Plants for Fitness Enhancement of a Coffee Leaf Miner Parasitoid
Abstract
1. Introduction
2. Materials and Methods
2.1. Coffee Leaf Miner Parasitoids Collection
2.2. Rearing of Proacrias Coffeae
2.3. Survival Experiments
2.4. Egg Load Experiment
2.5. Statistical Analysis
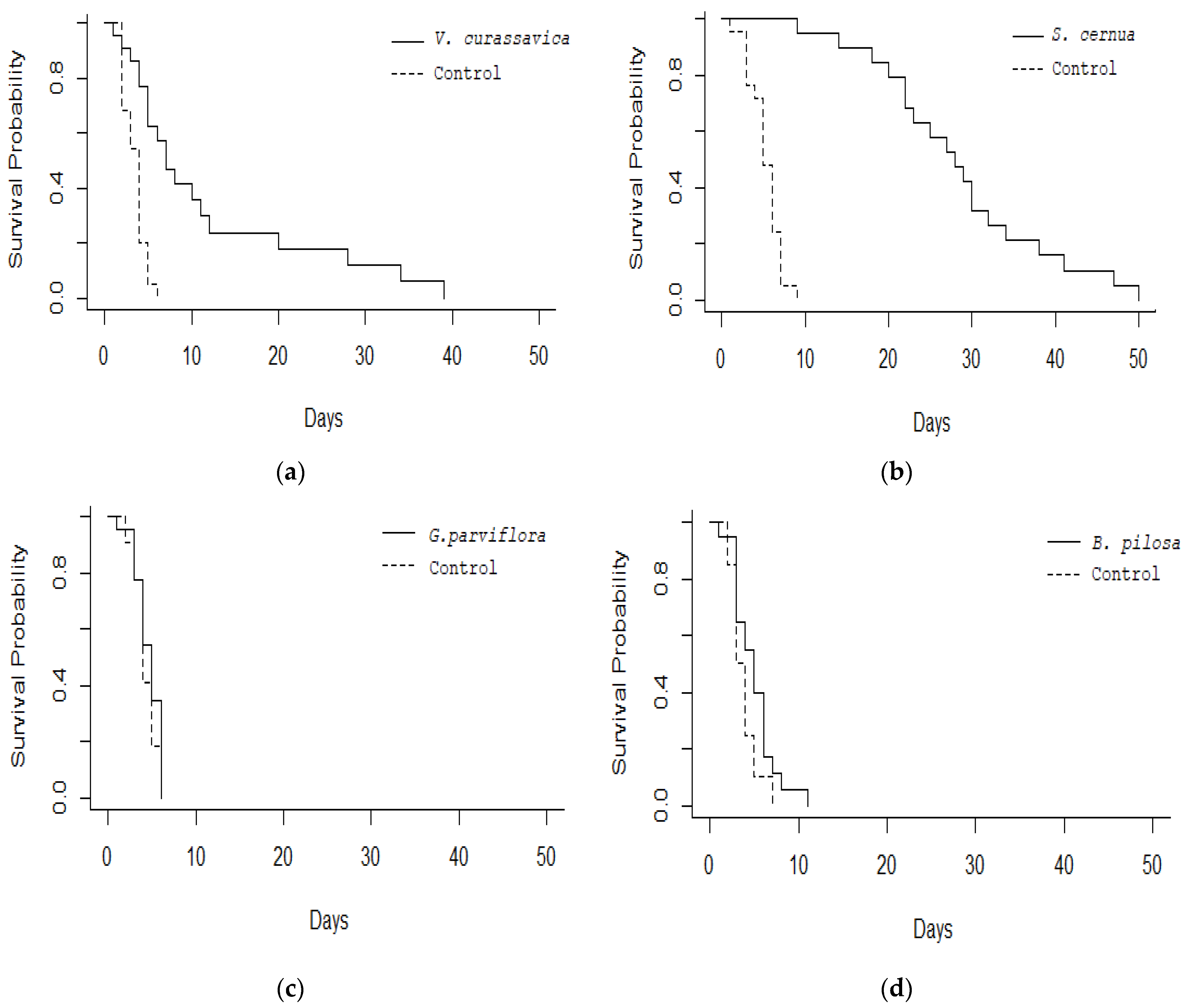
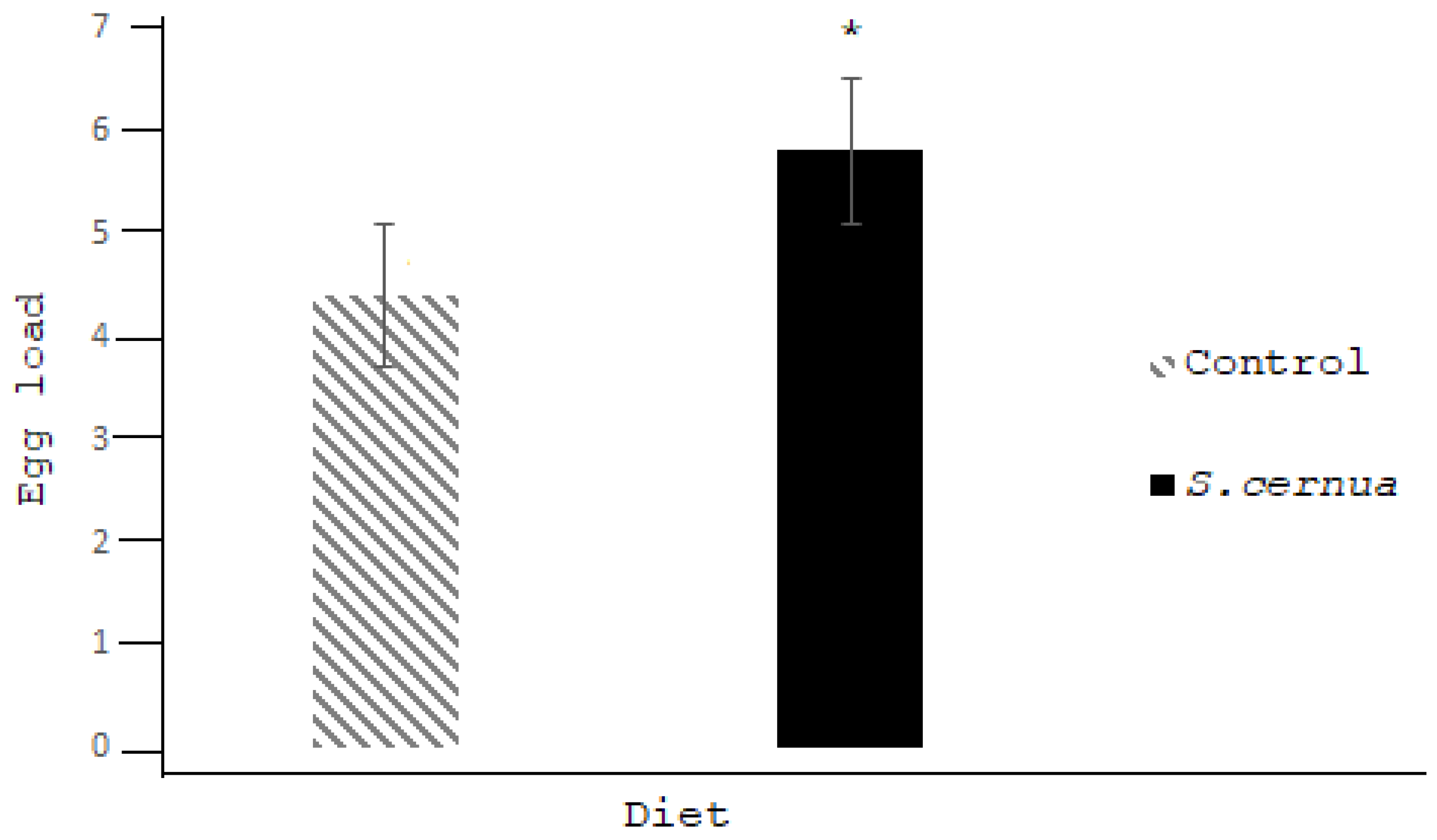
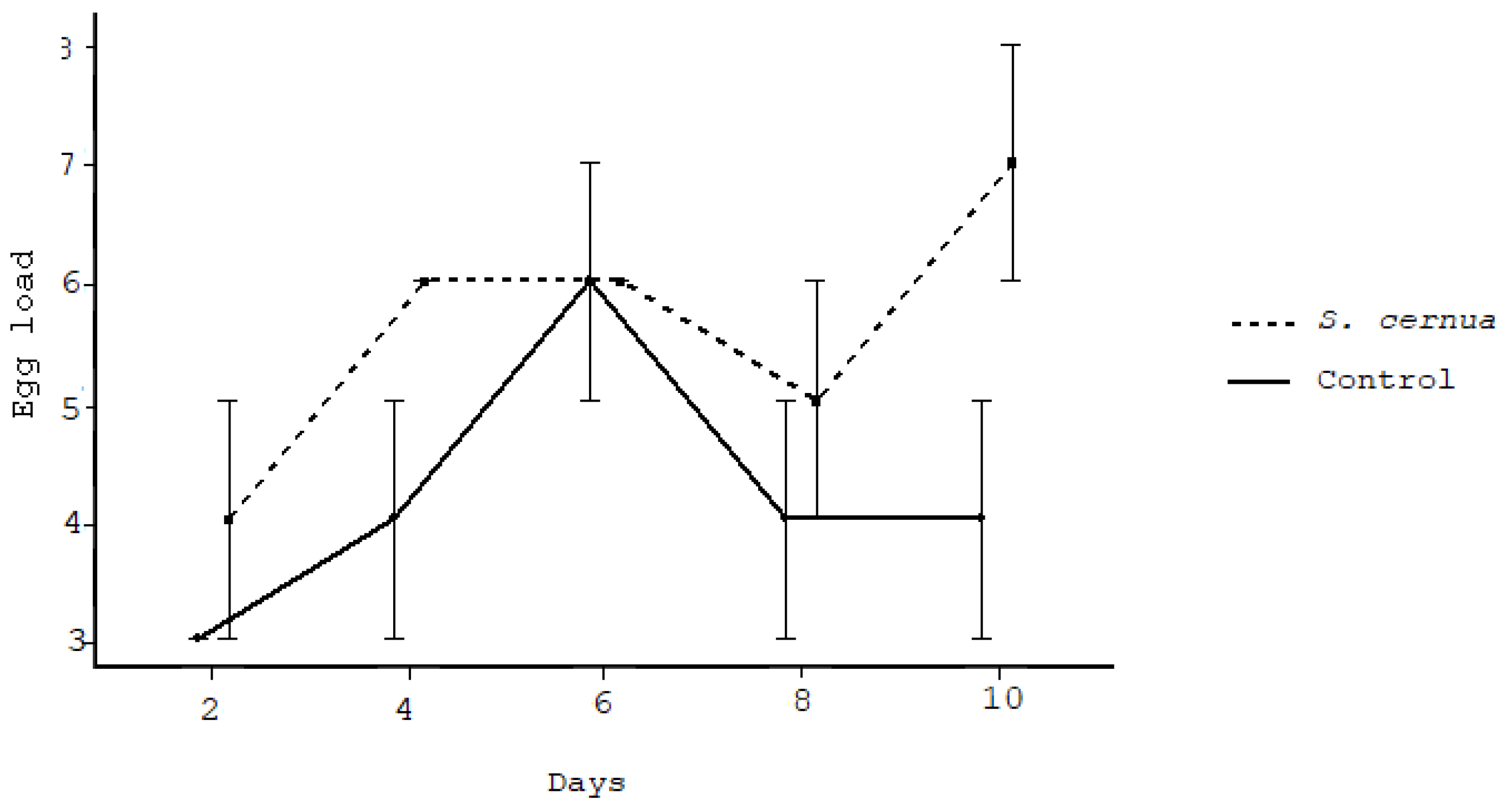
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wyckhuys, K.A.G.; Lu, Y.H.; Morales, H.; Vazquez, L.L.; Legaspi, J.C.; Eliopoulos, P.A.; Hernandez, L.M. Current status and potential of conservation biological control for agriculture in the developing world. Biol. Control 2013, 65, 152–167. [Google Scholar] [CrossRef]
- Venzon, M.; Amaral, D.S.S.L.; Togni, P.H.B.; Chiguachi, J.A.M. Interactions of natural enemies with non-cultivated plants. In Natural Enemies of Insect Pests in Neotropical Agroecosystems, 1st ed.; Souza, B., Vázquez, L., Marucci, R.C., Eds.; Springer: Cham, Switzerland, 2019; pp. 15–26. [Google Scholar]
- Blassioli-Moraes, M.C.; Venzon, M.; Silveira, L.C.P.; Gontijo, L.M.; Togni, P.H.B.; Sujii, E.R.; Haro, M.M.; Borges, M.; Michereff, M.F.F.; Santos de Aquino, M.F.; et al. Companion and smart plants: Scientific background to promote conservation biological control. Neotrop. Entomol. 2022, 51, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Shields, M.W.; Johnson, A.C.; Pandey, S.; Cullen, R.; González-Chang, M.; Wratten, S.D.; Gurr, G.M. History, current situation and challenges for conservation biological control. Biol. Control 2019, 131, 25–35. [Google Scholar] [CrossRef]
- Venzon, M.; Sujii, E.R. Conservative biological control. Inf. Agropecu. 2019, 30, 7–16. [Google Scholar]
- Wäckers, F.L.; Van Rijn, P.C. Pick and mix: Selecting flowering plants to meet the requirements of target biological control insects. In Biodiversity and Insect Pests: Key Issues for Sustainable Management; Gurr, G.M., Wratten, S.D., Snyder, W.E., Eds.; John Wiley & Sons: New York, NY, USA, 2012; pp. 139–165. [Google Scholar]
