Abstract
Interest in white lupine has increased in recent years in animal nutrition due to its balanced protein and lipid content, phytochemical compounds, and low alkaloid content. Agronomic traits, nutrients, and antioxidant phenols of six white lupin genotypes grown in a Mediterranean area were explored. Genotypes significantly differed in growth stages and life cycle length (from 172 to 204 days after sowing), plant height (from 36.1 to 97.2 cm), seed yield (from 1.02 to 3.50 Mg ha−1), and yield components. Seed yield was positively correlated with the number of seeds per pod and the thousand-seed weight. Across the average of genotypes, a high protein content (397 g kg−1), a low fiber content (133 g kg−1), and oil content (116 g kg−1) with a high oleic acid (453–509 g kg−1) and low erucic acid content (8–17 g kg−1) were found. The n3/n6 ratio varied from 1:1 to 1:4. Ecotype G showed the highest TPC, DPPH•, and ABTS•+ and Multitalia showed the highest content of Apigenin 1 and 2 derivatives. The lowest alkaloid content was recorded in Volos, Luxor, and Lublanc. Overall, this species can be considered a suitable feed crop and a valuable ingredient in animal nutrition due to its overall nutritional profile. At present, only Volos, Luxor, and Lublanc can be suggested in animal nutrition due to the low alkaloid content. Nonetheless, interesting agronomic and quality traits in Multitalia and the two ecotypes suggest room for breeding to reduce their antinutritional factors.
1. Introduction
The European Union (EU) is heavily dependent on imported soybean for its domestic consumption, being widely exposed to risks associated with world trade. In this context, the identification of alternative plant protein sources for sustainable livestock farming, which is highly dependent on international soybean imports, is key to fulfill the gap between consumption and demand [,]. In this sense, the measures set in the recent Common Agricultural Policy (CAP) have already contributed to the expansion of the plant protein sector in the EU. In the Mediterranean area, there is a large diffusion of winter legume crops that, in addition to the reduction in feed costs for livestock, may provide a sustainable benefit for the environment against soil nitrogen impoverishment and disruption of biological cycles of pests and plant diseases [].
Among grain legumes, lupin species (Lupinus spp.) could represent a suitable alternative protein source in both monogastric and ruminant feeds, capable of replacing soy without loss of quantity and quality of livestock products [,]. Actually, lupins have been suggested as possible alternative crops and traceable protein sources in Europe. In addition to the nutritional value in animal feeding, lupins grain productivity is reasonable [], can adapt to less fertile soils [], are low-nutrients-demanding [], and can play an excellent role in crop rotation [].
Nowadays, a growing interest in the production of white lupin (Lupinus albus L.) for animal feed has been observed, due to its seed nutritional quality and the potential benefits for health [].
Ruminants are the largest consumers of lupins [,,] and its use in dairy cows has shown an increase in milk production, fat, and protein contents, and an improvement of the fatty acid profile [,]. Monogastrics, such as pigs and poultry, are the second animal consumers; however, the high level of non-starch polysaccharides and the low level of starch in lupin seeds [,] affect the utilization of energy and contribute to the reduction in feed intake and digestibility [,]. Lastly, significant nutritional advantages have been identified in fish diets supplemented with lupins, where there is an increasing demand for plant protein to replace protein fish meal [,]. In addition, lupins have some unique functional properties to contribute to aquafeed pellets [].
From a nutritional point of view, white lupin seeds have a high protein content, from 32.9% [,] up to more than 36.0% []. Oil content varies from 9% to 13% with a high concentration of PUFA, which represent a precious source of essential fatty acids []; moreover, it has a high n3/n6 polyunsaturated fatty acid ratio compared to other vegetable oils [,] appropriate for animal feeding [,,]. Moreover, white lupin is characterized by many valuable biologically active substances [], such as phenolic acids, flavones, and isoflavones [,,], that promote antioxidant activity [,,,] and improve health []. In fact, white lupine seeds have an interesting content of biologically valuable substances with a high antioxidant potential that allow them to be used as a suitable nutraceutical for the prevention and treatment of some diseases [,,]. It has been reported that white lupins can have a role in the pathogenesis of health disorders due to their influence on lipid and glucose metabolism. Furthermore, functionality effects on inflammatory processes and changes in the gut microbiome, with significant influence on several physiological parameters, including metabolism, nutrient absorption, and immune function, have also been demonstrated [,,].
However, a drawback of lupin is the presence of antinutritional factors (ANFs), mainly represented by quinolizidine alkaloids, which can reduce animal performances [,,]. Therefore, the suitability of this legume is closely related to the alkaloid content, which can be reduced by adopting sweet varieties with low alkaloid content [].
Nutritional characteristics linked to protein content, lipid accumulation, fatty acid quality, phytosterol composition, as well as antinutritional factors of seeds are strongly influenced by the variety [,,]; however, only a few researchers have explored the effect of genotypes on productive and qualitative traits in white lupin seeds.
To this end, the aim of the present research was to explore the productive, chemical, and fatty acid composition, the total phenolic content, the antioxidant activity, the content of phenolic compounds, and total alkaloids in six genotypes of white lupin, grown side-by-side in the Mediterranean environment, in order to evaluate the possibility of introducing this species in animal feeding.
2. Materials and Methods
2.1. Plant Materials and Field Experiment
Six genotypes of Lupinus albus L. were adopted in this study. Two recently released varieties (Volos and Luxor) were compared with two older varieties (Lublanc and Multitalia) and two ecotypes from Southern Italy (Ecotype F and Ecotype G).
Lupin genotypes were sown in a medium-textured loamy soil in Southern Italy (Calabria, 38°04′51″ N, 15°40′49″ E) with nearly neutral pH (6.75), low salinity (0.6 mS cm−1), poor organic matter (0.69%), low nitrogen (0.62 g kg−1) and exchangeable P2O5 (1.0 mg kg−1), and high assimilable K2O (285 mg kg−1). Plots of 8 m2 (4 × 2 m), three-times-replicated in a randomized block design, were adopted. Sowing was executed on 22 November 2013 with a plant density of 60 plant m−2 on a ploughed and fertilized soil with 100 kg ha−1 of P2O5. Weed control was performed just after sowing, on 25 March and on 7 May by hand. Seeds were harvested between May and June, according to the physiological maturity of genotypes. Plant height and yield components (pods plant−1, seeds pod−1, and thousand-seed weight) were evaluated using ten plants for each plot, while total yield was determined on the two central rows in each plot. The date of the main growth stages (i.e., sowing-emergence, emergence-flowering, flowering-seed setting, seed setting-seed maturation) was also recorded according to the scale proposed by Dracup and Kirby [].
After sowing, monthly maximum and minimum air temperature gradually decreased and reached a minimum of 6.7 °C in January (Figure 1). Subsequently, the air temperature had an increasing trend from March, up to the monthly maximum temperature of 34.2 °C in June. The rainfall was 562.2 mm between September 2013 and June 2014, and over 67% was concentrated between November and March. The wettest month was December with 109.4 mm, while the driest months were May and June (20 and 5 mm, respectively).
