Abstract
Rising air temperature is a major constraint for crop productivity under the current climate change scenario. Rice crops are known to be sensitive to high-temperature (HT) stress at anthesis and post-anthesis stages. Photosynthesis is an important metabolic process and is affected by HT stress. A pot study was planned to screen a set of seventy-three Indian rice accessions based upon changes in the rate of photosynthesis (Pn) and related gas exchange traits under HT, and to characterize the contrasting rice accessions for component traits of HT stress tolerance. All accessions were raised under ambient temperature (AT) until the booting stage and exposed to HT using controlled chambers at anthesis and post-anthesis. HT exposure led to a large reduction (up to 50%) in Pn, but stomatal conductance (gs) and the rate of transpiration (E) increased significantly across the rice accessions. Based on the photosynthetic response under HT, two contrasting rice accessions (IRGC 135883, tolerant, and IRGC 127222, sensitive) were selected and characterized for HT tolerance, along with an NL-44 check. Among them, Pn decreased marginally but gs and E showed significant increases under HT in the tolerant accession, while sensitive accession showed an up to 50% reduction in Pn and marginal increase in gs and E. No significant changes were recorded for chlorophyll fluorescence (Fv/Fm) in both the genotypes, but tissue temperature depression (TTD) was higher in IRGC 135883 accession under HT. Endogenous abscisic acid (ABA) content increased under HT in the flag leaf of both the accessions, and the highest increase was observed in the sensitive accession. Similarly, spikelet fertility and grain yield showed large reductions in sensitive rice accession under HT. A large increase in ABA concentration in the leaves of the sensitive rice accession might be affecting its gs and cooling capacity under an HT environment. Finally, the study concludes that tolerant rice accessions can be recommended as donors and exploited in future rice breeding programs for developing climate-resilient rice genotypes.
1. Introduction
Rice is an important staple food and is consumed by more than half of the global population. Its production is affected by various biotic and abiotic stress factors. High-temperature (HT) stress is an important form of abiotic stress, affecting reproductive development and metabolic processes in rice [1]. An IPCC (2022) report predicted that global air temperature may exceed 1.5 to 2 °C by the end of this century [2]. Peng et al. [3] reported that an increment of 1 °C air temperature can reduce rice crop yield up to 10%. On the other hand, major rice-growing regions are in the tropics and subtropics, where rice crops already experience HT during the crop season. Due to climate change, the air temperature may further increase and affect rice productivity [2].
Photosynthesis is an important metabolic process and contributes to the yield potential of crops [4,5]. Though it is a light-dependent process, it is sensitive to HT stress too [6,7]. Several studies have reported heat-tolerant rice varieties exhibiting higher photosynthetic rates (Pn) and improved membrane thermostability [8,9]. The adaptation in plants to HT environments for photosynthesis depends on the natural genetic variation within a population. Variability for Pn in plants can lead to changes in growth rate and productivity [10,11,12]. Developing climate-resilient genotypes with improved productivity is required under the current scenario [13]. The genotypes with better adaptations under an HT environment can be used as a source of breeding for manipulating leaf photosynthesis (Pn) and photosynthate partitioning. Stomatal conductance (gs) is a major limiting factor for carbon assimilation and is regulated through the opening and closing of stomata. Pn and gs are inhibited by moderate heat stress in many plant species due to decreases in the activation state of Rubisco [14,15,16]. Increased gs has also been reported under HT stress [17].
The role of abscisic acid (ABA) for improving drought stress tolerance is well documented and also reported as conferring heat tolerance in plants in various studies [18,19,20]. HT stress induces endogenous ABA levels, which promotes water balance and strengthens heat tolerance by regulating stomatal closure and enhancing antioxidant ability [21,22]. Islam et al. [23] reported that rice plants treated with ABA showed reduced gs and transpiration rate (E) in the leaves. This function of ABA can lead to higher leaf temperatures under heat stress and may cause damage to the plants. Thus, the association of ABA for HT stress tolerance may require a threshold level to induce thermotolerance [24]. ABA accumulation has been shown to negatively regulate heat acclimation in mutant hts plants with semi-rolled leaves by modulating energy homeostasis [25].
Thus, there is a need to identify rice genotypes with better adaptations for photosynthetic carbon assimilation and other gas exchange traits, such as E and gs, under an HT environment. Tolerant genotypes can be identified and recommended as donors for breeding HT-stress-tolerant rice genotypes for sustaining rice productivity under a changing climate. Therefore, this study was planned with the objectives (i) to phenotype/screen a set of seventy-three rice accessions for Pn, E, gS and related gas exchange traits under HT stress and (ii) physiological and biochemical characterization of the contrasting rice accessions for component traits of HT stress tolerance.
2. Materials and Methods
2.1. Plant Material and Growth Conditions
Experiment I
Two pot experiments were conducted at the Controlled Environment Facility of Phenomics Center, ICAR (Indian Agricultural Research Institute): New Delhi, India (28°35 N latitude, 77°12 E longitude), during Kharif 2019–2020 and 2020–2021. The seeds of the rice accessions were obtained from IRRI, Philippines, and their details are given in Supplementary Table S1 (ST-1). Seedlings were raised in the field nursery and transplanted into plastic pots during both the rice seasons (Kharif, 2019–20 and 2020–21). In experiment I (2019–20), the screening of 73 rice accessions was carried out based on changes in gas exchange traits and panicle yield under HT stress. For experiment II (2020–21), contrasting accessions (IRGC 135883, tolerant, and IRGC 127222, sensitive) were selected from the accessions used in experiment I and characterized for physiological and biochemical component traits associated with HT stress tolerance.
Both experiments were conducted using white plastic pots that were 14” in diameter and 12” in height. The pots were filled with 20 kg of clay-loam soil that was mixed with 800 g of farmyard manure. N:P:K fertilizers were applied to the soil in the following amounts: (NH4)2SO4 (0.375 g/kg), KCl (0.075 g/kg) and Single Superphosphate (0.075 g/kg). At the tillering stage of growth, an additional dose of N (0.125 g/kg) was added to the soil. Six biological replicates (pots) were maintained for each accession. Initially, three seedlings were planted in each pot and later were thinned to two plants per pot. Pots were kept flooded (water 3–5 cm above the soil surface) until two weeks before physiological maturity (30–38 days post-heading). Weeds, insects and pests were controlled using appropriate measures.
2.2. Exposure to HT Stress
All the pots were maintained in the natural environment at ambient temperature (AT) (32 °C/25 °C) until the booting stage, and later, half of them (set of three replications) were transferred into controlled-environment chambers for HT exposure (HT, 38 °C/25 °C) and continued until the plants reached physiological maturity. However, the rice accessions used in the study had different growth durations; thus, the accessions were transferred into the chambers at the onset of the booting in an individual accession. The real-time data on temperature and relative humidity were monitored continuously at 30 min time intervals using MINCER obtained from NIAES, Tsukuba, Japan, as described by [26] and depicted in Figure 1.
