The Influence of Konik Horses Grazing and Meteorological Conditions on Wetland Communities
Abstract
1. Introduction
2. Materials and Methods
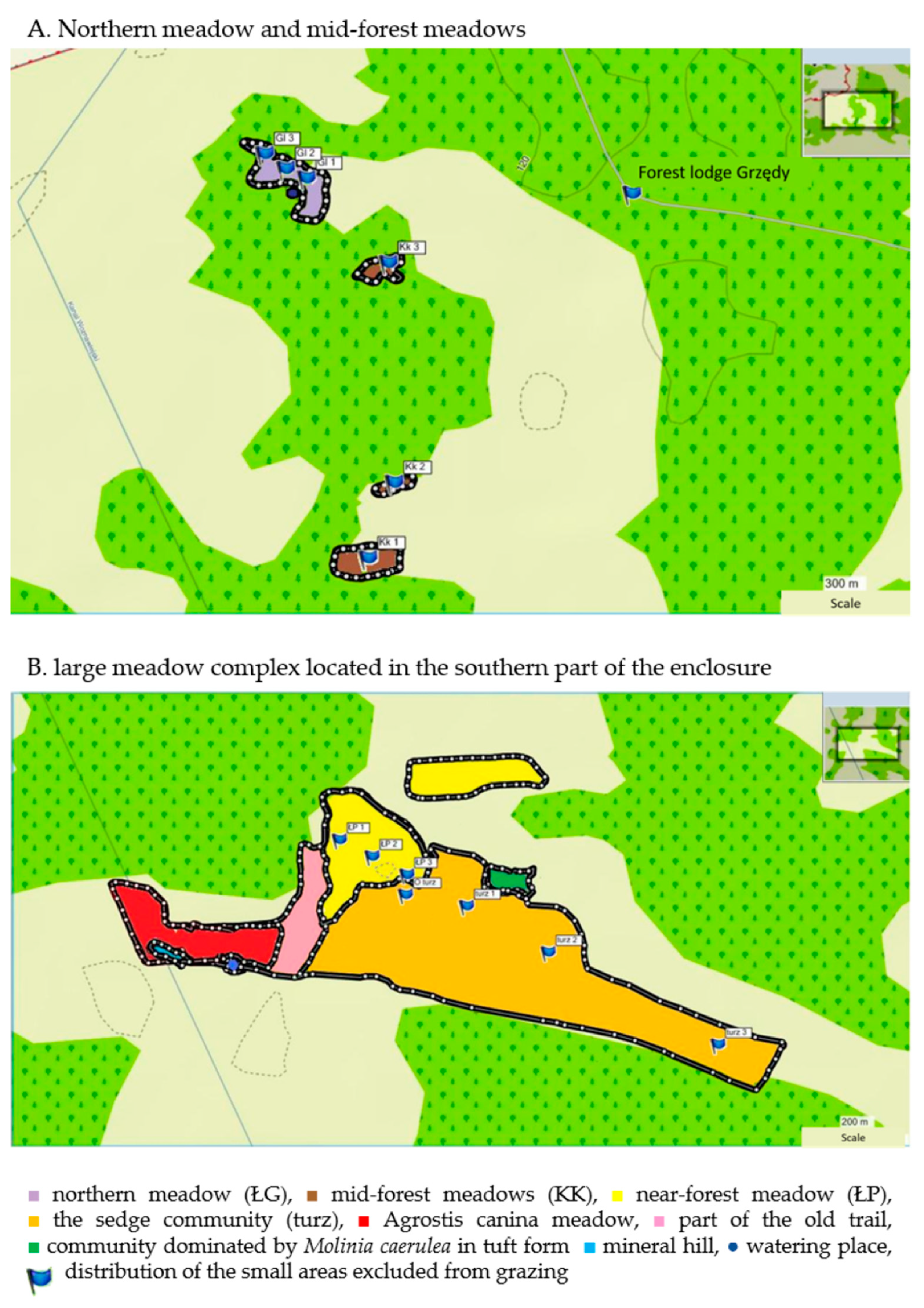
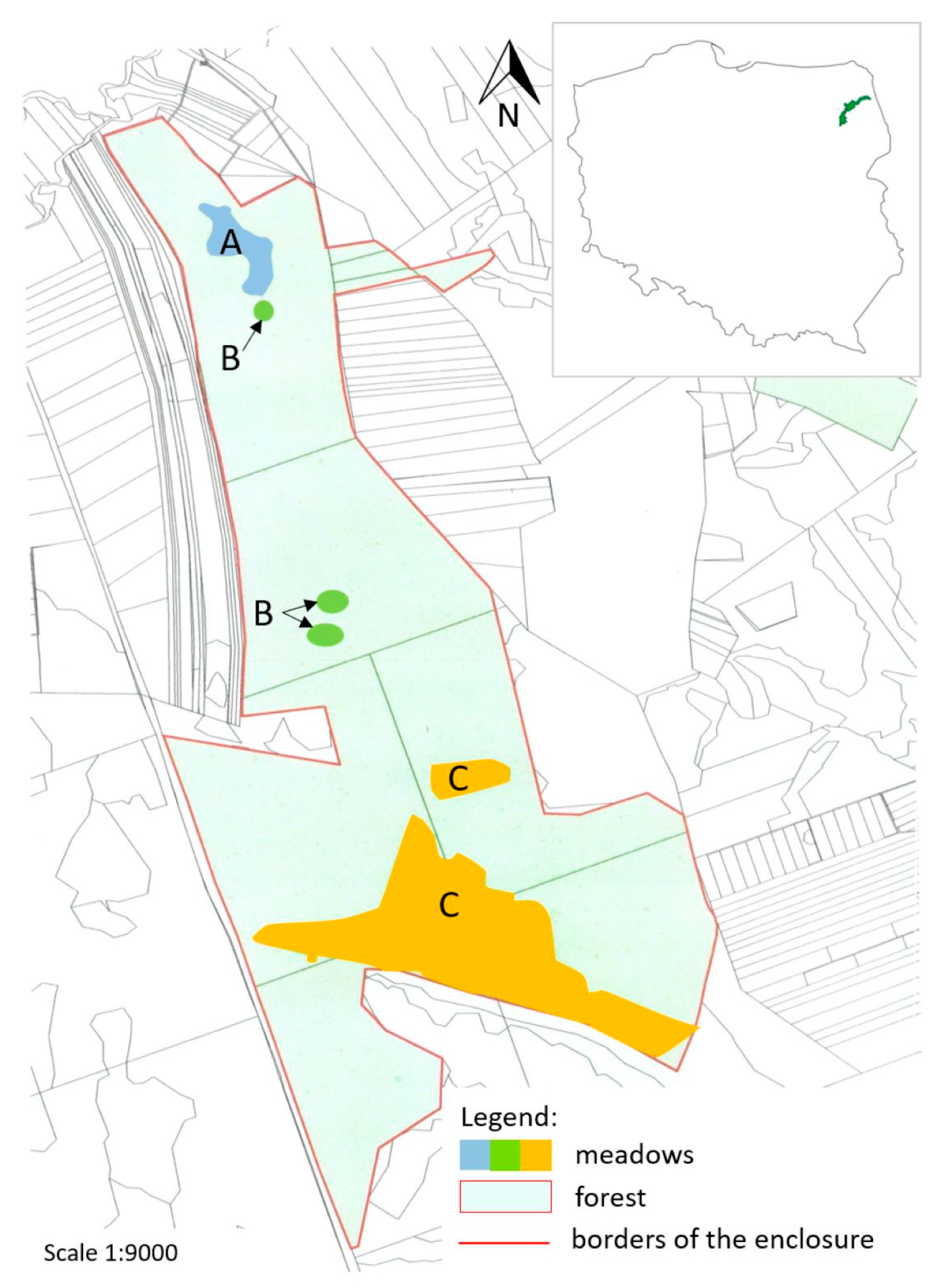
2.1. Study Site
2.2. Phytosociological Studies
2.3. Structure of the Sward
2.4. Botanical Analysis of the Sward
2.5. Statistical Analysis
3. Results
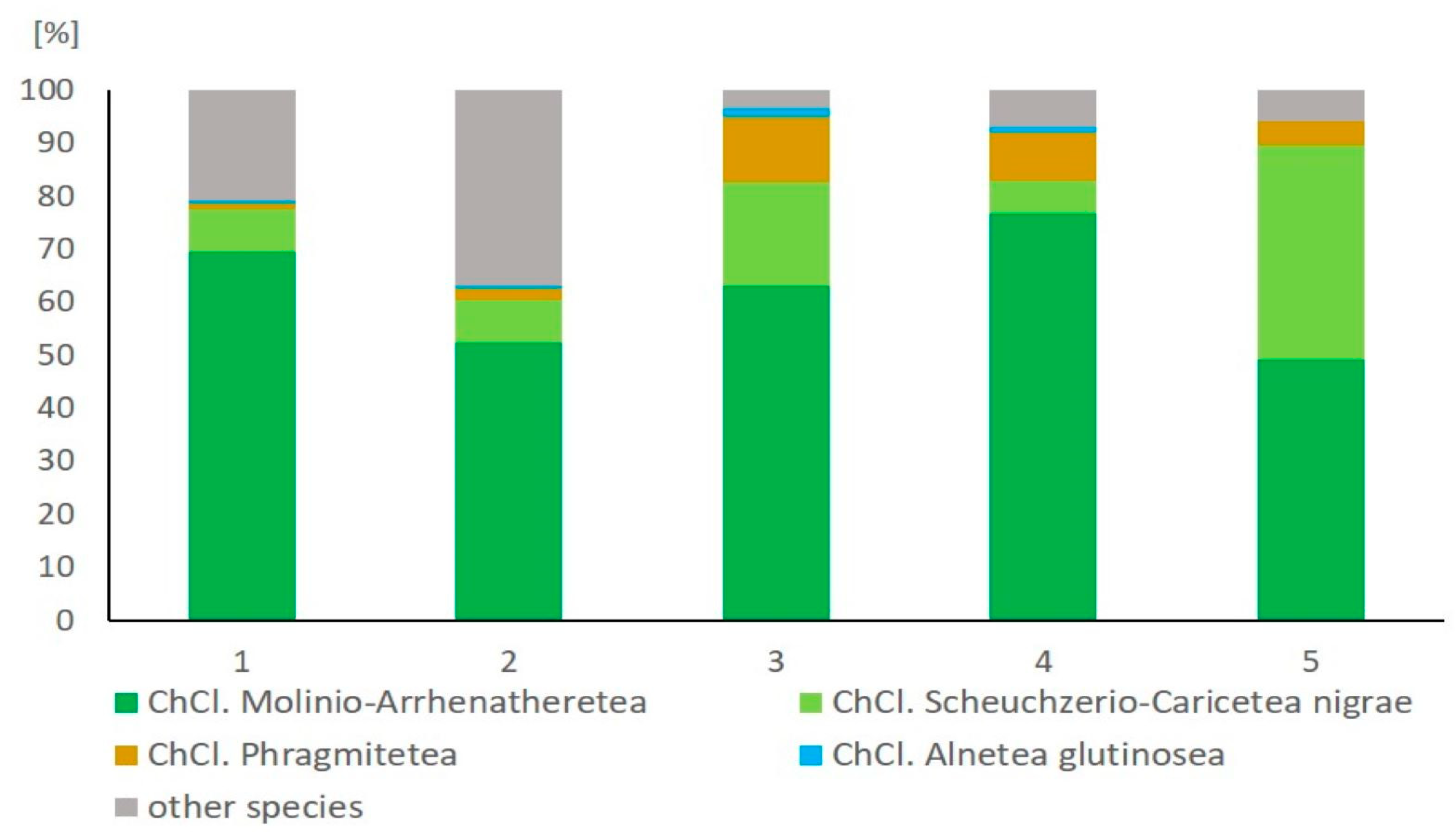
3.1. Phytosociological Studies
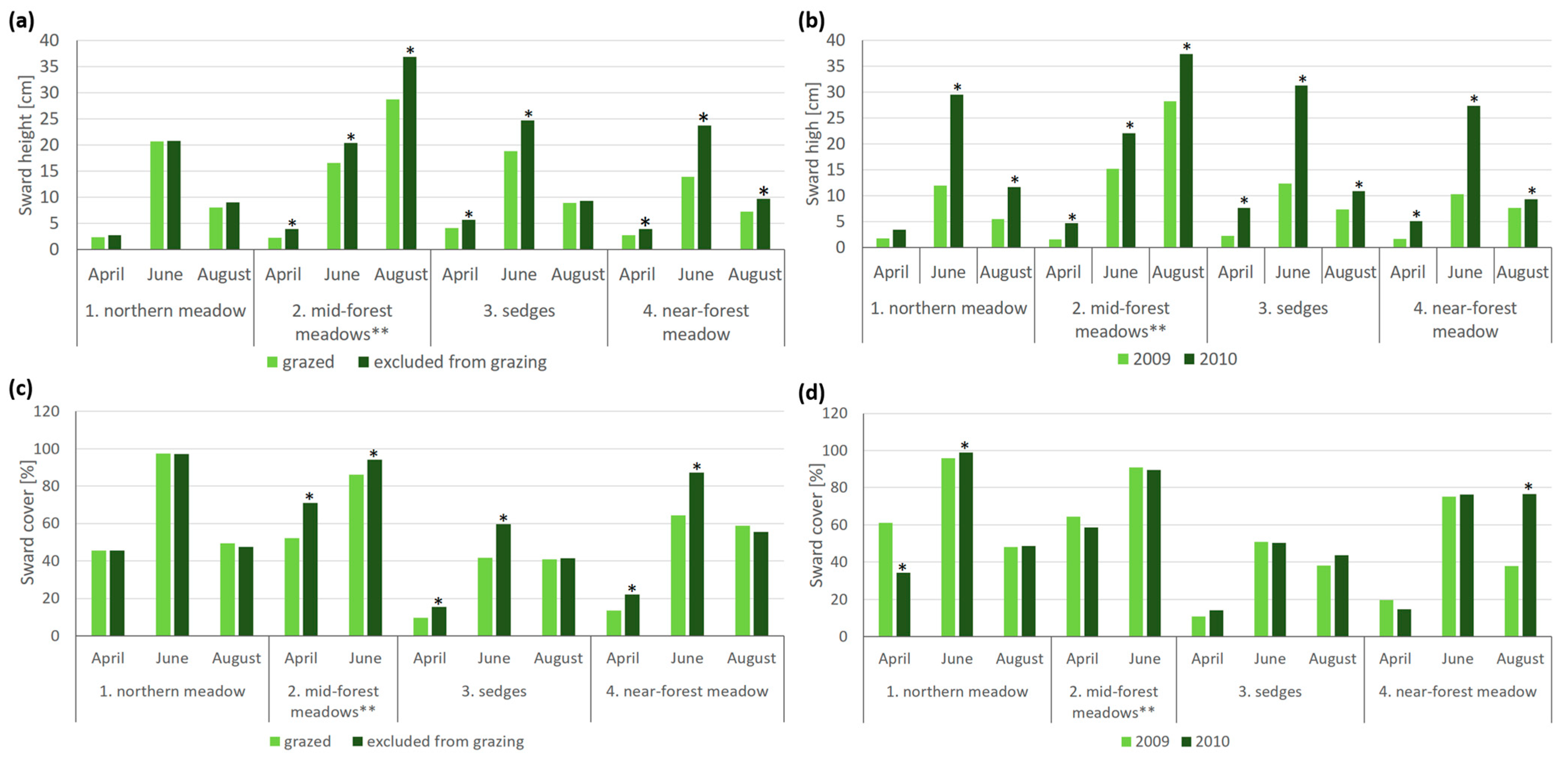
3.2. Sward Structure
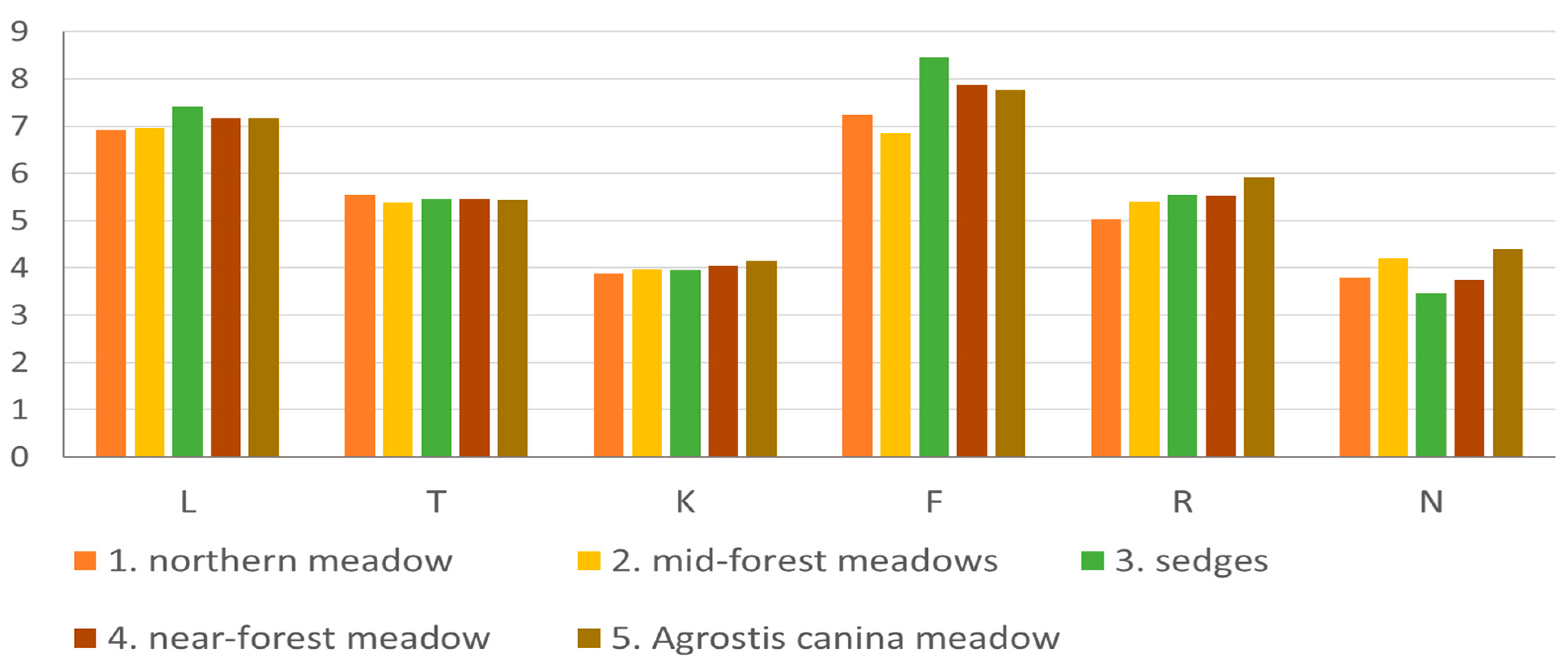
3.3. Botanical Analysis of the Sward
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Community | 1. Northern Meadow | 2. Mid-Forest Meadows | 3. Sedge Community | 4. Near-Forest Meadow | 5. Agrostis canina Meadow | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of relevés | 4 | 4 | 17 | 6 | 2 | |||||
| Sward cover [%] | 95 | 87.5 | 65 | 75.8 | 80 | |||||
| Synthetic indicators * | F | D | F | D | C | D | C | D | F | D |
| Ch, Dass. Molinietum caeruleae, Molinion | ||||||||||
| Carex panicea L. | 100 | 250 | 100 | 150 | V | 3368 | V | 2625 | 100 | 875 |
| Molinia caerulea (L.) Moench | 100 | 100 | 25 | 63 | V | 574 | V | 1875 | 100 | 1500 |
| Potentilla erecta (L.) Raeusch. | 100 | 4375 | 100 | 1188 | V | 1500 | 100 | 150 | ||
| Carex flava L. | V | 1047 | V | 875 | 100 | 250 | ||||
| Thalictrum flavum L. | 100 | 2875 | 75 | 88 | I | 32 | V | 75 | 50 | 25 |
| Lycopus europaeus L. | 25 | 13 | V | 224 | III | 58 | 100 | 250 | ||
| Gentiana pneumonanthe L. | 25 | 13 | 25 | 13 | III | 21 | III | 25 | 100 | 10 |
| Salix rosmarinifolia L. | 50 | 25 | 25 | 63 | IV | 91 | II | 17 | 100 | 150 |
| Inula britannica L. | I | 8 | 100 | 150 | ||||||
| Briza media L. | 75 | 88 | 50 | 25 | I | 8 | ||||
| Iris sibirica L. | 25 | 13 | ||||||||
| ChAll. Filipendulion | ||||||||||
| Filipendula ulmaria (L.) Maxim. | 100 | 1188 | 100 | 875 | V | 83 | 100 | 50 | ||
| Lysimachia vulgaris L. | 25 | 13 | V | 168 | IV | 133 | 100 | 150 | ||
| Valeriana officinalis L. | 75 | 138 | 75 | 38 | I | 1 | III | 45 | 50 | 25 |
| Lythrum salicaria L. | 25 | 13 | III | 54 | I | 8 | 100 | 50 | ||
| Stachys palustris L. | II | 24 | I | 8 | 50 | 25 | ||||
| ChAll. Calthion | ||||||||||
| Lathyrus palustris L. | 75 | 138 | 50 | 25 | II | 41 | I | 8 | 50 | 25 |
| Cirsium rivulare (Jacq.) All. | I | 8 | 50 | 25 | ||||||
| Myosotis palustris (L.) L. em. Rchb. | I | 2 | ||||||||
| Caltha palustris L. | 25 | 13 | III | 43 | 50 | 5 | ||||
| Polygonum bistorta L. | 25 | 63 | ||||||||
| ChAll. Cnidion dubii | ||||||||||
| Cnidium dubium (Schkuhr) Thell. | 25 | 13 | IV | 136 | V | 117 | 100 | 250 | ||
| ChO. Molinietalia | ||||||||||
