Abstract
Anthracnose disease is still a threat to avocado fruit quality, and the use of fungicide (Plochloraz®) for its control has generated safety concerns that necessitate the search for alternatives. Therefore, the efficiency of lactic acid bacteria (LAB) isolated from fresh fruits and vegetables as biocontrol agents against Colletotrichum gloeosporioides was investigated in this study. Weissella cibaria 21 (LAB 21), Leuconostoc pseudomesenteroides 56 (LAB 56), Weissella confusa 17 (LAB 17), Lactiplantibacillus plantarum 75 (LAB 75), and Lactiplantibacillus plantarum 171 (LAB 171) were evaluated in vitro as potential biocontrol agents to replace the Prochloraz® that is currently used in susceptible avocado (Persea americana Miller) Fuerte fruit. To confirm the biocontrol activity of the selected LAB strains, the antagonistic growth, spore germination, LAB recovery, nutrient competition, acid tolerance, and biofilm formation were assessed. In fruit treated with a LAB cell suspension, curatively inoculated with C. gloeosporioides, or naturally infected avocado cv Fuerte fruit, the epicatechin content and expression of defense-related genes (PAL, LOX, AVFADl 2–3, AVFAEL, and FLS) were compared with Prochloraz® and sterile water (control) treatments. With LAB 56, LAB 75, and LAB 21, significant inhibition of radial mycelial growth (MGI) (>90%) and spore germination (100%) was observed similar to those due to Prochloraz®. The MGI increased with a reduction in nutrient concentration. LAB strains reduced anthracnose disease incidence and severity compared with Prochloraz® and were the highest in LAB 21 and LAB 56. The LAB 21 and LAB 56 strains produced strong biofilms against C. gloeosporioides. In contrast to LAB 56, the control, and Prochloraz®, and LAB 21 had the highest epicatechin content (406 mg/g) and upregulated the PAL, AVFADl 2–3, AVFAEl, and FLS genes, thereby reducing the incidence of anthracnose in avocado fruit. As a result, LAB 21 suspensions can be used as an alternative to Prochloraz® in the control of anthracnose disease.
1. Introduction
Avocado (Persea americana Miller) is a major crop that is globally cultivated in both tropical and subtropical zones [1]. It is an economically important fruit that belongs to the Lauraceae family [2]. The avocado fruit has gained recognition as a heart-healthy fruit due to its high nutritional value and as a source of mono-unsaturated fatty acids, proteins, carbohydrates, minerals, and vitamins [3]. About 4.2 million tonnes of avocado fruit is produced annually and South Africa is one of the major global producers [4]. However, the availability of avocado fruit throughout the year is affected by the huge losses associated with postharvest diseases and poor postharvest management strategies [5].
Anthracnose and stem-end rot diseases caused by Colletotrichum gloeosporioides (Penz & Sacc.) and Lasiodipodia theobromae (Griffiths & Maubl.) are the two predominant postharvest diseases associated with avocado fruit [6,7]. However, the most common pathogen isolated from avocado rot is Colletotrichum gloeosporioides [7]. Anthracnose disease incidence in avocado fruit reduces their quality after harvest, which negatively influences the organoleptic traits and acceptability for markets, locally and internationally. In South Africa, Europe, and other avocado-exporting nations, Prochloraz® is used to manage anthracnose disease in the fruit supply chain. However, the use of Prochloraz® is not desirable in foods due to its deleterious effect on the environment and human health as a class 2 carcinogen [8]. Hence, it is necessary to seek an ecologically friendly, food-grade alternative that can promote food quality and health safety. In this light, the use of edible coatings, edible oils, chitosan loaded with essential oils and functionalized chitosan in the control of anthracnose was reported [9,10,11].
Although the biological control of anthracnose disease was reported, the availability and affordability of biocontrol agents is still a challenge, hence the need to develop more alternatives to ensure the effective control of C. gloeosporioides in fruits. Lactic acid bacteria (LAB) are a group of beneficial microorganisms that forms the microbial consortium in the fruit phyllosphere. Their ability for quick growth in the presence of mild acidic, anaerobic, or partially aerobic conditions [12], along with their safety status (GRAS), make them suitable for use in fruits and vegetables. Moreover, LAB are able to interfere with the microbial dynamics in fruits and vegetables by metabolizing substrates and producing antimicrobials against pathogens in food, hence their use as biocontrol agents against pathogens. Therefore, the use of LAB as biocontrol agents on fruits and vegetables could improve safety and extend the food shelf life.
The genera Lactobacillus, Leuconostoc, Weissella, Enterococcus, and Pediococcus are the most typically isolated LAB strains from raw fruits and vegetables [13,14] and could find application in the industry. Weissella cibaria, Weissella confuse, and Leuconostoc pseudomesenteroides are currently used in the food sector because of their ability to manufacture exopolysaccharides, which help to improve food’s sensory and rheological properties, as well as its shelf life [15]. Therefore the use of pure metabolites, cell-free supernatants, and beneficial microbial strains associated with fruit was found to be effective in the control of fruit postharvest diseases [16]. Hence, this study investigated the potential of selected LABs as a biocontrol agent against anthracnose disease in avocado cv Fuerte fruit. This study evaluated the efficacy of five different LAB strains as biocontrol agents against anthracnose disease in avocado cv Fuerte fruit through in vitro and in vivo assays. This study investigated the (i) effect of LAB strains on the inhibition of C. gloeosporioides mycelial growth and spore germination while deciphering its mechanism of inhibition, (ii) pathogen survival at varied nutrient and pH concentrations in an in vitro assay, (iii) effect of selected LAB strains on anthracnose disease incidence and severity in avocado fruit experimentally, (iv) effect of biocontrol agents on the fruit skin epicatechin content, and (v) effect of biocontrol agents on the expression of antifungal defense genes and biofilm formation.
2. Materials and Methods
2.1. Chemicals and Microbial Cultures
A Colletotrichum gloeosporioides culture formerly isolated from anthracnose-infected avocado fruit was obtained from the Fruit & Vegetable Postharvest Laboratory culture collection of Tshwane University of Technology, Pretoria, South Africa. The University of La Reunion QualiSud’s culture resources provided the LAB strains used in this investigation. Lactiplantibacillus plantarum 75 (LAB 75), Weissella confusa 17 (LAB 17), Weissella cibaria 21 (LAB 21), and Leuconostoc pseudomesenteroides 56 (LAB 56) were initially isolated from freshly sliced cabbage, while Lactiplantibacillus plantarum 171 (LAB 171) was isolated from tomato fruit. The LAB strains were previously isolated on MRS (de Man, Rogosa, and Sharpe) medium and identified using 16S-rRNA, recA, and pheS gene sequencing as earlier reported by Fessard et al. [14]. Avocado (Persea americana) Fuerte cultivar fruit were collected from an orchard packhouse in Tzaneen, Westfalia, Limpopo Province, South Africa. Oligonucleotides were synthesized at Inqaba Biotechnology Pty (Pretoria, South Africa) and biological reagents were supplied by Biolabs (New England). All other reagents were of analytical grade and supplied by Sigma Aldrich (Johannesburg, South Africa).
2.2. Preparation of Microbial Cultures
C. gloeosporioides spores were grown on potato dextrose agar (PDA, Merck) at 25 °C for 4–7 days. C. gloeosporioides agar plugs (6 mm) containing mycelia grown for 7 days were transferred into 30 mL of sterile water and were shaken for 2 min and allowed to stand for 5 min, after which the supernatant containing the conidia was collected. The resulting conidia suspension was assayed for its concentration and adjusted to 106 spores/mL after counting in a haemocytometer. Each LAB strain was reactivated in de Man, Rogosa, and Sharpe (MRS) broth medium, inoculated and sub-cultured on MRS agar, and incubated for 48 h at 30 °C. LAB cells were harvested via centrifugation (6000× g for 10 min), washed in sterile distilled water twice, and then re-suspended in water. The LAB cell concentration was quantified through the optical density (OD) determined at 720 nm using a UV-visible spectrophotometer (SPECTROstar Nano; BMG LABTECH GmbH, Ortenberg, Germany). Cell cultures were adjusted to 108 colony-forming units per millilitre (108 CFU/mL), as described by [17], and the concentration was confirmed via direct plating after serial dilutions [18].
2.3. Efficacy of LAB Strains and Mechanism of C. gloeosporioides Mycelial Growth Inhibition
The efficacy of LAB strains on the inhibition of C. gloeosporioides radial mycelial growth was evaluated in vitro using a double-layer contaminated substrate approach. Individual LAB strains (108 CFU/mL) were inoculated into the MRS agar and overlaid with PDA agar after solidification. From a 7 d old potato dextrose agar (PDA) plate culture of C. gloeosporioides, an agar plug (6 mm diameter) was placed at the centre of the medium plate on which LAB strains had been inoculated. The medium plate was incubated for seven days at 25 °C until the growth on the negative control (sterile distilled water) medium plate was covered by the fungi mycelia. The PDA plates containing C. gloeosporioides agar plugs on which Prochloraz® was added served as a positive control. Mycelial growth was measured for seven days and the mean radial mycelial growth diameter in two perpendicular directions was calculated. Equation (1) illustrates the calculation of the percentage of radial mycelial growth inhibition (MGI%), as provided by Zamani-Zadeh et al. [19]:
where T is the diameter of the colony developing on the medium inoculated with LAB strains and C is the diameter of the colony in the control test.
MGI% = [(C; − T)/C] × 100
To evaluate the fungicidal or fungistatic activities, the agar plugs used for the radial mycelia growth inhibition assay were transferred to a new PDA plate and cultured for 7 days at 25 °C [19]. After 7 days, the fungicidal activity was defined as the absence of further mycelia growth after incubation (final radial mycelia growth > initial radial mycelia growth in new plates), while those without further mycelia growth (final radial mycelia growth ≤ initial radial mycelia growth in new plates) were described as fungistatic. The experiment was done in five replicate samples. The LAB strains with fungicidal activities were further tested in vivo by intentionally or naturally infecting fruit with the pathogen to validate their efficacy in preventing the development of postharvest disease.
2.4. Inhibitory Effect of LAB Strains on Fungal Spore Germination
The inhibitory effect of LAB strains on spore germination was determined using the method described by [20]. A C. gloeosporioides spore suspension was filtered through a three-layer sterile cheese cloth and was quantified by counting at 40× magnification under a microscope using the “Assistenet” Neubauer-improved bright-line haemacytometer (Karl Hecht, Germany). The spore concentration was adjusted to the final concentration of 106 spores/mL. Spores (1 mL) were added individually to peptone water solution (10 mL) in test tubes containing 0.1 mL of 8 log CFU/mL of LAB 17, LAB 21, LAB 56, LAB 75, and LAB 171, while Prochloraz® and sterile distilled water were used as positive and negative controls, respectively. Afterwards, 100 µL of the resulting suspensions were placed at the center of a sterile glass slide, moistened, and incubated at 28 °C for 24 h. Each slide was fixed and stained with lactophenol cotton blue stain, and germinated spores were counted [20]. A spore was considered germinated when the developed germ tube was twice the original diameter of the spores. The experiment was done in five replicate determinations. The results were expressed as percent spore inhibition and percent spore germinated using Equations (2) and (3), respectively:
where Sc represents the average number of spores germinated in the control trials and St represents the average number of spores germinated in the LAB-treated samples.