- Vattala, H.D.; Wratten, S.D.; Phillips, C.B.; Wäckers, F.L. The influence of flower morphology and nectar quality on the longevity of a parasitoid biological control agent. Biol. Control 2006, 39, 179–185. [Google Scholar] [CrossRef]
- Wang, W.; Lu, S.L.; Liu, W.X.; Cheng, L.S.; Zhang, Y.B.; Wan, F.H. Effects of five naturally occurring sugars on the longevity, oogenesis, and nutrient accumulation pattern in adult females of the synovigenic parasitoid Neochrysocharis formosa (Hymenoptera: Eulophidae). Neotrop. Entomol. 2014, 43, 564–573. [Google Scholar] [CrossRef]
- Jamont, M.; Dubois-Pot, C.; Jaloux, B. Nectar provisioning close to host patches increases parasitoid recruitment, retention and host parasitism. Basic Appl. Ecol. 2014, 15, 151–160. [Google Scholar] [CrossRef]
- Olson, D.M.; Takasu, K.; Lewis, W.J. Food needs of adult parasitoids: Behavioral adaptations and consequences. In Plant-Provided Food for Carnivorous Insects: A Protective Mutualism and Its Applications; Wäckers, F.L., Van Rijn, P.C., Bruin, J., Eds.; Cambridge University Press: New York, NY, USA, 2005; pp. 137–148. [Google Scholar]
- Strand, M.R.; Casas, J. Parasitoid and host nutritional physiology in behavioral ecology. In Behavioral Ecology of Insect Parasitoids; Wajnberg, E., Bernstein, C., van Alphen, J., Eds.; Blackwell Publishing: Hong Kong, 2008; pp. 113–128. [Google Scholar]
- Papaj, D.R. Ovarian dynamics and host use. Annu. Rev. Entomol. 2000, 45, 423–448. [Google Scholar] [CrossRef]
- Jervis, M.A.; Heimpel, G.E.; Ferns, P.N.; Harvey, J.A.; Kidd, N.A. Life-history strategies in parasitoid wasps: A comparative analysis of ‘ovigeny’. J. Anim. Ecol. 2001, 70, 442–458. [Google Scholar] [CrossRef]
- Fernandes, L.G. Diversidade de Inimigos Naturais de Pragas do Cafeeiro em Diferentes Sistemas de Cultivo. Ph.D. Thesis, Universidade Federal de Lavras, Lavras, Brazil, 2013. [Google Scholar]
