Abstract
This study was conducted to test the hypothesis that supplementary blue light, feeding with selenium and iodine can improve the biochemical quality, macro- and micro-elements in the edible parts of fenugreek and, with the accumulation of selenium and iodine in plant tissue, the production of a biofortified crop. For this purpose, the effect of selenium (0, 2, and 4 mg L−1), iodine (0, 2, and 4 mg L−1), and supplementary blue light (no-blue light and blue light treatment) was tested in the form of a three-way factorial experiment based on a completely randomized design. The results showed that supplementary light treatment and feeding with iodine (2 mg L−1) increased the content of phenol, total protein, and vitamin C. The interaction of iodine (4 mg L−1) and blue lighting increased the content of iron and vitamin C. Irrespective of lighting conditions, iodine improved the iodine content. The combination of blue light and 4 mg L−1 selenium increased the nitrogen, iron, phosphorus, and magnesium compared to other treatments. The best conditions for the accumulation of selenium were a combination of blue light and 4 mg L−1 of selenium. Increasing the concentration of feeding with iodine and selenium was beneficial for the accumulation of flavonoids, carbohydrates, protein, and vitamin C. Although the triple effects of feeding with selenium and iodine under blue supplementary light affected some traits, it is difficult to elicit obvious results from them. In general, the application of iodine and selenium (4 mg L−1) under blue light is recommended to achieve the study objectives.
1. Introduction
Biofortification is a practical and cost-effective means of delivering micronutrients to populations that may have limited access to diverse diets and other micronutrient interventions [1]. The primary goal of biofortification is to reduce mortality and morbidity from micronutrient malnutrition and increase food security, productivity, and quality of life for poor populations in developing countries [2]. Since vegetables are relatively cheap and available to the majority of people, the biofortification of staple crops is a primary goal that can overcome the scarcity of rare elements that limit optimal growth. In addition, these elements also have functions in plants; it has been reported that the application of these rare elements led to the relief of various types of stress and increased yield [3], the promotion of plant metabolism, the increase of secondary metabolites, and the reduction of abiotic stress they follow [4]. The concentration of selenium in the soil varies widely in the earth’s crust [5]; therefore, selenium enrichment in places where there is a lack of selenium can lead to a reduction in damages caused by climate changes such as drought, salinity, heavy metals, and extreme temperature. Also, Se regulates the photosynthetic antenna complex and protects chlorophylls by increasing photosynthetic pigments [5]. Selenium (Se) is a nonmetallic or metalloid element that exists in a wide variety of forms, notably as selenide [Se(-II)], elemental selenium [Se(0)], selenite [Se(IV)], or selenate [Se(VI)], all of which occur naturally, are present in the environment and, accumulate in various organisms [6]. Selenate (Se [VI]) and selenite (Se [IV]), the two primary forms absorbable by plants [7,8], observed an increase in leaf photosynthesis, an increase in grain yield, and an increase in seed selenium content by foliar application of Fagopyrum esculentum Moench with 5 g/ha of selenium. In leafy vegetables such as spinach [9], lettuce [10], and basil [11], the application of selenium at the optimal concentration has caused a significant increase in Se content, generally without affecting the biomass and quality of leaves negatively. Iodine is another useful rare element in the human diet, and enriching plants with it can have practical aspects. While iodine deficiency was assumed to be eradicated in developed countries, problems related to it have resurfaced in many developed countries such as Australia, New Zealand, and a number of Western European countries. Andersson et al. [12] stated that approximately 30% of students worldwide have suboptimal dietary iodine intake. Studies by Caldwell et al. [13] in the United States and studies by Trumpff et al. [14] in European countries documented mild iodine deficiency in pregnant women. Meanwhile, the lack is certainly greater in developing countries. Considering that iodine is an important structural component of thyroid hormones such as thyroxine (T4) and triiodothyronine (T3) [15], iodine deficiency may lead to serious problems. In plants, the presence of low concentrations of iodine often has beneficial effects on plant growth, production, and stress resistance, while high concentrations are associated with toxic effects, especially in form I-, which is more phytotoxic than iodine (IO3-) [16]. Kiferle et al. [17] revealed that in Arabidopsis thaliana, iodine-free nutrient solutions jeopardized plant growth while adding iodine at micromolar concentrations (0.20 and 10 μM) was beneficial for biomass accumulation and led to flowering. This is premature. In addition, feeding these plants with iodine specifically regulates the expression of several genes that are mainly involved in plant defense response, indicating that iodine may be effective in alleviating biotic and abiotic stresses. Fenugreek (Trigonella foenum-graecum), belonging to the Fabaceae family, has been widely used for culinary as well as clinical purposes since ancient times. Folk medicine around the world especially uses this plant to boost immunity and combat digestive and reproductive disorders. This plant has a rich reservoir of various herbal compounds to which diverse medicinal effects are attributed [18]. Fenugreek is an important plant that has been recognized as an essential medicinal plant by various scientists around the world. This plant has been widely reported to be useful against several diseases such as cancer, high cholesterol, diabetes, and inflammation [19]. Also, in Iran, its dried powder is used as a spice in many dishes, and one of the important vegetables for cooking is the popular dish of Qorme Sabzi, which is prepared all over the country. Therefore, improving its quality can be important and a suitable option for the purpose of biofortification. In this study, our aim is to improve the quality components of the fenugreek plant, such as the content of minerals, selenium, and iodine content of the edible portion and seeds, the concentration of protein and carbohydrates, and total flavonoid vitamin C, phenolics, and anthocyanin content under the influence of root application of selenium (0, 2, and 4 mg L−1), iodine (0, 2, and 4 mg L−1), and supplementary light (no-supplementary light and addition of 15 µmol/m2/s blue light to the sunlight background). Our assumption was that these treatments would improve the quality characteristics of the edible parts of fenugreek in the greenhouse at optimal levels and enrich the edible part in terms of iodine and selenium.