2.5. Competition for Nutrients
The dual-culture approach was used to evaluate the ability of LAB strains to compete for nutrients with C. gloeosporioides at varied nutrient concentrations [21]. LAB 21 and LAB 56 were selected for further study based on their fungicidal activity on C. gloeosporioides, while sterile distilled water served as the control. PDA and MRS media were prepared at four different concentrations: full strength concentration (100%), half nutrient strength (50%), one-fourth of the full nutrient strength (25%), and one-tenth of the normal nutrient strength (10%). PDA medium was overlaid with MRS agar and the LAB strains (LAB 21 or LAB 56) were streaked on a side of the medium, while a 5 mm plug of C. gloeosporioides mycelia was placed on the other side of the plate. The plates were incubated in the dark at 28–30 °C for 7 days, the diameter of the mycelial growth was measured (mm) daily, and the mycelial growth inhibition (MGI) was calculated as described in equation 1.
2.6. Acid Tolerance of C. gloeosporioides
The acid tolerance of the C. gloeosporioides at various pHs (2.0, 3.0, 4.0, 4.5, 5.0, 6.0, 7.0, and 8.0) was investigated. Spores (106 CFU/mL) from a 7-day-old culture of C. gloeosporioides were suspended in sterile distilled water. The medium pH was adjusted to an appropriate pH with 1 M HCl through a digital pH meter (Meltter-Toledo Instruments Co., Shanghai, China), while a pH 7 medium was used as the control. Spores were inoculated on sterile PDA agar plates with different pHs and were incubated at 25 °C for 5 days. The number of colony-forming units per millilitre of fungal spores was calculated [22].
2.7. Determination of Biofilm Formation against C. gloeosporioides
The method described by Akinola et al. [23] was utilized in this study with slight modification. Cell turbidity was adjusted to 0.05 OD (106 CFU/mL) in a UV-visible spectrophotometer at 600 nm after LAB 56 and LAB 21 strains were cultured overnight at 30 °C in MRS broth. An aliquot of 200 μL broth was placed in 96-well polystyrene microtitre plates, comprising 100 μL of broth, 50 μL of LAB cultures (LAB 21 or LAB 56), and a 50 μL suspension of C. gloeosporioides spores in distilled water. Sterile distilled water served as the negative control (100 μL), and the positive control (100 μL) was C. gloeosporioides spore suspension. The microtitre plate was kept in a static state and incubated at 30 °C for 3, 24, 48, and 72 h. Afterwards, the wells were rinsed five times with phosphate buffer to eliminate loosely connected bacteria cells. For 45 min, the plate was air dried before adding 200 μL of 1% crystal violet solution and de-stained with 200 μL of an acetone–ethanol solution (4:1) prior to quantitative investigation of biofilm formation. The optical density of the solution in each well was determined using a microtitre plate reader at OD 570 nm (SPECTROstar Nano; BMG LABTECH GmbH, Ortenberg, Germany). The OD value was utilized to determine how the rate of biofilm production was influenced by the incubation time. The conditions stated by Akinola, Tshimpamba, Mwanza, and Ateba [23] for weak biofilm formation, intermediate biofilm formation, and robust biofilm production were used in this study. The experiment was conducted in six replicate wells.
where ODC is the OD of negative control, ODS is the OD of the sample, and OD is the optical density.
ODS < ODC denotes no biofilm formed, ODC < ODS < 2 × ODC denotes a weak biofilm formed, 2 × ODC < ODS < 4 × ODC denotes a moderate biofilm formed, and 4 × ODC > ODS denotes a strong biofilm formed,
2.8. Control of Anthracnose Incidence and Severity in Avocado cv Fuerte Fruit In Vivo
2.8.1. Fruit Preparation
A total of 344 avocado (Persea americana) “Fuerte” cultivar fruit that were free of decay were collected at the commercial maturity stage (25% dry matter) and were transported within 6 h of harvest at 14 °C to the Fruit & Vegetable Laboratory, Tshwane University of Technology, South Africa. The fruit were allowed to trigger ripen before use in the study. The surface of the fruit was cleaned by washing in 0.01% NaOCl for 5 min and then rinsed in sterile distilled water before air drying at 25 °C. The fruit were kept at 75% relative humidity (RH) for 48 h at 20 °C until they were slightly soft in firmness. Avocado fruit were randomly selected and allocated to each treatment.
2.8.2. Inoculation and Control of Anthracnose Disease in Avocado Fruit
The trigger-ripened fruit were wounded with a cork borer (6 mm diameter and 2 mm deep) at the equatorial section and were subjected to curative and preventative inoculation treatments. In the curative and preventative experimental study, each treatment contained three replicate boxes of six fruit, each totalling 72 fruit, while for the naturally infected study, a total of 200 fruit comprising five replicate boxes with 10 fruit each were used.
In the curative experiment, wounded fruit (72 fruit) were inoculated with 20 µL of C. gloeosporioides spore suspension (106 spores/mL). Thereafter, the inoculated fruit were held at 20 °C for 18 h to ensure penetration of the pathogen into the fruit. Afterwards, the inoculated fruit were divided into treatment groups comprising sterile distilled water (control), Prochloraz® (1000 mg/L) as the commercial control, LAB 21, and LAB 56. The fruit were dipped for 5 min in each LAB cell culture suspension (log 8 CFU/mL), Prochloraz®, and sterile water, and were held at 25 °C for five days at 75% RH, as described by Sellamuthu, Sivakumar, Soundy, and Korsten [9]. The fruit for the preventative experiment were dipped for 5 min in the four postharvest treatments mentioned above. The fruit were then air dried for 3 h at 25 °C to ensure the treatment gained access to the wounds. About 20 µL of C. gloeosporioides spores (106 spores/mL) was inoculated into the fruit. Thereafter, the fruit were held for 18 h at 25 °C and 75% RH, and stored at 7.5 °C for 28 days at 85% RH to model the shipment conditions on the sea. Afterwards, the fruit were held at 18 °C for 5 days while mimicking market shelf life conditions [10].
The effectiveness of LAB 21 and LAB 56 cell culture suspensions on the control of anthracnose disease was investigated in comparison to Prochloraz® in a natural infection experiment. A total of 30 trigger-ripened fruit per treatment comprising 5 replicate boxes of 10 fruit each was used in the experiment. The fruit were dipped in the treatments above, allowed to dry, and stored at 85% RH for 28 d at 6.5 °C. Afterwards, the fruit were kept at 18 °C for seven days in retail shelf-life conditions [9]. The percent anthracnose disease incidence in curative, preventative, and naturally infected and treated fruit was determined for 5 days, as described by Mokgalapa, Akinola, Shoko, Pillai, and Sivakumar [10]. The percentage of rotten fruit skin was used to evaluate the anthracnose severity in fruit. Fruit were assigned “1” when the fruit had 0% rotten pericarp and was considered as no severity, 25% of rotten fruit pericarp was regarded as low severity and assigned “2”, 26–50% of rotten pericarp was assigned “3” (moderate), 51–75% of rotten pericarp was assigned “4” (high), and 76–100% rot was assigned “5” (very high), as described by Hossain and Iqbal [24].
2.8.3. Recovery of the C. gloeosporioides and LAB Strains from Curative and Preventatively Inoculated “Fuerte” Avocado Fruit
The recovery of C. gloeosporioides and LAB survival in curative and preventatively inoculated avocado fruit was determined in fruit stored for five days using the standard plate count method [18]. After five days’ storage, the fruit were randomly selected, and a gram of pericarp was washed in ringer solution of quarter strength (Merck, Pretoria South Africa) and the surface effluent was filtered in 0.22 mm. An aliquot of filtrate from each treatment was serially diluted and spread-plated on PDA and MRS agar media in five replicates. The PDA and MRS agar plates were incubated at 28 °C for 5–7 days and at 30 °C for 48 h, respectively. The fungal and bacterial counts were evaluated as fungal or bacterial colony-forming units per gram of fruit pericarp.
2.9. Epicatechin Contents in Inoculated and Naturally Infected Avocado Fruit
The epicatechin content in the curatively inoculated and naturally infected treated fruit was quantified as described by Mokgalapa, Akinola, Shoko, Pillai, and Sivakumar [10] in an HPLC system (PerkinElmer, Waltham, MA, USA). Freeze-dried samples (0.5 g) were defatted using hexane before extraction and filtering for injection into the HPLC systems. Epicatechin peaks appeared at wavelengths of 280 nm and 320 nm and the confirmation of identity was achieved via a comparison of the retention times and absorption spectra with that of a pure standard. The resulting data were expressed as micrograms of epicatechin per gram of fruit (µg/g of dried weight).
2.10. Expression of Defence-Related Genes in Curatively Treated Avocado Fruit
2.10.1. Ribonucleic Acid (RNA) Extraction and Primer Design
Total RNA was isolated from both treated and untreated frozen avocado tissue (−80 °C) in the presence of liquid nitrogen using the Zymo Quick-RNATM Plant Miniprep kit (Zymo Research Corporation, Inqaba Biotech, South Africa) according to the manufacturer’s instructions. The RNA purity and quality were determined spectroscopically as described by Osondu, Akinola, Shoko, Pillai, and Sivakumar [11]. The primers for phenylalanine ammonia lyase (PAL), lipoxygenase (LOX), fatty acid elongase (AVFAEL), Δ12 fatty acid desaturase (AVFAD1 2–3), and flavanol synthase (FLS) were designed using Primer3 software. A housekeeping gene (actin) was used as a study reference gene and was constructed in relation to Persea americana messenger RNA coding for actin (GU272027.1).
2.10.2. Reverse Transcription and Quantitative Real-Time PCR
The LunaScriptTM RT SuperMix kit (New England Biolabs) was used to synthesize cDNA from 1 ng of RNA utilizing the RNA reverse transcription method in a CFX Connect Real-Time PCR Systems (Biorad Thermal Cycler, Singapore). The real-time qPCR (rt-qPCR) amplification was done using the Luna Universal qPCR Mastermix (New Englands Biolabs) and the SYBR-green fluorescence system in a two-step amplification of PAL, LOX, AVFAD1 2–3, AVFAEL, and FLS genes as described by Mokgalapa, Akinola, Shoko, Pillai, and Sivakumar [10]. The internal control was the no-reverse transcription mix (no–RT), while the negative control was the non-template (no RNA). A total reaction cocktail (20 µL) containing 1× qPCR Mastermix (10 µL) and 10 mM forward and reverse primers (0.5 µL) each, and a cDNA template (4 µL) was used for gene amplification. The PCR protocol included initial activation (95 °C for 1 min); denaturation (95 °C for 15 s); annealing and polymerization (55 °C for 30 s) for PAL, LOX, and actin genes; 53 °C for 30 s for AVFADl 2–3 and AVFAEL; and 54.5 °C for 30 s for FLS over 40 cycles. The melt curve analysis was utilized to determine the specificity of PCR products throughout a temperature range of 53.5 to 95 °C, with increments of 0.5 °C. Actin, which is a housekeeping gene in plant tissues, was used as the reference gene in the rt-qPCR. CFX Maestro software (version 4.12433.1219) was used to configure the requirements for the qPCR amplification system, with a threshold value (Ct) less than or equal to 38 (Ct ≤ 38) and efficiency (90–110%). The relative fold increase of genes (2−∆∆Ct) influenced by the treatments was computed using the untreated as a reference [11].
