Sweet Orange: Evolution, Characterization, Varieties, and Breeding Perspectives
Abstract
1. Introduction
2. A Single Hybrid Ancestor, Different Fruit Typologies
- (1)
- Common oranges, comprising many varieties that are different in origin, use, presence of seeds, and ripening time;
- (2)
- Navel oranges, in which a secondary fruitlet (navel), which develops within the primary fruit, occurs;
- (3)
- Pigmented or blood oranges, which accumulate moderate to high levels of anthocyanins in the flavedo and/or flesh during ripening;
- (4)
- Sugar or acidless oranges, which have very low acidity in the pulp, a flat flavor, and a consequent low diffusion and commercial importance.
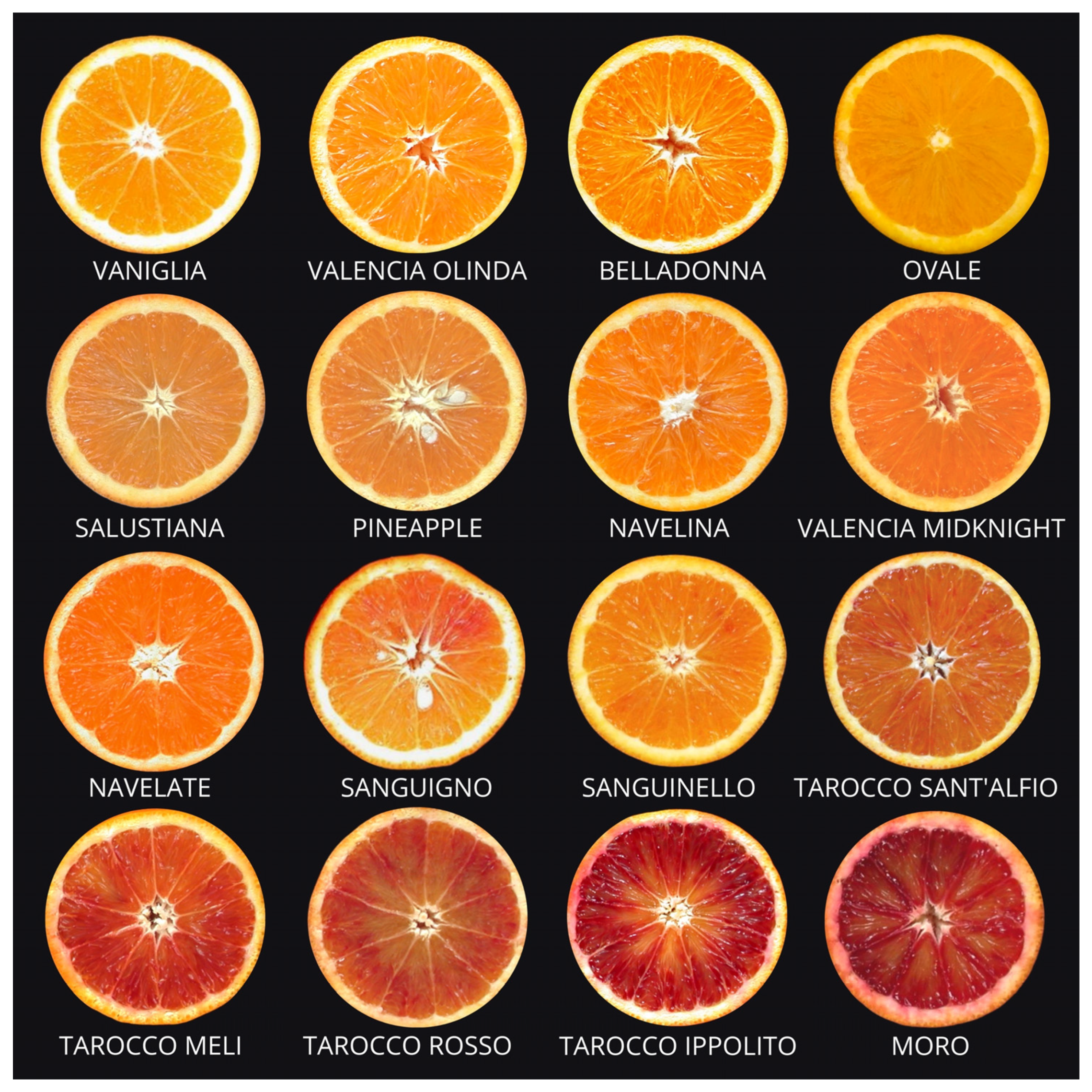
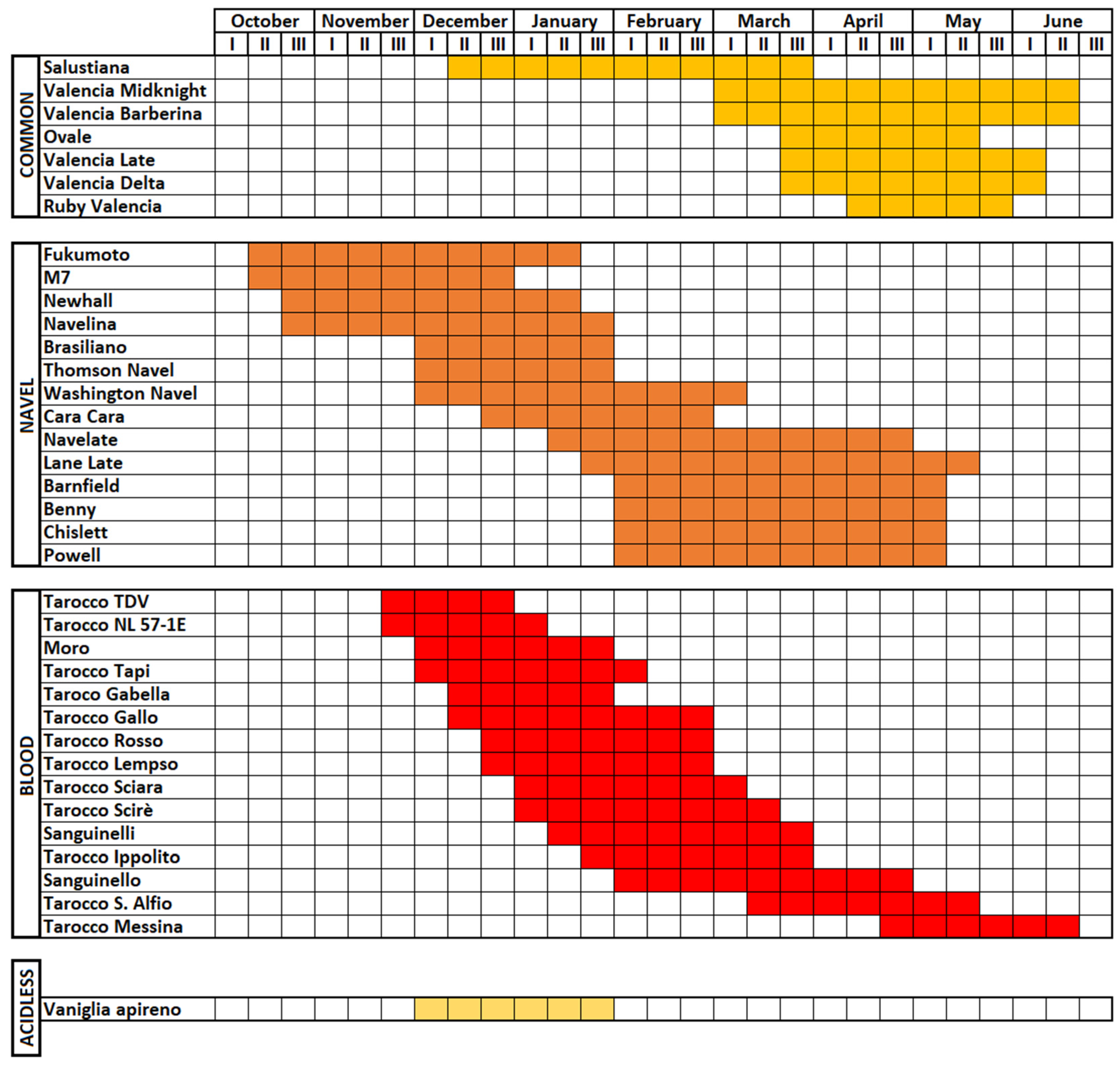
2.1. Common Oranges
2.2. Navel Oranges
2.3. Pigmented or Blood Oranges
- –
- Ordinary blood oranges: these comprise the three varieties selected and spread in Sicily (the “Sanguinello”, “Moro”, and “Tarocco”) and the “Maltese Sanguigno”, which is of unknown origin but was probably selected in Malta and subsequently spread throughout several North African areas [23];
- –
- Doble Fina varieties: these comprise a Spanish group originated from the “Doble Fina” variety from which several accessions were selected. The “Sanguinelli” variety, not to be confused with the Sicilian “Sanguinello”, belongs to this group. It was discovered in 1929 from a bud mutation of the “Doble Fina” in Castellón (Spain) and became widely popular due to its significantly higher levels of flesh and skin pigmentation compared to the original clone [34];
- –
- “Shamouti” or “Palestine Jaffa” blood oranges: these comprise a small group, including the “Shamouti Maouardi” and “Maouardi Beladi” varieties, that are all accessions with similar characteristics to the blond “Shamouti”, except for fruit pigmentation [35].
