Borated Fertilizations via Foliar and Soil for Peanut Production during the Sugarcane Reform
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Characterization
2.2. Plant Measurements
2.3. B Leaching Study
2.4. Data Processing and Statistical Analysis
3. Results
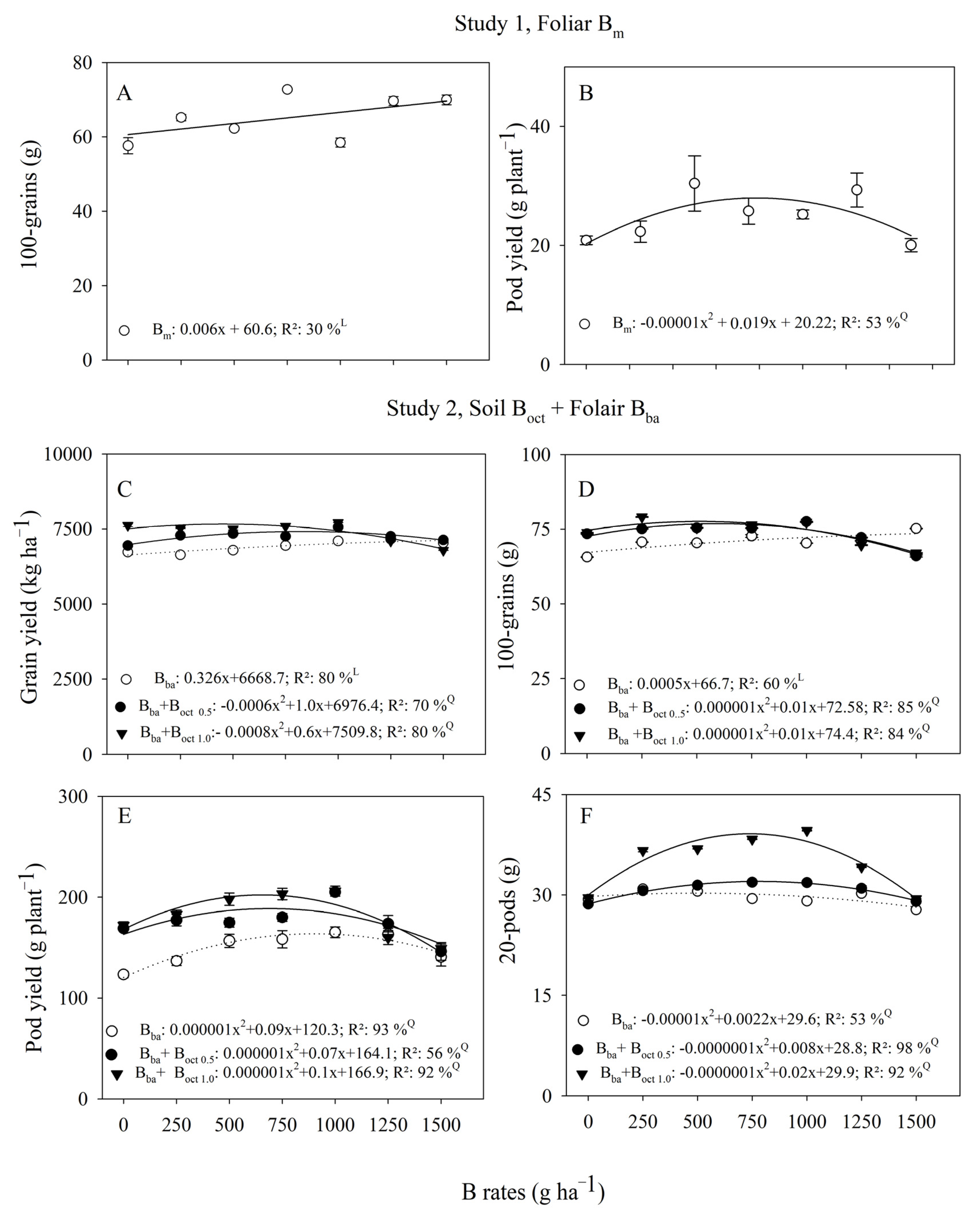
3.1. Peanut Yield
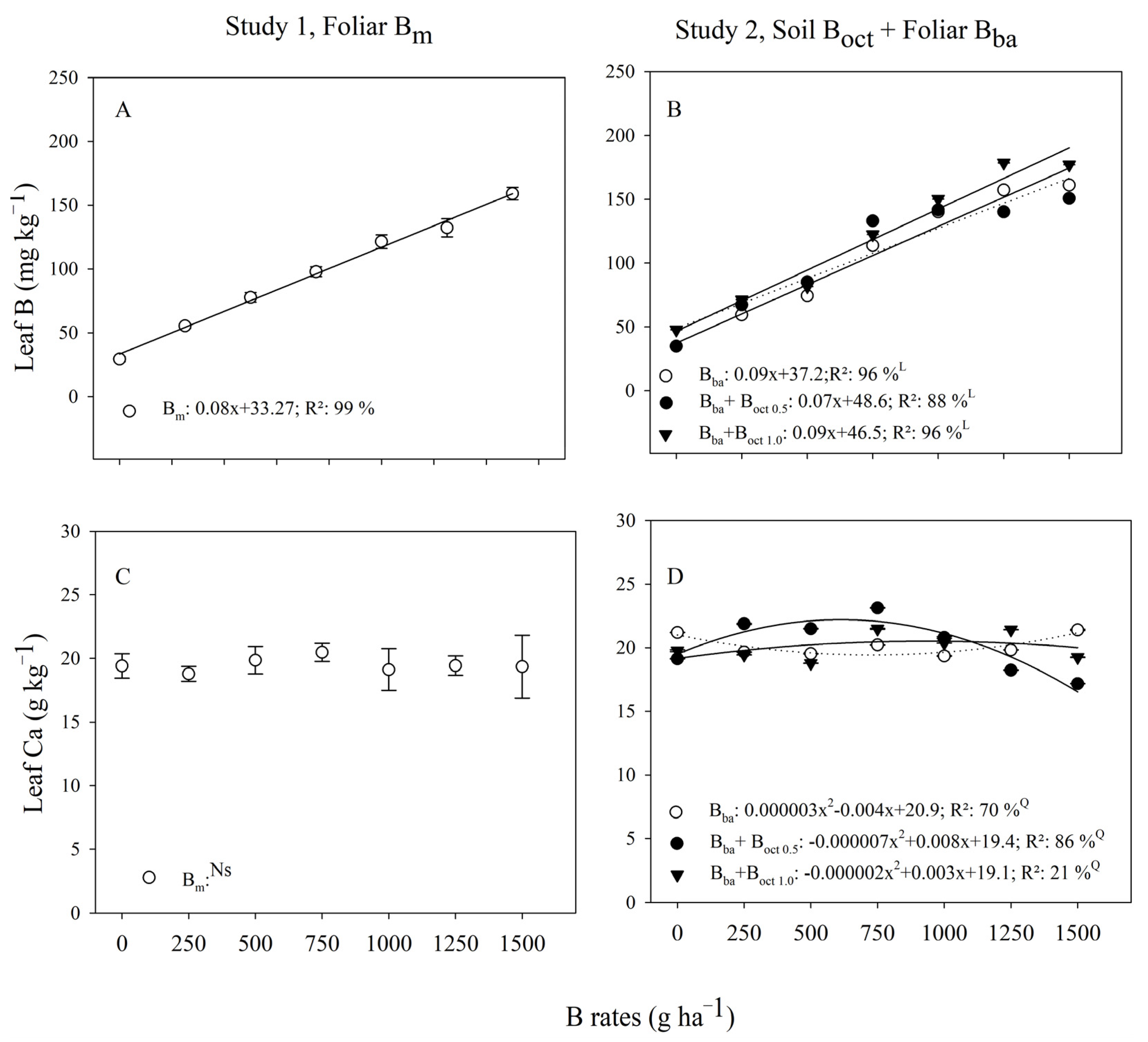
