Seed Fatty Acid Changes Germination Response to Temperature and Water Potentials in Six Sesame (Sesamum indicum L.) Cultivars: Estimating the Cardinal Temperatures
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Material
2.2. Test Specifications
2.3. Cardinal Temperature
- The dent-like temperature function with the abbreviation (D) is as follows (Equation (3)):
- The segmented function with the abbreviation (S) is as follows (Equation (4)):
- The beta function with the abbreviation (B) is as follows, and α is the shape parameter for the beta function that determines the curvature of the function (Equation (5)):
- Root Mean Square Error (RMSE)P and O are the predicted values of the germination rate using the model and the observed value, and n is the number of observations.
- R2 (Coefficient of determination) (Equation (7))where SSE and SSG are the sums of squared error and a sum of total squared, respectively.
- Simple linear regression coefficients (a and b) between predicted values and actual values. Coefficients a and b indicate the deviation of the regression line from the coordinate origin and the deviation of the regression line from the 1:1 line, respectively.
- Linear correlation coefficient (r) between observed and predicted germination days.
- Concordance correlation coefficient (rc) (Equation (8))where r is Pearson’s correlation coefficient, Cb is the accuracy of the model, rc is based on the predicted value of the germination rate using the model (X), the observed value (Y), and N (the number of observations), calculated based on the following Equation (9):
2.4. Seed Oil Extraction and Measurement of Fatty Acids Profile
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kozłowska, W.; Matkowski, A.; Zielińska, S. Light intensity and temperature effect on Salvia yangii (BT Drew) metabolic profile in vitro. Front. Plant Sci. 2022, 13, 888509. [Google Scholar] [CrossRef] [PubMed]
- Donohue, K.; Casas, R.R.D.; Burghardt, L.; Kovach, K.; Willis, C.G. Germination, post-germination adaptation, and species ecological ranges. Annu. Rev. Ecol. Syst. 2010, 41, 293–319. [Google Scholar] [CrossRef]
- Savaedi, Z.; Parmoon, G.; Moosavi, S.A.; Bakhshande, A. Light and Gibberellic Acid’s role in cardinal temperatures and thermal time required for the Charnushka (Nigella sativa) seed germination. Ind. Crops Prod. 2019, 132, 140–149. [Google Scholar] [CrossRef]
