Effects of Continuous Manure Application on the Microbial Community and Labile Organic Carbon Fractions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. In-Situ Experiment
2.3. Soil Sampling
2.4. Soil Properties
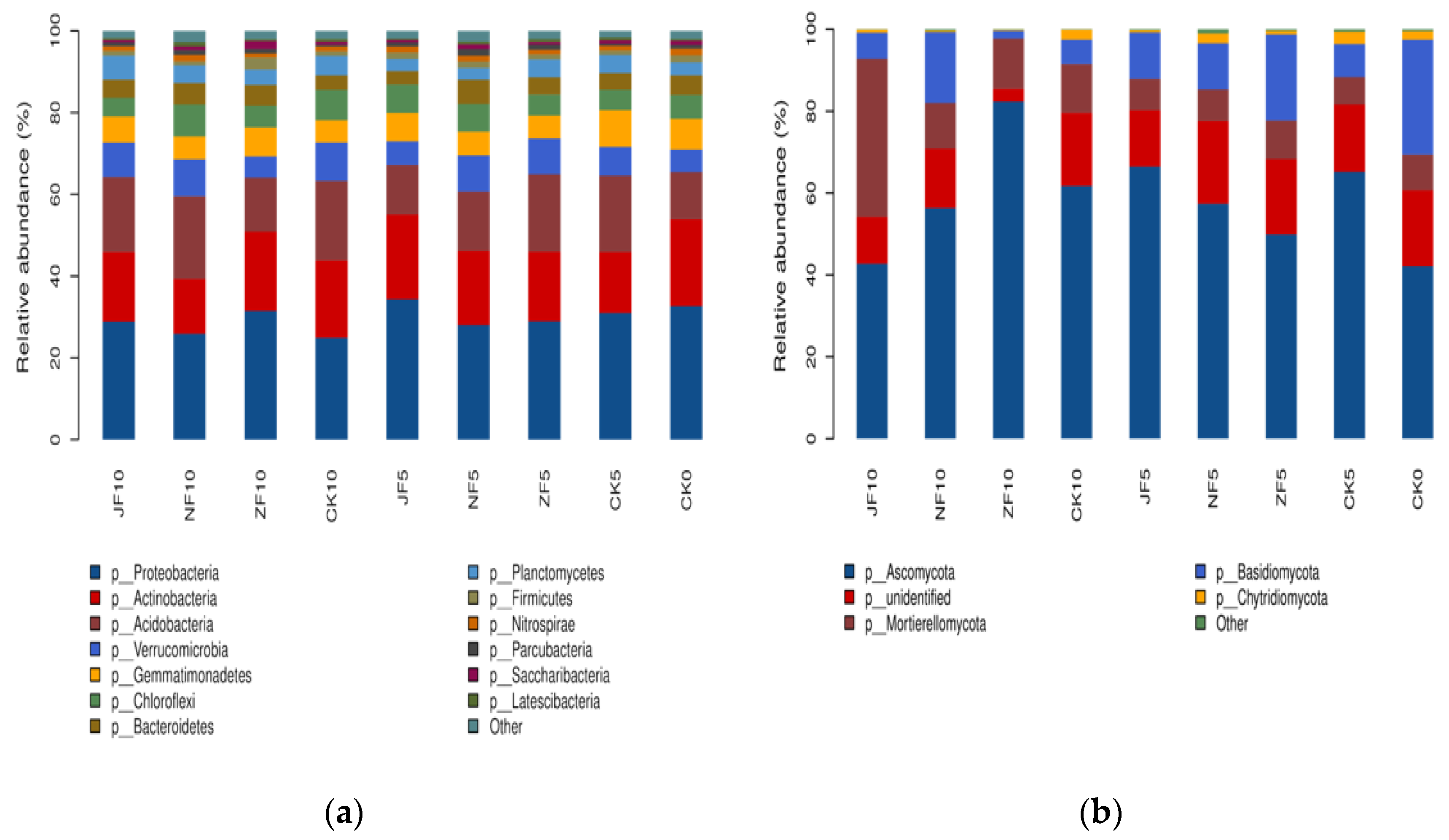
2.5. Microbial Community Structure and Diversity
2.6. Statistical Analysis
3. Results
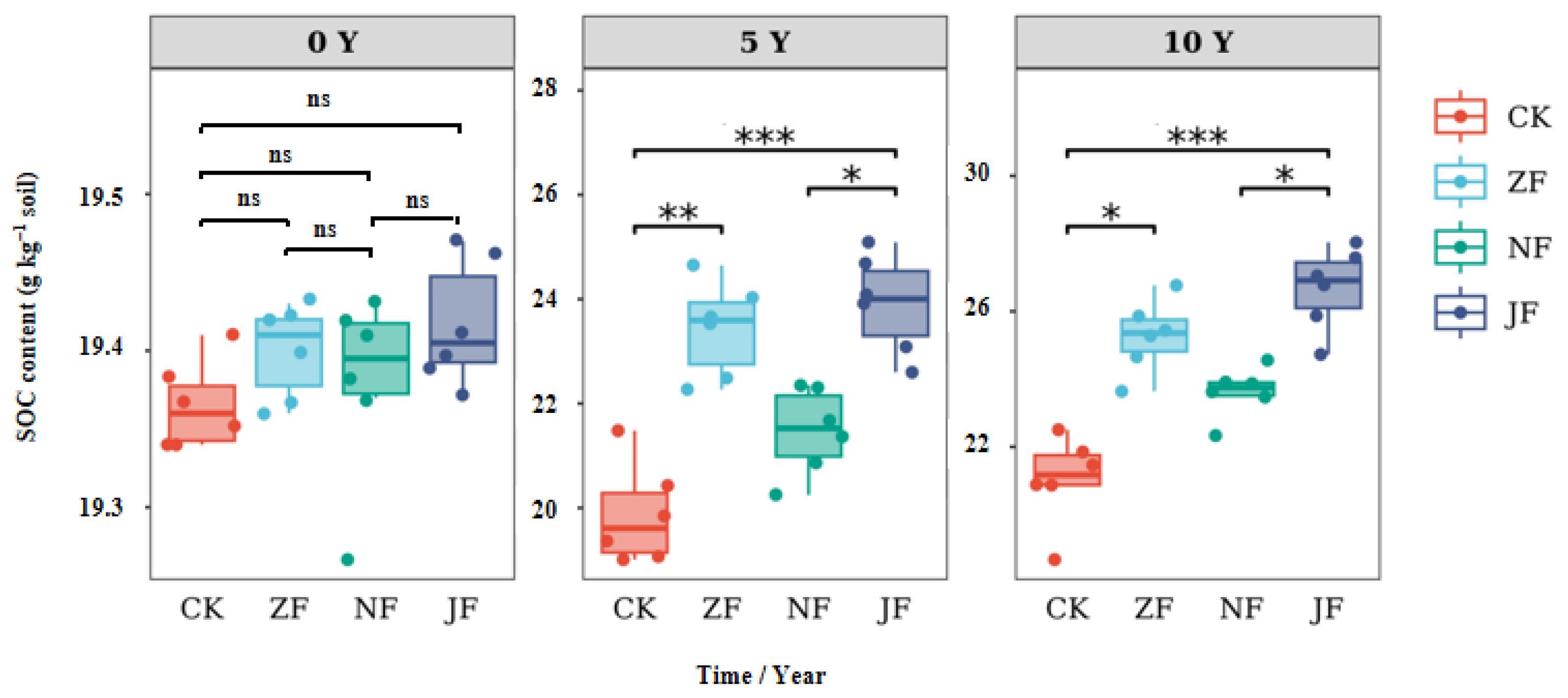
3.1. Changes in Total Soil Organic Carbon
3.2. Changes in Labile Organic Carbon Fractions
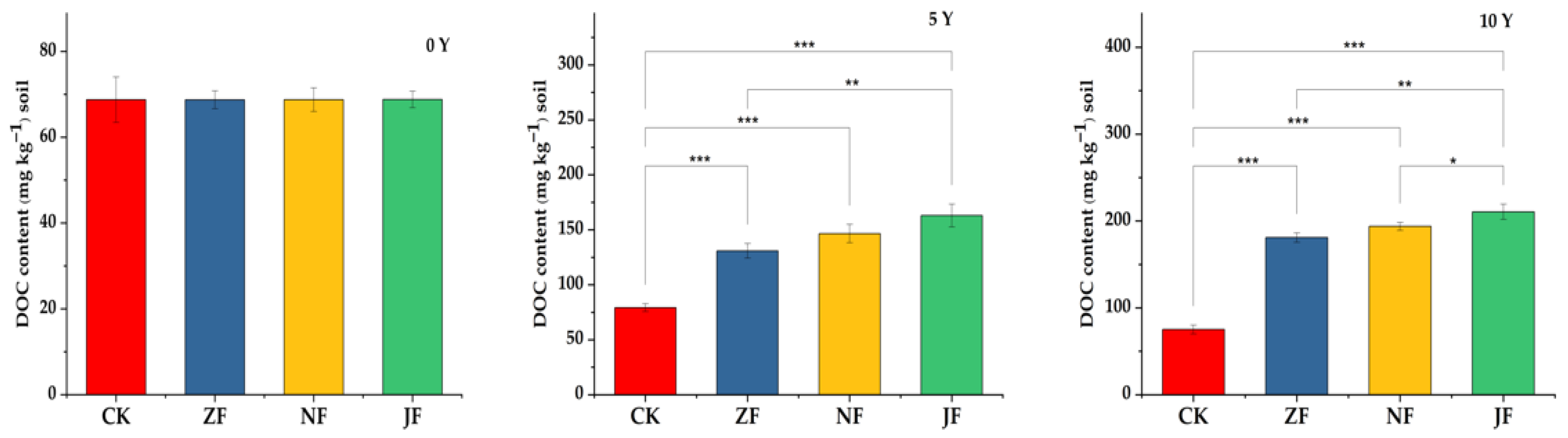
3.2.1. Changes in Dissolved Organic Carbon
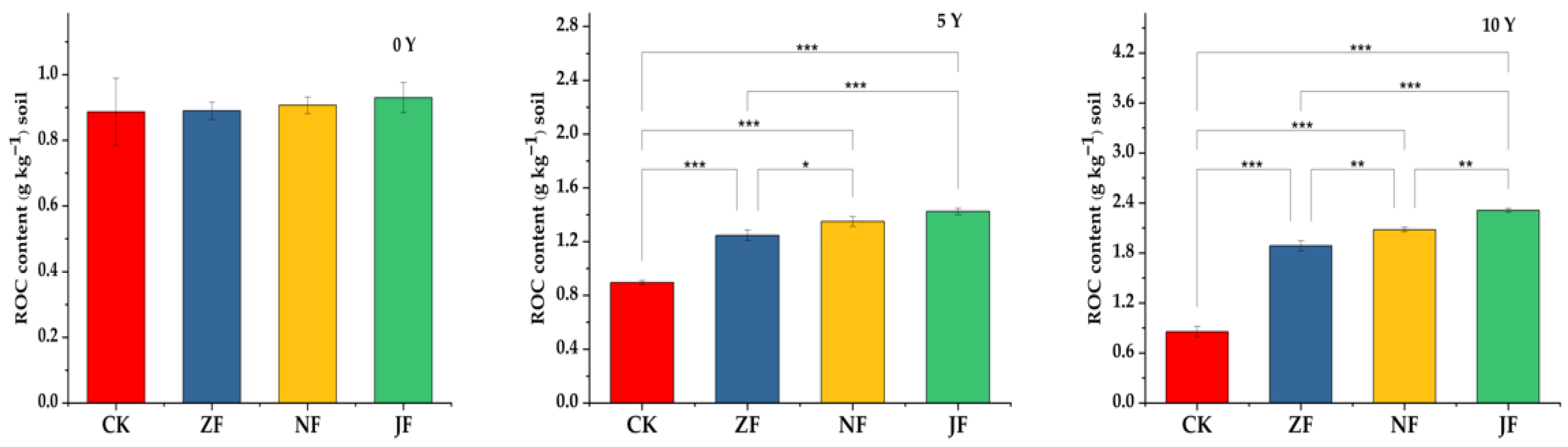
3.2.2. Changes in Readily Oxidizable Organic Carbon
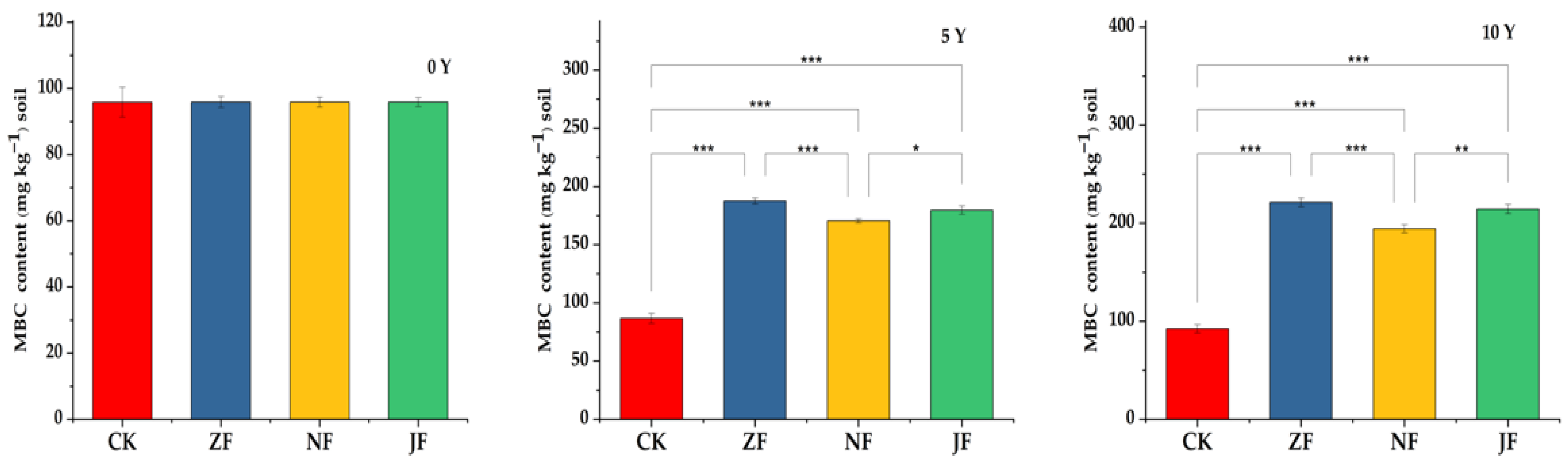
3.2.3. Changes in Microbial Biomass Carbon
3.2.4. Changes in Particulate Organic Carbon
3.3. Soil Microbial Community Diversity
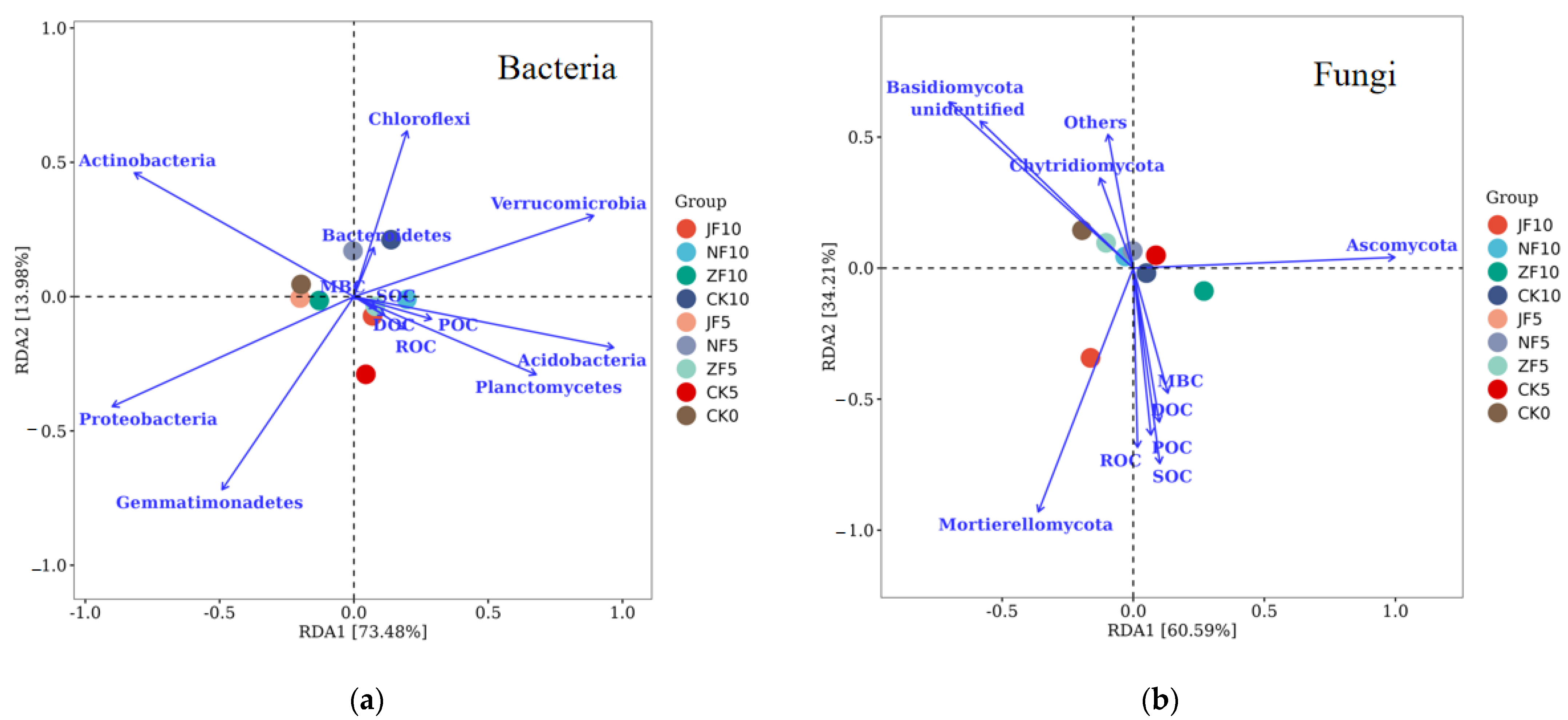
3.4. Relationships between Labile Organic Carbon Fractions and the Soil Microbial Community
4. Discussion
4.1. Effects of Continuous Manure Application on SOC Accumulation
4.2. Effects of Continuous Manure Application on Labile SOC Fractions
4.3. Effects of Soil Microbial Community on Labile SOC Fractions at Different Times
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, X.; Alexandre, J.; Sai, G. Soil protest communities form a dynamic hub in the soil microbiome. ISME J. 2017, 10, 634–638. [Google Scholar]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Liu, B.; Xia, H.; Jiang, C.C.; Muhammad, R.; Yang, L.; Chen, Y.F.; Fan, X.P.; Xia, X.G. 14 year applications of chemical fertilizers and crop straw effects on soil labile organic carbon fractions, enzyme activities and microbial community in rice-wheat rotation of middle China. Sci. Total Environ. 2022, 6, 156608. [Google Scholar] [CrossRef]
- Sul, W.J.; Asuming, B.S.; Wang, Q. Tropical agricultural land management influences on soil microbial communities through its effect on soil organic carbon. Soil Biol. Biochem. 2013, 65, 33–38. [Google Scholar] [CrossRef]
