An Appropriate Genetic Approach to Endangered Podolian Grey Cattle in the Context of Preserving Biodiversity and Sustainable Conservation of Genetic Resources
Abstract
1. Introduction
2. The Importance of Cattle Biodiversity
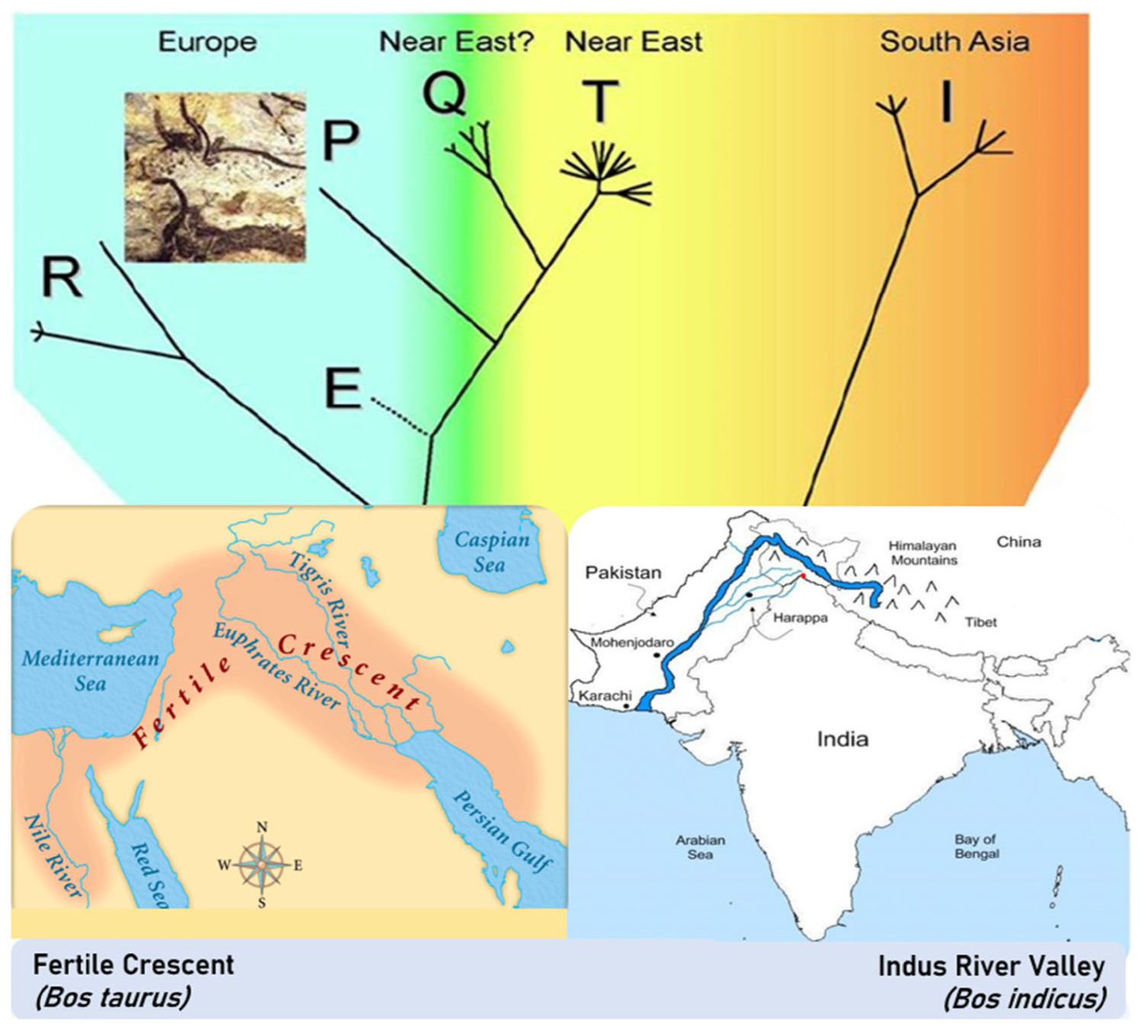
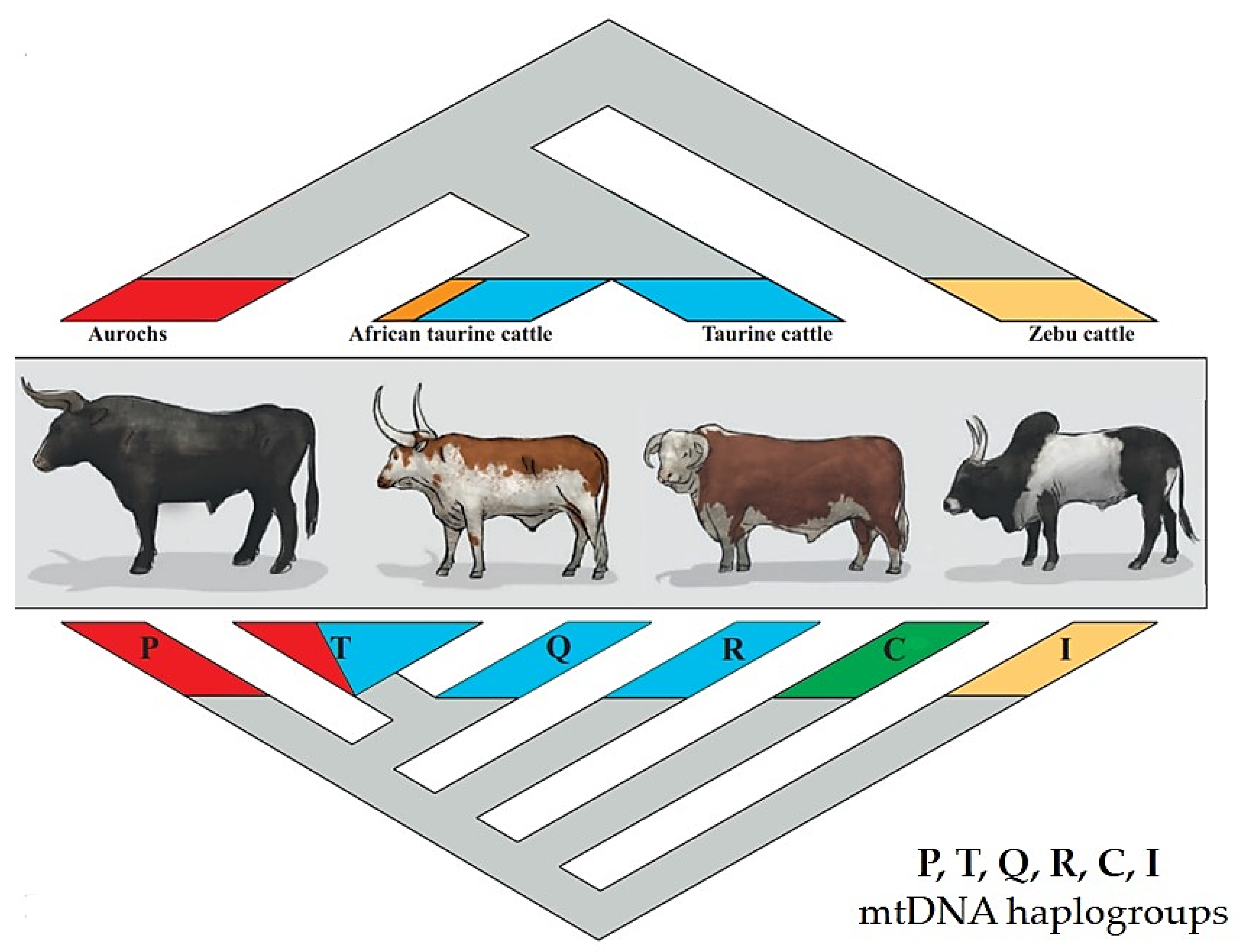
2.1. The Bovine Species: Origin, Evolution, and Domestication
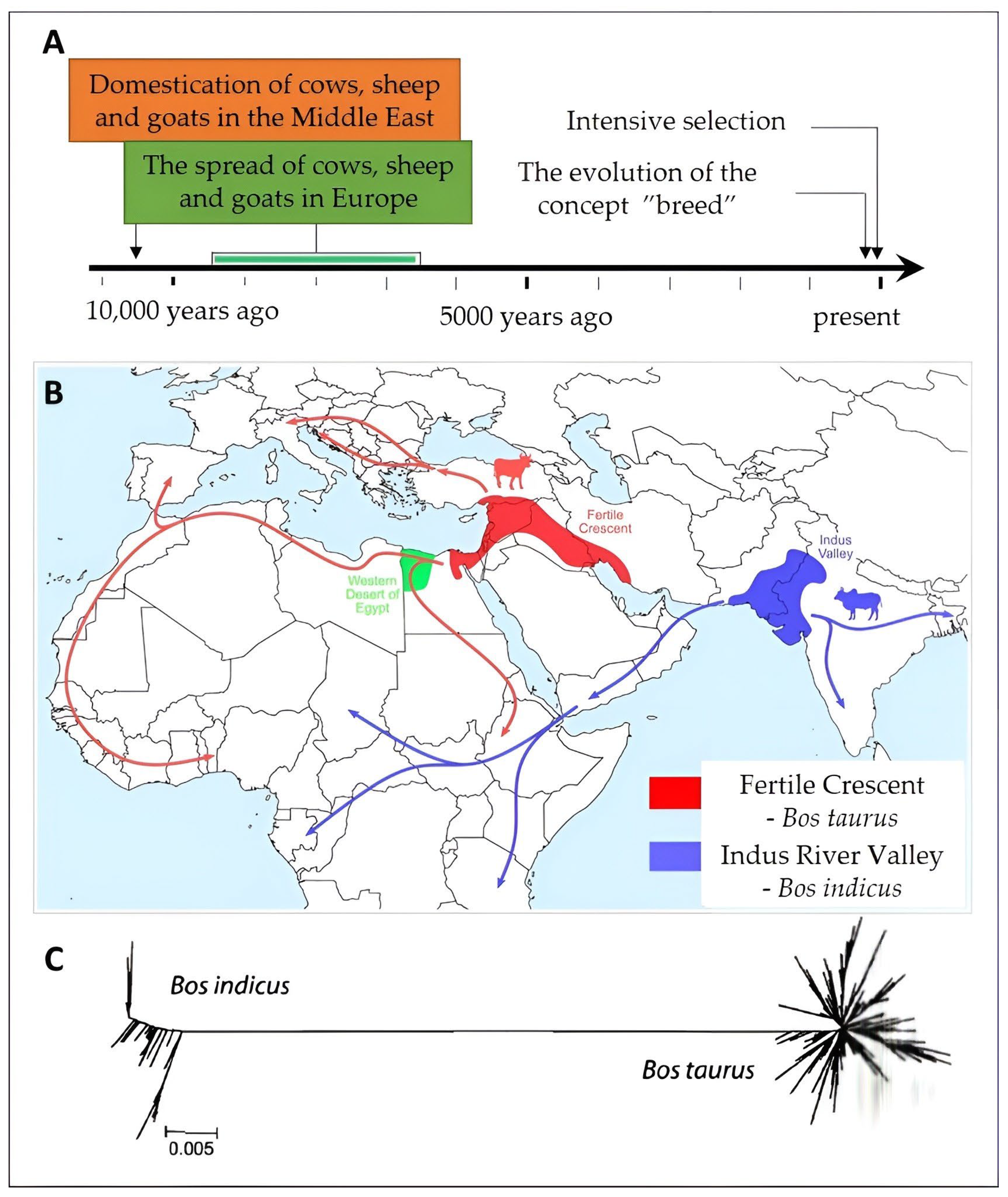
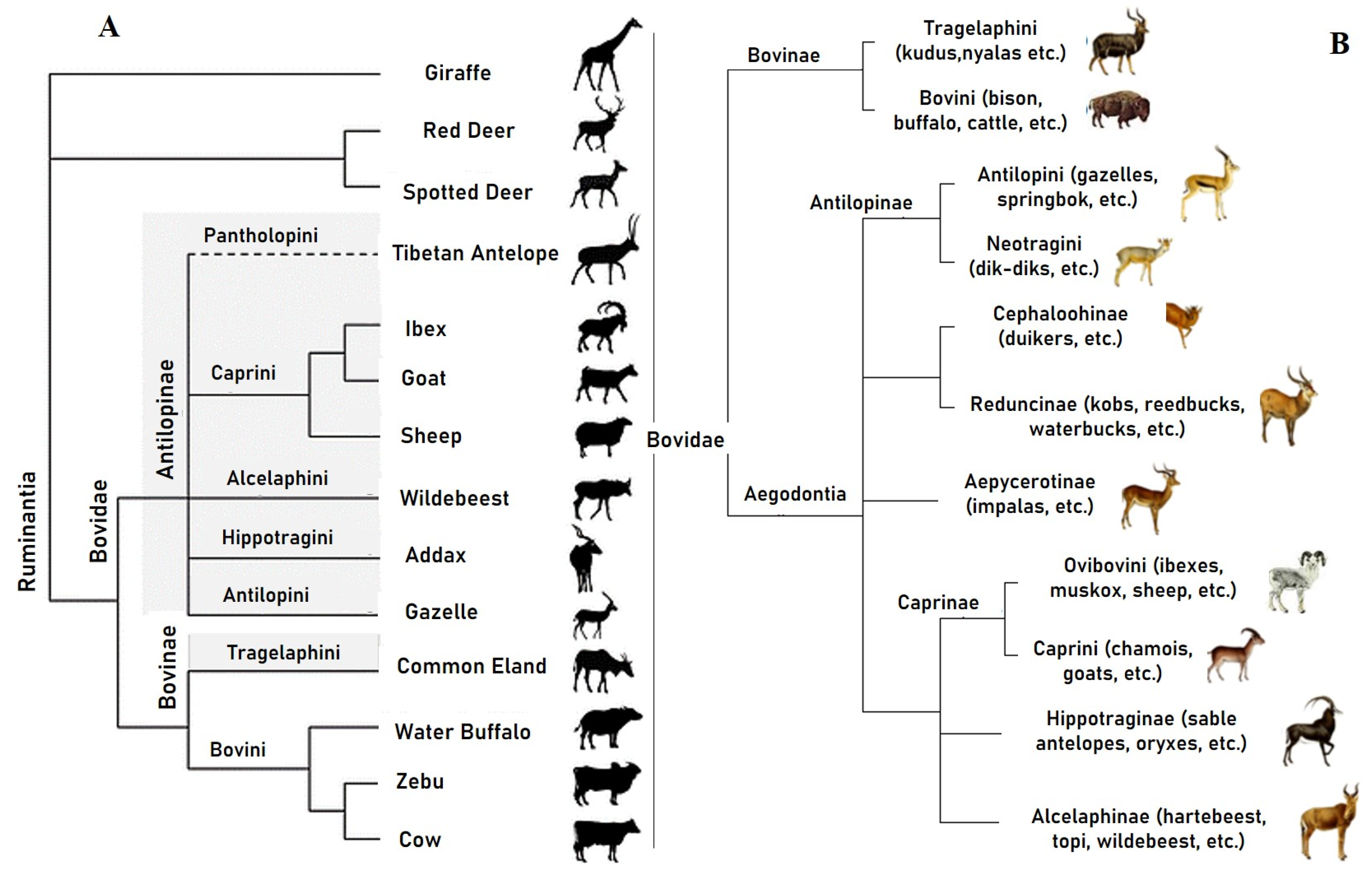
2.2. Systematics and Phylogeny of Cattle
3. The Origin and Phylogeny of the Podolian Cattle Breeds
3.1. Romanian Grey Steppe: Origin and Phylogeny
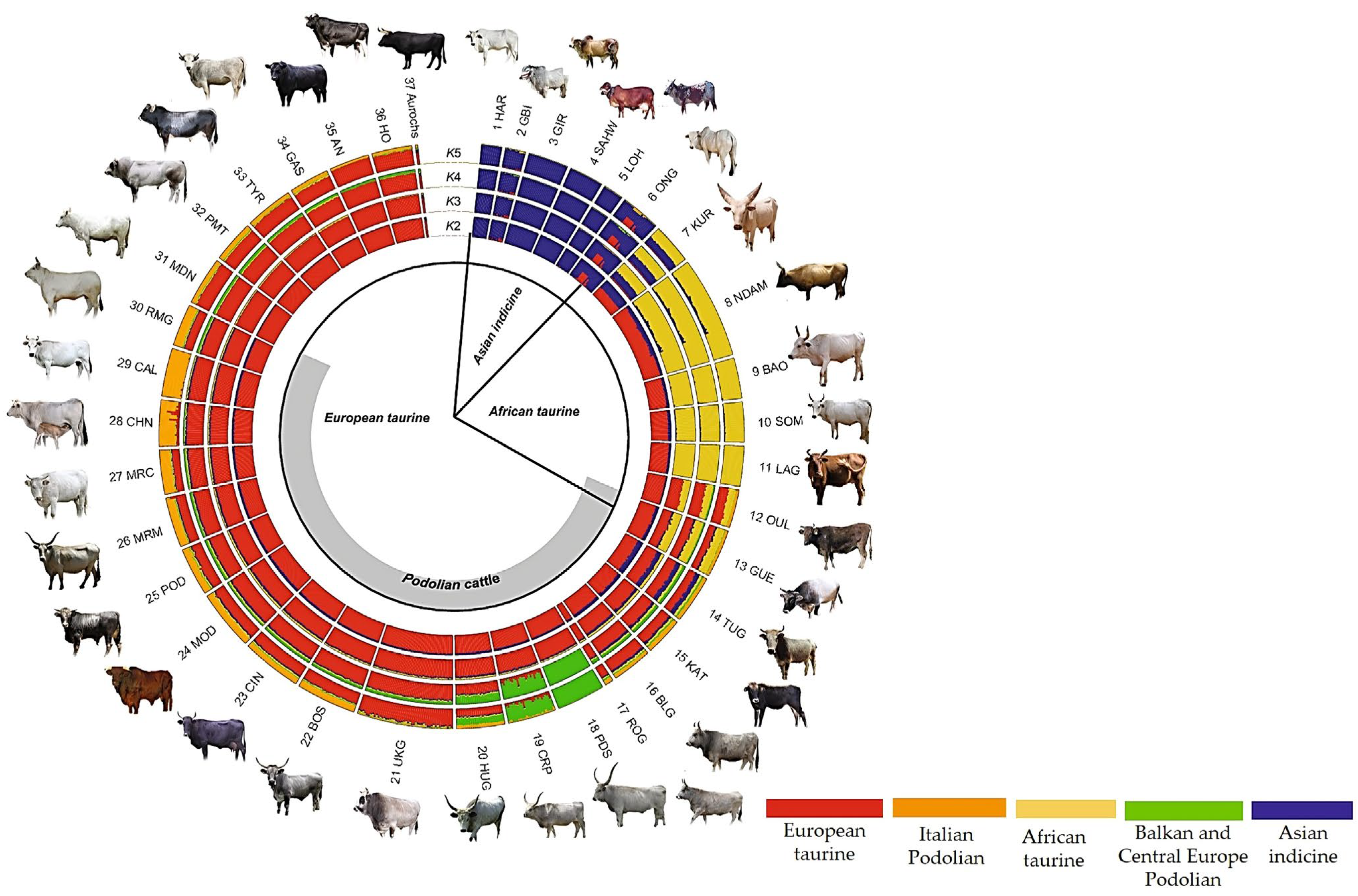
3.2. Genetic Relationships among Romanian Grey Cattle and Podolian Cattle Breeds
3.2.1. Genetic Diversity of Podolian Cattle Breeds
3.2.2. Podolian Cattle Breeds from Eastern Europe
- Romanian Grey Steppe Breed
- Ukrainian Grey Breed
- Hungarian Grey Breed
- Slavonian–Syrmian Podolian Breed
- Serbian (Srem) Podolian Breed
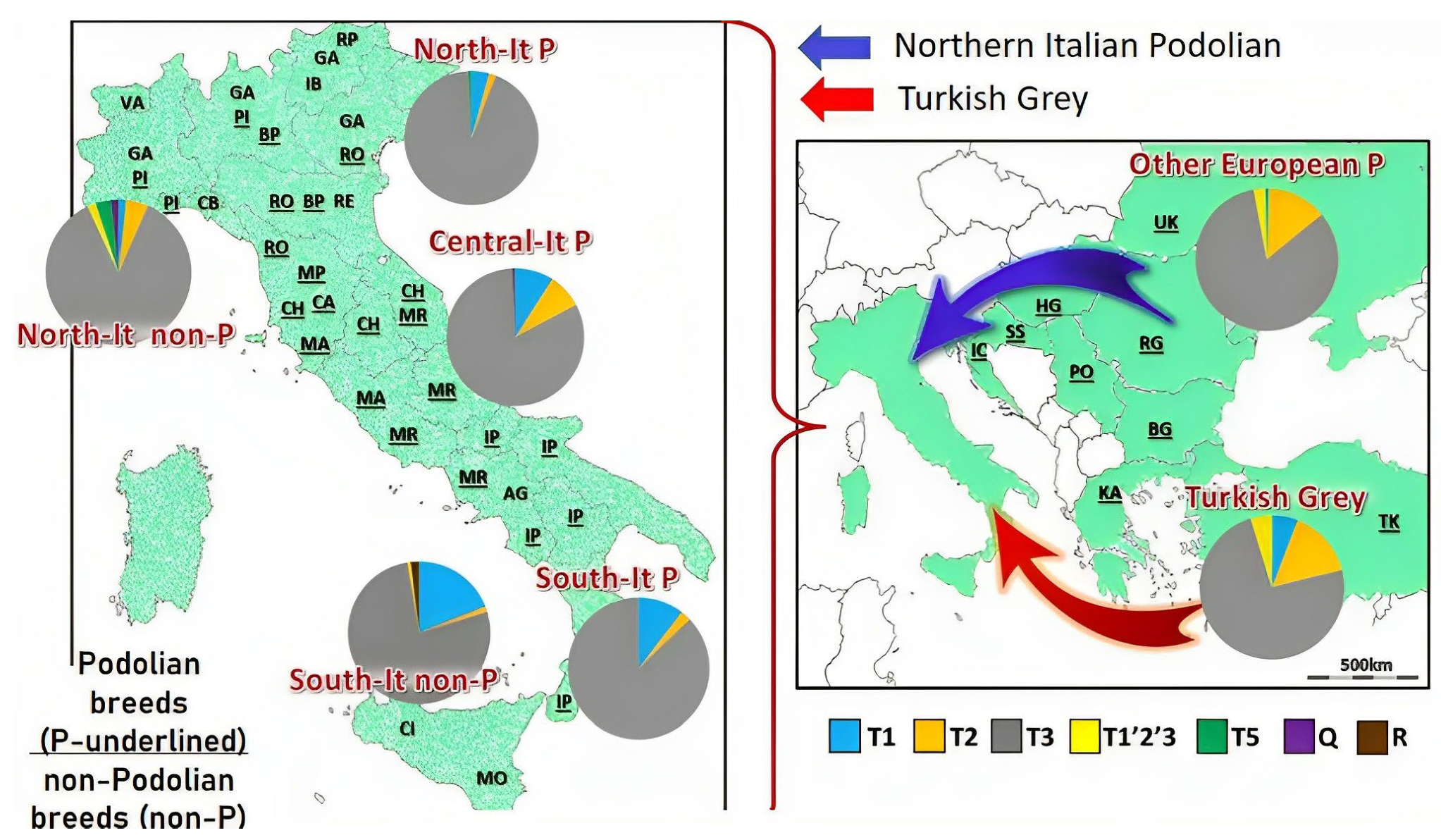
3.2.3. Podolian Cattle Breeds from Italy and Istria
- Boškarin or Istrian Breed
- Mursi Breed
- Istarsko Govedo Breed
- Maremana and Podolica Breeds
3.2.4. Podolian Cattle Breeds from Balkans and Anatolia
- Bulgarian Grey (Iskar) Breed
- Katerini Breed
- Sykia Cattle Breed
- Anatolian Grey Breed
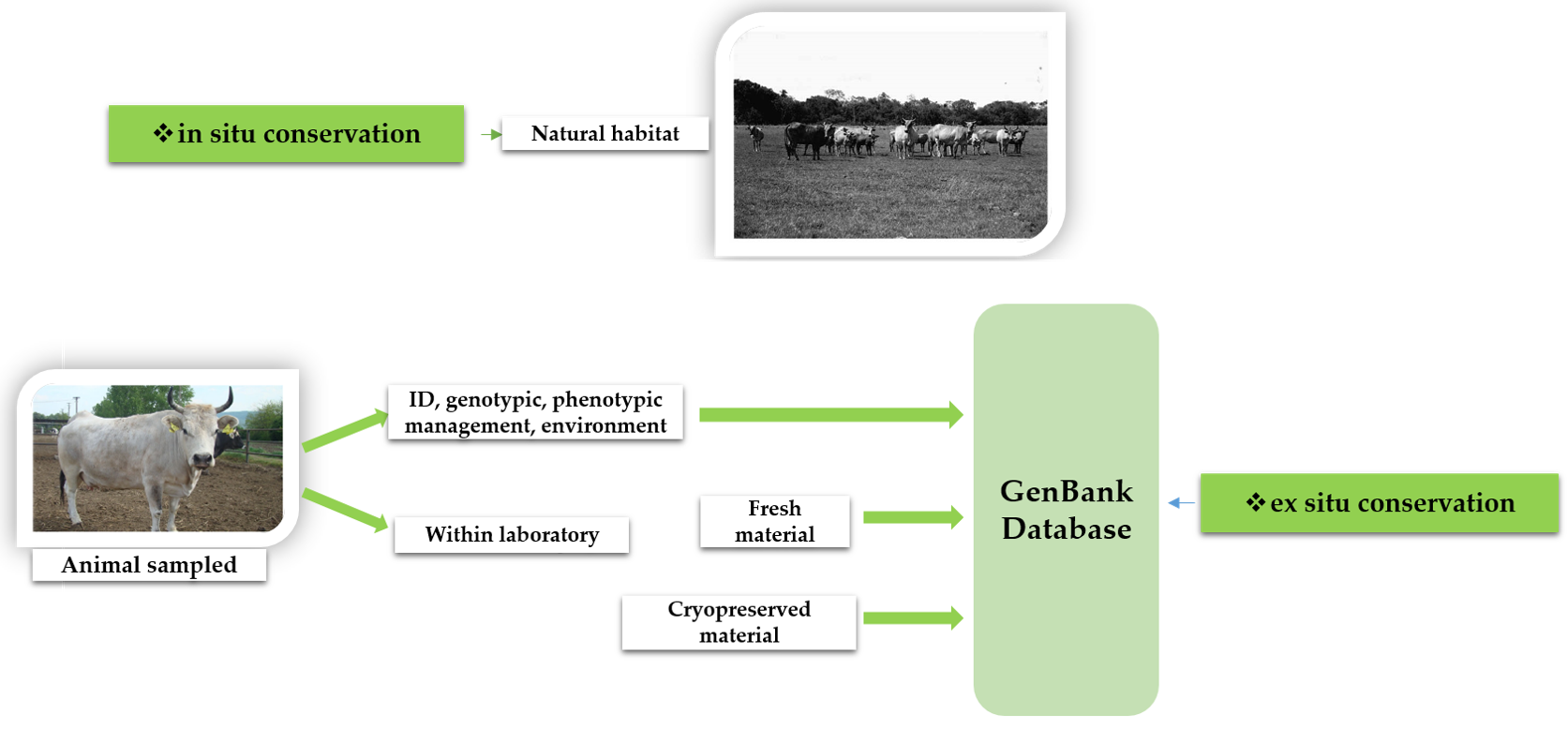
4. Trends and Management Recommendations in Genetic Conservation Programs, including the Endangered Grey Steppe Cattle
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Senczuk, G.; Guerra, L.; Mastrangelo, S.; Campobasso, C.; Zoubeyda, K.; Imane, M.; Marletta, D.; Kusza, S.; Karsli, T.; Gaouar, S.B.S.; et al. Fifteen Shades of Grey: Combined Analysis of Genome-Wide SNP Data in Steppe and Mediterranean Grey Cattle Sheds New Light on the Molecular Basis of Coat Color. Genes 2020, 11, 932. [Google Scholar] [CrossRef] [PubMed]
- Loftus, R.T.; MacHugh, D.E.; Bradley, D.G.; Sharp, P.M.; Cunningham, E.P. Evidence for two independent domestications of cattle. Proc. Natl. Acad. Sci. USA 1994, 91, 2757–2761. [Google Scholar] [CrossRef] [PubMed]
- MacHugh, D.E.; Shriver, M.D.; Loftus, R.T.; Cunningham, P.; Bradley, D.G. Microsatellite DNA variation and the evolution, domestication and phylogeography of taurine and zebu cattle (Bos taurus and Bos indicus). Genetics 1997, 146, 1071–1086. [Google Scholar] [CrossRef] [PubMed]
- Verdugo, M.P.; Mullin, V.E.; Scheu, A.; Mattiangeli, V.; Daly, K.G.; Maisano Delser, P.; Hare, A.J.; Burger, J.; Collins, M.J.; Kehati, R.; et al. Ancient cattle genomics, origins, and rapid turnover in the Fertile Crescent. Science 2019, 365, 173–176. [Google Scholar]
- Rieznykova, N.L. Grey Ukrainian cattle breed as the ancestor of Podolic (Podolian) group. J. Anim. Breed. Genet. 2021, 62, 165–190. [Google Scholar] [CrossRef]
- Maretto, F.; Ramljak, J.; Sbarra, F.; Penasa, M.; Mantovani, R.; Ivankovic, A. Genetic relationship among Italian and Croatian Podolian cattle breeds assessed by microsatellite markers. Livest. Sci. 2012, 13, 256. [Google Scholar] [CrossRef]
- D’Andrea, M.; Pariset, L.; Matassino, D.; Valentini, A.; Lenstra, A.J.; Maiorano, G.; Pilla, F. Genetic characterization and structure of the Italian Podolian cattle breed and its relationship with some major European breeds. Ital. J. Anim. Sci. 2011, 10, 237–243. [Google Scholar] [CrossRef]
- Hiendleder, S.; Lewalski, H.; Janke, A. Complete mitochondrial genomes of Bos taurus and Bos indicus provide new insights into intraspecies variation, taxonomy and domestication. Cytogenet. Genome Res. 2008, 120, 150–156. [Google Scholar] [CrossRef]
- Edwards, C.J.; Bollongino, R.; Scheu, A.; Chamberlain, A.; Tresset, A.; Vigne, J.D.; Baird, J.F.; Larson, G.; Ho, S.Y.; Heupink, T.H.; et al. Mitochondrial DNA analysis shows a Near Eastern Neolithic origin for domestic cattle and no indication of domestication of European aurochs. Proc. Biol. Sci. 2007, 274, 1377–1385. [Google Scholar] [CrossRef]