2.11. Statistical Analysis
The efficacy of the LAB strains on the fungal mycelia growth was measured using five replicates and in two independent experiments and the average data were acquired. Descriptive analysis was used to evaluate the means and percentages of values, while the analysis of variance in the Statistical Programme Genstat (version 11.1, International, Hempstead, UK) was used for the data analysis. Individual treatment means were compared using Fisher’s protected least significant difference (LSD) at a p ≤ 0.05 level of significance.
3. Results and Discussion
3.1. The Effects of LAB Strains on the Mycelial Growth Inhibition of C. gloeosporioides
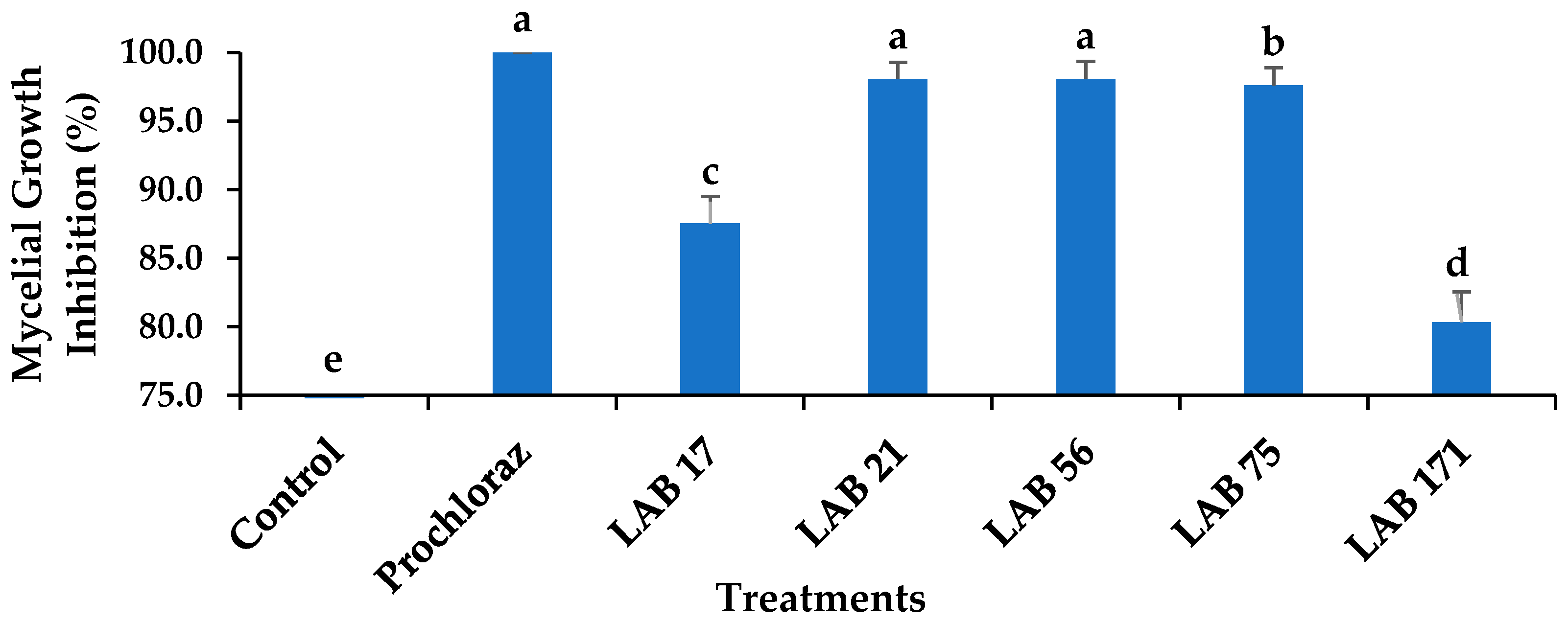
The radial mycelial growth inhibition of C. gloeosporioides by LABs compared with Prochloraz® ranged from 80.34% to 100% inhibition and was the lowest for LAB 17 (87.53%). Out of the five LABs strains tested, both LAB 21 and LAB 56 showed 98.08% inhibition of C. gloeosporioides mycelial growth and were not significantly different from each other and Prochloraz® (p ≤ 0.05), as shown in Figure 1. Inhibition of radial mycelial growth by LAB 21 and LAB 56 was comparable to that of a commercially available fungicide (Prochloraz®) that controls anthracnose disease in fruit. The production of lactic, acetic, and formic acids by LAB strains influences their ability to inhibit mycelial growth [18]. Konsue et al. [25] found that LAB strains significantly inhibit the mycelial growth of C. gloeosporioides isolated from mango fruit. In vitro, LAB 17, LAB 75, and LAB 171 exhibited fungistatic activity, whereas LAB 21 and LAB 56 showed fungicidal activity. Therefore, LAB 21 and LAB 56 were selected for further experiments in our study both in vitro and in vivo. The LAB strains could have produced antifungal metabolites that inhibited the growth of C. gloeosporioides by acidifying the growing medium. Furthermore, antimicrobial properties of LAB strains were attributed to the production of 2,3-butadione; reuterin (3-hydroxypropionaldehyde); acetaldehyde; hydrogen peroxide; hydroxyl radicals; and proteins, such as bacteriocins [26]. Weissella species are acid tolerant; can survive at a wide range of ideal growth temperatures; and can produce lactic acid, ethanol, butanol, and antibacterial compounds [27], as demonstrated in this study. Salah-Abbès et al. [28] observed that Ltp. plantarum inhibited Fusarium graminearum growth in vitro.
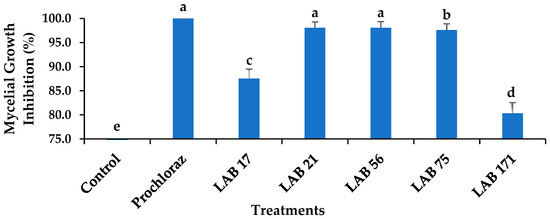
Figure 1.
Effects of LAB strains on the mycelial growth inhibition of Colletotrichum gloeosporioides after 7 days of incubation at 26 ± 2 °C. Each error bar represents the standard deviation of the mean of five replicates. Columns with different letters were significantly different at p ≤ 0.05 level. Keys: control—sterile distilled water; Prochloraz® (positive control); LAB 21—Weissella cibaria 21; LAB 17—Weissella confusa 17; LAB 56—Leuconostoc pseudomesenteroides 56; LAB 171—Ltp. plantarum 171; LAB 75—Ltp. plantarum 75.
3.2. Effects of LAB Strains on C. gloeosporioides Spore Germination
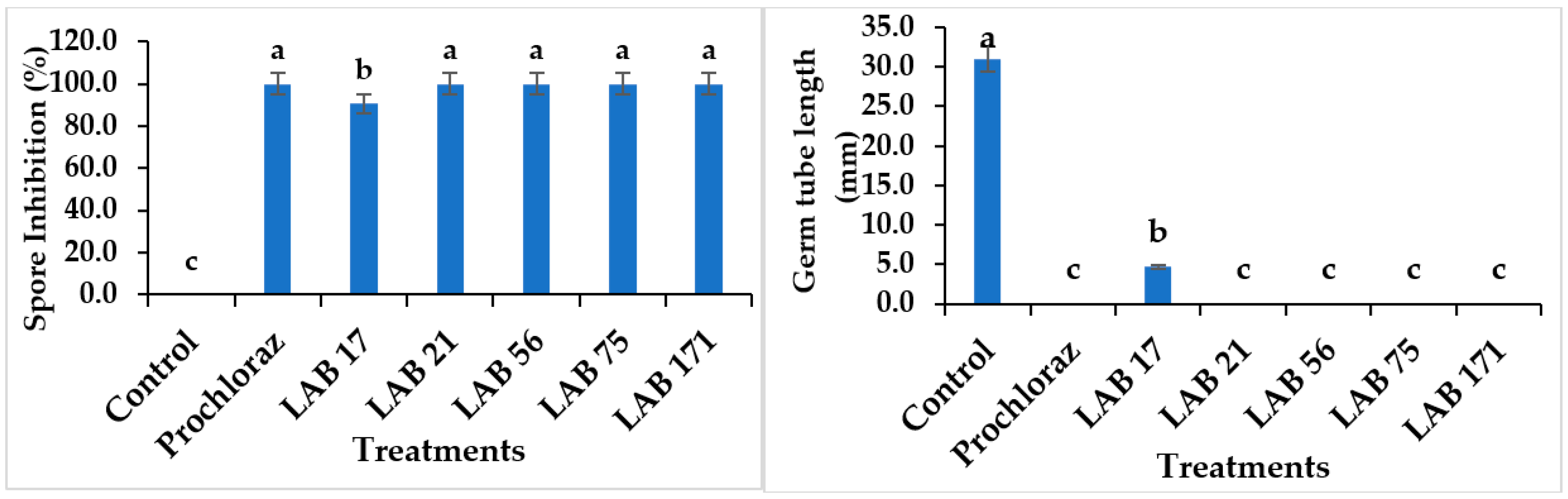
The in vitro percent inhibition of C. gloeosporioides spore germination in the LAB strains ranged from 90 to 100%, as shown in Figure 2. All LAB strains, except LAB 17 (90.76%), had a total inhibition of C. gloeosporioides spore germination within 24 h of incubation and the effects were not significantly different from Prochloraz® (p ≤ 0.05). However, the presence and length of the germ tube in the control suggest a 100% spore germination, while no germ tube was observed in LAB 21, LAB 56, LAB 75, LAB 171, and Prochloraz®, except LAB 17. The inhibition of C. gloeosporioides spores by LAB strains supported the previous report on the antifungal activities of Lb. paracasei against C. gloeosporioides spores [29]. However, our study reported a higher percentage of inhibition of C. gloeosporioides spores by LAB strains.
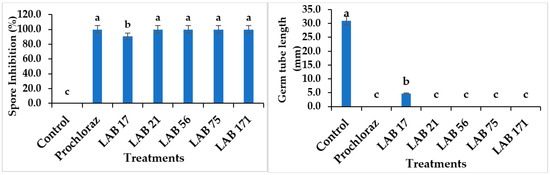
Figure 2.
Effects of LAB strains on the percent inhibition of C. gloeosporioides spores and length of the germ tube. Similar columns with different letters were significantly different at p ≤ 0.05 level. Keys: control—sterile distilled water; Prochloraz® (positive control); LAB 21—Weissella cibaria 21; LAB 17—Weissella confusa 17; LAB 56—Leuconostoc pseudomesenteroides 56; LAB 171—Ltp. plantarum 171; LAB 75—Ltp. plantarum 75.