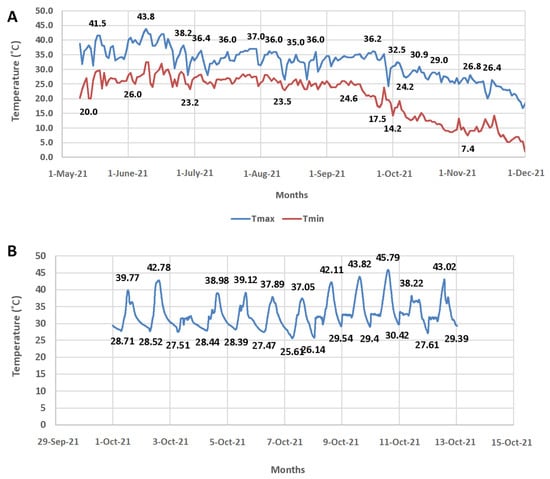
Figure 1.
The figure illustrates the ambient temperature (A) and high temperature (HT)maintained during the course of experiment (B). Each data point represents the average of three replications.
Experiment II
From 73 rice accessions phenotyped in experiment I, two contrasting accessions (IRGC 135883 and IRGC 127222) along with the check NL-44 were characterized for traits related to HT stress tolerance, such as chlorophyll fluorescence (Fv/Fm), tissue temperature depression (TTD), ABA concentration and leaf vein density, in addition to the traits analysed in experiment I. The methodology used for raising the plants in experiment II was similar to experiment I.
Out of six plants per treatment (two plants/pot), three plants were used for estimation of physiological traits (gas exchange traits and leaf Fv/Fm) and the remaining three plants were used for yield analysis. Five days after shifting pots inside the control chambers, physiological observations were recorded in flag leaves of both AT- and HT-treated plants. The middle part of the flag leaves was stored in distilled water and used for measuring leaf vein density. The remaining tissue was used for ABA analysis and stored at −80 °C until further analysis.
2.3. Observations
2.3.1. Rate of Photosynthesis and Other Gas Exchange Traits
In both experiments, the gas exchange traits viz. Pn, E and g and substomatal CO2 concentration (Ci) were recorded 5 days after initiation of high-temperature stress using a portable photosynthesis system (LI-6400 XT; LI-COR Inc., Lincoln, NE, USA) in both AT- and HT-exposed plants. Fully expanded flag leaf was selected from each replicate and analysed for gas exchange traits between 9:00 h and 12 h. The leaf chamber conditions were maintained during measurement as follows: light intensity at 1200 μmol m−2 s−1 PAR, flow rate was maintained at 500 μmol s−1, CO2 adjusted to 400 μmol mol−1 using CO2 mixer and the VPD was at 1.5 to 2.0 kPa. Photosynthetic Water Use Efficiency (pWUE) and Carboxylation Efficiency (CE) were calculated as the ratio of Pn and Ci (Pn/Ci), respectively [27].
2.3.2. Chlorophyll Fluorescence Parameters
Chlorophyll fluorescence (Fv/Fm) was measured in experiment II in the flag leaf using a modulated chlorophyll fluorometer (OS1p Chlorophyll Fluorometer; Opti-Sciences, Inc., Hudson, NH, USA). The measurements were taken for the maximum potential quantum efficiency of Photosystem II after a 30 min dark adaptation period.
2.3.3. Tissue Temperature Depression (TTD)
In experiment II, the surface temperature of the flag leaf was recorded on the 5th day of heat stress exposure, between 11.00 h and 14.00 h, using a hand-held infrared thermometer (IRT) (Crop Trak, Spectrum Technologies, Aurora, IL, USA). During measurements, the instrument was held at an angle of 30° to the horizontal plane, and the average value was used to calculate the TTD following Ayeneh et al. [28]. During the time of image capturing, mean air temperature was 38.1 °C (SD ± 1.28) and relative humidity was 68%.
2.3.4. Abscisic Acid (ABA) Estimation
ABA concentration in flag leaf samples was estimated using high-performance liquid chromatography (HPLC), following the methods from [29]. Leaf samples were snap-frozen in liquid nitrogen, and extraction was conducted with 10 mL of 80% v/v acetone (80 mL acetone, 1 mL glacial acetic acid and 100 mg of 2, 6 di-tert-butyl 4-methyl phenol). The tissue residue was homogenized and filtrate was vacuum evaporated and filtered through Whatman No. 1 filter. From the filtrate, acetone was removed using rotary flash vacuum evaporator. The lipid-soluble tissue extract was dissolved in 1% acetic acid solution. Before injecting into HPLC, the samples were filtered with a 0.45 mm Millipore filter. Then, 20 µL of standard ABA was injected into HPLC and the wavelength was set at 265 nm before injecting the sample.
2.3.5. Leaf Vein Density (LVD)
The sampled flag leaves were observed under a transmitted-light inverted imaging system (EVOS XL Core system) at 4× agnification. The abaxial side of the leaves was used for counting. To ensure accuracy, the widest middle section of the leaf was focused on for counting, as variations in vein density were observed in other areas of the leaf, as per Feldman [30]. Veins close to the midrib or on the edges of the leaf were not included in the count, as they tend to have higher vein density that does not accurately reflect the entire width of the leaf. For each accession, four images were captured at the right and left side of the midrib and manually counted using ImageJ software.
2.3.6. Spikelet Fertility and Yield Traits
For each accession, three plants were selected per treatment and measurements were taken for the following traits: grain weight per plant and spikelet fertility. Filled and unfilled grains were manually separated from the main culm and used for calculating the spikelet fertility percentage (filled grains/(filled + unfilled grains) × 100%) [31].
2.3.7. Statistical Analysis
Data on different parameters were analyzed using two-way analysis of variance with a completely randomized design. The statistical software SPSS 13.0 was used to compare the differences between accessions, treatments and their interaction. The results were further examined by comparing the least significant difference means at a 5% level using Tukey’s post hoc test.
3. Results
3.1. Experiment 1
3.1.1. Rate of Photosynthesis and Related Gas Exchange Traits under HT
In experiment 1, the response of gas exchange traits under AT and HT was determined in 73 pot-grown rice accessions. Significant differences (p < 0.05) existed among the rice accessions for all the traits studied under both the treatments (Figure 2a–e). Pn varied significantly among the accessions. The interaction between treatment and accessions (G × T) was statistically significant (p < 0.05). The overall mean of the Pnin control-grown plants of rice accessions was 18.84 μmol CO2 m−2 s−1 and reduced by 16% in HT-grown plants (Figure 2a). The gs and E showed significant (p < 0.05) G × T interaction and increased in plants grown under HT treatment. The average mean of gs across the accessions was 0.413 and 0.618 mol m−2 s−1 in plants grown in an AT and HT environment, respectively, while the genotypic mean of E ranged between 0.007 and 0.012 mol H2O m−2 s−1, respectively, under AT and HT conditions. Mean values of gs and E increased by 49 and 80%, respectively, due to high-temperature exposure (Figure 2b,c). The C showed significant (p < 0.05) G × T interaction and it was significantly impacted by HT treatment in all rice accessions, and overall mean of Ci increased by 12% under HT conditions (Figure 2d). pWUE showed a decreasing trend in HT conditions and the magnitude of reduction was 53% compared to AT-grown plants (Figure 2e). The width of the leaves under HT conditions was reduced by 5% compared to those grown under AT conditions, as shown in Figure 2f.
