Abstract
The world’s population continues to grow while available natural resources, such as arable land, water, and quality soil, are decreasing. Therefore, it is essential to implement environmentally friendly crop management strategies, which include the use of biostimulants. This study analysed the effects on strawberry plants of ActyseiTM and Phylgreen®, two commercial biostimulants based on extracts of the seaweed Ascophyllum nodosum. The study was conducted under field capacity (regular irrigation) and at 50% field capacity (mild water stress conditions) for 12 weeks. Different growth parameters of the aerial parts of the plants were measured weekly, such as the number of leaves, length of the longest leaf, leaf area, and the number of flowers and fruits produced, as well as the chlorophyll content, determined with a single-photon avalanche diode (SPAD) detector. At the end of the experiment, the plant material was collected, and the roots and aerial parts were weighed separately to obtain the fresh and dry weight of the samples. Fruit quality was assessed by analysing morphological parameters (weight and size) and some biochemical variables (proline, total soluble sugars, and antioxidant compounds contents). ActyseiTM application generally enhanced plant growth in control plants and under mild water stress conditions, even though root weight was reduced. In contrast, no significant effect of Phylgreen® on vegetative growth was observed, except for stimulating the root growth of plants watered at field capacity. Both biostimulants, Phylgreen® to a greater extent, showed an impact on the plants already seven weeks after their initial application, stimulating flower and fruit production, especially at field capacity.
1. Introduction
The demand for food is increasing as the world’s population grows. By 2050, the world’s population is expected to reach almost 10 × 109 people [1], while available arable land, water, and soil quality are decreasing [2]. One of the major challenges facing agriculture today is increasing crop production while maintaining environmental sustainability in light of this future scenario. It is, therefore, imperative to develop crop management strategies that do not have a negative impact on the environment. For this reason, the use of biostimulants is gaining importance worldwide. Biostimulants are considered environmentally friendly substances or microorganisms whose function is to stimulate natural processes that improve nutrient uptake and assimilation, alleviate abiotic stress, or improve some agronomic characteristics [3,4].
The use of seaweed in agriculture has been commonplace for a long time, but only recently have their biostimulant effects become known, representing at present more than 33% of the biostimulant market [5]. This is largely because seaweed extracts are considered environmentally friendly, readily biodegradable, non-toxic, and safe for humans, animals, and the environment [6]. The seaweed used in this study was Ascophyllum nodosum (L.) Le Jolis, which belongs to the brown algae group. It is the most widely used seaweed to manufacture agricultural products [7] and was the source of the first liquid seaweed extract commercialised [8]. Some studies indicate its stimulating action on seed germination, an invigorating action on seedlings, increased root growth, earlier flowering, and a delay in senescence. It is also involved in fruit ripening, increase in crop yield, and nutritional quality [5,7]. Other studies on different seaweed extracts reported increased activity of beneficial microorganisms in the rhizosphere and boosted plant tolerance to different types of abiotic stresses and plant defence against pests and diseases [7,8,9,10]. These functions are mainly attributed to the presence of bioactive compounds in the extracts. Most of these compounds are auxins and cytokinins, but other growth regulators, such as abscisic acid, gibberellins, salicylic acid, polyamines, and ethylene, have also been reported [11,12,13,14,15]. Polysaccharides are also bioactive compounds present in marine algae, constituting the structural component of their cell walls or food reserves. In brown algae, the polysaccharides found in the greatest quantities are alginates and fucoidans [9,16], which improve plant physiological processes related to growth and development [7]. They also boost immunity against pathogenic organisms and are involved in abiotic stress tolerance [17,18]. In addition to growth regulators and polysaccharides, seaweed extracts also contain amino acids; polyunsaturated fatty acids; vitamins B, C, E, and K; and sterols that have all been shown to activate defence mechanisms and aid crop growth [5,16,19]. Osmoprotectants have also been identified as beneficial for crops exposed to stresses with an osmotic component [20,21,22]. Seaweed extracts contain antioxidant compounds, such as flavonoids, phenolic compounds, tocopherols, ascorbic acid, terpenoids, and carotenoids [7], and also a large variety of macro- and micronutrients [19,23]. Using seaweeds as biodegradable, non-toxic, non-polluting, and non-hazardous plant biostimulants is auspicious, representing an area of active research [6,8,24].
Strawberry is one of the most important fruit crops, with a 20% increase in its cultivation area globally in the last decade [25]. Consumers’ demand steadily increases due to its exceptional sensorial quality, high nutritional value, and antioxidant benefits [26]. Strawberries require high external inputs regarding soil management, irrigation, and fertilisation [27], but have also been reported as one of the fruits with the highest pesticide residues [28]. Therefore, there is an increasing number of studies related to the beneficial use of biostimulants to enhance their production [25,29,30,31]. Nowadays, strawberries are grown all year round, thanks to greenhouses. However, their cultivation requires constant watering, which may be problematic in some areas due to the scarcity of this natural resource; this is likely to be worsened by global warming. Therefore, finding biostimulants with beneficial effects under reduced irrigation is of great interest. This study was undertaken to evaluate the growth and productivity of strawberry plants treated with two biostimulants based on Ascophyllum nodosum algae extracts and grown under two irrigation regimes, at field capacity and under mild water stress (50% field capacity). Vegetative and reproductive parameters, such as leaf area, leaf length, number of leaves, chlorophyll content, number of flowers and fruits, and the weight and final size of fruits, were analysed in the plants during and at the end of the trial. Biochemical analyses of fruits were also performed to quantify the concentrations of total soluble sugars, proline, total phenolics, and flavonoids.
2. Materials and Methods
2.1. Plant Material
Experiments were performed in 2021 on commercial strawberry, Fragaria × ananassa (Duchesne ex Weston) Duchesne ex Rozier, variety Camarosa, one of the most cultivated varieties worldwide due to its good adaptation to different climates. Moreover, it is a very early variety; it starts fruiting in December, is very productive, and its fruits are of high quality [32]. Plants were purchased from the nursery Planters Peris in Valencia, Spain, at the 2–3 true leaves seedling stage, and were transplanted individually into 2.5 L pots. The substrate used for all pots was a mixture of peat and perlite in a 3:1 ratio. After transplanting, the plants were separated into trays according to the treatment they were to undergo and watered until the water retention capacity of the substrate was exceeded, allowing them to drip.
2.2. Growing Conditions, Biostimulant Application and Water Stress Treatments
Plants were grown in a greenhouse under the conditions indicated in Table 1. Each treatment consisted of six pots, each with a strawberry plant, placed in a plastic tray. The trays were rotated weekly to receive the same amount of direct sunlight. The different treatments consisted of two irrigation levels: one at the field capacity, i.e., 70% of substrate moisture measured with a WET-2 Sensor (Delta T Devices, Cambridge, UK), and another one under (mild) water stress at 50% field capacity, i.e., 35% of substrate moisture. To ensure these levels, the substrate moisture was measured before each irrigation. The volume of water needed for each pot was applied according to the moisture level to be maintained. Watering was carried out weekly and before the application of the biostimulants to avoid their loss through leaching. Biostimulants were applied once all the plants had been watered so they were at the corresponding humidity. The two biostimulants used in this study are obtained from the brown algae Ascophylum nodosoum. Phylgreen® is a 100% (pure) seaweed extract obtained by natural cold extraction, containing 1.2% w/w (1.3% w/v) mannitol and 2% w/w (2.2% w/v) alginic acid, dry matter (from seaweed extract): 15% w/w (16.5% w/v). ActyseiTM is a formulation developed from an extract of the same seaweed, also containing 0.7% mannitol, 2% glycine, 1% organic N, 1% P, and 8% K. Both products were already available on the market during the trial and registered as special algae-based fertilisers according to Spanish fertiliser regulations.

