Hydrogen Peroxide Mitigates Cu Stress in Wheat
Abstract
1. Introduction
2. Results
2.1. Effects of Hydrogen Peroxide on Plant Height of Wheat under Cu Stress
2.2. Effects of Hydrogen Peroxide on Leaf Area of Wheat under Cu Stress
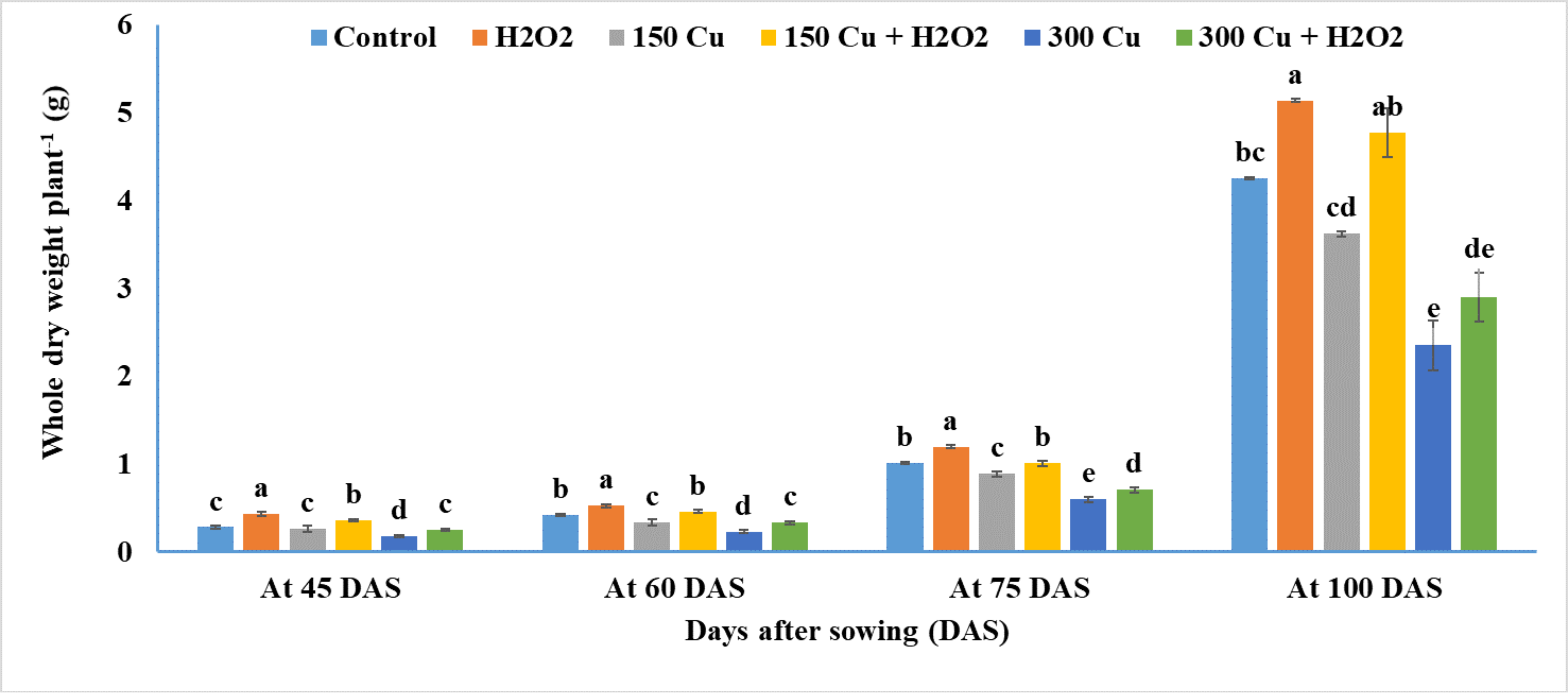
2.3. Effects of Hydrogen Peroxide on Whole Dry Weight of Wheat under Cu Stress
2.4. Effects of Hydrogen Peroxide on Total Chlorophyll of Wheat under Cu Stress
2.5. Effects of Hydrogen Peroxide on Net Photosynthesis and Stomatal Conductance of Wheat under Cu Stress
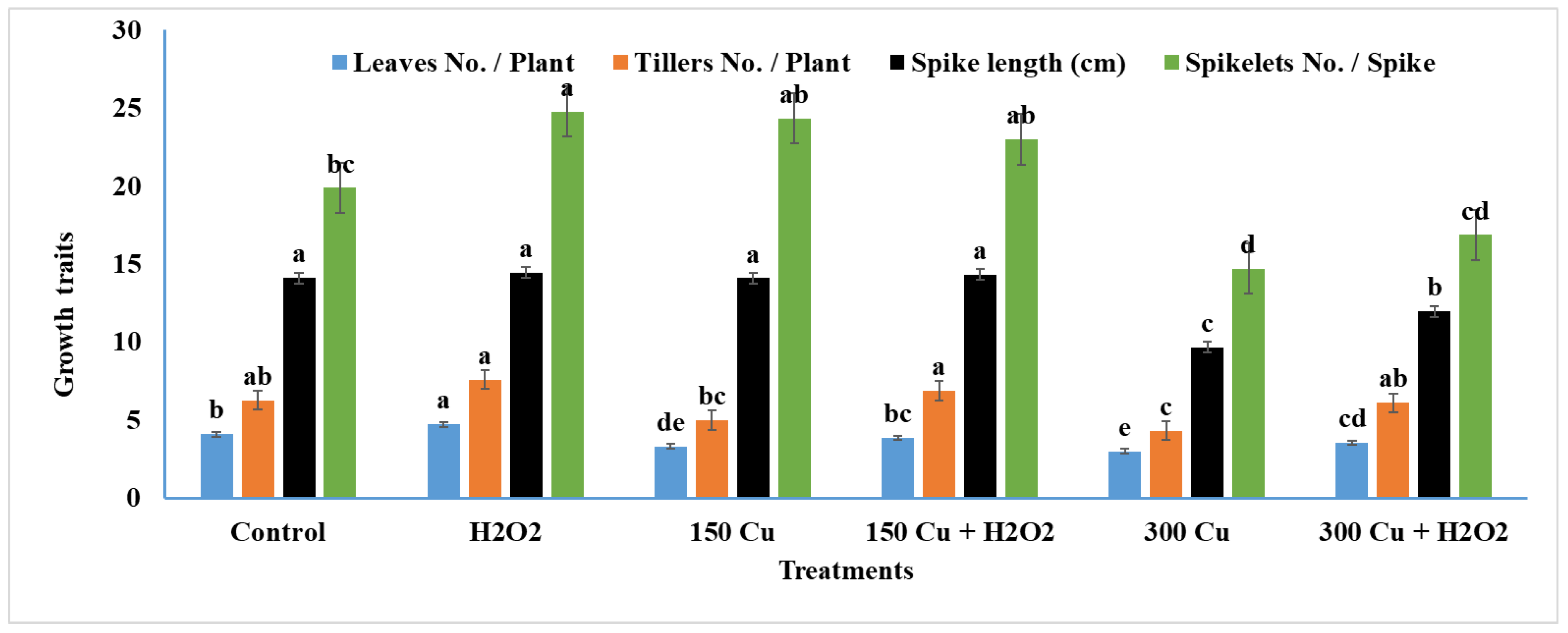
2.6. Effects of Hydrogen Peroxide on Growth Traits of Wheat under Cu Stress
2.7. Effects of Hydrogen Peroxide on Yield Traits of Wheat under Cu Stress
2.8. Effects of Hydrogen Peroxide on Cu, Zn, and Cd Concentration of Whole Wheat Plants Grown under Cu Stress
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Measurements
4.2.1. Plant Height and Leaf Area
4.2.2. Number of Leaves, Number of Tillers, and Whole Dry Weight per Plant
4.2.3. Total Chlorophyll
4.2.4. Gas Exchange Characteristics
4.2.5. Yield Traits
4.2.6. Elemental Analysis
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Seleiman, M.F.; Santanen, A.; Stoddard, F.L.; Mäkelä, P.S.A. Feedstock quality and growth of bioenergy crops fertilized with sewage sludge. Chemosphere 2012, 89, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Zhou, Y.; Chen, Z.; Jia, J.; Bao, X. Heavy metals and lead isotopes in soils, road dust and leafy vegetables and health risks via vegetable consumption in the industrial areas of Shanghai, China. Sci. Total Environ. 2018, 619, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Selim, S.; Jaakkola, S.; Mäkelä, P. Chemical composition and in vitro digestibility of whole-crop maize fertilized with synthetic fertilizer or digestate and harvested at two maturity stages in boreal growing conditions. Agric. Food Sci. 2017, 26, 47–55. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Kheir, A.M.S.; Al-Dhumri, S.; Alghamdi, A.G.; Omar, E.S.H.; Aboelsoud, H.M.; Abdella, K.A.; Abou El Hassan, W.H. Exploring optimal tillage improved soil characteristics and productivity of wheat irrigated with different water qualities. Agronomy 2019, 9, 233. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Santanen, A.; Kleemola, J.; Stoddard, F.L.; Mäkelä, P.S.A. Improved sustainability of feedstock production with sludge and interacting mychorriza. Chemosphere 2013, 91, 1236–1242. [Google Scholar] [CrossRef]
- Sofy, M.; Seleiman, M.F.; Alhammad, B.A.; Alharbi, B.A.; Mohamed, H.I. Minimizing adverse effects of Pb stress on maize yield, macro elements, and physiological and biochemical traits by combined treatment with jasmonic acid, salicylic acid, and proline. Agronomy 2020, 10, 699. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Mununga Katebe, F.; Raulier, P.; Colinet, G.; Ngoy Shutcha, M.; Mpundu Mubemba, M.; Jijakli, M.H. Assessment of Heavy Metal Pollution of Agricultural Soil, Irrigation Water, and Vegetables in and Nearby the Cupriferous City of Lubumbashi, (Democratic Republic of the Congo). Agronomy 2023, 13, 357. [Google Scholar] [CrossRef]