2.4. Sugar or Acidless Oranges
3. Pomological Qualitative Traits
Effect of Environment and Agronomical Practices on Fruit Quality
4. Fruit Bioactive Compounds
4.1. Primary Metabolites
4.2. Secondary Metabolites
5. Genetic Improvement in the Sweet Orange
| Gene/Marker | Trait | Reference |
|---|---|---|
| Ruby | Anthocyanin pigmentation | [36,39] |
| Noemi | Anthocyanin pigmentation and fruit acidity | [38] |
| CitPSY, CitPDS, CitZDS, CitLCYb, CitHYb, CitZEP, Csβ-LCY2, and CCD4b | Carotenoid accumulation | [142,143,144] |
| CsLOB1 and CsWRKY22 | Citrus canker development | [145,146,147,148] |
| AN1, NHX, and RAE1 | Fruit acidity | [128,130] |
| CsMIPs and CsTALEs | Response to biotic/abiotic stresses | [149,150] |
| CitRWP and CiRKD1 with a MITE insertion | Apomixis | [151,152] |
| VINV, CWINV1, CWINV2, SUS4, SUS5, SPS1, SPS2, VPP-1, and VPP-2 | Sugar accumulation in fruit juice sacs | [153] |
| SNP08 marker | Alternaria brown spot (ABS) resistance | [154] |
| CsERF74, CsNAC25, PGs, PMEs, CCOAMTs, OMT1, and CAD | Pulp tenderness | [155] |
6. Future Perspectives of Genetic Improvement
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- da Graça, J.V.; Douhan, G.W.; Halbert, S.E.; Keremane, M.L.; Lee, R.F.; Vidalakis, G.; Zhao, H. Huanglongbing: An overview of a complex pathosystem ravaging the world’s citrus. J. Integr. Plant Biol. 2016, 58, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y. Citrus Yellow Shoot Disease (Huanglongbing)—A Review. In Proceedings of the 4th International Citrus Congress, Tokyo, Japan, 9–12 November 1981; International Society of Citriculture: Tokyo, Japan, 1981; pp. 466–469. [Google Scholar]
- Texeira, D.C.; Ayres, J.; Kitajima, E.W.; Danet, L.; Jagoueix-Eveillard, S.; Saillard, C.; Bové, J.M. First Report of a Huanglongbing-Like Disease of Citrus in Sao Paulo State, Brazil and Association of a New Liberibacter Species, “Candidatus Liberibacter americanus”, with the Disease. Plant Dis. 2005, 89, 107. [Google Scholar] [CrossRef] [PubMed]
- Halbert, S.E.S. The Discovery of Huanglongbing in Florida. In Proceedings of the 2nd International Citrus Canker and Huanglongbing Research Workshop, Orlando, FL, USA, 9–11 November 2005. [Google Scholar]
- EPPO Global Database—Diaphorina Citri (DIAACI). Available online: https://gd.eppo.int/taxon/DIAACI/distribution (accessed on 23 November 2022).
- Spreen, T.H.; Gao, Z.; Fernandes, W.; Zansler, M.L. Global economics and marketing of citrus products. In The Genus Citrus; Woodhead Publishing: Sawston, UK, 2020; pp. 471–493. [Google Scholar] [CrossRef]
- Legge, J. The Chinese Classics; BoD–Books on Demand: Norderstedt, Germany, 1865. [Google Scholar]
- Wu, G.A.; Prochnik, S.; Jenkins, J.; Salse, J.; Hellsten, U.; Murat, F.; Perrier, X.; Ruiz, M.; Scalabrin, S.; Terol, J.; et al. Sequencing of diverse mandarin, pummelo and orange genomes reveals complex history of admixture during citrus domestication. Nat. Biotechnol. 2014, 32, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.A.; Terol, J.; Ibanez, V.; López-García, A.; Pérez-Román, E.; Borredá, C.; Domingo, C.; Tadeo, F.R.; Carbonell-Caballero, J.; Alonso, R.; et al. Genomics of the origin and evolution of Citrus. Nature 2018, 554, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Ollitrault, P.; Curk, F.; Krueger, R. Citrus Taxonomy. In The Genus Citrus; Woodhead Publishing: Sawston, UK, 2020. [Google Scholar]
- Debbabi, O.S.; Bouhlal, R.; Abdelaali, N.; Mnasri, S.; Mars, M. Pomological Study of Sweet Orange (Citrus sinensis L. Osbeck) Cultivars From Tunisia. Int. J. Fruit Sci. 2013, 13, 274–284. [Google Scholar] [CrossRef]
- Caruso, M.; Ferlito, F.; Licciardello, C.; Allegra, M.; Strano, M.C.; Di Silvestro, S.; Russo, M.P.; Pietropaolo, D.; Caruso, P.; Casas, G.L.; et al. Pomological diversity of the Italian blood orange germplasm. Sci. Hortic. 2016, 213, 331–339. [Google Scholar] [CrossRef]
- Demir, G.; Koyuncu, F. Evaluation of Pomological Properties of Some Orange Varieties from West Mediterranean, Turkey. Sci. Pap. 2018, 62, 135–139. [Google Scholar]
- Davies, F.S. The Navel Orange; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1986. [Google Scholar]
- Akyildiz, A.; Ağçam, E. Citrus Juices Technology. In Food Processing: Strategies for Quality Assessment; Food Engineering Series; Springer: New York, NY, USA, 2014. [Google Scholar]
- Domingues, A.R.; Marcolini, C.D.M.; Gonçalves, C.H.d.S.; de Resende, J.T.V.; Roberto, S.R.; Carlos, E.F. Rootstocks Genotypes Impact on Tree Development and Industrial Properties of ‘Valencia’ Sweet Orange Juice. Horticulturae 2021, 7, 141. [Google Scholar] [CrossRef]
- Hervalejo, A.; Arjona-López, J.; Ordóñez-Díaz, J.; Romero-Rodríguez, E.; Calero-Velázquez, R.; Moreno-Rojas, J.; Arenas-Arenas, F. Influence of Harvesting Season on Morphological and Sensory Quality, Bioactive Compounds and Antioxidant Activity of Three Late-Season Orange Cultivars ‘Barberina’, ‘Valencia Midknight’ and ‘Valencia Delta Seedless’. Agronomy 2021, 11, 673. [Google Scholar] [CrossRef]
- Rouse, R.E. Citrus Fruit Quality and Yield of Six Valencia Clones on 16 Rootstocks in the Immokalee Foundation Grove. Proc. Fla. State Hortic. Soc. 2000, 113, 112–114. [Google Scholar]
- Yildiz, E.; Demirkeser, T.H.; Kaplankiran, M. Growth, yield, and fruit quality of ‘Rhode red Valencia’ and ‘Valencia Late’ sweet oranges grown on three rootstocks in eastern Mediterranean. Chil. J. Agric. Res. 2013, 73, 16–17. [Google Scholar] [CrossRef]
- Zacarías-García, J.; Rey, F.; Gil, J.-V.; Rodrigo, M.J.; Zacarías, L. Antioxidant capacity in fruit of Citrus cultivars with marked differences in pulp coloration: Contribution of carotenoids and vitamin C. Food Sci. Technol. Int. 2021, 27, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Zacarías-García, J.; Pérez-Través, L.; Gil, J.-V.; Rodrigo, M.-J.; Zacarías, L. Bioactive Compounds, Nutritional Quality and Antioxidant Capacity of the Red-Fleshed Kirkwood Navel and Ruby Valencia Oranges. Antioxidants 2022, 11, 1905. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, U.; Alferez, F.; Zekri, M. 2022–2023 Florida Citrus Production Guide: Rootstock and Scion Selection; EDIS: Gainesville, FL, USA, 2022. [Google Scholar] [CrossRef]
- Barry, G.H.; Caruso, M.; Gmitter, F.G. Commercial scion varieties. In The Genus Citrus; Woodhead Publishing: Sawston, UK, 2020; pp. 83–104. [Google Scholar] [CrossRef]
- Scuderi, A. A Disorder of Fruits of “Ovale” Sweet Orange in Sicily. Phytopathol. Mediterr. 1970, 9, 182–184. [Google Scholar]
- Casella, D. L’agrumicoltura Siciliana; Annali della Regia Stazione Sperimentale di Agrumicoltura e Frutticoltura: Acireale, Italy, 1935; pp. 11–13. [Google Scholar]
- La Rosa, G.; Continella, A. Valutazione di cultivar tardive di arancio Navel in Sicilia. Riv. Di Fruttic. E Di Ortofloric. 2010, 1–2, 64–66. [Google Scholar]
- Scaramuzzi, G.; Catara, A.; Cartia, G. La Selezione Sanitaria Degli Agrumi per Le Virosi e Risultati Sperimentali Conseguiti in Sicilia. Riv. Di Patol. Veg. 1968, 4, 213–246. [Google Scholar]
- Merino, C.; Hervalejo, .; Salguero, A.; González, D.; Arenas-Arenas, F.J. Yield and Fruit Quality of Two Early Maturing Orange Cultivars, “Navelina” and “Fukumoto”, in Andalusia, Spain. Acta Hortic. 2015, 1065, 255–259. [Google Scholar] [CrossRef]
- Aleza, P.; Forner-Giner, M.A.; Del-Pino, A. El Panorama Varietal y Los Nuevos Patrones. Análisis de La Situación Actual. In Una Hoja de Ruta Para La Citricultura Española; Cajamar Caja Rural: San Luis de Sabinillas, Spain, 2020; pp. 151–166. [Google Scholar]
- Lee, H.S. Characterization of Carotenoids in Juice of Red Navel Orange (Cara Cara). J. Agric. Food Chem. 2001, 49, 2563–2568. [Google Scholar] [CrossRef]
- Lo Piero, A.R. The State of the Art in Biosynthesis of Anthocyanins and Its Regulation in Pigmented Sweet Oranges [(Citrus sinensis) L. Osbeck]. J. Agric. Food Chem. 2015, 63, 4031–4041. [Google Scholar] [CrossRef]
- Karp, D. The Bloods: Mutations, Chemistry and Flavor. Fruit Gard. 2007, 39, 16–22. [Google Scholar]
- Chapot, H. Quelques Oranges Sanguines. Cah. Rech. Agron. 1963, 18, 61–87. [Google Scholar]
- Costa, M.; Zaragoza, S.; Abad, V.; Domènech, E.; Bosch, A.; Ortiz, L.; Millo, E.P.; Bono, R.; Julià, J.F.; Caballer, V.; et al. Historia de La Naranja; Universidad Politecnica de Madrid: Madrid, Spain, 1991. [Google Scholar]