| Deschampsia caespitosa (L.) P. Beauv. | 100 | 513 | 100 | 513 | V | 77 | 100 | 875 | ||
| Lychnis flos-cuculi L. | 75 | 38 | 50 | 25 | II | 3 | 100 | 250 | ||
| Cirsium palustre (L.) Scop. | III | 25 | 50 | 25 | ||||||
| ChAll. Arrhenatherion | ||||||||||
| Galium mollugo L. | 25 | 63 | ||||||||
| Campanula patula L. | 25 | 3 | ||||||||
| ChO. Arrhenatheretalia | ||||||||||
| Daucus carota L. | 50 | 75 | 100 | 563 | ||||||
| Taraxacum officinale F.H.Wigg. | 25 | 13 | II | 10 | 50 | 5 | ||||
| ChO. Agropyro-Rumicion crispi | ||||||||||
| Ranunculus repens L. | I | 2 | 100 | 875 | ||||||
| Potentilla anserina L. | I | 3 | 100 | 250 | ||||||
| Lysimachia nummularia L. | 50 | 25 | 25 | 13 | I | 15 | I | 8 | 50 | 25 |
| Rumex crispus L. | 25 | 125 | ||||||||
| ChCl. Molinio-Arrhenatheretea | ||||||||||
| Poa pratensis L. | 100 | 1188 | 100 | 1338 | I | 1 | III | 25 | 100 | 10 |
| Rumex acetosa L. | 100 | 1188 | 75 | 188 | I | 2 | ||||
| Ranunculus acris L. | 100 | 563 | 100 | 513 | I | 1 | IV | 20 | 100 | 250 |
| Festuca rubra L. | 100 | 513 | 100 | 1025 | I | 3 | V | 183 | 100 | 250 |
| Cerastium holosteoides Fr. em. Hyl. | 75 | 188 | 100 | 250 | III | 25 | ||||
| Plantago lanceolata L. | 75 | 88 | ||||||||
| Cardamine pratensis L. | 75 | 38 | 25 | 13 | I | 1 | I | 2 | ||
| Vicia cracca L. | 50 | 125 | 50 | 125 | ||||||
| Holcus lanatus L. | 50 | 25 | 25 | 13 | 100 | 10 | ||||
| Leontodon hispidus L. | 25 | 63 | III | 44 | IV | 33 | 100 | 150 | ||
| Phleum pratense L. | 50 | 25 | ||||||||
| Avenula pubescens (Huds.) Dumort. | 25 | 13 | ||||||||
| Companion species | ||||||||||
| ChCl. Alnetea glutinosae | ||||||||||
| Betula pubescens Ehrh. | 75 | 88 | 25 | 63 | II | 26 | IV | 60 | 100 | 10 |
| Salix cinerea L. | 25 | 13 | III | 36 | I | 8 | ||||
| Salix aurita L. | 25 | 13 | III | 30 | ||||||
| Thelypteris palustris Schott. | II | 59 | ||||||||
| Betula humilis Schrank | II | 50 | ||||||||
| Frangula alnus Mill. | I | 2 | ||||||||
| ChCl. Phragmitetea | ||||||||||
| Galium palustre L. | 100 | 250 | 100 | 250 | V | 162 | V | 250 | 100 | 250 |
| Carex buxbaumii Wahlenb. | V | 385 | V | 292 | ||||||
| Carex elata All. | IV | 456 | I | 42 | 100 | 250 | ||||
| Phragmites australis (Cav.) Trin ex Steud | III | 85 | II | 83 | ||||||
| Scutellaria galericulata L. | II | 21 | I | 8 | ||||||
| Alisma plantago-aquatica L. | I | 15 | ||||||||
| Lysimachia thyrsiflora L. | I | 6 | ||||||||
| Poa palustris L. | 25 | 63 | III | 267 | 100 | 150 | ||||
| Glyceria fluitans (L.) R.Br. | 50 | 5 | ||||||||
| ChCl. Scheuchzerio-Caricetea nigrae | ||||||||||
| Agrostis canina L. | 100 | 825 | 100 | 875 | IV | 35 | V | 175 | 100 | 3750 |