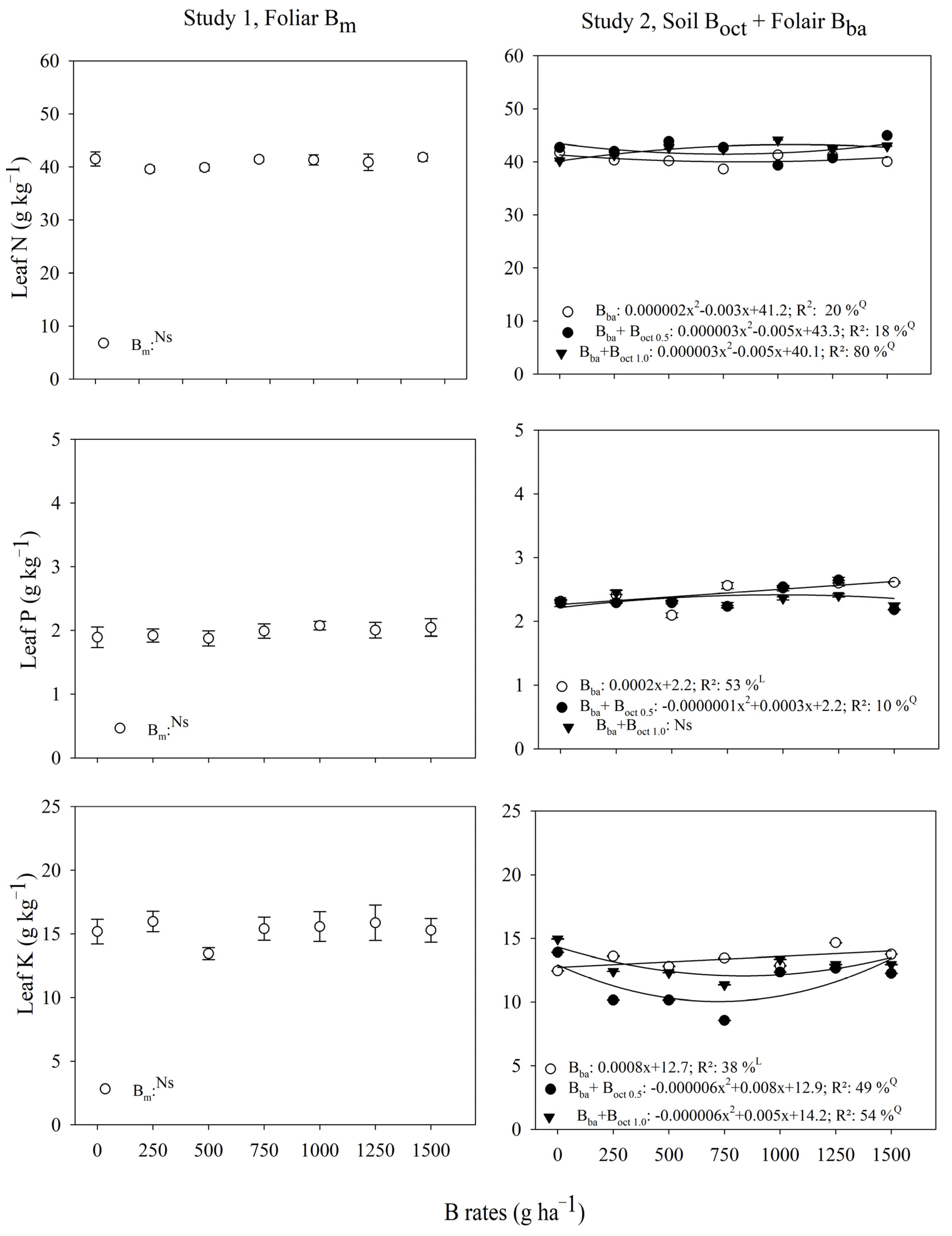
3.2. Leaf-Nutrients
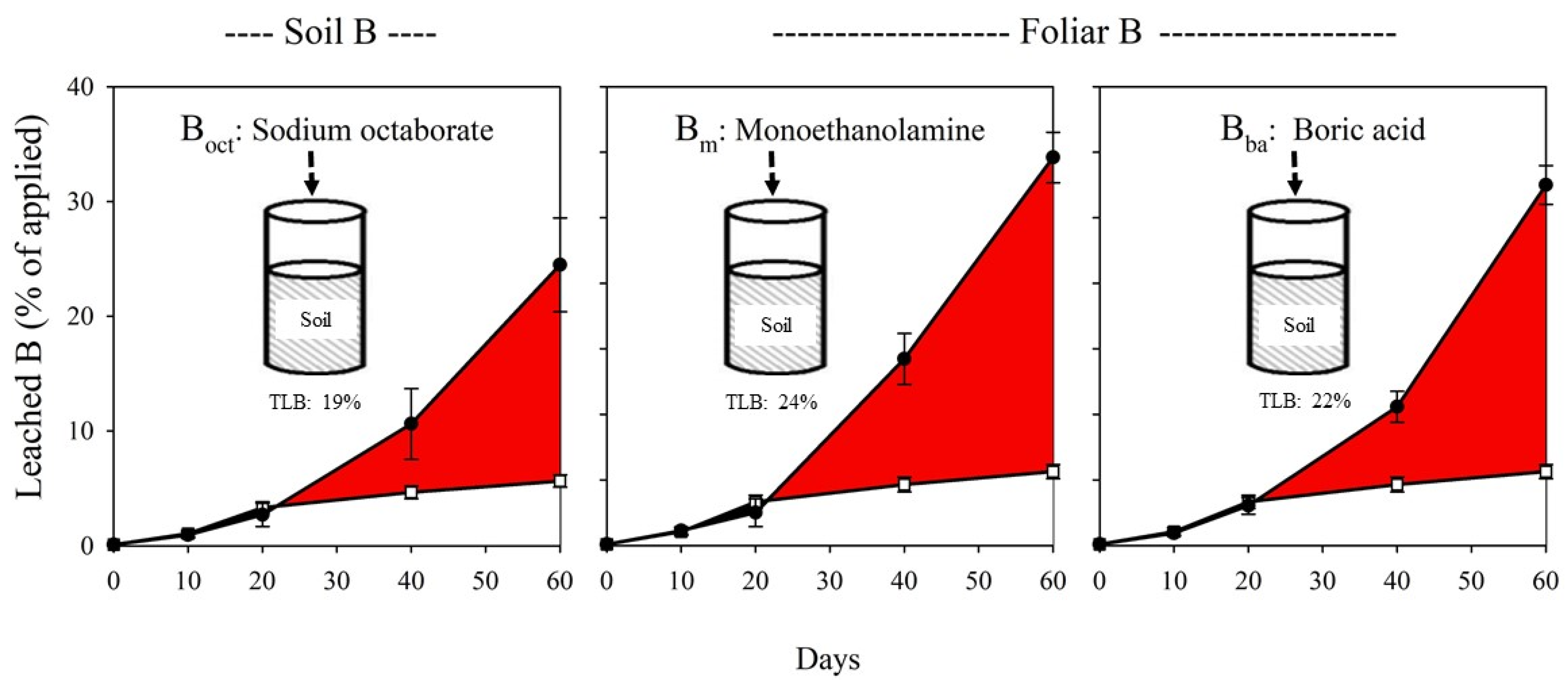
3.3. Leached B
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hauser, A. Peanuts. J. Agric. Food Inf. 2018, 19, 195–202. [Google Scholar] [CrossRef]
- CONAB. Conab—Boletim da Safra de Grãos, 8o Levantamento—Safra 2019/20. Available online: https://www.conab.gov.br/info-agro/safras/graos/boletim-da-safra-de-graos (accessed on 24 June 2020).
- EMBRAPA. Study Maps Peanut Production Areas in Brazil to Prevent Peanut Smut. Available online: https://www.embrapa.br/en/busca-de-noticias/-/noticia/40697528/estudo-mapeia-areas-de-producao-de-amendoim-do-brasil-para-prevenir-doenca-do-carvao (accessed on 20 January 2021).