- Reuther, W.; Batchelor, L.D.; Webber, H.J. The Citrus Industry. Vol. I. History, World Distribution, Botany and Varieties; CAB International: Wallingford, UK, 1967. [Google Scholar]
- Huang, D.; Wang, X.; Tang, Z.; Yuan, Y.; Xu, Y.; He, J.; Jiang, X.; Peng, S.-A.; Li, L.; Butelli, E.; et al. Subfunctionalization of the Ruby2–Ruby1 gene cluster during the domestication of citrus. Nat. Plants 2018, 4, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Catalano, C.; Ciacciulli, A.; Salonia, F.; Russo, M.P.; Caruso, P.; Caruso, M.; Russo, G.; Distefano, G.; Licciardello, C. Target-Genes Reveal Species and Genotypic Specificity of Anthocyanin Pigmentation in Citrus and Related Genera. Genes 2020, 11, 807. [Google Scholar] [CrossRef]
- Butelli, E.; Licciardello, C.; Ramadugu, C.; Durand-Hulak, M.; Celant, A.; Recupero, G.R.; Froelicher, Y.; Martin, C. Noemi Controls Production of Flavonoid Pigments and Fruit Acidity and Illustrates the Domestication Routes of Modern Citrus Varieties. Curr. Biol. 2019, 29, 158–164.e2. [Google Scholar] [CrossRef] [PubMed]
- Butelli, E.; Licciardello, C.; Zhang, Y.; Liu, J.; Mackay, S.; Bailey, P.; Reforgiato-Recupero, G.; Martin, C. Retrotransposons Control Fruit-Specific, Cold-Dependent Accumulation of Anthocyanins in Blood Oranges. Plant Cell 2012, 24, 1242–1255. [Google Scholar] [CrossRef]
- Strano, M.C.; Di Silvestro, S.; Allegra, M.; Russo, G.; Caruso, M. Effect of cold storage on the postharvest quality of different Tarocco sweet orange clonal selections. Sci. Hortic. 2021, 285, 110167. [Google Scholar] [CrossRef]
- Carmona, L.; Alquézar, B.; Marques, V.V.; Peña, L. Anthocyanin biosynthesis and accumulation in blood oranges during postharvest storage at different low temperatures. Food Chem. 2017, 237, 7–14. [Google Scholar] [CrossRef]
- Russo, M.; Bonaccorsi, I.L.; Arigò, A.; Cacciola, F.; De Gara, L.; Dugo, P.; Mondello, L. Blood orange (Citrus sinensis) as a rich source of nutraceuticals: Investigation of bioactive compounds in different parts of the fruit by HPLC-PDA/MS. Nat. Prod. Res. 2021, 35, 4606–4610. [Google Scholar] [CrossRef]
- Gasic, K.; Preece, J.E.; Karp, D. Register of New Fruit and Nut Cultivars List 50. HortScience 2020, 55, 1164–1201. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. United States Standards for Grades of Orange Juice; U.S. Department of Agriculture: Washington, DC, USA, 1983. [Google Scholar]
- Grosser, J.; Gmitter, F.; Wang, Y.; Casle, B. Working toward Better Orange Juice in the HLB World. Citrus Ind. 2019, 12–15. Available online: http://citrusindustry.net/2019/05/17/working-toward-better-orange-juice-in-the-hlb-world/ (accessed on 16 January 2023).
- Fellers, P.J. Florida’s Citrus Juice Standards for Grades and Their Differences From United States Standards for Grades and United States Food and Drug Administration Standards of Identity. Proc. Fla. State Hortic. Soc. 1990, 103, 260–264. [Google Scholar]
- Abouzari, A.; Nezhad, N.M. The Investigation of Citrus Fruit Quality. Popular Characteristic and Breeding. Acta Univ. Agric. Silvic. Mendel. Brun. 2016, 64, 725–740. [Google Scholar] [CrossRef]
- Tadeo, F.R.; Cercós, M.; Colmenero-Flores, J.M.; Iglesias, D.J.; Naranjo, M.A.; Ríos, G.; Carrera, E.; Ruiz-Rivero, O.; Lliso, I.; Morillon, R.; et al. Molecular Physiology of Development and Quality of Citrus. Adv. Bot. Res. 2008, 47, 147–223. [Google Scholar] [CrossRef]
- Gámbaro, A.; Roascio, A.; Hodos, N.; Migues, I.; Lado, J.; Heinzen, H.; Rivas, F. The impact of sensory attributes of mandarins on consumer perception and preferences. J. Agric. Food Res. 2021, 6, 100196. [Google Scholar] [CrossRef]
- Lado, J.; Rodrigo, M.J.; Zacarías, L. Maturity Indicators and Citrus Fruit Quality. Stewart Postharvest Rev. 2014, 10, 1–6. [Google Scholar]
- Erickson, L.C. The general physiology of citrus. In The Citrus Industry, 2nd ed.; Reuther, W., Batchelor, L.D., Webber, H.J., Eds.; University of California, Berkley: Berkeley, CA, USA, 1968; Volume II, pp. 86–126. [Google Scholar]
- UNECE Standard FFV-14; Concerning the Marketing and Commercial Quality Control of Citrus Fruit. United Nations: New York, NY, USA; Geneva, Switzerland, 2009.
- Farag, M.A.; Abib, B.; Ayad, L.; Khattab, A.R. Sweet and bitter oranges: An updated comparative review of their bioactives, nutrition, food quality, therapeutic merits and biowaste valorization practices. Food Chem. 2020, 331, 127306. [Google Scholar] [CrossRef]
- Francis, F.J. Quality as influenced by color. Food Qual. Prefer. 1995, 6, 149–155. [Google Scholar] [CrossRef]
- Kato, M. Mechanism of Carotenoid Accumulation in Citrus Fruit. J. Jpn. Soc. Hortic. Sci. 2012, 81, 219–233. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Alquézar, B.; Alós, E.; Lado, J.; Zacarías, L. Biochemical bases and molecular regulation of pigmentation in the peel of Citrus fruit. Sci. Hortic. 2013, 163, 46–62. [Google Scholar] [CrossRef]
- Jiménez-Cuesta, M.; Cuquerella, J.; Martinez-Javaga, J.M. Determination of a Color Index for Citrus Fruit Degreening. In Proceedings of the International Citrus Congress, Tokyo, Japan, 9–12 November 1981; International Society of Citriculture: Shimizu, Japan, 1981. [Google Scholar]
- Salvador, A.; Navarro, P.; Martinez-Javega, J.M. Manejo Postcosecha de Cítricos. In Proceedings of the XI Simposio Internacional de Citricultura, Mexico, 2007. [Google Scholar]
- Tadeo, F.R.; Terol, J.; Rodrigo, M.J.; Licciardello, C.; Sadka, A. Fruit growth and development. In The Genus Citrus; Woodhead Publishing: Sawston, UK, 2020; pp. 245–269. [Google Scholar] [CrossRef]
- Iwasaki, N.; Oogaki, C.; Iwamasa, M.; Matsushima, J.; Ishihata, K. Adaptability of Citrus Species Based on the Relationships between Climatic Parameters and Fruit Quality Characteristics. J. Jpn. Soc. Hortic. Sci. 1986, 55, 153–168. [Google Scholar] [CrossRef]
- Lado, J.; Alós, E.; Manzi, M.; Cronje, P.J.R.; Gómez-Cadenas, A.; Rodrigo, M.J.; Zacarías, L. Light Regulation of Carotenoid Biosynthesis in the Peel of Mandarin and Sweet Orange Fruits. Front. Plant Sci. 2019, 10, 1288. [Google Scholar] [CrossRef] [PubMed]
- Reuther, W.; Rasmussen, G.K.; Hilgeman, R.H.; Cahoon, G.A.; Cooper, W.C. A Comparison of Maturation and Composition of ‘Valencia’ Oranges in Some Major Subtropical Zones of the United States1. J. Am. Soc. Hortic. Sci. 1969, 94, 144–157. [Google Scholar] [CrossRef]
- Castle, W.S. A Career Perspective on Citrus Rootstocks, Their Development, and Commercialization. HortScience 2010, 45, 11–15. [Google Scholar] [CrossRef]
- Continella, A.; Pannitteri, C.; La Malfa, S.; Legua, P.; Distefano, G.; Nicolosi, E.; Gentile, A. Influence of different rootstocks on yield precocity and fruit quality of ‘Tarocco Scirè’ pigmented sweet orange. Sci. Hortic. 2018, 230, 62–67. [Google Scholar] [CrossRef]
- Bowman, K.D.; Joubert, J. Citrus Rootstocks. In The Genus Citrus; Woodhead Publishing: Sawston, UK, 2020; pp. 105–127. [Google Scholar]
- Caruso, M.; Continella, A.; Modica, G.; Pannitteri, C.; Russo, R.; Salonia, F.; Arlotta, C.; Gentile, A.; Russo, G. Rootstocks Influence Yield Precocity, Productivity, and Pre-Harvest Fruit Drop of Mandared Pigmented Mandarin. Agronomy 2020, 10, 1305. [Google Scholar] [CrossRef]
- Fellers, P.J. Citrus: Sensory Quality as Related to Rootstock, Cultivar, Maturity, and Season. In Evaluation of Quality of Fruits and Vegetables; Springer: Boston, MA, USA, 1985. [Google Scholar]
- Castle, W.S. Rootstock as a fruit quality factor in citrus and deciduous tree crops. N. Z. J. Crop Hortic. Sci. 1995, 23, 383–394. [Google Scholar] [CrossRef]
- Modica, G.; Pannitteri, C.; Di Guardo, M.; La Malfa, S.; Gentile, A.; Ruberto, G.; Pulvirenti, L.; Parafati, L.; Continella, A.; Siracusa, L. Influence of rootstock genotype on individual metabolic responses and antioxidant potential of blood orange cv. Tarocco Scirè. J. Food Compos. Anal. 2022, 105, 104246. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Huang, M.; Chen, J. Mechanisms for the Influence of Citrus Rootstocks on Fruit Size. J. Agric. Food Chem. 2015, 63, 2618–2627. [Google Scholar] [CrossRef]
- Alquézar, B.; Bennici, S.; Carmona, L.; Gentile, A.; Peña, L. Generation of Transfer-DNA-Free Base-Edited Citrus Plants. Front. Plant Sci. 2022, 13, 835282. [Google Scholar] [CrossRef]
- Bowman, K.D.; McCollum, G. US-942-Signed-Release-Notice; U.S. Department of Agriculture, ARS: Washington, DC, USA, 2010.