- Rawnsley, R.P.; Smith, A.P.; Christie, K.M.; Harrison, M.T.; Eckard, R.J. Current and future direction of nitrogen fertiliser use in Australian grazing systems. Crop Pasture Sci. 2019, 70, 1034–1043. [Google Scholar] [CrossRef]
- Langworthy, A.D.; Rawnsley, R.P.; Freeman, M.J.; Pembleton, K.G.; Corkrey, R.; Harrison, M.T.; Lane, P.A.; Henry, D.A. Potential of summer-active temperate (C3) perennial forages to mitigate the detrimental effects of supraoptimal temperatures on summer home-grown feed production in south-eastern Australian dairying regions. Crop Pasture Sci. 2018, 69, 808–820. [Google Scholar] [CrossRef]
- Phelan, D.C.; Harrison, M.T.; McLean, G.; Cox, H.; Pembleton, K.G.; Dean, G.J.; Parsons, D.; do Amaral Richter, M.E.; Pengilley, G.; Hinton, S.J.; et al. Advancing a farmer decision support tool for agronomic decisions on rainfed and irrigated wheat cropping in Tasmania. Agric. Syst. 2018, 167, 113–124. [Google Scholar] [CrossRef]
- Ara, I.; Turner, L.; Harrison, M.T.; Monjardino, M.; deVoil, P.; Rodriguez, D. Application, adoption and opportunities for improving decision support systems in irrigated agriculture: A review. Agric. Water Manag. 2021, 257, 107161. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Kheir, A.S. Maize productivity, heavy metals uptake and their availability in contaminated clay and sandy alkaline soils as affected by inorganic and organic amendments. Chemosphere 2018, 204, 514–522. [Google Scholar] [CrossRef]
- Abdullahi, N.; Igwe, E.C.; Dandago, M.A. Heavy metal uptake and stress in food crops: A Review. Agric. Sci. Technol. 2021, 13, 323–332. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Santanen, A.; Mäkelä, P.S.A. Recycling Sludge on Cropland as Fertilizer–Advantages and Risks. Resour. Conserv. Recycl. 2020, 155, 104647. [Google Scholar] [CrossRef]
- Ibrahim, A.; Harrison, M.; Meinke, H.; Fan, Y.; Johnson, P.; Zhou, M. A regulator of early flowering in barley (Hordeum vulgare L.). PLoS ONE 2018, 13, e0200722. [Google Scholar] [CrossRef]
- Harrison, M.T.; Roggero, P.P.; Zavattaro, L. Simple, efficient and robust techniques for automatic multi-objective function parameterisation: Case studies of local and global optimisation using APSIM. Environ. Model. Softw. 2019, 117, 109–133. [Google Scholar] [CrossRef]
- Liu, K.; Harrison, M.T.; Shabala, S.; Meinke, H.; Ahmed, I.; Zhang, Y.; Tian, X.; Zhou, M. The State of the Art in Modeling Waterlogging Impacts on Plants: What Do We Know and What Do We Need to Know. Earth’s Future 2020, 8, e2020EF001801. [Google Scholar] [CrossRef]
- Muchuweti, M.; Birkett, J.; Chinyanga, E.; Zvauya, R.; Scrimshaw, M.D.; Lester, J. Heavy metal content of vegetables irrigated with mixtures of wastewater and sewage sludge in Zimbabwe: Implications for human health. Agric. Ecosyst. Environ. 2006, 112, 41–48. [Google Scholar] [CrossRef]