2. Materials and Methods
2.1. Culture Conditions and Experimental Design
In this study, fenugreek seeds were obtained from Pakan Company (Isfahan, Iran) and rinsed well before planting and treated with benomyl fungicide (2 g per 1000 L of water) for 20 min, followed by 3 rinses with distilled water. After disinfection, fenugreek seeds (10 seeds) were planted in a 4 L pot containing cocopeat/perlite substrate (1:1 ratio) on 15 March 2022, and continued to grow for 5 months after planting. After the stage of 4 true leaves, thinning was conducted and five seedlings were kept. At this stage, they were fed with Hoagland nutrient solution supplemented with sodium selenate (Na2SeO4; 0, 2, and 4 mg L−1) and potassium iodate (KIO3; 0, 2, and 4 mg L−1). The electrical conductivity of the food solution was 1.8–2.0 millisiemens/cm2 and its acidity was 5.8–6.4. The composition of the food solution was as follows: Ca(NO3)2·4H2O, 945 mg L−1; KNO3, 607 mg L−1; MgSO4·7H2O, 493 mg L−1; NH4H2PO4, 115 mg L−1; Na2Fe- EDTA, 30 mg L−1; MnSO4·H2O, 2.13 mg L−1; CuSO4·5H2O, 0.08 mg L−1; ZnSO4·7H2O, 0.22 mg L−1; H3BO3, 2.86 mg L−1; and (NH4)6Mo6O24·4H2O, 0.02 mg L−1. Two hours of blue supplementary light irradiation (intensity 15 μmol m−2 s−1) started at dawn. Two hours of blue light exposure were set by an analog timer. LED lamps were purchased from Iran Guru Light Company (Tehran, Iran). Next, the light intensity was adjusted by adjusting the distance between the lamps and the plants. Light intensity and light quality (Figure 1) were confirmed by a Sekonic light meter made in Japan. The greenhouse environment containing the pots had a relative humidity of 60–70%, the setting sun radiation during planting was 600 µmol m−2 s−1, and the average daily temperature during planting was 22–28 degrees Celsius. The experimental treatments were arranged as a three-factor factorial based on a completely randomized design. Each experimental treatment had three replicates, and the average of three plants per pot was considered as one replicate. Leaf samples were taken at the beginning of fruit formation and stored in a −20 freezer for subsequent analysis.
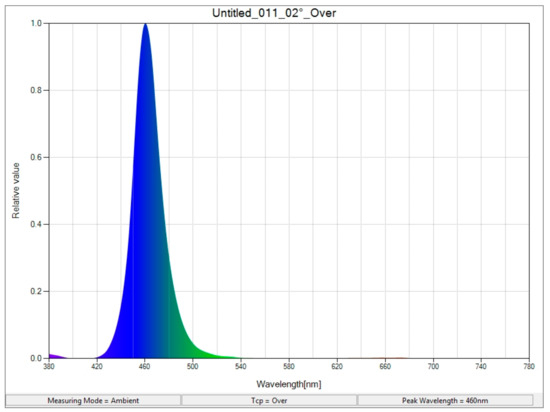
Figure 1.
Light quality quantification (peak length = 450 nm).
2.2. Measurements
2.2.1. Biochemical Determination
Total phenol content. The total phenolic content (TPC) was determined quantitatively using the Folin–Ciocalteu reagent, with gallic acid as the standard [20]. To prepare the extract, 2 g of the plant leaves were crushed and 5 mL of the extraction solution was added to it, and it was placed at room temperature for one hour; after centrifugation, the floating solution (the upper part) was removed using Whatman filter paper, and it was used to measure the amount of phenol. Reading the amount of absorption, 100 µL of the extract was brought to a volume of 8 mL with distilled water, and then 500 µL of diluted Folin–Ciocalteu (50%) was added to it; after one minute of adding Folin–Ciocalteu, 1500 µL of sodium carbonate (20%) was added. The samples were held for two hours at room temperature and dark conditions, and the absorbance was read at the 760 nm wavelength. To prepare the standard solution, a stock solution of gallic acid (0.1 g gallic acid with pure methanol, volume 100 mL), Folin–Ciocalteu (5 mL Folin–Ciocalteu with distilled water, volume 50 mL), and sodium carbonate 5.7% (1.5 g sodium carbonate in 20 mL distilled water) were prepared. Volumes of 10, 15, 20, 25, and 30 µL of gallic acid were added into small glass containers, and 2.5 mL of Folin–Ciocalteu and 2 mL of 7.5% sodium carbonate were added to each of them. The absorbance of the solutions at the wavelength of 760 nm was read using a spectrophotometer (Jenway model, England). Then, the standard curve was drawn from the model absorption. The amount of total phenol was expressed using the sample and standard absorption based on mg/g of wet weight.
Total flavonoid content: Total flavonoid values were determined using the aluminum nitrate method. First, 11.5 mL of 30% ethanol and 0.7 mL of 5% sodium nitrite were added to one mL of the plant extract solution. After 5 min of the reaction, the solution was mixed with 0.7 mL of aluminum nitrate, and after 6 min, 5 mL of 5% sodium hydroxide was added to the existing mixture. Ten minutes later, the absorbance of the samples was read at a wavelength of 760 nm using a spectrophotometer [21].
Total sugar content: The amount of total soluble sugar was determined by the anthrone sulfuric acid colorimetric method [22]. First, fresh samples (0.5 g) of the plant aerial parts (leaves) were heated in a hot water bath, including 10 mL of distilled water, for 30 min. Then, 0.1 mL of supernatant was mixed with 1.9 mL of distilled water, 0.5 mL of anthrone ethyl acetate, and 5 mL of sulfuric acid. After shaking the solution, the soluble sugar was determined using a UV spectrophotometer at a 630 nm wavelength.
Total soluble protein: The Bradford [23] method evaluated the total soluble protein content using the Coomassie Brilliant Blue G-250 dye. At first, 8 mL of distilled water was added to 0.5 g of fresh sample (leaves). The resulting homogenous solution was centrifuged at 3000 rpm for ten minutes at 4 °C. Then, 0.2 mL of the supernatant was combined with 0.8 mL of distilled water and 5 mL of Coomassie Brilliant Blue G-250 dye (0.1 g L−1). After 5 min, the protein content of the solution was determined using a spectrophotometer at a wavelength of 595 nm.
Vitamin C: To determine the amount of vitamin C in the leaves, a titrimetric method with iodine, potassium iodide, and potassium iodate was performed in the presence of a starch reagent [24]; the following solution was prepared with sodium thiosulfate (10 mmol/dm3), potassium iodide (5 mmol/dm3), and potassium iodate (1 mmol/dm3). Titration was conducted using sodium thiosulfate solution in an acidic environment in the presence of a starch reagent. The endpoint of the titration was determined by discoloration (from the initial deep purple color). Vitamin C was calculated by moles of iodine titrated with sodium thiosulfate solution and reported as milligrams per 100 milliliters [25]. The titration process was repeated with a solution containing specific concentrations of standard vitamin C (10, 20, 40, 80, and 160 mg 100 mL−1), and the standard curve was drawn.