| Carex nigra Reichard | 100 | 250 | 50 | 125 | IV | 265 | III | 92 | 100 | 250 |
| Viola palustris L. | 50 | 438 | 25 | 63 | II | 12 | IV | 133 | 100 | 10 |
| Comarum palustre L. | 50 | 75 | 25 | 13 | V | 1044 | V | 117 | 100 | 150 |
| Veronica scutellata L. | 25 | 13 | 50 | 75 | I | 8 | 100 | 10 | ||
| Juncus articulatus L. em. K. Richt. | V | 219 | II | 83 | ||||||
| Ranunculus flammula L. | V | 84 | I | 8 | 100 | 1500 | ||||
| Calamagrostis stricta (Timm) Koeler | IV | 121 | ||||||||
| Menyanthes trifoliata L. | II | 34 | ||||||||
| Stellaria palustris Retz. | 25 | 13 | II | 7 | I | 8 | 100 | 30 | ||
| Eriophorum angustifolium Honck. | I | 16 | ||||||||
| Other | ||||||||||
| Geum rivale L. | 100 | 2063 | 100 | 2938 | I | 2 | ||||
| Anthoxanthum odoratum L. | 100 | 1500 | 100 | 1438 | I | 3 | V | 42 | ||
| Luzula multiflora (Retz.) Lej. | 100 | 250 | 75 | 188 | III | 58 | ||||
| Viola sp. | 100 | 100 | 50 | 75 | II | 50 | IV | 133 | 100 | 250 |
| Glechoma hederacea L. | 75 | 38 | 25 | 13 | ||||||
| Veronica chamaedrys L. | 50 | 75 | 50 | 125 | I | 15 | ||||
| Epilobium palustre L. | 50 | 25 | I | 18 | III | 58 | 50 | 25 | ||
| Stellaria graminea L. | 25 | 63 | 50 | 75 | 100 | 10 | ||||
| Mentha aquatica L. | 25 | 63 | V | 238 | V | 250 | 100 | 250 | ||
| Dactylorhiza incarnata (L.) Soó | I | 1 | ||||||||
| Cirsium arvense (L.) Scop. | 75 | 138 | V | 142 | 100 | 250 | ||||
| Plantago major L. | III | 12 | ||||||||
| Ranunculus sceleratus L. | 50 | 25 | ||||||||
| Urtica dioica L. | 50 | 125 | ||||||||
| Betula sp. | 50 | 75 | ||||||||
References
- Report from the Commission to the Council and the European Parliament: The State of Nature in the European Union 2020. Available online: https://www.eea.europa.eu/publications/state-of-nature-in-the-eu-2020 (accessed on 20 December 2022).
- Schils, R.L.; Bufe, C.; Rhymer, C.M.; Francksen, R.M.; Klaus, V.H.; Abdalla, M.; Milazzo, F.; Lellei-Kovács, E.; Berge, H.T.; Bertora, C.; et al. Permanent grasslands in Europe: Land use change and intensification decrease their multifunctionality. Agric. Ecosyst. Environ. 2022, 330, 107891. [Google Scholar] [CrossRef]
- Verhoeven, J.T.A. Wetlands in Europe: Perspectives for restoration of a lost paradise. Ecol. Eng. 2014, 66, 6–9. [Google Scholar] [CrossRef]
- Török, P.; Janišová, M.; Kuzemko, A.; Rūsiņa, S.; Stevanović, Z.D. Grasslands, their threats and management in eastern Europe. In Grasslands of the World: Diversity, Management and Conservation; Squires, V.R., Dengler, J., Hua, L., Feng, H., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 64–88. ISBN 978-036-778-093-7. [Google Scholar]