- Shahid, M.J.; Ali, S.; Shabir, G.; Siddique, M.; Rizwan, M.; Seleiman, M.F.; Afzal, M. Comparing the performance of four macrophytes in bacterial assisted floating treatment wetlands for the removal of trace metals (Fe, Mn, Ni, Pb, and Cr) from polluted river water. Chemosphere 2020, 243, 125353. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.J.; Parker, D.R.; Clarke, J.M. Metals and micronutrients-food safety issues. Field Crops Res. 1999, 60, 143–163. [Google Scholar] [CrossRef]
- Khan, I.; Seleiman, M.F.; Chattha, M.U.; Jalal, R.S.; Mahmood, F.; Hassan, F.A.S.; Izzet, W.; Alhammad, B.A.; Ali, E.F.; Roy, R.; et al. Enhancing antioxidant defense system of mung bean with a salicylic acid exogenous application to mitigate cadmium toxicity. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12303. [Google Scholar] [CrossRef]
- Wang, B.; Chu, C.; Wei, H.; Zhang, L.; Ahmad, Z.; Wu, S.; Xie, B. Ameliorative effects of silicon fertilizer on soil bacterial community and pakchoi (Brassica chinensis L.) grown on soil contaminated with multiple heavy metals. Environ. Pollut. 2020, 267, 115411. [Google Scholar] [CrossRef]
- Shar, G.Q.; Kazi, T.G.; Shah, F.A.; Shar, A.H.; Soomro, F.M. Variable uptake and accumulation of essential and heavy metals in maize (Zea mays L.) grains of six maize varieties. Aust. J. Basic Appl. Sci. 2011, 5, 117–121. [Google Scholar]
- Stobrawa, K.; Lorenc-Plucinska, G. Changes soilin carbohydrate metabolism in fine roots of the native European black poplar (Populus nigra L.) in a heavy metal-polluted environment. Sci. Total Environ. 2007, 373, 157–165. [Google Scholar] [CrossRef]
- Mir, A.R.; Pichtel, J.; Hayat, S. Copper: Uptake, toxicity and tolerance in plants and management of Cu-contaminated soil. Biometals 2021, 34, 737–759. [Google Scholar] [CrossRef]
- Adnan, M.; Fahad, S.; Saleem, M.H.; Ali, B.; Mussart, M.; Ullah, R.; Amanullah, Jr.; Arif, M.; Ahmad, M.; Shah, W.A.; et al. Comparative efficacy of phosphorous supplements with phosphate solubilizing bacteria for optimizing wheat yield in calcareous soils. Sci. Rep. 2022, 12, 11997. [Google Scholar] [CrossRef]
- Shabbir, Z.; Sardar, A.; Shabbir, A.; Abbas, G.; Shamshad, S.; Khalid, S.; Murtaza, G.; Dumat, C.; Shahid, M. Copper uptake, essentiality, toxicity, detoxification and risk assessment in soil-plant environment. Chemosphere 2020, 259, 127436. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Rizwan, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Zia-urRehman, M.; Irshad, M.K.; Bharwana, S.A. The effect of excess copper on growth and physiology of important food crops: A review. Environ. Sci. Pollut. Res. 2015, 22, 8148–8162. [Google Scholar] [CrossRef]
- Ouzounidou, G. Effect of copper on germination and seedling growth of Minuartia, Silene, Alyssum and Thlaspi. Plant Biol. 1995, 37, 411–416. [Google Scholar] [CrossRef]
