Abstract
Soil salinization has a major impact on crop revenue, which may be counteracted by harnessing the microbiota that the soil itself harbors. This study aimed to explore the application of fungi exudates (Trametes versicolor and Pleurotus sajor caju) on the potential relief of salt stress in the performance of Lens culinaris in two different soils (a silvopastoral agroforestry soil and LUFA 2.2). Three salinity levels (8, 16, and 26 mS·cm−1) and three levels of each fungal exudate (1%, 6%, and 12%) were evaluated in a 96-h cross-design experiment. Seed germination was checked daily, and after 96-h, seedling radicle and hypocotyl were measured, along with fresh and dry weights and soil phosphatase activity. The sole application of exudates from neither fungus affected the germination and seedling development of L. culinaris. Salinity alone induced a dose-effect response in all L. culinaris tested endpoints, as expected because conductivities above 8 mS·cm−1 are known to be detrimental for many crop species. Application of exudates to salinized soils improved germination rates at 16 and 26 mS·cm−1 compared to the single respective salinity treatment alone, but mainly in assays carried out in natural soil. In addition, under the same conditions (26 mS·cm−1 and all fungi %), there was an improvement in natural soil acid phosphatase activity. The application of rot fungi exudates demonstrates potential in what might prove to be a sustainable measure to reclaim salinized soils and improve crop productivity, which is consistent with current soil protection policies.
1. Introduction
Climate changes have harshly impacted soils worldwide. Among the many causes for soil loss or low productivity, salinization is currently of the utmost interest, e.g., [1,2]. Whether derived from sea level rise (primary salinization) or man-induced (secondary salinization through the usage of poor-quality water for irrigation from salt-affected aquifers), effects on soil biota (plants, animals, fungi), microbial community, physicochemical properties (nutrient cycle, organic matter decomposition) and structure have already been extensively documented [3,4,5]. Concurrently with soil salinization, the world faces an alarming populational growth, which, combined with salinization, might end up in food supply collapse or shortage. That being so, salinization has been targeted as a major challenge in the coming years in the agenda of organizations and soil policies/strategies, which must be addressed by the development and employment of sustainable solutions for the reclamation and/or mitigation of salt-affected soil, as for example, those fromthe European Parliament, resolution P9_TA (2021)0143, 2021 or from theFood and Agriculture Organization of the United Nations, 2021).
Those same resolutions have pinpointed (although briefly and not in much detail) that harnessing and protecting the micro-biodiversity that soil itself harbors is a step towards that achievement, with great potential to contribute to soil resilience under climate change stress and/or pollution. The soils microbiome (which includes arbuscular mycorrhizal fungi—AMF; plant growth promoting bacteria—PGPR; and rot fungi—RT) can greatly enhance organic matter recycling, nutrient recycling/efficient usage, improve soils water and carbon retention, and benefit the plants they interact with (either by promoting their growth, by helping in the control of phytopathogens, or by providing protection against abiotic stress), e.g., [6,7,8,9,10]. Such characteristics have motivated research mainly aimed at the use of AMF and/or PGPR with the objective of increasing soil resilience to biotic/abiotic stressors and/or pollution at the expense of RT. However, the focus on the use of the two first groups (AMF and PGPR) has some shortcomings to be considered, and that might be overcome by RT, such as AMFs obligatory interaction with a host-plant for the fungi’s growth and reproduction, e.g., [11], and the PGPRs possible incapacity of surviving alone in disturbed soils, which can seriously affect their resilience, along with its limited dispersal ability (for instance it may happen, in few cases, the plant with which they interact might be more tolerant to stress and thus, provide protection to the bacteria and not the other way around), e.g., [12,13]. Rot fungi offer new opportunities for the development of more efficient microbial agents harmonized with the aforementioned policies and strategies for soil protection. However, despite the efforts implemented in this line of investigation, to date and our knowledge, robust experimental evidence is still lacking on the efficacy of methodologies using fungi to restore soils [14]. Some recent studies have addressed the role of white rot fungi as cross-functional organisms, i.e., with the ability to participate in several soil functions, such as biological nitrogen fixation and the phosphorous cycle [15,16]. Rot fungi might tolerate exceptional levels of abiotic stress, namely salinity, being amongst the most tolerant edaphic organisms while comprising almost 70% of soil biomass due to great hyphal growth and extension and with single abilities relative to cellulose degradation and on recalcitrant C conversion to labile C, e.g., [17,18,19]. Some studies point out the tolerance of fungi to abiotic stress. For instance, Venâncio et al. [20] have shown the resilience of four rot fungi species under strongly saline environments. For instance, the growth of Lentinus sajor caju, the most sensitive species, was reduced by 50% at a conductivity of 23.7 mS·cm−1, whereas the growth of Phanerochaete chrysosporium, the most tolerant, was reduced by 50% at a conductivity corresponding to over ½ the conductivity of natural seawater (37.4 mS·cm−1). Field studies have also highlighted that rot fungi are highly resilient to salinity increments. For instance, Rath et al. [4] evaluated the fungal and bacterial biomass in two salinity gradients (one in an agricultural area—ca. 0.1 to 3 mS·cm−1—and one in a natural vegetation area—ca. 0.3 to 10 mS·cm−1), verified that despite bacterial growth being strongly inhibited by salinity, the fungal biomass was similar across the salinity gradient, indicating that these fungi are able to thrive under very harsh conditions [4].
The resilience of fungi under strong salinity levels is related to the overproduction of metabolites in the fungi mycelia responsible for the adaptation of the fungi to saline stress [20]. Some of these metabolites may include phosphatases, which may play a crucial role in plant growth in the vicinity of fungi since phosphorous is often a limited resource in soils, as well as its main roles in many cellular functions [21]. Thus, fungi may also indirectly contribute to plant growth through the production of these metabolites. To highlight that the P nutrient cycle is still poorly understood under abiotic stress, particularly that induced by salts, e.g., [22]. However, considering that seawater contains phosphorous, salinization may potentially affect the activity of phosphatase enzymes and alter the cycling of phosphorus in the soil [23]. However, the specific impact of seawater on phosphatase activity in soils would depend on a variety of factors, such as the soil type, the amount of seawater present, and the length of time the soil is exposed to seawater, points still to be determined and taking into consideration as a side objective in this work.
This work aimed to verify whether rot fungi exudates, in addition to salt-affected soils, could improve soil productivity. Therefore, it was studied the effect of the addition of two white-rot fungi exudates (Pleurotus sajor caju and Trametes versicolor) had in the germination and growth of a legume species, Lens culinaris when exposed to two soils differing in their properties (for instance, organic matter) different origin soils with three increasing salinization levels. The plant choice was based, aside from their small dimensions and ease of development under laboratory conditions, on the emerging interest that lentils have created as a functional food due to their high nutritional value, presence of bioactive components, antioxidants, and other phytochemicals that render them health properties, e.g., [24,25,26]. Moreover, it is also a legume with beneficial properties for the soil, namely by fixing atmospheric nitrogen in endosymbiotic association with bacteria, which reduces the need to use fertilizers and fossil fuels, and its ability to increase soil carbon sequestration [27].
The following hypotheses were pursued to understand the results: (i) the salinity factor alone will affect the traits of the plants in a concentration-dependent manner and independently of soil type; (ii) the sole addition of fungi exudates to the different soils will not induce changes compared to control soils; and, (iii) the addition of fungi exudates to the salinized soils will increase both soil function expressed by means of plant germination and growth (increased productivity), as well as highlight on the potential increased on phosphorous availability to plants (assessed by indirect measurement of phosphatase activity).
2. Materials and Methods
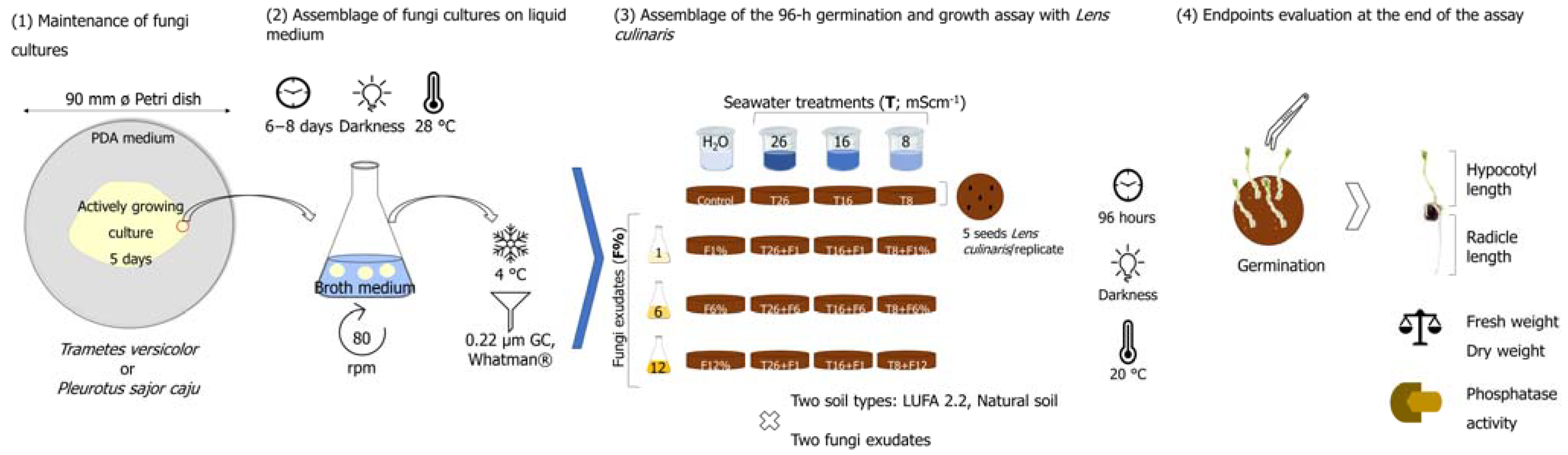
A schematic illustration summarizing the main steps of the methods employed is presented in Figure 1.
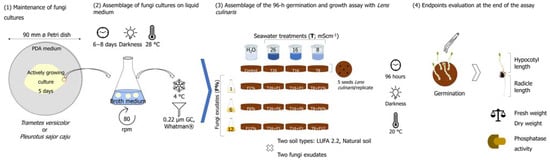
Figure 1.
Schematic illustration summarizing the main steps of the methods employed in this study, from the establishment of fungi cultures in a liquid medium, through the experimental setup and finalizing in the endpoints evaluated at the end of the 96-h assay with Lens culinaris.
2.1. Test Species
Two white-rot fungi belonging to the group Basidiomycota were used to produce the filtrates employed in this work: Trametes versicolor and Pleurotus sajor caju. Individual cultures of each species of fungi were maintained in sterilized Petri dishes in potato dextrose agar (PDA, 39 gL−1, Merck, Darmstadt, Germany), in the dark, at an optimal growth temperature of 28 °C. Replication of the fungi cultures was performed once a week. The individual liquid-growing fungi cultures were then established in sterilized 250 mL Erlenmeyer flasks (20 min, at 121 °C, 1 bar; Uniclave 88, AJC) by inoculating previously sterilized broth medium (100 mL, NutriSelect® Basic, Merck & Co., St Louis, MO, USA) with 6–8 discs (7 mm ø; average biomass of each disc of 73 mg and 80 mg for T. versicolor and P. sajor caju, respectively) from actively growing cultures of each fungus. Liquid-growing cultures were left to grow for approximately 8 days in an orbital shaker (80 rpm) at 28 °C in total darkness. After this period, the exudates were collected and filtered through 0.22 µm GF/C 55 mm ø filters (Whatman, Munich) and maintained under refrigerated conditions (4 °C), for less than a week, until use in the germination and seedling growth experiments [28]. This was considered the exudate at 100%, which was then diluted according to the fungi exudate percentages that were intended.