- Buttar, I.; Solounias, N. Specialized Position of the Horns, and Frontal and Parietal Bones in Bos taurus (Bovini, Artiodactyla), and Notes on the Evolution of Bos. Ann. Zool. Fenn. 2021, 58, 49–63. [Google Scholar] [CrossRef]
- Davidescu, M.A.; Grădinaru, A.C.; Creangă, Ș. Endangered Romanian cattle breeds—Between traditional breeding and genetic conservation. In Proceedings of the International Scientific Congress—Life Sciences, a Challenge for the Future, Iași, Romania, 17–18 October 2019; pp. 339–349. [Google Scholar]
- Dascălu, D.L.; Creangă, Ș.; Borș, S.I.; Doliș, M.G.; Simeanu, D.; Donosă, R. The Romanian Grey Steppe characterization—The breed evolution in the last century from the numerical and morphological point of view. Danub. Anim. Genet. Resour. 2017, 2, 70–75. [Google Scholar]
- Scherf, B.D. World Watch List for Domestic Animal Diversity, 3rd ed.; FAO: Rome, Italy, 2000; Available online: http://www.fao.org/docrep/009/x8750e/x8750e00.htm (accessed on 14 February 2023).
- Teneva, A.; Todorovska, E.; Tyufekchiev, N.; Kozelov, L.; Atanassov, A.; Foteva, S.; Ralcheva, S.; Zlatarev, S. Molecular characterization of Bulgarian livestock genetic resources: Genetic diversity in Bulgarian grey cattle as revealed by microsatellite markers. Biotechnol. Anim. Husb. 2005, 21, 35–42. [Google Scholar] [CrossRef]
- Pariset, L.; Mariotti, M.; Nardone, A.; Soysal, M.I.; Ozkan, E.; Williams, J.L.; Dunner, S.; Leveziel, H.; Maroti-Agots, A.; Bodo, I.; et al. Relationships between Podolic cattle breeds assessed by single nucleotide polymorphisms (SNPs) genotyping. J. Anim. Breed. Genet. 2010, 127, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Creangă, Ş.; Maciuc, V.; Pîntea, M. Genetic Polymorphism in Cattle Breeding in Moldova; Ion Ionescu de la Brad: Iaşi, Romania, 2008; pp. 86–87. ISBN 978-973-147024-5. [Google Scholar]
- Creangă, S.; Maciuc, V. Romanian Grey Steppe; Alfa: Iaşi, Romania, 2010; pp. 145–147. ISBN 978-606-540-034-4. [Google Scholar]
- Mastrangelo, S.; Tolone, M.; Ben Jemaa, S.; Sottile, G.; Di Gerlando, R.; Cortés, O.; Senczuk, G.; Portolano, B.; Pilla, F.; Ciani, E. Refining the genetic structure and relationships of European cattle breeds through meta-analysis of worldwide genomic SNP data, focusing on Italian cattle. Sci. Rep. 2020, 10, 14522. [Google Scholar] [CrossRef]
- Creangă, S.; Chelmu, S.S.; Maciuc, V.; Bâlteanu, V.A. Molecular characterization of Grey Steppe cattle breed for its genetic bio-preservation. Rom. Biotechnol. Lett. 2013, 18, 8893–8900. [Google Scholar]
- Maciuc, V.; Radu-Rusu, R.M. Assessment of Grey Steppe cattle genetic and phenotypic traits as valuable resources in preserving biodiversity. Environ. Eng. Manag. J. 2018, 17, 2741–2748. [Google Scholar]
- Bâlteanu, A.V.; Pop, D.F.; Vlaic, A.; Carsai, C.T.; Creangă, Ş.; Rusu, R.A. Characterization of the άs1 Casein IRV allele provides evidence for phylogeny of the ancient Romanian Grey Steppe cattle, Moldavian strain. Int. J. Vet. Sci. Anim. Husb. 2010, 53, 167–172. [Google Scholar]
- Bâlteanu, V.A.; Vlaic, A.; Rusu, A.R.; Creangă, Ş.; Pop, F.D.; Odagiu, A.; Pântea, M.L.; Hâncu, V. Milk proteins polymorphism in Romanian Grey Steppe cattle studied by isoelectric focusing technique (IEF). Identification of a new allele alpha S1-casein allele: Alpha S1 IRV. Bull. USAMV-CN Anim. Sci. Biotechnol. 2007, 63, 304–310. [Google Scholar]
- Ilie, D.E.; Cean, A.; Cziszter, L.T.; Gavojdian, D.; Ivan, A.; Kusza, S. Microsatellite and Mitochondrial DNA study of native Eastern European cattle populations: The case of the Romanian Grey. PLoS ONE 2015, 10, e0138736. [Google Scholar] [CrossRef]
- Baumung, R.; Sölkner, J. Analysis of pedigrees of Tux-Zillertal, Carinthian Blond and Original Pinzgau cattle population in Austria. J. Anim. Breed. Genet. 2002, 119, 175–181. [Google Scholar] [CrossRef]