- Ke, W.; Xiong, Z.; Xie, M.; Luo, Q. Accumulation, subcellular localization and ecophysiological responses to copper stress in two Daucus carota L. populations. Plant Soil 2007, 292, 291–304. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Almeida, J.M.; Fidalgo, F.; Confraria, A.; Santos, A.; Pires, H.; Santos, I. Effect of hydrogen peroxide on catalase gene expression, isoform activities and levels in leaves of potato sprayed with homobrassinolide and ultrastructural changes in mesophyll cells. Funct. Plant Biol. 2005, 32, 707–720. [Google Scholar] [CrossRef]
- Nurnaeimah, N.; Mat, N.; Mohd, K.S.; Badaluddin, N.A.; Yusoff, N.; Sajili, M.H.; Mahmud, K.; Adnan, A.F.M.; Khandaker, M.M. The Effects of Hydrogen Peroxide on Plant Growth, Mineral Accumulation, as Well as Biological and Chemical Properties of Ficus deltoidei. Agronomy 2020, 10, 599. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Abdel-Aal, S.M.; Ibrahim, M.E.; Monneveux, P. Variation of yield and milling, technological and rheological characteristics in some Egyptian bread wheat (Triticum aestivum L.) cultivars. Emir. J. Food Agric. 2010, 22, 84–90. [Google Scholar] [CrossRef]
- Seleiman, M.; Abdel-Aal, M. Response of growth, productivity and quality of some Egyptian wheat cultivars to different irrigation regimes. Egypt. J. Agron. 2018, 40, 313–330. [Google Scholar] [CrossRef]
- Al-Ashkar, I.; Alderfasi, A.; El-Hendawy, S.; Al-Suhaibani, N.; El-Kafafi, S.; Seleiman, M.F. Detecting salt tolerance in doubled haploid wheat lines. Agronomy 2019, 9, 211. [Google Scholar] [CrossRef]
- Ding, Z.; Ali, E.F.; Elmahdy, A.M.; Ragab, K.; Seleiman, M.F.; Kheir, A.M.S. Modeling the combined impacts of deficit irrigation, rising temperature and compost application on wheat yield and water productivity. Agric. Water Manag. 2021, 244, 106626. [Google Scholar] [CrossRef]
- Ding, Z.; Kheir, A.S.; Ali, O.A.; Hafez, E.; Elshamey, E.A.; Zhou, Z.; Wang, B.; Lin, X.; Ge, Y.; Fahmy, A.E.; et al. A vermicompost and deep tillage system to improve saline-sodic soil quality and wheat productivity. J. Environ. Manag. 2021, 277, 111388. [Google Scholar] [CrossRef]
- Yan, H.; Harrison, M.T.; Liu, K.; Wang, B.; Feng, P.; Fahad, S.; Meinke, H.; Yang, R.; Liu, D.L.; Archontoulis, S.; et al. Crop traits enabling yield gains under more frequent extreme climatic events. Sci. Total Environ. 2022, 808, 152170. [Google Scholar] [CrossRef] [PubMed]
- Shahpari, S.; Allison, J.; Harrison, M.T.; Stanley, R. An integrated economic, environmental and social approach to agricultural land-use planning. Land 2021, 10, 364. [Google Scholar] [CrossRef]
- FAOSTAT. 2020. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 20 February 2023).
- Chaves, M.S.; Martinelli, J.A.; Wesp-Guterres, C.; Graichen, F.A.S.; Brammer, S.P.; Scagliusi, S.M.; da Silva, P.R.; Wiethölter, P.; Torres, G.A.M.; Lau, E.Y.; et al. The importance for food security of maintaining rust resistance in wheat. Food Secur. 2013, 5, 157–176. [Google Scholar] [CrossRef]
- Chenu, K.; Porter, J.R.; Martre, P.; Basso, B.; Chapman, S.C.; Ewert, F.; Bindi, M.; Asseng, S. Contribution of crop models to adaptation in wheat. Trends Plant Sci. 2017, 22, 472–490. [Google Scholar] [CrossRef]