- Sienkiewicz–Paderewska, D.; Paderewski, J.; Suwara, I.; Kwasowski, W. Fen grassland vegetation under different land uses (Biebrza National Park, Poland). Glob. Ecol. Conserv. 2020, 23, e01188. [Google Scholar] [CrossRef]
- Joyce, C.B. Ecological consequences and restoration potential of abandoned wet grasslands. Ecol. Eng. 2013, 66, 91–102. [Google Scholar] [CrossRef]
- Grzybowski, M.; Glińska-Lewczuk, K. The principal threats to the peatlands habitats, in the continental bioregion of Central Europe—A case study of peatland conservation in Poland. J. Nat. Conserv. 2019, 53, 125778. [Google Scholar] [CrossRef]
- Kryszak, J.; Kryszak, A. Floristic changes in meadow swards after suspension of utilization. Grassland Sci. Europ. 2005, 1, 272–275. [Google Scholar]
- Sand-Jensen, K.; Jørgensen, H.; Larsen, J.R. Long-term influence of hay-cutting on plant species richness, biodiversity and soil fertility in a Danish fen. Ecol. Eng. 2019, 134, 93–100. [Google Scholar] [CrossRef]
- Sýkora, K.; Ten Brink, D.-J.; Buis, E.; van Haaften, E.-J.; Klimkowska, A. Pattern in plant communities in the Lower Basin of the Biebrza National Park. In Grazing as a Conservation Management Tool in Peatland. Report of a Workshop Held 22–26 April 2002 in Goniadz; Bokdam, J., van Braeckel, A., Werpachowski, C., Znaniecka, M., Eds.; Biebrza National Park: Goniądz, Poland, 2002; pp. 37–44. [Google Scholar]
- Kotowski, W.; Jabłońska, E.; Banaszuk, H. Conservation management in fens: Do large tracked mowers impact functional plant diversity? Biol. Conserv. 2013, 167, 292–297. [Google Scholar] [CrossRef]
- Middleton, B.; Holsten, B.; Diggelen, R.V. Biodiversity management of fens and fen meadows by grazing, cutting and burning. App. Veg. Sci. 2006, 9, 279–284. [Google Scholar] [CrossRef]
- Stammel, B.; Kiehl, K.; Pfadenhauer, J. Alternative management on fens: Response variation to grazing and mowing. Appl. Veg. Sci. 2003, 6, 245–254. [Google Scholar] [CrossRef]
- Nagy, G.; Nyakas, A.; Tóth, C.; Vinczeffy, I. Sward composition of natural grasslands in relation to the method of utilization on Puszta Hortobágy. Grassland Sci. Europ. 2001, 2, 107–109. [Google Scholar]
- Fløjgaard, C.; Brunbjerg, A.K.; Andersen, D.K.; Dalby, L.; Lehmann, L.J.; Bruun, H.H.; Ejrnæs, R. Nibble, cut, stomp and burn: Biodiversity effects of disturbances in fen grassland. Ecol. Eng. 2022, 25, e12666. [Google Scholar] [CrossRef]
- Faser, M.D.; Theobald, V.J.; Dhanoa, M.S.; Davies, O.D. Impact on sward composition and stock performance of grazing Molinia-dominant grassland. Agric. Ecosyst. Environ. 2011, 144, 102–106. [Google Scholar] [CrossRef]
- Seer, F.K.; Schrautzer, J. Status, future prospects, and management recommendation for alkaline fens in an agricultural landscape: A comprehensive survey. J. Nat. Conserv. 2014, 22, 358–368. [Google Scholar] [CrossRef]