3.3. Competition for Nutrients between LAB Strains and C. gloeosporioides
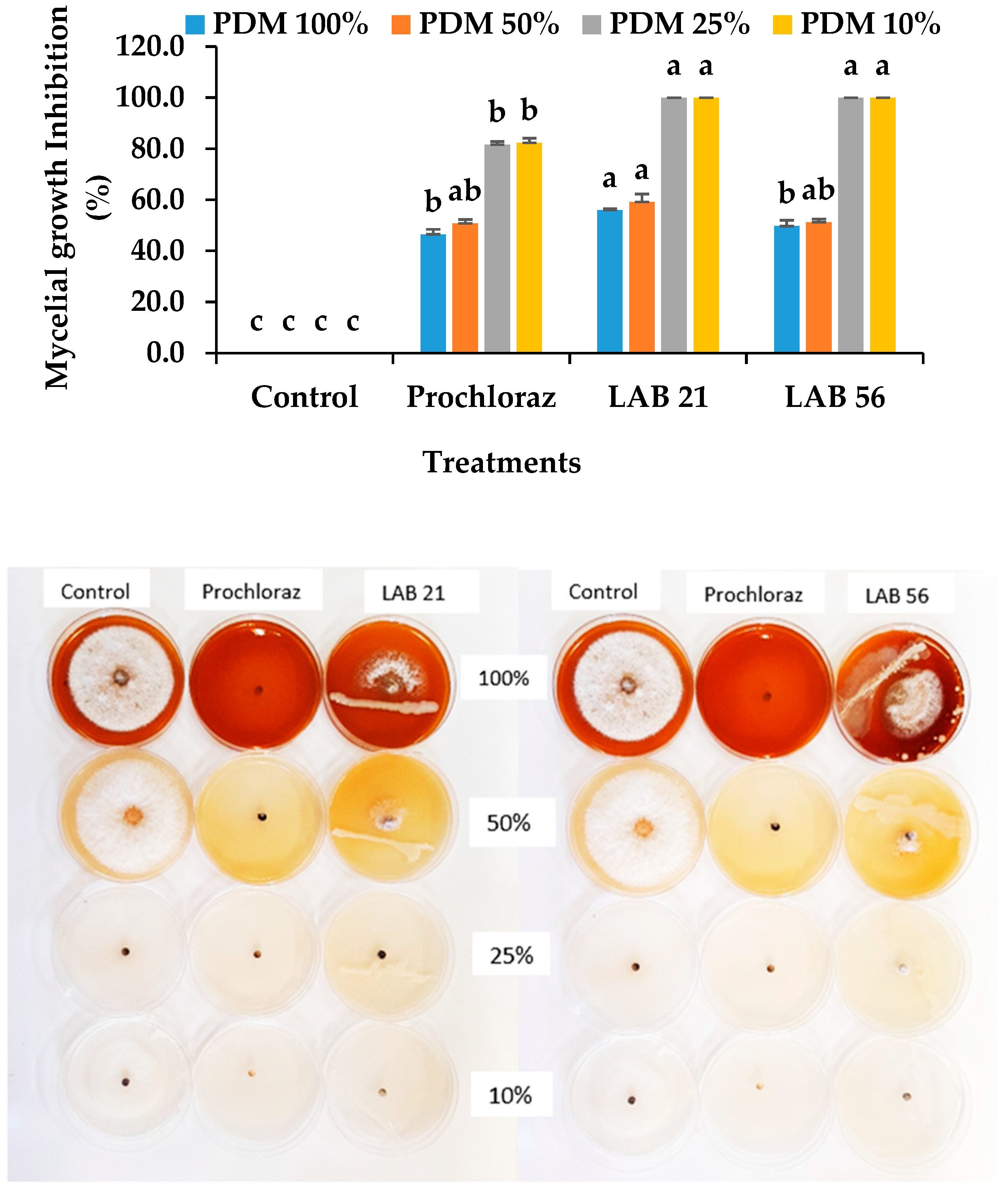
At lower nutrient concentrations, the LAB 21 and LAB 56 strains totally (100%) inhibited the mycelial growth of C. gloeosporioides and was at the maximum in one-fourth (PDM 25%) and one-tenth percent (PDM 10%) of the typical PDA concentration (Figure 3). The LAB 21 and LAB 56 inhibition of C. gloeosporioides mycelial growth was significantly different from the control but not from Prochloraz®. There was no significant difference between LAB 21 and 56 at PDM 25% and PDM 10% (p ≥ 0.05), while a significant difference existed at full strength (PDM 100%) concentrations (p ≤ 0.05). At the half-strength (PDM 50%) concentration, LAB 21 and 56 were only slightly different from each other but different from the control (p ≥ 0.05). This suggested the ability of LAB 21 and 56 to survive with low nutrients, compete and efficiently deplete the growth medium, and thereby limit the pathogen’s access to nutrients. As a defence against the pathogen, these LABs might have produced some metabolites that inhibited the growth of C. gloeosporioides. Our findings were consistent with the report of the inhibition of fungal pathogen growth due to the use-up of glucose by lactic acid bacteria [30]. Similarly, Konsue, Dethoup, and Limtong [25] reported a maximum inhibition of C. gloeosporioides when the LAB strains were dual-cultured on a PDA plate at one-tenth of the normal medium concentration.
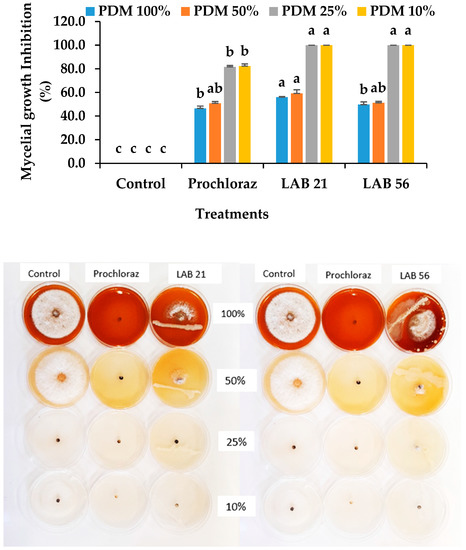
Figure 3.
Effects of the LAB strains on nutrient competition and inhibition of C. gloeosporioides mycelial growth. Similar columns with the same alphabetic letter were not significantly different (p ≤ 0.05). Keys: control—sterile distilled water; LAB 21—Weissella cibaria 21; LAB 56—Leu. pseudomesenteroides 56; PDM 100%—full strength (100%); PDM 50%—half-strength (50%); PDM 25%—one-fourth concentration (25%); PDM 10%—one-tenth concentration (10%).
3.4. Acid Tolerance of C. gloeosporioides
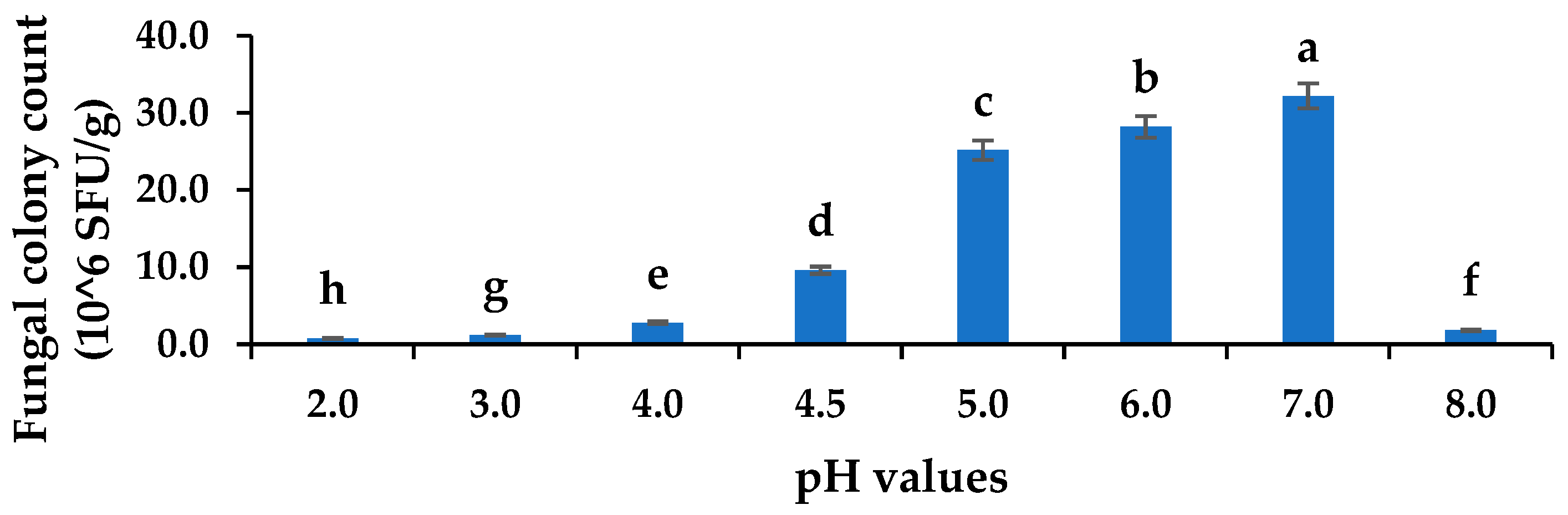
The pathogen colony counts at 72 h of cultivation ranged from (0.75 to 32.2) × 106 SFU/g. There was a significant difference between the fungal counts at different pHs (p ≤ 0.05). The population of C. gloeosporioides decreased in a highly acidic or alkaline medium, and the C. gloeosporioides colonies were the most abundant at normal pH 7 (Figure 4). Moreover, the alkaline pH 8 (1.8 × 106 SFU/g) did not favour the growth of C. gloeosporioides, and neither did the strongly acidic pHs 2, 3, and 4 as the colony count declined. Thus, the fungus could tolerate mild acidity, allowing them to grow in fruits. Furthermore, the LAB 21 and 56 strains have the potential to produce organic acids (lactic acid), which invariably lower the pH levels and inhibit C. gloeosporioides. A similar observation of unfavourable conditions for fungal growth in highly acidic pH and pH 9–11 (alkaline) was reported by Ali et al. [31], thus supporting the finding on C. gloeosporioides in this study. Therefore, the observed increase in mycelia inhibition of C. gloeosporioides could be due to the release of organic acids by the LABs during metabolism thereby causing the inhibition of the fungal pathogen.
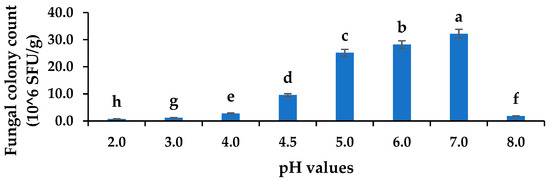
Figure 4.
Acid tolerances of C. gloeosporioides at various pH concentrations. Columns with different letters were significantly different at the p ≤ 0.05 level. Keys: SFU/g—spore forming units per gram.
