Waterlogging Effects on Soybean Physiology and Hyperspectral Reflectance during the Reproductive Stage
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions and Plant Materials
2.2. Waterlogging Treatments
2.3. Physiological Measurements
2.4. Leaf Hyperspectral Reflectance
2.5. Growth and Reproduction Measurements
2.6. Nutrients Analysis
2.7. Statistical Analysis
3. Results
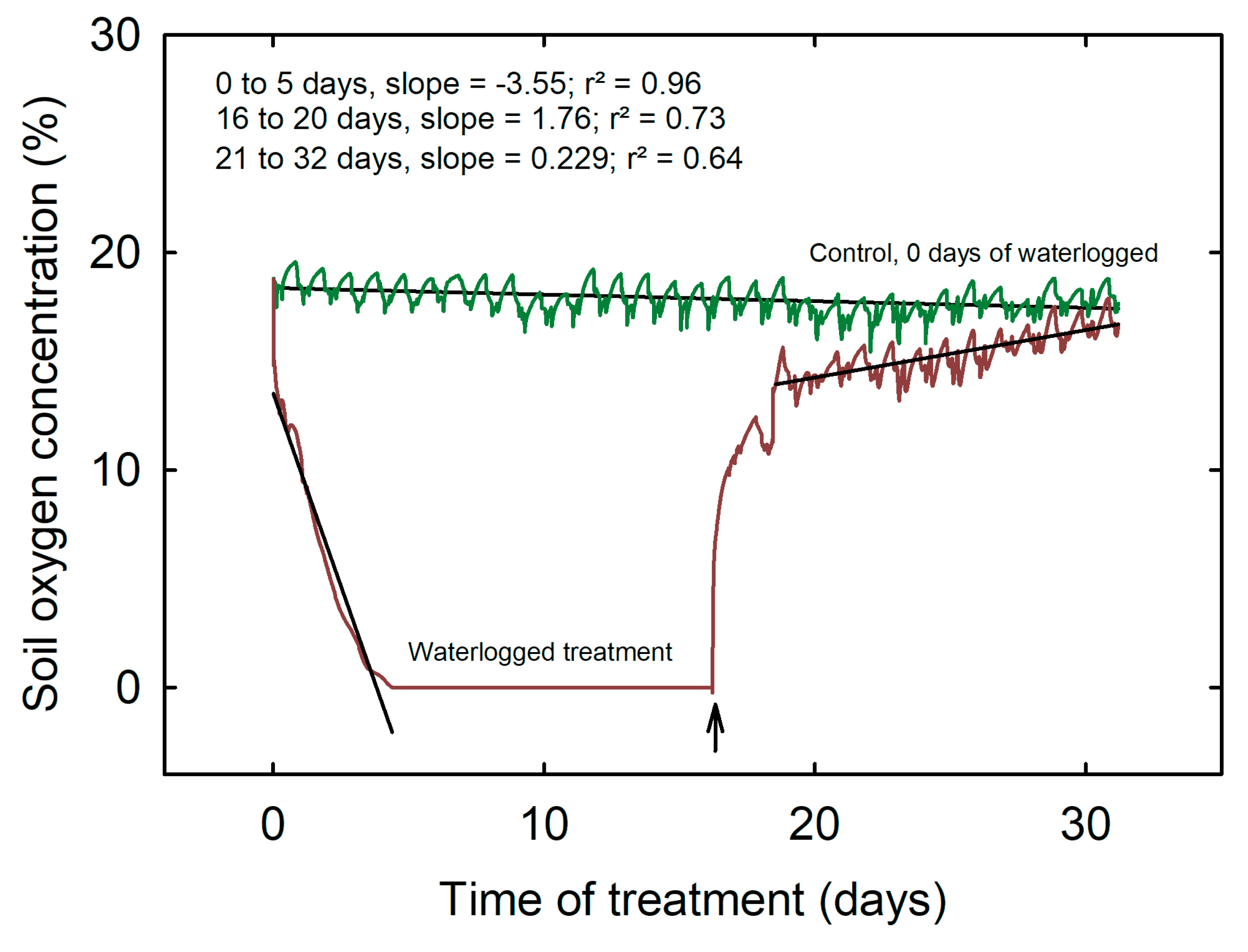
3.1. Soil Oxygen Concentration
3.2. Leaf Gas Exchange Parameters
3.3. Mineral and Nutrients Analysis
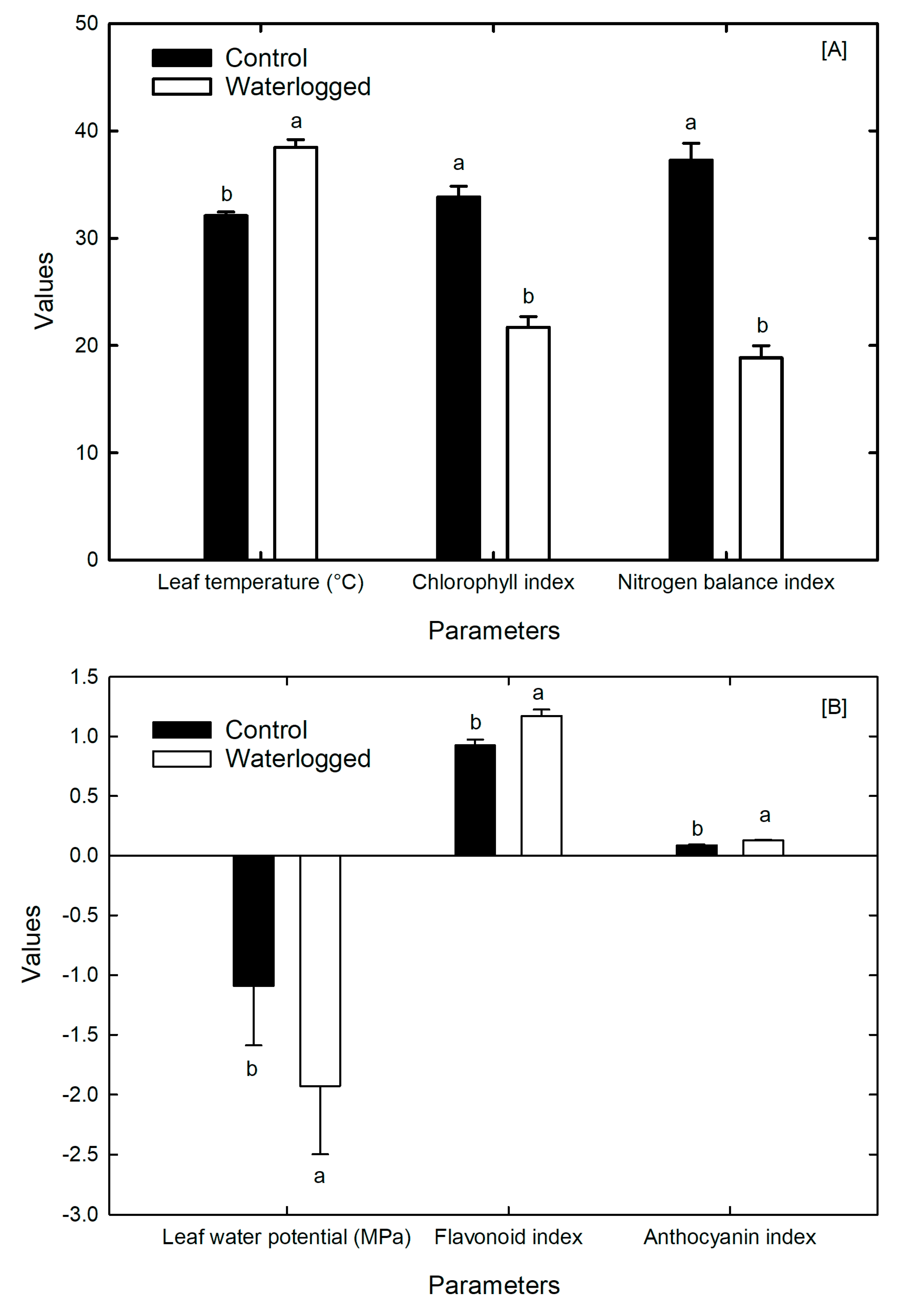
3.4. Leaf Temperature and Plant Pigments
3.5. Growth, Development, and Biomass Components
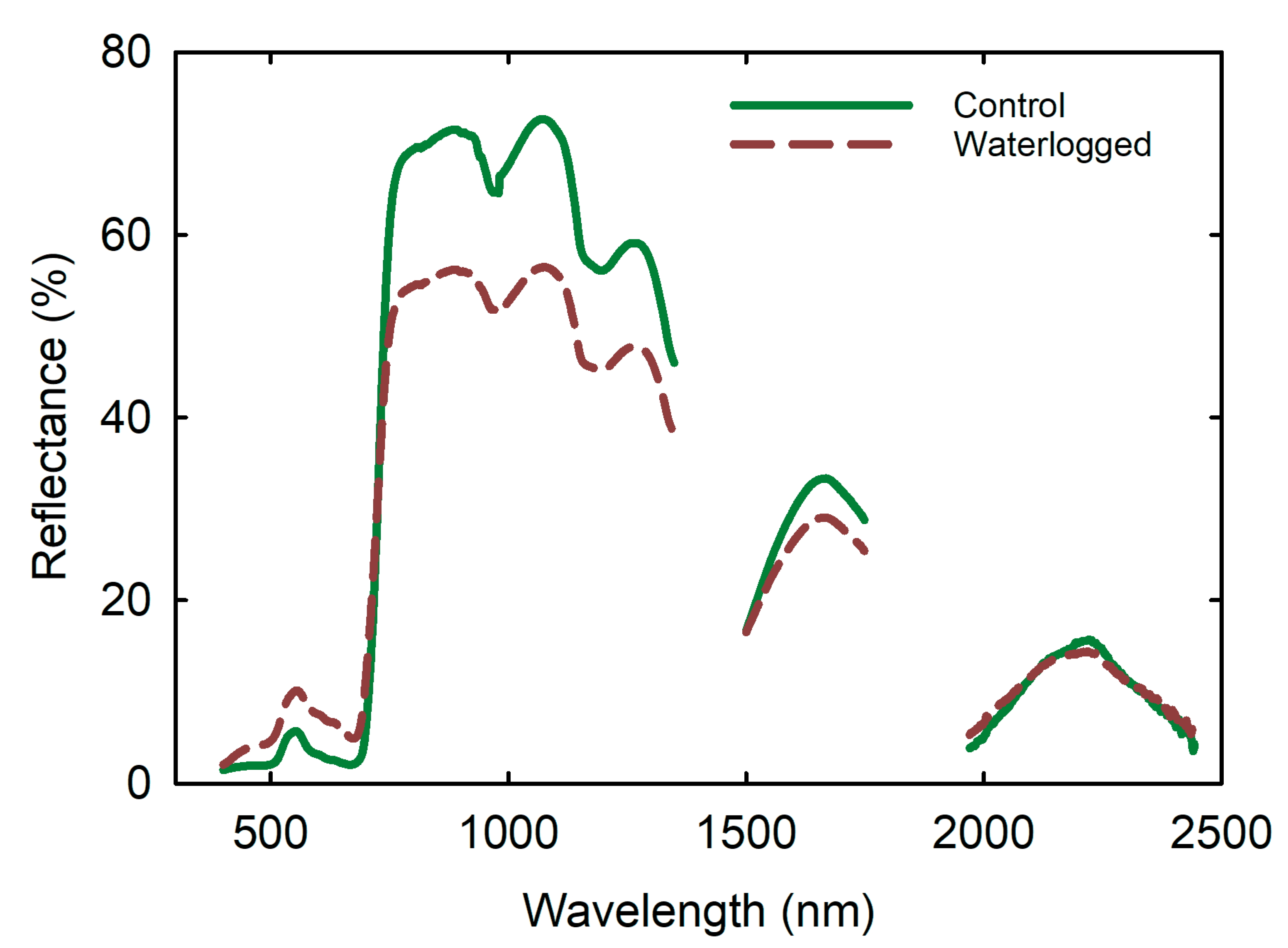
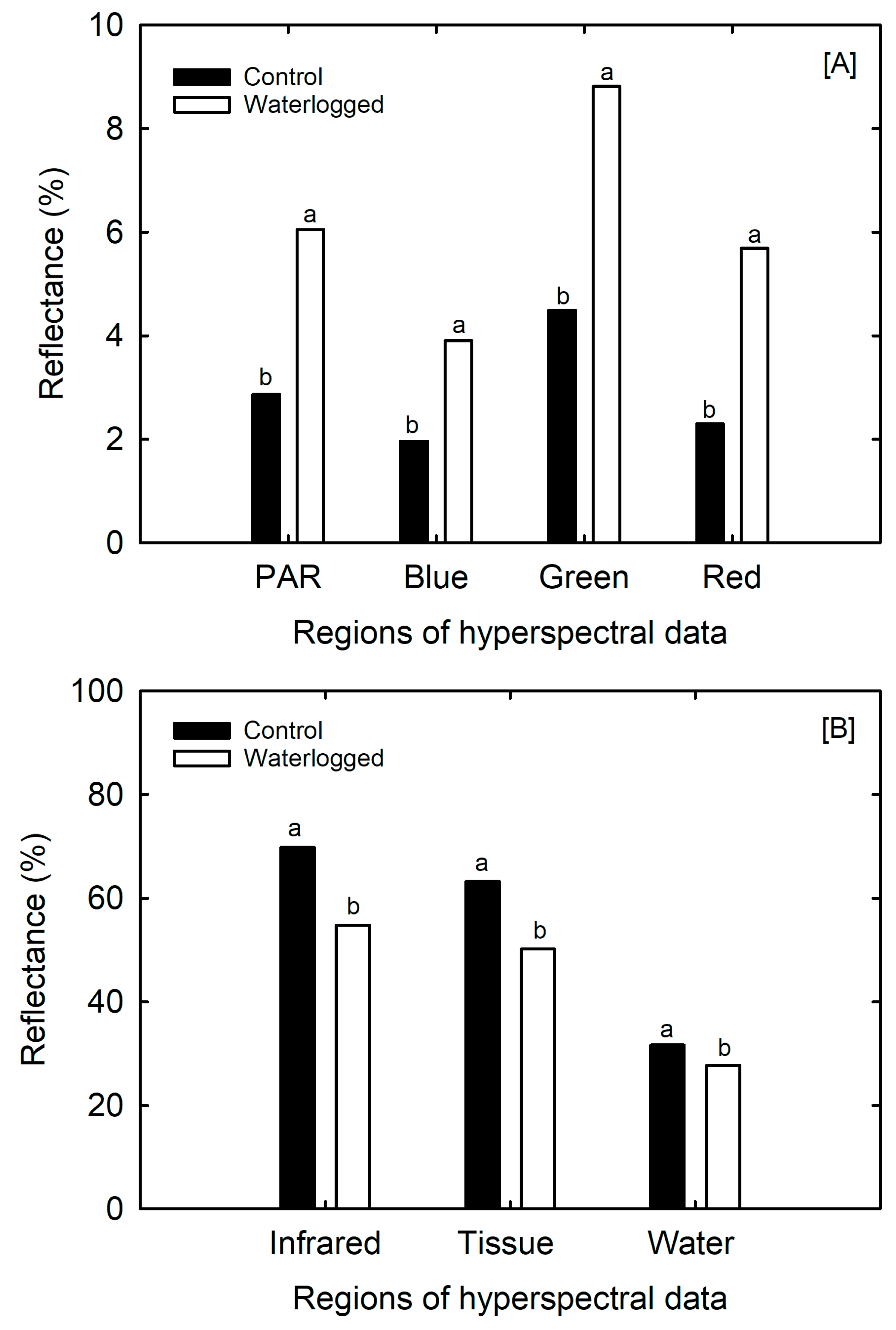