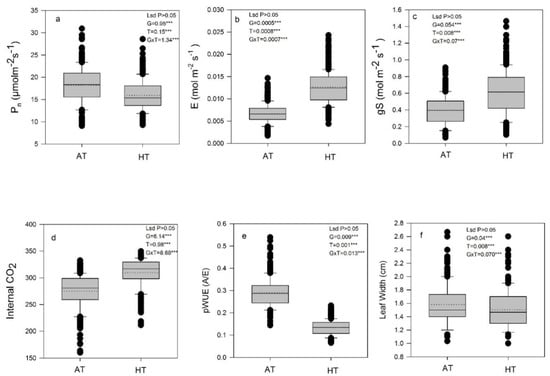
Figure 2.
The box plot illustrates the phenotypic plasticity of (a) photosynthetic rate (Pn), (b) transpiration rate (E), (c) stomatal conductance (gs), (d) substomatal CO2 concentration (Ci), (e) photosynthetic water use efficiency (pWUE) and (f) Leaf width in rice accessions under both ambient-temperature (AT) and high-temperature (HT) conditions in experiment 1. The box plot represents the median values (n = 3) for each trait of the 73 rice accessions. The black solid and dotted lines indicate the median and mean of all the rice accessions used in the study, respectively. Significance values were calculated and represented as p < 0.001 denoted by ***. Results that were not statistically significant are indicated as ns. Outliers are represented by closed circles.
3.1.2. Analysis of Percent Reductions in Leaf Pn under HT
The percentage reduction in Pnwas calculated for all the 73 rice accessions ranged between −0.5 and 50% (Figure 3). The maximum reduction in Pnwas was observed in accessions IRGC 127720, IRGC 127222 and IRGC 125890, whereas minimum reduction in Pnwas was observed in NL44, IRGC 135883 and IRGC 132387 accessions. Based upon percent reductions in Pn, IRGC 135883 (11% reduction) and IRGC 127222 (50%) were categorised as tolerant and sensitive to HT. Both accessions, along with a check (NL-44), were further characterized for heat tolerance using component physiology traits.
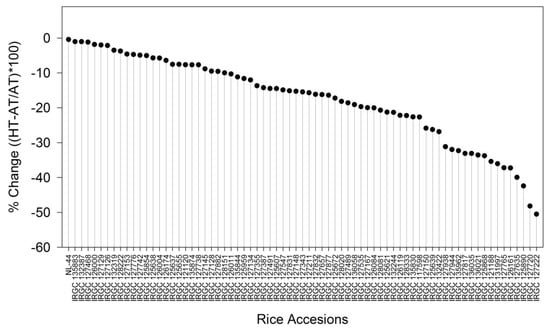
Figure 3.
The figure illustrates the response index of 73 rice accessions determined based on their photosynthetic rate (Pn) under high-temperature (HT) conditions in comparison to ambient-temperature (AT) conditions. The accessions are arranged from least responsive to most responsive for the trait, as indicated by the vertical lines in the figure.
3.2. Experiment II
3.2.1. Gas Exchange Traits and Fv/Fm under HT in Contrasting Accessions
A significant G × T (p < 0.05) effect was recorded for Pn and other gas exchange traits in two contrasting rice accessions and the check NL-44 (Figure 4a–f). Pn showed a significant reduction (p < 0.05) under HT in the sensitive accession IRGC 127222. This accession showed higher Pn than the tolerant accession, IRGC 135883, under AT conditions, but under HT stress, it showed an almost 50% reduction, while IRGC 135883 showed only a marginal reduction of 7%. These changes in Pn were comparable to NL-44 (Figure 4a). HT treatment showed a significant genotypic (p < 0.05) effect for stomatal conductance (gs) (Figure 4b). The differences in gs were not significant between the contrasting accessions under AT conditions, but under HT conditions, they increased by 41% and 9% in both IRGC 135883 and IRGC127222 accessions, respectively. On the other hand, NL-44 showed the highest increase (70%) in gs under HT conditions. Similarly, a significant genotypic (p < 0.01) treatment (p < 0.001) and genotype × treatment (p < 0.01) effect were recorded for E and increased significantly (60%) under HT in the tolerant accession IRGC 135883 as well as in the check NL-44 (Figure 4c). In contrast, the increase in E in the sensitive accession, IRGC 127222, was relatively marginal when exposed to HT. Under AT treatment, the level of E was almost similar, in both the rice accessions and check.
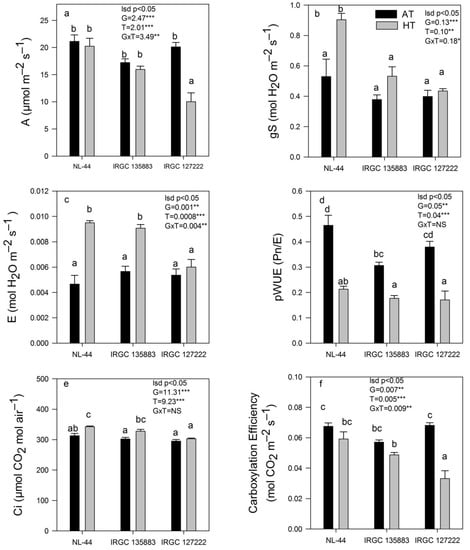
Figure 4.
The effect of ambient-temperature (AT) and high-temperature (HT) exposure on (a) photosynthetic rate (Pn), (b) stomatal conductance (gs), (c) transpiration rate (E), (d) photosynthetic water use efficiency (pWUE), (e) substomatal CO2 concentration (Ci) and (f) carboxylation efficiency (CE) of contrasting rice accessions during the reproductive stage was measured. Means of three replicates are indicated by the vertical columns in the figure. Error bars indicate the ± SEM. The least significant difference test was used to determine significance. Means with the different letters are significantly different at level * p < 0.05, ** p < 0.01,*** p < 0.001, NS non-significant.
pWUE significantly differed among both the accessions. Due to HT stress, pWUE decreased in both the accessions, and NL-44 showed the highest pWUE followed by IRGC 135883 (0.18 μmolmol−1) and IRGC 127222 (0.17 μmolmol−1) (Figure 4d). Ci did not change significantly due to HT stress treatment in both the rice accessions (Figure 4e). In the check (NL-44) and the tolerant accession, IRGC 135883, Ci increased marginally under HT stress, but in the sensitive accession (IRGC127222), it did not change. In general, the CE decreased under HT treatment in all accessions but the reduction was lowest in tolerant accession IRGC 135883 and check NL-44, whereas IRGC127222 showed a greater than 50% reduction in CE under HT (Figure 4f).
3.2.2. Chlorophyll Fluorescence (Fv/Fm), Tissue Temperature Depression (TTD) and Leaf Width
Under HT conditions, a significant decrease in flag leaf Fv/Fm was observed, and the rate of reduction was highest in sensitive accession IRGC127222 (21%), followed by a 14% reduction in IRGC 135883 and 9% in NL-44. The level of Fv/Fm under HT conditions was almost similar in accession IRGC 135883 and check (NL-44) (Figure 5a). The highest TTD was recorded under HT in check NL-44 (4.68 °C), followed by IRGC 135883 (3.08 °C) and IRGC 127222 at (0.48 °C) (Figure 5b). For leaf width, there was no significant difference between the treatment and accessions (Figure 5c).