3.5. Effect of Incubation Time on the Production of Biofilms against Colletotrichum gloeosporioides
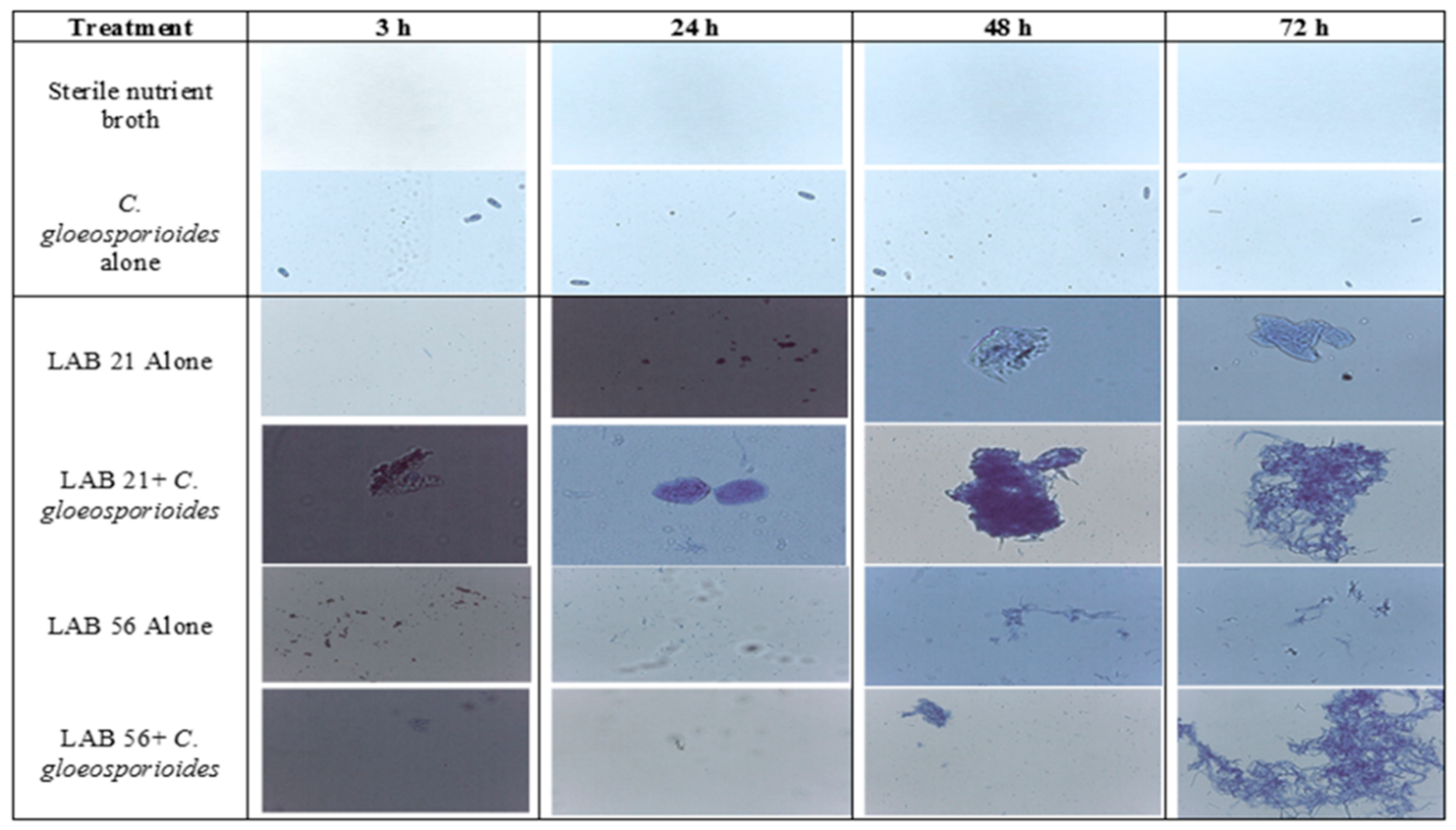
The optical densities (ODs) of selected LABs and C. gloeosporioides on their own ranged from 0.09 to 0.36 and the co-cultures of LABs and C. gloeosporioides ranged from 0.16–2.35 (Table S1), while the microscopic observation of biofilm formation, as influenced by incubation time, is presented in Figure 5. The optical density of LAB 21 and LAB 56 increased with incubation time and was highest in the co-culture treatments, especially in the LAB 21 + C. gloeosporioides (OD: 2.35) co-culture. The biofilm formation using LAB 21 or LAB 56 in a C. gloeosporioides co-culture was much higher in biomass than in LAB 21 or LAB 56 single cultures compared with the control and C. gloeosporioides as standalone samples. LAB 21 and LAB 56 were identified to have the potential to produce biofilm [32]. LAB 21 and LAB 56 showed a rapid biofilm formation against C. gloeosporioides as the incubation time increased. The level of biofilm formation ranged from no biofilm in the control and C. gloeosporioides treatments to a strong biofilm in the co-culture of LAB 21 or LAB 56 and C. gloeosporioides (Table S1). These findings were substantiated via observation on a 40× light microscope (Leica DM 3000, Germany’s Leica Microsystem), as shown in Figure 5.
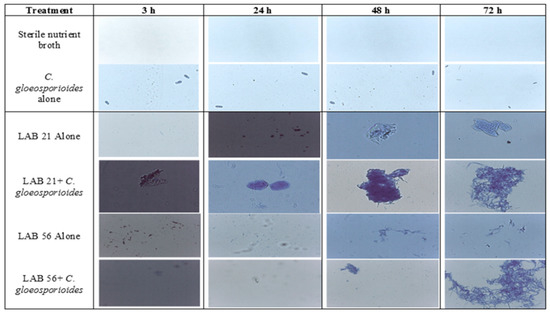
Figure 5.
Effects of the incubation time on biofilm formation by LAB strains against C. gloeosporioides. Keys: control—sterile distilled water; LAB 21—Weissella cibaria; LAB 56—Leu. pseudomesenteroides 56; LAB 21 + C. gloeosporioides—Weissella cibaria 21 + C. gloeosporioides; LAB 56 + C. gloeosporioides—Leu. pseudomesenteroides + C. gloeosporioides; C. gloeosporioides (fungal pathogen).
The LAB strains showed the ability to produce biofilm as a strategy of protection and antagonistic activity against the pathogen. Biofilm production was reported as one of the methods by which LABs suppress pathogen development and survive within matrixes. Similar results were indicated in the study that was conducted by Fan et al. [33] on the biofilm production potential as a means of survival of Lactobacillus species in a co-culture with Saccharomyces cerevisiae. From Figure 5, the observation of C. gloeosporioides under the light microscope suggested no attachment or aggregation of biofilms by the pathogen with increasing incubation time; however, germ tube formation was observed at 72 h compared with LAB 21 and LAB 56, which had biofilm formation at 48 h and 72 h of incubation. Moreover, the co-culture of LAB 21 or LAB 56 and C. gloeosporioides showed a significant aggregation of biofilms after 48 h of incubation relative to the standalone C. gloeosporioides culture and nutrient broth. This suggested that the LABs used biofilm aggregation and entrapments as a strategy against the growth of C. gloeosporioides.
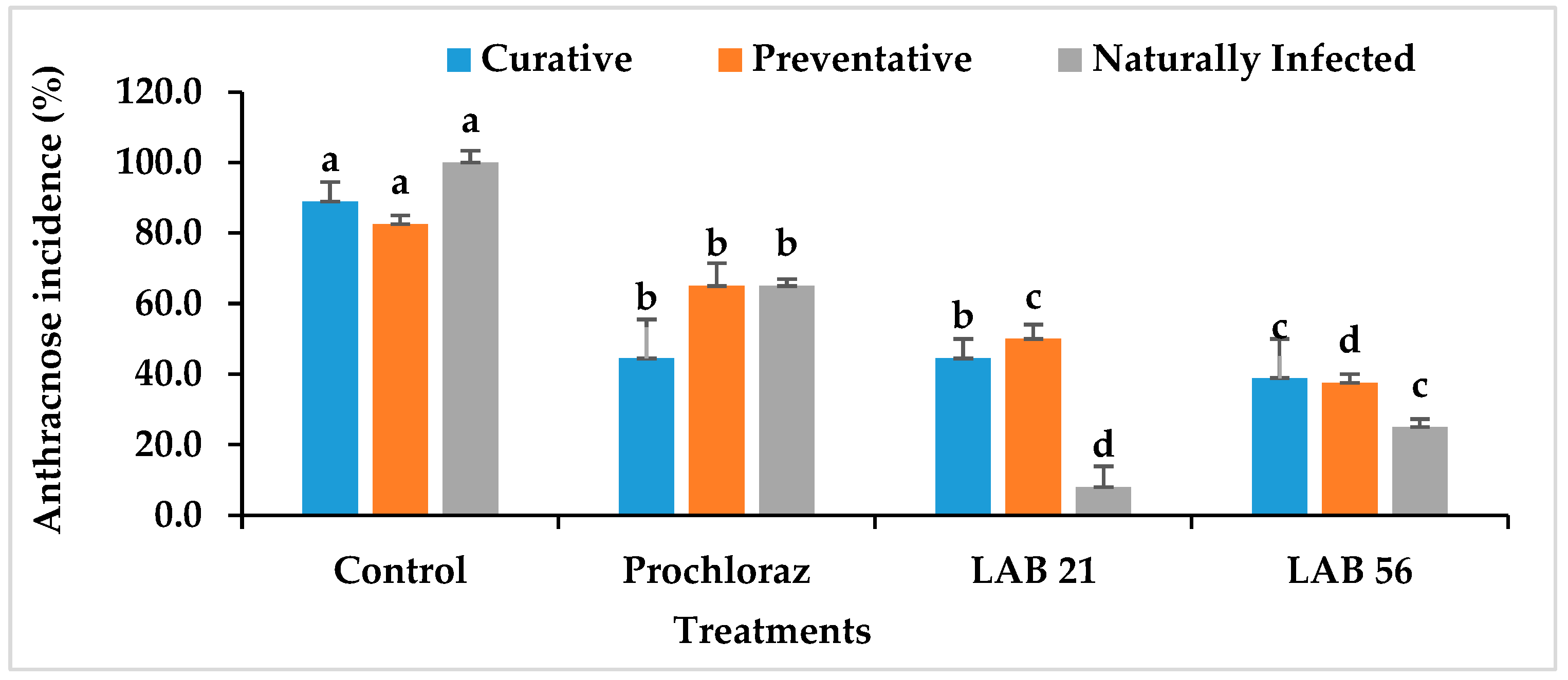
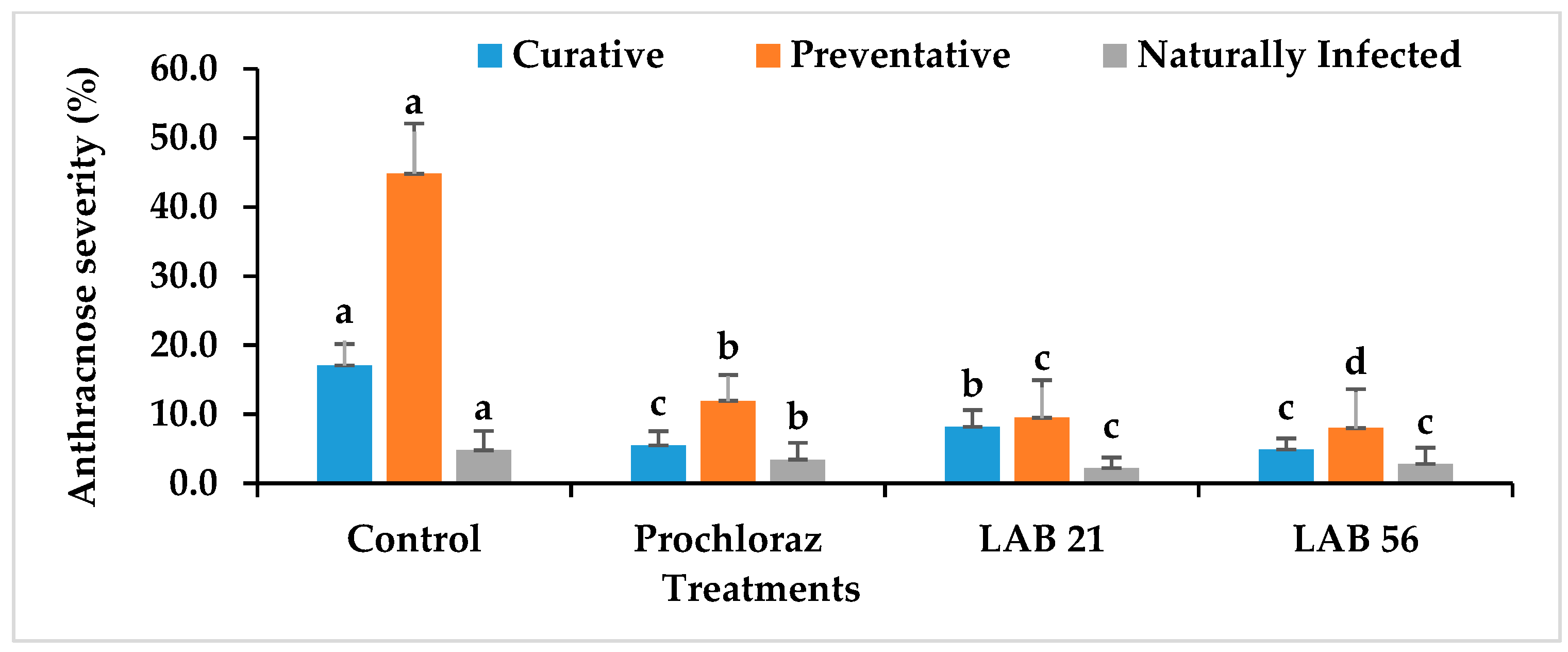
3.6. Disease Incidence and Severity in LAB-Treated Avocado Fruit
The anthracnose disease incidence and severity in preventatively, curatively, or naturally infected avocado fruit due to LAB strain treatment is presented in Figure 6. The anthracnose disease incidence and severity were significantly reduced in LAB-strain—treated avocado fruit compared with the control and Prochloraz®. However, LAB 56 had the lowest anthracnose disease incidence (38.8%) compared with Prochloraz® (44.4%) and the control (88.8%) in the curatively treated fruit. The anthracnose disease incidence in LAB-21-treated avocado (44.4%) was not significantly different from Prochloraz® (p ≥ 0.05). The anthracnose disease severity ranged from 3.8 to 17.05% and was the lowest with LAB 56 (3.8%). The disease severity in LAB-56-treated fruit was not significantly different from those treated with Prochloraz® (5.5%) in the curatively treated avocado fruit.
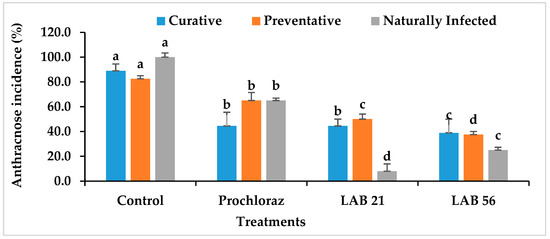
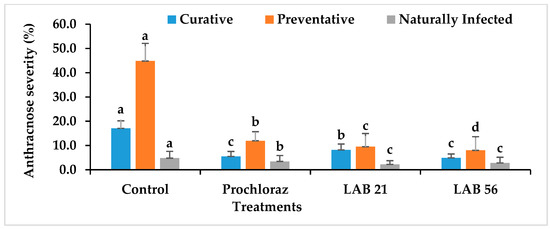
Figure 6.
Effects of LAB strains on anthracnose disease incidence and severity in curatively, preventatively, and naturally infected fruit. Similar columns with different letters were significantly different at the p ≤ 0.05 level. Keys: control—sterile distilled water; Prochloraz® (positive control); LAB 21—Weissella cibaria 21; LAB 56—Leuconostoc pseudomesenteroides 56.
