Effect of Different Levels of Calcium and Addition of Magnesium in the Diet on Garden Snails’ (Cornu aspersum) Condition, Production, and Nutritional Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Experimental and Analytical Procedures
2.3. Statistical Analysis
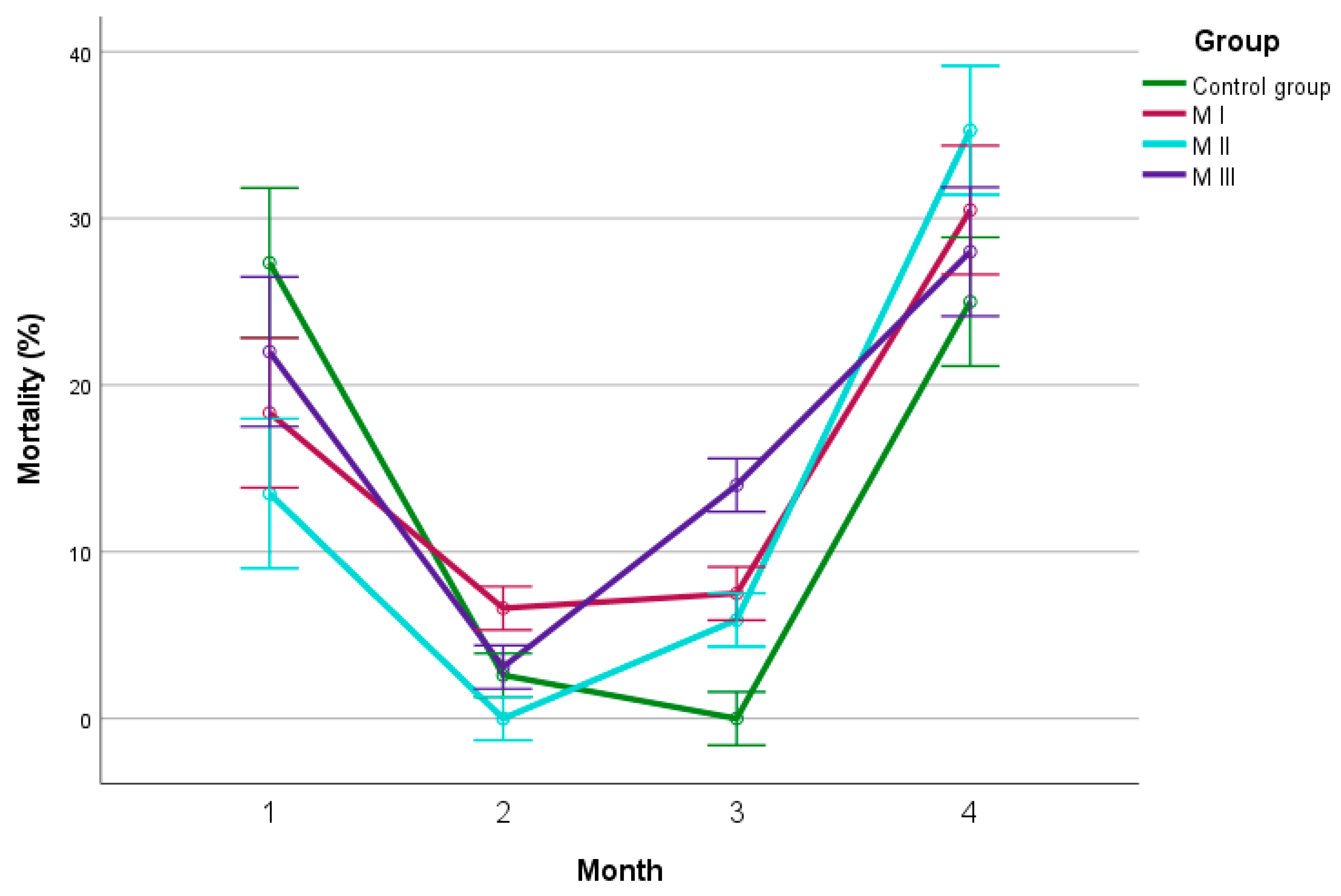
3. Results
4. Discussion
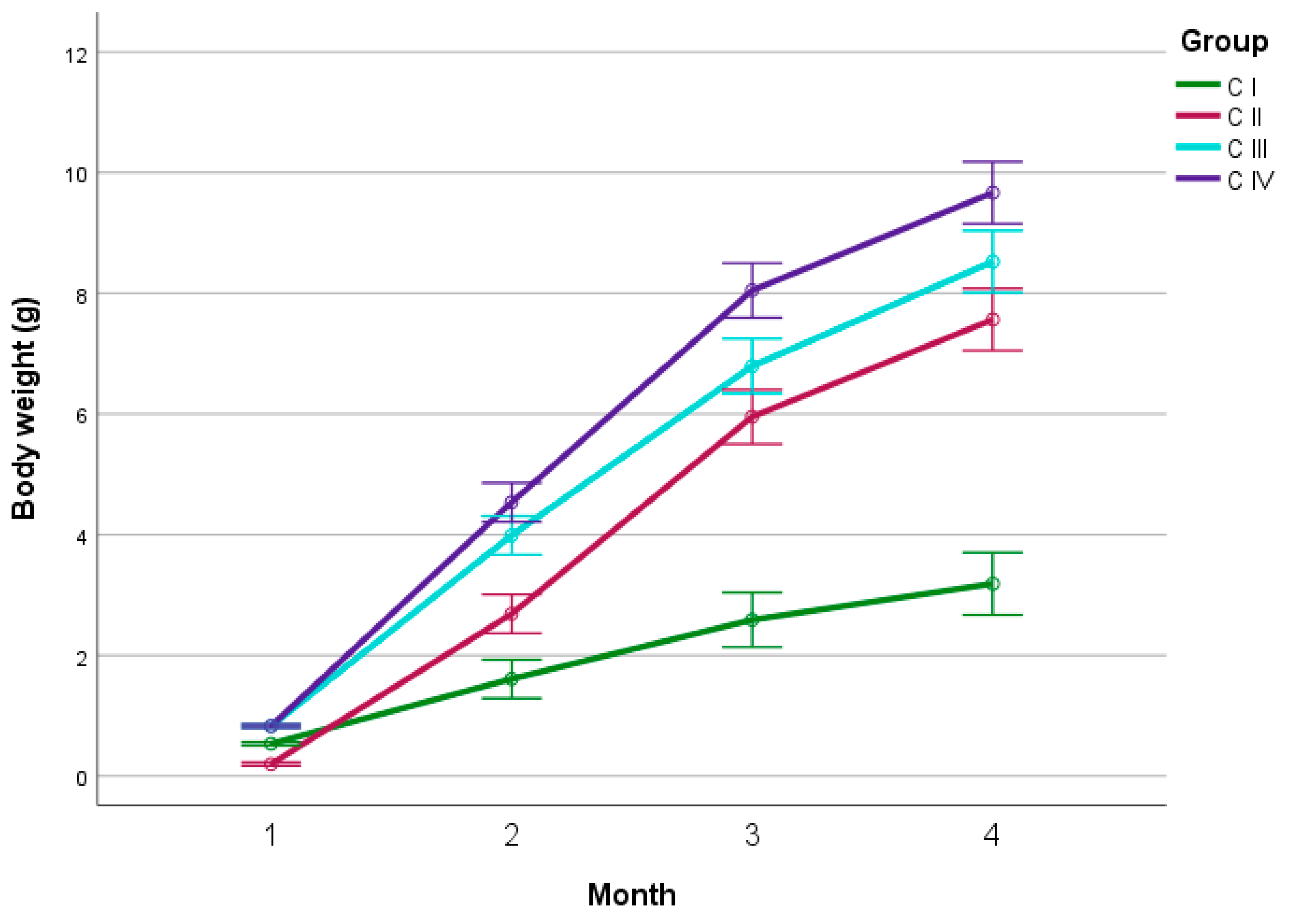
4.1. Growth Rates during Fattening
4.1.1. Experiment I
4.1.2. Experiment II
4.2. Carcass Characteristics
4.2.1. Experiment I
4.2.2. Experiment II
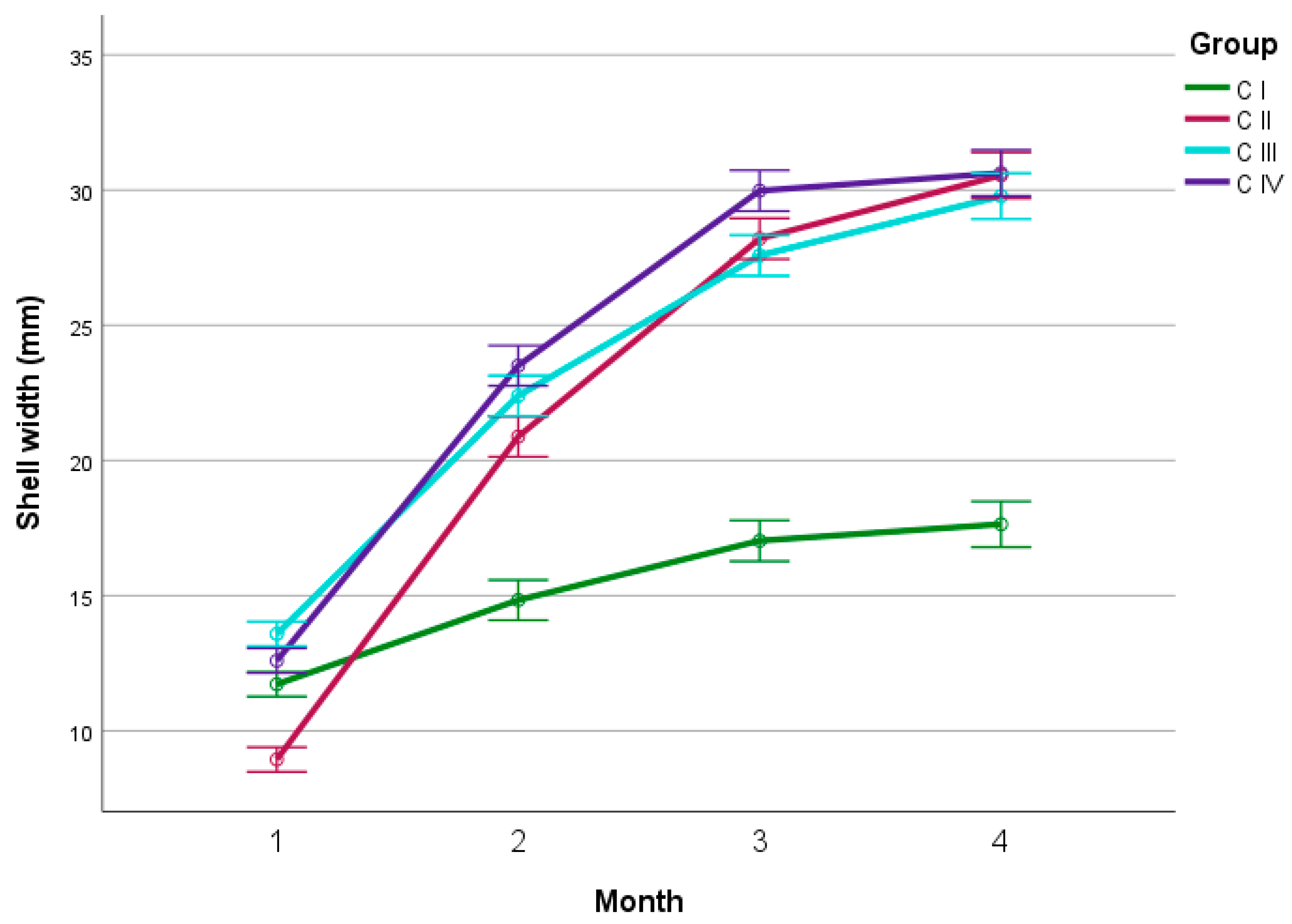
4.3. Characteristics of Shells
4.3.1. Experiment I
4.3.2. Experiment II
4.4. Impact of Ca and Mg
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zucaro, A.; Forte, A.; De Vico, G.; Fierro, A. Environmental Loading of Italian Semi-Intensive Snail Farming System Evaluated by Means of Life Cycle Assessment. J. Clean. Prod. 2016, 125, 56–67. [Google Scholar] [CrossRef]
- Hatziioannou, M.; Issari, A.; Neofitou, C.; Aifadi, S.; Matsiori, S. Economic Analysis and Production Techniques of Snail Farms in Southern Greece. World J. Agric. Res. 2014, 2, 276–279. [Google Scholar] [CrossRef]
- Ogunniyi, L.T. Economic Analysis of Snail Production IN Ibadan, Oyo Stat. Int. J. Agric. Econ. Rural. Dev. 2009, 2, 26–34. [Google Scholar]
- Zagata, L.; Sutherland, L.A. Deconstructing the ‘Young Farmer Problem in Europe’: Towards a Research Agenda. J. Rural Stud. 2015, 38, 39–51. [Google Scholar] [CrossRef]
- Elmslie, L. Snail Collection and Small-Scale Production in Africa and Europe. In Ecological Implications of Minilivestock: Potential of Insects, Rodents, Frogs, and Snails; Paoletti, M.G., Ed.; Science Publishers: New Hampshire, UK, 2005; pp. 93–121. ISBN 9781482294439. [Google Scholar]
- Rygało-Galewska, A.; Zglińska, K.; Niemiec, T. Edible Snail Production in Europe. Animals 2022, 12, 2732. [Google Scholar] [CrossRef]
- Ireland, M.P. The Effect of Dietary Calcium on Growth, Shell Thickness and Tissue Calcium Distribution in the Snail Achatina fulica. Comp. Biochem. Physiol. A Physiol. 1991, 98, 111–116. [Google Scholar] [CrossRef]
- Berillis, P.; Hatziioannou, M.; Panagiotopoulos, N.; Neofitou, C. Similar Shell Features between Rear and Wild Cornu aspersum Snails. World J. Agric. Res. 2013, 1, 1–4. [Google Scholar]
- Murphy, B. Breeding and Growing Snails Commercially in Australia; Rural Industries Research Development Corporation: Wagga Wagga, Australia, 2001; ISBN 0-642-58219-X.
- Lehmann, U.; Hillmer, G. Bezkręgowce Kopalne; Wydawnictwo Geologiczne: Warszawa, Poland, 1991. [Google Scholar]
- Zhang, C.; Zhang, R. Matrix Proteins in the Outer Shells of Molluscs. Mar. Biotechnol. 2006, 8, 572–586. [Google Scholar] [CrossRef]
- Sowiński, G.; Wąsowski, R. Chów Ślimaków. Pielęgnacja, Żywienie, Zarys Chorób z Profilaktyką Oraz Kulinaria; Wydawnictwo Uniwersytetu Warmińsko-Mazurskiego: Olsztyn, Poland, 2000; ISBN 83-88343-40-8. [Google Scholar]
- Ligaszewski, M. Opracowanie Metody Oceny Jakości Produkcji Ślimaków Jadalnych z Gatunku Helix Aspersa z Różnych Warunków Hodowlanych i Środowiskowych na Podstawie Analizy cech Jakościowych I Ilościowych ich Muszli; Instytut Zootechniki Pib: Balice, Poland, 2008. [Google Scholar]
- Fournié, J.; Chétail, M. Calcium Dynamics in Land Gastropods. Am. Zool. 1984, 24, 857–870. [Google Scholar] [CrossRef]
- Pu, F.; Chen, N.; Xue, S. Calcium Intake, Calcium Homeostasis and Health. Food Sci. Hum. Wellness 2016, 5, 8–16. [Google Scholar] [CrossRef]
- Radwińska, J.; Zarczyńska, K. Effects of Mineral Deficiency on the Health of Young Ruminants. J. Elem. 2014, 19, 915–928. [Google Scholar]
- Jaiswal, J.K. Calcium—How and Why? J. Biosci. 2001, 26, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Jatto, O.E.; Asia, I.O.; Medjor, W.E. Proximate and Mineral Composition of Different Species of Snail Shell. Pac. J. Sci. Technol. 2010, 11, 416–419. [Google Scholar]
- Beeby, A.; Richmond, L. Magnesium and the Deposition of Lead in the Shell of Three Populations of the Garden Snail Cantareus aspersus. Environ. Pollut. 2011, 159, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Hotopp, K.P. Land Snails and Soil Calcium in Central Appalachian Mountain Forest. Southeast. Nat. 2002, 1, 27–44. [Google Scholar] [CrossRef]
- Tompa, A.S.; Wilbur, K.M. Calcium Mobilisation during Reproduction in Snail Helix aspersa. Nature 1977, 270, 53–54. [Google Scholar] [CrossRef]
- Emelue, G.U.; Omonzogbe, E.A. Growth Performance of African Giant Land Snails (Archachatina marginata) Fed with Feed Formulated with Different Calcium Sources. Malays. J. Sustain. Agric. 2018, 2, 1–4. [Google Scholar] [CrossRef]
- Voelker, J. Der Chemische Einfluss von Kalzimkarbonat auf Wachstum, Entwicklung und Gehausebau von Achatina fulica Bowdich (Pulmonata). Mitteilungen Hambg. Zool. Mus. Inst. Univ. Hambg. 1959, 57, 37–78. [Google Scholar]
- Chevalier, L.; Desboquois, C.; Papineau, J.; Charrier, M. Influence of Inorganic Compounds on Food Selection by the Brown Garden Snail Cornu aspersum (Müller) (Gastropoda: Pulmonata). J. Moll. Stud. 2000, 66, 61–68. [Google Scholar] [CrossRef]
- Oluokun, J.A.; Omole, A.J.; Fapounda, O. Effect of Increasing the Level of Calcium Supplementation in the Diets of Growing Snail on Performance Characteristics. Res. J. Agric. Biol. Sci. 2005, 1, 76–79. [Google Scholar]
- Gouveia, A.R.; Pearce-Kelly, P.; Quicke, D.L.J.; Leather, S.R. Effects of Different Calcium Concentrations Supplemented on the Diet of Partula gibba on Their Morphometric Growth Parameters, Weight and Reproduction Success. Malacologia 2011, 54, 139–146. [Google Scholar] [CrossRef]
- Marin, F.; Roy, N.L.; Marie, B. The Formation and Mineralization of Mollusk Shell. Front. Biosci. 2012, 4, 1099–1125. [Google Scholar] [CrossRef] [PubMed]