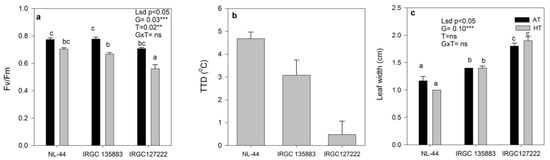
Figure 5.
The effect of ambient-temperature (AT) and high-temperature (HT) exposure on (a) PS II efficiency (Fv/Fm), (b) tissue temperature depression (TTD) and (c) leaf width of contrasting rice accessions at the reproductive stage. Means of three replicates are indicated by the vertical columns in the figure. Error bars indicate the ± SEM. The least significant difference test was used to determine significance. Means with the different letters are significantly different at level: ** p < 0.01, *** p < 0.001, ns non-significant.
3.2.3. Endogenous ABA Content in Flag Leaf under HT
The endogenous ABA content varied significantly among the accessions and treatment (genotype (p < 0.05); treatment (p < 0.001); genotype X treatment (p < 0.01)). The ABA concentration in the flag leaves significantly increased, irrespective of the rice accessions under HT stress (Figure 6). Under AT, the ABA content was minimum in sensitive accession IRGC 127222 (5.95 µg g−1 FW), and it was at par in NL-44 (6.86 µg g−1 FW) and IRGC 135883 (7.38 µg g−1 FW). However, in response to reproductive-stage HT stress, it increased in all the accessions and the highest increase was observed in sensitive accession IRGC 127222 (115.41% increase), followed by IRGC 135883 (40% increase) and NL-44 (29.22% increase).
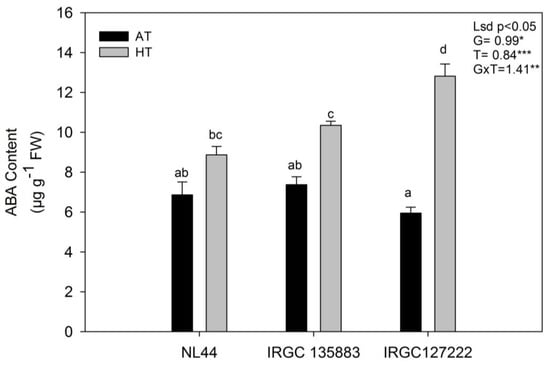
Figure 6.
The effect of ambient-temperature (AT) and high-temperature (HT) exposure on abscisic acid (ABA) content of contrasting rice accessions at the reproductive stage. Means of three replicates are indicated by the vertical columns in the figure. Error bars indicate the ± SEM. The least significant difference test was used to determine significance. Means with the different letters are significantly different at level: * p < 0.05,** p < 0.01, *** p < 0.001.
3.2.4. Spikelet Fertility and Grain Yield under HT
HT stress exposure resulted in a reduction in spikelet fertility, and there was significant (p < 0.001) G × T interaction for spikelet fertility. Among two rice accessions, IRGC 127222 recorded the highest percent reduction (31%) in spikelet fertility over AT-grown plants, while IRGC 135883 showed a lower (21%) reduction in spikelet fertility under heat stress, as compared to AT. The check NL-44 showed a 13% reduction in spikelet fertility due to HT compared to AT (Figure 7a). Similarly, a significant interaction among accessions (p < 0.001), treatment (p < 0.001), as well as G × T (p < 0.001) was observed for grain yield (gplant−1) (Figure 7b). HT exposure decreased the grain yield significantly (58%) in the IRGC 127222 accession, while the reduction in grain yield of IRGC 135883 accession was 11%, and it was at par with NL-44 (9% reduction) under HT treatment. A significant positive correlation was observed between photosynthesis and yield parameters, viz. grain yield (p < 0.001) and spikelet fertility (p < 0.05) and Fv/Fm (<0.05) (Figure 8), while a negative correlation existed between photosynthesis and ABA (<0.05). Similarly, negative correlation was observed between ABA and grain yield (<0.05), SF (0.01) and Fv/Fm (<0.05). However, Fv/Fm showed a positive correlation with TTD (<0.01) (Figure 8).
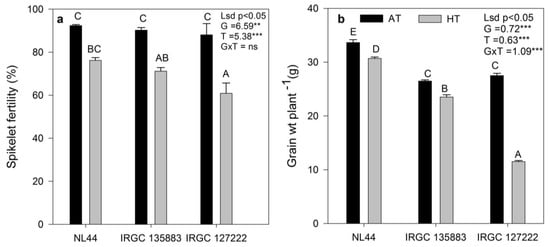
Figure 7.
The effect of ambient-temperature (AT) and high-temperature (HT) exposure on (a) spikelet fertility, (b) grain weight per plant of contrasting rice accessions at the reproductive stage. Means of three replicates are indicated by the vertical columns in the figure. Error bars indicate the ± SEM. The least significant difference test was used to determine significance. Means with the different letter are significantly different at level: ** p < 0.01, *** p < 0.001, ns non-significant.
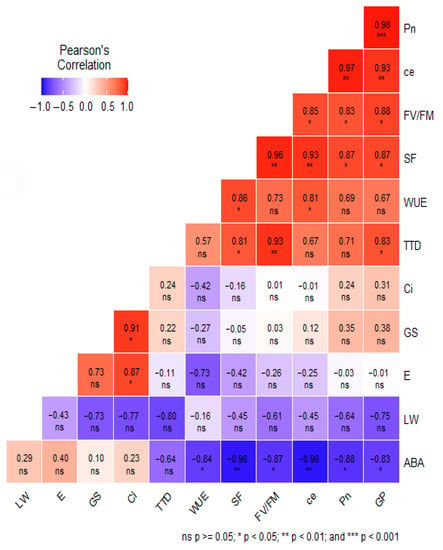
Figure 8.
Pearson correlation matrix for physiological and grain yield traits in ambient-temperature (AT) and high-temperature (HT) treatments. ***, **, * correlation is significant at the 0.001, 0.01 and 0.05 level, respectively, with photosynthetic rate (Pn), transpiration rate (E), stomatal conductance (gs), substomatal CO2 concentration (Ci), photosynthetic water use efficiency (pWUE), carboxylation efficiency (CE), photosystem II efficiency (Fv/Fm), tissue temperature depression (TTD), abscisic acid content (ABA), leaf width (LW), spikelet fertility (SF) and grain yield per plant (GP).
4. Discussion
Photosynthesis is an important physiological process affected by HT stress [7]. The photosynthetic products are the major determinants of plant productivity, and they are widely recognized as a key trait to improving crop yield [12,32,33]. In the current study, we observed large variability among 73 Indian rice accessions for gas exchange parameters, including Pn under AT and HT environments. In general, Pn decreased across the accessions and the percent reduction ranged between 0.5 and 51% (Figure 2a). On the other hand, gs and E increased in all accessions under HT. Reductions in Pn under HT can occur due to sensitivity of photochemical reactions and carbon assimilation [16,34,35]. Similar reductions in Pn have been reported under moderate increases in growth temperature (35–40 °C), which were attributed to higher rates of photorespiration under HT [7,36]. Photochemical reactions of Pn in thylakoid lamellae and carbon reduction in the stroma have been known as most sensitive to HT effects [35,37].