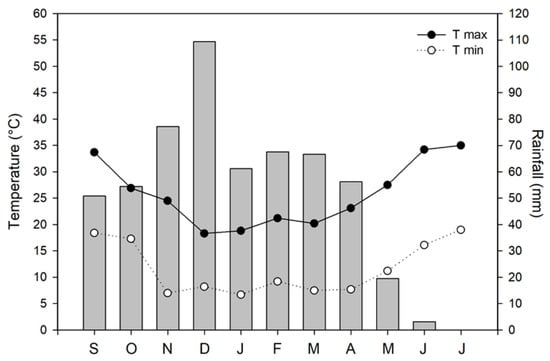
Figure 1.
Meteorological trend (maximum and minimum air temperature and rainfall) during the growing season of 2013/2014 in Southern Italy (Calabria, 38°04′51″ N, 15°40′49″ E).
2.2. Sample Preparation
Before all analyses, lupin seeds were ground using a 1.1 mm sieve (SM 100, Retsch, Haan, Germany). Analytical determinations were carried out in triplicate.
2.3. Proximate Chemical Analysis
Proximate chemical analyses of the lupin seed samples, such as moisture, crude protein, ether extract, crude fiber, and ash, were carried out following the standard procedures from the Association of Official Analytical Chemists []: for moisture content (method n. 930.15), for crude protein (method n. 2001.11) by means of the Kjeldahl procedure using a Kjeltec system (FOSS, Padua, Italy), for ether extract (method n. 920.39) by using the Soxtec™ 8000 Extraction Systems (FOSS, Padua, Italy), for crude fiber (CF) (method n. 978.10) using a Fibertec™ 2010 (FOSS, Padua, Italy) after acid (with H2SO4) and basic (with NaOH) hydrolyses, and for ash content (method n. 942.05). All determinations were expressed as fed, and the seed moisture content ranged from 80 g kg−1 in Multitalia and Ecotype G to 96 g kg−1 in Volos.
2.4. Analysis of Fatty Acids, Nutritional, and Quality Indices Calculation
Fatty acids (FAs) of lupin seeds were analyzed after oil extraction and their conversion into fatty acid methyl esters (FAMEs) according to the Christie method []. After the extraction, the FAMEs were analyzed by means of a TRACE 1310 gas chromatography-flame ionization detector (GC-FID—Thermo Fisher Scientific, Milan, Italy). Quali-quantitative FAs determination was performed using the analytical method previously reported by Oteri et al. []. The individual fatty acid concentration was expressed in g kg−1 (1 kg is the sum of identified FAME areas).
The atherogenic (AI) and thrombogenic (TI) nutritional indices [], the hypocholesterolemic/hypercholesterolemic ratio (H/H) [], and the peroxidation index (PI) [] were calculated.
2.5. Extraction and Identification of Alkaloids
The alkaloids from the sample were extracted as described by Muzquiz et al. [] and Oboh et al. [] and analyzed by high-resolution gas chromatography/mass spectrometry (HRGC/MS) [] using a Shimadzu TQ8030 HRGC-MS/MS (Shimadzu Italia, Milan, Italy), equipped with a Shimadzu AOC-20s autosampler and a Supelco (Supelco, Bellefonte, PA, USA) SLB-5MS capillary column (5% polydiphenylsiloxane, 95% polydimethylsiloxane) of 30 m × 0.25 mm, 0.25 μm (L × I.D., film thickness), operating in electronic ionization (EI) mode. The instrumental conditions were performed as described by Calabrò et al. [] and Musco et al. []. For the quantification of alkaloids, the internal standard method was adopted (concentration of caffeine equal to 0.5 mg mL−1) []. The limit of quantification was 0.1 mg kg−1 for all alkaloids.
2.6. Total Phenolic and Antioxidant Activity Analyses
The method described by López-Mejía et al. [] was used for the quantification of the total phenolic content (TPC) and the extraction yields (%) were calculated gravimetrically with the weight of the seed and the extracts.
The TPC was determined following the Karamać et al. method [] and the results expressed as mg of GAE (gallic acid equivalents) per g of seeds (mg g−1).
The antioxidant capacity of polyphenols was measured by DPPH• and ABTS•+ assays. The scavenging activity of both DPPH• [] and ABTS•+ [] was evaluated spectrophotometrically (Cary 60 UV-Vis spectrophotometer, Agilent Technologies, Santa Clara, CA, USA) and the results were expressed for DPPH• as EC50 (concentration of dried extract mg mL−1 solution) and for ABTS•+ as µmol TE (Trolox equivalents) g−1 of seeds.
2.7. HPLC Analysis of Phenolic Compounds
The liquid chromatographic (LC) analyses were carried out on two different instruments. The quantification of Apigenin derivatives was performed using HPLC-DAD equipment and based on the Apigenin calibration curve, while their identification was carried out using an HPLC-MS system PDA (Waters Corp., Milford, CT, USA) [].
The quantification of Apigenin derivatives was performed using the calibration curve obtained with an external standard. HPLC-MS analyses were carried out on a Waters Fraction Link autopurification system equipped with a Waters 2487 UV detector, Waters 2525 binary pump, and Waters Micromass ZQ detector operating in ESI+ mode. The chromatograms obtained show several peaks between two and five, of which only two were identified as Apigenin derivatives by comparing the UV spectra with the external standards (ST). The compounds were quantified using the Apigenin calibration curve and the amounts of derivatives were expressed as Apigenin equivalents (mg g−1 seeds).
Figure 2 reports an HPLC chromatogram characterized by the presence of two dominant peaks (1 and 2) with retention times of 22.6 and 27.7 min, respectively. Both compounds possessed similar UV spectra with λmax at 335 nm, and a retention time shorter than that of the Apigenin standard, which suggests that they are Apigenin glycosides. Compound 1, showing a molecular ion [M+H]+ at m/z = 595 (base peak) in the mass spectrum, was identified as Apigenin-6,8-di-C-beta-glucopyranoside according to Siger et al. [], while compound 2 was not chemically identified.
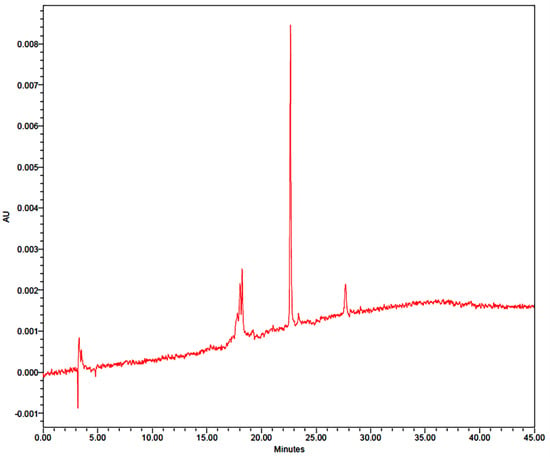
Figure 2.
HPLC chromatogram of phenolic compounds of Lupinus albus L. seed extract at 335 nm.
2.8. Statistical Analysis
Plant height was analyzed by one-way ANOVA with repeated measures, where the days after sowing represents the within-factor and the genotype represents the between-factor (PASW Statistics 18, SPSS Inc., South Wacker Drive, Chicago, USA). Seed yield, yield components, and seed quality were analyzed by a one-way ANOVA according to the randomized block design using the Bartlett’s test to verify the assumption of homogeneity of variances. Tukey’s test (p ≤ 0.05) was used for mean separation, and percentage values were previously arcsin √%-transformed.