- Niu, L.; Liao, W. Hydrogen peroxide signaling in plant development and abiotic responses: Crosstalk with nitric oxide and calcium. Front. Plant Sci. 2016, 7, 230. [Google Scholar] [CrossRef]
- Bagheri, M.; Gholami, M.; Baninasab, B. Hydrogen peroxide-induced salt tolerance in relation to antioxidant systems in pistachio seedlings. Sci. Hortic. 2019, 243, 207–213. [Google Scholar] [CrossRef]
- Andrade, C.A.; de Souza, K.R.; de Oliveira Santos, M.; da Silva, D.M.; Alves, J.D. Hydrogen peroxide promotes the tolerance of soybeans to waterlogging. Sci. Hortic. 2018, 232, 40–45. [Google Scholar] [CrossRef]
- Wu, D.; Chu, H.Y.; Jia, L.X.; Chen, K.M.; Zhao, L.Q. A feedback inhibition between nitric oxide and hydrogen peroxide in the heat shock pathway in arabidopsis seedlings. Plant Growth Regul. 2015, 75, 503–509. [Google Scholar] [CrossRef]
- Chen, Y.; Guerschman, J.; Shendryk, Y.; Henry, D.; Harrison, M.T. Estimating pasture biomass using sentinel-2 imagery and machine learning. Remote Sens. 2021, 13, 603. [Google Scholar] [CrossRef]
- Sándor, R.; Ehrhardt, F.; Grace, P.; Recous, S.; Smith, P.; Snow, V.; Soussana, J.F.; Basso, B.; Bhatia, A.; Brilli, L.; et al. Ensemble modelling of carbon fluxes in grasslands and croplands. Field Crops Res. 2020, 252, 107791. [Google Scholar] [CrossRef]
- Taylor, C.A.; Harrison, M.T.; Telfer, M.; Eckard, R. Modelled greenhouse gas emissions from beef cattle grazing irrigated leucaena in northern Australia. Anim. Prod. Sci. 2016, 56, 594–604. [Google Scholar] [CrossRef]
- Nazir, F.; Hussain, A.; Fariduddin, Q. Hydrogen peroxide modulate hotosynthesis and antioxidant systems in tomato (Solanum lycopersicum L.) plants under copper stress. Chemosphere 2019, 230, 544–558. [Google Scholar] [CrossRef]
- Cruz, F.J.R.; da Cruz Ferreira, R.L.; Conceição, S.S.; Lima, E.U.; de Oliveira Neto, C.F.; Galvão, J.R.; da Cunha Lopes, S.; Viegas, I.D.J.M. Copper toxicity in plants: Nutritional, physiological, and biochemical aspects. In Advances in Plant Defense Mechanisms; IntechOpen: London, UK, 2022. [Google Scholar]
- Hossain, M.S.; Abdelrahman, M.; Tran, C.D.; Nguyen, K.H.; Chu, H.D.; Watanabe, Y.; Hasanuzzaman, M.; Mohsin, S.M.; Fujita, M.; Tran, L.S.P. Insights into acetate-mediated copper homeostasis and antioxidant defense in lentil under excessive copper stress. Environ. Pollut. 2020, 258, 113544. [Google Scholar] [CrossRef]
- Yuan, M.; Li, X.; Xiao, J.; Wang, S. Molecular and functional analyses of COPT/Ctr-type copper transporter-like gene family in rice. BMC Plant Biol. 2011, 11, 69. [Google Scholar] [CrossRef]
- Marques, D.M.; Veroneze Júnior, V.; da Silva, A.B.; Mantovani, J.R.; Magalhães, P.C.; de Souza, T.C. Copper toxicity on photosynthetic responses and root morphology of Hymenaea courbaril L. (Caesalpinioideae). Water Air Soil Pollut. 2018, 229, 138. [Google Scholar] [CrossRef]