To attest to soil productivity, a legume species was used, Lens culinaris (also known as Lens sculenta). The seeds of L. culinaris used in the germination and seedling growth assays were obtained from Germline (Les grivelles, France), which produced the seeds through organic farming.
2.2. Characterization of the Soils Used in the Assays
Considering the influence that soil structure and texture might have in the life traits of biota, as well as its reaction to the salinity stress, assays were carried out in two soils: a silvopastoral agroforestry oak woodland soil and LUFA 2.2 soil (for further information on both soils, please see Jurburg et al. [29] and Land- und Forstwirtschaftliche Untersuchungsanstalt LUFA Speyer that in Germany, respectively). Briefly, both soils were collected from sustainably managed soils from Germany and Portugal (LUFA and NS, respectively), in which the addition of pesticides or chemical fertilizers ceased (>5 years). The LUFA has pH and water holding capacity (WHC) values slightly higher than those of the NS but a lower nitrogen and organic matter (OM) content than NS. Both soils can be classified as natural and sandy loam soils, with very similar values for the other endpoints, such as organic carbon content (Corg) and the percentages of sand, silt, and clay (Supplementary Table S1). However, for simplification purposes, from this point onwards, they will be denominated as Natural Soil (NS, for that from the silvopastoral agroforestry origin) and LUFA (as a designation for the soil LUFA 2.2). The characteristics of each soil are listed in Supplementary Table S1.
2.3. Experimental Design: Salinity Levels and Fungi Exudates Percentages
Accordingly, and following the classification of Zaman et al. [30], which combines crop tolerance and the categorization of the soil based on its EC, three salinity levels were established to conduct this work: 8 mS·cm−1 (which would correspond to the highest EC in a soil classified as slightly saline and already inducing some restrictions to crops’ yield), 16 mS·cm−1 (moderately saline soil in which crops yield are strongly restricted), and 26 mS·cm−1 (a strongly saline soil in which only a few, very tolerant crops are able to show satisfactory yields). To simulate the salinized soils, an artificial seawater (SW) solution was preferred instead of a surrogate salt (e.g., NaCl) since it better resembles what would happen under sea level rise scenarios [5,20]. The SW solution was obtained by directly dissolving seawater salts (Ocean Nature Premium Sea Salt, Queensland, Australia) into distilled water, with continuous monitoring of the conductivity using a portable meter (LF 330/SET, best nr. 300 204, Mettler Toledo®, Greifensee, Switzerland), until reaching the desired salinity levels of 8, 16, and 26 mS·cm−1 (onwards designated as T8, T16, and T26).
To obtain the fungi exudates, cultures were established in a liquid PDA medium (afore-described). This was considered the best way to guarantee the application of all the metabolites secreted by the fungi (e.g., polysaccharides, proteins, e.g., [20]) posteriorly to the soil. Fungal exudates are therefore referred to herein as the liquid medium in which fungi were allowed to grow. The posterior defined dilutions percentages of 1%, 6%, and 12% (later presented as F1%, F6%, and F12%, and obtained by dilution of the 100% exudate with distilled water) were established based on previous works focused on the same approach using rot fungi exudates employed to remediate contaminated soils [31]. The volume of the test solution to be added to the soil was calculated by adjusting soil moisture to 45% of its water-holding capacity (WHCmax, Supplementary Table S1) following Venâncio et al. [5], which corresponded to approximately 3 mL of exudate per plate. The conductivity (LF 330/SET portable meter, Mettler Toledo®, Greifensee, Switzerland) and pH (WTW pH 330i portable meter, Mettler Toledo®, Greifensee, Switzerland) values of the solution added to the soils [fungi exudate mixed with the respective SW dilution (or water in case of control replicates) are presented at Supplementary Table S2].
The salinity levels (T8, T16, T26) and the fungi exudates levels (F1%, F6%, and F12%) were considered as factors. To break down and ease the interpretation of the results obtained, first, the influence of each factor alone (within each fungi species and type of soil) was analyzed, and then the interaction between the two factors.
2.4. Germination and Seedling Growth Assay with Lens Culinaris
A 96-h germination and seedling growth assay was carried out with L. culinaris to assess the productivity of the two tested soils under salt stress and supplemented with exudates obtained from the two fungi species (T. versicolor and P. sajor caju). Facing the lack of experiments considering rot fungi exudates and soil salinization and attempting to rapidly discriminate several treatments and combinations with a substantial number of endpoints as an outcome, this assay was chosen since it is considered very sensitive and cost-effective. Since no universal seed germination and seedling growth assay has been adopted yet, previous published experimental designs were adopted, e.g., [32,33]. Moreover, L. culinaris has a fast germination rate and growth within the period of the established test.
Considering the salinity levels above and the percentages of exudates, a complete crossover design was elaborated, with two additional treatments [one composed only of salinity treatments and the other only with exudates (%)], in addition to a control condition (only moistened with distilled water). Each treatment had three replicates; each replicate was set in 60 mm ø Petri dishes (mean dish height of 1.8 mm), each containing approximately 18–20 g of soil. After preparing all treatments with filtrates obtained from both fungal species, five seeds of L. culinaris were assigned to each Petri dish, in which they were allowed to germinate. The assay lasted 96 h, with each Petri dish checked for germinated seeds every 24 h. In the end, the root and shoot sizes of the developed seedlings were measured with the help of a ruler, and their fresh weight was determined (AND HR 250 AZ). After these measurements, the seedlings were placed in an oven (60 °C, 24 h) to assess the dry weight further.
Three randomly picked soil samples were also selected from each treatment to proceed with pH and conductivity measurements at the end of the assays, using soil-water suspensions in a 1:5 w:v proportion. Deionized water was used for this purpose with a pH >5.2 and conductivity < 0.2 mS·cm−1. For pH assessment, soils samples were magnetically stirred for 15 min and left to rest for another 60 min, after which the pH was measured using a WTW pH 330i meter (Mettler Toledo®, Greifensee, Switzerland) [34], while for conductivity, soils samples were mechanically shaken for 15 min and left to rest overnight so that the bulk sediment could settle [35]. Conductivity was measured the day after with an LF 330/SET meter (Mettler Toledo®, Greifensee, Switzerland).
2.5. Alkaline and Acid Phosphatase Activity
Since phosphorus (P) is often sparse in soils, phosphatases’ activity (as an indirect measure of P in soil) was analyzed to further contribute to the understanding of the results obtained. Two phosphatase enzymes were thus complementarily analyzed in this study with differential optimal pH values of activity: the alkaline phosphatase (pH = 11) and the acid phosphatase (pH = 6). The method followed was previously described by Eivazi and Tabatabai [36]. Briefly, 1 g of previously cold storage soil samples were mixed with 1 mL of substrate 4-Nitrophenyl phosphate disodium salt hexahydrate (1 g/L; CAS number 333338-18-4, high purity, Merck & Co., Inc., St Louis, MO, USA), plus 125 µL of toluene (CAS number 108-88-3, ≥99.5% purity, Merck & Co., Inc., St Louis, MO, USA) and vortexed (10 s) [37]. The 4-Nitrophenyl phosphate disodium salt hexahydrate stock solution was prepared in a universal buffer with pH adjusted according to the enzyme (pH 6 for acid phosphatase and pH 11 for alkaline phosphatase). Then, samples were incubated in an orbital shaker (150 rpm) for 60 min at 37 °C. Afterward, the samples were centrifuged at 10,000× g for 8 min, the supernatant was carefully collected (avoiding resuspension or collection of soil particulates that could interfere with absorbance reading), and the reaction product (p-nitrophenol, pNP) was read at 420 nm (Multiskan Microplate Photometer, Thermo Scientific). The pNP concentration was calculated using a calibration curve made with a standard solution of pNP (10 mg/mL; CAS number 100-02-7 ReagentPlus®, ≥99%, Merck & Co., Inc., St Louis, MO, USA), and results expressed in µg pNP/g soil/h.
2.6. Data Analysis
Data analysis was carried out independently for each soil type and species of fungi. To germination datasets (presented in %), an Anscombe arcsine transformation was first applied. Then, datasets were checked for normality (Shapiro-Wilk test) and homoscedasticity (Brown-Forsythe test), followed by Dunnett’s post hoc test (p < 0.05). Whenever datasets failed normality and homoscedasticity assumptions, a non-parametric ANOVA was carried out, followed by Dunn’s post hoc test (p < 0.05).
The weight and length datasets were first checked for normality and homoscedasticity (Shapiro-Wilk and Brown-Forsythe tests, respectively). A one-way ANOVA was carried out to assess possible differences between (i) control condition (soil only moistened with H2O) vs. soil moistened with SW at the three salinity levels of 8, 16, and 26 mS·cm−1; (ii) control condition vs. soil moistened with fungi exudate at the three different percentages of 1%, 6%, and 12%. To understand if there was an interaction between factors (considering salinity level and fungi exudate percentages as factors), a two-way ANOVA followed by Holm-Sidak all pairwise comparison (p < 0.05) was applied to each dataset. The same outline was applied to the phosphatase enzyme datasets.
3. Results
3.1. Germinatoon and Seedling Growth of L. culinaris in a Salinized Natural Soil
As expected, soil salinity increased in both soils according to the salinity level tested. A slight decrease in the pH was also noticed when conductivity increased. A summary of the conductivity and pH levels of the soils at the end of the assays is available in Supplementary Table S3).
The results from the assay carried out with L. culinaris in NS soil with T. versicolor and P. sajor caju are summarized in Figure 2 and Figure 3 and Supplementary Figure S1. In both assays, as expected, the salinity factor influenced all the endpoints evaluated during the 96-h germination and seedling assay with L. culinaris (Figure 2 and Figure 3 and Supplementary Figure S1). For instance, after 24 h, germination (%) was statistically different between the CTR and all three salinity levels in both fungi [Holm-Sidak, p < 0.01; Figure 2, graphics (A) through (D) for T. versicolor, and E) through H) for P. sajor caju]. At the end of the assay (96 h), the differences in the cumulative germination between the CTR and T8 (the lower salinity treatment) disappeared, with these two groups forming a homogeneous group. At 96 h, the two other salinity treatments (T26 and T16) remained statistically different from the CTR and T8 and different from each other (Holm-Sidak, p ≤ 0.009 for T26 and Holm-Sidak, p ≤ 0.013 for T16; Figure 2). Likewise, salinity affected the other evaluated endpoints in a conductivity-dependent manner. The CTR and the T26 treatment belonged in all endpoints and the two assays (different fungus) to different homogeneous groups (Figure 3 and Figure S1; Holm-Sidak, p < 0.05). For both assays, in the length measurements, the T26 and T16 often shared the same homogenous group (Figure 3), while in the weight measurements, this tendency was not observed with the two treatments belonging to distinct homogenous groups (Figure S1).