The increase in gs is considered as one of the major strategies in plants to acclimatize to an HT environment [38]. We observed significant increase in gs and E in rice accessions while experiencing moderate reductions in Pn that could be considered as tolerant accessions (Figure 2a–c). This ability to maintain high gs and E under HT allows for the diffusion of carbon dioxide into the leaf, which increases the Ci, as recorded in the tolerant accessions (Figure 4e, 8). However, closure of stomata under HT can impede photosynthesis and Ci, which can have a negative impact on plant survival [39,40].
Based upon the photosynthetic response, we selected a top-performing accession (IRGC 135883, no reduction in Pn under HT) and a worst-performing accession (IRGC 127222, up to 50% reduction in Pn under HT).We conducted another study to analyse the component traits associated with HT tolerance and exposed the plants to HT using similar methodology (Figure 3). Based upon the above response to Pn under HT, we considered IRGC 135883 as a tolerant accession, while IRGC 127222 was considered a sensitive type. The findings of this experiment confirmed the similar response of the accessions in terms of changes in Pn and related gas exchange traits under HT (Figure 3). Accession IRGC 127222 showed large reductions (50%) in Pn under HT when compared with IRGC 135883 and check (NL-44) (Figure 4). NL-44 is recognized as a potential donor for improving heat tolerance in both vegetative and reproductive stages of rice [9,41]. NERICA lines are a cross between African rice (Oryza glaberrima Steud.) and Asian rice (O. sativa) that inherit high-yielding characteristics from the Asian parent and the ability to thrive in harsh environments from the African parent [9]. NL-44 was found to maintain higher spikelet fertility under a controlled-environment temperature of 38 °C. It exhibited superior antioxidant scavenging abilities in both vegetative tissue and spikelets, and produced a higher grain yield [9].
In our study, we evaluated the relationship between pWUE and HT tolerance in rice accessions. pWUE, which is the ratio of carbon assimilation to gs, is a key determinant of how gross primary productivity responds to climate change [42,43,44]. Some studies show that decreased gs and increased Pn, or a combination of both, can lead to an increase in pWUE [45,46]. In this study, we observed that irrespective of the HT tolerance levels, rice accessions exhibited a decrease in gs and an increase in Pn under HT conditions, resulting in a reduction in pWUE (Figure 4d).
Chlorophyll fluorescence, the ratio of variable fluorescence to maximum fluorescence (Fv/Fm) and the base fluorescence (F0), is a physiological trait that is correlated with heat tolerance [47,48,49]. Changes in canopy temperature have been used as an indicator of a plant’s ability to balance moisture loss and heat stress [50]. We observed a significant difference in TTD between sensitive and tolerant rice accessions (Figure 5b). Tolerant accessions had higher TTD than sensitive accessions, indicating their greater ability to maintain a cooler canopy under HT (Figure 5a). Photosystem II (PSII) has been reported to be the most sensitive to HT, and the Fv/Fm ratio indicates the maximum quantum efficiency of PS II [51]. In our study, the accessions did not significantly vary for Fv/Fm values under HT treatment (Figure 5a).
Photosynthetic adaptation for long-term high-temperature exposure may occur, partly due to structural changes in the leaf tissues [52,53,54]. Leaf vein traits, including vein density and the size of xylem conduits within bundle sheaths, can influence leaf hydraulic conductance [55,56,57,58]. A clear relationship between plant hydraulic conductance and photosynthesis is shown in rice [59]. In the present study, there were no significant differences in vein density under HT in either accession (unpublished). Rice leaf vein density is strongly influenced by leaf width [57,60]. Tabassum et al. [61] found that water deficit caused by polyethylene glycol led to rice leaf narrowing and an increase in leaf density. Xiong et al. [62] observed significant variations in leaf morphological traits among the genotypes. In the present study, genotypic variation in leaf width was observed under AT and HT conditions, but no variation between the treatments was found (Figure 5c).
ABA has been reported to play a significant role in HT tolerance of plants by increasing hydrogen peroxide levels and inducing antioxidant capacity and heat shock proteins [18,21,63,64,65,66]. Higher leaf ABA concentration has been found to induce thermotolerance in maize and bromegrass [24,67]. We found an increased ABA concentration in HT-exposed plants of both the accessions and the sensitive accession showed a higher increase in ABA but a reduction in gas exchange traits (Figure 6 and Figure 8). The negative effects of ABA in plants under stress may not occur solely due to increased concentrations but may depend on the plant types, such as in rolled leaf plants [25]. Rolled leaf plants consume more energy due to a higher respiration rate under heat stress and may experience damage when sprayed with ABA under heat stress, possibly due to ABA-induced stomatal closure, resulting in lower net Pn and E but higher leaf temperatures [18,25].
HT above 35 °C during the flowering stage can negatively impact spikelet fertility in rice by causing poor anther dehiscence and incomplete fertilization [68,69,70]. HT treatment can also affect pollen germination and its growth [71,72]. In our study, we observed that the sensitive accession (IRGC 127222) showed higher reductions in spikelet fertility and grain yield under HT stress (Figure 7a,b). An HT-induced reduction in leaf photosynthesis rate and increased spikelet sterility negatively affect rice yield and productivity [73,74]. We observed that the tolerant accessions (IRGC 135883 and NL-44) showed higher spikelet fertility and grain yield, as well as maintaining higher Pn and cooler canopy temperatures (Figure 7a,b and Figure 8).
5. Conclusions
The findings of this study showed that large variability exists among Indian rice accessions for Pn (0–50% reductions) under an HT stress environment. On the other hand, a significant increase in E and gs under HT indicates their better cooling capacity. Increased gs and E are the adaptive features of tolerant rice accessions and contributed in their photosynthetic performance under an HT environment. Rice accessions showing no reductions in Pn under HT can be considered as tolerant types. This rice accession also showed higher TTD and marginal reductions in the Fv/Fm ratio. Increased ABA concentration in the flag leaves and gs suggested that ABA is involved in stomatal regulation. However, a large increase in ABA concentration in the leaves of the sensitive rice accession might affect its gs and cooling capacity under an HT environment. Finally, the findings of this study conclude that the identified tolerant rice accession can be recommended as donors and exploited in future rice breeding programs for developing climate-resilient rice genotypes.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agriculture13030545/s1, Table S1: Details of the rice accessions used in experiment 1.