- Wesemael, B. Agricultural management explains historic changes in regional soil carbon stocks. Proc. Natl. Acad. Sci. USA 2010, 107, 14926–14930. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.C.; Wang, M.V.; Hu, S.J. Economics- and policy-driven organic carbon input enhancement dominates soil organic carbon accumulation in Chinese croplands. Proc. Natl. Acad. Sci. USA 2018, 115, 4045–4050. [Google Scholar] [CrossRef] [PubMed]
- Ling, N.; Sun, B.J.; Ma, J.H. Response of the bacterial diversity and soil enzyme activity in particle-size fractions of Mollisol after different fertilization in a long-term experiment. Biol. Fertil. Soils 2014, 50, 901–911. [Google Scholar] [CrossRef]
- Hu, X.; Gu, H.; Liu, J.; Wei, D.; Zhu, P.; Cui, X.; Zhou, B.; Chen, X.; Jin, J.; Liu, X.; et al. Metagenomics reveals divergent functional profiles of soil carbon and nitrogen cycling under long-term addition of chemical and organic fertilizers in the black soil region. Geoderma 2022, 418, 115846. [Google Scholar] [CrossRef]
- Plaza, C.; Courtier-Murias, D.; Fernández, J.M.; Polo, A.; Simpson, A.J. Physical, chemical, and biochemical mechanisms of soil organic matter stabilization under conservation tillage systems: A central role for microbes and microbial by-products in C sequestration. Soil Biol. Biochem. 2013, 57, 124–134. [Google Scholar] [CrossRef]
- Bengtson, P.; Barer, J.; Grayston, S.J. Evidence of a strong coupling between root exudation, C and N availability, and stimulated SOM decomposition caused by rhizosphere priming effects. Ecol. Evol. 2012, 2, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Yuan, J.C.; Wu, J.G.; Cai, H.G. Effects of straw maize on the bacterial community and carbon stability at different soil depths. Agriculture 2023, 13, 1307. [Google Scholar] [CrossRef]
- Guenet, B.; Juarez, S.; Bardoux, G.; Abbadie, L.; Chenu, C. Evidence that stable C is as vulnerable to priming effect as is more labile C in soil. Soil Biol. Biochem. 2012, 52, 43–48. [Google Scholar] [CrossRef]
