New Advances in Nano-Enabled Weed Management Using Poly(Epsilon-Caprolactone)-Based Nanoherbicides: A Review
Abstract
1. Introduction
2. Weed Control System
3. Challenges in Weed Management
3.1. Herbicide Resistance
3.2. Herbicide Residues
4. Current Weed Management Approaches
5. Nanotechnology in Weed Management
6. Types of Nanomaterials for Assembling Nanoherbicides
6.1. Nanoherbicides Based on Inorganic Nanomaterials
6.2. Nanoherbicides Based on Organic Nanomaterials
6.3. Nanoherbicides Based on Organic/Inorganic (Hybrid) Nanomaterials
7. PCL Polymer as an Ecofriendly Nanocarrier for Herbicides
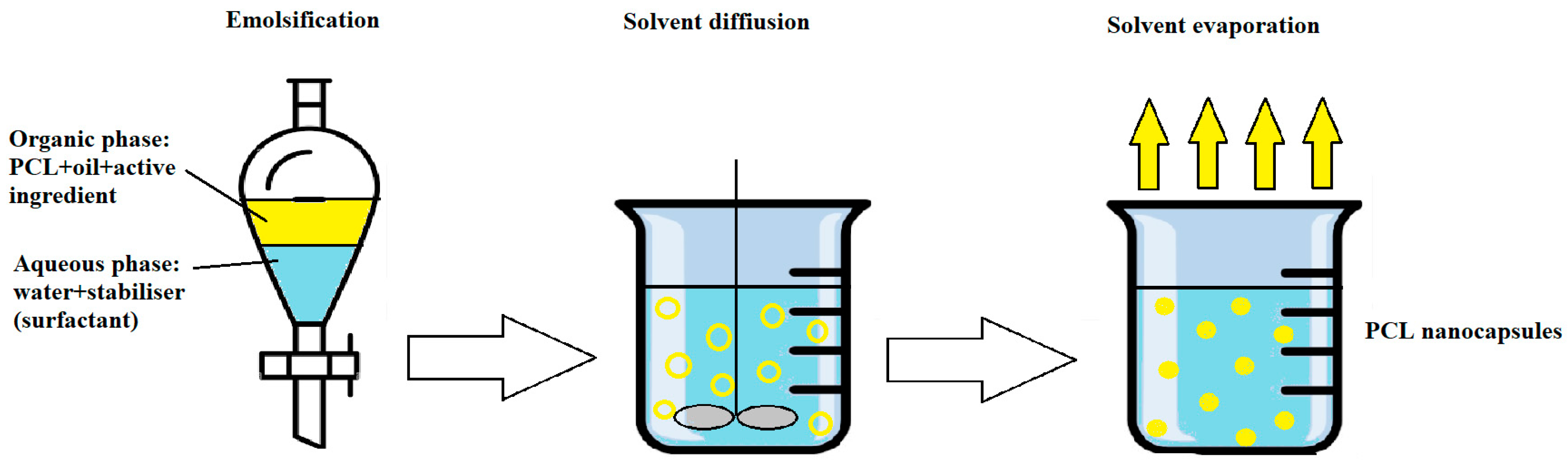
8. Classical Methods for Preparation of PCL-Based Nanocapsules
9. PCL-Based Nanoherbicides
9.1. Metribuzin-PCL Nanoherbicide
9.2. Atrazine-PCL Nanoherbicides
9.3. Pretilachlor-PCL Nanoherbicide
10. Behavior of PCL-Based Nano-Enabled Herbicides in Plant Systems
11. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zargar, M.; Bayat, M.; Romanova, E.; Izadi-Darbandi, E. POST herbicide programs utilizing tribenuron for cleavers (Galium aparine L.) control in winter wheat cultivars. Arch. Agron. Soil Sci. 2020, 66, 1235–1243. [Google Scholar] [CrossRef]
- Zargar, M.; Bayat, M.; Astarkhanova, T. Study of postemergence-directed herbicides for redroot pigweed (Amaranthus retroflexus L.) control in winter wheat in southern Russia. J. Plant Prot. Res. 2020, 60, 7–13. [Google Scholar] [CrossRef]
- Saxena, A.; Jain, A.; Upadhyay, P.; Gauba, P.G. Applications of nanotechnology in agriculture. J. Nanosci. Nanoeng. Appl. 2018, 8, 20–27. [Google Scholar]
- Yadav, A.S.; Srivastava, D.S. Application of nanotechnology in weed management: A Review. RRJoCST 2015, 4, 21–23. [Google Scholar]
- Emamverdian, A.; Ghorbani, A.; Li, Y.; Pehlivan, N.; Barker, J.; Ding, Y.; Liu, G.; Zargar, M. Responsible mechanisms for the restriction of heavy metal toxicity in plants via the co-foliar spraying of nanoparticles. Agronomy 2023, 13, 1748. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ghorbani, A.; Pehlivan, N.; Alwahibi, M.S.; Elshikh, M.S.; Liu, G.; Li, Y.; Barker, J.; Zargar, M.; Chen, M. Co-application of melatonin and zeolite boost bamboo tolerance under cadmium by enhancing antioxidant capacity, osmolyte accumulation, plant nutrient availability, and decreasing cadmium absorption. Sci. Hortic. 2023, 322, 112433. [Google Scholar] [CrossRef]
- Khatem, R.; Bakthi, A.; Hermosín, M.C. Comparison of the systemic nanoherbicide Imazamox-LDH obtained by direct synthesis and reconstruction: Preliminary results. In Proceedings of the Nanotech France 2016 International Conference & Exhibition, Paris, France, 1–3 June 2016. [Google Scholar]
- Choukaife, H.; Doolaanea, A.; Alfatama, M. Alginate Nanoformulation: Influence of Process and Selected Variables. Pharmaceuticals 2020, 13, 335. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, A.; Kaur, H.; Mehta, S.; Husen, A. Smart nanomaterial and nanocomposite with advanced agrochemical activities. Nanoscale Res. Lett. 2021, 16, 156. [Google Scholar] [CrossRef] [PubMed]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture (Review). Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef]
- Wang, D.; Saleh, N.B.; Byro, A.; Zepp, R.; Sahle-Demessie, E.; Luxton, T.P.; Ho, K.T.; Burgess, R.M.; Flury, M.; White, J.C.; et al. Nano-enabled pesticides for sustainable agriculture and global food security. Nat. Nanotechnol. 2022, 17, 347–360. [Google Scholar] [CrossRef]
- Zargar, M.; Kavhiza, N.J.; Bayat, M.; Pakina, E. Wild Mustard (Sinapis arvensis) Competition and Control in Rain-Fed Spring Wheat (Triticum aestivum L.). Agronomy 2021, 11, 2306. [Google Scholar] [CrossRef]
- Bombo, A.B.; Pereira, A.E.S.; Lusa, M.G.; Oliveira, E.M.; Oliveira, J.L.; Campos, E.V.R.; Jesus, M.B.; Oliveira, H.C.; Fraceto, L.F.; Mayer, J.L.S. A Mechanistic View of Interactions of a Nanoherbicide with Target Organism. J. Agric. Food Chem. 2019, 67, 4453–4462. [Google Scholar] [CrossRef]
- Kubiak, A.; Wolna-Maruwka, A.; Niewiadomska, A.; Pilarska, A.A. The Problem of Weed Infestation of Agricultural Plantations vs. the Assumptions of the European Biodiversity Strategy. Agronomy 2022, 12, 1808. [Google Scholar] [CrossRef]
- Rojas, S.; Rodríguez-Diéguez, A.; Horcajada, P. Metal–Organic Frameworks in Agriculture. ACS Appl. Mater. Interfaces 2022, 14, 16983–17007. [Google Scholar] [CrossRef] [PubMed]
- Stybayev, G.; Zargar, M.; Serekpayev, N.; Zharlygassov, Z.; Baitelenova, A.; Nogaev, A.; Mukhanov, N.; Elsergani, M.I.M.; Abdiee, A.A.A. Spring-Planted Cover Crop Impact on Weed Suppression, Productivity, and Feed Quality of Forage Crops in Northern Kazakhstan. Agronomy 2023, 13, 1278. [Google Scholar] [CrossRef]
- Mundargi, R.C.; Babu, V.R.; Rangaswamy, V.; Patel, P.; Aminabhavi, T.M. Nano/micro technologies for delivering macromolecular therapeutics using poly(D,L-lactide-co-glycolide) and its derivatives. JCR 2008, 125, 193–209. [Google Scholar] [CrossRef]
- Heap, I. The International Herbicide-Resistant Weed Database. 2023. Available online: https://www.weedscience.org/Home.aspx (accessed on 1 March 2022).