- Roinel, N.; Morel, F.; Istin, M. Etude des Granules Calcifiés du Manteau des Lamellibranches à L’aide de la Microsonde Électronique. Calcif. Tissue Res. 1973, 11, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Fleury, C.; Marin, F.; Marie, B.; Luquet, G.; Thomas, J.; Josse, C.; Serpentini, A.; Lebel, J.M. Shell Repair Process in the Green Ormer Haliotis tuberculata: A Histological and Microstructural Study. Tissue Cell 2008, 40, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Simkiss, K.; Watkins, B. The Influence of Gut Microorganisms on Zinc Uptake in Helix aspersa. Environ. Pollut. 1990, 66, 263–271. [Google Scholar] [CrossRef]
- Ireland, M.P.; Marigomez, I. The Influence of Dietary Calcium on the Tissue Distribution of Cu, Zn, Mg & P and Histological Changes in the Digestive Gland Cells of the Snail Achatina fulica Bowdichm. J. Molluscan Stud. 1992, 58, 157–168. [Google Scholar] [CrossRef]
- Kouassi Kouadio, D.; Jean-Baptiste, A.; Mamadou, K. Growth Performance of Archachatina marginata Bred on the Substrate Amended with Industrial Calcium: Mikhart. Int. J. Sci. Res. 2016, 5, 582–586. [Google Scholar] [CrossRef]
- Aman, J.B.; Adou, C.F.D.; Karamoko, M.; Otchoumou, A. Effect of Source and Amendment Rate of Rearing Substrate on the Growth and Yield of Archachatina marginata. J. Res. Ecol. 2019, 7, 2546–2554. [Google Scholar]
- Crowell, H.H. Laboratory Study of Calcium Requirements of the Brown Garden Snail, Helix aspersa Müller. J. Molluscan Stud. 1973, 40, 491–503. [Google Scholar] [CrossRef]
- Wacker, A.; Baur, B. Effects of Protein and Calcium Concentrations of Artificial Diets on the Growth and Survival of the Land Snail Arianta arbustorum. Invertebr. Reprod. Dev. 2004, 46, 47–53. [Google Scholar] [CrossRef]
- Tchakounte, F.M.; Kana, J.R.; Azine, P.C.; Meffowoet, C.P.; Djuidje, V.P. Growth and Reproductive Performances of African Giant Snail (Archachatina marginata) as Affected by Dietary Calcium Levels. Sci. J. Vet. Adv. 2019, 8, 263–271. [Google Scholar]
- Otchoumou, A.; Dupont-Nivet, M.; Dosso, H. Effects of Diet Quality and Dietary Calcium on Reproductive Performance in Archachatina ventricosa (Gould 1850), Achatinidae, under Indoor Rearing Conditions. Invertebr. Reprod. Dev. 2012, 56, 14–20. [Google Scholar] [CrossRef]
- Lobo, H.L.; Santos, A.G. Níveis de Cálcio em Rações para Escargot (Cornu aspersum). Bachelor’s Thesis, Federal University of São João del-Rei, São João del-Rei, Brazil, 2017; p. 32. [Google Scholar]
- Drapała, T. Chemia Ogólna Nieorganiczna; Pwn: Warszawa, Poland, 1986. [Google Scholar]
- Karmanska, A.; Stanczak, A.; Karwowski, B. Magnez Aktualny Stan Wiedzy. Bromatol. Chem. Toksykol. 2015, 48, 677–689. [Google Scholar]
- Rubin, H. Central Role for Magnesium in Coordinate Control of Metabolism and Growth in Animal Cells. Proc. Natl. Acad. Sci. USA 1975, 72, 3551–3555. [Google Scholar] [CrossRef] [PubMed]
- Banai, S.; Haggroth, L.; Epstein, S.E.; Casscells, W. Influence of Extracellular Magnesium on Capillary Endothelial Cell Proliferation and Migration. Circ. Res. 1990, 67, 645–650. [Google Scholar] [CrossRef]
- Wolf, F.I.; Cittadini, A. Magnesium in Cell Proliferation and Differentiation. Front. Biosci. 1999, 4, 607–617. [Google Scholar] [CrossRef]
- Maier, J.A.M.; Bernardini, D.; Rayssiguier, Y.; Mazur, A. High Concentrations of Magnesium Modulate Vascular Endothelial Cell Behaviour In Vitro. Biochim. Biophys. Acta-Mol. Basis Dis. 2004, 1689, 6–12. [Google Scholar] [CrossRef]
- Chandra, A.K.; Sengupta, P.; Goswami, H.; Sarkar, M. Effects of Dietary Magnesium on Testicular Histology, Steroidogenesis, Spermatogenesis and Oxidative Stress Markers in Adult Rats. Indian J. Exp. Biol. 2013, 51, 37–47. [Google Scholar]
- Gaál, K.K.; Sáfár, O.; Gulyás, L.; Stadler, P. Magnesium in Animal Nutrition. J. Am. Coll. Nutr. 2004, 23, 754s–757s. [Google Scholar] [CrossRef]
- Cowan, J.A. Introduction to the Biological Chemistry of Magnesium Ion. In The Biological Chemistry of Magnesium; Cowan, J.A., Ed.; Vch Publishers: New York, NY, USA, 1995; pp. 1–23. ISBN 1560816279. [Google Scholar]
- Rubin, H. The Logic of the Membrane, Magnesium, Mitosis (MMM) Model for the Regulation of Animal Cell Proliferation. Arch. Biochem. Biophys. 2007, 458, 16–23. [Google Scholar] [CrossRef]
- Vink, R.; Cernak, I. Regulation of Intracellular Free Magnesium in Central Nervous System Injury. Front. Biosci. 2000, 5, A541. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, K.; Kocot, J.; Horecka, A. The Biochemistry of Magnesium. J. Elementol. 2010, 15, 601–616. [Google Scholar] [CrossRef]
- Cefaratti, C.; Romani, A.; Scarpa, A. Differential Localization and Operation of Distinct Mg2+ Transporters in Apical and Basolateral Sides of Rat Liver Plasma Membrane. J. Biol. Chem. 2000, 275, 3772–3780. [Google Scholar] [CrossRef]
- Garfinkel, D.; Garfinkel, L. Magnesium and Regulation of Carbohydrate Metabolism at the Molecular Level. Magnesium 1988, 7, 249–261. [Google Scholar]
- Tammaro, P.; Smith, A.L.; Crowley, B.L.; Smirnov, S.V. Modulation of the Voltage-Dependent K+ Current By Intracellular Mg2+ in Rat Aortic Smooth Muscle Cells. Cardiovasc. Res. 2005, 65, 387–396. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shi, J.; Krishnamoorthy, G.; Yang, Y.; Hu, L.; Chaturvedi, N.; Harilal, D.; Qin, J.; Cui, J. Mechanism of Magnesium Activation of Calcium-Activated Potassium Channels. Nature 2002, 418, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Agus, Z.S.; Morad, M. Modulation of Cardiac Ion Channels by Magnesium. Annu. Rev. Physiol. 1991, 53, 299–307. [Google Scholar] [CrossRef]
- Mcdonald, P.; Edwards, R.A.; Greenhalgh, J.F.D.; Morgan, C.A.; Sinclair, L.A.; Wilkinson, R.G. Animal Nutrition, 7th ed.; Longman Scientific and Technical: Madison, WI, USA, 2011; ISBN 9781408204238. [Google Scholar]