Table 1.
Greenhouse temperature and relative humidity conditions during the experimental period.
The study included six experimental variants: three treatments at field capacity (i.e., 70% substrate moisture), including plants not treated with biostimulants (control), treated with ActyseiTM or with Phylgreen®, and the same three groups of plants grown at 50% of field capacity (i.e., 35% substrate moisture). In all treatments, the number of replicates was six individual plants. The applied dose of each biostimulant was 3 mL L−1, as recommended by the manufacturer. Due to the density of the products and for better absorption by the plants, the required volume of each biostimulant was thoroughly mixed with 50 mL of water per pot and applied directly to the pot’s substrate. The same volume of water was added to the pots from the treatments without biostimulants to avoid possible differences in water stress levels.
2.3. Chlorophyll Measurement and Analyses of Physical Parameters
Chlorophyll content was measured using the Konica Minolta SPAD-502 Plus, calculating the average of ten measurements per plant. Flowers and fruits produced were counted weekly. As they ripened, the fruits were weighed, and length and width measurements were taken for each fruit with the help of a digital calliper. After 12 weeks, all plants were removed from the pots and washed with water to separate the peat and perlite adhering to the roots. Once the substrate was removed, photographs of the whole plant were taken to obtain the leaf area and the final length of each plant. Both measurements were performed with the Digimizer v.4.6.1 software (MedCalc Software, Ostend, Belgium, 2005–2016). Each plant was separated into aerial and root parts and weighed with a precision balance. Once the fresh weights of each plant part were recorded, they were transferred to an oven maintained at a constant temperature of 60 °C for seven days for subsequent measurement of dry weights.
2.4. Biochemical Analyses of Fruits
Total soluble sugars (TSS), proline (Pro), total phenolic compounds (TPC), and total flavonoids (TF) were analysed in fruits produced by the strawberry plants and stored at −20 °C. Fruits were sampled gradually throughout the experiment and stored at −20 °C in separate pools for each treatment. For biochemical analysis, ten fruits were randomly taken from each group. Methanolic extracts were prepared to quantitatively estimate the fruits’ TSS, TPC, and TF contents. Fresh fruit samples (0.5 g) were homogenised in 750 µL of 80% methanol (v/v), and extraction was allowed to take place overnight at 4 °C, followed by centrifugation at 13,300× g for 10 min. The clear supernatant was collected, stored at −80 °C, and subsequently used for biochemical assays.
The method described by Dubois et al. [33] was used to determine TSS. To the methanolic extracts, 5% phenol (v/v) and concentrated sulphuric acid were added to induce caramelisation of the extracted sugars via an exothermic reaction. After incubation for 20 min at room temperature, TSS were quantified spectrophotometrically by measuring the absorbance at 490 nm. A standard curve was prepared using known glucose concentrations, and the TSS content in the samples was expressed as equivalents of glucose (mg eq. glucose g−1 FW).
Pro was quantified according to the classical method by Bates et al. [34]. Freshly ground fruit material (0.5 g) was extracted in 3% aqueous sulphosalicylic acid (w/v) and mixed with acid ninhydrin. Afterwards, samples were incubated in a water bath for one hour at 98 °C and subsequently cooled on ice, followed by extraction with toluene. Spectrophotometric estimation of Pro concentration was performed by measuring the absorbance of the organic phase at 520 nm. Solutions with known Pro concentrations were assayed in parallel to obtain a standard curve. Pro concentration was finally calculated in nmol g−1 FW.
Methanolic extracts were used for the spectrophotometric analyses of antioxidant compounds, TPC and TF. The TPC content in the sample was determined following the protocol of Blainski et al. [35], based on the reaction with the Folin–Ciocalteu reagent (FCR) and sodium bicarbonate (Na2CO3). The samples were incubated at room temperature for 90 min in the dark, and the absorbance was measured at 765 nm. Gallic acid (GA) was used to prepare a standard curve, and TPC contents were expressed as mg eq. GA g−1 DW. The TF content was quantified spectrophotometrically using the method developed by Zhishen et al. [36]. To the methanolic extract of each sample, sodium nitrite (NaNO2) was added, leading to the nitration of aromatic rings containing a catechol group, followed by the addition of aluminium chloride (AlCl3) at basic pH. The absorbance of the samples was measured at 510 nm, and the TF contents were expressed as equivalents of the standard catechin (mg eq. C g−1 DW).
2.5. Statistical Analyses
The analysis of variance of the data was performed using IBM SPSS Statistics v. 23.0 for Windows (IBM Corp., Armonk, NY, USA) software. To analyse the influence of the biostimulants on the plants’ vegetative and reproductive parameters, a one-way ANOVA was conducted independently for plants grown at 70% and 35% substrate moisture. The Tukey Honestly significant difference (HSD) post hoc test was applied to identify statistically significant differences between the treatments’ mean values, with a significance level of p < 0.05. The effect of substrate moisture, the biostimulant treatment, and their interactions were analysed by performing a two-way ANOVA with all analysed traits.
Finally, a principal component analysis (PCA) was performed using Statgraphics Centurion package version XVII (Statgraphics Technologies, The Plains, VA, USA) to explain the variation of the dataset under the two levels of substrate moisture and three treatments (control and two biostimulants) using mean values of all analysed morphological and biochemical traits. The dimensionality of the multivariate data was reduced to the first two principal components and expressed graphically in a PCA biplot, indicating both variable loadings and sample scores.
3. Results
3.1. Vegetative Growth
The effect of the two applied biostimulants on the plants grown under two different irrigation regimes (as indicated in Section 2.2), was studied compared with control plants without biostimulants. Although some growth parameters were determined throughout the 12 weeks of the experiment, the statistical analysis was performed with the final data when the plants were sampled. The results of a two-way ANOVA considering the effect on growth parameters of the field capacity, the biostimulant treatment, and their interaction are shown in Table 2. Watering at 50% field capacity caused very mild water stress on the plants, reflected only in significant changes in the fresh weight of roots (RFW) and chlorophyll content in leaves, not affecting any of the additional growth variables analysed (Table 2). On the contrary, the biostimulant treatments significantly affected all growth parameters, except the leaf number, compared to the control treatment. The interaction between the water deficit and the biostimulant treatments was significant only for the leaf fresh and dry weight (Table 2).

Table 2.