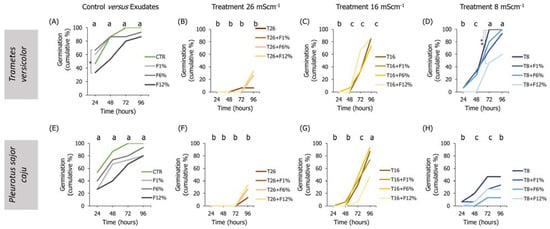
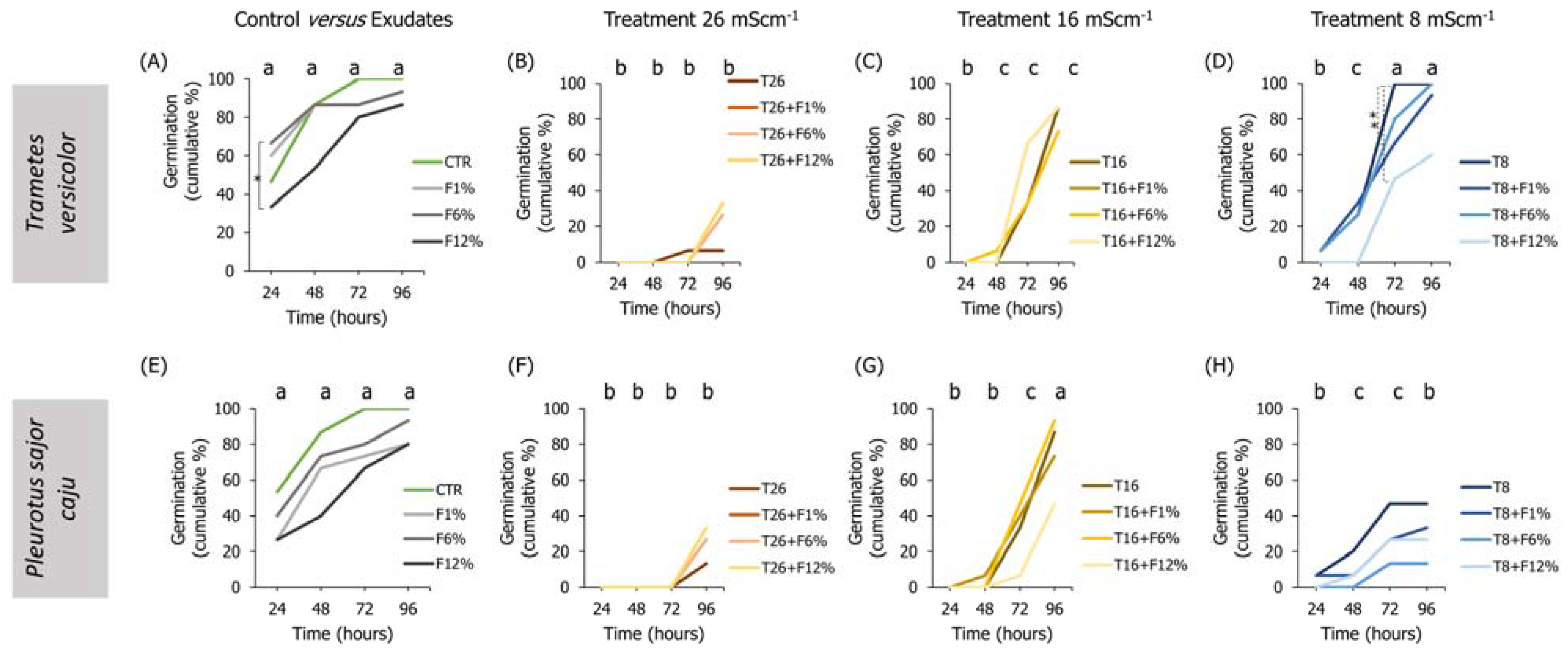
Figure 2.
Lens culinaris germination rates, at 96 h, in natural soil salinized at three different levels (8, 16, and 26 mS·cm−1, designated as T8, T16, and T26, respectively) and supplemented with fungi exudates at three levels (1%, 6, and 12%, designated as F1%, F6%, and F12%, respectively). Graphics (A–D) present the results obtained from soil moistened with Trametes versicolor exudates, and graphics (E–H) present the results obtained from soil moistened with Pleurotus sajor caju exudates. CTR stands for control soil. Letters (a, b, c) stand for homogenous groups assessed only between CTR and the salinity treatments within each observation time for the germination endpoint (24, 48, 72, or 96 h; Holm-Sidak, p < 0.05). The * stands for statistical differences between treatments within each time period (Holm-Sidak, p < 0.05).
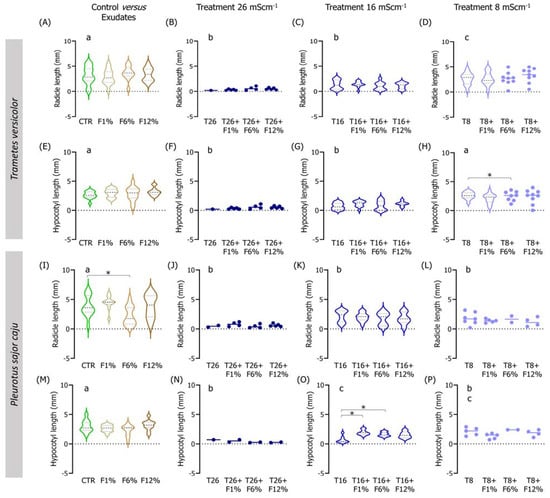
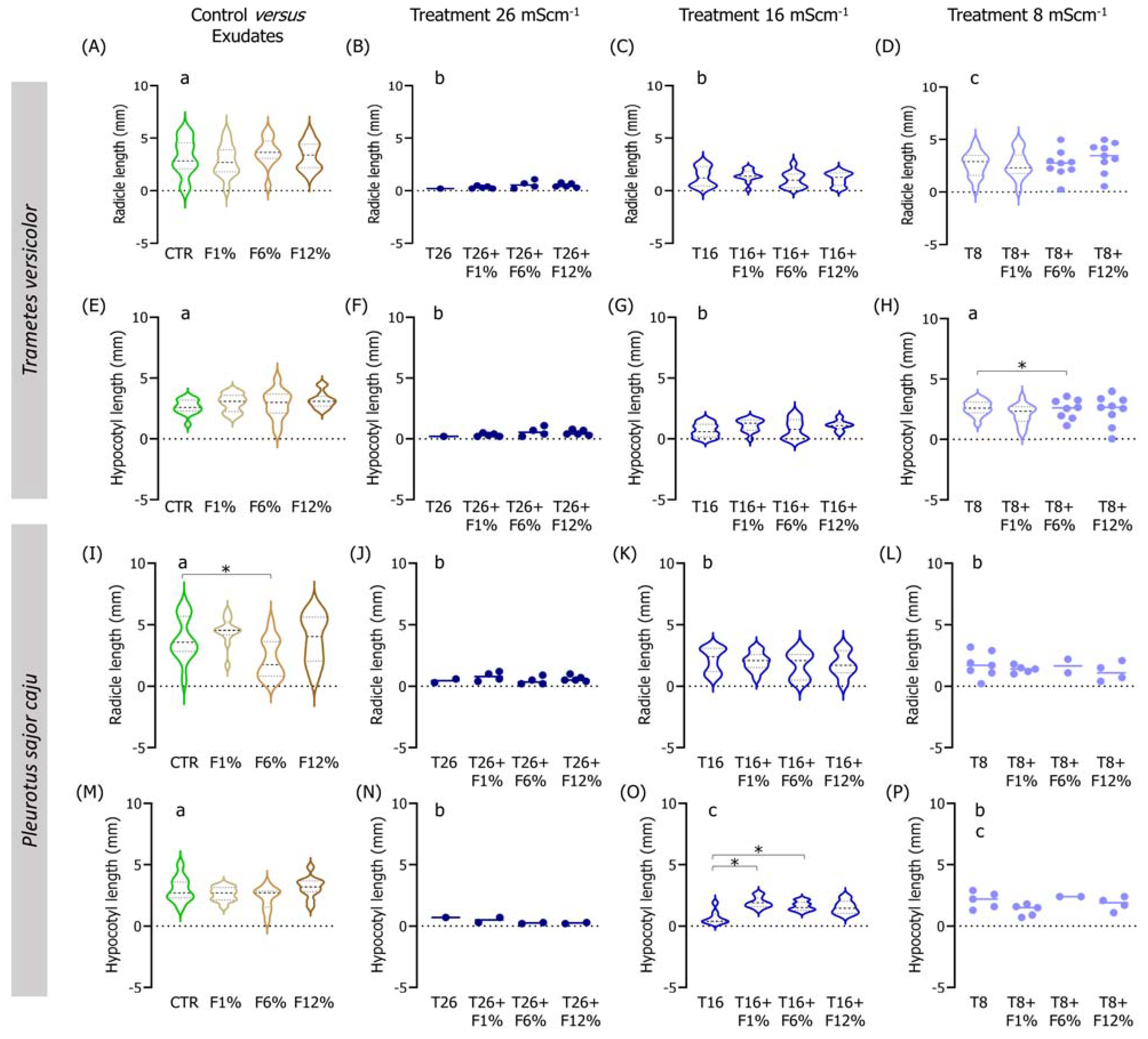
Figure 3.
Violin plots of the seedling length of Lens culinaris germinated in natural soil salinized at three different levels (8, 16, and 26 mS·cm−1, designated as T8, T16, and T26, respectively) and supplemented with fungi exudates at three levels (1%, 6, and 12%, designated as F1%, F6%, and F12%, respectively). Graphics (A–H) present the results obtained from soil moistened with Trametes versicolor exudates, and graphics (I–P) present the results obtained from soil moistened with Pleurotus sajor caju exudates. CTR stands for control soil. Letters (a, b, c) stand for homogenous groups assessed only between CTR and the salinity treatments at 96-h in each assessed endpoint (Holm-Sidak, p < 0.05). The * stands for statistical differences between treatments (Holm-Sidak, p < 0.05). Note: Data represented only by points means that no sufficient points were available for the construction of the violin.
Regarding the influence of the sole addition of fungi exudates on the germination and seedling growth of L. culinaris in NS soil, no clear trends were observed for any of the fungi species, tested exudates percentages, or evaluated endpoints (Figure 2).
When analyzing the interactions between factors (salinity and fungi exudate percentages), statistical differences were observed, especially in the lowest salinity treatment (T8) when employing T. versicolor exudates in the NS soil (Figure 2). For instance, L. culinaris fresh weight was improved in the T8 + F1%, T8 + F6%, and T8 + F12% fungi treatments (Holm-Sidak, p ≤ 0.01; Figure S1D), and the dry weight in all fungi treatments comparatively to the T8 treatment (Holm-Sidak, p ≤ 0.025; Figure S1H). Regarding the length measurements, only the seedlings in the T8 + F6% treatment had a slightly longer hypocotyl than the T8 seedlings (Holm-Sidak, p = 0.009; Figure 3H).