- Sölkner, I.; Filipcic, L.; Hampshire, N. Genetic variability of populations and similarity of subpopulations in Austrian cattle breeds determined by analysis of pedigrees. Anim. Sci. 2010, 67, 249–256. [Google Scholar] [CrossRef]
- Hristov, P.; Teofanova, D.; Neov, B.; Shivachev, B.; Radoslavov, G. Mitochondrial diversity in autochthonous cattle breeds from the Balkan Peninsula. Czech. J. Anim. Sci. 2015, 60, 311–318. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Patel, J.B.; Ankuya, K.J.; Chauhan, H.D.; Pawar, M.M.; Gupta, J.P. Conservation of Indigenous Cattle Breeds. J. Anim. Res. 2019, 1, 1–12. [Google Scholar] [CrossRef]
- Barnes, K.; Collins, T.; Dion, S.; Reynolds, H.; Riess, S.; Stanzyk, A.; Wolfe, A.; Lonergan, S.; Boettcher, P.; Charrondiere, U.R.; et al. Importance of cattle biodiversity and its influence on the nutrient composition of beef. Anim. Front. 2012, 2, 54–60. [Google Scholar] [CrossRef][Green Version]
- Zeder, M.A.; Bradley, D.; Emshwiller, E.; Smith, B.D. Documenting Domestication New Genetic and Archaeological Paradigms, 1st ed.; University of California Press: Berkeley, CA, USA, 2006; p. 375. ISBN 9780520246386. [Google Scholar]
- Clutton-Brock, J. A Natural History of Domesticated Mammals, 2nd ed.; Cambridge University Press: Cambridge, UK, 1999; pp. 114–127. ISBN 0521634954. [Google Scholar]
- Diamond, J. Evolution, consequences and future of plant and animal domestication. Nature 2002, 418, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Groeneveld, L.F.; Lenstra, J.A.; Eding, H.; Toro, M.A.; Scherf, B.; Pilling, D. Genetic diversity in farm animals—A review. Anim. Genet. 2010, 41, 6–31. [Google Scholar] [CrossRef] [PubMed]
- Achilli, A.; Olivieri, A.; Pellecchia, M.; Uboldi, C.; Colli, L. Mitochondrial genomes of extinct aurochs survive in domestic cattle. Curr. Biol. 2008, 18, 157–158. [Google Scholar] [CrossRef]
- Achilli, A.; Bonfiglio, S.; Olivieri, A.; Malusà, A.; Pala, M. The multifaceted origin of taurine cattle reflected by the mitochondrial genome. PLoS ONE 2009, 4, e5753. [Google Scholar] [CrossRef]
- Hassanin, A.; Ropiquet, A. Molecular phylogeny of the tribe Bovini (Bovidae, Bovinae) and the taxonomic status of the Kouprey, Bos sauveli Urbain. Mol. Phylogenet. Evol. 2005, 33, 896–907. [Google Scholar] [CrossRef]
- Götherström, A.; Anderung, C.; Hellborg, L.; Elburg, R.; Smith, C.; Bradley, D.G.; Ellegren, H. Cattle domestication in the Near East was followed by hybridization with aurochs bulls in Europe. Proc. Biol. Sci. 2005, 22, 1579–2345. [Google Scholar] [CrossRef]
- Pitt, D.; Sevane, N.; Nicolazzi, E.L.; MacHugh, D.E.; Park, S.D.E.; Colli, L.; Martinez, R.; Bruford, M.W.; Orozco-terWenge, P. Domestication of cattle: Two or three events? Evol. Appl. 2019, 12, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Meadow, R.H. Animal Domestication in the Middle East: A Revised View from the Eastern Margin, 2nd ed.; Possehl, G.L., Ed.; Harappan Civilization: A Recent Perspective; American Institute of Indian Studies and Oxford & IBH Pub.: New Delhi, India, 1993; pp. 295–321. ISBN 8120407792. [Google Scholar]
- Park, S.D.E.; Magee, D.A.; McGettigan, P.A.; Teasdale, M.D.; Edwards, C.J.; Lohan, A.J.; Murphy, A.; Braud, M.; Donoghue, M.T.; Liu, Y.; et al. Genome sequencing of the extinct Eurasian wild aurochs, Bos primigenius, illuminates the phylogeography and evolution of cattle. Genome Biol. 2015, 16, 234. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, M.R.; Chen, W.; Lenstra, J.A.; Goderie, C.R.J.; MacHugh, D.E.; Park, S.D.E.; Magee, D.A.; Matassino, D.; Ciani, F.; Megens, H.J.; et al. Genetic origin, admixture and population history of aurochs (Bos primigenius) and primitive European cattle. Heredity 2017, 118, 169–176. [Google Scholar] [CrossRef]