3.6. Remote Sensing Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, K. Chemistry and nutritional value of soybean components. In Soybeans: Chemistry, Technology, and Utilization; Liu, K., Ed.; Springer US: Boston, MA, USA, 1997; pp. 25–113. ISBN 978-1-4615-1763-4. [Google Scholar]
- FAO. FAOSTAT Database. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 31 May 2022).
- Council for Agricultural Science and Technology. Sustainability of U.S. Soybean Production: Conventional, Transgenic, and Organic Production Systems; Council for Agricultural Science and Technology: Ames, IA, USA, 2009; ISBN 978-1-887383-32-5. [Google Scholar]
- Van Ee, J.H. Soy constituents: Modes of action in low-density lipoprotein management. Nutr. Rev. 2009, 67, 222–234. [Google Scholar] [CrossRef]
- Shea, M.K.; Booth, S.L. Update on the role of vitamin K in skeletal health. Nutr. Rev. 2008, 66, 549–557. [Google Scholar] [CrossRef]
- Franke, A.A.; Custer, L.J.; Wang, W.; Shi, C.Y. HPLC Analysis of Isoflavonoids and Other Phenolic Agents from Foods and from Human Fluids. Proc. Soc. Exp. Boil. Med. 1998, 217, 263–273. [Google Scholar] [CrossRef]
- De Mejia, E.G.; Bradford, T.; Hasler, C. The anticarcinogenic potential of soybean lectin and lunasin. Nutr. Rev. 2003, 61, 239–246. [Google Scholar] [CrossRef]
- Bandillo, N.B.; Anderson, J.E.; Kantar, M.B.; Stupar, R.M.; Specht, J.E.; Graef, G.L.; Lorenz, A.J. Dissecting the Genetic Basis of Local Adaptation in Soybean. Sci. Rep. 2017, 7, 17195. [Google Scholar] [CrossRef]
- Zachary Shea Soybean Production, Versatility, and Improvement. In Legume Crops; Singer, W.M., Ed.; IntechOpen: Rijeka, Croatia, 2020; p. Ch. 3. ISBN 978-1-83968-274-2. [Google Scholar]
- Linkemer, G.; Board, J.E.; Musgrave, M.E. Water logging effects on growth and yield components in late planted soybean. Crop Sci. 1998, 38, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Ploschuk, R.A.; Miralles, D.J.; Striker, G.G. A quantitative review of soybean responses to waterlogging: Agronomical, morpho-physiological and anatomical traits of tolerance. Plant Soil 2022, 475, 237–252. [Google Scholar] [CrossRef]
- USDA United States Department of Agriculture (USDA), National Agricultural Statistics Services. Available online: https://www.nass.usda.gov/Publications/AgCensus/2012/Online_Resources/Ag_Census_Web_Maps/Data_download/index.php/ (accessed on 4 April 2023).
