller, E.; Van Dijk, J.; Kenyon, F.; et al. Global change and helminth infections in grazing ruminants: Impacts, trends and sustainable solutions. Agriculture 2013, 3, 484–502. [Google Scholar] [CrossRef]
- Carroll, R.I.; Forbes, A.; Graham, D.A.; Messam, L.L. McV. The impact of liver fluke infection on steers in Ireland: A meta-analytic approach. Prev. Vet. Med. 2020, 174, 104807. [Google Scholar] [CrossRef]
- Beesley, N.J.; Caminade, C.; Charlier, J.; Flynn, R.J.; Hodgkinson, J.E.; Martinez-Moreno, A.; Martinez-Valladares, M.; Perez, J.; Rinaldi, L.; Williams, D.J.L. Fasciola and fasciolosis in ruminants in Europe: Identifying research needs. Transbound. Emerg. Dis. 2018, 65, 199–216. [Google Scholar] [CrossRef]
- Jackson, F.; Miller, J. Alternative approaches to control—Quo vadit? Vet. Parasitol. 2006, 139, 371–384. [Google Scholar] [CrossRef]
- Vercruysse, J.; Dijk, V.; Morgan, J.; Geary, E.R.; Samson-Himmelstjerna, V.; Claerebout, E. Control of helminth ruminant infect.ions by 2030. Parasitology 2018, 145, 1655–1664. [Google Scholar] [CrossRef]
- Fissiha, W.; Kinde, M.Z. Anthelmintic resistance and its mechanism: A review. Infect. Drug. Resist. 2021, 14, 5403–5410. [Google Scholar] [CrossRef]
- Gasbarre, L.C. Anthelmintic resistance in cattle nematodes in the US. Vet. Parasitol. 2014, 204, 3–11. [Google Scholar] [CrossRef]
- Mederos, A.E.; Carracelas, B.; Minho, A.P.; Fernández, S.; Sánchez, J. Prevalence and factors associated with anthelmintic resistance in gastrointestinal nematodes of cattle: A systematic review and meta-analysis. J. Vet. Med. Health 2018, 2, 2. [Google Scholar]
- Income, N.; Tongshoob, J.; Taksinoros, S.; Adisakwattana, P.; Rotejanaprasert, C.; Maneekan, P.; Kosoltanapiwat, N. Helminth infections in cattle and goats in Kanchanaburi, Thailand, with focus on strongyle nematode infections. Vet. Sci. 2021, 8, 324. [Google Scholar] [CrossRef]
- European Commission. Factsheet on the 2014–2022 Rural Development Programme for Slovenia. (August 2021). Available online: https://ec.europa.eu/info/sites/default/files/food-farming-fisheries/key_policies/documents/rdp-factsheet-slovenia_en.pdf (accessed on 12 August 2022).
- Uredba o Ukrepu Dobrobit Živali iz Programa Razvoja Podeželja Republike Slovenije za Obdobje 2014–2020 v Letu 2022. Uradni List RS, št. 203/21: 12959. Available online: http://www.pisrs.si/Pis.web/pregledPredpisa?id=URED8412 (accessed on 20 January 2023).
- Thienpont, D.; Rochette, F.; Vanparijs, O.F.J. Diagnosing Helminthiasis by Coprological Examination, 3rd ed.; Janssen Research Foundation: Beerse, Belgium, 2003; pp. 31–34. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Kedem, B.; Fokianos, K. Regression Models for Time Series Analysis; Wiley Series in Probability and Statistics; Wiley: Hoboken, NJ, USA, 2005; ISBN 978-0-471-46168-5. [Google Scholar]
- Control Of Worms Sustainably (COWS) Integrated Parasite Control on Cattle Farms. Available online: https://www.cattleparasites.org.uk/app/uploads/2020/06/integrated-control-190520-FINAL.pdf (accessed on 12 August 2022).
- National Animal Disease Information Service (NADIS). Parasite Forecasts. Available online: https://nadis.org.uk/parasite-forecast/ (accessed on 12 August 2022).