- Horky, P.; Sochor, J.; Skladanka, J.; Klusonova, I.; Nevrkla, P. Effect of Selenium, Vitamins E and C on Antioxidant Potential and Quality of Boar Ejaculate. J. Anim. Feed Sci. 2016, 25, 29–36. [Google Scholar] [CrossRef]
- Freitag, J.J.; Martin, K.J.; Conrades, M.B.; Bellorin-Font, E.; Teitelbaum, S.; Klahr, S.; Slatopolsky, E. Evidence for Skeletal Resistance to Parathyroid Hormone in Magnesium Deficiency: Studies in Isolated Perfused Bone. J. Clin. Investig. 1979, 64, 1238–1244. [Google Scholar] [CrossRef]
- Schlingmann, K.P.; Gudermann, T. A Critical Role of TRPM Channel-Kinase for Human Magnesium Transport. J. Physiol. 2005, 566, 301–308. [Google Scholar] [CrossRef]
- Monteilh-Zoller, M.K.; Hermosura, M.C.; Nadler, M.J.S.; Scharenberg, A.M.; Penner, R.; Fleig, A. TRPM7 Provides an Ion Channel Mechanism for Cellular Entry of Trace Metal Ions. J. Gen. Physiol. 2003, 121, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Okorodudu, A.O.; Yang, H.; Elghetany, M.T. Ionized Magnesium in the Homeostasis of Cells: Intracellular Threshold for Mg in Human Platelets. Clin. Chim. Acta 2001, 303, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Féray, J.C.; Garay, R. A One-to-One Mg2+:Mn2+ Exchange in Rat Erythrocytes. J. Biol. Chem. 1987, 262, 5763–5768. [Google Scholar] [CrossRef] [PubMed]
- Goytain, A.; Quamme, G.A. Functional Characterization of the Human Solute Carrier, SLC41A2. Biochem. Biophys. Res. Commun. 2005, 330, 701–705. [Google Scholar] [CrossRef]
- Schmitz, C.; Perraud, A.-L.; Johnson, C.O.; Inabe, K.; Smith, M.K.; Penner, R.; Kurosaki, T.; Fleig, A.; Scharenberg, A.M. Regulation of Vertebrate Cellular Mg2+ Homeostasis by TRPM7. Cell 2003, 114, 191–200. [Google Scholar] [CrossRef]
- Matsuda, H. Magnesium Gating of the Inwardly Rectifying K+ Channel. Annu. Rev. Physiol. 1991, 53, 289–298. [Google Scholar] [CrossRef]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef]
- Shils, M.E. Experimental Human Magnesium Depletion. Medicine 1969, 48, 61–85. [Google Scholar] [CrossRef]
- Whang, R.; Flink, E.B.; Dyckner, T.; Wester, P.O.; Aikawa, J.K.; Ryan, M.P. Magnesium Depletion as a Cause of Refractory Potassium Repletion. Arch. Intern. Med. 1985, 145, 1686–1689. [Google Scholar] [CrossRef]
- Chang, C.; Bloom, S. Interrelationship of Dietary Mg Intake and Electrolyte Homeostasis in Hamsters: I. Severe Mg Deficiency, Electrolyte Homeostasis, and Myocardial Necrosis. J. Am. Coll. Nutr. 1985, 4, 173–185. [Google Scholar] [CrossRef]
- Goytain, A.; Quamme, G.A. Functional Characterization of ACDP2 (Ancient Conserved Domain Protein), a Divalent Metal Transporter. Physiol. Genom. 2005, 22, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Toba, Y.; Kajita, Y.; Masuyama, R.; Takada, Y.; Suzuki, K.; Aoe, S. Nutrient Metabolism Dietary Magnesium Supplementation Affects Bone Metabolism and Dynamic Strength of Bone in Ovariectomized Rats. J. Nutr. 2000, 130, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Clark, I. Effects of Magnesium Ions on Calcium and Phosphorus Metabolism. Am. J. Physiol. 1968, 214, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Scanlan, B.J.; Tuft, B.; Elfrey, J.E.; Smith, A.; Zhao, A.; Morimoto, M.; Chmielinska, J.J.; Tejero-Taldo, M.I.; Mak, I.T.; Weglicki, W.B.; et al. Intestinal Inflammation Caused by Magnesium Deficiency Alters Basal and Oxidative Stress-Induced Intestinal Function. Mol. Cell. Biochem. 2007, 306, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Gunther, T.; Hollriegl, V.; Vormann, J.; Bubeck, J.; Classen, H.G. Increased Lipid Peroxidation in Rat Tissues by Magnesium Deficiency and Vitamin E Depletion. Magnes. Bull. 1994, 16, 38–43. [Google Scholar]
- Rude, R.K.; Gruber, H.E.; Wei, L.Y.; Frausto, A.; Mills, B.G. Magnesium Deficiency: Effect on Bone and Mineral Metabolism In the Mouse. Calcif. Tissue Int. 2003, 72, 32–41. [Google Scholar] [CrossRef]
- Rude, R.K.; Kirchen, M.E.; Gruber, H.E.; Stasky, A.A.; Meyer, M.H. Magnesium Deficiency Induces Bone Loss in the Rat. Miner. Electrolyte Metab. 1998, 24, 314–320. [Google Scholar] [CrossRef]
- Lipiński, K.; Stasiewicz, M.; Purwin, C.; Zuk-Gołaszewska, K. Effects of Magnesium on Pork Quality. J. Elem. 2011, 16, 325–337. [Google Scholar] [CrossRef]
- Vormann, J.; Günther, T.; Höllriegl, V.; Schumann, K. Pathobiochemical Effects of Graded Magnesium Deficiency in Rats. Z. Ernahrungswissenschaft 1998, 37 (Suppl. S1), 92–97. [Google Scholar]
- Quamme, G.A.; De Rouffignac, C. Epithelial Magnesium Transport and Regulation by the Kidney. Front. Biosci. 2000, 5, D694–D711. [Google Scholar] [CrossRef]
- Elin, R.J. Magnesium: The Fifth but Forgotten Electrolyte. Am. J. Clin. Pathol. 1994, 102, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Weaver, V.M.; Welsh, J. 1,25-Dihydroxycholecalciferol Supplementation Prevents Hypocalcemia in Magnesium-Deficient Chicks. J. Nutr. 1993, 123, 764–771. [Google Scholar] [CrossRef]
- Yoshimura, T.; Tamenori, Y.; Kawahata, H.; Suzuki, A. Fluctuations of Sulfate, S-Bearing Amino Acids and Magnesium in a Giant Clam Shell. Biogeosciences 2014, 11, 3881–3886. [Google Scholar] [CrossRef]
- Gomot, A.; Gomot, L.; Boukraa, S.; Bruckert, S. Influence of Soil on the Growth of the Land Snail Helix aspersa. An Experimental Study of the Absorption Route for the Stimulating Factors. J. Molluscan Stud. 1989, 55, 1–7. [Google Scholar] [CrossRef]
- Ligaszewski, M.; Pol, P. Wybrane Zagadnienia z Dziedziny Helikultury; Zespół Wydawnictw i Poligrafii iz Pib: Kraków, Poland, 2019; ISBN 9788376073927. [Google Scholar]
- Mayaki, O.M.; Ozumba, A.U.; Aderele, A.A.; Daramola, A.O. Effect of Sources of Fibre on Performance of Growing Snail. Niger. Food J. 2013, 31, 28–32. [Google Scholar] [CrossRef]