Pearson’s correlation test (p ≤ 0.05) was executed to assess the significance of correlation coefficients among growth stage (vegetative and reproductive stages), morphology, yield, and yield components. Relationships between plant height and growth stages were modeled through non-linear regressions. Coefficients were considered significant at p ≤ 0.05. Residuals for normality were verified by the Shapiro–Wilk test, and the goodness of fit was assessed by the R2 (SigmaPlot11, Systat Software Inc., San Jose, CA, USA).
3. Results
3.1. Phenology and Plant Height
Lupin genotypes showed different life cycle lengths and growth stages (Figure 3A). Ecotypes F and G reached seed maturity 204 days after sowing (DAS), Luxor and Multitalia reached it 185 DAS, and Volos and Lublanc reached it 172 DAS. Volos showed the longest sowing-emergence interval (30 DAS) but the shortest emergence-flowering (13 DAS) overall. This latter interval was the longest in Ecotypes F and G (70 and 67 DAS, respectively). The flowering-seed setting interval was the shortest in Multitalia, Ecotype G, and Luxor (on average, 23.5 DAS), and the longest in Lublanc (27 DAS) and Volos (29 DAS). The seed setting-seed maturity interval was the longest in Multitalia, Luxor, and Volos (on average 99.5 DAS), and the shortest in Ecotype F and Lublanc (averaged, 83 DAS).
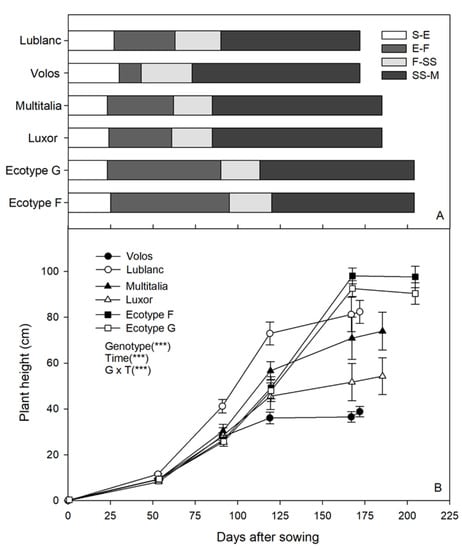
Figure 3.
Main growth stages (A) and plant height (B) of six white lupin (Lupinus albus L.) genotypes. Growth stage intervals: sowing-emergence (S-E), emergence-flowering (E-F), flowering-seed setting (F-SS), seed setting-seed maturity (SS-M). Effects statistically significant at p ≤ 0.001 (***).
Plant height was also different among genotypes and it was significantly affected by the time (Figure 3B). Generally, there were no significant differences among genotypes at 100 DAS (on average, 32 cm), except for Lublanc (~10 cm taller). Lublanc was the tallest genotype up to 124 DAS (72.9 cm); however, it gained only 9 cm thereafter (at 172 DAS). Volos was the shortest genotype and reached the maximum height (36.1 cm) at 124 DAS. On the other hand, plant elongation was rapid in Ecotype G and F from 124 DAS up to the maximum values of 90.1 and 97.2 cm at 168 DAS. Multitalia and Luxor had a similar trend up to 90 DAS; however, the former was taller than the latter at harvest (72.9 and 53.2 cm, respectively).
3.2. Seed Yield and Yield Component
Yield components, namely the number of pods per plant, the number of seeds per pod, and the thousand-seed weight, as well as seed yield were significantly different among lupin genotypes (p ≤ 0.05).
The number of pods per plant was the significantly highest in Multitalia, Ecotype F, Ecotype G, and Lublanc (on average, 4.5), and the lowest in Luxor (3.4). Volos differed neither from the highest nor from the lowest values (Figure 4A). The number of seeds per pod was the significantly highest in Luxor, Ecotype G, and Lublanc (on average 3.5), and the lowest in Volos (2.4). Ecotype F and Multitalia differed neither from the highest nor from the lowest values (Figure 4B). The thousand-seed weight was the significantly highest in Multitalia and Lublanc (on average, 426.3 g), and the lowest in Luxor, Ecotype F, Ecotype G, and Volos (on average, 326.7 g) (Figure 4C). The seed yield was the significantly highest in Lublanc (3.5 Mg ha−1) and the lowest in Volos (1.02 Mg ha−1). Multitalia differed neither from Lublanc nor from Luxor. Ecotype G differed neither from Luxor nor from Ecotype F (Figure 4D).
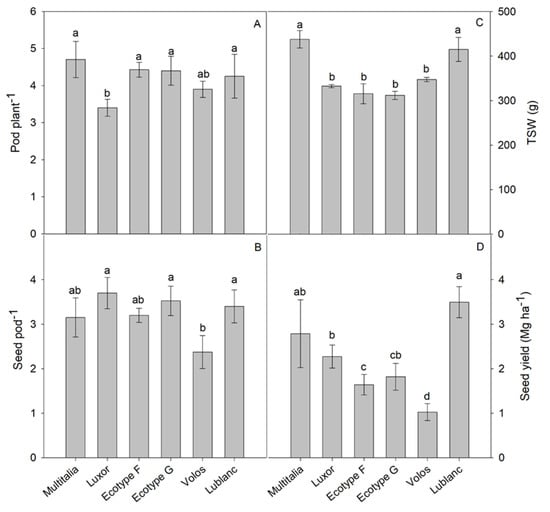
Figure 4.
Number of pods per plant (A), number of seeds per pod (B), thousand-seed weight (C), and seed yield (D) of six white lupin (Lupinus albus L.) genotypes. Mean values ± standard deviation with different letters differ significantly (p ≤ 0.05).
3.3. Correlations and Relationships
Several significant correlations among morphology, growth stages, yield, and yield components of white lupin genotypes were observed (Table 1). Plant height was positively correlated with the vegetative stage, the number of pods per plant, and the number of seeds per pod. Vegetative and reproductive stages were negatively correlated; the vegetative stage was negatively correlated with the thousand-seed weight, as was the reproductive stage with plant height. Seed yield was positively correlated with the number of seeds per pod and the thousand-seed weight.

Table 1.
Pearson correlation coefficients (r) among growth stage (vegetative and reproductive stages, VS and RS, respectively), morphology (plant height), yield, and yield components of six white lupin genotypes. Significant per p ≤ 0.001 (***), p ≤ 0.01 (**), p ≤ 0.05 (*).
A positive relationship was found between plant height at harvest and the vegetative growth of white lupins (Figure 5A). The increasing trend was almost linear from a plant height of 38 cm up to around 85 cm, which was reached at vegetative growth from 43 to 80 days. Afterward, plant height reached a plateau with further vegetative growth. On the contrary, plant height and reproductive growth showed a negative relationship (Figure 5B). Plant height was the highest and almost stable at the lowest interval of reproductive stages (from 109 to 117 days). Beyond 120 days, the plant height quickly dropped and the reduction was almost linear with the length of the reproductive growth.