- Rezende, M.Q. Extrafloral Nectary-Bearing Trees Enhance Pest Control and Increase Fruit Weight in Associated Coffee Plants. Ph.D. Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 2014. [Google Scholar]
- Marques, K.B.S.C. Infestação e Parasitismo de Leucoptera coffeella (Guérin-Mèneville & Perrottet, 1842) em Cafeeiros em Transição Agroecológica. Master’s Thesis, Universidade Federal de Lavras, Lavras, Brazil, 2017. [Google Scholar]
- Martins, E.F. Conservation Biological Control of Coffee Leaf Miner: Role of Green Lacewings and Parasitoids. Ph.D. Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 2021. [Google Scholar]
- Rezende, M.Q.; Venzon, M.; Dos Santos, P.S.; Cardoso, I.M.; Janssen, A. Extrafloral nectary-bearing leguminous trees enhance pest control and increase fruit weight in associated coffee plants. Agric. Ecosyst. Environ. 2021, 319, 107–538. [Google Scholar] [CrossRef]
- Leite, S.A.; Guedes, R.N.C.; Santos, M.P.D.; Costa, D.R.D.; Moreira, A.A.; Matsumoto, S.N.; Castellani, M.A. Profile of coffee crops and management of the neotropical coffee leaf miner, Leucoptera coffeella. Sustainability 2020, 12, 8011. [Google Scholar] [CrossRef]
- Ferreira, F.Z.; Silveira, L.C.P.; Haro, M.M. Families of Hymenoptera parasitoids in organic coffee cultivation Santo Antonio do Amparo, MG, Brazil. Coffee Sci. 2013, 8, 1–4. [Google Scholar]
- Melo, T.L.; Castellani, M.A.; Nascimento, M.D.L.D.; Junior, M.; De Oliveira, A.; Ferreira, G.F.P.; Lemos, O.L. Comunidades de parasitóides de Leucoptera coffeella (Guérin-Mèneville & Perrottet, 1842) (Lepidoptera: Lyonetiidae) em cafeeiros nas regiões Oeste e Sudoeste da Bahia. Ciência Agrotecnologia 2007, 31, 966–972. [Google Scholar]
- Ramiro, Z.A.; Costa, V.A.; Dias, A.M.; Oliveira, D.A. Estudo da fauna de Braconidae (Hymenoptera, Ichneumonoidea) em cultura de café (Coffea arabica L., Rubiaceae), no Estado de São Paulo. In Simpósio de Pesquisa dos Cafés do Brasil; São Paulo, Brazil, 2007. [Google Scholar]