- Scheu, A.; Hartz, S.; Schmölcke, U.; Tresset, A.; Burger, J.; Bollongino, R. Ancient DNA provides no evidence for independent domestication of cattle in Mesolithic Rosenhof, Northern Germany. J. Archaeol Sci. 2008, 35, 1257–1264. [Google Scholar] [CrossRef]
- Taberlet, P.; Valentini, A.; Rezaei, H.R.; Naderi, S.; Pompanon, F.; Negrini, R.; Ajmone-Marsan, P. Are cattle, sheep, and goats endangered species? Mol. Ecol. 2008, 17, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Taberlet, P.; Coissac, E.; Pansu, J.; Pompanon, F. Conservation genetics of cattle, sheep, and goats. C. R. Biol. 2011, 334, 247–254. [Google Scholar] [CrossRef]
- Bradley, D.G.; Magee, D.A. Genetics and the Origins of Domestic Cattle. In New Genetics and Archaeological Paradigms; University of California Press, Ltd.: Berkeley, CA, USA, 2006; pp. 317–328. [Google Scholar] [CrossRef]
- Sinding, M.H.S.; Gilbert, M.T.P. The Draft Genome of Extinct European Aurochs and its Implications for De-Extinction. Open Quat. 2016, 2, 7. [Google Scholar] [CrossRef]
- Bollongino, R.; Edwards, C.J.; Alt, K.W.; Burger, J.; Bradley, D.G. Early history of European domestic cattle as revealed by ancient DNA. Biol. Lett. 2006, 2, 155–159. [Google Scholar] [CrossRef]
- Bollongino, R.; Elsner, J.; Vigne, J.D.; Burger, J. Y-SNPs do not indicate hybridisation between European aurochs and domestic cattle. PLoS ONE 2008, 3, e3418. [Google Scholar] [CrossRef]
- Vigne, J.D.; Helmer, D.; Peters, J. New archaeozoological approaches. In First Steps of Animal Domestication, Proceedings of the 9th Conference of the International Council of Archaeozoology; Oxbow Books: Durham, UK, 2005; pp. 1–41. ISBN 1842171216. [Google Scholar]
- Savage, D.E.; Russell, D.E. Mammalian Paleofaunas of the World; Addison-Wesley Publishing Company: Boston, MA, USA, 1983; p. 432. ISBN 0201064944. [Google Scholar] [CrossRef]
- Elsik, C.G.; Tellam, R.L.; Worley, K.C.; Gibbs, R.A.; Muzny, D.M.; Weinstock, G.M. The genome sequence of taurine cattle: A window to ruminant biology and evolution. Science 2009, 324, 522–528. [Google Scholar] [CrossRef]
- Dekel, Y.; Machluf, Y.; Ben-Dor, S.; Yifa, O.; Stoler, A.; Ben-Shlomo, I.; Bercovich, D. Dispersal of an ancient retroposon in the TP53 promoter of Bovidae: Phylogeny, novel mechanisms, and potential implications for cow milk persistency. BMC Genom. 2015, 16, 53. [Google Scholar] [CrossRef] [PubMed]
- Arif, I.A.; Bakir, M.A.; Khan, H.A. Inferring the Phylogeny of Bovidae Using Mitochondrial DNA Sequences: Resolving Power of Individual Genes Relative to Complete Genomes. Evol. Bioinform. 2012, 8, 139–150. [Google Scholar] [CrossRef]
- Bibi, F. A multi-calibrated mitochondrial phylogeny of extant Bovidae (Artiodactyla, Ruminantia) and the importance of the fossil record tosystematics. BMC Evol. Biol. 2013, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Matthee, C.A.; Davis, S.K. Molecular insights into the evolution of the family Bovidae: A nuclear DNA perspective. Mol. Biol. Evol. 2001, 18, 1220. [Google Scholar] [CrossRef]
- Feldhamer, G.; Drickamer, L.; Vessey, S.; Merritt, J.; Krajewski, C. Mammalogy: Adaptation, Diversity, Ecology, 1st ed.; The Johns Hopkins University Press: Baltimore, MD, USA, 2007; pp. 156–158. ISBN 1421436523. [Google Scholar]
- Gentry, A. Bovidae. In Paleontology and Geology of Laetoli: Human Evolution in Context: Fossil Hominins and the Associated Fauna; Harrison, T., Ed.; Springer: New York, NY, USA, 2011; Volume 2, pp. 363–465. [Google Scholar] [CrossRef]
- Hernandez-Fernandez, M.; Vrba, E.S. A complete estimate of the phylogenetic relationships in Ruminantia: A dated species-level super tree of the extant ruminants. Biol. Rev. 2005, 80, 269–302. [Google Scholar] [CrossRef] [PubMed]
- Vrba, E.S.; Schaller, G.B. Phylogeny of Bovidae based on behavior, glands, skulls, and postcrania. In Antelopes, Deer, and Relatives; Yale University Press: New Haven, CT, USA; London, UK, 2000; pp. 203–222. [Google Scholar]