- Feigl, G.; Kumar, D.; Lehotai, N.; Tugyi, N.; Molnár, Á.; Ördög, A.; Szepesi, Á.; Gémes, K.; Laskay, G.; Erdei, L.; et al. Physiological and morphological responses of the root system of Indian mustard (Brassica juncea L. Czern.) and rapeseed (Brassica napus L.) to copper stress. Ecotoxicol. Environ. Saf. 2013, 94, 179–189. [Google Scholar] [CrossRef]
- Mocquot, B.; Vangronsveld, J.; Clijsters, H.; Mench, M. Copper toxicity in young maize (Zea mays L.) plants: Effects on growth, mineral and chlorophyll contents, and enzyme activities. Plant Soil 1998, 182, 287–300. [Google Scholar] [CrossRef]
- Chatterjee, J.; Chatterjee, C. Phytotoxicity of cobalt, chromium and copper in cauliflower. Environ. Pollut. 2000, 109, 69–74. [Google Scholar] [CrossRef]
- Shahbaz, M.; Tseng, M.H.; Stuiver, C.E.E.; Koralewska, A.; Posthumus, F.S.; Venema, J.H.; Parmar, S.; Schat, H.; Hawkesford, M.J.; De Kok, L.J. Copper exposure interferes with the regulation of the uptake, distribution and metabolism of sulfate in Chinese cabbage. J. Plant Physiol. 2010, 167, 438–446. [Google Scholar] [CrossRef]
- Ali, S.; Shahbaz, M.; Shahzad, A.N.; Khan, H.A.A.; Anees, M.; Haider, M.S.; Fatima, A. Impact of copper toxicity on stone-head cabbage (Brassica oleracea var. capitata) in hydroponics. PeerJ 2015, 3, 1119. [Google Scholar] [CrossRef]
- Zengin, F.K.; Kirbag, S. Effects of copper on chlorophyll, proline, protein and abscisic acid level of sunflower (Helianthus annuus L.) seedlings. J. Environ. Biol. 2007, 28, 561. [Google Scholar] [PubMed]
- Zlobin, I.E.; Kholodova, V.P.; Rakhmankulova, Z.F.; Kuznetsov, V.V. Brassica napus responses to short-term excessive copper treatment with decrease of photosynthetic pigments, differential expression of heavy metal homeostasis genes including activation of gene NRAMP4 involved in photosystem II stabilization. Photosynth. Res. 2015, 125, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Rozentsvet, O.A.; Nesterov, V.N.; Sinyutina, N.F. The effect of copper ions on the lipid composition of subcellular membranes in Hydrilla verticillata. Chemosphere 2012, 89, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Liu, L.; Wang, Q.; Saleem, M.H.; Bashir, S.; Ullah, S.; Peng, D. Copper environmental toxicology, recent advances, and future outlook: A review. Environ. Sci. Pollut. Res. 2019, 26, 18003–18016. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Qiu, H.; Chu, W.; Fu, Y.; Cai, S.; Min, H.; Sha, S. Copper ultrastructural localization, subcellular distribution, and phytotoxicity in Hydrilla verticillata (Lf) Royle. Environ. Sci. Pollut. Res. 2013, 20, 8672–8679. [Google Scholar] [CrossRef] [PubMed]
- Panou-Filotheou, H.; Bosabalidis, A.M.; Karataglis, S. Effects of copper toxicity on leaves of oregano (Origanum vulgare subsp. hirtum). Ann. Bot. 2001, 88, 207–214. [Google Scholar] [CrossRef]
- Feigl, G.; Kumar, D.; Lehotai, N.; Pető, A.; Molnár, Á.; Rácz, É.; Ördög, A.; Erdei, L.; Kolbert, Z.; Laskay, G. Comparing the effects of excess copper in the leaves of Brassica juncea (L. Czern) and Brassica napus (L.) seedlings: Growth inhibition, oxidative stress and photosynthetic damage. Acta Biol. Hung. 2015, 66, 205–221. [Google Scholar] [CrossRef]