- Ferraz-Almeida, R.; da Mota, R.P. Routes of soil use and conversions with the main crops in Brazilian Cerrado: A scenario from 2000 to 2020. Land 2021, 10, 1135. [Google Scholar] [CrossRef]
- Salata, B.L.; Ferraz-Almeida, R. Perfil da produção agrícola na região de São Carlos, SP: Um balanço dos últimos 12 anos. Rev. Acta Ambient. Catarin. 2021, 18, 55. [Google Scholar]
- Zhang, X.; Chen, P.; Du, Q.; Zhou, Y.; Ren, J.; Jin, F.; Yang, W.; Yong, T. Effects of maize/soybean and maize/peanut intercropping systems on crops nitrogen uptake and nodulation nitrogen fixation. Chin. J. Eco-Agric. 2019, 27, 1183–1194. [Google Scholar] [CrossRef]
- Htoon, W.; Kaewpradit, W.; Vorasoot, N.; Toomsan, B.; Akkasaeng, C.; Puppala, N.; Wongkaew, S.; Jogloy, S. Relationships between Nutrient Uptake and Nitrogen Fixation with Aflatoxin Contamination in Peanut under Terminal Drought. Agronomy 2019, 9, 419. [Google Scholar] [CrossRef]
- Salome, J.R.E.; Sakai, R.H.E.; Ambrosano, E. Viabilidade econômica da rotação de adubos verdes com cana-de-açúcar. Rev. Bras. Agroecol. 2007, 2, 116–119. [Google Scholar]
- Ambrosano, E.J.; Trivelin, P.C.O.; Cantarella, H.; Ambrosano, G.M.B.; Schammass, E.A.; Guirado, N.; Rossi, F.; Mendes, P.C.D.; Muraoka, T. Sugarcane yield on consecutive cuts after pre-cultivation of green manure species. Rev. Agric. 2015, 89, 235. [Google Scholar]
- Begum, R.A.; Fry, S.C. Boron bridging of rhamnogalacturonan-II in Rosa and arabidopsis cell cultures occurs mainly in the endo-membrane system and continues at a reduced rate after secretion. Ann. Bot. 2022, 130, 703–715. [Google Scholar] [CrossRef]
- Archana, V.; Pandey, N. Reproductive development and pollen-stigma interaction in sunflower plants receiving boron deficient and toxic supply. J. Plant Nutr. 2021, 44, 2157–2166. [Google Scholar] [CrossRef]
- Galeriani, T.M.; Neves, G.O.; Santos Ferreira, J.H.; Oliveira, R.N.; Oliveira, S.L.; Calonego, J.C.; Crusciol, C.A.C. Calcium and Boron Fertilization Improves Soybean Photosynthetic Efficiency and Grain Yield. Plants 2022, 11, 2937. [Google Scholar] [CrossRef]
- Sarker, M.M.H.; Kashem, M.A.; Ali, S. Role of Vermicomposts Quality on Zinc and Boron Nutrition and Growth of Cauliflower. Agric. Res. 2021, 10, 205–214. [Google Scholar] [CrossRef]
- Moghadam, N.K.L.; Behnam, A.E.; Ghorbanpour, M. Boron Tolerance in Plants. In Metalloids in Plants: Advances and Future Prospects; Wiley: Hoboken, NJ, USA, 2020; pp. 301–314. [Google Scholar]
- Crusciol, C.A.C. Adubação de Cultivares de Amendoim do Tipo Rasteiro; Jaboticabal: São Paulo, Brazil, 2017; pp. 1–5. [Google Scholar]
- Rahman, N.E.; Suntoro, S.E.; Sakya, A.T. Peanut Growth and Gynophore Formation on Boron and Phosphor Applications. J. Soil Sci. Agroclimatol. 2019, 16, 57–66. [Google Scholar]
- Mantovani, J.P.M.; Calonego, J.C.; Foloni, J.S.S. Adubação foliar de boro em diferentes estádios fenológicos da cultura do amendoim. Rev. Ceres 2013, 60, 270–278. [Google Scholar] [CrossRef]
- Kabir, R.; Yeasmin, S.; Islam, A.K.M.M.; Sarkar, M.R. Effect of Phosphorus, Calcium and Boron on the Growth and Yield of Groundnut (Arachis hypogea L.). Int. J. Bio-Sci. Bio-Technol. 2013, 5, 51–60. [Google Scholar]
- Vishwakarma, A.K.; Singh, B.; Pathak, K.A.; Ramesh, S.; Singh, A.L. Effect of different sources of boron application on productivity of groundnut in Mizoram. Int. J. Trop. Agric. 2008, 26, 157–159. [Google Scholar]
- Han, C.; Li, W.; Wang, J.; Huang, Z. Boron leaching: Creating vacancy-rich Ni for enhanced hydrogen evolution. Nano Res. 2022, 15, 1868–1873. [Google Scholar] [CrossRef]
- Camargo, O.D.; Moniz, A.C.; Jorge, J.A.; Valadares, J.M.A.S. Métodos de Análise Química, Mineralógica e Física de Solo, 1st ed.; Instituto Agronômico de Campinas: Campinas, Brazil, 2009. [Google Scholar]
- Van Raij, B. Análise Química para Avaliação da Fertilidade de Solos Tropicais; Instituto Agronômico de Campinas: Campinas, Brazil, 2001. [Google Scholar]
- EMBRAPA. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Rio de Janeiro, Brazil, 2018. [Google Scholar]
- Soil Survey Staf. Soil Taxonomy. Keys to Soil Taxonomy; United States Department of Agriculture Natural Resources Conservation Service: Washington, DC, USA, 2022.
- Malavolta, E.E.; Vitti, G.C.; de Oliveira, G.C.V.E.S. Avaliação do Estado Nutricional das Plantas: Princípios e Aplicações, 2nd ed.; Potafos: Piracicaba, Brazil, 1997. [Google Scholar]
- Kappes, C.E.G.; Carvalho, A.L.E.; Camillo, M.A. Doses and periods of boron foliar applications on agronomic characteristics and quality of soybean seeds. Sci. Agrar. 2008, 9, 291–297. [Google Scholar]
- Bergmann, H.; Eckert, H.; Meisgeier, G. Effect of monoethanolamine on yield and water use efficiency of spring barley. Z. Pflanz. Bodenkd. 1990, 153, 21–24. [Google Scholar] [CrossRef]
- Varanda, M.A.F.; Capone, A.; Menegon, M.Z.; Almeida, M.P.; Barros, H.B. Produtividade de soja submetida a diferentes fontes de boro via foliar em várzea irrigada no estado do Tocantins. Nucleus 2018, 15, 117–128. [Google Scholar] [CrossRef]
- Chitdeshwari, T.E.; Poongothai, S. Yield Of Groundnut And Its Nutrient Uptake As Influenced By Zinc, Boron And Sulphur. Agric. Sci. Dig. 2003, 23, 263–266. [Google Scholar]
- Li, M.; Zhao, Z.; Zhang, Z.; Zhang, W.; Zhou, J.; Xu, F.; Liu, X. Effect of boron deficiency on anatomical structure and chemical composition of petioles and photosynthesis of leaves in cotton (Gossypium hirsutum L.). Sci. Rep. 2017, 7, 4420. [Google Scholar] [CrossRef] [PubMed]
- Instituto Agronômico (IAC). Culturas Econômicas. Amendoim Arachis hypogaea; Instituto ed. Campinas: São Paulo, Brazil, 2014; pp. 22–27. [Google Scholar]
- Pierre, A.K.; Mulvaney, M.; Rowland, D.; Tillman, B.; Grey, T.; Iboyi, J.; Leon, R.; Perondi, D.; Wood, C. Foliar Fertilization as a Strategy to Increase the Proportion of Mature Pods in Peanut (Arachis hypogaea L.). Peanut Sci. 2019, 46, 140–147. [Google Scholar] [CrossRef]
- Peerzada, A.M.E.; Ali, H.H.E.; Chauhan, B.S. Weed management in sorghum [Sorghum bicolor (L.) Moench] using crop competition: A review. Crop Prot. 2017, 95, 74–80. [Google Scholar] [CrossRef]
- Herrera-Rodríguez, M.B.; González-Fontes, A.; Rexach, J.; Camacho-Cristobal, J.J. Role of Boron in Vascular Plants and Response Mechanisms to Boron Stresses, 1st ed.; Global Science Books: Carrollton, GA, USA, 2010. [Google Scholar]
- Singh, A.E.; Lal, A.E.; Misra, R.S. Boron Fertilization is a Must to Enhance Peanut Production in India. Available online: https://escholarship.org/uc/item/9z63872f (accessed on 19 January 2021).