- Foster, J.B.; Magdoff, F. Liebig, Marx, and the Depletion of Soil Fertility: Relevance for Today’s Agriculture; Foster, M.J.B., Buttel, F., Eds.; Hungry for profit; NYU Press: New York, NY, USA, 2000; pp. 43–60. [Google Scholar]
- Zargar, M.; Pakina, E. Reduced rates of herbicide combined with biological components suppressing weeds in wheat fields of Moscow, Russia. Res. Crops 2014, 15, 332–338. [Google Scholar] [CrossRef]
- Bayat, M.; Engeribo, A.; Meretukov, Z.; Dubrovina, T.; Zargar, M. Response of common lambsquarters (Chenopodium album L.) to chemical weed control programs. Res. Crops. 2019, 20, 859–863. [Google Scholar]
- McElroy, J.S. Vavilovian mimicry: Nikolai Vavilov and his little-known impact on weed science. Weed Sci. 2014, 62, 16. [Google Scholar] [CrossRef]
- Loos, R.; Tavazzi, S.; Mariani, G.; Suurkuusk, G.; Paracchini, B.; Umlauf, G. Analysis of emerging organic contaminants in water, fish and suspended particulate matter (SPM) in the joint Danube survey using solid-phase extraction followed by UHPLC-MS-MS and GC–MS analysis. Sci. Total Environ. 2017, 607, 1201–1212. [Google Scholar] [CrossRef]
- Béranger, R.; Billoir, E.; Nuckols, J.R.; Blain, J.; Millet, M.; Bayle, M.L.; Combourieu, B.; Philip, T.; Schüz, J.; Fervers, B. Agricultural and domestic pesticides in house dust from different agricultural areas in France. Environ. Sci. Pollut. Res. 2019, 26, 19632–19645. [Google Scholar] [CrossRef]
- Ronco, A.E.; Marino, D.J.G.; Abelando, M.; Almada, P.; Apartin, C.D. Water quality of the main tributaries of the Parana basin: Glyphosate and AMPA in surface water and bottom sediments. Environ. Monit. 2016, 188, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Annabestani, M.; Izadi-Darbandi, E.; Vidacovic, M.; Zargar, M. Chemical weed management programs for cycloxydim-tolerant maize in Iran. J. Crop Prot. 2020, 9, 471–481. [Google Scholar]
- Compaore, H.; Ilboudo, S.; Bama Nati, A.D.; Dama-Balima, M.M. Pratiques paysannes de gestion des pesticides dans les bas-fonds rizicoles: Risques sanitaires et environnementaux (Dano, Burkina Faso). In Risques Climatiques et Agriculture en Afrique de l’Ouest; Sultan, B., Bossa, A.Y., Salack, S., Sanon, M., Eds.; IRD Éditions: Paris, France, 2020; Chapter 14. [Google Scholar] [CrossRef]
- Tillitt, D.E.; Papoulias, D.M.; Whyte, J.J.; Richter, C.A. Atrazine reduces reproduction in fathead minnow (Pimephales promelas). Aquat. Toxicol. 2010, 99, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.; Santos, S. Sustainable Approach to Weed Management: The Role of Precision Weed Management. Agronomy 2022, 12, 118. [Google Scholar] [CrossRef]
- Tulkubayeva, S.A.; Tulayev, Y.V.; Somova, S.V.; Vykhodtsev, V.A. The infl uence of fi eld crop rotations on the contamination of crops in the conditions of the Kostanay region. J. Agric. Sci. 2023, 2, 67–74. [Google Scholar]
- Bayat, M.; Zargar, M. Field bindweed (Convolvulus arvensis) control and winter wheat response to post herbicides application. J. Crop Sci. Biotech. 2020, 23, 149–155. [Google Scholar] [CrossRef]
- Muchhadiya, R.M.; Kumawat, P.D.; Sakarvadia, H.L.; Muchhadiya, P.M. Weed management with the use of nano-encapsulated herbicide formulations: A review. J. Pharm. Innov. 2022, 11, 2068–2075. [Google Scholar]
- Wani, S.; Bhat, S.A. Nano-Technology Vis-A-Vis Weed Management. Just Agric. 2023, 3, 6. [Google Scholar]
- Viji, N.; Chinnamuthu, C.R. Nanoparticle effect on degradation of vanillic acid, a germination inhibiting dormancy factor present in Cyperus rotundus. Indian J. Weed Sci. 2019, 51, 98–100. [Google Scholar] [CrossRef]
- Brindha, K.; Chinnamuthu, R. Zinc oxide nanorods to degrade phenolics and stored starch of Cyperus rotundus tubers management. J. Crop Weed 2017, 13, 184–188. [Google Scholar]
- Choudhary, S.K. Novel nanotechnological tools for weed management–A review. Chem. Sci. Rev. Lett. 2020, 9, 886–894. [Google Scholar]
- Tripathi, S.; Mahra, S.; Victoria, J.; Tiwari, K.; Rana, S.; Tripathi, D.K.; Sharma, S.; Sahi, S. Recent Advances and Perspectives of Nanomaterials in Agricultural Management and Associated Environmental Risk: A Review. Nanomaterials 2023, 13, 1604. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Sarkar, B.; Kumar, A.; Thongmee, S. Chapter 18—Regulatory affairs, commercialization, and economic aspects of nanomaterials used for agriculture. In Agricultural Nanobiotechnology, Biogenic Nanoparticles, Nanofertilizers and Nanoscale Biocontrol Agents; Woodhead: Cambridge, UK, 2022; pp. 479–502. [Google Scholar]
- Pontes, M.S.; Santos, J.S.; da Silva, J.L.; Miguel, T.B.; Miguel, E.C.; Souza Filho, A.G.; Felipe Santiago, E. Assessing the Fate of Superparamagnetic Iron Oxide Nanoparticles Carrying Usnic Acid as Chemical Cargo on the Soil Microbial Community. ACS Nano 2023, 17, 7417–7430. [Google Scholar] [CrossRef]
- de Sousa, G.F.M.; Gomes, D.G.; Campos, E.V.R.; Oliveira, J.L.; Fraceto, L.F.; Stolf-Moreira, R.; Oliveira, H.C. Post-Emergence Herbicidal Activity of Nanoatrazine Against Susceptible Weeds. Front. Environ. Sci. 2018, 6, 12. [Google Scholar] [CrossRef]