2.2.2. Mineral Quantification
The corresponding fully expanded leaves were harvested and washed three times, dehydrated at 60 °C after 72 h, and ground for nutrient quantification. The nitrogen content of the powdered samples was determined by the Kjeldahl method [20]. In order to determine the content of phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), iron (Fe), copper (Cu), manganese (Mn), and zinc (Zn) from the tissue of leaf samples first, the powdered samples were ashed at 500 °C for 6 h. Then, 1 normal hydrochloric acid (HCl) was used to elicit them [26]. The concentrations of K, Ca, Mg, P, Fe, Mn, Zn, and Cu were quantified by inductively coupled plasma (ICP) emission spectrometry (Agilent Technologies, Santa Clara, United States). Also, the estimation of the phosphorus concentration of the samples was determined by the vanadomolybdophosphoric acid colorimetric method [27] and using a spectrophotometer (ShimadzoUV2401 PC).
2.2.3. Selenium and Iodine Quantification
To measure total selenium, 5 g of plant samples (dry weight) were first digested in 25 mL of a mixture of nitric acid and perchloric acid (volume ratio, 4:1) at 130 °C for one hour. After cooling, 5 mL of hydrochloric acid was added and heated at 115 °C for 20 min. After digesting the samples and cooling them at the laboratory temperature, the extracts were transferred to 50 mL tubes and twice distilled water was added to gain a defined volume; this was used to determine the total selenium content using an atomic emission spectrometer device [28].
The plant samples were ashed at 550 °C. Then, 20 mL of distilled water, 20 mL of one molar sulfuric acid, and 10 mL of hydrogen peroxide (10%) were added to one g of ash in a small cup. The mixture was slowly boiled and filtered using a Buchner funnel placed on a vacuum pump. The filtrated material was transferred into a small separator funnel and mixed with 3 mL of tetra-chloromethane. The purple layer of the mixture was moved into a 250 mL flask, and this process was repeated several times to ensure the extraction of all iodine. For calibration, 0.01 M of iodine solution was transferred to a 50 mL flask, and then 25 mL of this solution was kept, and the rest was diluted to draw the calibration graph. The absorbance of the samples was read at a wavelength of 450 nm [29].
2.3. Data Analysis
A least significant difference (LSD) procedure and analysis of variance (ANOVA, GLM proc) of a three-way factorial based on a completely randomized design were fulfilled using SAS 9.4. The results were shown as mean ± standard error (SE). Differences at 5% probability were considered substantial.
3. Results
This study was conducted with the aim of evaluating the effects of root application of selenium and iodine under water supplemental light on quality characteristics, antioxidant compounds, accumulation of iodine and selenium trace elements in edible parts, and absorption of minerals in fenugreek plants. In the following, we will examine the results of the main, two-way, and three-way effects of test factors on different traits in fenugreek plants.
3.1. Evaluation of the Main Effects of Selenium, Iodine, and Supplementary Lighting on Different Traits
With the application of selenium, the copper content was significantly absorbed by plants. With the increase of Se feeding level from 0 to 4 mg L−1, the accumulation of copper in the leaves of plants also increased (Figure 2). Iodine treatment also significantly improved total protein concentration (Figure 3). The highest total protein content was associated with a root application of 4 mg L−1 of iodine. Meanwhile, the lowest total protein content was observed under control and 2 mg L−1 iodine treatment (Figure 3). As presented in Figure 4, root application of iodine significantly positively regulated the phenolic content in the leaves of fenugreek plants. The results of comparing the averages showed that the highest total phenol was accumulated in the plants that were fed with potassium iodide (2 and 4 mg L−1), while the lowest phenolic content was observed in the absence of iodine root application (Figure 4). As shown in Figure 5, 2 h of supplementary blue lighting enhanced protein levels in the foliage of fenugreek compared to the no-supplementary blue light conditions (Figure 5). We also studied Mn accumulation in the leaf tissue of plants under supplemental light conditions. The results showed a significant increase in the manganese content under supplementary light conditions compared to the no-supplementary lighting (Figure 6). The response of vitamin C accumulation to supplementary blue light was also significant; therefore, the supplemental blue light at dawn was an effective condition for vitamin C accumulation compared to the absence of supplemental light (Figure 7). The phenol content was also significantly affected by supplementary light (Figure 8). The results showed that the content of phenolic compounds decreases under the application of supplementary blue light compared to the no-supplementary blue lighting (Figure 8). In general, it is recommended to use 2 h supplementary blue lighting and feeding with iodine to improve the biochemical quality of plants.
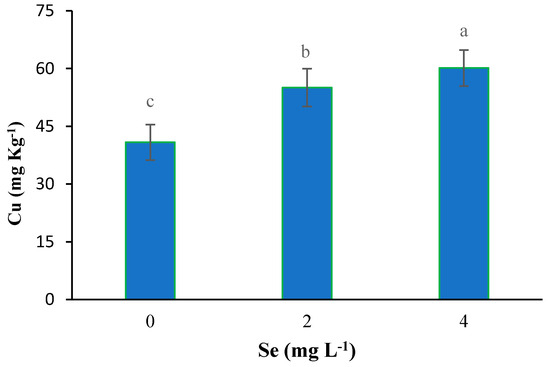
Figure 2.
Main effect of selenium on the Cu content in fenugreek. Means followed by different letters are significantly different by Tukey adjusted means comparisons at p ≤ 0.05.
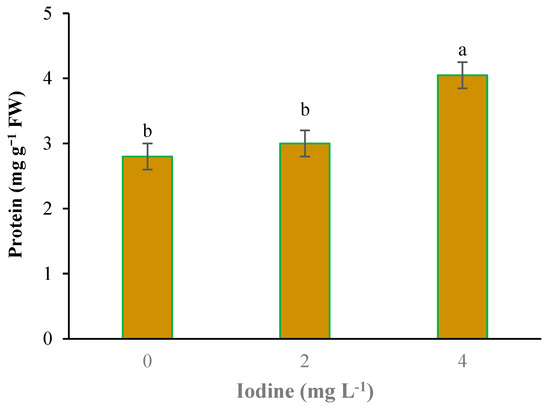
Figure 3.
Main effect of iodine on the protein content in fenugreek. Means followed by different letters are significantly different by Tukey adjusted means comparisons at p ≤ 0.05.
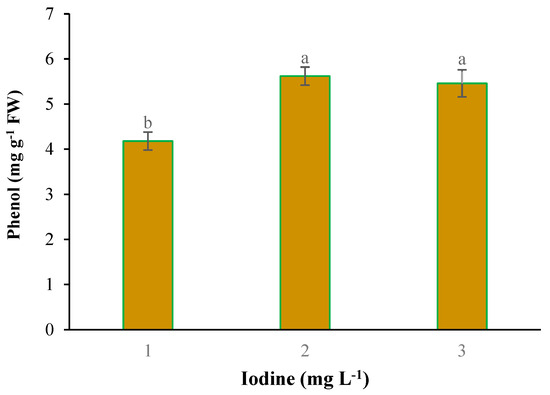
Figure 4.