- Pörtner, H.-O.; Roberts, D.C.; Adams, H.; Adelekan, I.; Adler, C.; Adrian, R.; Aldunce, P.; Ali, E.; Ara Begum, R.; Bednar-Friedl, B.; et al. IPCC, 2022: Climate Change 2022: Impacts, Adaptation, and Vulnerability: Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Armstrong, W. Aeration in Higher Plants. In Advances in Botanical Research; Woolhouse, H.W., Ed.; Academic Press: Cambridge, MA, USA, 1980; Volume 7, pp. 225–332. ISBN 0065-2296. [Google Scholar]
- Ploschuk, R.A.; Miralles, D.J.; Colmer, T.D.; Ploschuk, E.L.; Striker, G.G. Waterlogging of Winter Crops at Early and Late Stages: Impacts on Leaf Physiology, Growth and Yield. Front. Plant Sci. 2018, 9, 1863. [Google Scholar] [CrossRef]
- Ponnamperuma, F.N. The chemistry of submerged soils. In Advances in Agronomy; Brady, N.C., Ed.; Academic Press: Cambridge, MA, USA, 1972; Volume 24, pp. 29–96. ISBN 0065-2113. [Google Scholar]
- Shaw, R.E.; Meyer, W.S.; McNeill, A.; Tyerman, S.D. Waterlogging in Australian agricultural landscapes: A review of plant responses and crop models. Crop. Pasture Sci. 2013, 64, 549–562. [Google Scholar] [CrossRef]
- Gibbs, J.; Greenway, H. Review: Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct. Plant Biol. 2003, 30, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Sairam, R.K.; Kumutha, D.; Ezhilmathi, K.; Chinnusamy, V.; Meena, R.C. Waterlogging induced oxidative stress and antioxidant enzyme activities in pigeon pea. Biol. Plant. 2009, 53, 493–504. [Google Scholar] [CrossRef]
- Garcia, N.; Da-Silva, C.J.; Cocco, K.L.T.; Pomagualli, D.; de Oliveira, F.K.; da Silva, J.V.L.; de Oliveira, A.C.B.; Amarante, L.D. Waterlogging tolerance of five soybean genotypes through different physiological and biochemical mechanisms. Environ. Exp. Bot. 2020, 172, 103975. [Google Scholar] [CrossRef]
- Malik, A.I.; Ailewe, T.I.; Erskine, W. Tolerance of three grain legume species to transient waterlogging. AoB Plants 2015, 7, plv040. [Google Scholar] [CrossRef] [PubMed]
- Araki, H.; Hamada, A.; Hossain, M.A.; Takahashi, T. Waterlogging at jointing and/or after anthesis in wheat induces early leaf senescence and impairs grain filling. Field Crop. Res. 2012, 137, 27–36. [Google Scholar] [CrossRef]
- Kaur, G.; Zurweller, B.A.; Nelson, K.A.; Motavalli, P.P.; Dudenhoeffer, C.J. Soil Waterlogging and Nitrogen Fertilizer Management Effects on Corn and Soybean Yields. Agron. J. 2017, 109, 97–106. [Google Scholar] [CrossRef]
- Yamauchi, T.; Colmer, T.D.; Pedersen, O.; Nakazono, M. Regulation of Root Traits for Internal Aeration and Tolerance to Soil Waterlogging-Flooding Stress. Plant Physiol. 2017, 176, 1118–1130. [Google Scholar] [CrossRef]
- Thomas, A.L.; Carmello-Guerreiro, S.M.; Sodek, L. Aerenchyma Formation and Recovery from Hypoxia of the Flooded Root System of Nodulated Soybean. Ann. Bot. 2005, 96, 1191–1198. [Google Scholar] [CrossRef]
- Scott, H.D.; DeAngulo, J.; Daniels, M.B.; Wood, L.S. Flood Duration Effects on Soybean Growth and Yield. Agron. J. 1989, 81, 631–636. [Google Scholar] [CrossRef]
- Rhine, M.D.; Stevens, G.; Shannon, G.; Wrather, A.; Sleper, D. Yield and nutritional responses to waterlogging of soybean cultivars. Irrig. Sci. 2010, 28, 135–142. [Google Scholar] [CrossRef]
- Kim, K.H.; Cho, M.J.; Kim, J.-M.; Lee, T.; Heo, J.H.; Jeong, J.Y.; Lee, J.; Moon, J.-K.; Kang, S. Growth Response and Developing Simple Test Method for Waterlogging Stress Tolerance in Soybean. J. Crop. Sci. Biotechnol. 2019, 22, 371–378. [Google Scholar] [CrossRef]
- Zhao, D.; Reddy, K.R.; Kakani, V.G.; Read, J.J.; Carter, G.A. Corn (Zea mays L.) growth, leaf pigment concentration, photosynthesis and leaf hyperspectral reflectance properties as affected by nitrogen supply. Plant Soil 2003, 257, 205–218. [Google Scholar] [CrossRef]