- Xiang, S.; Lv, X.; He, L.; Shi, H.; Liao, S.; Liu, C.; Sun, X. Dual-action pesticide carrier that continuously induces plant resistance, enhances plant anti-tobacco mosaic virus activity, and promotes plant growth. J. Agric. Food Chem. 2019, 67, 10000–10009. [Google Scholar] [CrossRef]
- de Oliveira, H.C.; Stolf, M.R.; Martinez, C.B.; Grillo, R.; de Jesus, M.B.; Fraceto, L.F. Nanoencapsulation enhances the post-emergence herbicidal activity of atrazine against mustard plants. PLoS ONE 2015, 10, 0132971. [Google Scholar] [CrossRef] [PubMed]
- Kah, M.; Tufenkji, N.; White, J.C. Nano-enabled strategies to enhance crop nutrition and protection. Nat. Nanotechnol. 2019, 14, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Forini, M.M.L.; Antunes, D.R.; Cavalcante, L.A.F.; Pontes, M.S.; Biscalchim, É.R.; Sanches, A.O.; Santiago, E.F.; Fraceto, L.F.; Grillo, R. Fabrication and characterization of a novel herbicide delivery system with magnetic collectability and its phytotoxic effect on photosystem II of aquatic macrophyte. J. Agric. Food Chem. 2020, 68, 11105–11113. [Google Scholar] [CrossRef]
- Reddy, S.S.; Chhabra, V. Nanotechnology: Its scope in agriculture. J. Phys. Conf. Ser. 2022, 2267, 012112. [Google Scholar] [CrossRef]
- Soltani Nejad, M.; Samandari Najafabadi, N.; Aghighi, S.; Pakina, E.; Zargar, M. Evaluation of Phoma sp. Biomass as an Endophytic Fungus for Synthesis of Extracellular Gold Nanoparticles with Antibacterial and Antifungal Properties. Molecules 2022, 27, 1181. [Google Scholar] [CrossRef]
- Cartwright, A.; Jackson, K.; Morgan, C.; Anderson, A.; Britt, D.W. A review of metal and metal-oxide nanoparticle coating technologies to inhibit agglomeration and increase bioactivity for agricultural applications. Agronomy 2020, 10, 1018. [Google Scholar] [CrossRef]
- Wen, Y.; Zhang, L.; Chen, Z.; Sheng, X.; Qiu, J.; Xu, D. Co-exposure of silver nanoparticles and chiral herbicide imazethapyr to Arabidopsis thaliana: Enantioselective effects. Chemosphere 2016, 145, 207–214. [Google Scholar] [CrossRef]
- Cao, L.; Zhou, Z.; Niu, S.; Cao, C.; Li, X.; Shan, Y.; Huang, Q. Positive-charge functionalized mesoporous silica nanoparticles as nanocarriers for controlled 2,4- dichlorophenoxy acetic acid sodium salt release. J. Agric. Food Chem. 2018, 66, 6594–6603. [Google Scholar] [CrossRef] [PubMed]
- Ke, M.; Qu, Q.; Peijnenburg, W.J.G.M.; Li, X.; Zhang, M.; Zhang, Z.; Lu, T.; Pan, X.; Qian, H. Phytotoxic effects of silver nanoparticles and silver ions to Arabidopsis thaliana as revealed by analysis of molecular responses and of metabolic pathways. Sci. Total Environ. 2018, 644, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Sharif, S.N.M.; Hashim, N.; Isa, M.d.I.; Mamat, M.; Mohd Ali, N.; Suriani, A.B.; Hussein, M.; Mustafar, S. The intercalation behaviour and physico-chemical characterization of novel intercalated nanocomposite from zinc/aluminium layered double hydroxides and broadleaf herbicide clopyralid. Chem. Chem. Technol. 2020, 14, 38–46. [Google Scholar] [CrossRef]
- Rebitski, E.; Darder, M.; Aranda, P. Layered double hydroxide/ sepiolite hybrid nanoarchitectures for the controlled release of herbicides. Beilstein J. Nanotechnol. 2019, 9, 1679–1690. [Google Scholar] [CrossRef] [PubMed]
- Ghazali, S.A.I.S.M.; Sarijo, S.H.; Hussein, M.Z. New synthesis of binate herbicide interleaved anionic clay material: Synthesis, characterization and simultaneous controlled- release properties. J. Porous Mater. 2021, 28, 495–505. [Google Scholar] [CrossRef]
- Lima, P.H.C.; Tavares, A.A.; de Lima Silva, S.M.; de Moura, M.R.; Aouada, F.A.; Grillo, R. Recent advances on nanohybrid systems constituting clay–chitosan with organic molecules–a review. Appl. Clay Sci. 2022, 226, 106548. [Google Scholar] [CrossRef]
- Hermosin, M.C.; Calderon, M.J.; Aguer, J.P.; Cornejo, J. Organoclays for controlled release of the herbicide fenuron. Pest. Manag. Sci. 2001, 57, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Bayat, M.; Zargar, M.; Murtazova, K.S.; Nakhaev, M.R.; Shkurkin, S.I. Ameliorating Seed Germination and Seedling of Nano-Primed Wheat and Flax Seeds Using Seven Biogenic Metal-Based Nanoparticles. Agronomy 2022, 12, 811. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Sheng, M.; Cui, S.; Wang, Z.; Zhang, X.; Xu, Q. Enhanced adhesion of carbon nanotubes by dopamine modification. Langmuir 2019, 35, 4527–4533. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Wang, J.; Chu, C.; Chen, W.; Wu, C.; Liu, G. Metal–organic framework-based stimuli-responsive systems for drug delivery. Adv. Sci. 2019, 6, 1801526. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Su, X.; Yan, S.; Shen, J. Multifunctional Nanoparticles and Nanopesticides in Agricultural Application. Nanomaterials 2023, 13, 1255. [Google Scholar] [CrossRef] [PubMed]