- Resende, A.L.S.; dos Santos, C.M.A.; Campos, J.M.; Menezes, E.B.; de Lima Aguiar-Menezes, E. Ocorrência de parasitóides do bicho mineiro infestando seis cultivares de café arábica em sistema orgânico com e sem arborização. Rev. Bras. Agroecol. 2007, 2, 921–924. [Google Scholar]
- Tango, M.F.; Fernandes, D.R.; Paz, C.C.; Lara, R.I.; Perioto, N.W. Orgilinae (Hymenoptera: Braconidae) en cultivo de café de Cravinhos, SP, Brasil. Rev. Colomb. Entomol. 2014, 40, 25–33. [Google Scholar]
- Togni, P.H.B.; Venzon, M.; Lagôa, A.C.G.; Sujii, E.R. Brazilian legislation leaning towards fast registration of biological control agents to benefit organic agriculture. Neotrop. Entomol. 2019, 48, 175–185. [Google Scholar] [CrossRef]
- Dantas, J.; Motta, I.O.; Vidal, L.A.; Nascimento, E.F.; Bilio, J.; Pupe, J.M.; Veiga, A.; Carvalho, C.; Lopes, R.B.; Rocha, T.L.; et al. A comprehensive review of the coffee leaf miner Leucoptera coffeella (Lepidoptera: Lyonetiidae)—A major pest for the coffee crop in Brazil and others neotropical countries. Insects 2021, 12, 1130. [Google Scholar] [CrossRef]
- Amaral, D.S.; Venzon, M.; Pallini, A.; Lima, P.C.; De Souza, O. A diversificação da vegetação reduz o ataque do bicho-mineiro-do-cafeeiro Leucoptera coffeella (Guérin-mèneville) (Lepidoptera: Lyonetiidae). Neotrop Entomol. 2010, 39, 543–548. [Google Scholar] [CrossRef]
- Carvalho, C.F.; Carvalho, S.M.; Souza, B. Coffee. In Natural Enemies of Insect Pests in Neotropical Agroecosystems, 1st ed.; Souza, B., Vázquez, L.L., Marucci, R.C., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Rezende, M.Q.; Venzon, M.; Silva, A.F.; Abrão, M.M.; Calderón-Arroyo, C. Associational defense in coffee crops promoted by plants bearing extrafloral nectaries. In Controle Alternativo de Pragas e Doenças: Opção ou Necessidade? 1st ed.; Venzon, M., Neves, W.S., Júnior, T.J.P., Pallini, A., Eds.; EPAMIG: Belo Horizonte, Brazil, 2021; pp. 88–93. [Google Scholar]