- Marangoni, F.F.; Otto, R.; de Almeida, R.F.; Casarin, V.; Vitti, G.C.; Tiritan, C.S. Soluble Sources of Zinc and Boron on Sugarcane Yield in Southeast Brazil. Sugar Tech 2019, 21, 917–924. [Google Scholar] [CrossRef]
- Yin, A.; Huang, B.; Xie, J.; Huang, Y.; Shen, C.; Xin, J. Boron decreases cadmium influx into root cells of Capsicum annuum by altering cell wall components and plasmalemma permeability. Environ. Sci. Pollut. Res. 2021, 28, 52587–52597. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Sanders, D.C.; Nelson, P.V.; Lengnick, L.; Sperry, W.J. Boron improves growth, yield, quality, and nutrient content of tomato. J. Am. Soc. Hortic. Sci. 2003, 128, 441–446. [Google Scholar] [CrossRef]
- Bethke, G.; Thao, A.; Xiong, G.; Li, B.; Soltis, N.E.; Hatsugai, N.; Hillmer, R.A.; Katagiri, F.; Kliebenstein, D.J.; Pauly, M.; et al. Pectin biosynthesis is critical for cell wall integrity and immunity in arabidopsis thaliana. Plant Cell 2016, 28, 537–556. [Google Scholar] [CrossRef]
- Power, P.P.E.; Woods, W.G. The chemistry of boron and its speciation in plants. Plant Soil 1997, 193, 1–14. [Google Scholar] [CrossRef]
- El-Kader, A.E.; Mona, G. Effect of Sulfur Application and Foliar Spraying with Zinc and Boron on Yield, Yield Components, and Seed Quality of Peanut (Arachis hypogaea L.). J. Agric. Biol. Sci. 2013, 9, 127–135. [Google Scholar]
- Azevedo, W.R.; Faquin, E.; Valdemar, E.; Fernandes, L.A. Boron adsorption in lowland soils from Southern of the State of Minas Gerais, Brazil. Pesqui. Agropecu. Bras. 2001, 36, 957–964. [Google Scholar] [CrossRef]

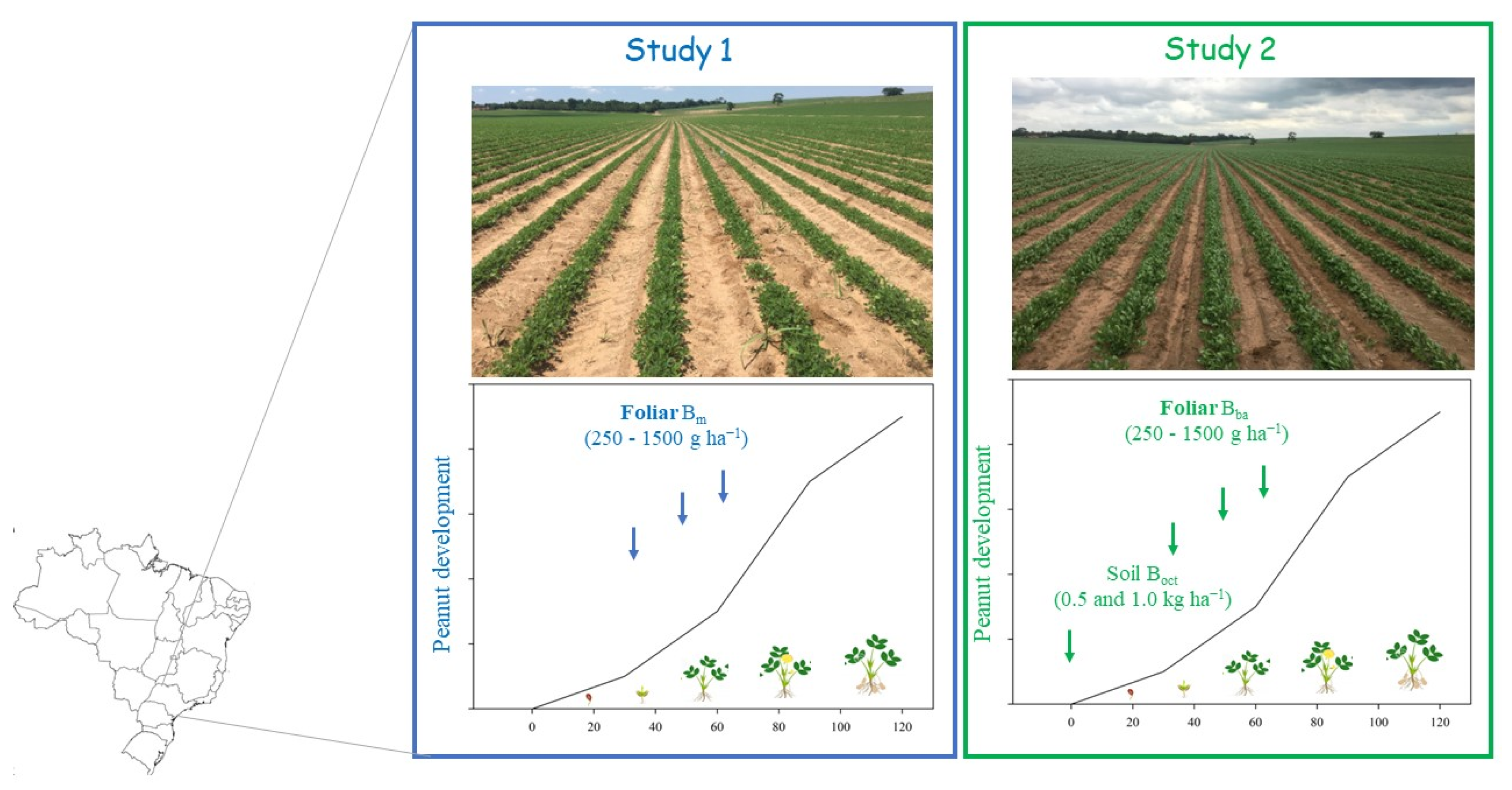

| Soil Layer | pH | OM | P | K | Ca | Mg |
| (m) | CaCl2 | mg dm−3 | mmolc dm−3 | |||
| 0.0–0.25 | 6.3 ± 0.9 | 14.0 ± 2.7 | 9.0 ± 4.0 | 1.5 ± 0.4 | 28.0 ± 11.0 | 19.0 ± 7.0 |
| 0.25–0.50 | 4.2 ± 0.6 | 8.0 ± 1.5 | 2.0 ± 0.9 | 0.7 ± 0.2 | 8.0 ± 3.1 | 6.0 ± 2.2 |
| B | Cu | Fe | Mn | Zn | Al | |
| mg dm−3 | mmolc dm−3 | |||||
| 0.0–0.25 | <0.1 | 1.2 ± 0.1 | 27 ± 0.7 | 3.7 ± 0.1 | 0.4 ± 0.1 | 0.0 ± 0.0 |
| 0.25–0.50 | <0.1 | 0.9 ± 0.1 | 25 ± 0.7 | 4.1 ± 0.1 | 0.2 ± 0.0 | 3.0 ± 0.1 |
| Fertilizers | Form | B | Density | Solubility | pH | |
| % | g cm 3 | g 100 mL−1 | - | |||
| Boric acid, H3BO3 | Dust | 17 (w/w) | 1.4 ± 0.1 | 2.5 | 5.1 ± 0.4 | |
| Sodium octaborate, Na2B8O14 H2O | Dust | 21(w/w) | 1.1 ± 0.0 | 5.3 | 7.5 ± 0.6 | |
| Monoethanolamine, C2H10BNO4 | CS | 150 (w/v) | 1.3 ± 0.1 | - | 8.2 ± 0.6 | |
| B Rates | Study 1, Foliar | Study 2, Foliar + Soil | ||