Two-way ANOVA (F values) considering the effect of the substrate moisture (A), biostimulant treatment (B), and their interaction (A × B) on growth parameters measured in strawberry plants after 12 weeks of treatment. Abbreviations: LN, number of leaves; LLL, length of the longest leaf; LA, leaf area; Chl, chlorophyll content in leaves (SPAD); RFW, root fresh weight, LFW, leaf fresh weight; RDW, root dry weight; LDW, leaf dry weight.
The number of leaves (LN) at field capacity showed a non-significant variation between the treatments (Figure 1a). At 50% field capacity, the biostimulants, especially Phylgreen®, increased the average number of leaves compared to control plants, but the differences in LN between treatments were not statistically significant (Figure 1a, Table 2). On the contrary, treatment with ActyseiTM caused a significant increase in the two other foliar parameters analysed, namely, the length of the longest leaf (LLL) (Figure 1b) and the leaf area (LA) (Figure 1c), whereas Phylgreen® treatment showed no significant effect (Figure 1b,c). Similarly, ActyseiTM (but not Phylgreen®) treatment increased the mean value of chlorophyll contents, although the difference with control plants was significant only at 50% field capacity (Figure 1d).
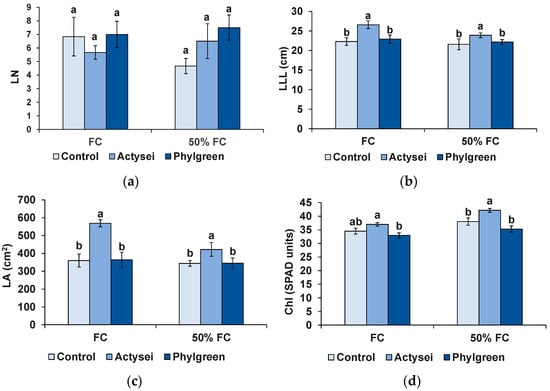
Figure 1.
Foliar parameters after 12 weeks of the indicated treatments: (a) leaf number (LN); (b) length of the longest leaf (LLL); (c) leaf area (LA); (d) chlorophyll content (Chl) in SPAD units. Bars represent means with SD (n = 6). Different lowercase letters over the bars indicate significant differences between treatments according to the Tukey test (p < 0.05), at field capacity (FC) or 50% field capacity (50% FC).
The effects of the tested biostimulants on plant biomass at the end of the 12-week treatments are shown in Figure 2. In agreement with the above data on foliar parameters (Figure 1b,c), ActyseiTM treatments with irrigation at field capacity led to a significant increase in the biomass of the plants’ aerial part, both in terms of leaf fresh weight (ca. 1.7-fold higher than the control) and dry weight (1.6-fold). Reducing field capacity to 50% resulted in non-significant differences in LFW and LDW between the ActyseiTM-treated and control plants (Figure 2b,d). Interestingly, this biostimulant had the opposite effect on root biomass, reducing the average fresh and dry weight of the roots at field capacity and 50% FC; the differences between ActyseiTM-treated and control plants were statistically significant in all cases, except for the root DW at FC (Figure 2a,c). The biostimulant Phylgreen®, on the other hand, showed no effect on plant biomass accumulation, except for a slight (but significant) increase in root FW at FC compared to the corresponding control plants (Figure 2a).
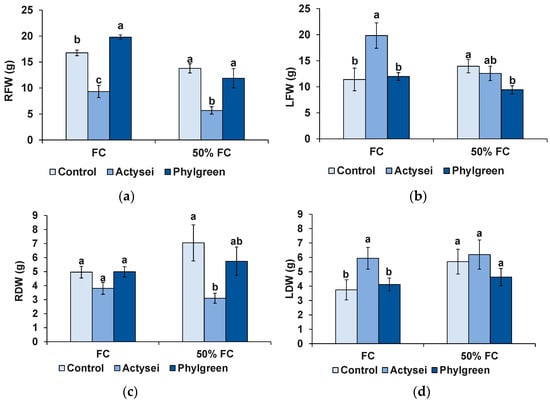
Figure 2.
Biomass of plants after 12 weeks of the indicated treatments: (a) root fresh weight (RFW); (b) leaf fresh weight (LFW); (c) root dry weight (RDW); (d) leaf dry weight (RDW). Bars represent means with SD (n = 6). Different lowercase letters over the bars indicate significant differences between treatments according to the Tukey test (p < 0.05), at field capacity (FC) or 50% field capacity (50% FC).
3.2. Reproductive Traits
The number of flowers and fruits produced by the plants was recorded weekly (Figure 3). In all treatments, two peaks were registered for flowering, the first in the second week and the second in the eighth week. By the end of the first half of the experiment, i.e., in the sixth week, flowering almost ceased in all treatments, but a second period started in the following week. Flowering phenology was similar in all plants that received abundant irrigation at field capacity (Figure 3a), and flower production showed similar variations throughout the 12 weeks. However, the number of flowers registered was higher in plants treated with biostimulants during the whole period of the trial, as can be observed in Figure 3a. For example, in the eighth week, the number of flowers reached a maximum in the three treatments, but ActyseiTM-treated plants produced 30% more flowers than the control.
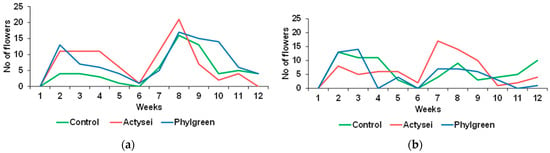
Figure 3.
Total weekly production of flowers during the 12 weeks of plants from the indicated treatments: (a) plants watered at field capacity; (b) plants watered to half of the field capacity.
Flower production was also maintained in plants that received half the amount of water. The floral phenology was similar to that of plants watered at field capacity, with two peaks of flowering, the first in the second and third weeks, followed by a drastic reduction in the number of flowers by the middle of the test (weeks 5 and 6). In the second half of the experiment, plants started to produce new flowers, and again ActyseiTM clearly stimulated flowering. For example, in the eighth week, plants from this treatment almost doubled, and in the ninth week tripled, the flower production in control plants.
The production of fruits followed the same trend as flowering, with two peaks separated by an intermediate period (weeks 5 to 7) marked by the production of very few fruits. Comparing the plants watered at field capacity, a higher production was generally observed in plants treated with biostimulants (Figure 4a). The highest number of mature fruits was registered by the end of the trial in the 10th week for ActyseiTM-treated plants, with 60% more fruits than in control plants, and in the 11th week for plants treated with Phylgreen®, which doubled those produced in control plants.
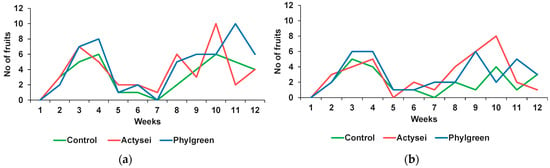
Figure 4.
Total weekly production of mature fruits during the 12 weeks by plants from the indicated treatments: (a) plants watered at field capacity; (b) plants watered to half of the field capacity.