- Albrecht, U.; McCollum, G.; Bowman, K.D. Influence of rootstock variety on Huanglongbing disease development in field-grown sweet orange (Citrus sinensis [L.] Osbeck) trees. Sci. Hortic. 2012, 138, 210–220. [Google Scholar] [CrossRef]
- Bowman, K.D.; McCollum, G.; Albrecht, U. Performance of ‘Valencia’ orange (Citrus sinensis [L.] Osbeck) on 17 rootstocks in a trial severely affected by huanglongbing. Sci. Hortic. 2016, 201, 355–361. [Google Scholar] [CrossRef]
- Chen, J.; Yuan, Z.; Zhang, H.; Li, W.; Shi, M.; Peng, Z.; Li, M.; Tian, J.; Deng, X.; Cheng, Y.; et al. Cit1,2RhaT and two novel CitdGlcTs participate in flavor-related flavonoid metabolism during citrus fruit development. J. Exp. Bot. 2019, 70, 2759–2771. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Suh, J.H.; Gmitter, F.G.; Wang, Y. Differentiation between Flavors of Sweet Orange (Citrus sinensis) and Mandarin (Citrus reticulata). J. Agric. Food Chem. 2018, 66, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Lado, J.; Gambetta, G.; Zacarias, L. Key determinants of citrus fruit quality: Metabolites and main changes during maturation. Sci. Hortic. 2018, 233, 238–248. [Google Scholar] [CrossRef]
- Breksa, A.P.; Kahn, T.; Zukas, A.A.; Hidalgo, M.B.; Yuen, M.L. Limonoid content of sour orange varieties. J. Sci. Food Agric. 2011, 91, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Ranganna, S.; Govindarajan, V.S.; Ramana, K.V.R.; Kefford, J.F. Citrus fruits—Varieties, chemistry, technology, and quality evaluation. Part II. Chemistry, technology, and quality evaluation. A. Chemistry. Crit. Rev. Food Sci. Nutr. 1983, 18, 313–386. [Google Scholar] [CrossRef]
- Nour, V.; Trandafir, I.; Ionica, M.E. HPLC Organic Acid Analysis in Different Citrus Juices under Reversed Phase Conditions. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 44–48. [Google Scholar]
- Proteggente, A.R.; Saija, A.; De Pasquale, A.; Rice-Evans, C.A. The Compositional Characterisation and Antioxidant Activity of Fresh Juices from Sicilian Sweet Orange (Citrus sinensis L. Osbeck) Varieties. Free Radic. Res. 2003, 37, 681–687. [Google Scholar] [CrossRef]
- Aghajanian, P.; Hall, S.; Wongworawat, M.D.; Mohan, S. The Roles and Mechanisms of Actions of Vitamin C in Bone: New Developments. J. Bone Miner. Res. 2015, 30, 1945–1955. [Google Scholar] [CrossRef]
- Chambial, S.; Dwivedi, S.; Shukla, K.K.; John, P.J.; Sharma, P. Vitamin C in Disease Prevention and Cure: An Overview. Indian J. Clin. Biochem. 2013, 28, 314–328. [Google Scholar] [CrossRef]
- Iqbal, K.; Khan, A.; Khattak, M.M.A.K. Biological Significance of Ascorbic Acid (Vitamin C) in Human Health—A Review. Pak. J. Nutr. 2004, 3, 5–13. [Google Scholar] [CrossRef]
- Cebadera-Miranda, L.; Domínguez, L.; Dias, M.I.; Barros, L.; Ferreira, I.C.F.R.; Igual, M.; Martínez-Navarrete, N.; Fernández-Ruiz, V.; Morales, P.; Cámara, M. Sanguinello and Tarocco (Citrus sinensis [L.] Osbeck): Bioactive compounds and colour appearance of blood oranges. Food Chem. 2019, 270, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Licciardello, C.; Caruso, P.; Russo, M.P.; Pietropaolo, D.; Recupero, G.R.; Rapisarda, P.; Ballistreri, G.; Fabroni, S.; Caruso, M. New CREA citrus hybrids. Citrus Res. Technol. 2016, 37, 98–101. [Google Scholar] [CrossRef]
- Waheed, A.; Mahmud, S.; Saleem, M.; Ahmad, T. Fatty acid composition of neutral lipid: Classes of Citrus seed oil. J. Saudi Chem. Soc. 2009, 13, 269–272. [Google Scholar] [CrossRef]
- Yoshida, Y.; Niki, E. Antioxidant Effects of Phytosterol and Its Components. J. Nutr. Sci. Vitaminol. 2003, 49, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Rozner, S.; Garti, N. The activity and absorption relationship of cholesterol and phytosterols. Colloids Surfaces A Physicochem. Eng. Asp. 2006, 282–283, 435–456. [Google Scholar] [CrossRef]
- Khan, M.K.; Zill-E-Huma; Dangles, O. A Comprehensive Review on Flavanones, the Major Citrus Polyphenols. J. Food Compos. Anal. 2014, 33, 85–104. [Google Scholar] [CrossRef]
- Tapiero, H.; Tew, K.D.; Ba, G.N.; Mathé, G. Polyphenols: Do they play a role in the prevention of human pathologies? Biomed. Pharmacother. 2002, 56, 200–207. [Google Scholar] [CrossRef]
- Ahmed, O.M.; AbouZid, S.F.; Ahmed, N.A.; Zaky, M.Y.; Liu, H. An Up-to-Date Review on Citrus Flavonoids: Chemistry and Benefits in Health and Diseases. Curr. Pharm. Des. 2021, 27, 513–530. [Google Scholar] [CrossRef]
- Hwang, S.-L.; Shih, P.-H.; Yen, G.-C. Neuroprotective Effects of Citrus Flavonoids. J. Agric. Food Chem. 2012, 60, 877–885. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Bautista, R.J.H.; Sandhu, M.A.; Hussein, O.E. Beneficial Effects of Citrus Flavonoids on Cardiovascular and Metabolic Health. Oxid. Med. Cell. Longev. 2019, 2019, 5484138. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid Composition of Citrus Juices. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef]
- Tung, Y.-C.; Chou, Y.-C.; Hung, W.-L.; Cheng, A.-C.; Yu, R.-C.; Ho, C.-T.; Pan, M.-H. Polymethoxyflavones: Chemistry and Molecular Mechanisms for Cancer Prevention and Treatment. Curr. Pharmacol. Rep. 2019, 5, 98–113. [Google Scholar] [CrossRef]
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M. HL-60 Differentiating Activity and Flavonoid Content of the Readily Extractable Fraction Prepared from Citrus Juices. J. Agric. Food Chem. 1999, 47, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Nagy, S. Vitamin C contents of citrus fruit and their products: A review. J. Agric. Food Chem. 1980, 28, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Epifano, F.; Fiorito, S.; Taddeo, V.A.; Genovese, S. 4′-Geranyloxyferulic acid: An overview of its potentialities as an anti-cancer and anti-inflammatory agent. Phytochem. Rev. 2015, 14, 607–612. [Google Scholar] [CrossRef]
- Mohamed, R.A.; Yousef, Y.M.; El-Tras, W.F.; Khalafallaa, M.M. Dietary essential oil extract from sweet orange (Citrus sinensis) and bitter lemon (Citrus limon) peels improved Nile tilapia performance and health status. Aquac. Res. 2021, 52, 1463–1479. [Google Scholar] [CrossRef]
- Gross, J. Pigments in Fruits; Academic Press: London, UK, 1987. [Google Scholar]
- Alquézar, B.; Rodrigo, M.J.; Zacarías, L. Carotenoid Biosynthesis and Their Regulation in Citrus Fruits. Tree For. Sci. Biotechnol. 2008, 2, 23–37. [Google Scholar]
- Ma, G.; Zhang, L.; Sugiura, M.; Kato, M. Citrus and Health. In The Genus Citrus; Woodhead Publishing: Sawston, UK, 2020. [Google Scholar]
- Liu, Q.; Xu, J.; Liu, Y.; Zhao, X.; Deng, X.; Guo, L.; Gu, J. A novel bud mutation that confers abnormal patterns of lycopene accumulation in sweet orange fruit (Citrus sinensis L. Osbeck). J. Exp. Bot. 2007, 58, 4161–4171. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Hasegawa, S.; Miyake, M. Biochemistry and biological functions of citrus limonoids. Food Rev. Int. 1996, 12, 413–435. [Google Scholar] [CrossRef]