- Soltani Nejad, M.; Samandari Najafabadi, N.; Aghighi, S.; Zargar, M.; Stybayev, G.; Baitelenova, A.; Kipshakbayeva, G. Application of biosynthesized silver nanoparticles from oak fruit exudates against Pectobacterium carotovorum subsp. carotovorum causing postharvest soft rot disease in vegetables. Agronomy 2023, 13, 1624. [Google Scholar] [CrossRef]
- Prudnikova, S.V.; Boyandin, A.N.; Kalacheva, G.S.; Sinskey, A.J. Degradable polyhydroxyalkanoates as herbicide carriers. J. Polym. Environ. 2013, 21, 675–682. [Google Scholar] [CrossRef][Green Version]
- Zhang, W.; He, S.; Liu, Y.; Geng, Q.; Ding, G.; Guo, M.; Cao, Y. Preparation and characterization of novel functionalized prochloraz microcapsules using silica–alginate–elements as controlled release carrier materials. ACS Appl. Mater. Interfaces 2014, 6, 11783–11790. [Google Scholar] [CrossRef]
- Xu, C.; Shan, Y.; Bilal, M.; Xu, B.; Cao, L.; Huang, Q. Copper ions chelated mesoporous silica nanoparticles via dopamine chemistry for controlled pesticide release regulated by coordination bonding. J. Chem. Eng. 2020, 395, 125093. [Google Scholar] [CrossRef]
- Shan, Y.; Cao, L.; Xu, C.; Zhao, P.; Cao, C.; Li, F.; Xu, B.; Huang, Q. Sulfonate functionalized mesoporous silica nanoparticles as carriers for controlled herbicide diquat dibromide release through electrostatic interaction. Int. J. Mol. Sci. 2019, 20, 1330. [Google Scholar] [CrossRef]
- Chen, X.; Wang, T. Preparation and characterization of atrazine-loaded biodegradable PLGA nanospheres. J. Integr. Agric. 2019, 18, 1035–1041. [Google Scholar] [CrossRef]
- Takeshita, V.; de Sousa, B.T.; Preisler, A.C.; Carvalho, L.B.; Pereira, A.D.E.S.; Tornisielo, V.L.; Dalazen, G.; Oliveira, H.C.; Fraceto, L.F. Foliar absorption and field herbicidal studies of atrazine-loaded polymeric nanoparticles. J. Hazard. Mater. 2021, 418, 126350. [Google Scholar] [CrossRef]
- de Oliveira, J.L.; Campos, E.V.R.; da Silva, C.M.G.; Pasquoto, T.; Lima, R.; Fraceto, L.F. Solid lipid nanoparticles co-loaded with simazine and atrazine: Preparation, characterization, and evaluation of herbicidal activity. J. Agric. Food Chem. 2015, 63, 422–432. [Google Scholar] [CrossRef]
- Heydari, M.; Yousefi, A.R.; Nikfarjam, N.; Rahdar, A.; Kyzas, G.Z.; Bilal, M. Plantbased nanoparticles prepared from protein containing tribenuron-methyl: Fabrication, characterization, and application. Chem. Biol. Technol. Agric. 2021, 8, 53. [Google Scholar] [CrossRef]
- Maes, C.; Brostaux, Y.; Bouquillon, S.; Fauconnier, M.L. Use of New Glycerol-Based Dendrimers for Essential Oils Encapsulation: Optimization of Stirring Time and Rate Using a Plackett-Burman Design and a Surface Response Methodology. Foods 2021, 10, 207. [Google Scholar] [CrossRef]
- Lima, P.H.C.d.; Antunes, D.R.; Forini, M.M.d.L.; Pontes, M.d.S.; Mattos, B.D.; Grillo, R. Recent advances on lignocellulosic-based nanopesticides for agricultural applications. Front. Nanotechnol. 2021, 3, 809329. [Google Scholar] [CrossRef]
- Kumar, N.; Kumbhat, S. Essentials in Nanoscience and Nanotechnology; Carbon-Based Nanomaterials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 189–236. [Google Scholar]
- Lim, C.J.; Basri, M.; Omar, D.; Abdul Rahman, M.B.; Salleh, A.B.; Raja Abdul Rahman, R.N.Z. Green nanoemulsion-laden glyphosate isopropylamine formulation in suppressing creeping foxglove (A. gangetica), slender button weed (D. ocimifolia) and buffalo grass (P. conjugatum). Pest. Manag. Sci. 2013, 69, 104–111. [Google Scholar] [CrossRef]
- Lim, C.J.; Basri, M.; Omar, D.; Abdul Rahman, M.B.; Salleh, A.B.; Raja Abdul Rahman, R.N.Z. Physicochemical characterization and formation of glyphosate-laden nano-emulsion for herbicide formulation. Ind. Crop. Prod. 2012, 36, 607–613. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, Q.; Yan, W.; Li, B.; Qian, K.; Li, T.; Xiao, W.; He, L. Controlled release of acetochlor from poly (butyl methacrylate-diacetone acrylamide) based formulation prepared by nanoemulsion polymerisation method and evaluation of the efficacy. Int. J. Environ. Anal. Chem. 2014, 1094, 1001–1012. [Google Scholar] [CrossRef]
- Zainuddin, N.J.; Ashari, S.E.; Salim, N.; Asib, N.; Omar, D.; Lian, G.E.C. Optimization and characterization of palm oil-based nanoemulsion loaded with parthenium hysterophorus crude extract for natural herbicide formulation. J. Oleo Sci. 2019, 68, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Shakiba, S.; Astete, C.E.; Paudel, S.; Sabliov, C.M.; Rodrigues, D.F.; Louie, S.M. Emerging investigator series: Polymeric nanocarriers for agricultural applications: Synthesis, characterization, and environmental and biological interactions. Environ. Sci. Nano 2020, 7, 37–67. [Google Scholar] [CrossRef]
- Lu, B.; Lv, X.; Le, Y. Chitosan-modified PLGA nanoparticles for control-released drug delivery. Polymers 2019, 11, 304. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle-based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef]
- Ayoub, H.A.; Khairy, M.; Elsaid, S.; Rashwan, F.A.; Abdel-Hafez, H.F. Pesticidal activity of nanostructured metal oxides for generation of alternative pesticide formulations. J. Agric. Food Chem. 2018, 66, 5491–5498. [Google Scholar] [CrossRef]