- He, Y.T.; He, X.H.; Xu, M.G.; Zhang, W.J.; Yang, X.Y.; Huang, S.M. Long-term fertilization increases soil organic carbon and alters its chemical composition in three wheat-maize cropping sites across central and south China. Soil Tillage Res. 2018, 177, 79–87. [Google Scholar] [CrossRef]
- Shi, L.H.; Li, C.; Tang, H.M. Effects of long-term fertilizer management on soil labile organic carbon fractions and hydrolytic enzyme activity under a double-cropping rice system of southern China. Chin. J. Appl. Ecol. 2021, 32, 921–930. (In Chinese) [Google Scholar]
- Frederik, B.; Jakob, M.; Lars, S. Long term Pand K fertilization strategies and balances affect soil availability indices, crop yield depression risk and Nuse. Euro. J. Agron. 2017, 86, 12–23. [Google Scholar]
- Liu, C.; Lu, M.; Cui, J. Effects of straw carbon input on carbon dynamics in agricultural soils: A meta analysis. Glob. Chang. Biol. 2014, 5, 1366–1381. [Google Scholar]
- Ogunniyi, J.E.; Guo, C.; Tian, X.H. The effects of three mineral nitrogen sources and zinc on maize and wheat straw decomposition and soil organic carbon. J. Inte. Agric. 2014, 12, 2768–2777. [Google Scholar]
- Guo, Z.Y.; Wang, J.G.; Wang, Y.F. Rhizosphere isoflavones (daidzein and genistein) levels and their relation to the microbial community structure of mono-cropped soybean soil. Soil Biol. Biochem. 2011, 43, 2257–2264. [Google Scholar] [CrossRef]
- Paul, E.A. The nature and dynamics of soil organic matter: Plant inputs, microbial transformations, and organic matter stabilization. Soil Biol. Biochem. 2016, 98, 109–126. [Google Scholar] [CrossRef]
- Xie, H.; Li, J.; Zhu, P.; Peng, C.; Wang, J.K.; He, H.B.; Zhang, X.D. Long-term manure amendments enhance neutral sugar accumulation in bulk soil and particulate organic matter in a Mollisol. Soil Biol. Biochem. 2014, 78, 45–53. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed.; Natural Resources Conservation Service and USDA: Washington, DC, USA, 1999. [Google Scholar]