- Ejaz, S.; Ejaz, A.; Matsuda, K.; Lim, C.W. Limonoids as cancer chemopreventive agents. J. Sci. Food Agric. 2006, 86, 339–345. [Google Scholar] [CrossRef]
- Rahman, A.K.M.S.; Chowdhury, A.K.A.; Ali, H.-A.; Raihan, S.Z.; Ali, M.S.; Nahar, L.; Sarker, S.D. Antibacterial activity of two limonoids from Swietenia mahagoni against multiple-drug-resistant (MDR) bacterial strains. J. Nat. Med. 2009, 63, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Abdelgaleil, S.A.M.; Hashinaga, F.; Nakatani, M. Antifungal activity of limonoids from Khaya ivorensis. Pest Manag. Sci. 2005, 61, 186–190. [Google Scholar] [CrossRef]
- Manners, G.D. Citrus Limonoids: Analysis, Bioactivity, and Biomedical Prospects. J. Agric. Food Chem. 2007, 55, 8285–8294. [Google Scholar] [CrossRef]
- Siddiqui, A.N.; Kulkarni, D.N.; Kulkarni, K.D.; Mulla, M.Z. Studies on Debittering of Sweet Orange Juice. World J. Dairy Food Sci. 2013, 8, 185–189. [Google Scholar]
- Chandler, B.V.; Johnson, R.L. New sorbent gel forms of cellulose esters for debittering citrus juices. J. Sci. Food Agric. 1979, 30, 825–832. [Google Scholar] [CrossRef]
- Shaw, P.E.; Wilson, C.W. Debittering Citrus Juices with ?-Cyclodextrin Polymer. J. Food Sci. 1983, 48, 646–647. [Google Scholar] [CrossRef]
- Puri, M.; Marwaha, S.S.; Kothari, R.M.; Kennedy, J.F. Biochemical Basis of Bitterness in Citrus Fruit Juices and Biotech Approaches for Debittering. Crit. Rev. Biotechnol. 1996, 16, 145–155. [Google Scholar] [CrossRef]
- Purewal, S.S.; Sandhu, K.S. Debittering of citrus juice by different processing methods: A novel approach for food industry and agro-industrial sector. Sci. Hortic. 2021, 276, 109750. [Google Scholar] [CrossRef]
- Suntar, I.; Khan, H.; Patel, S.; Celano, R.; Rastrelli, L. An Overview onCitrus aurantiumL.: Its Functions as Food Ingredient and Therapeutic Agent. Oxid. Med. Cell. Longev. 2018, 2018, 7864269. [Google Scholar] [CrossRef] [PubMed]
- de Lima, L.P.; de Paula Barbosa, A. A review of the lipolytic effects and the reduction of abdominal fat from bioactive compounds and moro orange extracts. Heliyon 2021, 7, e07695. [Google Scholar] [CrossRef] [PubMed]
- Maccarone, E.; Campisi, S.; Fallico, B.; Rapisarda, P.; Sgarlata, R. Flavor Components of Italian Orange Juices. J. Agric. Food Chem. 1998, 46, 2293–2298. [Google Scholar] [CrossRef]
- Jackson, L.K.; Futch, S.H. Ambersweet Orange; University of Florida Cooperative Extension Service, Institute of Food and Agriculture Sciences, EDIS: Gainesville, FL, USA, 1993. [Google Scholar]
- Cheftel, J.C. Food and nutrition labelling in the European Union. Food Chem. 2005, 93, 531–550. [Google Scholar] [CrossRef]
- Cross, N.U.S. Food Standards and Food Grades. In Handbook of Food Science, Technology, and Engineering; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Stover, E.; Gmitter, F.G.; Grosser, J.; Baldwin, E.; Wu, G.A.; Bai, J.; Wang, Y.; Chaires, P.; Motamayor, J.C. Rationale for reconsidering current regulations restricting use of hybrids in orange juice. Hortic. Res. 2020, 7, 38. [Google Scholar] [CrossRef]
- Castle, W.S.; Baldwin, J.C. Young-tree Performance of Juvenile Sweet Orange Scions on Swingle Citrumelo Rootstock. HortScience 2011, 46, 541–552. [Google Scholar] [CrossRef]
- Navarro, L.; Aleza, P.; Cuenca, J.; Juárez, J.; Pina, J.A.; Ortega, C.; Navarro, A.; Ortega, V. The Mandarin Triploid Breeding Program in Spain. Acta Hortic. 2015, 1065, 389–396. [Google Scholar] [CrossRef]
- Bai, J.; Baldwin, E.; Hearn, J.; Driggers, R.; Stover, E. Volatile Profile Comparison of USDA Sweet Orange-like Hybrids versus ‘Hamlin’ and ‘Ambersweet’. HortScience 2014, 49, 1262–1267. [Google Scholar] [CrossRef]
- Stover, E.; Driggers, R.; Hearn, C.J.; Bai, J.; Baldwin, E.; Mccollum, T.G.; Hall, D.G. Breeding “sweet oranges” at the USDA U.S. Horticultural Research Laboratory. Acta Hortic. 2016, 1127, 41–44. [Google Scholar] [CrossRef]
- Albrecht, U.; Alferez, F.; Zekri, M. 2019–2020 Florida Citrus Production Guide: Rootstock and Scion Selection; EDIS: Gainesville, FL, USA, 2019. [Google Scholar]
- Wang, L.; Huang, Y.; Liu, Z.; He, J.; Jiang, X.; He, F.; Lu, Z.; Yang, S.; Chen, P.; Yu, H.; et al. Somatic variations led to the selection of acidic and acidless orange cultivars. Nat. Plants 2021, 7, 954–965. [Google Scholar] [CrossRef]
- Raveh, E.; Goldenberg, L.; Porat, R.; Carmi, N.; Gentile, A.; La Malfa, S. Conventional Breeding of Cultivated Citrus Varieties. In The Citrus Genome; Compendium of Plant Genome; Springer: Cham, Switzerland, 2020; pp. 33–48. [Google Scholar] [CrossRef]
- Strazzer, P.; Spelt, C.E.; Li, S.; Bliek, M.; Federici, C.T.; Roose, M.L.; Koes, R.; Quattrocchio, F.M. Hyperacidification of Citrus fruits by a vacuolar proton-pumping P-ATPase complex. Nat. Commun. 2019, 10, 744. [Google Scholar] [CrossRef] [PubMed]
- Caruso, M.; Casas, G.L.; Scaglione, D.; Gattolin, S.; Rossini, L.; Distefano, G.; Cattonaro, F.; Catara, A.; Licciardello, G.; Morgante, M.; et al. Detection of natural and induced mutations from next generation sequencing data in sweet orange bud sports. Acta Hortic. 2019, 1230, 119–124. [Google Scholar] [CrossRef]
- Kamatyanatt, M.; Singh, S.K.; Sekhon, B.S. Mutation Breeding in Citrus—A Review. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 1–8. [Google Scholar]
- Vardi, A.; Levin, I.; Carmi, N. Induction of Seedlessness in Citrus: From Classical Techniques to Emerging Biotechnological Approaches. J. Am. Soc. Hortic. Sci. 2008, 133, 117–126. [Google Scholar] [CrossRef]
- Hearn, C.J. Development of Seedless Orange and Grapefruit Cultivars through Seed Irradiation. J. Am. Soc. Hortic. Sci. 1984, 109, 270–273. [Google Scholar] [CrossRef]
- Deng, X.X. Citrus Cultivars Released during the Past 10 Years in China. In Proceedings of the International Society of Citriculture; International Society of Citriculture: Riverside, CA, USA, 2000; pp. 37–38. [Google Scholar]
- Cimen, B.; Yesiloglu, T.; Incesu, M.; Yilmaz, B. Studies on mutation breeding in citrus: Improving seedless types of ‘Kozan’ common orange by gamma irradiation. Sci. Hortic. 2021, 278, 109857. [Google Scholar] [CrossRef]
- Gmitter, F.G.; Soneji, J.R.; Rao, M.N. Citrus Breeding. In Breeding Plantation Tree Crops: Temperate Species; Springer: New York, NY, USA, 2009; pp. 105–134. [Google Scholar]
- Grosser, J.; Gmitter, F.J. New Sweet Orange Cultivars. Citrus Ind. Mag. 2016, 96, 10–18. [Google Scholar]
- Caruso, M.; Smith, M.W.; Froelicher, Y.; Russo, G.; Gmitter, F.G. Traditional Breeding. In The Genus Citrus; Woodhead Publishing: Sawston, UK, 2020; pp. 129–148. [Google Scholar]
- Tribulato, E.; la Rosa, G. L’arancio ‘Tarocco’ ed i suoi cloni. Riv. Fruttic. 1994, 11, 9–14. [Google Scholar]
- Tribulato, E.; la Rosa, G. ‘Ippolito’: Un nuovo clone di ‘Tarocco’. Riv. Fruttic. Ortofloric. 2000, 62, 34–35. [Google Scholar]