In the preventatively inoculated avocado fruit, there was a reduction in anthracnose incidence in the LAB-strain-treated fruit (Figure 6). The percent disease incidence ranged from 25% with LAB 56 to 100% in the control fruit. LAB 56 demonstrated a pronounced inhibition of anthracnose disease in infected avocado fruit compared with Prochloraz® and other treatments. The anthracnose disease severity was the lowest with LAB 56 (8%) and highest in the control (44.83%) in the preventative experiment (Figure 6). The LAB strain treatments significantly reduced the disease severity in preventatively infected avocado fruit compared with Prochloraz® and sterile distilled water. Fruit treated with LAB 21 (9.5%) and LAB 56 (8%) had significantly lower disease severity relative to Prochloraz® and the control (p ≤ 0.05). The control had the highest disease severity when compared with other treatments.
Lower anthracnose incidence was observed in fruit treated with LAB 21 (8%) and LAB 56 (25%) compared with the control (100%) in the naturally infected fruit. The anthracnose disease incidence and severity were drastically reduced by LAB 21 and LAB 56 treatments than the control (Figure 6). The anthracnose severity was observed in both the control and Prochloraz® on day 2, while both the LAB-21- and LAB-56-treated fruit displayed disease on day 4 (data not shown) and day 5 in the naturally infected fruit. The control had the highest significant disease incidence and severity compared with the Prochloraz®-, LAB-21-, and LAB-56-treated fruit (p ≤ 0.05).
The application of biocontrol agents against fruit deterioration is a more environmentally friendly substitute to the use of chemical fungicides [34] in the control of fruit rot in avocado fruit. The inhibitory activities of selected LAB strains against anthracnose disease could be linked to the primary role of LABs in food preservation systems [35]. The inhibitory activities of the LAB strains against the anthracnose-causing pathogen in avocado fruit corroborated the observations made in the in vitro assays (MGI, spore germination, nutrient competition, C. gloeosporioides, and LAB survival at various pHs). A similar observation was reported by Trias Mansilla et al. [36], where W. cibaria significantly reduced fungal rot infection levels by 50% in golden delicious apples. Thus, LAB 21 and LAB 56 could have utilized their ability to produce antimicrobial compounds, such as the organic phenylacetic, fumaric, malic, acetic, citric, and lactic acids [27], coupled with its biofilm entrapment in the inhibition of C. gloeosporioides in avocado fruit. Therefore, LAB 21 and LAB 56 could serve as a replacement to the currently used fungicide (Prochloraz®) in the avocado fruit industry.
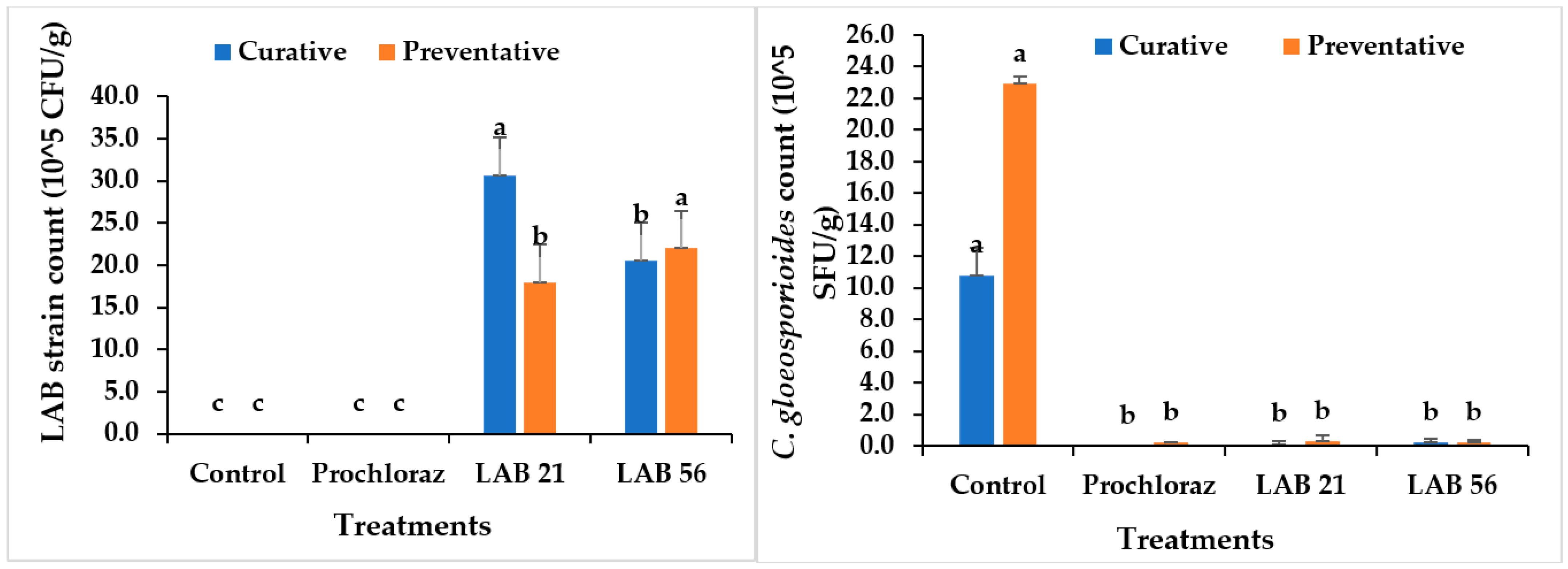
3.7. LAB and Fungal Pathogen Recovery in Infected Avocado Fruit
Selected LAB strains and C. gloeosporioides were recovered in curatively and preventatively treated avocado fruit, as indicated in Figure 7. In avocado fruit subjected to postharvest treatments, the LAB and C. gloeosporioides populations recovered ranged from 0 to 30.6 × 105 CFU/mL and 0 to 10.8 × 105 SFU/g, respectively, in the curative experiment. LAB 21 (30.6 × 105 CFU/g) was the highest recovered in the curative experiment on day 5 (p ≤ 0.05), while the control had the highest recovery of C. gloeosporioides (10.8 × 105 SFU/g). There was no significant difference in C. gloeosporioides recovery between treatments, except for the control, as shown in Figure 7 (p ≥ 0.05). C. gloeosporioides had no growth in Prochloraz®-, LAB-21-, and LAB-56-treated fruit in the curative experiment, thus suggesting a fungicidal activity of the LAB strains and Prochloraz®. Furthermore, in the preventatively inoculated avocado fruit, the C. gloeosporioides recovery ranged from (0.2 to 22.9) × 105 SFU/g and was the highest in the control similar to the observation in the curative experiment, while LAB 21, LAB 56, and Prochloraz® were not significantly different from each other (p ≥ 0.05). Likewise, LAB 56 showed the highest LAB recovery when compared with each other treatments. The observation by Trias Mansilla, Bañeras Vives, Montesinos Seguí, and Badosa Romañó [36] on the maintained population of LAB strains in wounded fruit after storage corroborates the observation in this study. Hence, this study clearly showed that the LAB 21 and LAB 56 treatments were effective at controlling anthracnose disease in avocado fruit and can be used as an alternative to Prochloraz® in the control of anthracnose disease in Fuerte avocado fruit.