Main effect of iodine on the phenol content in fenugreek. Means followed by different letters are significantly different by Tukey adjusted means comparisons at p ≤ 0.05.
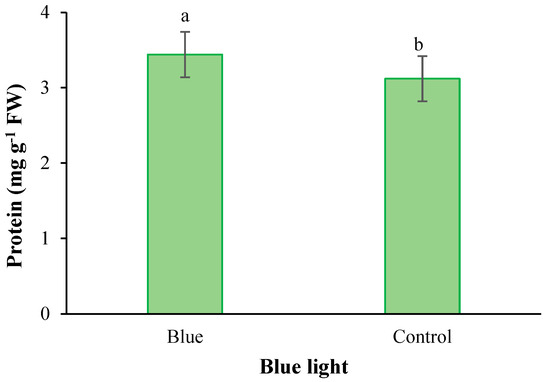
Figure 5.
Main effect of supplementary blue light on the protein content in fenugreek. Means followed by different letters are significantly different by Tukey adjusted means comparisons at p ≤ 0.05.
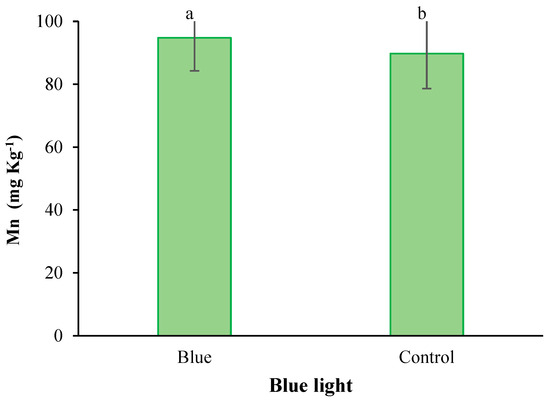
Figure 6.
Main effect of supplementary blue light on the Mn content in fenugreek. Means followed by different letters are significantly different by Tukey adjusted means comparisons at p ≤ 0.05.
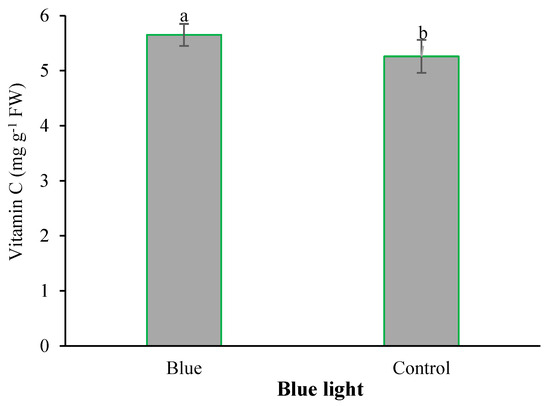
Figure 7.
Main effect of supplementary blue light on the vitamin C content in fenugreek. Means followed by different letters are significantly different by Tukey adjusted means comparisons at p ≤ 0.05.
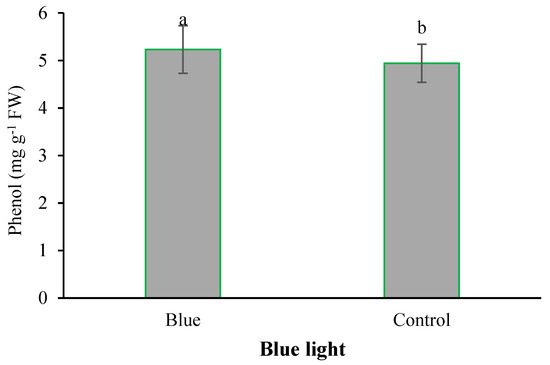
Figure 8.
Main effect supplementary blue light on the phenol content in fenugreek. Means followed by different letters are significantly different by Tukey adjusted means comparisons at p ≤ 0.05.
3.2. Evaluation of the Interaction Effects of Iodine and Blue Light Supplementary Lighting on Different Traits
The response of total carbohydrates, total protein, and vitamin C to the interaction of iodine and supplementary light was significant. The increase in carbohydrate content was the highest under the interaction of blue supplemental light*0 and 2 mg L−1 of iodine and the absence of supplemental light*2 and 4 mg L−1 of selenium. Meanwhile, the lowest content of total carbohydrates was observed under no application of iodine and supplemental blue light (Table 1). In general, a logical trend was not observed in relation to the carbohydrate content under the interaction of iodine and supplementary lighting. The interaction of supplemental blue light with 4 mg L−1 iodine was the most efficient condition for promoting the vitamin C content (Table 1). No significant difference was observed between other treatments. The total protein content was also positively improved under the interaction of supplementary blue light with 4 mg L−1 iodine compared to other treatments (Table 1).

Table 1.
Effects of supplementary blue light and iodine on Fe, Se, protein, carbohydrate, iodine, and vitamin C in fenugreek.
Among the nutritional elements studied, the accumulation of copper and iron was significantly affected by the interaction of iodine and the supplementary illumination of blue light. Although the response of copper to the interaction of iodine and complementary blue light was significant, a logical trend was not observed in this regard (Table 1). In contrast, supplemental blue light and root feeding of 4 mg L−1 of iodine was the most useful treatment for iron accumulation in plant leaves (Table 1). As expected, the concentration of iodine was significantly affected by iodine root application, and regardless of the supplementary illumination of blue light, with the increase in potassium iodate root application, the accumulation of iodine in plant tissue increased (Table 1). Selenium accumulation was also evaluated. Selenium content was lower than other treatments under the interaction of photo-supplemented water with 4 mg L−1 iodine. Among other treatments, no difference was observed in relation to selenium accumulation (Table 1).