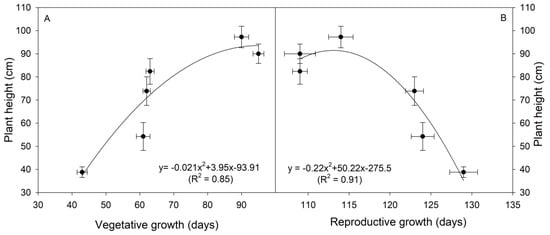
Figure 5.
Relationships between plant height and the vegetative growth (A), and plant height and the reproductive growth (B) of six white lupin (Lupinus albus L.) genotypes.
3.4. Nutritional composition of lupins
Table 2 shows the mean values of the chemical composition in the six genotypes of white lupin seed. Volos showed the significantly (p ≤ 0.05) highest crude protein, crude fiber, and ash content, and the lowest oil content. Lublanc and Multitalia showed the highest oil and the lowest ash content. Lublanc also had the lowest crude protein and Multitalia had the lowest crude fiber. Both Ecotype F and G showed the highest crude fiber and ash, but the lowest crude protein (Ecotype F) and oil content (Ecotype G). Alkaloids were significantly lower in Volos, Luxor, and Lublanc (0.05–0.19 g kg−1) than in Multitalia and Ecotype F and G (2.0–2.5 g kg−1).

Table 2.
Chemical composition (g kg−1, as fed) of the six genotypes of Lupinus albus L. (n = 18).
3.5. Fatty Acids profiles, Nutritional, and Quality Indices
Fatty acid composition in the six genotypes of white lupin seed is presented in Table 3. In descending order, oleic (C18:1n9), linoleic (C18:2n6), linolenic (C18:3n3), and palmitic (C16:0) acids were the dominant fatty acids in all samples. The oleic acid showed a significantly (p ≤ 0.05) higher value in the Luxor, Lublanc, and Multitalia than in Volos and Ecotype F and G. The linoleic and linolenic acids were the highest in Volos and the lowest in Luxor. The palmitic acid was higher in Luxor compared to the remaining genotypes. The erucic acid (C22:1n9) was the lowest in Luxor and the highest in Ecotypes F and G; intermediate values were observed in the other genotypes.

Table 3.
Fatty acid profile (g kg−1, as fed) in the six genotypes of Lupinus albus L. (n = 18).
Fatty acid classes, fatty acid ratios, and the atherogenic (AI) and thrombogenic (TI) nutritional indices are shown in Table 4. The Ecotype G showed the significantly highest value of SFAs. MUFAs resulted as higher in Luxor, Lublanc, and Multitalia than the remaining genotypes. Volos showed the highest value of PUFAs, both n6 and n3-PUFAs, and the lowest SFAs and MUFAs. Ecotype G also showed the highest SFA/UFA ratio, while Volos, Lublanc, and Multitalia showed the lowest one. Volos showed also the lowest n3/n6 ratio. With regard to the nutritional indices, strictly related to the fatty acid profile, Luxor showed the best AI; Luxor and Ecotype G showed the best TI; Lublanc showed the best HH but the lowest PI. The remaining genotypes showed significantly higher and not statistically different PI values.

Table 4.
Fatty acid classes (g kg−1, as fed), ratios, and quality indices in the six genotypes of Lupinus albus L. (n = 18).
3.6. Total Phenolic Content and Antioxidant Activity
Total phenolic content, antioxidant properties, and phenolic compounds in the six white lupin genotypes are shown in Table 5. Ecotypes G and F had the significantly highest TPC and ABST•+ content. DPPH• was also the highest in Ecotype F, while the remaining genotypes were not significantly different. Multitalia exhibited the highest content of both phenolic compounds, Apigenin 1 and 2. Apigenin 2 was also at the highest levels in both Ecotypes F and G, and in Luxor. However, Apigenin 1 was the lowest in these latter three genotypes.

Table 5.
Total phenolic content, antioxidant properties, and phenolic compounds in the six genotypes of Lupinus albus L. (n = 18).
4. Discussion
The growing season was quite favorable for lupin growth, with air temperature always above the base temperature [] and rainfalls well distributed throughout the main phenophases. The life cycle length was within the range reported by López-Bellido et al. [] in other similar Mediterranean environments, where 222 days and 173 days were recorded for autumn and winter sowing, respectively. A shorter crop cycle (153 days) was recorded in Luxor and Rosetta white lupin varieties by Gresta et al. [] in a similar environment; an earlier flowering was also recorded in Luxor.
In addition to the length of the growth cycle (32 days shorter in the earliest Volos and Lublanc as compared to the latest maturity group, ecotypes F and G), it is worth mentioning that local ecotypes F and G showed about a 30-days-longer vegetative phase (i.e., the interval sowing-emergence and emergence-flowering) than the improved genotypes Lublanc, Multitalia, and Luxor and even 50 days longer than that of Volos. However, the reproductive phase (i.e., the interval flowering-seed setting and seed setting-maturation) was approximately 10–20 days shorter in ecotypes F and G as compared to the improved white lupin genotypes. The longest vegetative phase resulted also in the tallest plants. Indeed, a positive regression was observed between the plant height and the length of the vegetative phase (R2 = 0.85), while a negative regression emerged between the plant height and the length of the reproductive phase (R2 = 0.91). Plant height resulted in agreement with values obtained by Gresta et al. [], in which Luxor reached a height of 94 cm.
Seed yield was outstanding in two out of the six genotypes (Lublanc and Multitalia) and the thousand-seed weight (r = 0.69, p ≤ 0.001) and, secondarily, the number of seeds per pod (r = 0.41, p ≤ 0.05) markedly influenced yield formation. Although seed yield and growth stages were not significantly correlated, the thousand-seed weight was negatively correlated with the vegetative stage; hence, it can be speculated that the longer the vegetative stage, the lower the seed yield.
The seed yield of Lublanc was similar to the yield of 3.48 Mg ha−1 of cultivar Multolupa reported by López-Bellido et al. [] in the most favorable growing conditions and with the same plant density. On the other hand, the seed yield of Volos was even lower than that achieved in most limiting growing conditions []. Gresta et al. [] reported a slightly higher seed yield (3.7 Mg ha−1) for Luxor and Rosetta white lupins. The seed yield of the local ecotypes (F and G) was likely affected by the longer vegetative development that favored biomass accumulation over nutrient translocation to the seed. Nonetheless, this suggests that there is room for improvement by manipulating the growing cycle of these locally adapted ecotypes.
With regard to the proximate composition, lupin genotypes showed interesting quality traits; on average, the high protein content (397 g kg−1, dry matter), the low fiber content (133 g kg−1, dry matter), and the oil content (116 g kg−1, dry matter) with an interesting fatty acid profile make lupin seeds a valuable protein and energy source for animal feeding. The chemical composition appears similar to soybean and of higher value than other legumes, such as pea (Pisum sativum) and fava bean (Vicia faba var. minor), which are widely cultivated as soybean alternatives in the Mediterranean countries []. Proximate composition results are within the range reported in the literature for white lupin grown in the Mediterranean environment [,,]. On the other hand, the oil content was higher than that observed by Chiofalo et al. [] in white lupin Luxor and Rosetta grown in a different Mediterranean area. Among investigated genotypes, Multitalia showed the most valuable chemical composition in terms of protein and oil content (highest values) and crude fiber (lowest values). Conversely, Luxor showed the lowest values of protein and oil and the highest level of crude fiber.