- Sampelayo, M.R.S.; Fonolla, J.; Extremera, F.G. Factors Affecting the Food Intake, Growth and Protein Utilization in the Helix Aspersa Snail. Protein Content of the Diet and Animal Age. Lab. Anim. 1991, 25, 291–298. [Google Scholar] [CrossRef]
- Bonnet, J.-C.; Vrillon, J.-L.; Aupinel, P. L’escargot Helix aspersa; Inra: Paris, France, 1992; ISBN 9782759201976. [Google Scholar]
- García, A.; Perea, J.; Martín, R.; Acero, R.; Mayoral, A.; Peña, F.; Luque, M. Effect of Two Diets on the Growth of the Helix aspersa Müller During the Juvenile Stage. In Proceedings of the 56th Annual Meeting EAAP, Uppsala, Sweden, 5–8 June 2005; pp. 1–9. [Google Scholar]
- Cameron, R.A.D.; Carter, M.A. Intra- and Interspecific Effects of Population Density on Growth and Activity in Some Helicid Land Snails (Gastropoda: Pulmonata). J. Anim. Ecol. 1979, 48, 237. [Google Scholar] [CrossRef]
- Staikou, A.; Dimitriadou, L. Effect of Crowding on Growth and Mortality in the Edible Snail Helix lucorum (Gastropoda: Pulmonata) in Greece. Isr. J. Zool. 1989, 36, 1–9. [Google Scholar]
- Guo, Y.; Zhang, G.; Yuan, J.; Nie, W. Effects of Source and Level of Magnesium and Vitamin E on Prevention of Hepatic Peroxidation and Oxidative Deterioration of Broiler Meat. Anim. Feed Sci. Technol. 2003, 107, 143–150. [Google Scholar] [CrossRef]
- Lee, S.; Britton, W.M. Magnesium Toxicity: Effect on Phosporus Utilization by Broiler Chicks. Poult. Sci. 1980, 59, 1989–1994. [Google Scholar] [CrossRef]
- Hess, J.; Britton, W. Effects of Dietary Magnesium Excess in White Leghorn Hens. Poult Sci 1997, 76, 703–710. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists; AOAC Intl.: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Uchiyama, M.; Mihara, M. Determination of Malonaldehyde Precursor in Tissues by Thiobarbituric Acid Test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Chevallier, H. La Variabilité de L’escargot Petit-Gris Hélix aspersa Muller. Bull. Muséum Natl. D’histoibe Nat. 1977, 3, 425–442. [Google Scholar]
- Cooke, A.S. Shell Thinning in Avian Eggs by Environmental Pollutants. Environ. Pollut. 1973, 4, 85–152. [Google Scholar] [CrossRef]
- Ligaszewski, M.; Surówka, K.; Stekla, J. The Shell Features of Cornu aspersum (Synonym Helix aspersa) And Helix pomatia: Characteristics and Comparison. Am. Malacol. Bull. 2009, 27, 173–181. [Google Scholar] [CrossRef]
- Fournie, J.; Chetail, M. Evidence for a Mobilization of Calcium Reserves for Reproduction Requirements in Deroceras reticulatum (Syn: Agriolimax reticulatus) (Gastropoda: Pulmonata). Malacologia 1980, 22, 285–291. [Google Scholar]
- Cowie, R.H.; Cain, A.J. Laboratory Maintenance and Breeding of Land Snails, with an Example from Helix aspersa. J. Molluscan Stud. 1983, 49, 176–177. [Google Scholar] [CrossRef]
- Dan, N.A. Studies on the Growth and Ecology of Helix asposa Muller. Ph.D. Thesis, University of Manchester, Manchester, UK, 1978. [Google Scholar]
- Milinsk, M.C.; Padre, R.D.G.; Hayashi, C.; De Oliveira, C.C.; Visentainer, J.V.; De Souza, N.E.; Matsushita, M. Effects of Feed Protein and Lipid Contents on Fatty Acid Profile of Snail (Helix aspersa maxima) Meat. J. Food Compos. Anal. 2006, 19, 212–216. [Google Scholar] [CrossRef]
- Desbuquois, C. Influence of Egg Cannibalism on Growth, Survival and Feeding in Hatchlings of the Land Snail Helix aspersa Müller (Gastropoda, Pulmonata, Stylommatophora). Reprod. Nutr. Dev. 1997, 37, 191–202. [Google Scholar] [CrossRef]
- Suttle, N. Mineral Nutrition of Livestock; Suttle, N., Ed.; Cabi: Cambridge, UK, 2010; ISBN 9781845934736. [Google Scholar]
- Srinivasan, V.; Bhavan, P.S.; Rajkumar, G.; Satgurunathan, T.; Muralisankar, T. Dietary Supplementation of Magnesium Oxide (MgO) Nanoparticles for Better Survival and Growth of the Freshwater Prawn Macrobrachium rosenbergii Post-Larvae. Biol. Trace Elem. Res. 2017, 177, 196–208. [Google Scholar] [CrossRef]
- Jahan, I.; Reddy, A.K.; Sudhagar, S.A.; Harikrishna, V.; Singh, S.; Varghese, T.; Srivastava, P.P. The Effect of Fortification of Potassium and Magnesium in the Diet and Culture Water on Growth, Survival and Osmoregulation of Pacific White Shrimp, Litopenaeus vannamei Reared in Inland Ground Saline Water. Turk. J. Fish Aquat. Sci. 2018, 18, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Wacker, A. Lipids in the Food of a Terrestrial Snail. Invertebr. Reprod. Dev. 2005, 47, 205–212. [Google Scholar] [CrossRef]
- Egonmwan, R.I. Effects of Dietary Calcium on Growth and Oviposition of the African Land Snail Limicolaria flammea (Pulmonata: Achatinidae). Rev. Biol. Trop. 2008, 56, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Jess, S.; Marks, R.J. Population Density Effects on Growth in Culture of the Edible Snail Helix aspersa Var. Maxima. J. Molluscan Stud. 1995, 61, 313–323. [Google Scholar] [CrossRef]
- Daguzan, J.; Bonnet, J.C.; Perrin, Y.; Perrin, E.; Rouet, H. Contribution à L’élevage de L’escargot Petit-Gris: Helix aspersa Müller (Mollusque Gastéropode Pulmoné Stylommatophore). I.—Reproduction et Éclosion des Jeunes, en Bâtiment et en Conditions Thermohygrométriques Contrôlées. Ann. Zootech. 1981, 30, 249–272. [Google Scholar] [CrossRef]
- Milinsk, M. Influence of Diets Enriched with Different Vegetable Oils on the Fatty Acid Profiles of Snail Helix aspersa maxima. Food Chem. 2003, 82, 553–558. [Google Scholar] [CrossRef]
- Nicolai, A.; Filser, J.; Lenz, R.; Bertrand, C.; Charrier, M. Adjustment of Metabolite Composition in the Haemolymph to Seasonal Variations in the Land Snail Helix pomatia. J. Comp. Physiol. B 2011, 181, 457–466. [Google Scholar] [CrossRef]
- Dimitriadis, V.K.; Domouhtsidou, G.P. Carbohydrate Cytochemistry of the Intestine and Salivary Glands of the Snail Helix lucorum: Effect of Starvation and Hibernation. J. Molluscan Stud. 1995, 61, 215–224. [Google Scholar] [CrossRef]