- Venzon, M. Agro-Ecological Management of Coffee Pests in Brazil. Front. Sustain. Food Syst. 2021, 5, 721117. [Google Scholar] [CrossRef]
- Rezende, M.Q.; Venzon, M.; Perez, A.L.; Cardoso, I.M.; Janssen, A. Extrafloral nectaries of associated trees can enhance natural pest control. Agric. Ecosyst. Environ. 2014, 188, 198–203. [Google Scholar] [CrossRef]
- Silva, M.A.A.D.; Almeida, W.L.D.; Andrade, M.V.S.; Ribeiro, J.G.; Araújo, F.C.D.; Silva, T.O.; Roncho, C.P. Levantamento da infestação de plantas daninhas, durante o verão, em lavoura de café arábica fertirrigada do Alto Paranaiba-MG. In Proceedings of the VI Simposio de Pesquisa dos Cafés do Brasil, Espírito Santo, Brazil, 18 March 2009. [Google Scholar]
- Budumajji, U.; Solomon Raju, A.J. Pollination ecology of Bidens pilosa L. (Asteraceae). Taiwania 2018, 63, 89–100. [Google Scholar]
- Moreira, H.D.C.; Bragança, H.B.N. Manual de Identificação de Plantas Infestantes; FMC Agricultural Products: Campinas, Brazil, 2011. [Google Scholar]
- Oliveira, L.D.G.R.D.; Garcia, F.C.P. Senna (Leguminosae-Caesalpinioideae) in Minas Gerais state, Brazil. Rodriguésia 2021, 72, 1–57. [Google Scholar] [CrossRef]
- Bristot, S.F.; Della Colle, M.P.; Rossato, A.E.; Citadini-Zanette, V. Uso medicinal de Varronia curassavica Jacq. “erva-baleeira” (Boraginaceae): Estudo de caso no sul do Brasil. Braz. J. Anim. Environ. Res. 2021, 4, 170–182. [Google Scholar] [CrossRef]
- Hoeltgebaum, M.P.; Montagna, T.; Lando, A.P.; Puttkammer, C.; Orth, A.I.; Guerra, M.P.; Reis, M.S. Reproductive Biology of Varronia curassavica Jacq. (Boraginaceae). An. Acad. Bras. Cienc. 2018, 90, 59–71. [Google Scholar] [CrossRef]
- Miranda, N.F. Parasitoides (Hymenoptera: Eulophidae) de bicho-mineiro Leucoptera coffeella (Guérin-Mèneville) (Lepidoptera Lyonetiidae). Master’s Thesis, Universidade Estadual Paulista, Jaboticabal, Brazil, 2009. [Google Scholar]