- Grillo, R.; Clemente, Z.; de Oliveira, J.L.; Campos, E.V.R.; Chalupe, V.C.; Jonsson, C.M.; Fraceto, L.F. Chitosan nanoparticles loaded the herbicide paraquat: The influence of the aquatic humic substances on the colloidal stability and toxicity. J. Hazard. Mater. 2015, 286, 562–572. [Google Scholar] [CrossRef]
- Liang, W.; Yu, A.; Wang, G.; Zheng, F.; Hu, P.; Jia, J.; Xu, H. A novel water-based chitosan-La pesticide nanocarrier enhancing defense responses in rice (Oryza sativa L.) growth. Carbohydr. Polym. 2018, 199, 437–444. [Google Scholar] [CrossRef]
- Sun, C.J.; Wang, Y.; Zhao, X. Progress on categories and synergistic mechanisms of nanopesticides. Chin. J. Pestic. Sci. 2020, 22, 205–213. [Google Scholar]
- Yang, S.; Gu, N.; Peng, M.; Jiang, Q.; Liu, E.; Li, Z.; Dong, M. A preparation method of nano-pesticide improves the selective toxicity toward natural enemies. Nanomaterials 2022, 12, 2419. [Google Scholar]
- Mahajan, R.; Selim, A.; Neethu, K.; Sharma, S.; Shanmugam, V.; Jayamurugan, G. A systematic study to unravel the potential of using polysaccharides based organic-nanoparticles versus hybrid-nanoparticles for pesticide delivery. Nanotechnology 2021, 32, 47. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Y.; Tian, D.; Shen, F.; Wan, X.; Xu, L.; Chen, Y.; Zhang, H.; Hu, J.; Shen, F. Fabrication and characterization of lignin–xylan hybrid nanospheres as pesticide carriers with enzyme-mediated release property. Soft Matter. 2020, 16, 9083–9093. [Google Scholar] [CrossRef]
- Sinisi, V.; Pelagatti, P.; Carcelli, M.; Migliori, A.; Mantovani, L.; Righi, L.; Leonardi, G.; Pietarinen, S.; Hubsch, C.; Rogolino, D. A green approach to copper-containing pesticides: Antimicrobial and antifungal activity of brochantite supported on lignin for the development of biobased plant protection products. ACS Sustain. Chem. Eng. 2019, 7, 3213–3221. [Google Scholar] [CrossRef]
- Bayat, M.; Zargar, M.; Chudinova, E.; Astarkhanova, T.; Pakina, E. In Vitro Evaluation of Antibacterial and Antifungal Activity of Biogenic Silver and Copper Nanoparticles: The First Report of Applying Biogenic Nanoparticles against Pilidium concavum and Pestalotia sp. Fungi. Molecules 2021, 26, 5402. [Google Scholar] [CrossRef] [PubMed]
- Almasi, H.; Jafarzadeh, P.; Mehryar, L. Fabrication of novel nanohybrids by impregnation of CuO nanoparticles into bacterial cellulose and chitosan nanofibers: Characterization, antimicrobial and release properties. Carbohydr. Polym. 2018, 186, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Bayat, M.; Zargar, M.; Astarkhanova, T.; Pakina, E.; Ladan, S.; Lyashko, M.; Shkurkin, S.I. Facile Biogenic Synthesis and Characterization of Seven Metal-Based Nanoparticles Conjugated with Phytochemical Bioactives Using Fragaria ananassa Leaf Extract. Molecules 2021, 26, 3025. [Google Scholar] [CrossRef] [PubMed]
- Diyanat, M.; Saeidian, H. The metribuzin herbicide in polycaprolactone nanocapsules shows less plant chromosome aberration than non-encapsulated metribuzin. Environ. Chem. Lett. 2019, 17, 1881–1888. [Google Scholar] [CrossRef]
- Pereira, A.E.; Grillo, R.; Mello, N.F.; Rosa, A.H.; Fraceto, L.F. Application of poly(epsilon-caprolactone) nanoparticles containing atrazine herbicide as an alternative technique to control weeds and reduce damage to the environment. J. Hazard. Mater. 2014, 268, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Bansal, K.; Sasso, L.; Makwana, H.; Awwad, S.; Brocchini, S.; Alexander, C. Chapter 10: Nanopharmacy: Exploratory Methods for Polymeric Materials. In Pharmaceutical Nanotechnology: Innovation and Production, 1st ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2017; ISBN 9783527340545. [Google Scholar]
- Sinha, V.R.; Bansal, K.; Kaushik, R.; Kumria, R.; Trehan, A. Poly-ε-caprolactone microspheres and nanospheres: An overview. Int. J. Pharm. 2004, 278, 1–23. [Google Scholar] [CrossRef]
- Fessi, H.; Devissaguet, J.P.; Puisieux, F.; Thies, C. Procédé de Préparation de Systèmes Colloidaux Dispersibles d’une Substance, sous Forme du Nanoparticules. Patent EP 0275796A1, 20 July 1988. [Google Scholar]
- Pulingam, T.; Foroozandeh, P.; Chuah, J.-A.; Sudesh, K. Exploring Various Techniques for the Chemical and Biological Synthesis of Polymeric Nanoparticles. Nanomaterials 2022, 12, 576. [Google Scholar] [CrossRef]
- Lino, R.C.; de Carvalho, S.M.; Noronha, C.M.; Sganzerla, W.G.; da Rosa, C.G.; Nunes, M.R.; Barreto, P.L.M. Development and Characterization of Poly-ε-caprolactone Nanocapsules Containing β-carotene Using the Nanoprecipitation Method and Optimized by Response Surface Methodology. Braz. Arch. Biol. Technol. 2020, 63, 1–12. [Google Scholar] [CrossRef]
- Salvo, P.; Verplancke, R.; Bossuyt, F.; Latta, D.; Vandecasteele, B.; Liu, C.; Vanfleteren, J. Adhesive bonding by SU-8 transfer for assembling microfluidic devices. Microfluid. Nanofluidics 2012, 13, 987–991. [Google Scholar] [CrossRef]
- Leroux, J.C.; Allemann, E.; Doelker, E.; Gurny, R. New approach for the preparation of nanoparticles by an emulsification-diffusion method. Eur. J. Pharm. Biopharm. 1995, 41, 14–18. [Google Scholar]