- Bao, S.D. Soil Agrochemical Analysis; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Jiang, P.K.; Xu, Q.F.; Xu, Z.H.; Cao, Z.H. Seasonal changes in soil labile organic carbon pools within a Phyllostachys praecox stand under high rate fertilization and winter mulch in subtropical China. For. Ecol. Manag. 2006, 236, 30–36. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Elliott, E.T. Particulate soil organic matter changes across a grassland cultivation sequence. Soil Sci. Soc. Am. J. 1992, 56, 777–783. [Google Scholar] [CrossRef]
- Blair, G.J.; Lefroy, R.D.B.; Lise, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Mi, W.H.; Sun, Y.; Xia, X.Q. Effect of inorganic fertilizers with organic amendments on soil chemical properties and rice yield in a low-productivity paddy soil. Geoderma 2018, 320, 23–29. [Google Scholar] [CrossRef]
- Nobile, C.M.; Bravin, M.N.; Becquer, T. Phosphorus sorption and availability in an andosol after a decade of organic or mineral fertilizer applications: Importance of pH and organic carbon modifications in soil as compared to phosphorus accumulation. Chemosphere 2020, 239, 124709. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Bei, S.K.; Li, B.S. Organic fertilizer, but not heavy liming, enhances banana biomass, increases soil organic carbon and modifies soil microbiota. Appl. Soil Ecol. 2018, 136, 67–79. [Google Scholar] [CrossRef]
- Will, C.; Thürmer, A.; Wollherr, A. Horizon-specific bacterial community composition of german grassland soils, as revealed by pyrosequencing-based analysis of 16S rRNA genes. Appl. Enviro. Microbiol. 2010, 76, 6751–6759. [Google Scholar] [CrossRef]
- Acosta, M.V.; Dowd, S.E.; Sun, Y.; Wester, D. Pyrosequencing analysis for characterization of soil bacterial populations as affected by an integrated livestock-cotton production system. Appl. Soil Ecol. 2010, 45, 13–25. [Google Scholar] [CrossRef]
- Abrar, M.M.; Xu, M.; Shah, S.A.A.; Aslam, M.W.; Aziz, T.; Mustafa, A.; Ashraf, M.N.; Zhou, B.K.; Ma, X.Z. Variations in the profile distribution and protection mechanisms of organic carbon under long-term fertilization in a Chinese Mollisol. Sci. Total Environ. 2020, 723, 138181. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.T.; Li, Z.H.; Wang, S. Effects of in-situ straw decomposition on composition of humus and structure of humic acid at different soil depths. J. Soils Sediments 2017, 17, 2391–2399. [Google Scholar] [CrossRef]