Unlike ruminants, the use of lupins in the diet of monogastrics could be detrimental for their performance and production. The lack of endogenous enzymes for degradation of lupins’ structural carbohydrates in their digestive system can reduce feed intake, digestible energy, and digestibility of nutrients []. However, based on research and commercial experience, the inclusion of white lupine in the diet of monogastrics, recommended according to the species and physiological state, has no negative effects on the digestibility of nutrients [].
It is worth mentioning that lupin oil content is low as compared to other crops (soybean, sunflower, etc.) []; nonetheless, its fatty acid profile is noteworthy for animal nutrition [,,]. Among fatty acids, oleic acid (C18: 1n9) was the most represented (476 g kg−1, on average), making up almost 50% of the total fatty acids identified, and 86%, on average, of the monounsaturated, with the highest content in Luxor, Lublanc, and Multitalia (509 g kg−1, 494 g kg−1, and 493 g kg−1, respectively). Our results are similar to those reported in white lupin seeds by Uzun et al. [] (476 g kg−1, on average), Calabrò et al. [] (478 g kg−1, on average), and Musco et al. [] (466 g kg−1, on average), and slightly higher than those of Chiofalo et al. [] (449 g kg−1, on average). Furthermore, among the monounsaturated, the erucic acid, considered an anti-quality factor, showed values (8–17 g kg−1) much lower than those observed by Bhardwaj et al. [] (23.6–27.3 g kg−1), Boschin et al. [] (39–53 g kg−1), and Volek and Marounek [] (36.9 g kg−1). Nonetheless, it was within the range reported in the literature in different white lupin genotypes grown in the Mediterranean area [,,].
Alkaloids content in Volos (5.2 mg per 100 g) and Luxor (6.1 mg per 100 g) were much lower than the limit of toxicity (20 mg per 100 g) for human and animal consumption released by the health authorities of the United Kingdom, France, and Australia [], and Lublanc was close to the upper limit (19.4 mg per 100 g), while Multitalia and Ecotype F and G had a total content of alkaloids (227, 250, and 201 mg per 100 g, respectively) well beyond the toxicity limit. Hence, the large difference of alkaloid can be ascribed to the genotype, which confirms the irreplaceable role of genetic breeding to improve varieties with low antinutritional factors in lupin species.
With regard to the polyunsaturated fatty acids, the high content of essential fatty acids (C18:2n6 and C18:3n3) found in seed oil, specifically in Volos, is typical of many legume species []. From this point of view, Luxor and Multitalia resulted in outstanding genotypes, owing to the highest n3/n6 PUFA ratio, which is considered optimal in animal nutrition and able to positively influence the productive performance, antioxidant properties, immune response, semen quality, and product quality with health benefits also for the consumers []. Concerning the nutritional indices, literature information is scarce for atherogenic and thrombogenic indices of white lupin seeds. Both indices give a measurement of the level of the atherogenicity and of thrombogenicity of a feed/food []. In addition, no reference was found for hypocholesterolemic/hypercholesterolemic fatty acid ratio (H/H), even if the ability of lupin seed oil to lower plasma cholesterol level as well as to slow down the lipid peroxidation process and to enhance the antioxidant enzyme activity is widely recognized []. The present results showed values within the range reported in the literature for AI and TI [,,]. Lublanc showed, on average, the best values (0.08, 0.14, and 11.27, respectively) of all the nutritional indices, making this genotype very interesting from a nutritional point of view. On the contrary, Luxor showed the worst values (0.11, 0.18, and 8.64, respectively), making its lipid fraction less suitable for animal and human nutrition.
The favorable proximate composition, as well as the interesting lipid fraction, has opened up the possibility of using white lupin seed in livestock nutrition, especially in food chains aimed at supporting animal health and the quality and safety of the productions. Zraly et al. [] obtained a significantly lower content of palmitic acid and a significant increase in oleic acid and n3-PUFA, as well as in the alpha-linolenic (C18:3n3) acid content in the meat of pigs fed with white lupin (20% inclusion of genotype Amiga) with a beneficial effect on human health. In addition, Mieczkowska and Smulikowska [] have reported that a diet for broiler chickens containing white lupin Bardo increased the levels of oleic (C18:1n9) and alpha-linolenic acids in chicken fat tissue and concluded that the white lupin seed can be used as a source of alpha-linolenic acid in a balanced chicken diet and may favorably modify the fatty acid composition of carcass lipids and, thus, the health attributes of broiler meat. Furthermore, Podolian young bulls fed with white lupin Multitalia, as a soybean substitute, showed a similar fatty acid profile in the meat, confirming white lupin seed as an alternative protein source to soybean []. Finally, Volek and Marounek [] have shown an improvement in the AI and TI in the meat of rabbits when sunflower meal was substituted with white lupin (Amiga) in their diet.
However, considering the alkaloid content, among the examined lupin species, only Volos, Luxor, and Lublanc with a mean value of 0.1 g kg−1 could represent a good ingredient for feed. As known, pigs appear more sensitive to alkaloids compared to poultry and ruminants, showing a delay in gastric emptying and a reduction in feed intake []. As the alkaloid content of recent varieties of sweet lupin ranges from 0.1 to 0.4 g kg−1, with a mean content of 0.2 g kg−1, poor pig performance due to alkaloids in sweet lupin varieties is unlikely [].
In the present study, the peroxidation index (PI) was significantly influenced by the genotype effect. The PI expresses a measure of the peroxidation susceptibility and peroxidative lipid damage, which is reflected by the different PUFA content. Unfortunately, no data on this index were found in the literature. Luxor showed the lowest value and, therefore, a high oil stability, whereas Volos had the highest value and, therefore, a low oil stability, in relation to its lower and higher PUFA content, respectively.
With regard to the different content of phenolic compounds, the present findings agree with Oomah et al. [] who described a dominant effect of genotype on total phenolics. The concentration and the proportions of polyphenolic compounds in plants are affected by many factors, such as soil type, sun exposure, air temperature and rainfall, ripeness at the time of harvest, processing, and storage []. This might explain the different range of values obtained in our samples when compared with values found in the literature.
The antioxidant activity of lupin seed is similar [] or lower to other grain legumes []. To date, it has not been determined whether this activity is affected by lupin genotype or environmental conditions []. Our results highlight an effect of genotypes on antioxidant properties of lupin seeds grown in the same environmental conditions, confirming what has been reported by Wang et al. [].
The most abundant phenolic compounds detected in lupin seeds belong to the subclasses of phenolic acids, flavones, and isoflavones []. In this study, two Apigenin glycosides were identified, a group of flavones already observed in various species of lupin seeds [,]. Apigenin 1 and 2 derivatives showed the highest values in Multitalia. However, this genotype did not show the highest antioxidant activity; this agrees with the observations of Karamać et al. [], who reported that the antioxidant capacities of lupin seeds are not strictly correlated with the content of the di-C-glycosides of Apigenin. On the whole, the contents of Apigenin-6,8-di-C-beta-glucopyranoside in the six genotypes of lupin seeds resulted in a higher range (13–27 mg 100 g−1, dry matter) than that reported by Khan et al. [] in white lupin seed (12–14 mg 100 g−1, dry matter). It is worth emphasizing the presence of Apigenin−6,8-di-C-glucoside in lupin seeds, because this type of flavone glycoside is not often found in dietary plants []. However, recent studies have shown a wide variety of beneficial health effects from Apigenin derivatives, including antioxidant, anti-inflammatory, hypoglycemic, and hypocholesterolemic activities [,].