| (g ha−1) | Bm | Bba | Bba + Boct 0.5 | Bba + Boct 1.0 |
| Grain yield (kg ha−1) | ||||
| 0 | 6824.2 ± 390.4 | 6727.7 ± 13.9 C | 6943.7 ± 5.8 B | 7623.2 ± 52.2 A |
| 250 | 6677.7 ± 173.3 | 6636.2 ± 26.4 C | 7284.2 ± 5.6 B | 7531.0 ± 18.5 A |
| 500 | 6834.2 ± 223.9 | 6790.0 ± 3.5 C | 7346.0 ± 5.3 B | 7507.0 ± 36.8 A |
| 750 | 7178.5 ± 444.0 | 6944.2 ± 4.4 C | 7253.0 ± 26.4 B | 7593.0 ± 6.5 A |
| 1000 | 7200.0 ± 161.3 | 7098.7 ± 3.1 C | 7562.0 ± 2.9 B | 7716.0 ± 3.9 A |
| 1250 | 6941.0 ± 366.2 | 7159.7 ± 4.5 B | 7252.2 ± 7.9 A | 7099.0 ± 7.2 C |
| 1500 | 6518.5 ± 297.0 | 7036.7 ± 8.0 B | 7129.7 ± 4.4 A | 6789.7 ± 4.3 C |
| 100-grains (g) | ||||
| 0 | 57.6 ± 2.1 | 65.6 ± 0.1 B | 73.4 ± 0.1 A | 73.8 ± 0.1 A |
| 250 | 65.2 ± 0.8 | 70.6 ± 0.1 C | 75.1 ± 0.1 B | 79.1 ± 0.1 A |
| 500 | 62.2 ± 0.5 | 70.4 ± 0.1 B | 75.4 ± 0.1 A | 75.5 ± 0.1 A |
| 750 | 72.7 ± 0.4 | 72.7 ± 0.5 C | 75.3 ± 0.1 B | 76.4 ± 0.1 A |
| 1000 | 58.5 ± 1.2 | 70.3 ± 0.1 B | 77.5 ± 0.1 A | 77.3 ± 0.0 A |
| 1250 | 69.6 ± 1.1 | 71.1 ± 0.1 B | 72.2 ± 0.1 A | 69.7 ± 0.1 C |
| 1500 | 69.9 ± 1.3 | 75.2 ± 0.1 A | 66.0 ± 0.4 C | 67.0 ± 0.2 B |
| Pod yield (g plant−1) | ||||
| 0 | 20.9 ± 0.7 | 123.3 ± 3.1 B | 169.0 ± 2.3 A | 172.0 ± 3.7 A |
| 250 | 22.3 ± 1.8 | 136.7 ± 4.4 B | 177.2 ± 5.7 A | 182.7 ± 4.6 A |
| 500 | 30.4 ± 4.6 | 156.6 ± 6.6 C | 174.5 ± 4.5 B | 198.0 ± 6.2 A |
| 750 | 25.8 ± 2.2 | 158.2 ± 8.6 C | 180.0 ± 3.9 B | 203.5 ± 5.7 A |
| 1000 | 25.2 ± 0.7 | 165.2 ± 5.3 B | 205.0 ± 3.9 A | 206.0 ± 5.0 A |
| 1250 | 29.3 ± 2.9 | 163.2 ± 6.0 A | 160.0 ± 8.1 A | 173.7 ± 7.0 A |
| 1500 | 20.1 ± 1.1 | 140.7 ± 9.0 A | 146.2 ± A8.8 | 149.0 ± 4.7 A |
| 20-pods (g) | ||||
| 0 | 29.2 ± 0.8 | 29.3 ± 0.1 A | 28.6 ± 0.1 B | 29.5 ± 0.1 A |
| 250 | 24.3 ± 0.9 | 30.9 ± 0.1 B | 30.6 ± 0.0 B | 36.6 ± 0.2 A |
| 500 | 31.2 ± 0.8 | 30.5 ± 0.1 C | 31.4 ± 0.1 B | 36.9 ± 0.0 A |
| 750 | 31.5 ± 1.5 | 29.1 ± 0.1 C | 31.8 ± 0.0 B | 39.6 ± 0.0 A |
| 1000 | 26.7 ± 1.6 | 29.1 ± 0.1 C | 31.8 ± 0.0 B | 39.6 ± 0.1 A |
| 1250 | 26.4 ± 0.0 | 30.2 ± 0.1 C | 31.0 ± 0.4 B | 34.2 ± 0.1 A |
| 1500 | 29.3 ± 1.5 | 27.8 ± 0.1 C | 29.0 ± 0.2 A | 29.4 ± 0.0 A |
| ANOVA 1 | Grains | 100-grains | Pods | 20-pods |
| pBm | 0.58 | <0.01 | <0.05 | 0.50 |
| p Boct andp Bab andp Boct ∗ Bab | <0.01 | <0.01 | <0.01 | <0.01 |
| B Rates | Study 1, Foliar | Study 2, Foliar + Soil | ||
| (g ha−1) | Bm | Bba | Bba + Boct 0.5 1 | Bba + Boct 1.0 1 |
| Leaf B (mg kg−1) | ||||