- Banger, K.; Toor, G.S.; Biswas, A.; Sidhu, S.S.; Sudhir, K. Soil organic carbon fractions after 16-years of applications of fertilizers and organic manure in a Typic Rhodalfs in semi-arid tropics. Nut. Cycl. Agroe. 2010, 86, 391–399. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, X.; Liusui, Y.; Han, C.; Zhao, C.; Liu, H. Variation of soil aggregation and intra-aggregate carbon by long-term fertilization with aggregate formation in a grey desert soil. Catena 2017, 10, 437–445. [Google Scholar] [CrossRef]
- Wang, H.B.; Jin, J.; Yu, P.Y.; Fu, W.J.; Morrison, L.; Lin, H.P.; Meng, M.J.; Zhou, X.F.; Lv, Y.L.; Wu, J.S. Converting evergreen broad-leaved forests into tea and Moso bamboo plantations affects labile carbon pools and the chemical composition of soil organic carbon. Sci. Total Environ. 2020, 711, 135225. [Google Scholar] [CrossRef]
- Dou, S.; Shan, J.; Song, X.Y.; Cao, G.R.; Wu, M.; Li, C.L.; Guan, S. Are humic substances soil microbial residues or unique synthesized compounds? A perspective on their distinctiveness. Pedosphere 2020, 30, 159–167. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Dou, S.; Ndzelu, B.S.; Ma, R.; Zhang, D.D.; Zhang, X.W.; Ye, S.F.; Wang, H.R. Effects of returning corn straw and fermented corn straw to fields on the soil organic carbon pools and humus composition. Soil 2022, 8, 605–619. [Google Scholar] [CrossRef]
- Catharine, M.; Pschenyckyj, J.M.; Clark, L.J.; Shaw, R.I.; Griffiths, C.D.E. Effects of acidity on dissolved organic carbon in organic soil extracts, pore water and surface litters. Sci. Total Environ. 2020, 703, 215–221. [Google Scholar]
- Paulina, B.; Ramírez, S.F.A.; Beatriz, D.; Ignacio, V.; Carlos, A.B. Soil microbial community responses to labile organic carbon fractions in relation to soil type and land use along a climate gradient. Soil Biol. Biochem. 2020, 141, 243–254. [Google Scholar]