The same trend of fruit production was observed in plants watered at half the field capacity, again with two separate phases, the first with a peak by the fourth week and the second by the 10th week, with almost no fruit formation during weeks 5 to 7. The highest number of fruits was registered in the 10th week, with AcyseiTM-treated plants doubling the fruit production in control (Figure 4b).
When comparing the total number of mature fruits collected throughout the 12 weeks, it was higher in the plants that were watered at field capacity, with the highest number in Phylgreen®-treated plants (53), followed by ActyseiTM-treated plants (45), whereas the lowest fruit production was found in control plants (37). At 50% field capacity, almost an equal number of mature fruits were sampled from the plants treated with the two biostimulants (36), but only 24 in control plants.
The size (length and width) and weight of the fruits collected from the strawberry plants were determined after the different treatments (Table 3, Figure 5). A significant difference was registered between the fruits produced at field capacity and those produced at 50% field capacity, the former with significantly higher weight and width. The interaction of the two factors (substrate moisture and biostimulant treatment) was also significant, as Phylgreen®-treated plants showed higher fruit length and fresh weight at 50% field capacity compared to control and ActyseiTM-treated plants (Table 3).

Table 3.
Two-way ANOVA (F values) considering the effect of the substrate moisture (A), biostimulant treatment (B), and their interaction (A × B) on fruit parameters measured in strawberry plants. Abbreviations: Fruit width (FW); fruit length (FL); fruit fresh weight (FFW); proline (Pro); total soluble sugars (TSS); total phenolic compounds (TPC); total flavonoids (TF).
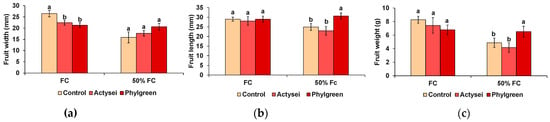
Figure 5.
Morphological evaluation of the sampled fruits after 12 weeks of the indicated treatments: (a) Fruit width; (b) fruit length; (c) fruit fresh weight (bars represent means with SD (n = 20). Different lowercase letters over the bars indicate significant differences between treatments according to the Tukey test (p < 0.05) at field capacity (FC) and 50% field capacity (50% FC).
When analysing the two watering levels separately, at field capacity, the only significant difference was the width of the fruits, which was larger in control plants than in those treated with the biostimulants (Figure 5a). On the contrary, at 50% field capacity, the plants treated with Phylgreen® produced significantly bigger fruits in terms of length (Figure 5b) and weight (Figure 5c). ActyseiTM treatment did not affect significantly the size or weight of the fruits under any tested experimental condition (Figure 5).
3.3. Biochemical Analyses of the Fruits
The concentration of four types of biochemical compounds generally involved in the responses of plants to abiotic stress, including drought, was determined in the mature strawberry fruits; namely, proline and total soluble sugars (TSS), two common osmolytes, and total phenolics and flavonoids, representative examples of antioxidant compounds (Table 3, Figure 6). Amongst all analysed compounds, only proline showed a significant variation between the treatments, as a significant increase was found in the fruits from the treatment with ActyseiTM grown at 50% field capacity. The proline concentration was ~2-fold and 2.5-fold higher than that recorded in fruits harvested from control plants at field capacity and 50% field capacity, respectively (Figure 6a). Furthermore, at 50% field capacity, the fruits produced by the same plants showed slightly higher concentrations of TSS (Figure 6b), total phenolics (Figure 6c), and total flavonoids (Figure 6d); however, their variations were not statistically significant, as indicated by the two-way ANOVA (Table 3).
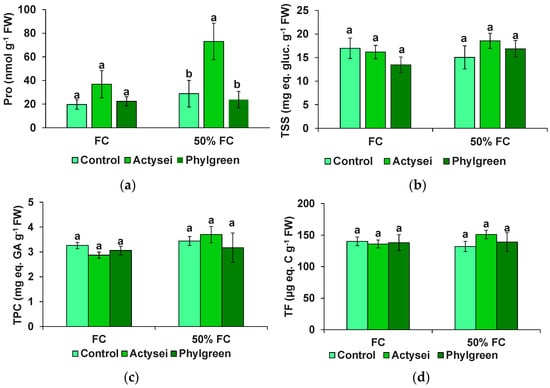
Figure 6.
Biochemical evaluation of the sampled fruits after 12 weeks of the indicated treatments: (a) proline (Pro); (b) total soluble sugars (TSS); (c) total phenolic compounds (TPC); (d) total flavonoids. Bars represent means with SD (n = 10). Different lowercase letters over the bars indicate significant differences between treatments according to the Tukey test (p < 0.05) at the field capacity and 50% field capacity.
3.4. Principal Component Analysis
A principal component analysis (PCA) was performed with the mean values of all analysed traits in the vegetative parts of the plants and the fruits (Figure 7). Four variables had an eigenvalue higher than one, summing up to 97% of the total variation (Table 4). The first principal component, explaining 41.75% of the variability, was strongly positively correlated with chlorophyll concentration (Chl) in the leaves, the leaf dry weight (LDW), and the concentration of proline (Pro) in the fruits, and negatively correlated to the root fresh weight (RFW). The second component explained an additional 28.92% of the variation and was positively correlated with the leaf area (LA), length of the longest leaf (LLL), and leaf fresh weight (LFW), and negatively correlated mostly with the total phenolic compounds (TPC) contents in fruits.
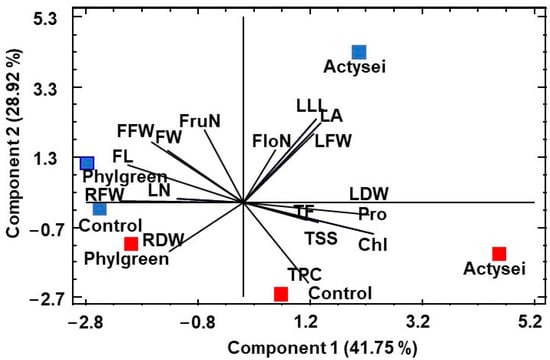
Figure 7.
Principal component analysis. Loading and scatter plots of the PCA scores were conducted with all the analysed traits in strawberry plants treated with two biostimulants (ActyseiTM and Phylgreen®) and without biostimulant treatment (control) grown during 12 weeks at field capacity (blue) and 50% field capacity (red). Abbreviations: Number of leaves (LN); length of the longest leaf (LLL); leaf area (LA); (d) chlorophyll content (Chl); root fresh weight (RFW); root dry weight (RDW); leaf fresh weight (LFW); leaf dry weight (LDW); number of flowers (FloN); number of fruits (FruN), fruit width (FW); fruit length (FL); fruit fresh weight (FFW); proline (Pro); total soluble sugars (TSS); total phenolic compounds (TPC); total flavonoids (TF).

Table 4.