| 0 | 29.3 ± 2.1 | 34.9 ± 0.0 B | 34.9 ± 0.0 B | 47.8 ± 0.1 A |
| 250 | 55.4 ± 2.0 | 59.4 ± 0.1 C | 67.2 ± 0.1 B | 71.3 ± 0.1 A |
| 500 | 77.7 ± 3.8 | 74.3 ± 0.2 C | 85.2 ± 0.2 A | 81.7 ± 0.0 B |
| 750 | 97.8 ± 3.9 | 113.8 ± 0.0 C | 133.0 ± 0.1 A | 122.4 ± 0.0 B |
| 1000 | 121.4 ± 5.4 | 140.1 ± 0.1 C | 141.6 ± 0.0 B | 150.2 ± 0.1 A |
| 1250 | 132.3 ± 7.1 | 157.3 ± 0.2 B | 140.1 ± 0.1 C | 178.6 ± 0.2 A |
| 1500 | 159.0 ± 4.9 | 161.1 ± 0.0 B | 150.7 ± 0.1 B | 177.0 ± 0.0 A |
| Leaf Ca (g kg−1) | ||||
| 0 | 19.4 ± 1.0 | 21.2 ± 0.0 A | 19.1 ± 0.0 C | 19.7 ± 0.1 B |
| 250 | 18.8 ± 0.8 | 19.6 ± 0.0 B | 21.8 ± 0.0 A | 19.4 ± 0.0 C |
| 500 | 19.8 ± 0.5 | 19.5 ± 0.0 B | 21.5 ± 0.0 A | 18.7 ± 0.0 C |
| 750 | 20.5 ± 0.9 | 20.2 ± 0.0 C | 23.1 ± 0.0 A | 21.5 ± 0.0 B |
| 1000 | 19.1 ± 1.2 | 19.3 ± 0.0 C | 20.8 ± 0.1 A | 20.4 ± 0.0 B |
| 1250 | 19.4 ± 1.4 | 19.8 ± 0.0 B | 18.2 ± 0.0 C | 21.4 ± 0.0 A |
| 1500 | 19.3 ± 0.9 | 21.4 ± 0.0 A | 17.2 ± 0.0 C | 19.2 ± 0.0 B |
| ANOVA 2 | ||||
| Leaf B | Leaf Ca | |||
| pBm | <0.001 | 0.94 | ||
| p Boct | <0.001 | <0.001 | ||
| p Bba | <0.001 | <0.001 | ||
| p Boct ∗ Bba | <0.001 | <0.001 | ||
| B Rates | Study 1, Foliar | Study 2, Foliar + Soil | ||
| (g ha−1) | Bm | Bba | Bba + Boct 0.5 1 | Bba + Boct 1.0 1 |
| Leaf N (g kg−1) | ||||
| 0 | 41.5 ± 1.4 | 41.7 ± 0.1 B | 42.7 ± 0.1 A | 40.2 ± 0.1 C |
| 250 | 39.5 ± 0.6 | 40.3 ± 0.2 C | 41.9 ± 0.1 A | 41.3 ± 0.1 B |
| 500 | 39.9 ± 0.6 | 40.2 ± 0.1 C | 43.8 ± 0.2 A | 42.7 ± 0.2 B |
| 750 | 41.4 ± 0.5 | 38.6 ± 0.2 B | 42.7 ± 0.1 A | 42.4 ± 0.1 A |
| 1000 | 41.3 ± 1.0 | 41.3 ± 0.1 B | 39.4 ± 0.1 C | 44.1 ± 0.0 A |
| 1250 | 40.8 ± 1.6 | 41.1 ± 0.0 B | 40.7 ± 0.1 C | 42.4 ± 0.1 A |
| 1500 | 41.8 ± 0.7 | 40.0 ± 0.1 C | 44.9 ± 0.1 A | 42.9 ± 0.0 B |
| Leaf P (g kg−1) | ||||
| 0 | 1.9 ± 0.2 | 2.3 ± 0.0 A | 2.3 ± 0.1 A | 2.3 ± 0.0 A |
| 250 | 1.9 ± 0.1 | 2.4 ± 0.1 A | 2.3 ± 0.0 B | 2.4 ± 0.0 A |
| 500 | 1.8 ± 0.1 | 2.1 ± 0.0 B | 2.3 ± 0.0 A | 2.3 ± 0.0 A |
| 750 | 1.9 ± 0.1 | 2.5 ± 0.0 A | 2.2 ± 0.0 B | 2.2 ± 0.0 B |
| 1000 | 2.1 ± 0.1 | 2.5 ± 0.0 A | 2.5 ± 0.0 A | 2.3 ± 0.0 B |
| 1250 | 2.0 ±0.1 | 2.6 ± 0.0 A | 2.6 ± 0.0 A | 2.4 ± 0.0 B |
| 1500 | 2.0 ± 0.1 | 2.6 ± 0.0 A | 2.2 ± 0.0 B | 2.2 ± 0.1 B |
| Leaf K (g kg−1) | ||||
| 0 | 15.2 ± 1.0 | 12.4 ± 0.0 A | 13.9 ± 0.0 B | 14.9 ± 0.0 A |
| 250 | 15.9 ± 0.8 | 13.6 ± 0.0 A | 10.1 ± 0.0 C | 12.4 ± 0.0 B |
| 500 | 13.4 ± 0.5 | 12.8 ± 0.0 A | 10.1 ± 0.0 C | 12.3 ± 0.0 B |
| 750 | 15.4 ± 0.9 | 13.4 ± 0.0 A | 8.5 ± 0.0 C | 11.3 ± 0.0 B |