- Yan, S.S.; Song, J.M.; Fan, J.S.; Yan, C.; Dong, S.K.; Ma, C.M.; Gong, Z.P. Changes in soil organic carbon fractions and microbial community under rice straw return in Northeast China. Global Ecol. Cons. 2020, 22, 111–117. [Google Scholar] [CrossRef]
- Mi, W.H.; Sun, Y.; Gao, Q.; Liu, M.Y.; Wu, L.H. Changes in humus carbon fractions in paddy soil given different organic amendments and mineral fertilizers. Soil Tillage Res. 2019, 195, 201–213. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Liu, X.F.; Wang, Z.L.; Zhao, S.W. Soil organic carbon fractions and sequestration across a 150-yr secondary forest chrono sequence on the Loess Plateau, China. Catena 2015, 133, 367–371. [Google Scholar] [CrossRef]
- Johnson, E.N.; Malhi, S.S.; Hall, L.M.; Phelps, S. Effects of nitrogen fertilizer application on seed yield, N uptake, N use efficiency, and seed quality of Brassica carinata. Cana. J. Plant Sci. 2013, 93, 1073–1081. [Google Scholar] [CrossRef]
- Émilie, M.; Denis, A.A. Carbon accumulates in organo-mineral complexes after long-term liquid dairy manure application. Agric. Ecosyst. Environ. 2015, 202, 108–119. [Google Scholar]
- Li, X.H. Characteristics of Organic Carbon Pool Changes in Black Soil of Continuous Soybean with Different Organic Materials; Jilin Agricultural University: Jilin, China, 2020. [Google Scholar]
- Haoan, L.; Wei, G. Partial substitution of chemical fertilizer with organic amendments affects soil organic carbon composition and stability in a greenhouse vegetable production system. Soil Tillage Res. 2019, 191, 185–196. [Google Scholar]
- Marcote, I.; Hernández, T.; Garcíndez, T. Influence of one or two successive annual applications of organic fertilizers on the enzyme activities of a soil under barley cultivation. Biores. Tech. 2001, 79, 147–154. [Google Scholar] [CrossRef]
- Fugen, D.; Alan, L.W.; Rao, S. Soil enzyme activities and organic matter composition affected by 26 years of continuous cropping. Soil Sci. Soc. 2016, 26, 618–625. [Google Scholar]