To a certain degree, associations between the factors (salinity and fungi exudate percentage) when employing P. sajor caju fungi exudates in the NS soil (Figure 3) were found mostly in the intermediate salinity treatment (T16; Figure 3). In the weight measurements, there was a trend for an increment in the fresh and dry weights of the seedlings, despite only the T16 + F12% being statistically different from T16 in both endpoints (Holm-Sidak, p ≤ 0.09; Figure S1K). Regarding the lengths, only in the hypocotyl, a statistically significant increase was found between the T16 + F1% or T16 + F6% and T16 (Holm-Sidak, p ≤ 0.013; Figure 3O).
3.2. Germination and Seedling Growth of L. culinaris in Salinized LUFA Soil
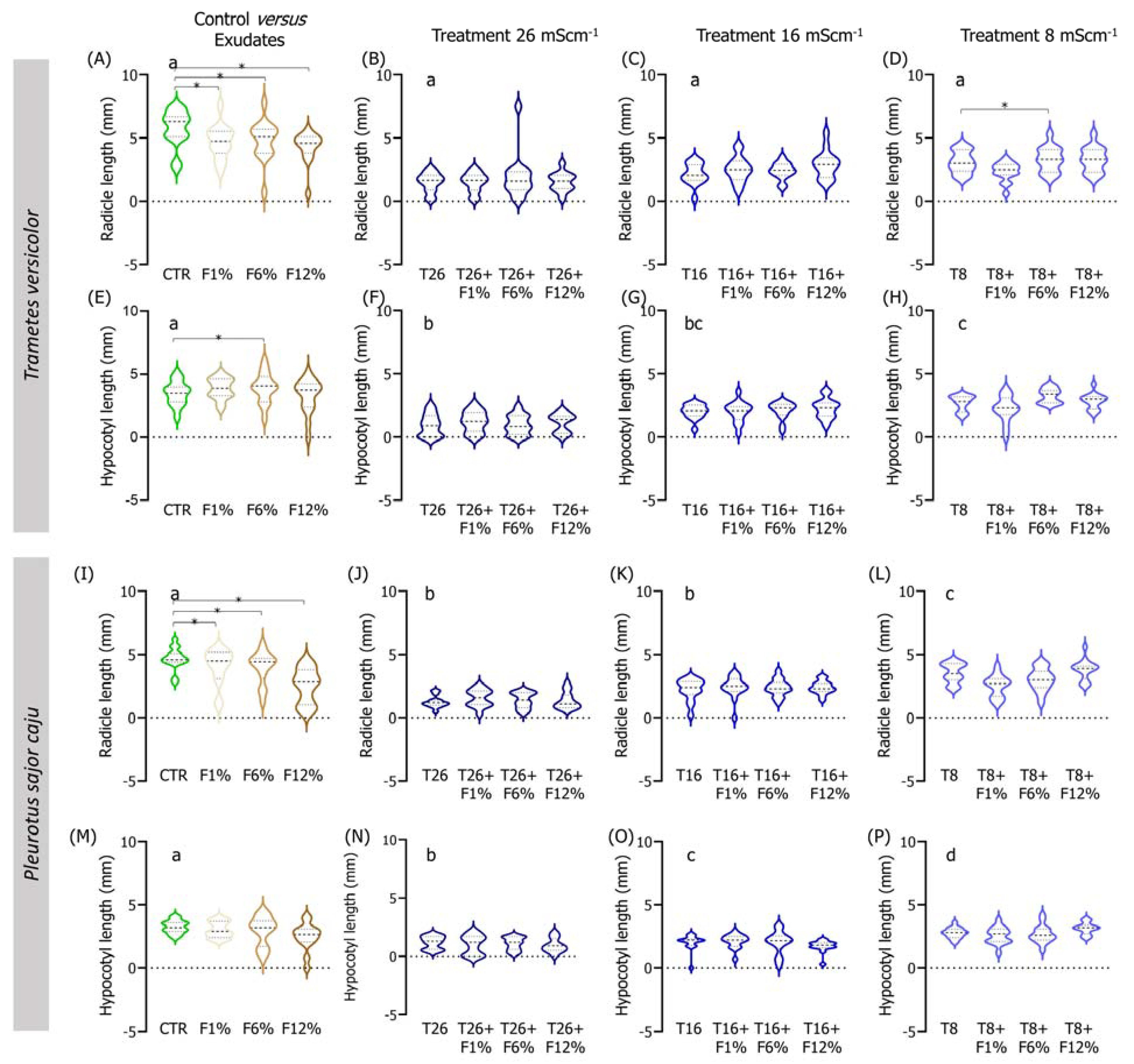
Similar to assays carried out in NS soil, the salinity factor alone affected the germination and seedling growth of L. culinaris when assays were conducted using LUFA soil. First, regarding germination, soil salinity had an effect in a concentration-dependent manner until 48 h of exposure, especially in T16 and T8, compared to the CTR (Figure 4). Until the end of the assay (96 h), differences between the CTR and T16 and T8 disappeared, with these three groups performing a single homogenous group, while T26 remained statistically different from CTR and the other salinity treatments (Figure 4). In the other endpoints, the CTR treatment was distinct from the highest salinity treatment (T26) in several endpoints [fresh and dry weight; Figure S2, graphics (A) versus (B), (E) versus (F), (I) versus (J), (M) versus (N)]), while frequently sharing the same homogenous group with the lowest salinity treatment (T8; fresh weight, radicle length, or dry weight, e.g., Figure 5 and Figure S2).
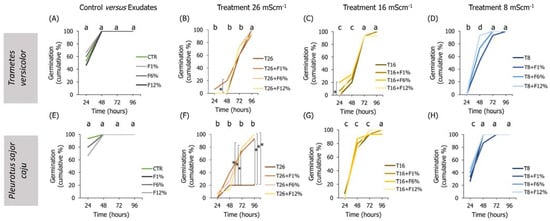
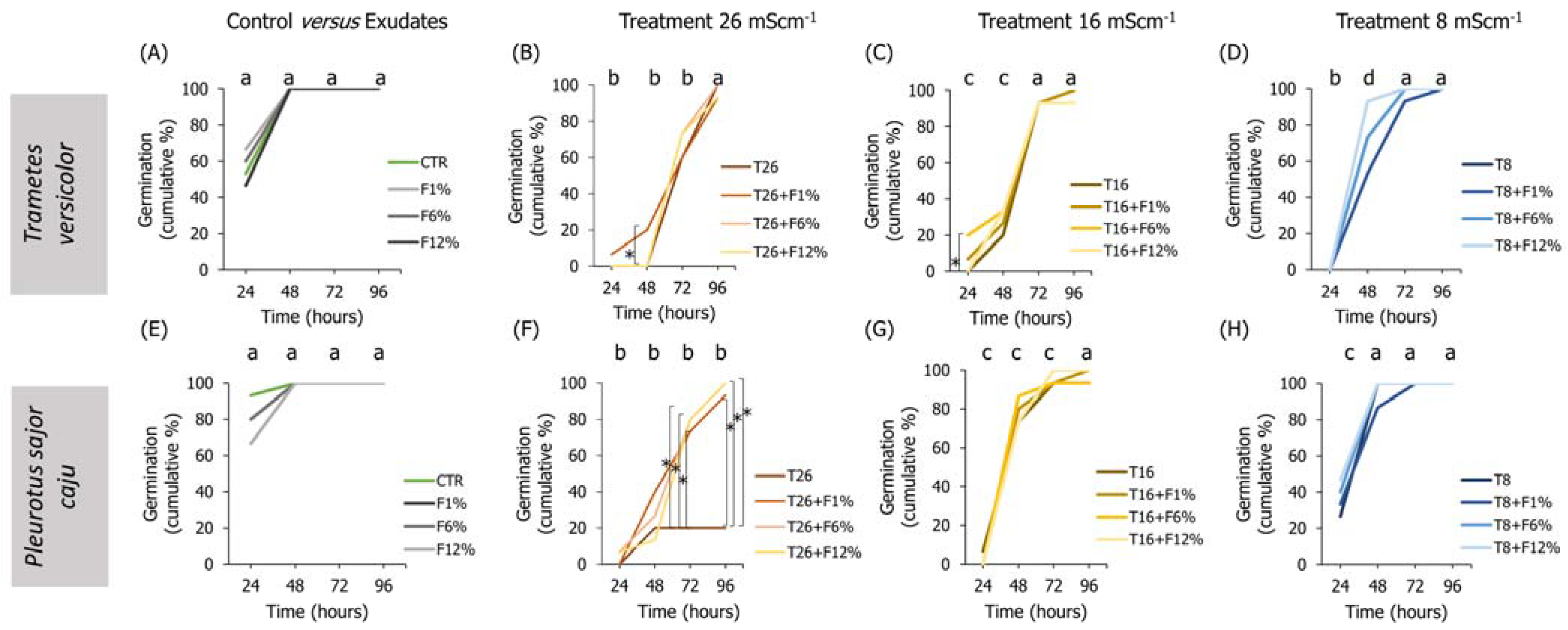
Figure 4.
Lens culinaris germination rates, at 96 h, in LUFA 2.2 soil salinized at three different levels (8, 16, and 26 mS·cm−1, designated as T8, T16, and T26, respectively) and supplemented with fungi exudates at three levels (1%, 6, and 12%, designated as F1%, F6%, and F12%, respectively). Graphics (A–D) present the results obtained from soil moistened with Trametes versicolor exudates, and graphics (E–H) present the results obtained from soil moistened with Pleurotus sajor caju exudates. CTR stands for control soil. Letters (a, b, c, d) stand for homogenous groups assessed only between CTR and the salinity treatments within each observation time for the germination endpoint (24, 48, 72, or 96 h; Holm-Sidak, p < 0.05). The * stands for statistical differences between treatments within each time period (Holm-Sidak, p < 0.05).
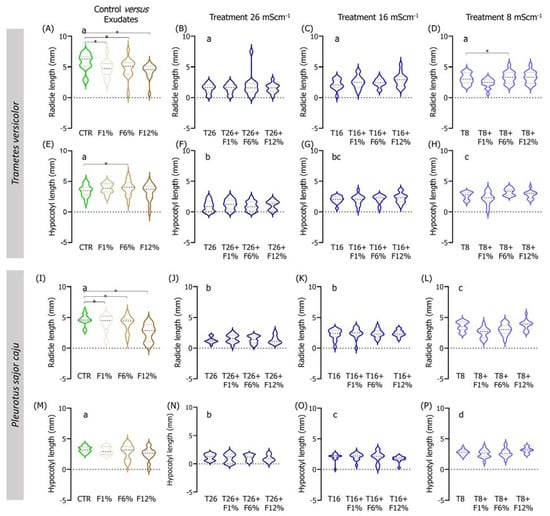
Figure 5.