- Wilson, D.E.; Reeder, D.A.M. Mammal Species of the World, 3rd ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2005; p. 2142. ISBN 978-0-8018-8221-0. [Google Scholar]
- Cotterill, F.P.D. The Upemba lechwe, Kobus anselli: An antelope new to science emphasizes the conservation importance of Katanga, Democratic Republic of Congo. J. Zool. 2005, 265, 113–132. [Google Scholar] [CrossRef]
- Harrison, T. Paleontology and Geology of Laetoli Human Evolution in Context; Springer: Dordrecht, The Netherlands, 2011; pp. 363–465. ISBN 978-9048-199-624. [Google Scholar]
- Demiguel, D.; Sánchez, I.M.; Alba, D.M.; Galindo, J.; Robles, J.M.; Moyà-Solà, S. First evidence of Azanza and Morales, (Ruminantia, Bovidae), in the Miocene of the Vallès-Penedès Basin (Spain). J. Vertebr. Paleontol. 2012, 32, 57–62. [Google Scholar] [CrossRef]
- Geraads, D.; El Boughabi, S.; Zouhri, S. A new caprin bovid (Mammalia) from the late Miocene of Morocco. Palaeontol. Afr. 2012, 47, 19–24. [Google Scholar]
- Li, M.H.; Tapio, I.; Vilkki, J.; Ivanova, Z.; Kiselyova, T.; Marzanov, N.; Ćinkulov, M.; Stojanović, S.; Ammosov, I.; Popov, R.; et al. The genetic structure of cattle populations (Bos taurus) in northern Eurasia and the neighbouring Near Eastern regions: Implications for breeding strategies and conservation. Mol. Ecol. 2007, 16, 3839–3853. [Google Scholar] [CrossRef]
- Soysal, M.I.; Kök, S. The last survivors of Grey cattle resisting extinction. A case study of characteristics and sustainability of traditional systems of native Grey cattle breeds, in Olaizola. Mediterr. Livest. Prod. Uncertain. Oppor. 2008, 78, 55–63. [Google Scholar]
- Davidescu, M.-A.; Simeanu, D.; Gorgan, D.-L.; Ciorpac, M.; Creanga, S. Analysis of Phylogeny and Genetic Diversity of Endangered Romanian Grey Steppe Cattle Breed, a Reservoir of Valuable Genes to Preserve Biodiversity. Agriculture 2022, 12, 2059. [Google Scholar] [CrossRef]
- Yilmaz, O.; Akin, O.; Yener, S.; Ertugrul, M.; Wilson, R. The domestic livestock resources of Turkey: Cattle local breeds and types and their conservation status. Anim. Genet. Resour. 2012, 50, 65–73. [Google Scholar] [CrossRef]
- Palova, N.; Krusheva, D. Conservation of genetic resources in livestock breeding in the Experimental Station of Agriculture—Sredets. Türk Tarım ve Doğa Bilimleri Dergisi 2014, 6, 1106–1111. [Google Scholar]
- Senczuk, G.; Mastrangelo, S.; Ajmone-Marsan, P.; Becskei, Z.; Colangelo, P.; Colli, L.; Ferretti, L.; Karsli, T.; Lancioni, H.; Lasagna, E.; et al. On the origin and diversification of Podolian cattle breeds: Testing scenarios of European colonization using genome-wide SNP data. Genet. Sel. 2021, 53, 48. [Google Scholar] [CrossRef] [PubMed]
- Curik, I.; Ferenčaković, M.; Karapandza, N.; Cubric Curik, V.; Sölkner, V. Estimation of inbreeding and effective population size in Istrian cattle using molecular information. Acta Agrar. Debr. 2014, 18, 30–34. [Google Scholar]
- Ramljak, J.; Ivanković, A.; Veit-Kensch, C.E.; Förster, M.; Medugorac, I. Analysis of genetic and cultural conservation value of three indigenous Croatian cattle breeds in a local and global context. J. Anim. Breed. Genet. 2010, 128, 73–84. [Google Scholar] [CrossRef]
- Keros, T.; Jemeršić, L.; Prpić, J.; Brnić, D. Molecular Characterization of Autochtonus Slavonian Syrmian Podolian Cattle. Acta Vet. 2015, 65, 89–98. [Google Scholar] [CrossRef][Green Version]
- Karatosidi, D.; Marsico, G.; Ligda, C.; Tarricone, S. Assessment of the meat quality of Italian Podolian and Greek Katerini cattle. Anim. Genet. Resour. 2013, 53, 141–146. [Google Scholar] [CrossRef]
- Antonopoulos, D.; Vougiouklaki, D.; Laliotis, G.P.; Tsironi, T.; Valasi, I.; Chatzilazarou, A.; Halvatsiotis, P.; Houhoula, D. Identification of Polymorphisms of the CSN2 Gene Encoding β-Casein in Greek Local Breeds of Cattle. Vet. Sci. 2021, 8, 257. [Google Scholar] [CrossRef]