- Sanehkoori, F.H.; Pirdashti, H.; Bakhshandeh, E. Quantifying water stress and temperature effects on camelina (Camelina sativa L.) seed germination. Environ. Exp. Bot. 2021, 186, 104450. [Google Scholar] [CrossRef]
- Saeed, S.; Ullah, A.; Ullah, S.; Noor, J.; Ali, B.; Khan, M.N.; Hashem, M.; Mostafa, Y.S.; Alamri, S. Validating the impact of water potential and temperature on seed germination of wheat (Triticum aestivum L.) via hydrothermal time model. Life 2022, 12, 983. [Google Scholar] [CrossRef] [PubMed]
- Sghaier, H.; Tarnawa, A.; Khaeim, H.; Kovács, G.P.; Gyuricza, C.; Kende, Z. The effects of temperature and water on rapeseed seed germination and seedling development (Brassica napus L.). Plants 2022, 11, 2819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chen, D.; Liu, H.; Guo, C.; Tang, L.; Wang, H.; Chen, Y.; Luo, K. Effect of temperature and water potential on the germination of seeds from three different populations of Bidens pilosa as a potential Cd hyperaccumulator. BMC Plant Biol. 2022, 22, 487. [Google Scholar] [CrossRef]
- Khan, W.; Shah, S.; Ullah, A.; Ullah, S.; Amin, F.; Iqbal, B.; Ahmad, N.; Abdel-Maksoud, M.A.; El-Zaidy, M.; Al-Qahtani, W.H.; et al. Utilizing hydrothermal time models to assess the effects of temperature and osmotic stress on maize (Zea mays L.) germination and physiological responses. BMC Plant Biol. 2023, 23, 414. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, K.; Li, X.; Chen, X.; Liu, W.; Wang, J. Factors affecting seed germination and emergence of Aegilops tauschii. Weed Res. 2020, 60, 171–181. [Google Scholar] [CrossRef]
- Bewley, J.; Bradford, K.; Hilhorst, H.; Nonogaki, H. Seeds: Physiology of Development, Germination, and Dormancy, 3rd ed.; Springer: Cham, Switzerland, 2013; pp. 85–246. [Google Scholar]
- Bello, P.; Bradford, K.J. Relationships of Brassica seed physical characteristics with germination performance and plant blindness. Agriculture 2021, 11, 220. [Google Scholar] [CrossRef]
- Ostadian Bidgoly, R.; Balouchi, H.; Soltani, E.; Moradi, A. Effect of temperature and water potential on Carthamus tinctorius L. Seed germination: Quantification of the cardinal temperature and modeling using hydrothermal time. Ind. Crops Prod. 2018, 113, 121–127. [Google Scholar] [CrossRef]
- Rowse, H.R.; Finch-Savage, W.E. Hydrothermal threshold models can describe the germination response of carrot (Daucus carota) and onion (Allium cepa) seed populations across both sub-and supra-optimal temperatures. New Phytol. 2003, 158, 101–108. [Google Scholar] [CrossRef]