3.3. Evaluation of the Interaction Effects of Selenium and Supplementary Blue Lighting on Different Traits
What is interesting is that the interaction of root application of selenium with iodine significantly affected the absorption of nutrients including nitrogen, phosphorus, potassium, magnesium, and iron. The nitrogen content increased under interactions of blue light and an application of 4 mg L−1 compared to other treatments (Table 2). The phosphorus content was also responsible for the interaction of supplementary blue light and root application of selenium so that in the absence of supplementary light and feeding with 2 mg L−1 of selenium, the phosphorus content decreased following the application of supplementary light and 4 mg L−1 of selenium. Other treatments had a higher phosphorus content with no significant difference (Table 2). Supplemental blue light treatment and root application of 2 mg L−1 selenium increased the potassium accumulation in the leaf tissue. The lowest potassium content was related to the absence of supplementary light application and the root application of 4 mg L−1 of selenium (Table 2). Blue supplemental light and root application of 4 mg L−1 of selenium were the best conditions for magnesium absorption following the same light conditions and root application of 2 mg L−1 of selenium (Table 2). We also considered the iron content. The results showed that the root application of 4 mg L−1 of selenium under both lighting conditions increases the iron content in the plant tissue. The lowest iron content was observed in leaves not treated with selenium (Table 2). These results show that feeding plants with 4 mg L−1 of selenium and supplementary blue light is probably a suitable condition for the absorption of nutrients by fenugreek.

Table 2.
Effects of supplementary blue light and selenium on N, P, K, Mg, Fe, and Se in fenugreek.
The selenium content was also analyzed. As expected, the selenium content of plant leaves increased with the increase in selenium feeding concentration, but the best condition for selenium accumulation in the leaves of fenugreek plants was under supplemental blue light and feeding with 4 mg L−1 of selenium (Table 2). As a result, the complementary blue light could probably help the absorption of rare elements.
3.4. Evaluation of the Interaction Effects of Selenium and Iodine on Different Traits
The content of biochemicals including flavonoid, carbohydrate, protein, and vitamin C was significantly responsive to the interaction of iodine and selenium (Table 3 and Table 4). The accumulation of carbohydrates was also significantly affected, but a logical trend was not observed among the treatments (Table 3). The content of vitamin C seemed to be mainly affected by iodine concentration and increased, and its lowest amount was observed when iodine and selenium were not applied (Table 3). In general, it can be concluded that adding 4 mg L−1 of iodine and 4 milligrams per liter of selenium to the food solution can be useful for the accumulation of biochemicals. These results show that trace elements may synergistically help the absorption of other essential nutrients. The contents of iron and manganese were also significantly affected by the interaction of selenium and iodine, while a logical trend was not found in relation to the concentration of nutrition with trace elements (Table 3).

Table 3.
Effects of selenium and iodine on Mn, iodine, Fe, N, K, carbohydrate, and vitamin C in fenugreek.

Table 4.
Effects of selenium and iodine on flavonoid, protein, and Mg in fenugreek.
The accumulation of nitrogen, potassium, magnesium, iron, and manganese was significantly affected by iodine and selenium feeding. In general, the content of nitrogen and potassium increased with increasing concentrations of selenium and iodine in the food solution (Table 3). The magnesium content was also improved by increasing the concentration of trace elements such as selenium and iodine (Table 4). In general, the total protein content increased with increasing selenium and iodine concentrations (Table 4). The accumulation of flavonoids was the highest with the application of 2 mg L−1 of iodine after feeding with 4 mg L−1 of iodine regardless of the selenium concentration (Table 4).
3.5. Evaluation of the Effects on the Interaction of Iodine, Selenium, and Supplementary Light on Different Traits
The effect of selenium, iodine, and supplementary blue light on the content of flavonoid and phenolic compounds was significant (Table 5). Despite this significance, no logical trend can be observed in relation to the different levels of treatments used (Table 5). This trend can also be seen in relation to the absorption of nutrients including phosphorus, zinc, and calcium. In general, it can be said that the triple effects in this study cannot express the efficiency of the interaction over the interaction of iodine, selenium, and supplementary light.

Table 5.
Effects of selenium, iodine, and supplementary blue light on flavonoid, P, Zn, Ca, phenol, and Mg in fenugreek 40 days after seed planting.
4. Discussions
While iodine is part of the structure of the two main thyroid hormones, selenium is present in selenocysteine, which contains glutathione peroxidase, which intervenes in the protection of the thyroid gland [30]. Due to this strong relationship between iodine and selenium in metabolic processes in the human body, selenium deficiency often jeopardizes the positive effect of efforts to iodize table salt. Therefore, many researchers seek to develop methods for the simultaneous supply of selenium and iodine in food [31].
Our assumption in this test was that feeding with selenium and iodine under blue supplementary light would improve the quality characteristics and antioxidant compounds, and with the accumulation of rare elements required by the human body (including selenium and iodine), a biofortified product will be produced. Based on this, biochemicals, macro- and micronutrients, and the content of selenium and iodine in the leaves of fenugreek plants were analyzed. Total phenolic and protein contents were improved in plants fed with iodine. In line with our results, Kiferle et al. [32] showed that iodine-containing compounds improve the antioxidant power, total phenol, rosmarinic acid, and cinnamic acid in basil. Also, in a recent study, Kiferle et al. [17] showed that iodine is incorporated in proteins, especially proteins associated with chloroplasts, and has a function in photosynthesis, which indicates the involvement of iodine in plant nutrition. Supplemental blue light increased the phenolic compounds and vitamin C content in plant tissues. In line with this finding, Larsen et al. [33] revealed that blue light stimulates the accumulation of phenolic compounds such as flavonoids and anthocyanins from the phenylpropanoid pathway. Flavonoids, anthocyanins, and phenolic acids are strong absorbers of reactive oxygen species (ROS). The increase of phenolic compounds under blue light is well documented [34,35,36]. Also, Kang et al. [37] showed that blue light irradiation increases the content of ascorbic acid and the expression of genes related to its biosynthesis in Chinese cabbage, as the results of this study showed. Blue light also increased the content of copper and manganese in the leaves of fenugreek plants. Also, the interaction of iodine and blue light increased the iron content. Different studies have shown that under blue light, the contents of N, P, and K in leaves and false stems of garlic (Allium) sativum L.) increase [38]. In the Gynostemma pentaphyllum plant, red and blue light have also been reported to be useful for the absorption and enrichment of Ca, Fe, Zn, Cu, and Se [39]. However, blue light treatment can also increase the uptake of iron, copper, zinc, and magnesium in lettuce (Lactuca sativa) leaves [40].