- Li, M.L.; Gu, J.; Gao, H. Effect of different organic fertilizers on soybean plant traits, quality and yield. Northwest Univ. Agric. Fores. J. (Nat. Sci. Edit.) 2007, 9, 67–72. [Google Scholar]
- Fukumasu, J.; Liz, J.; Shaw, B. The role of macro-aggregation in regulating enzymatic depolymerization of soil organic nitrogen. Soil Biol. Biochem. 2017, 115, 100–108. [Google Scholar] [CrossRef]
- Cong, P.; Wang, J.; Lia, Y.Y.; Liu, N.N.; Dong, J.X.; Pang, H.C.; Zhang, L.; Gao, Z.J. Changes in soil organic carbon and microbial community under varying straw incorporation strategies. Soil Tillage Res. 2020, 204, 104735. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, W.; Yang, L. Antibiotic resistance genes and bacterial communities in cornfield and pasture soils receiving swine and dairy manure. Environ Pollut. 2019, 248, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Mayrberger, J.M. Studies of Genera Cytophaga-Flavobacterium in Context of the Soil Carbon Cycle. Ph.D. Thesis, Michigan State University, East Lansing, MI, USA, 2011. [Google Scholar]
- Brundrett, M.C.; Ashwath, N. Glomeromycotan mycorrhizal fungi from tropical Australia III Measuring diversity in natural and disturbed habitats. Plant Soil 2013, 370, 419–430. [Google Scholar] [CrossRef]
- Al-Dossary, M.A.; Raheem, S.S.; Almyah, M.K. Molecular identification of five species of family Chaetomiaceae (Sordariomycetes, Ascomycota) from Iraqi soil. Biod. Biol. Diver. 2021, 22, 1277–1284. [Google Scholar] [CrossRef]
- Lopatto, E.; Choi, J.; Colina, A. Characterizing the soil microbiome and quantifying antibiotic resistance gene dynamics in agricultural so il following swine CAFO manure application. PLoS ONE 2019, 14, e0220770. [Google Scholar] [CrossRef]
- Kinnunen, A.; Maijala, P.; Jarvinen, P. Improved Efficiency in Screening for Lignin Modifying Peroxidases and Laccases of Basidiomycetes. Current Biot. 2017, 6, 105–115. [Google Scholar] [CrossRef]

| Soil | pH | SOC (g kg−1) | Total N (g kg−1) | Available P (mg kg−1) | Available K (mg kg−1) | Sand (%) | Silt (%) | Clay (%) |
|---|---|---|---|---|---|---|---|---|