- UCana, K.; Killi, F. Effects of different irrigation programs on flower and capsule numbers and shedding percentage of sesame. Agric. Water Manag. 2010, 98, 227–233. [Google Scholar] [CrossRef]
- Rostami, M.; Farzaneh, V.; Boujmehrani, A.; Mohammadi, M.; Bakhshabadi, H. Optimizing the extraction process of sesame seedís oil using response surface method on the industrial scale. Ind. Crops Prod. 2014, 58, 160–165. [Google Scholar] [CrossRef]
- Were, B.A.; Onkware, A.O.; Gudu, S.; Welander, M.; Carlsson, A.S. Seed oil content and fatty acid composition in East African sesame (Sesamum indicum L.) accessions evaluated over three years. Field Crops Res. 2006, 97, 254–260. [Google Scholar] [CrossRef]
- Ahmed, I.A.M.; AlJuhaimi, F.; Özcan, M.M.; Ghafoor, K.; Şenay Şimşek, S.; Babiker, E.E.; Osman, M.A.; Gassem, M.A.; Salih, H.A.A. Evaluation of chemical properties, amino acid contents, and fatty acid compositions of sesame seed provided from different locations. J. Oleo Sci. 2020, 69, 795–800. [Google Scholar] [CrossRef]
- Dossa, K.; Li, D.; Zhou, R.; Yu, J.; Wang, L.; Zhang, Y.; Zhang, X. The genetic basis of drought tolerance in the high oil crop Sesamum indicum. Plant Biotechnol. J. 2019, 17, 1788–1803. [Google Scholar] [CrossRef]
- Langyan, S.; Yadava, P.; Sharma, S.; Gupta, N.C.; Bansal, R.; Yadav, R.; Kalia, S.; Kumar, A. Food and nutraceutical functions of sesame oil: An underutilized crop for nutritional and health benefits. Food Chem. 2022, 389, 132990. [Google Scholar] [CrossRef]
- Belo, R.G.; Tognetti, J.; Benech-Arnold, R.; Izquierdo, N.G. Sunflowers’ seed oil composition affects germination responses to temperature and water potential. Ind. Crops Prod. 2014, 62, 537–544. [Google Scholar] [CrossRef]
- Michel, B.E.; Kaufmann, M.R. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973, 51, 914–916. [Google Scholar] [CrossRef]
- Soltani, A.; Robertson, M.; Torabi, B.; Yousefi-Daz, M.; Sarparast, R. Modeling seedling emergence in chickpeas as influenced by temperature and sowing depth. Agric. For. Meteorol. 2006, 138, 156–167. [Google Scholar] [CrossRef]
- Yol, E.; Toker, R.; Golukcu, M.; Uzun, B. Oil content and fatty acid characteristics in Mediterranean sesame core collection. Crop Sci. 2015, 55, 2177–2185. [Google Scholar] [CrossRef]
- Azadmard-Damirchi, S.; Habibi-Nodeh, F.; Hesari, J.; Nemati, M.; Fathi Achachlouei, B. Effect of pretreatment with microwaves on oxidative stability and nutraceuticals content of oil from rapeseed. Food Chem. 2010, 121, 1211–1215. [Google Scholar] [CrossRef]