- Baby, R.L.; Hasan, I.; Kabir, K.A.; Naser, M.N. Nutrient Analysis of Some Commercially Important Molluscs of Bangladesh. J. Sci. Res. 2010, 2, 390–396. [Google Scholar] [CrossRef]
- Çağıltay, F. Amino Acid, Fatty Acid, Vitamin and Mineral Contents of the Edible Garden Snail (Helix aspersa). J. Fishscicom. 2011, 5, 354–363. [Google Scholar] [CrossRef]
- Mikkelsen, F.F.; Weber, R.E. Oxygen Transport and Hemocyanin Function in the Pulmonate Land Snail, Helix pomatia: Physiological and Molecular Implications of Polyphasic Oxygen-Binding Curves. Physiol. Zool. 1992, 65, 1057–1073. [Google Scholar] [CrossRef]
- Tufts, E.V.; Greenberg, D.M. The Biochemistry of Magnesium Deficiency. J. Biol. Chem. 1938, 122, 693–714. [Google Scholar] [CrossRef]
- Bunce, G.E.; Chiemchaisri, Y.; Phillips, P.H. The Mineral Requirements of the Dog IV. Effect of Certain Dietary and Physiologic Factors upon the Magnesium Deficiency Syndrome. J. Nutr. 1962, 76, 23–29. [Google Scholar] [CrossRef]
- Nugara, D.; Edwards, H.M. Influence of Dietary Ca and P Levels on the Mg Requirement of the Chick. J. Nutr. 1963, 80, 181–184. [Google Scholar] [CrossRef]
- Gomot, A. Biochemical Composition of Helix Snails: Influence of Genetic and Physiological Factors. J. Molluscan Stud. 1998, 64, 173–181. [Google Scholar] [CrossRef]
- Çelik, M.Y.; Duman, M.B.; Sariipek, M.; Uzun Gören, G.; Kaya Öztürk, D.; Kocatepe, D.; Karayücel, S. Comparison of Fatty Acids and Some Mineral Matter Profiles of Wild and Farmed Snails, Cornu aspersum Müller, 1774. Molluscan Res. 2019, 39, 234–240. [Google Scholar] [CrossRef]
- Engmann, F.; Afoakwah, N.A.; Darko, P.O.; Sefah, W. Proximate and Mineral Composition of Snail (Achatina achatina) Meat; any Nutritional Justification for Acclaimed Health Benefits? J. Basic Appl. Sci. Res. 2013, 3, 8–15. [Google Scholar]
- Gomot, A.; Pihan, F. Comparison of the Bioaccumulation Capacities of Copper and Zinc in Two Snail Subspecies (Helix). Ecotoxicol. Environ. Saf. 1997, 38, 85–94. [Google Scholar] [CrossRef]
- Niemiec, T.; Łozicki, A.; Pietrasik, R.; Pawęta, S.; Rygało-Galewska, A.; Matusiewicz, M.; Zglińska, K. Impact of Ag Nanoparticles (AgNPs) and Multimicrobial Preparation (EM) on the Carcass, Mineral and Fatty Acid Composition of Cornu aspersum aspersum Snails. Animals 2021, 11, 1926. [Google Scholar] [CrossRef]
- Özogul, Y.; Özogul, F.; Olgunoglu, A.I. Fatty Acid Profile and Mineral Content of the Wild Snail (Helix pomatia) from the Region of the South of the Turkey. Eur. Food Res. Technol. 2005, 221, 547–549. [Google Scholar] [CrossRef]
- Fagbuaro, O.; Oso, J.A.; Edward, J.B.; Ogunleye, R.F. Nutritional Status of Four Species of Giant Land Snails in Nigeria. J. Zhejiang Univ. Sci. B 2006, 7, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Ligaszewski, M.; Pol, P. Ocena Wpływu Różnych Systemów Chowu Ślimaka Szarego (Helix aspersa) na Wartość Odżywczą i Wydajność Jego Mięsa. Wiadomości Zootech. 2016, 54, 18–34. [Google Scholar]
- Rico, M.C.; Lerma, A.; Planells, E.; Aranda, P.; Gonzalez, J.L. Changes in the Nutritive Utilization of Protein Induced by Mg Deficiency in Rats. Int. J. Vitam. Nutr. Res. 1995, 65, 122–126. [Google Scholar]
- Bara, M.; Guiet-Bara, A.; Durlach, J. Regulation of Sodium and Potassium Pathways by Magnesium in Cell Membranes. Magnes. Res. 1993, 6, 167–177. [Google Scholar] [PubMed]
- Nica, D.V.; Bordean, D.M.; Hărmănescu, M.; Bura, M.; Gergen, I. Interactions among Heavy Metals (Cu, Cd, Zn, Pb) and Metallic Macroelements (K, Ca, Na, Mg) in Roman Snail (Helix pomatia) Soft Tissues. Acta Met.–MEEMB 2014, 11, 65. [Google Scholar]
- Beeby, A.; Richmond, L. Magnesium and the Regulation of Lead in Three Populations of the Garden Snail Cantareus aspersus. Environ. Pollut. 2010, 158, 2288–2293. [Google Scholar] [CrossRef]
- Liu, Y.X.; Guo, Y.M.; Wang, Z. Effect of Magnesium on Reactive Oxygen Species Production in the Thigh Muscles of Broiler Chickens. Br. Poult. Sci. 2007, 48, 84–89. [Google Scholar] [CrossRef]
- Woerpel, H.R.; Balloun, S.L. Effect of Iron and Magnesium on Manganese Metabolism. Poult. Sci. 1964, 43, 1134–1142. [Google Scholar] [CrossRef]
- Sanchez-Morito, N.; Planells, E.; Aranda, P.; Llopis, J. Magnesium-Manganese Interactions Caused by Magnesium Deficiency in Rats. J. Am. Coll. Nutr. 1999, 18, 475–480. [Google Scholar] [CrossRef]
- Lease, J.G.; Williams, W.P. The Effect of Added Magnesium on the Availability of Zinc with Some High-Protein Feedstuffs. Poult. Sci. 1967, 46, 242–248. [Google Scholar] [CrossRef]
- Planells, E.; Aranda, P.; Lerma, A.; Llopis, J. Changes in Bioavailability and Tissue Distribution of Zinc Caused by Magnesium Deficiency in Rats. Br. J. Nutr. 1994, 72, 315–323. [Google Scholar] [CrossRef]
- Planells, E.; Llopis, J.; Peran, F.; Aranda, P. Changes in Tissue Calcium and Phosphorus Content and Plasma Concentrations of Parathyroid Hormone and Calcitonin after Long-Term Magnesium Deficiency in Rats. J. Am. Coll. Nutr. 1995, 14, 292–298. [Google Scholar] [CrossRef] [PubMed]
- O’dell, B.L. Magnesium Requirement and Its Relation to other Dietary Constitunents. Fed. Proc. 1960, 19, 648–654. [Google Scholar] [PubMed]
- Afanas’ev, I.B.; Suslova, T.B.; Cheremisina, Z.P.; Abramova, N.E.; Korkina, L.G. Study of Antioxidant Properties of Metal Aspartates. Analyst 1995, 120, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Onderci, M.; Sahin, N.; Gulcu, F.; Yildiz, N.; Avci, M.; Kucuk, O. Responses of Quail to Dietary Vitamin E and Zinc Picolinate at Different Environmental Temperatures. Anim. Feed Sci. Technol. 2006, 129, 39–48. [Google Scholar] [CrossRef]
- Al-Saad, K.M.; Al-Sadi, H.I.; Abdul-Majeed, M.O. Clinical, Hematological, Biochemical and Pathological Studies on Zinc Deficiency (Hypozincemia) in Sheep. Vet. Res. 2010, 3, 14–20. [Google Scholar]