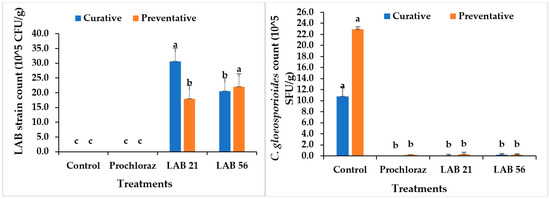
Figure 7.
Recovery of LAB strains and C. gloeosporioides in curatively and preventatively inoculated avocado fruit. Similar columns with different letters were significantly different at the p ≤ 0.05 level. Keys: control—sterile distilled water; Prochloraz® (positive control); LAB 21—Weissella cibaria 21; LAB 56—Leuconostoc pseudomesenteroides 56.
3.8. Effects of LAB Strains on the Epicatechin Content in Treated Avocado Fruit
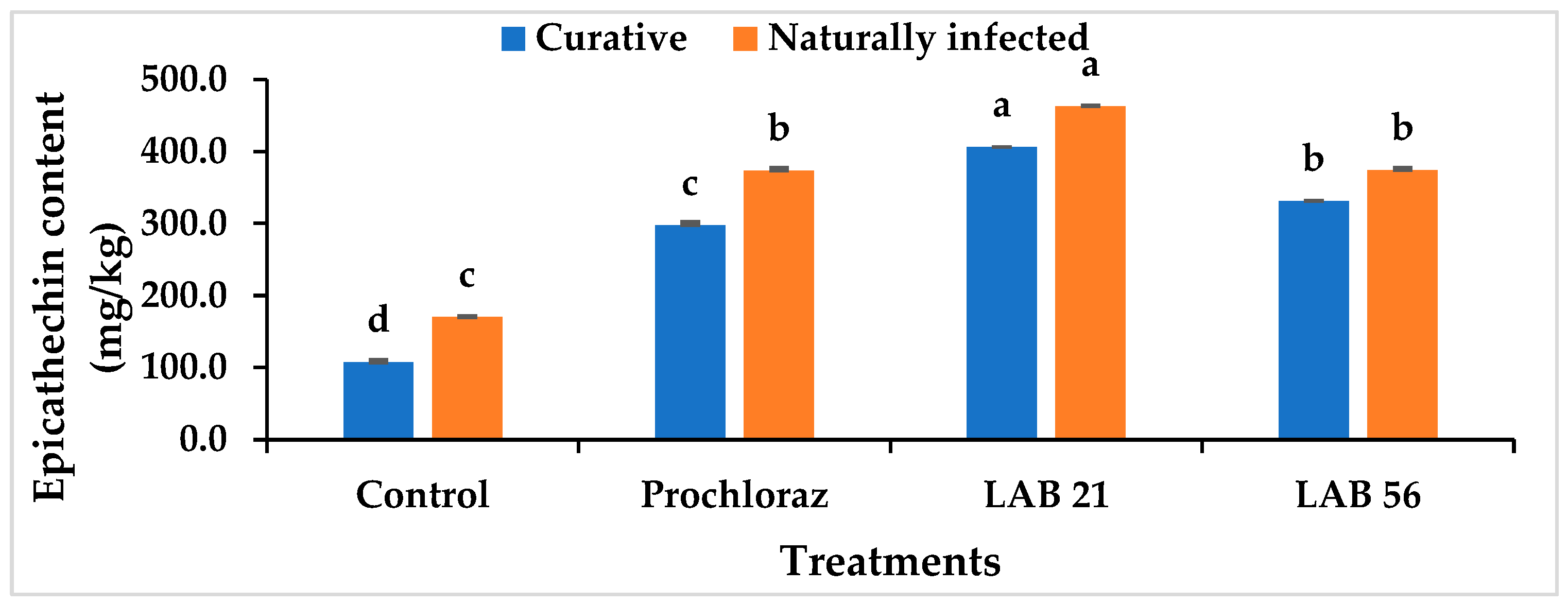
Figure 8 shows the skin epicatechin content of the curatively and naturally infected fruit. The skin epicatechin level in the pericarp of curatively treated and naturally infected fruit was significantly higher after the LAB 21 treatment compared with the LAB 56 and Prochloraz® treatments and the untreated fruit. In naturally infected fruit treated with LAB 21, the epicatechin content increased to 462.49 mg/kg, while the fruit treated with Prochloraz® showed a content of 373.28 mg/kg. Compared with the other three postharvest treatments, the epicatechin content of the control fruit was significantly lower in curatively (107.02 mg/kg) and naturally infected fruit (169.82 mg/kg). Untreated fruit had a decline in epicatechin content due to changes in ethylene emission and ripening, which resulted in lipoxygenase activation and a reduction in epicatechin concentration in the fruit pericarp [37]. Reduced quantities of epicatechin in the fruit peel have a direct regulatory effect on the levels of antifungal substances, such as AFD (1-acetoxy-2-hydroxy-4-oxoheneicosa-12,15-diene), and can increase the fruit’s susceptibility to C. gloeosporioides [38].
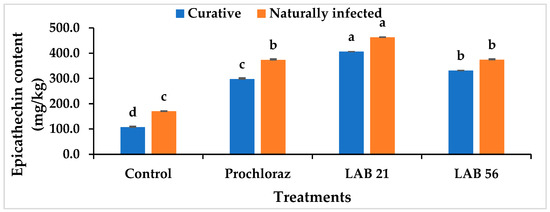
Figure 8.
Effects of LAB strain treatments on the skin epicatechin content in inoculated and naturally infected avocado cv. “Fuerte” fruit. Similar columns with different letters were significantly different at the p ≤ 0.05 level. Keys: control—sterile distilled water; Prochloraz®: positive control; LAB 21—Weissella cibaria 21; LAB 56—Leuconostoc pseudomesenteroides 56.
3.9. Effects of LAB Treatments on the Expression of Defense-Related Genes in Avocado Fruit
The PAL, FLS, AVFADL 2–3, and AVFAEL genes were significantly upregulated in curatively inoculated avocado fruit after the postharvest treatment with LAB 21 (Table 1). In contrast, LOX genes were downregulated with the LAB 21 treatment. The untreated fruit that was curatively inoculated showed the highest upregulation of the LOX gene compared with the fruit that was treated with all of the postharvest treatments. It was shown that inhibition of LOX activity delays the formulation of the latent pathogens responsible for avocado rot after harvest [10,11,20]. Furthermore, the activities of AVFAD1 2–3 and AVFAEL genes were related to the antifungal diene AFD activation [39]. The FLS gene controls the epicatechin accumulation in pericarp tissues along the flavonoid biosynthesis pathway. Therefore, the upregulation of the FLS gene in this study corroborated the observation of epicathechin accumulation in Camellia sinensis [40] and Ginkgo biloba [41] upon the upregulation of the FLS gene.

Table 1.
Expression of antifungal defense genes in curatively inoculated avocado fruit treated with LAB strains.
4. Conclusions
It was evident from this study that LAB 21 suspension was capable of controlling the development of anthracnose disease better than Prochloraz® during storage by utilizing acidity, biofilm entrapment, epicatechin accumulation, and the upregulation of antifungal defense genes along the phenylpropanoid pathway. LAB 21 exerted antifungal activity against C. gloeosporioides. Therefore, LAB 21 was shown to be effective at controlling anthracnose decay in the highly susceptible avocado cv Fuerte fruit with the potential to replace Prochloraz® in the future.
Supplementary Materials
The following supporting information can be downloaded from https://www.mdpi.com/article/10.3390/agriculture13020269/s1. Table S1: Biofilm formation by LAB 21 and LAB 56 against C. gloeosporioides.
Author Contributions
Conceptualization, A.S.M., S.A.A. and D.S.; methodology, S.A.A., T.S. and D.S.; validation, S.A.A. and D.S.; formal analysis, A.S.M. and S.A.A.; investigation, A.S.M., S.A.A., T.S. and D.S.; resources, F.R. and D.S.; data curation, S.A.A., T.S. and D.S.; writing—original draft preparation, A.S.M. and S.A.A.; writing—review and editing, S.A.A. and D.S.; visualization, S.A.A. and D.S.; supervision, S.A.A. and D.S.; project administration, A.S.M.; funding acquisition, D.S. All authors read and agreed to the published version of the manuscript.