Violin plots of the seedling length of Lens culinaris germinated in LUFA 2.2 soil salinized at three different levels (8, 16, and 26 mS·cm−1, designated as T8, T16, and T26, respectively) and supplemented with fungi exudates at three levels (1%, 6, and 12%, designated as F1%, F6%, and F12%, respectively). Graphics (A–H) present the results obtained from soil moistened with Trametes versicolor exudates, and graphics (I–P) present the results obtained from soil moistened with Pleurotus sajor caju exudates. CTR stands for control soil. Letters (a, b, c, d) stand for homogenous groups assessed only between CTR and the salinity treatments at 96-h in each assessed endpoint (Holm-Sidak, p < 0.05). The * stands for statistical differences between treatments (Holm-Sidak, p < 0.05). Note: Data represented only by points means that no sufficient points were available for the construction of the violin.
In the treatments of exudates addition alone to the LUFA soil, it was observed that, overall, seedlings radicles from all T. versicolor fungi exudates treatments were significantly smaller than seedlings from the CTR condition (Holm-Sidak, p ≤ 0.013; Figure 5A). This tendency was also observed in seedlings grown in treatments supplemented with P. sajor caju filtrates (Holm-Sidak, p ≤ 0.009; Figure 5I). In addition, in the soil supplemented with P. sajor caju exudates, it was observed that seedlings grown in LUFA soil supplemented with F12% were heavier than those from CTR (Holm-Sidak, p ≤ 0.009; Figure S2I) and seedlings from F1% presented higher dry weight than those from the CTR (Holm-Sidak, p ≤ 0.009; Figure S2H).
Analyzing both factors, it was observed that the addition of 12% and 6% of T. versicolor exudate to the LUFA soil moistened with seawater at 8 mS·cm−1 significantly increased seedlings dry weight and seedling radicle comparatively to T8 (Holm-Sidak, p = 0.009; Holm-Sidak, p = 0.013, respectively; Figure S2H and Figure 5D). The addition of P. sajor caju exudate to the LUFA soil greatly increased the germination rate at the highest tested salinity treatment (T26), with statistically significant differences at 72 h and 96 h (Figure 4F). For instance, at 96 h, average germination in T26 was 21%, while it was 81%, 90%, and 90% in T26 + F1%, T26 + F6%, and T26 + F12% (Holm-Sidak, p ≤ 0.01; Figure 4F), with no other relevant changes observed on other evaluated parameters (Figure 5 and Figure S2).
3.3. Soils Phosphatase Activity
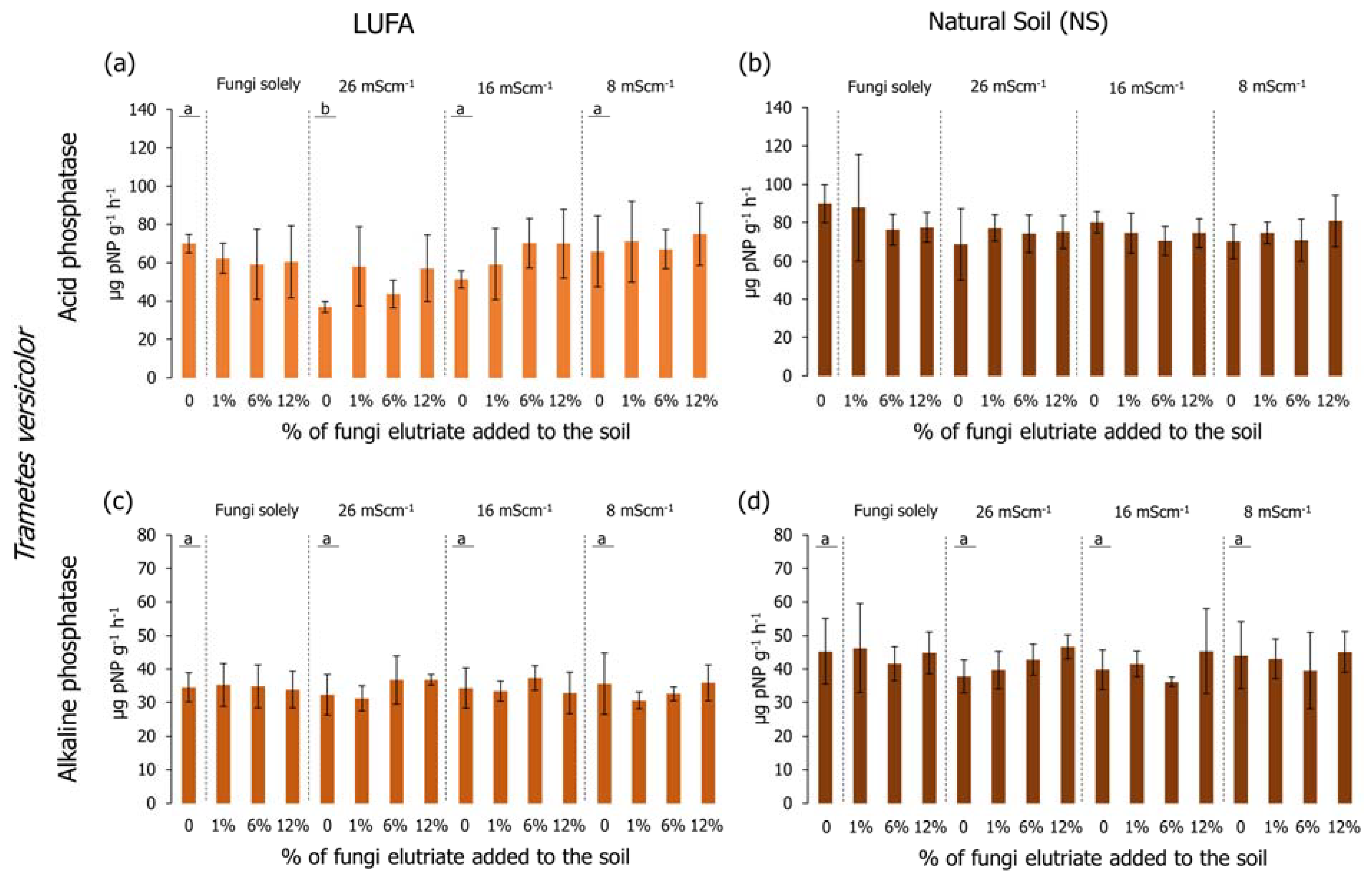
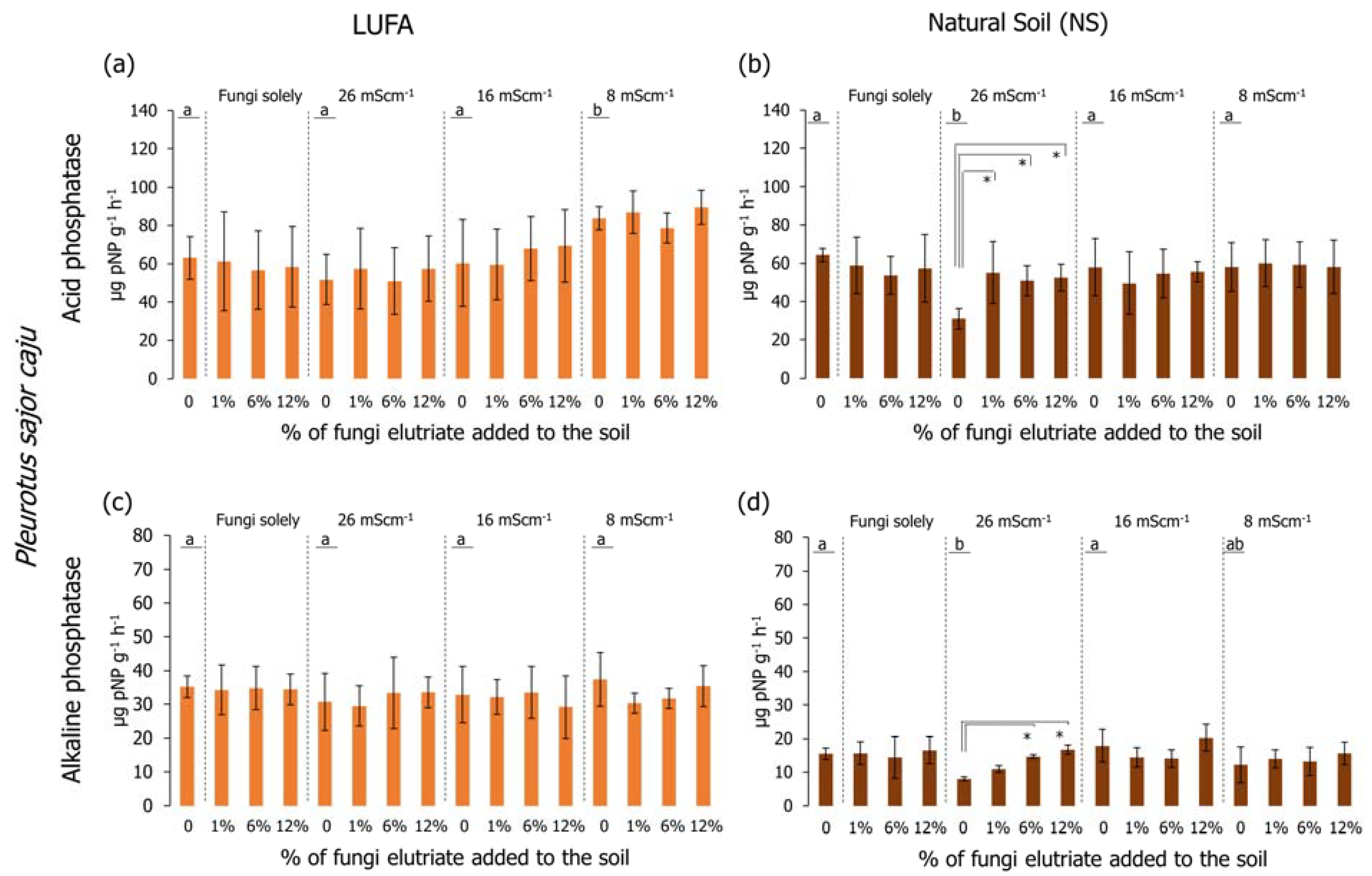
Regarding the determination of the acid phosphatase activity in LUFA soil, considering only salinized soil (no fungi added) (Figure 6a and Figure 7a), it was possible to observe that in the first assay (Figure 6a), the activity in the T26 treatment was significantly lower than the CTR and the other salinity treatments (Holm-Sidak, p < 0.05); while in the second assay (Figure 7a), despite the lower activity of the acid phosphatases in the T26 treatment, it was not significantly different from CTR or T16 (Holm-Sidak, p > 0.05). Despite this, in the second assay, the acid phosphatase activity in the T8 treatment was significantly higher than in the CTR, T26, and T16 (Figure 7a, Holm-Sidak, p < 0.05). When considering the exudate addition solely or interaction between the factors (salinity level and exudate %), no significant differences were observed in acid phosphatases in LUFA soil (Figure 6a and Figure 7a). Still, in LUFA soil and considering the activity of the alkaline phosphatases, no significant alterations were detected, either looking at the factors isolated or in combination (Figure 6c and Figure 7c). However, in the first assay, a tendency was observed for increased activity of acid phosphatases at T26 when T. versicolor exudates were added to the soil, although due to high data variability, this was not statistically relevant (Figure 6a). When assays were carried out in NS, acid phosphatase activity was not different between salinity levels in the first assay (Figure 6a), although a statistically significant decrease was detected in the second assay in T26 relative to CTR, T16, and T8 (Holm-Sidak, p < 0.05; Figure 7b). The sole addition of fungi exudates (either T. versicolor or P. sajor caju) did not influence acid phosphatase activity in NS (Figure 6b and Figure 7b). When taking into consideration both factors, the addition of T. versicolor did not influence the activity of acid phosphatases in NS (Figure 6b), although the addition of P. sajor caju exudates (at all % levels) significantly increased acid phosphatases activity relatively to T26 treatment and up to levels equivalent to control conditions (Holm-Sidak, p < 0.05; Figure 7b).