- Azadmard-Damirchi, S.; Dutta, P.C. A novel solid-phase extraction method separates 4-desmethyl-, 4-monomethyl-, and 4, 4′-dimethyl sterols in vegetable oils. J. Chromatogr. A 2006, 1108, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Teimori, H.; Balouchi, H.; Moradi, A.; Soltani, E. Effect of seed aging and water potential on seed germination and biochemical indices of Fenugreek (Trigonella foenum-graecum) at different temperatures. Iran. J. Seed Res. 2019, 5, 105–128. [Google Scholar] [CrossRef]
- Tajlil, A.H.; Pazoki, A.; Eradatmand Asli, D. Effects of seed priming by mannitol and zinc sulfate on biochemical parameters and seed germination of chickpea. Int. J. Farming Allied Sci. 2014, 3, 294–298. [Google Scholar]
- Hoseini, A.; Salehi, A.; Sayyed, R.Z.; Balouchi, H.; Moradi, A.; Piri, R.; Fazeli-Nasab, B.; Poczai, P.; Ansari, M.J.; Obaid, S.A.; et al. Efficacy of biological agents and fillers seed coating in improving drought stress in anise. Front. Plant Sci. 2022, 13, 955512. [Google Scholar] [CrossRef]
- Khalili, N.; Soltani, A.; Zeinali, E.; Ghaderi far, F. Evaluation of nonlinear regression models to quantify barley germination rate response to temperature and water potential. J. Crop Prod. 2015, 7, 40–23. [Google Scholar]
- Torabi, B.; Soltani, E.; Archontoulis, S.V.; Rabii, A. Temperature and water potential effects on Carthamus tinctorius L. Seed germination: Measurements and modeling using hydrothermal and multiplicative approaches. Braz. J. Bot. 2016, 39, 427–436. [Google Scholar] [CrossRef]
- Soltani, E.; Oveisi, M.; Soltani, A.; Galeshi, S.; Ghaderifar, F.; Zeinali, E. Affected by temperature and water Seed germination modeling of volunteer canola as potential: Hydrothermal time model. Weed Res. J. 2014, 6, 23–38. [Google Scholar]
- Soltani, E.; Soltani, A.; Galeshi, S.; Ghaderi-Far, F.; Zeinali, E. Seed germination modeling of wild mustard (Sinapis arvensis L.) as affected by temperature and water potential: Hydrothermal time model. J. Plant Prod. Res. 2013, 20, 19–34. [Google Scholar]
- Parmoon, G.; Moosavi, A.; Akbari, H.; Ebadi, A. Quantifying cardinal temperatures and thermal time required for germination of Silybum marianum seed. Crop J. 2015, 3, 145–151. [Google Scholar] [CrossRef][Green Version]
- Nozari-Nejad, M.; Zeinali, E.; Soltani, A.; Soltani, E.; Kamkar, B. Quantify wheat germination rate response to temperature and water potential. J. Crop Prod. 2014, 6, 117–135. [Google Scholar]