- Miao, X.; Sun, W.; Fu, Y.; Miao, L.; Cai, L. Zinc Homeostasis in the Metabolic Syndrome and Diabetes. Front. Med. 2013, 7, 31–52. [Google Scholar] [CrossRef]
- Alves, C.X.; Vale, S.H.L.; Dantas, M.M.G.; Maia, A.A.; Franca, M.C.; Marchini, J.S.; Leite, L.D.; Brandao-Neto, J. Positive Effects of Zinc Supplementation on Growth, Gh, Igf1, and Igfbp3 in Eutrophic Children. J. Pediatr. Endocrinol. Metab. 2012, 25, 881–887. [Google Scholar] [CrossRef]
- Kincaid, R.L. Assessment of Trace Mineral Status of Ruminants: A Review. J. Anim. Sci. 2000, 77, 1. [Google Scholar] [CrossRef]
- El-Far, A.H. Biochemical Alterations in Zinc Deficient Sheep Associated by Hyperlactatemia. Am. J. Anim. Vet. Sci. 2013, 8, 112–116. [Google Scholar] [CrossRef]
- Walter, T.; Olivares, M.; Pizarro, F.; Muñoz, C. Iron, Anemia, and Infection. Nutr. Rev. 1997, 55, 111–124. [Google Scholar] [CrossRef]
- Benito, P.; Miller, D. Iron Absorption and Bioavailability: An Updated Review. Nutr. Res. 1998, 18, 581–603. [Google Scholar] [CrossRef]
- Planells, E.; Aranda, P.; Peran, F.; Llopis, J. Changes in Calcium and Phosphorus Absorption and Retention during Long-Term Magnesium Deficiency in Rats. Nutr. Res. 1993, 13, 691–699. [Google Scholar] [CrossRef]
- Sanchez-Morito, N.; Planells, E.; Aranda, P.; Llopis, J. Influence of Magnesium Deficiency on the Bioavailability and Tissue Distribution of Iron in the Rat. J. Nutr. Biochem. 2000, 11, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, Z.; Chen, Y.; Qiao, J.; Gao, M.; Yuan, J.; Nie, W.; Guo, Y. Magnesium Deficiency Enhances Hydrogen Peroxide Production and Oxidative Damage in Chick Embryo Hepatocyte In Vitro. Biometals 2006, 19, 71–81. [Google Scholar]
- Bussière, F.I.; Zimowska, W.; Gueux, E.; Rayssiguier, Y.; Mazur, A. Stress Protein Expression cDNA Array Study Supports Activation of Neutrophils During Acute Magnesium Deficiency in Rats. Magnes. Res. 2002, 15, 37–42. [Google Scholar] [PubMed]
- Weglicki, W.B.; Dickens, B.F.; Wagner, T.L.; Chmielinska, J.J.; Phillips, T.M. Immunoregulation by Neuropeptides in Magnesium Deficiency: Ex Vivo Effect of Enhanced Substance P Production on Circulating T Lymphocytes from Magnesium-Deficient Mice. Magnes. Res. 1996, 9, 3–11. [Google Scholar]
- Haag, J.R.; Palmer, L.S. The Effect of Variations in the Proportions of Calcium, Magnesium, and Phosphorus Contained in the Diet. J. Biol. Chem. 1928, 76, 367–389. [Google Scholar] [CrossRef]
- Ligaszewski, M. Kształtowanie się Wskaźników Wartości Użytkowej Muszli Ślimaków Jadalnych z Rodzaju Helix w Zróżnicowanych Warunkach Hodowlanych i Środowiskowych. Rocz. Nauk. Zootech. 2005, 19, 1–104. [Google Scholar]
- Ligaszewski, M.; Pol, P. Edible Snails Breeding in Poland. Zagadnienia Doradz. Rol. 2021, 1, 67–85. [Google Scholar]
- Glass, N.H.; Darby, P.C. The Effect of Calcium and Ph on Florida Apple Snail, Pomacea paludosa (Gastropoda: Ampullariidae), Shell Growth and Crush Weight. Aquat. Ecol. 2009, 43, 1085–1093. [Google Scholar] [CrossRef]
- Łozicki, A.; Niemiec, T.; Pietrasik, R.; Pawęta, S.; Rygało-Galewska, A.; Zglińska, K. The Effect of Ag Nanoparticles and Multimicrobial Preparation as Factors Stabilizing the Microbiological Homeostasis of Feed Tables for Cornu aspersum (Müller) Snails on Snail Growth and Quality Parameters of Carcasses and Shells. Animals 2020, 10, 2260. [Google Scholar] [CrossRef] [PubMed]
- Atteh, J.O.; Leeson, S. Influence of Increasing Dietary Calcium and Magnesium Levels on Performance, Mineral Metabolism, and Egg Mineral Content of Laying Hens. Poult. Sci. 1983, 62, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Shastak, Y.; Rodehutscord, M. A Review of the Role of Magnesium in Poultry Nutrition. World Poult. Sci. J. 2019, 71, 125–137. [Google Scholar] [CrossRef]
- Holder, D.P.; Huntley, D.M. Influence of Added Manganese, Magnesium, Zinc, and Calcium Level on Egg Shell Quality. Poult. Sci. 1978, 57, 1629–1634. [Google Scholar] [CrossRef]
- Pati, S.G.; Panda, F.; Samanta, L.; Paital, B. Spatio-Temporal Changes in Oxidative Stress Physiology Parameters in Apple Snail Pila globosa as a Function of Soil Mg, Ca, Organic Carbon and Aquatic Physico-Chemical Factors. Environ. Geochem. Health 2023, 45, 2591–2610. [Google Scholar] [CrossRef]
- Ondina, P.; Mato, S.; Hermida, J.; Outeiro, A. Importance of Soil Exchangeable Cations and Aluminium Content on Land Snail Distribution. Appl. Soil Ecol. 1998, 9, 229–232. [Google Scholar] [CrossRef]
- Schwartzkopf, C. Potassium, Calcium, Magnesium—How They Relate to Plant Growth. Usga Green Sect. Rec. 1972, 10, 1–2. [Google Scholar]
- Huber, D.M.; Jones, J.B. The Role of Magnesium in Plant Disease. Plant Soil 2013, 368, 73–85. [Google Scholar]
- Kobayashi, H.; Masaoka, Y.; Sato, S. Effects of Excess Magnesium on the Growth and Mineral Content of Rice and Echinochloa. Plant Prod. Sci. 2005, 8, 38–43. [Google Scholar] [CrossRef]
- Franklin, W.T.; Olsen, J.S.; Soltanpour, P.N. Effects of Excessive Magnesium in Irrigation Waters on Wheat and Corn Growth. Commun. Soil Sci. Plant Anal. 1991, 22, 49–61. [Google Scholar] [CrossRef]
- Razowska-Jaworek, L. Calcium and Magnesium in Groundwater Occurrence and Significance for Human Health, 1st ed.; Razowska-Jaworek, L., Ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2019. [Google Scholar]
- Rapant, S.; Cvečková, V.; Fajčíková, K.; Sedláková, D.; Stehlíková, B. Impact of Calcium and Magnesium in Groundwater and Drinking Water on the Health of Inhabitants of the Slovak Republic. Int. J. Environ. Res. Public Health 2017, 14, 278. [Google Scholar] [CrossRef]
- Clark, R.B. Physiological Aspects of Calcium, Magnesium, and Molybdenum Deficiencies in Plants. Soil Acidity Liming 2015, 12, 99–170. [Google Scholar] [CrossRef]