Author Contributions
Conceptualization, data curation, methodology, formal analysis, writing—original draft, M.K.M., S.K. (Sourabh Karwa), P.P. and M.P.; project administration, supervision, formal analysis, writing—review and editing, M.P., S.K. (Sourabh Karwa), P.K., S.N., M.K., S.K. (Sudhir Kumar) and V.C.; funding acquisition, writing—review and editing, S.K. (Sourabh Karwa), R.P. and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the financial support received from the Indian Council of Agricultural Research (ICAR), New Delhi.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mittler, R.; Blumwald, E. Genetic Engineering for Modern Agriculture: Challenges and Perspectives. Annu. Rev. Plant Biol. 2010, 61, 443–462. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.R.; Skea, J.; Slade, R.; Al Khourdajie, A.; van Diemen, R.; McCollum, D.; Pathak, M.; Some, S.; Vyas, P.; Fradera, R.; et al. (Eds.) IPCC, Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2022. [Google Scholar]
- Peng, S.; Huang, J.; Sheehy, J.E.; Laza, R.C.; Visperas, R.M.; Zhong, X.; Centeno, G.S.; Khush, G.S.; Cassman, K.G. Rice Yields Decline with Higher Night Temperature from Global Warming. Proc. Natl. Acad. Sci. USA 2004, 101, 9971–9975. [Google Scholar] [CrossRef] [PubMed]
- Long, S.P.; Marshall-Colon, A.; Zhu, X.-G. Meeting the Global Food Demand of the Future by Engineering Crop Photosynthesis and Yield Potential. Cell 2015, 161, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Ort, D.R.; Merchant, S.S.; Alric, J.; Barkan, A.; Blankenship, R.E.; Bock, R.; Croce, R.; Hanson, M.R.; Hibberd, J.M.; Long, S.P.; et al. Redesigning Photosynthesis to Sustainably Meet Global Food and Bioenergy Demand. Proc. Natl. Acad. Sci. USA 2015, 112, 8529–8536. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.V.V.; Bheemanahalli, R.; Jagadish, S.V.K. Field Crops and the Fear of Heat Stress—Opportunities, Challenges and Future Directions. Field Crop. Res. 2017, 200, 114–121. [Google Scholar] [CrossRef]
- Moore, C.E.; Meacham-Hensold, K.; Lemonnier, P.; Slattery, R.A.; Benjamin, C.; Bernacchi, C.J.; Lawson, T.; Cavanagh, A.P. The Effect of Increasing Temperature on Crop Photosynthesis: From Enzymes to Ecosystems. J. Exp. Bot. 2021, 72, 2822–2844. [Google Scholar] [CrossRef]
- Scafaro, A.P.; Haynes, P.A.; Atwell, B.J. Physiological and Molecular Changes in Oryza Meridionalis Ng., a Heat-Tolerant Species of Wild Rice. J. Exp. Bot. 2010, 61, 191–202. [Google Scholar] [CrossRef]
- Bahuguna, R.N.; Jha, J.; Pal, M.; Shah, D.; Lawas, L.M.F.; Khetarpal, S.; Jagadish, K.S. V Physiological and Biochemical Characterization of NERICA-L-44: A Novel Source of Heat Tolerance at the Vegetative and Reproductive Stages in Rice. Physiol. Plant. 2015, 154, 543–559. [Google Scholar] [CrossRef]
- Flood, P.J.; Harbinson, J.; Aarts, M.G.M. Natural Genetic Variation in Plant Photosynthesis. Trends Plant Sci. 2011, 16, 327–335. [Google Scholar] [CrossRef]
- Gu, J.; Yin, X.; Stomph, T.-J.; Struik, P.C. Can Exploiting Natural Genetic Variation in Leaf Photosynthesis Contribute to Increasing Rice Productivity? A Simulation Analysis. Plant. Cell Environ. 2014, 37, 22–34. [Google Scholar] [CrossRef]
- Furbank, R.T.; Sharwood, R.; Estavillo, G.M.; Silva-Perez, V.; Condon, A.G. Photons to Food: Genetic Improvement of Cereal Crop Photosynthesis. J. Exp. Bot. 2020, 71, 2226–2238. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Ort, D.R. How Do We Improve Crop Production in a Warming World? Plant Physiol. 2010, 154, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.; Rodríguez, P.; Dell’Amico, J.; Nicolás, E.; Torrecillas, A.; Sánchez-Blanco, M.J. High-Temperature Preconditioning and Thermal Shock Imposition Affects Water Relations, Gas Exchange and Root Hydraulic Conductivity in Tomato. Biol. Plant. 2003, 47, 203. [Google Scholar] [CrossRef]
- Perdomo, J.A.; Capó-Bauçà, S.; Carmo-Silva, E.; Galmés, J. Rubisco and Rubisco Activase Play an Important Role in the Biochemical Limitations of Photosynthesis in Rice, Wheat, and Maize under High Temperature and Water Deficit. Front. Plant Sci. 2017, 8, 490. [Google Scholar] [CrossRef]
- Qu, Y.; Sakoda, K.; Fukayama, H.; Kondo, E.; Suzuki, Y.; Makino, A.; Terashima, I.; Yamori, W. Overexpression of Both Rubisco and Rubisco Activase Rescues Rice Photosynthesis and Biomass under Heat Stress. Plant. Cell Environ. 2021, 44, 2308–2320. [Google Scholar] [CrossRef]
- Urban, J.; Ingwers, M.; McGuire, M.A.; Teskey, R.O. Stomatal Conductance Increases with Rising Temperature. Plant Signal. Behav. 2017, 12, e1356534. [Google Scholar] [CrossRef]
- Rezaul, I.M.; Baohua, F.; Tingting, C.; Weimeng, F.; Caixia, Z.; Longxing, T.; Guanfu, F. Abscisic Acid Prevents Pollen Abortion under High-Temperature Stress by Mediating Sugar Metabolism in Rice Spikelets. Physiol. Plant. 2019, 165, 644–663. [Google Scholar] [CrossRef]
- Wu, J.-R.; Wang, L.-C.; Lin, Y.-R.; Weng, C.-P.; Yeh, C.-H.; Wu, S.-J. The Arabidopsis Heat-Intolerant 5 (Hit5)/Enhanced Response to Aba 1 (Era1) Mutant Reveals the Crucial Role of Protein Farnesylation in Plant Responses to Heat Stress. New Phytol. 2017, 213, 1181–1193. [Google Scholar] [CrossRef]
- Bi, H.; Zhao, Y.; Li, H.; Liu, W. Wheat Heat Shock Factor TaHsfA6f Increases ABA Levels and Enhances Tolerance to Multiple Abiotic Stresses in Transgenic Plants. Int. J. Mol. Sci. 2020, 21, 3121. [Google Scholar] [CrossRef]
- Hsieh, E.-J.; Cheng, M.-C.; Lin, T.-P. Functional Characterization of an Abiotic Stress-Inducible Transcription Factor AtERF53 in Arabidopsis thaliana. Plant Mol. Biol. 2013, 82, 223–237. [Google Scholar] [CrossRef]
- Hu, X.; Liu, R.; Li, Y.; Wang, W.; Tai, F.; Xue, R.; Li, C. Heat Shock Protein 70 Regulates the Abscisic Acid-Induced Antioxidant Response of Maize to Combined Drought and Heat Stress. Plant Growth Regul. 2010, 60, 225–235. [Google Scholar] [CrossRef]
- Islam, M.R.; Feng, B.; Chen, T.; Tao, L.; Fu, G. Role of Abscisic Acid in Thermal Acclimation of Plants. J. Plant Biol. 2018, 61, 255–264. [Google Scholar] [CrossRef]