- Nave, A.; Gonçalves, F.; Crespí, A.L.; Campos, M.; Torres, L. Evaluation of native plant flower characteristics for conservation biological control of Prays oleae. Bull. Entomol. Res. 2016, 106, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Winkler, K.; Wäckers, F.L.; Kaufman, L.V.; Larraz, V.; Van Lenteren, J.C. Nectar exploitation by herbivores and their parasitoids is a function of flower species and relative humidity. Biol. Control 2009, 50, 299–306. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.D.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Zeitschrift. 2013, 22, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Burks, R.A. Key to the Nearctic Genera of Eulophidae, Subfamilies Entedoninae, Euderinae, and Eulophinae (Hymenoptera Chalcidoidea). World Wide Web Electronic Publication. Available online: http//faculty.ucr.edu/%7Eheraty/Eulophidae/,2003 (accessed on 15 November 2021).
- Navarro-Gutiérrez, P.; Gallardo-Covas, F. Host instar preference of Mirax insularis (Muesebeck) (Hymenoptera: Braconidae), a Koinobiont Parasitoid of Leucoptera coffeella Guerin-Meneville (Lepidoptera: Lyonetiidae). J. Agric. Univ. P.R. 2009, 93, 139–142. [Google Scholar] [CrossRef]
- Wade, M.R.; Wratten, S.D. Excised or intact inflorescences? Methodological effects on parasitoid wasp longevity. Biol. Control 2007, 40, 347–354. [Google Scholar] [CrossRef]
- Core Team, R. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Patt, J.M.; Hamilton, G.C.; Lashomb, J.H. Foraging success of parasitoid wasps on flowers: Interplay of insect morphology, floral architecture and searching behavior. Entomol. Exp. Appl. 1997, 83, 21–30. [Google Scholar] [CrossRef]
- Stang, M.; Klinkhamer, P.G.; Van Der Meijden, E. Size constraints and flower abundance determine the number of interactions in a plant–flower visitor web. Oikos 2006, 112, 111–121. [Google Scholar] [CrossRef]