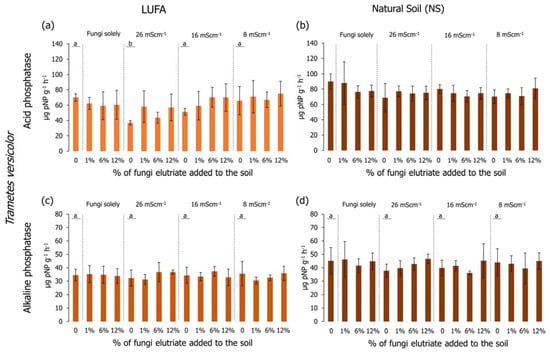
Figure 6.
Acid (graphics a,b) and alkaline (graphics c,d) phosphatase activity (pNP/g soil/h) at the end of the 96-h germination and seedling growth assay with Lens culinaris in which two different soils at three salinity levels (mS·cm−1) were supplemented with Trametes versicolor rot fungus exudates. Vertical bars represent the standard deviation. Letters indicate homogenous groups between salinity levels solely (Holm-Sidak, p < 0.05).
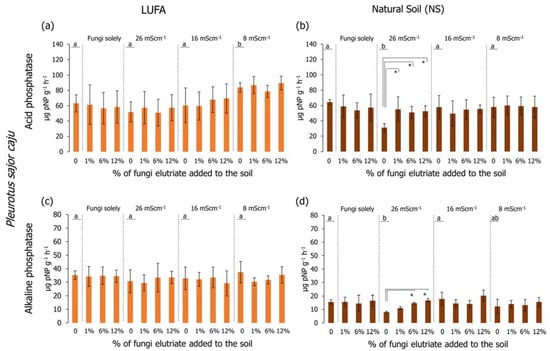
Figure 7.
Acid (graphics a,b) and alkaline (graphics c,d) phosphatase activity (pNP/g soil/h) at the end of the 96-h germination and seedling growth assay with Lens culinaris in which two different soils at three salinity levels (mS·cm−1) were supplemented with Pleurotus sajor caju rot fungus exudates. Vertical bars represent the standard deviation. Letters indicate homogenous groups between salinity levels solely (Holm-Sidak, p < 0.05). Asterisks (*) indicate significant statistical differences between treatments and the respective salinity treatments (Dunnett’s, p < 0.05).
Looking at the results of the alkaline phosphatases, no significant alterations were detected when carrying out the assays in LUFA soil using any of the rot fungi exudates (Figure 6c and Figure 7c). However, when conducting the assays in NS, considering the salinity factor solely, alkaline phosphatase activity was significantly lower in the T26 treatment compared to CTR and T16 (Figure 7d), although not different from T8. The activity of alkaline phosphatases in the first assay was higher than in the second assay (Figure 6d and Figure 7d). Relatively to the combination of both factors (salinity and fungi exudates), the addition of T. versicolor exudates did not result in any alteration in alkaline phosphatases activity, whereas when adding P. sajor caju exudates (6% and 12%) to soil salinized at 26 mS·cm−1 (T26) resulted in a significantly higher activity of the enzymes (Holm-Sidak, p < 0.05; Figure 7d).
4. Discussion
The objective of this work was to understand if the addition of exudates produced by rot fungi could improve the germination and seedling growth of a legume species (Lens culinaris) in soils salinized at three different degrees (slightly salinized—8 mS·cm−1, moderately salinized—16 mS·cm−1, and highly salinized—26 mS·cm−1). To date and to our knowledge, the opportunity offered by rot fungi to protect climate-change-affected soils due to their potential high tolerance to abiotic stress is still poorly addressed and understood [14], for which this work aims at providing the first results.
First, we analyzed if the application of the fungi filtrates themselves could induce any effect on L. culinaris germination and seedling development. A significant delay in the first 24 h in the assay with T. versicolor applied at 12% was recorded compared to the control (CTR), although from this observation point onwards, this difference disappeared, and germination reached over 80% in the F12% treatment at the end of the assay. Similarly, a significant difference was observed in the radicle length of lentils cultivated in soil moistened with P. sajor caju at F6%, compared to the CTR. However, in the general panorama, among the several plant parameters analyzed, these were the only significant changes observed and may be related to some degree of competition between the plant and some portion of the fungus mycelium that went with the filtrate, for example, for water. Fungi are extremely hygroscopic, meaning they quickly and freely absorb and exchange water molecules with surrounding soil particles, e.g., [38,39]; on the other hand, the presence of water is crucial in imbibition, identified as the first stage of water absorption by the seed for its germination and related to later seedling growth [40]. Moreover, despite not being significant, it is important to highlight, for instance, that in assays covering the addition of T. versicolor exudates, there was a tendency for improvement in the weight and length parameters of L. culinaris. This last observation may be related to the supplementation of polysaccharides upon the addition of the exudates to the soils since both fungi species are known to actively excrete to the surroundings these types of compounds (for instance, glucose, mannose, or galactose [41,42]), which may be then taken up by the plant.
The effects of salinity levels alone were also investigated. As expected, the effects on germination and seedling development of L. culinaris followed an expected dose-response relationship. Twenty-four hours after the start of the trials, germination rates under CTR conditions were already close to or greater than 50%, while germination speed in the higher salinity treatment (26 mS·cm−1) was severely and significantly delayed (less than 20% of the seeds germinated in natural soil, although this was not observed in LUFA soil). In the other two salinity treatments (8 and 16 mS·cm−1), despite an initial delay (in the first 24 h to 48 h), they emerged quickly and simultaneously, reaching germination rates approximately to those of the natural soil CTR treatment until the end of the trial. As also expected, delays in germination had repercussions later, on seedling growth (length and weight), with the two treatments with higher salinity almost always creating a different homogeneous group from the CTR. These results agree with the available literature on the effects of salinity on the germination and growth of other plant species, namely from the Fabaceae group. For example, Singh et al. [43], when studying 162 wild lentil genotypes (Lens sp.), verified that increasing soil conductivity from ~4.6 to 14 mS·cm−1 (originally 40 to 120 mM NaCl) led to a suppression of lentils germination rate up to 60–70%. Likewise, Das and Islam [44], also using five different varieties of L. culinaris, verified that 150 mM of NaCl (~17.3 mS·cm−1) completely inhibited the germination of two out of the five studied varieties, while at 100 mM NaCl (~11.6 mS·cm−1) germination rates were below 40% in all varieties. The reduction in seedling germination and growth rates happens because increasing the salinity of the surrounding soil leads to the plant having less availability and access to water present in the soil [45]. The uptake of water by the plant takes place through a process of osmosis (therefore, in a normal situation, the water would move in favor of the water potential, i.e., into the plant, where a higher concentration of solutes would be found). If there are more salts outside, the plant not only cannot absorb water, but it can even lose it if the levels of external salinity are too high [45].
Plants have developed an array of features/mechanisms (from the cellular to the tissular level) that allow them to cope with salinity, for instance, diminishing ion uptake by reducing the membranes transporters with an affinity for sodium, vacuolization of the excessive number of ions, production of reactive oxygen species, or even hormonal regulation, e.g., reviewed by [46,47]. Despite that, when beyond their optimal salinity threshold (species-specific), those coping mechanisms might no longer be suitable and, therefore, decrease the plants/crop productivity substantially, even putting at risk its viability [48]. Considering what was previously analyzed, soil salinization can bring serious drawbacks to plants/crops productivity, and such a problem has fueled research in the development of environmentally friendly techniques aiming at maintaining or increasing crop productivity under salinity stress. In the present study, this outlook was pursued by supplementing salinized soils with two rot fungi exudates due to their primary function in soils’ nutrient recycling, availability, and facilitation to other edaphic organisms similar to plants e.g., [49]. Under salinized soils, the addition of fungi exudates improved some features of L. culinaris. To highlight that germination in a natural soil salinized at 26 mS·cm−1 was approximately 10%, while in a natural soil at the same salinity level but supplemented with T. versicolor exudate, germination rates increased by approximately 30%. As well, in seedlings’ fresh and dry weight (in the same conditions, natural soil salinized at 26 mS·cm−1 and supplemented with T. versicolor), there was a tendency for a higher expression of the weight parameters relative to salinized natural soil (26 mS·cm−1), although not statistically significant due to the low number of points in the dataset. The same trend in germination was observed at the salinity level of 26 mS·cm−1 when natural soil was moistened with P. sajor caju, whereas at the salinity of 16 mS·cm−1, a tendency was recorded for improvement of seedlings’ fresh and dry weight (although only significantly different at the 12% fungi exudate P. sajor caju). Such results corroborate the beneficial role of rot fungi in plants exposed to salinity, most probably at the expense of exopolymer production. As evidenced by Venâncio et al. [20], under salinity stress, these fungi invest in the production of these polymers as they may act as osmoprotectors, providing a shield against water loss and, thus, dehydration. By producing these osmolytes, which are released at random to the vicinity, they may reach the seed (later the root of the seedling) and, in the same way, act as a protective layer during seed germination, which is a critical developmental stage that is highly water-dependent [42]. The differential protective role of fungi at different salinity levels may be further related to exudate elemental composition. For instance, T. versicolor exudates are known to be rich mostly in sugars, with mannose and glucose as major components, followed by galactose. For P. sajor caju, exudates, though sharing a very similar composition with those of T. versicolor, have different sugar proportions, with fucose and galactose as keys exopolysaccharides, followed by glucose and mannose [41,42]. While mannitol (derived from mannose) is related to increased tolerance to abiotic stress [50], galactose plays a major role in signaling pathways by intervening in Ca2+ concentrations in the cytosol [50].
At the lowest salinity level, the trend was to worsen the performance of seed germination and seedling growth in natural soil moistened with fungi exudates compared to treatment with the same salinity level alone. Such a result in lower conductivities may be explained by the composition of seawater itself. The results of Venâncio et al. [5] with four forage plant species showed that SW induced less toxicity in germination and growth compared to the surrogate salt (NaCl); moreover, L. perenne soil moistened only with SW (at a conductivity of 52 mS·cm−1) did not induce any effect, improving plants’ growth in about 30%. Considering the higher germination rates, for instance, in the salinity treatment alone of 8 mS·cm−1 comparatively to the same salinity treatments plus fungi exudates, the SW composition must be considered. Seawater has a complex composition in which many ions, probably present in concentrations below their threshold of toxicity for these plants, play fundamental roles in cellular functions. Let us consider the role of calcium (Ca2+) as an example. It is one of the major molecules in signaling cascades playing a major role in membrane integrity and ionic regulation/transport and, particularly in plants, regulates stomatal aperture, thus also helping the plants to prevent further water loss under salt stress, e.g., [51,52]. Moreover, Ca2+ is involved in the activation of a particular group of genes—Salt Overly Sensitive (SOS)—which mediate cell signaling under salt stress [53]. Therefore, this small input of ions could have been used as additives by the plants, explaining the results obtained and in accordance with what is reported in the literature, e.g., [53,54]. When adding the fungi, the latter may have outcompeted the plants for the same resources to grow. This may explain the lower germination rates, for instance, in T8 + F% treatments comparatively to the salinity treatment alone (8 mS·cm−1).