Correlation coefficients between morphological and biochemical traits and the two first principal components of strawberry plants treated with two biostimulants (ActyseiTM and Phylgreen®) and without biostimulant treatment, grown during 12 weeks at field capacity and 50% field capacity. Abbreviations: Number of leaves (LN); length of the longest leaf (LLL); leaf area (LA); chlorophyll content (Chl); root fresh weight (RFW); root dry weight (RDW); leaf fresh weight (LFW); leaf dry weight (LDW); number of flowers (FloN); number of fruits (FruN), fruit width (FW); fruit length (FL); fruit fresh weight (FFW); proline (Pro); total soluble sugars (TSS); total phenolic compounds (TPC); total flavonoids (TF).
The PCA clearly separated the score plots along the second component according to field capacity, with those at field capacity (70% soil moisture) in the positive upper part of the graph and those at 50% field capacity (35% soil moisture) in the negative lower part. The scatterplots of the three treatments (Control, ActyseiTM, and Phylgreen®) were separated along the first principal component. The most dispersed scores were that of ActyseiTM, at field capacity positively correlated with the leaf area (LA), the length of the longest leaf (LLL), the leaf fresh weight (LFW), and the number of flowers produced (NFlo), whereas at 50% field capacity with the concentration of chlorophyll (Chl), proline (Pro), and total flavonoids (TF). The smallest dispersion, indicating the most similar responses under the two watering regimes, was found for plants treated with Phylgreen®, which was primarily related to the root fresh weight (RFW) at field capacity and with the root dry weight (RDW) at 50% field capacity. The score of control plants (without biostimulants) at 50% field capacity was primarily characterised by their higher concentrations of total phenolics (TPC) (Figure 7).
4. Discussion
Due to the exceptional economic importance of Fragaria × ananassa and the urgency to develop environmentally friendly agricultural practices, the use of biostimulants in this crop is continuously increasing. There are numerous reports on the effects of different types of biostimulants on strawberries, such as plant hydrolysates, chitosan, or silicon [37], animal protein hydrolysates [38], probiotic bacteria [39], mycorrhizal fungi and root endophytes [40], amongst many others. Seaweeds have been used in agriculture for centuries [5], with records of their use as mulch and manure by Romans in the 1st century. However, their extensive use as animal feed, soil additive, and agrochemical dates back to 1950 [8]. Nevertheless, it was in this century, with the shift to “green agriculture”, that biostimulants obtained from seaweed boomed, reaching ~95% of the market share of all biostimulants extracted from plants [8]. Seaweed extracts show great variability depending on the extraction procedure, the species used, and the place and season of seaweed harvesting, which can significantly influence their biochemical composition [8].
The two biostimulants used in this study positively affected vegetative development and plant productivity, but some differences were observed. Phylgreen® had a greater effect on plant roots, with a significant increase in root fresh weight (RFW) at field capacity (70% substrate moisture), whereas ActyseiTM enhanced leaf development in terms of leaf size and weight. The beneficial effects of seaweed derivatives in stimulating root growth in different crops are well-known [5]. A detailed analysis of root systems in different strawberry varieties, including Camarosa (the one used in our study) treated with an alkaline powdered extract derived from A. nodosum, indicated stimulation of root length, total root area, total root volume, and root number [41], secondary root density [42], or fresh and dry weight [27]. On the other hand, there are reports of biostimulants obtained from the same brown algae improving leaf development in strawberries [41,43,44] even more than the root system [45], but there are also publications reporting no significant effects of this type of biostimulants on leaf area or weight [27]. Seaweed extracts contain a multitude of substances that are difficult to identify and quantify but seem to play synergic effects when used as biostimulants. A. nodosum belongs to the category of algae that withstands periods of immersion and periods at the water surface, following the tidal cycles. Therefore, as it is more exposed to thermic, hydric, and saline stresses, its biochemical composition includes compounds directly involved in stress tolerance. The two biostimulants used in this study were obtained by the cold extraction method without using chemical solvents or high temperatures; therefore, the algae’s active components remained unaltered. Phylgreen® is reported by the producer [46] as effective in favouring the vegetative growth and especially the development of the root system, improving essential nutrients absorption, and enhancing tolerance to stress. ActyseiTM is also reported as a natural activator of biological and physiological processes in plants, formulated from an extract of A. nodosum with nitrogen and potassium [47]. It can be easily absorbed, favouring the uptake of nutrients by plants. In this study, we detected only the improvement of the root system at field capacity in Phylgreen®-treated plants. However, there was a clear effect of ActyseiTM at the foliar level, improving the length of the longest leaf, mean leaf area and chlorophyll content under both irrigation conditions and leaf fresh and dry weight in plants watered at field capacity. This biostimulant has a high K content, an essential nutrient for plant growth, photosynthesis, regulating stomatal opening, enhancing cell turgor, and is involved in the translocation of sugars and starch [48,49,50]. A direct correlation between K nutritional status and plant drought resistance has been reported [51,52]. In addition, ActyseiTM contains glycine, the smallest amino acid that is an ideal chelating agent that helps mineral uptake by roots. It also has a stimulating effect on photosynthesis by favouring chlorophyll synthesis [53]. The chlorophyll concentrations in our experimental conditions were higher in plants treated with ActyseiTM under both watering levels. Several reports indicate that using products from A. nodosum can increase the chlorophyll levels in various crops [48,49,50,51,54,55,56,57], including strawberries [27]. Improved chlorophyll content, related to increased yields, may also be associated with betaine and betaine-like compounds present in A. nodosum extracts [58]. In addition to their role as compatible solutes, betaines have been implicated in reducing chlorophyll degradation and may represent a nitrogen source [5]
The present study reports a positive effect of seaweed-derived biostimulants on flower and fruit production. Seaweed extracts contain plant hormones, such as cytokinins and auxins, which advance cell division during the early stages of plant growth and can induce early and more vigorous flower formation [41]. There are many reports on the beneficial effects of A. nodosum extracts on reproductive phenology, fruit production, and quality in different crops [59,60], including that of Phylgreen® [61,62]. In our study, the two biostimulants tested favoured flowering, increasing the number of flowers formed in plants irrigated at field capacity. The same trend was found concerning fruit set at the same field capacity. In both cases, Phylgreen® was more effective, inducing increases of about 50% and 40% in the number of flowers and fruits, respectively, with respect to the control plants. The corresponding values for ActyseiTM-treated plants were approximately 40% and 20%. Several researchers reported yield increases of strawberries treated with A. nodosum-derived products [27,41,42,63,64] (yield and fruit size), and there is increasing information on the positive effect of the algae extract on different crops, as discussed in several recent reviews [5,6,24,57,65,66,67,68,69]. Seaweed extracts have also been reported to increase the fruit size and fruit weight in strawberries [64], along with an increase in the concentrations of TSS, TPC [43], fructose, sucrose, and quercetin [70]. However, there are also many studies in which no significant effect was observed in terms of carbohydrates, phenolic compounds, and flavonoids [64], or in general, no effect on fruit quality [27], which is consistent with our experiment on plants irrigated at field capacity. The two biostimulants are reported by the producers to increase the plants’ natural phytohormonal levels and improve metabolic action, resulting in better sprouting, flowering, development, fruit growth, fruit quality, and seed germination [71].