- Jervis, M.A.; Kidd, N.A.C.; Fitton, M.G.; Huddleston, T.; Dawah, H.A. Flower-visiting by hymenopteran parasitoids. J. Nat. Hist. 1993, 27, 67–105. [Google Scholar] [CrossRef]
- Schmalhofer, V.R. Tritrophic interactions in a pollination system: Impacts of species composition and size of flower patches on the hunting success of a flower-dwelling spider. Oecologia 2001, 129, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Villa, V.; Vibrans, H.; Uscanga-Mortera, E.; Aguirre-Jaimes, A. Floral visitors and pollinator dependence are related to floral display size and plant height in native weeds of central Mexico. Flora 2020, 262, 151505. [Google Scholar] [CrossRef]
- Torres, C.; Galetto, L. Are nectar sugar composition and corolla tube length related to the diversity of insects that visit Asteraceae flowers? Plant Biol. 2002, 4, 360–366. [Google Scholar] [CrossRef]
- Martins, E.F. Interações ecológicas da erva-baleeira (Varronia curassavica Jacq.) e seus artrópodes visitantes. Master’s Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 2017. [Google Scholar]
- Brandão, D.S.; Mendes, A.D.R.; Santos, R.R.; Rocha, S.M.G.; Leite, G.L.D.; Martins, E.R. Biologia floral e sistema reprodutivo da erva-baleeira (Varronia curassavica Jacq.). Rev. Bras.Plantas Med. 2015, 17, 562–569. [Google Scholar] [CrossRef]
- Ramos, D.L.; Cunha, W.L.; Evangelista, J.; Lira, L.A.; Rocha, M.V.C.; Gomes, P.A.; Frizzas, M.R.; Togni, P.H.B. Ecosystem services provided by insects in Brazil: What do we really know? Neotrop. Entomol. 2020, 49, 783–794. [Google Scholar] [CrossRef]
- Noriega, J.A.; Hortal, J.; Azcárate, F.M.; Berg, M.P.; Bonada, N.; Briones, M.J.; Del Toro, I.; Goulson, D.; Ibanez, S.; Landis, D.A.; et al. Research trends in ecosystem services provided by insects. Basic Appl. Ecol. 2018, 26, 8–23. [Google Scholar] [CrossRef]
- Géneau, C.E.; Wäckers, F.L.; Luka, H.; Daniel, C.; Balmer, O. Selective flowers to enhance biological control of cabbage pests by parasitoids. Basic Appl. Ecol. 2012, 13, 85–93. [Google Scholar] [CrossRef]