- Mirski, P. Space use by semi-free-ranging cows on wetlands and its implication as a conservation management tool. Restor. Ecol. 2022, 30, e13533. [Google Scholar] [CrossRef]
- Vulink, J.T. Hungry Herds: Management of Temperate Lowlands Wetlands by Grazing; Rijksuniversiteit Groningen: Groningen, The Netherlands, 2001; pp. 1–394. ISBN 978-90-3691-258-7. [Google Scholar]
- Menard, C.; Duncan, P.; Fleurance, G.; Georges, J.; Lila, M. Comparative foraging and nutrition of horses and cattle in European wetlands. J. Appl. Ecol. 2002, 39, 120–133. [Google Scholar] [CrossRef]
- Batáry, P.; Dicks, L.V.; Kleijn, D.; Sutherland, W.J. The role of agri-environment schemes in conservation and environmental management. Conserv. Biol. 2015, 29, 1006–1016. [Google Scholar] [CrossRef]
- Pasicka, E. Polish Konik horse—Characteristics and historical background of native descendants of tarpan. Acta Sci. Pol. Med. Vet. 2013, 12, 25–38. [Google Scholar]
- Chodkiewicz, A. Advantages and disadvantages of Polish primitive horse grazing on valuable nature areas—A review. Glob. Eco. Conserv. 2020, 21, e00879. [Google Scholar] [CrossRef]
- Górniak, A. Klimat i termika wód powierzchniowych Kotliny Biebrzańskiej. In Kotlina Biebrzańska i Biebrzański Park Narodowy. Aktualny Stan, Walory, Zagrożenia i Potrzeby Czynnej Ochrony Środowiska; Banaszuk, H., Ed.; Wydawnictwo Ekonomia i Środowisko: Białystok, Poland, 2004; pp. 345–362. ISBN 978-838-877-149-1. [Google Scholar]
- Kossowska-Cezak, U.; Olszewski, K.; Przybylska, G. Klimat Kotliny Biebrzańskiej. Zesz. Prob. Post. Nauk Rol. 1991, 372, 119–159. [Google Scholar]
- Grygoruk, M.; Biereżnej-Bazille, U.; Mazgajski, M.; Sienkiewicz, J. Climate-induced challenges for wetlands: Reveailing the background for the adaptive ecosystem management in the Biebrza Valley, Poland. In Managing Protected Areas in Central and Eastern Europe under Climate Change; Rannow, S., Neubert, M., Eds.; Springer: Cham, Switzerland, 2014; Volume 58, pp. 209–249. ISBN 978-94-024-0302-2. [Google Scholar]
- Wiśniewski, S.; Marszałek, M.; Fabjański, K.; Wiśniewska, M. Koncepcja Programowo-przestrzenna Renaturyzacji Sieci Hydrograficznej rzek Jerzgni i Ełku w Rejonie Basenu Środkowego Biebrzańskiego Parku Narodowego. Biebrza National Park, Pracownia Architektury Żywej „Paź”. Available online: http://www.renaturyzacja.biebrza.org.pl/aktualizacja/data/pliki/814_Koncepcja_Programowo_Przestrzenna_Renaturyzacji_Jegrzni_i_Elku.pdf (accessed on 20 December 2022).