- Krutyakov, Y.A.; Mukhina, M.T.; Shapoval, O.A.; Zargar, M. Effect of foliar treatment with aqueous dispersions of silver nanoparticles on legume-Rhizobium symbiosis and yield of soybean (Glycine max L. Merr.). Agronomy 2022, 12, 1473. [Google Scholar] [CrossRef]
- Abigail, E.A.; Chidambaram, R. Nanotechnology in Herbicide Resistance. Nanostructured Materials—Fabrication to Applications; Seehra, M.S., Ed.; InTech.: London, UK, 2017; ISBN 978-953-51-3372-8. [Google Scholar]
- Grillo, R.; dos Santos, N.Z.P.; Maruyama, C.R.; Rosa, A.H.; de Lima, R.; Fraceto, L.F. Poly(epsilon-caprolactone) nanocapsules as carrier systems for herbicides: Physico-chemical characterization and genotoxicity evaluation. J. Hazard. Mater. 2012, 231, 1–9. [Google Scholar] [CrossRef]
- Grillo, R.; Rosa, A.H.; Fraceto, L.F. Poly(ε-caprolactone) nanocapsules carrying the herbicide atrazine: Effect of chitosan-coating agent on physicochemical stability and herbicide release profile. Int. J. Environ. Sci. Technol. 2014, 11, 1691–1700. [Google Scholar] [CrossRef]
- de Oliveira, H.C.; Stolf-Moreira, R.; Martinez, C.B.R.; Sousa, G.F.M.; Grillo, R.; de Jesus, M.B.; Fraceto, L.F. Evaluation of the side effects of poly(epsilon-caprolactone) nanocapsules containing atrazine toward maize plants. Front. Chem. 2015, 3, 61. [Google Scholar] [CrossRef]
- de Sousa, B.T.; Pereira, A.D.E.S.; Fraceto, L.F.; de Oliveira, H.C.; Dalazen, G. Effectiveness of nanoatrazine in post-emergent control of the tolerant weed Digitaria insularis. J. Plant Prot. Res. 2020, 60, 185–192. [Google Scholar] [CrossRef]
- Preisler, A.C.; Pereira, A.E.; Campos, E.V.; Dalazen, G.; Fraceto, L.F.; Oliveira, H.C. Atrazine nanoencapsulation improves pre-emergence herbicidal activity against Bidens pilosa without enhancing long-term residual effect on Glycine max. Pest. Manag. Sci. 2020, 76, 141–149. [Google Scholar] [CrossRef]
- Wu, J.; Zhai, Y.; Abdolahpur Monikh, F.; Arenas-Lago, D.; Grillo, R.; Vijver, M.G.; Peijnenburg, W.J.G.M. The Differences between the Effects of a Nanoformulation and a Conventional Form of Atrazine to Lettuce: Physiological Responses, Defense Mechanisms, and Nutrient Displacement. J. Agric. Food Chem. 2021, 69, 12527–12540. [Google Scholar] [CrossRef]
- Moore, A.J.S.; Dean, L.S.N.; Yam, A.N.K.; de Lima, R.; Fraceto, L.F.; Tetley, T.D. Bioreactivity of a novel poly(epsilon-caprolactone) nanocapsule containing atrazine with human lung alveolar epithelial cells. Environ. Sci. Nano 2022, 9, 2134. [Google Scholar] [CrossRef]
- de Sousa, B.T.; Pereira, A.D.E.S.; Fraceto, L.F.; Oliveira, H.C.; Dalazen, G. Post-emergence herbicidal activity of nanoatrazine against Alternanthera tenella Colla plants compared to other weed species. Heliyon 2022, 8, 7. [Google Scholar] [CrossRef]
- Takeshita, V.; Carvalho, L.B.; Galhardi, J.A.; Munhoz-Garcia, G.V.; Pimpinato, R.F.; Oliveira, H.C.; Tornisielo, V.L.; Fraceto, L.F. Development of a Preemergent Nanoherbicide: From Efficiency Evaluation to the Assessment of Environmental Fate and Risks to Soil Microorganisms. ACS Nanosci. Au 2022, 2, 307–323. [Google Scholar] [CrossRef]
- Takeshita, V.; Munhoz-Garcia, G.V.; Werk Pinácio, C.; Cardoso, B.C.; Nalin, D.; Tornisielo, V.L.; Fraceto, L.F. Availability of Metribuzin-Loaded Polymeric Nanoparticles in Different Soil Systems: An Important Study on the Development of Safe Nanoherbicides. Plants 2022, 11, 3366. [Google Scholar] [CrossRef]
- Diyanat, M.; Saeidian, H.; Baziar, S.; Mirjafary, Z. Preparation and characterization of polycaprolactone nanocapsules containing pretilachlor as a herbicide nanocarrier. Environ. Sci. Pollut. Res. 2019, 26, 21579–21588. [Google Scholar] [CrossRef]
- Clemente, Z.; Grillo, R.; Jonsson, M.; Santos, N.Z.; Feitosa, L.O.; Lima, R.; Fraceto, L.F. Ecotoxicological evaluation of poly(epsilon-caprolactone) nanocapsules containing triazine herbicides. J. Nanosci. Nanotechnol. 2014, 14, 4911–4917. [Google Scholar] [CrossRef]
- Mahé, I.; Gauvrit, C.; Angevin, F.; Chauvel, B. Quels enseignements tirer du retrait de l’atrazine dans le cadre de l’interdiction prévue du glyphosate. Cah. Agric. 2020, 29, 29. [Google Scholar] [CrossRef]
- Jablonowski, N.D.; Schäffer, A.; Burauel, P. Still present after all these years: Persistence plus potential toxicity raise questions about the use of atrazine. Environ. Sci. Pollut. Res. 2011, 18, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Giupponi, C. The substitution of hazardous molecules in production processes: The atrazine case study in italian agriculture. SSRN Electron. J. 2001, 18. [Google Scholar] [CrossRef]
- Recker, R.; Mitchell, P.; Stoltenberg, D.; Lauer, J.; Davis, V. Late-Season Weed Escape Survey Reveals Discontinued Atrazine Use Associated with Greater Abundance of Broadleaf Weeds. Weed Technol. 2015, 29, 451–463. [Google Scholar] [CrossRef]