Watering at half the field capacity, maintaining substrate moisture at about 35% during plant growth resulted in mild water stress for the plants. Under these conditions, their vegetative growth was not substantially impaired, but the flower and especially fruit set were much lower than in those plants grown at field capacity. Strawberries require large amounts of water because of their shallow rooting system, large leaf area, and high fruit water content [72]. As water availability in many areas where they are cultivated is shrinking [72], finding methods to improve their productivity under reduced irrigation has become a priority. Algae-based biostimulants target the plants main stress mitigation mechanisms, potentially affecting membrane stability, synthesis of compatible solutes and osmoprotective compounds, and stimulating antioxidant ROS scavenging mechanisms [17]. Seaweed extracts have effectively mitigated the effects of different abiotic stressors, such as salinity, drought, and temperature extremes [5,14,24]. It is difficult to fully understand how seaweed extracts increase plant stress tolerance due to the high number of genes involved in such complex processes and the difficulty of separating direct effects from secondary ones [73]. However, it is generally agreed that biostimulants enhance plant stress tolerance due to the bioactive molecules they contain, such as betaines [58] and cytokinins [74]. In plants treated with algal extracts, endogenous levels of proline, phenolics, flavonoids and other stress-related molecules, and the activity of antioxidant enzymes have been reported to increase [74,75,76]. Both biostimulants have a higher concentration of alginates, mannitol, and polyphenols than traditional seaweed extracts. Alginates are present in the cell walls of A. nodosum and play a role in cellular water balance and protection against salt stress. Mannitol is a polyol that acts as a compatible solute, enhancing cellular water retention and protecting plants from osmotic stress. In addition, mannitol, and polyphenols, present at high concentrations in these biostimulants, are powerful ROS scavengers, acting as antioxidants and preventing metabolic damage produced by oxidative stress [77]. Applying glycine was reported to enhance enzymatic and non-enzymatic defence mechanisms against salt stress, stimulating the expression of genes related to salt tolerance such as AOX, NHX1, and SOS1 in wheat plants [78].
In the present work, differences were observed between the effect of the two tested biostimulants on plants irrigated at 50% field capacity. Phylgreen® induced significantly higher mean fruit weight and mean fruit width than control plants. On the other hand, ActyseiTM triggered the highest accumulation of proline, TSS, TPC, and flavonoids, although only the variation in proline was significant compared to the control.
The most abundant flavonoids in strawberries are kaempferol, quercetin, and catechin, all with potent antioxidant properties. A diet rich in fruits and vegetables with a high antioxidant content may reduce cardiovascular disease incidence by preventing LDL cholesterol oxidation, fostering plaque stability, enhancing arterial endothelial function, and reducing the propensity for thrombosis [79]. The healthy properties of its fruits increase the interest in improving strawberry crop yields under limited irrigation.
5. Conclusions
The two biostimulants applied had a beneficial effect on strawberry plants under control conditions (watering at field capacity) and mild water stress (watering at half field capacity). However, their effects on plants were different. ActyseiTM enhanced the development of the foliar part of the plants, as indicated by significant increases in the length of the longest leaf, the mean leaf area, the concentration of chlorophyll, and the leaf’s fresh and dry weight. In contrast, Phylgreen® did not significantly influence vegetative growth except for the increase of the fresh weight of roots in plants watered at field capacity. Applying the two biostimulants was mostly beneficial regarding reproductive traits, as they stimulated the production of flowers and fruits under both irrigation regimes. The total number of fruits produced was higher in the plants treated with the biostimulants than in the control, untreated plants, and the plants watered at 50% field capacity produced larger fruits when treated with Phylgreen®.
Author Contributions
Conceptualization, M.V. and M.B.; methodology, E.C., R.S., M.M., and N.T.-P.; software, I.L.; validation, M.M., O.V., M.V., and M.B.; formal analysis, R.S.; investigation, E.C. and R.S.; resources, M.V. and O.V.; data curation, R.S., E.C., and N.T.-P.; writing—original draft preparation, R.S. and M.B.; writing—review and editing, O.V., M.M., I.L., and M.V.; visualization, R.S. and N.T.-P.; supervision, M.B. and M.V.; project administration, M.V.; funding acquisition, M.V. and O.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly funded by the company SEIPASA S.A. and partly by the Polytechnic University of Valencia through internal funds.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
R.S. is thankful to the Department of Science and Technology, Government of India, for providing a SIRE fellowship (SIR/2022/000435).
Conflicts of Interest
The authors declare no conflict of interest.
References
- United Nations. World Population Prospects: The 2019 Revision Population Database. Available online: https://population.un.org/wpp/ (accessed on 15 June 2023).
- Intergovernmental Panel on Climate Change. Special Report on Climate Change and Land: Summary for Policymakers; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.; Menon, U.; Subramanian, S.; Jithesh, M.; Rayorath, P.; Hodges, D.; Crithcley, A.; Craigie, J.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth. Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- El Boukhari, M.E.M.; Barakate, M.; Bouhia, Y.; Lyamlouli, K. Trends in seaweed extract based biostimulants: Manufacturing process and beneficial effect on soil-plant systems. Plants 2020, 9, 359. [Google Scholar] [CrossRef]
- Espinosa-Antón, A.; Hernández-Herrera, R.; González, M. Extractos bioactivos de algas marinas como bioestimulantes del crecimiento y la protección de las plantas. Biot. Veg. 2020, 20, 257–282. [Google Scholar]
- Stirk, W.A.; Rengasamy, K.R.R.; Kulkarni, M.G.; van Staden, J. Plant biostimulants from seaweed. In The Chemical Biology of Plant Biostimulants; Geelen, D., Xu, L., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 31–55. [Google Scholar]
- Vera, J.; Castro, J.; González, A.; Moenne, A. Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Mar. Drugs 2011, 9, 2514–2525. [Google Scholar] [CrossRef]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef]