- Gilhaus, K.; Hölzel, N. Seasonal variations of fodder quality and availability as constraints for stocking rates in year-round grazing schemes. Agric. Ecosyst. Environ. 2016, 234, 5–15. [Google Scholar] [CrossRef]
- Kabała, C.; Charzynski, P.; Chodorowski, J.; Drewnik, M.; Glina, B.; Greinert, A.; Hulisz, P.; Jankowski, M.; Jonczak, J.; Łabaz, B.; et al. Polish Soil Classification, 6th edition—Principles, classification scheme and correlations. Soil Sci. Annu. 2019, 70, 71–97. [Google Scholar] [CrossRef]
- Chrzanowski, S. Hydrogeniczne siedliska glebotwórcze w dolinie Biebrzy Środkowej. In Kotlina Biebrzańska i Biebrzański Park Narodowy. Aktualny Stan, Walory, Zagrożenia i Potrzeby Czynnej Ochrony Środowiska; Banaszuk, H., Ed.; Wydawnictwo Ekonomia i Środowisko: Białystok, Poland, 2004; pp. 292–312. ISBN 978-838-877-149-1. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie. Grundzűge der Vegetationskunde, 3rd ed.; Springer: Wien, Austria, 1964; pp. 1–866. [Google Scholar] [CrossRef]
- Mirek, Z.; Piękoś-Mirkowa, H.; Zając, A.; Zając, M. Flowering Plants and Pteridophytes of Poland. A Checklist. In Biodiversity of Poland; Mirek, Z., Ed.; W. Szafer Institute of Botany, Polish Academy of Sciences: Cracow, Poland, 2002; Volume 1, pp. 1–442. [Google Scholar]
- Matuszkiewicz, W. Przewodnik do Oznaczania Zbiorowisk Roślinnych Polski (Polish Plant Communities’ Guidebook), 3rd ed.; PWN: Warszawa, Poland, 2006; pp. 1–540. ISBN 978-830-116-707-3. [Google Scholar]
- Magurran, A. Ecological Diversity and Its Measurement; Springer: Dordrecht, The Netherlands, 1988; pp. 1–179. [Google Scholar] [CrossRef]
- Filipek, J. Project of classification of meadow and pasture plants based on the numbers of utility value. Post. Nauk Rol. 1973, 20, 59–68. [Google Scholar]
- Ellenberg, H.; Weber, H.D.; Wirth, V.; Werner, W.; Paulissen, D. Zeigerwerte von Pflanzen in Mitteleuropa. Scr. Geobot. 1992, 18, 1–258. [Google Scholar]
- Diekmann, M. Species indicator values as an important tool in applied plant ecology—A review. Basic and Appl. Ecol. 2003, 4, 493–506. [Google Scholar] [CrossRef]
- Barthram, G.T. Experimental Techniques: The HFRO Sward Stick; Biennial Report; Hill Farming Research Organization: Midlothian, Scotland, 1985; pp. 29–30. [Google Scholar]
- West, O. The significance of percentage area determinations yielded by the percentage area or density list method of pasture analysis. J. Ecol. 1938, 26, 210–217. [Google Scholar] [CrossRef]
- Mannetje, L.; Haydock, K.P. The dry-weight-rank method for botanical analysis of pasture. Grass Forage Sci. 2006, 18, 268–275. [Google Scholar] [CrossRef]
- Ward, J.H., Jr. Hierarchical Grouping to Optimize an Objective Function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 20 December 2022).