- Török, P.; Valkó, O.; Deák, B.; Kelemen, A.; Tóth, E.; Tóthmérész, B. Managing for species composition or diversity? Pastoral and free grazing systems in alkali steppes. Agric. Ecosyst. Environ. 2016, 234, 23–30. [Google Scholar] [CrossRef]
- Tóth, E.; Deák, B.; Valkó, O.; Kelemen, A.; Miglécz, T.; Tóthmérész, B.; Török, P. Livestock Type is More Crucial Than Grazing Intensity: Traditional Cattle and Sheep Grazing in Short-Grass Steppes. Land Degrad. Dev. 2018, 29, 231–239. [Google Scholar] [CrossRef]
- Öner, Y.; Yılmaz, O.; Eriş, C.; Ata, N.; Ünal, C.; Koncagül, S. Genetic diversity and population structure of Turkish native cattle breeds. S. Afr. J. Anim. 2019, 49, 628–635. [Google Scholar] [CrossRef]
- Demir, E.; Balcioğlu, M.S. Genetic diversity and population structure of four cattle breeds raised in Turkey using microsatellite markers. Czech J. Anim. Sci. 2019, 64, 411–419. [Google Scholar] [CrossRef]
- Mokhnachova, N.; Suprovich, T.; Dobrynska, M.; Fursa, N. Characteristics of Ukrainian Grey cattle by DNA-markers. J. Anim. Breed. 2018, 51, 283–289. [Google Scholar] [CrossRef]
- Svishcheva, G.; Babayan, O.; Lkhasaranov, B.; Tsendsuren, A.; Abdurasulov, A.; Stolpovsky, Y. Microsatellite Diversity and Phylogenetic Relationships among East Eurasian Bos taurus Breeds with an Emphasis on Rare and Ancient Local Cattle. Animals 2020, 10, 1493. [Google Scholar] [CrossRef] [PubMed]
- Beja-Pereira, A.; Caramelli, D.; Lalueza-Fox, C.; Vernesi, C.; Ferrand, N.; Casoli, A.; Goyache, F.; Royo, L.J.; Conti, S.; Lari, M.; et al. The origin of European cattle: Evidence from modern and ancient DNA. Proc. Natl. Acad. Sci. USA 2006, 21, 8113–8118. [Google Scholar] [CrossRef]
- Pellecchia, M.; Negrini, R.; Colli, L.; Patrini, M.; Milanesi, E.; Achilli, A.; Bertorelle, G.; Cavalli-Sforza, L.L.; Piazza, A.; Torroni, A.; et al. The mystery of Etruscan origins: Novel clues from Bos taurus mitochondrial DNA. Proc. R. Soc. Biol. Sci. 2007, 274, 1175–1179. [Google Scholar] [CrossRef]
- Di Lorenzo, P.; Lancioni, H.; Ceccobelli, S.; Colli, L.; Cardinali, I.; Karsli, T.; Capodiferro, M.R.; Sahin, E.; Ferretti, L.; Ajmone Marsan, P.; et al. Mitochondrial DNA variants of Podolian cattle breeds testify for a dual maternal origin. PLoS ONE 2018, 13, e0192567. [Google Scholar] [CrossRef]
- Carșai, T.C.; Vlaic, A.; Coșier, V.; Bâlteanu, A.V. Research on the Polymorphism at the Leptin Gene Locus for Selection Purposes Assisted by Genetic Markers in Cattle; Bioflux: Cluj-Napoca, Romania, 2008; pp. 47–84. [Google Scholar]
- Creangă, Ş.; Dascălu, D.; Ruginosu, E.; Borş, I.; Ilie, D.E.; Cean, A. Demographic Study on the total Grey Steppe Breed Population in Romania. Agron. Res. Mold. 2013, 46, 85–97. [Google Scholar]
- Georgescu, S.E.; Manea, M.A.; Zaulet, M.; Costache, M. Genetic diversity among Romanian cattle breeds with a special focus on the Romanian Grey Steppe Breed. Rom. Biotechnol. Lett. 2009, 14, 4194–4200. [Google Scholar]
- Gradinaru, A.C.; Petrescu-Mag, I.V.; Oroian, F.C.; Balint, C.; Oltean, I. Milk Protein Polymorphism Characterization: A Modern Tool for Sustainable Conservation of Endangered Romanian Cattle Breeds in the Context of Traditional Breeding. Sustainability 2018, 10, 534. [Google Scholar] [CrossRef]
- Bâlteanu, A.V.; Vlaic, A.; Pop, F.D.; Rusu, A.R.; Martin, P.; Miranda, G.; Creangă, Ș. Characterization at protein level of the new αs1 casein allele IRV discovered in Romanian Grey Steppe cattle breed Moldavian variety. Sci. Pap. Anim. Husb. Biotechnol. Ser. 2008, 41, 1–10. [Google Scholar]
- Ilie, D.E.; Creangă, Ș.; Grădinaru, A.C.; Borș, S.I.; Dascălu, D.L.; Chirilă, D.; Cean, A. Genetic diversity of Romanian Grey Steppe cattle based on milk protein polymorphism. J. Biotechnol. 2014, 185, S49. [Google Scholar] [CrossRef]