| Mollisol | 6.76 ± 0.19 | 19.37 ± 0.43 | 1.13 ± 0.08 | 33.91 ± 1.12 | 93.12 ± 1.84 | 16.27 ± 0.05 | 68.19 ± 0.92 | 15.54 ± 0.32 |
| Material | pH | Organic Carbon (g·kg−1) | Total N (g·kg−1) | Total P (g·kg−1) | Total K (g·kg−1) | C/N |
|---|---|---|---|---|---|---|
| pig manure | 7.04 ± 0.12 | 267.93 ± 0.48 | 21.14 ± 0.14 | 7.05 ± 0.18 | 8.32 ± 0.13 | 12.67 ± 0.21 |
| cow manure | 7.25 ± 0.08 | 175.41 ± 0.43 | 13.89 ± 0.14 | 3.62 ± 0.12 | 8.37 ± 0.09 | 12.63 ± 0.23 |
| chicken manure | 8.06 ± 0.31 | 242.45 ± 0.51 | 17.05 ± 0.19 | 8.81 ± 0.23 | 14.11 ± 0.22 | 14.21 ± 0.13 |
| Time (year) | Treatments | Bacterial Community | Fungal Community | ||
|---|---|---|---|---|---|
| Chao1 Index | Shannon Index | Chao1 Index | Shannon Index | ||
| 0 | CK | 4647.02 c | 9.67 b | 916.15 c | 3.56 b |
| 5 | CK | 4704.96 c | 9.91 b | 980.02 c | 5.21 d |
| ZF | 4716.14 c | 9.92 b | 1030.53 b | 5.60 c | |
| NF | 4969.69 a | 10.14 a | 1079.36 b | 6.12 b | |
| JF | 4890.91 b | 9.97 ab | 1119.78 a | 6.32 a | |
| 10 | CK | 4893.20 c | 9.96 c | 1019.69 c | 5.43 c |
| ZF | 5014.52 b | 10.00 bc | 1109.31 b | 6.11 b | |
| NF | 5055.35 a | 10.22 a | 1122.65 b | 6.17 b | |
| JF | 5055.70 a | 10.04 b | 1286.15 a | 6.39 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Fan, W.; Wu, J. Effects of Continuous Manure Application on the Microbial Community and Labile Organic Carbon Fractions. Agriculture 2023, 13, 2096. https://doi.org/10.3390/agriculture13112096
Yan H, Fan W, Wu J. Effects of Continuous Manure Application on the Microbial Community and Labile Organic Carbon Fractions. Agriculture. 2023; 13(11):2096. https://doi.org/10.3390/agriculture13112096
Chicago/Turabian StyleYan, Han, Wei Fan, and Jinggui Wu. 2023. "Effects of Continuous Manure Application on the Microbial Community and Labile Organic Carbon Fractions" Agriculture 13, no. 11: 2096. https://doi.org/10.3390/agriculture13112096
APA StyleYan, H., Fan, W., & Wu, J. (2023). Effects of Continuous Manure Application on the Microbial Community and Labile Organic Carbon Fractions. Agriculture, 13(11), 2096. https://doi.org/10.3390/agriculture13112096