- Crouch, I.J.; van Staden, J. Evidence for the presence of plant growth regulators in commercial seaweed products. Plant Growth Reg. 1993, 13, 21–29. [Google Scholar] [CrossRef]
- Tarakhovskaya, E.R.; Maslov, Y.I.; Shishova, M.F. Phytohormones in algae. Russ. J. Plant Physiol. 2007, 54, 163–170. [Google Scholar] [CrossRef]
- Yokoya, N.S.; Stirk, W.A.; van Standen, J.; Novák, O.; Turečková, V.; Pĕnčík, A.; Strnad, M. Endogenous cytokinins, auxins and abscisic acid in red algae from Brazil. J. Phycol. 2010, 46, 1198–1205. [Google Scholar] [CrossRef]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Yalçin, S.; Okudan, E.S.; Karakaș, O.; Önem, A.N.; Bașkan, K.S. Identification and quantification of some phytohormones in seaweeds using UPLC-MS/MS. J. Liq. Chromatogr. Relat. Technol. 2019, 42, 475–484. [Google Scholar] [CrossRef]
- Nabti, E.; Jha, B.; Hartmann, A. Impact of seaweeds on agricultural crop production as biofertilizer. Int. J. Environ. Sci. Technol. 2017, 14, 1119–1134. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Zou, P.; Lu, X.; Zhao, H.; Yuan, Y.; Meng, L.; Zhang, C.; Li, Y. Polysaccharides derived from the brown algae Lessonia nigrescens enhance salt stress tolerance to wheat seedlings by enhancing the antioxidant system and modulating intracellular ion concentration. Front. Plant Sci. 2019, 10, 48. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Seca, A.M.L.; Pinto, D.C.G.A.; Michalak, I.; Trincone, A.; Mishra, A.P.; Nigam, M.; Zam, W.; Martins, N. Current trends on seaweeds: Looking at chemical composition, phytopharmacology, and cosmetic applications. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef]
- Sharma, H.S.; Fleming, C.; Selby, C.; Rao, J.R.; Martin, T. Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 2014, 26, 465–2014. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babbohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Di Stasio, E.; Van Oosten, M.J.; Silletti, S.; Raimondi, G.; Carrillo, P.; Maggio, A. Ascophyllum nodosum-based algal extracts act as enhancers of growth, fruit quality, and adaptation to stress in salinized tomato plants. J. Appl. Phycol. 2018, 30, 2675–2686. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Ruiz-López, M.A.; Norrie, J.; Hernández-Carmona, G. Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.). J. Appl. Phycol. 2014, 26, 619–2014. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Sangiorgio, D.; Cellini, A.; Spinelli, F.; Donati, I. Promoting strawberry (Fragaria × ananassa) stress resistance, growth, and yield using native bacterial biostimulants. Agronomy 2023, 13, 529. [Google Scholar] [CrossRef]
- Ganhão, R.; Pinheiro, J.; Tino, C.; Faria, H.; Gil, M.M. Characterization of nutritional, physicochemical, and phytochemical composition and antioxidant capacity of three strawberry “Fragaria × ananassa Duch.” cultivars (“Primoris”, “Endurance”, and “Portola”) from western region of Portugal. Foods 2019, 8, 682. [Google Scholar] [CrossRef]
- Spinelli, F.; Fiori, G.; Noferini, M.; Sprocatti, M.; Costa, G. A novel type of seaweed extract as a natural alternative to the use of iron chelates in strawberry production. Sci. Hortic. 2010, 125, 263–269. [Google Scholar] [CrossRef]
- Soltaniband, V.; Brégard, A.; Gaudreau, L.; Dorais, M. Biostimulants promote plant development, crop productivity, and fruit quality of protected strawberries. Agronomy 2022, 12, 1684. [Google Scholar] [CrossRef]
- Bogunovic, I.; Duralija, B.; Gadze, J.; Ivica, K. Biostimulant usage for preserving strawberries to climate damages. Hort. Sci. 2015, 42, 132–140. [Google Scholar] [CrossRef]
- Tomić, J.; Pešaković, M.; Milivojević, J.; Karaklajić-Stajić, Ž. How to improve strawberry productivity, nutrients composition, and beneficial rhizosphere microflora by biofertilization and mineral fertilization? J. Plant Nutr. 2018, 41, 2009–2021. [Google Scholar] [CrossRef]
- Saidimoradi, D.; Ghaderi, N.; Javadi, T. Salinity stress mitigation by humic acid application in strawberry (Fragaria × ananassa Duch.). Sci. Hortic. 2019, 256, 108594. [Google Scholar] [CrossRef]
- Eurosemillas.com. Lo que pide la tierra. Available online: http://www.eurosemillas.com/es/variedades/fresa/item/99-camarosa.html (accessed on 29 June 2023).
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.; de Mello, J. Application and analysis of the Folin Ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Matteazzi, A.; Andreotti, C. Foliar applications of biostimulants promote growth, yield and fruit quality of strawberry plants grown under nutrient limitation. Agronomy 2019, 9, 483. [Google Scholar] [CrossRef]
- Marfa, O.; Caceres, R.; Polo, J.; Rodenas, J. Animal protein hydrolysate as a biostimulant for transplanted strawberry plants subjected to cold stress. Acta Hortic. 2009, 842, 315–318. [Google Scholar] [CrossRef]
- Rahman, M.; Sabir, A.A.; Mukta, J.A.; Khan, M.M.A.; Mohi-Ud-Din, M.; Miah, M.G.; Rahman, M.; Islam, M.T. Plant probiotic bacteria Bacillus and Paraburkholderi improve growth, yield and content of antioxidants in strawberry fruit. Sci. Rep. 2018, 8, 2504. [Google Scholar] [CrossRef]
- Sinclair, G.; Charest, C.; Dalpe, Y.; Khanizadeh, S. Influence of colonization by arbuscular mycorrhizal fungi and a root endophyte on selected strawberry cultivars under salt conditions. Can. J. Plant Sci. 2014, 93, 997–999. [Google Scholar] [CrossRef]
- Alam, M.; Braun, G.; Norrie, J.; Hodges, D. Effect of Ascophyllum extract application on plant growth, fruit yield and soil microbial communities of strawberry. Can. J. Plant Sci. 2013, 93, 23–36. [Google Scholar] [CrossRef]
- Mattner, S.W.; Milinkovic, M.; Arioli, T. Increased growth response of strawberry roots to a commercial extract from Durvillaea potatorum and Ascophyllum nodosum. J. Appl. Phycol. 2018, 30, 2943–2951. [Google Scholar] [CrossRef]
- Ashour, M.; Al-Souti, A.S.; Hassan, S.M.; Ammar, G.A.G.; Goda, A.M.A.-S.; El-Shenody, R.; Abomohra, A.E.-F.; El-Haroun, E.; Elshobary, M.E. Commercial seaweed liquid extract as strawberry biostimulants and bioethanol production. Life 2023, 13, 85. [Google Scholar] [CrossRef]
- El-Miniawy; Ragab, M.E.; Youssef, S.; Metwally, A. Influence of foliar spraying of seaweed extract on growth, yield and quality of strawberry plants. J. Appl. Sci. Res. 2014, 10, 88–94. [Google Scholar]
- Dong, C.; Wang, G.; Du, M.; Niu, C.; Zhang, P.; Zhang, X.; Ma, D.; Ma, F.; Bao, Z. Biostimulants promote plant vigor of tomato and strawberry after transplanting. Sci. Hortic. 2020, 267, 109355. [Google Scholar] [CrossRef]
- Phylgreen. Tradecorp. Bionutrición-Ecológicos. Available online: https://tradecorp.es/producto/phylgreen/ (accessed on 24 October 2023).
- Actisey. Vademecum de Productos Fitosanitarios y Nutricionales España Carlos de Liñán. Portal TecnoAgrícola. Available online: https://www.buscador.portaltecnoagricola.com/vademecum/esp/producto/13411/ACTYSEI (accessed on 24 October 2023).