- Gong, M.; Li, Y.-J.; Chen, S.-Z. Abscisic Acid-Induced Thermotolerance in Maize Seedlings Is Mediated by Calcium and Associated with Antioxidant Systems. J. Plant Physiol. 1998, 153, 488–496. [Google Scholar] [CrossRef]
- Li, G.; Zhang, C.; Zhang, G.; Fu, W.; Feng, B.; Chen, T.; Peng, S.; Tao, L.; Fu, G. Abscisic Acid Negatively Modulates Heat Tolerance in Rolled Leaf Rice by Increasing Leaf Temperature and Regulating Energy Homeostasis. Rice 2020, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Karwa, S.; Bahuguna, R.N.; Chaturvedi, A.K.; Maurya, S.; Arya, S.S.; Chinnusamy, V.; Pal, M. Phenotyping and Characterization of Heat Stress Tolerance at Reproductive Stage in Rice (Oryza sativa L.). Acta Physiol. Plant. 2020, 42, 29. [Google Scholar] [CrossRef]
- Kiran, T.V.; Rao, Y.V.; Subrahmanyam, D.; Rani, N.S.; Bhadana, V.P.; Rao, P.R.; Voleti, S.R. Variation in Leaf Photosynthetic Characteristics in Wild Rice Species. Photosynthetica 2013, 51, 350–358. [Google Scholar] [CrossRef]
- Ayeneh, A.; van Ginkel, M.; Reynolds, M.P.; Ammar, K. Comparison of Leaf, Spike, Peduncle and Canopy Temperature Depression in Wheat under Heat Stress. Field Crop. Res. 2002, 79, 173–184. [Google Scholar] [CrossRef]
- Zeevaart, J.A. Changes in the Levels of Abscisic Acid and Its Metabolites in Excised Leaf Blades of Xanthium Strumarium during and after Water Stress. Plant Physiol. 1980, 66, 672–678. [Google Scholar] [CrossRef]
- Feldman, A.B.; Murchie, E.H.; Leung, H.; Baraoidan, M.; Coe, R.; Yu, S.-M.; Lo, S.-F.; Quick, W.P. Increasing Leaf Vein Density by Mutagenesis: Laying the Foundations for C4 Rice. PLoS ONE 2014, 9, e94947. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Boote, K.J.; Allen, L.H.; Sheehy, J.E.; Thomas, J.M.G. Species, Ecotype and Cultivar Differences in Spikelet Fertility and Harvest Index of Rice in Response to High Temperature Stress. Field Crop. Res. 2006, 95, 398–411. [Google Scholar] [CrossRef]
- Zhu, X.-G.; Long, S.P.; Ort, D.R. Improving Photosynthetic Efficiency for Greater Yield. Annu. Rev. Plant Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Peng, S.; Li, Y. Variation of Photosynthesis during Plant Evolution and Domestication: Implications for Improving Crop Photosynthesis. J. Exp. Bot. 2022, 73, 4886–4896. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, R.; Ślesak, I.; Orzechowska, A.; Kruk, J. Physiological and Biochemical Responses to High Light and Temperature Stress in Plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar] [CrossRef]
- Cochavi, A.; Amer, M.; Stern, R.; Tatarinov, F.; Migliavacca, M.; Yakir, D. Differential Responses to Two Heatwave Intensities in a Mediterranean Citrus Orchard Are Identified by Combining Measurements of Fluorescence and Carbonyl Sulfide (COS) and CO2 Uptake. New Phytol. 2021, 230, 1394–1406. [Google Scholar] [CrossRef] [PubMed]
- Sage, R.F.; Way, D.A.; Kubien, D.S. Rubisco, Rubisco Activase, and Global Climate Change. J. Exp. Bot. 2008, 59, 1581–1595. [Google Scholar] [CrossRef]
- Wise, R.R.; Olson, A.J.; Schrader, S.M.; Sharkey, T.D. Electron Transport Is the Functional Limitation of Photosynthesis in Field-Grown Pima Cotton Plants at High Temperature. Plant. Cell Environ. 2004, 27, 717–724. [Google Scholar] [CrossRef]
- Crawford, A.J.; McLachlan, D.H.; Hetherington, A.M.; Franklin, K.A. High Temperature Exposure Increases Plant Cooling Capacity. Curr. Biol. 2012, 22, R396–R397. [Google Scholar] [CrossRef]
- Ashraf, M.; Hafeez, M. Thermotolerance of Pearl Millet and Maize at Early Growth Stages: Growth and Nutrient Relations. Biol. Plant. 2004, 48, 81–86. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Ye, C.; Li, X.; Redoña, E.; Ishimaru, T.; Jagadish, K. Genetics and Breeding of Heat Tolerance in Rice BT. In Rice Improvement: Physiological, Molecular Breeding and Genetic Perspectives; Ali, J., Wani, S.H., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 203–220. ISBN 978-3-030-66530-2. [Google Scholar]
- Hatfield, J.L.; Dold, C. Water-Use Efficiency: Advances and Challenges in a Changing Climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef]
- Parry, M.A.J.; Flexas, J.; Medrano, H. Prospects for Crop Production under Drought: Research Priorities and Future Directions. Ann. Appl. Biol. 2005, 147, 211–226. [Google Scholar] [CrossRef]
- Petrik, P.; Petek-Petrik, A.; Konôpková, A.; Fleischer, P.; Stojnic, S.; Zavadilova, I.; Kurjak, D. Seasonality of PSII Thermostability and Water Use Efficiency of in Situ Mountainous Norway Spruce (Picea abies). J. For. Res. 2022, 34, 197–208. [Google Scholar] [CrossRef]
- Ashraf, M.; Bashir, A. Relationship of Photosynthetic Capacity at the Vegetative Stage and during Grain Development with Grain Yield of Two Hexaploid Wheat (Triticum aestivum L.) Cultivars Differing in Yield. Eur. J. Agron. 2003, 19, 277–287. [Google Scholar] [CrossRef]
- Van Den Boogaard, R.; Alewijnse, D.; Veneklaas, E.J.; Lambers, H. Growth and Water-Use Efficiency of 10 Triticum Aestivum Cultivars at Different Water Availability in Relation to Allocation of Biomass. Plant. Cell Environ. 1997, 20, 200–210. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Kjær, K.H.; Rosenqvist, E.; Ottosen, C.-O.; Wu, Z. Screening and Validation of Tomato Genotypes under Heat Stress Using Fv/Fm to Reveal the Physiological Mechanism of Heat Tolerance. Environ. Exp. Bot. 2015, 118, 1–11. [Google Scholar] [CrossRef]
- Zhou, R.; Jiang, F.; Niu, L.; Song, X.; Yu, L.; Yang, Y.; Wu, Z. Increase Crop Resilience to Heat Stress Using Omic Strategies. Front. Plant Sci. 2022, 13, 891861. [Google Scholar] [CrossRef]
- Chaudhary, S.; Devi, P.; HanumanthaRao, B.; Jha, U.C.; Sharma, K.D.; Prasad, P.V.V.; Kumar, S.; Siddique, K.H.M.; Nayyar, H. Physiological and Molecular Approaches for Developing Thermotolerance in Vegetable Crops: A Growth, Yield and Sustenance Perspective. Front. Plant Sci. 2022, 13, 878498. [Google Scholar] [CrossRef]
- Pradhan, A.; Aher, L.; Hegde, V.; Jangid, K.K.; Rane, J. Cooler Canopy Leverages Sorghum Adaptation to Drought and Heat Stress. Sci. Rep. 2022, 12, 4603. [Google Scholar] [CrossRef]