Interestingly, the interaction of selenium and supplementary blue light increased the content of nutrients including nitrogen, potassium, and magnesium in the tissues of the samples. Selenium absorption from food solution increased under supplementary blue light. The beneficial effect of selenium (Se) on plants is related to the improvement of nitrogen (N) assimilation and its role as an abiotic stress reducer by inhibiting reactive oxygen species (ROS) that are increased by antioxidant metabolism [5]. Many studies have been conducted on the effect of feeding with selenium on reducing the absorption of heavy metals such as cadmium [41], arsenic [42], and mercury [43] with different plants. However, there is little data regarding the antagonistic or synergistic effects of selenium feeding on macro and micronutrients. Chen et al. [44] showed that high nitrogen fertilization can increase Se uptake and translocation in wheat in soils with low selenium concentration. In this study, along with the nitrogen content, the highest selenium content was observed under blue supplementary light treatment and feeding with 4 mg L−1 of selenium, which is consistent with the findings of Chen et al. [44].
Also, the results showed that the interaction of supplementary blue light and selenium increases the total carbohydrate content. Fan et al. [45] showed that blue light significantly accelerates the concentration of carbohydrates in stored plant tissues and is considered to be a regulator of photosynthesis [45]. Contrary to our results under blue light, a decrease in carbohydrate content was also reported by Ajdanian et al. [46]. However, in the present study, supplementary blue light was used in the form of two-hour radiation in the presence of the full spectrum of the sun. Therefore, the use of blue light along with other spectrums may lead to an increase in the carbohydrate content. Das et al. [47] also reported that selenium increases the accumulation of total soluble sugar and the levels of reducing and non-reducing sugars in rice hulls, as we observed in the present study in fenugreek.
The interaction of selenium and iodine also increased the content of nitrogen and potassium in line with the increase of protein content under the same treatment. This result shows that an increase in nitrogen absorption may have led to an increase in total protein because nitrogen is needed in plants to make amino acids, which are building blocks for protein biosynthesis. In the potato plant, the addition of iodine, selenium, and salicylic acid to nutrient solutions did not significantly change the number or total mass of potato tubers. Meanwhile, this application increased the content of nitrogen, potassium, and sodium in potato tubers and decreased the content of manganese and zinc in the roots [48].
One of the most important goals of this study was the biofortification of fenugreek products with selenium and iodine. As we expected, selenium and iodine contents increased in fenugreek foliage. Cipriano et al. [49] reported that the addition of selenium sources to the soil increases the accumulation of selenium in the leaves and seeds of sorghum. Puccinelli et al. [50] also showed that adding selenium to a basil planting medium can increase the selenium concentration in the biomass of basil and lettuce. The biofortification of iodine through a hydroponic system has shown promising results in spinach [51] and lettuce [52]. In all cases, the bioaccumulation efficiency of iodine from hydroponic solutions was superior to other exposure methods. Cakmak et al. [53] also reported that iodine root application during the growth period resulted in a higher iodine content in wheat.
The triple effects of experimental factors including selenium, iodine, and supplementary light on traits such as flavonoid and phenolic compounds and phosphorus, zinc, and calcium were significant. However, it is difficult to draw a clear conclusion from them.
5. Conclusions
This study was conducted with the aim of investigating the effects of feeding with selenium and iodine under blue supplemental light on antioxidant compounds on the enrichment of the trace elements iodine and selenium in edible parts and absorption of minerals in fenugreek plants. The evaluation of the main effects showed that the application of 2 h of supplementary light and root feeding with iodine (2 mg L−1) is useful for improving the content of phenol, total protein, and vitamin C. The interaction of iodine (4 mg L−1) and supplemental blue light illumination improved the iron and vitamin C content. The iodine concentration increased with the increase of potassium iodate feeding concentration in plant tissue regardless of the supplementary illumination of blue light. The content of nitrogen, iron, phosphorus, and magnesium increased under the interactions of blue light and the application of 4 mg L−1 of selenium compared to other treatments. The best conditions for the accumulation of selenium in the leaves of fenugreek plants were under supplementary blue light and feeding with 4 mg L−1 of selenium. Increasing the concentration of feeding with iodine and selenium was beneficial for the accumulation of flavonoids, carbohydrates, proteins, and vitamin C. Although the triple effects of feeding with selenium and iodine under blue supplementary light affected some traits, it is difficult to draw a conclusion from them, which may be due to the inefficiency of the triple effects of iodine, selenium, and blue light. In general, the application of 4 mg L−1 of iodine and selenium under blue light conditions is recommended to achieve the test objective. For further studies, it is recommended to evaluate the antagonistic and synergistic effects between iodine or selenium with other essential elements.
Author Contributions
Conceptualization, S.R.; methodology, M.Z. and M.B.; software, B.Y.; validation, D.R.; formal analysis, S.R. and E.P.; investigation, B.Y. and M.B.; data curation, E.P.; writing—original draft, S.R., D.R. and M.B.; writing—review and editing, M.Z. and E.P.; supervision, D.R. and M.Z.; project administration, B.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