- Werpachowski, C. List of Vascular Plants of the Biebrza Valley, 3rd ed.; Biebrza National Park: Goniądz, Poland, 2003; pp. 1–99. [Google Scholar]
- Regulation of the Ministry of the Environment. Rozporządzenie Ministra Środowiska z Dnia 9 Października 2014 r. w Sprawie Ochrony Gatunkowej Roślin (Dz. U. Poz. 1409); Ministry of the Environment: Warsaw, Poland, 2014. [Google Scholar]
- Chodkiewicz, A.; Stypiński, P. The grazing selectivity of Konik horses on grasslands located in Biebrza National Park. Grassland Sci. Europ. 2010, 15, 1024–1027. [Google Scholar]
- Kulik, M. Changes of biodiversity and species composition of Molinia meadow depending on use method. Pol. J. Environ. Stud. 2014, 23, 773–782. [Google Scholar]
- Sienkiewicz-Paderewska, D.; Borawska-Jarmułowicz, B.; Mastalerczuk, G.; Chodkiewicz, A.; Stypiński, P. Effects of mowing cessation on Molinietum caerulae meadow vegetation. Water-Environ. Rural. Areas 2012, 12, 167–179. [Google Scholar]
- Kącki, Z.; Michalska-Hejduk, D. Assessment of biodiversity in Molinia meadows in the Kampinoski National Park based on biocenotic indicators. Pol. J. Environ. Stud. 2010, 19, 351–362. [Google Scholar]
- Ziaja, M.; Wójcik, T.; Wrzesień, M. Conservation status and trends in the transformation of Molinia meadows in the Łąki w Komborni Natura 2000 site, SE Poland. Acta Agrobot. 2017, 70, 1718. [Google Scholar] [CrossRef]
- Havlová, M. Syntaxonomical revision of the Molinion meadows in the Czech Republic. Preslia 2006, 78, 87–101. [Google Scholar]
- Zelnik, I.; Čarni, A. Wet meadows of the alliance Molinion and their environmental gradients in Slovenia. Biologia 2008, 63, 187–196. [Google Scholar] [CrossRef]
- Čop, J.; Vidrih, M.; Hacin, J. Influence of cutting regime and fertilizer application on the botanical composition, yield and nutritive value of herbage of wet grasslands in Central Europe. Grass Forage Sci. 2009, 64, 454–465. [Google Scholar] [CrossRef]
- Kulik, M.; Baryła, R.; Urban, D.; Grzywaczewski, G.; Bochniak, A.; Różycki, A.; Tokarz, E. Vegetation and birds species changes in meadow habitats in Polesie National Park, Eastern Poland. Annu. Set Environ. Prot. 2017, 19, 211–229. [Google Scholar]
- van der Hoek, D.; Sýkora, K.V. Fen-meadow succession in relation to spatial and temporal differences in hydrological and soil conditions. Appl. Veg. Sci. 2006, 9, 185–194. [Google Scholar] [CrossRef]
- Michalska-Hejduk, D.; Kopeć, D. Dynamics of semi-natural vegetation with a focus on Molinion meadows after 50 years of strict protection. Pol. J. Environ. Stud. 2012, 21, 1731–1741. [Google Scholar]
- Nowak, T.; Węgrzynek, B.; Tokarska-Guzik, B. Assets and threats to Molinia meadows (Molinia caeruleae alliance) on chosen NATURA 2000 areas in the eastern part of the Silesian Upland. Acta Sci. Pol. Agric. 2015, 14, 49–61. [Google Scholar]
- Kryszak, A. Różnorodność florystyczna zespołów łąk i pastwisk klasy Molinio-Arrhenatheretea w Wielkopolsce w aspekcie ich wartości gospodarczej. Rocz. AR Pozn. Rozpr. Nauk. 2001, 314, 1–175. [Google Scholar]
- Łuczaj, Ł.; Sadowska, B. Edge effect in different groups of organisms: Vascular plant, bryophyte and fungi species richness across a forest-grassland border. Folia Geobot. 1997, 32, 343–353. [Google Scholar] [CrossRef]
- Wlizło, B.; Szwed, W. Roślinność Ostoi Konika Polskiego w Roztoczańskim Parku Narodowym. Monography; Katedra Podstaw Leśnictwa; Akademia Rolnicza im. Augusta Cieszkowskiego: Poznań, Poland, 2007; pp. 1–117. [Google Scholar]
- Pałczyński, A. Kierunki przemian szaty roślinnej i siedlisk zatorfionych dolin rzecznych pod wpływem ingerencji człowieka. Zesz. Prob. Post. Nauk Rol. 1975, 169, 87–101. [Google Scholar]
- Okruszko, H.; Szuniewicz, J.; Kamiński, J.; Chrzanowski, S. Charakterystyka środowiska oraz zakres jego renaturyzacji w Basenie Środkowym Biebrzy. Zesz. Prob. Post. Nauk Rol. 1996, 432, 9–32. [Google Scholar]
- Gore, A.J.P.; Urquhart, C. The effects of waterlogging on the growth of Molinia caerulea and Eriophorum vaginatum. J. Ecol. 1966, 54, 617–633. [Google Scholar] [CrossRef]
- Kluse, J.S.; Allen Diaz, B.H. Importance of soil moisture and its interaction with competition and clipping for two mountain meadow grasses. Plant Ecol. 2005, 176, 87–99. [Google Scholar] [CrossRef]
- Kundzewicz, Z.W.; Matczak, P. Climate change regional review: Poland. Wires Clim. Change 2012, 3, 297–311. [Google Scholar] [CrossRef]
- Mioduszewski, W.; Ślesicka, A.; Querner, E. Warunki zasilania doliny Dolnej Biebrzy. Water-Environ. Rural 2004, 4, 67–78. [Google Scholar]
- Budny, M.L.; Benscoter, B.W. Shrub encroachment increases transpiration water loss from a Subtropical Wetland. Wetlands 2016, 36, 631–638. [Google Scholar] [CrossRef]
- Kołos, A.; Banaszuk, P. Mowing as a tool for wet meadows restoration: Effect of long-term managemet on species richness and composition of sedge-dominated wetland. Ecol. Eng. 2013, 55, 23–28. [Google Scholar] [CrossRef]