- Bączek-Kwinta, R.; Kozieł, A.; Seidler-Łożykowska, K. Are the Fluorescence Parameters of German Chamomile Leaves the First Indicators of the Anthodia Yield in Drought Conditions? Photosynthetica 2011, 49, 87–97. [Google Scholar] [CrossRef]
- Sage, R.F.; Kubien, D.S.; Porter, J.R.; Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R.; Jones, P.D.; New, M.; Parker, D.E.; et al. Inhibition of Photosynthesis by Heat Stress: The Activation State of Rubisco as a Limiting Factor in Photosynthesis. Plant. Cell Environ. 2007, 61, 7–30. [Google Scholar] [CrossRef]
- Yamori, W.; Hikosaka, K.; Way, D.A. Temperature Response of Photosynthesis in C3, C4, and CAM Plants: Temperature Acclimation and Temperature Adaptation. Photosynth. Res. 2014, 119, 101–117. [Google Scholar] [CrossRef]
- Yang, D.; Peng, S.; Wang, F. Response of Photosynthesis to High Growth Temperature Was Not Related to Leaf Anatomy Plasticity in Rice (Oryza sativa L.). Front. Plant Sci. 2020, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Scoffoni, C.; Gago, J.; Sack, L. Leaf Mesophyll Conductance and Leaf Hydraulic Conductance: An Introduction to Their Measurement and Coordination. J. Exp. Bot. 2013, 64, 3965–3981. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Flexas, J.; Yu, T.; Peng, S.; Huang, J. Leaf Anatomy Mediates Coordination of Leaf Hydraulic Conductance and Mesophyll Conductance to CO2 in Oryza. New Phytol. 2017, 213, 572–583. [Google Scholar] [CrossRef]
- Ye, M.; Wu, M.; Zhang, H.; Zhang, Z.; Zhang, Z. High Leaf Vein Density Promotes Leaf Gas Exchange by Enhancing Leaf Hydraulic Conductance in Oryza sativa L. Plants. Front. Plant Sci. 2021, 12, 693815. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; Feild, T.S.; Jordan, G.J. Leaf Maxmum Photosynthetic Rate and Venation Are Linked by Hydraulics. Plant Physiol. 2007, 144, 1890–1898. [Google Scholar] [CrossRef] [PubMed]
- Taylaran, R.D.; Adachi, S.; Ookawa, T.; Usuda, H.; Hirasawa, T. Hydraulic Conductance as Well as Nitrogen Accumulation Plays a Role in the Higher Rate of Leaf Photosynthesis of the Most Productive Variety of Rice in Japan. J. Exp. Bot. 2011, 62, 4067–4077. [Google Scholar] [CrossRef]
- Baird, A.S.; Taylor, S.H.; Pasquet-Kok, J.; Vuong, C.; Zhang, Y.; Watcharamongkol, T.; Scoffoni, C.; Edwards, E.J.; Christin, P.-A.; Osborne, C.P.; et al. Developmental and Biophysical Determinants of Grass Leaf Size Worldwide. Nature 2021, 592, 242–247. [Google Scholar] [CrossRef]
- Tabassum, M.A.; Ye, Y.; Yu, T.; Zhu, G.; Rizwan, M.S.; Wahid, M.A.; Peng, S.; Li, Y. Rice (Oryza sativa L.) Hydraulic Conductivity Links to Leaf Venation Architecture under Well-Watered Condition Rather than PEG-Induced Water Deficit. Acta Physiol. Plant. 2016, 38, 92. [Google Scholar] [CrossRef]
- Xiong, D.; Yu, T.; Zhang, T.; Li, Y.; Peng, S.; Huang, J. Leaf Hydraulic Conductance Is Coordinated with Leaf Morpho-Anatomical Traits and Nitrogen Status in the Genus Oryza. J. Exp. Bot. 2015, 66, 741–748. [Google Scholar] [CrossRef]
- Cho, S.H.; von Schwartzenberg, K.; Quatrano, R. The Role of Abscisic Acid in Stress Tolerance. Annu. Plant Rev. 2009, 36, 282–297. [Google Scholar]
- Hu, X.-J.; Chen, D.; Lynne Mclntyre, C.; Fernanda Dreccer, M.; Zhang, Z.-B.; Drenth, J.; Kalaipandian, S.; Chang, H.; Xue, G.-P. Heat Shock Factor C2a Serves as a Proactive Mechanism for Heat Protection in Developing Grains in Wheat via an ABA-Mediated Regulatory Pathway. Plant. Cell Environ. 2018, 41, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Li, H.A.O.; Liu, S.-S.; Yi, C.-Y.; Wang, F.; Zhou, J.I.E.; Xia, X.-J.; Shi, K.A.I.; Zhou, Y.-H.; Yu, J.-Q. Hydrogen Peroxide Mediates Abscisic Acid-Induced HSP70 Accumulation and Heat Tolerance in Grafted Cucumber Plants. Plant. Cell Environ. 2014, 37, 2768–2780. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Bassil, E.; Hamilton, J.S.; Inupakutika, M.A.; Zandalinas, S.I.; Tripathy, D.; Luo, Y.; Dion, E.; Fukui, G.; Kumazaki, A.; et al. ABA Is Required for Plant Acclimation to a Combination of Salt and Heat Stress. PLoS ONE 2016, 11, e0147625. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.J.; Ishikawa, M.; Gusta, L.V.; MacKenzie, S.L. Abscisic Acid-Induced Heat Tolerance in Bromus Inermis Leyss Cell-Suspension Cultures (Heat-Stable, Abscisic Acid-Responsive Polypeptides in Combination with Sucrose Confer Enhanced Thermostability). Plant Physiol. 1994, 105, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Jagadish, S.V.K.; Muthurajan, R.; Oane, R.; Wheeler, T.R.; Heuer, S.; Bennett, J.; Craufurd, P.Q. Physiological and Proteomic Approaches to Address Heat Tolerance during Anthesis in Rice (Oryza sativa L.). J. Exp. Bot. 2010, 61, 143–156. [Google Scholar] [CrossRef]
- Shi, W.; Yang, J.; Kumar, R.; Zhang, X.; Impa, S.M.; Xiao, G.; Jagadish, S.V.K. Heat Stress During Gametogenesis Irreversibly Damages Female Reproductive Organ in Rice. Rice 2022, 15, 32. [Google Scholar] [CrossRef]
- Hein, N.T.; Ciampitti, I.A.; Jagadish, S.V.K. Bottlenecks and Opportunities in Field-Based High-Throughput Phenotyping for Heat and Drought Stress. J. Exp. Bot. 2021, 72, 5102–5116. [Google Scholar] [CrossRef]
- Matsui, T.; Kobayasi, K.; Yoshimoto, M.; Hasegawa, T.; Tian, X. Dependence of Pollination and Fertilization in Rice (Oryza sativa L.) on Floret Height within the Canopy. Field Crop. Res. 2020, 249, 107741. [Google Scholar] [CrossRef]
- Satake, T.; Yoshida, S. High Temperature-Induced Sterility in Indica Rices at Flowering. Jpn. J. Crop Sci. 1978, 47, 6–17. [Google Scholar] [CrossRef]
- Salvucci, M.E.; Crafts-Brandner, S.J. Inhibition of Photosynthesis by Heat Stress: The Activation State of Rubisco as a Limiting Factor in Photosynthesis. Physiol. Plant. 2004, 120, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Henry, A.; Sreenivasulu, N. Rice Yield Formation under High Day and Night Temperatures—A Prerequisite to Ensure Future Food Security. Plant. Cell Environ. 2020, 43, 1595–1608. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).