Differences between soils must be further discussed as well. In their majority, improvements in the evaluated endpoints were in natural soil, which was slightly more acidic and with higher organic matter content than LUFA but with very similar values to LUFA in the other parameters (please see Supplementary Table S1). Since fungi use soil organic matter as a substrate, this may be the main driver for the results obtained [55], but it should also be noted that small increments in organic matter percentage may improve soil cation exchange capacity and immobilize excessive deleterious ions, e.g., [56].
The results obtained, particularly at 26 mS·cm−1, regarding the germination rates are still very important achievements since the germination phase is a critical stage for the establishment of seedlings and, therefore, influencing the growth and productivity of the crop/plant, i.e., if the seed is not able to first germinate, then consequently the growth of the seedlings and plants will be suppressed. In the present study, germination hindering at the highest level of salinity appeared to be relieved or facilitated by the presence of the fungal exudates, a result that might be explained by two hypotheses. The first hypothesis to explore is the potential ability of fungal hyphae to bind and accumulate from the soil, intracellularly, ions present in concentrations that would be otherwise toxic to plants, limiting ions availability, e.g., [57,58]. The second hypothesis is related to the presence of salts in the soil that may have stimulated the fungi’s enzymatic production, as when stressed, they tend to overcompensate through the release of extracellular enzymes, which help catalyze the breakdown of recalcitrant compounds to promote the release of simple sugars, necessary for their maintenance and growth, as already evidenced by Venâncio et al. [20] through FT-IR analyses to the mycelia composition of four white-rot fungi pre-exposed to low levels of salinity. Although when doing this, it is possible that they also release nutrients to the surroundings, thus helping the plants to obtain nutrients that they need to cope with and thrive under a salinized environment. For instance, in a study by Yin et al. [15] in which the objective was to assess the potential of the white-rot fungi Ceriporia lacerata as a soil probiotic, specifically in the improvement of biological nitrogen fixation by Vicia faba, the authors verified that the fungus released to not only the culture solution phytohormones (such as gibberellins and brassinosteroids) but also iron-binding ligands that promoted the mobilization of insoluble iron (into soluble forms, readily available to the plant; [15]). In the same line of evidence, Sui et al. [16], using the same white-rot fungi species (C. lacerata, strain HG2011), verified that its presence greatly improved the mobilization of phosphorous (P) on soil (often the most limiting factor in plants/crops growth and productivity). As a result, not only soil phosphatase activity was improved, but crop P uptake and overall yield increased [16], thus reducing the quantity of chemical fertilizer applied without later implications on crop productivity [16]. In the present study and in line with that reported by Sui et al. [16], it is also important to highlight that in natural soil, the addition of P. sajor caju exudates to soils salinized at 26 mS·cm−1 significantly improved acid phosphatase activity. Despite the assays short-term duration, the screening of different levels of both factors (salinity and fungi exudates), as well as the exploration of different fungi species and soils in these assays, allowed for the perception of future directions in which research may be channeled for the delivery and screening of other markers related to the stress imposed on the plant. Examples may include malondialdehyde, the assessment of the activity of antioxidant enzymes (superoxide dismutase, catalase, and peroxidase), and the analysis of the extracellular polymeric substances produced by the white rot fungi under salinity stress. Nonetheless, it should be noted that information on this field of research is still very scarce, and this is thus a first step in discriminating salinity values to be remediated, potential target fungi/crop species, and parameters to be consider.
5. Conclusions
Rot fungi can play a role in the process of recovery of salt-affected soils. Due to their high salinity tolerance, they may thrive in these environments producing and releasing nutrients to the soil, which may be then used by plants in the vicinity to subsist.
In this, the approach of supplying the soil with exudates from white-rot fungi was proven to be a feasible measure to at least improve crop germination in slightly and moderately saline soils (herein tested conductivities of 8 and 16 mS·cm−1). In some cases (e.g., addition of P. sajor caju exudates in LUFA soil), boosting germination up to 60%. This is the first step to understanding what level of salinity these fungi may assist in the recovery of salinized soils. In addition, some other positive outcomes in other tested endpoints were also observed. For instance, the seed weight and length were improved when soil moistened at 8 mS·cm−1 was supplemented with 6% and 12% of T. versicolor exudates.
Exploring the potential of rot fungi can thus be of added value and needs to be explored quickly and thoroughly since this is a promising and sustainable technique for future applications. In the face of the lack of literature on the role of rot fungi in salt-affected soils, it should be noted that more research is still needed to determine its applications on other potential and important crops. Considering the above, the potential of applying rot fungi as soil probiotics to aid crop productivity under salinized environmentsshould be recognized as a win-win approach.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture13020382/s1, Table S1: Characteristics of the soil used in the germination and seedling growth assays with Lens culinaris. Presented as average values. Corg—organic carbon content; N—nitrogen content; OM—organic matter content (%); WHCmax—maximum water holding capacity. Table S2: Physicochemical parameters (conductivity—mS·cm−1 and pH) of the exudates obtained from the liquid culture of the two white rot fungi (Trametes versicolor and Pleurotus sajor caju) mixed with the seawater solutions that were later applied to the soils in the germination and seedling growth assay with Lens culinaris. Table S3: Physicochemical parameters (conductivity—mScm−1 and pH) of three random soil samples (average ± standard deviation) collected at the end of the germination and seedling growth assay with Lens culinaris. CTR—control; T—salinity treatment; F—fungi elutriate %. Figure S1. Violin plots of the seedling weight of Lens culinaris germinated in natural soil salinized at three different levels (8, 16, and 26 mS·cm−1, designated as T8, T16, and T26, respectively) and supplemented with fungi exudates at three levels (1%, 6, and 12%, designated as F1%, F6%, and F12%, respectively). CTR stands for control soil. Letters (a, b, c, d) stand for homogenous groups assessed only between CTR and the salinity treatments within each observation point for the germination endpoint (24, 48, 72, or 96 h) and for 96-h with respect to the remaining endpoints (Holm-Sidak, p < 0.05). The * stands for statistical differences between treatments (Holm-Sidak, p < 0.05). Note: Data represented only by points means that no sufficient points were available for the construction of the violin. Figure S2. Violin plots of the seedling weight of Lens culinaris germinated in LUFA 2.2 soil salinized at three different levels (8, 16, and 26 mS·cm−1, designated as T8, T16, and T26, respectively) and supplemented with fungi exudates at three levels (1%, 6, and 12%, designated as F1%, F6%, and F12%, respectively). CTR stands for control soil. Letters (a, b, c, d) stand for homogenous groups assessed only between CTR and the salinity treatments within each observation point for the germination endpoint (24, 48, 72, or 96 h) and for 96-h with respect to the remaining endpoints (Holm-Sidak, p < 0.05). The * stands for statistical differences between treatments (Holm-Sidak, p < 0.05). Note: Data represented only by points means that no sufficient points were available for the construction of the violin.
Author Contributions
Conceptualization, I.L. and C.V.; methodology, J.B., P.C. and C.V.; validation, J.B., P.C., I.L, E.F. and C.V.; formal analysis, J.B., P.C., I.L, E.F. and C.V.; investigation, J.B., P.C. and C.V.; writing—original draft preparation, J.B. and C.V.; writing—review and editing, J.B., P.C., I.L., E.F. and C.V.; visualization, J.B., I.L. and C.V.; supervision, I.L. and C.V.; funding acquisition, I.L. and C.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work acknowledges the financial support to CESAM by FCT/MCTES (UIDP/50017/2020+UIDB/50017/2020+LA/P/0094/2020), and the projects SALTFREE (PTDC/AAC-CLI/111706/2009) and SALTFREE II (POCI-01–0145-FEDER-031022) and to LabEx DRIIHM—Dispositif de Recherche Interdisciplinaire sur les Interactions Hommes-Milieux and OHMI—Observatoire Hommes-Millieux International Estarreja for funding the project “CLEAR-Resorting to microbial Consortia to restore metal contaminated soils for the area of EstArReja”.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Raw data are available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Daliakopoulos, I.N.; Tsanis, I.K.; Koutroulis, A.; Kourgialas, N.N.; Varouchakis, A.E.; Karatzas, G.P.; Ritsema, C.J. The threat of soil salinity: A European scale review. Sci. Total Environ. 2016, 573, 727–739. [Google Scholar] [CrossRef]
- Sahab, S.; Suhani, I.; Srivastava, V.; Chauhan, P.S.; Singh, R.P.; Prasad, V. Potential risk assessment of soil salinity to agroecosystem sustainability: Current status and management strategies. Sci. Total Environ. 2021, 764, 144164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.W.; Chong, W.A.N.G.; Rui, X.U.E.; Wang, L.J. Effects of salinity on the soil microbial community and soil fertility. J. Integr. Agric. 2019, 18, 1360–1368. [Google Scholar] [CrossRef]
- Rath, K.M.; Murphy, D.N.; Rousk, J. The microbial community size, structure, and process rates along natural gradients of soil salinity. Soil Biol. Biochem. 2019, 138, 107607. [Google Scholar] [CrossRef]
- Venâncio, C.; Pereira, R.; Lopes, I. The influence of salinization on seed germination and plant growth under mono and polyculture. Environ. Pollut. 2020, 260, 113993. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.D.; Elliott, E.T.; Paustian, K.; Peterson, G.A. Fungal translocation as a mechanism for soil nitrogen inputs to surface residue decomposition in a no-tillage agroecosystem. Soil Biol. Biochem. 2000, 32, 689–698. [Google Scholar] [CrossRef]