- Senczuk, G.; Mastrangelo, S.; Ciani, E.; Battaglini, L.; Cendron, F.; Ciampolini, R.; Crepaldi, P.; Mantovani, R.; Bongioni, G.; Pagnacco, G.; et al. The genetic heritage of Alpine local cattle breeds using genomic SNP data. Genet. Sel. Evol. 2020, 52, 40. [Google Scholar] [CrossRef] [PubMed]
- Kushniraand, A.V.; Glazko, V.I. Gray Ukrainian Cattle and Their Closely Related Forms. Contemp. Probl. Ecol. 2009, 2, 288–295. [Google Scholar] [CrossRef]
- Holló, G.; Nuernberg, K.; Somogyi, T.; Anton, I.; Holló, I. Comparison of fattening performance and slaughter value of local Hungarian cattle breeds to international breeds. Arch. Anim. Breed. 2012, 55, 1–12. [Google Scholar] [CrossRef]
- Zsolnai, A.; Maróti-Agóts, A.; Kovács, A.; Bâlteanu, A.V.; Kaltenecker, E.; Anton, I. Genetic position of Hungarian Grey among European cattle and identification of breed-specific markers. Animal 2002, 14, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Poljak, F.; Ilkić, J.; Čuklić, D.; Pintić, V.; Ernoić, M. Basic external characteristics and measurements of Slavonian-Sirmian Podolian cattle. Stočarstvo 2002, 56, 105–116. [Google Scholar]
- Caput, P.; Ivanković, A.; Konjačić, M. Genome typing of autochthonous breeds of domestic animals in Croatia. Stočarstvo 2004, 58, 265–293. [Google Scholar]
- Bogdanovic, V.; Djedovic, R.; Perisic, P.; Petrovic, M.M. Cattle breeding goals and programmes in Serbia. Biotechnol. Anim. Husb. 2005, 21, 15–21. [Google Scholar] [CrossRef]
- Terefe, E.; Haile, A.; Mulatu, W.; Dessie, T.; Mwai, O. Phenotypic characteristics and trypanosome prevalence of Mursi cattle breed in the Bodi and Mursi districts of South Omo Zone, southwest Ethiopia. Trop. Anim. Health Prod. 2015, 47, 485–493. [Google Scholar] [CrossRef]
- Moioli, B.; Napolitano, F.; Catillo, G. Genetic Diversity between Piedmontese, Maremmana, and Podolica Cattle Breeds. J. Hered. 2004, 95, 250–256. [Google Scholar] [CrossRef]
- Ivanković, A.; Caput, P.; Prekalj, G.; Kelava, N.; Konjačić, M.; Šubara, G.; Šuran, E. Characteristics of the Istrian cattle in beef production. Stočarstvo 2010, 64, 91–99. [Google Scholar]
- Blackburn, H.D.; Wilson, C.S.; Krehbiel, B. Conservation and Utilization of Livestock Genetic Diversity in the United States of America through Gene Banking. Diversity 2019, 11, 244. [Google Scholar] [CrossRef]
- Neov, B.; Teofanova, D.; Zagorchev, L.; Radoslavov, G.; Hristov, P. Milk protein polymorphism in bulgarian grey cattle population. Bulg. J. Agric. Sci. 2013, 19, 194–196. [Google Scholar]
- Hunter, P. The genetics of domestication: Research into the domestication of livestock and companion animals sheds light both on their “evolution” and human history. EMBO Rep. 2018, 19, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Ozsensoy, Y.; Kurar, E. Genetic diversity of native Turkish cattle breeds: Mantel, AMOVA and bottleneck analysis. J. Adv. Vet. Anim. Res. 2014, 1, 86–93. [Google Scholar] [CrossRef]
- Cosentino, C.; D’Adamo, C.; Naturali, N.; Pecora, G.; Paolino, R.; Musto, M.; Adduci, F.; Freschi, P. Podolian cattle: Reproductive activity, milk and future prospects. Ital. J. Agron. 2018, 13, 982. [Google Scholar] [CrossRef]
- Napolitano, F.; Pacelli, C.; DeRosa, G.; Braghieri, A.; Girolami, A. Sustainability and welfare of Podolian cattle. Livest. Prod. Sci. 2005, 92, 323–331. [Google Scholar] [CrossRef]
- Napolitano, F.; Girolami, A.; Pacelli, C.; Braghieri, A. Activity Budgets and Forage Selection of Podolian Cattle, a Semiwild Bovine Breed. Int. Sch. Res. Netw. 2011, 2011, 972804. [Google Scholar] [CrossRef]
- Ginja, C.; Gama, L.T.; Cortés, O.; Burriel, I.M.; Vega-Pla, J.L.; Penedo, C.; Sponenberg, P.; Cañón, J.; Sanz, A.; do Egito, A.A.; et al. The Genetic Ancestry of American Creole Cattle Inferred from Uniparental and Autosomal Genetic Markers. Sci. Rep. 2019, 9, 11486. [Google Scholar] [CrossRef] [PubMed]