- Charlier, J.; Meyns, T.; Soenen, K.; Vercruysse, J. Monitoring gastrointestinal nematode and liver fluke infections in Belgium by bulk tank milk ELISA: Are we making progress in parasite control? Vlaams Diergeneeskd. Tijdschr. 2013, 82, 17–22. [Google Scholar] [CrossRef]
- George, M.M.; Paras, K.L.; Howell, S.B.; Kaplan, R.M. Utilization of composite fecal samples for detection of anthelmintic resistance in gastrointestinal nematodes of cattle. Vet. Parasitol. 2017, 240, 24–29. [Google Scholar] [CrossRef]
- Maurizio, A.; di Regalbono, A.F.; Cassini, R. Quantitative monitoring of selected groups of parasites in domestic ruminants: A comparative review. Pathogens 2021, 10, 1173. [Google Scholar] [CrossRef]
- Raue, K.; Heuer, L.; Böhm, C.; Wolken, S.; Epe, C.; Strube, C. 10-year parasitological examination results (2003 to 2012) of faecal samples from horses, ruminants, pigs, dogs, cats, rabbits and hedgehogs. Parasitol. Res. 2017, 116, 3315–3330. [Google Scholar] [CrossRef]
- May, K.; Raue, K.; Blazejak, K.; Jordan, D.; Strube, C. Pasture rewetting in the context of nature conservation shows no long-term impact on endoparasite infections in sheep and cattle. Parasites Vectors 2022, 15, 33. [Google Scholar]
- Tan, T.K.; Panchadcharam, C.; Low, V.L.; Lee, S.C.; Ngui, R.; Sharma, R.S.; Lim, Y. Co-infection of Haemonchus contortus and Trichostrongylus spp. among livestock in Malaysia as revealed by amplification and sequencing of the internal transcribed spacer II DNA region. BMC Vet. Res. 2014, 10, 38. [Google Scholar] [CrossRef]
- Chiu-Chen, H.; Lian-Chen, W.; Chien-Hung, P.; Cheng-Hsiung, Y.; Cheng-Hung, L. Investigation of gastrointestinal parasites of dairy cattle around Taiwan. J. Microbiol. Immunol. Infect. 2014, 47, 70–74. [Google Scholar]
- Pinilla León, J.C.; Delgado, U.N.; Florez, A.A. Prevalence of gastrointestinal parasites in cattle and sheep in three municipalities in the Colombian Northeastern Mountain. Vet. World 2019, 12, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Obi, C.F.; Akata, M.C.; Ezubelu, O.J. Prevalence of gastrointestinal helminth parasites of trade cattle in Aguata and Orumba South Local Government Areas, Southeastern Nigeria. J. Parasit. Dis. 2020, 44, 546–552. [Google Scholar] [CrossRef]
- Haymanot, F.; Kaba, T. Prevalence and associated factors of gastrointestinal helminthiasis of lactating cow and effect of strategic deworming on milk quantity, fat, and protein in Kucha, Ethiopia. BMC Vet. Res. 2022, 18, 150. [Google Scholar] [CrossRef] [PubMed]
- Craig, T.M. Helminth parasites of the ruminant gastrointestinal tract. Food Anim. Pract. 2009, 5, 78–91. [Google Scholar]
- Irie, T.; Sakaguchi, K.; Ota-Tomita, A.; Tanida, M.; Hidaka, K.; Kirino, Y.; Nonaka, N.; Horii, Y. Continuous Moniezia benedeni infection in confined cattle possibly maintained by an intermediate host on the farm. J. Vet. Med. Sci. 2013, 75, 1585–1589. [Google Scholar] [CrossRef]
- Thamsborg, S.; Ketzis, J.; Horii, Y.; Matthews, J.B. Strongyloides spp. infections of veterinary importance. Parasitology 2017, 144, 274–284. [Google Scholar] [CrossRef]
- Vercruysse, J.; Claerebout, E. Treatment vs non-treatment of helminth infections in cattle: Defining the threshold. Vet. Parasitol. 2001, 98, 195–214. [Google Scholar] [CrossRef]
- Bidovec, A. Preučevanje Endohelmintov iz Prebavil Divjih Prežvekovalcev v Sloveniji: Doktorska Disertacija; BF VTOZD za Veterinarstvo: Ljubljana, Slovenia, 1984. [Google Scholar]
- Kopitar, M. Preučevanje Endohelmintov iz Prebavil Drobnice v Sloveniji: Doktorska Diseratcija; BF VTOZD za Veterinarstvo: Ljubljana, Slovenia, 1984. [Google Scholar]
- Brglez, J. Načrtovano zatiranje zajedavskih bolezni pri drobnici. In Zbornik Možnosti Razvoja Reje Drobnice v Sloveniji; Kmetijska Založba: Slovenj Gradec, Slovenia, 1996; Volume 1, pp. 198–202. [Google Scholar]
- Bešvir, J. Examination of Endohelminths from the Digestive Tract of Cattle in Slovenia. Doctoral Thesis, BF VTOZD za Veterinarstvo, Ljubljana, Slovenia, 1988. [Google Scholar]
- Charlier, J.; Höglund, J.; Morgan, E.R.; Geldhof, P.; Vercruysse, J.; Claerebout, E. Biology and epidemiology of gastrointestinal nematodes in cattle. Vet. Clin. North Am. Food. Anim. Pract. 2020, 36, 1–15. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).