- Purkis, S.J.; Klema, V.V. Remote Sensing and Global Environmental Change; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 1444340255. [Google Scholar]
- Zhao, D.; Reddy, K.R.; Kakani, V.G.; Read, J.J.; Koti, S. Selection of Optimum Reflectance Ratios for Estimating Leaf Nitrogen and Chlorophyll Concentrations of Field-Grown Cotton. Agron. J. 2005, 97, 89–98. [Google Scholar] [CrossRef]
- Wijewardana, C.; Alsajri, F.A.; Irby, J.T.; Krutz, L.J.; Golden, B.; Henry, W.B.; Gao, W.; Reddy, K.R. Physiological assessment of water deficit in soybean using midday leaf water potential and spectral features. J. Plant Interactions 2019, 14, 533–543. [Google Scholar] [CrossRef]
- Plant, R.E.; Munk, D.S.; Roberts, B.R.; Vargas, R.N.; Travis, R.L.; Rains, D.W.; Hutmacher, R.B. Application of remote sensing to strategic questions in cotton management and research. J. Cotton. Sci. 2001, 5, 30–41. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. 1950, 347, 32. [Google Scholar]
- Walne, C.H.; Reddy, K.R. Developing Functional Relationships between Soil Waterlogging and Corn Shoot and Root Growth and Development. Plants 2021, 10, 2095. [Google Scholar] [CrossRef] [PubMed]
- Poudel, S.; Vennam, R.R.; Shrestha, A.; Reddy, K.R.; Wijewardana, N.K.; Reddy, K.N.; Bheemanahalli, R. Resilience of soybean cultivars to drought stress during flowering and early-seed setting stages. Sci. Rep. 2023, 13, 1277. [Google Scholar] [CrossRef]
- Reddy, K.R.; Matcha, S.E. Quantifying nitrogen effects on castor (Ricinus communis L.) development, growth, and photosynthesis. Ind. Crop. Prod. 2010, 31, 185–191. [Google Scholar] [CrossRef]
- Zhao, D.; Reddy, K.R.; Kakani, V.G.; Reddy, V.R. Nitrogen deficiency effects on plant growth, leaf photosynthesis, and hyperspectral reflectance properties of sorghum. Eur. J. Agron. 2005, 22, 391–403. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Nitrogen Analysis of Soil and Plant Tissues. J. AOAC Int. 1980, 63, 770–778. [Google Scholar] [CrossRef]
- SAS Institute SAS Guide to Macro Processing; SAS Institute: Cary, NC, USA, 2011; Volume 11.
- Pereira, Y.C.; da Silva, F.R.; da Silva, B.R.S.; Cruz, F.J.R.; Marques, D.J.; Lobato, A.K.D.S. 24-epibrassinolide induces protection against waterlogging and alleviates impacts on the root structures, photosynthetic machinery and biomass in soybean. Plant Signal. Behav. 2020, 15, 1805885. [Google Scholar] [CrossRef]
- Lapaz, A.D.M.; de Camargos, L.S.; Yoshida, C.H.P.; Firmino, A.C.; de Figueiredo, P.A.M.; Aguilar, J.V.; Nicolai, A.B.; Paiva, W.D.S.D.; Cruz, V.H.; Tomaz, R.S. Response of soybean to soil waterlogging associated with iron excess in the reproductive stage. Physiol. Mol. Biol. Plants 2020, 26, 1635–1648. [Google Scholar] [CrossRef]
- Jackson, M.B.; Hall, K.C. Early stomatal closure in waterlogged pea plants is mediated by abscisic acid in the absence of foliar water deficits. Plant Cell Environ. 1987, 10, 121–130. [Google Scholar] [CrossRef]
- Olorunwa, O.J.; Adhikari, B.; Shi, A.; Barickman, T.C. Screening of cowpea (Vigna unguiculata (L.) Walp.) genotypes for waterlogging tolerance using morpho-physiological traits at early growth stage. Plant Sci. 2022, 315, 111136. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Plant Responses to Flooding of Soil. Bioscience 1984, 34, 162–167. [Google Scholar] [CrossRef]
- Yordanova, R.Y.; Popova, L.P. Flooding-induced changes in photosynthesis and oxidative status in maize plants. Acta Physiol. Plant. 2007, 29, 535–541. [Google Scholar] [CrossRef]
- Pang, J.; Zhou, M.; Mendham, N.; Shabala, S. Growth and physiological responses of six barley genotypes to waterlogging and subsequent recovery. Aust. J. Agric. Res. 2004, 55, 895–906. [Google Scholar] [CrossRef]