Funding
This research was funded by the SARChI Research Chair grant for Phytochemical Food Network to Improve Nutritional Quality for Consumers, which was from the National Research Foundation (NRF), grant number 98352.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ramírez-Gil, J.G.; Ramelli, E.G.; Osorio, J.G.M. Economic impact of the avocado (cv. Hass) wilt disease complex in Antioquia, Colombia, crops under different technological management levels. Crop Prot. 2017, 101, 103–115. [Google Scholar] [CrossRef]
- Li, M.; Feng, W.; Yang, J.; Gao, Z.; Zhang, Z.; Zhang, W.; Wang, S.; Wang, W.; Gong, D.; Hu, M. First report of anthracnose caused by Colletotrichum siamense on avocado fruits in China. Crop Prot. 2022, 155, 105922. [Google Scholar] [CrossRef]
- Guan, V.X.; Neale, E.P.; Probst, Y.C. Consumption of avocado and associations with nutrient, food and anthropometric measures in a representative survey of Australians: A secondary analysis of the 2011–2012 National Nutrition and Physical Activity Survey. Br. J. Nutr. 2022, 128, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, T.; Mebrate, M.A.; Gibril, M.; Ferede, E.; Limeneh, D.Y.; Kong, F. Beneficiation of avocado processing industry by-product: A review on future prospect. Curr. Res. Green Sustain. Chem. 2022, 5, 100253. [Google Scholar] [CrossRef]
- de Oliveira, T.S.; Costa, A.M.M.; Cabral, L.M.C.; Freitas-Silva, O.; Rosenthal, A.; Tonon, R.V. Anthracnose Controlled by Essential Oils: Are Nanoemulsion-Based Films and Coatings a Viable and Efficient Technology for Tropical Fruit Preservation? Foods 2023, 12, 279. [Google Scholar] [CrossRef]
- Sharma, M.; Kulshrestha, S. Colletotrichum gloeosporioides: An anthracnose causing pathogen of fruits and vegetables. Biosci. Biotechnol. Res. Asia 2015, 12, 1233–1246. [Google Scholar] [CrossRef]
- Ramírez-Gil, J.G.; Henao-Rojas, J.C.; Morales-Osorio, J.G. Postharvest diseases and disorders in avocado cv. Hass and their relationship to preharvest management practices. Heliyon 2021, 7, e05905. [Google Scholar] [CrossRef]
- Tian, F.; Qiao, C.; Wang, C.; Pang, T.; Guo, L.; Li, J.; Pang, R.; Xie, H. Dissipation behavior of prochloraz and its metabolites in grape under open-field, storage and the wine-making process. J. Food Compos. Anal. 2022, 114, 104846. [Google Scholar] [CrossRef]
- Sellamuthu, P.S.; Sivakumar, D.; Soundy, P.; Korsten, L. Essential oil vapours suppress the development of anthracnose and enhance defence related and antioxidant enzyme activities in avocado fruit. Postharvest Biol. Technol. 2013, 81, 66–72. [Google Scholar] [CrossRef]
- Mokgalapa, N.; Akinola, S.A.; Shoko, T.; Pillai, S.K.; Sivakumar, D. Chitosan molecular weights affect anthracnose incidence and elicitation of defence-related enzymes in avocado (Persea americana) cultivar ‘Fuerte’. Int. J. Food Microbiol. 2022, 366, 109561. [Google Scholar] [CrossRef]
- Osondu, H.A.A.; Akinola, S.A.; Shoko, T.; Pillai, S.K.; Sivakumar, D. Coating properties, resistance response, molecular mechanisms and anthracnose decay reduction in green skin avocado fruit (‘Fuerte’) coated with chitosan hydrochloride loaded with functional compounds. Postharvest Biol. Technol. 2022, 186, 111812. [Google Scholar] [CrossRef]
- Pranaw, K.; Dutta, D.; Singh, S.; Khare, S.K. Lactic Acid Bacteria for Production of Platform Chemicals: A Dark Horse in the Field of Industrial Biotechnology. In Advances in the Domain of Environmental Biotechnology; Springer: Singapore, 2021; pp. 3–25. [Google Scholar] [CrossRef]
- So, Y.M.; Seo, Y.J. Characterization of the Psychrotrophic Lactic Acid Bacterium Leuconostoc gelidum subsp. aenigmaticum LS4 Isolated from Kimchi Based on Comparative Analyses of Its Genomic and Phenotypic Properties. Foods 2021, 10, 1899. [Google Scholar] [CrossRef]
- Fessard, A.; Kapoor, A.; Patche, J.; Assemat, S.; Hoarau, M.; Bourdon, E.; Bahorun, T.; Remize, F. Lactic fermentation as an efficient tool to enhance the antioxidant activity of tropical fruit juices and teas. Microorganisms 2017, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Bibi, A.; Xiong, Y.; Rajoka, M.S.R.; Mehwish, H.M.; Radicetti, E.; Umair, M.; Shoukat, M.; Khan, M.K.I.; Aadil, R.M. Recent Advances in the Production of Exopolysaccharide (EPS) from Lactobacillus spp. and its application in the food industry: A Review. Sustainability 2021, 13, 12429. [Google Scholar] [CrossRef]
- Granada, D.; Lopez-Lujan, L.; Ramirez-Restrepo, S.; Morales, J.; Pelaez-Jaramillo, C.; Andrade, G.; BEDOYA-PÉREZ, J.C. Bacterial extracts and bioformulates as a promising control of fruit body rot and root rot in avocado cv. Hass. J. Integr. Agric. 2020, 19, 748–758. [Google Scholar] [CrossRef]
- Managa, M.G.; Akinola, S.A.; Remize, F.; Garcia, C.; Sivakumar, D. Physicochemical parameters and bioaccessibility of lactic acid bacteria fermented chayote Leaf (Sechium edule) and pineapple (Ananas comosus) smoothies. Front. Nutr. 2021, 8, 649189. [Google Scholar] [CrossRef] [PubMed]
- Cele, N.P.; Akinola, S.A.; Manhivi, V.E.; Shoko, T.; Remize, F.; Sivakumar, D. Influence of Lactic Acid Bacterium Strains on Changes in Quality, Functional Compounds and Volatile Compounds of Mango Juice from Different Cultivars during Fermentation. Foods 2022, 11, 682. [Google Scholar] [CrossRef]
- Zamani-Zadeh, M.; Soleimanian-Zad, S.; Sheikh-Zeinoddin, M.; Goli, S.A.H. Integration of Lactobacillus plantarum A7 with thyme and cumin essential oils as a potential biocontrol tool for gray mold rot on strawberry fruit. Postharvest Biol. Technol. 2014, 92, 149–156. [Google Scholar] [CrossRef]
- Obianom, C.; Sivakumar, D. Differential response to combined prochloraz and thyme oil drench treatment in avocados against the control of anthracnose and stem-end rot. Phytoparasitica 2018, 46, 273–281. [Google Scholar] [CrossRef]
- Zhang, H.; Mahunu, G.K.; Castoria, R.; Apaliya, M.T.; Yang, Q. Augmentation of biocontrol agents with physical methods against postharvest diseases of fruits and vegetables. Trends Food Sci. Technol. 2017, 69, 36–45. [Google Scholar] [CrossRef]
- Sadishkumar, V.; Jeevaratnam, K. In vitro probiotic evaluation of potential antioxidant lactic acid bacteria isolated from idli batter fermented with Piper betle leaves. Int. J. Food Sci. Technol. 2017, 52, 329–340. [Google Scholar] [CrossRef]
- Akinola, S.A.; Tshimpamba, M.E.; Mwanza, M.; Ateba, C.N. Biofilm Production Potential of Serovars Isolated from Chickens in North West Province, South Africa. Pol. J. Microbiol. 2020, 69, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Iqbal, A. Effect of shrimp chitosan coating on postharvest quality of banana (Musa sapientum L.) fruits. Int. Food Res. J. 2016, 23, 277–283. [Google Scholar]
- Konsue, W.; Dethoup, T.; Limtong, S. Biological control of fruit rot and anthracnose of postharvest mango by antagonistic yeasts from economic crops leaves. Microorganisms 2020, 8, 317. [Google Scholar] [CrossRef]
- Schnürer, J.; Magnusson, J. Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci. Technol. 2005, 16, 70–78. [Google Scholar] [CrossRef]
- Yu, Y.-J.; Chen, Z.; Chen, P.T.; Ng, I.-S. Production, characterization and antibacterial activity of exopolysaccharide from a newly isolated Weissella cibaria under sucrose effect. J. Biosci. Bioeng. 2018, 126, 769–777. [Google Scholar] [CrossRef]
- Salah-Abbès, J.B.; Mannai, M.; Belgacem, H.; Zinedine, A.; Abbès, S. Efficacy of lactic acid bacteria supplementation against Fusarium graminearum growth in vitro and inhibition of Zearalenone causing inflammation and oxidative stress in vivo. Toxicon 2021, 202, 115–122. [Google Scholar] [CrossRef]
- Barrios-Roblero, C.; Rosas-Quijano, R.; Salvador-Figueroa, M.; Gálvez-López, D.; Vázquez-Ovando, A. Antifungal lactic acid bacteria isolated from fermented beverages with activity against Colletotrichum gloeosporioides. Food Biosci. 2019, 29, 47–54. [Google Scholar] [CrossRef]
- Honoré, A.H.; Aunsbjerg, S.D.; Ebrahimi, P.; Thorsen, M.; Benfeldt, C.; Knøchel, S.; Skov, T. Metabolic footprinting for investigation of antifungal properties of Lactobacillus paracasei. Anal. Bioanal. Chem. 2016, 408, 83–96. [Google Scholar] [CrossRef]
- Ali, S.R.; Fradi, A.J.; Al-Aaraji, A.M. Effect of some physical factors on growth of five fungal species. Eur. Acad. Res 2017, 2, 1069–1078. [Google Scholar]
- Jurášková, D.; Ribeiro, S.C.; Silva, C.C. Exopolysaccharides produced by lactic acid bacteria: From biosynthesis to health-promoting properties. Foods 2022, 11, 156. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Huang, X.; Chen, J.; Han, B. Formation of a mixed-species biofilm is a survival strategy for unculturable lactic acid bacteria and Saccharomyces cerevisiae in Daqu, a Chinese traditional fermentation starter. Front. Microbiol. 2020, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Lahlali, R.; Hamadi, Y.; Drider, R.; Misson, C.; El Guilli, M.; Jijakli, M.H. Control of citrus blue mold by the antagonist yeast Pichia guilliermondii Z1: Compatibility with commercial fruit waxes and putative mechanisms of action. Food Control 2014, 45, 8–15. [Google Scholar] [CrossRef]
- Diana Andrushia, A.; Trephena Patricia, A. Artificial bee colony based feature selection for automatic skin disease identification of mango fruit. In Nature Inspired Optimization Techniques for Image Processing Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 215–233. [Google Scholar] [CrossRef]
- Trias Mansilla, R.; Bañeras Vives, L.; Montesinos Seguí, E.; Badosa Romañó, E. Lactic acid bacteria from fresh fruit and vegetables as biocontrol agents of phytopathogenic bacteria and fungi. Int. Microbiol. 2008, 11, 231–236. [Google Scholar] [CrossRef]
- Prusky, D.; Barad, S.; Luria, N.; Ment, D. pH Modulation of host environment, a mechanism modulating fungal attack in postharvest pathogen interactions. In Post-Harvest Pathology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 11–25. [Google Scholar] [CrossRef]
- Prusky, D.; Ardi, R.; Kobiler, I.; Beno-Moalem, D.; Leikin, A. Mechanism of resistance of avocado fruits to Colletotrichum gloeosporioides attack. In Proceedings of the ACIAR Proceedings; 1998; pp. 63–71. [Google Scholar]
- Xoca-Orozco, L.-Á.; Cuellar-Torres, E.A.; González-Morales, S.; Gutiérrez-Martínez, P.; López-García, U.; Herrera-Estrella, L.; Vega-Arreguín, J.; Chacón-López, A. Transcriptomic analysis of avocado hass (Persea americana Mill) in the interaction system fruit-chitosan-Colletotrichum. Front. Plant Sci. 2017, 8, 956. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Ding, Z.; Liu, F. The dynamic changes of catechins and related genes in tea (Camellia sinensis) flowers. Acta Physiologiae Plantarum 2019, 41, 30. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, J.; Wang, T.; Cao, F.; Wang, G. Metabolomic and transcriptomic analyses of mutant yellow leaves provide insights into pigment synthesis and metabolism in Ginkgo biloba. BMC Genom. 2020, 21, 858. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).