- Yasari, E.; Miri, M.; Atashi, S.; Jamali, M. Application of hydrothermal time model to determine the cardinal temperatures for seed germination in crops (A case study; velvetleaf (Abutilon theophrasti Med.)). Iran. J. Seed Sci. Technol. 2018, 7, 85–94. [Google Scholar]
- Queiroz, M.S.; Oliveira, E.S.; Steiner, F.; Zuffo, A.M.; Zoz, T.; Vendruscolo, E.P.; Silva, M.V.; Mello, F.F.R.; Cabral, R.C.; Menis, F.T. Drought stresses seed germination and early growth of maize and sorghum. J. Agric. Sci. 2019, 11, 310–318. [Google Scholar] [CrossRef]
- Sun, M.; Spears, J.F.; Isleib, T.G.; Jordan, D.L.; Penny, B.; Johnson, D.; Copeland, S. Effect of production environment on seed quality of normal and high-oleate large seeded Virginia-type peanut (Arachis hypogaea L.). Peanut Sci. 2014, 41, 90–99. [Google Scholar] [CrossRef]
- Moosavi, S.A.; Siadat, S.A.; Poshtdar, A.; Direkvand, F. Ultrasonic-assisted seed priming to alleviate aging damages to milk thistle (Silybum marianum) seeds. Not. Sci. Biol. 2018, 10, 275–281. [Google Scholar] [CrossRef][Green Version]
- Asadi Aghbolaghi, M.; Sedghi, M.; Sharifi, R.S.; Dedicova, B. Germination and the biochemical response of pumpkin seeds to different concentrations of humic acid under cadmium stress. Agriculture 2022, 12, 374. [Google Scholar] [CrossRef]
- Ghadri-Far, F.; Soltani, E. Evaluation of sesame cultivars germination on response to temperature: Determination of cardinal temperatures and thermal tolerance. Iran. J. Field Crop Sci. 2015, 46, 473–483. [Google Scholar] [CrossRef]
- Oteng, A.; Kersten, S. Mechanisms of action of trans fatty acids. Adv. Nutr. 2020, 11, 697–708. [Google Scholar] [CrossRef]
- Islam, S.; Carmen, R.C.; Garner, J.O. Fatty acid compositions in ungerminated (whole seed), cotyledon, and embryo tissues of cowpea (Vigna unguiculata L. Walp) seed grown under different temperatures. J. Food Agric. Environ. 2007, 5, 190–196. [Google Scholar]
- Yeilaghi, H.; Arzani, A.; Ghaderian, M.; Fotovat, R.; Feizi, M.; Pourdad, S.S. Effect of salinity on seed oil content and fatty acid composition of safflower (Carthamus tinctorius L.) genotypes. Food Chem. 2012, 130, 618–625. [Google Scholar] [CrossRef]
- Martinez-Rivas, L.M.; Gracia-Diaz, M.T.; Mancha, M. Temperature and oxygen regulation of microsomal oleate desaturase (FAD2) from sunflower. Biochem. Soc. 2000, 28, 890–892. [Google Scholar] [CrossRef]