- Osemwota, I.O.; Omueti, J.A.I.; Ogboghodo, A.I. Effect of Calcium/Magnesium Ratio in Soil on Magnesium Availability, Yield, and Yield Components of Maize. Commun. Soil Sci. Plant Anal. 2007, 38, 2849–2860. [Google Scholar] [CrossRef]

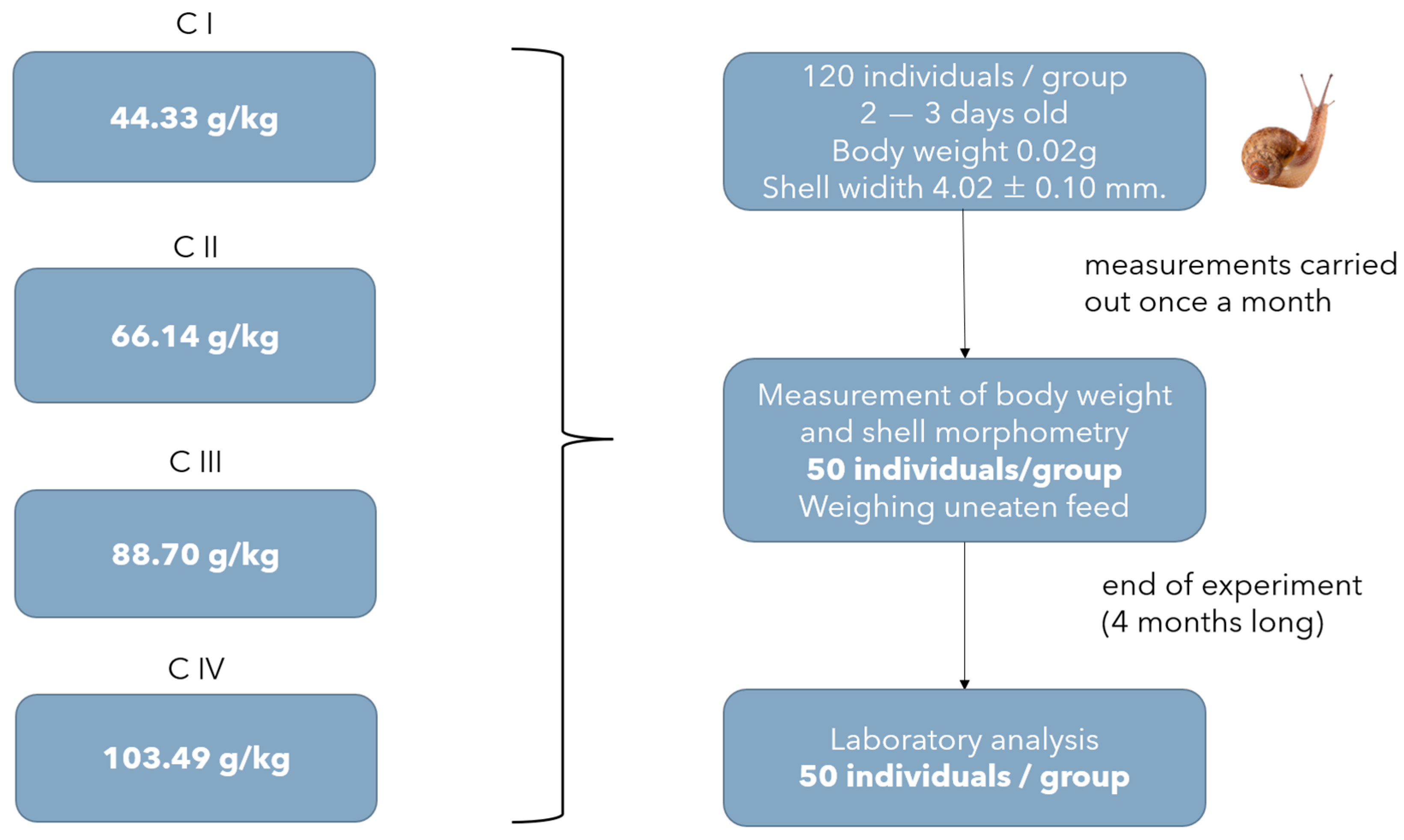




| Ingredients (%) | Experiment I | Experiment II | ||||||
|---|---|---|---|---|---|---|---|---|
| C I | C II | C III | C IV | CON | M I | M II | M III | |
| Corn meal | 53.0 | 50.0 | 45.0 | 43.3 | 43.8 | 43.5 | 43.3 | 43.1 |
| Post-extraction soybean meal 460 | 18.0 | 19.0 | 21.0 | 21.0 | 21.0 | 21.0 | 21.0 | 21.0 |
| Wheat bran | 10.0 | 8.0 | 6.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Fodder yeast | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| Rapeseed oil | 2.0 | 2.0 | 2.5 | 2.5 | 2.0 | 2.0 | 2.0 | 2.0 |
| Monocalcium phosphate | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| NaCl | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Vitamin premix | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Ca carbonate | 8.9 | 13.2 | 17.3 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Mg oxide (g) | - | - | - | - | - | 0.8 | 3.1 | 4.7 |
| Composition (% DM) | ||||||||
| Crude protein | 18.22 ± 0.53 | 18.86 ± 0.76 | 18.72 ± 0.44 | 18.30 ± 0.68 | 18.40 ± 0.58 | 18.74 ± 0.63 | 19.03 ± 0.71 | 18.82 ± 0.42 |
| Ether extracts | 2.93 ± 0.09 | 3.11 ± 0.12 | 3.32 ± 0.11 | 3.31 ± 0.07 | 3.01 ± 0.10 | 3.33 ± 0.13 | 3.15 ± 0.10 | 3.12 ± 0.08 |
| Crude fibre | 3.62 ± 0.09 | 4.05 ± 0.09 | 3.47 ± 0.09 | 3.30 ± 0.09 | 3.33 ± 0.09 | 3.62 ± 0.09 | 3.59 ± 0.09 | 3.81 ± 0.09 |
| Crude ash | 21.15 ± 0.62 | 23.48 ± 0.73 | 25.48 ± 0.93 | 27.30 ± 1.03 | 27.24 ± 0.79 | 26.30 ± 0.81 | 27.00 ± 0.53 | 27.60 ± 0.86 |
| Nitrogen-free extract | 54.08 ± 2.09 | 50.50 ± 1.83 | 49.01 ± 1.12 | 47.78 ± 1.06 | 48.02 ± 1.66 | 48.01 ± 0.67 | 47.23 ± 1.34 | 46.65 ± 1.12 |
| Ca | 4.43 ± 0.13 | 6.61 ± 0.21 | 8.87 ± 0.32 | 10.35 ± 0.39 | 10.35 ± 0.22 | 10.35 ± 0.31 | 10.35 ± 0.40 | 10.35 ± 0.25 |
| Mg | 0.26 ± 0.01 | 0.25 ± 0.01 | 0.26 ± 0.01 | 0.24 ± 0.01 | 0.25 ± 0.01 | 0.33 ± 0.01 | 0.56 ± 0.02 | 0.72 ± 0.02 |
| Calculated BE (MJ) | 13.21 ± 0.52 | 12.79 ± 0.44 | 12.59 ± 0.49 | 12.32 ± 0.37 | 12.26 ± 0.42 | 12.43 ± 0.50 | 12.28 ± 0.38 | 12.14 ± 0.45 |
| Experiment I | |||||||
| Indices | n | Experimental Groups | SEM | p-Value | |||
| C I | C II | C III | C IV | ||||
| Carcass weight (g) | 50 | 2.89 ± 1.95 a | 6.87 ± 1.40 b | 7.41 ± 1.39 b | 8.27 ± 1.68 c | 0.23 | <0.0001 |
| Shell weight (g) | 50 | 0.29 ± 0.22 a | 0.70 ± 0.34 b | 1.12 ± 0.21 c | 1.40 ± 0.24 d | 0.03 | <0.0001 |
| Share of the carcass in total body weight (%) | 50 | 91.24 ± 0.93 c | 90.78 ± 1.12 c | 86.87 ± 2.32 b | 85.46 ± 2.20 a | 0.24 | <0.0001 |
| Shell shape index | 50 | 1.44 ± 0.36 | 1.39 ± 0.08 | 1.44 ± 0.07 | 1.43 ± 0.12 | 0.03 | 0.5572 |
| Solidity index (g/cm2) × 100 | 50 | 11.19 ± 2.43 a | 10.25 ± 2.10 a | 18.08 ± 2.85 b | 21.15 ± 3.55 c | 0.39 | <0.0001 |
| Crushing force of the shells (N) | 15 | 12.44 ± 6.68 a | 17.47 ± 8.01 a | 32.85 ± 13.46 b | 54.83 ± 17.39 c | 3.38 | <0.0001 |
| TBARS (nmol/g lyophilisate) | 6 | 56.94 ± 0.65 ab | 58.87 ± 1.47 c | 58.02 ± 1.84 bc | 55.59 ± 0.38 a | 0.45 | <0.0001 |
| Mature individuals (%) | 50 | 4 ± 0.02 a | 20 ± 0.04 b | 20 ± 0.04 b | 46 ± 0.05 c | 0.06 | <0.0001 |
| Total FCR (kg DM/kg) | 3 | 0.86 ± 0.32 a | 1.10 ± 0.14 ab | 0.94 ± 0.10 a | 1.28 ± 0.05 b | 0.09 | 0.0009 |
| Total feed intake (g/individual) | 3 | 11.09 ± 0.31 | 8.34 ± 0.03 | 8.75 ± 0.51 | 10.64 ± 0.25 | 0.92 | 0.1647 |
| Experiment II | |||||||
| Indices | n | Control Group | Experimental Groups | SEM | p-Value | ||
| M I | M II | M III | |||||
| Carcass weight (g) | 46 | 8.23 ± 1.30 b | 8.18 ± 1.51 b | 8.45 ± 1.53 b | 7.47 ± 1.65 a | 0.22 | 0.0134 |
| Shell weight (g) | 46 | 1.39 ± 0.23 a | 1.50 ± 0.35 ab | 1.49 ± 0.28 a | 1.61 ± 0.27 b | 0.04 | 0.0047 |
| Share of the carcass in total body weight (%) | 46 | 85.45 ± 2.12 c | 84.52 ± 2.07 b | 84.92 ± 1.87 bc | 81.98 ± 3.04 a | 0.30 | <0.0001 |
| Shell shape index | 46 | 1.43 ± 0.10 c | 1.29 ± 0.08 b | 1.27 ± 0.08 ab | 1.24 ± 0.11 a | 0.02 | <0.0001 |
| Solidity index (g/cm2) × 100 | 46 | 21.17 ± 3.13 a | 20.26 ± 3.52 a | 20.51 ± 3.03 a | 23.58 ± 3.84 b | 0.51 | <0.0001 |
| Crushing force of the shells (N) | 15 | 57.29 ± 17.31 | 59.38 ± 23.48 | 61.41 ± 22.61 | 49.73 ± 10.73 | 5.77 | 0.5123 |
| TBARS (nmol/g lyophilisate) | 6 | 55.59 ± 0.35 a | 55.33 ± 0.32 a | 55.90 ± 1.36 a | 57.65 ± 1.96 b | 0.39 | 0.0246 |
| Mature individuals (%) | 46 | 46.00 ± 0.41 | 63.00 ± 0.49 | 65.00 ± 0.48 | 59.00 ± 0.52 | 0.07 | 0.2287 |
| Total FCR (kg DM/kg) | 3 | 1.27 ± 0.06 b | 1.10 ± 0.25 ab | 0.94 ± 0.17 a | 1.61 ± 0.12 c | 0.09 | 0.0049 |
| Total feed intake (g/individual) | 3 | 11.09 ± 0.29 | 8.34 ± 0.23 | 8.75 ± 0.88 | 10.64 ± 3.04 | 0.91 | 0.1643 |
| Experiment I | ||||||
| Item | Experimental Groups | SEM | p-Value | |||
| C I | C II | C III | C IV | |||
| Crude protein | 60.26 ± 1.33 c | 58.06 ± 1.56 b | 53.38 ± 1.79 a | 59.97 ± 1.96 bc | 0.69 | <0.0001 |
| Ether extracts | 4.66 ± 0.62 c | 3.14 ± 2.41 b | 1.49 ± 0.26 a | 1.48 ± 0.24 a | 0.51 | 0.0008 |
| Crude ash | 14.07 ± 0.72 c | 14.30 ± 1.38 c | 11.09 ± 1.26 b | 9.20 ± 0.62 a | 0.43 | <0.0001 |
| Experiment II | ||||||
| Item | Control Group | Experimental Groups | SEM | p-Value | ||
| M I | M II | M III | ||||
| Crude protein | 59.97 ± 1.92 b | 52.16 ± 1.72 a | 52.48 ± 1.00 a | 52.14 ± 0.76 a | 0.56 | <0.0001 |
| Ether extracts | 1.56 ± 0.28 a | 1.68 ± 0.72 a | 2.29 ± 1.51 ab | 2.86 ± 0.25 b | 0.33 | 0.0442 |
| Crude ash | 9.20 ± 0.58 | 9.22 ± 0.24 | 9.01 ± 0.39 | 8.79 ± 0.97 | 0.25 | 0.6056 |
| Experiment I | |||||||
| Item | Experimental Groups | SEM | p-Value | ||||
| C I | C II | C III | C IV | ||||
| Ca (%) | Meat | 1.36 ± 0.23 | 1.31 ± 0.09 | 1.45 ± 0.05 | 1.40 ± 0.12 | 0.07 | 0.5847 |