5. Conclusions
This study proved the potential of white lupin as an alternative winter legume crop for Mediterranean environments. However, genotype selection remains a fundamental issue for introducing this crop into existing rainfed cropping systems.
At present, only Volos, Luxor, and Lublanc can be recommended as protein feed for animal nutrition, due to the low antinutritional factors. In addition, these varieties resulted also well balanced from a chemical and nutritional point of view, except for Volos, also from a productive point of view.
Although Multitalia and local ecotypes (F and G) are characterized by interesting productive traits (pod number per plant, seeds per pod, thousand-seed weight, and grain yield), along with seed nutritional and antioxidant values, the high content of alkaloids negatively affect their use in animal nutrition. They could be considered a good material to be investigated in future breeding programs to reduce alkaloid content.
Overall, white lupin can be recommended as an alternative to soybean and an emerging Mediterranean legume crop; nonetheless, further investigations on current genotypes and further landraces on agronomic traits, phenolic patrimony, antioxidant potential, and biological activities, along with low antinutritional factors, will be key to its use in the livestock sector and to tackling sustainability challenges.
Author Contributions
Conceptualization, F.G. and B.C.; methodology, F.G., M.O., D.S. and B.C.; software, D.S.; validation, F.G. and D.S.; formal analysis, M.O., A.C., R.A. and G.M.; investigation, F.G.; resources, F.G.; data curation, F.G., D.S. and B.C.; writing—original draft preparation, D.S. and M.O.; writing—review and editing, F.G. and B.C.; supervision, F.G. and B.C.; funding acquisition, B.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by PON-MISE I&C 2014-2020 FESR, ASSE I—Innovazione e Azione, Azione 1.1.3. D.M. 05/03/2018, project codex N. F/200078/01-03/X45, CUP B41B20000280005, Title of the project “BIO=C=O”, Scientific Responsible Biagina Chiofalo.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- FEFAC. Fédération Européenne des Fabricants d’aliments Composés EuropäIscher Verband der Mischfutterindustrie European Feed Manufacturers Federation (18) PR 8 final. Position on the development of a European Protein Plan. 2018. Available online: https://fefac.eu/wp-content/uploads/2020/07/18_PR_1-1.pdf (accessed on 24 May 2022).
- European Commission. Report from the Commission to the Council and the European Parliament on the Development of Plant Proteins in the European Union. COM 757, Brussels. 2018. Available online: https://ec.europa.eu/info/sites/default/files/food-farming-fisheries/plants_and_plant_products/documents/report-plant-proteins-com2018-757-final_en.pdf (accessed on 24 March 2022).
- Caballero, R. Castile-La Mancha: A once traditional and integrated cereal-sheep farming system under change. Am. J. Altern. Agric. 1999, 14, 188–192. [Google Scholar] [CrossRef]
- Sedláková, K.; Straková, E.; Suchý, P.; Krejcarová, J.; Herzig, I. Lupin as a perspective protein plant for animal and human nutrition-A review. Acta Vet. Brno 2016, 85, 165–175. [Google Scholar] [CrossRef]
- White, C.L.; Staines, V.E. A review of the nutritional value of lupins for dairy cows. Aust. J. Agric. Res. 2007, 58, 185–202. [Google Scholar] [CrossRef]
- Chiofalo, B.; Lo Presti, V.; Chiofalo, V.; Gresta, F. The productive traits, fatty acid profile and nutritional indices of three lupin (Lupinus spp.) species cultivated in a Mediterranean environment for the livestock. Anim. Feed Sci. Technol. 2012, 171, 230–239. [Google Scholar] [CrossRef]
- Gresta, F.; Wink, M.; Prins, U.; Abberton, M.; Capraro, J.; Scarafoni, A.; Hill, G. Lupins in European cropping systems. In Legumes in Cropping Systems; Murphy-Bokern, F., Stoddard, C., Watson, D., Eds.; CABI: Wallingford, UK, 2017; pp. 88–108. [Google Scholar]
- Bolland, M.D.A.; Brennan, R.F. Comparing the phosphorus requirements of wheat, lupine, and canola. Aust. J. Agric. Res. 2008, 59, 983–998. [Google Scholar] [CrossRef]
- Fumagalli, P.; Comolli, R.; Ferre, C.; Ghiani, A.; Gentili, R.; Citterio, S. The rotation of white lupin (Lupinus albus L.) with metal-accumulating plant crops: A strategy to increase the benefits of soil phytoremediation. J. Environ. Manag. 2014, 145, 35–42. [Google Scholar] [CrossRef]
- Písaříková, B.; Zralý, Z. Nutritional Value of Lupine in the Diets for Pigs (a Review). Acta Vet. Brno 2009, 78, 399–409. [Google Scholar] [CrossRef]
- Petterson, D.S. The Use of Lupins in Feeding Systems—Review. Asian-Australas. J. Anim. Sci. 2000, 13, 861–882. [Google Scholar] [CrossRef]
- Department of Health and Ageing Office of the Gene Technology Regulator. Australian Government, 2013. The Biology of Lupinus L. (Lupin or Lupine). Available online: http://www.ogtr.gov.au (accessed on 24 March 2022).
- Palander, S.; Laurinen, P.; Perttilä, S.; Valaja, J.; Partanen, K. Protein and amino acid digestibility and metabolizable energy value of pea (Pisum sativum), faba bean (Vicia faba) and lupin (Lupinus angustifolius) seeds for turkeys of different age. Anim. Feed Sci. Technol. 2006, 127, 89–100. [Google Scholar] [CrossRef]
- Steenfeldt, S.; González, E.; Knudsen, K.E.B. Effects of inclusion with blue lupins (Lupinus angustifolius) in broiler diets and enzyme supplementation on production performance, digestibility and dietary AME content. Anim. Feed Sci. Technol. 2003, 110, 185–200. [Google Scholar] [CrossRef]
- Abraham, E.; Ganopoulos, I.; Madesis, P.; Mavromatis, A.; Mylona, P.; Nianiou-Obeidat, I.; Parissi, Z.; Polidoros, A.; Tani, E.; Vlachostergios, D. The Use of Lupin as a Source of Protein in Animal Feeding: Genomic Tools and Breeding Approaches. Int. J. Mol. Sci. 2019, 20, 851. [Google Scholar] [CrossRef] [PubMed]
- Zraly, Z.; Pisarikova, B.; Trckova, M.; Herzig, I.; Juzl, M.; Simeonova, J. Effect of lupin and amaranth on growth efficiency and health, carcass characteristics and meat quality of market pigs. Acta Vet. Brno 2007, 75, 451–460. [Google Scholar] [CrossRef]
- Glencross, B. Feeding Lupins to Fish: A Review of the Nutritional and Biological Value of Lupins in Aquaculture Feeds; The Department of Fisheries, Government of Western Australia (DFWA): Perth, WA, Australia, 2001. [Google Scholar]
- Glencross, B. In Proceedings of the Third Workshop for Seeding a Future for Grains in Aquaculture Feeds; Department of Fisheries: Perth, WA, Australia, 2005.