- Rabiei, Z.; Tahmasebi, E.S.; Vannozzi, G.P. Regulation of polyunsaturated fatty acids accumulation and characterization of linolenic acid after sunflower seed germination. Helia 2007, 30, 175–182. [Google Scholar] [CrossRef]
- Sogut, T.; Ozturk, F.; Kizil, S. Effect of sowing time on peanut (Arachis hypogaea L.) cultivars: I.e.; yield, yield components, oil, and protein content. Sci. Pap. Ser. A Agron. 2016, 59, 415–420. [Google Scholar] [CrossRef]
- Sehgal, A.; Sita, K.; Siddique, K.H.M.; Kumar, R.; Bhogireddy, S.; Varshney, R.K.; HanumanthaRao, B.; Nair, R.M.; Prasad, P.V.V.; Nayyar, H. Drought and Heat-Stress Effects on Seed Filling in Food Crops: Impacts on Functional Biochemistry, Seed Yields, and Nutritional Quality. Front. Plant Sci. 2018, 9, 1705. [Google Scholar] [CrossRef]
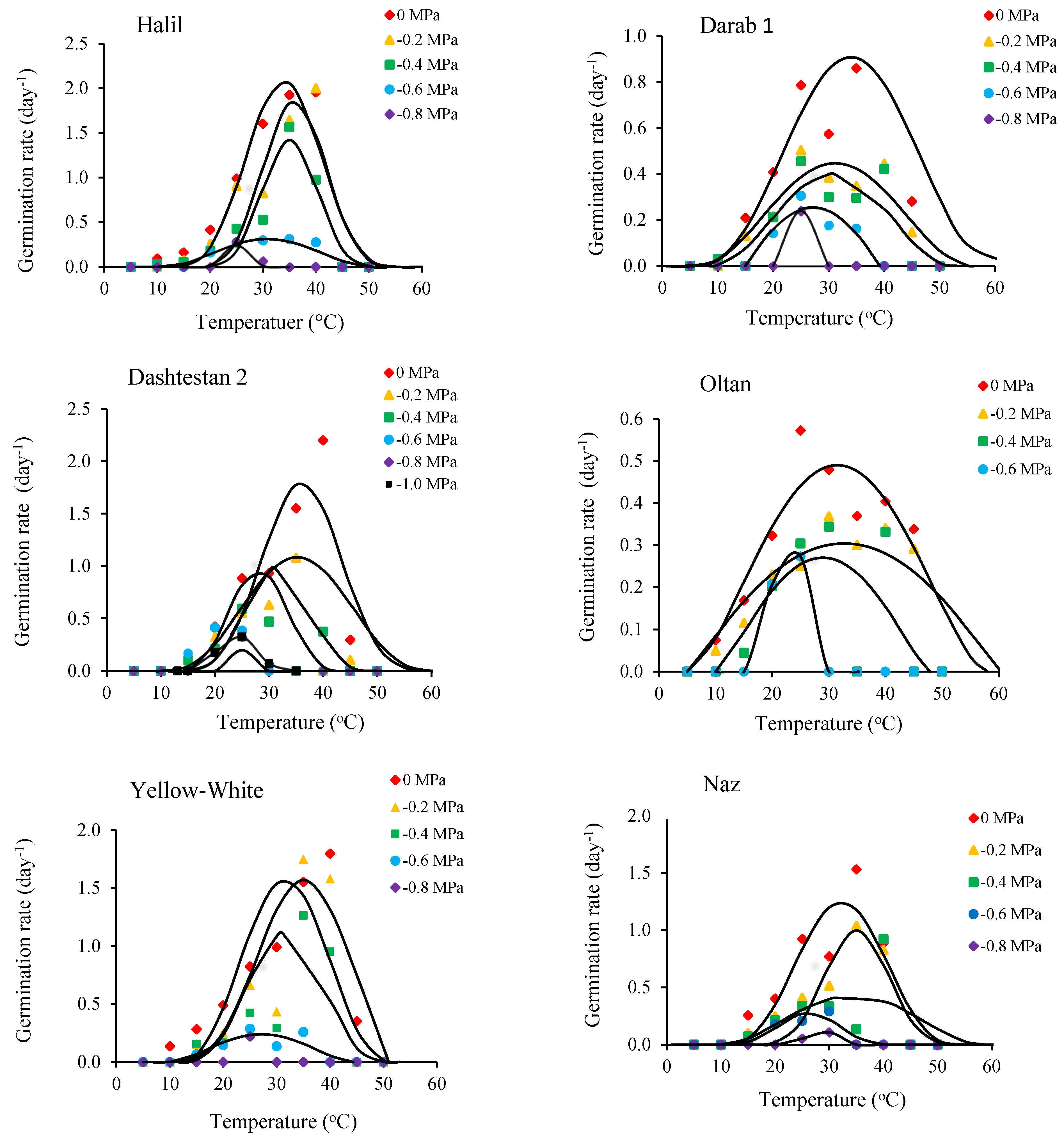


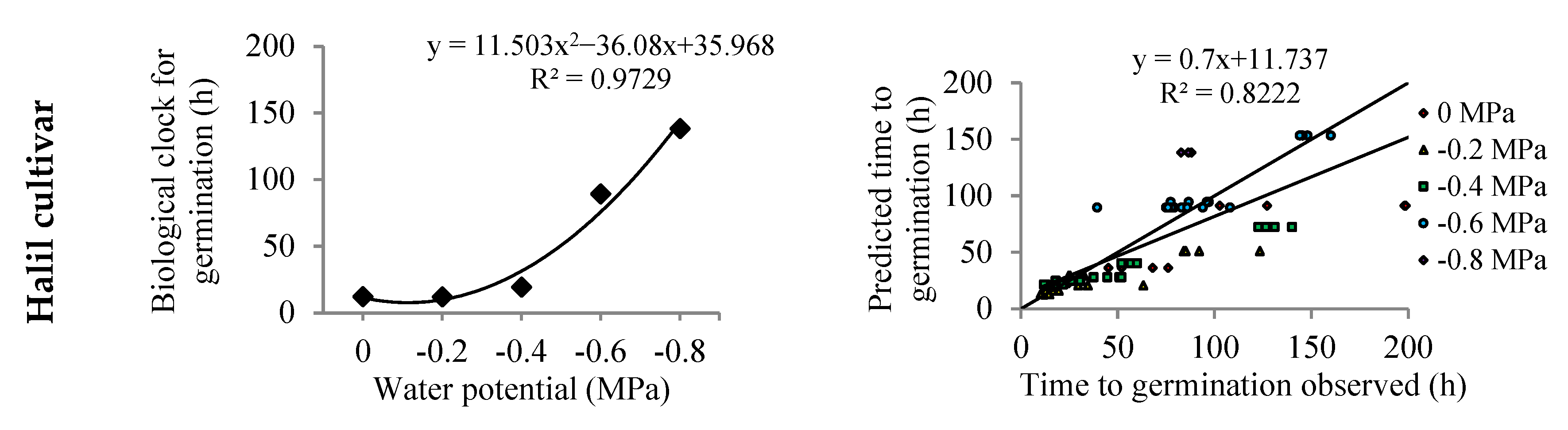
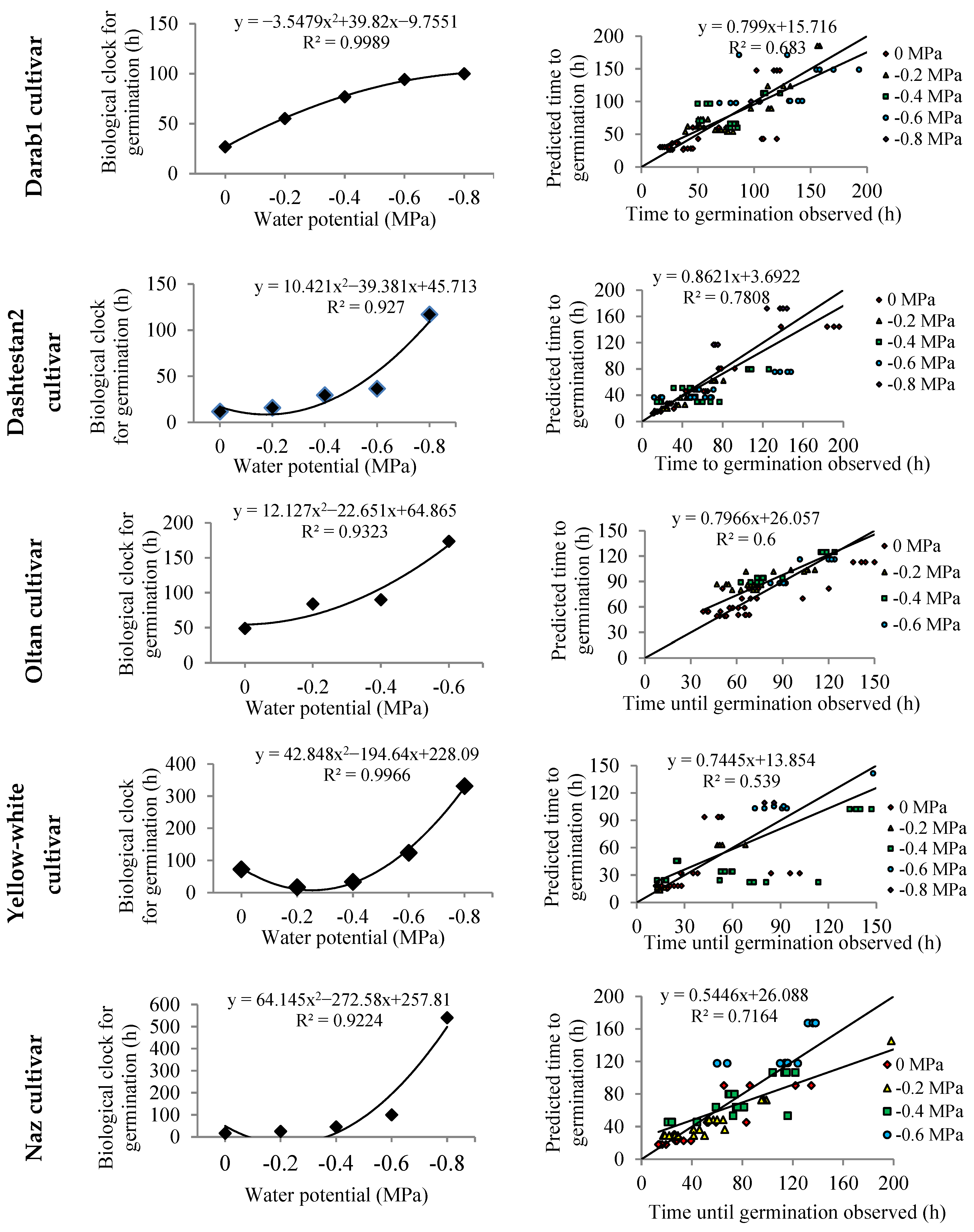
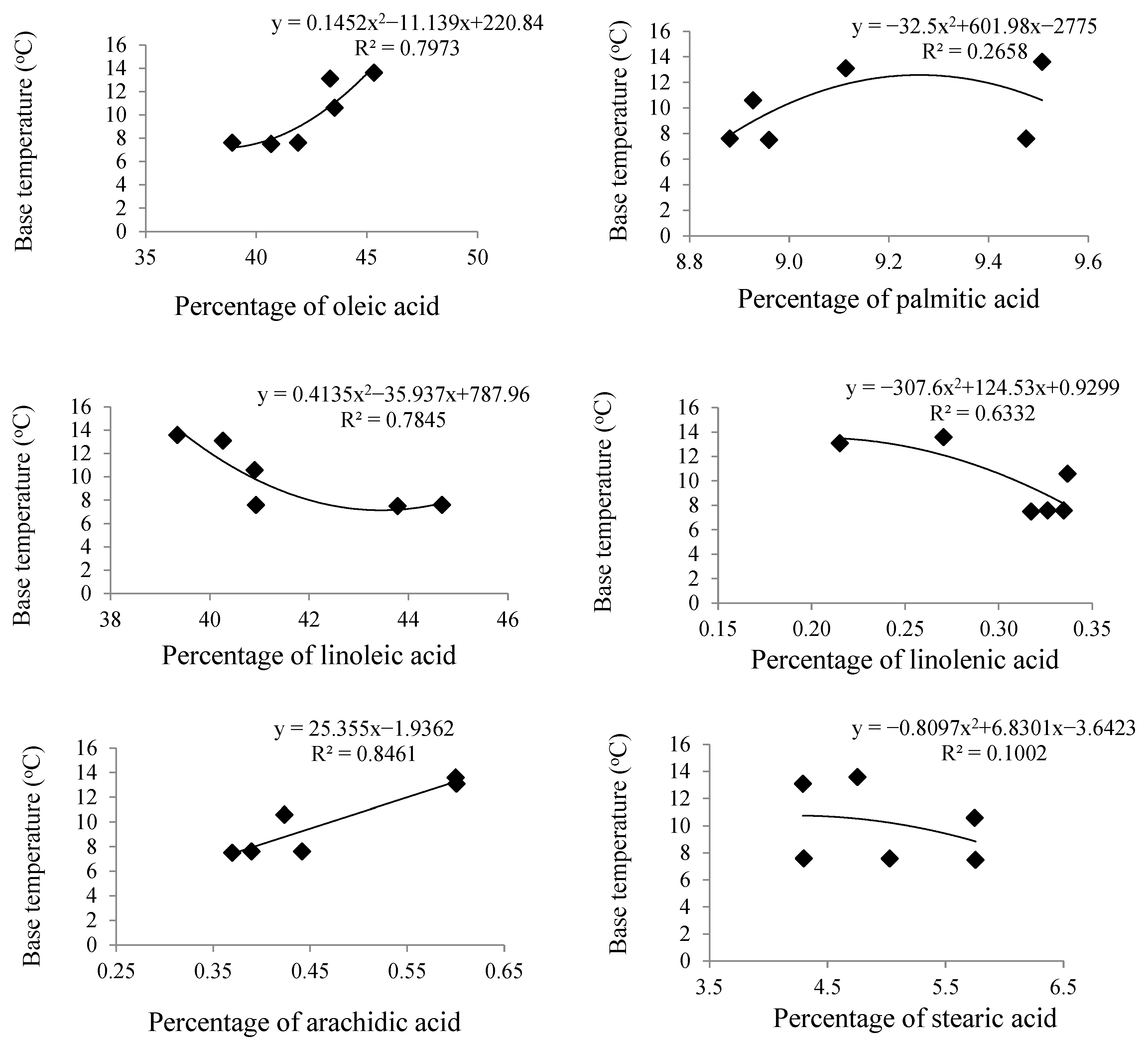
| Cultivars. | Halil | Darab1 | Dashtestan2 | Oltan | Yellow-White | Naz |
|---|---|---|---|---|---|---|
| Year of introduction | 2013 | 2009 | 2006 | 1999 | 2006 | 2001 |
| Branching | Branched | Branched | Branched | Branched | Branched | Single branch |
| Seed color | Brown | light brown | light brown | dark brown | Light cream | Cream |
| Production Year | 2017–2018 | 2017–2018 | 2017–2018 | 2017–2018 | 2017–2018 | 2017–2018 |
| Cultivars | Germination Percentages | Thousand Seed Weight (g) | Seed Moisture Content (%) | Oil Percentages |
|---|---|---|---|---|
| Halil | 99 | 3.8 | 6.07 | 58.25 |
| Darab1 | 98 | 3.12 | 5.15 | 54.77 |
| Dashtestan2 | 99 | 3.44 | 5.37 | 65.05 |
| Oltan | 98 | 3.25 | 4.87 | 57.54 |
| Yellow-white | 99 | 2.91 | 5.85 | 52.66 |
| Naz | 99 | 2.85 | 5.16 | 54.09 |
| Variety (Model) | Water Potential (MPa) | rc | RMSE | R2 | a | b | r |
|---|---|---|---|---|---|---|---|
| Halil (Dent-like) | 0 | 0.87 | 0.0067 | 0.96 | 0.0015 | 0.96 ** | 0.98 |
| −0.2 | 0.0112 | 0.86 | 0.0041 | 0.86 ** | 0.93 | ||
| −0.4 | 0.0102 | 0.74 | 0.0051 * | 0.74 ** | 0.86 | ||
| −0.6 | 0.0032 | 0.62 | 0.0025 ** | 0.61 ** | 0.78 | ||
| −0.8 | 0.0022 | 0.54 | 0.0003 | 0.55 ** | 0.73 | ||
| Darab1 (Beta) | 0 | 0.83 | 0.0080 | 0.62 | 0.0083 ** | 0.63 ** | 0.79 |
| −0.2 | 0.0032 | 0.71 | 0.0038 ** | 0.68 ** | 0.84 | ||
| −0.4 | 0.0033 | 0.68 | 0.0034 ** | 0.64 ** | 0.82 | ||
| −0.6 | 0.0022 | 0.67 | 0.0022 * | 0.67 ** | 0.82 | ||
| −0.8 | 0.0002 | 0.99 | 0.000007 | 1.01 ** | 1 | ||