- Zoltan, B.D.; Chytry, M.; Hájková, P.; Havlová, M. Vegetation of lowland wet meadows along a climatic continentality gradient in Central Europe. Preslia 2005, 77, 89–111. [Google Scholar]

| Year | Total Number of Horses * [Heads] | Stocking Rate * (LSU/ha) ** |
|---|---|---|
| 2008 | 34 | 0.11 |
| 2009 | 36 | 0.12 |
| 2010 | 26 | 0.09 |
| Meadow | Phytosociological Classification | Area * | |
|---|---|---|---|
| [ha] | [%] | ||
| 1. Northern meadow | community with Potentilla erecta and Thalictrum flavum from the Molinion alliance | 2.7 | 1.3 |
| 2. Mid-forest meadows | Molinio-Arrhenatheretea class | 0.3 | 0.1 |
| 3. Sedge community | alliance Molinion var. Carex panicea | 15.6 | 7.5 |
| 4. Near-forest meadow | association Molinietum caeruleae | 5.4 | 2.6 |
| 5. Agrostis canina meadow | association Molinietum caeruleae var. Agrostis canina | 3.4 | 1.6 |
| Total area of non-forest communities | 27.4 | - | |
| Communities | Total Number of Species | Species Richness (S) | Shannon–Wiener Index (H’) | Fodder Value Score (FVS) |
|---|---|---|---|---|
| 1. Northern meadow | 46 | 32.0 | 2.58 | 4.5 |
| 2. Mid-forest meadows | 50 | 27.5 | 2.53 | 4.0 |
| 3. Sedge community | 48 | 22.5 | 2.17 | 3.2 |
| 4. Near-forest meadow | 59 | 30.5 | 2.41 | 3.2 |
| 5. Agrostis canina meadow | 48 | 42.5 | 2.77 | 4.6 |
| Month | Year | Community | Management | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trait | April | June | August | 2009 | 2010 | 1 | 2 * | 3 | 4 | Grazed | Excluded from Grazing |
| Sward height [cm] | 3.51 a | 20.00 c | 14.75 b | 8.82 a | 16.69 b | 10.64 a | 18.19 c | 11.95 b | 10.24 a | 11.25 a | 14.26 b |
| Sward cover [%] | 34.74 a | 78.46 c | 61.63 b | 57.80 a | 58.75 a | 64.48 c | 83.88 d | 34.73 a | 50.02 b | 54.92 a | 61.642 b |
| Characteristic | Group of Plants [% DW] | ||
|---|---|---|---|
| Sedges | Grasses | Other Monocot and Dicot Species | |
| Community | |||
| 1. Northern meadow | 26.49 c | 25.89 c | 47.62 a |
| 2. Mid-forest meadows | 13.95 c | 36.33 d | 49.71 a |
| 3. Sedge community | 77.16 b | 15.13 b | 7.71 b |
| 4. Near-forest meadow | 52.68 a | 27.02 a | 20.30 b |
| Year | |||
| 2009 | 37.05 a | 25.39 a | 37.56 a |
| 2010 | 48.09 b | 26.80 a | 25.12 b |
| Management | |||
| Grazed | 43.92 a | 24.45 a | 31.64 a |
| Excluded from grazing | 41.22 a | 27.74 a | 31.04 a |
| Community | Year | Carex sp. | Poaceae sp. | Other Species | Dominating Species (Two-Year Average) |
|---|---|---|---|---|---|
| 1. Northern meadow | 2009 | 46.06 a | 25.53 a | 58.66 a | Carex panicea, C. elata, C. flava (46%), Molinia caerulea (18%), Potentilla erecta (12%), Agrostis canina (4%) |
| 2010 | 59.30 ac | 26.26 a | 36.59 b | Carex panicea, C. elata, C. flava (59%), Molinia caerulea (8%), Potentilla erecta (2%), Agrostis canina (13%) | |
| 2. Mid-forest meadows | 2009 | 15.82 b | 33.61 ab | 55.95 a | Carex panicea, C. elata, C. flava (16%), Agrostis canina (24%), Molinia caerulea (4%), Potentilla erecta (3%) |
| 2010 | 37.15 a | 39.06 b | 43.48 ab | Carex panicea, C. flava (37%), Thalictrum flavum (20%), Molinia caerulea (3%), Agrostis canina (5%) | |
| 3. Sedge community | 2009 | 75.89 c | 16.38 a | 7.72 c | Carex panicea, C. flava (76%), Molinia caerulea (11%), Agrostis canina (3%) |
| 2010 | 78.43 c | 13.87 a | 7.70 c | Carex panicea, C. flava (79%), Molinia caerulea (4%), Agrostis canina (2%) | |
| 4. Near-forest meadow | 2009 | 10.44 b | 26.04 a | 27.90 b | Carex panicea, C. elata, C. flava (10%), Potentilla erecta (11%), Molinia caerulea (9%), Agrostis canina (15%) |
| 2010 | 17.46 b | 28.01 ab | 12.70 bc | Carex panicea, C. flava (16%), Molinia caerulea (8%), Agrostis canina (8%), Thalictrum flavum (18%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chodkiewicz, A.; Stypiński, P.; Studnicki, M.; Borawska-Jarmułowicz, B. The Influence of Konik Horses Grazing and Meteorological Conditions on Wetland Communities. Agriculture 2023, 13, 325. https://doi.org/10.3390/agriculture13020325
Chodkiewicz A, Stypiński P, Studnicki M, Borawska-Jarmułowicz B. The Influence of Konik Horses Grazing and Meteorological Conditions on Wetland Communities. Agriculture. 2023; 13(2):325. https://doi.org/10.3390/agriculture13020325
Chicago/Turabian StyleChodkiewicz, Anna, Piotr Stypiński, Marcin Studnicki, and Barbara Borawska-Jarmułowicz. 2023. "The Influence of Konik Horses Grazing and Meteorological Conditions on Wetland Communities" Agriculture 13, no. 2: 325. https://doi.org/10.3390/agriculture13020325
APA StyleChodkiewicz, A., Stypiński, P., Studnicki, M., & Borawska-Jarmułowicz, B. (2023). The Influence of Konik Horses Grazing and Meteorological Conditions on Wetland Communities. Agriculture, 13(2), 325. https://doi.org/10.3390/agriculture13020325