- Loftus, R.; Scherf, B. World Watch List for Domestic Animal Diversity, 1st ed.; FAO/UNEP: Rome, Italy, 1993; Available online: https://www.fao.org/3/y0741t/y0741t.pdf (accessed on 25 January 2023).
- Rischkowsky, B.; Pilling, D. The First Report on the State of the World’s Animal Genetic Resources for Food and Agriculture; FAO: Rome, Italy, 2007; Available online: https://www.fao.org/3/a1260e/a1260e.pdf (accessed on 18 February 2023).
- FAO. Commission on Genetic Resources for Food and Agriculture Assessments; FAO: Rome, Italy, 2015; Available online: https://www.fao.org/cgrfa/en (accessed on 22 February 2023).

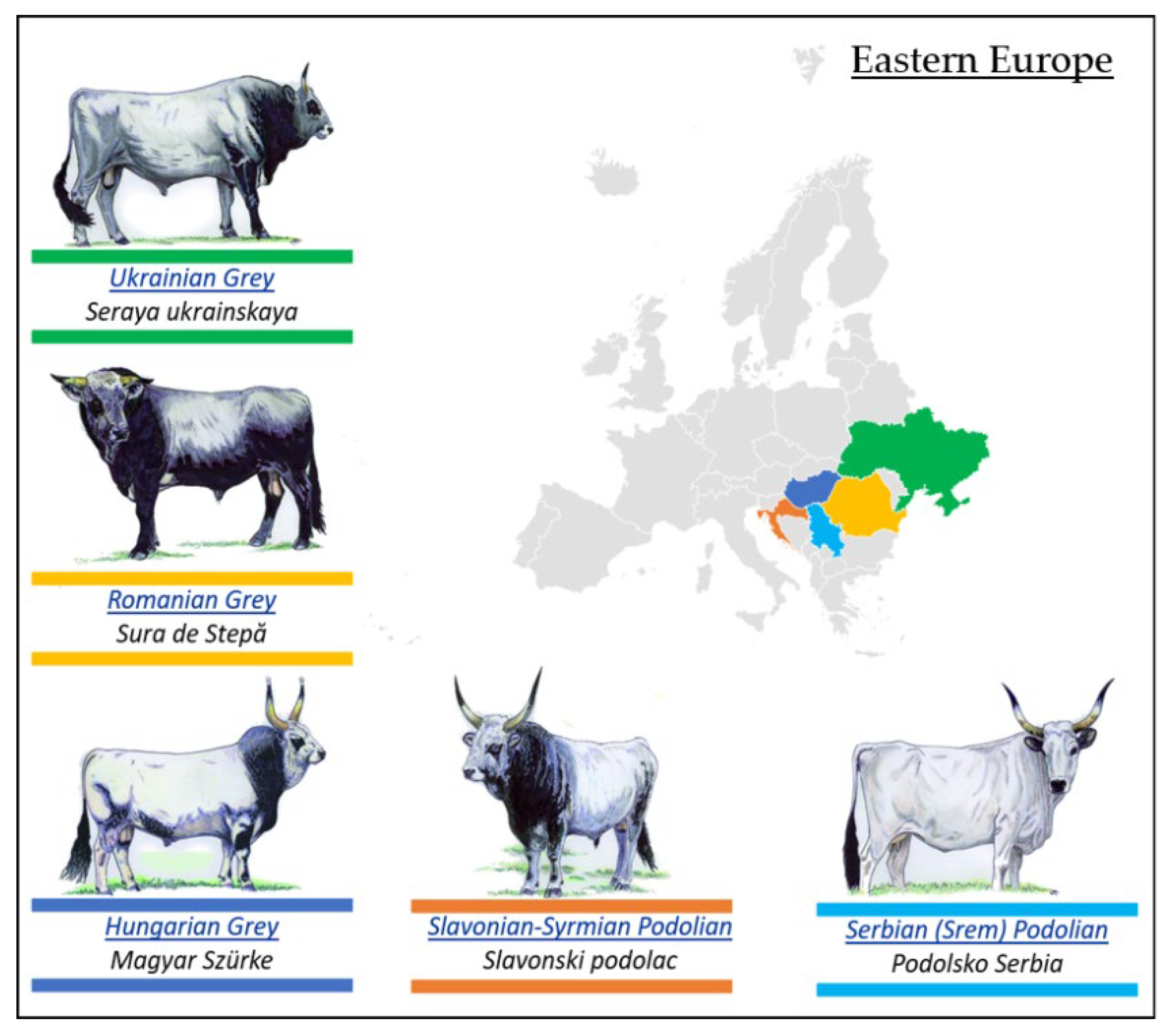
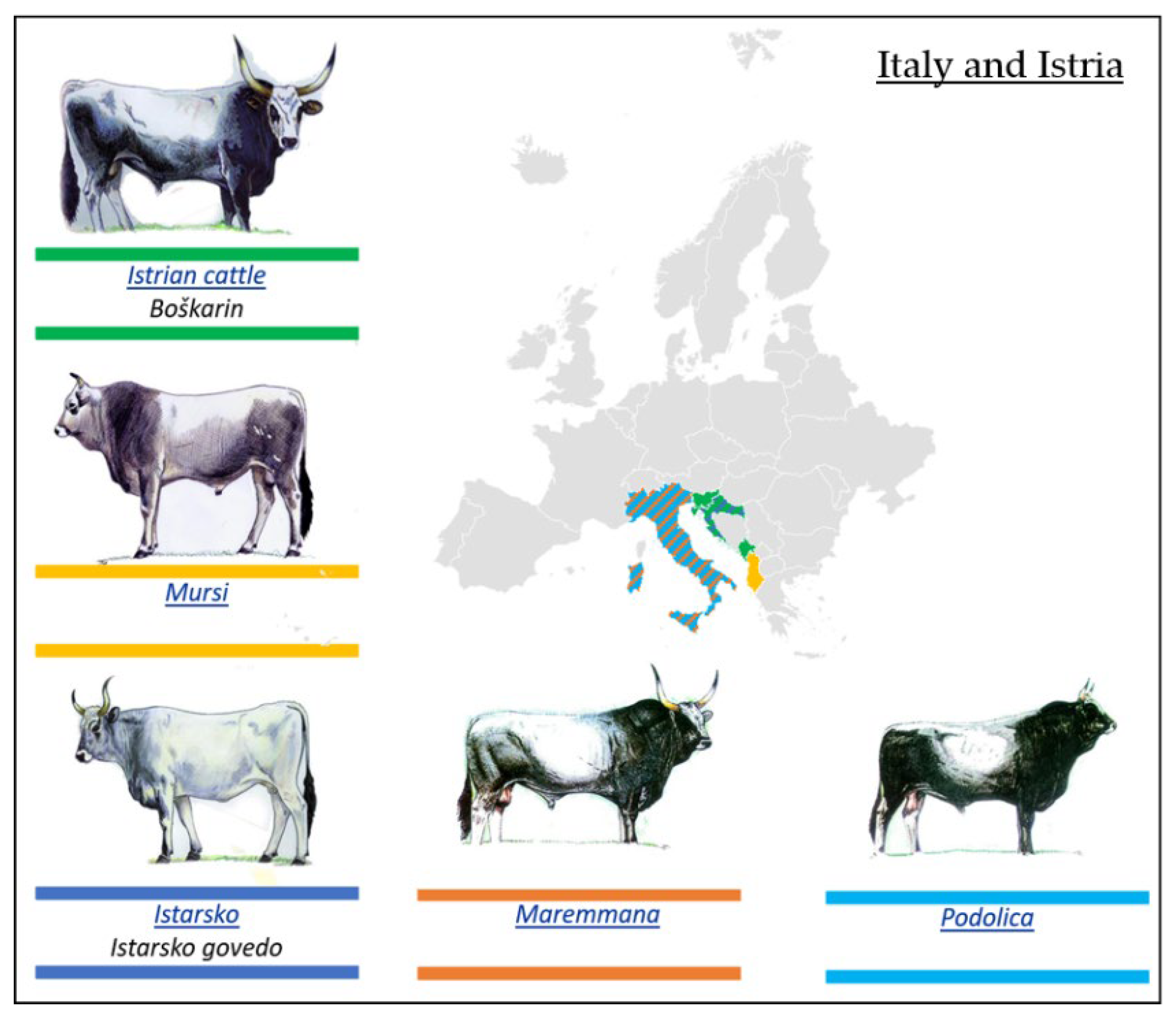
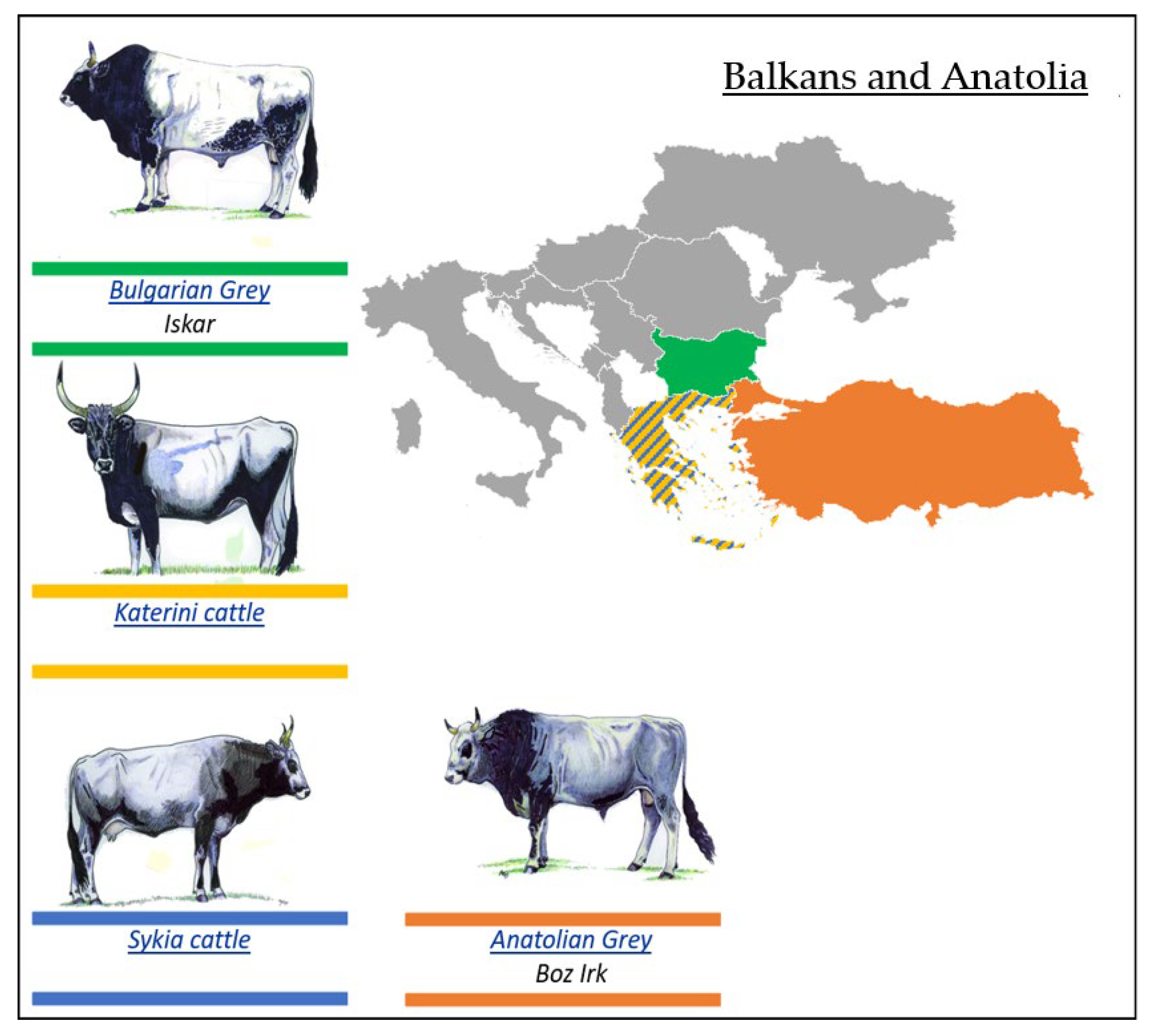
| Podolian Cattle Breeds/Geographical Distribution | ||
|---|---|---|
| 1. Eastern Europe | 2. Italy and Istria | 3. Balkans and Anatolia |
| Ukrainian Grey (Seraya Ukrainskaya) | Istrian cattle (Boškarin) | Bulgarian Grey (Iskar) |
| Romanian Grey (Sură Stepă) | Mursi | Katerini cattle |
| Hungarian Grey (Magyar Szűrke) | Istarsko (Istarsko govedo) | Sykia cattle |
| Slavonian–Syrmian Podolian (Slavonsky Podolac) | Maremmana | Anatolian Grey (Boz Irk) |
| Serbian (Srem) Podolian (Podolsko Serbia) | Podolica | |
| Podolian Cattle Breeds and Their Photo | Average Adult Weight (kg) | Average Wither Height (cm) | References | |||
|---|---|---|---|---|---|---|
| Males | Females | Males | Females | |||
 | Romanian Grey Steppe (Sura de Stepa) | 780 | 480 | 137 | 129 | [64,65,66] |
 | Iskar Grey (Bulgarian Grey) | 750 | 350 | 140 | 118 | [67,68] |
 | Istrian Grey (Boskarin) | 900 | 625 | 148 | 138 | [69,70] |
 | Slavonian Podolian (Slavonian–Syrmian) | 600–800 (1000 for oxen) | 470 | 135–145 | 128 | [71,72] |
 | Katerini cattle | 400 | 285 | 123 | 113 | [73,74] |
 | Hungarian Grey Steppe (Hungarian Grey) | 900 | 600 | 150 | 140 | [75,76] |
 | Turkish Grey Steppe (Boz Irk/Boz Step) | 470 | 375 | 126 | 117 | [77,78] |
 | Ukrainian Grey | 780 | 480 | 137 | 129 | [79,80] |
Northern Italy | Central Italy | Southern Italy |
|---|---|---|
| Piedmontese—PI | Romagnola—RO | Italian Podolian—IP |
| Bianca di Val Padana—BP | Mucco Pisano—MP | Agerolese—AG |
| Valdostana—VA | Calvana—CA | Cinisara—CI |
| Grey Alpine—GA | Chianina—CH | Modicana—MO |
| Italian Brown—IB | Maremmana—MA | |
| Italian Red Pied—RP | Marchigiana—MR | |
| Cabannina—CB | ||
| Reggiana—RE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davidescu, M.-A.; Pânzaru, C.; Usturoi, A.; Radu-Rusu, R.-M.; Creangă, Ș. An Appropriate Genetic Approach to Endangered Podolian Grey Cattle in the Context of Preserving Biodiversity and Sustainable Conservation of Genetic Resources. Agriculture 2023, 13, 2255. https://doi.org/10.3390/agriculture13122255
Davidescu M-A, Pânzaru C, Usturoi A, Radu-Rusu R-M, Creangă Ș. An Appropriate Genetic Approach to Endangered Podolian Grey Cattle in the Context of Preserving Biodiversity and Sustainable Conservation of Genetic Resources. Agriculture. 2023; 13(12):2255. https://doi.org/10.3390/agriculture13122255
Chicago/Turabian StyleDavidescu, Mădălina-Alexandra, Claudia Pânzaru, Alexandru Usturoi, Răzvan-Mihail Radu-Rusu, and Șteofil Creangă. 2023. "An Appropriate Genetic Approach to Endangered Podolian Grey Cattle in the Context of Preserving Biodiversity and Sustainable Conservation of Genetic Resources" Agriculture 13, no. 12: 2255. https://doi.org/10.3390/agriculture13122255
APA StyleDavidescu, M.-A., Pânzaru, C., Usturoi, A., Radu-Rusu, R.-M., & Creangă, Ș. (2023). An Appropriate Genetic Approach to Endangered Podolian Grey Cattle in the Context of Preserving Biodiversity and Sustainable Conservation of Genetic Resources. Agriculture, 13(12), 2255. https://doi.org/10.3390/agriculture13122255