- Aly, A.A.; Mohamed, A.A. The impact of copper ion on growth, thiol compounds and lipid peroxidation in two maize cultivars (‘Zea mays’ L.) grown‘in vitro’. Aust. J. Crop Sci. 2012, 6, 541–549. [Google Scholar]
- Ambrosini, V.G.; Rosa, D.J.; Basso, A.; Borghezan, M.; Pescador, R.; Miotto, A.; Melo, G.W.B.D.; Soares, C.R.F.D.S.; Comin, J.J.; Brunetto, G. Liming as an ameliorator of copper toxicity in black oat (Avena strigosa Schreb.). J. Plant Nutr. 2017, 40, 404–416. [Google Scholar] [CrossRef]
- Ke, W.; Xiong, Z.T.; Chen, S.; Chen, J. Effects of copper and mineral nutrition on growth, copper accumulation and mineral element uptake in two Rumex japonicus populations from a copper mine and an uncontaminated field sites. Environ. Exp. Bot. 2007, 59, 59–67. [Google Scholar] [CrossRef]
- Cambrollé, J.; García, J.L.; Figueroa, M.E.; Cantos, M. Evaluating wild grapevine tolerance to copper toxicity. Chemosphere 2015, 120, 171–178. [Google Scholar] [CrossRef]
- Wang, L.; Yang, L.; Yang, F.; Li, X.; Song, Y.; Wang, X.; Hu, X. Involvements of H2O2 and metallothionein in NO-mediated tomato tolerance to copper toxicity. J. Plant Physiol. 2010, 167, 1298–1306. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, S.; Xu, H.; Hou, D.; Li, Y.; Wang, F. H₂O₂ is involved in the metallothionein-mediated rice tolerance to copper and cadmium toxicity. Int. J. Mol. Sci. 2017, 18, 2083. [Google Scholar] [CrossRef]
- Chen, B.T.; Avshalumov, M.V.; Rice, M.E. H2O2 is a novel, endogenous modulator of synaptic dopamine release. J. Neurophysiol. 2001, 85, 2468–2476. [Google Scholar] [CrossRef]
- Barba-Espín, G.; Diaz-Vivancos, P.; Job, D.; Belghazi, M.; Job, C.; Hernández, J.A. Understanding the role of H2O2 during pea seed germination: A combined proteomic and hormone profiling approach. Plant Cell Environ. 2011, 34, 1907–1919. [Google Scholar] [CrossRef]
- Ma, F.; Wang, L.; Li, J.; Samma, M.K.; Xie, Y.; Wang, R.; Wang, J.; Zhang, J.; Shen, W. Interaction between HY1 and H2O2 in auxin-induced lateral root formation in Arabidopsis. Plant Mol. Biol. 2014, 85, 49–61. [Google Scholar] [CrossRef]
- Ge, X.M.; Cai, H.L.; Lei, X.; Zou, X.; Yue, M.; He, J.M. Heterotrimeric G protein mediates ethylene-induced stomatal closure via hydrogen peroxide synthesis in Arabidopsis. Plant J. 2015, 82, 138–150. [Google Scholar] [CrossRef]
- Ashfaque, F.; Khan, M.I.; Khan, N.A. Exogenously applied H2O2 promotes proline accumulation., water relations., photosynthetic efficiency and growth of wheat (Triticum aestivum L.) under salt stress. Annu. Res. Rev. Biol. 2014, 4, 105–120. [Google Scholar] [CrossRef]
- Nazir, F.; Fariduddin, Q.; Khan, T. Hydrogen peroxide as a signalling molecule in plants and its crosstalk with other plant growth regulators under heavy metal stress. Chemosphere 2020, 252, 126486. [Google Scholar] [CrossRef]
- Ellouzi, H.; Sghayar, S.; Abdelly, C. H2O2 seed priming improves tolerance to salinity; drought and their combined effect more than mannitol in Cakile maritima when compared to Eutrema salsugineum. J. Plant Physiol. 2017, 210, 38–50. [Google Scholar] [CrossRef]