- Kato, M.; Ikoma, Y.; Matsumoto, H.; Sugiura, M.; Hyodo, H.; Yano, M. Accumulation of Carotenoids and Expression of Carotenoid Biosynthetic Genes during Maturation in Citrus Fruit. Plant Physiol. 2004, 134, 824–837. [Google Scholar] [CrossRef]
- Alquézar, B.; Zacarías, L.; Rodrigo, M.J. Molecular and functional characterization of a novel chromoplast-specific lycopene β-cyclase from Citrus and its relation to lycopene accumulation. J. Exp. Bot. 2009, 60, 1783–1797. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhu, K.; Sun, Q.; Zhang, W.; Wang, X.; Cao, H.; Tan, M.; Xie, Z.; Zeng, Y.; Ye, J.; et al. Natural Variation in CCD4 Promoter Underpins Species-Specific Evolution of Red Coloration in Citrus Peel. Mol. Plant 2019, 12, 1294–1307. [Google Scholar] [CrossRef] [PubMed]
- Peng, A.; Chen, S.; Lei, T.; Xu, L.; He, Y.; Wu, L.; Yao, L.; Zou, X. Engineering canker-resistant plants through CRISPR/Cas9-targeted editing of the susceptibility gene CsLOB1 promoter in citrus. Plant Biotechnol. J. 2017, 15, 1509–1519. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Y.; Wang, N. Highly Efficient Generation of Canker-Resistant Sweet Orange Enabled by an Improved CRISPR/Cas9 System. Front. Plant Sci. 2022, 12, 769907. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Peng, A.; Xie, Z.; He, Y.; Zou, X. CRISPR/Cas9-mediated editing of CsWRKY22 reduces susceptibility to Xanthomonas citri subsp. citri in Wanjincheng orange (Citrus sinensis (L.) Osbeck). Plant Biotechnol. Rep. 2019, 13, 501–510. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Jia, H.; Sosso, D.; Li, T.; Frommer, W.B.; Yang, B.; White, F.F.; Wang, N.; Jones, J.B. Lateral organ boundaries 1 is a disease susceptibility gene for citrus bacterial canker disease. Proc. Natl. Acad. Sci. USA 2014, 111, E521–E529. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.D.P.S.; Pedrosa, A.M.; Du, D.; Gonçalves, L.P.; Yu, Q.; Gmitter, F.G.; Costa, M.G.C. Genome-Wide Characterization and Expression Analysis of Major Intrinsic Proteins during Abiotic and Biotic Stresses in Sweet Orange (Citrus sinensis L. Osb.). PLoS ONE 2015, 10, e0138786. [Google Scholar] [CrossRef]
- Peng, W.; Yang, Y.; Xu, J.; Peng, E.; Dai, S.; Dai, L.; Wang, Y.; Yi, T.; Wang, B.; Li, D.; et al. TALE Transcription Factors in Sweet Orange (Citrus sinensis): Genome-Wide Identification, Characterization, and Expression in Response to Biotic and Abiotic Stresses. Front. Plant Sci. 2022, 12, 3215. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, Y.; Zhang, S.; Cao, L.; Huang, Y.; Cheng, J.; Wu, G.; Tian, S.; Chen, C.; Liu, Y.; et al. Genomic analyses of primitive, wild and cultivated citrus provide insights into asexual reproduction. Nat. Genet. 2017, 49, 765–772. [Google Scholar] [CrossRef]
- Shimada, T.; Endo, T.; Fujii, H.; Nakano, M.; Sugiyama, A.; Daido, G.; Ohta, S.; Yoshioka, T.; Omura, M. MITE insertion-dependent expression of CitRKD1 with a RWP-RK domain regulates somatic embryogenesis in citrus nucellar tissues. BMC Plant Biol. 2018, 18, 166. [Google Scholar] [CrossRef]
- Hussain, S.B.; Guo, L.-X.; Shi, C.-Y.; Khan, M.A.; Bai, Y.-X.; Du, W.; Liu, Y.-Z. Assessment of sugar and sugar accumulation-related gene expression profiles reveal new insight into the formation of low sugar accumulation trait in a sweet orange (Citrus sinensis) bud mutant. Mol. Biol. Rep. 2020, 47, 2781–2791. [Google Scholar] [CrossRef] [PubMed]
- Arlotta, C.; Ciacciulli, A.; Strano, M.C.; Cafaro, V.; Salonia, F.; Caruso, P.; Licciardello, C.; Russo, G.; Smith, M.W.; Cuenca, J.; et al. Disease Resistant Citrus Breeding Using Newly Developed High Resolution Melting and CAPS Protocols for Alternaria Brown Spot Marker Assisted Selection. Agronomy 2020, 10, 1368. [Google Scholar] [CrossRef]
- Feng, G.; Ai, X.; Yi, H.; Guo, W.; Wu, J. Genomic and transcriptomic analyses of Citrus sinensis varieties provide insights into Valencia orange fruit mastication trait formation. Hortic. Res. 2021, 8, 218. [Google Scholar] [CrossRef] [PubMed]
- Grosser, J.W.; Gmitter, F.G. Protoplast fusion for production of tetraploids and triploids: Applications for scion and rootstock breeding in citrus. Plant Cell Tissue Organ Cult. 2011, 104, 343–357. [Google Scholar] [CrossRef]
- Cuenca, J.; Garcia-Lor, A.; Navarro, L.; Aleza, P. Citrus Genetics and Breeding. In Advances in Plant Breeding Strategies: Fruits; Springer: Cham, Switzerland, 2018; Volume 3. [Google Scholar]
- Gmitter, F.G.; Xiao, S.Y.; Huang, S.; Hu, X.L.; Garnsey, S.M.; Deng, Z. A localized linkage map of the citrus tristeza virus resistance gene region. Theor. Appl. Genet. 1996, 92, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Xiao, S.; Huang, S.; Gmitter, F.G. Development and characterization of SCAR markers linked to the citrus tristeza virus resistance gene from Poncirus trifoliata. Genome 1997, 40, 697–704. [Google Scholar] [CrossRef]
- Ling, P.; Duncan, L.W.; Deng, Z.; Dunn, D.; Hu, X.; Huang, S.; Gmitter, F.G. Inheritance of citrus nematode resistance and its linkage with molecular markers. Theor. Appl. Genet. 2000, 100, 1010–1017. [Google Scholar] [CrossRef]
- Fang, D.Q.; Federici, C.T.; Roose, M.L. Development of molecular markers linked to a gene controlling fruit acidity in citrus. Genome 1997, 40, 841–849. [Google Scholar] [CrossRef]
- García, R.; Asíns, M.J.; Forner, J.; Carbonell, E.A. Genetic analysis of apomixis in Citrus and Poncirus by molecular markers. Theor. Appl. Genet. 1999, 99, 511–518. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, L.-L.; Ruan, X.; Chen, D.; Zhu, A.; Chen, C.; Bertrand, D.; Jiao, W.-B.; Hao, B.-H.; Lyon, M.P.; et al. The draft genome of sweet orange (Citrus sinensis). Nat. Genet. 2013, 45, 59–66. [Google Scholar] [CrossRef]
- Cervera, M.; Juarez, J.; Navarro, A.; Pina, J.A.; Duran-Vila, N.; Navarro, L.; Peña, L. Genetic transformation and regeneration of mature tissues of woody fruit plants bypassing the juvenile stage. Transgen. Res. 1998, 7, 51–59. [Google Scholar] [CrossRef]
- Gutiérrez, M.A.; Luth, D.; Moore, G.A. Factors affecting Agrobacterium-mediated transformation in Citrus and production of sour orange (Citrus aurantium L.) plants expressing the coat protein gene of citrus tristeza virus. Plant Cell Rep. 1997, 16, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Fagoaga, C.; Rodrigo, I.; Conejero, V.; Hinarejos, C.; Tuset, J.J.; Arnau, J.; Pina, J.A.; Navarro, L.; Peña, L. Increased tolerance to Phytophthora citrophthora in transgenic orange plants constitutively expressing a tomato pathogenesis related protein PR-5. Mol. Breed. 2001, 7, 175–185. [Google Scholar] [CrossRef]
- Fagoaga, C.; López, C.; Moreno, P.; Navarro, L.; Flores, R.; Peña, L. Viral-Like Symptoms Induced by the Ectopic Expression of the p23 Gene of Citrus tristeza virus Are Citrus Specific and Do Not Correlate with the Pathogenicity of the Virus Strain. Mol. Plant-Microbe Interact. 2005, 18, 435–445. [Google Scholar] [CrossRef]