This work was supported by RUDN University Strategic Academic Leadership Program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bouis, H.E. Biofortification: An agricultural tool to address mineral and vitamin deficiencies. In Food Fortification in a Globalized World; Academic Press: Cambridge, MA, USA, 2018; pp. 69–81. [Google Scholar]
- Kavhiza, N.J.; Zargar, M.; Prikhodko, S.I.; Pakina, E.N.; Murtazova, K.M.-S.; Nakhaev, M.R. Improving Crop Productivity and Ensuring Food Security through the Adoption of Genetically Modified Crops in Sub-Saharan Africa. Agronomy 2022, 12, 439. [Google Scholar] [CrossRef]
- Danso, O.P.; Asante-Badu, B.; Zhang, Z.; Song, J.; Wang, Z.; Yin, X.; Zhu, R. Selenium biofortification: Strategies, progress and challenges. Agriculture 2023, 13, 416. [Google Scholar] [CrossRef]
- Bayat, M.; Zargar, M.; Murtazova, K.M.-S.; Nakhaev, M.R.; Shkurkin, S.I. Ameliorating Seed Germination and Seedling Growth of Nano-Primed Wheat and Flax Seeds Using Seven Biogenic Metal-Based Nanoparticles. Agronomy 2022, 12, 811. [Google Scholar] [CrossRef]
- Lanza, M.G.D.B.; Dos Reis, A.R. Roles of selenium in mineral plant nutrition: ROS scavenging responses against abiotic stresses. Plant Physiol. Biochem. 2021, 164, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, C.B.; Chandrajith, R. Introduction to Medical Geology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Qu, L.; Xu, J.; Dai, Z.; Elyamine, A.M.; Huang, W.; Han, D.; Dang, B.; Xu, Z.; Jia, W. Selenium in soil-plant system: Transport, detoxification and bioremediation. J. Hazard. Mater. 2023, 452, 131272. [Google Scholar] [CrossRef]
- Tao, J.; Leng, J.; Lei, X.; Wan, C.; Li, D.; Wu, Y.; Yang, Q.; Wang, P.; Feng, B.; Gao, J. Effects of selenium (Se) uptake on plant growth and yield in common buckwheat (Fagopyrum esculentum Moench). Field Crops Res. 2023, 302, 109070. [Google Scholar] [CrossRef]
- Saffaryazdi, A.; Lahouti, M.; Ganjeali, A.; Bayat, H. Impact of selenium supplementation on growth and selenium accumulation on spinach (Spinacia oleracea L.) plants. Not. Sci. Biol. 2012, 4, 95–100. [Google Scholar] [CrossRef]
- Goicoechea, N.; Garmendia, I.; Fabbrin, E.G.; Bettoni, M.M.; Palop, J.A.; Sanmartín, C. Selenium fertilization and mycorrhizal technology may interfere in enhancing bioactive compounds in edible tissues of lettuces. Sci. Hortic. 2015, 195, 163–172. [Google Scholar] [CrossRef]
- Mezeyová, I.; Hegedusova, A.; Andrejiová, A.; Hegedus, O.; Golian, M. Phytomass and content of essential oils in Ocimum basilicum after foliar treatment with selenium. J. Int. Sci. Publ. 2016, 4, 19–27. [Google Scholar]
- Andersson, M.; Karumbunathan, V.; Zimmermann, M.B. Global iodine status in 2011 and trends over the past decade. J. Nutr. 2012, 142, 744–750. [Google Scholar] [CrossRef]
- Caldwell, K.L.; Pan, Y.; Mortensen, M.E.; Makhmudov, A.; Merrill, L.; Moye, J. Iodine status in pregnant women in the National Children’s Study and in US women (15–44 years), National Health and Nutrition Examination Survey 2005–2010. Thyroid 2013, 23, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Trumpff, C.; De Schepper, J.; Tafforeau, J.; Van Oyen, H.; Vanderfaeillie, J.; Vandevijvere, S. Mild iodine deficiency in pregnancy in Europe and its consequences for cognitive and psychomotor development of children: A review. J. Trace Elem. Med. Biol. 2013, 27, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Turan, E.; Karaaslan, O. The relationship between iodine and selenium levels with anxiety and depression in patients with euthyroid nodular goiter. Oman Med. J. 2020, 35, e161. [Google Scholar] [CrossRef] [PubMed]
- Incrocci, L.; Carmassi, G.; Maggini, R.; Poli, C.; Saidov, D.; Tamburini, C.; Kiferle, C.; Perata, P.; Pardossi, A. Iodine accumulation and tolerance in sweet basil (Ocimum basilicum L.) with green or purple leaves grown in floating system technique. Front. Plant Sci. 2019, 10, 1494. [Google Scholar] [CrossRef]
- Kiferle, C.; Martinelli, M.; Salzano, A.M.; Gonzali, S.; Beltrami, S.; Salvadori, P.A.; Hora, K.; Holwerda, H.T.; Scaloni, A.; Perata, P. Evidences for a nutritional role of iodine in plants. Front. Plant Sci. 2021, 12, 616868. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Yadav, S.S.; Kumar, S.; Narashiman, B. Ethnopharmacological, phytochemical and clinical studies on Fenugreek (Trigonella foenum-graecum L.). Food Biosci. 2022, 46, 101546. [Google Scholar] [CrossRef]
- Syed, Q.A.; Rashid, Z.; Ahmad, M.H.; Shukat, R.; Ishaq, A.; Muhammad, N.; Rahman, H.U.U. Nutritional and therapeutic properties of fenugreek (Trigonella foenum-graecum): A review. Int. J. Food Prop. 2020, 23, 1777–1791. [Google Scholar] [CrossRef]
- McDonald, S.; Prenzler, P.D.; Autolovich, M.; Robards, K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001, 73, 73–84. [Google Scholar] [CrossRef]
- Bayat, M.; Zargar, M.; Astarkhanova, T.; Pakina, E.; Ladan, S.; Lyashko, M.; Shkurkin, S.I. Facile Biogenic Synthesis and Characterization of SevenMetal-Based Nanoparticles Conjugated with Phytochemical Bioactives Using Fragaria ananassa Leaf Extract. Molecules 2021, 26, 3025. [Google Scholar] [CrossRef]
- Bayat, M.; Engeribo, A.; Meretukov, Z.; Aigerim, A.; Temewei, A.G.; Dubrovina, T.; Zargar, M. Response of common lambsquarters (Chenopodium album L.) to chemical weed control programs. Res. Crops 2019, 20, 859–863. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Brater, M.D. The United States Pharmacopoeia (USP) 25 and National Formulary (NF) 20; Webcom Limited: Toronto, ON, Canada, 2002; p. 163. [Google Scholar]
- Skinner, J. Experiments in Chemistry, Measuring the amount of vitamin C in fruit drinks. In Microscale Chemistry; Royal Society of Chemistry: London, UK, 1997; p. 67. [Google Scholar]
- Savvas, D.; Gizas, G. Response of hydroponically grown gerbera to nutrient solution recycling and different nutrient cation ratios. Sci. Hortic. 2002, 96, 267–280. [Google Scholar] [CrossRef]
- Soltani Nejad, M.; Samandari Najafabadi, N.; Aghighi, S.; Pakina, E.; Zargar, M. Evaluation of Phoma sp. Biomass as an Endophytic Fungus for Synthesis of Extracellular Gold Nanoparticles with Antibacterial and Antifungal Properties. Molecules 2022, 27, 1181. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.L.; Gu, Z.X. Selenium accumulation in different brown rice cultivars and its distribution in fractions. J. Agric. Food Chem. 2009, 57, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Liprot, G.F. Modem Inorganic Chemistry, 2nd ed.; Mills & Boon Ltd.: London, UK, 1971; p. 339. [Google Scholar]