- Lindahl, B.; Finlay, R.; Olsson, S. Simultaneous, bidirectional translocation of 32P and 33P between wood blocks connected by mycelial cords of Hypholoma fasciculare. New Phytol. 2001, 150, 189–194. [Google Scholar] [CrossRef]
- Frey, S.D.; Six, J.; Elliott, E.T. Reciprocal transfer of carbon and nitrogen by decomposer fungi at the soil–litter interface. Soil Biol. Biochem. 2003, 35, 1001–1004. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-tolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef]
- Evelin, H.; Devi, T.S.; Gupta, S.; Kapoor, R. Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: Current understanding and new challenges. Front. Plant Sci. 2019, 10, 470. [Google Scholar] [CrossRef]
- Basiru, S.; Mwanza, H.P.; Hijri, M. Analysis of arbuscular mycorrhizal fungal inoculant benchmarks. Microorganisms 2021, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Cardoso, P.; Freitas, R.; Figueira, E. Salt tolerance of rhizobial populations from contrasting environmental conditions: Understanding the implications of climate change. Ecotoxicology 2015, 24, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.; Cardoso, P.; Figueira, E.; Lopes, I.; Venâncio, C. Forward-looking on new microbial consortia: Combination of rot fungi and rhizobacteria on plant growth-promoting abilities. Appl. Soil Ecol. 2023, 182, 104689. [Google Scholar] [CrossRef]
- Yin, J.; Sui, Z.; Li, Y.; Yang, H.; Yuan, L.; Huang, J. A new function of white-rot fungi Ceriporia lacerata HG2011: Improvement of biological nitrogen fixation of broad bean (Vicia faba). Microbiol. Res. 2022, 256, 126939. [Google Scholar] [CrossRef]
- Sui, Z.; Yin, J.; Huang, J.; Yuan, L. Phosphorus mobilization and improvement of crop agronomic performances by a new white-rot fungus Ceriporia lacerata HG2011. J. Sci. Food Agric. 2021, 102, 1640–1650. [Google Scholar] [CrossRef]
- Gao, D.; Du, L.; Yang, J.; Wu, W.M.; Liang, H. A critical review of the application of white rot fungus to environmental pollution control. Crit. Rev. Biotechnol. 2010, 30, 70–77. [Google Scholar] [CrossRef]
- de Vries, F.T.; Caruso, T. Eating from the same plate? Revisiting the role of labile carbon inputs in the soil food web. Soil Biol. Biochem. 2016, 102, 4–9. [Google Scholar] [CrossRef]
- Venâncio, C.; Ribeiro, R.; Lopes, I. Seawater intrusion: An appraisal of taxa at most risk and safe salinity levels. Biol. Rev. 2021, 97, 361–382. [Google Scholar] [CrossRef]
- Venâncio, C.; Pereira, R.; Freitas, A.C.; Rocha-Santos, T.A.P.; da Costa, J.P.; Duarte, A.C.; Lopes, I. Salinity induced effects on the growth rates and mycelia composition of basidiomycete and zygomycete fungi. Environ. Pollut. 2017, 231, 1633–1641. [Google Scholar] [CrossRef]
- Salvachúa, D.; Martínez, A.T.; Tien, M.; López-Lucendo, M.F.; García, F.; De Los Ríos, V.; Martínez, M.J.; Prieto, A. Differential proteomic analysis of the secretome of Irpex lacteus and other white-rot fungi during wheat straw pretreatment. Biotechnol. Biofuels 2013, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Margalef, O.; Sardans, J.; Maspons, J.; Molowny-Horas, R.; Fernández-Martínez, M.; Janssens, I.A.; Richter, A.; Ciais, P.; Obersteiner, M.; Peñuelas, J. The effect of global change on soil phosphatase activity. Glob. Change Biol. 2021, 27, 5989–6003. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Yang, J.; Gao, S.; Yao, R.; Wang, X. The Effect and Influence Mechanism of Soil Salinity on Phosphorus Availability in Coastal Salt-Affected Soils. Water 2022, 14, 2804. [Google Scholar] [CrossRef]
- Faris, M.E.A.I.E.; Takruri, H.R.; Issa, A.Y. Role of lentils (Lens culinaris L.) in human health and nutrition: A review. Mediterr. J. Nutr. Metab. 2013, 6, 3–16. [Google Scholar] [CrossRef]
- Aslani, Z.; Alipour, B.; Mirmiran, P.; Bahadoran, Z. Lentil’s (Lens culinaris L.) functional properties in prevention and treatment of non-communicable chronic diseases: A review. Int. J. Nutr. Food Sci. 2015, 4, 15–20. [Google Scholar] [CrossRef]
- Mo’ez Al-Islam, E.F.; Mohammad, M.G.; Soliman, S. Lentils (Lens culinaris L.): A candidate chemopreventive and antitumor functional food. Funct. Foods Cancer Prev. Ther. 2020, 99–120. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Wang, X.; Fu, S. Legume-soil interactions: Legume addition enhances the complexity of the soil food web. Plant Soil 2014, 385, 273–286. [Google Scholar] [CrossRef]
- Louarn, J.; Carbonne, F.; Delavault, P.; Becard, G.; Rochange, S. Reduced germination of Orobanche cumana seeds in the presence of arbuscular mycorrhizal fungi or their exudates. PLoS ONE 2012, 7, e49273. [Google Scholar] [CrossRef]
- Jurburg, S.D.; Natal-da-Luz, T.; Raimundo, J.; Morais, P.V.; Sousa, J.P.; Van Elsas, J.D.; Salles, J.F. Bacterial communities in soil become sensitive to drought under intensive grazing. Sci. Total Environ. 2018, 618, 1638–1646. [Google Scholar] [CrossRef]
- Zaman, M.; Shahid, S.A.; Heng, L. Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer Nature: Berlin/Heidelberg, Germany, 2018; p. 164. [Google Scholar]
- Xu, P.; Lai, C.; Zeng, G.; Huang, D.; Chen, M.; Song, B.; Peng, X.; Wan, J.; Hu, L.; Duan, A.; et al. Enhanced bioremediation of 4-nonylphenol and cadmium co-contaminated sediment by composting with Phanerochaete chrysosporium inocula. Bioresour. Technol. 2018, 250, 625–634. [Google Scholar] [CrossRef]
- Balestri, E.; Menicagli, V.; Ligorini, V.; Fulignati, S.; Galletti, A.M.R.; Lardicci, C. Phytotoxicity assessment of conventional and biodegradable plastic bags using seed germination test. Ecol. Indic. 2019, 102, 569–580. [Google Scholar] [CrossRef]
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D. Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects. Waste Manag. 2018, 71, 109–114. [Google Scholar] [CrossRef] [PubMed]
- ISO 17512-1; International Organization for Standardization. Soil Quality: Avoidance Test for Testing the Quality of Soils and the Toxicity of Chemicals- Test with Earthworms (Eisenia fetida). International Organization for Standard: Geneva, Switzerland, 2008.
- FAOUN. Food and agriculture organization of the United Nations e physical and chemical methods of soil and water analysis. Soils Bull. 1984, 10, 1e275. [Google Scholar]
- Eivazi, F.; Tabatabai, M.A. Phosphatases in soils. Soil Biol. Biochem. 1977, 9, 167–172. [Google Scholar] [CrossRef]
- Le Bayon, R.C.; Weisskopf, L.; Martinoia, E.; Jansa, J.; Frossard, E.; Keller, F.; Föllmi, K.B.; Gobat, J.M. Soil phosphorus uptake by continuously cropped Lupinus albus: A new microcosm design. Plant Soil 2006, 283, 309–321. [Google Scholar] [CrossRef]
- Thybring, E.E. Water relations in untreated and modified wood under brown-rot and white-rot decay. Int. Biodeterior. Biodegrad. 2017, 118, 134–142. [Google Scholar] [CrossRef]
- Ringman, R.; Beck, G.; Pilgård, A. The importance of moisture for brown rot degradation of modified wood: A critical discussion. Forests 2019, 10, 522. [Google Scholar] [CrossRef]
- Vertucci, C.W. The kinetics of seed imbibition: Controlling factors and relevance to seedling vigor. Seed Moisture 1989, 14, 93–115. [Google Scholar]
- Gangola, M.P.; Ramadoss, B.R. Sugars play a critical role in abiotic stress tolerance in plants. In Biochemical, Physiological and Molecular Avenues for Combating Abiotic Stress Tolerance in Plants; Academic Press: Cambridge, MA, USA, 2018; pp. 17–38. [Google Scholar]
- Afzal, S.; Chaudhary, N.; Singh, N.K. Role of soluble sugars in metabolism and sensing under abiotic stress. In Plant Growth Regulators; Springer: Cham, Switzerland, 2021; pp. 305–334. [Google Scholar]
- Singh, D.; Singh, C.K.; Kumari, S.; Singh Tomar, R.S.; Karwa, S.; Singh, R.; Singh, R.B.; Sarkar, S.K.; Pal, M. Discerning morpho-anatomical, physiological and molecular multiformity in cultivated and wild genotypes of lentil with reconciliation to salinity stress. PLoS ONE 2017, 12, e0177465. [Google Scholar] [CrossRef]
- Das, S.K.; Islam, A.R. Effects of salinity on germination and seedling growth of lentil (Lens culinaris MEDIK) varieties in Bangladesh. Barishal Univ. J. Part 1 2018, 5, 141–151. [Google Scholar]
- Gavrilescu, M. Water, soil, and plants interactions in a threatened environment. Water 2021, 13, 2746. [Google Scholar] [CrossRef]
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651. [Google Scholar] [CrossRef]
- Krishna, M.P.; Mohan, M. Litter decomposition in forest ecosystems: A review. Energy Ecol. Environ. 2017, 2, 236–249. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in plants under abiotic stresses: New insights into a classical phenomenon. Planta 2020, 251, 1–17. [Google Scholar] [CrossRef]
- Courchesne, W.E.; Vlasek, C.; Klukovich, R.; Coffee, S. Ethanol induces calcium influx via the Cch1-Mid1 transporter in Saccharomyces cerevisiae. Arch. Microbiol. 2011, 193, 323–334. [Google Scholar] [CrossRef]
- Tuteja, N.; Mahajan, S. Calcium signaling network in plants: An overview. Plant Signal. Behav. 2007, 2, 79–85. [Google Scholar] [CrossRef]
- Mahajan, S.; Pandey, G.K.; Tuteja, N. Calcium-and salt-stress signaling in plants: Shedding light on SOS pathway. Arch. Biochem. Biophys. 2008, 471, 146–158. [Google Scholar] [CrossRef]
- Xu, D.; Wang, W.; Gao, T.; Fang, X.; Gao, X.; Li, J.; Bu, H.; Mu, J. Calcium alleviates decreases in photosynthesis under salt stress by enhancing antioxidant metabolism and adjusting solute accumulation in Calligonum mongolicum. Conserv. Physiol. 2017, 5, cox060. [Google Scholar] [CrossRef]
- Roy, P.R.; Tahjib-Ul-Arif, M.; Polash, M.A.S.; Hossen, M.Z.; Hossain, M.A. Physiological mechanisms of exogenous calcium on alleviating salinity-induced stress in rice (Oryza sativa L.). Physiol. Mol. Biol. Plants 2019, 25, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Nichols, K.A.; Wright, S.F. Contributions of Fungi to Soil Organic Matter in Agroecosystems. Soil Organic Matter in Sustainable Agriculture; CRC Press: Boca Raton, FL, USA, 2004; pp. 179–198. [Google Scholar]
- Lwin, C.S.; Seo, B.H.; Kim, H.U.; Owens, G.; Kim, K.R. Application of soil amendments to contaminated soils for heavy metal immobilization and improved soil quality—A critical review. Soil Sci. Plant Nutr. 2018, 64, 156–167. [Google Scholar] [CrossRef]
- Kasotia, A.; Varma, A.; Tuteja, N.; Choudhary, D.K. Amelioration of soybean plant from saline-induced condition by exopolysaccharide producing Pseudomonas-mediated expression of high affinity K+-transporter (HKT1) gene. Curr. Sci. 2016, 111, 1961–1967. [Google Scholar] [CrossRef]
- Scharnagl, K.; Scharnagl, A.; von Wettberg, E. Nature’s potato chip: The role of salty fungi in a changing world. Am. J. Bot. 2017, 104, 641–644. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).