- Glencross, B. Harvesting the benefits of lupin meals in aquaculture feeds. In Proceedings of the 12th International Lupin Conference, Fremantle, Western Australia, 14–18 September 2008. [Google Scholar]
- Martínez-Villaluenga, C.; Frias, J.; Vidal-Valverde, C. Functional lupin seeds (Lupinus albus L. and Lupinus luteus L.) after extraction of α-galactosides. Food Chem. 2006, 98, 291–299. [Google Scholar] [CrossRef]
- Straková, E.; Suchy, P.; Večerek, V.; Šerman, N.; Mas, M.; Jůzl, M. Nutritional composition of seeds of the genus Lupinus. Acta Vet. Brno 2006, 75, 489–493. [Google Scholar] [CrossRef]
- Sujak, A.; Kotlarz, A.; Strobel, W. Compositional and nutritional evaluation of several lupin seeds. Food Chem. 2006, 98, 711–719. [Google Scholar] [CrossRef]
- Boschin, G.; D’Agostina, A.; Annicchiarico, P.; Arnoldi, A. The fatty acid composition of the oil from Lupinus albus cv. Luxe affected by environmental and agricultural factors. Eur. Food Res. Technol. 2007, 225, 769–776. [Google Scholar] [CrossRef]
- Mierlita, D.; Simeanu, D.; Pop, I.M.; Criste, F.; Pop, C.; Simeanu, C.; Lup, F. Chemical composition and nutritional evaluation of the lupine seeds (Lupinus albus L.) from low-alkaloid varieties. Rev. Chim. 2018, 69, 453–458. [Google Scholar] [CrossRef]
- Singh, C.K.; Robinson, P.H.; McNiven, M.A. Evaluation of raw and roasted lupin seeds as protein supplements for lactating cows. Anim. Feed Sci. Technol. 1995, 52, 73–76. [Google Scholar] [CrossRef]
- Vicenti, A.; Toteda, F.; Di Turi, L.; Cocca, C.; Perrucci, M.; Melodia, L.; Ragni, M. Use of sweet lupin (Lupinus albus L. var. Multitalia) in feeding for Podolian young bulls and influence on productive performances and meat quality traits. Meat Sci. 2009, 82, 247–251. [Google Scholar] [CrossRef]
- Lampart-Szczapa, E.; Siger, A.; Trojanowska, K.; Nogala-Kałucka, M.; Małecka, M.; Pachołek, B. Chemical composition and antibacterial activities of lupin seeds extracts. Nahrung/Food 2003, 47, 286–290. [Google Scholar] [CrossRef]
- Dueñas, M.; Hernandez, T.; Estrella, I.; Fernandez, D. Germination as a process to increase the polyphenol content and antioxidant activity of lupin seeds (Lupinus angustifolius L.). Food Chem. 2009, 117, 599–607. [Google Scholar] [CrossRef]
- Ranilla, L.G.; Genovese, M.I.; Lajolo, F.M. Isoflavones and antioxidant capacity of Peruvian and Brazilian lupin cultivars. J. Food Compos. Anal. 2009, 22, 397–404. [Google Scholar] [CrossRef]
- Siger, A.; Czubiński, J.; Kachlicki, P.; Dwiecki, K.; Lampart-Szczapa, E.; Nogala-Kałucka, M. Antioxidant activity and phenolic content in three lupin species. J. Food Compos. Anal. 2012, 25, 190–197. [Google Scholar] [CrossRef]
- Khan, M.K.; Karnpanit, W.; Nasar-Abbas, S.M.; Huma, Z.; Jayasena, V. Phytochemical composition and bioactivities of lupin: A review. Food Sci. Technol. 2015, 50, 2004–2012. [Google Scholar] [CrossRef]
- Pastor-Cavada, E.; Juan, R.; Pastor, J.E.; Alaiz, M.; Vioque, J. Antioxidant activity in the seeds of four wild Lupinus species from Southern Spain. J. Food Biochem. 2010, 34, 149–160. [Google Scholar] [CrossRef]
- Gresta, F.; Avola, G.; Abbate, V.; Magazzù, G.; Chiofalo, B. Lupin seed for crop-livestock food chains. Ital. J. Agron. 2010, 4, 333–340. [Google Scholar] [CrossRef]
- Calabrò, S.; Cutrignelli, M.I.; Lo Presti, V.; Tudisco, R.; Chiofalo, V.; Grossi, M.; Infascelli, F.; Chiofalo, B. Characterization and effect of year of harvest on the nutritional properties of three varieties of white lupine (Lupinus albus L.). J. Sci. Food Agric. 2015, 95, 3127–3136. [Google Scholar] [CrossRef]
- Musco, N.; Cutrignelli, M.I.; Calabrò, S.; Tudisco, R.; Infascelli, F.; Grazioli, R.; Lo Presti, V.; Gresta, F.; Chiofalo, B. Comparison of nutritional and antinutritional traits among different species (Lupinus albus L., Lupinus luteus L., Lupinus angustifolius L.) and varieties of lupin seeds. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1227–1241. [Google Scholar] [CrossRef]
- Dronne, Y. L’approvisionnement en protéines de la France dans son contexte européen et mondial. Fourrages 2003, 174, 107–128. [Google Scholar]
- Dracup, M.; Kirby, J.M. Lupin Development Guide; University of Western Australia Press: Perth, WA, Australia, 1996; p. 97. [Google Scholar]
- AOAC. Official Methods of Analysis, 21st ed.; Association of Official Analytical Chemists International: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Christie, W.W. Preparation of Ester derivatives of fatty acids for chromatographic analysis. In Advances in Lipid Methodology-Two; Christie, W.W., Ed.; Oily Press: Dundee, Scotland, 1993; pp. 69–111. [Google Scholar]
- Oteri, M.; Gresta, F.; Costale, A.; Lo Presti, V.; Meineri, G.; Chiofalo, B. Amaranthus hypochondriacus L. as a Sustainable Source of Nutrients and Bioactive Compounds for Animal Feeding. Antioxidants 2021, 10, 876. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Santos-Silva, J.; Bessa, R.J.B.; Santos-Silva, F. Effect of genotype, feeding system and slaughter weight on the quality of light lambs. II. Fatty acid composition of meat. Livest. Prod. Sci. 2002, 77, 187–192. [Google Scholar] [CrossRef]
- Luciano, G.; Pauselli, M.; Servili, M.; Mourvaki, E.; Serra, A.; Monahan, F.J.; Lanza, M.; Priolo, A.; Zinnai, A.; Mele, M. Dietary olive cake reduces the oxidation of lipids, including cholesterol, in lamb meat enriched in polyunsaturated fatty acids. Meat Sci. 2013, 93, 703–714. [Google Scholar] [CrossRef]
- Muzquiz, M.; Cuadrado, C.; Ayet, G.; de la Cuadra, C.; Burbao, C.; Osagie, A. Variation of alkaloid component of lupine seeds in 49 genotypes of Lupinus albus L. from different countries and locations. J. Agric. Food Chem. 1994, 42, 1447–1450. [Google Scholar] [CrossRef]