- Schomburg, L.; Köhrle, J. On the importance of selenium and iodine metabolism for thyroid hormone biosynthesis and human health. Mol. Nutr. Food Res. 2008, 52, 1235–1246. [Google Scholar] [CrossRef]
- Duborská, E.; Šebesta, M.; Matulová, M.; Zvěřina, O.; Urík, M. Current Strategies for Selenium and Iodine Biofortification in Crop Plants. Nutrients 2022, 14, 4717. [Google Scholar] [CrossRef] [PubMed]
- Kiferle, C.; Ascrizzi, R.; Martinelli, M.; Gonzali, S.; Mariotti, L.; Pistelli, L.; Flamini, G.; Perata, P. Correction: Effect of Iodine treatments on Ocimum basilicum L.: Biofortification, phenolics production and essential oil composition. PLoS ONE 2020, 15, e0229016. [Google Scholar] [CrossRef] [PubMed]
- Larsen, D.H.; Li, H.; Shrestha, S.; Verdonk, J.C.; Nicole, C.; Marcelis, L.F.; Woltering, E.J. Lack of blue light regulation of antioxidants and chilling tolerance in Basil. Front. Plant Sci. 2022, 13, 852654. [Google Scholar] [CrossRef]
- Son, K.H.; Oh, M.M. Growth, photosynthetic and antioxidant parameters of two lettuce cultivars as affected by red, green, and blue light-emitting diodes. Hortic. Environ. Biotechnol. 2015, 56, 639–653. [Google Scholar] [CrossRef]
- Qian, H.; Liu, T.; Deng, M.; Miao, H.; Cai, C.; Shen, W.; Wang, Q. Effects of light quality on main health-promoting compounds and antioxidant capacity of Chinese kale sprouts. Food Chem. 2016, 196, 1232–1238. [Google Scholar] [CrossRef]
- Taulavuori, K.; Hyöky, V.; Oksanen, L.; Taulavuori, E.; Julkunen-Tiitto, R. Species-specific differences in synthesis of flavonoids and phenolic acids under increasing periods of enhanced blue light. Environ. Exp. Bot. 2016, 121, 145–150. [Google Scholar] [CrossRef]
- Kang, C.H.; Yoon, E.K.; Muthusamy, M.; Kim, J.A.; Jeong, M.J.; Lee, S.I. Blue LED light irradiation enhances L-ascorbic acid content while reducing reactive oxygen species accumulation in Chinese cabbage seedlings. Sci. Hortic. 2020, 261, 108924. [Google Scholar] [CrossRef]
- Yang, X.J. Effects of Light Quality on the Physiological Characteristics and Quality in Garlic Seedling. Ph.D. Thesis, Shandong Agricultural University, Tai’an, China, 2011. (In Chinese). [Google Scholar]
- Lee, H.J.; Ha, J.H.; Kim, S.G.; Choi, H.K.; Kim, Z.H.; Han, Y.J.; Kim, J.I.; Oh, Y.; Fragoso, V.; Shin, K.; et al. Stem-piped light activates phytochrome B to trigger light responses in Arabidopsis thaliana roots. Sci. Signal. 2016, 9, ra106. [Google Scholar] [CrossRef] [PubMed]
- Xu, L. Effect of Light Quality on Physiological Characteristics and Quality of Leaf Lettuce. Ph.D. Thesis, Shandong Agricultural University, Tai’an, China, 2007. (In Chinese). [Google Scholar]
- Affholder, M.C.; Flöhr, A.; Kirchmann, H. Can Cd content in crops be controlled by Se fertilization? A meta-analysis and outline of Cd sequestration mechanisms. Plant Soil 2019, 440, 369–380. [Google Scholar] [CrossRef]
- Camara, A.Y.; Wan, Y.; Yu, Y.; Wang, Q.; Li, H. Effect of selenium on uptake and translocation of arsenic in rice seedlings (Oryza sativa L.). Ecotox. Environ. Saf. 2018, 148, 869–875. [Google Scholar] [CrossRef]
- Wang, Y.; Dang, F.; Evans, R.D.; Zhong, H.; Zhao, J.; Zhou, D. Mechanistic understanding of MeHg-Se antagonism in soil-rice systems: The key role of antagonism in soil. Sci. Rep. 2016, 6, 19477. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, D.; Song, W.; Lei, L.; Yu, D.; Miao, S. Effects of nitrogen application on selenium accumulation, translocation and distribution of winter wheat at different growth periods. J. Plant Nutr. Fert. 2016, 22, 395–402. (In Chinese) [Google Scholar]
- Fan, X.X.; Xu, Z.G.; Liu, X.Y.; Tang, C.M.; Wang, L.W.; Han, X.L. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Ajdanian, L.; Babaei, M.; Aroiee, H. Investigation of photosynthetic effects, carbohydrate and starch content in cress (Lepidium sativum) under the influence of blue and red spectrum. Heliyon 2020, 6, e05628. [Google Scholar] [CrossRef]
- Das, D.; Seal, P.; Biswas, A.K. Influence of selenium on growth, antioxidants production and physiological parameters of rice (Oryza sativa L.) seedlings and its possible reversal by coapplication of sulphate. Am. J. Plant Sci. 2019, 10, 2236–2278. [Google Scholar] [CrossRef]
- Smoleń, S.; Kowalska, I.; Skoczylas, Ł.; Liszka-Skoczylas, M.; Grzanka, M.; Halka, M.; Sady, W. The effect of salicylic acid on biofortification with iodine and selenium and the quality of potato cultivated in the NFT system. Sci. Hortic. 2018, 240, 530–543. [Google Scholar] [CrossRef]
- Cipriano, P.E.; da Silva, R.F.; de Lima, F.R.D.; de Oliveira, C.; de Lima, A.B.; Celante, G.; Dos Santos, A.A.; Archilha, M.V.; Pinatto-Botelho, M.F.; Faquin, V.; et al. Selenium biofortification via soil and its effect on plant metabolism and mineral content of sorghum plants. J. Food Compos. Anal. 2022, 109, 104505. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Pintimalli, L.; Rosellini, I.; Pezzarossa, B. Biofortification of lettuce and basil seedlings to produce selenium enriched leafy vegetables. Horticulturae 2022, 8, 801. [Google Scholar] [CrossRef]
- Weng, H.-X.; Yan, A.-L.; Hong, C.-L.; Xie, L.-L.; Qin, Y.-C.; Cheng, C.Q. Uptake of Different Species of Iodine by Water Spinach and Its Effect to Growth. Biol. Trace Elem. Res. 2008, 124, 184–194. [Google Scholar] [CrossRef]
- Blasco, B.; Rios, J.J.; Cervilla, L.; Ruiz, J.; Romero, L.; Sánchez-Rodrigez, E. Iodine biofortification and antioxidant capacity of lettuce: Potential benefits for cultivation and human health. Ann. Appl. Biol. 2008, 152, 289–299. [Google Scholar] [CrossRef]
- Cakmak, I.; Marzorati, M.; Van den Abbeele, P.; Hora, K.; Holwerda, H.T.; Yazici, M.A.; Savasli, E.; Neri, J.; Du Laing, G. Fate and bioaccessibility of iodine in food prepared from agronomically biofortified wheat and rice and impact of cofertilization with zinc and selenium. J. Agric. Food Chem. 2020, 68, 1525–1535. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).