| Dashtestan2 (Dent-like) | 0 | 0.88 | 0.0074 | 0.94 | 0.0021 | 0.94 ** | 0.97 |
| −0.2 | 0.0089 | 0.86 | 0.0039 | 0.84 ** | 0.93 | ||
| −0.4 | 0.0088 | 0.59 | 0.0069 ** | 0.58 ** | 0.77 | ||
| −0.6 | 0.0083 | 0.47 | 0.0079 ** | 0.41 ** | 0.69 | ||
| −0.8 | 0.0024 | 0.58 | 0.0011 ** | 0.55 ** | 0.76 | ||
| Oltan (Beta) | 0 | 0.79 | 0.0029 | 0.74 | 0.0039 ** | 0.72 ** | 0.86 |
| −0.2 | 0.0026 | 0.24 | 0.0076 ** | 0.22 ** | 0.49 | ||
| −0.4 | 0.0031 | 0.42 | 0.0039 ** | 0.40 ** | 0.65 | ||
| −0.6 | 0.0004 | 0.99 | 0.00002 | 0.99 ** | 1 | ||
| −0.8 | - | - | - | - | - | ||
| Yellow-white (Beta) | 0 | 0.73 | 0.0127 | 0.73 | 0.0033 | 0.83 ** | 0.86 |
| −0.2 | 0.0184 | 0.43 | 0.0157 ** | 0.57 ** | 0.66 | ||
| −0.4 | 0.0139 | 0.36 | 0.0104 ** | 0.52 ** | 0.60 | ||
| −0.6 | 0.0027 | 0.42 | 0.0033 ** | 0.40 ** | 0.65 | ||
| −0.8 | 0.0016 | 0.72 | 0.0003 | 0.72 ** | 0.85 | ||
| Naz (Segmented) | 0 | 0.75 | 0.0065 | 0.89 | 0.0039 * | 0.86 ** | 0.94 |
| −0.2 | 0.0061 | 0.80 | 0.0051 ** | 0.74 ** | 0.89 | ||
| −0.4 | 0.0052 | 0.53 | 0.0062 ** | 0.43 ** | 0.73 | ||
| −0.6 | 0.0022 | 0.64 | 0.0017 ** | 0.59 ** | 0.80 | ||
| −0.8 | 0.0006 | 0.12 | 0.0008 ** | 0.06 | 0.34 |
| Cultivar (Top Model) | Water Potential (MPa) | a | Tb (°C) | To1 (°C) | To2 (°C) | Tc (°C) | Rmax (day−1) | G0 (h) |
|---|---|---|---|---|---|---|---|---|
| Halil (Dent-like) | 0 | - | 11.7 | 36.1 | 40.0 | 45.0 | 1.94 | 12.3 |
| −0.2 | - | 13.2 | 40.0 | 42.1 | 45.0 | 1.99 | 12.0 | |
| −0.4 | - | 13.8 | 36.9 | 38.7 | 45.0 | 1.22 | 19.4 | |
| −0.6 | - | 12.0 | 25.7 | 40.0 | 45.0 | 0.17 | 89.3 | |
| −0.8 | - | 14.8 | 25.0 | 34.1 | 39.1 | 0.17 | 138.3 | |
| Average | - | - | 13.1 | 32.7 | 39.0 | 43.8 | 1.13 | 54.2 |
| Darab1 (Beta) | 0 | 2.91 | 4.0 | 31.8 | - | 64.0 | 0.89 | 26.9 |
| −0.2 | 1.87 | 6.0 | 28.4 | - | 56.3 | 0.43 | 55.2 | |
| −0.4 | 1.35 | 8.0 | 23.7 | - | 53.2 | 0.31 | 76.9 | |
| −0.6 | 1.00 | 14.9 | 26.6 | - | 39.3 | 0.26 | 94.3 | |
| −0.8 | 1.00 | 20.0 | 25.0 | - | 30.0 | 0.24 | 100.0 | |
| Average | - | - | 10.6 | 27.1 | - | 48.6 | 0.43 | 70.7 |
| Dashtestan2 (Dent-like) | 0 | - | 12.7 | 41.5 | 42.3 | 45.4 | 2.04 | 11.7 |
| −0.2 | - | 13.1 | 40.0 | 43.3 | 45.1 | 1.51 | 15.9 | |
| −0.4 | - | 11.1 | 35.0 | 40.0 | 45.0 | 0.82 | 29.6 | |
| −0.6 | - | 11.0 | 29.5 | 35.0 | 44.0 | 0.65 | 36.6 | |
| −0.8 | - | 12.4 | 23.6 | 25.0 | 40.8 | 0.19 | 117.1 | |
| Average | - | - | 12.0 | 33.9 | 37.1 | 44.1 | 1.06 | 42.2 |
| Oltan (Beta) | 0 | 1.76 | 5.0 | 32.0 | - | 58.0 | 0.48 | 49.0 |