- Steffens, D.; Hütsch, B.W.; Eschholz, T.; Lošák, T.; Schubert, S. Water logging may inhibit plant growth primarily by nutrient deficiency rather than nutrient toxicity. Plant Soil Environ. 2005, 51, 545–552. [Google Scholar] [CrossRef]
- Huang, B.; Johnson, J.W.; Nesmith, S.; Bridges, D.C. Growth, physiological and anatomical responses of two wheat genotypes to waterlogging and nutrient supply. J. Exp. Bot. 1994, 45, 193–202. [Google Scholar] [CrossRef]
- Arduini, I.; Baldanzi, M.; Pampana, S. Reduced Growth and Nitrogen Uptake During Waterlogging at Tillering Permanently Affect Yield Components in Late Sown Oats. Front. Plant Sci. 2019, 10, 1087. [Google Scholar] [CrossRef]
- Board, J.E. Waterlogging Effects on Plant Nutrient Concentrations in Soybean. J. Plant Nutr. 2008, 31, 828–838. [Google Scholar] [CrossRef]
- Bacanamwo, M.; Purcell, L.C. Soybean dry matter and N accumulation responses to flooding stress, N sources and hypoxia. J. Exp. Bot. 1999, 50, 689–696. [Google Scholar] [CrossRef]
- Cartelat, A.; Cerovic, Z.G.; Goulas, Y.; Meyer, S.; Lelarge, C.; Prioul, J.-L.; Barbottin, A.; Jeuffroy, M.-H.; Gate, P.; Agati, G.; et al. Optically assessed contents of leaf polyphenolics and chlorophyll as indicators of nitrogen deficiency in wheat (Triticum aestivum L.). Field Crop. Res. 2005, 91, 35–49. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Environmental Significance of Anthocyanins in Plant Stress Responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Huang, B. Nutrient Accumulation and Associated Root Characteristics in Response to Drought Stress in Tall Fescue Cultivars. Hortscience 2001, 36, 148–152. [Google Scholar] [CrossRef]
- Ransom, J. Flooding/Waterlogging Will Affect Crop Development. Available online: https://www.ag.ndsu.edu/news/newsreleases/2011/may-30-2011/flooding-waterlogging-will-affect-crop-development (accessed on 10 July 2022).
- Reicosky, D.C.; Smith, R.C.G.; Meyer, W.S. Foliage temperature as a means of detecting stress of cotton subjected to a short-term water-table gradient. Agric. For. Meteorol. 1985, 35, 193–203. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, Y.; Yue, Z.; Chen, X.; Cao, X.; Ai, X.; Jiang, B.; Xing, Y. The leaf-air temperature difference reflects the variation in water status and photosynthesis of sorghum under waterlogged conditions. PLoS ONE 2019, 14, e0219209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhu, K.; Wang, Y.Q.; Zhang, Z.P.; Lu, F.; Yu, H.Q.; Zou, J.Q. Changes in photosynthetic and chlorophyll fluorescence characteristics of sorghum under drought and waterlogging stress. Photosynthetica 2019, 57, 1156–1164. [Google Scholar] [CrossRef]
- Yang, F.; Liu, T.; Wang, Q.; Du, M.; Yang, T.; Liu, D.; Li, S.; Liu, S. Rapid determination of leaf water content for monitoring waterlogging in winter wheat based on hyperspectral parameters. J. Integr. Agric. 2021, 20, 2613–2626. [Google Scholar] [CrossRef]
- Yang, F.; Liu, S.; Wang, Q.; Liu, T.; Li, S. Assessing Waterlogging Stress Level of Winter Wheat from Hyperspectral Imagery Based on Harmonic Analysis. Remote Sens. 2022, 14, 122. [Google Scholar] [CrossRef]
- Jiang, J.; Steven, M.D.; He, R.; Chen, Y.; Du, P.; Guo, H. Identifying the spectral responses of several plant species under CO2 leakage and waterlogging stresses. J. Greenh. Gas Control. 2015, 37, 1–11. [Google Scholar] [CrossRef]
- Kovar, M.; Brestic, M.; Sytar, O.; Barek, V.; Hauptvogel, P.; Zivcak, M. Evaluation of Hyperspectral Reflectance Parameters to Assess the Leaf Water Content in Soybean. Water 2019, 11, 443. [Google Scholar] [CrossRef]