| Shell | 37.99 ± 0.96 bc | 36.16 ± 0.97 a | 37.05 ± 1.15 ab | 38.96 ± 0.22 c | 0.45 | 0.0048 | |
| Cu (mg/kg) | Meat | 36.70 ± 1.19 a | 56.99 ±4.04 c | 45.33 ±1.58 b | 60.33 ± 3.90 c | 1.49 | <0.0001 |
| Shell | 6.12 ± 2.60 b | 3.47 ± 0.47 a | 3.51 ± 0.72 a | 1.88 ± 0.02 a | 0.68 | 0.0072 | |
| Fe (mg/kg) | Meat | 357.52 ± 31.75 d | 312.01 ± 28.83 c | 218.19 ± 30.51 b | 127.82 ± 9.70 a | 13.38 | <0.0001 |
| Shell | 196.76 ± 35.77 bc | 129.26 ± 50.56 a | 202.39 ±23.17 c | 135.82 ± 26.76 ab | 17.41 | 0.0181 | |
| K (mg/kg) | Meat | 7097.36 ± 430.28 b | 7718.49 ± 275.80 c | 6960.40 ± 529.03 b | 6220.12 ± 214.21 a | 191.53 | 0.0012 |
| Shell | 806.19 ± 184.37 | 791.41 ±289.89 | 726.69 ± 126.45 | 1096.67 ± 48.90 | 92.06 | 0.0624 | |
| Mg (mg/kg) | Meat | 2948.14 ± 425.59 a | 3532.52 ± 122.51 b | 2670.22 ± 308.84 a | 2684.13 ± 186.15 a | 142.78 | 0.0026 |
| Shell | 508.48 ± 32.46 b | 322.13 ± 9.54 a | 356.97 ± 26.00 a | 320.12 ±5.89 a | 10.74 | <0.0001 | |
| Mn (mg/kg) | Meat | 25.98 ± 2.90 | 23.31 ± 2.65 | 24.57 ± 2.08 | 21.82 ± 0.94 | 1.14 | 0.1148 |
| Shell | 14.58 ± 0.68 c | 10.98 ± 0.78 b | 9.99 ± 0.56 a | 9.76 ± 0.20 a | 0.30 | <0.0001 | |
| Na (mg/kg) | Meat | 5915.44 ± 886.60 a | 7934.27 ± 175.55 b | 5467.21 ± 783.89 a | 5299.06 ± 298.35 a | 308.26 | <0.0001 |
| Shell | 1198.09 ± 169.30 c | 806.95 ± 242.83 ab | 752.42 ± 117.97 a | 1050.68 ± 27.87 bc | 79.87 | 0.0057 | |
| P (mg/kg) | Meat | 11,109.90 ± 1289.60 b | 11,616.93 ± 265.68 b | 10,415.35 ± 880.25 ab | 9271.07 ± 415.49 a | 409.35 | 0.0089 |
| Shell | 896.51 ± 55.47 c | 558.19 ± 14.21 b | 532.45 ± 47.52 b | 428.37 ± 16.78 a | 18.92 | <0.0001 | |
| Si (mg/kg) | Meat | 57.49 ± 15.46 bc | 36.40 ± 9.59 a | 45.59 ± 14.02 ab | 66.00 ± 10.35 c | 6.30 | 0.0293 |
| Shell | 182.30 ± 45.08 c | 93.11 ± 27.66 b | 190.42 ± 36.81 c | 24.98 ± 3.69 a | 16.13 | <0.0001 | |
| Zn (mg/kg) | Meat | 105.27 ± 11.43 b | 104.58 ± 8.85 b | 95.25 ± 7.30 b | 57.26 ± 3.06 a | 4.12 | <0.0001 |
| Shell | 6.40 ± 1.04 a | 6.28 ± 2.25 a | 5.26 ± 1.16 a | 17.91 ± 1.75 b | 0.77 | <0.0001 | |
| Experiment II | |||||||
| Item | Control Group | Experimental Groups | SEM | p-Value | |||
| M I | M II | M III | |||||
| Ca (%) | Meat | 1.38 ± 0.11 b | 1.16 ± 0.05 a | 1.29 ± 0.09 ab | 1.26 ± 0.23 ab | 0.05 | 0.0274 |
| Shell | 38.96 ± 0.21 | 33.78 ± 6.48 | 38.70 ± 2.39 | 37.64 ± 1.08 | 1.48 | 0.0977 | |
| Cu (mg/kg) | Meat | 60.33 ± 3.88 b | 48.69 ± 4.43 a | 57.20 ± 2.94 b | 48.86 ± 2.72 a | 1.78 | 0.0013 |
| Shell | 1.90 ± 0.02 | 3.06 ± 1.53 | 3.84 ±1.45 | 4.98 ± 2.66 | 0.82 | 0.1048 | |
| Fe (mg/kg) | Meat | 127.82 ± 9.72 a | 228.16 ± 8.39 c | 223.68 ± 18.68 c | 174.40 ± 7.05 b | 5.93 | <0.0001 |
| Shell | 135.80 ± 26.76 a | 184.22 ± 2.28 b | 200.70 ± 25.37 b | 131.80 ± 10.40 a | 8.78 | <0.0001 | |
| K (mg/kg) | Meat | 6220.12 ± 214.23 | 6102.19 ± 233.64 | 5829.50 ± 443.64 | 5759.94 ± 228.28 | 147.78 | 0.1424 |
| Shell | 1096.70 ± 27.92 b | 750.43 ± 90.35 a | 882.32 ± 138.52 a | 827.05 ± 140.74 a | 53.64 | 0.0043 | |
| Mg (mg/kg) | Meat | 2684.13 ± 186.13 c | 2279.38 ± 44.22 a | 2500.01 ± 154.35 bc | 2381.87 ± 92.34 ab | 65.65 | 0.0055 |
| Shell | 320.10 ± 5.78 b | 271.84 ± 2.85 a | 367.77 ± 42.97 c | 408.85 ± 16.86 d | 11.62 | <0.0001 | |
| Mn (mg/kg) | Meat | 21.82 ± 0,92 a | 22.77 ± 1,10 a | 25.34 ± 1.80 b | 21.17 ± 1.30 a | 0.66 | 0.0043 |
| Shell | 9.80 ± 0.22 b | 9.18 ± 0.47 ab | 9.25 ± 0.58 b | 8.42 ± 0.46 a | 0.21 | 0.0058 | |
| Na (mg/kg) | Meat | 5299.06 ± 298,35 c | 4899.43 ± 142,00 b | 4644.27 ± 262,32 ab | 4497.35 ± 112,38 a | 109.15 | 0.0013 |
| Shell | 1050.70 ± 26.98 c | 738.78 ± 32.79 a | 849.92 ± 101.69 ab | 941.13 ± 180.83 bc | 52.60 | 0.0082 | |
| P (mg/kg) | Meat | 9271.07 ± 415.42 b | 7905.68 ± 332.83 a | 8164.64 ± 330.69 a | 7958.51 ± 327.05 a | 176.73 | <0.0001 |
| Shell | 428.40 ± 16.79 b | 244.33 ± 24.08 a | 259.35 ± 39.55 a | 296.59 ± 38.89 a | 15.10 | <0.0001 | |
| Si (mg/kg) | Meat | 66.00 ± 10.32 a | 102.26 ± 19.74 b | 119.27 ± 10.67 b | 104.25 ± 3.47 b | 6.24 | <0.0001 |
| Shell | 25.00 ± 3.66 a | 179.36 ± 24.08 b | 178.05 ± 39.55 b | 149.24 ± 38.89 b | 29.65 | 0.0094 | |
| Zn (mg/kg) | Meat | 57.26 ± 3.09 c | 49.63 ± 1.24 a | 53.86 ± 1.85 b | 53.35 ± 1.71 b | 1.04 | 0.0022 |
| Shell | 17.90 ± 1.72 c | 4.52 ± 0.96 ab | 4.42 ± 0.62 a | 6.63 ± 1.74 b | 0.62 | <0.0001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rygało-Galewska, A.; Zglińska, K.; Roguski, M.; Roman, K.; Bendowski, W.; Bień, D.; Niemiec, T. Effect of Different Levels of Calcium and Addition of Magnesium in the Diet on Garden Snails’ (Cornu aspersum) Condition, Production, and Nutritional Parameters. Agriculture 2023, 13, 2055. https://doi.org/10.3390/agriculture13112055
Rygało-Galewska A, Zglińska K, Roguski M, Roman K, Bendowski W, Bień D, Niemiec T. Effect of Different Levels of Calcium and Addition of Magnesium in the Diet on Garden Snails’ (Cornu aspersum) Condition, Production, and Nutritional Parameters. Agriculture. 2023; 13(11):2055. https://doi.org/10.3390/agriculture13112055
Chicago/Turabian StyleRygało-Galewska, Anna, Klara Zglińska, Mateusz Roguski, Kamil Roman, Wiktor Bendowski, Damian Bień, and Tomasz Niemiec. 2023. "Effect of Different Levels of Calcium and Addition of Magnesium in the Diet on Garden Snails’ (Cornu aspersum) Condition, Production, and Nutritional Parameters" Agriculture 13, no. 11: 2055. https://doi.org/10.3390/agriculture13112055
APA StyleRygało-Galewska, A., Zglińska, K., Roguski, M., Roman, K., Bendowski, W., Bień, D., & Niemiec, T. (2023). Effect of Different Levels of Calcium and Addition of Magnesium in the Diet on Garden Snails’ (Cornu aspersum) Condition, Production, and Nutritional Parameters. Agriculture, 13(11), 2055. https://doi.org/10.3390/agriculture13112055