- Ma, H.; Lu, H.; Han, H.; Yu, Q.; Powles, S. Metribuzin resistance via enhanced metabolism in a multiple herbicide resistant Lolium rigidum population. Pest. Manag. Sci. 2020, 76, 3785–3791. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Lee, J.S.; Hwang, I.C.; Park, H.J. Evaluation of Penetration of Nanocarriers into Red Pepper Leaf Using Confocal Laser Scanning Microscopy. Crop Prot. 2014, 66, 61–66. [Google Scholar] [CrossRef]


| Nanomaterials | Effect on Crop Production System | Ref. |
|---|---|---|
| Polymeric nanomaterials | Efficient release of agrochemicals; Outstanding biocompatibility; Reduce the effect on nontargeted organisms. | [8] |
| Silver nanomaterials | Boost plant growth; Act as anti-microbial property; Decrease in pesticide concentration while increasing its efficiency. | [9] |
| Nano-alumino-silicates | Improve the effectiveness of pesticides. | [10] |
| Titanium dioxide | Enhance chlorophyll content, nutrient uptake, activity of Rubisco, and antioxidant enzymes. | [9] |
| Carbon nanomaterials (graphene, graphene oxide, and carbon dots) | Enhance plant seed germination and vigor index, lower transport and leaching potential of pesticides in soil. | [9,11] |
| Metal-based nanoparticles | Improve percent germination, regulate plant uptake of phosphorus and nitrogen. | [9] |
| Active Ingredient | Studied Subjects | Main Results | Reference |
|---|---|---|---|
| Triazine class (ametryn, atrazine, and simazine) | Preparation and characterization of three nanoherbicides; stability assessment; in vitro release kinetics studies; evaluation of genotoxicity on Allium cepa. | Encapsulation efficiency greater than 80%; stable solutions over 270 days. Reduced genotoxicity of ametryn, atrazine, and simazine on Allium cepa; reduction in the dose of application; increases in their absorption by plant; controlled-release mechanism is relaxation of polymer chains. | [101] |
| Atrazine | To modify surface of PCL-atrazine nanocapsules with chitosan and investigate the effect of coating on physico-chemical properties of the nanocapsules. | With addition of chitosan, the zeta potential of nanocapsules shifted from negative to positive amounts, improving their adhesion to the target substrate. | [102] |
| Preparation and characterization of nano-PCL-atrazine and evaluation of its herbicidal activity on Brassica sp. and its genotoxicity on Allium cepa. | Increased herbicidal activity of atrazine; reduced mobility of atrazine in the soil; important root development; reduced genotoxicity (chromosomal aberration) in Allium cepa. | [91] | |
| Preparation and characterization of nano-PCL-atrazine and evaluation of herbicidal activity on Brassica juncea. | Average particle size of 240.7 nm; 10-fold increase in efficacy of commercial atrazine in controlling mustard plants. | [42] | |
| Analyzing the effect of PCL-atrazine on growth, physiological, and oxidative stress parameters of maize plants (Zea mays L.) grown in soil. | PCL-atrazine with concentration of 0.1 mg/mL was effective for weed control; no effects were detected, even shortly after application. Pre- and post-emergence treatment with PCL-atrazine and PCL resulted in no development of any macroscopic symptoms in maize leaves and no impacts on shoot growth. | [103] | |
| Preparation and application of nano-PCL-atrazine and evaluation of its herbicidal activity in field against Amaranthus viridis and Bidens Pilosa. | Increase in the efficiencies of atrazine (more than 50% for both species compared to control with 40% herbicidal efficiency); 10-times-diluted concentration (200 g/ha) of nanoherbicide also showed the same results as commercial one. | [40] | |
| PCL-atrazine nanocapsules in post-emergent control of an atrazine-tolerant weed, sourgrass (D. insularis), in greenhouse. | Faster and greater inhibition of sourgrass photosystem II activity and greater enhancement in dry weight for nanoformulation-treated plants (compared with commercial herbicide). | [104] | |
| Pre-emergence activity of atrazine via nanoencapsulation with PCL against Bidens pilosa; residual effects of nano-PCL-atrazine and conventional atrazine on soybean plants after different periods of soil treatment. | Higher seedling mortality of B. pilosa in soil treatment with nano-PCL-atrazine than in soil treatment with atrazine, even after a 10-fold dilution; greater short-term toxicity effects of nano-atrazine than atrazine, but similar intense toxicity of nano- and non-nano-atrazine in a long-term treatment of soil on soybean. | [105] | |
| Morphoanatomical changes in mustard (B. juncea) leaves based on the foliar uptake of nano-PCL-atrazine in a post-emergent treatment; phytotoxicity and nanoparticle uptake. | Nano-PCL-atrazine stuck to the leaf surface, penetrated mesophyll, and transported through the vascular tissue into the cells and degraded the chloroplasts causing herbicidal activity. | [13] | |
| Comparing the effects of nano-PCL-atrazine and pure atrazine at different concentrations on defense mechanisms, physiological responses, and nutrient displacement in lettuce (Lactuca sativa) as a non-target plant. | In short-term exposure, the growth inhibition of nano-PCL-atrazine was similar to that of atrazine; in long-term exposure, high concentrations of nano-PCL-atrazine had greater negative effects on the end points of ROS production, protein content, and alteration in enzyme activities; nano-PCL-atrazine and atrazine differently affected displacement of nutrients, such as, Cu, K, and Fe, for plant growth. | [106] | |