- Patt, J.M.; Rohrig, E. Laboratory evaluations of the foraging success of Tamarixia radiata (Hymenoptera: Eulophidae) on flowers and extrafloral nectaries: Potential use of nectar plants for conservation biological control of Asian citrus psyllid (Hemiptera: Liviidae). Fla. Entomol. 2017, 100, 149–156. [Google Scholar] [CrossRef]
- Benelli, G.; Giunti, G.; Tena, A.; Desneux, N.; Caselli, A.; Canale, A. The impact of adult diet on parasitoid reproductive performance. J. Pest Sci. 2017, 90, 807–823. [Google Scholar] [CrossRef]
- Jervis, M.A.; Ellers, J.; Harvey, J.A. Resource acquisition, allocation, and utilization in parasitoid reproductive strategies. Ann. Rev. Entomol. 2008, 53, 361–385. [Google Scholar] [CrossRef] [PubMed]
- Damien, M.; Barascou, L.; Ridel, A.; Van Baaren, J.; Le Lann, C. Food or host: Do physiological state and flower type affect foraging decisions of parasitoids? Behav. Ecol. Sociobiol. 2019, 73, 1–12. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón-Arroyo, C.; Togni, P.H.B.; Pantoja, G.M.; Saenz, A.S.; Venzon, M. Plants for Fitness Enhancement of a Coffee Leaf Miner Parasitoid. Agriculture 2023, 13, 244. https://doi.org/10.3390/agriculture13020244
Calderón-Arroyo C, Togni PHB, Pantoja GM, Saenz AS, Venzon M. Plants for Fitness Enhancement of a Coffee Leaf Miner Parasitoid. Agriculture. 2023; 13(2):244. https://doi.org/10.3390/agriculture13020244
Chicago/Turabian StyleCalderón-Arroyo, Carolina, Pedro H. B. Togni, Gabriel M. Pantoja, Angela S. Saenz, and Madelaine Venzon. 2023. "Plants for Fitness Enhancement of a Coffee Leaf Miner Parasitoid" Agriculture 13, no. 2: 244. https://doi.org/10.3390/agriculture13020244
APA StyleCalderón-Arroyo, C., Togni, P. H. B., Pantoja, G. M., Saenz, A. S., & Venzon, M. (2023). Plants for Fitness Enhancement of a Coffee Leaf Miner Parasitoid. Agriculture, 13(2), 244. https://doi.org/10.3390/agriculture13020244