- Emengini, E.J.; Blackburn, G.A.; Theobald, J.C. Discrimination of plant stress caused by oil pollution and waterlogging using hyperspectral and thermal remote sensing. JARS 2013, 7, 073476. [Google Scholar] [CrossRef]
- Serrano-Calvo, R.; Cutler, M.E.J.; Bengough, A.G. Spectral and Growth Characteristics of Willows and Maize in Soil Contaminated with a Layer of Crude or Refined Oil. Remote Sens. 2021, 13, 3376. [Google Scholar] [CrossRef]
- Zahir, S.A.D.M.; Omar, A.F.; Jamlos, M.F.; Azmi, M.A.M.; Muncan, J. A review of visible and near-infrared (Vis-NIR) spectroscopy application in plant stress detection. Sens. Actuators A Phys. 2022, 338, 113468. [Google Scholar] [CrossRef]


| Parameters. | Treatment | Time | Treatment * Time |
|---|---|---|---|
| Photosynthesis (µmol m−2 s−1) | *** | *** | *** |
| Stomatal conductance (mol CO2 m−2 s−1) | *** | *** | *** |
| Electron transport rate (µmol electron m−2 s−1) | *** | *** | *** |
| Transpiration rate (mmol H2O m−2 s−1) | *** | *** | *** |
| The ratio of internal to external CO2 concentration (unitless) | *** | *** | *** |
| Water use efficiency (µmol CO2 mmol−1 H2O) | * | ns | ns |
| Canopy temperature (°C) | *** | *** | *** |
| Chlorophyll content (µg cm−2) | *** | *** | *** |
| Flavonoid index | *** | ns | ** |
| Anthocyanin index | *** | *** | *** |
| Nitrogen balance index | *** | *** | *** |
| Treatments | Nitrogen (%) | Phosphorous (%) | Potassium (%) | Calcium (%) | Magnesium (%) | Iron (mg kg1) | Manganese (mg kg−1) | Zinc (mg kg1) | Copper (mg kg−1) | Boron (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 3.7 a | 0.62 b | 2.19 b | 1.25 a | 0.36 a | 98 b | 60 b | 36 b | 1.6 b | 88 a |
| Waterlogged | 2.8 b | 0.87 a | 2.5 a | 1.22 a | 0.27 b | 114 a | 81 a | 42 a | 1.85 a | 92 a |
| Parameter | Treatments | % Change | |
|---|---|---|---|
| Control | Waterlogged | ||
| Plant height, cm | 68.5 a | 54.1 b | −21 |
| Main stem leaves, no. plant−1 | 17.7 a | 15.1 b | −15 |
| Pods, no. plant−1 | 308 a | 66 b | −79 |
| Leaf area, cm2 plant−1 | 12,524 a | 3731 b | −70 |
| Leaf dry weight, g plant−1 | 55 a | 18 b | −67 |
| Stem dry weight, g plant−1 | 86 a | 29 b | −67 |
| Root dry weight, g plant−1 | 28 a | 12 b | −57 |
| Pod dry weight, g plant−1 | 30 a | 6 b | −81 |
| Above-ground dry weight, g plant−1 | 172 a | 53 b | −69 |
| Total dry weight, g plant−1 | 200 a | 65 b | −68 |
| Root/shoot | 0.17 b | 0.25 a | 49 |
| Pod production efficiency, g kg−1 | 151 a | 85 b | −44 |
| Pod no./total dry weight, no. g−1 | 1.55 a | 1.02 b | −34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adegoye, G.A.; Olorunwa, O.J.; Alsajri, F.A.; Walne, C.H.; Wijewandana, C.; Kethireddy, S.R.; Reddy, K.N.; Reddy, K.R. Waterlogging Effects on Soybean Physiology and Hyperspectral Reflectance during the Reproductive Stage. Agriculture 2023, 13, 844. https://doi.org/10.3390/agriculture13040844
Adegoye GA, Olorunwa OJ, Alsajri FA, Walne CH, Wijewandana C, Kethireddy SR, Reddy KN, Reddy KR. Waterlogging Effects on Soybean Physiology and Hyperspectral Reflectance during the Reproductive Stage. Agriculture. 2023; 13(4):844. https://doi.org/10.3390/agriculture13040844
Chicago/Turabian StyleAdegoye, Grace A., Omolayo J. Olorunwa, Firas A. Alsajri, Charles H. Walne, Chaturika Wijewandana, Swatantra R. Kethireddy, Krishna N. Reddy, and K. Raja Reddy. 2023. "Waterlogging Effects on Soybean Physiology and Hyperspectral Reflectance during the Reproductive Stage" Agriculture 13, no. 4: 844. https://doi.org/10.3390/agriculture13040844
APA StyleAdegoye, G. A., Olorunwa, O. J., Alsajri, F. A., Walne, C. H., Wijewandana, C., Kethireddy, S. R., Reddy, K. N., & Reddy, K. R. (2023). Waterlogging Effects on Soybean Physiology and Hyperspectral Reflectance during the Reproductive Stage. Agriculture, 13(4), 844. https://doi.org/10.3390/agriculture13040844