- Hu, R.; Wei, H.; Chen, S.-C.; He, Y.-R. Construction of the plant expression vector with hepatitis a capsid protein fusion gene and genetic transformation of Citrus. Sinensis Osbeck. Yi Chuan = Hered./Zhongguo Yi Chuan Xue Hui Bian Ji 2004, 26, 425–431. [Google Scholar]
- Gonzalez-Ramos, J.; Graham, J.; Mirkov, T. Transformation of Citrus Cultivars with Genes Encoding Potential Resistance to Citrus Canker (Xanthomonas axonopodis Pv. Citri). Phytopathology 2005, 95, S35. [Google Scholar]
- Omar, A.A.; Song, W.-Y.; Grosser, J.W. Introduction of Xa21, a Xanthomonas-resistance gene from rice, into ‘Hamlin’ sweet orange [Citrus sinensis (L.) Osbeck] using protoplast-GFP co-transformation or single plasmid transformation. J. Hortic. Sci. Biotechnol. 2007, 82, 914–923. [Google Scholar] [CrossRef]
- Barbosa-Mendes, J.M.; Mourão Filho, F.D.A.A.; Filho, A.B.; Harakava, R.; Beer, S.V.; Mendes, B.M.J. Genetic transformation of Citrus sinensis cv. Hamlin with hrpN gene from Erwinia amylovora and evaluation of the transgenic lines for resistance to citrus canker. Sci. Hortic. 2009, 122, 109–115. [Google Scholar] [CrossRef]
- Yang, L.; Hu, C.; Li, N.; Zhang, J.; Yan, J.; Deng, Z. Transformation of sweet orange [Citrus sinensis (L.) Osbeck] with pthA-nls for acquiring resistance to citrus canker disease. Plant Mol. Biol. 2011, 75, 11–23. [Google Scholar] [CrossRef]
- Fu, X.-Z.; Chen, C.-W.; Wang, Y.; Liu, J.-H.; Moriguchi, T. Ectopic expression of MdSPDS1 in sweet orange (Citrus sinensis Osbeck) reduces canker susceptibility: Involvement of H2O2 production and transcriptional alteration. BMC Plant Biol. 2011, 11, 55. [Google Scholar] [CrossRef]
- Attílio, L.B.; Martins, P.K.; Gómez-Krapp, L.M.; Machado, M.A.; Freitas-Astúa, J. Genetic transformation of sweet oranges to over-express SABP2 gene. BMC Proc. 2014, 8, P109. [Google Scholar] [CrossRef]
- Dutt, M.; Barthe, G.; Irey, M.; Grosser, J. Correction: Transgenic Citrus Expressing an Arabidopsis NPR1 Gene Exhibit Enhanced Resistance against Huanglongbing (HLB; Citrus Greening). PLoS ONE 2016, 11, e0147657. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Duan, Y.; Olivares-Fuster, O.; Wu, Z.; Arias, C.R.; Burns, J.K.; Grosser, J.W. Protoplast transformation and regeneration of transgenic Valencia sweet orange plants containing a juice quality-related pectin methylesterase gene. Plant Cell Rep. 2005, 24, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Pons, E.; Alquézar, B.; Rodríguez, A.; Martorell, P.; Genovés, S.; Ramón, D.; Rodrigo, M.J.; Zacarías, L.; Peña, L. Metabolic engineering of β-carotene in orange fruit increases its in vivo antioxidant properties. Plant Biotechnol. J. 2014, 12, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, Y.; Shen, S.; Yin, X.; Klee, H.; Zhang, B.; Chen, K. Transcription factor CitERF71 activates the terpene synthase gene CitTPS16 involved in the synthesis of E-geraniol in sweet orange fruit. J. Exp. Bot. 2017, 68, 4929–4938. [Google Scholar] [CrossRef]
- Wong, W.S.; Li, G.G.; Ning, W.; Xu, Z.F.; Hsiao, W.L.W.; Zhang, L.Y.; Li, N. Repression of chilling-induced ACC accumulation in transgenic citrus by over-production of antisense 1-aminocyclopropane-1-carboxylate synthase RNA. Plant Sci. 2001, 161, 969–977. [Google Scholar] [CrossRef]
- Hasan, N.; Kamruzzaman, M.; Islam, S.; Hoque, H.; Bhuiyan, F.H.; Prodhan, S.H. Development of partial abiotic stress tolerant Citrus reticulata Blanco and Citrus sinensis (L.) Osbeck through Agrobacterium-mediated transformation method. J. Genet. Eng. Biotechnol. 2019, 17, 14. [Google Scholar] [CrossRef]
- Salonia, F.; Ciacciulli, A.; Poles, L.; Pappalardo, H.D.; La Malfa, S.; Licciardello, C. New Plant Breeding Techniques in Citrus for the Improvement of Important Agronomic Traits. A Review. Front. Plant Sci. 2020, 11, 1234. [Google Scholar] [CrossRef]
- Jia, H.; Wang, N. Xcc-facilitated agroinfiltration of citrus leaves: A tool for rapid functional analysis of transgenes in citrus leaves. Plant Cell Rep. 2014, 33, 1993–2001. [Google Scholar] [CrossRef]
- Jia, H.; Wang, N. Targeted Genome Editing of Sweet Orange Using Cas9/sgRNA. PLoS ONE 2014, 9, e93806. [Google Scholar] [CrossRef]
- Salonia, F.; Ciacciulli, A.; Pappalardo, H.D.; Poles, L.; Pindo, M.; Larger, S.; Caruso, P.; Caruso, M.; Licciardello, C. A dual sgRNA-directed CRISPR/Cas9 construct for editing the fruit-specific β-cyclase 2 gene in pigmented citrus fruits. Front. Plant Sci. 2022, 13, 975917. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Aka Kacar, Y.; Cristofani-Yaly, M.; Curtolo, M.; Machado, M.A. Markers, Maps, and Marker-Assisted Selection. In The Citrus Genome; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Curtolo, M.; Cristofani-Yaly, M.; Gazaffi, R.; Takita, M.A.; Figueira, A.; Machado, M.A. QTL mapping for fruit quality in Citrus using DArTseq markers. BMC Genom. 2017, 18, 289. [Google Scholar] [CrossRef]
- Minamikawa, M.F.; Nonaka, K.; Kaminuma, E.; Kajiya-Kanegae, H.; Onogi, A.; Goto, S.; Yoshioka, T.; Imai, A.; Hamada, H.; Hayashi, T.; et al. Genome-wide association study and genomic prediction in citrus: Potential of genomics-assisted breeding for fruit quality traits. Sci. Rep. 2017, 7, 4721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, Z.; Zong, Y.; Wang, Y.; Liu, J.; Chen, K.; Qiu, J.-L.; Gao, C. Efficient and transgene-free genome editing in wheat through transient expression of CRISPR/Cas9 DNA or RNA. Nat. Commun. 2016, 7, 12617. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, W.; Katin-Grazzini, L.; Ding, J.; Gu, X.; Li, Y.; Gu, T.; Wang, R.; Lin, X.; Deng, Z.; et al. A method for the production and expedient screening of CRISPR/Cas9-mediated non-transgenic mutant plants. Hortic. Res. 2018, 5, 13. [Google Scholar] [CrossRef]
- Lin, C.-S.; Hsu, C.-T.; Yang, L.-H.; Lee, L.-Y.; Fu, J.-Y.; Cheng, Q.-W.; Wu, F.-H.; Hsiao, H.C.-W.; Zhang, Y.; Zhang, R.; et al. Application of protoplast technology to CRISPR/Cas9 mutagenesis: From single-cell mutation detection to mutant plant regeneration. Plant Biotechnol. J. 2018, 16, 1295–1310. [Google Scholar] [CrossRef] [PubMed]
- Veillet, F.; Perrot, L.; Chauvin, L.; Kermarrec, M.-P.; Guyon-Debast, A.; Chauvin, J.-E.; Nogué, F.; Mazier, M. Transgene-Free Genome Editing in Tomato and Potato Plants Using Agrobacterium-Mediated Delivery of a CRISPR/Cas9 Cytidine Base Editor. Int. J. Mol. Sci. 2019, 20, 402. [Google Scholar] [CrossRef]
- Zhang, Y.; Iaffaldano, B.; Qi, Y. CRISPR ribonucleoprotein-mediated genetic engineering in plants. Plant Commun. 2021, 2, 100168. [Google Scholar] [CrossRef]
- Lassoued, R.; Phillips, P.W.B.; Macall, D.M.; Hesseln, H.; Smyth, S.J. Expert opinions on the regulation of plant genome editing. Plant Biotechnol. J. 2021, 19, 1104–1109. [Google Scholar] [CrossRef]
 male,
male,  female, red arrow: phylogenetic relationship].