- Grzebisz, W.; Gransee, A.; Szczepaniak, W.; Diatta, J. The effects of potassium fertilization on water-use efficiency in crop plants. J. Plant Nutr. Soil Sci. 2013, 176, 355–374. [Google Scholar] [CrossRef]
- Sustr, M.; Soukup, A.; Tylova, E. Potassium in root growth and development. Plants 2019, 8, 435. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.F.; Wu, W.H. Potassium and phosphorus transport and signaling in plants. J. Integr. Plant Biol. 2021, 63, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Mosa, W.; Salem, M.; Al-huqail, A.; Ali, H. Application of glycine, folic acid, and moringa extract as biostimulants for enhancing the production of ‘Flame Seedless’ grape cultivar. BioResources 2021, 16, 3391–3410. [Google Scholar] [CrossRef]
- Goñi, O.; Quille, P.; O’Connell, S. Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef]
- Ali, J.; Jan, I.; Ullah, H.; Ahmed, N.; Alam, M.; Ullah, R.; El-Sharnouby, M.; Kesba, H.; Shukry, M.; Sayed, S.; et al. Influence of Ascophyllum nodosum extract foliar spray on the physiological and biochemical attributes of okra under drought stress. Plants 2022, 11, 790. [Google Scholar] [CrossRef]
- Kumari, S.; Sehrawat, K.D.; Phogat, D.; Sehrawat, A.R.; Chaudhary, R.; Sushkova, S.N.; Voloshina, M.S.; Rajput, V.D.; Shmaraeva, A.N.; Marc, R.A.; et al. Ascophyllum nodosum (L.) Le Jolis, a pivotal biostimulant toward sustainable agriculture: A comprehensive review. Agriculture 2023, 13, 1179. [Google Scholar] [CrossRef]
- Blunden, G.; Jenkins, T.; Liu, Y. Enhanced leaf chlorophyll levels in plants treated with seaweed extract. J. Appl. Phycol. 1997, 8, 535543. [Google Scholar] [CrossRef]
- Pohl, A.; Grabowska, A.; Kalisz, A.; Sękara, A. The eggplant yield and fruit composition as affected by genetic factor and biostimulant application. Not. Bot. Horti. Agrobot. 2019, 47, 929–938. [Google Scholar] [CrossRef]
- Carmody, N.; Goñi, O.; Łangowski, Ł.; O’Connell, S. Ascophyllum nodosum extract biostimulant processing and its impact on enhancing heat stress tolerance during tomato fruit set. Front. Plant Sci. 2020, 25, 807. [Google Scholar] [CrossRef]
- De Clercq, P.; Pauwels, E.; Top, S.; Steppe, K.; Van Labeke, M.-C. Effect of seaweed-based biostimulants on growth and development of Hydrangea paniculata under continuous or periodic drought stress. Horticulturae 2023, 9, 509. [Google Scholar] [CrossRef]
- Di Mola, I.; Ottaiano, L.; Cozzolino, E.; Marra, R.; Vitale, S.; Pironti, A.; Fiorentino, N.; Mori, M. Yield and quality of processing tomato as improved by biostimulants based on Trichoderma sp. and Ascophyllum nodosum and biodegradable mulching films. Agronomy 2023, 13, 901. [Google Scholar] [CrossRef]
- Weber, N.; Schmitzer, V.; Jakopic, J.; Stampar, F. First fruit in season: Seaweed extract and silicon advance organic strawberry (Fragaria × ananassa Duch.) fruit formation and yield. Sci. Hortic. 2018, 242, 103–109. [Google Scholar] [CrossRef]
- Roussos, P.; Denaxa, N.-K.; Damvakaris, T. Strawberry fruit quality attributes after application of plant growth stimulating compounds. Sci. Hortic. 2009, 119, 138–146. [Google Scholar] [CrossRef]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant biostimulants: Importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress—A review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants research in some horticultural plant species-A review. Food Energy Secur. 2018, 8, e00162. [Google Scholar] [CrossRef]
- Garza-Alonso, C.A.; Olivares-Sáenz, E.; González-Morales, S.; Cabrera-De la Fuente, M.; Juárez-Maldonado, A.; González-Fuentes, J.A.; Tortella, G.; Valdés-Caballero, M.V.; Benavides-Mendoza, A. Strawberry biostimulation: From mechanisms of action to plant growth and fruit quality. Plants 2022, 11, 3463. [Google Scholar] [CrossRef] [PubMed]
- Righini, H.; Roberti, R.; Baraldi, E. Use of algae in strawberry management. J. Appl. Phycol. 2018, 30, 3551–3564. [Google Scholar] [CrossRef]
- Nanda, S.; Kumar, G.; Hussain, S. Utilization of seaweed-based biostimulants in improving plant and soil health: Current updates and future prospective. Int. J. Environ. Sci. Technol. 2022, 19, 12839–12852. [Google Scholar] [CrossRef]
- Kapur, B.; Sarıdaș, M.A.; Çeliktopuz, E.; Kafkas, E.; Paydaș Kargı, S. Health and taste related compounds in strawberries under various irrigation regimes and bio-stimulant application. Food Chem. 2018, 263, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Actisey. Bioestimulantes Agrícolas. Seipasa. Available online: https://www.seipasa.com/es_ES/extracto-de-algas-actysei/ (accessed on 24 October 2023).
- Martínez-Ferri, E.; Soria, C.; Ariza, M.T.; Medina, J.J.; Miranda, L.; Domíguez, P.; Muriel, J.L. Water relations, growth and physiological response of seven strawberry cultivars (Fragaria × ananassa Duch.) to different water availability. Agric. Water Manag. 2016, 164, 73–82. [Google Scholar] [CrossRef]
- De Saeger, J.; Van Praet, S.; Vereecke, D.; Park, J.; Jacques, S.; Han, T.; Depuydt, S. Toward the molecular understanding of the action mechanism of Ascophyllum nodosum extracts on plants. J. Appl. Phycol. 2020, 32, 573–597. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.H. Cytokinin-containing seaweed and humic acid extracts associated with creeping bentgrass leaf cytokinins and drought resistance. Crop Sci. 2004, 44, 1737–1745. [Google Scholar] [CrossRef]
- Aziz, N.G.A.; Mahgoub, M.H.; Siam, H.S. Growth, flowering and chemical constituents performance of Amaranthus tricolor plants as influenced by seaweed (Ascophyllum nodosum) extract application under salt stress conditions. J. Appl. Sci. Res. 2011, 7, 1472–1484. [Google Scholar]
- Lola-Luz, T.; Hennequart, F.; Gaffney, M. Enhancement of phenolic and flavonoid compounds in cabbage (Brassica oleracea) following application of commercial seaweed extracts of the brown seaweed (Ascophyllum nodosum). Agric. Food Sci. 2013, 22, 288–295. [Google Scholar] [CrossRef]
- Iwamoto, K.; Shiraiwa, Y. Salt-regulated mannitol metabolism in algae. Mar. Biotechnol. 2005, 7, 407–415. [Google Scholar] [CrossRef]
- Badran, E.G.; Abogadallah, G.M.; Nada, R.M.; Nemat Alla, M.M. Role of glycine in improving the ionic and ROS homeostasis during NaCl stress in wheat. Protoplasma 2015, 252, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Hannum, S.M. Potential impact of strawberries on human health: A review of the science. Crit. Rev. Food Sci. Nutr. 2004, 44, 1–17. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).