- Oboh, H.A.; Muzquiz, M.; Burbano, C.; Cuadrado, C.; Pedrosa, M.M.; Ayet, G.; Osagie, A.U. Anti-nutritional constituents of six underutilized legumes grown in Nigeria. J. Chromatogr. A 1998, 823, 307–312. [Google Scholar] [CrossRef]
- Nossack, A.C.; Vilegas, J.H.Y.; von Baer, D.; Lancas, F.M. Supercritical fluid extraction and chromatographic analysis (HRGC-FID and HRGC-MS) of Lupinus spp. alkaloids. J. Braz. Chem. Soc. 2000, 11, 495–501. [Google Scholar] [CrossRef]
- López-Mejía, O.A.; López-Malo, A.; Palou, E. Antioxidant capacity of extracts from amaranth (Amaranthus Hypochondriacus L.) seeds or leaves. Ind. Crops Prod. 2014, 53, 55–59. [Google Scholar] [CrossRef]
- Karamać, M.; Gai, F.; Longato, E.; Meineri, G.; Janiak, M.A.; Amarowicz, R.; Peiretti, P.G. Antioxidant Activity and Phenolic Composition of Amaranth (Amaranthus caudatus) during Plant Growth. Antioxidants 2019, 8, 173. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free-radical method to evaluated antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Grillo, G.; Boffa, L.; Talarico, S.; Solarino, R.; Binello, A.; Cavaglià, G.; Bensaid, S.; Telysheva, G.; Cravotto, G. Batch and Flow Ultrasound-Assisted Extraction of Grape Stalks: Process Intensification Design up to a Multi-Kilo Scale. Antioxidants 2020, 9, 730. [Google Scholar] [CrossRef] [PubMed]
- López-Bellido, L.; Fuentes, M.; Lhamby, J.C.B.; Castillo, J.E. Growth and yield of white lupin (Lupinus albus) under Mediterranean conditions: Effect of sowing date. Field Crops Res. 1994, 36, 87–94. [Google Scholar] [CrossRef]
- López-Bellido, L.; Fuentes, M.; Castillo, J.E. Growth and Yield of White Lupin Under Mediterranean Conditions. J. Agron. 2000, 92, 200–205. [Google Scholar] [CrossRef]
- Martinelli, F.; Vollheyde, A.; Cebriän-Piqueras, M.A.; von Haaren, C.; Lorenzetti, E.; Barberi, P.; Loreto, F.; Piergiovanni, A.R.; Totev, V.V.; Bedini, A.; et al. LEGU-MED: Developing Biodiversity-Based Agriculture with Legume Cropping Systems in the Mediterranean Basin. Agronomy 2022, 12, 132. [Google Scholar] [CrossRef]
- Suchy, P.; Strakova, E.; Kroupa, L.; Vecerek, V. The fatty acid content of oil from seeds of some lupin varieties. In Proceedings of the 12th International Lupin Conference. Fremantle, Western Australia, 14–18 September 2008. [Google Scholar]
- Uzun, B.; Arslan, C.; Karhan, M.; Toker, C. Fat and fatty acids of white lupin (Lupinus albus L.) in comparison to sesame (Sesamum indicum L.). Food Chem. 2007, 102, 45–49. [Google Scholar] [CrossRef]
- Bhardwaj, H.L.; Hamama, A.A.; van Santen, E. Fatty acids and oil content in white lupin seed as affected by production practices. J. Am. Oil. Chem. Soc. 2004, 81, 1035–1038. [Google Scholar] [CrossRef]
- Volek, Z.; Marounek, M. Effect of feeding growing-fattening rabbits a diet supplemented with whole white lupin (Lupinus albus cv. Amiga) seeds on fatty acid composition and indexes related to human health in hind leg meat and perirenal fat. Meat Sci. 2011, 87, 40–45. [Google Scholar] [CrossRef]
- Boschin, G.; D’agostina, S.; Annicchiarico, P.; Arnoldi, A. Effect of genotype and environment on fatty acid composition of Lupinus albus L. seed. Food Chem 2008, 108, 600–606. [Google Scholar] [CrossRef]
- Bağci, E. Fatty acid composition of some Astragalus species from Turkey. Chem. Nat. Compd. 2006, 42, 645–648. [Google Scholar] [CrossRef]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Abd El-Hack, M.E.; Khafaga, A.F.; Taha, A.E.; Tiwari, R.; Yatoo, M.I.; Bhatt, P.; Khurana, S.K.; et al. Omega-3 and Omega-6 Fatty Acids in Poultry Nutrition: Effect on Production Performance and Health. Animals 2019, 9, 573. [Google Scholar] [CrossRef]
- Osman, M.; Mahmoud, G.I.; Romeilah, R.M.; Fayed, S.A. Lupin seeds lower plasma lipid concentrations and normalize antioxidant parameters in rats. Grasas Aceites 2011, 62, 162–170. [Google Scholar] [CrossRef]
- Mieczkowska, A.; Smulikowska, S. The influence of white lupin seeds in diets supplemented with fats of animal or plant origin on the fatty acid composition of broiler tissues. J. Anim. Feed Sci. 2005, 1, 93–107. [Google Scholar] [CrossRef]
- Kim, J. Feeding lupins to Pigs. Copyright© Western Australian Agriculture Authority. 2013. 3 Baron-Hay Court South Perth WA 6151. Available online: https://www.agric.wa.gov.au (accessed on 25 January 2023).
- Oomah, B.D.; Tiger, N.; Olson, M.; Balasubramanian, P. Phenolics and Antioxidative Activities in Narrow-Leafed Lupins (Lupinus angustifolius L.). Plant Foods Hum. Nutr. 2006, 61, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Clements, J. Antioxidant activities of Lupin seeds. In Proceedings of the 12th International Lupin Conference. Fremantle, Western Australia, 14–18 September 2008. [Google Scholar]
- Karamać, M.; Orak, H.H.; Amarowicz, R.; Orak, A.; Piekoszewski, W. Phenolic contents and antioxidant capacities of wild and cultivated white lupin (Lupinus albus L.) seeds. Food Chem. 2018, 1, 258. [Google Scholar] [CrossRef] [PubMed]
- Elbandy, M.; Rho, J.R. New flavone-di-C-glycosides from the seeds of Egyptian lupin (Lupinus termis). Phytochem. Lett. 2014, 9, 127–131. [Google Scholar] [CrossRef]
- Arnoldi, A.; Boschin, G.; Zanoni, C.; Aiello, G.; Lammi, C. The Health Benefits of Lupin in Cardiovascular Prevention: Ten Years of Successful Investigations. In Proceedings of the XIV International Lupin Conference, Milan, Italy. 2015. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1756464615003965 (accessed on 2 October 2022).
- Kim, M.K.; Yun, K.J.; Lim, D.H.; Kim, J.; Jang, Y.P. Anti-inflammatory properties of flavone di-C-glycosides as active principles of Camellia mistletoe, Korthalsella japonica. Biomol. Ther. 2016, 24, 630–637. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).