| −0.2 | 1.83 | 5.0 | 39.1 | - | 60.4 | 0.29 | 84.0 | |
| −0.4 | 1.11 | 10.0 | 27.0 | - | 47.9 | 0.26 | 90.1 | |
| −0.6 | 71.10 | 9.9 | 25.4 | - | 35.2 | 0.14 | 173.6 | |
| Average | - | - | 7.5 | 30.9 | - | 50.4 | 0.29 | 99.1 |
| Yellow- white (Beta) | 0 | 17.37 | 5.0 | 49.0 | - | 65.0 | 0.34 | 72.5 |
| −0.2 | 4.90 | 10.0 | 35.0 | - | 53.2 | 1.44 | 16.7 | |
| −0.4 | 8.51 | 10.0 | 38.1 | - | 53.2 | 0.72 | 33.3 | |
| −0.6 | 3.60 | 10.0 | 32.9 | - | 44.3 | 0.19 | 123.9 | |
| −0.8 | 241.8 | 3.2 | 23.6 | - | 46.8 | 0.07 | 331.1 | |
| Average | - | - | 7.6 | 35.7 | - | 52.5 | 0.55 | 115.5 |
| Naz (Segmented) | 0 | - | 10.0 | 36.9 | - | 45.0 | 1.42 | 16.8 |
| −0.2 | - | 10.0 | 39.1 | - | 45.0 | 0.96 | 24.9 | |
| −0.4 | - | 5.0 | 40.0 | - | 44.8 | 0.53 | 45.7 | |
| −0.6 | - | 8.0 | 27.9 | - | 41.7 | 0.24 | 100.4 | |
| −0.8 | - | 5.0 | 30.0 | - | 43.9 | 0.05 | 540.5 | |
| Average | - | - | 7.6 | 34.8 | - | 44.1 | 0.65 | 145.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balouchi, H.; Soltani Khankahdani, V.; Moradi, A.; Gholamhoseini, M.; Piri, R.; Heydari, S.Z.; Dedicova, B. Seed Fatty Acid Changes Germination Response to Temperature and Water Potentials in Six Sesame (Sesamum indicum L.) Cultivars: Estimating the Cardinal Temperatures. Agriculture 2023, 13, 1936. https://doi.org/10.3390/agriculture13101936
Balouchi H, Soltani Khankahdani V, Moradi A, Gholamhoseini M, Piri R, Heydari SZ, Dedicova B. Seed Fatty Acid Changes Germination Response to Temperature and Water Potentials in Six Sesame (Sesamum indicum L.) Cultivars: Estimating the Cardinal Temperatures. Agriculture. 2023; 13(10):1936. https://doi.org/10.3390/agriculture13101936
Chicago/Turabian StyleBalouchi, Hamidreza, Vida Soltani Khankahdani, Ali Moradi, Majid Gholamhoseini, Ramin Piri, Seyedeh Zahra Heydari, and Beata Dedicova. 2023. "Seed Fatty Acid Changes Germination Response to Temperature and Water Potentials in Six Sesame (Sesamum indicum L.) Cultivars: Estimating the Cardinal Temperatures" Agriculture 13, no. 10: 1936. https://doi.org/10.3390/agriculture13101936
APA StyleBalouchi, H., Soltani Khankahdani, V., Moradi, A., Gholamhoseini, M., Piri, R., Heydari, S. Z., & Dedicova, B. (2023). Seed Fatty Acid Changes Germination Response to Temperature and Water Potentials in Six Sesame (Sesamum indicum L.) Cultivars: Estimating the Cardinal Temperatures. Agriculture, 13(10), 1936. https://doi.org/10.3390/agriculture13101936