- Xiong, J.; Yang, Y.; Fu, G.; Tao, L. Novel roles of hydrogen peroxide (H2O2) in regulating pectin synthesis and demethylesterification in the cell wall of rice (Oryza sativa) root tips. New Phytol. 2015, 206, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.M.; Farooq, S.H.; Iqbal, N.A.; Arshad, R.U. Influence of exogenous application of hydrogen peroxide on root and seedling growth on wheat (Triticum aestivum L.). Int. J. Agric. Biol. 2004, 6, 366–369. [Google Scholar]
- Sairam, R.K.; Srivastava, G.C. Induction of oxidative stress and antioxidant activity by hydrogen peroxide treatment in tolerant and susceptible wheat genotypes. Biol. Plant. 2000, 43, 381–386. [Google Scholar] [CrossRef]
- Khan, M.I.; Khan, N.A.; Masood, A.; Per, T.S.; Asgher, M. Hydrogen peroxide alleviates nickel- inhibited photosynthetic responses through increase in useefficiency of nitrogen and sulfur, and glutathione production in mustard. Front. Plant Sci. 2016, 7, 44. [Google Scholar] [CrossRef]
- Kumari, S.; Verma, V.K. Hydrogen peroxide mediates suberization, root thickness and stomatal movement in wheats. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2448–2454. [Google Scholar] [CrossRef]
- An, Y.; Feng, X.; Liu, L.; Xiong, L.; Wang, L. ALA-induced flavonols accumulation in guard cells is involved in scavenging H2O2 and inhibiting stomatal closure in Arabidopsis cotyledons. Front. Plant Sci. 2016, 7, 1713. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Khan, T.A.; Yusuf, M. Hydrogen peroxide mediated tolerance to copper stress in the presence of 28-homobrassinolide in Vigna radiata. Acta Physiol. Plant. 2014, 36, 2767–2778. [Google Scholar] [CrossRef]
- Guzel, S.; Terzi, R. Exogenous hydrogen peroxide increases dry matter production., mineral content and level of osmotic solutes in young maize leaves and alleviates deleterious effects of copper stress. Bot. Stud. 2013, 54, 26. [Google Scholar] [CrossRef]
- Hasan, S.A.; Irfan, M.; Masrahi, Y.S.; Khalaf, M.A.; Hayat, S. Growth, photosynthesis, and antioxidant responses of Vigna unguiculata L. treated with hydrogen peroxide. Cogent Food Agric. 2016, 2, 1155331. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhammad, B.A.; Seleiman, M.F.; Harrison, M.T. Hydrogen Peroxide Mitigates Cu Stress in Wheat. Agriculture 2023, 13, 862. https://doi.org/10.3390/agriculture13040862
Alhammad BA, Seleiman MF, Harrison MT. Hydrogen Peroxide Mitigates Cu Stress in Wheat. Agriculture. 2023; 13(4):862. https://doi.org/10.3390/agriculture13040862
Chicago/Turabian StyleAlhammad, Bushra Ahmed, Mahmoud F. Seleiman, and Matthew Tom Harrison. 2023. "Hydrogen Peroxide Mitigates Cu Stress in Wheat" Agriculture 13, no. 4: 862. https://doi.org/10.3390/agriculture13040862
APA StyleAlhammad, B. A., Seleiman, M. F., & Harrison, M. T. (2023). Hydrogen Peroxide Mitigates Cu Stress in Wheat. Agriculture, 13(4), 862. https://doi.org/10.3390/agriculture13040862