| 1000 | 15.6 ± 1.2 | 12.8 ± 0.0 B | 12.3 ± 0.0 C | 13.3 ± 0.0 A |
| 1250 | 15.9 ± 1.4 | 14.6 ± 0.0 A | 12.6 ± 0.0 C | 12.9 ± 0.0 B |
| 1500 | 15.3 ± 0.9 | 13.7 ± 0.0 A | 12.2 ± 0.0 C | 12.9 ± 0.0 B |
| ANOVA 2 | ||||
| Leaf N | Leaf P | Leaf K | ||
| pBm | 0.65 | 0.83 | 0.49 | |
| p Boct | <0.001 | <0.001 | <0.001 | |
| p Bba | <0.001 | <0.001 | <0.001 | |
| pBoct∗ Bba | <0.001 | <0.001 | <0.001 | |
| B Rates | Study 2, Foliar + Soil | ||
| (g ha−1) | Bba | Bba + Boct 0.5 1 | Bba + Boct 1.0 1 |
| Maturation (%) | |||
| 0 | 54.2 ± 0.2 C | 59.4 ± 0.1 B | 64.6 ± 0.0 A |
| 250 | 59.2 ± 0.1 C | 62.2 ± 0.1 B | 66.2 ± 0.1 A |
| 500 | 57.8 ± 0.0 C | 63.4 ± 0.1 B | 73.0 ± 0.1 A |
| 750 | 58.5 ± 0.1 C | 62.7 ± 0.1 B | 69.4 ± 0.1 A |
| 1000 | 58.3 ± 0.1 C | 62.9 ± 0.1 B | 64.2 ± 0.1 A |
| 1250 | 63.2 ± 0.1 A | 62.8 ± 0.1 A | 58.6 ± 0.1 B |
| 1500 | 66.7 ± 1.0 A | 64.9 ± 0.1 B | 53.3 ± 0.2 C |
| Normal seedlings (%) | |||
| 0 | 65.5 ± 6.7 B | 85.5 ± 4.8 A | 87.0 ± 1.9 A |
| 250 | 66.5 ± 1.9 B | 92.7 ± 2.9 A | 97.5 ± 1.3 A |
| 500 | 85.2 ± 2.6 A | 88.7 ± 1.7 A | 85.7 ± 4.2 A |
| 750 | 80.7 ± 3.1 B | 90.5 ± 2.5 A | 74.5 ± 4.3 B |
| 1000 | 85.2 ± 3.9 B | 95.0 ± 2.1 A | 80.0 ± 2.2 B |
| 1250 | 73.2 ± 5.4 B | 93.5 ± 1.3 A | 75.2 ± 4.6 B |
| 1500 | 56.0 ± 4.2 C | 78.0 ± 2.4 B | 88.0 ± 0.8 A |
| ANOVA 2 | |||
| Maturation | Normal seedlings | ||
| p Boct | <0.001 | <0.001 | |
| p Bba | <0.001 | <0.001 | |
| pBoct∗ Bba | <0.001 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betiol, R.A.B.; Ferraz-Almeida, R.; Otto, R.; Cesar Vitti, G. Borated Fertilizations via Foliar and Soil for Peanut Production during the Sugarcane Reform. Agriculture 2023, 13, 347. https://doi.org/10.3390/agriculture13020347
Betiol RAB, Ferraz-Almeida R, Otto R, Cesar Vitti G. Borated Fertilizations via Foliar and Soil for Peanut Production during the Sugarcane Reform. Agriculture. 2023; 13(2):347. https://doi.org/10.3390/agriculture13020347
Chicago/Turabian StyleBetiol, Ruan Aparecido Biagi, Risely Ferraz-Almeida, Rafael Otto, and Godofredo Cesar Vitti. 2023. "Borated Fertilizations via Foliar and Soil for Peanut Production during the Sugarcane Reform" Agriculture 13, no. 2: 347. https://doi.org/10.3390/agriculture13020347
APA StyleBetiol, R. A. B., Ferraz-Almeida, R., Otto, R., & Cesar Vitti, G. (2023). Borated Fertilizations via Foliar and Soil for Peanut Production during the Sugarcane Reform. Agriculture, 13(2), 347. https://doi.org/10.3390/agriculture13020347