female, red arrow: phylogenetic relationship].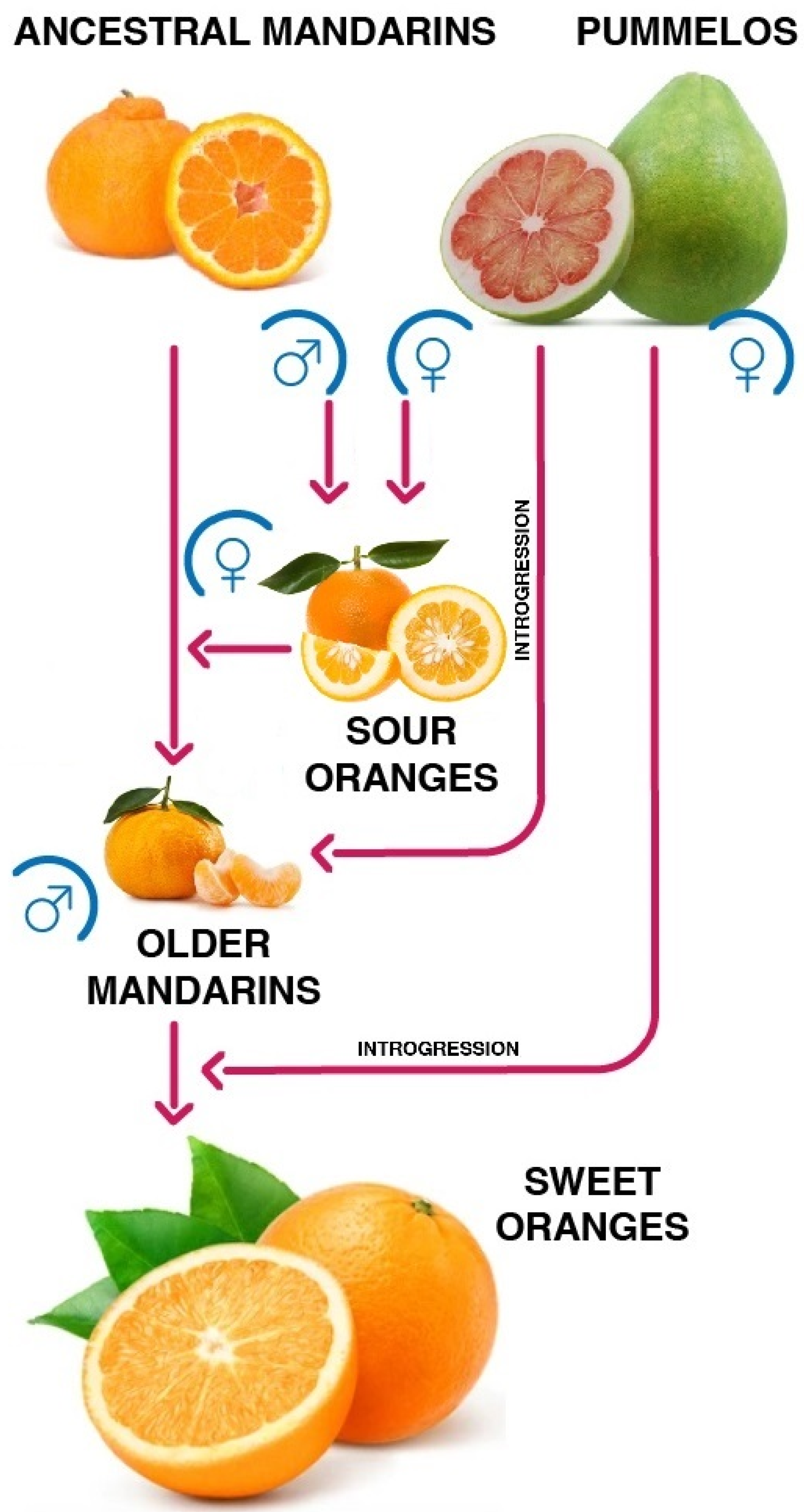



| Minimum Juice Content (%) | Minimum Sugar/Acid Ratio | |
|---|---|---|
| Blood oranges | 30 | 6.5:1 |
| Navel group | 33 | 6.5:1 |
| Other varieties | 35 | 6.5:1 |
| “Mosambi”, “Sathgudi”, and “Pacitan”(with more than one fifth of green color) | 33 | |
| Other varieties (with more than one fifth of green color) | 45 |
| Compound Class | Compound Name | Sanguinello | Moro | Tarocco |
| Hydroxycinnamic acids | Chlorogenic acid (mg/L) | 1.40 ± 0.26 | 4.80 ± 4.15 | 5.45 ± 5.49 |
| p-Coumaric acid (mg/L) | 1.40 ± 0.26 | 1.42 ± 1.91 | 1.61 ± 1.31 | |
| Ferulic + sinapic acid (mg/L) | 5.91 ± 1.11 | 4.57 ± 3.76 | 3.68 ± 1.55 | |
| Flavanone glycosides | Narirutin (mg/L) | 17.22 ± 3.24 | 18.25 ± 2.79 | 14.17 ± 2.25 |
| Hesperidin (mg/L) | 189.20 ± 35.59 | 174.28 ± 13.13 | 217.77 ± 48.19 | |
| Didymin (mg/L) | 6.60 ± 1.24 | 6.80 ± 1.05 | 5.54 ± 0.38 | |
| Anthocyanidin glycosides | Cyanidin-3-glucoside (mg/L) | 5.18 ± 3.99 | 46.30 ± 19.88 | 10.33 ± 11.63 |
| Cyanidin-3-(6″-malonyl)-glucoside (mg/L) | 7.33 ± 1.55 | 53.98 ± 1.06 | 25.08 ± 6.36 | |
| Vitamin C (ascorbic acid (mM)) | 3.27 ± 0.27 | 3.32 ± 0.25 | 3.11 ± 0.07 | |
| Compound Class | Compound Name | W. Navel | Valencia | Ovale |
| Hydroxycinnamic acids | Chlorogenic acid (mg/L) | 1.94 ± 0.12 | 1.84 ± 0.03 | 2.32 ± 0.59 |
| p-Coumaric acid (mg/L) | 0.16 ± 0.04 | 0.15 ± 0.04 | 0.47 ± 0.30 | |
| Ferulic + sinapic acid (mg/L) | 0.76 ± 0.01 | 1.11 ± 0.03 | 1.31 ± 0.61 | |
| Flavanone glycosides | Narirutin (mg/L) | 5.96 ± 0.21 | 4.57 ± 0.96 | 10.17 ± 4.49 |
| Hesperidin (mg/L) | 100.75 ± 10.35 | 52.05 ± 13.22 | 121.73 ± 27.57 | |
| Didymin (mg/L) | 2.80 ± 0.01 | 1.86 ± 0.38 | 4.80 ± 2.20 | |
| Anthocyanidin glycosides | Cyanidin-3-glucoside (mg/L) | nd | nd | nd |
| Cyanidin-3-(6″-malonyl)-glucoside (mg/L) | nd | nd | nd | |
| Vitamin C (ascorbic acid (mM)) | 2.62 ± 0.30 | 2.21 ± 0.17 | 3.01 ± 0.07 |
| Selection | Female Parent | Male Parent |
|---|---|---|
| FF-1-64-97 | “Ambersweet” | “Tunis” sour orange × “Succory” sweet orange |
| FF-1-65-55 | “Ambersweet” | “Tunis” sour orange × “Succory” sweet orange |
| FF-1-75-55 | “Ambersweet” | “Wilking” × “Valencia” |
| FF-1-76-50 | “Ambersweet” | “Wilking” × “Valencia” |
| FF-1-76-52 | “Ambersweet” | “Wilking” × “Valencia” |
| Submitter | Cultivar | Sequencing Technology | Assembly Name | Assembly Level | Contig N50 (lb) | Size (Mb) | Submission Date | Bioproject | Biosample ID |
|---|---|---|---|---|---|---|---|---|---|
| China sweet orange genome project | “Valencia” | Illumina | Csi_valencia_1.0 | Chromosome | 49.9 | 327.7 | 12/12/2012 | PRJNA86123 | SAMN02981414 |
| DOE-Joint Genome Institute | “Ridge Pineapple” | 454 GS-FLX Titanium, 454 FLX Standard, and ABI 3739 | Citrus_sinensis_v1.0 | Scaffold | 6.6 | 319.2 | 30/05/2014 | PRJNA225968 | SAMN02389851 |
| Huazhong Agriculture University | “Valencia” | PacBio and Illumina GAII | ASM1810434v1 | Scaffold | 2102.1 | 338.4 | 20/04/2021 | PRJNA347609 | SAMN05893359 |
| Huazhong Agriculture University | HZAU_DHSO_2021 | Oxford Nanopore | ASM1810577v1 | Chromosome | 24,160.9 | 334.3 | 23/04/2021 | PRJNA347609 | SAMN16516428 |
| Huazhong Agriculture University | SO3 | PacBio Sequel | ASM1914366v1 | Chromosome | 246.2 | 310.6 | 06/07/2021 | PRJNA321100 | SAMN07311581 |
| Huazhong Agriculture University | TCPS1 | PacBio Sequel | ASM1914415v1 | Chromosome | 266.1 | 346.5 | 06/07/2021 | PRJNA321100 | SAMN07313349 |
| Huazhong Agriculture University | NW | Oxford Nanopore | ASM1914418v1 | Chromosome | 1932.8 | 322.6 | 06/07/2021 | PRJNA321100 | SAMN07313221 |
| Huazhong Agriculture University | NHE | PacBio Sequel | ASM1914419v1 | Chromosome | 251.3 | 315.1 | 06/07/2021 | PRJNA321100 | SAMN05412752 |
| Huazhong Agriculture University | BT2 | Oxford Nanopore | ASM1914422v1 | Chromosome | 1218.0 | 330.2 | 06/07/2021 | PRJNA321100 | SAMN07311744 |
| Huazhong Agriculture University | UKXC | Oxford Nanopore | ASM1914424v1 | Chromosome | 1693.9 | 328.7 | 06/07/2021 | PRJNA321100 | SAMN07313355 |
| Clemson University | “Valencia” | PacBio Sequel II | DVS_A1.0 | Chromosome | 32,942.3 | 299.0 | 11/02/2022 | PRJNA736174 | SAMN19611724 |
| Clemson University | “Valencia” | PacBio Sequel II | DVS_B1.0 | Chromosome | 32,342.9 | 299.6 | 11/02/2022 | PRJNA736176 | SAMN19611724 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seminara, S.; Bennici, S.; Di Guardo, M.; Caruso, M.; Gentile, A.; La Malfa, S.; Distefano, G. Sweet Orange: Evolution, Characterization, Varieties, and Breeding Perspectives. Agriculture 2023, 13, 264. https://doi.org/10.3390/agriculture13020264
Seminara S, Bennici S, Di Guardo M, Caruso M, Gentile A, La Malfa S, Distefano G. Sweet Orange: Evolution, Characterization, Varieties, and Breeding Perspectives. Agriculture. 2023; 13(2):264. https://doi.org/10.3390/agriculture13020264
Chicago/Turabian StyleSeminara, Sebastiano, Stefania Bennici, Mario Di Guardo, Marco Caruso, Alessandra Gentile, Stefano La Malfa, and Gaetano Distefano. 2023. "Sweet Orange: Evolution, Characterization, Varieties, and Breeding Perspectives" Agriculture 13, no. 2: 264. https://doi.org/10.3390/agriculture13020264
APA StyleSeminara, S., Bennici, S., Di Guardo, M., Caruso, M., Gentile, A., La Malfa, S., & Distefano, G. (2023). Sweet Orange: Evolution, Characterization, Varieties, and Breeding Perspectives. Agriculture, 13(2), 264. https://doi.org/10.3390/agriculture13020264