| Preparation and characterization of nano-PCL-atrazine; to study nanoherbicide–leaf relationship and effects of this system in field and greenhouse on mustard and understand nanoherbicide’s mode of action using radiometric techniques. | Nanocapsule size about 200–300 nm; increased efficiency of atrazine uptake by mustard leaves (40% increase); a 50% reduction in atrazine rate for post-emergence control of R. raphanistrum plants under greenhouse and field conditions; increased inhibition of photosystem II (PSII) activity; improvement in the distribution of the herbicide in the plant; two-fold higher weed control in field compared to conventional formulation. | [66] | |
| Preparation of nano-PCL-atrazine and evaluation of its effect on alveolar epithelial human lung cells. | Nano-PCL-atrazine was more toxic to human lung cells than atrazine or PCL nanocapsules. | [107] | |
| To study post-emergence herbicidal efficiency of PCL-atrazine (at 200 g a. i. ha−1) against Alternanthera tenella Colla in comparison to other weed species. | Nanoformulation showed higher inhibition of maximum quantum efficiency of photosystem II (up to 39%) than commercial atrazine with same concentration. | [108] | |
| Metribuzin | Synthesis of nano-PCL-metribuzin and using it in control of Portulaca oleraceae; evaluation of its toxicity on Allium cepa for pre-emergence applications in soybean. | Particle size of 150–250 nm; encapsulation efficiency of 83.2%; low vertical movement of nanoherbicide in soil (leaching); increased stability of metribuzin; increased herbicidal activity on purslane; nanoherbicide caused less plant chromosome aberration than non-encapsulated metribuzin. | [90] |
| Preparation of nano-PCL-metribuzin and application on control of Ipomoea grandifolia; evaluation of behavior of nanoherbicide in 3 types of soil; comparison of the environmental fate of nanoherbicide with that of commercial metribuzin. | Nanoparticle size of 195 ± 35 nm; encapsulation efficiency of 74.8 ± 0.5%; higher efficiency of nanoherbicide, even at the lowest dose of 48 g a.i. per ha; nosuppressive effects on soil enzymatic activities; lower retention in soil than its commercial analogue; no difference was found in the half-life of metribuzin. | [109] | |
| Trace nano-PCL-metribuzin in different soils; to investigate mobility and retention dynamics of PCL-metribuzin in comparison with conventional formulation. | In deep soils containing fresh organic materials, nano-PCL-metribuzin was sorbed more than commercial formulation (14.61 ± 1.41% and 9.72 ± 1.81%, respectively). | [110] | |
| Polycaprolactone | Preparation of nanoformulation and using it on barnyard grass; to study its effects on rice as anon-target plant); evaluation of its genotoxicity effect. | Particle size 70–200 nm; encapsulation efficiency of 99.5 ± 1.3%; upon genotoxicity experiments, nanoherbicide was less toxic than commercial herbicide; nanoherbicide had no negative effect on rice plant, but a significant effect on barnyard grass. | [111] |
| Ametryn | Ecotoxicological evaluation of PCL-ametryn and triazin class of herbicides; evaluation of their toxicityto aquatic organisms and in cytogenetic tests employing human lymphocyte cultures. | The nanoformulations showed lower toxicity than the commercial ones.Nanoherbicides resulted in lower toxicity to the algae and higher toxicity to the microcrustacean than the herbicides alone. The cytogenetic tests showed that the nanoformulations were less toxic than conventional herbicides. | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zargar, M.; Bayat, M.; Saquee, F.S.; Diakite, S.; Ramzanovich, N.M.; Akhmadovich, K.A.S. New Advances in Nano-Enabled Weed Management Using Poly(Epsilon-Caprolactone)-Based Nanoherbicides: A Review. Agriculture 2023, 13, 2031. https://doi.org/10.3390/agriculture13102031
Zargar M, Bayat M, Saquee FS, Diakite S, Ramzanovich NM, Akhmadovich KAS. New Advances in Nano-Enabled Weed Management Using Poly(Epsilon-Caprolactone)-Based Nanoherbicides: A Review. Agriculture. 2023; 13(10):2031. https://doi.org/10.3390/agriculture13102031
Chicago/Turabian StyleZargar, Meisam, Maryam Bayat, Francess Sia Saquee, Simbo Diakite, Nakhaev M. Ramzanovich, and Khasukhadzhiev A. S. Akhmadovich. 2023. "New Advances in Nano-Enabled Weed Management Using Poly(Epsilon-Caprolactone)-Based Nanoherbicides: A Review" Agriculture 13, no. 10: 2031. https://doi.org/10.3390/agriculture13102031
APA StyleZargar, M., Bayat, M., Saquee, F. S., Diakite, S., Ramzanovich, N. M., & Akhmadovich, K. A. S. (2023). New Advances in Nano-Enabled Weed Management Using Poly(Epsilon-Caprolactone)-Based Nanoherbicides: A Review. Agriculture, 13(10), 2031. https://doi.org/10.3390/agriculture13102031







